Chapter 14 Brachytherapy
Brachytherapy, a term from the Greek language, means “therapy at a short distance.” It was increasingly used in the treatment of malignant tumors shortly after the discovery of radium-226 (226Ra) by Marie Curie. In 1960, Henschke1 published the first paper on afterloading low-dose-rate (LDR) brachytherapy in gynecologic malignant disease and later in other tumors. Following soon was a publication describing the Fletcher-Suit afterloading applicators.2
Brachytherapy, used as an integral part of cancer treatment for almost a century, sustained a rapid growth with the development of afterloading devices and the introduction of artificial radionuclides.3,4 In 1961, Henschke5 developed the first high-dose-rate (HDR) machine using small cobalt sources of high activity. He and his associates stated, “On the basis of our limited experience with such short treatment times in the last 3 years, we feel that they may be used with impunity if the total dose is divided into more fractions.”6 In later reports, they reasoned that moving source remote afterloaders could be used with all gamma-emitting radioisotopes, but cesium-137 (137Cs) appeared most suitable, except in the case of short treatment times, for which cobalt-60 (60Co) and iridium-192 (192Ir) would be preferable because of their higher specific activities. New isotopes were available for brachytherapy, including gold-198 (198Au), 60Co, 137Cs, and 192Ir; a few years later iodine-125 (125I) and californium-252 (252Cf), and more recently, americium-241 (241Am), palladium-103 (103Pd), ytterbium-169 (169Yb), selenium-75 (75Se), and samarium-145 (145Sm) have been added to our armamentarium. The widespread use of remote afterloading devices has enhanced the clinical applications of brachytherapy and has practically eliminated radiation exposure to the operators. Furthermore, in many parts of the world, HDR brachytherapy has supplanted LDR brachytherapy with equivalent clinical results. According to the International Commission on Radiation Units (ICRU) Report No. 38,7 dose rates of 0.4 to 2 Gy/h are referred to as LDR, those in the range of 2 to 12 Gy/h are medium-dose-rate (MDR), and those greater than 12 Gy/h are HDR.
Interstitial Brachytherapy
Afterloading
The flexible carrier method was first used with radon seeds by Hames8 in 1937 and later by Morton and associates,9 who used cobalt sources. Afterloading was systematized by Henschke and colleagues at Memorial Sloan-Kettering Cancer Center (MSKCC) in New York City. Since the early 1960s, Pierquin and colleagues10 in Europe popularized the afterloading Henschke techniques with personal modifications and contributed the use of “hairpins” for afterloading with thicker iridium wires mainly for lesions of the oral cavity and oropharynx. Several reports in the literature describe techniques and instrumentation for the use of afterloading interstitial therapy with radium and other isotopes such as tantalum wires and 192Ir and 125I seeds.11–19
Removable Iridium-192 Hairpin Technique
The physical characteristics of the Paris technique were described by Pierquin and colleagues in 1964.10 Metallic gutter guides have been constructed to facilitate insertion of the iridium wires. The usual separation of the legs is 1.2 cm, although 0.9- or 1.5-cm separation can be used. The standard gutter length is 2.5, 3, 4, or 5 cm. Iridium wire ends are inserted along the gutters and held in place with a fine-tip clamp while the gutter guide is removed. Gutter guides should allow for a predictable insertion of the hairpin, which results in an acceptable geometry and homogeneous dose distribution of the implant. The gutter guide technique is used primarily in smaller tumors of the oral cavity and in the anal region.
Removable Iodine 125 Plastic Tube Implants
Clarke and colleagues20,21 at William Beaumont Hospital (WBH) described a temporary removable 125I plastic tube implant technique. Iodine-125 seeds, 4.5 mm in length, were used with interseed spacing within the ribbons (from seed center to seed center) ranging from 4.5 mm (seeds back to back) to 12.5 mm (8-mm spacers). The operative technique using hollow stainless-steel 17-gauge trocars is identical to the 192Ir implant procedure. Iodine-125 dosimetry is somewhat more complex because iodine seed dose distributions are more anisotropic, fall off more rapidly with distance, and are more sensitive to tissue heterogeneities than those of 192Ir sources. However, the 125I tubes must have a greater diameter to house the 125I seed ribbons, which are larger than the 192Ir ribbons. The seed ribbons are prepared by loading loose seeds into the hollow ribbons; the seeds are separated by spacers and held in position by a “pusher.” The open end of the seed ribbon is heated for sealing. The seed separation varies depending on the activity, the geometry of the implant, and the desired dose rate, which is individualized for each patient and determined after the procedure in the operating room is completed. The most common clinical applications of temporary 125I seeds are episcleral plaque therapy for ocular melanoma and volume implants in the breast, brain, soft tissue sarcomas of the extremities, and head and neck. Of particular relevance is its use in pediatric brachytherapy to decrease the dose to growing neighboring organs.
Permanent Interstitial Iodine-125 Implants
A system with 10 125I seeds contained within a braided synthetic absorbable carrier has been developed for implants in a shallow plane of tissue or for a tumor site that is inaccessible to standard implant devices.22,23 The 125I seeds are spaced at 1-cm intervals, center to center. The carrier retains a half-circle, taper-point surgical needle. Each strand of 10 seeds is contained within a stainless-steel tubular ring, which effectively shields radiation. The unopened package has a surface dose rate of less than 0.2 mR/h for a loading of ten 0.5-mCi seeds. Consequently, it can be handled and stored without additional shielding.
In circumstances in which the supplied surgical needle is unsuitable, it can be replaced by a tie-on needle (e.g., a French spring-eye needle). The placement of the strands and spacing of the seeds should follow appropriate dosimetric considerations. Martinez and associates described a methodology to insert seeds into an absorbable suture24 and to sterilize them for intraoperative use.25 The absorbable carrier material and 125I seeds are implanted in the tumor tissues by successive advancing of the needle and gentle pulling of the carrier. The carrier material is absorbed by body tissue; the rate depends on the nature of the implanted tissue. Intramuscular implantation studies in rats showed that the absorption of the carrier is minimal until the 40th postoperative day. Absorption is essentially complete after 60 to 90 days.
Goffinet and coworkers26 at Stanford University reported on 64 intraoperative 125I implants with absorbable Vicryl suture carriers performed in 53 patients with head and neck cancers, many of them recurrent after initial definitive radiation therapy. Among 14 patients who had received no prior therapy, local control was achieved in 10 (71%), and 5 (40%) were alive 2 to 45 months after therapy. Among 34 patients who had received prior therapy, local control was achieved in 20 (59%), and no recurrences developed in any head and neck site in 13 (38%). Complications were noted in 7 (50%) of 14 patients treated definitively, including skin ulceration and intraoral and intrapharyngeal ulceration, which usually healed. Of 34 patients who had 125I suture implants after prior therapy, 7 (20%) had complications after the procedure. In a variation of this technique, the 125I suture material is sewed through Gelfoam, which in turn is secured to the tumor bed with special clips.
Templates
Syed-Neblett Templates
Prostatic Template
Puthawala and associates27 described a prostate template used to guide the insertion of metallic source guides transperineally. The template consists of two concentric rings with radii of 1 and 2 cm, containing 6 and 12 guide holes, respectively. Up to 18 metallic source guides (17-gauge, 20-cm-long needles) are inserted transperineally through the prostate and seminal vesicles as indicated. The tips of the guides are usually 1 cm above the level of the bladder neck. The template is fixed to the perineum by 00 silk sutures, and the space between the perineum and the template is filled with gauze soaked in antibiotic cream.
Modified Perineal Template
Hockel and Muller28 described a modified Syed-Neblett type of perineal template for HDR interstitial brachytherapy of gynecologic malignant disease. The template can easily be disassembled after insertion of the central needles into the pelvis, allowing cystoscopic and rectoscopic control of the needle position. Needles penetrating the bladder or the rectum can be repositioned before reassembling the template, eliminating a high-irradiation zone in tumor-free bladder and rectum walls.
Martinez Universal Perineal Interstitial Template
The Martinez Universal Perineal Interstitial Template (MUPIT) was designed to treat locally advanced or recurrent tumors in the prostate, anorectal, perineal, or gynecologic area. The device consists of two acrylic cylinders, one that can be placed in the vagina and the other in the rectum, an acrylic template with an array of holes that allows placement of the metallic guides in the tissues to be implanted, and a cover plate.29 The cylinders are placed in the vagina or rectum, or both, and fastened to the templates so that a fixed geometric relationship among the tumor volume, normal structures, and source placement is preserved throughout the course of the implantation. When the MUPIT interstitial template is used, no central intracavitary sources are inserted, except in some patients requiring an intrauterine tandem (beyond the volume treated with the interstitial sources). Differential loading (using 192Ir seeds of different activity) has always been used for the MUPIT implants to optimize LDR dose distribution.
Template versus Intracavitary Brachytherapy in Locally Advanced Gynecologic Tumors
In an effort to improve on these results, LDR transperineal interstitial brachytherapy techniques were developed as a supplement to external beam radiation therapy (EBRT). Prempree30 used radium needle implants in conjunction with a tandem in 49 patients with stage IIIB cervical carcinoma. All patients were followed for a minimum of 5 years. The control rate in the cervix, vagina, and parametrium was 84% and the major complication rate was 8%. Gaddis and colleagues31 have reported the results of treating 75 patients with primary cervical carcinoma using the Syed-Neblett template. With a median follow-up of 17 months, the overall pelvic control rate was 71% (77% in stage IE/IIA, 80% in stage IIB, 54% in stage III, and 0% in stage IV). It is difficult to compare interstitial brachytherapy with traditional intracavitary treatment owing to differences in patient populations, length of follow-up, and methods of reporting results.
Monk and associates32 have reported results in 70 patients with stage II, III, or IVA cervical cancer treated with interstitial therapy using the Syed-Neblett template retrospectively compared with 61 patients with similar disease treated with intracavitary treatment. They reported similar results in stage III and IVA patients; however, patients with stage II disease had a significantly improved 5-year disease-free survival (DFS) rate (50% vs. 21%) and 5-year local control rate (61% vs. 32%) with intracavitary treatment. A greater percentage of the patients in the interstitial group had unknown tumor size (27% vs. 7%), and the stage distribution was slightly in favor of the intracavitary group.
Gupta and associates33 reviewed the outcomes of patients with locally advanced or recurrent gynecologic cancers who were treated with LDR brachytherapy using the MUPIT. The 3-year actuarial local control rate, DFS rate, and overall survival (OS) rate for all patients were 60%, 55%, and 41%, respectively. For patients with primary cervical cancer, the 3-year actuarial local control rate was 44%. However, 12 of the 30 patients with primary cervical cancer had a disease volume of 100 cm3 or greater. In patients with recurrent disease, the control rate was 68%. The overall complication rate was 13%. These results suggest that in patients with locally advanced or recurrent disease, interstitial implants using the MUPIT applicator can produce acceptable results with acceptable rates of toxicity.
Some concern exists regarding operator variability for interstitial implants. Although patient numbers are small, Gupta and associates33 retrospectively analyzed local control with respect to physician performing the implant. No difference in outcome was found for those patients undergoing this procedure by the senior author versus the other four operators.
In a report by Gupta and associates,33 41% of the patients had recurrent disease, 22% had received prior irradiation, and 26% had disease greater than 100 cm3 in volume. The 3-year local control rate with a disease volume of 100 cm3 or less was 89%. The 3-year actuarial local control rate in the 15 patients who had received prior irradiation was 49%. The major complication rate using interstitial treatment in this group of patients was only 13%.
Russell and colleagues34 have reported their results with re-irradiation of recurrent gynecologic cancers using intracavitary treatment in 25 patients. They report crude local control rates of 56% and major complication rates of 50%. Similar results have been reported by Puthawala and colleagues35 using interstitial implants for recurrent pelvic malignant tumors. After a minimum follow-up of 2 years in 40 patients, they reported a pelvic control rate of 67% and a grade 4 complication rate of 15%.
Dose-Rate Delivery Issues
LDR Implants: Manual versus Remote Afterloading
Few studies compare results of intracavitary therapy with LDR remote afterloading implants with those for manual afterloading systems because there are no significant changes in isotopes or dose rates. Battermann and Szabol36 reported their experience with the LDR Selectron afterloading machine for patients with cancer of the cervix using the same treatment policy as previously used for manual afterloading. Local tumor control and complications were the same for both groups.
HDR Remote Afterloading Implants: Potential Advantages Over LDR
Fractionation and adjustment of total dose are crucial factors in lowering the frequency of complications without compromising the results of therapy with HDR systems.37 Scalliet and associates38 compared HDR and LDR in gynecologic brachytherapy, especially regarding the conversion of LDR total dose into equivalent HDR dose per fraction and total dose. Calculation of biologically equivalent schedules requires knowledge of repair capacity and repair kinetics of tumors and normal tissues, both of which influence the biologic effect of any radiation dose. The emerging clinical experience with HDR is equivalent to that of classic LDR. However, although treatment with LDR has proven to be quite tolerant to a lack of absolute precision, that would be disastrous with HDR techniques due to large dosages per fraction.
Pulsed-Dose-Rate Brachytherapy
Pulsed-dose-rate (PDR) brachytherapy was proposed39 to exploit the advantages of HDR computer-controlled remote afterloading technology. It was noted that by varying the dwell times of the stepping source, dose optimization could be achieved, maintaining the potential benefits of LDR, including improved radiation protection. The inactive source times, when the sources are in the safe between pulses, should allow for improved nursing care and patient visiting. The pulse delivers about 0.5 Gy per 10-minute exposure every hour. As the dose rate gradually decreases because of radioactive decay of the source, somewhat longer periods of pulsed times are required.
Pulsed LDR brachytherapy has been made possible with the development of commercial devices modified from HDR intracavitary brachytherapy applications. A single high-activity source that can be programmed for a dwell time in each position at appropriate periods, to reflect the required differential loading of activity, and with a dose rate of 0.5 Gy/h, has been used at a few institutions. Using the linear-quadratic formula, Brenner and Hall39 determined the pulse lengths and frequencies based on radiobiologic data that were equivalent to conventional continuous LDR irradiation. They determined that for a regimen of 30 Gy in 60 hours, a 1-hour period between 10-minute pulses might produce up to a 2% increase in late effects probability.
Visser and associates40 described a radiobiologic model and equations to determine the PDR or HDR schedules equivalent to certain LDR schedules, similar to that proposed by Brenner and Hall,39 by applying probable ranges for the values for alpha/beta ratio and repair time. They concluded that eight fractions of 1 to 1.5 Gy per 24 hours, up to 3 hours apart, would be equivalent to commonly used LDR treatment schedules. Hall pointed out that Visser and associates40 showed that the more different the proposed regimen is from continuous LDR, the longer the overall treatment time needs to be extended to preserve the therapeutic ratio.
Erickson and Shadley,41 using in vitro irradiation experiments on rodent tumor cell lines, showed that there was a slight increase in cell killing with PDR relative to continuous LDR irradiation of hourly 5-, 10-, or 20-minute pulses, or a 20-minute pulse every 2 hours. In no case was the increased killing statistically significant, and the cells did appear to be clinically indistinguishable as determined by the Brenner and Hall criteria.
In an editorial, Hall and Brenner42 noted that although the linear-quadratic model has been widely used and accepted, it has not been tested in extreme cases and that the biologic data needed for the model calculations are not well known. Armour, White, and colleagues43,44 at WBH developed a reproducible rat model for comparing late rectal toxicity from different brachytherapy techniques, that is, LDR, HDR, and PDR. Later, Armour and coworkers, using the same rat model, reported PDR results with a very strong dependence of late rat rectal injury on radiation pulse size.45
PDR has a significant potential for paradigm shift in brachytherapy. Nonetheless, knowledge of tissue repair kinetics is paramount for adequate selection of dose per pulse and interpulse interval. Therapeutic ratio can be improved by adjusting interpulse intervals to the repair half-times for normal tissues. Likewise, superfractionated schedules with low dose per pulse can be explored in conditions of tumor hypoxia, thanks to the predicted hypersensitivity at low dose per fraction. The use of chemical agents (nicotinamide and others) concurrent with this superfractionated schedule is foreseen in controlled clinical trials.46
Quality Assurance, Radiation Safety, Implant Removal
Dosimetry
When manual intracavitary afterloading is used, for the sake of prompt patient loading, the various cesium tubes are color-coded. The attending physician or resident (after verifying the source loading) and the dosimetrist/physicist load the applicator in the patient. The loading time is documented by the physician, and the physicist measures the radiation exposure levels around the patient and arranges lead shields appropriately. The nursing division is also actively involved in checking every 3 to 4 hours that applicators or sources do not become dislodged over the course of treatment. For further discussion of LDR brachytherapy quality assurance techniques and programs, the reader is referred to a review by Williamson47 and published AAPM recommendations.
Brachytherapy Techniques For Specific Sites
Brain Implants
Brachytherapy may allow delivery of interstitial irradiation boosts to primary brain tumors after conventional EBRT or may be used to treat recurrent brain tumors. Patients with primary malignant brain tumors who received initial doses of more than 50 Gy of EBRT to the whole brain survived 20.5 weeks longer than did patients treated by surgery only. Walker and colleagues,48 analyzing the Brain Tumor Study Group data, showed stepwise increments in survival in patients receiving 50, 55, or 60 Gy. At the same time, it is well known that higher irradiation doses may significantly increase the risk of brain necrosis.
Prados and coworkers49 reported results in 56 patients with glioblastoma multiforme and 32 patients with anaplastic glioma treated with temporary 125I interstitial implants. Patients received EBRT (median, 59.4 Gy), most with concomitant hydroxyurea, followed by interstitial implant. Eight patients (14%) survived 3 years or longer, and 16 (29%) survived 2 years or longer. A second operation was necessary in 50% of patients to remove symptomatic necrosis produced by the implant. Prolonged steroid use was necessary in many patients.
Another technique described was developed at Washington University using radioactive sources placed in Teflon catheters inserted into the brain under direct CT monitoring.50 Multiple burr holes are made in the brain at 1-cm intervals (with the patient under local anesthesia). The locations of the burr holes are determined using a template, which is attached to a stereotactic frame and to the patient’s head. The template used is a thick acrylic block containing a 7- × 7-cm array of 49 holes spaced at 1-cm intervals. The holes along the diagonal axis of the template have slightly larger diameters to provide a method of orientation for each CT slice. The tumor is outlined on the CT screen with the aid of intravenously administered contrast material. The template is placed against the scalp at the site allowing best access to the tumor, usually a lateral surface. Intravenous contrast material is administered and scanning is performed with the scan plane parallel to the rows of the template. The target volume for the implant is the contrast-enhancing ring seen on CT scans, with a 1-cm margin. The number of catheters required to encompass the target at each level is determined at the CT console. Seventeen-gauge catheters, 15 cm long spaced at 1-cm intervals, are then placed through the template into the brain to the desired depths with CT monitoring. Following the grid pattern, under CT observation, the Teflon angiocatheters with a metallic stylet are inserted through the burr holes into the brain substance to ensure straight and parallel insertion. After the tumor volume is implanted, the length of the radioactive sources is determined, and films, with the distribution of the catheters, are obtained for dosimetry calculations. Dummy seeds and ribbons are loaded in each of the catheters. Once the catheters are secured, the patient is transferred to the intensive care unit, where the dummy sources are replaced by ribbons of active 192Ir seed with a specific activity of about 0.6 mCi per seed. Metal buttons are attached to the catheters to fasten them to the scalp.
Careful records are maintained of the position and length of all the catheters. Computer-generated isodose calculations are used to determine the dose and distribution in the implant volume. The dose rate ranges from 0.5 to 0.8 Gy/h at 0.5 to 1 cm. In general, the implant duration is 70 to 100 hours to deliver a 60- to 70-Gy total dose to the entire tumor. Verification dosimetry with thermoluminescent dosimeters placed in catheters disclosed an agreement of ± 5% to 10% between the computer calculations and the actual doses at any point within the irradiated volume. This method has been used in more than 70 patients at Washington University, most of them with glioblastoma multiforme, sometimes recurrent after EBRT, and in a few patients with solitary brain metastasis.50 Fatal intracranial bleeding has been rare (<5%), and edema is not severe enough to represent a significant management problem. Brain necrosis has been observed in about 25% of patients.
GliaSite Radiation System
The GliaSite is an inflatable balloon catheter that is placed in the resection cavity at the time of tumor debulking. Low-dose-rate radiation is delivered with an aqueous solution of organically bound 125I (lotrex [sodium-3-(125I)-iodo-4-hydroxybenzenesulfonate]), which is temporarily introduced into the balloon portion of the device via a subcutaneous port. A dosage of 40 to 60 Gy is generally delivered to all tissues within the target volume. One to 2 weeks later, the device is filled with Iotrex for 3 to 6 days, following which the device is explanted. The indications are for adults with recurrent high-grade malignant gliomas who are able to undergo resection.51 The largest retrospective series from 10 centers reported on 95 patients with recurrent grade 3 or 4 gliomas, a median age of 51 years, and a median Karnofsky performance status score of 80. All patients had previously undergone resection and had received EBRT as part of their initial treatment. After recurrence, each patient underwent maximal surgical debulking of the recurrence followed by GliaSite insertion. The balloon was afterloaded with liquid I (Iotrex) to deliver a median dose of 60 Gy to an average depth of 1 cm with a median dose rate of 52.3 Gy/h. The median survival time for all patients, measured from date of GliaSite placement, was 36.3 weeks, with an estimated 1-year survival of 31.1%. The median survival was 35.9 weeks for patients with an initial diagnosis of glioblastoma multiforme and 43.6 weeks for those with non–glioblastoma multiforme malignant gliomas.52
GliaSite was also evaluated in the treatment of resected single brain metastases. A prospective multi-institutional phase II study of GliaSite brachytherapy was reported in which 71 patients were enrolled and 54 received 60 Gy prescribed at a depth of 1 cm after maximally safe resection of a single brain metastasis. No whole brain EBRT was given. The local control rate was 85%, with a median survival time and duration of functional independence of 40 weeks, similar to patients treated with postsurgical whole brain irradiation.53
Ocular Implants
Episcleral Plaque Therapy
The Collaborative Ocular Melanoma Study (COMS) was conducted as a multicenter randomized phase III clinical trial comparing eye plaque therapy to enucleation with survival and preservation of vision as endpoints. The study enrolled 1317 patients and demonstrated an equivalent 5-year OS rate of 81% in both arms54 (see Chapter 29). In the brachytherapy arm, visual acuity decreased over time, with vision of 20/200 or worse in 43% of treated eyes. At the interval of 5 years following plaque therapy, enucleation was needed in 10% of patients because of tumor relapse and in 3% due to treatment-related complications (see Chapter 29).
In a recent COMS report with 12 years of follow-up, 471 of 1317 patients died.55 Of 515 patients eligible for 12 years of follow-up, 231 (45%) were alive and clinically cancer free 12 years after treatment. For patients in both treatment arms, 5- and 10-year all-cause mortality rates were 19% and 35%, respectively; by 12 years, the cumulative all-cause mortality rate was 43% among patients in the 125I brachytherapy arm and 41% among those in the enucleation arm. Five-, 10-, and 12-year rates of death with histopathologically confirmed melanoma metastasis were 10%, 18%, and 21%, respectively, in the 125I brachytherapy arm and 11%, 17%, and 17%, respectively, in the enucleation arm. Older age and larger maximum basal tumor diameter were the primary predictors of time to death from all causes and death with melanoma metastasis.
Historically, plaque therapy has been delivered using the 60Co plaque system originally developed by Stallard56 for treatment of retinoblastoma. These plaques are available in a limited range of sizes (8- to 12-mm diameters). Both circular and semicircular notched plaques are available for treatment of posterior lesions abutting the optic nerve. Although easy to prepare and use, 60Co plaques do not allow for customization of the dose distribution, shielding of critical structures, or treatment of eye tumors on an outpatient basis.
131Cs has been studied in the past few years as an alternative to iodine or palladium, given its higher mean photon energy resulting in increased energy penetration and lower outer scleral doses.57,58 Clinical trials using 131Cs are under way.
Pterygium Therapy
It is common practice to administer radiation therapy after surgical resection of the pterygium because of the high local recurrence rate (20% to 69%).59,60 In most institutions, a 90Sr β-ray applicator is used for treatment of these patients. The overall diameter of the applicator is 12.7 mm; the center is a circular radioactive disk 5 mm in diameter containing the isotope. The dose rate is generally about 5 Gy/min. Irradiation is begun within 24 hours after resection because local relapse increases with greater time delays.60
A lid retractor is inserted to hold the eye open. The cornea and conjunctiva are anesthetized with a few drops of 0.5% to 1% lidocaine. The applicator is carefully applied on the surface of the resected sclera. Doses of about 10 Gy are delivered. The application is repeated in three consecutive weekly fractions for a total of 30 Gy. If a larger area of resection is to be irradiated, it may require application to two contiguous areas, each receiving the same dose. The lens, which is located at the depth of 3.5 to 5 mm from the surface, receives less than 5% of the dose.61
Maxillary Sinus Therapy
Rosenblatt and colleagues62 described the use of a surgical obturator made of vinyl polysiloxane as a carrier for after-loading 192Ir seed ribbons to treat patients with maxillary antrum tumors after partial or total maxillectomy. Two weeks after the surgical procedure, an impression was made of the maxillary cavity, and the obturator mold was built. Multiple nylon catheters were inserted, depending on the geometry and dosimetry of the implant. Prescribed doses were 45 to 70 Gy at 0.5 cm from the outermost source plane. The obturator mold previously loaded with 192Ir was carefully coated with acylmethacrylate resin to secure it in place and prevent disturbance of the dosimetry once inserted in the surgical cavity.
Nasal Vestibule Implants
Small lesions of the nasal vestibule can be adequately treated with either external or interstitial irradiation, whereas more advanced lesions require a combination of both modalities. Irradiation is an excellent alternative to surgery in the treatment of these tumors because tumor control can be very good and cosmetic results are better than with surgery.63,64
These tumors are implanted with single- or double-plane techniques using rigid radium or cesium needles or 192Ir nylon tubing techniques. According to Mendenhall and associates, the distal vertical needles (perpendicular to the dorsum of the nose) in each plane may be mounted in a nylon bar in order to stabilize them.63,64
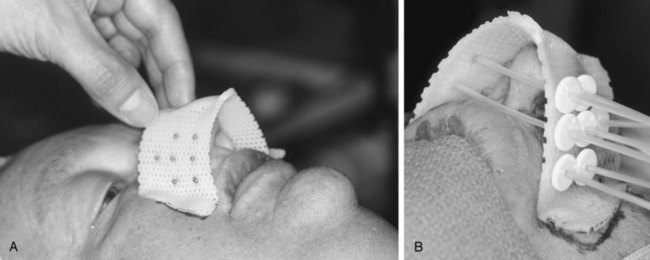
For small or large primaries, at WBH we always recommend the use of a cast and/or template to improve the geometric coverage of the implant. Both afterloading, LDR, and HDR treatment techniques can be used. It is important to pack all empty spaces to minimize the cavities. Figure 14-1 illustrates a patient treatment with the cast technique.
Skin and Lip Implants
Interstitial single- or double-plane implants could be performed to encompass the tumor with a safe margin. Doses of 50 to 70 Gy are delivered in 5 to 7 days. Carcinoma of the skin has been treated with surface molds or interstitial brachytherapy.65 Jorgensen and coworkers66 reported on 869 patients with squamous cell carcinoma of the lip for whom irradiation was the initial form of treatment in all but 25. Radium implants were used in 766 patients, with local tumor control rates of 93% in T1, 87% in T2, and 75% in T3 tumors. Brachytherapy is recommended over surgery in lesions at or close to the commissure. Functional results are superior to surgery without the postsurgical drooling of saliva.
Nasopharyngeal Implants
Some investigators have used interstitial techniques, which are more difficult to perform because of difficulty in positioning the needles/seeds in the tumor area and limitation of effective depth dose. Palatal fenestration may be required in patients with lesions in the superior and high posterior nasopharyngeal walls, which are more difficult to reach through the nasal or oral cavities.67,68 The use of 103Pd seeds for permanent implant of nasopharyngeal tumors has been described by Porrazzo and associates.69
Wang70 described the use of intracavitary brachytherapy alone or combined with EBRT to boost the dose to the nasopharynx. Two pediatric endotracheal tubes with inner and outer diameters of 5 mm and 6.9 mm, respectively, each loaded with two 20-mg RaEq 137Cs sources, are used. Local anesthesia of the nasal cavity is achieved using cocaine. The endotracheal tubes are introduced through the nares into the nasopharynx with the head hyperextended. Under fluoroscopic control on the simulator, the tips of the cesium sources are placed at the free edge of the soft palate posteriorly and behind the posterior wall of the maxillary sinus anteriorly. A 5-mL balloon attached to the distal end of the endotracheal tube is inflated for anchoring purposes. The dose reference point is 0.5 cm below the mucosa of the nasopharyngeal vault; the dose rate is approximately 1.2 Gy/h.
Levendag and colleagues described a nasopharyngeal applicator for HDR brachytherapy, currently commercially available (Nucletron™, Venendaal, The Netherlands), used as a boost.71,72 The Dutch investigators evaluated different high-dose, high-precision techniques as a boost in T1 to T4 nasopharyngeal cancers treated with EBRT.73 HDR brachytherapy was found to offer optimal sparing of organs at excellent target coverage for T1 to T2b tumors, whereas IMRT with stereotactic radiosurgery boost was the preferred technique for T3 to T4 lesions.
A recent publication from the MSKCC showed that re-irradiation of locally recurrent nasopharyngeal cancer resulted in significantly fewer grade 3 or higher events when an intracavitary brachytherapy boost was employed in conjunction with IMRT compared with IMRT alone (8% vs. 73%; p <.005), with the same local control and OS rates.74
Oral Cavity Implants
Implants of the Floor of the Mouth and Tongue
Marcus and associates75 described a template for floor-of-mouth implants made of aluminum, stainless steel, or nylon that is individually customized to fit the lesion of each patient. The device is inserted into the floor of the mouth under general anesthesia and is secured by one suture through the submental area, which is tied to a cotton cigarette roll. The active ends of the radium needles may be positioned above the level of the mucosa to ensure an adequate surface dose. Crossing is accomplished by placing a needle parallel to the mucosal surface on the implant device. The system is not afterloaded, but the procedure can be performed rapidly with predictable geometry so irradiation exposure to the operating staff is lower than with the hairpin technique. According to the investigators, the advantages of this technique over use of iridium hairpins is that all needles with the template are rigidly fixed in relationship to one another and that the isodose distributions can be calculated before the procedure or can be modified if necessary by adjusting the arrangement of the needles.
Implants of the Tonsillar Region, Including the Faucial Arch
Iridium 192 hairpin or plastic tube techniques have been used by Mazeron and coworkers.76 The nylon tube technique also may be used to implant the soft palate.77 The iridium hairpin technique is used with one gutter guide placed in the soft palate in the transverse plane and additional gutter guides placed vertically into the anterior tonsillar pillars, depending on the extent of the lesion. Iridium hairpins are afterloaded into the gutter guides, which are removed as described earlier.
Mendenhall and associates78 reviewed the techniques for implantation of the anterior tonsillar pillars, soft palate, or tonsillar region using two nylon bars, each containing three full-intensity, 2- to 3-cm active-length radium or cesium needles implanted into the anterior tonsillar pillar and the other 1 cm medial to the tonsillar pillar bar, in the base of the tongue. A crossing needle is sometimes included in the anterior pillar bar to ensure adequate mucosal dose. Goffinet and associates79 developed a method for intraoral tonsillopalatine implants.
Breast Implants as a Boost
Interstitial irradiation of the breast has been used as a boost in conjunction with conservation surgery after the whole organ is given EBRT (45 to 50 Gy). Bartelink and Borger in Europe80 and Martinez and Goffinet in the United States81 pioneered this technique. Selection of patients for this technique is limited to those with an adequate breast volume and lesions less than 5 cm in diameter.
Mansfield and associates82 described an intraoperative technique at the time of the breast tumor excision. The plastic tubes were loaded with the active sources within 6 hours of surgery. The dose rate was 0.3 to 0.5 Gy/h; the usual dose was 20 Gy delivered in 50 to 60 hours. Ten days later breast irradiation was begun with tangential fields, 6-MV photons, to deliver 45 Gy at 1.8 Gy per day. The 10-year local tumor control rates for stage T1 and T2 tumors were 93% and 87%, respectively, and the 10-year DFS rates were 82% and 75%, respectively.
Mazeron and associates83 used a technique for interstitial brachytherapy of the breast with rigid metallic needles inserted through a template in single or double planes. After EBRT (45 Gy in 25 fractions), a boost of 37 Gy to the primary tumor was prescribed at the 85% basal dose rate (Paris system). Implanted volume was adapted to tumor extent by varying the number of sources and active length according to the Paris system rules. Fifty-eight patients were treated with single-plane and 340 with two-plane implants. Local recurrence rates were 10% for T1 (2 of 20), 15% for T2a (21 of 138), 23% for T2b (30 of 129), and 25% for T3 (13 of 53). The local tumor control rates at 15 years were 76% for T1 and T2a lesions and 70% for T2b and T3 lesions. Mean dose rates were 0.53 Gy/h for patients with local recurrence and 0.56 Gy/h for recurrence-free patients (p <.01). Local tumor control correlations with dose rate and tumor size were shown.
Accelerated Partial Breast Irradiation
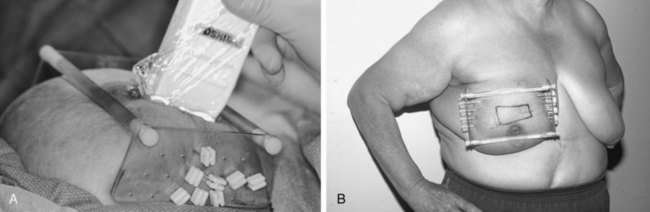
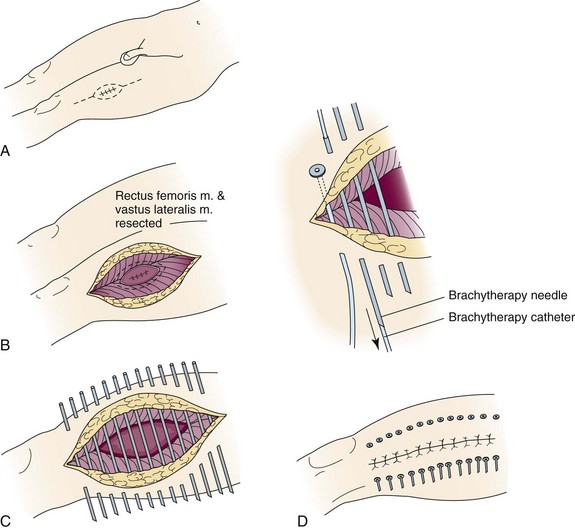
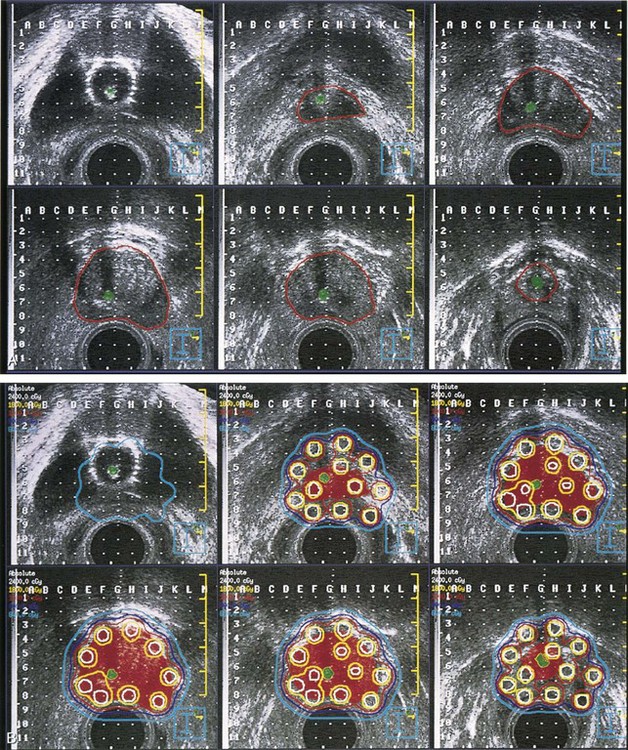
In an effort to improve the accessibility, convenience, and logistics of breast-conserving therapy at WBH, we initiated pilot trials to test the technical feasibility and acute toxicity of interstitial brachytherapy directed only to the tumor bed after lumpectomy in selected patients with early-stage breast cancer treated with breast-conserving therapy.84 An LDR APBI trial was initiated in 1991; in 1995 we switched to HDR interstitial breast brachytherapy and started an HDR APBI trial. Figure 14-2 demonstrates the application and use of one particular template system.
The interim findings of our in-house protocol treating the tumor bed alone after lumpectomy with LDR interstitial brachytherapy in selected patients with early-stage breast cancer treated with breast-conserving therapy were published by Vicini and associates.85 From March 1, 1993, through January 1, 1995, 50 women with early-stage breast cancer were entered into a protocol of tumor bed irradiation alone using an interstitial LDR implant. Patients were eligible if their tumor was an infiltrating ductal carcinoma 3 cm or smaller in diameter, surgical margins were clear by at least 2 mm, the tumor did not contain an extensive intraductal component, the axilla was surgically staged with three or fewer nodes involved with cancer, and a postoperative mammogram was performed. Implants were positioned using a template guide delivering 50 Gy over 96 hours to the lumpectomy bed plus a 1- to 2-cm margin. With a relatively short follow-up time, rates of local control, cosmetic outcome, and complications were encouraging
Vicini and associates86 published results in 199 breast cancer patients treated with APBI interstitial brachytherapy at WBH using either LDR or HDR treatment. At 5 years, the results (local control, cosmesis) are as good as those obtained with the more standard 6 weeks of whole breast EBRT. Chen and colleagues87 updated the toxicity analysis in these 199 patients with a mean follow-up of 5.4 years. The long-term rate of toxicity was as low as that for conventional 6-week treatment. Benitez and colleagues88 reported on the acute and long-term surgical complications of APBI with interstitial brachytherapy and found no increase in intraoperative, perioperative, or postoperative complications.
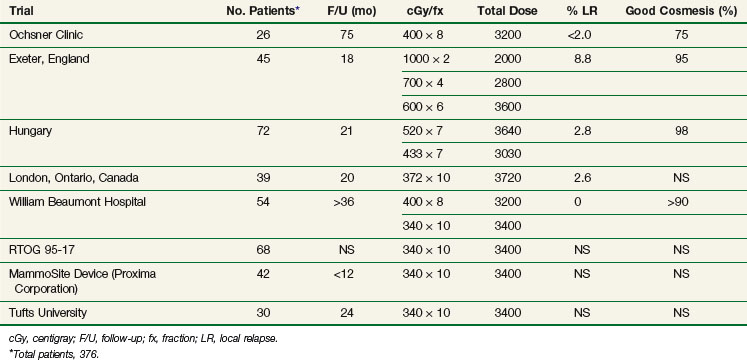
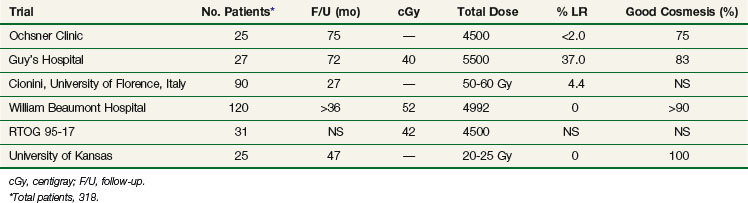
Results in various series using APBI with either HDR or LDR techniques are shown in Tables 14-1 and 14-2. Table 14-1 summarizes the phase I/II APBI using interstitial HDR breast brachytherapy, and Table 14-2 describes the phase I/II APBI using interstitial LDR breast brachytherapy.
In the only randomized trial completed to date, comparing whole breast irradiation (WBRT) to APBI with HDR multicatheter brachytherapy in 258 patients, there was no difference in 5-year rates of OS, DFS, or cancer-specific survival (CSS) between the two treatment modalities. However, there was a significantly better cosmesis achieved with HDR brachytherapy (APBI) as opposed to WBRT (excellent and good scores, 87% vs. 63%, respectively; p = .009).89
A single-institution matched-pair analysis was recently published by our group, examining the patterns of failure in patients treated with APBI versus WBRT with 10 years of follow-up.90 The rates of ipsilateral breast tumor recurrence were 4% in the WBRT group versus 5% in the APBI group (p = .48), and, therefore, WBRT would not confer an advantage in terms of ipsilateral breast tumor recurrence over APBI in well-selected patients.
Polgar and colleagues91 recently published data on the 12-year clinical outcome of one of the earliest experiences with interstitial HDR brachytherapy for suitable breast cancer patients (N = 45). Four (8.9%) ipsilateral breast tumor recurrences were observed, for a 5-, 10-, and 12-year actuarial rate of 4.4%, 9.3%, and 9.3%, respectively. A total of two regional nodal failures were observed for a 12-year actuarial rate of 4.4%. The 12-year rates of DFS, CSS, and OS were 75.3%, 91.1%, and 88.9%, respectively. Grade 3 fibrosis was observed in one patient (2.2%). No patient developed grade 3 telangiectasia. Fat necrosis requiring surgical intervention occurred in one woman (2.2%). Cosmetic results were rated as excellent or good in 35 patients (77.8%).
The Beaumont group confirmed the excellent cosmesis obtained with multicatheter HDR APBI, with over 95% of patients having excellent or good cosmesis with long follow-up. Most importantly, it was noted that cosmetic results stabilized at 2 years with a mild improvement thereafter.92
A new applicator, the single-lumen MammoSite balloon, was introduced as an alternative to the multiple-catheter brachytherapy technique. Edmundson and associates93 reported the dosimetry and excellent conformality in dose distribution. Keish and colleagues94 published the preliminary results using the MammoSite balloon technique.
Lung and Mediastinum Implants
Lung Implants
The group at MSKCC has published several reports95 on the use of 125I seeds and 198Au grains for permanent perioperative brachytherapy in patients with persistent or recurrent bronchogenic carcinoma after EBRT or for residual disease after surgical resection. The radioactive seeds or grains are directly implanted in the tumor at the time of thoracotomy under general anesthesia.
Temporary removable implants of the mediastinum with or without resection followed by a moderate dose of postoperative EBRT (35 to 40 Gy) have been used alone or combined with 125I implantation of the known primary tumor. The MSKCC report described local tumor control in 78% of patients with stage I and II tumors and 67% of those with stage III lesions.95 Furthermore, patients with microscopic residual tumor have significantly better tumor control and survival than those with gross residual disease.
Although lobectomy is the standard for lung cancer because a wedge resection has a three to five times greater incidence of local recurrence, poor pulmonary function may preclude lobectomy. Segmentectomy or wedge resection along with brachytherapy delivered via a vicryl mesh implant imbedded with 125I is a novel therapeutic modality to treat early-stage lung cancer. This modality is being evaluated in a large national prospective randomized trial (ACOSOG Z4032).96
However, there are concerns with exposure of operating room personnel and patient contacts to unnecessary radioactivity risks. Accordingly, HDR brachytherapy with afterloader catheters placed at the time of wedge resection has been recently reported.97 The radiation dose was 24.5 Gy (3.5 Gy per fraction over seven fractions twice daily for 4 days), prescribed to 1 cm deep to the stapled line.
Intrabronchial Site Implants
Intrabronchial insertion of LDR or HDR radioactive sources has gained popularity for treatment of patients with symptoms related to malignant airway obstruction. Figure 14-3 illustrates the use of a bronchoscopically placed endobronchial catheter.
Lo and colleagues98 described results in 110 patients (group 1) treated with LDR brachytherapy (133 procedures) and 59 patients (group 2) treated with HDR brachytherapy (161 procedures). In group 1, patients were treated with one or two sessions of 30 to 60 Gy each, calculated at a 1-cm radius. In patients in group 2, three weekly sessions of 7 Gy each, calculated at a 1-cm radius, were used. EBRT had previously been given to 88% of patients in group 1 and to 85% of patients in group 2. Laser bronchoscopy was performed in 36% of patients in group 1 and in 24% of patients in group 2 before brachytherapy. Clinical or bronchoscopic improvement was noted in 72% of patients in group 1 and in 85% of patients in group 2 (p >.05). Complication rates were low and equivalent in both groups. Survival rates were similar in both groups (median, <6 months).
Esophageal Implants
Flores and coworkers99 outlined the advantages of intracavitary brachytherapy. Radiation sources can be easily placed at the desired tumor site and subsequently removed. Normal anatomy is preserved. Radiation dose to the tumor is higher than to the adjacent tissues. With remote afterloading, radiation exposure to the staff can be eliminated. The insertion technique can be performed as an outpatient procedure under mild sedation. A soft rubber bougie or French catheter (No. 24 to 26) is inserted, preferably through the nose. The cut-end feeding tube is removed, and the esophageal stricture is dilated to 32-French by a balloon dilator (2 minutes required). The esophageal bougie containing dummy markers for intracavitary treatment is placed and secured in the desired position using fluoroscopy. After the position of the dummy sources is verified on radiograph, the patient is taken to the treatment room where the remote-controlled afterloading device is connected for treatment. If LDR sources are used, the usual dose rate is 0.4 Gy/h at 0.5 to 1 cm. Depending on the EBRT dose given, the total intracavitary dose is prescribed to complete 65 to 70 Gy to the tumor volume. With higher dose rates, corresponding lower treatment times and total doses are used.
Syed and colleagues100 described a comparable technique used in 47 patients with carcinoma of the esophagus (37 with primary and 10 with recurrent lesions). After completion of EBRT, patients received intraluminal brachytherapy to deliver 30 to 40 Gy at 0.5 cm from the surface of the applicator in two applications, 2 weeks apart. In patients with minimum residual tumor, only one application was used to deliver a 20- to 25-Gy minimal tumor dose. Most patients also received concomitant 5-fluorouracil infusion. Intraluminal application was performed with a Syed-Puthawala-Hedger esophageal applicator. The total length of the applicator is 65 cm. Marked rings are present at 10-cm intervals from the tip of the applicator for identification on localization films. The central nasogastric tube can be used for both feeding and suction. The procedure is performed under either general anesthesia or deep sedation and local anesthesia. Extent of the tumor, stricture, ulceration, and impending tracheoesophageal fistula are evaluated by endoscopy. Determination is made of the proximal and distal end of the tumor from the level of the incisor teeth, on endoscopy, and on review of the initial barium swallow x-ray films. Orthogonal anteroposterior and lateral x-ray films of the chest are obtained after inactive dummy sources have been inserted into the afterloading catheters in the applicator. The location of the tumor is marked on the x-ray films, and appropriate margins are determined to perform the dose calculations. Radioactive sources are spaced 0.5 to 1 cm apart, and margins of 3 cm above and below the tumor are allowed.
The main indication for brachytherapy in esophageal cancers is palliation of dysphagia in locally advanced or recurrent disease. A Dutch randomized trial comparing single-dose brachytherapy versus self-expanding metal stents used for palliation of esophageal obstruction due to inoperable cancer recruited 209 patients in nine institutions.101 Primary outcome was relief of dysphagia during follow-up, and secondary outcomes were complications, treatment for persistent or recurrent dysphagia, health-related quality of life, and costs. Dysphagia improved more rapidly after stent placement than after brachytherapy, but long-term relief of dysphagia was better after brachytherapy. Stent placement had more complications than brachytherapy (33% vs. 21%; p = .02), which was mainly due to an increased incidence of late hemorrhage (13% vs. 5%; p = .05). Groups did not differ for persistent or recurrent dysphagia (p = .81), or for median survival (p = .23). Quality-of-life scores were in favor of brachytherapy compared with stent placement. and total medical costs were the same.
Pancreatic Implants
In 98 patients described by Peretz and associates,102 the mean matched peripheral dose was 136.6 Gy. The mean activity of the implant was 35 mCi, and the mean volume was 53 cm3. Ten patients (10%) survived more than 18 months, and three patients are long-term survivors. Twenty-eight of 68 patients (45%) who had one or more follow-up radiographic studies to assess tumor response showed 30% or more reduction in tumor size. Significant pain relief was observed in 37 (65%) of 57 patients. Nineteen patients (20%) experienced postoperative complications; one patient died with a pancreatic fistula and generalized sepsis, and eight patients (8%) experienced major complications that included fistula formation, gastrointestinal bleeding, gastrointestinal obstruction, and intra-abdominal abscess. Similar survival results have been reported from other institutions. Because of potential biologic disadvantages of 125I (long half-life and LDR), Peretz and associates introduced 103Pd (half-life of 17 days and 20 to 23 KeV) as a new isotope for pancreatic implants.102
Biliary Tree Implants
An increasingly popular technique is the insertion of radioactive sources via Teflon catheters in the biliary tree under fluoroscopic conditions. The main objective is to drain bile and palliate obstructive jaundice. Intracatheter irradiation is best delivered as a boost supplement to EBRT, which is delivered to a larger target volume, including nodal drainage.103
An alternative method of insertion for the radioactive sources is via an endoscopic approach under fluoroscopic control. This has become the preferred technique in institutions at which liver transplant is considered after planned preoperative chemoradiation, because it prevents potential tumor implantation in the catheter tract that transgresses the chest or abdominal wall (see Chapter 47).
Meerwaldt and associates104 reported on 42 patients with bile duct tumors treated with one or two brachytherapy sessions plus EBRT, alone or combined with palliative resection. A dose of 15 Gy was delivered at each of two sessions or 25 Gy in one session, calculated at 2 cm from the wire, combined with EBRT (40 Gy in 16 fractions). Fourteen percent of the 42 patients survived for 2 years or more. Median and long-term survival rates were best in the 11 patients who had a noncurative resection in addition to irradiation (median survival of 15 months versus 8 months; 3-year OS rate of 36% vs. 6%; p = .06). Fever occurred shortly after insertion of the 192Ir wire in 6 of 38 brachytherapy sessions; it was usually controlled with antibiotics.
Fritz and colleagues105 reported on 18 patients with carcinoma of the hepatic duct bifurcation and 12 patients with carcinoma of the common duct or the common hepatic duct treated with EBRT and intraluminal HDR brachytherapy alone or combined with noncurative resection. Nine patients received radiation therapy after palliative tumor resection, and 21 patients were primarily irradiated. Twenty-five patients completed the full course of radiation therapy. EBRT dose varied from 30 to 45 Gy and brachytherapy doses from 20 to 45 Gy. Biliary drainage after irradiation was achieved using percutaneous catheters, endoprostheses, or stents. The median survival time for the entire group was 10 months. The actuarial survival rate was 34% after 1 year, 18% after 2 and 3 years, and 8% after 5 years. Three patients were still living without evidence of disease at 35 to 69 months. Major complications such as bacterial cholangitis could be lowered from 37% to 28% through exchange of percutaneous transhepatic catheters to endoprosthesis or stents. The longest lasting drainage was achieved using stents. The incidence of radiogenic ulcers was lowered from 23% to 7.6% after the total dose of HDR afterloading boost was reduced to 20 Gy.
Mayo Clinic investigators published results in a series of 24 patients with unresectable bile duct cancers treated with EBRT plus 192Ir alone or with concomitant 5-fluorouracil during EBRT.106 EBRT was delivered at a dose of 45 to 54 Gy in 25 to 30 fractions over 5.5 to 6 weeks, and the brachytherapy dose was 20 to 30 Gy at a 1-cm radius. Three of the 24 patients were 5-year survivors; results appeared best in the nine patients who received concomitant 5-fluorouracil during EBRT (5-year OS of 22% vs. 8%).
Soft Tissue Sarcomas
Because of the use of irradiation, wound closure requires extra planning and care to avoid undue tension predisposing to wound breakdown. To further diminish wound complications, the loading of the 192Ir ribbon or 125I seeds is delayed until 3 or 5 days after surgery (3 days is adequate if there is no wound tension).107 Before loading, anteroposterior and lateral radiographs with radiopaque dummy sources in the plastic tubes provide the information necessary for computerized dosimetry calculations and dose-rate determination. CT planned dosimetry is recommended.
The LDR dose for tumor bed implants is 45 to 50 Gy in 4 to 5 days if used as the sole therapy for high-grade sarcomas. For HDR implants the dose is 4 Gy × 101 in a twice-daily schedule or 15 to 20 Gy if used as a supplement to EBRT doses of 45 to 50 Gy and a marginal gross total resection. At the MSKCC, EBRT is now combined with brachytherapy for high-grade sarcomas with positive margins of resection and for all low-grade lesions. In such cases the implant boost dose is 15 to 30 Gy, supplemented by 45 to 50 Gy of EBRT (15 to 20 Gy for narrow or microscopic positive margins, 30 Gy for grossly positive margins). For additional technical details, the reader is referred to the textbook by Hilaris and colleagues.108
Pisters and coworkers109 reported on 164 patients with soft tissue sarcomas randomized to receive or not receive brachytherapy as the sole method of irradiation after complete wide local tumor resection at the MSKCC (78 and 86 patients in either group, respectively). A target region in the tumor bed was identified by adding 2 cm to the superior and inferior dimensions and 1.5 to 2 cm in the medial and lateral directions. Afterloading catheters were placed approximately 1 cm apart and were fixed in treatment position with absorbable sutures, secured to the skin at the catheter exit site with buttons and nonabsorbable sutures. Implant dose was 42 to 50 Gy over 4 to 6 days using 192Ir. Sources were loaded on the fifth or sixth postoperative day to decrease interference with wound healing. There were 13 local recurrences in 78 patients (16%) who received brachytherapy and 25 (29%) in 86 patients treated with surgery only. Actuarial estimates of local recurrence at 60 months were 18% in the brachytherapy group and 31% in the no-irradiation group. It is highly likely that the prescribed dose of irradiation was not adequate to eliminate microscopic disease, and higher doses (55 to 60 Gy) would have been more effective. Subsequent evaluations of the MSKCC data show inadequate local control of low-grade sarcomas using brachytherapy alone and of high-grade lesions with positive margins of resection. Such patients are now treated with combined EBRT and brachytherapy.
Potter and colleagues110 reported on 12 patients with soft tissue sarcomas treated with HDR or PDR brachytherapy. Brachytherapy was part of the recurrence treatment regimen in eight patients and part of the primary treatment alone or combined with EBRT in four patients.
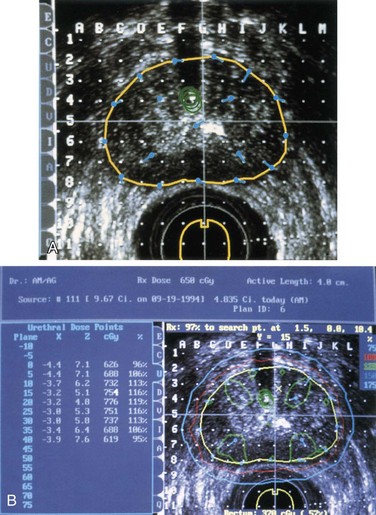
Brachytherapy (15 to 20 Gy with LDR) was combined with EBRT (45 to 50 Gy) either preoperatively or postoperatively by Schray and associates at the Mayo Clinic.111 Three-dimensional reconstruction of the tumor or tumor bed was accomplished by CT scan or MRI. Margins beyond the tumor were 5 to 10 cm axially or along tissue planes and 2 to 4 cm radially or perpendicular to tissue planes for the EBRT component of treatment. Brachytherapy was performed with standard nylon afterloading tubes positioned to encompass the boost volume with a 2- to 4-cm margin (Fig. 14-4). The boost volume was considered to be the tumor bed after preoperative irradiation and the surgical bed and incision if no previous irradiation had been given. With rare exceptions (distal extremities, groin), needles were placed transversely to the incision (and axis of the extremity) under direct visualization of the tumor bed and with entry and exit points outside the tumor and surgical bed. Nylon tubes were commonly placed in contact with bone and neurovascular structures and were maintained in place by skin fascia and muscle, sutures being rarely used. After the implant was in place, the skin was closed with sutures. Implants were loaded 72 to 96 hours postoperatively with 192Ir; the sources were placed 1 cm deep to the skin surface along the tube axis. Average implant activity was 107 mCi, and a portion of the implants used two or more planes. The average dose rate was 48.6 cGy/h, average time was 44.2 hours, and average dose was 20.52 Gy. In 63 patients, 65 brachytherapy procedures were performed. With median follow-up of 20 months, there were two local failures in 56 patients (4%) treated initially and in 3 of 9 patients treated for recurrent tumors. Only one of the five local recurrences was within the implanted volume. Two of 40 implants (5%) performed at initial resection followed by postoperative EBRT led to wound complications, in contrast to 4 of 16 implants (25%) performed at resection after preoperative EBRT.
One of the largest series of patients with high-grade soft tissue sarcomas of the extremities treated with surgery and immediate adjuvant HDR brachytherapy followed by EBRT was reported from the University of Florence, in Italy.112 One hundred twelve patients were enrolled, and the median follow-up time was 75 months (minimum, 11 months). The OS was 77.5% and 71.1% at 5 and 7 years, respectively, and the DFS was 63.3% and 58.4% at 5 and 10 years, respectively. Local control was achieved in 91.5% and 87% at 5 and 10 years, respectively. Fourteen patients (12.5%) had local complications attributed to radiotherapy. Eleven patients developed wound healing problems that needed further surgery.
The addition of brachytherapy after surgery for primary or recurrent sarcomas of the extremities yields good results with local control in excess of 88% and a relatively low rate of complications (below 15%).113,114,115,116,117
Uterine Cervical Implants
If ideally inserted in the patient, the tandem should be in the midline or as nearly as possible equidistant from the lateral pelvic wall, and the vaginal colpostats should be symmetrically positioned against the cervix in relation to the tandem. The tandem should be kept along the sagittal axis of the pelvis, equidistant from the pubis, sacral promontory, and lateral pelvic wall, as allowed by the geometry of the patient and the tumor to avoid overdosage to the bladder, rectosigmoid, or either ureter. Corn and colleagues,118 in a retrospective evaluation of the technical quality of brachytherapy procedures with respect to ovoid and tandem placement, demonstrated a significantly worse outcome for patients whose implants were judged to be unacceptable.
Brachytherapy Systems for Carcinoma of the Cervix
The Manchester intracavitary system, introduced by Tod and Meredith119 in 1938, was the first applicator and loading system designed to meet certain dosimetric specifications, and it used a dosimetric field quantity, total exposure at point A, to prescribe treatment, rather than milligram hours. Point A was defined as being 2 cm above the mucous membrane of the lateral vaginal fornix and 2 cm lateral to the center of the uterine canal. Allegedly this area corresponded to the paracervical triangle, in the medial edge of the broad ligament, where the uterine vessels cross the ureter. A subsequent arbitrary convention defined point A as being 2 cm above the external cervical os and 2 cm lateral to the midline. Yet another definition located point A as being 2 cm above the distal end of the lowest source in the cervical canal and 2 cm lateral to the tandem.
Mini-colpostats have a diameter of 1.6 cm and a flat inner surface to allow their insertion in patients in whom the only alternative would be protruding vaginal sources in the tandem.120 Some mini-ovoids have no shielding; therefore, the surface dose is significantly higher than with the regular ovoids (with 3M cesium sources, the surface dose is 9.8 cGy/mg/h with the mini-ovoids, in contrast to 6.3 cGy with the 2-cm-diameter ovoids), and they are usually loaded with 10-mg sources. The 3M mini-ovoids have internal shielding. However, phantom measurements did not demonstrate a significant decrease in dose for the newer mini-colpostats with rectal shielding for a source separation of 3 cm, which potentially could allow undue user confidence in the doses delivered.
Henschke Applicator
The Henschke applicator and other applicators are commercially available.121 The basic configuration of the ovoids is hemispheres that are inserted parallel to the lateral wall of the vaginal vault and the intrauterine tandem. Three ovoid diameters and various tandems are available. Although this applicator’s configuration conforms better to a narrow vaginal vault, the radioactive sources are placed parallel to the long axis of the bladder and the rectum and do not have any shielding, therefore potentially delivering a higher dose to these organs. Users should familiarize themselves with the dosimetric aspects of these devices. Delclos and colleagues120 emphasized that the dosimetry with the Fletcher colpostats is unique and that treatment techniques and tables derived for this applicator should not be used with other applicators, because this might result in significantly higher doses to the vagina, bladder, or rectum. Appropriate source loading and dose prescription produce satisfactory clinical results.
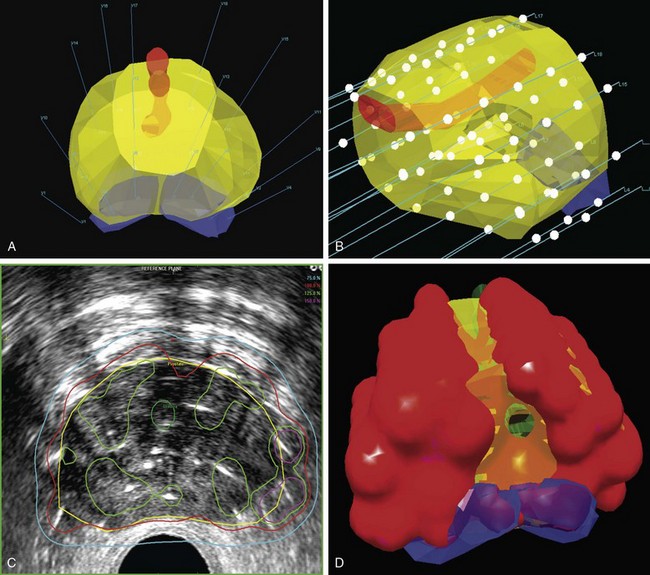
Image-Guided Cervical Brachytherapy
MRI has become in the last decade the best and most accurate imaging modality for a number of clinical sites, including cervical cancer. Due to its superior contrast resolution for soft tissues, resulting in better definition of tumor size, volume, parametrial infiltration, and distinction from the normal uterus, MRI is superior to CT or ultrasound in delineating cervical cancer target volumes as well as the organs at risk.122
In the late 1990s, there was an increasing interest in using MRI for 3D brachytherapy planning.123–125 The early clinical experience with MRI-based 3D HDR or LDR brachytherapy was reported by the University of Vienna and Institut Gustave Roussy.126–129 In 2000, GEC-ESTRO (Groupe Européen de Curiethérapie and European Society for Therapeutic Radiology and Oncology) decided to support a 3D imaging-based, 3D treatment planning approach in cervical cancer brachytherapy with the creation of a Working Group. Because the gross tumor volume (GTV) and clinical target volume (CTV) for brachytherapy change significantly during treatment, the time frame for assessment of GTV and CTV for brachytherapy is specified in this report: At the time of diagnosis, it is noted as GTV(D) and CTV(D) and at the time of brachytherapy as GTV(B) and CTV(B). Furthermore, the CTV for brachytherapy is defined related to the risk for recurrence (high-risk CTV and intermediate-risk CTV).130 Recommendations from the same European working group were formulated regarding 3D dose-volume parameters for brachytherapy of cervical carcinoma with emphasis on MRI assessment of GTV and CTV.131 In one seminal report from Vienna, Potter and associates were able to demonstrate not only a significant decrease in grade 3 and 4 gastrointestinal and genitourinary toxicities from 10% to 2% with the use of MR-based brachytherapy but, more importantly, a significantly better local control rate (90% vs. 71%) and better 3-year OS for tumors greater than 5 cm (58% vs. 28%).132 In a follow-up report, the Vienna group reiterated the importance of treatment planning based on dose-volume histograms and the importance of dose-response relationships for local control, especially for the high-risk clinical target volume (HR-CTV) group.133 They found a significant dependence between local control and dose coverage of high-risk CTV, expressed as D100 and D90. A D90 for HR-CTV greater than 87 Gy resulted in an LR incidence of 4% compared with 20% for a D90 less than 87 Gy. They concluded that tumor control rates of more than 95% can be achieved if the D90 (EQD2) for HR-CTV is 87 Gy or greater.
Positron emission tomography combined with CT (PET/CT) has also been described as a useful tool in 3D-based adaptive brachytherapy.134,135 However, its more recognized imaging role is to determine whether nodes are metastatically involved135 (see web-only expanded discussion, available on the Expert Consult website![]() ).
).
Image-guided brachytherapy for cervical cancer has the potential for significant growth in the United States, as shown by a recent poll of the American Brachytherapy Society.136
Interstitial Implants for Cervical Carcinoma
Metallic needles containing 226Ra, 60Co, or 137Cs or afterloading metallic guides or Teflon catheters for insertion of 192Ir wires or seeds have been implanted in the parametrium or cervix, using a transvaginal or transperineal approach (sometimes in lieu of intracavitary insertions when the cervical canal cannot be identified) and frequently with the aid of templates.137 The procedure is similar to that followed for intracavitary insertions. The cervix should always be held firmly with a tenaculum. For implants in the cervix itself, the needles or nylon catheters with metallic guides (5 to 6 cm long) are inserted straight, about 1.2 cm apart, following the position of the uterus (which can be verified with a finger in the rectum).
Martinez and associates,137 Aristizabal and coworkers,138 and Feder and colleagues139 have popularized the use of interstitial implants, using perineal templates with introduction of metallic needles through the perineum into the parametrial tissues. Iridium 192 seeds are inserted in an afterloading fashion. Aristizabal and colleagues138 modified their technique by deleting three anteriorly and three posteriorly placed needles in the central row; the central tandem was also omitted in an effort to decrease an initial high incidence of vesicovaginal or rectovaginal fistula. The investigators reported about 75% pelvic tumor control in 118 patients with stages IIB and III carcinoma of the uterine cervix. The major complication rate was 6% with less than 4500 mg/h, 16% with 4500 to 4999 mg/h, 28% with 5500 mg/h, and 87% with higher intracavitary doses (combined with 45 to 50 Gy to the whole pelvis).
Martinez and coworkers29 described results in 104 patients with locally advanced or recurrent pelvic tumor using a universal perineal template (32 to 35 Gy at a dose rate of 2.75 cGy/h) combined with EBRT (36 Gy to the whole pelvis and 14 Gy to the pelvic side wall with midline block using four-field techniques, 4- or 10-MV photons). Local tumor control was obtained in 82% of 63 patients with gynecologic lesions. The major complication rate requiring surgical intervention was 3.2%.
Leborgue and colleagues140 reported their experience with MDR brachytherapy (1 to 12 Gy/h). In carcinoma of the cervix, EBRT with a central block was given to the pelvis (40 Gy at 2 Gy/fraction), and patients with stage IIB disease received an additional 20 Gy to the whole pelvis without central shielding. A control group of 102 patients was treated with LDR brachytherapy (average dose rate was 0.44 Gy/h, two 32.5-Gy fractions to point A in 74 hours each, 2 weeks apart). The MDR group was treated at 1.6 to 1.7 Gy/h to point A. Dose fractionation schedules for MDR were derived using the linear-quadratic equation to arrive at a biologically equivalent dose. Grade 2 and 3 sequelae were noted in 1 of 102 patients treated with LDR brachytherapy, in 25 (83%) of 30 patients treated with MDR brachytherapy with a 5% dose reduction compared with LDR therapy (61.75 Gy), and in 4 (40%) of 10 and 0 of 38 patients treated with 3 or 6 MDR fractions for a total of 58 or 55.5 Gy to point A, respectively. The average nominal biologically effective dose for the various groups ranged from 78 to 124 Gy. The incidence of the late rectal complications was zero for patients receiving rectal biologically effective doses of less than 50 Gy, 24% to 36% for 50 Gy to 199 Gy, and 67% for 200 Gy or greater. The investigators concluded that the safest schedule was to deliver 18 Gy to the whole pelvis with EBRT, plus brachytherapy delivering a dose rate to point A of 1.6 Gy/h, in six fractions of 8 Gy, two in each treatment day, 10 days apart. Two fractions are given on a single day, 6 hours apart, to reduce the number of insertions to three. This study emphasizes the importance of conducting prospective dose fractionation studies based on sound biologic data.
LDR versus HDR Brachytherapy Implants
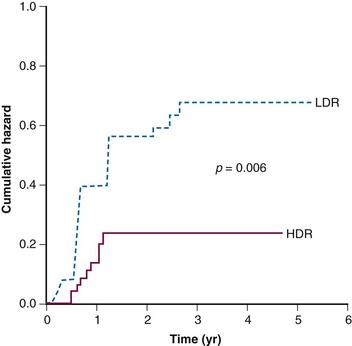
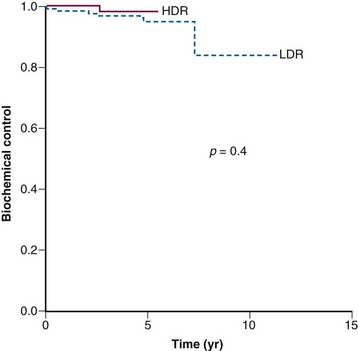
There is still a matter of controversy as to whether HDR brachytherapy may be associated with a higher risk of long-term complications than LDR brachytherapy. Nonetheless, multiple retrospective comparisons, a few randomized trials, and a recent meta-analysis have failed to demonstrate that one type of brachytherapy is better than the other.141,142,143–145
Endometrial Implants
The dose of irradiation delivered with this system is somewhat empirically derived. In general, in preoperative insertions we use 3500 mg/h in the uterine cavity; however, cavities larger than 8 cm receive doses of approximately 4000 mg/h. Doses of about 65 Gy to the mucosal surface of the vagina are delivered (1900 to 2000 mg/h) with 2-cm-diameter vaginal ovoids. Grigsby and colleagues146 reported higher survival rates and fewer pelvic recurrences and distant metastases in patients with stage I poorly differentiated endometrial carcinoma when doses higher than 3500 mg/h were delivered in the uterus. A lesser beneficial impact was noted in moderately differentiated tumors.
Chao and colleagues147 described the medical complications associated with 150 intracavitary implants performed in 96 patients treated with irradiation alone for inoperable carcinoma of the endometrium. General anesthesia was used in 98 implants, spinal anesthesia in 26 implants, local anesthesia in 25 implants, and epidural anesthesia in 1 implant. Preventive measures included low-dose cutaneous heparin in 55 patients and intermittent pneumatic compression boots in 29. Four patients (4.2%) developed life-threatening complications (myocardial infarction in two patients, congestive heart failure in one patient, and pulmonary embolism in one patient). Two patients died (of myocardial infarction and pulmonary embolism).
Rotte148 reported an incidence of 7.5% thromboembolic complications in 106 patients with carcinoma of the endometrium undergoing LDR implants. In contrast, none of the patients treated at the institution with HDR devices had thromboembolic phenomena. It is important to identify patients at high risk for thromboembolic complications, such as those with trauma to the lower extremities or pelvis, obesity, advanced age, and a history of prior thromboembolism. For these patients, outpatient HDR brachytherapy is the recommended intracavitary treatment.
Implants of the Vagina, Vulva, and Female Urethra
Indications for and techniques of interstitial therapy for carcinoma of the vagina, vulva, and urethra have been described.32,137,149 These areas are potentially vulnerable to severe complications because of the reported lower tolerance of the surrounding tissues to irradiation and because these areas are exposed to the constant irritation of perspiration, urine, and, occasionally, feces; therefore, it is important to minimize irradiation to the surrounding normal areas.
Implants for Tumors of the Vulva or Distal Urethra
Vulvar or distal urethral tumors can be treated with similar brachytherapy techniques. Erickson150 published a historical review of interstitial implants for vulvar carcinoma.
The patient is placed in a lithotomy position, and ring, double-plane, or volume implants can be designed around the urethra or in the vulvar labia. When the procedure is completed, cystoscopy is performed to ascertain the position of the catheters in the bladder and an indwelling three-way catheter is inserted. If there is intravesical bleeding, periodic irrigation of the bladder is necessary while the implant is in place, and it is preferable to leave a three-way catheter in place for a few days (up to 1 week) to facilitate bladder irrigation and avoid clot formation with bladder neck obstruction. When the vulva is involved, the sources must protrude into the perineum. If the tumor extends into the vagina, an intravaginal cylinder with some sources may be necessary to increase the dose to the vaginal mucosa. Gupta and associates33 reported excellent local controls with minimal urinary incontinence. The design of the implant, placement of the radioactive sources, and tumor doses are similar to those for comparable lesions in the vagina.
Anal Canal and Rectum Implants
Papillon and coworkers151 reported on 221 patients with epidermoid carcinoma of the anal canal treated with EBRT 7 combined with 5-fluorouracil and mitomycin C, followed by a 192Ir implant 2 months later. The implants were performed with either a plastic template or a steel fork, using four to eight wires, 5 to 7 cm long, adapted to the tumor extent covering the quadrants of the anal circumference involved by the tumor. A minimum dose of 15 to 20 Gy was delivered in 15 to 28 hours. Of 179 patients followed for 5 years, 118 (65.9%) were alive and well and 110 (61.4%) had anal preservation. Thirty-three patients (18.4%) died of cancer. Of patients with tumors measuring less than 4 cm, 50 (75.7%) of 66 were alive with anal preservation at the time of the report, and only 5 (7.5%) died of cancer.
Papillon and colleagues151 also reported on 90 patients with T1 or T2 rectal carcinoma treated with contact x-ray endocavitary therapy followed by a 192Ir implant with an iridium fork. Doses were similar to those administered to the patients with anal carcinoma. The 5-year OS was 77.8%; 67 (74%) were alive with anal preservation, and only 10 (11.1%) died of cancer. They also reported on a third group of patients with more advanced, moderately infiltrating, low-lying T2 or T3 tumors, who would have been treated by abdominoperineal resection but because of age or poor operative risk were treated with radiation therapy, including interstitial implants. At 4 years, 37 (59.6%) of 62 patients were alive, and 36 (58%) had anal preservation. Only nine patients (14.5%) died of cancer; three had unresectable lesions and one died after major surgery.
Puthawala and colleagues152 reported on 40 patients with anorectal cancer who were treated with EBRT (40 to 50 Gy in 25 to 30 fractions) followed by two 192Ir implants using the Syed template to deliver a total tumor dose of 65 to 75 Gy. Local tumor control was achieved in 70% of tumors, with 20% major morbidity.
A nonrandomized comparison between EBRT boost versus brachytherapy was done in a cohort of 162 patients with invasive nonmetastatic anal squamous cell carcinoma treated with a split course of EBRT to 45.1 Gy followed by either an EBRT boost or brachytherapy to a mean dose of 17.9 Gy. Boost modality and overall treatment time were the only significant prognostic variables in multivariate analysis.153 In the subgroup of patients with an overall treatment time of less than 80 days, the 5-year cumulative rate of local recurrence was significantly decreased with the brachytherapy boost (brachytherapy versus EBRT, 9% vs. 28%; p = .03). In the case of an overall treatment time of 80 days or more, the 5-year rate of local recurrence was not affected by the boost technique (brachytherapy vs. EBRT, 29% vs. 38%; p = .21).
Bladder Implants
Van der Werf-Messing154 popularized bladder implants with radium or cesium needles. Apart from surgery and general anesthesia, disadvantages of this technique were radiation safety, impaired wound healing because of acute side effects, and, sometimes, difficulties in removing the radioactive sources. In a series of 160 patients, the mean hospitalization time was 36 days after the operation. In 10% of the patients, the abdomen had to be reopened to remove one or more needles. Van der Werf-Messing and associates used radium implants in 328 patients with T2 tumors and 63 patients with T3 tumors after a small dose of preoperative irradiation (3.5 Gy for three fractions). The recurrence rates were 16% for the T2 tumors and 28% for the T3 tumors. The DFS was 75% and 62%, respectively. Battermann and Tierie,155 using a similar technique, obtained local tumor control in 69 (81%) of 85 patients with T2 tumors, and a 10-year DFS of 56%. Subsequently, van der Werf-Messing and van Putten156 used 40-Gy EBRT followed by 137Cs implants in 48 patients with T2 bladder tumors and 42 patients with T3 tumors. The 5-year DFS was 70%.
A different method using iridium wires was designed in France and subsequently modified by Battermann and Boon157 to overcome most of the disadvantages of the rigid needle technique. After a lower abdominal incision, the bladder is opened to visualize the tumor area. Plastic carriers consisting of a hollow part and a thinner leading end are inserted 1.5 cm apart. The tubes penetrate the abdominal wall, are tunneled in the bladder muscle through the tumor and out of the bladder, and penetrate the abdominal wall again. The catheters should be placed in such a way that removal is feasible without a second laparotomy, although in more complex cases this may be necessary. Dummy sources are introduced in the carriers to visualize the length of the source to be used while the bladder is still open. After the position of the sources is checked, the bladder is closed, and subsequently the abdomen is closed. A Foley catheter is placed for drainage. After film localization, the dose distribution is determined. The carriers are connected to the MicroSelectron (LDR or HDR), and the radioactive phase of the procedure is started. The tubes are well tolerated and, after completion of irradiation, can be removed easily. All patients receive preoperative EBRT to prevent tumor seeding during operation (30 Gy). A dose of 40 Gy is given by brachytherapy at a dose rate of 0.3 to 0.5 Gy/h.
Although EBRT combined with chemotherapy is a well-established alternative to surgery in selected bladder cancer patients in an effort to preserve the organ or in inoperable cases, the combination of EBRT and brachytherapy was studied less in this setting. A multi-institutional Dutch retrospective case-control study comparing the outcome of surgery and brachytherapy-based radiotherapy in patients with solitary T1G3 to T2 bladder tumors with a diameter of up to 5 cm was recently published.158 Eighty-nine patients (mean age, 68.4 years) underwent transurethral tumor resection (TURT) followed by a course of EBRT and interstitial brachytherapy, and 179 patients underwent TURT followed by cystectomy. Their technique was previously described and consisted of placing needles at the time of cystectomy and using 137Cs, and after 1996, afterloading catheters using 192Ir.159 The dose delivered with brachytherapy was either 55 Gy in combination with pelvic EBRT of 304 fractions of 3.5 Gy or 30 Gy with brachytherapy followed by 20 Gy of EBRT in 10 fractions. With a median follow-up of over 5 years, there was no difference in disease-specific survival rates between the groups (5- and 10-year rates of 71% and 66% in the brachytherapy group and 60% and 57% in the cystectomy group, respectively). Five-year OS were 57% in the brachytherapy group and 52% in the cystectomy group; however, the 10-year OS was better in the cystectomy group than in the brachytherapy group (42% and 33%, respectively). This is caused by the significant age difference in favor of the cystectomy group. The cystectomy-free survival rate in the brachytherapy group was 70%. These results, obtained in a multi-institutional setting, suggest that in a selected patient population a bladder-sparing treatment including brachytherapy (i.e., a combination of TURT, EBRT, and interstitial brachytherapy) can be applied successfully.
Implants of the Penis
The treatment of penile squamous cell carcinoma is generally surgical. In selected cases (T1 to T2 tumors <4 cm, no penile shaft invasion), brachytherapy yields excellent results with good erectile function preservation (see Chapter 55).160,161,162,163–165
The most common technique uses a pair of Plexiglas templates and rigid needles.160,165 Iridium 192 wires are manually loaded. A dose of 60 Gy is delivered over 4 to 5 days. Pulse-dose-rate therapy is an alternative to 192Ir wires, with the added benefit of radioprotection. Pretreatment circumcision is essential for good cosmesis and adequate follow-up.
The 5-year penile preservation rates are 70% to 88%, and at 10 years, 67% to 72%. In one of the largest published series from France, the 10-year painful ulceration and stenosis risk rates were 26% and 29%, respectively.161 The 10-year CSS was 92%.
Prostate Implants
Permanent Iodine-125 Implants
Hilaris and colleagues166 popularized the use of 125I seeds for treatment of stage A (T1), B (T2), or, occasionally, early C (T3) prostate carcinoma. The 125I seeds were implanted permanently in the prostate through an open retropubic laparotomy incision with the patient in a modified lithotomy position.
Critz and associates,167 in a report of 239 patients with clinical T1 or T2 surgically negative-node carcinoma of the prostate treated with 125I prostate implants (80 Gy MPD [minimal peripheral dose]) after pelvic EBRT (10- by 8-cm portals, bilateral 120-degree arcs, 45 Gy in 5 weeks), described a good correlation between the prostate volume and amount of 125I required for implant (15 to 20 mCi for a 30-cm3 prostate volume versus 25 mCi for a 60-cm3 prostate volume). Five patients developed rectal ulcers that were treated conservatively, and one patient required a colostomy. One patient had superficial urethral necrosis and two urethral strictures (history of previous transurethral resection). Sexual potency was maintained at 5 years in 75% of patients.
The treatment of organ-confined carcinoma of the prostate with permanent radioisotopes by the retropubic method has generated variable and controversial results. Advances in radioisotope development, computer-based dosimetry, and transrectal ultrasound and CT imaging have fostered techniques of closed transperineal implantation that produce more homogeneous, reproducible, and larger-volume implants with a higher peripheral dose than was possible in the past.168 With a median follow-up of 37 months (range, 12 to 78 months), 93% of 291 early-stage A and B patients treated with 125I or 103Pd alone had a normal posttreatment prostate-specific antigen (PSA) level (median value, 0.4). Of 160 more advanced, stage A to C patients treated with EBRT and implant boost, 85% had a normal PSA level (median value, 0.3). The elimination of surgery with these techniques permits outpatient treatment, resulting in high patient acceptance. If longer follow-up substantiates the favorable early results, these methods may offer the least morbid and least expensive method of treatment for early-stage carcinoma of the prostate.169
Transperineal Implants: Ultrasound Guided
Implants are performed on an outpatient basis under spinal anesthesia. With the patient in an exaggerated pelvic tilt lithotomy position, the 7.5-MHz biplanar ultrasound probe is inserted in the rectum to guide the physician in the positioning of the needle insertion. Transverse images are recorded at 5-mm increments from the base of the prostatic apex. The optimal seed distribution is determined by superimposing the computer-generated isodose distribution over the target volume. Disposable guides preloaded with sources and absorbable spacers and/or seeds in a strand of absorbable suture are introduced through the appropriate template holes as indicated in the plan. Alternatively, the Mick applicator (Mick Radio-Nuclear Instruments, Inc., Bronx, New York) may be used. Each needle is guided to its predetermined position within the prostate under direct ultrasound visualization. Figure 14-5 demonstrates the use of ultrasound volume studies in treatment planning for prostate implants. The onscreen template coordinates of the ultrasound unit allow accurate visualization of the needle position in the prostate. Some institutions have used CT-based fluoroscopic methods for monitoring needle insertion. CT-based dosimetry is performed on every patient.
The recommendation for use of permanent implants is based on both patient and disease factors. These include prostate volume less than 60 cm3, no severe pubic arch interference, no severe urinary obstructive symptoms, and clinically intracapsular disease; prior transurethral resection of the prostate is discouraged. The Gleason score should be 6 or less and the PSA level 10 or less; the patient should be stage T1 or T2a. In addition, one can use either 125I or 103Pd, alone or as a boost, after EBRT. Use of 125I and/or 103Pd seeds embedded in a stiffened Vicryl carrier has decreased the possibility of seeds migrating and causing lung embolization. Indeed, in a small randomized study of 64 patients comparing loose seeds with stranded seeds, more of the patients with loose seeds experienced seed loss (47%) than those with stranded seeds (23%).170
Wallner and associates,171 in a review of 65 patients treated with a transperineal 125I implant for T1 and T2 prostatic carcinoma, noted that a greater incidence of urinary grades 2 and 3 morbidity was associated with the maximum central urethral dose, length of urethra that received more than 300 Gy, and large prostate volume. Rectal ulceration was associated with irradiation of the rectal wall to doses greater than 100 Gy. Efforts should be made to keep the central urethral dose below 400 Gy and rectal surface dose below 100 Gy to decrease toxicity.
Long-term outcome with permanent seed implants for localized prostate cancer were published on both sides of the Atlantic. One of the largest series based on a multi-institutional pooled analysis was reported by Zelefski and colleagues.172 The study population included 2,693 patients treated in 11 U.S. institutions with permanent interstitial brachytherapy monotherapy for T1 to T2 prostate cancer between 1988 and 1998, with a minimum follow-up of 5 years. The PSA relapse-free survival rate at 8 years was 92% for patients achieving a nadir PSA level of less than 0.5 ng/mL and 67% if the nadir PSA level was more than 2 ng/mL. Once again, the implant quality reflected by the dose delivered to 90% of the prostate (D90) emerged as a significant predictor of biochemical outcome, along with tumor stage, Gleason score, and pretreatment PSA level. Notably, patients treated with 125I with a D90 of 130 Gy or more had an 8-year PSA relapse-free survival rate of 93% compared with 76% for those with a D90 of less than 130 Gy.
A large European, single-institution series of brachytherapy monotherapy using 125I was reported in 921 prostate cancer patients treated between 1989 and 2004 with a T stage of T2c or less.173 Only 232 patients were classified as low risk, 369 patients as intermediate risk, and the remaining 320 patients as high risk. The median follow-up was close to 6 years. Average 5- and 10-year rates of biochemical no evidence of disease (bNED) were 79% and 57%, respectively. Average 10-year rates of bNED by risk group were 88% for low-risk disease, 61% for intermediate-risk disease, and 30% for high-risk disease. The average 10-year OS and disease-specific survival (DSS) rates were 59% and 82%, respectively. Ten-year rates of OS and DSS by risk group were, respectively, 68% and 96% for low-risk disease, 64% and 87% for intermediate-risk disease, and 49% and 69% for high-risk disease. In multivariate Cox regression analysis, both risk group and treatment era were independent predictors of bNED and survival.
Similar results were reported by Dattoli and coworkers in 282 prostate cancer patients with intermediate-risk and high-risk disease.174 With a median follow-up of 9.5years, the 14-year PRFS was 87% for the intermediate-risk group and 72% for the high-risk group.
Lee and colleagues reported the preliminary results of the RTOG 0019 phase II study examining the rate of biochemical recurrence and late grade 3 or higher genitourinary and gastrointestinal toxicity after treatment with EBRT and brachytherapy in a multi-institutional, cooperative group setting; 138 patients were enrolled, and 130 were eligible for the analysis.175 The median follow-up for surviving patients was 49 months (range, 20 to 60 months). The 48-month estimate of late grade 3 or higher genitourinary or gastrointestinal toxicity was 15% and the 48-month estimate of biochemical recurrence was 14% according to the Phoenix definition (nadir + 2 ng/mL).
Removable Interstitial Implants with Iridium-192
Syed176 and Martinez177 and their colleagues developed an interstitial implant LDR technique using removable 192Ir sources with a transperineal template for treatment of carcinoma of the prostate. After the patient has recovered from anesthesia, anteroposterior and lateral x-ray films of the pelvis are obtained for dose computations. Later, 192Ir seeds, about eight per ribbon with 0.3-mCi RaEq, are inserted in the guides (dose rate, 0.7 to 0.8 Gy/h to the periphery of the gland). Minimum tumor doses of 30 to 35 Gy are delivered. This is combined with 40 Gy (in 2Gy/fraction) to the prostate or the whole pelvis as required.
In an update of the initial publication, Stromberg and associates178 described implantation of the prostate with a transperineal template (MUPIT). A bilateral staging pelvic lymphadenopathy is performed; the length of metallic guides is determined by palpation, and palpable tumor margins are identified with inactive gold seeds. In a modification of the technique, the first needle is placed with the guidance of a finger in the rectum to prevent piercing of that organ. A rectal tube is inserted to help position the template to allow for proper spacing of the rest of the needles. The template is carefully aligned parallel to the floor of the pelvis, not conforming to the perineal slope, to avoid posterior angling of the needles. The needles are differentially loaded with 192Ir seeds on nylon ribbons of varying activity, number of seeds, and the use of ribbon spacers. The posterior needles, in particular, which are primarily directed to the seminal vesicles, are loaded only in the superior half. The implant dose is 33 Gy, which is combined with EBRT (5 Gy in one dose before implantation and 30 Gy in 17 fractions after implantation). Local control rates of 100% by clinical examination and 84.5% by biopsy and actuarial disease-free survival rates of 89% have been reported.
Image-Guided Intensity-Modulated High-Dose-Rate Prostate Brachytherapy
Image-guided intensity-modulated prostate brachytherapy began in 1988 at Kiel University in Germany and soon after, in 1991, at the WBH in Royal Oak, Michigan, and the Seattle Tumor Institute.179 This section will be subdivided between intensity-modulated HDR prostate brachytherapy (IMBT) as a boost and as monotherapy.
Intensity-Modulated HDR Brachytherapy Boost
With ultrasound guidance and the interactive online dosimetry system, organ motion and setup inaccuracies (as compared with EBRT) are insignificant because they can be corrected during the procedure without increasing target volume margins. Common pitfalls of brachytherapy, including operator dependence and difficulty with reproducibility, have been eliminated with the intraoperative online planning system180,181 (Fig. 14-6).
From November 1991 through November 1995, 58 patients received 45.6-Gy pelvic EBRT and three HDR 192Ir boost implants of 5.5 to 6.5 Gy each.182 They were compared with 278 similarly staged patients treated from January 1987 through December 1991 with EBRT to prostate-only fields (median dose, 66.6 Gy).183 No patient received androgen deprivation. Patient outcome was analyzed for biochemical control. Biochemical failure was defined as a PSA level higher than 1.5 ng/mL and rising on two consecutive values. If serial posttreatment PSA levels were showing a continuous downward trend, failure was not scored. Median follow-up was 43 months for the conventionally treated group and 26 months for the IMBT boost group. The median pretreatment PSA level was 14.3 ng/mL for the EBRT-alone group and 14 ng/mL for the IMBT boost group. The median Gleason scores were 6 and 7, respectively, for the two groups. The biochemical control rate was significantly higher in the IMBT boost treatment group. Three-year actuarial biochemical control rates were 85% and 52% for the conformal IMBT boost patients and conventionally treated patients, respectively. In a multivariate analysis, the use of IMBT boost and the pretreatment PSA level were significant prognostic determinants of biochemical control. The 3-year actuarial rates of biochemical control for conformal IMBT boost versus conventionally treated patients, respectively, were 83% and 72% for a pretreatment PSA level of 4.1 to 10 ng/mL, 85% and 47% for a PSA level of 10.1 to 20 ng/mL, and 89% and 29% for a PSA level higher than 10 ng/mL. When the analysis was limited to patients in both groups with a minimum 12-month follow-up, the IMBT boost group continued to show a higher biochemical control rate than the conventional radiation group (3-year actuarial rates of 86% vs. 53%).183
Martinez and associates184 updated the series with an analysis of 207 patients treated on the dose-escalation IMBT-HDR prostate brachytherapy trial. It was demonstrated to be a precise and accurate dose delivery system and a very effective treatment for patients with unfavorable prostate cancer. The improved outcomes coupled with the low risk of complications and the advantages over permanent seed of not being radioactive after implantation, define a new standard for treatment. Using the same database, Brenner, Martinez, and associates185 reported a low α/β ratio of 1.2 showing high sensitivity to fractionation similar to the late-responding tissues.
With longer follow-up and a larger number of patients, Martinez and associates published the long-term results of the WBH prostate HDR dose-escalation trial.186 Data demonstrated that a conformal IMBT HDR brachytherapy boost improves biochemical control and CSS in patients with prostate cancer and poor prognostic factors. At the 2005 ASTRO annual meeting, Vargas and colleagues187 from WBH reported the final analysis of the HDR boost dose-escalation trial. For the first time, an improvement in biochemical control led to a decrease in metastatic rate and improved OS.
Galalee and colleagues179 reported on the collaborative trial between Kiel University in Germany, the Seattle Tumor Institute, and the WBH on long-term outcomes by risk factor using a conformal HDR brachytherapy boost for patients with localized prostate cancer during the PSA era. Similar results were found at the three institutions in 611 patients with prostate cancer harboring intermediate-risk and high-risk factors. With a mean follow-up of 5 years, the 5- and 10-year biochemical control rate was 77% and 73%, the DFS was 67% and 49%, and the CSS was 96% and 92%, respectively. The similarity in results at the three institutions gives credence to the reproducibility of the brachytherapy boost treatment. Dose escalation greater than 95 Gy resulted in better 5-year biochemical control for conformal HDR boost (59% vs. 85%; p <.001) for the entire cohort of hormonal-naïve men. Discriminating by risk factors, a striking dose-escalation effect was seen in the group of patients with two or three poor prognostic factors (p = .02 and p <.001). This unfavorable group has a remarkable 5-year biochemical control rate of 85%.
These excellent results were confirmed by others, showing that HDR prostate brachytherapy is a robust, safe, and reproducible treatment method.188,189,190,191
Two randomized trials demonstrated the superiority of adding HDR boost to EBRT compared with EBRT.
In a study by Sathya and coworkers, 104 patients with T2 and T3 prostate cancer with no evidence of metastatic disease were randomly assigned to EBRT of 66 Gy in 33 fractions during 6.5 weeks or to HDR boost of 35 Gy delivered to the prostate during 48 hours plus EBRT of 40 Gy in 20 fractions during 4 weeks.192 The median follow-up was 8.2 years. In the HDR boost plus EBRT arm, 17 patients (29%) experienced biochemical failure (BCF) compared with 33 patients (61%) in the EBRT arm (p = .0024). Eighty-seven patients (84%) had a postradiation biopsy; 10 (24%) of 42 in the HDR boost plus EBRT arm had biopsy positivity compared with 23 (51%) of 45 in the EBRT arm (odds ratio, 0.30; p = .015). The OS was over 90% for both treatment regimens.
Hoskin and colleagues randomized 220 patients with prostate cancer and PSA levels less than 50 ng/mL to receive either standard EBRT with 55 Gy in 20 fractions over 4 weeks or a combined schedule of EBRT with 35.75 Gy in 13 fractions over 2.5 weeks followed by a temporary HDR afterloading implant delivering 17 Gy in 2 fractions over 24 hours.193 With a median follow-up of 30 months, a significant improvement in actuarial bRFS was seen in favor of the combined EBRT/brachytherapy schedule (p = .03). A lower incidence of acute rectal discharge was seen in the EBRT/brachytherapy group (p = .025), and other acute and late toxicities were equivalent. Patients randomized to brachytherapy had a significantly better Functional Assessment of Cancer Therapy–Prostate (FACT-P) score at 12 weeks (p = .02).
Martinez and associates194 looked at the question of effects on long-term survival with a short course (≤6 months) of adjuvant androgen deprivation when a very high radiation dose was delivered to 934 patients treated with an IMBT-HDR brachytherapy boost in a hypofractionated regimen. At 8 years, the addition of a course of 6 months or less of androgen-deprivation therapy (ADT) to a very high hypofractionated radiation dose had not conferred a therapeutic advantage but added side effects and cost. Furthermore, for the most unfavorable group of patients harboring all three poor risk factors, there was a higher rate of distant metastasis and more prostate cancer-related deaths. This result questions the value of a short course of ADT and the impact on delaying curative treatment.
Intensity-Modulated HDR Brachytherapy as Monotherapy
The twice-per-day accelerated hypofractionated regime was selected based on HDR favorable radiobiologic considerations and physical dose delivery advantages of transrectal ultrasound (TRUS) guidance,195 with conformal intensity-modulated real-time dosimetry of prostate HDR brachytherapy. The Beaumont HDR dose schedule is 38 Gy in four fractions of 9.5 Gy delivered twice-daily via a single implant. This is biologically equivalent to 74 Gy in daily fractions of 2 Gy of EBRT. At the California Endocuritherapy (CET) Cancer Center, the HDR dose schedule is 42 Gy in six fractions twice daily in two separate implants 1 week apart. The biologic equivalence is 76 Gy in 2-Gy daily fractions of EBRT.
Patients with clinical stage II (T1c to T2a) disease, a Gleason score of less than 7 (unilobar, 3 + 4, no perineural invasion) and pretreatment PSA levels of less than 12 ng/mL were treated with monotherapy. The majority of patients presented with what would be considered low-risk or favorable prostate cancer. Patients were offered either HDR or LDR brachytherapy as treatment options, and then the patient selected the brachytherapy modality. A short course of neoadjuvant androgen deprivation (<6 months) was used for downsizing the gland volume in 31% of WBH patients, in equal proportions between permanent seeds and HDR and in 30% of the CET Cancer Center patients.196 All procedures were done under spinal anesthesia. Figure 14-7 depicts an HDR intraoperative implant using the Nucletron SWIFT guidance system.
For the implant procedure and for pain control during the entire treatment time, spinal anesthesia was administered following placement of an epidural catheter for analgesia. Dosimetry was continuously updated in real time based on the actual location of needles to compensate for organ distortion and motion and to ensure conformal coverage of the gland.180,181 Gold seed markers were then placed under TRUS guidance at the base and at the apex of the prostate to assess and measure possible interfraction needle displacement. Before delivery of the radiation, the entire prostate was imaged again, with final needle and urethral positions captured by TRUS, and a final treatment plan was created.
The toxicity profile of IMBT-HDR monotherapy was first described by Grills and colleagues197 from WBH, demonstrating less acute and chronic toxicity with HDR when compared with permanent seeds with 103Pd. Also, the impotency rate was decreased in the HDR group of treated patients by half compared with the rate with permanent seeds. The following toxicity analysis is an updated report from the combined experience from WBH and CET.198 The median follow-up for all patients was 4.1 years (range, 0.8 to 12.3 years).
Potency
Regardless of the use of adjuvant hormonal therapy, all cases were included for which complete pretreatment and post-treatment information was available. This included 61 patients treated with HDR brachytherapy alone and 81 patients treated with permanent 103Pd. The 5-year probability of impotency was 38% for all patients with available data. As shown in Figure 14-8, the probability was 49% for LDR patients and 21% for HDR patients (p = .006). The mean times to impotency for the HDR and LDR treatments were 3.9 and 3.2 years, respectively.
Survival Outcomes
The 5-year actuarial outcomes for monotherapy showed no difference in terms of ASTRO definitions for biochemical failure, cancer mortality, or overall survival between HDR alone versus permanent seeds. In Figure 14-9, the actuarial 5-year biochemical control curves for HDR and LDR are depicted. No difference in biochemical control ASTRO definition can be seen by treatment modality.
Updated Analyses
An update of the largest prostate HDR monotherapy series of 248 patients from WBH and CET was published in 2009, with a median follow-up of 5 years.198 The 5-year Phoenix definition for biochemical control was 91%. In a comparable cohort of patients treated with permanent seed implants (Pd103), the biochemical control was similar; nonetheless, the HDR patients experienced significantly less acute and chronic genitourinary and gastrointestinal toxicities.
Endovascular Brachytherapy
A new application of brachytherapy was intended to prevent restenosis after coronary angioplasty, stenting, peripheral vascular bypass surgery, or access procedures for renal dialysis, because restenosis after coronary angioplasty and stenting was reported in about 30% to 60% of patients. Most restenosis occurs 3 to 9 months after the angioplasty procedure; also, restenosis of aortofemoral bypasses has been reported in 15% to 30% of patients with arterial and vein bypass and in 40% to 50% with prosthetic bypasses. The results of treatment for coronary restenosis and peripheral vascular restenosis were traditionally poor until endovascular brachytherapy began to be tested in animal studies199–202 (see web-only expanded discussion, available on the Expert Consult website![]() ). and subsequent multi-institutional clinical trials.203,204 Phase III trials subsequently demonstrated an advantage in patients who were treated versus not treated with endovascular brachytherapy205,206,207,208–211 (see Human Studies, web-only expanded discussion, available on the Expert Consult website
). and subsequent multi-institutional clinical trials.203,204 Phase III trials subsequently demonstrated an advantage in patients who were treated versus not treated with endovascular brachytherapy205,206,207,208–211 (see Human Studies, web-only expanded discussion, available on the Expert Consult website![]() ). Coronary brachytherapy is now used infrequently, as the recently approved drug-eluting stents have virtually eliminated in-stent restenosis, thereby significantly decreasing the need for endovascular brachytherapy212 (see Current Indications, web-only expanded discussion, available on the Expert Consult website
). Coronary brachytherapy is now used infrequently, as the recently approved drug-eluting stents have virtually eliminated in-stent restenosis, thereby significantly decreasing the need for endovascular brachytherapy212 (see Current Indications, web-only expanded discussion, available on the Expert Consult website![]()
![]() ).
).
Animal Studies
Animal experiments in pigs199,200 using coronary endovascular brachytherapy with 192Ir at doses ranging from 7 to 25 Gy have shown a significant reduction in the incidence and degree of restenosis after injury of the swine coronary vessels with a stretching balloon. Most of the studies have been done at 1 month after the procedure and irradiation. Wiedermann and colleagues201 with 20 Gy at 1.5 mm, observed a 70% reduction in restenosis. Waksman and coworkers,202 with doses of 7 or 14 Gy at 2 mm, noted a reduction of 30% and 67%, respectively, in restenosis at 6 months, and Raizner200 observed not only a dose effect but a dose-rate effect as well with regard to restenosis.
In addition to endothelial proliferation, there is smooth muscle degeneration and hyperplasia, which contribute to restenosis after angioplasty and stenting. Waksman and colleagues134,135 have shown experimentally that intravascular irradiation modifies this response, inhibiting early adventitial cell proliferation and modifying the production of α-actinin by the adventitia myofibroblasts at later times after injury. Intravascular irradiation may be expected to reduce the growth of the restenosis lesion and has the potential to positively affect vascular remodeling.
Human Studies
Schopohl and colleagues203 used a Nucletron MicroSelectron HDR device, a 5-French catheter, and a 9-French ReKa catheter to deliver 12 Gy at 3 mm from the 192Ir source. With a follow-up of 8 to 69 months, of 29 patients treated after repair (stent) of femoral restenosis, 21 remained without restenosis, 4 had reocclusion, 1 died at 36 months of ovarian cancer, and 2 had no follow-up because they had moved and had no forwarding address.
Condado and coworkers204 treated 21 patients using HDR 192Ir to deliver 20 to 25 Gy. Three of 11 patients treated with 20 Gy developed restenosis in contrast with no restenosis in 10 patients treated with 25 Gy. One patient developed a coronary aneurysm. Initially, the dose was prescribed at 25 Gy, but review of the dosimetry showed that the actual dose delivered was 54 Gy.
Scripps Clinic investigators conducted a randomized study comparing sham radiation with coronary intravascular irradiation with LDR.205,206,207 Eligibility for the study included patients with stenosed vessels who had previous successful stenting or angioplasty in lesions in coronary vessels larger than 3 mm. The iridium source had a strength of 100 mCi, and the mean treatment time was approximately 35 minutes. Maximum vessel and the minimum dose in the adventitia was 80 Gy (average). One patient died, and one required restenting. Results were reported in 26 patients assigned to 192Ir and 29 assigned to a placebo. Angiographically identified restenosis (>50% of luminal diameter) was noted in 17% of the 192Ir group and 54% of patients not irradiated (p = .01). The mean luminal diameter was larger with irradiation (2.43 mm) than with placebo (1.85 mm). Updated 3-year angiographic restenosis rates favored irradiated patients (64% with placebo vs. 33% with irradiation, p <.05), and the target lesion revascularization (TLR) rate at 5-year follow-up was significantly lower in irradiated patients (48% vs. 23%, p = .05).
Subsequently, three multi-institutional phase III trials were performed that demonstrated the advantage of endovascular radiation versus placebo (GAMMA I, START, and INHIBIT).208–211 The GAMMA I trial208 was a multi-institutional phase III trial of 252 patients (12 institutions) testing the value of 192Ir (gamma radiation) in preventing coronary in-stent restenosis. At 6-month follow-up, the rate of angiographic restenosis was reduced from 50.5% to 21.5% in favor of irradiated patients, and the TLR rate at 9 months was reduced from 45% to 24% (p = .03). The START trial209 was a multi-institutional phase III trial, involving 476 patients (50 centers) with in-stent restenosis, which tested 90Sr/Y (beta radiation). At 8-month follow-up, the angiographic in-stent restenosis rate was significantly decreased from 41% with placebo to 14% with irradiation, and the TLR rate was decreased from 22% to 13% (p <.008). The INHIBIT trial210,211 randomized 332 patients (27 institutions) with in-stent restenosis to placebo versus irradiation with 32P (beta radiation). The in-stent angiographic restenosis rate at 9 months was decreased from 49% with placebo to 16% with irradiation (p <.0001), and the rate of major adverse cardiac events was decreased from 31% to 15% (p = .0006).
Although the above reported trials demonstrated a favorable response to endovascular irradiation, the complex interactions of two specialties (cardiology, radiation oncology) coupled with a difficult delivery system led the cardiologist to seek other treatment alternatives. Drug-eluting stents were developed and tested. The results of the drug-eluting stent trials demonstrated equal effectiveness in preventing restenosis with a much simpler cardiology approach.212
Full list of cited references (black and red numbers) is published online at www.expertconsult.com. The full list for this chapter contains 212 references.
29 Martinez AM, Edmundson GK, Cox RS, et al. Combination of external beam irradiation and multiple-site perineal applicator (MUPIT) for treatment of locally advanced or recurrent prostatic, anorectal, and gynecologic malignancies. Int J Radiat Oncol Biol Phys. 1985;11:391-398.
33 Gupta AK, Vicini FA, Frazier AI, et al. Iridium 192 transperineal interstitial brachytherapy for locally advanced or recurrent gynecological malignancies. Int J Radiat Oncol Biol Phys. 1999;43:1055-1060.
42 Hall EJ, Brenner DJ. Pulsed dose rate brachytherapy. Can we take advantage of new technology? [Editorial.]. Int J Radiat Oncol Biol Phys. 1996;34:511-512.
46 Polo A. Pulsed dose rate brachytherapy. Clin Transl Oncol. 2008;10:324-333.
52 Gabayan AJ, Green SB, Sanan A, et al. GliaSite brachytherapy for treatment of recurrent malignant gliomas. A retrospective multi-institutional analysis. Neurosurgery. 2006;58:701-709.
55 The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. V. Twelve-year mortality rates and prognostic factors. COMS report No. 28. Arch Ophthalmol. 2006;124:1684-1693.
57 Zhang H, Martin D, Chiu-Tsao ST, et al. A comprehensive dosimetric comparison between (131)Cs and (125)I brachytherapy sources for COMS eye plaque implant. Brachytherapy. 2010;9(4):362-372.
67 Erickson BA, Wilson JF. Nasopharyngeal brachytherapy. Am J Clin Oncol. 1993;16:424-443.
73 Levendag PC, Lagerwaard FJ, de PC, et al. High-dose, high-precision treatment options for boosting cancer of the nasopharynx. Radiother Oncol. 2002;63:67-74.
74 Koutcher L, Lee N, Zelefsky M, et al. Reirradiation of locally recurrent nasopharynx cancer with external beam radiotherapy with or without brachytherapy. Int J Radiat Oncol Biol Phys. 2010;76:130-137.
82 Mansfield CM, Domamicky LT, Schwartz GF, et al. Perioperative implantation of iridium 192 as the boost technique for stage I and II breast cancer. Results of a 10-year study of 655 patients. Radiology. 1994;192:33-36.
86 Vicini FA, Kestin L, Chen P, et al. Limited-field radiation therapy in the management of early-stage breast cancer. JNCI. 2003;95:1205-1211.
87 Chen P, Vicini FA, Benitez P, et al. Long-term cosmetic results and toxicity after accelerated partial-breast irradiation. A method of radiation delivery by interstelial brachytherapy for the treatment of early-stage breast carcinoma. Cancer. 2006;166:991-999.
89 Polgar C, Fodor J, Major T, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma—5-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:694-702.
91 Polgar C, Major T, Fodor J, et al. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy. 12-year update of a prospective clinical study. Radiother Oncol. 2010;94:274-279.
92 Chen PY, Vicini FA, Benitez P, et al. Long-term cosmetic results and toxicity after accelerated partial-breast irradiation. A method of radiation delivery by interstitial brachytherapy for the treatment of early-stage breast carcinoma. Cancer. 2006;106:991-999.
106 Foo M, Gunderson LL, Bender C, Buskirk S. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:929-935.
109 Pisters PWT, Harrison LB, Leung DHY, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859-868.
111 Schray MF, Gunderson LL, Sim FH, et al. Soft tissue sarcoma. Integration of brachytherapy, resection, and external irradiation. Cancer. 1990;66:451-456.
115 Mierzwa ML, McCluskey CM, Barrett WL, et al. Interstitial brachytherapy for soft tissue sarcoma. A single institution experience. Brachytherapy. 2007;6:298-303.
122 Barkati M, van DS, Foroudi F, et al. The use of magnetic resonance imaging for image-guided brachytherapy. J Med Imaging Radiat Oncol. 2010;54:137-141.
130 Haie-Meder C, Potter R, Van LE, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I). Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235-245.
131 Potter R, Haie-Meder C, Van LE, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67-77.
132 Potter R, Dimopoulos J, Georg P, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol. 2007;83:148-155.
133 Dimopoulos JC, Potter R, Lang S, et al. Dose-effect relationship for local control of cervical cancer by magnetic resonance image-guided brachytherapy. Radiother Oncol. 2009;93:311-315.
134 Haie-Meder C, Mazeron R, Magne N. Clinical evidence on PET-CT for radiation therapy planning in cervix and endometrial cancers. Radiother Oncol. 2010;96:351-355.
137 Martinez A, Cox RS, Edmundson GK. A multiple-site perineal applicator, MUPIT, for the treatment of prostatic, anorectal, and gynecological malignancies. Int J Radiat Oncol Biol Phys. 1984;10:297-305.
141 Wang X, Liu R, Ma B, et al: High dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancer. Cochrane Database Syst Rev 7:CD007563, 2010.
142 Narayan K, Van DS, Bernshaw D, et al. Comparative study of LDR (Manchester system) and HDR image-guided conformal brachytherapy of cervical cancer. Patterns of failure, late complications, and survival. Int J Radiat Oncol Biol Phys. 2009;74:1529-1535.
153 Hannoun-Levi JM, Ortholan C, Resbeut M, et al. High-dose split-course radiation therapy for anal cancer. Outcome analysis regarding the boost strategy (CORS-03 Study). Int J Radiat Oncol Biol Phys. Jul 7, 2010. [Epub ahead of print.]
158 van der Steen-Banasik EM, Ploeg M, Witjes JA, et al. Brachytherapy versus cystectomy in solitary bladder cancer. A case control, multicentre, East-Netherlands study. Radiother Oncol. 2009;93:352-357.
160 Crook J. Radiation therapy for cancer of the penis. Urol Clin North Am. 2010;37:435-443.
162 Rozan R, Albuisson E, Giraud B, et al. Interstitial brachytherapy for penile carcinoma: a multicentric survey (259 patients). Radiother Oncol. 1995;36:83-93.
170 Reed DR, Wallner KE, Merrick GS, et al. A prospective randomized comparison of stranded vs. loose 125I seeds for prostate brachytherapy. Brachytherapy. 2007;6:129-134.
172 Zelefsky MJ, Kuban DA, Levy LB, et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys. 2007;67:327-333.
173 Hinnen KA, Battermann JJ, van Roermund JG, et al. Long-term biochemical and survival outcome of 921 patients treated with I-125 permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2010;76:1433-1438.
174 Dattoli M, Wallner K, True L, et al. Long-term outcomes after treatment with brachytherapy and supplemental conformal radiation for prostate cancer patients having intermediate and high-risk features. Cancer. 2007;110:551-555.
175 Lee WR, Bae K, Lawton C, et al. Late toxicity and biochemical recurrence after external-beam radiotherapy combined with permanent-source prostate brachytherapy. Analysis of Radiation Therapy Oncology Group study 0019. Cancer. 2007;109:1506-1512.
179 Galalae RM, Martinez AA, Mate T, et al. Long-term outcome by risk factors using conformal HDR brachytherapy boost with or without neoadjuvant androgen deprivation suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:1048-1055.
185 Brenner D, Martinez A, Edmundson G, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low api ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6-13.
186 Martinez AA, Gonzalez J, Spencer W, et al. Conformal high dose rate brachytherapy improves biochemical control and causes specific survival in patients with prostate cancer and poor prognostic factors. J Urol. 2003;169:974-980.
187 Vargas C, Martinez AA, Bioke T, et al. Long-term survival benefit of a prospective dose escalation trial using high dose rate (HDR) brachytherapy boost. Int J Radiat Oncol Biol Phys. 2005;63:S-37.
188 Bachand F, Martin AG, Beaulieu L, et al. An eight-year experience of HDR brachytherapy boost for localized prostate cancer. Biopsy and PSA outcome. Int J Radiat Oncol Biol Phys. 2009;73:679-684.
191 Duchesne GM, Williams SG, Das R, et al. Patterns of toxicity following high-dose-rate brachytherapy boost for prostate cancer. Mature prospective phase I/II study results. Radiother Oncol. 2007;84:128-134.
193 Hoskin PJ, Motohashi K, Bownes P, et al. High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer. Initial results of a randomised phase three trial. Radiother Oncol. 2007;84:114-120.
194 Martinez AA, Demanes J, Galalae RM, et al. Lack of benefit from a short course of androgen deprivation for unfavorable prostate cancer patients treated with an accelerated hypofractionated regimen. Int J Radiat Oncol Biol Phys. 2005;62:1322-1331.
197 Grills IS, Martinez AA, Hollander M, et al. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared with low dose rate palladium seeds. J Urol. 2004;171:1098-1104.
198 Martinez AA, Demanes J, Vargas C, et al. High-dose-rate prostate brachytherapy. An excellent accelerated-hypofractionated treatment for favorable prostate cancer. Am J Clin Oncol. 2009;33(5):481-486.
207 Teirstein PS, Masullo V, Jani S, et al. Three-year clinical and angiographic follow-up after coronary radiation. Results of a randomized clinical trial. Circulation. 2000;101:360-365.
212 Waugh J, Wagstaff AJ. The paclitaxel (TAXUS)-elating stent. A review of its use in the management of de novo coronary artery lesions. Am J Cardiovasc Drugs. 2004;4:257-268.
1 Henschke UK. Afterloading applicator for radiation therapy of carcinoma of the uterus. Radiology. 1960;74:834.
2 Fletcher GH. Cervical radium applicators with screening in the direction of bladder and rectum. Radiology. 1953;60:77-84.
3 Henschke UK. Interstitial implantation with radioisotopes. In: Hahan PF, editor. Therapeutic Use of Artificial Radioisotopes. New York: Wiley, 1956.
4 Batley F, Constable WE. The use of the “Manchester System” for treatment of cancer of the uterine cervix with modern afterloading radium applicators. J Can Assoc Radiol. 1967;18:396.
5 Henschke UK. Afterloading applicator for radiation therapy of carcinoma of the uterus. Radiology. 1960;74:834.
6 Henschke UK, Hilaris BS, Mahan GD. Afterloading in interstitial and intracavitary radiation therapy. AJR Am J Roentgenol. 1963;90:386-395.
7 International Commission on Radiation Units (ICRU). ICRU Report No. 38. Dose and Volume Specification for Reporting Intracavitary Therapy in Gynecology. Bethesda, MD: ICRU; 1985. pp 1-16
8 Hames F. A new method in the use of radon gold seeds. Am J Surg. 1937;38:235.
9 Morton JL, Callendine GWJr, Myers WG. Radioactive cobalt 60 in plastic tubing for interstitial radiation therapy. Radiology. 1951;56:553.
10 Pierquin B, Chassagne D, Bailiet F, et al. The place of implantation in tongue and floor of mouth cancer. JAMA. 1971;215:961.
11 Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal 192Ir brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys. 1994;28:945-951.
12 Bier R, Small RC, Leake DL, et al. An afterloading technique for radium needles in the treatment of carcinoma of the oral cavity. Radiology. 1973;108:711.
13 Delclos L. Are interstitial radium applications passé? Front Radiat Ther Oncol. 1978;12:42.
14 Hilaris BS, Henschke UK, Holt JG. Clinical experience with long half-life and low-energy encapsulated radioactive sources in cancer radiation therapy. Radiology. 1968;91:1163.
15 Henschke UK. Artificial radioisotopes in nylon ribbons for implantation in neoplasms. In: International Conferences on the Peaceful Uses of Atomic Energy. New York: United Nations; 1956.
16 Hilaris BS. Handbook of Interstitial Brachytherapy. Acton, MS: Acton Publishing; 1975.
17 Morphis OL. Teflon tube method of radium implantation. AJR Am J Roentgenol. 1960;83:455.
18 Mowatt KS, Stevens KA. Afterloading. A contribution to the protection problem. J Fac Radiol. 1956;8:28.
19 Paine CH. Modem afterloading methods for interstitial radiotherapy. Clin Radiol. 1972;23:263.
20 Clarke DH, Edmundson GK, Martinez A, et al. The utilization of I-125 seeds as a substitute for Ir-192 seeds in temporary interstitial implants. An overview and a description of the William Beaumont Hospital technique. Int J Radiat Oncol Biol Phys. 1988;15:1027-1033.
21 Clarke DH, Edmundson GK, Martinez A, et al. The clinical advantages of I-125 seeds as a substitute for Ir-192 seeds in temporary plastic tube implants. Int J Radiat Oncol Biol Phys. 1989;17:859-863.
22 Harter DJ, Delclos L. Sealed sources in synthetic absorbable suture. Radiology. 1975;116:727.
23 Scott WP. Simplified interstitial therapy technique (Vicryl) for unresectable lung cancer. Radiology. 1975;117:734.
24 Palos B, Pooler D, Goffinet DR, Martinez A. A method for inserting I-125 seeds into the absorbable sutures for permanent implantation in tissue. Int J Radiat Oncol Biol Phys. 1980;6:381-385.
25 Martinez A, Goffinet DR, Palos B, et al. Sterilization of 125-iodine seeds encased in Ethicon Vicryl sutures for permanent interstitial implantation. Int J Radiat Oncol Bio Phys. 1979;5:411-413.
26 Goffinet DR, Martinez A, Fee WEJr. 125I Vicryl suture implants as a surgical adjuvant in cancer of the head and neck. Int J Radiat Oncol Biol Phys. 1985;11:399.
27 Puthawala AA, Syed AM, Tansey LA, et al. Temporary iridium 192 implant in the management of carcinoma of the prostate. Endocurie Hypertherm Oncol. 1985;1:25.
28 Hockel M, Muller T. A new perineal template assembly for high-dose rate interstitial brachytherapy of gynecologic malignancies. Radiother Oncol. 1994;31:262-264.
29 Martinez AM, Edmundson GK, Cox RS, et al. Combination of external beam irradiation and multiple-site perineal applicator (MUPIT) for treatment of locally advanced or recurrent prostatic, anorectal, and gynecologic malignancies. Int J Radiat Oncol Biol Phys. 1985;11:391-398.
30 Prempree T. Parametrial implant in stage IIIB cancer of the cervix. III. A five-year study. Cancer. 1983;52:748.
31 Gaddis OJr, Morrow CP, Klement V, et al. Treatment of cervical carcinoma employing a template for transperineal interstitial 192Ir brachytherapy. Int J Radiat Oncol Biol Phys. 1983;9:819-827.
32 Monk BJ, Tewari K, Burger RA, et al. A comparison of intracavitary versus interstitial irradiation in the treatment of cervical cancer. Gynecol Oncol. 1997;67:241-247.
33 Gupta AK, Vicini FA, Frazier AI, et al. Iridium 192 transperineal interstitial brachytherapy for locally advanced or recurrent gynecological malignancies. Int J Radiat Oncol Biol Phys. 1999;43:1055-1060.
34 Russell AH, Koh WJ, Markette K, et al. Radical reirradiation for recurrent or second primary carcinoma of the female reproductive tract. Gynecol Oncol. 1987;27:226-232.
35 Puthawala AA, Syed AM, Fleming PA, et al. Re-irradiation with interstitial implant for recurrent pelvic malignancies. Cancer. 1982;50:2810-2814.
36 Battermann JJ, Szabol B. Preliminary results of radiation therapy for carcinoma of the uterine cervix, using the Selectron after-loading machine. In: Mould RF, editor. Brachytherapy 2. Leersum, The Netherlands: Nucletron International BV; 1989:229-234.
37 Gupta BD, Ayyagari S, Sharma SC, et al. Carcinoma of the cervix. Optimal time-dose fractionation of HDR brachytherapy and comparison with conventional dose-rate brachytherapy. In: Mould RF, editor. Brachytherapy 2. Leerum, The Netherlands: Nucletron International BV; 1989:307-308.
38 Scalliet P, Gerbaulet A, Dubray B. HDR versus LDR in gynecological brachytherapy revisited. Radiother Oncol. 1993;28:118-126.
39 Brenner DJ, Hall EJ. Conditions for the equivalence of continuous to pulsed low-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 1991;20:181-190.
40 Visser AG, van den Aardweg GJMJ, Levenday PC. Pulsed-dose-rate and fractionated high-dose-rate brachytherapy. Choice of brachytherapy schedules to replace low-dose-rate treatments. Int J Radiat Oncol Biol Phys. 1996;34:497-505.
41 Erickson BA, Shadley JD. In vitro test of the cytotoxic equivalence between pulsed dose rate and continuous low dose rate. Radiat Oncol Invest. 1996;3:217-244.
42 Hall EJ, Brenner DJ. Pulsed dose rate brachytherapy. Can we take advantage of new technology? [Editorial.]. Int J Radiat Oncol Biol Phys. 1996;34:511-512.
43 Armour E, White J, DeWitt C, et al. Effects of continuous low-dose-rate brachytherapy on the rectum of the rat. Radiat Res. 1996;145:474-480.
44 White JR, Armour EP, Armin AR, et al. Reproducible rat model for comparing late rectal toxicity from different brachytherapy techniques. Int J Radiat Oncol Biol Phys. 1997;37:1155-1161.
45 Armour EP, White JR, Armin AR, et al. Pulsed low-dose-rate brachytherapy in a rat model: dependence of late rectal injury on radiation pulse size. Int J Radiat Oncol Biol Phys. 1997;38:825-834.
46 Polo A. Pulsed dose rate brachytherapy. Clin Transl Oncol. 2008;10:324-333.
47 Williamson JF. Practical quality assurance in low-dose-rate brachytherapy. In: Proceedings of American College of Medical Physics-Sponsored Symposium on Quality Assurance in Radiotherapy Physics. Madison, WI: Medical Physics Publishing; 1991:139-182.
48 Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1733.
49 Prados MD, Gutin PH, Philips TL, et al. Interstitial brachytherapy for newly diagnosed patients with malignant gliomas. The UCSF experience. Int J Radiat Oncol Biol Phys. 1992;24:593-597.
50 Perez CA, Zwicker RD, Li Z. Clinical application of low-dose rate and medium dose rate brachytherapy. In: Lewitt SH, Brady LW, Purdy JA, Perez CA, editors. Technical basis of radiation therapy: practical clinical applications. ed 4. New York: Springer; 2008:322-324.
51 Tatter SB, Shaw EG, Rosenblum ML, et al. An inflatable balloon catheter and liquid 125I radiation source (GliaSite Radiation Therapy System) for treatment of recurrent malignant glioma: multicenter safety and feasibility trial. J Neurosurg. 2003;99:297-303.
52 Gabayan AJ, Green SB, Sanan A, et al. GliaSite brachytherapy for treatment of recurrent malignant gliomas. A retrospective multi-institutional analysis. Neurosurgery. 2006;58:701-709.
53 Rogers LR, Rock JP, Sills AK, et al. Results of a phase II trial of the GliaSite radiation therapy system for the treatment of newly diagnosed, resected single brain metastases. J Neurosurg. 2006;105:375-384.
54 The Collaborative Ophthalmology Study Group. The Collaborative Ocular Melanoma Study randomized trial of iodine-125 brachytherapy for choroidal melanoma, III. Initial mortality findings. COMS report No 18. Arch Ophthalmol. 2001;119:969.
55 The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. V. Twelve-year mortality rates and prognostic factors. COMS report No. 28. Arch Ophthalmol. 2006;124:1684-1693.
56 Stallard HB. Malignant melanoma of the choroid treated with radioactive applicators. Trans Ophthalmol Soc UK. 1959;79:373-392.
57 Zhang H, Martin D, Chiu-Tsao ST, et al. A comprehensive dosimetric comparison between (131)Cs and (125)I brachytherapy sources for COMS eye plaque implant. Brachytherapy. 2010;9(4):362-372.
58 Rivard MJ, Melhus CS, Sioshansi S, et al. The impact of prescription depth, dose rate, plaque size, and source loading on the central axis using 103Pd, 125I, and 131Cs. Brachytherapy. 2008;7:327-335.
59 Cameron ME. Pterygium Throughout the World. Springfield, Illinois: Charles C. Thomas; 1965.
60 van den Brenk HAS. Results of prophylactic postoperative irradiation in 1300 cases of pterygium. AJR Am J Roentgenol. 1968;103:723-733.
61 Greenberg M. Eye: Choroidal melanomas and pterygium. In: Pierquin B, Wilson J-F, Chassagne D, editors. Modern brachytherapy. New York: Masson; 1987:301-314.
62 Rosenblatt E, Rachmiel A, Blumenfeld I, et al. Intracavitary mould brachytherapy in malignant tumors of the maxilla. Endocurie Hypertherm Oncol. 1996;12:25-34.
63 Mendenhall NP, Parsons JT, Cassisi NJ, et al. Carcinoma of the nasal vestibule. Int J Radiat Oncol Biol Phys. 1984;10:627-637.
64 Mendenhall NP, Parsons JT, Cassisi NJ, et al. Carcinoma of the nasal vestibule treated with radiation therapy. Laryngoscope. 1987;97:626-632.
65 Marchese MI, Nori D, Anderson LL, et al. A versatile permanent planar implant technique utilizing iodine 125 seeds imbedded in Gel-foam. Int J Radiat Oncol Biol Phys. 1984;10:747.
66 Jorgensen K, Elbrond O, Andersen AP. Carcinoma of the lip. A series of 869 cases. Acta Radiol Ther Phys Biol. 1973;12:177-190.
67 Erickson BA, Wilson JF. Nasopharyngeal brachytherapy. Am J Clin Oncol. 1993;16:424-443.
68 Wang CC, Busse J, Gitterman M. A simple afterloading applicator for intracavitary irradiation of carcinoma of the nasopharynx. Radiology. 1975;115:737-738.
69 Porrazzo MS, Hilaris BS, Moorthy CR, et al. Permanent interstitial implantation using palladium 103. The New York Medical College preliminary experience. Int J Radiat Oncol Biol Phys. 1992;23:1033-1036.
70 Wang CC. Re-irradiation of recurrent nasopharyngeal carcinoma. Treatment techniques and results. Int J Radiat Oncol Biol Phys. 1987;13:953-956.
71 Levendag PC, Peters R, Meeuwis CA, et al. A new applicator design for endocavitary brachytherapy of cancer in the nasopharynx. Radiother Oncol. 1997;45:95-98.
72 Levendag PC, Lagerwaard FJ, Noever I, et al. Role of endocavitary brachytherapy with or without chemotherapy in cancer of the nasopharynx. Int J Radiat Oncol Biol Phys. 2002;52:755-768.
73 Levendag PC, Lagerwaard FJ, de PC, et al. High-dose, high-precision treatment options for boosting cancer of the nasopharynx. Radiother Oncol. 2002;63:67-74.
74 Koutcher L, Lee N, Zelefsky M, et al. Reirradiation of locally recurrent nasopharynx cancer with external beam radiotherapy with or without brachytherapy. Int J Radiat Oncol Biol Phys. 2010;76:130-137.
75 Marcus RBJr, Million RR, Mitchell TP. A preloaded, custom-designed implantation device for stage Tl-T2 carcinoma of the floor of mouth. Int J Radiat Oncol Biol Phys. 1980;6:111-113.
76 Mazeron N, Lusichini A, Marinello G, et al. Interstitial radiation therapy for squamous cell carcinoma of the tonsillar region. The Creteil experience (1971-1981). Int J Radiat Oncol Biol Phys. 1986;12:895-900.
77 Esche BA, Haie CM, Gerbaulet AP, et al. Interstitial and external radiotherapy in carcinoma of the soft palate and uvula. Int J Radiat Oncol Biol Phys. 1988;15:619-625.
78 Mendenhall WM, Parsons IT, Mendenhall JP, et al. Brachytherapy in head and neck cancer. Oncology. 1991;5:44-54.
79 Goffinet DR, Martinez A, Palos B, et al. A method of interstitial tonsillopalatine implants. Int J Radiat Oncol Biol Phys. 1977;22:155-162.
80 Bartelink H, Borger JH. Breast conservation therapy. Current and future role of interstitial boost irradiation. In: Mould RF, editor. Brachytherapy 2. Leersum, The Netherlands: Nucletron International BV; 1989:497-502.
81 Martinez A, Goffinet DR. Irradiation with external beam and interstitial radioactive implant as primary treatment for early breast cancer. Surg Gynecol Obstet. 1981;152:285-290.
82 Mansfield CM, Domamicky LT, Schwartz GF, et al. Perioperative implantation of iridium 192 as the boost technique for stage I and II breast cancer. Results of a 10-year study of 655 patients. Radiology. 1994;192:33-36.
83 Mazeron JJ, Simon JM, Crook J, et al. Influence of dose rate on local control of breast carcinoma treated by external beam irradiation plus iridium 192 implant. Int J Radiat Oncol Biol Phys. 1991;21:1173-1177.
84 Martinez AA, Chen PY, Gustafson G, et al: Irradiation of the tumor bed alone after lumpectomy with high-dose-rate brachytherapy. In Proceedings of the 19th Annual Meeting of the American Brachytherapy Society, Palm Beach, Florida, 1997.
85 Vicini FA, Baglan KL, Kestin LL, et al. Accelerated treatment of breast cancer. J Clin Oncol. 2001;19:1993-2001.
86 Vicini FA, Kestin L, Chen P, et al. Limited-field radiation therapy in the management of early-stage breast cancer. JNCI. 2003;95:1205-1211.
87 Chen P, Vicini FA, Benitez P, et al. Long-term cosmetic results and toxicity after accelerated partial-breast irradiation. A method of radiation delivery by interstelial brachytherapy for the treatment of early-stage breast carcinoma. Cancer. 2006;166:991-999.
88 Benitez PR, Chen PY, Vicini FA, et al. Surgical considerations in the treatment of early-stage breast cancer with accelerated partial breast irradiation (APBI) in breast conserving therapy via interstitial brachytherapy. Am J Surg. 2004;188:355-364.
89 Polgar C, Fodor J, Major T, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma—5-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:694-702.
90 Antonucci JV, Wallace M, Goldstein NS, et al. Differences in patterns of failure in patients treated with accelerated partial breast irradiation versus whole-breast irradiation. A matched-pair analysis with 10-year follow-up. Int J Radiat Oncol Biol Phys. 2009;74:447-452.
91 Polgar C, Major T, Fodor J, et al. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy. 12-year update of a prospective clinical study. Radiother Oncol. 2010;94:274-279.
92 Chen PY, Vicini FA, Benitez P, et al. Long-term cosmetic results and toxicity after accelerated partial-breast irradiation. A method of radiation delivery by interstitial brachytherapy for the treatment of early-stage breast carcinoma. Cancer. 2006;106:991-999.
93 Edmundson GK, Vicini FA, Chen P, et al. Dosimetric characteristics of the MammoSite RTS, a new breast brachytherapy applicator. Int J Radiat Oncol Biol Phys. 2002;52:1132-1139.
94 Keish M, Vicini F. Applying innovations in surgical and radiation oncology to breast conservation. Breast J. 2005;11(Suppl 1):S24-S29.
95 Hilaris BS, Gomez J, Nori D, et al. Combined surgery, intraoperative brachytherapy, and postoperative external radiation in stage III non-small cell lung cancer. Cancer. 1985;55:1226-1231.
96 Smith RP, Schuchert M, Komanduri K, et al. Dosimetric evaluation of radiation exposure during I-125 vicryl mesh implants: implications for ACOSOG z4032. Ann Surg Oncol. 2007;14:3610-3613.
97 McKenna RJJr, Mahtabifard A, Yap J, et al. Wedge resection and brachytherapy for lung cancer in patients with poor pulmonary function. Ann Thorac Surg. 2008;85:S733-S736.
98 Lo TC, Girshovich L, Healey GA, et al. Low-dose-rate versus high-dose-rate intraluminal brachytherapy for malignant endobronchial tumors. Radiother Oncol. 1995;35:193-197.
99 Flores AD, Nelems B, Evans K, et al. Impact of new radiotherapy modalities on the surgical management of cancer of the esophagus and cardia. Int J Radiat Oncol Biol Phys. 1989;17:937-944.
100 Syed AMN, Puthawala AA, Severance SR, et al. Intraluminal irradiation in the treatment of esophageal cancer. Endocurie Hypertherm Oncol. 1987;3:105-113.
101 Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer. Multicentre randomised trial. Lancet. 2004;364:1497-1504.
102 Peretz T, Nori D, Hilaris B, et al. Treatment of primary unresectable carcinoma of the pancreas with I-125 implantation. Int J Radiat Biol Phys. 1989;17:931-935.
103 Fields JN, Emami B. Carcinoma of the extrahepatic biliary system. Results of primary and adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 1987;13:331-338.
104 Meerwaldt JH, Veeze-Kuijpers B, Visser AG, et al. Combined modality radiotherapy in the treatment of bile duct carcinoma. In: Mould RF, editor. Brachytherapy 2. Leersum, The Netherlands: Nucletron International BV; 1989:577-583.
105 Fritz P, Brambs HJ, Schraube P, et al. Combined external beam radiotherapy and intraluminal high-dose-rate brachytherapy on bile duct carcinomas. Int J Radiat Oncol Biol Phys. 1994;29:855-861.
106 Foo M, Gunderson LL, Bender C, Buskirk S. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:929-935.
107 Lacerna M, Irwin R, Vicini F, et al. Iodine 125 temporary interstitial Implants and limb conserving surgery in the management of extremity soft tissue sarcomas. Endocurie Hypertherm Oncol. 1996;12:191-198.
108 Hilaris BS, Nori D, Anderson LL. Brachytherapy for soft tissue sarcomas. In: Hilaris BS, Nori D, Anderson LL, editors. Atlas of Brachytherapy. New York: Macmillan; 1988:170-182.
109 Pisters PWT, Harrison LB, Leung DHY, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859-868.
110 Potter R, Knocke TH, Kovacs G, et al. Brachytherapy in the combined modality treatment of pediatric malignancies. Principles and preliminary experience with treatment of soft tissue sarcoma (recurrence) and Ewing’s sarcoma. Klin Paediatr. 1995;207:164-173.
111 Schray MF, Gunderson LL, Sim FH, et al. Soft tissue sarcoma. Integration of brachytherapy, resection, and external irradiation. Cancer. 1990;66:451-456.
112 Beltrami G, Rudiger HA, Mela MM, et al. Limb salvage surgery in combination with brachytherapy and external beam radiation for high-grade soft tissue sarcomas. Eur J Surg Oncol. 2008;34:811-816.
113 Bolling T, Schuller P, Distelmaier B, et al. Perioperative high-dose rate brachytherapy using a bendy applicator (flab). Treatment results of 74 patients. Anticancer Res. 2008;28:3885-3890.
114 Laskar S, Bahl G, Puri A, et al. Perioperative interstitial brachytherapy for soft tissue sarcomas. Prognostic factors and long-term results of 155 patients. Ann Surg Oncol. 2007;14:560-567.
115 Mierzwa ML, McCluskey CM, Barrett WL, et al. Interstitial brachytherapy for soft tissue sarcoma. A single institution experience. Brachytherapy. 2007;6:298-303.
116 Torres MA, Ballo MT, Butler CE, et al. Management of locally recurrent soft-tissue sarcoma after prior surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67:1124-1129.
117 Aronowitz JN, Pohar SS, Liu L, et al. Adjuvant high dose rate brachytherapy in the management of soft tissue sarcoma. A dose-toxicity analysis. Am J Clin Oncol. 2006;29:508-513.
118 Corn BW, Hanlon AL, Pajak TF, et al. Technically accurate intracavitary insertions improve pelvic control and survival among patients with locally advanced carcinoma of the uterine cervix. Gynecol Oncol. 1994;53:294-300.
119 Tod MC, Meredith WJ. A dosage system for use in the treatment of cancer of the uterine cervix. Br J Radiol. 1938;11:809.
120 Delclos L, Fletcher GH, Sampiere V, et al. Can the Fletcher gamma ray colpostat system be extrapolated to other systems? Cancer. 1978;41:970.
121 Henschke UK. Afterloading applicator for radiation therapy of carcinoma of the uterus. Radiology. 1960;74:834.
122 Barkati M, van DS, Foroudi F, et al. The use of magnetic resonance imaging for image-guided brachytherapy. J Med Imaging Radiat Oncol. 2010;54:137-141.
123 Thomas L. [Contribution of imaging in radiotherapy planning in uterine cervix cancers]. Cancer Radiother. 2000;4:127-132.
124 Hatano K, Sekiya Y, Araki H, et al. Evaluation of the therapeutic effect of radiotherapy on cervical cancer using magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 1999;45:639-644.
125 Knutsen BH, Skretting A, Hellebust TP, et al. Determination of 3D dose distribution from intracavitary brachytherapy of cervical cancer by MRI of irradiated ferrous sulphate gel. Radiother Oncol. 1997;43:219-227.
126 Muschitz S, Petrow P, Briot E, et al. Correlation between the treated volume, the GTV and the CTV at the time of brachytherapy and the histopathologic findings in 33 patients with operable cervix carcinoma. Radiother Oncol. 2004;73:187-194.
127 Wachter-Gerstner N, Wachter S, Reinstadler E, et al. Bladder and rectum dose defined from MRI based treatment planning for cervix cancer brachytherapy. Comparison of dose-volume histograms for organ contours and organ wall, comparison with ICRU rectum and bladder reference point. Radiother Oncol. 2003;68:269-276.
128 Haie-Meder C, de CR, Petrow P, et al. [Brachytherapy in cervix cancers. Development of techniques and concepts]. Cancer Radiother. 2003;7:42-49.
129 Potter R, Knocke TH, Fellner C, et al. Definitive radiotherapy based on HDR brachytherapy with iridium 192 in uterine cervix carcinoma. Report on the Vienna University Hospital findings (1993-1997) compared to the preceding period in the context of ICRU 38 recommendations. Cancer Radiother. 2000;4:159-172.
130 Haie-Meder C, Potter R, Van LE, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I). Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235-245.
131 Potter R, Haie-Meder C, Van LE, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67-77.
132 Potter R, Dimopoulos J, Georg P, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol. 2007;83:148-155.
133 Dimopoulos JC, Potter R, Lang S, et al. Dose-effect relationship for local control of cervical cancer by magnetic resonance image-guided brachytherapy. Radiother Oncol. 2009;93:311-315.
134 Haie-Meder C, Mazeron R, Magne N. Clinical evidence on PET-CT for radiation therapy planning in cervix and endometrial cancers. Radiother Oncol. 2010;96:351-355.
135 Magne N, Chargari C, Vicenzi L, et al. New trends in the evaluation and treatment of cervix cancer. The role of FDG-PET. Cancer Treat Rev. 2008;34:671-681.
136 Viswanathan AN, Erickson BA. Three-dimensional imaging in gynecologic brachytherapy. A survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2010;76:104-109.
137 Martinez A, Cox RS, Edmundson GK. A multiple-site perineal applicator, MUPIT, for the treatment of prostatic, anorectal, and gynecological malignancies. Int J Radiat Oncol Biol Phys. 1984;10:297-305.
138 Aristizabal SA, Valencia A, Ocampo G, et al. Interstitial parametrial irradiation in cancer of the cervix stage IIB-IIIB. Endocurie Hypertherm Oncol. 1985;1:41.
139 Feder BH, Syed AM, Neblett D. Treatment of extensive carcinoma of the cervix with the transperineal parametrial butterfly. A preliminary report. Int J Radiat Oncol Biol Phys. 1978;4:735-742.
140 Leborgue F, Fowler JF, Leborgue JH, et al. Fractionation in medium-dose-rate brachytherapy of cancer of the cervix. Int J Radiat Oncol Biol Phys. 1996;35:907-914.
141 Wang X, Liu R, Ma B, et al: High dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancer. Cochrane Database Syst Rev 7:CD007563, 2010.
142 Narayan K, Van DS, Bernshaw D, et al. Comparative study of LDR (Manchester system) and HDR image-guided conformal brachytherapy of cervical cancer. Patterns of failure, late complications, and survival. Int J Radiat Oncol Biol Phys. 2009;74:1529-1535.
143 Viani GA, Manta GB, Stefano EJ, et al. Brachytherapy for cervix cancer. Low-dose rate or high-dose rate brachytherapy—a meta-analysis of clinical trials. J Exp Clin Cancer Res. 2009;28:47.
144 Falkenberg E, Kim RY, Meleth S, et al. Low-dose-rate vs. high-dose-rate intracavitary brachytherapy for carcinoma of the cervix. The University of Alabama at Birmingham (UAB) experience. Brachytherapy. 2006;5:49-55.
145 Lertsanguansinchai P, Lertbutsayanukul C, Shotelersuk K, et al. Phase III randomized trial comparing LDR and HDR brachytherapy in treatment of cervical carcinoma. Int J Radiat Oncol Biol Phys. 2004;59:1424-1431.
146 Grigsby PW, Perez CA, Kuten A, et al. Clinical stage I endometrial cancer. Results of adjuvant irradiation and patterns of failure. Int J Radiat Oncol Biol Phys. 1991;21:379-385.
147 Chao CKS, Grigsby PW, Perez CA, et al. Brachytherapy-related complications for medically inoperable stage I endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1995;31:37-42.
148 Rotte K. Technique and results of HDR afterloading in cancer of the endometrium. In: Martinez AA, Orton CG, Mould RF, editors. Brachytherapy: HDR and LDR. Columbia, Maryland: Nucletron Corporation; 1990:68-79.
149 Perez CA, Kuske R, Glasgow GP. Review of brachytherapy for gynecologic tumors. Endocurie Hypertherm Oncol. 1985;1:153.
150 Erickson BA. Interstitial implantation of vulvar malignancies. An historical perspective. Endocurie Hypertherm Oncol. 1996;12:101-112.
151 Papillon J, Montbarbon JR, Gerard JP, et al. Interstitial curietherapy in the conservative treatment of anal and rectal cancers. Int J Radiat Oncol Biol Phys. 1989;17:1161-1169.
152 Puthawala AA, Syed AMN, Gates TC, et al. Definitive treatment of extensive anorectal cancer by external and interstitial irradiation. Cancer. 1982;50:1746-1750.
153 Hannoun-Levi JM, Ortholan C, Resbeut M, et al. High-dose split-course radiation therapy for anal cancer. Outcome analysis regarding the boost strategy (CORS-03 Study). Int J Radiat Oncol Biol Phys. Jul 7, 2010. [Epub ahead of print.]
154 van der Werf-Messing B. Interstitial radium therapy of superficial bladder cancer (category T1NxM9 and T2NxM0). In: Smith PH, Prout GBJr, editors. Bladder cancer. London: Butterworth; 1984:191-202.
155 Batterman JJ, Tierie AH. Results of implantation for T1 and T2 bladder tumors. Radiother Oncol. 1986;5:85-90.
156 van der Werf-Messing B, van Putten WLJ. Carcinoma of the urinary bladder category T2, 3NxM0 treated by 40 Gy external irradiation followed by cesium 137 implant at reduced dose (50%). Int J Radiat Oncol Biol Phys. 1989;16:369.
157 Battermann JJ, Boon TA. Treatment of T2 bladder tumors with interstitial therapy. The role of lymph node dissection. In: Mould RF, editor. Brachytherapy 2. Leersum, The Netherlands: Nucletron International BV; 1989:187-191.
158 van der Steen-Banasik EM, Ploeg M, Witjes JA, et al. Brachytherapy versus cystectomy in solitary bladder cancer. A case control, multicentre, East-Netherlands study. Radiother Oncol. 2009;93:352-357.
159 Van der Steen-Banasik EM, Visser AG, Reinders JG, et al. Saving bladders with brachytherapy. Implantation technique and results. Int J Radiat Oncol Biol Phys. 2002;53:622-629.
160 Crook J. Radiation therapy for cancer of the penis. Urol Clin North Am. 2010;37:435-443.
161 de Creviosier R, Slimane K, Sanfilippo N, et al. Long-term results of brachytherapy for carcinoma of the penis confined to the glans (N- or NX). Int J Radiat Oncol Biol Phys. 2009;74:1150-1156.
162 Rozan R, Albuisson E, Giraud B, et al. Interstitial brachytherapy for penile carcinoma: a multicentric survey (259 patients). Radiother Oncol. 1995;36:83-93.
163 Crook J, Grimard L, Tsihlias J, et al. Interstitial brachytherapy for penile cancer. An alternative to amputation. J Urol. 2002;167:506-511.
164 Crook JM, Jezioranski J, Grimard L, et al. Penile brachytherapy. Results for 49 patients. Int J Radiat Oncol Biol Phys. 2005;62:460-467.
165 Crook J, Jezioranski J, Cygler JE. Penile brachytherapy. Technical aspects and postimplant issues. Brachytherapy. 2010;9:151-158.
166 Hilaris BS, Whitmore WF, Batata MA, et al. Behavioral patterns of prostate adenocarcinoma following an I-125 implant and pelvic node dissection. Int J Radiat Oncol Biol Phys. 1977;2:631.
167 Critz FA, Tarlton RS, Holladay DA. Prostate-specific antigen-monitored combination radiotherapy for patients with prostate cancer. I-125 implant followed by external beam irradiation. Cancer. 1995;75:2383.
168 Blasko JC, Radge H, Schumacher D. Transperineal percutaneous iodine 125 implantation for prostatic carcinoma using transrectal ultrasound and template guidance. Endocurie Hypertherm Oncol. 1987;3:131-139.
169 Blasko JC, Grimm PD, Ragde H. Brachytherapy and organ preservation in the management of carcinoma of the prostate. Semin Radiat Oncol. 1993;3:240-249.
170 Reed DR, Wallner KE, Merrick GS, et al. A prospective randomized comparison of stranded vs. loose 125I seeds for prostate brachytherapy. Brachytherapy. 2007;6:129-134.
171 Wallner K, Roy J, Harrison L. Dosimetry guidelines to minimize urethral and rectal morbidity following transperineal I-125 prostate brachytherapy. Int J Radiat Oncol Biol Phys. 1995;32:465-471.
172 Zelefsky MJ, Kuban DA, Levy LB, et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys. 2007;67:327-333.
173 Hinnen KA, Battermann JJ, van Roermund JG, et al. Long-term biochemical and survival outcome of 921 patients treated with I-125 permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2010;76:1433-1438.
174 Dattoli M, Wallner K, True L, et al. Long-term outcomes after treatment with brachytherapy and supplemental conformal radiation for prostate cancer patients having intermediate and high-risk features. Cancer. 2007;110:551-555.
175 Lee WR, Bae K, Lawton C, et al. Late toxicity and biochemical recurrence after external-beam radiotherapy combined with permanent-source prostate brachytherapy. Analysis of Radiation Therapy Oncology Group study 0019. Cancer. 2007;109:1506-1512.
176 Syed AMN, Puthawala AA, Tansey LA, et al. Temporary iridium 192 implantation in the management of carcinoma of the prostate. In: Hilaris BS, Batata MA, editors. Brachytherapy oncology-1983. New York: Memorial Sloan-Kettering Cancer Center; 1983:83-91.
177 Martinez AM, Benson RC, Edmundson GE, et al. Pelvic lymphadenectomy combined with transperineal interstitial implantation of iridium 192 and external beam radiation for locally advanced prostatic carcinoma. Technical description. Int J Radiat Oncol Biol Phys. 1985;11:841-847.
178 Stromberg J, Martinez A, Benson R, et al. Improved local control and survival for surgically staged patients with locally advanced prostate cancer treated with low-dose-rate iridium 192 prostate implantation and external beam irradiation. Int J Radiat Oncol Biol Phys. 1994;28:171-177.
179 Galalae RM, Martinez AA, Mate T, et al. Long-term outcome by risk factors using conformal HDR brachytherapy boost with or without neoadjuvant androgen deprivation suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:1048-1055.
180 Edmundson GK, Yan D, Martinez AA, et al. Intra-operative optimization of needle placement and dwell times for conformal prostate brachytherapy. Int J Radiat Oncol Biol Phys. 1995;33:1257-1263.
181 Edmundson GK, Rizzo NR, Teahan M, et al. Concurrent treatment planning for out-patient high dose rate brachytherapy prostate template implants. Int J Radiat Oncol Biol Phys. 1993;27:1215-1221.
182 Martinez A, Gonzalez J, Stromberg J, et al. Conformal prostate brachytherapy. Initial experience of a phase I/II dose escalating trial. Int J Radiat Oncol Biol Phys. 1995;33:1019-1027.
183 Kestin LL, Martinez AA, Stromberg JS, et al. Matched-pair analysis of conformal high-dose-rate brachytherapy boost versus external-beam radiation therapy alone for locally advanced prostate cancer. J Clin Oncol. 2000;18:2869-2880.
184 Martinez AA, Gustafson G, Gonzalez J, et al. Dose escalation using conformal high dose rate brachytherapy improves the outcome in unfavorable prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:316-327.
185 Brenner D, Martinez A, Edmundson G, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low api ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6-13.
186 Martinez AA, Gonzalez J, Spencer W, et al. Conformal high dose rate brachytherapy improves biochemical control and causes specific survival in patients with prostate cancer and poor prognostic factors. J Urol. 2003;169:974-980.
187 Vargas C, Martinez AA, Bioke T, et al. Long-term survival benefit of a prospective dose escalation trial using high dose rate (HDR) brachytherapy boost. Int J Radiat Oncol Biol Phys. 2005;63:S-37.
188 Bachand F, Martin AG, Beaulieu L, et al. An eight-year experience of HDR brachytherapy boost for localized prostate cancer. Biopsy and PSA outcome. Int J Radiat Oncol Biol Phys. 2009;73:679-684.
189 Pellizzon AC, Salvajoli J, Novaes P, et al. Updated results of high-dose rate brachytherapy and external beam radiotherapy for locally and locally advanced prostate cancer using the RTOG-ASTRO Phoenix definition. Int Braz J Urol. 2008;34:293-301.
190 Kalkner KM, Wahlgren T, Ryberg M, et al. Clinical outcome in patients with prostate cancer treated with external beam radiotherapy and high dose-rate iridium 192 brachytherapy boost: a 6-year follow-up. Acta Oncol. 2007;46:909-917.
191 Duchesne GM, Williams SG, Das R, et al. Patterns of toxicity following high-dose-rate brachytherapy boost for prostate cancer. Mature prospective phase I/II study results. Radiother Oncol. 2007;84:128-134.
192 Sathya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. 2005;23:1192-1199.
193 Hoskin PJ, Motohashi K, Bownes P, et al. High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer. Initial results of a randomised phase three trial. Radiother Oncol. 2007;84:114-120.
194 Martinez AA, Demanes J, Galalae RM, et al. Lack of benefit from a short course of androgen deprivation for unfavorable prostate cancer patients treated with an accelerated hypofractionated regimen. Int J Radiat Oncol Biol Phys. 2005;62:1322-1331.
195 Martinez AA, Pataki I, Edmundson G, et al. Phase II prospective study of the use of conformal high-dose rate brachytherapy as monotherapy for the treatment of favorable stage prostate cancer. A feasibility report. Int J Radiat Oncol Biol Phys. 2001;49:61-69.
196 Demanes DJ, Rodriguez RR, Altieri GA, et al. High dose rate prostate brachytherapy. The California Endocurietherapy (CET) method. Radiother Oncol. 2000;57:289-296.
197 Grills IS, Martinez AA, Hollander M, et al. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared with low dose rate palladium seeds. J Urol. 2004;171:1098-1104.
198 Martinez AA, Demanes J, Vargas C, et al. High-dose-rate prostate brachytherapy. An excellent accelerated-hypofractionated treatment for favorable prostate cancer. Am J Clin Oncol. 2009;33(5):481-486.
199 Raizner AE. Endovascular irradiation in animals. What have we learned? Presented at the First Annual Conference on Interventional Endovascular Brachytherapy. New York, May 30, 1996.
200 Wiedermann JG, Marboe CH, Amols H, et al. Intracoronary irradiation markedly reduces restenosis after balloon angioplasty in swine. J Am Coll Cardiol. 1995;25:1451-1456.
201 Waksman R, Robinson KA, Crocker IR, et al. Endovascular low-dose irradiation inhibits neointima formation after coronary artery balloon injury in swine. Circulation. 1995;91:1953-1959.
202 Waksman R, Robinson KA, Crocker IR, et al. Intracoronary radiation before stent implantation inhibits neointima formation in stented porcine coronary arteries. Circulation. 1995;92:1383-1386.
203 Schopohl B, Juling-Pohliat L, Boettcher HD, et al. Endovascular irradiation for an avoidance or recurrent stenosis after stent implantation in peripheral arteries. Five years’ follow-up. Presented at Discoveries in Radiation for Restenosis. Atlanta, GA, January 11-12, 1996.
204 Condado JA, Gurdiel O, Espinosa R, et al. Late follow-up after percutaneous transluminal coronary angioplasty (PTCA) and intracoronary radiation therapy (ICRT). Presented at Discoveries in Radiation for Restenosis. Atlanta, GA, January 11-12, 1996.
205 Teirstein PS, Masullo V, Jani S, et al. Catheter-based radiotherapy to inhibit restenosis after coronary stenting. N Engl J Med. 1997;336:1697-1703.
206 Terstein PS, Massullo V, Jani S, et al. Two-year follow-up after catheter-based radiotherapy to inhibit coronary restenosis. Circulation. 1999;99:243-247.
207 Teirstein PS, Masullo V, Jani S, et al. Three-year clinical and angiographic follow-up after coronary radiation. Results of a randomized clinical trial. Circulation. 2000;101:360-365.
208 Leon MB, Teirstein PS, Moses JW, et al. Localized intracoronary gamma-radiation therapy to inhibit the recurrence of restenosis after stenting. N Engl J Med. 2001;344:250-256.
209 Popma J, Heuser R, Suntharalingam M, et al. for the START Investigators: Late clinical and angiographic outcomes after use of 90Sr/Y beta radiation for the treatment of in-stent restenosis. J Am Coll Cardiol. 2000;36:311-312.
210 Waksman R, Raizner A, Chiu K, et al. Beta radiation to inhibit recurrence of in-stent restenosis. Clinical and angiographic results of the multicenter, randomized double blind study Circulation Online. 2000;102:e9046.
211 Waksman R, Ajani A, White RL, et al. Two year follow-up after beta and gamma intracoronary radiation therapy for patients with diffuse in-stent restenosis. Am J Cardiol. 2001;88:4325-4428.
212 Waugh J, Wagstaff AJ. The paclitaxel (TAXUS)-elating stent. A review of its use in the management of de novo coronary artery lesions. Am J Cardiovasc Drugs. 2004;4:257-268.