14 Botulinum Toxin Injections in Myofascial Pain Disorders
This chapter will discuss the uses of botulinum toxin for pain management in three of the myofascial pain disorders: cervical dystonia, myofascial pain syndrome and the piriformis syndrome. Although the uses for botulinum toxin that are licensed by the Food and Drug Administration (FDA) do not include pain management, it has been used by a range of medical specialists to address pain control of various etiologies. The purpose of this chapter is to provide clinical insights for clinicians regarding the use of botulinum toxin in three disorders that involve myofascial pain, based on the authors’ experience and literature review. As with all medications and procedures, clinicians must obtain the necessary training and knowledge to achieve the most effective and safe outcome. Accordingly, one should know essential features about this toxin before treating patients for pain. These vital elements include: mechanism of action1,2; concept of median lethal dose (LD50); dosing and administration3; basic neuromuscular anatomy and physiology; conjunctive therapy with botulinum toxin for optimal treatments;4,5 and contraindications, of using this toxin. Because it is beyond the scope of this chapter to address all of the topics just listed, the authors urge the reader to visit other informational sources listed at the end of this chapter.
Botulinum Toxin and Its Clinical Use
Justinus Kerner, a German physician and poet, provided the first accurate and complete description of the clinical symptoms of food-borne botulism. He described 230 patients in the 1820s who suffered all the muscular and autonomic symptoms including gastrointestinal disturbances, dry eyes, dry skin, and weakness associated with the ingestion of contaminated meats.6 Kerner termed this condition “sausage poison” and “fatty poison”. He went on to perform animal experiments and experiments on himself. He administered botulinum toxin extracted from contaminated sausages to birds, cats, rabbits, frogs, flies, locusts, and snails.7 Kerner developed hypotheses from his experiments on the pathophysiology of the toxin. In his monograph he stated: “The nerve conduction is brought by the toxin into a condition in which its influence on the chemical process of life is interrupted. The capacity of nerve conduction is interrupted by the toxin in the same way as in an electrical conductor by rust.” Later in the monograph, he conceives of the idea of using the toxin for therapeutic uses.7
The term botulism was derived from the Latin term, “botulus”, which means sausage. Edward Schantz first isolated the toxin and he and Alan Scott began work on a standardized botulinum toxin preparation in the late 1960s.6 Scott first used botulinum toxin type A in monkey experiments in 1973 and he first used botulinum toxin type A in humans to treat strabismus in 1980.6 Botulinum toxin type A was approved by the Food and Drug Administration (FDA) in 1989 for the treatment of strabismus, blepharospasm, and hemifacial spasm in patients more than 12 years of age. Botulinum toxin type B received FDA approval for treatment of cervical dystonia in 2000, and botulinum toxin type A was approved by the FDA to treat severe primary axillary hyperhydrosis and moderate-to-severe glabellar frown lines in 2002.
In addition to the approved uses in the United States, there are other published uses of botulinum toxin,8 which include painful or potentially painful conditions such as: achalasia; anismus (painful); bladder detrusor hyperactivity; essential tremor; myofascial pain syndrome (painful); focal dystonias (sometimes painful); muscle spasm (often painful); piriformis syndrome (painful); spasmodic dysphonia; spasticity (sometimes painful); whiplash (painful); chronic focal painful neuropathies; migraines and other headache disorders; temporomandibular joint pain disorders; gastrointestinal dysmotility disorders; chronic low back pain.
Aside from publications, noteworthy medical organizations have commented on the effectiveness and safety of botulinum toxin. The National Institutes of Health (NIH) Consensus Development Conference published a statement in 1990 that summarized the indications and contraindications of botulinum toxin usage for the treatment of a variety of conditions.9 The NIH conference endorsed the use of the neurotoxin as safe and effective for the symptomatic treatment of adductor spasmodic dysphonia, blepharospasm, cervical dystonia, hemifacial spasm, jaw-closing oromandibular dystonia, and strabismus. The same year, the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology further endorsed the use of botulinum toxin for the symptomatic treatment of these conditions.10
Botulinum toxin is held to be one of the deadliest poisons known to mankind, yet by harnessing this activity, it has resulted in great advances in the treatment of numerous conditions.2 The understanding of the mechanism of action of botulinum toxin had its beginnings in 1949 when Burgen’s group discovered that botulinum toxin blocks neuromuscular transmission.11 In the 1950s, researchers discovered that injecting overactive muscles with small quantities of botulinum toxin type A resulted in decreased muscle activity by blocking the release of acetylcholine (Ach) at the neuromuscular junction.11 The understanding of this mechanism of action led to the application of botulinum toxin in most clinical settings. Since then, significant advances have occurred in the understanding of the mechanisms of action of botulinum toxin other than muscle paralysis—and this is leading to additional applications.
There are seven serotypes (A, B, C1, D, E, F and G) of botulinum toxin that act by inhibiting the exocytosis of Ach from presynaptic boutons of cholinergic neurons. Botulinum toxin is synthesized as a single-chain polypeptide (~150 kDa) that is activated by proteolytic cleavage into a 100-kDa heavy chain and 50-kDa light chain linked by a disulfide bond. The heavy chain domain is involved in cellular uptake into the presynaptic terminal by binding to extracellular receptors and in the transport of the neurotoxin through the lipid bilayer. The toxin is now intracellular but is sequestered in a membranous organelle without access to its targets, which reside in the cytoplasm. It is one of the functional domains (amino) of the heavy chain of the toxin that undergoes a conformational change facilitating translocation of the light chain through the lipid bilayer into the cytoplasm. Finally, the disulfide bond between the heavy and light chain is cleaved, allowing the light chain access to the cytoplasm. The light chain is a zinc-dependent endoprotease and the targets of the protease are presynaptic proteins required for intracellular trafficking of acetylcholine vesicles into the synaptic cleft. The presynaptic proteins targeted by the protease are called SNARE proteins (soluble N-ethylmaleimide-sensitive fusion protein attachment receptor) and are critical for the exocytosis of synaptic vesicles. It is this step, the proteolytic cleavage of the SNARE proteins, that is best understood in the intoxication by botulinum neurotoxin.12
The release of Ach into the synaptic cleft requires docking of the Ach-containing vesicle to the presynaptic membrane.12 The docking of the vesicle requires various proteins that are located in the wall of the vesicle, in the presynaptic membrane or in the cytoplasm. This family of proteins (SNAREs) includes vesicle-associated membrane protein (VAMP)/synaptobrevin, syntaxin, and SNAP-25. These proteins form a complex that allows the vesicle to fuse with the motor nerve terminus membrane and release the acetylcholine into the synaptic cleft. The site of action of botulinum toxin type A is SNAP-25 (synaptosome-associated protein with a molecular weight of 25 kDa), which is a presynaptic membrane protein. Botulinum toxin type B targets VAMP (vesicle-associated membrane protein), which is located in the wall of the vesicle. Cleavage of the proteins prevents the assembly of the fusion complex and thereby blocks the docking of the vesicle and the release of the Ach leading to relaxation of the muscle cell and a state of chemical denervation.12
Of significant clinical importance is the duration of action of the toxin effect. In general terms, the clinical effects of botulinum toxin injections are delayed a day or two with the maximal effects of functional muscular weakness peaking at about 2 weeks.1,5 The therapeutic effect of botulinum toxin-induced neuromuscular blockade usually lasts 3 to 4 months and ranges 2 to 6 months following an injection.13 Several factors likely contribute to the duration of action of the toxin. The best understood factor and most often quoted is the synaptic remodeling; however, other factors such as the duration of protease activity in the nerve terminal, the rate of replacement of the cleaved SNARE proteins, and the activity of the cleavage products may also influence the rate of function recovery.6
The development of neutralizing antibodies to botulinum toxin can be a therapeutic problem for patients and physicians.14 Patients who initially respond well to treatment with botulinum toxin can become nonresponders.15 The incidence rates of neutralizing antibody development and the exact cause in individual patients remains unknown.14 More frequent injections, more “booster injections,” and higher doses are possible risk factors, thus extending the time between injections, minimizing the dose, and avoiding booster injections may decrease the development of neutralizing antibodies.15
Some authors have noted that the pain relief preceded muscle decontraction and exceeded the degree and duration expected as a consequence of its neuromuscular actions.13 These observations suggest that botulinum toxin may have antinociceptive properties independent of the muscle relaxation. These observations and the expanding body of literature examining botulinum toxin for primary headache disorders and muscle conditions including a pain component led to further investigation into botulinum toxin in pain.16
A number of in vitro experiments have provided evidence that botulinum toxin inhibits neurogenic inflammation by attenuation of the release of neurotransmitters.17 Botulinum toxin was found to inhibit substance P release from cultured embryonic dorsal root ganglion neurons and to reduce stimulated release of calcitonin gene-related peptide from cultured trigeminal ganglia neurons.18
Additional support has come from animal experiments demonstrating reduction in nociceptive behaviors in animal models of inflammatory and traumatic neuropathic pain following peripheral injections of botulinum toxin.17 In rats with induced trigeminal neuropathy, intradermal injection of botulinum toxin in the area of the infraorbital branch of the trigeminal nerve alleviated the mechanical allodynia and reduced the exaggerated neurotransmitter release.19 In another experiment by Cui and colleagues, a rat formalin model of inflammatory pain was inhibited by subcutaneous administration of botulinum toxin injection and this inhibition was associated with a reduction in neurotransmitter release from the peripheral terminals of nociceptive sensory neurons. 20 It was Cui’s study in 2002 that provided the first evidence that botulinum toxin had an effect on nociceptive sensory nerves in vivo.20 In studies by Aoki, botulinum toxin inhibited several of the neurophysiologic and neurochemical effects of formalin in the rat formalin-pain model including glutamate release, Fos-LI in the dorsal horn, and evoked-activity of WDR neurons in the spinal cord.16
The efficacy of botulinum toxin in neuropathic pain initially was suggested in small anecdotal case studies and small open-label trials.21 An open study of botulinum toxin in 13 volunteers with trigeminal neuralgia found a reduction in visual analog scale scores and surface area of pain.22 Tsai and coworkers conducted an open label, prospective pilot study using botulinum toxin injected intracarpally in five patients with primary carpal tunnel syndrome.23 Their data suggested a long-acting antinociceptive effect of botulinum toxin.
More recently, two well designed clinical trials evaluated the efficacy of botulinum toxin for chronic neuropathic pain and diabetic neuropathic pain.17,24 Ranoux and colleagues published a randomized, double-blind, placebo-controlled, parallel group study providing evidence in support of the efficacy of botulinum toxin for the pain associated with focal neuropathies such as postherpetic neuralgia and posttraumatic or postoperative neuropathy.17 The study included 29 patients with focal painful neuropathies and mechanical allodynia, and treatment consisted of a one-time intradermal administration of botulinum toxin into the painful area. Outcome measures included average spontaneous pain intensity, quantified testing of thermal and mechanical perception of pain, allodynia to brush and decreased pain threshold to cold; the measures were evaluated at baseline, 4, 12, and 24 weeks. The results indicated that botulinum toxin treatment, relative to placebo, was associated with persistent effects on spontaneous pain intensity from 2 weeks after the injection to 14 weeks.12 A recent study by Yuan and associates report the results of a double-blind, placebo-controlled, crossover trial of intradermal botulinum toxin for diabetic neuropathic pain in 18 patients.24 The authors found a significant reduction in visual analog scale of pain at 1, 4, 8, and 12 weeks after botulinum toxin injection when compared to the placebo group. Specifically, within the botulinum toxin group, 44.4% of the participants experienced a reduction of the visual analog scale greater than or equal to 3 within 3 months after the injection, in contrast to the placebo group that reported no similar response. In addition, the authors evaluated sleep quality using the Chinese version of the Pittsburgh Sleep Quality Index and found a difference in the improvement in sleep quality between the botulinum toxin treatment group and the placebo group. The difference between the groups reached significance (P < .05) only 4 weeks after the initial injection, but did not support sleep improvement with botulinum toxin at week 12, which was the endpoint of the study design.24
Both reports (Yuan and colleagues24 and Ranoux and associates17) are small studies but each support a trend of reduced pain perception beginning at 1 week postinjection and extending to 12 weeks in the Yuan and colleagues’ study and 14 weeks in the Ranoux and associates’ study.17,24 Both studies report essentially no adverse events and this is supported by the general botulinum toxin literature. Although this suggests a promising approach to the treatment of neuropathy, additional larger, well designed, multicenter clinical trials with longer periods of follow-up are necessary.
Cervical Dystonia
Cervical dystonia (CD), also known as spasmodic torticollis and torsion dystonia, is a common form of focal dystonia manifesting as involuntary contraction and twisting of the neck muscles.25,26 These features lead to abnormal postures and movements of the head. The deviation of the head can be multidirectional and is described as torticollis (the most common form of CD with patient’s head turned to one side); laterocollis (lateral flexion of the neck), anterocollis (flexion of the neck) and retrocollis (extension of the neck). It is possible that one can have a combination of these forms. The prevalence of CD was reported to be 8.9/1,000,00027 and it is recently estimated by the Dystonia Medical Research Foundation that 250,000 people suffer from CD in the United States. It is believed that 66% to 75% of the patients with CD are disabled from the pain associated with CD.27–30 CD is mostly idiopathic and about 12% of those affected have a family history.31 Idiopathic CD is the most common form of adult-onset focal dystonia slowly developed over several years in patients 30 to 50 years old.32 Cervical dystonia can be caused by any injury or inflammation of the cervical muscles or cranial nerves from various disease processes, including head, neck, and shoulder trauma33 or from taking dopaminergic block agents.34 There is evidence indicating that CD arises from basal ganglia circuit abnormalities leading to dopaminergic dysfunction, which in turn causes disinhibited thalamocortical output and dystonic postures.31,35–37
Patients with CD can have a wide spectrum of symptoms involving the head, neck, upper extremity, and other body parts with sustained painful muscle contraction, pulling, and/or stiffness.28,31,37 The severity of the pain is usually relative to the intensity of the dystonia and muscle spasms.28 Jahanshahi and colleagues reported progression of dystonic symptoms to extranuchal, but still cervical, innervated sites such as hand, arm, and oromandibular region in one third of the 72 patients with adult-onset cervical dystonia.38 Patients with CD may develop neck pain from the muscle contraction and muscle strain from correcting the abnormal posture. The chronic abnormal posture may also lead to degenerative changes in the cervical spine leading to facet pain, radiculopathy, or spinal stenosis. Headaches associated with CD are common.39 Acute posttraumatic CD is different and the symptoms include immediate local pain after trauma, followed by a significant limitation in cervical range of motion, and abnormal posture of the head and shoulder. Trapezius hypertrophy may occur as well. Those changes often result in abnormal muscle contraction and pain.28,31 The abnormal posture and pain in patients with CD are part of their functional limitation and interfere with the patients’ activities of daily living.31 Rondot and coworkers40 found that 99% of the 220 patients they studies had various functional difficulties. Dysphasia and subclinical swallowing motility disturbances were reported in those patients.41 Permanent disability from the decreased cervical range of motion, involuntary movements, and intractable pain may occur.42 The diagnosis of CD is clinical and inspection is usually enough. However, a though physical examination should be conducted to rule out “pseudodystonia” due to structural abnormalities43 and secondary dystonia.
Treatment of Cervical Dystonia with Botulinum Toxin
There are various treatment options for CD including medications,32,44–47 surgery,48–56 rehabilitation,57 deep brain stimulation,58,59 and injections. The goal of the treatments is to reduce the discomfort and pain; improve function and quality of life; and prevent complications. Other than botulinum toxin (BTX) injection, the other treatment modalities are beyond the scope of this chapter.
The care of CD patients was drastically improved since 1989 when botulinum toxin was introduced in the United States. The FDA approved the indication of botulinum toxin injection for CD treatment in 2000. It is generally considered that BTX injection is the treatment of choice for patients with CD.60–62 The effectiveness of local injections of botulinum toxin is supported by evidence-based reviews and meta-analysis.45,54,63,64 Jankovic and Schwartz65 followed 202 out of 232 patients who received botulinum toxin type A injection for medically intractable CD for at least 3 months and up to 4 years. Seventy one percent of those patients had improved symptoms and 76% had almost complete relief of pain. Hsiung and colleagues published that 63% of patients treated with botulinum toxin injection reported benefit at 5 years.66 In a 20-week multicenter double-blind, randomized, controlled study in the United States involving 80 patients, the botulinum toxin group demonstrated improvement in the total Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) score than did the placebo group at weeks 4, 8, and 12 weeks.67 The side effects of the two groups were similar except for blurred vision and weakness in the botulinum group. The Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology concluded that botulinum toxin is safe and effective for the treatment of CD.68 There are numerous studies using botulinum toxin type B for CD treatments.69–71 Pappert and Germanson compared the CD treatment with botulinum toxin type A and botulinum toxin type B in a randomized, double-blind study, and found that both types of botulinum toxin are effective and safe for the treatment of CD in botulinum toxin-naïve patients.72 There is disagreement regarding the study conclusion.73,74 In a Cochrane Reviews, Costa and associates concluded that single injections of botulinum toxin type A and type B are effective and safe for treating CD, and long-term, uncontrolled studies suggested that further injection cycles continue to work for most patients.63,75 In another Cochrane Review, these authors concluded that it was not possible to make a definitive comparison between botulinum toxin type A and type B for CD treatment.76
Because of the complexity of the neck, it is critical for the clinician to be familiar with the anatomic landmarks, the vital structure, and the muscles for a successful treatment of CD with botulinum toxin injection. Attention should be paid to the following structures/organs when injecting botulinum toxin: the brachial plexus, carotid sheath, pharynx, esophagus, and the apex of the lung. The muscles involved in various forms of CD are listed in Table 14-1. Although the use of electromyography (EMG) for botulinum toxin injection for CD management remains controversial,68 the authors recommend using EMG-guided injection whenever possible to ensure the effective targeting of the affected muscles77 and the motor end plates within those muscles78–80 to potentiate neurotoxin effects. It is essential to have the patients as relaxed as possible to eliminate the false motor action potentials on EMG. If palpation alone is used to identify affected muscles, injection into either the mid-belly or several sites of the muscle is generally recommended.
| CD Type | Muscles Involved |
|---|---|
| Torticollis |
CD, Cervical dystonia.
Adapted from Brashear A: Botulinum toxin type A in the treatment of patients with cervical dystonia. Biologics 3:1-7, 2009.
Because of the variation of muscle involvement, there are no clear guidelines for the appropriate dose of botulinum toxin for the treatment of CD. A wide range of dosages was reported in CD treatments with botulinum toxin type A81–90 and type B.69–71 Dressler found that 20 units of botulinum toxin type A reduced the dystonic activity in the sternocleidomastoid muscle and larger doses offered minimal additional effect.91 Koller and colleagues92 noted that fixed-dose, fixed-muscle controlled studies of botulinum toxin for the clinical management of CD did not produce the same effects. Based on our experiences and the literature review, we think that, in general, patients should receive as few doses of botulinum toxin as possible as long as their symptoms are managed; the dose of botulinum toxin should be individualized; and the lowest effective dosage of botulinum toxin should be administered for treatment of CD to protect the patient from becoming immune to its therapeutic effect.93,94
Myofascial Pain Syndrome
Myofascial pain syndrome (MPS) is a painful disorder that is characterized by the presence of the palpable taut band of muscle fibers and myofascial trigger points (MTrP).95 The International Association for the Study of Pain defines the trigger point as a discrete tender point palpable in the taut muscle band.96 A myofascial pain syndrome caused by MTrPs characteristically results from either an acute episode of muscle overload or a chronic and/or repetitive muscle overload.97 In addition, there are autonomic phenomena (piloerection, localized sweating, or even regional temperature changes in the skin because of altered blood flow), concomitant disorders, psychosocial factors, and psychiatric presentations including depression and anxiety.98,99 There are different opinions about what MPS represents. Some scholars think that it should cover all the regional pain caused by any soft tissue pathology. Thus, MPS may be considered as a primary local or original pain syndrome or a secondary syndrome caused by other disorders.100,101
Research studies indicate that the clinical characteristics of an MTrP can be explained by hypercontracted muscle fibers located at and produced by a region of muscle with multiple dysfunctional motor end plates (neuromuscular junctions). The dysfunction is a markedly excessive continuous release of the normal synaptic transmitter, Ach. The noise-like potentials and spikes that are strongly associated with MTrPs102–104 were first interpreted as coming from muscle spindles.103 However, electromyographic studies clearly identify these noise-like and spike potentials as motor endplate potentials of skeletal muscle fibers.105 Electromyographers generally recognize these end-plate potentials as normal.105–108 However, physiologists have distinguished these potentials from normal miniature end-plate potentials and have shown that they represent a pathologic increase in spontaneous release of Ach.109–112 The end-plate noise component can result from mechanical strain of the neuromuscular junction caused by stresses applied to the nerve terminal112 or produced by muscle overload. The end-plate noise component of end-plate potentials appears to be present before the needle examination and is commonly caused by stressful activity of the muscle, especially in latent MTrPs that cause no clinical pain complaint. The end-plate spikes, however, are often induced by the presence of the needle113 and are more likely to appear in more active MTrPs.
Histologically, MTrPs show large, darkly stained, round myofibers in cross section in canine,114 equine115 and in human116,117 studies. Longitudinal sections of myofibers several hundred microns in length of canine muscle show hypercontracted fibers (also called “contraction knots”). The integrated hypothesis for the pathophysiology of MTrPs attributes these contraction knots to the observed depolarization of the post-junctional membrane that continuously releases calcium from the sarcoplasmic reticulum. This hypothesis identifies contraction knots as limiting circulation because the strong contraction of the sarcomeres is sustained within the hypercontracted fiber, whereas local energy consumption is increased. The resulting energy crisis should exhibit severe local hypoxia, demonstrated in the MTrPs.118 Increased tension of involved muscle fibers accounts for the palpable taut band consistently associated with an MTrP. The energy crisis and local hypoxia that was observed to extend for several millimeters could account for the release of substances that sensitize local nociceptors, causing the local and referred pain characteristic of MTrPs.97 An interesting study examined rabbit muscle after a marker (iron deposit) was placed at precisely the location where an active trigger point was identified by twitch response, taut band, and spontaneous electrical activity. Small C nerve fibers (most likely nerves that carry pain information) were found in the immediate vicinity.107 Taken together, these data support the idea that MTrPs are related to abnormal motor end-plate activity and subsequent hypercontraction of the associated myofibers.119
MTrPs are identified on physical examination by palpating a localized tender spot in a nodular portion of a taut band of muscle fibers. Pressure (usually with the examiner’s fingertip) over a trigger point elicits pain at that area and may also elicit pain at a distance from the point under the fingertip. This is known as referred pain. Another important feature of the trigger point is that the elicited pain mirrors the patient’s experience. Insertion of a needle, snapping palpation, or even a brisk tap with the fingertip directly over the MTrP may elicit a brief muscle contraction detectable by the examiner. This brisk contraction of muscle fibers of the taut band is termed a local twitch response.97 There is a burst of electrical activity within the muscle band that has the twitch response (end-plate noise), and such activity is not observed in the other muscle bands. This type of end-plate noise is found more prevalently in myofascial trigger points than in sites that are outside the MTrP but still within the end-plate zone.120 Animal studies102,121,122 and a human study123 have shown that this response is propagated as a spinal reflex. This response is a valuable indicator that the needle being injected into an MTrP has effectively reached at least one necessary target in the MTrP. Demonstration of a local twitch is additional confirmation of the diagnosis. In addition, passive stretch range of motion of the muscle is limited by pain, and both maximum contraction in the shortened position and maximum voluntary contraction are likely to be inhibited or to be associated with pain.
If abnormal end-plate activity is responsible for MTrPs, then a powerful rationale exists for the use of neuromuscular blocking agents, such as botulinum toxin, in the treatment of myofascial pain syndrome.124 Injection of muscles with botulinum toxin can be appropriate therapy for myofascial pain caused by MTrP but is unlikely to be so for treatment of myofascial pain of unspecified origin, when that term is used in the general sense.125 Myofascial pain with muscle tenderness versus specific MTrPs responsible for the clinical pain is an ambiguous diagnosis. The tender spots may be due to fibromyalgia, bursitis, or one of many other diagnoses that do not justify injection with this product.
At present, no routine laboratory test or routine imaging test is available to confirm the presence of MTrPs. Usually two objective tests can be used to confirm the presence of MTrPs. One requires electrodiagnostic technique and the other uses ultrasound imaging. Both animal and human research studies have shown that MTrPs are characterized by electrically active loci that exhibit end-plate noise and often spikes.107
Treatment of Myofascial Pain Syndrome with Botulinum Toxin
The previous chapter in the second edition included a Medline search conducted in 1997 for the headings “botulinum toxin,” “myofascial pain,” and “pain” for the period 1966 to September 1997 and resulted in 18 references.126 An updated Medline search for the period September 1997 to April 2009 was conducted using the headings “botulinum toxin” and “myofascial pain syndrome” and resulted in 45 references. Of these, 21 studies were in English and included “pain” or “myofascial pain” within the article title.127–147
A few words of caution before considering using botulinum toxin in the treatment of a patient with myofascial pain: Recall that the approved indications for the use of botulinum toxin in the United States are for six conditions: strabismus, blepharospasm, hemifacial spasm, cervical dystonia, primary axillary hyperhidrosis, and moderate-to-severe glabellar frown lines.6 Use of botulinum toxin for myofascial pain is off-label and should be considered only for patients with conditions that failed conservative treatment or for patients judged inappropriate for conservative treatment.145
Two studies were designed to compare trigger point injections in the management of patients with myofascial pain and headaches.127,135 The studies included a total of 74 patients that were randomly assigned to one of three groups: dry needling, lidocaine injection, or botulinum toxin injection. Venancio and colleagues assessed their subjects during a 12-week period based on levels of pain intensity, frequency and duration, local post-injection sensitivity, obtainment time and duration of relief, and the use of rescue medication.127 In contrast, Kamanli and coworkers’ assessment measures included cervical range of motion, trigger point pain pressure threshold, pain scores, visual analog scales for pain, fatigue, and work disability at entry and at 4 weeks.135 In addition, depression, anxiety, and quality of life were assessed. Venancio and coworkers found that, statistically, all the groups showed favorable results for the evaluated requisites, except for the use of rescue medication and local post-injection sensitivity (botulinum toxin group showed better results). Similarly in the study by Kamanli and coworkers, all three groups showed a significant improvement in pain pressure thresholds and pain scores; however, the lidocaine injection group’s pain pressure threshold values were significantly higher than the dry needling group and their pain scores were significantly lower than either the dry needling or botulinum toxin injection groups.135 Both studies concluded that lidocaine could be adopted as a substance of choice considering its reduced cost and botulinum toxin should be reserved for refractory cases.
A study by Porta compared the effects of botulinum toxin type A with the steroid methylprednisolone in146 patients with chronic myofascial pain in the piriformis, iliopsoas, or scalenus anterior muscles.146 Both groups of patients had a significant pain reduction at 30 days post-injection but there was no significant difference between the groups. At 60 days following the injection the pain scores were statistically significantly lower in the botulinum toxin group when compared to the steroid group. In addition, the pain scores in the botulinum toxin group were lower at 60 days than at 30 days post injection.146
Six of the twenty-one studies had “myofascial pain syndrome” and “neck” or “back” pain in the title including a review by Porta and Maggioni.128,132,134,136,139,148 Gobel’s report was a randomized, double-blind, placebo-controlled, multicenter study including 145 patients with moderate-to-severe myofascial pain syndrome affecting cervical and/or shoulder muscles.132 The injections were made into the 10 most tender trigger points (40 units per site). Compared to placebo, botulinum toxin resulted in more patients with mild or no pain at week 5, a significantly greater change from baseline in pain intensity during weeks 5 through 8, and significantly fewer days per week without pain between weeks 5 and 12.132 Another randomized, double-blind, placebo-controlled study with 29 subjects with neck and upper back pain of myofascial origin compared botulinum toxin injections to placebo (saline injections).148 The botulinum toxin injection used 50 units and the outcome measures were the visual analog scale for pain, the neck disability index, and the medical outcome study 36-item short form health survey.148 Improvements were seen in the visual analog scale and the neck disability index but were not significant when compared to the controls. The outcomes as measured by the 36-item bodily pain short form and the 36-item mental health short form did reach statistical significance in the treatment group at 2 and 4 months and at 1 month, respectively.148 Braker and colleagues evaluated the effectiveness of botulinum toxin injections compared to placebo in 20 patients with cervical myofascial pain following a whiplash injury (2 to 48 weeks following injury).128 Subjects received 200 units of botulinum toxin or placebo at four tender points and were seen in follow-up at 3, 6, 9, 12, and 24 weeks.128 Outcome measures included pain intensity evaluated by the visual analog scale and verbal rating scale, quality of life measured by the SF-36 questionnaire, treatment efficacy as per the global assessment of the physician and patient, and intensity of pain response to mechanical pressure and cervical range of motion. Both groups had a time-dependent improvement in all parameters, which was consistently larger in the botulinum toxin group, although it did not reach significance.148
Wheeler and colleagues conducted a randomized, double-blind study comparing the effects of botulinum toxin injections to normal saline in 33 subjects randomly assigned to either 50 or 100 units of botulinum toxin or normal saline.136 The subjects were evaluated over a 4-month period by assessment of pain and disability and pressure algometer readings. This study did not find any statistically significant benefit of botulinum toxin over placebo.136 The trial by Ojala and colleagues was designed to determine the effect of small doses of botulinum toxin (5 units per site).134 The authors found that no statistically significant changes in neck pain and pressure pain threshold values occurred between the low-dose botulinum toxin and saline groups. Ferrante and his colleagues149 conducted a randomized, double-blind, placebo-controlled study with 132 patients with cervicothoracic myofascial pain with trigger points. They concluded that the injection of botulinum toxin type A directly into the trigger points did not improve the cervicothoracic myofascial pain. In a comment on this study, Abram indicated that the negative results of the study by Ferrante and colleagues149 were unexpected because there is reason to predict reduction of pain given the pharmacologic effects attributed to botulinum toxin.150 He commented the notion from Ferrante and colleagues149 that the site of trigger point injection may not be the optimal site for botulinum toxin injection of the muscle. Abram150 stated that although the motor end plate is the most effective location for injection and the site is not known for many of the muscles in myofascial pain, botulinum toxin should diffuse throughout the muscle and provide some effect at the higher dose of the study.150 He also expressed his concern about the accuracy of the diagnosis and the possible heterogeneity of the cohort selected in the Ferrante and colleagues’149 study. Abram150 stated that not all patients with muscle tenderness have myofascial pain syndrome; and some patients’ myofascial pain can be secondary to other painful disorders such as radiculopathy, facet arthropathy, or complex regional pain syndrome, which are much more resistant to treatment than primary myofascial pain syndrome. He suggested selecting patients who experience complete but temporary relief from local anesthetic trigger point injection for botulinum toxin injection or study.150 The authors think that Abram’s opinions are validated and they could be applied to other studies or treatments using botulinum toxin for patients with myofascial pain syndrome.
A consensus statement on botulinum toxin in myofascial pain by Reilich and colleagues states that botulinum toxin should be considered if the patient has an assured chronic myofascial pain syndrome that has been resistant to less invasive measures.138 Their conclusions state that botulinum toxin injections should be performed in institutions with extensive experience in therapy of myofascial pain and treatment with botulinum toxin. Furthermore, the injections should be part of a multimodal therapeutic program including physical therapy and relaxation exercises. Thus, botulinum toxin injections seem to be an effective supplemental treatment, although the authors state further clinical evidence is needed from double-blind controlled trials.138
In MTrP injection, targeting is critical for attaining optimal and effective therapeutic goal. The trigger point injection (TPI) with botulinum toxin is similar to the TPI with other agents. Most practitioners use the direct injection technique. The skin overlying the MTrP is prepped and a needle is advanced to the point of maximum tenderness. Usually this will reproduce the patient’s myofascial pain or the referred pain. The botulinum toxin is injected after negative aspiration for blood. Usual side effects as with other forms of botulinum toxin injection and TPI may occur, although the risks are fewer. Lang reported a novel grid-pattern technique for injecting botulinum toxin in myofascial pain.151 He reported that 65% of the 72 patients had good-to-excellent pain relief, whereas 24% had fair, and 12% had poor pain relief. Of note, the median dose of botulinum toxin used in this study was 200 units.
If the initial acute MTrP pain was not properly diagnosed or not treated effectively, it may lead to the chronic myofascial pain syndrome. The unresolved perpetuating mechanical or systemic factors may maintain the chronicity of the myofascial pain.97 Injecting these chronic MTrPs with botulinum toxin (or other agents) can be expected to provide relief only for a limited time if the perpetuating factors are not identified and resolved. It is important to remember that the commonly occurring, acute, single MTrP often reverts from active to latent MTrP without specific treatment if the individual simply avoids the muscle overload situation that activated the MTrP and proceeds with daily activities within limits that are not painful. This daily activity, which includes actively stretching the involved muscle gently but repeatedly, is an effective treatment. The recovery from acute MTrP syndromes is expedited and the likelihood of lasting relief is greatly improved if the patient learns to perform slow, gentle, active, full range-of-motion exercises specifically for the involved muscles at least once daily. Following injection of chronic MTrPs, the authors consider these exercises essential for optimum results.
Piriformis Syndrome
Piriformis syndrome (PS) is a myofascial pain disorder that manifests with a spectrum of symptoms, including buttock pain, referred pain to the ipsilateral lower extremity, and/or low back pain, caused by piriformis muscle inflammation, spasms, contracture, anatomic anomalies, or irritation to the sciatic nerve.152–154 The piriformis is from the Latin words pirum (pear) and forma (shape). It is a flat muscle originating from the anterior lateral surface of sacrum (second to fourth vertebrae); exiting through the greater sciatic foramen; and inserting onto the superomedial greater trochanter. It neighbors with five hip rotator muscles: the superior and inferior gemelli, the obturator externus and internus, and the quadratus femoris. During lower extremity weight bearing or ambulation, the piriformis muscle contracts to prevent rapid internal rotation of the hip. It externally rotates the hip when the hip and knee are extended, whereas it abducts the hip when it is flexed to 90 degrees. The sciatic nerve, posterior femoral cutaneous nerve, gluteal nerves, and the gluteal vessels pass below the piriformis muscle. However, there are anatomic variations of the sciatic nerve to the piriformis muscle: superior to, divided by, or piercing through the piriformis muscle.
There were numerous studies in the past in attempting to find out the cause of low back pain and sciatica. Yeoman dissected 100 cadavers to find the relationship of the sacroiliitis and sciatica.155 In 1934, Freiberg and Vinke assumed that the sacroiliac joint (SIJ) lesion caused inflammation in the piriformis muscle, its fascia, and the lumbosacral plexus leading to the irritation of sciatic nerve.156 Beaton and Anson related the anomalies of the piriformis muscle to sciatica.157 The term PS was first used by Robinson in 1947 when he described this syndrome with158 six cardinal features to describe this syndrome. The six key features are (1) a history of trauma or fall to the buttock; (2) gluteal or SIJ pain with radiation to the lower extremity; (3) gluteal atrophy; (4) a palpable sausage-shaped mass; (5) positive Lasègue’s sign (pain on voluntary flexion, adduction, and internal rotation of the hip); and (6) exacerbation of pain with bending or lifting.
The PS remains a controversial diagnosis as to whether it is a distinct clinical diagnosis of the tender piriformis muscle with the pathology of muscle spasms or hypertrophy, or the compression of the sciatic nerve by the piriformis muscle causing neuropathic pain; and is complicated with the pain from five neighboring short external rotators.159–161 However, the authors think that the two entities could be the manifestation of the same diagnosis with different stage or severity of the disorder. The incidence of PS ranges from 0.33% to 6%162–164 and accounts for 6% to 8% of the yearly cases of low back pain and sciatica in the United States.165 Patients with PS are typically 30 to 40 years old, female (three to six times more than their male counterparts)166–170 with a recent history of trauma to the buttocks or pelvis (usually from a fall), exacerbated activity intensity, or changes of low back, pelvis and lower extremity biomechanics due to lower extremity injuries, leg length discrepancy (≤0.5 inch), hip joint replacement, gait disturbances, even pregnancy.152,171–173 It is also seen more in long distance truck drivers, cyclists, tennis players, ballet dancers, and the like. The typical symptoms of PS include buttock pain with or without radiation to the ipsilateral lower extremity, to or below the knee. These symptoms may be exacerbated with ipsilateral hip adduction, internal rotation or prolonged sitting (sitting intolerance), intercourse or bowel movement.157,162,169,172,174,175 Severe cases of PS may lead to gluteal atrophy,174,176 motor weakness of the S1 innervated muscles,168,169,176 foot drop,176,177 and sensory deficits.164,168,169,175,176 The diagnosis of PS was once thought to be a diagnosis of exclusion and because of the aforementioned symptoms, PS is very often misdiagnosed as sacroiliitis or lumbosacral radiculopathy.178 Therefore, careful evaluation to rule out the other causes is essential for the correct diagnosis and effective treatment. Patients with PS often have tenderness at the buttock region overlying the greater sciatic notch or the piriformis muscle or a taut muscle band (with or without trigger point) to palpation, with or without radiation to the ipsilateral lower radiation reproducing the patients’ symptoms.158,178 Several provocative maneuvers have been used to aid the diagnosis of PS, including the FADIR test (hip Flexion, ADduction and Internal Rotation, also known as FAIR, which is also a therapeutic position),97,169,179,180 the Pace test,162 the Freiberg test,156 and the Beatty test.166 Because the piriformis muscle is so deep, palpation of the muscle and the trigger point can be properly performed only by rectal or vaginal examination.162,174–176 Imaging studies, such as computed tomography (CT), ultrasound, or magnetic resonance imaging (MRI) are employed to rule out other potential sources of compression at or near the sciatic notch, such as intrapelvic abscess, occult tumor, or hematoma181–183 or in the lumbosacral spine. One helpful diagnostic aid is EMG. In the case of lumbosacral radiculopathy, the EMG examination may reveal abnormal spontaneous electrical activity in the lumbar paraspinal muscles, whereas in piriformis syndrome, no such abnormal electrical activity should be seen.184 Because the site of nerve compression is distal to the nerve root,185 other investigators have reported that H-wave studies are delayed when comparing the patient’s extended painful leg to the same leg in a position of adduction, internal rotation, and flexion.186 Conduction may be delayed when the muscle is at its thickest in the shortened position.
The injury to the piriformis muscle may lead to the inflammation and muscle spasms, and the irritation of the sciatic nerve.162,187 Histamine, serotonin, prostaglandin, and bradykinin from the inflammation may irritate the sciatic nerve resulting in a cycle of pain, spasms, inflammation, and irritation.188,189 The characteristic signs and symptoms are sometimes caused by pain referred from piriformis MTrPs153,185,190,191 and sometimes are caused by compression of the sciatic nerve between the bony rim of the sciatic foramen and a hypertrophied piriformis muscle.192 Pace and Nagle suggested that focal irritation of the piriformis muscle leads to myofascial pain syndrome that can be treated with trigger point injection.162 The nodular MTrP and its taut band can provide the increased muscle bulk and tension. Pain in these patients may come from the nerve entrapment and the referred pain from the piriformis MTrPs. An additional source of pain in these patients is the tendency for compression of motor nerves to activate MTrPs in the muscles supplied by that nerve. Although some clinicians think that this diagnosis is controversial, numerous peer-reviewed articles clearly define clinical, anatomic, and electrophysiologic evidence for this distinct condition causing low back and leg pain.152–154,180,186,193–195 The attachment of the muscle at the greater trochanter is prone to be equally involved and tender and is readily available for palpation. It is very useful to identify the central TrP tenderness in the region of the mid-belly as well as the attachment tenderness at both ends. This strengthens the MTrP diagnosis considerably. Contraction of muscles with MTrPs appears to be most painful when the muscle is voluntarily contracted in the shortened position. If the enthesopathy is sensitive enough, the muscle hurts when forcefully loaded in any position but particularly in the shortened position.
Treatment of Piriformis Syndrome with Botulinum Toxin
In most cases, the conservative treatments, such as physical therapy with piriformis muscle stretching, NSAIDs, muscle relaxants, ultrasound, vapo-coolant spray coupled with soft tissue stretching, are effective in reducing the inflammation, spasms, and pain.152,153,164,172,174 It is also reported that repeated piriformis muscle injections with clonidine and bupivacaine provide long-lasting pain relief in patients with PS.196 For patients who failed to improve with conservative treatments, local injections of anesthetics and/or corticosteroids may be considered and has been shown to be effective.186 However, some patients may gain short-term benefits from local MTrP injections but remain refractory to other treatment for long-term pain control. This subset of patients has demonstrated clear benefit from botulinum toxin treatment, especially if the piriformis muscle shows EMG evidence of involuntary muscle contraction.165,197–202
Porta conducted a comparative trial of botulinum toxin type A and methylprednisolone in group of 40 subjects included 23 patients with piriformis syndrome.146 Subjects were randomized to receive methylprednisolone or botulinum toxin type A (100 units). The 23 patients received injections in the piriformis muscle. At 60 days, the botulinum toxin group demonstrated statistically significant pain reductions as determined by their visual analog pain scale scores (P <0.0001). In addition, a trend was noted at 30 days post-injection with the botulinum toxin group having lower pain scores but this did not reach statistical significance. The exact benefit derived from the piriformis muscle injection is uncertain because the data was not subanalyzed by the muscle injected.146
Childers and colleagues199 conducted one of the earliest studies to examine the effectiveness of intramuscular botulinum toxin injections as a treatment for piriformis muscle syndrome. The authors examined a convenient sample of three consecutive patients. All patients presented with findings consistent with a diagnosis of piriformis syndrome and all had failed a trial of conservative management including NSAIDs, stretching, ultrasound, and piriformis trigger point injections.197 H-reflexes and segmental nerve conduction studies in all patients confirmed conduction block along the sciatic nerve above the gluteal fold, consistent with the diagnosis.186,203 The involved piriformis muscle in each patient was injected under fluoroscopic guidance with 100 units of botulinum toxin type A reconstituted in 5 mL of preservative-free saline.197 Pain reduction was assessed through pretreatment to post-treatment differences on a patient self-reporting instrument that recorded visual analog pain scale scores of pain intensity, psychological distress from pain, spasm frequency, and interference with daily activities. Results of this open label case series demonstrated that the average pain scores decreased from 6.1 to 3.4 and were 3.6 two weeks later.197 Twelve weeks later, two out of three patients had returned to their previous pain levels, whereas one patient sustained longer lasting benefit.197 The same author was the first to report a randomized, double-blind, placebo-controlled crossover trial of the effectiveness of botulinum toxin type A injections for refractory piriformis syndrome.198 Ten women initially enrolled and nine women completed the study. The subjects were randomized to receive either 100 units of botulinum toxin injection or saline under the guidance of fluoroscopy or EMG.198 The primary outcome measure of interest was differences between the pre-injection and post-injection visual analog pain scale scores (VAS). After injection with the vehicle, decreases were detected, but only in one of the four categories (distress), in contrast to the botulinum toxin group in which decreases were observed under all VAS categories.198
In 2002, Fishman and colleagues evaluated the efficacy of botulinum toxin type A used in conjunction with physical therapy for piriformis syndrome in 67 subjects.165 The results in this double-blind, placebo-controlled trial were measured on the visual analog scale. The patients injected with botulinum toxin experienced more relief from pain than the subjects receiving placebo (P = .001) and more relief than patients receiving lidocaine with steroid (P < .05).165
Yoon and colleagues conducted a prospective, single site, open label trial to evaluate the efficacy of botulinum toxin type A (150 units) in patients with piriformis syndrome resistant to conventional therapy.202 Twenty patients had botulinum toxin injected using CT guidance and nine patients served as active controls receiving an injection of dexamethasone (5 mg) and 1% lidocaine. Pain intensity scores, using a numeric rating system, were significantly lower at 4, 8, and 12 weeks after treatment than at baseline (P < .0001).202
Fishman and coworkers performed the first dose finding study for botulinum toxin type B in a cohort of eight consecutive patients receiving injections with 5000, 7500, 10,000 or 12,500 units.201 Visual analog pain scale scores were assessed at 0, 2, 4, 8, and 12 weeks.200 The subjects receiving the 12,500 units of botulinum toxin type B had the greatest clinical improvement at 3 weeks postinjection but then regressed over the following 5 weeks.200 Lang conducted a single center, open label study to evaluate the clinical safety and efficacy of botulinum toxin type B in patients with piriformis syndrome in 2004.201 Twenty patients were enrolled in the study and the injections were performed under electromyographic guidance with 5000 units of botulinum toxin type B. The study did not have controls and the single examiner was not blinded. Significant reductions in mean visual analog scale scores for buttock and hip pain were noted at 4, 12, and 16 weeks and low back pain at weeks 2, 12, and 16.
When injecting myofascial trigger points in the piriformis muscle, it is difficult to localize accurately in such a deep muscle and the trigger points are located in the end-plate zone in the mid-belly region of the muscle. In this case, use of EMG guidance to inject the neurotoxin specifically where end-plate potentials are observed will ensure optimal placement of the product.198 Because all or part of the sciatic nerve may occasionally traverse this part of the muscle, this use of EMG guidance is of additional importance.
The local anesthetic, corticosteroid, and botulinum toxin injections in the aforementioned reports165,197–202 were all performed under some sort of guidance, including EMG, ultrasound, fluoroscopy, and CT. Additional studies also demonstrated the convenience, accuracy, and efficacy in the injection of piriformis muscle.204–207 Therefore, the authors strongly recommend that the piriformis injection therapy should be conducted under the guidance of one of the proven methods to ensure the safety, accuracy, and efficacy.
Although there are associated disadvantages such as radiation exposure, contrast reaction, and increased cost, guided piriformis injections are widely used to ensure accurate placement of the medications and to avoid nerve injury around the piriformis muscle. After cadaver study, Benzon and colleagues208 used the lower border of the SIJ as their reference for the steroid injection to the piriformis muscle and near the sciatic nerve for better symptom relief. They inserted the needle 2 cm lateral and 1 cm caudal to the SIJ—guided with a fluoroscopy while patients were prone. The insulated needle was advance until a motor response was elicited in the foot with a nerve stimulator. The needle was then withdrawn until the elicited motor response disappeared and the steroid was injected near the sciatic nerve. The needle was then further pulled back to the piriformis muscle belly, confirmed with contrast (Fig. 14-1), and followed with steroid injection to the muscle. Similar technique was reported by Betts209 and confirmed with a cadaver study.207 In a 10-year study of the diagnosis, treatment, and outcome of the PS, Fishman and colleagues210 inserted the needle at a point one third the distance from the greater trochanter to the area of maximum tenderness in the buttock at the depth of approximately 3 to 5 cm. Electromyographic localization of the piriformis muscle was conducted only when the location or depth of the needle placement was uncertain. We use a fluoroscopically guided technique published by one of the authors (MKC).198,211 The patient is placed in the prone position, the skin over the largest bulk of the buttocks is prepped and draped in a sterile fashion. The greater trochanter of the femur, the body of the sacrum, and the sciatic notch are identified using fluoroscopy. The skin is marked at the midway on a line that crosses the middle of the sciatic notch between the sacrum and the greater trochanter of the hip. A dual-purpose injection/electromyographic needle is inserted in an angle slightly lateral to medial until the ileum is encountered. It is then withdrawn slightly, and the injection site is visualized by fluoroscopy. To further verify the needle placement within the piriformis muscle, an electromyographic electrode is connected to the hub of the needle and a ground and reference electrode is secured to the lateral upper thigh. The patient is instructed to externally rotate the thigh to activate the piriformis muscle until brisk motor unit action potentials are observed.
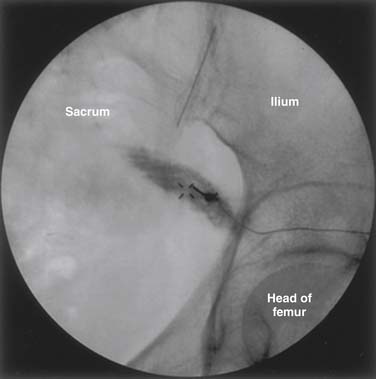
Figure 14-1 Piriformis muscle and needle placement confirmation with contrast.
(From Benzon HT, Katz JA, Benzon HA, Iqbal MS: Piriformis syndrome: Anatomic considerations, a new injection technique, and a review of the literature. Anesthesiology 98:1442-1448, 2003.)
Smith and coworkers212 reported in detail a technique of piriformis muscle injection with ultrasound guidance. The patient was placed in a prone position. The posterior superior iliac spine (PSIS) was palpated and scanned. The transducer was then placed horizontally across the PSIS, and then moved inferiorly while kept horizontally. The lateral sacrum was visualized medially and the posterior inferior iliac spine (PIIS) laterally. After the image was optimized, the following were observed “(1) medial: the lateral, hyperechoic bony margin of the sacrum; (2) superficial: hyperechoic skin, mixed echogenicity subcutaneous fat, and the hypoechoic marbled appearance of the overlying gluteus maximus; and (3) deep: the piriformis muscle appearing deep to the lateral sacral border (whereas the gluteus maximus is superficial to the sacrum), and traversing from cephalomedial to caudolateral beneath the gluteus maximus” (Fig. 14-2). The sciatic nerve was then identified, which was an oval, mixed-echogenicity structure lying superficial to the quadratus femoris and deep to the gluteus maximus (Fig. 14-3). After the sciatic nerve and its course and relationship with the piriformis muscle including the variation were verified, the piriformis muscle was again localized and the skin overlying the muscle was marked and prepared. Under ultrasound guidance, the needle was advanced medial to lateral, parallel to the long axis of the piriformis and the transducer, to the lateral sacrum. They stated that the operator can feel and visualize when the needle abuts the muscle sheath. The needle is then passed into the sheath or the muscle. In this process, the depth was controlled on the ultrasound image. The injectate in the piriformis muscle was collected as a bolus or tracked laterally between the multiple slips of the piriformis. The needle placement was also verified with contrast and real-time fluoroscopy. They indicated that this technique offers advantages compared to traditional imaging methods, “including accessibility, compact size, lack of ionizing radiation exposure, and direct visualization of neuromuscular structures”.
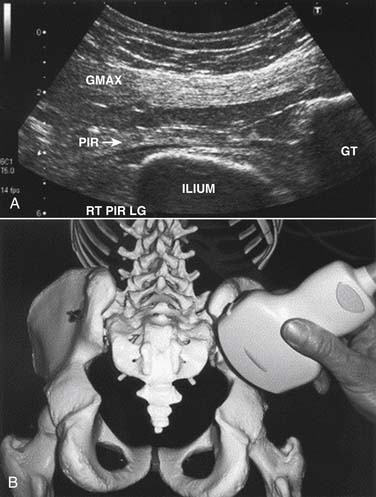
Figure 14-2 Longitudinal ultrasound view of the piriformis muscle. A, “The piriformis (PIR) muscle-tendon is visualized as a thin, hyperechoic band passing medial (left) to lateral (right) toward the greater trochanter (GT). The piriformis travels deep to the gluteus maximus (GMAX) as it passes out of the superior greater sciatic foramen, formed anteriorly by the ilium. In this image, the lateral sacrum would lie just off-screen to the left.” B, “Transducer position is relative to skeletal landmarks to obtain ultrasound image shown in A. RT PIR LG, right piriformis, longitudinal view (parallel to the long axis of the muscle).”208
(From Smith J, Hurdle MF, Locketz AJ, Wisniewski SJ: Ultrasound-guided piriformis injection: Technique description and verification. Arch Phys Med Rehabil 87:1664-1667, 2006.)
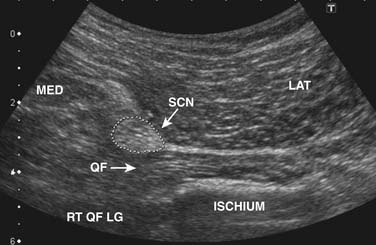
Figure 14-3 Transverse ultrasound view of the sciatic nerve. “The transducer is placed in an anatomic transverse plane at the level of the distal-lateral aspect of the ischial tuberosity. Here, the conspicuity of the hyperechoic sciatic nerve is increased as it passes between the hypoechoic quadratus femoris (deep) and gluteus maximus (superficial). The nerve can then be traced cranially to visualize its passage deep to the piriformis.” LAT, Lateral; MED, Medial; QF, quadratus femoris; RT QF LG, Right side, quadratus femoris, longitudinal view (parallel to the long axis of the muscle); SCN, Sciatic nerve.”208
(From Smith J, Hurdle MF, Locketz AJ, Wisniewski SJ: Ultrasound-guided piriformis injection: Technique description and verification. Arch Phys Med Rehabil 87:1664-1667, 2006.)
Huerto and associates204 used a similar technique plus a motor stimulator for perisciatic and piriformis muscle injection and concluded that this technique is accurate, safe, and reproducible for piriformis injection. Reus and colleagues used the inferior gluteal artery as the landmark for localizing the sciatic nerve in their ultrasound-guided perisciatic steroid injection.205 The sciatic nerve was found slightly lateral to the inferior gluteal artery on the longitudinal section and the skin was marked and prepared. The direction of the needle was controlled by longitudinal section of the sciatic nerve. The piriformis muscle was not injected or mentioned in this technique. Using the technique by Smith and coworkers212 Finnoff and colleagues213 used a cadaveric model to claim that ultrasound-guided piriformis injections were significantly more accurate than fluoroscopically guided contrast-controlled injections. They indicated that despite the use of bony landmarks and contrast, most of the fluoroscopically attempted piriformis injections were placed superficially within the gluteus maximus. However, we think that more studies need to be conducted to support such a claim. Nevertheless, the success of using the fluoroscopy-guided and the ultrasound-guided techniques is evident in previous publications. Pitfalls may occur in cases using both techniques and are most likely operator dependent.
Fanucci and coworkers199 used CT to guide their percutaneous botulinum toxin injection for PS treatment. With the success of delivering injectate to the piriformis muscle and significant PS symptom relief with the other techniques mentioned earlier, we do not recommend routine use of this technique to avoid unnecessary cost and radiation exposure to the patients.
1. Coffield J.A., Considine R.B., Simpson L.L. The site and mechanism of action of botulinum neurotoxin. In: Jankovic J., Hallett M., editors. Therapy with Botulinum Toxin. New York: Marcel Dekker, 1994.
2. Hallett M. One man’s poison—clinical applications of botulinum toxin. N Engl J Med. 1999;341:118-120.
3. Mellanby J. Comparative activities of tetanus and botulinum toxins. Neuroscience. 1984;11:29-34.
4. Childers M.K., Biswas S.S., Petroski G., et al. Inhibitory casting decreases a vibratory inhibition index of the H-reflex in the spastic upper limb. Arch Phys Med Rehabil. 1999;80:714-716.
5. Hesse S., Jahnke M.T., Luecke D., et al. Short-term electrical stimulation enhances the effectiveness of botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci Lett. 1995;201:37-40.
6. Stacy M.A. Handbook of Dystonia. New York: Informa Healthcare; 2007.
7. Erbguth F.J., Naumann M. Historical aspects of botulinum toxin: Justinus Kerner (1786-1862) and the “sausage poison”. Neurology. 1999;53:1850-1853.
8. Childers M.K. Use of botulinum toxin type A in pain management. Columbia, Mo: AIS; 1999.
9. Consensus conference. Clinical use of botulinum toxin. National Institutes of Health. Conn Med. 1991;55:471-477.
10. Training guidelines for the use of botulinum toxin for the treatment of neurologic disorders. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1994;44:2401-2403.
11. Simpson L.L. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol. 2004;44:167-193.
12. Mense S. Neurobiological basis for the use of botulinum toxin in pain therapy. J Neurol. 2004;251(suppl 1):I1-7.
13. Wheeler A.H. Myofascial pain disorders: Theory to therapy. Drugs. 2004;64:45-62.
14. Brashear A. Botulinum toxin type A in the treatment of patients with cervical dystonia. Biologics. 2009;3:1-7.
15. Greene P., Fahn S., Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord. 1994;9:213-217.
16. Aoki K.R. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26:785-793.
17. Ranoux D., Attal N., Morain F., et al. Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann Neurol. 2008;64:274-283.
18. Durham P.L., Cady R., Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: Implications for migraine therapy. Headache. 2004;44:35-42.
19. Kitamura Y., Matsuka Y., Spigelman I., et al. Botulinum toxin type A (150 kDa) decreases exaggerated neurotransmitter release from trigeminal ganglion neurons and relieves neuropathy behaviors induced by infraorbital nerve constriction. Neuroscience. 2009;159:1422-1429.
20. Cui M., Khanijou S., Rubino J., et al. Subcutaneous administration of botulinum toxin type A reduces formalin-induced pain. Pain. 2004;107:125-133.
21. Apfel S.C. Botulinum toxin for neuropathic pain? Neurology. 2009;72:1456-1457.
22. Piovesan E.J., Teive H.G., Kowacs P.A., et al. An open study of botulinum-A toxin treatment of trigeminal neuralgia. Neurology. 2005;65:1306-1308.
23. Tsai C.P., Liu C.Y., Lin K.P., et al. Efficacy of botulinum toxin type A in the relief of carpal tunnel syndrome: A preliminary experience. Clin Drug Investig. 2006;26:511-515.
24. Yuan R.Y., Sheu J.J., Yu J.M., et al. Botulinum toxin for diabetic neuropathic pain: a randomized double-blind crossover trial. Neurology. 2009;72:1473-1478.
25. Fahn S. The varied clinical expressions of dystonia. Neurol Clin. 1984;13:541-544.
26. Markham C.H. The dystonias. Curr Opin Neurol Neurosurg. 1992;5:301-307.
27. Nutt J.G., Muenter M.D., Melton L.J.3rd, et al. Epidemiology of dystonia in Rochester, Minnesota. Adv Neurol. 1988;50:361-365.
28. Chan J., Brin M.F., Fahn S. Idiopathic cervical dystonia: Clinical characteristics. Mov Disord. 1991;6:119-126.
29. Kutvonen O., Dastidar P., Nurmikko T. Pain in spasmodic torticollis. Pain. 1997;69:279-286.
30. Lowenstein D.H., Aminoff M.J. The clinical course of spasmodic torticollis. Neurology. 1988;38:530-532.
31. Jankovic J., Leder S., Warner D., Schwartz K. Cervical dystonia: Clinical findings and associated movement disorders. Neurology. 1991;41:1088-1091.
32. Dauer W.T., Burke R.E., Greene P., Fahn S. Current concepts on the clinical features, aetiology and management of idiopathic cervical dystonia. Brain. 1998;121:547-560.
33. Tarsy D. Comparison of acute- and delayed-onset posttraumatic cervical dystonia. Mov Disorder. 1998;13:481-485.
34. Geyer H.L., Bressman S.B. The diagnosis of dystonia. Lancet Neurol. 2006;5:780-790.
35. Naumann M., Pirker W., Reiners K., et al. Imaging the pre- and postsynaptic side of striatal dopaminergic synapses in idiopathic cervical dystonia: A SPECT study using [123I] epidepride and [123I] beta-CIT. Mov Disord. 1998;13:319-323.
36. Perlmutter J.S., Stambuk M., Markham J. Quantified binding of [F18] spiperone in focal dystonia. Mov Disord. 1996;11:819.
37. Horstink C.A., Booij J., Berger H.J.C. Striatal D2 receptor loss in writer’s cramp. Mov Disord. 1996;11:784.
38. Jahanshahi M., Marion M.H., Marsden C.D. Natural history of adult-onset idiopathic torticollis. Arch Neurol. 1990;47:548-552.
39. Galvez-Jimenez N., Lampuri C., Patino-Picirrillo R., et al. Dystonia and headaches: Clinical features and response to botulinum toxin therapy. Adv Neurol. 2004;94:321-328.
40. Rondot P., Marchand M.P., Dellatolas G. Spasmodic torticollis—review of 220 patients. Can J Neurol Sci. 1991;18:143-151.
41. Comella C.L., Tanner C.M., DeFoor-Hill L., Smith C. Dysphagia after botulinum toxin injections for spasmodic torticollis: Clinical and radiologic findings. Neurology. 1992;42:1307-1310.
42. Jankovic J., Brin M.F. Botulinum toxin: Historical perspective and potential new indications. Muscle Nerve Suppl. 1997:S129-145.
43. Weiner W.J., Lang A.E. Idiopathic torsion dystonia. In: Movement Disorders: A Comprehensive Survey. New York: Futura; 1987.
44. Adler C.H., Kumar R. Pharmacological and surgical options for the treatment of cervical dystonia. Neurology. 2000;55:S9-14.
45. Balash Y., Giladi N. Efficacy of pharmacological treatment of dystonia: Evidence-based review including meta-analysis of the effect of botulinum toxin and other cure options. Eur J Neurol. 2004;11:361-370.
46. Ohara S., Hayashi R., Momoi H., et al. Mexiletine in the treatment of spasmodic torticollis. Mov Disord. 1998;13:934-940.
47. Lucetti C., Nuti A., Gambaccini G., et al. Mexiletine in the treatment of torticollis and generalized dystonia. Clin Neuropharmacol. 2000;23:186-189.
48. Bang M.S., Lee S.U. Cervical Dystonia. Essentials of Physical Medicine and Rehabilitation. Philadelphia: Saunders; 2008.
49. Bronte-Stewart H. Surgical therapy for dystonia. Curr Neurol Neurosci Rep. 2003;3:296-305.
50. Celayir A.C. Congenital muscular torticollis: Early and intensive treatment is critical. A prospective study. Pediatr Int. 2000;42:504-507.
51. Goto S., Mita S., Ushio Y. Bilateral pallidal stimulation for cervical dystonia. An optimal paradigm from our experiences. Stereotact Funct Neurosurg. 2002;79:221-227.
52. Kiss Z.H., Doig K., Eliasziw M., et al. The Canadian multicenter trial of pallidal deep brain stimulation for cervical dystonia: Preliminary results in three patients. Neurosurg Focus. 2004;17:E5.
53. Krauss J.K. Deep brain stimulation for dystonia in adults. Overview and developments. Stereotact Funct Neurosurg. 2002;78:168-182.
54. Langlois M., Richer F., Chouinard S. New perspectives on dystonia. Can J Neurol Sci. 2003;30(suppl 1):S34-S44.
55. Lozano A.M., Abosch A. Pallidal stimulation for dystonia. Adv Neurol. 2004;94:301-308.
56. Yu S.W., Wang N.H., Chin L.S., Lo W.H. Surgical correction of muscular torticollis in older children. Zhonghua Yi Xue Za Zhi (Taipei). 1995;55:168-171.
57. West H.H. Treatment of spasmodic torticollis with amantadine: A double-blind study. Neurology. 1997;27:198-199.
58. Hung S.W., Hamani C., Lozano A.M., et al. Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology. 2007;6:457-459.
59. Kupsch A., Benecke R., Müller J., et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;9:1978-1990.
60. Jankovic J., Esquenazi A., Fehlings D., et al. Evidence-based review of patient-reported outcomes with botulinum toxin type A. Clin Neuropharmacol. 2004;27:234-244.
61. Jankovic J. Treatment of cervical dystonia with botulinum toxin. Mov Disord. 2004;19:S109-S115.
62. Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75:951-957.
63. Costa J., Espirito-Santo C., Borges A., et al. Botulinum toxin type B for cervical dystonia. Cochrane Database Syst Rev. 2005.
64. Brefel-Courbon C., Simonetta-Moreau M., More C., et al. A pharmacoeconomic evaluation of botulinum toxin in the treatment of spasmodic torticollis. Clin Neuropharmacol. 2000;23:203-207.
65. Jankovic J., Schwartz K. Botulinum toxin injections for cervical dystonia. Neurology. 1990;40:277-280.
66. Hsiung G.Y., Das S.K., Ranawaya R., et al. Long-term efficacy of botulinum toxin A in treatment of various movement disorders over a 10-year period. Mov Disord. 2002;17:1288-1293.
67. Truong D., Duane D.D., Jankovic J., et al. Efficacy and safety of botulinum type A toxin (Dysport) in cervical dystonia: Results of the first US randomized, double-blind, placebo-controlled study. Mov Disord. 2005;20:783-791.
68. Simpson D.M., Blitzer A., Brashear A., et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699-1706.
69. Lew M.F., Adornato B.T., Duane D.D., et al. Botulinum toxin type B: A double-blind, placebo-controlled, safety and efficacy study in cervical dystonia. Neurology. 1997;49:701-707.
70. Brashear A., Lew M.F., Dykstra D.D., et al. Safety and efficacy of NeuroBloc (botulinum toxin type B) in type A-responsive cervical dystonia. Neurology. 1999;53:1439-1446.
71. Brin M.F., Lew M.F., Adler C.H., et al. Safety and efficacy of NeuroBloc (botulinum toxin type B) in type A-resistant cervical dystonia. Neurology. 1999;53:1431-1438.
72. Pappert E.J., Germanson T., Myobloc/Neurobloc European Cervical Dystonia Study Group. Botulinum toxin type B vs. type A in toxin-naïve patients with cervical dystonia: Randomized, double-blind, noninferiority trial. Mov Disord. 2008;23:510-517.
73. Mueller J. Reply: Botulinum toxin type B vs. type A in toxin-naïve patients with cervical dystonia: Randomized, double-blind, noninferiority trial. Mov Disord. 2009;24:1098-1099.
74. Pappert E.J. Reply: Botulinum toxin type B vs. type A in toxin-naïve patients with cervical dystonia: Randomized, double-blind, noninferiority trial. Mov Disord. 2009;24:1100.
75. Costa J., Espirito-Santo C., Borges A., et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2005.
76. Costa J., Espirito-Santo C., Borges A., et al. Botulinum toxin type A versus botulinum toxin type B for cervical dystonia. Cochrane Database Syst Rev. 2005.
77. Comella C.L., Buchman A.S., Tanner C.M., et al. Botulinum toxin injection for spasmodic torticollis: Increased magnitude of benefit with electromyographic assistance. Neurology. 1992;42:878-882.
78. Childers M.K., Kornegay J.N., Aoki R., et al. Evaluating motor end-plate-targeted injections of botulinum toxin type A in a canine model. Muscle Nerve. 1998;21:653-655.
79. Childers M.K. The importance of electromyographic guidance and electrical stimulation for injection of botulinum toxin. Phys Med Rehabil Clin N Am. 2003;14:781-792.
80. Childers M.K. Targeting the neuromuscular junction in skeletal muscles. Am J Phys Med Rehabil. 2004;83:S38-S44.
81. Jankovic J., Schwartz K.S. Clinical correlates of response to botulinum toxin injections. Arch Neurol. 1991;48:1253-1256.
82. Brans J.W., Lindeboom R., Snoek J.W., et al. Botulinum toxin versus trihexyphenidyl in cervical dystonia: A prospective, randomized, double-blind controlled trial. Neurology. 1996;46:1066-1072.
83. Greene P., Kang U., Fahn S., et al. Double-blind, placebo-controlled trial of botulinum toxin injections for the treatment of spasmodic torticollis. Neurology. 1990;40:1213-1218.
84. Jankovic J., Orman J. Botulinum A toxin for cranial-cervical dystonia: A double-blind, placebo-controlled study. Neurology. 1987;37:616-623.
85. Lorentz I.T., Subramaniam S.S., Yiannikas C. Treatment of idiopathic spasmodic torticollis with botulinum toxin A: A double-blind study on twenty-three patients. Mov Disord. 1991;6:145-150.
86. Moore A.P., Blumhardt L.D. A double blind trial of botulinum toxin “A” in torticollis, with one year follow up. J Neurol Neurosurg Psychiatry. 1991;54:813-816.
87. Odergren T., Hjaltason H., Kaakkola S., et al. A double blind, randomised, parallel group study to investigate the dose equivalence of Dysport and Botox in the treatment of cervical dystonia. J Neurol Neurosurg Psychiatry. 1998;64:6-12.
88. Ranoux D., Gury C., Fondarai J., et al. Respective potencies of Botox and Dysport: A double blind, randomised, crossover study in cervical dystonia. J Neurol Neurosurg Psychiatry. 2002;72:459-462.
89. Kessler K.R., Skutta M., Benecke R. Long-term treatment of cervical dystonia with botulinum toxin A: Efficacy, safety, and antibody frequency. German Dystonia Study Group. J Neurol. 1999;246:265-274.
90. Poewe W., Deuschl G., Nebe A., et al. What is the optimal dose of botulinum toxin A in the treatment of cervical dystonia? Results of a double blind, placebo controlled, dose ranging study using Dysport. German Dystonia Study Group. J Neurol Neurosurg Psychiatry. 1998;64:13-17.
91. Dressler D. Electromyographic evaluation of cervical dystonia for planning of botulinum toxin therapy. Eur J Neurol. 2000;7:713-718.
92. Koller W., Vetere-Overfield B., Gray C., Dubinsky R. Failure of fixed-dose, fixed muscle injection of botulinum toxin in torticollis. Clin Neuropharmacol. 1990;13:355-358.
93. Kessler K.R., Benecke R. The EBD test—a clinical test for the detection of antibodies to botulinum toxin type A. Mov Disord. 1997;12:95-99.
94. Velickovic M., Benabou R., Brin M.F. Cervical dystonia pathophysiology and treatment options. Drugs. 2001;61:1921-1943.
95. Childers M.K., Feldman J.B., Guo H.M. Myofascial pain syndrome. In: Frontera W.R., Silver J.K., Rizzo T.D., editors. Essentials of Physical Medicine and Rehabilitation. Philadelphia: Saunders; 2008:591-597.
96. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-226.
97. Simons D.G., Travell J.G., Simons L.S. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual, 2nd ed. Baltimore: Williams & Wilkins; 1999.
98. Escobar P.L., Ballesteros J. Myofascial pain syndrome. Orthop Rev. 1987;16:708-713.
99. Bohr T.W. Fibromyalgia syndrome and myofascial pain syndrome. Do they exist? Neurol Clin. 1995;13:365-384.
100. Borg-Stein J., Simons D.G. Focused review: Myofascial pain. Arch Phys Med Rehabil. 2002;83:S40-49.
101. Aronoff G.M. Myofascial pain syndrome and fibromyalgia: A critical assessment and alternate view. Clin J Pain. 1998;14:74-85.
102. Hong C.Z., Yu J. Spontaneous electrical activity of rabbit trigger spot after transection of spinal cord and peripheral nerve. J Musculoskel Pain. 1998;6:45-58.
103. Hubbard D.R., Berkoff G.M. Myofascial trigger points show spontaneous needle EMG activity. Spine. 1993;18:1803-1807.
104. Shaari C.M., Sanders I. Quantifying how location and dose of botulinum toxin injections affect muscle paralysis. Muscle Nerve. 1993;16:964-969.
105. Wiederholt W.C. “End-plate noise” in electromyography. Neurology. 1970;20:214-224.
106. Hong C.Z., Simons D.G. Histological findings of responsive loci in a myofascial trigger spot of rabbit skeletal muscle from where localized twitch responses could be elicited [abstract]. Arch Phys Med Rehabil. 1996;77:962.
107. Hong C.Z., Simons D.G. Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points. Arch Phys Med Rehabil. 1998;79:863-872.
108. Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice, 2nd ed. Philadelphia, F.A.: Davis; 1989.
109. DeBassio W.A., Schnitzler R.M., Parsons R.L. Influence of lanthanum on transmitter release at the neuromuscular junction. J Neurobiol. 1971;2:263-278.
110. Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971;179:247-260.
111. Ito Y., Miledi R., Vincent A. Transmitter release induced by a “factor” in rabbit serum. Proc R Soc Lond B Biol Sci. 1974;187:235-241.
112. Liley A.W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956;132:650-666.
113. Dumitru D., King J.C., McCarter R.J. Single muscle fiber discharge transformations: Fibrillation potential to positive sharp wave. Muscle Nerve. 1998;21:1759-1768.
114. Simons D.G., Stolov W.C. Microscopic features and transient contraction of palpable bands in canine muscle. Am J Phys Med. 1976;55:65-88.
115. Macgregor J., Graf von Schweinitz D. Needle electromyographic activity of myofascial trigger points and control sites in equine cleidobrachialis muscle—an observational study. Acupunct Med. 2006;24:61-70.
116. Janssens L.A. Trigger points in 48 dogs with myofascial pain syndromes. Vet Surg. 1991;20:274-278.
117. Windisch A., Reitinger A., Traxler H., et al. Morphology and histochemistry of myogelosis. Clin Anat. 1999;12:266-271.
118. Bruckle W., Suckfull M., Fleckenstein W., et al. [Tissue pO2 measurement in taut back musculature (m. erector spinae)]. [German]. Z Rheumatol. 1990;49:208-216.
119. Cazzato G., Walton J.N. The pathology of the muscle spindle. A study of biopsy material in various muscular and neuromuscular diseases. J Neurol Sci. 1968;7:15-70.
120. Simons D.G., Hong C.Z., Simons L.S. Endplate potentials are common to midfiber myofascial trigger points. Am J Phys Med Rehabil. 2002;81:212-222.
121. Hong C.Z., Torigoe Y. Electrophysiological characteristics of localized twitch responses in responsive taut bands of rabbit skeletal muscle. J Musculoskel Pain. 1995;1:15-34.
122. Hong C.Z., Torigoe Y. Electrophysiological characteristics of localized twitch responses in responsive taut bands of rabbit skeletal muscle. J Musculoskel Pain. 1994;2:17-43.
123. Hong C.Z. Persistence of local twitch response with loss of conduction to and from the spinal cord. Arch Phys Med Rehabil. 1994;75:12-16.
124. Childers M.K., Wilson D.J., Galate J.F., et al. Treatment of painful muscle syndromes with botulinum toxin: a review. J Back Musculoskel Reh. 1998;10:89-96.
125. Simons D.G. Myofascial pain syndrome: One term but two concepts: A new understanding [editorial]. J Musculoskel Pain. 1995;3:7-13.
126. Lennard T.A. Pain Procedures in Clinical Practice, 2nd ed. Philadelphia: Hanley & Belfus; 2000.
127. Venancio R.A., Alencar F.G.Jr., Zamperini C. Botulinum toxin, lidocaine, and dry needling injections in patients with myofascial pain and headaches. Cranio. 2009;27:46-53.
128. Braker C., Yariv S., Adler R., et al. The analgesic effect of botulinum-toxin A on postwhiplash neck pain. Clin J Pain. 2008;24:5-10.
129. Casale R., Tugnoli V. Botulinum toxin for pain. Drugs R D. 2008;9:11-27.
130. Srinivasan A.K., Kaye J.D., Moldwin R. Myofascial dysfunction associated with chronic pelvic floor pain: Management strategies. Curr Pain Headache Rep. 2007;11:359-364.
131. Clark G.T., Stiles A., Lockerman L.Z., et al. A critical review of the use of botulinum toxin in orofacial pain disorders. Dent Clin North Am. 2007;51:245-261.
132. Gobel H., Heinze A., Reichel G., et al. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: Results from a randomized double-blind placebo-controlled multicentre study. Pain. 2006;125:82-88.
133. Lang A.M. Considerations for the use of botulinum toxin in pain management. Lippincotts Case Manag. 2006;11:279-282.
134. Ojala T., Arokoski J.P., Partanen J. The effect of small doses of botulinum toxin A on neck-shoulder myofascial pain syndrome: A double-blind, randomized, and controlled crossover trial. Clin J Pain. 2006;22:90-96.
135. Kamanli A., Kaya A., Ardicoglu O., et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
136. Wheeler A.H., Goolkasian P., Gretz S.S. A randomized, double-blind, prospective pilot study of botulinum toxin injection for refractory, unilateral, cervicothoracic, paraspinal, myofascial pain syndrome. Spine. 1998;23:1662-1666.
137. Aoki K.R. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache. 2003;43(suppl 1):S9-15.
138. Reilich P., Fheodoroff K., Kern U., et al. Consensus statement: botulinum toxin in myofascial [corrected] pain. J Neurol. 2004;251(suppl 1):I36-I38.
139. Porta M., Maggioni G. Botulinum toxin (BoNT) and back pain. J Neurol, suppl 1. 2004:I15-I18.
140. De Andres J., Cerda-Olmedo G., Valia J.C., et al. Use of botulinum toxin in the treatment of chronic myofascial pain. Clin J Pain. 2003;19:269-275.
141. Lang A.M. Botulinum toxin type A therapy in chronic pain disorders. Arch Phys Med Rehabil. 2003;84(3 suppl 1):S69-73.
142. Argoff C.E. A focused review on the use of botulinum toxins for neuropathic pain. Clin J Pain. 2002;18(Suppl 6):S177-181.
143. Smith H.S., Audette J., Royal M.A. Botulinum toxin in pain management of soft tissue syndromes. Clin J Pain. 2002;18(Suppl 6):S147-154.
144. Lang A.M. Botulinum toxin therapy for myofascial pain disorders. Curr Pain Headache Rep. 2002;6:355-360.
145. Criscuolo C.M. Interventional approaches to the management of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5:407-411.
146. Porta M. A comparative trial of botulinum toxin type A and methylprednisolone for the treatment of myofascial pain syndrome and pain from chronic muscle spasm. Pain. 2000;85:101-105.
147. Diaz J.H., Gould H.J. Management of post-thoracotomy pseudoangina and myofascial pain with botulinum toxin. Anesthesiology. 1999;91:877-879.
148. Lew H.L., Lee E.H., Castaneda A., et al. Therapeutic use of botulinum toxin type A in treating neck and upper-back pain of myofascial origin: A pilot study. Arch Phys Med Rehabil. 2008;89:75-80.
149. Ferrante F.M., Bearn L., Rothrock R., King L. Evidence against trigger point injection technique for the treatment of cervicothoracic myofascial pain with botulinum toxin type A. Anesthesiology. 2005;103:377-383.
150. Abram S.E. Does botulinum toxin have a role in the management of myofascial pain? Anesthesiology. 2005;103:223-224.
151. Foster L., Clapp L., Erickson M., Jabbari B. Botulinum toxin A and chronic low back pain: A randomized double-blind study. Neurology. 2001;56:1290-1293.
152. Hallin R.P. Sciatic pain and the piriformis muscle. Postgrad Med. 1983;74:69-72.
153. Simons D.G., Travell J.G. Myofascial origins of low back pain. 3. Pelvic and lower extremity muscles. Postgrad Med. 1983;73:99-105.
154. Solheim L.F., Siewers P., Paus B. The piriformis muscle syndrome. Sciatic nerve entrapment treated with section of the piriformis muscle. Acta Orthop Scand. 1981;52:73-75.
155. Yeoman W. The relation of arthritis of the sacroiliac joint to sciatica, with an analysis of 100 cases. Lancet. 1928;2:1119-1122.
156. Freiberg A.H., Vinke T.H. Sciatica and the sacroiliac joint. L Bone Joint Surg. 1934;16:126-136.
157. Beaton L.E., Anson B.J. The sciatic nerve and the piriformis muscle: Their interrelation a possible cause of coccygodynia. J Bone Joint Surg Am. 1938;20:686-688.
158. Robinson D.R. Pyriformis syndrome in relation to sciatic pain. Am J Surg. 1947;73:355-358.
159. Stewart J.D. The piriformis syndrome is overdiagnosed. Muscle Nerve. 2003;27:644-646.
160. Fishman L.M., Schaefer M.P. The piriformis syndrome is underdiagnosed. Muscle Nerve. 2003;27:646-649.
161. Pećina M. Contribution to the etiological explanation of the piriformis syndrome. Acta Anat (Basel). 1979;105:181-187.
162. Pace J.B., Nagle D. Piriformis syndrome. West J Med. 1976;124:435-439.
163. Bernard T.N.Jr., Kirkaldy-Willis W.H. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res. 1987;217:266-280.
164. Parziale J.R., Hudgins T.H., Fishman L.M. The piriformis syndrome. Am J Orthop. 1996;25:819-823.
165. Fishman L.M., Anderson C., Rosner B. Botox and physical therapy in the treatment of piriformis syndrome. Am J Phys Med Rehabil. 2002;81:936-942.
166. Beatty R.A. The piriformis muscle syndrome: A simple diagnostic maneuver. Neurosurgery. 1994;34:512-514.
167. Foster M.R. Piriformis syndrome. Orthopedics. 2002;25:821-825.
168. Beauchesne R.P., Schutzer S.F. Myositis ossificans of the piriformis muscle: An unusual cause of piriformis syndrome. A case report. J Bone Joint Surg Am. 1997;79:906-910.
169. Benson E.R., Schutzer S.F. Posttraumatic piriformis syndrome: Diagnosis and results of operative treatment. J Bone Joint Surg Am. 1999;81:941-949.
170. Bonica J.J. Definitions and taxonomy of pain. In: Bonica J.J., editor. The Management of Pain. Philadelphia: Lea & Febiger, 1990.
171. McCrory P., Bell S. Nerve entrapment syndromes as a cause of pain in the hip, groin and buttock. Sports Med. 1999;27:261-274.
172. Hughes S.S., Goldstein M.N., Hicks D.G., Pellegrini V.D.Jr. Extrapelvic compression of the sciatic nerve. An unusual cause of pain about the hip: Report of five cases. J Bone Joint Surg Am. 1992;74:1553-1559.
173. Noftal F. The piriformis syndrome. Can J Surg. 1988;31:210.
174. Barton P.M. Piriformis syndrome: A rational approach to management. Pain. 1991;47:345-352.
175. Hanania M., Kitain E. Perisciatic injection of steroid for the treatment of sciatica due to piriformis syndrome. Reg Anesth Pain Med. 1998;23:223-228.
176. Chen W.S. Bipartite piriformis muscle: An unusual cause of sciatic nerve entrapment. Pain. 1994;58:269-272.
177. Carmody C., Prietto C. Entrapment of the sciatic nerve as a late sequela of injury to the hamstring muscles. A case report. J Bone Joint Surg Am. 1995;77:1100-1102.
178. Kirkaldy-Willis W.H., Hill R.J. A more precise diagnosis for low-back pain. Spine. 1979;4:102-109.
179. Dobrusin R. An osteopathic approach to conservative management of thoracic outlet syndromes. J Am Osteopath Assoc. 1989;89:1046-1050.
180. Steiner C., Staubs C., Ganon M., et al. Piriformis syndrome: Pathogenesis, diagnosis, and treatment. J Am Osteopath Assoc. 1987;87:318-323.
181. Chen W.S. Sciatica due to piriformis pyomyositis. Report of a case. J Bone Joint Surg Am. 1992;74:1546-1548.
182. Kinahan A.M., Douglas M.J. Piriformis pyomyositis mimicking epidural abscess in a parturient. Can J Anaesth. 1995;42:240-245.
183. Ku A., Kern H., Lachman E., et al. Sciatic nerve impingement from piriformis hematoma due to prolonged labor. Muscle Nerve. 1995;18:789-790.
184. LaBan M.M., Meerschaert J.R., Taylor R.S. Electromyographic evidence of inferior gluteal nerve compromise: An early representation of recurrent colorectal carcinoma. Arch Phys Med Rehabil. 1982;63:33-35.
185. Papadopoulos S.M., McGillicuddy J.E., Albers J.W. Unusual cause of “piriformis muscle syndrome”. Arch Neurol. 1990;47:1144-1146.
186. Fishman L.M., Zybert P.A. Electrophysiologic evidence of piriformis syndrome. Arch Phys Med Rehabil. 1992;73:359-364.
187. Sayson S.C., Ducey J.P., Maybrey J.B., et al. Sciatic entrapment neuropathy associated with an anomalous piriformis muscle. Pain. 1994;59:149-152.
188. Lynn B. Cutaneous hyperalgesia. Br Med Bull. 1977;33:103-108.
189. Jankiewicz J.T., Hennrikus W.L., Houkom J.A. The appearance of the piriformis muscle in computed tomography and magnetic resonance imaging: A case report and review of the literature. Clin Orthop Rel Res. 1991;262:205-209.
190. Simons D.G., Travell J.G. Myofascial origins of low back pain. 1. Principles of diagnosis and treatment. Postgrad Med. 1983;73:66-70.
191. Simons D.G., Travell J.G. Myofascial origins of low back pain. 2. Torso muscles. Postgrad Med. 1983;73:81-92.
192. Chen W.S., Wan Y.L. Sciatica caused by piriformis muscle syndrome: Report of two cases. J Formos Med Assoc. 1992;91:647-650.
193. Jankiewicz J.J., Hennrikus W.L., Houkom J.A. The appearance of the piriformis muscle syndrome in computed tomography and magnetic resonance imaging. A case report and review of the literature. Clin Orthop Rel Res. 1991;262:205-209.
194. Julsrud M.E. Piriformis syndrome. J Am Podiatr Med Assoc. 1989;79:128-131.
195. Noftal F. The piriformis syndrome. Can J Surg. 1988;31:210.
196. Naja Z., Al-Tannir M., El-Rajab M., et al. The effectiveness of clonidine-bupivacaine repeated nerve stimulator-guided injection in piriformis syndrome. Clin J Pain. 2009;25:199-205.
197. Galate J.F., Childers M.K., Gnatz S. Effectiveness of botulinum toxin in refractory piriformis muscle syndrome [abstract]. Arch Phys Med Rehabil. 1997;78:1041.
198. Childers M.K., Wilson D.J., Gnatz S.M., et al. Botulinum toxin type A use in piriformis muscle syndrome: A pilot study. Am J Phys Med Rehabil. 2002;81(10):751-759.
199. Fanucci E., Masala S., Sodani G., et al. CT-guided injection of botulinic toxin for percutaneous therapy of piriformis muscle syndrome with preliminary MRI results about denervative process. Eur Radiol. 2001;11:2543-2548.
200. Fishman L.M., Konnoth C., Rozner B. Botulinum neurotoxin type B and physical therapy in the treatment of piriformis syndrome: A dose-finding study. Am J Phys Med Rehabil. 2004;83:42-50.
201. Lang A.M. Botulinum toxin type B in piriformis syndrome. Am J Phys Med Rehabil. 2004;83:198-202.
202. Yoon S.J., Ho J., Kang H.Y., et al. Low-dose botulinum toxin type A for the treatment of refractory piriformis syndrome. Pharmacotherapy. 2007;27:657-665.
203. Childers M.K., Biswas S.S., Petroski G., et al. Inhibitory casting decreases a vibratory inhibition index of the H-reflex in the spastic upper limb. Arch Phys Med Rehabil. 1999;80:714-716.
204. Huerto A.P., Yeo S.N., Ho K.Y. Piriformis muscle injection using ultrasonography and motor stimulation—report of a technique. Pain Physician. 2007;10:687-690.
205. Reus M., de Dios Berná J., Vázquez V., et al. Piriformis syndrome: A simple technique for US-guided infiltration of the perisciatic nerve. Preliminary results. Eur Radiol. 2008;18:616-620.
206. Spiller J. Acupuncture, ketamine and piriformis syndrome—a case report from palliative care. Acupunct Med. 2007;25:109-112.
207. Gonzalez P., Pepper M., Sullivan W., Akuthota V. Confirmation of needle placement within the piriformis muscle of a cadaveric specimen using anatomic landmarks and fluoroscopic guidance. Pain Physician. 2008;11:327-331.
208. Benzon H.T., Katz J.A., Benzon H.A., Iqbal M.S. Piriformis syndrome: anatomic considerations, a new injection technique, and a review of the literature. Anesthesiology. 2003;98:1442-1448.
209. Betts A. Combined fluoroscopic and nerve stimulator technique for injection of the piriformis muscle. Pain Physician. 2004;7:279-281.
210. Fishman L.M., Dombi G.W., Michaelsen C., et al. Piriformis syndrome: Diagnosis, treatment, and outcome—a 10-year study. Arch Phys Med Rehabil. 2002;83:295-301.
211. Childers M.K., Kornegay J.N., Aoki R., et al. Evaluating motor end-plate-targeted injections of botulinum toxin type A in a canine model. Muscle Nerve. 1998;21:653-655.
212. Smith J., Hurdle M.F., Locketz A.J., Wisniewski S.J. Ultrasound-guided piriformis injection: Technique description and verification. Arch Phys Med Rehabil. 2006;87:1664-1667.
213. Finnoff J.T., Hurdle M.F., Smith J. Accuracy of ultrasound-guided versus fluoroscopically guided contrast-controlled piriformis injections: a cadaveric study. J Ultrasound Med. 2008;27:1157-1163.