CHAPTER 292 Bone Graft Options, Bone Graft Substitutes, and Bone Harvest Techniques
In 1998, individuals spent $91 billion (1% of the U.S. gross domestic product) to treat back pain directly and $26 billion (2.5% of national health care expenditures) on other expenditures attributable to back pain.1 Compression of neural elements, malalignment, and abnormal motion across intervertebral segments are three surgically correctable causes of back pain. Unlike limited neural decompression, such as hemilaminotomy for removal of a herniated disk fragment, more extensive decompression can compromise the structural integrity of a spinal segment, thereby necessitating fusion, or arthrodesis, across that motion segment. Spinal arthrodesis is also required in situations of gross spinal instability as occurs in traumatic fractures, pathologic fractures, and rheumatologic disorders. Despite significant advances in spinal instrumentation technology and surgical techniques, without solid bony fusion, all spinal instrumentation will eventually fail, highlighting the essential role of bone grafting for successful arthrodesis.
History of Spinal Arthrodesis
Autogenous bone graft was first used to induce spinal fusion in 1911 by Albee2 and Hibbs.3 Albee used tibial grafts placed between spinous processes for patients with Pott’s disease, and Hibbs used laminar and spinous process bone fragments for dorsal fusion along decorticated facet joints. Their idea of creating a rigid union between vertebral segments to correct segmental instability was the basis of further advancements in spinal fusion over the next several decades.
Beginning in 1933, Burns4 used an anterior approach for lumbar interbody graft placement for spondylolisthesis. Cloward,5 among others, used a posterior approach to lumbar interbody graft placement. From the 1930s through the 1960s metallic internal fixation devices were developed by pioneering spine surgeons such as Harrington, Luque, Judet, Roy-Camille, Magerl, and Wiltse, revolutionizing spinal stabilization. More recently, advances in biomechanics, metallurgic sciences (metal alloys), bone allograft material, and instrumentation design have continued to improve fusion rates.
A growing understanding of bone physiology also played a role in the advancement of spinal fusion techniques and the achievement of higher fusion rates, particularly in patients at higher risk of nonunion. In the 1960s a new set of cell-signaling proteins, bone morphogenetic proteins (BMPs), was discovered and characterized by Urist.6,7 These proteins have since been shown to enhance spinal arthrodesis, thus lessening the need for autograft harvesting and reducing the morbidity associated with it.8 As science continues to elucidate the biology of bone formation, the techniques of bone grafting and spinal fusion will continue to evolve.
Principles of Bone Physiology
Bone is a dynamic tissue in a continuous state of metabolic activity (deposition, resorption, and remodeling). In fact, the human body replaces its skeletal mass once every 10 years.9 There are several cell types important in bone growth and formation: osteogenic precursor cells, osteoblasts, osteoclasts, osteocytes, and hematopoietic elements of bone. Osteogenic precursor cells are located in the deep layer of the periosteum and are present on all nonresorptive bone surfaces, serving as a major component of the covering of bone.10 Osteoblasts are metabolically active, mature bone-forming cells. They primarily secrete an unmineralized organic matrix (osteoid), which mineralizes to give the bone strength and rigidity. As osteoblasts secrete osteoid and become trapped within bone matrix, they mature and become osteocytes. Osteocytes are key players in the control of extracellular concentrations of calcium and phosphorus, as well as in adaptive remodeling of bone.11 Osteoclasts are bone-resorbing cells affected by hormonal and cellular mechanisms.
These cellular components are the building blocks for the three types of bone: woven bone, cortical bone, and cancellous bone. Woven bone is present during embryonic development and fracture healing (callus formation).12 Comprised of randomly arranged collagen bundles and vascular spaces lined with osteoblasts, woven bone is remodeled and eventually replaced with cortical (lamellar) or cancellous (trabecular) bone. Cortical bone’s primary structural unit is an osteon, or haversian system. The packing density of osteons determines the mechanical strength of cortical bone. Cancellous bone fills the spaces between cortical bone surfaces with a network of honeycombed spaces, known as bony trabeculae, and contains hematopoietic elements.
In addition to the cellular components of bone, the extracellular matrix provides an important part of its structural integrity. Twenty percent of bone is water, with the dry weight consisting of 35% organic and 65% inorganic substances. The organic components, secreted by the osteoblasts, consist of collagen (principally type I collagen) and glycosaminoglycans (chondroitin sulfate, hyaluronic acid, and keratan sulfate). The inorganic matrix consists of 85% calcium phosphate, 10% calcium carbonate, 5% fluoride derivatives, and trace elements. These compounds mineralize the organic osteoid and enhance the rigidity and hardness of bone.13
Bone metabolism is regulated heavily by hormones, including parathyroid hormone (PTH), calcitonin, and vitamin D. PTH acts on osteoblasts, which leads to the release of osteoclast-stimulating factor and thereby activates the resorptive process. PTH analogues that influence this interaction have been used to treat osteoporosis.14 Calcitonin, released by parafollicular cells in response to an increased plasma calcium concentration, inhibits calcium-dependent cellular processes. Osteopenia and osteoporosis are also treated with calcitonin supplementation, with evidence of a decreased number of fractures in patients so treated.15 Vitamin D stimulates increased renal and intestinal absorption of calcium. In cases of vitamin D deficiency, children develop rickets and adults develop osteomalacia.
In addition to hormones, growth factors have a profound effect on the maturation and formation of bone. Growth factors such as BMPs, tumor growth factor (TGF), and platelet-derived growth factor (PDGF) all have the ability to induce stem cell differentiation and chemotaxis. BMPs are one of the key components of inducing bone formation by activating the transcription of genes that mediate cell migration, proliferation, and differentiation.16 TGF and PDGF are other potential osteoinductive agents, and both peptides are released in high concentrations in inflammation and after tissue injury to stimulate osteogenesis, angiogenesis, fibroblast migration, and deposition of matrix. Osteoblast proliferation and collagen synthesis are also stimulated by PDGF.17
Osteogenesis, osteoconduction, and osteoinduction are the three main properties that are desirable in bone grafts. Osteogenesis refers to the cellular component of fusion, which gives the graft the ability to form new bone. Bone marrow aspirates and fresh autografts contain these cells. Osteoconduction refers to the presence of a solid matrix for new bone formation. Autograft, allograft, tricalcium phosphate, and collagen matrix may serve as good osteoconductors. Osteoinduction refers to the process by which precursors of osteoblasts are actively induced to differentiate into mature bone-forming cells. Growth factors (BMPs and PDGF) are the largest components of osteoinduction.18
Bone Healing
There are three phases of bone healing: early inflammation, repair, and remodeling. Early inflammation occurs during the first 2 weeks and consists of hematoma formation with subsequent inflammatory cell and fibroblast infiltration. Inflammatory cytokines and growth factors then lead to vascularization and the formation of granulation tissue (procallus). Between the fourth and sixth weeks, the repair phase is characterized by continued vascular ingrowth and the secretion of osteoid and fibrocollagenous fibers. This cartilaginous callus has limited mechanical strength. Over the next several months, the organic osteoid matrix is mineralized, converting the cancellous bone to compact bone. The remodeling phase continues over months to years as the fractured bone is restored to its normal size, shape, and strength. In 6 months, adequate bone strength develops.10
Proper bone healing may be limited by a number of clinical factors. Because the healing process is dependent on the early boost in inflammatory cytokines and growth factors, anti-inflammatory agents (including steroids and nonsteroidal anti-inflammatory drugs) inhibit overall fusion rates.19,20 The presence of nicotine, not just smoking, also results in decreased healing rates owing to impaired vascularization of tissues.21 In addition, systemic factors such as radiation, diabetes, rheumatoid arthritis, and osteoporosis are all known to increase pseudarthrosis rates.10 Advanced age is also a predictor of pseudarthrosis. The ratio of stem cells present in bone marrow decreases as one ages,22 and the postmenopausal state itself is associated with increased bone resorption in women.13
Biomechanical Considerations
It is important to understand several basic biomechanical principles when planning spinal arthrodesis. Wolff’s law states that bone will form in places where stress requires its presence.23 Improper placement of rigid instrumentation, brace therapy, and bed rest may shield a bone graft from mechanical stress (stress shielding) and ultimately weaken bony fusion.24 Load sharing refers to the instrumentation or graft sharing the load with the patient’s spinal elements; load bearing refers to the instrumentation or graft bearing the entire load, removing all stress from the spinal elements. To maintain the appropriate load-bearing capacity, the 80-20 rule of Harms should be considered, which states that the anterior spinal column supports 80% of the axial load, and the remaining 20% is transmitted through the posterior column.
Assessment of Fusion
Fusion success does not always correlate with patient outcome; patients with pseudarthrosis may still have a good outcome. However, it is important to evaluate for pseudarthrosis in a patient who does not have a good outcome following a spinal arthrodesis procedure. The “gold standard” for fusion assessment is direct surgical assessment.25,26 Plain radiographs, the most common noninvasive method of evaluation, are accurate in only about two thirds of patients when compared with surgical exploration and palpation.27 Static radiographs have a positive predictive value of 76% and a negative predictive value of 54%.28 Dynamic (flexion-extension) radiographs have greater accuracy, with a positive predictive value of 70% and a negative predictive value of 86%.29
Computed tomography (CT) improves the detail with which observers can assess spinal fusion, and it is more sensitive to the detection of pseudarthrosis than static films and flexion-extension radiographs.27 Many investigators have determined that thin-cut CT scans are even better at detecting pseudarthrosis.30 In posterior lumbar interbody fusion procedures, CT has been demonstrated to be more sensitive for detecting abnormalities than plain radiographs.31,32
Technetium 99m bone scanning has been used for the evaluation of pseudarthrosis; however, one study showed that its positive predictive value was only 40% and it negative predictive value was 95%,33 suggesting that bone scanning does not reliably diagnose pseudarthrosis following lumbar fusion surgery.
Roentgen stereophotogrammetric analysis evaluates bony fusion by using radiopaque markers implanted into each vertebral level incorporated into the fusion at the time of surgery. Postoperatively, the patient undergoes computerized radiographic assessment with biplanar roentgen tubes in different positions to detect movement.34 Small trials have demonstrated the ability of this imaging modality to detect motion between adjacent vertebral bodies. In one such study, 10 patients underwent roentgen stereophotogrammetric analysis following lumbar fusion, and the findings were confirmed in all 10 patients via open exploration.35 Major drawbacks of this method of analysis are that it is invasive and not widely available.
Magnetic resonance imaging (MRI) has been of limited use in the evaluation of pseudarthrosis because of poor visualization of bone; however, MRI may add unique information to detect pseudarthrosis in specific cases that CT scanning does not.36 Other minor techniques, such as ultrasonography and polytomography, have also been used to assess bony fusion.29,37
Autograft
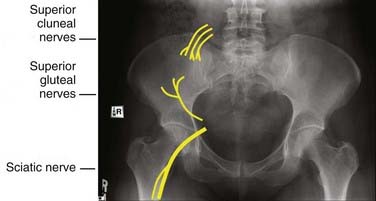
Autogenous bone graft is the gold standard against which other bone graft materials are compared (Table 292-1). It contains the natural combination of osteogenic, osteoconductive, and osteoinductive properties that allow optimal bone integration and fusion. Given the autogenous source, there is no additional risk of disease transmission or a deleterious host-graft reaction. Disadvantages of autograft include its limited availability, harvest site morbidity, possible additional incision, and increased operative time. Harvested autograft can consist of cancellous bone, cortical bone, or a combination of both.
Cancellous autograft contains a trabecular scaffold with matrix proteins, collagen, mineral, and bone marrow cells, creating an almost ideal environment for new bone growth. Cancellous autograft is incorporated into new bone relatively rapidly compared with larger segments of cortical autograft (e.g., fibular strut graft), which may take years to incorporate.38 As with autograft in general, there is a limited amount of cancellous autograft available to harvest, and it has a low weight-bearing capacity. Indications for cancellous autograft include the need for a nonstructural graft, such as an onlay graft or facet joint packing. It can also be used to fill a structural graft, such as an interbody cage or cortical strut graft.
Autograft can be harvested in a variety of forms, such as strut graft, tricortical graft, bicortical graft, unicortical graft, and cancellous pieces. Depending on the form of graft required, the anatomy of the patient, and the location of the primary operation, potential donor sites include the iliac crest, rib, fibula, and locally collected pieces of bone from the bony decompression. Iliac crest is the most common source for both cortical and cancellous autograft for spine surgery.39
Harvest Sites
Anterior Iliac Crest
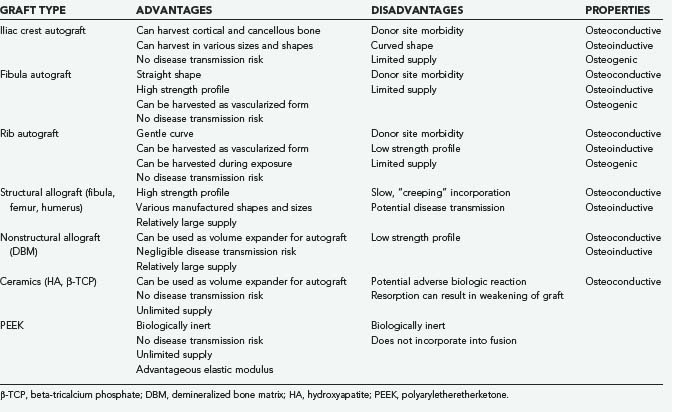
The anterior iliac crest is well suited for procedures in which the patient is in the supine position. The anterior iliac crest, specifically the anterior superior iliac spine (ASIS), acts as an attachment site for various muscles as well as the inguinal ligament. A skin incision should be made parallel to the iliac crest and started approximately 4 cm lateral to the ASIS to avoid the lateral femoral cutaneous nerve (Fig. 292-1). Variations in the course of the lateral femoral nerve have been studied extensively in human cadavers.40,41 The nerve’s normal course is inferomedial to the ASIS; however, but it may be superolateral in up to 13% of patients, where it is within 2 cm of the ASIS 98% of the time.40–44
Posterior Iliac Crest
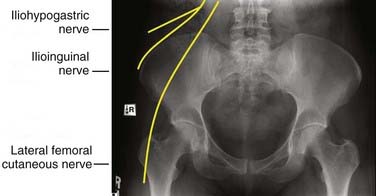
The posterior iliac crest is another common autograft harvest site for spine surgery, especially for posterior fusion procedures. The posterior iliac crest can be accessed through a separate incision, parallel and overlying the lateral portion of the crest, made at least 8 cm lateral to midline to avoid injury to the cluneal nerves (Fig. 292-2). In the case of posterior lumbar fusion, the dissection in the fascial plane can be performed from the midline lumbar incision laterally to access the posterior iliac crest. The attachment of the deep posterior fascia is identified and then dissected off the iliac crest. The gluteal muscle can also be dissected off the lateral aspect of the crease to expose the required amount of crest. The autograft can then be harvested with a combination of drills, osteotomes, and curets. Once inside the cancellous marrow space of the ileum, a significant amount of cancellous autograft can be obtained by harvesting in all four directions. One needs to keep in mind the medial location of the sacroiliac joint to avoid injury to it. Once the harvest is complete, the donor cavity and wound are closed as previously described.
Fibula
A fibular graft’s shape, strength, and relative ease of harvest make it well suited for use in anterior cervical arthrodesis as a structural graft. It can be harvested in both a vascularized and nonvascularized form. The former is much more technically demanding to harvest and implant and is therefore recommended in high-demand situations such as failed prior fusions, cervical arthrodesis of three or more levels, osteomyelitis, and tumor cases in which radiation has been or will be used.45
Rib
Rib bone is another source of autograft that can be harvested in a vascularized and nonvascularized fashion. It can be harvested during a standard thoracotomy exposure of the anterior thoracic spine, from the posterior thoracic cage when the patient is in the prone position, or during minimally invasive thoracoscopic spine surgery.46 Although the rib has a curve similar to the cervical lordotic curve, its low strength profile prevents it from being used as a structural graft.
Complications
Even though autogenous bone graft is the gold standard, it is not without drawbacks. The devascularization of the bone that occurs during harvesting can result in cell death of the graft.47 The reported pseudarthrosis rates with autograft used for spinal fusion procedures are 5% to 44%.47 In addition, the quality and quantity of the autograft is donor specific, with characteristics such as age, anatomy, gender, and presence of osteoporosis and other medical conditions having significant effects. Donor site morbidity can include superficial or deep infection, transient or permanent sensory loss, transient or chronic donor site pain, herniation of muscle or viscera into the donor bed, deep hematoma, joint destabilization, instability fracture, heterotrophic bone formation, damage to major vascular structures, and bowel perforation.
Superficial nerves can be transected and suffer retraction injury during dissection or may be entrapped by scar formation. The lateral femoral cutaneous nerve is the most commonly injured nerve during anterior iliac crest autograft harvest. Injury can result in a sensory deficit or meralgia paresthetica. Temporary sensory impairment rates range from 0% to 20%, and permanent impairment rates are 0% to 5%.40 A temporary neural deficit is best explained by a neurapraxic injury caused by direct or excessive retraction, whereas a permanent lesion is most likely an axonemetic injury from direct laceration. A meticulous and systematic dissection at least 2 cm lateral to the ASIS can help avoid the lateral femoral cutaneous nerve and identify an anomalous course of the nerve or a branch. Slight flexion of the hip may help avoid retraction injury. The cluneal nerves can be encountered 6 to 8 cm lateral to the posterior superior iliac spine, and care must be taken not to extend the incision too lateral. Other nerves potentially at risk are the ilioinguinal, iliohypogastric, genitofemoral, superior gluteal, femoral, and sciatic nerves; however, injuries to these nerves are rare and are usually associated with extensive muscle-splitting techniques for larger autograft harvests.
The incidence of chronic iliac donor site pain ranges from 26% to 34%, with approximately 3% to 10% of patients reporting severe chronic pain.48,49 Resnick50 studied 31 patients in a randomized, controlled fashion and found that reconstruction of the iliac crest with tricalcium phosphate bone void filler significantly decreased the severity of postoperative pain 6 weeks after surgery.
Iliac crest fractures can result in pelvic instability and severe pain syndromes. Older osteoporotic women are at particularly high risk for iliac graft site fracture, and additional care should be exercised in this population.51 The ASIS is the attachment site of several large muscle groups, so removing a large quantity of autograft can cause significant weakening, predisposing the patient to stress fractures. It is recommended that the harvest site for the anterior iliac crest be at least 3 cm posterior to the anterior border of the anterior iliac spine.52 Some reports recommend the use of an oscillating saw rather than an osteotome when obtaining iliac crest grafts to reduce the risk of iliac fracture.53
Allograft
Allograft is absorbed and remodeled into new bone by a process termed creeping substitution, in which the allograft is slowly replaced in a process similar to fracture healing or bone infarct.54 Allograft is incorporated at a slower pace than autograft, with a transplanted cortical allograft consisting of as much as 50% to 90% necrotic bone at 5 years.55 Allograft can be used as a substitute for autograft or as an extender by adding volume to a given amount of autograft. Advances in donor screening and processing have greatly decreased the risk of disease transmission associated with allograft use. The American Association of Tissue Banks and the Food and Drug Administration require serologic testing for antibodies to human immunodeficiency virus (HIV) types 1 and 2, hepatitis B and C, syphilis, and human T-lymphotropic virus types I and II, as well as nucleic acid testing for HIV-1 and hepatitis C virus. Additional tests may include screening for HIV Ag p24, cytomegalovirus, and West Nile virus.
Allograft routinely used for spinal fusion is preserved by one of two methods.55 Fresh-frozen grafts are washed with an antibiotic solution and frozen to −70° C, with a shelf life of 5 years. This process leaves the graft with antigenic properties and a potential HIV transmission risk, although the BMPs are preserved. Freeze-dried grafts are washed with an antibiotic solution and frozen to −70° C. The water content is then reduced to less than 5% (lyophilization), leaving the graft with the ability to be stored at room temperature. Freeze-dried bone is less immunogenic and has a negligible viral transmission risk. However, the BMPs in the graft are destroyed during lyophilization. Although the freeze-drying process weakens the biomechanical strength of the bone graft, markedly diminishing its torsional and bending strength, it does not significantly affect the compressive or tensile strength.56,57 The combination of freeze-drying and subsequent gamma irradiation further weakens bone allograft.58,59
Demineralized Bone Matrix
Demineralized bone matrix (DBM) is formed by exposing allograft bone to acid, which extracts the mineral component. DBM is manufactured in several forms, including gel, putty, and pliable sheets. DBM is commonly used as a volume expander by mixing it with autograft. The preparation of DBM is thought to increase the bioavailability of BMPs within the graft material, thereby giving DBM greater osteoinductive properties than standard allograft.60 The optimal DBM-to-autograft ratio is 3 : 1.61 Sassard and coworkers62 compared patients who underwent posterolateral fusion with either Grafton gel (DBM-glycerol composite) and local harvested autograft or iliac crest autograft. They found a similar fusion rate (60% and 56%, respectively, for Grafton and controls) in the two groups.
Xenograft
Xenograft is tissue harvested from one individual species, processed, and then implanted in an individual of a different species. Xenografts have had very limited clinical use largely due to histocompatibility problems, which have also been noted in other animal-derived products used in surgery. Several host-donor reactions can occur, with the most rapid and violent being the hyperacute reaction. Human xenoreactive natural antibodies target specific terminal carbohydrate epitopes, called Gal-1,3Gal, which are synthesized by a specific form of the enzyme galactosyltransferase and expressed on nonprimate cells.63
Synthetic Bone Graft Substitutes
Ceramics
Despite DBM’s frequent use as a bone graft extender, there has been significant recent interest in synthetic materials such as hydroxyapatite (HA) and beta-tricalcium phosphate (beta-TCP). These synthetics have become a popular alternative to biologic tissue because of their unlimited supply and lack of disease transmission. Polymethyl methacrylate (PMMA) has also been tried but is not incorporated into fusion masses.64 When mixed with autograft, HA and beta-TCP can successfully augment instrumented and noninstrumented posterolateral lumbar fusions.65
Polyaryletheretherketone
Polyaryletheretherketone (PEEK) is a high-temperature thermoplastic polymer developed in the 1980s that has been increasingly used as a radiolucent biomaterial to replace metal implant components for trauma, orthopedic, and spine procedures.66 The PEEK material can be specifically tailored to exhibit an elastic modulus ranging from 3 to 18 Gpa* (that of cortical bone) to 110 GPa (that of titanium alloy) by creating carbon fiber–reinforced composites with varying carbon fiber content and orientation.67 Because PEEK is a biologically inert material, biomaterials such as HA and beta-TCP have been added to create a more bioactive composite. Although HA and beta-TCP increase the elastic modulus of the PEEK composite, they have a relatively weak affinity to the PEEK matrix and thus substantially reduce the strength and toughness of the composite.66
Fusion Enhancers
Bone Morphogenetic Proteins
Urist discovered in 1965 that implanting DBM in the muscle of rabbits and other small animals could induce new bone formation.6 Further characterization and extraction of the noncollagenous proteins that make up the organic matrix of bone led to the discovery of a group of glycoproteins collectively called bone morphogenetic proteins that were members of the TGF-β protein superfamily. Because hundreds of kilograms of allograft bone are required to extract milligrams of BMP, and because an immunogenic reaction is possible with anything less than 100% purified BMP product, recombinant technology has been employed to a provide an unlimited, quality-controlled source of BMP. Fourteen different BMPs have been characterized thus far68; however, researchers have had the most success inducing bone formation with BMP-269 and BMP-770 (osteogenic protein-1 [OP-1]). These two are currently the only BMPs approved by the U.S. Food and Drug Administration for use in spine surgery. Specifically, rhBMP-2 has been approved to enhance anterior interbody spinal fusions,69 and rhBMP-7 (rhOP-1) has been approved under a humanitarian device exemption as an alternative to autograft in compromised patients (e.g., smokers, diabetics, osteoporotics) requiring revision posterolateral lumbar fusion or in patients in whom autogenous bone harvest is not feasible or is not expected to promote fusion.
Although there is strong evidence in support of BMP use, several potential complications have been reported. The off-label use of rhBMP-2 in anterior cervical fusions has been associated with clinically significant soft tissue swelling, making its use in this procedure relatively contraindicated.71 The use of rhBMP-2 in transforaminal lumbar interbody fusion has also been reported to lead to significant vertebral bone resorption, which could lead to graft subsidence and nonunion.72 The routine use of BMPs can be cost-prohibitive, and it is generally recommended that the patient’s age, bone quality, spinal disease process, planned operation, medical comorbidities, and risk of nonunion be considered before the use of BMPs.
Direct-Current Electrical Stimulation
Direct-current electrical stimulation uses an implanted generator to deliver a constant current to the fusion bed and has demonstrated efficacy in dorsolateral spine fusion. One study indicated an increased fusion rate of approximately 85% to 95% for pedicle screw instrumented dorsolateral fusion, and an increase in smokers from 66% to 83%.73
Clinical Applications of Bone Graft Materials
Cervicothoracic Anterior Fusion
Anterior cervical and upper thoracic discectomies and corpectomies may necessitate an anterior fusion to reconstruct the height and structural support of the decompressed region, including the use of an interbody graft, strut graft, or fusion cage to achieve a solid bony fusion across the affected vertebral segments. Tricortical iliac crest autograft has been widely used for anterior column reconstruction because its cortical component is strong enough to act as a structural graft, and its cancellous portion promotes graft incorporation and fusion. Owing to the morbidity associated with harvesting iliac crest, the use of allograft has also been extensively studied. Multiple series have demonstrated equivalent fusion rates and clinical outcomes with tricortical fibula allograft and autogenous iliac crest graft in single and multilevel anterior cervical fusions.74–76 Long-segment structural allografts have also been successful in anterior cervical spinal reconstructions following multilevel corpectomy.77 In addition, a variety of synthetic interbody fusion cages, manufactured from titanium or carbon fiber polymers, can be used in the cervical and thoracic spine and have demonstrated comparable efficacy to autograft.78,79
Anterior plate fixation has gained widespread acceptance as a supplement to cervical interbody fusion. The function of anterior cervical plates is to load-share with the interbody graft and increase the segmental stiffness of a construct, thereby increasing its translational stability. Although fusion rates for common procedures such as single or two-level anterior cervical discectomy and fusion (ACDF), performed without plating, are generally satisfactory,80 there is evidence that superior fusion rates can be attained with plate fixation for multilevel constructs. One large retrospective analysis reported fusion rates of 96% and 91% for one- and two-level ACDF with plate fixation, respectively, compared with 90% and 72% for one- and two-level procedures without plating.81 Other studies have consistently demonstrated fusion rates in excess of 90% for one- and two-level ACDF procedures using plate fixation, regardless of the type of interbody graft used.75,74,78 In addition to anterior plate fixation, external pulsed electromagnetic field stimulation has recently been shown to increase arthrodesis rates in ACDF.82 This adjunct has proved particularly useful in augmenting fusion in patients undergoing multilevel (two- to four-level) ACDF and in smokers, both factors typically associated with an increased risk of nonunion.
Thoracolumbar Anterior Fusion
Analogous to the anterior plating constructs used in the cervical region, ventral and ventrolateral instrumentation is often used to provide rigid internal fixation as well as to load-share with a strut graft following thoracic and lumbar corpectomy or after anterior lumbar discectomy and interbody fusion. Many different options exist. Stand-alone anterior thoracolumbar fusion using an interbody graft in conjunction with ventrolateral instrumentation consistently demonstrates arthrodesis rates greater than 90% without the need for supplemental posterior instrumentation.83,84
Thoracolumbar Posterior Fusion
Posterior thoracolumbar fusion is generally more difficult to achieve than posterior fusion in the cervical or upper thoracic spine. The thoracolumbar segments are subjected to the highest compressive loads in the spine, and the distance between adjacent posterior segments is greater, necessitating larger quantities of graft material. In addition, the normal range of motion permitted by the thoracolumbar segments makes it more difficult to obtain rigidity in a fusion construct. Because the fusion rate is affected by the mechanical stability of the involved segments, it is advantageous to create a rigid fusion construct. In planning a construct to limit motion, the concept of instantaneous axis of rotation (IAR) for the lower thoracic and lumbar spine needs to be recognized. The IAR can be considered the axis around which the vertebral body rotates, and it is generally located within the posterior aspect of the vertebral body in the lumbar spine. As described by White and Panjabi,85 a fusion mass placed at a maximal distance from the IAR is most effective in preventing movement around that IAR. Biomechanical studies have demonstrated that a lumbar fusion mass incorporating the dorsal elements and transverse processes bilaterally provides more rigidity than an interbody fusion mass alone; this is because it is located at a greater distance from, and thus has greater leverage in preventing motion around, the IAR.86
Cleveland M, Bosworth D. Pseudoarthrosis in the lumbosacral spine. J Bone Joint Surg Am. 1948;30:302-312.
Daftari TK, Whitesides TEJr, Heller JG, et al. Nicotine on the revascularization of bone graft. An experimental study in rabbits. Spine. 1994;19:904-911.
Kowalski RJ, Ferrara LA, Benzel EC. Biomechanics of bone fusion. Neurosurg Focus. 2001;10:E2.
Prendergast PJ, Huiskes R. The biomechanics of Wolff’s law: recent advances. Ir J Med Sci. 1995;164:152-154.
Sawin PD, Dickman CA, Crawford NR, et al. The effects of dexamethasone on bone fusion in an experimental model of posterolateral lumbar spinal arthrodesis. J Neurosurg. 2001;94:76-81.
Service RF. Tissue engineers build new bone. Science. 2000;289:1498-1500.
1 Luo X, Pietrobon R, Sun SX, et al. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine. 2004;29:79-86.
2 Albee FH. Transplantation of a portion of the tibia into the spine for Pott’s disease. JAMA. 1911;57:885-886.
3 Hibbs RA. An operation for progressive spinal deformities. N Y State J Med. 1911;93:1013.
4 Burns BH. An operation for spondylolisthesis. Lancet. 1933;224:1233-1239.
5 Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10:154-168.
6 Urist MR. Bone: formation by autoinduction. Science. 1965;150:893-899.
7 Urist MR. Bone: formation by autoinduction. 1965. Clin Orthop Relat Res. 2002;395:4-10.
8 Carlisle E, Fischgrund JS. Bone morphogenetic proteins for spinal fusion. Spine J. 2005;5:240S-249S.
9 Frost HM. Tetracycline-based histological analysis of bone remodeling. Calcif Tissue Res. 1969;3:211-237.
10 Kalfas IH. Principles of bone healing. Neurosurg Focus. 2001;10:E1.
11 Dee R. Bone Healing. New York: McGraw-Hill; 1988.
12 Recker R. Embryology, Anatomy, and Microstructure of Bone. New York: Raven Press; 1992.
13 Fawcett D. Bloom and Fawcett: A Textbook of Histology, 12th ed. New York: Chapman and Hall; 1994.
14 Morley P, Whitfield JF, Willick GE. Parathyroid hormone: an anabolic treatment for osteoporosis. Curr Pharm Des. 2001;7:671-687.
15 Kanis JA, McCloskey EV. Effect of calcitonin on vertebral and other fractures. QJM. 1999;92:143-149.
16 Candia AF, Watabe T, Hawley SH, et al. Cellular interpretation of multiple TGF-beta signals: intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development. 1997;124:4467-4480.
17 Helm GA, Dayoub HJr, Jane JA. Gene-based therapies for the induction of spinal fusion. Neurosurg Focus. 2001;10:E5.
18 Pilitsis JG, Lucas DR, Rengachary SS. Bone healing and spinal fusion. Neurosurg Focus. 2002;13:E1.
19 Reuben SS, Ablett D, Kaye R. High dose nonsteroidal anti-inflammatory drugs compromise spinal fusion. Can J Anaesth. 2005;52:506-512.
20 Sawin PD, Dickman CA, Crawford NR, et al. The effects of dexamethasone on bone fusion in an experimental model of posterolateral lumbar spinal arthrodesis. J Neurosurg. 2001;94:76-81.
21 Daftari TK, Whitesides TEJr, Heller JG, et al. Nicotine on the revascularization of bone graft. An experimental study in rabbits. Spine. 1994;19:904-911.
22 Service RF. Tissue engineers build new bone. Science. 2000;289:1498-1500.
23 Prendergast PJ, Huiskes R. The biomechanics of Wolff’s law: recent advances. Ir J Med Sci. 1995;164:152-154.
24 Kowalski RJ, Ferrara LA, Benzel EC. Biomechanics of bone fusion. Neurosurg Focus. 2001;10:E2.
25 Cleveland M, Bosworth D. Pseudoarthrosis in the lumbosacral spine. J Bone Joint Surg Am. 1948;30:302-312.
26 Hilibrand AS, Dina TS. The use of diagnostic imaging to assess spinal arthrodesis. Orthop Clin North Am. 1998;29:591-601.
27 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion. J Neurosurg Spine. 2005;2:653-657.
28 Kant AP, Daum WJ, Dean SM, Uchida T. Evaluation of lumbar spine fusion. Plain radiographs versus direct surgical exploration and observation. Spine. 1995;20:2313-2317.
29 Brodsky AE, Kovalsky ES, Khalil MA. Correlation of radiologic assessment of lumbar spine fusions with surgical exploration. Spine. 1991;16:S261-S265.
30 Chafetz N, Cann CE, Morris JM, et al. Pseudarthrosis following lumbar fusion: detection by direct coronal CT scanning. Radiology. 1987;162:803-805.
31 Siambanes D, Mather S. Comparison of plain radiographs and CT scans in instrumented posterior lumbar interbody fusion. Orthopedics. 1998;21:165-167.
32 Shah RR, Mohammed S, Saifuddin A, Taylor BA. Comparison of plain radiographs with CT scan to evaluate interbody fusion following the use of titanium interbody cages and transpedicular instrumentation. Eur Spine J. 2003;12:378-385.
33 Bohnsack M, Gosse F, Ruhmann O, Wenger K. The value of scintigraphy in the diagnosis of pseudarthrosis after spinal fusion surgery. J Spinal Disord. 1999;12:482-484.
34 Johnsson R, Selvik G, Stromqvist B, Sunden G. Mobility of the lower lumbar spine after posterolateral fusion determined by roentgen stereophotogrammetric analysis. Spine. 1990;15:347-350.
35 Pape D, Fritsch E, Kelm J, et al. Lumbosacral stability of consolidated anteroposterior fusion after instrumentation removal determined by roentgen stereophotogrammetric analysis and direct surgical exploration. Spine. 2002;27:269-274.
36 Lang P, Chafetz N, Genant HK, Morris JM. Lumbar spinal fusion. Assessment of functional stability with magnetic resonance imaging. Spine. 1990;15:581-588.
37 Jacobson JA, Starok M, Pathria MN, Garfin SR. Pseudarthrosis: US evaluation after posterolateral spinal fusion: work in progress. Radiology. 1997;204:853-858.
38 Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10-27.
39 Wolfe SA, Kawamoto HK. Taking the iliac-bone graft. J Bone Joint Surg Am. 1978;60:411.
40 Mischkowski RA, Selbach I, Neugebauer J, et al. Lateral femoral cutaneous nerve and iliac crest bone grafts—anatomical and clinical considerations. Int J Oral Maxillofac Surg. 2006;35:366-372.
41 Murata Y, Takahashi K, Yamagata M, et al. The anatomy of the lateral femoral cutaneous nerve, with special reference to the harvesting of iliac bone graft. J Bone Joint Surg Am. 2000;82:746-747.
42 Aszmann OC, Dellon ES, Dellon AL. Anatomical course of the lateral femoral cutaneous nerve and its susceptibility to compression and injury. Plast Reconstr Surg. 1997;100:600-604.
43 de Ridder VA, de Lange S, Popta JV. Anatomical variations of the lateral femoral cutaneous nerve and the consequences for surgery. J Orthop Trauma. 1999;13:207-211.
44 Hospodar PP, Ashman ES, Traub JA. Anatomic study of the lateral femoral cutaneous nerve with respect to the ilioinguinal surgical dissection. J Orthop Trauma. 1999;13:17-19.
45 Lee MJ, Ondra SL, Mindea SA, et al. Indications and rationale for use of vascularized fibula bone flaps in cervical spine arthrodeses. Plast Reconstr Surg. 2005;116:1-7.
46 Picetti GD3rd, Ertl JP, Bueff HU. Endoscopic instrumentation, correction, and fusion of idiopathic scoliosis. Spine J. 2001;1:190-197.
47 Sandhu HS, Grewal HS, Parvataneni H. Bone grafting for spinal fusion. Orthop Clin North Am. 1999;30:685-698.
48 Heary RF, Schlenk RP, Sacchieri TA, et al. Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery. 2002;50:510-516.
49 Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134-139.
50 Resnick DK. Reconstruction of anterior iliac crest after bone graft harvest decreases pain: a randomized, controlled clinical trial. Neurosurgery. 2005;57:526-529.
51 Hu RW, Bohlman HH. Fracture at the iliac bone graft harvest site after fusion of the spine. Clin Orthop Relat Res. 1994;309:208-213.
52 Porchet F, Jaques B. Unusual complications at iliac crest bone graft donor site: experience with two cases. Neurosurgery. 1996;39:856-859.
53 Jones AA, Dougherty PJ, Sharkey NA, Benson DR. Iliac crest bone graft. Osteotome versus saw. Spine. 1993;18:2048-2052.
54 Bauer TW. An overview of the histology of skeletal substitute materials. Arch Pathol Lab Med. 2007;131:217-224.
55 Ehrler DM, Vaccaro AR. The use of allograft bone in lumbar spine surgery. Clin Orthop Relat Res. 2000;371:38-45.
56 Triantafyllou N, Sotiropoulos E, Triantafyllou JN. The mechanical properties of the lyophylized and irradiated bone grafts. Acta Orthop Belg. 1975;41(suppl 1):35-44.
57 Pelker RR, Friedlaender GE, Markham TC. Biomechanical properties of bone allografts. Clin Orthop Relat Res. 1983;174:54-57.
58 Randall RL, Pelker RR, Friedlaender GE, et al. Sequential dependence of freeze-drying and irradiation on biomechanical properties of rat bone. Am J Orthop. 2002;31:129-134.
59 Cornu O, Banse X, Docquier PL, et al. Effect of freeze-drying and gamma irradiation on the mechanical properties of human cancellous bone. J Orthop Res. 2000;18:426-431.
60 Edwards JT, Diegmann MH, Scarborough NL. Osteoinduction of human demineralized bone: characterization in a rat model. Clin Orthop Relat Res. 1998;357:219-228.
61 Morone MA, Boden SD. Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel. Spine. 1998;23:159-167.
62 Sassard WR, Eidman DK, Gray PM, et al. Augmenting local bone with Grafton demineralized bone matrix for posterolateral lumbar spine fusion: avoiding second site autologous bone harvest. Orthopedics. 2000;23:1059-1064.
63 Candinas D, Adams DH. Xenotransplantation: postponed by a millennium? QJM. 2000;93:63-66.
64 Hochschuler SH, Guyer RD, Stith WJ, et al. Proplast reconstruction of iliac crest defects. Spine. 1988;13:378-379.
65 Epstein NE. Efficacy of different bone volume expanders for augmenting lumbar fusions. Surg Neurol. 2008;69:16.
66 Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845.
67 Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;235:224.
68 Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 85, 2003. 1544-1542
69 Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376-381.
70 Vaccaro AR, Patel T, Fischgrund J, et al. A pilot safety and efficacy study of OP-1 putty (rhBMP-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur Spine J. 2003;12:495-500.
71 Smucker JD, Rhee JM, Singh K, et al. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006;31:2813-2819.
72 McClellan JW, Mulconrey DS, Forbes RJ, et al. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech. 2006;19:483-486.
73 Kucharzyk DW. A controlled prospective outcome study of implantable electrical stimulation with spinal instrumentation in a high-risk spinal fusion population. Spine. 1999;24:465-468.
74 Samartzis D, Shen FH, Matthews DK, et al. Comparison of allograft to autograft in multilevel anterior cervical discectomy and fusion with rigid plate fixation. Spine J. 2003;3:451-459.
75 Samartzis D, Shen FH, Goldberg EJ, An HS. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine. 2005;30:1756-1761.
76 Young WF, Rosenwasser RH. An early comparative analysis of the use of fibular allograft versus autologous iliac crest graft for interbody fusion after anterior cervical discectomy. Spine. 1993;18:1123-1124.
77 Singh K, DeWald CJ, Hammerberg KW, DeWald RL. Long structural allografts in the treatment of anterior spinal column defects. Clin Orthop Relat Res. 2002;394:121-129.
78 Zevgaridis D, Thome C, Krauss JK. Prospective controlled study of rectangular titanium cage fusion compared with iliac crest autograft fusion in anterior cervical discectomy. Neurosurg Focus. 2002;12:E2.
79 Vaccaro AR, Robbins MM, Madigan L, et al. Early findings in a pilot study of anterior cervical fusion in which bioabsorbable interbody spacers were used in the treatment of cervical degenerative disease. Neurosurg Focus. 2004;16:E7.
80 Assietti R, Beretta F, Arienta C. Two-level anterior cervical discectomy and cage-assisted fusion without plates. Neurosurg Focus. 2002;12:E3.
81 Kaiser MG, Haid RWJr, Subach BR, et al. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50:229-236.
82 Foley KT, Mroz TE, Arnold PM, et al. Randomized, prospective, and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J. 2008;8:436-442.
83 Sasso RC, Best NM, Reilly TM, McGuire RAJr. Anterior-only stabilization of three-column thoracolumbar injuries. J Spinal Disord Tech. 2005;18(suppl):S7-S14.
84 Rauzzino MJ, Shaffrey CI, Wagner J, et al. Surgical approaches for the management of idiopathic thoracic scoliosis and the indications for combined anterior-posterior technique. Neurosurg Focus. 1999;6:E6.
85 White AA, Panjabi MM. Clinical biomechanics of the spine. Philadelphia: JB Lippincott; 1990.
86 Esses SI, Doherty BJ, Crawford MJ, Dreyzin V. Kinematic evaluation of lumbar fusion techniques. Spine. 1996;21:676-684.