Brain
Methods of imaging the brain
1. Computed tomography (CT). This is the technique of choice for the investigation of serious head injury; for suspected intracranial haemorrhage, stroke, infection and other acute neurological emergencies. CT is quick, efficient and safer to use in the emergency situation than MRI.
2. Magnetic resonance imaging (MRI). This is the best and most versatile imaging modality for the brain, constrained only by availability, patient acceptability, and the logistics and safety of patient handling in emergency situations. New protocols and higher field strength magnets have raised the sensitivity of MRI in epilepsy imaging, acute stroke, aneurysm detection and follow-up post treatment of neoplastic and vascular disorders. It is the only effective way of diagnosing multiple sclerosis.
3. Angiography. This is very important in intracranial haemorrhage (ICH), especially subarachnoid haemorrhage (SAH) and, increasingly, in intra-arterial management of ischaemic stroke. However, with the widespread availability of multi-detector CT scanners, CT angiography (CTA) is now preferentially used in ischaemic stroke, SAH and ICH. Angiography is still requested for pre-operative assessment of tumours, vascular malformations and angiographic expertise is vital for the performance of many neurointerventional procedures.
4. Radionuclide imaging. There are two principal methods. The first is regional cerebral blood flow scanning, still more used in research than in clinical management, especially in the dementias and in movement disorders such as Parkinsonism; second is positron emission tomography (PET). By this method focal hyper-metabolism may be shown using 18F fluorodeoxyglucose (18FDG), for example in epilepsy, and cell turnover may be shown using 11C-methionine, for example in tumour studies.
5. Ultrasound (US). This is particularly helpful in neonates and during the first year of life to image haemorrhagic and ischaemic syndromes, developmental malformations, and hydrocephalus using the fontanelles as acoustic windows. In adults, transcranial Doppler may be used for intracerebral arterial velocity studies to assess the severity of vasospasm.
6. Plain films of the skull. These are of little value except in head injury.
Computed tomography of the brain
Indications
1. Following major head injury (if the patient has lost consciousness, has impaired consciousness, or has a neurological deficit). The presence of a skull fracture also justifies the use of CT. NICE (National Institute for Health and Care Excellence) guidance has been issued on the use of imaging for head injuries for adults and children, specifically CT, listing the criteria for assessment based on best relevant data and consensus recommendations.
2. In suspected intracranial infection (the use of contrast enhancement is recommended).
3. For suspected intracranial haemorrhage and cases of ischaemic and haemorrhagic stroke.
4. In suspected raised intracranial pressure, and as a precaution before lumbar puncture once certain criteria are fulfilled. These would include reduced consciousness (a Glasgow coma score of less than 15), definite papilloedema, focal neurological deficit, immune suppression and bleeding dyscrasias.
5. In other situations, such as epilepsy, migraine, suspected tumour, demyelination, dementia and psychosis, CT is a poorer-quality tool. If imaging can be justified, MRI is greatly preferable and is recommended by NICE in these situations except for the first episode of psychosis.
Technique
1. Most clinical indications are adequately covered by 3-mm sections parallel to the floor of the anterior cranial fossa, from the foramen magnum to the midbrain, with 7-mm sections to the vertex (or contigious 3-mm slices throughout). In all trauma cases, window width and level should be adjusted to examine bone and any haemorrhagic, space-occupying lesions. Review of all trauma studies should be done on brain windows, bone and ‘blood windows’ (i.e. W175 L75).
2. In suspected infection, tumours, vascular malformations and subacute infarctions, the sections should be repeated following intravenous (i.v.) contrast enhancement, if MR is not available. Standard precautions with regard to possible adverse reactions to contrast medium should be taken.
3. Dynamic studies using iodinated contrast are increasingly being used as a routine in high-velocity head trauma, the assessment of intracerebral bleeding in young patients, aneurysmal SAH, ruptured arteriovenous shunts and dural venous sinus thrombosis. CT angiography (CTA) on a typical 64-slice multidetector scanner is performed using 70–100 ml of contrast and 50 ml saline chaser, injected at 4 ml s−1 with a delay of 15 s or triggered by bolus tracking with ROI in the aortic arch. Overlapping slices of 0.75–1.25 mm are reconstructed. CT venography (CTV) involves injecting 90–100 ml of contrast with a delay of 40 s. Images are usually reviewed both as three-dimensional rendered data and multiplanar reformats (MPRs).
Magnetic resonance imaging of the brain
Technique
1. Long TR sequences. The whole brain can be examined with 4-mm sections with 1-mm interspaces using T2-weighted turbo spin echo imaging. Proton density sequences, long TR and short TE, are used mostly for the assessment of demyelination and intra-articular disc changes in the temporomandibular joints.
2. Short TR sequences. T1-weighted sequences are used for the demonstration of detailed anatomy but gadolinium chelate contrast agents are required to view pathology. Common practice is to obtain a sagittal or coronal T1-weighted sequence as part of a standard brain study. Volumetric sequences pre and post contrast are used for image guidance software interfaces for epilepsy imaging, insertion of deep brain stimulators for movement disorders and the removal of intra- and extra-axial tumours.
3. Gradient-echo T2-weighted sequences and susceptibility weighted imaging. Although suffering from various artifacts, the sensitivity of these sequences to susceptibility effects makes them very sensitive to the presence of blood products, as in cases of previous head injury, SAH and cavernomas. Haemosiderin produces marked focal loss of signal in such cases, and all patients with a history of head injury or other causes of haemorrhage should be imaged with this sequence. These sequences identify abnormal mineral deposition and can be used in deposition disorders.
4. FLAIR sequences (FLuid Attenuated Inversion Recovery) provide very good contrast resolution in the detection of demyelinating plaques and infarcts, and have the advantage that juxta-ventricular pathology contrasts with dark CSF, and is not lost by proximity to the intense brightness of the ventricular CSF, as in spin-echo T2-weighted studies. There are usually obtained in the sagittal or coronal plane.
5. Angiographic sequences. There are many methods, of which ‘time-of flight’ is one of the more commonly used. This is a very short TR, T1-weighted gradient echo three-dimensional sequence, with sequential presaturation of each partition so that only non-presaturated inflowing blood gives a high signal. Image display is by so-called ‘MIP’ or maximum intensity projection, giving a three-dimensional model of the intracranial vessels. As it uses the T1 properties, high signal from blood products in the subarachnoid space may reduce the sensitivity to aneurysms. Phase contrast MR angiography uses velocity encoding flow and is useful to detect flow in small and tortuous vessels. Contrast-enhanced MR angiography requires a pump injector and is less susceptible to flow artifacts. Timing of image acquisition is crucial and it is very useful in neck vessel imaging.
6. Echoplanar or diffusion weighted imaging (DWI). This sequence is becoming widely available on scanners. Most units perform DWI on all patients with suspected stroke, vasculitis, encephalitis, abscesses and in the workup of intracranial tumours. DWI examines the free movement, or Brownian motion, of water molecules at a cellular level. In acute infarcts cytotoxic oedema prevents free movement of water, whereas in tumours there is no restriction. All DWI should be reviewed together with conventional sequences and apparent diffusion coefficient (ADC) maps. Acute infarcts are hyperintense on DWI and hypointense on ADC. DWI is a useful technique in assessment of cholesteatoma both in detecting cholesteatoma if CT is equivocal and is ideal in evaluation of recurrent disease.
Imaging of intracranial haemorrhage
Haacke, EM, Mittal, S, Wu, Z, et al. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. Am J Neuroradiol. 2009; 30(1):19–30.
Mittal, S, Wu, Z, Neelavalli, J, et al. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. Am J Neuroradiol. 2009; 30(2):232–252.
Wada, R, Aviv, RI, Fox, AJ, et al. CT angiography ‘spot sign’ predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007; 38(4):1257–1262.
Westerlaan, HE, Gravendeel, J, Fiore, D, et al. Multislice CT angiography in the selection of patients with ruptured intracranial aneurysms suitable for clipping or coiling. Neuroradiology. 2007; 49(12):997–1007.
Imaging of gliomas
Jain, R. Perfusion CT imaging of brain tumours: an overview. Am J Neuroradiol. 2011; 32(9):1570–1577.
Al-Okaili, RN, Krejza, J, Wang, S, et al. Advanced MR imaging techniques in the diagnosis of intraaxial brain tumors in adults. RadioGraphics. 2006; 26:S173–S189.
Osborn, AG. Osborn’s Brain: Imaging, pathology, anatomy. Salt Lake City: Amirsys, Inc; 2012.
Stadlbauer, A, Gruber, S, Nimsky, C, et al. Preoperative grading of gliomas by using metabolite quantification with high-spatial-resolution proton MR spectroscopic imaging. Radiology. 2006; 238(3):958–969.
Radionuclide imaging of the brain
Regional cerebral blood flow imaging
Radiopharmaceuticals
1. 99mTc-hexamethylpropyleneamineoxime (HMPAO or exametazime), 500 MBq (5 mSv ED). The most commonly used agent, HMPAO is a lipophilic complex that crosses the blood–brain barrier and localizes roughly in proportion to cerebral blood flow. It is rapidly extracted by the brain, reaching a peak of 5–6% of injected activity within a minute or so, with minimal redistribution (about 86% remains in the brain at 24 h).
2. 99mTc-ethyl cysteinate dimer (ECD), 500 MBq (5 mSv ED). This localizes rapidly in proportion to cellular metabolism rather than blood flow, and the distribution has some differences to that of HMPAO, which may need to be taken into consideration for clinical diagnosis.1,2 It currently has the advantage of greater stability than HMPAO and can be used for up to 6 h after reconstitution, which is of particular benefit for ictal epilepsy studies where an injection is only given once a seizure occurs.
Additional techniques
1. If blood flow in the carotid and major cerebral arteries is of interest, a dynamic study during injection is performed.
2. Three-dimensional mapping of activity distributions onto standard brain atlases and image registration with MRI, CT and quantitative analysis are areas of increasing interest, particularly with the development of SPECT-CT scanners.
Positron emission tomography
Technique
1. 18FDG -up to the UK limit of 400 MBq i.v. (10 mSv ED) is administered.
2. To reduce muscle uptake of 18FDG, patients should remain in a relaxed environment such as lying in a darkened room, without talking if head and neck area are being imaged, between injection and scan.
3. Image at 1 h post injection. Later imaging has been reported to enhance tumour conspicuity due to a higher tumour to background ratio.
201Thallium brain scanning
References
1. Asenbaum, S, Brücke, T, Pirker, W, et al. Imaging of cerebral blood flow with technetium-99m-HMPAO and technetium-99m-ECD: a comparison. J Nucl Med. 1998; 39(4):613–618.
2. Koyama, M, Kawashima, R, Ito, H, et al. SPECT imaging of normal subjects with technetium-99m-HMPAO and technetium-99m-ECD. J Nucl Med. 1997; 38(4):587–592.
Ultrasound of the infant brain
Equipment
4–7-MHz, sector transducer. A 7.5- or 10-MHz transducer to visualize superficial structures.
Coronal
1. The orbital roofs and cribriform plate (these combine to produce a ‘steer’s head’ appearance); anterior interhemispheric fissure; frontal lobes
2. Greater wings, lesser wings and body of sphenoid (these produce a ‘mask’ appearance); the cingulate sulcus; frontal horns of lateral ventricles
3. Pentagon view: five-sided star formed by the internal carotid, middle cerebral and anterior cerebral arteries
4. Frontal horns of lateral ventricles; cavum septum pellucidum; basal ganglia. Bilateral C-shaped echoes from the parahippocampal gyri; thalami
5. Trigones of lateral ventricles containing choroid plexus; echogenic inverted V from the tentorium cerebelli; cerebellum
6. Occipital horns of lateral ventricles; parietal and occipital cortex; cerebellum.
Cerebral angiography
Contraindications
1. Patients with unstable neurology (usually following subarachnoid haemorrhage or stroke)
2. Patients unsuitable for surgery
3. Patients in whom vascular access would be impossible or relatively risky
4. Iodine allergy. Relative contraindication as angiography could be performed with gadolinium chelates.
Technique
Catheter flushing solutions should consist of heparinized saline (2500 IU l−1 normal saline). Using standard percutaneous catheter introduction techniques, the femoral artery is catheterized. It is a common practice to deliver an access sheath through which catheters are introduced and exchanged if necessary. There is a wide range of catheters available and there are proponents of many types. In patients up to middle age without major hypertension, there will be little difficulty with any standard catheter and a simple 4F polythene catheter with a slightly curved tip or 45° bend will suffice in the majority. Older patients and those with atherosclerotic disease may need catheters offering greater torque control such as the JB2 or Simmons (Figs 13.1, 13.2) as appropriate. Catheter control will be better if passed through an introducer set, and this is also indicated where it is anticipated that catheter exchange may be required. Selective studies of the common carotids and the vertebrals are preferable to super-selective studies of the internal and external carotids unless absolutely necessary. The following points should be noted:
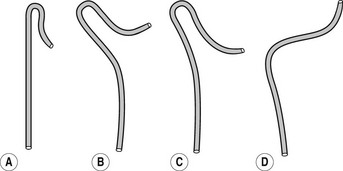
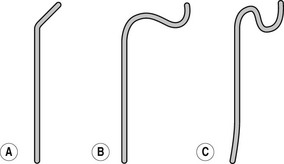
Figure 13.2 Simmons (sidewinder) catheters. (a) For narrow aorta. (b) For moderately narrow aorta. (c) For wide aorta. (d) For elongated aorta. End holes: 1 Side holes: 0.
1. The hazards of cerebral angiography are largely avoidable; they consist of the complications common to all forms of angiography (see Chapter 9) and those which are particularly related to cerebral angiography.
2. Any on-table ischaemic event has an explanation. Cerebral angiography does not possess an inherently unavoidable complication rate as has been suggested in the past. If an ischaemic event occurs there has been a complication, and its cause must be identified.
3. The most significant complications are embolic in origin, and the most important emboli are particulate. Air bubbles should be avoided as part of good angiographic practice, but are unlikely to cause a severe neurological complication.
4. Emboli may come from the injected solutions. Avoid contamination from glove powder, and dried blood or clot on gloves. Take care to avoid blood contamination of the saline or of the contrast medium, which is always dangerous. Avoid exposure of solutions to air. Contrast medium or heparin/saline in an open bowl is bad practice.
5. Emboli may arise from dislodgement of plaque or thrombus. Never pass a catheter or guidewire through a vessel that has not been visualized by preliminary injection of contrast medium. Use appropriate angled, hydrophilic guidewires. Do not try to negotiate excessively acute bends in vessels. The splinting effect of the catheter will cause spasm and arrest the flow in the artery. Dissections could occur which may lead to occlusion. Newer catheter designs tend to be softer and allow further distal access for more complex procedures. Sharp curves can otherwise be safely negotiated with microcatheters.
6. Emboli may arise from the formation of thrombus within a catheterized vessel. Always ensure that there is free blood flow past the catheter, and avoid forceful passage of a guidewire or catheter in such a way as may damage the intima of the vessel and cause thrombus formation.
7. Emboli may arise within the catheter. Do not allow blood to flow back into the catheter, or if it does occur then flush regularly or by continuous infusion. Never allow a guidewire to remain within a catheter for more than 1 min without withdrawal and flushing, and never introduce a guidewire into a contrast-filled catheter, but fill the catheter with heparinized saline first.
8. Keep study time to a minimum, but not at the expense of the diagnostic usefulness of the study.