Chapter 145 Brachytherapy for Choroidal Melanoma
Treatment of choroidal melanoma with brachytherapy – suturing a radiation source to the eye – was first reported by Moore in 1930.1 These early studies by Moore with radon seeds paved the way for the use of other forms of radiation at clinical centers around the world, including cobalt-60,2–5 ruthenium-106,6–8 gold-198,9 iodine-125,10–18 and palladium-103.19–21 Iodine-125 is currently the most commonly used isotope in brachytherapy for choroidal melanoma in the USA, whereas ruthenium-106 and palladium-103 continue to be popular at some centers. Brachytherapy offers an alternative to enucleation in the treatment of choroidal melanoma, allowing globe-salvaging with the possibility of maintaining useful vision. The Collaborative Ocular Melanoma Study (COMS) was a prospective, randomized study that provided clinicians with statistically sound evidence supporting the use of radiation in the treatment of choroidal melanoma. The study investigated more than 1300 patients with medium-sized melanoma and randomized to iodine-125 brachytherapy or enucleation.22 After 12 years of follow-up, there was no statistically significant difference between these two modalities of treatment with regard to mortality.22,23 Although treatment protocols and selection of tumors for plaque treatment must be individualized and differ among clinical centers, generally acceptable indications for plaque brachytherapy include: (1) selected small choroidal melanomas exhibiting growth or malignant transformation; (2) medium-sized choroidal and ciliary body melanomas in eyes with visual potential; (3) large melanomas with dimensions up to 16 mm in diameter and 8–10 mm in thickness; (4) larger melanomas, especially in monocular patients. However, despite success in sparing enucleation, radiation has profound effects on the surrounding retina and optic nerve, with vision limited by the resulting radiation retinopathy and optic neuropathy.24–28 These complications can have profound effects on quality of life with 209 patients from the COMS reporting no significant difference in visual function 3–5 years post-treatment for plaque brachytherapy versus enucleation.29 Continued research and investigation are needed to identify efficacious supplemental therapies for these universally common radiation-related complications.
Dosimetry
The optimal tumoricidal radiation dose for uveal melanoma remains unclear,30,31 but doses range between 50 and 100 Gy, with doses less than 50 Gy being associated with significant treatment failures.30 Kindy-Degnan et al.32 used helium ion radiation and reported on tumor apex dosages ranging between 50 and 80 Gy, showing that regardless of whether 50, 60, 70, or 80 Gy was used, there were no differences in tumor regression, survival, complications, or visual outcomes. Initially, the COMS decided to deliver 100 Gy to the tumor apex with a delivery rate of 50–125 cGy/h (tumors <5 mm in height are managed as if they were 5 mm thick).33 However, in 1996, based on newer dosimetric measurements and the recommendations of the American Association of Physicists in Medicine (AAPM), the COMS modified the prescription dose to deliver 85 Gy to the tumor apex with a delivery rate of 43–105 cGy/hour.34 Based on these modifications, with the AAPM-recommended calculation and revised prescription dose, the actual dose delivered to the tumor is the same. As a result, when analyzing studies prior to 1986 that utilized iodine-125, the actual dose to the tumor is 85% of the stated dose. Furthermore, the American Brachytherapy Society has presented the COMS dose recommendation as the guideline for the treatment of choroidal melanoma.35 As more accurate estimates for some of the assumptions used and better calculation algorithms have become available, dosimetry has been further refined and recalculated incorporating anisotropy, line source approximation, and Silastic and gold shield attenuation. Tables of dose to the tumor apex and various other ocular structures have been presented for the various size plaques.36 On average, the dose to the apex of the tumor appears to be 0.89 of the 85 Gy that has been assumed using the AAPM formalism.36 The physics of brachytherapy has been reviewed in several publications.36–38
Isotope selection
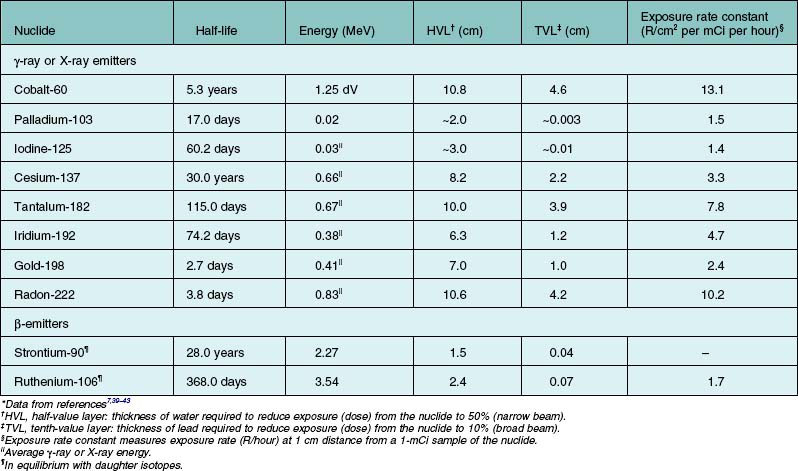
Brachytherapy is the application of radiation from isotopes over very short distances in contact with a surface. The radiation is distributed over a short distance from a surface or within the target tissue. Table 145.1 presents physical characteristics for the commonly used isotopes in brachytherapy. The table breaks isotopes into two broad categories: (1) those that emit γ-rays or X-rays; (2) those that emit β-particles (electrons). Table 145.1 footnotes explain some of the simplifications. The half-value (HVL) in water for a narrow beam is useful in comparing isotopes for the penetration of their radiations in tissue. The tenth-value layer (TVL) in lead for a broad beam is useful in evaluating penetration in shielding materials.
The energy of γ-rays or X-rays relates to their penetration in matter in a complex way. The more energetic rays penetrate to a greater extent and are more difficult to shield; these two factors are dealt with in the last two columns of Table 145.1.
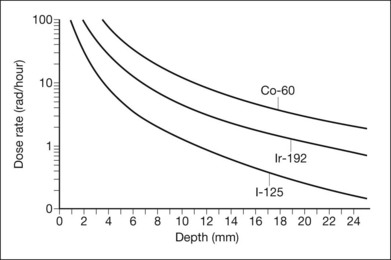
The HVL in water is an index of the extent to which the energy of the radiation is absorbed in water (or tissue). At short distances, important for application in localized tumors of the eye, the inverse-square law largely governs the radiation penetration in tissue. Figure 145.1 displays the depth–dose curves for iodine-125, iridium-192, and cobalt-60. Because of the small height (up to approximately 1 cm) of the tumors treated, this inverse-square law is far more important in determining scleral (or choroidal blood vessel) dose for a given dose to the tumor apex. The dose rate falls more rapidly near the source than further away. Thus, the scleral dose relative to the dose at the apex of the tumor may be reduced by simply introducing a space between the source and the sclera. In the orbit, only size constraints limit the magnitude of the space, with 1 mm being the maximum in plaques used for ocular brachytherapy. With greater distances and less energetic rays, tissue absorption begins to play a more important role. For the β-emitters, absorption in tissues, which is complex and related to the energy of the β-particle, must be measured.
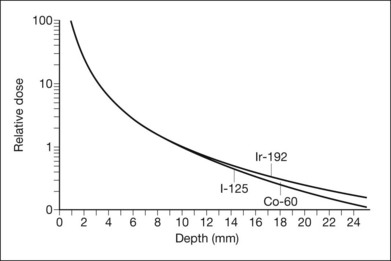
The exposure rate constant is an expression of dose rate at a standard distance from a specified amount of isotope. More atoms of an isotope with a low constant can achieve the same dose rate as one with a higher constant, the other characteristics being equal. The curves of dose rate versus depth in tissue for different isotopes can nearly be superimposed by adjusting the amount of each isotope in inverse ratio to the ratio of the exposure rate constants (Fig. 145.2).44
Beta-emitters, such as ruthenium-106, have been shown to be successful in tumor control for small to medium tumors, potentially up to 16 mm in diameter and up to 8 mm in thickness when used alone with calculated apex doses, or combined with transpupillary thermotherapy (TTT) when apex doses are insufficient.45 The presumed advantages of these lower-dose radiation sources are a decrease in the potential risk to adjacent structures as well as decrease the risk of radiation retinopathy.46 However, studies have not shown a reduction in treatment-related visual decline,45 as doses are thought to still be above those maximally tolerated by the retina, vasculature, and optic nerve.
Plaque design
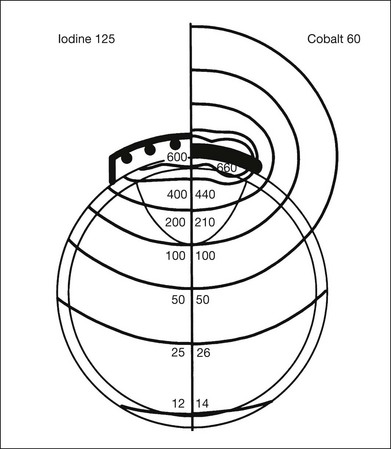
The iodine-125 plaque design used in the COMS evolved from that used by Rotman et al.47 and Robertson et al.,18 and consists of a gold plaque that is approximately 0.4 mm thick with a lip around its perimeter that resembles a smooth bottle cap (Fig. 145.3).44 The shielding device decreases lateral spread of radiation and results in a collimated beam of radiation. Plaques may be customized with notches or indentations of the gold rim to allow placement adjacent to the optic nerve sheath for juxtapapillary tumors. Notched plaques attempt to minimize the likelihood of plaque tilting secondary to placement next to the optic nerve sheath, as this tilt may result in excess radiation to the optic nerve, while decreasing the dose to the tumor apex. Other unique plaque designs and concepts include slotted plaques,48 which may aid in the treatment of juxtapapillary tumors, including circumpapillary tumors, which prove difficult to treat. Within the gold plaque is a soft, pliable plastic (Silastic) seed carrier insert with evenly spaced troughs that accept the iodine-125 seeds. The carrier is designed so that the seeds are adjacent to the gold. The thickness of the seed carrier separates the seeds from the sclera surface by 1 mm. The prepared loops facilitate anchoring of the plaque to the sclera with sutures. Eyelet placement proves important to minimize the potential for plaque tilt, with those with circumferential eyelets posing decreased likelihood of tilting. The relative dose distribution associated with a particular radioactive plaque is basically determined by the arrangement (distribution) of the seeds in the plaque. The dose distribution from seeds within a plaque, such as shown in Figure 145.3, is demonstrated by the schematic in Figure 145.4. By distributing iodine seeds over the area of the plaque and having a 1 mm spacer between the seeds and the sclera, the scleral dose delivered by an iodine-125 plaque is approximately the same as that delivered by a cobalt-60 plaque. Monte Carlo calculations by Chiu-Tsao et al.49 demonstrate a lower scleral dose for iodine-125 compared to cobalt-60, for a point source model.
Indications for treatment
Medium tumors
The Collaborative Ocular Melanoma Study (COMS), a prospective, randomized study funded by the National Eye Institute, compared survival in patients with medium-sized tumors randomized to brachytherapy or enucleation.33 The rationale for this study was outlined by Robertson in 1989.50 Results of the COMS medium-size tumor trial became available in 2001.22 Medium tumors were defined as tumors with a thickness between 2.5 and 10 mm, as well as a maximal basal diameter <16 mm. Stringent inclusion and exclusion criteria were used, including patient-related factors, such as a history of any prior cancer, comorbidities that may influence survival, and immunosuppressive therapy, as well as tumor characteristics such as juxtapapillary tumors (tumors within 1 mm of the optic nerve), diffuse (ring) melanomas, and melanomas with a primary ciliary body location. With 1317 patients enrolled and randomized between enucleation and iodine-125 brachytherapy in the medium-size COMS study, mortality rates were not statistically different up to 12 years after treatment. The trial had >80% power to conclude that neither treatment altered the mortality by as much as 25% from each other. The COMS study group refined survival rates to include 12-year data.23 Overall, cumulative all-cause mortality at 12 years for patients treated with either enucleation or plaque radiotherapy was 41% and 43%, respectively. For melanoma-specific mortality, or death confirmed histopathologically with melanoma metastasis, rates for iodine-125 were 10%, 18%, and 21% at 5, 10, and 12 years, respectively. For patients that received primary enucleation, melanoma-specific death rates were 11%, 17%, and 17%. These most recently updated statistics emphasize that even at longer follow-up, there were no statistically significant differences in survival between iodine-125 plaque brachytherapy and enucleation.
Long-term studies utilizing plaques other than iodine-125 have demonstrated success in the treatment of choroidal melanoma. For palladium-103, Finger et al.51 reported on 400 patients treated with plaque brachytherapy and a mean follow-up of 51 months. Metastatic rates were found to be 6%, with estimated 5- and 10-year risks of metastasis of 7.3% and 13.4%, respectively. For ruthenium-106 plaques, Verschueren et al.45 reported on 425 patients treated with plaque brachytherapy, with and without TTT, and a median follow-up of 50 months. Failure of local tumor control was 3.9%, with estimated metastasis rates of 24.5% and 30.9% at 5 and 10 years, respectively, and overall survival of 79.6% and 68.2%. Investigators utilizing ruthenium-106 plaques have shown that tumors >5 mm had increased risk of local recurrence.52 As a result, for tumors >5 mm or those tumors with insufficient doses to the tumor apex, adjuvant TTT is suggested;45,52 therefore, tumors up to 8 mm in thickness and 16 mm in diameter may potentially be treated with ruthenium-106.45
The extended COMS report that included 12-year mortality rates also assessed prognostic factors for metastasis and death, reporting a correlation with advanced age at baseline, as well as larger maximum basal tumor diameter. Shields et al.53 conducted an in-depth analysis of tumor size and risk for metastasis, reporting on the millimeter-by-millimeter risk for metastasis. In a series of 8033 patients with ciliochoroidal melanoma, tumor size was significantly associated with rates of metastasis. For small melanomas, rates of metastasis were 6%, 12%, and 20% at 5, 10, and 20 years, respectively. These rates were shown to increase based on tumor thickness, with large tumors metastasizing in 25%, 49%, and 67% of patients at 5, 10, and 20 years. Of note, advanced baseline age and tumor size were confirmed as risk factors for metastatic disease, as well as ciliary body location and clinical findings such as a brown tumor, subretinal fluid, and extraocular extension.
Small tumors
Curtin et al.54 reported on the observation of small choroidal melanomas in an era of enucleation as the gold standard. In a series of 46 patients observed over 14 years, melanoma-specific mortality was 6.5%, with no patients that were observed dying of melanoma-related causes. Other authors have reported on similar rates of melanoma-related mortalities for tumors that received either prompt treatment or delayed treatment.55,56 The COMS classified small melanomas as tumors with an apical height of 1–3 mm, and a maximal basal diameter between 5 and 16 mm.57,58 The COMS included 204 patients that did not fit the inclusion criteria for the medium-sized tumor trial. Upon study enrollment, 16 patients (8%) had treatment, while 67 patients (33%) required treatment at follow-up. Estimates of the need for treatment of small melanomas were 21%, 33%, and 38% at 2, 5, and 7 years, respectively. Overall, there were 27 deaths, with six melanoma-related. Five- and 8-year melanoma-specific mortality was 1% and 3.7%, respectively. Importantly, the study identified risk factors that were found to be predictive of tumor growth, including initial tumor thickness, the presence of orange pigment, the absence of drusen, and the absence of RPE changes surrounding the tumor (absence of halo). Other risk factors for the malignant transformation of small melanomas include subretinal fluid, patient symptoms, margins close to the optic nerve, and ultrasound hollowness.53,59
The COMS small tumor trial was not designed with mortality as a primary outcome, and initially was designed to be the framework for a larger, randomized trial to determine significant differences between observation, with deferred treatment upon documented growth compared with immediate treatment of small melanomas. Singh et al.60 reported on the proposed inclusion criteria for the aforementioned trial. Based on subgroup analysis from the COMS with identification of risk factors for tumor growth, the authors determined criteria to be: (1) apical height between 1 and 3 mm, and maximum basal diameter between 5 and 16 mm; (2) presence of any of three risk factors, including basal diameter >12 and <16 mm; tumor thickness between 2 and 3 mm, and the presence of orange pigment. To date, a randomized, controlled trial investigating these treatment strategies for small melanomas has not been undertaken. A more recent series by Sobrin et al.,61 reported on 154 patients with small choroidal melanomas who were observed for signs of malignant transformation (tumor growth or presence of orange pigment). A total of 45 patients (29%) who were observed needed treatment, with a mean interval to treatment of 4.1 years. All patients with observed transformation were treated with iodine-125 plaque brachytherapy utilizing intraoperative ultrasound for plaque localization. Of those treated, 4.5% developed metastasis, with only one death during the follow-up period. Additionally, one patient (2%) had local tumor recurrence necessitating enucleation. Notably, for those patients with small melanomas that continued to be observed for a mean of 8.1 years, no patient developed metastasis or died secondary to melanoma. Vision at baseline was 20/25, with final visual acuities of 20/30 for patients who were observed, and 20/50 at 2 years for patients that were treated with plaque brachytherapy. Radiation retinopathy and optic neuropathy were observed in 56% and 31% of patients, respectively.
Finally, transpupillary thermotherapy (TTT) has been proposed for the treatment of small choroidal melanomas that have a tumor height <4 mm. Numerous authors have reported on TTT in these lesions, with rates of tumor recurrence ranging from 8% to 56%, without standard inclusion criteria.39,62–69 A systematic review of the literature by Singh et al.70 reported a mean local tumor recurrence rate of 17% including 7% with extrascleral extension at a mean follow-up of 37 months. Although TTT may cause less visual morbidity in the treatment of small choroidal melanomas, the sole use of this therapy has the potential for high recurrence rates, which have been shown to be associated with an increased risk of metastasis.71 As a result, the sole use of TTT in the treatment of small choroidal melanomas should be carefully considered.
Large tumors
With the COMS establishing the widespread use of plaque brachytherapy for medium-sized choroidal melanoma, most eyes are salvaged, but not without severe visual morbidity secondary to the effects of radiation on the eye. The maximum tumor size that can be effectively treated with radiation without causing severe radiation complications is not known with certainty. Gragoudas72 suggested that eyes harboring tumors that involve up to 30% of the eye can usually be treated and salvaged after radiation. Char et al.73 reported information that suggested an upper size limit for tumors treated with radiation. Nearly 50% of the eyes with tumor thickness >10 mm eventually required enucleations after treatment with helium ions. Castro et al.74 noted that the enucleation rate for complications from helium ions increased as the tumor thickness increased beyond 6 mm. Munzenrider et al.75 reported that the enucleation rate following proton beam irradiation was significantly higher if the tumor thickness was >8 mm and/or if the tumor basal diameter was >16 mm. The COMS reported that enucleations were more common for tumors with greater thicknesses and greater base dimensions, although percentages were not given.71 If one adheres to the guidelines suggested by the COMS that radioactive plaques should extend 2 mm beyond the tumor margin, the maximum basal dimension of a tumor treatable with brachytherapy is approximately 21 mm, since for practical purposes it becomes technically difficult to place an episcleral plaque of more than 25 mm in diameter on the eye surface.
By COMS definition, large melanomas are tumors that are >10 mm in thickness, or >2 mm in thickness with basal diameter >16 mm. The COMS investigated the use of radiation pre-enucleation versus primary enucleation for large choroidal melanomas. At 10 years, survival rates did not show statistically significant differences, with melanoma-specific death rates of 40% for enucleation, whereas rates were 45% for patients that received radiation prior to enucleation.76 Although the COMS only looked at brachytherapy for medium-sized tumors, several studies have investigated the use of brachytherapy for large melanomas as an alternative to enucleation.77–79 Wilson et al. reported on 124 patients with large tumors of the ciliary body and choroid treated with iodine-125 brachytherapy and concluded that tumor thickness >8 mm in height or 16 mm in maximal basal diameter can be effectively treated with a favorable expectation of globe salvage, but limited vision conservation. Puusaari et al.78 came to a similar conclusion based on 96 eyes with large choroidal melanomas meeting one of three possible criteria: (1) largest basal diameter >16 mm provided that thickness was >2 mm; (2) height >10 mm regardless of basal diameter; (3) peripapillary tumor >8 mm in height and located <2 mm from the optic disc. The authors found that they had a fair chance of globe conservation with acceptable cosmesis, as well as a reasonable chance of conserving useful vision for 1–2 years. Shields et al.79 reported on 354 large choroidal melanomas (>8 mm in thickness) that were treated with plaque brachytherapy. Failure in local tumor control was estimated in 9% and 13% of tumors at 5 and 10 years, respectively. Metastasis of melanoma was estimated in 30% and 55% at 5 and 10 years, respectively. Notably, estimates of poor visual acuity (20/200 or worse) were 97% at 15 years.
Ruthenium-106 has also been used to treat large choroidal melanomas. Despite the finding that ruthenium-106 was a risk factor for local tumor recurrence,79 Kaiserman et al.80 reported on 63 large tumors treated with ruthenium-106. With a mean follow-up of 69.6 months and mean tumor thickness of 9.29 mm, 23.8% failed local tumor control. Estimated rates of metastasis were 22.5% and 48.1% at 5 and 10 years, respectively, while melanoma-specific mortality was found to be 20.5% and 46.2%. Interestingly, 70.8% of patients maintained vision better than 20/200. Other investigators suggest the use of adjuvant TTT in cases of tumors treated with ruthenium-106.81,82 Large melanomas present a unique situation, and were not included in the COMS for treatment with plaque brachytherapy. Investigators have shown that large tumors can be effectively treated with brachytherapy, but visual morbidity is high secondary to the large amounts of radiation to obtain effective tumor control.
Juxtapapillary tumors
Notched plaques are designed with an indentation in the plaque rim to allow flush placement adjacent to the optic nerve sheath. In addition, novel designs incorporating a slot in the plaque may allow for the successful treatment of juxtapapillary, and even circumpapillary tumors.48 In 1994, De Potter et al.83 reported on 127 patients with juxtapapillary melanomas treated with either enucleation or plaque brachytherapy. Despite being a non-randomized, retrospective study with a small percentage of patients treated with plaque brachytherapy (28%), the authors found that the treatment modality did not affect risk for metastasis. The same group also reported success using iodine-125 notched plaques in patients with juxtapapillary tumors overhanging the disc with tumor recurrence rates of 10%, a rate of metastasis of 13%, and 3% mortality.84 As expected for radiation treatment in close proximity to the optic nerve and fovea, 77% of patients had a visual acuity of 20/200 or less. Sagoo et al.85 also reported on the use of notched iodine-125 plaques in the treatment of circumpapillary tumors (tumors encircling the optic nerve). Recurrence rates were shown to be 14%, while the metastatic rate was 4% with no melanoma-specific deaths observed with a mean of 52 months follow-up. Preservation of visual acuity was poor, with greater than 60% manifesting with vision less than 20/200. Emphasizing the importance of notched plaques with placement confirmation by intraoperative ultrasound, Hui et al.86 reported on control rates of 100% at 30 months using notched plaques, compared to 89% at 25 months for non-notched plaques. Despite the promising results of notched plaque brachytherapy for juxtapapillary choroidal melanomas, studies with long-term follow-up are lacking. However, a recently published study87 reported on 650 juxtapapillary tumors treated with notched plaque brachytherapy with or without TTT with a mean follow-up of 52 months. Overall incidence of tumor recurrence was 11%, while metastasis was 10% with a melanoma-specific death rate of 3%. Kaplan–Meier estimates of tumor recurrence at 10 years were shown to be 21%, with metastasis and death of 24% and 9%, respectively.
In addition to the use of notched plaques with placement aided by intraoperative ultrasound, some have advocated the use of TTT as an adjunctive treatment for juxtapapillary tumors that have historically had higher rates of tumor recurrence. In the prior study by Sagoo et al.,87 TTT was utilized as an adjunctive treatment in 56% of eyes. Overall, tumor recurrences with TTT were 9% (compared to 14% for no TTT), metastasis was 9% (compared to 10%), with a death rate of 2% (compared to 5%). However, differences in recurrences and metastasis were not statistically significant when TTT was used as an adjuvant treatment in juxtapapillary tumors.
In conclusion, juxtapapillary tumors may be treated with plaque brachytherapy, with notched plaques and intraoperative ultrasound contributing to successful treatment. Proton beam irradiation (PBI) has also been shown to be efficacious in the treatment of peripapillary and parapapillary melanomas,88 as compared to notched plaques for juxtapapillary tumors.89 Additionally, TTT may be used as an adjunctive therapy with uncertain benefits. However, as a result of the close proximity to the macula and optic nerve, rates of visual compromise are high, with the majority of patients being legally blind.
Plaque placement technique
With advancements in ophthalmic photography, including wide-angle photography and digital software measurements, tumors are more often captured in toto as montage images. Tumor dimensions can then be determined by approximating the basal tumor diameter with regards to disc diameters or use of calipers, with the horizontal diameter of the optic nerve approximated at 1.5 mm. Wide-angle photography has been shown to be more accurate than ultrasound in measuring the basal diameter, as it potentially captures those margins or pigmentation that are not determined on ultrasound secondary to lack of elevation.90,91 Using this estimate, basal tumor diameter can be determined, with ultrasound (quantitative A-scan) aiding in the measurement of the tumor thickness and presence or absence of extraocular extension. For tumors that are unable to be captured completely by photographs, tumors can be measured using a technique first described by Hilton,92 where a grid is placed over the 20-D lens with each square representing 1 disc diameter. The tumor is viewed monocularly through the grid, allowing an approximate calculation of the basal diameter. The pre-surgical tumor estimates are important to pre-select plaque sizes to ensure that the plaque covers the entire tumor base along with 2 mm or more of tumor-free perimeter. For example, a tumor with a base diameter of 12 mm is treated with a 16 mm in diameter plaque.
The choice of anesthesia is left to the discretion of the surgeon. After an adequate peritomy, the surface of the sclera corresponding to the tumor is exposed. If extraocular extension is observed within a pseudoencapsulated focus ≤2 mm in diameter, the plaque is placed over the tumor base and the extension. Because an extraocular extension of <2 mm may not worsen the prognosis for survival,93 proceeding with brachytherapy as intended is reasonable. If extraocular extension is in excess of that noted, enucleation with tenonectomy must be considered. As an alternative, an iodine-125 plaque without a gold shield could be placed over the extension to allow radiation of orbital tissues adjacent to the visible extension. Ultrasound frequently detects extensions >2 mm,94 with extensions of this size occurring infrequently.93 As a result, discovery of large extraocular extensions (>2 mm) at surgery should rarely occur. For example, there have been virtually no such instances for globes enucleated in COMS.
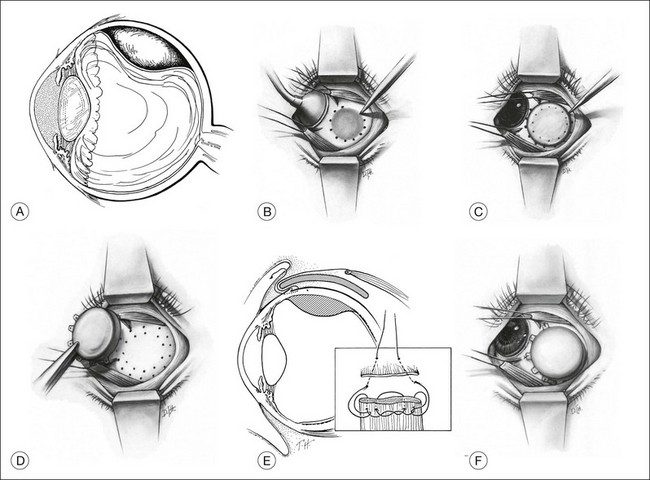
At surgery, the tumor base is localized with standard techniques used for retinal breaks or transillumination (or both). Sometimes the shadow of the tumor can be clearly outlined on the sclera while the globe is transilluminated through the cornea (Fig. 145.5A). The perimeter of the tumor base should be verified with indirect ophthalmoscopy. The MIRA diathermy-transillumination unit may assist in outlining the tumor base (MIRA 1 electrode handle with fiberoptic bundle and coaxial cable with scleral transilluminator). The intense light of the angled fiberoptic transilluminator allows the operator to visualize the tumor with indirect ophthalmoscopy while simultaneously visualizing the transsclerochoroidal retinal illumination from the fiberoptic light tip as it is moved to localize the perimeter of the tumor. Indirect ophthalmoscopy with scleral depression can also aid in identifying tumor margins. With the fiberoptic light at the perimeter of the tumor, a low-intensity diathermy mark can be made on the sclera. This mark can be made darker with a surgical marking pen (Fig. 145.5B). The tumor diameter can be measured and recorded using calipers, followed by estimation of 2 mm perimeter beyond the tumor borders. A transparent acrylic dummy plaque with a diameter equal to that of the therapeutic plaque is used to facilitate localization and later placement of the opaque therapeutic plaque. The dummy plaque is placed on the sclera, covering the scleral marks that identify the tumor perimeter. The dummy plaque must completely cover the base of the tumor as well as a tumor-free perimeter of 2 mm or more (Fig. 145.5C). After the perimeter of the dummy plaque has been marked on the sclera with pencil or diathermy, it is removed, and the opaque therapeutic plaque is placed within this ring of scleral marks and anchored with two or three intrascleral sutures (Fig. 145.5D,E). If the therapeutic plaque is the same size or smaller than the boundary of the tumor base as marked on the sclera, the procedure is terminated, and a larger plaque is prepared and placed on the eye later. If the plaque diameter is 3 mm or more greater than the diameter of the tumor base, it is sutured in place.
The location of the radioactive plaque can be confirmed with a fiberoptic light source, using a technique similar to that described by Robertson et al.95 The MIRA transillumination diathermy probe combined with indirect ophthalmoscopy can be used to provide transscleral transillumination around the boundary of the plaque. As the probe moves along the plaque, the light can be visualized in the choroid and its location relative to the tumor base can be readily seen. For anteriorly located tumors, transscleral illumination around the plaque boundary is ordinarily satisfactory, as standard B-scan ultrasonography proves difficult in discerning anterior segment structures. However, newer technologies such as ultrasound biomicroscopy (UBM) and anterior segment optical coherence tomography may open new avenues for anterior segment image acquisition.96 Confirmation of the relationship of the tumor and plaque position can be obtained with B-scan ultrasonograpy.97–99 Harbour et al.97 showed that standard techniques for localizing plaque placement were suboptimal in 21% (14% posterior, 7% juxtapapillary) of patients, primarily in tumors located posteriorly and juxtapapillary. Intraoperative ultrasound allowed for identification of malpositioned plaques, affording the opportunity to reposition the plaque while still in the operating room. However, despite successful repositioning, Almony et al.100 reported on plaque tilting at time of plaque removal. The authors found that whereas only 9% of patients had plaque tilt >1 mm at insertion, approximately 53% of plaques had tilt >1 mm at removal. Plaque tilt was also shown to be associated with a reduction in radiation doses to the tumor apex. Interestingly, three patients were found to have local tumor failure and were associated with plaque tilt of >2 mm at removal. As observed in prior studies, posteriorly located plaques, as well as juxtapapillary tumors necessitating notched plaques were more likely to have significant tilt at removal. Of note, episcleral hematoma was also identified as a risk factor for plaque tilt and often associated with disinsertions of rectus muscles. As a result, intraoperative ultrasound should be commonly used in combination with other localization techniques to ensure sufficient plaque placement and identify suboptimal or tilted plaques to allow for immediate repositioning. Although no long-term studies have been reported, intraoperative ultrasound may minimize the risk of local treatment failures.
A notable complication associated with plaque manipulation is hemorrhage in and around the tumor. Several investigators have reported on this complication,2,17 along with the possibility of vitreous hemorrhage. These instances may be aided with the use of intraoperative ultrasound to assist plaque placement secondary to media opacities and inability to utilize standard techniques.
Tumors with margins close to the optic nerve present a unique situation regarding plaque placement. Although the diameter of the intrascleral portion of the optic nerve measures 1.5 mm, the diameter of the optic nerve sheath upon exiting the eye increases to 3–4 mm. As a result, for tumors that are within 2 mm from the optic nerve, applying a plaque with 2 mm tumor-free margins proves a difficult task. A standard round plaque may be situated with the posterior aspect placed against the optic nerve beyond the posterior edge of the tumor. However, plaques abutting the optic nerve have a high incidence of plaque tilt, resulting in decreased apical tumor dose with an associated risk of tumor failure, as well as an increased radiation dose to the optic nerve with risk of optic neuropathy.97,100 These findings have been demonstrated by Williams et al.101 using MRI scans of plaques placed in a juxtapapillary position in cadavers. An alternative approach involves the design of plaques with an indentation to fit around the optic nerve. Additionally, the rim of the plaque may be removed to allow lateral radiation to provide treatment to those margins that are unable to be covered completely by the plaque. However, this latter option exposes the optic nerve to a higher dose of radiation with increased likelihood of significant visual morbidity.
Rectus muscles may be detached to facilitate plaque placement and minimize pressure on the globe, with understanding that these procedures pose the risk of hematomas and associated plaque tilt which may increase the likelihood of local failure. These findings suggest the use of intraoperative ultrasound following plaque placement and at removal to identify plaques that may have tilted significantly and placing the patient at risk for local tumor control failure.100 After placement of the plaque, the rectus muscle may be sutured to the original insertion or anchored to the side of the plaque or anterior to the plaque. An adjustable suture placed at the time of plaque placement may be preferable if the muscle must remain detached during the brachytherapy period, as this will facilitate reattachment of the muscle at the time of plaque removal. Muscles are usually engaged with a double-armed absorbable suture if it must be detached at the time of plaque placement. The ends of the double-armed suture are passed intrasclerally 1.5 mm behind the insertion and then through the conjunctiva, where they are anchored in the cul-de-sac so that they can be retrieved at the time of plaque removal. Alternatively, the ends of the double-armed suture can be loosely tied and placed under the conjunctiva for later access when the plaque is removed. The muscle is allowed to retract and remain detached until the time of plaque removal (Fig. 145.5F).
Close evaluation of the tissue area of plaque placement must be emphasized, as any episcleral tissue around the optic nerve sheath, inferior oblique muscle, and posterior ciliary vessels and nerves may create added distance of the plaque away from the tumor.102–106 These observations must be relayed to the radiation oncologist, as it may warrant recalculation of radiation doses or durations. On completion of plaque placement, the conjunctiva is closed over the plaque with sutures, although risk for complications is low for anteriorly located tumors where the conjunctiva is not able to be closed completely over the plaque or conjunctiva that retracts postoperatively.
Radiation plaques are kept in place for 4–5 days, with patients often staying as inpatients during the course of treatment. With conclusion of plaque treatment, plaque removal is usually performed with local anesthesia. There may be some utility to the use of ultrasound prior to plaque removal to identify those plaques that may have sustained significant tilt.100 Once identified, discourse between the treating surgeon and the radiation oncologist is important to determine the possible need for a longer course of treatment or whether adjuvant TTT is warranted. Following the removal of the plaque, muscles that have been transected are reattached by approximating the sides of the muscle to near the original insertion, allowing the central portion to drape posteriorly (in general, a slight recession of the muscle approximately 1.5 mm may compensate for the tendency of the shortened reattached muscle to cause overaction). For preplaced sutures, the muscle is drawn up to the site of the intrascleral suture passage and secured.
Postoperative observations
Serial follow-up examinations are conducted between 6 weeks and 3 months and usually at 6-month intervals thereafter. Documentation with wide-angle photography to measure tumor basal dimensions is necessary to enable early detection of tumor growth. Standardized A-scan and B-scan echography is obtained to determine tumor thickness. Occasionally, tumors exhibit swelling on the first postoperative visit following plaque removal, although tumor shrinkage may also be observed. In general, tumor shrinkage does not occur until 6–9 months after treatment, and some tumors may never demonstrate a decrease in size. Echographic changes following plaque brachytherapy include diminished tumor height and increased tissue reflectivity within the tumor. Additionally, a hypoechoic space corresponding to the site of prior plaque placement is frequently observed. This hypoechoic space may be exaggerated in cases of fungal granulomas, an unusual complication that has been reported following ruthenium-106 plaque treatment.107
The ophthalmologist must also be vigilant during follow-up visits to identify early indicators of adverse effects of plaque brachytherapy, namely radiation retinopathy (maculopathy and optic neuropathy). These effects can present with subtle findings on optical coherence tomography (OCT) prior to clinical signs of radiation complications. Horgan et al.108 report on the incidence of OCT changes in 70% of 135 patients with a mean time to onset of 12 months. Current treatment of radiation complications has not proven successful once an eye manifests with signs of radiation changes. Future treatment strategies will need to focus on early identification of the radiation effects, followed by targeted treatment in an effort to maintain visual acuity.
Local tumor response
Most choroidal melanomas treated with brachytherapy show evidence of shrinkage, with almost half of all tumors decreasing 50% from the pretreatment thickness. Despite the shrinkage seen in medium and large tumors, they will rarely regress to a flat scar. Shrinkage may not be observed at first, but usually progresses over 2 or more years, then remain stable thereafter. With tumor regression, echographic findings change concurrently, showing an increase in the internal reflectivity along with a decrease in tumor vascularity. Choroidal melanomas that have broken through Bruch’s membrane to form a collar-button configuration have a distinct regression pattern.109 The body of these tumors tends to shrink while the collar-button portion becomes more prominent and darker, and may shed pigmented debris into the vitreous cavity. Histopathologically, this debris usually consists of pigment-laden macrophages, melanoma cells, or a combination of both.
With the exception of the first postoperative visit where tumors may swell and increase in size, choroidal melanomas following plaque brachytherapy should be observed at regular intervals for any signs of growth. If the tumor thickness increases by 300 µm, or if any of the tumor borders advance by 250 µm, the tumor is highly suspected of expansion. These tumors should be observed more frequently and if continued growth, 300 µm increase in thickness or 250 µm increase of any border, is observed, plaque brachytherapy is considered a failure and further treatment is recommended. Although several investigators have reported successful retreatment with brachytherapy leading to local tumor control,8,12,110 definitive enucleation should be recommended. Tumors that recur following plaque brachytherapy may have a greater likelihood of metastasis; therefore, maximizing primary treatment response is imperative.
Recurrences
Failure to control tumor growth after plaque brachytherapy has been shown to vary from 1.7% to 16%,12,103,111–113 depending on the institution, isotope, and the length of follow-up. Wilson and Hungerford114 observed a recurrence rate of only 4% with iodine-125, compared to 5% with PBI and 11% with ruthenium-106. In addition, Fontanesi et al.115 reported a recurrence rate of 2.3% for 144 patients treated with iodine-125. The COMS enucleated 10% of eyes secondary to suspected or documented tumor recurrence.71 More recently, a recurrence rate of 1.7% was reported for 117 patients with choroidal melanoma treated with iodine-125 plaque brachytherapy.113 The study emphasized the importance of confirming proper plaque placement using intraoperative ultrasound at the time of surgery. Data from other plaques, ruthenium-106 and palladium-103, have also demonstrated low rates of local tumor failure at 3.9%45 and 3%,51 respectively.
Efforts to reduce the rate of recurrences following plaque brachytherapy prove exceedingly important, as tumors with local recurrences, despite undergoing enucleation, have an increased risk of metastasis. Gragoudas et al.72 reported a risk ratio of 2.44 among patients treated with proton beam irradiation (PBI). Vrabec et al.112 reported nearly three times greater estimated 5-year mortality (42% versus 13%) among patients treated with cobalt-60. Harbour et al.116 reported a 5-year actuarial rate of local recurrence of 10%, with metastasis occurring in 19% of patients with horizontal (marginal) recurrences (relative risk 2.2), and increasing to 49% if the recurrence was vertical and diffuse (relative risk of 5.1). In the COMS, tumors with local recurrence had a calculated risk ratio of 1.5% for metastasis.
Histopathologic findings in eyes treated with brachytherapy indicate that physical necrosis and tumor shrinkage are not uniformly observed. MacFaul and Morgan117 reviewed the histologic changes in 27 eyes treated with cobalt-60 and found clear evidence of radiation effect in all eyes, but tissue necrosis was not evident in 12 eyes. Necrosis was a predominant feature in only two eyes. Char et al.4 observed significant necrosis in only one of four eyes, whereas Rotman et al.47 reported no recognizable viable tumor cells in two of three eyes enucleated after treatment. Saornil et al.118 reported on the histopathologic features of 22 eyes that underwent enucleation after treatment with iodine-125 plaque brachytherapy. The authors found that tumor necrosis was extremely variable with 50% of eyes not showing evidence of necrosis, and only 14% of eyes showing necrosis in 50% or more of the tumor. Additional findings within the tumors were varying degrees of inflammation, fibrosis, tumor blood vessel damage, and increased number of balloon cells. Changes in the surrounding retina were characterized by atrophy, hyperplasia, and fibrous metaplasia of the retinal pigment epithelium, retinal gliosis, protein, lipid and blood deposits within the retina, and retinal blood vessel abnormalities characterized by thickening, thrombosis, and neovascularizations. These changes are similar to those seen after proton beam irradiation.118 Finally, Avery et al.119 reported on the histopathologic findings in 75 eyes from the COMS that were enucleated after plaque brachytherapy and compared with eyes that received treatment with primary enucleation. Tumors treated with iodine-125 showed significantly less mitotic activity, marked inflammation, invasion of the retina and scleral extension, as well as vitreous hemorrhage. Additionally, 55% of eyes showed effects on the retinal vasculature consistent with changes seen with radiation retinopathy. Finally, only 60% of eyes showed evidence of tumor growth or extrascleral extension in tumors enucleated secondary to presumed failure of local control.
Visual results
Early reports by Stallard5 in 1968 appeared to indicate a satisfactory 5-year survival rate among 100 patients treated with cobalt-60; however, other early studies identified severe ocular complications that were disturbingly prevalent. MacFaul40 reported that among 78 patients alive at 17 years post-radiation with cobalt-60, 35% of eyes treated required enucleation secondary to tumor growth or radiation complications. For those eyes that were not severe enough to necessitate enucleation, 35% had vision that was severely affected (hand movement or less), while only 39% had a visual acuity of 6/18 or better. These early studies also observed that exudative retinopathy sometimes did not appear until 7 years after treatment, and microvascular abnormalities were occasionally delayed 15 years.5 Additional studies with cobalt-60 showed evidence of radiation retinopathy, optic neuropathy, cataracts, and failure of local tumor control.3,4,24,41 Complications following radiation were commonly seen after 2 years, but may present earlier.
The complications of radiation maculopathy and optic neuropathy are dependent on the total amount of radiation delivered to the macula and optic nerve, respectively. The resultant dose received by these structures depends on the tumor size and location, with increased risk in tumors with increased height and basal diameter, and located in close proximity to the fovea and optic nerve. In addition to dose-related effects, radiation complications are also associated with the size of the fractions for fractionated doses, increasing in severity for larger fractions. Other factors, such as concomitant use of chemotherapeutic agents and diabetes mellitus, may lower the threshold for developing radiation damage. Biologic variability also likely serves as another factor. Histopathologic studies have shown selective loss of endothelial cells that line blood vessels, resulting in capillary drop-out, microaneurysms, and other changes similar to those seen with diabetic retinopathy. Patients may present with macular edema, microaneurysms, telangiectatic vessels, capillary non-perfusion as seen on fluorescein angiography, neovascularization, or vitreous hemorrhages.42,43
Visual prognosis has been shown to be less favorable when foveal or optic nerve radiation is in excess of 5000 Gy.27 Brown et al.24,25 indicated that the lowest doses of radiation generally causing macular retinopathy were 45 Gy from cobalt-60 plaques and 36 Gy from external beam radiation. Optic neuropathy was observed to occur from doses as low as 35 Gy from cobalt-60 and 36 Gy from external beam radiation. The same group27 reported severe visual loss from radiation doses to the fovea as low as 21, 23, and 30 Gy. These cases showed visual loss from nonproliferative radiation retinopathy or exudative retinal detachments.
In 1976, Sealy et al.120 first reported the use of iodine-125 in the management of melanoma. The isotope was chosen because of its advantageous physical characteristics, which allow the radiation to be almost completely shielded by a thin sheet (0.4 mm) of lead or gold. Packer et al.14 reported preliminary evidence on the use of iodine-125, followed by animal studies reported by Robertson et al.17,18 Studies by Garretson et al.11 found that visual acuity of 26 patients treated with iodine-125 remained within 2 Snellen lines in 54% of patients. Of those that developed radiation changes, mean duration to onset was approximately 32 months.
Although the proponents of iodine-125 had hoped that the lower energy emission, combined with greater space between source and sclera and lateral shielding, would delay or lower the incidence of radiation complications, long-term results have been disappointing, with almost 50% of eyes treated with brachytherapy being legally blind at 5 years. For patients treated with iodine-125 and with at least 3 years median follow-up, rates for radiation maculopathy range between 13–52%,106,121–125 while rates of optic neuropathy range between 0–46%.11,106,110,115,121–126 The upper range for radiation complications was shown in large tumors as based on COMS criteria.125 Krohn et al.121 found that patients with radiation maculopathy had a median dose of 49 Gy to the macula, while Stack et al.122 found that patients who received >90 Gy to the macula had a 63% risk of maculopathy. Although radiation is known to significantly affect the retina, a recent report by Boldt et al.127 reported that 49.2% of patients with choroidal melanomas had abnormalities in the posterior pole prior to plaque treatment, with rates increasing to >90% at 5 and 8 years post-treatment. Proliferative radiation retinopathy was found to occur in 5.2% of patients at 5 years, while optic neuropathy had an incidence of 27.4%. These observations suggest that the inflammatory and angiogenic factors of tumors may contribute to retinopathy prior to additive treatment effects of plaque brachytherapy. The visual outcome in eyes treated with iodine-125 is similar to that reported for other forms of radiation therapy (helium ion, 53% 20/200 or less,128 PBI 42% 20/200 or less129) suggesting that research should continue to be directed toward ways to reduce or treat radiation complications. The COMS estimated that nearly 50% of patients treated with iodine-125 would lose substantial vision by 3 years (loss of ≥6 lines of vision), with 43% having visual acuity less than 20/200.22 Other recent studies with at least 3 years follow-up report between 42–74% of patients treated with plaque brachytherapy lose ≥2 lines of vision.121–123,130 The COMS presented quality of life data for 209 patients treated with either enucleation or brachytherapy.29 At 2 years, patients treated with brachytherapy reported peripheral vision and vision for driving that was significantly better than patients who were treated with enucleation. However, following 3–5 years, these findings were not significantly different. This timing corresponds to the visual decline observed for brachytherapy-treated eyes secondary to complications of radiation retinopathy.
Studies with different isotopes are unlikely to be fruitful in decreasing radiation complications, but research continues in this field. Although the physical characteristics of certain isotopes may allow better shielding with a greater differential radiation dose between tumor apex and sclera, the γ-rays, X-rays, and β-particles must still obey physical laws. Besides iodine-125, two of the most commonly used isotopes for plaque brachytherapy are palladium-103 and ruthenium-106. Ruthenium-106 is a β-emitter and first reported to be efficacious in 11 melanomas by Lommatzsch in 1974.131 A later series of 309 patients treated with ruthenium-106 with a mean follow-up of 6.7 years, showed efficacy of treatment in 70% of eyes, with 23% retaining vision 6/12 or better.132 In a smaller subset of patients with juxtapapillary melanomas treated with ruthenium-106, Lommatzsch et al. showed that the probability of developing complete radiation optic neuropathy was 23% and 53% at 5 and 10 years, respectively. Additionally, the probability of retaining 20/40 vision was 38% and 26% at 5 and 10 years. Verschueren et al.45 reported on 425 patients with small to medium-sized choroidal melanomas treated with ruthenium-106. The authors found that rates of radiation complications (maculopathy, retinopathy, and optic neuropathy) were 40% and 65% at 2 and 5 years, respectively. In addition, almost 38% had a decrease in vision to legal blindness, or <0.10.
For palladium-103, the Palladium-103 for Choroidal Melanoma Study Group51 reported on 400 patients treated with plaque brachytherapy. With regards to visual acuity, 79% and 69% of patients had vision 20/200 or better at 5 and 10 years, respectively. Finger et al.133 also correlated the incidence of radiation maculopathy with tumor location, tumor height, and radiation doses in 384 patients treated with palladium-103. Tumors in an anterior location were much less likely to manifest with maculopathy versus tumors with a posterior location (7% compared with 41%). Additionally, with radiation doses to the fovea <35 Gy, doses between 35–70 Gy had a risk of 1.74, while those >70 Gy had a risk of 2.43. This study compares similarly with the incidence of maculopathy following iodine-125. Palladium-103 emits lower-energy photons than iodine-125, resulting in radiation that is more readily absorbed by the tumor and less absorbed by tissues in close proximity to the plaque.134–136 Disadvantages of palladium-103 compared with iodine-125 include an increased scleral dose directly beneath the plaque (a disadvantage in treating tumors at or very near the fovea), a relatively short half-live (17 versus 60 days), and less available information on the dosimetry of palladium-103.
Despite use of lower-energy isotopes and modern plaque design with shielding to minimize radiation exposure to vital structures, radiation-related complications are commonly encountered, necessitating further research into their treatment and prevention. Although studies have shown that radiation retinopathy occurs over longer periods of time, 70% of patients treated with iodine-125 showed signs of retinopathy on OCT upon 2 years follow-up, with a mean onset of 12 months. Notably, only 38% of patients had clinical signs of radiation retinopathy and median visual acuity at onset of 20/40.108 These earliest effects manifest as macular edema secondary to the leakage from capillaries with poor endothelial support. With the advent of optical coherence tomography, earlier identification of radiation complications can be attained.
Vascular endothelial growth factor (VEGF) has been shown to be elevated in eyes with choroidal melanoma, with the highest levels being found in those treated with radiation.137,138 VEGF is a potent vascular permeability factor that may contribute to macular edema and has been suggested to play a role in radiation maculopathy.108 Additional cytokines may also factor into the retinal response to radiation toxicity. Although current studies using bevacizumab, ranibizumab, and triamcinolone have not shown dramatic effects in the sustained treatment of radiation maculopathy,139–143 there is some evidence that early identification followed by prompt treatment with anti-VEGF agents may decrease the progression of radiation retinopathy; therefore, stabilizing visual acuity. Horgan et al.144 reported on the use of periocular triamcinolone in 108 patients at the time of plaque placement followed by repeat dosing at 4 and 8 months, finding that at 18 months, there was a significant decrease in the risk of macular edema and the risk of moderate to severe vision loss was decreased from 48% to 31%. Side-effects included increased intraocular pressure as well as progression of cataracts. Further studies with longer follow-up are needed to assess the effect of VEGF inhibitors, corticosteroids, or combination of the two on maculopathy, with focus on the early identification with OCT and early treatment prior to significant visual decline.
Laser photocoagulation has also been investigated in the treatment of radiation retinopathy. As with the treatment of clinically significant macular edema (CSME) from diabetes, focal laser therapy of 19 patients was shown to have an initial effect with improvement in vision and CME at 6 months; however, at 12 and 24 months, there was no significant difference between treated and untreated eyes.145 In another study with a mean follow-up of 39 months, 12 patients treated with focal laser for CME resulted in 67% with improvement in visual acuity and 50% with resolution in CME.146 With a mean follow-up of 109 months, 38 patients with CSME were treated with focal laser therapy, revealing that although both treated and untreated groups had a decrease in visual acuity post-plaque treatment, those treated with laser showed a better final vision.147
In an alternative strategy using laser photocoagulation, Finger and Kurli treated 45 patients that developed radiation retinopathy following plaque brachytherapy with sector laser photocoagulation on the surface of the tumor and 2–3 mm of the margins.148 With a mean follow-up of 48 months, approximately 65% had regression of radiation retinopathy and 47% had regression of maculopathy. However, almost 47% had loss of ≥3 lines of vision. Interestingly, when 16 tumors were treated prior to the development of radiation retinopathy or maculopathy, only three patients developed radiation complications which regressed with additional laser. Of note, compared with the group that received treatment after development of retinopathy, no (0) patients treated with prophylactic laser lost >3 lines of vision. Laser photocoagulation may have a role in the treatment and prevention of radiation retinopathy, and this study emphasizes the importance of early identification and treatment of radiation complications. Larger studies with additional patients are needed to determine the efficacy of laser treatment for radiation retinopathy.
A novel approach to the prevention of radiation complications has been investigated by Oliver et al.149 on cadavers. The authors showed that using silicone oil, perfluorocarbons, and other vitreous substitutes, the effective radiation dose could be attenuated, potentially minimizing the effects to the vital eye structures. Although these are only proof of concept studies on cadavers, vitreous attenuators warrant further studies on the potential for protective effects.
Adjuvant therapy
To increase the number of neoplastic cells made nonviable within a choroidal melanoma during treatment and to reduce unwanted complications of radiation, some investigators have suggested the use of adjuvant therapies, including hyperthermia, chemotherapy, and antiangiogenic agents. Transpupillary thermotherapy (TTT) uses a diode laser and near-infrared irradiation. TTT may induce tumor cell necrosis up to 3 mm in depth, and has been investigated as primary treatment for small melanomas;62 however, 22% had failure of local tumor control at 3 years, with evidence of extraocular extension in some cases.150 As a result, TTT has been used more frequently as an adjuvant therapy. Authors propose that adjuvant TTT may allow the use of lower radiation doses in order to decrease the risk for radiation complications, treat larger melanomas, as well as treat juxtapapillary tumors with margins close to the optic nerve precluding adequate plaque coverage of these margins. Additionally, TTT may be used for tumors that fail local control. Shields et al.151 reported on 270 patients with combined plaque brachytherapy and TTT, showing that failure of local control was estimated to be 2% and 3% at 2 and 5 years, respectively. TTT was applied at 4-month intervals in three sessions following treatment with plaque brachytherapy. Other authors have reported on the efficacy of combined treatment,45,152,153 with a slight trend toward better visual acuity for those treated with adjuvant TTT.45 Additionally, adjuvant TTT combined with plaque brachytherapy may be beneficial for juxtapapillary tumors as discussed above.87
Choroidal melanoma has been shown to have a unique spatial distribution of vasculature with mature vessels found in the basal aspects of tumors, and immature neovessels radiating apically and peripherally. Vessel maturation was also shown to be associated with histopathologic predictors of poor prognosis. However, with the rich vasculature of these tumors, treatment with antiangiogenic agents has been proposed.154 Additionally, cell lines of choroidal melanoma have been shown to secrete VEGF,155 while others have shown increased concentrations of VEGF in the vitreous of eyes with choroidal melanoma.138 Missotten et al.137 reported increased concentrations of VEGF in choroidal melanomas compared to controls, with significant correlations with increasing tumor height and basal diameter. Interestingly, VEGF concentrations were found to be higher after treatment with plaque brachytherapy. As primary treatment for choroidal melanoma, a small case series reported that antiangiogenic agents when used alone were not effective at delaying tumor progression.156 However, an interesting pilot study reported on the use of systemic administration of bevacizumab. The two patients treated had complete resolution of exudative retinal detachments, as well as significant decreases (>50%) in tumor height. Neither patient had local or systemic recurrence of choroidal melanoma at 40 and 35 months.157 Adverse effects were mild, including hypertension, with no heart attacks, strokes, or deaths. Some investigators have proposed adjuvant treatment of choroidal melanoma with intravitreal bevacizumab combined with plaque brachytherapy. However, timing may prove to be an important factor for the efficacy of this treatment. Although treatment prior to plaque placement may result in tumor size reduction, this strategy may be associated with closure of tumor vasculature with resultant hypoxia of tumor cells. Hypoxic cells have been shown to be resistant to radiation therapy and chemotherapy. Other investigators have shown a reduction in tumor size and resolution of exudative retinal detachment when melanomas were treated with intravitreal bevacizumab upon plaque removal. Further studies with more patients and longer follow-up are needed to determine the efficacy and safety of antiangiogenic inhibitors in the treatment of choroidal melanoma.
Conclusion
The COMS Medium Tumor Trial established the use of iodine-125 plaque brachytherapy as a primary globe-salvaging treatment for choroidal melanoma. Plaque brachytherapy and enucleation were shown to have similar rates of melanoma-specific deaths. In addition, small, large, and juxtapapillary tumors have been successfully treated with plaque brachytherapy. Despite eye preservation, visual morbidity is high secondary to radiation-related complications, including radiation maculopathy and optic neuropathy. Alternative radioisotopes have been investigated to minimize treatment-related effects without significant reductions in visual loss. As a result, a better understanding of the mechanisms involved in radiation damage is needed, along with the development of treatment strategies for these visually devastating complications. Current studies emphasize the early identification and treatment of radiation retinopathy in hopes of maintaining useful vision. Radiation therapy, both plaque brachytherapy and proton beam irradiation, have been shown to have comparable results regarding local tumor control, survival/metastasis, and globe-salvage.74,158–168 However, rates of radiation complications with resultant visual loss remain high regardless of treatment type. Previous studies97,100 have shown that intraoperative ultrasound provides valuable information regarding plaque placement. Ultrasound may allow for the repositioning of plaques that have tilted, shifted, or inaccurately placed. There has been much debate about the learning curve for plaque placement, including whether ultrasound confirmation is needed in the hands of an experienced ocular oncologist. A study by Shah et al.169 analyzed the learning curve associated with plaque placement for uveal melanoma over a 20-year period. The authors found that, initially, 21% of plaques required repositioning. This rate decreased to 12% after a period of 10 years, and further decreased to 4% after approximately 20 years. The study estimated that 1275 plaque cases were needed to obtain a precision rate of 90%. A study by Chang et al.170 demonstrated this learning curve with a report that of 150 patients treated with plaque brachytherapy for uveal melanoma, 36% of patients required plaque repositioning secondary to inadequate placement. Intraoperative ultrasound provides a valuable tool to aid in plaque placement and maximizes the likelihood of local tumor control.
1 Moore RF. Choroidal sarcoma treated by the intraocular insertion of radon seeds. Br J Ophthalmol. 1930;14:145–152.
2 Brady LW, Shields JA, Augsburger JJ, et al. Malignant intraocular tumors. Cancer. 1982;49:578–585.
3 Shields JA, Augsburger JJ, Brady LW, et al. Cobalt plaque therapy of posterior uveal melanomas. Ophthalmology. 1982;89:1201–1207.
4 Char DH, Lonn LI, Margolis LW. Complications of cobalt plaque therapy of choroidal melanomas. Am J Ophthalmol. 1977;84:536–541.
5 Stallard HB. Malignant melanoblastoma of the choroid. Bibl Ophthalmol. 1968;75:16–38.
6 Lommatzsch PK, Kirsch IH. 106Ru/106Rh plaque radiotherapy for malignant melanomas of the choroid. With follow-up results more than 5 years. Doc Ophthalmol. 1988;68:225–238.
7 Lommatzsch PK. Treatment of choroidal melanomas with 106Ru/106Rh beta-ray applicators. Trans Ophthalmol Soc UK. 1977;97:428–429.
8 Muller RP, Busse H, Potter R, et al. Results of high dose 106-ruthenium irradiation of choroidal melanomas. Int J Radiat Oncol Biol Phys. 1986;12:1749–1755.
9 Moura RA, McPherson AR, Easley J. Malignant melanoma of the choroid: treatment with episcleral 198Au plaque and xenon-arc photocoagulation. Ann Ophthalmol. 1985;17:114–125.
10 Char DH, Castro JR, Quivey JM, et al. Uveal melanoma radiation. 125I brachytherapy versus helium ion irradiation. Ophthalmology. 1989;96:1708–1715.
11 Garretson BR, Robertson DM, Earle JD. Choroidal melanoma treatment with iodine 125 brachytherapy. Arch Ophthalmol. 1987;105:1394–1397.
12 Lean EK, Cohen DM, Liggett PE, et al. Episcleral radioactive plaque therapy: initial clinical experience with 56 patients. Am J Clin Oncol. 1990;13:185–190.
13 Packer S, Rotman M. Radiotherapy of choroidal melanoma with iodine-125. Ophthalmology. 1980;87:582–590.
14 Packer S, Rotman M, Fairchild RG, et al. Irradiation of choroidal melanoma with iodine 125 ophthalmic plaque. Arch Ophthalmol. 1980;98:1453–1457.
15 Packer S, Rotman M, Salanitro P. Iodine-125 irradiation of choroidal melanoma. Clinical experience. Ophthalmology. 1984;91:1700–1708.
16 Packer S, Stoller S, Lesser ML, et al. Long-term results of iodine 125 irradiation of uveal melanoma. Ophthalmology. 1992;99:767–773. discussion 774
17 Robertson DM, Earle J, Anderson JA. Preliminary observations regarding the use of iodine-125 in the management of choroidal melanoma. Trans Ophthalmol Soc UK. 1983;103:155–160.
18 Robertson DM, Fountain KS, Anderson JA, et al. Radioactive iodine-125 as a therapeutic radiation source for management of intraocular tumors. Trans Am Ophthalmol Soc. 1981;79:294–306.
19 Finger PT. Microwave plaque thermoradiotherapy for choroidal melanoma. Br J Ophthalmol. 1992;76:358–364.
20 Finger PT. Microwave thermoradiotherapy for uveal melanoma: results of a 10-year study. Ophthalmology. 1997;104:1794–1803.
21 Finger PT, Berson A, Ng T, et al. Palladium-103 plaque radiotherapy for choroidal melanoma: an 11-year study. Int J Radiat Oncol Biol Phys. 2002;54:1438–1445.
22 Diener-West M, Earle JD, Fine SL, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001;119:969–982.
23 The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol. 2006;124:1684–1693.
24 Brown GC, Shields JA, Sanborn G, et al. Radiation retinopathy. Ophthalmology. 1982;89:1494–1501.
25 Brown GC, Shields JA, Sanborn G, et al. Radiation optic neuropathy. Ophthalmology. 1982;89:1489–1493.
26 Char DH, Kroll S, Quivey JM, et al. Long term visual outcome of radiated uveal melanomas in eyes eligible for randomisation to enucleation versus brachytherapy. Br J Ophthalmol. 1996;80:117–124.
27 Cruess AF, Augsburger JJ, Shields JA, et al. Visual results following cobalt plaque radiotherapy for posterior uveal melanomas. Ophthalmology. 1984;91:131–136.
28 Wen JC, McCannel TA. Treatment of radiation retinopathy following plaque brachytherapy for choroidal melanoma. Curr Opin Ophthalmol. 2009;20:200–204.
29 Melia M, Moy CS, Reynolds SM, et al. Quality of life after iodine 125 brachytherapy vs enucleation for choroidal melanoma: 5-year results from the Collaborative Ocular Melanoma Study: COMS QOLS Report No. 3. Arch Ophthalmol. 2006;124:226–238.
30 Overgaard J, Overgaard M, Hansen PV, et al. Some factors of importance in the radiation treatment of malignant melanoma. Radiother Oncol. 1986;5:183–192.
31 Trott KR. The optimal radiation dose per fraction for the treatment of malignant melanomas. Int J Radiat Oncol Biol Phys. 1991;20:905–907.
32 Kindy-Degnan NA, Char DH, Castro JR, et al. Effect of various doses of radiation for uveal melanoma on regression, visual acuity, complications, and survival. Am J Ophthalmol. 1989;107:114–120.
33 National Institutes of Health. Guide for grants and contracts. Protocol of the Collaborative Ocular Melanoma Study. Bethesda: NIH; 1986. 15
34 Nath R, Anderson LL, Luxton G, et al. Dosimetry of interstitial brachytherapy sources: recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med Phys. 1995;22:209–234.
35 Nag S, Quivey JM, Earle JD, et al. The American Brachytherapy Society recommendations for brachytherapy of uveal melanomas. Int J Radiat Oncol Biol Phys. 2003;56:544–555.
36 Krintz AL, Hanson WF, Ibbott GS, et al. A reanalysis of the Collaborative Ocular Melanoma Study Medium Tumor Trial eye plaque dosimetry. Int J Radiat Oncol Biol Phys. 2003;56:889–898.
37 Glasgow JP, Perez CA. Physics of brachytherapy. In: Perez CA, Brady LW. Principles and practice of radiation oncology. Philadelphia: Lippincott, 1987.
38 Phillips TL, Char DH, McCormick B, et al. Eye tumors: brachytherapy experience. In: Nath R, Anderson LL, Luxton G, et al. Interstitial brachytherapy: physical, biological, and clinical considerations. New York: Raven Press, 1990.
39 Harbour JW, Meredith TA, Thompson PA, et al. Transpupillary thermotherapy versus plaque radiotherapy for suspected choroidal melanomas. Ophthalmology. 2003;110:2207–2214. discussion 2215
40 MacFaul PA. Local radiotherapy in the treatment of malignant melanoma of the choroid. Trans Ophthalmol Soc UK. 1977;97:421–427.
41 Davidorf FH, Makley TA, Lang JR. Radiotherapy of malignant melanoma of the choroid. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976;81:849–861.
42 Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy–clinical, histopathological, ultrastructural and experimental correlations. Eye (Lond). 1991;5:239–251.
43 Amoaku WM, Archer DB. Fluorescein angiographic features, natural course and treatment of radiation retinopathy. Eye (Lond). 1990;4:657–667.
44 Earle J, Kline RW, Robertson DM. Selection of iodine 125 for the Collaborative Ocular Melanoma Study. Arch Ophthalmol. 1987;105:763–764.
45 Verschueren KM, Creutzberg CL, Schalij-Delfos NE, et al. Long-term outcomes of eye-conserving treatment with Ruthenium(106) brachytherapy for choroidal melanoma. Radiother Oncol. 2010;95:332–338.
46 Wilkinson DA, Kolar M, Fleming PA, et al. Dosimetric comparison of 106Ru and 125I plaques for treatment of shallow (<or=5 mm) choroidal melanoma lesions. Br J Radiol. 2008;81:784–789.
47 Rotman M, Long RS, Packer S, et al. Radiation therapy of choroidal melanoma. Trans Ophthalmol Soc UK. 1977;97:431–435.
48 Finger PT. Finger’s “slotted” eye plaque for radiation therapy: treatment of juxtapapillary and circumpapillary intraocular tumours. Br J Ophthalmol. 2007;91:891–894.
49 Chiu-Tsao ST, O’Brien K, Sanna R, et al. Monte Carlo dosimetry for 125I and 60Co in eye plaque therapy. Med Phys. 1986;13:678–682.
50 Robertson DM. A rationale for comparing radiation to enucleation in the management of choroidal melanoma. Am J Ophthalmol. 1989;108:448–451.
51 Finger PT, Chin KJ, Duvall G. Palladium-103 ophthalmic plaque radiation therapy for choroidal melanoma: 400 treated patients. Ophthalmology. 2009;116:790–796. 796 e791
52 Papageorgiou KI, Cohen VM, Bunce C, et al. Predicting local control of choroidal melanomas following (106)Ru plaque brachytherapy. Br J Ophthalmol. 2011;95:166–170.
53 Shields CL, Furuta M, Thangappan A, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989–998.
54 Curtin VT. Natural course of small malignant melanomas of choroid and ciliary body. Trans Ophthalmol Soc N Z. 1978;30:61.
55 Packard RB. In malignant choroidal melanoma will a delay in radical treatment influence prognosis? Trans Ophthalmol Soc UK. 1983;103:49–53.
56 Augsburger JJ. Is observation really appropriate for small choroidal melanomas. Trans Am Ophthalmol Soc. 1993;91:147–168. discussion 169–175
57 Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No. 5. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol. 1997;115:1537–1544.
58 Mortality in patients with small choroidal melanoma. COMS report no. 4. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol. 1997;115:886–893.
59 Shields CL, Furuta M, Berman EL, et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol. 2009;127:981–987.
60 Singh AD, Bena JF, Mokashi AA, et al. Growth of small tumors. Ophthalmology. 2006;113:1061. e1061–e1064
61 Sobrin L, Schiffman JC, Markoe AM, et al. Outcomes of iodine 125 plaque radiotherapy after initial observation of suspected small choroidal melanomas: a pilot study. Ophthalmology. 2005;112:1777–1783.
62 Shields CL, Shields JA, Perez N, et al. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology. 2002;109:225–234.
63 De Potter P, Jamart J. Adjuvant indocyanine green in transpupillary thermotherapy for choroidal melanoma. Ophthalmology. 2003;110:406–413. discussion 413–404
64 Cajigal-Morales C, Valverde-Almohalla S, Encinas-Martin JL. [Transpupillary thermotherapy in the primary management of choroidal melanoma]. Arch Soc Esp Oftalmol. 2005;80:171–178.
65 Langmann G, Lechner H, Wenzel E, et al. [Transpupillary thermotherapy (TTT) for uveal melanomas. Long term results of a single TTT with an adapter for a conventional infrared laser]. Ophthalmologe. 2005;102:1162–1167.
66 Spire M, Devouassoux MS, Kodjikian L, et al. Primary transpupillary thermotherapy for 18 small posterior pole uveal melanomas. Am J Ophthalmol. 2006;141:840–849.
67 Stoffelns BM. [Tumor regression and visual outcome after transpupillary thermotherapy (TTT) for malignant choroidal melanoma]. Klin Monbl Augenheilkd. 2006;223:74–80.
68 Win PH, Robertson DM, Buettner H, et al. Extended follow-up of small melanocytic choroidal tumors treated with transpupillary thermotherapy. Arch Ophthalmol. 2006;124:503–506.
69 Pan Y, Diddie K, Lim JI. Primary transpupillary thermotherapy for small choroidal melanomas. Br J Ophthalmol. 2008;92:747–750.
70 Singh AD, Kivela T, Seregard S, et al. Primary transpupillary thermotherapy of “small” choroidal melanoma: is it safe? Br J Ophthalmol. 2008;92:727–728.
71 Jampol LM, Moy CS, Murray TG, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report No. 19. Ophthalmology. 2002;109:2197–2206.
72 Gragoudas E. Proton beam therapy: proton therapy for uveal melanoma – 20 years experience. New Orleans: American Academy of Ophthalmology; 2001.
73 Char DH, Quivey JM, Castro JR, et al. Helium ions versus iodine 125 brachytherapy in the management of uveal melanoma. A prospective, randomized, dynamically balanced trial. Ophthalmology. 1993;100:1547–1554.
74 Castro JR, Char DH, Petti PL, et al. 15 years experience with helium ion radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 1997;39:989–996.
75 Munzenrider JE, Gragoudas ES, Seddon JM, et al. Conservative treatment of uveal melanoma: probability of eye retention after proton treatment. Int J Radiat Oncol Biol Phys. 1988;15:553–558.
76 Hawkins BS. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am J Ophthalmol. 2004;138:936–951.
77 Wilson MW, Alejandro KC, Cantrell JE, et al. 125-I episcleral plaque brachytherapy in the management of large malignant melanomas of the ciliary body and/or choroid: scientific poster 76, 2002. Joint Meeting American Academy of Ophthalmology and Pan-American Association of Ophthalmology, Orlando, Florida. 2002.
78 Puusaari I, Heikkonen J, Summanen P, et al. Iodine brachytherapy as an alternative to enucleation for large uveal melanomas. Ophthalmology. 2003;110:2223–2234.
79 Shields CL, Naseripour M, Cater J, et al. Plaque radiotherapy for large posterior uveal melanomas (>or =8-mm thick) in 354 consecutive patients. Ophthalmology. 2002;109:1838–1849.
80 Kaiserman N, Kaiserman I, Hendler K, et al. Ruthenium-106 plaque brachytherapy for thick posterior uveal melanomas. Br J Ophthalmol. 2009;93:1167–1171.
81 Bergman L, Nilsson B, Lundell G, et al. Ruthenium brachytherapy for uveal melanoma, 1979–2003: survival and functional outcomes in the Swedish population. Ophthalmology. 2005;112:834–840.
82 Kreusel KM, Bechrakis N, Riese J, et al. Combined brachytherapy and transpupillary thermotherapy for large choroidal melanoma: tumor regression and early complications. Graefes Arch Clin Exp Ophthalmol. 2006;244:1575–1580.
83 De Potter P, Shields CL, Shields JA, et al. Impact of enucleation versus plaque radiotherapy in the management of juxtapapillary choroidal melanoma on patient survival. Br J Ophthalmol. 1994;78:109–114.
84 Sagoo MS, Shields CL, Mashayekhi A, et al. Plaque radiotherapy for juxtapapillary choroidal melanoma overhanging the optic disc in 141 consecutive patients. Arch Ophthalmol. 2008;126:1515–1522.
85 Sagoo MS, Shields CL, Mashayekhi A, et al. Plaque radiotherapy for choroidal melanoma encircling the optic disc (circumpapillary choroidal melanoma). Arch Ophthalmol. 2007;125:1202–1209.
86 Hui JI, Murray TG. Radioactive plaque therapy. Int Ophthalmol Clin. 2006;46:51–68.
87 Sagoo MS, Shields CL, Mashayekhi A, et al. Plaque radiotherapy for juxtapapillary choroidal melanoma: tumor control in 650 consecutive cases. Ophthalmology. 2011;118:402–407.
88 Lane AM, Kim IK, Gragoudas ES. Proton irradiation for peripapillary and parapapillary melanomas. Arch Ophthalmol. 2011;129:1127–1130.
89 Houston SK, 3rd., Markoe AM, Boldt HC, et al. Juxtapapillary uveal melanomas: patient outcomes after treatment with proton irradiation for peripapillary and parapapillary melanomas. Arch Ophthalmol. 2011;129:1218–1220.
90 Pe’er J, Sancho C, Cantu J, et al. Measurement of choroidal melanoma basal diameter by wide-angle digital fundus camera: a comparison with ultrasound measurement. Ophthalmologica. 2006;220:194–197.
91 Kim Y, Hwang TS, Choi D, et al. Comparison of digital fundus photographic and echographic measurements for maximal linear dimension from eyes with choroidal melanoma. Retina. 2009;29:1321–1327.
92 Hilton GF. Measuring grid for indirect ophthalmoscopy. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:530–531.
93 Pach JM, Robertson DM, Taney BS, et al. Prognostic factors in choroidal and ciliary body melanomas with extrascleral extension. Am J Ophthalmol. 1986;101:325–331.
94 Martin JA, Robertson DM. Extrascleral extension of choroidal melanoma diagnosed by ultrasound. Ophthalmology. 1983;90:1554–1559.
95 Robertson DM, Fuller DG, Anderson RE. A technique for accurate placement of episcleral iodine-125 plaques. Am J Ophthalmol. 1987;103:63–65.
96 Bianciotto C, Shields CL, Guzman JM, et al. Assessment of anterior segment tumors with ultrasound biomicroscopy versus anterior segment optical coherence tomography in 200 cases. Ophthalmology. 2011;118:1297–1302.
97 Harbour JW, Murray TG, Byrne SF, et al. Intraoperative echographic localization of iodine 125 episcleral radioactive plaques for posterior uveal melanoma. Retina. 1996;16:129–134.
98 Pavlin CJ, Japp B, Simpson ER, et al. Ultrasound determination of the relationship of radioactive plaques to the base of choroidal melanomas. Ophthalmology. 1989;96:538–542.
99 Williams DF, Mieler WF, Lewandowski M, et al. Echographic verification of radioactive plaque position in the treatment of melanomas. Arch Ophthalmol. 1988;106:1623–1624.
100 Almony A, Breit S, Zhao H, et al. Tilting of radioactive plaques after initial accurate placement for treatment of uveal melanoma. Arch Ophthalmol. 2008;126:65–70.
101 Williams DF, Mieler WF, Jaffe GJ, et al. Magnetic resonance imaging of juxtapapillary plaques in cadaver eyes. Br J Ophthalmol. 1990;74:43–46.
102 Char DH. Management of orbital tumors. Mayo Clin Proc. 1993;68:1081–1096.
103 Karlsson UL, Augsburger JJ, Shields JA, et al. Recurrence of posterior uveal melanoma after 60Co episcleral plaque therapy. Ophthalmology. 1989;96:382–388.
104 De Potter P, Shields CL, Shields JA, et al. Plaque radiotherapy for juxtapapillary choroidal melanoma. Visual acuity and survival outcome. Arch Ophthalmol. 1996;114:1357–1365.
105 Quivey JM, Augsburger J, Snelling L, et al. 125I plaque therapy for uveal melanoma. Analysis of the impact of time and dose factors on local control. Cancer. 1996;77:2356–2362.
106 Quivey JM, Char DH, Phillips TL, et al. High intensity 125-iodine (125I) plaque treatment of uveal melanoma. Int J Radiat Oncol Biol Phys. 1993;26:613–618.
107 Okera S, Dodd T, Selva D, et al. Fungal granuloma following ruthenium plaque radiotherapy of a choroidal melanoma. Clin Experiment Ophthalmol. 2003;31:159–161.
108 Horgan N, Shields CL, Mashayekhi A, et al. Early macular morphological changes following plaque radiotherapy for uveal melanoma. Retina. 2008;28:263–273.
109 Robertson DM. Choroidal melanomas with a collar-button configuration: response pattern after iodine 125 brachytherapy. Arch Ophthalmol. 1999;117:771–775.
110 Bosworth JL, Packer S, Rotman M, et al. Choroidal melanoma: I-125 plaque therapy. Radiology. 1988;169:249–251.
111 Beitler JJ, McCormick B, Ellsworth RM, et al. Ocular melanoma: total dose and dose rate effects with Co-60 plaque therapy. Radiology. 1990;176:275–278.
112 Vrabec TR, Augsburger JJ, Gamel JW, et al. Impact of local tumor relapse on patient survival after cobalt 60 plaque radiotherapy. Ophthalmology. 1991;98:984–988.
113 Tabandeh H, Chaudhry NA, Murray TG, et al. Intraoperative echographic localization of iodine-125 episcleral plaque for brachytherapy of choroidal melanoma. Am J Ophthalmol. 2000;129:199–204.
114 Wilson MW, Hungerford JL. Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology. 1999;106:1579–1587.
115 Fontanesi J, Meyer D, Xu S, et al. Treatment of choroidal melanoma with I-125 plaque. Int J Radiat Oncol Biol Phys. 1993;26:619–623.
116 Harbour JW, Char DH, Kroll S, et al. Metastatic risk for distinct patterns of postirradiation local recurrence of posterior uveal melanoma. Ophthalmology. 1997;104:1785–1792. discussion 1792–1783
117 MacFaul PA, Morgan G. Histopathological changes in malignant melanomas of the choroid after cobalt plaque therapy. Br J Ophthalmol. 1977;61:221–228.
118 Saornil MA, Fisher MR, Campbell RJ, et al. Histopathologic study of eyes after iodine I 125 episcleral plaque irradiation for uveal melanoma. Arch Ophthalmol. 1997;115:1395–1400.
119 Avery RB, Diener-West M, Reynolds SM, et al. Histopathologic characteristics of choroidal melanoma in eyes enucleated after iodine 125 brachytherapy in the collaborative ocular melanoma study. Arch Ophthalmol. 2008;126:207–212.
120 Sealy R, le Roux PL, Rapley F, et al. The treatment of ophthalmic tumours with low-energy sources. Br J Radiol. 1976;49:551–554.
121 Krohn J, Monge OR, Skorpen TN, et al. Posterior uveal melanoma treated with I-125 brachytherapy or primary enucleation. Eye (Lond). 2008;22:1398–1403.
122 Stack R, Elder M, Abdelaal A, et al. New Zealand experience of I125 brachytherapy for choroidal melanoma. Clin Experiment Ophthalmol. 2005;33:490–494.
123 Jensen AW, Petersen IA, Kline RW, et al. Radiation complications and tumor control after 125I plaque brachytherapy for ocular melanoma. Int J Radiat Oncol Biol Phys. 2005;63:101–108.
124 Lumbroso-Le Rouic L, Charif Chefchaouni M, Levy C, et al. 125I plaque brachytherapy for anterior uveal melanomas. Eye (Lond). 2004;18:911–916.
125 Puusaari I, Heikkonen J, Kivela T. Ocular complications after iodine brachytherapy for large uveal melanomas. Ophthalmology. 2004;111:1768–1777.
126 Mameghan H, Karolis C, Fisher R, et al. Iodine-125 irradiation of choroidal melanoma: clinical experience from the Prince of Wales and Sydney Eye Hospitals. Australas Radiol. 1992;36:249–252.
127 Boldt HC, Melia BM, Liu JC, et al. I-125 brachytherapy for choroidal melanoma photographic and angiographic abnormalities: the Collaborative Ocular Melanoma Study: COMS Report No. 30. Ophthalmology. 2009;116:106–115. e101
128 Linstadt D, Castro J, Char D, et al. Long-term results of helium ion irradiation of uveal melanoma. Int J Radiat Oncol Biol Phys. 1990;19:613–618.
129 Gragoudas ES, Seddon JM, Egan K, et al. Long-term results of proton beam irradiated uveal melanomas. Ophthalmology. 1987;94:349–353.
130 Jones R, Gore E, Mieler W, et al. Posttreatment visual acuity in patients treated with episcleral plaque therapy for choroidal melanomas: dose and dose rate effects. Int J Radiat Oncol Biol Phys. 2002;52:989–995.
131 Lommatzsch P. Treatment of choroidal melanomas with 106Ru/106Rh beta-ray applicators. Surv Ophthalmol. 1974;19:85–100.
132 Lommatzsch PK. Results after beta-irradiation (106Ru/106Rh) of choroidal melanomas: 20 years’ experience. Br J Ophthalmol. 1986;70:844–851.
133 Finger PT, Chin KJ, Yu GP. Risk factors for radiation maculopathy after ophthalmic plaque radiation for choroidal melanoma. Am J Ophthalmol. 2010;149:608–615.
134 Finger PT, Moshfeghi DM, Ho TK. Palladium 103 ophthalmic plaque radiotherapy. Arch Ophthalmol. 1991;109:1610–1613.
135 Finger PT, Lu D, Buffa A, et al. Palladium-103 versus iodine-125 for ophthalmic plaque radiotherapy. Int J Radiat Oncol Biol Phys. 1993;27:849–854.
136 Chiu-Tsao ST, Anderson LL. Thermoluminescent dosimetry for 103Pd seeds (model 200) in solid water phantom. Med Phys. 1991;18:449–452.
137 Missotten GS, Notting IC, Schlingemann RO, et al. Vascular endothelial growth factor a in eyes with uveal melanoma. Arch Ophthalmol. 2006;124:1428–1434.
138 Boyd SR, Tan D, Bunce C, et al. Vascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: identification of a potential therapeutic window. Br J Ophthalmol. 2002;86:448–452.
139 Mason JO, 3rd., Albert MA, Jr., et al. Intravitreal bevacizumab treatment for radiation macular edema after plaque radiotherapy for choroidal melanoma. Retina. 2007;27:903–907.
140 Finger PT, Chin K. Anti-vascular endothelial growth factor bevacizumab (Avastin) for radiation retinopathy. Arch Ophthalmol. 2007;125:751–756.
141 Gupta A, Muecke JS. Treatment of radiation maculopathy with intravitreal injection of bevacizumab (Avastin). Retina. 2008;28:964–968.
142 Finger PT. Radiation retinopathy is treatable with anti-vascular endothelial growth factor bevacizumab (Avastin). Int J Radiat Oncol Biol Phys. 2008;70:974–977.
143 Finger PT, Chin KJ. Intravitreous ranibizumab (Lucentis) for radiation maculopathy. Arch Ophthalmol. 2010;128:249–252.
144 Horgan N, Shields CL, Mashayekhi A, et al. Periocular triamcinolone for prevention of macular edema after plaque radiotherapy of uveal melanoma: a randomized controlled trial. Ophthalmology. 2009;116:1383–1390.
145 Hykin PG, Shields CL, Shields JA, et al. The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology. 1998;105:1425–1429.
146 Kinyoun JL, Zamber RW, Lawrence BS, et al. Photocoagulation treatment for clinically significant radiation macular oedema. Br J Ophthalmol. 1995;79:144–149.
147 Kinyoun JL. Long-term visual acuity results of treated and untreated radiation retinopathy (an AOS thesis). Trans Am Ophthalmol Soc. 2008;106:325–335.
148 Finger PT, Kurli M. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol. 2005;89:730–738.
149 Oliver SC, Leu MY, DeMarco JJ, et al. Attenuation of iodine 125 radiation with vitreous substitutes in the treatment of uveal melanoma. Arch Ophthalmol. 2010;128:888–893.
150 Singh AD, Eagle RC, Jr., Shields CL, et al. Clinicopathologic reports, case reports, and small case series: enucleation following transpupillary thermotherapy of choroidal melanoma: clinicopathologic correlations. Arch Ophthalmol. 2003;121:397–400.
151 Shields CL, Cater J, Shields JA, et al. Combined plaque radiotherapy and transpupillary thermotherapy for choroidal melanoma: tumor control and treatment complications in 270 consecutive patients. Arch Ophthalmol. 2002;120:933–940.
152 Bartlema YM, Oosterhuis JA, Journee-De Korver JG, et al. Combined plaque radiotherapy and transpupillary thermotherapy in choroidal melanoma: 5 years’ experience. Br J Ophthalmol. 2003;87:1370–1373.
153 Damato B, Patel I, Campbell IR, et al. Local tumor control after 106Ru brachytherapy of choroidal melanoma. Int J Radiat Oncol Biol Phys. 2005;63:385–391.
154 Pina Y, Cebulla CM, Murray TG, et al. Blood vessel maturation in human uveal melanoma: spatial distribution of neovessels and mature vasculature. Ophthalmic Res. 2009;41:160–169.
155 Ijland SA, Jager MJ, Heijdra BM, et al. Expression of angiogenic and immunosuppressive factors by uveal melanoma cell lines. Melanoma Res. 1999;9:445–450.
156 Lima BR, Schoenfield LR, Singh AD. The impact of intravitreal bevacizumab therapy on choroidal melanoma. Am J Ophthalmol. 2011;151:323–328. e322
157 Newman H, Finger PT, Chin KJ, et al. Systemic bevacizumab (Avastin) for exudative retinal detachment secondary to choroidal melanoma. Eur J Ophthalmol. 2011;21:796–801.
158 Gragoudas ES, Lane AM, Munzenrider J, et al. Long-term risk of local failure after proton therapy for choroidal/ciliary body melanoma. Trans Am Ophthalmol Soc. 2002;100:43–48. discussion 48–49
159 Gragoudas ES. Proton beam irradiation of uveal melanomas: the first 30 years. The Weisenfeld Lecture. Invest Ophthalmol Vis Sci. 2006;47:4666–4673.
160 Egger E, Zografos L, Schalenbourg A, et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 2003;55:867–880.
161 Egger E, Schalenbourg A, Zografos L, et al. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int J Radiat Oncol Biol Phys. 2001;51:138–147.
162 Kodjikian L, Roy P, Rouberol F, et al. Survival after proton-beam irradiation of uveal melanomas. Am J Ophthalmol. 2004;137:1002–1010.
163 Damato B, Lecuona K. Conservation of eyes with choroidal melanoma by a multimodality approach to treatment: an audit of 1632 patients. Ophthalmology. 2004;111:977–983.
164 Damato B, Kacperek A, Chopra M, et al. Proton beam radiotherapy of choroidal melanoma: the Liverpool-Clatterbridge experience. Int J Radiat Oncol Biol Phys. 2005;62:1405–1411.
165 Dendale R, Lumbroso-Le Rouic L, Noel G, et al. Proton beam radiotherapy for uveal melanoma: results of Curie Institut-Orsay proton therapy center (ICPO). Int J Radiat Oncol Biol Phys. 2006;65:780–787.
166 Fuss M, Loredo LN, Blacharski PA, et al. Proton radiation therapy for medium and large choroidal melanoma: preservation of the eye and its functionality. Int J Radiat Oncol Biol Phys. 2001;49:1053–1059.
167 Hocht S, Bechrakis NE, Nausner M, et al. Proton therapy of uveal melanomas in Berlin. 5 years of experience at the Hahn-Meitner Institute. Strahlenther Onkol. 2004;180:419–424.
168 Caujolle JP, Mammar H, Chamorey E, et al. Proton beam radiotherapy for uveal melanomas at nice teaching hospital: 16 years’ experience. Int J Radiat Oncol Biol Phys. 2010;78:98–103.
169 Shah NV, Houston SK, Murray TG, et al. Evaluation of surgical learning curve for I-125 episcleral plaque placement for the treatment of posterior uveal melanoma: a two decade review. Clin Ophthalmol. 2012;6:447–452.
170 Chang MY, Kamrava M, Demanes DJ, et al. Intraoperative ultrasonography-guided positioning of Iodine-125 plaque brachytherapy in the treatment of choroidal melanoma. Ophthalmology. 2012;119:1073–1077.