40 Bowel Obstructions
• Complete small bowel obstructions are at high risk for strangulation and typically require surgical treatment.
• Partial small bowel obstructions are at lower risk for strangulation and are generally managed nonoperatively.
• Closed-loop bowel obstructions have a higher likelihood of concomitant vascular compromise and strangulation.
• Strangulation may lead to bowel wall necrosis, perforation, peritonitis, sepsis, and death. Fever in a patient with bowel obstruction suggests strangulation and perforation.
• Plain radiographs can be used initially to evaluate cases of suspected bowel obstruction. Computed tomography may be necessary to confirm the diagnosis when plain radiographic findings are nondiagnostic. Computed tomography can exclude the diagnosis of closed-loop obstruction.
• Obstructions that complicate the first 30 days after laparotomy are often managed nonoperatively, whereas obstructions that occur after laparoscopy generally require surgery.
Perspective
Bowel obstructions develop from mechanical blockage of normal intestinal transit. Blockages may result from intraluminal matter (e.g., foreign bodies), intramural wall thickening (tumors, hernia, inflammation), or extraluminal compression (hernias, masses, adhesions). Bowel proximal to the obstruction progressively dilates, which leads to pain, obstipation (inability to pass flatus), and vomiting. Dehydration and electrolyte abnormalities ensue. As the bowel wall becomes edematous, increasing pressure causes collapse of the capillary bed and subsequent tissue ischemia, a condition known as strangulation. Strangulation may lead to bowel wall necrosis, perforation, peritonitis, sepsis, and death. Patients with small bowel obstruction have a 5% to 42% incidence of strangulation, which carries a mortality of 20% to 37%.1
Differential Diagnosis
Potential causes of mechanical small or large bowel obstruction are summarized in Box 40.1.
Diagnostic Testing
Laboratory Testing
Laboratory abnormalities are not diagnostic of bowel obstruction but instead may indicate complications of obstruction. A complete blood count may demonstrate leukocytosis with a left shift in a patient with strangulation; serum chemistry evaluations may show dehydration, hypokalemia, and acid-base disturbances. The serum lactate concentration can be elevated in the setting of strangulation, but its measurement is neither sensitive nor specific.2
Radiographs
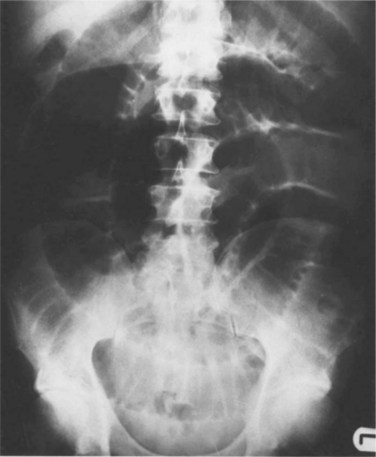
Plain supine and upright radiographs of the abdomen are the most commonly ordered initial diagnostic study for bowel obstruction because of their widespread availability and the low cost of radiographic evaluation (Fig. 40.1).
Sigmoid volvulus is characterized by a massively distended loop of large bowel arising out of the pelvis, sometimes described as a “bent inner tube,” with accompanying proximal large bowel dilation. (The large bowel is identified by widely spaced haustral markings that do not completely traverse the bowel lumen.) A competent ileocecal valve prevents decompression into the small bowel, thereby leading to massive dilation of the large bowel (Fig. 40.2).
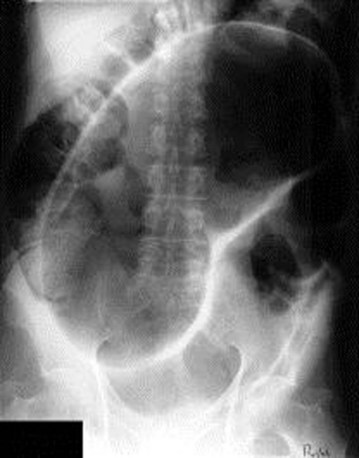
Fig. 40.2 Sigmoid volvulus. Note U-shaped, massively dilated, and ahaustral sigmoid.
(From Kahi CJ, Rex DK. Bowel obstruction and pseudo-obstruction. Gastroenterol Clin North Am 2003;32:1229-47.)
Large bowel obstruction resulting from mechanical causes must be differentiated from pseudoobstruction, a colonic motility disorder that is treated nonoperatively. Enemas of a water-soluble contrast agent (WSCA) (used instead of barium to avoid chemical peritonitis in the setting of perforation) can differentiate obstruction from pseudoobstruction. A contrast agent enema improves the sensitivity and specificity for mechanical obstruction from 84% and 72% to 96% and 98%, respectively, by using a criterion standard of laparotomy findings and clinical follow-up.3 In addition, a contrast agent enema is helpful in confirming the diagnosis of sigmoid and cecal volvulus, with a tapering “bird’s beak” appearance at the end of the column of contrast agent as it reaches the base of the colonic twist.
Studies evaluating the effectiveness of plain radiography in diagnosing obstruction are limited by their methodology. Researchers have used combinations of discharge diagnosis, surgical reports, enteroclysis, and clinical follow-up as criterion standards. A review of these studies shows that plain radiographs are of limited value because they are diagnostic in only 50% to 60% of cases4 and have a reported sensitivity of just 46% to 76%.5 A study of plain radiographs interpreted by gastrointestinal radiologists had a sensitivity of 66%; 21% of radiographic studies reported as showing normal bowel were actually images of obstructions.6 Despite the limitations related to false-negative interpretations, plain radiography is still recommended as the initial test of choice for the evaluation of possible bowel obstruction because the classic radiographic findings, when present, are useful for quickly confirming the diagnosis.
Computed Tomography
CT of the abdomen is commonly used for the diagnosis of bowel obstruction (1) to confirm a clinically suspected obstruction not identified on a plain radiograph; (2) to determine the level, severity, and cause of the obstruction; (3) to characterize a closed-loop obstruction; (4) to demonstrate signs of strangulation7; and (5) to identify alternative causes of acute abdominal pain when obstruction is not present.
CT has a sensitivity of 64% to 100% and a specificity of 71% to 100% for the diagnosis of bowel obstruction.8 Signs of small bowel obstruction on CT consist of small bowel dilation to a caliber of 2.5 cm or greater with a distinct transition zone and a collapsed distal bowel lumen. The “small bowel feces sign”—the presence of intraluminal particulate material in dilated small bowel—can be helpful in confirming the diagnosis of small bowel obstruction.9 CT correctly identifies the cause of obstruction in 73% to 95% of patients.10
A completely fluid-filled closed-loop obstruction is more easily diagnosed with CT than with plain radiography. Characteristics of closed-loop obstruction include a C- or U-shaped configuration of the dilated loops, a radial distribution of the dilated loops toward the site of obstruction, a radial distribution and engorged mesenteric vessels toward the site of obstruction (the “beak and whirl” sign), and the presence of two collapsed bowel lumens adjacent to the site of obstruction.1
Intravenous contrast-enhanced CT has a sensitivity for the diagnosis of strangulation of 56% to 85% and a specificity exceeding 90%.1,11,12 Strangulation is suggested by a characteristic configuration of the obstructed loop, bowel wall thickening and reduced contrast enhancement, mesenteric vascular changes, gas in the bowel wall, and the presence of free peritoneal fluid. Combinations of clinical, laboratory, and radiographic features have been used in an attempt to identify patients with strangulation who need urgent surgery. One study suggested that the presence of any three of six variables (duration of pain longer than 4 days, abdominal guarding, C-reactive protein level higher than 75 mg/L, white blood cell count greater than 10,000/mm3, presence of more than 500 mL of intraabdominal fluid on CT, or reduced wall contrast enhancement on CT) had a sensitivity of 68% for resection of small bowel.13 Another study found that the presence of two of four clinical criteria (tachycardia, leukocytosis, fever, and tenderness) increased the specificity of the CT criteria.14
CT enteroclysis (delivery of contrast agent to the small bowel via a tube so that it bypasses the pylorus) combines the anatomic information provided by CT with the intraluminal detail of enteroclysis. Although this technique is more labor-intensive because of the need to intubate the small bowel, it may play a role in the future evaluation of small bowel obstruction.15 CT enteroclysis is a useful test for confirming the diagnosis of superior mesenteric artery syndrome, a rare cause of proximal small bowel obstruction that occurs after rapid weight loss.
Orally administered WSCA can help distinguish partial small bowel obstruction from complete obstruction. Two separate metaanalyses have shown that identification of oral contrast agent in the colon 4 to 24 hours after administration predicts the success of conservative management with a pooled positive likelihood ratio of 25 to 40 and that the absence of contrast agent predicts failure of conservative therapy with a pooled negative likelihood ratio of 0.03 to 0.04.16,17
Treatment
Conservative Management of Small Bowel Obstruction
“Don’t let the sun set on a bowel obstruction.” This adage has guided surgeons since the advent of operative therapy for this condition inasmuch as clinical and radiographic predictors of strangulation were poor before the advent of CT. In the modern era, however, nearly half of all patients with acute small bowel obstruction improve with conservative therapy.18 The mainstay of nonoperative treatment is gastric decompression via nasogastric suctioning. The nasogastric tube is easy to insert, requires no fluoroscopic guidance to place, and reduces the risk for aspiration pneumonia by effectively relieving the gastric distention. Nasointestinal (long) tubes that cross the pylorus are not recommended for ED use.
Small bowel obstructions caused by postoperative adhesions in the first 30 days after laparotomy complicate up to 10% of procedures. These early adhesions represent inflammatory reactions, unlike the fibrous adhesions that develop later in the postoperative course. Obstructions occurring in the early postlaparotomy period can usually be managed nonoperatively because more than 85% resolve spontaneously.19 Conversely, early postlaparoscopic small bowel obstructions generally require surgery because they are typically caused by herniation of bowel through the peritoneal defect made by the trochar.20
Oral contrast agents have been postulated to have a therapeutic role in resolving small bowel obstructions because WSCAs are hyperosmolar and draw water into the small bowel lumen while decreasing bowel wall edema, but studies have demonstrated conflicting results. A metaanalysis of four trials demonstrated no significant difference between WSCAs and placebo in successful nonoperative management of acute small bowel obstruction; however, hospital stay was shorter in patients given WSCAs than in patients receiving placebo.16
Surgical Management of Small Bowel Obstruction
Laparoscopic treatment of small bowel obstruction appears to be appropriate for carefully selected patients.21 The most significant complication of laparoscopy is iatrogenic perforation of bowel, which occurs in 1% to 16% of patients. Between 8% and 40% of cases initially planned as laparoscopic procedures are converted to laparotomy intraoperatively. Prospective studies are needed to further compare the risks and benefits of laparoscopic and open surgery for acute small bowel obstruction (Fig. 40.3).
Management of Large Bowel Obstruction
Sigmoid volvulus is initially treated by nonoperative decompression, followed by elective surgery to prevent recurrence after colonoscopy excludes the possibility of carcinoma. Decompression can be performed via rigid or flexible endoscopic detorsion of the twisted segment.22 Endoscopy is contraindicated in patients with suspected intestinal necrosis. A rectal tube may be left in place before surgery to facilitate further decompression and prevent recurrence during hospitalization.
Cecal volvulus is managed surgically, with most surgeons choosing a right colectomy as definitive therapy. Cecopexy, or manual detorsion and subsequent correction of the underlying hypermobile right colon, is associated with a high rate of recurrent volvulus.23
Pseudoobstruction can initially be managed nonsurgically with a combination of decompression, colonoscopy, and promotility agents, including neostigmine.24 Medications that slow colonic motility should be discontinued, and suspected infectious causes should be treated appropriately. Surgical management is indicated when conservative therapy fails or in cases of suspected colonic ischemia, perforation, or sepsis. Because of the large number of potential causes of pseudoobstruction, inpatient management with surgical and gastroenterologic consultation is recommended.
Abbas S, Bissett IP, Parry BR. Oral water soluble contrast for the management of adhesive small bowel obstruction. Cochrane Database Syst Rev. 3, 2007. CD004651
Maglinte DDT, Heitkamp DE, Howard TJ, et al. Current concepts in imaging of small bowel obstruction. Radiol Clin North Am. 2003;41:263–283.
1 Balthazar EJ, Birnbaum BA, Megibow AJ, et al. Closed-loop and strangulating intestinal obstruction: CT signs. Radiology. 1992;185:769–775.
2 Murray MJ, Gonze MD, Nowak LR, et al. Serum D-lactate levels as an aid to diagnosing acute intestinal ischemia. Am J Surg. 1994;167:575–578.
3 Chapman AH, McNamara M, Porter G. The acute contrast enema in suspected large bowel obstruction: value and technique. Clin Radiol. 1992;46:273–278.
4 Maglinte DDT, Heitkamp DE, Howard TJ, et al. Current concepts in imaging of small bowel obstruction. Radiol Clin North Am. 2003;41:263–283.
5 Daneshmand S, Hedley CG, Stain SC. The utility and reliability of computed tomography scan in the diagnosis of small bowel obstruction. Am Surg. 1999;65:922–926.
6 Shrake PD, Rex DK, Lappas JC, et al. Radiographic evaluation of suspected small bowel obstruction. Am J Gastroenterol. 1991;86:175–178.
7 Balthazar EJ. CT of small-bowel obstruction. AJR Am J Roentgenol. 1994;162:255–261.
8 Megibow AJ, Balthazar EJ, Cho KC, et al. Bowel obstruction: evaluation with CT. Radiology. 1991;180:313–318.
9 Lazarus DE, Slywotsky C, Bennett GL, et al. Frequency and relevance of the “small-bowel feces” sign on CT in patients with small-bowel obstruction. AJR Am J Roentgenol. 2004;183:1361–1366.
10 Maglinte DDT, Reyes BL, Harmon BH, et al. Reliability and role of plain film radiography and CT in the diagnosis of small bowel obstruction. AJR Am J Roentgenol. 1996;167:1451–1455.
11 Frager D, Baer JW, Medwid SW, et al. Detection of intestinal ischemia in patients with acute small-bowel obstruction due to adhesions or hernia: efficacy of CT. AJR Am J Roentgenol. 1996;166:67–71.
12 Jancelewicz T, Vu LT, Shawo AE, et al. Predicting strangulated small bowel obstruction: an old problem revisited. J Gastrointest Surg. 2009;13:93–99.
13 Schwenter F, Poletti PA, Platon A, et al. Clinicoradiologic score for predicting the risk of strangulated small bowel obstruction. Br J Surg. 2010;97:1119–1125.
14 Kim JH, Ha HK, Kim JK, et al. Usefulness of known computed tomography and clinical criteria for diagnosing strangulation in small-bowel obstruction: analysis of true and false interpretation groups in computed tomography. World J Surg. 2004;28:63–68.
15 Gollub MJ. Multidetector computed tomography enteroclysis of patients with small bowel obstruction—a volume-rendered “surgical perspective.”. J Comput Assist Tomogr. 2005;29:401–407.
16 Abbas S, Bissett IP, Parry BR. Oral water soluble contrast for the management of adhesive small bowel obstruction. Cochrane Database Syst Rev. 3, 2007. CD004651
17 Branco BC, Barmparas G, Schnueriger B, et al. Systematic review and meta-analysis of the diagnostic and therapeutic role of water-soluble contrast agent in adhesive small bowel obstruction. Br J Surg. 2010;97:470–478.
18 Williams SB, Greenspon J, Young HA, et al. Small bowel obstruction: conservative vs. surgical management. Dis Colon Rectum. 2005;48:1140–1146.
19 Pickleman J, Lee RM. The management of patients with suspected early postoperative small bowel obstruction. Ann Surg. 1989;210:216–219.
20 Velasco JM, Vallina VL, Bonomo SR, et al. Postlaparoscopic small bowel obstruction—rethinking its management. Surg Endosc. 1998;12:1043–1045.
21 Franklin ME, Jr., Gonzalez JJ, Jr., Miter DB, et al. Laparoscopic diagnosis and treatment of intestinal obstruction. Surg Endosc. 2004;18:26–30.
22 ASGE Standards of Practice Committee. The role of endoscopy in the management of patients with known and suspected colonic obstruction and pseudo-obstruction. Gastrointest Endosc. 2010;71:669–679.
23 Rabinovici R, Simansky DA, Kaplan O, et al. Cecal volvulus. Dis Colon Rectum. 1990;33:765–769.
24 Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137–141.