CHAPTER 352 Blunt Cerebrovascular Injury
Blunt injury to the carotid or vertebral vessels (blunt cerebrovascular injury) is uncommon and is diagnosed in about 1 of 1000 (0.1%) patients hospitalized for trauma in the United States.1 This incidence increases to 1% when all blunt trauma patients are screened.2 In patients presenting to a trauma center for major trauma, the incidence of blunt carotid injury is 0.08% to 0.67%.3–5 Most of these injuries, however, are diagnosed only after symptoms (e.g., cerebral ischemia) develop. Consequently, the mortality and morbidity associated with blunt cerebrovascular injury are often high.3 Several injury patterns can occur that cause both immediate and long-term symptoms; these include intimal flap or dissection, pseudoaneurysm formation, thrombosis, fistula formation, complete transection, and progressive narrowing of the lumen, contributing to vessel occlusion, thrombosis, or continued thromboembolism.6 Both blunt and penetrating injuries to the vertebral artery can occur and include similar injuries to those seen in the carotid artery.7–9 The incidence of vertebral artery injury after blunt trauma is 0.20% to 0.77%.10
Pathology
Location and Risk Factors
Most injuries that involve the ICA occur at the base of the skull.11 Extradural arteries (i.e., the cervical ICA or the vertebral artery) are at greater risk for dissection than the intradural portions because they are more mobile, are not protected by the skull, and may be damaged by surrounding bony structures. In particular, injury may occur where a mobile vessel segment traverses a fixed location, such as the skull base foramina. In addition, injury such as dissection may be seen extending from above the relatively fixed common carotid artery bifurcation along the ICA to the foramen lacerum and carotid canal. The vertebral artery is mobile in its proximal (V1) and distal (V3) extradural segments. The foraminal or V2 portion of the vertebral artery is less commonly injured because it is less mobile and is protected by the bony walls of the transverse foramina. Intradural vertebral artery injuries (V4) may occur from extension from the V3 segment.
The risk for injury may be increased among patients with certain connective tissue disorders, such as fibromuscular dysplasia (FMD), Marfan’s syndrome, or Ehlers-Danlos syndrome (type IV), among others. Redundancy and loops of the cervical ICA also may increase the risk for injury, including spontaneous dissection.12
Mechanisms of Injury
There are a number of causes of injury to the carotid artery, but generally it results after overt head and neck trauma. In particular, blunt vascular injuries occur with cervical hyperextension and rotation, hyperflexion, or a direct blow.2 However, dissection also may occur with apparently trivial traumatic events, such as nose blowing or head turning.13 One of the most common mechanisms of blunt vascular injury is a motor vehicle collision (MVC). During an MVC, the injury results from rapid deceleration, with the neck hyperextended or flexed and rotated. The carotid arteries are immobile at the skull base, and the traumatic motion stretches the carotid artery over the upper cervical vertebrae and tears the vessel intima. Other mechanisms of blunt carotid injury include strangulation, basilar skull fractures, and facial fractures. Extradural vertebral artery dissections or injuries are associated with cervical spine fractures, particularly those with a rotational component.
The carotid or vertebral artery occasionally may suffer iatrogenic injury during neurosurgical or angiographic procedures that involve vessel exposure, passage of hardware near or through the artery, or direct manipulation of the vessel.14 Specific procedures during which ICA injury may occur include proximal vascular control during aneurysm surgery, exposure for anterior cervical spine procedures, placement of posterior high cervical hardware (e.g., C1 lateral mass screw), or transsphenoidal surgery.14–18 The vertebral arteries also are at risk for iatrogenic injury during neurosurgical procedures; during procedures on the cervical spine, posterior or anterior iatrogenic injury occurs in 0.3% to 0.5% of cases.19–24 The vessels also may be damaged during endovascular procedures when catheters are passed through the common or internal carotid artery or balloons are deployed in the vessel. Iatrogenic arterial dissection occurs in 0.07% to 0.3% of patients undergoing diagnostic cerebral angiograms.25,26
Clinical Presentation
Clinical features of extradural blunt cerebrovascular injury and, in particular, of dissection include headache, neck pain, transient ischemic attack (TIA), ischemic stroke, pulsatile tinnitus, and cranial nerve palsy. Intradural injuries most often present with either ischemic stroke or subarachnoid hemorrhage (SAH). The patient may present with acute symptoms or in a subacute fashion, or symptoms may develop in a delayed fashion (hours to weeks). In addition, many patients with blunt cerebrovascular injuries are completely asymptomatic.5,27 Some studies (retrospective), however, suggest a reduced risk for stroke when patients are treated before ischemia develops (6%), whereas 50% of untreated patients develop a stroke.28 Other studies have failed to demonstrate this effect, and no randomized trial has been performed.28 Consequently, there is debate about which trauma patients should be screened for blunt cerebrovascular injury.
There are limited data to make firm recommendations on screening, but it is reasonable to screen asymptomatic patients with significant blunt head trauma and the following risk factors for carotid injury: Glasgow Coma Scale (GCS) score of 8 or greater, petrous bone fracture, diffuse axonal injury, and LeFort II or III facial fractures.29,30 Overall, among these patients, traumatic cerebrovascular injuries may be identified in up to 44% of screened patients using angiography,30 but this incidence may be as high as 90% when all carotid risk factors are present. Risk factors for vertebral artery injuries include cervical spine fractures, particularly those associated with severe flexion or extension; upper cervical spine injuries; and fractures through the foramen transversarium.29,30 The frequency of vertebral artery injury when these risk factors are present is between 11% and 24%.31,32 In addition, any patient with a neurologic abnormality that is not explained by the injury or with epistaxis from a suspected arterial source following injury should be screened. Patients who are victims of near hanging, those with seat belt abrasions of the neck, and those with soft tissue swelling of the anterior neck are also candidates for screening.
Symptoms and Signs
Extradural Carotid Injury
Nonspecific headache is a common presentation of blunt carotid injury in the awake trauma patient. Similarly, patients may present with neck pain, ear ache, or facial pain. Ophthalmologic manifestations, including an ipsilateral Horner’s syndrome, are frequent.33 Emboli from the carotid injury may cause optic neuropathy or central retinal artery infarction. Other cranial nerve findings are less frequent. Cerebral ischemia may occur in up to half of patients34 from hemodynamic or embolic factors, and the presentation can range from hemispheric transient ischemic events, to a cerebrovascular accident (CVA), to coma or brain death. Epistaxis may occur in some cavernous carotid injuries.
Intradural Vertebral Artery Injury
Headache and less commonly vertigo, tinnitus, nausea, and vomiting are the usual symptoms in the awake patient. Lateral medullary syndrome is the most common stroke, although other strokes that involve the brainstem may occur. Intradural injuries and in particular vertebral artery dissections are associated with SAH and aneurysm formation35 more frequently than ICA injuries.
Physical Findings
On physical examination, patients may present with a neck hematoma, bruits, pulsatile neck mass, or a palpable thrill (Table 352-1).36,37 One third of trauma patients with blunt carotid injury may have other associated cerebral vascular injuries.11 Also, many patients will have associated traumatic injuries, in particular to the head or chest.38,39 There are conflicting reports on whether the type of injury or the presence of associated injuries increases the likelihood of a patient having a blunt carotid injury.11,39
| MANIFESTATION | FREQUENCY (%) |
|---|---|
| Headache | 58-92 |
| Cerebral ischemia | 63-90 |
| Horner’s syndrome | 9-75 |
| Neck pain | 18-46 |
| Scalp tenderness | 8-27 |
| Bruit | 12-39 |
| Carotid tenderness | 8-19 |
| Cranial nerve palsy | 5-12 |
| Syncope | 11 |
| Amaurosis fugax | 4-6 |
| Dysgeusia | 3 |
Modified from Zetterling M, Carlstrom C, Konrad P. Review article: internal carotid artery dissection. Acta Neurol Scand. 2000;101:1-7; and Singh RR, Barry MC, Ireland A, Bouchier Hayes D. Current diagnosis and management of blunt internal carotid artery injury. Eur J Vasc Endovasc Surg. 2004;27:577-584.
Imaging and Diagnosis
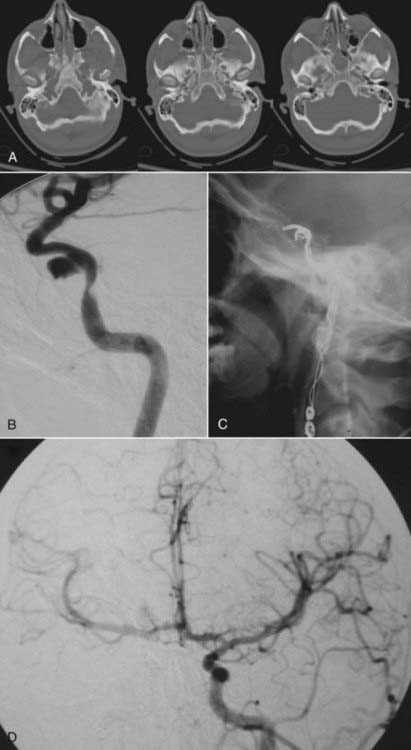
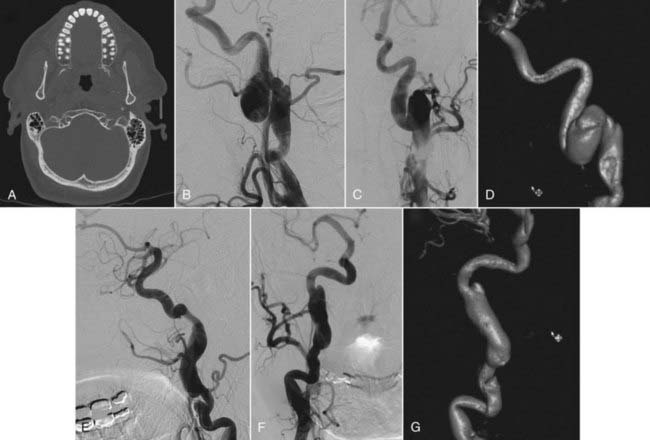
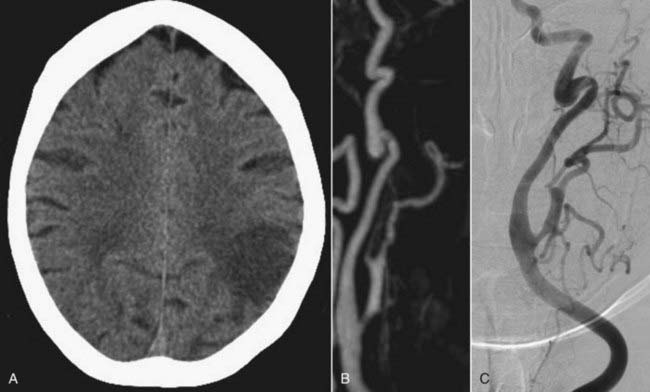
Head computed tomography (CT) scans are almost universally obtained in patients admitted with a head injury (Figs. 352-1A, 352-2A, and 352-3A). Blunt cerebrovascular injury should be considered when there is a neurologic deficit that cannot be explained by the head CT scan, particularly when it is normal, or by the initial injury. In some series of blunt cerebrovascular injuries, this is how 18% to 34% of patients present.4,11
Because blunt cerebrovascular injury can be progressive, it is important to identify these lesions early and to have effective follow-up. There should be a high index of suspicion in the patients who have a mechanism of injury that places the ICA or vertebral artery at risk.3 Blunt cerebrovascular injury may be diagnosed noninvasively using ultrasonography, computed tomography angiography (CTA), magnetic resonance imaging (MRI), and magnetic resonance angiography (MRA). Duplex ultrasound alone is not an adequate test to diagnose traumatic cerebrovascular injury because of limited sensitivity, although Duplex ultrasonography may be used for screening.3,5,27 Transcranial Doppler may be used to listen for distal emboli. Recent advances in noninvasive imaging have made MRI or MRA, followed by CT or CTA, the initial screening study of choice in most patients with suspected dissection. The “gold standard” imaging modality to detect injury of the cerebral cervicocranial vessels is digital subtraction angiography (DSA). The use of these CT- and particularly MRI-based modalities may be limited in trauma patients. Therefore, DSA retains a major role in both diagnosis and treatment (Figs. 352-1B; 352-2B, C, E, and F; and 352-3C). The various imaging modalities, however, have yet to be systematically compared.
Noninvasive Imaging
Contrast-enhanced CT imaging of the neck or CT angiography may be used to detect symptomatic or occult lesions.40,41 In particular, multislice (eight or more slices), multidetector CTA may be a reasonable screening modality to use instead of DSA because their detection rates are similar. However, CTA using four of fewer slices is an inadequate imaging study. On CT, patients will have a focal area of low density within the vessel, and the dissection may be further visualized and defined with CTA (Fig. 352-2D and G).
MRA, because it is noninvasive and requires no contrast administration, is used as an alternative to DSA to image carotid injury (Fig. 352-3B). When compared with DSA, MRA has 95% sensitivity and 99% specificity in some studies.42 On T1-weighted fat-saturated MRI, intramural hematoma will appear as a crescent of intensity encircling the carotid, with narrowing of the adjacent lumen compared with the contralateral carotid. The extent of injury can further be defined with MRA (see Fig. 352-3B). MRI and MRA are less effective when used to evaluate extradural vertebral artery dissection or injury, which often requires angiographic evaluation. Furthermore, noninvasive imaging suggests rather than confirms the diagnosis of intradural dissection. Consequently, DSA often is necessary to evaluate most suspected intradural dissections. After diagnosis, MRI and MRA may provide the information necessary for medical management during follow-up.
Angiography
Angiographic examination of suspected blunt cerebrovascular injury requires a four-vessel angiographic study. Even if the injury is extradural, the intracranial circulation requires study. Radiographic features to examine on intracranial views include intradural extension of an extradural dissection and evidence of emboli, collaterals, slow flow, or stagnation of contrast. Luminal irregularity, stenosis, and less often occlusion are the most frequent findings when there is extradural dissection. The stenosis of dissection differs in its configuration and location from that seen with atherosclerotic disease. Typically, dissection of the ICA spares the common carotid and begins about 2 cm distal to the common origin, enabling differentiation from atherosclerotic disease in which the carotid bulb is most frequently involved. Most often, dissection-related occlusion demonstrates a tapered or flame-shaped distal extent in the acute phase. The lumen often may appear normal after the skull base. Other angiographic features may include an intimal flap, double lumen, intraluminal filling defects, and dissecting aneurysms. Other vascular disorders, such as FMD, may be visible in patients with blunt cerebrovascular injury. In symptomatic patients (including unexplained neurologic deficits after trauma or arterial epistaxis) who undergo four-vessel studies, injury is observed in 40% to 100% of those who also undergo DSA.2,29,43,44
Classification of Blunt Carotid Injury
Carotid injuries were first classified by Cogbill and colleagues27 and then modified by Biffl and coworkers.29,45 The lesions are graded from I to V (Table 352-2). Higher grade is associated with higher mortality, greater stroke risk, and greater likelihood that the lesion will persist or progress. Patients with grade I disease have a 3% stroke risk and a 7% risk for angiographic progression to higher grade. The risk for progression is 70% in grade II lesions, and grade III lesions tend to persist unless treated. Grade IV lesions have a 44% risk for stroke and, like grade III lesions, persist if not treated. Grade V lesions are nearly always fatal.4,46
| GRADE | DESCRIPTION |
|---|---|
| I | Irregularity on angiography or dissection with <25% stenosis |
| II | Dissection with >25% luminal narrowing or a raised intimal flap |
| III | Pseudoaneurysm |
| IV | Complete occlusion |
| V | Transection of the carotid artery with free extravasation of contrast |
Modified from Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47:845-853; and Nunnink L: Blunt carotid artery injury. Emerg Med. 2002;14:412-421.
Vertebral Artery Injury
Evaluation of both vertebral arteries is important. Bilateral dissection is observed more frequently in vertebral artery injury than ICA injury47 and is usually centered at the C1-2 region. Angiographic findings of intradural vertebral artery dissection include segmental narrowing called the “string sign” or the “pearl-and-string sign” when narrowed segments alternate with adjacent segments of vessel dilation. Other angiographic findings include aneurysms (fusiform or saccular), a double lumen, and tapered narrowing with vessel occlusion. An angiographic pattern of stenosis usually is associated with an ischemic presentation, whereas aneurysms, including the pearl-and-string sign, more frequently present with SAH.48 An intradural vertebral artery dissection, even without a history of trauma, should be considered when SAH that involves the posterior fossa is present without a visible aneurysm. In these cases, both intradural vertebral arteries must be visualized angiographically to exclude dissection as a cause of the SAH.
Management
Blunt cerebrovascular injury may be treated with several different modalities, the choice of which depends on the location and type of lesion, the mechanism of the injury, and the patient’s clinical condition.38 Treatment options include surgery, endovascular techniques, anticoagulation, antiplatelet agents, and observation. There are no definitive guidelines on how best to treat blunt cerebrovascular injury, and most information is derived from retrospective case series. Management is aimed at identifying patients with potential ICA or vertebral artery injuries to stabilize the patients and potentially to prevent new neurological deficits. The full benefit of treatment is not precisely defined, but there appears to be a trend toward better outcome in patients who receive “early” treatment.
Treatment of blunt cerebrovascular injury can be guided by the injury grade (see Table 352-2). Grade I and II injuries can be treated with anticoagulation (e.g., heparin, low-molecular-weight heparin, or warfarin sodium [Coumadin]) or antiplatelet agents (e.g., aspirin), unless otherwise contraindicated. Extradural pseudoaneurysms (grade III) may also be treated with anticoagulation or antithrombotic agents provided intradural extension is excluded. Dissecting aneurysms most often resolve with observation or medical therapy, so direct repair using either surgical or endovascular techniques should usually be considered only when medical treatment is ineffective. When there is vessel occlusion, surgery or interventional techniques may be considered to restore flow if the patient presents with an early neurological deficit clearly related to hypoperfusion and an accessible lesion. Grade V lesions often are fatal but may be directly repaired or reconstructed using an interposition graft in rare instances.
Anticoagulation and Antithrombotic Agents
Low-grade (grade I or II) lesions or inaccessible lesions may be treated with full anticoagulation alone or with antiplatelet agents. Patients who present with a dense neurological deficit, however, are unlikely to be helped by anticoagulation. Heparin infusion should be started without a bolus and titrated to an activated partial thromboplastin time (aPTT) of 50 to 60 seconds. The heparin is then converted to warfarin sodium titrated to an INR of 2 to 3 and continued for 3 to 6 months. About 90% of dissections may improve with anticoagulation, but pseudoaneurysms can enlarge or remain.49 Complications that include SAH, bleeding gastritis, and heparin-induced thrombocytopenia may complicate the course in 1 of 10 patients. In addition, because many patients also have other injuries, heparin or anticoagulation may be contraindicated in the acute phase of trauma. This may occur in nearly two thirds of patients.50 When anticoagulation is contraindicated, treatment normally involves the use of antiplatelet agents (e.g., aspirin) or low-molecular-weight dextran.11 Follow-up is important to assess the response to therapy.
Surgery
Surgery is not commonly needed for most injuries, particularly grade I and II injuries. Factors that favor surgical repair include increased thrombogenic potential, incomplete collateral circulation, presence of an expanding hematoma, and clinical worsening despite anticoagulation.36 Some lesions, particularly those located lower in the neck, often are surgically accessible and can be treated with direct repair, commonly using vein interposition grafts. Other surgical options include vessel ligation to prevent extension of the lesion or thrombus, extracranial-intracranial bypass to reestablish blood flow, and aneurysm resection with vessel reconstruction. Among patients who present with a profound neurological deficit, revascularization does not improve outcome. When patients have no or a minor deficit alone and require surgery, revascularization and repair usually are associated with a better outcome than ligation.51,52
Endovascular Techniques
Some lesions can be treated with endovascular stenting or coil obliteration (Fig. 352-1C and D). Endovascular treatment can be particularly effective for fistulas or pseudoaneurysms and for lesions that occur in the high internal carotid artery (e.g., symptomatic extradural carotid injury). When there is symptomatic stenosis associated with dissection, angioplasty and a stent may be used to restore the true lumen to more normal size and so improve flow.53,54 Because the radial force required to restore lumen diameter after a dissection is relatively low, primary angioplasty often is not required. The stent, however, should cross the entire length of the dissection, which if long may require multiple overlapping stents. The initial stent should then be placed at the proximal margin of the dissection to eliminate the in-flow zone of the false lumen.
The choice of treatment with surgery or with endovascular techniques has not been fully defined.36 Because there is a risk for stroke from catheter manipulation within an acutely injured vessel, some authors recommend that an injured artery should not be treated using endovascular techniques until 48 to72 hours after the event. Others recommend waiting 1 week before stenting an injured vessel.55 In addition, evidence of thrombosis in the affected vessel or in patients with a completed CVA is considered a relative contraindication when using endovascular treatment. It is thought that in these patients, emboli may cause a stroke, or opening the vessel may increase the risk for or aggravate a stroke through hemorrhagic conversion. After endovascular stents are placed, patients require full systemic anticoagulation because antiplatelet agents alone may not be sufficient to prevent stent thrombosis.29,56,57
Management of Extradural Internal Carotid Artery Lesions
Traumatic extradural aneurysms, when asymptomatic, often do not require surgical intervention because some may resolve spontaneously or at least remain stable; however, these aneurysms may require direct treatment when they are symptomatic either from distal emboli or mass effect in the neck, are enlarging, or are located in the skull base or middle ear, where they may expose the patient to a hemorrhage risk. In these circumstances, aneurysms may be resected, followed by ICA reconstruction using a saphenous vein graft or primary reanastomosis.1,58,59 Stent placement alone or used to provide a scaffold for coil embolization may also be used for symptomatic dissection-associated aneurysms. Exactly how a traumatic aneurysm is treated using surgical or endovascular techniques depends in large part on its location and morphology and the presence of other vessel disease. For example, endovascular techniques may be difficult when there is excessive vessel tortuosity or FMD.
Management of Vertebral Artery Lesions
There is come controversy about the optimal treatment of these lesions and about whether treatment is needed at all.31 There currently are no specific guidelines. Several authors advocate the use of anticoagulation after blunt vertebral artery injuries to reduce the rate of stroke,60 whereas others have failed to show a clear benefit of this therapy.61–63 Some vertebral artery injuries might require repair using surgical or endovascular techniques. The primary goal of such procedures is to control bleeding.64–68 Because the aim often is to close the vessel, particularly when there are two adequate vertebral arteries, or to close a fistula and allow the collateral circulation to reconstitute the distal blood flow, endovascular coiling may be particularly effective for some vertebral injuries.15,69–71 Stents also can be used in vertebral dissections.72–75 Alternatively, vessel reconstruction or bypass may be required.
Surgical or Endovascular Lesions
Iatrogenic injuries that occur during surgical procedures can be treated with tamponade, vessel ligation, primary repair, and in some instances, endovascular closure.15,19,20,24,76–82 Occasionally, vessel injury may occur during endovascular procedures. Most patients with endovascular-induced injuries can be treated with anticoagulation alone, and surgery is generally not needed.
Follow-Up
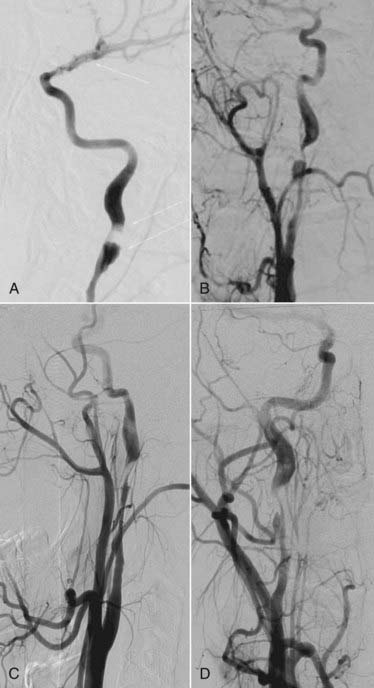
There is a dynamic nature to ICA and vertebral artery dissections and injuries, and the lesions can change over a short period of time (Fig. 352-4). Follow-up angiography, therefore, is recommended in grade I to III injuries. Alternatively, if the lesion is well seen on MRA or CTA, these techniques may be used for follow-up. Biffl and coworkers observed that among grade I and II injuries, some healed, whereas other formed a pseudoaneurysm.83 Pseudoaneurysms often form within 7 to 10 days, and these authors also observed that angiogram-related complications were greater when the study was performed within the first week of injury. It has been recommended, therefore, that follow-up studies be performed at least 7 days after injury. Follow-up imaging should be repeated again after 3 months of therapy when patients are treated using medical management. If there is normal luminal configuration, therapy may be discontinued. If repeat studies show luminal stenosis or irregularities, treatment should be continued for 3 more months with repeat imaging studies. There does not appear to be a benefit to therapy beyond 1 year, even when a radiographic abnormality remains. If a pseudoaneurysm forms and enlarges, surgery or endovascular techniques may be needed.
Outcome
Outcome after blunt carotid injury often may be poor; between 5% and 26% of patients with these types of injury may die.11,28,39,49 The outcome can be good for the many patients who survive the initial injury, although it varies according to the study. Carrillo and colleagues found that 86% of survivors had no neurological deficit or only a minor deficit.11 Kraus and associates found a good or fair outcome in 69% of patients.5 Outcome after carotid injury is associated with age and the segment of carotid artery affected. Worse outcome is observed with increasing age, distal location along the carotid, and multiple affected areas.28 Although ICA injuries account for most of the poor outcomes, blunt vertebral artery injuries are not benign and are associated with a 14% to 24% stroke rate and an 18% to 27% incidence of severe functional deficits.49,56,60,83,84
Ahn JY, Han IB, Kim TG, et al. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. ANJR Am J Neuroradiol. 2006;27:1514-1520.
Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47:845-853.
Biffl WL, Moore EE, Offner PJ, et al. Optimizing screening for blunt cerebrovascular injuries. Am J Surg. 1999;178:517-522.
Biffl WL, Ray CEJr, Moore EE, et al. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg. 2002;235:699-706.
Cogbill TH, Moore EE, Meissner M, et al. The spectrum of blunt injury to the carotid artery: a multicenter perspective. J Trauma Injury Infect Crit Care. 1994;37:473-479.
Dziewas R, Konrad C, Drager B, et al. Cervical artery dissection: clinical features, risk factors, therapy and outcome in 126 patients. [see comment]. J Neurol. 2003;250;:1179-1184.
Englund R, Harris JP, May J. Blunt trauma to the internal carotid artery. Ann Vasc Surg. 1988;2:363-366.
Fabian TC, Patton JHJr, Croce MA, et al. Blunt carotid injury. Importance of early diagnosis and anticoagulant therapy. Ann Surg. 1996;223:513-522.
Flis CM, Jager HR, Sidhu PS. Carotid and vertebral artery dissections: clinical aspects, imaging features and endovascular treatment. Eur Radiol. 2007;17:820-834.
Higashida RT, Halbach VV, Tsai FY, et al. Interventional neurovascular treatment of traumatic carotid and vertebral artery lesions: results in 234 cases. AJR Am J Roentgenol. 1989;153:577-582.
Inamasu J, Guiot BH. Iatrogenic carotid artery injury in neurosurgery. Neurosurg Rev. 2005;28:239-247.
Jeffery P, Immelman E, Beningfield S. A review of the management of vertebral artery injury. Eur J Vasc Endovasc Surg. 1995;10:391-393.
Karlin RM, Marks C. Extracranial carotid artery injury: Current surgical management. Am J Surg. 1983;146:225-227.
Kerwin AJ, Bynoe RP, Murray J, et al. Liberalized screening for blunt carotid and vertebral artery injuries is justified. J Trauma Injury Infect Crit Care. 2001;51:308-314.
Kraus RR, Bergstein JM, DeBord JR. Diagnosis, treatment, and outcome of blunt carotid arterial injuries. Am J Surg. 1999;178:190-193.
Miller PR, Fabian TC, Bee TK, et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma Injury Infect Crit Care. 2001;51:279-285.
Miller PR, Fabian TC, Croce MA, et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236:386-393.
Ramadan F, Rutledge R, Oller D, et al. Carotid artery trauma: a review of contemporary trauma center experiences. J Vasc Surg. 1995;21:46-55.
Sankaran S. Penetrating wounds of the neck: principles and some controversies. Surg Clin North Am. 1977;57:139-149.
Singh RR, Barry MC, Ireland A, Bouchier Hayes D. Current diagnosis and management of blunt internal carotid artery injury. Eur J Vasc Endovasc Surg. 2004;27:577-584.
Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. [review]. Postgrad Med J. 2005;81;:383-388.
Zetterling M, Carlstrom C, Konrad P. Review article: internal carotid artery dissection. Acta Neurol Scand. 2000;101:1-7.
1 Candon E, Marty-Ane C, Pieuchot P, Frerebeau P. Cervical-to-petrous internal carotid artery saphenous vein in situ bypass for the treatment of a high cervical dissecting aneurysm: technical case report. Neurosurgery. 1996;39:863-866.
2 Biffl WL, Moore EE, Offner PJ, et al. Optimizing screening for blunt cerebrovascular injuries. Am J Surg. 1999;178:517-522.
3 Davis JW, Holbrook TL, Hoyt DB, et al. Blunt carotid artery dissection: incidence, associated injuries, screening, and treatment. J Trauma Injury Infect Crit Care. 1990;30:1514-1517.
4 Fabian TC, Patton JHJr, Croce MA, et al. Blunt carotid injury. Importance of early diagnosis and anticoagulant therapy. Ann Surg. 1996;223:513-522.
5 Kraus RR, Bergstein JM, DeBord JR. Diagnosis, treatment, and outcome of blunt carotid arterial injuries. Am J Surg. 1999;178:190-193.
6 Mokri B, Piepgras DG, Houser OW. Traumatic dissections of the extracranial internal carotid artery. Neurosurgery. 1988;68:189-197.
7 Flis CM, Jager HR, Sidhu PS. Carotid and vertebral artery dissections: clinical aspects, imaging features and endovascular treatment. Eur Radiol. 2007;17:820-834.
8 Jeffery P, Immelman E, Beningfield S. A review of the management of vertebral artery injury. Eur J Vasc Endovasc Surg. 1995;10:391-393.
9 Naito I, Iwai T, Sasaki T. Management of intracranial vertebral artery dissections initially presenting without subarachnoid hemorrhage. Neurosurgery. 2002;51:930-937.
10 Inamasu J, Guiot BH. Vertebral artery injury after blunt cervical trauma: an update. Surg Neurol. 2006;65:238-245.
11 Carrillo EH, Osborne DL, Spain DA, et al. Blunt carotid artery injuries: difficulties with the diagnosis prior to neurologic event. [see comment]. J Trauma Injury Infect Crit Care. 1999;46;:1120-1125.
12 Barbour PJ, Castaldo JE, Rae-Grant AD, et al. Internal carotid artery redundancy is significantly associated with dissection. [see comment]. Stroke. 1994;25;:1201-1206.
13 Caplan LR, Biousse V. Cervicocranial arterial dissections. J Neuroophthalmol. 2004;24:299-305.
14 Inamasu J, Guiot BH. Iatrogenic carotid artery injury in neurosurgery. Neurosurg Rev. 2005;28:239-247.
15 Choi J-W, Lee J-K, Moon K-S, et al. Endovascular embolization of iatrogenic vertebral artery injury during anterior cervical spine surgery: report of two cases and review of the literature. Spine. 2006;31:E891-E894.
16 Currier BL, Todd LT, Maus TP, et al. Anatomic relationship of the internal carotid artery to the C1 vertebra: A case report of cervical reconstruction for chordoma and pilot study to assess the risk of screw fixation of the atlas. Spine. 2003;28:E461-E467.
17 Fukushima T, Maroon JC. Repair of carotid artery perforations during transsphenoidal surgery. Surg Neurol. 1998;50:174-177.
18 Sakai N, Murai Y, Suzuki N, et al. [A case of iatrogenic carotid artery dissection treated with radial artery graft]. No Shinkei Geka. 2001;29:837-841.
19 Burke JP, Gerszten PC, Welch WC. Iatrogenic vertebral artery injury during anterior cervical spine surgery. Spine J. 2005;5:508-514.
20 Golfinos JG, Dickman CA, Zabramski JM, et al. Repair of vertebral artery injury during anterior cervical decompression. Spine. 1994;19:2552-2556.
21 Inamasu J, Guiot BH. Iatrogenic vertebral artery injury. Acta Neurol Scand. 2005;112:349-357.
22 Neo M, Sakamoto T, Fujibayashi S, Nakamura T. The clinical risk of vertebral artery injury from cervical pedicle screws inserted in degenerative vertebrae. Spine. 2005;30:2800-2805.
23 Prabhu VC, France JC, Voelker JL, Zoarski GH. Vertebral artery pseudoaneurysm complicating posterior C1-2 transarticular screw fixation: case report. Surg Neurol. 2001;55:29-33.
24 Smith MD, Emery SE, Dudley A, et al. Vertebral artery injury during anterior decompression of the cervical spine. A retrospective review of ten patients. J Bone Joint Surg Br. 1993;75:410-415.
25 Cloft HJ, Jensen ME, Kallmes DF, Dion JE. Arterial dissections complicating cerebral angiography and cerebrovascular interventions. Am J Neuroradiol. 2000;21:541-545.
26 Willinsky RA, Taylor SM, TerBrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2899 procedures and review of the literature. Radiology. 2003;227:522-528.
27 Cogbill TH, Moore EE, Meissner M, et al. The spectrum of blunt injury to the carotid artery: a multicenter perspective. J Trauma Injury Infect Crit Care. 1994;37:473-479.
28 Nanda A, Vannemreddy PSSV, Willis BK, et al. Management of carotid artery injuries: Louisiana State University Shreveport experience. Surg Neurol. 2003;59:184-190.
29 Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47:845-853.
30 Kerwin AJ, Bynoe RP, Murray J, et al. Liberalized screening for blunt carotid and vertebral artery injuries is justified. J Trauma Injury Infect Crit Care. 2001;51:308-314.
31 Anonymous. Management of vertebral artery injuries after nonpenetrating cervical trauma. Neurosurgery. 2002;50:S173-S178.
32 Friedman D, Flanders A, Thomas C, Millar W. Vertebral artery injury after acute cervical spine trauma: rate of occurrence as detected by MR angiography and assessment of clinical consequences. AJR Am J Roentgenol. 1995;164:443-447.
33 Biousse V, Touboul PJ, D’Anglejan-Chatillon J, et al. Ophthalmologic manifestations of internal carotid artery dissection. Am J Ophthalmol. 1998;126:565-577.
34 Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. [review]. Postgrad Med J. 2005;81;:383-388.
35 Kaplan JA. Delayed fatal hemothorax due to traumatic carotid dissection: a case report of a previously unreported cause of death. J Forens Sci. 1994;39:552-556.
36 Singh RR, Barry MC, Ireland A, Bouchier Hayes D. Current diagnosis and management of blunt internal carotid artery injury. Eur J Vasc Endovasc Surg. 2004;27:577-584.
37 Zetterling M, Carlstrom C, Konrad P. Review article: internal carotid artery dissection. Acta Neurol Scand. 2000;101:1-7.
38 Fabian TC, George SMJr, Croce MA, et al. Carotid artery trauma: management based on mechanism of injury. J Trauma Injury Infect Crit Care. 1990;30:953-961.
39 Parikh AA, Luchette FA, Valente JF, et al. Blunt carotid artery injuries. J Am Coll Surg. 1997;185:80-86.
40 Ofer A, Nitecki SS, Braun J, et al. CT angiography of the carotid arteries in trauma to the neck. Eur J Vasc Endovasc Surg. 2001;21:401-407.
41 Rogers FB, Baker EF, Osler TM, et al. Computed tomographic angiography as a screening modality for blunt cervical arterial injuries: preliminary results. J Trauma. 1999;46:380-385.
42 Levy C, Laissy JP, Raveau V, et al. Carotid and vertebral artery dissections: three-dimensional time-of-flight MR angiography and MR imaging versus conventional angiography. Radiology. 1994;190:97-103.
43 Sanzone AG, Torres H, Doundoulakis SH. Blunt trauma to the carotid arteries. Am J Emerg Med. 1995;13:327-330.
44 Watridge CB, Muhlbauer MS, Lowery RD. Traumatic carotid artery dissection: diagnosis and treatment. [see comment]. J Neurosurg. 1989;71;:854-857.
45 Nunnink L. Blunt carotid artery injury. Emerg Med. 2002;14:412-421.
46 Englund R, Harris JP, May J. Blunt trauma to the internal carotid artery. Ann Vasc Surg. 1988;2:363-366.
47 Dziewas R, Konrad C, Drager B, et al. Cervical artery dissection: clinical features, risk factors, therapy and outcome in 126 patients. [see comment]. J Neurol. 2003;250;:1179-1184.
48 Shin JH, Suh DC, Choi CG, Leei HK. Vertebral artery dissection: spectrum of imaging findings with emphasis on angiography and correlation with clinical presentation. Radiographics. 2000;20:1687-1696.
49 Miller PR, Fabian TC, Bee TK, et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma Injury Infect Crit Care. 2001;51:279-285.
50 Wahl WL, Brandt M-M, Thompson BG, et al. Antiplatelet therapy: an alternative to heparin for blunt carotid injury. J Trauma Injury Infect Crit Care. 2002;52:896-901.
51 Karlin RM, Marks C. Extracranial carotid artery injury: Current surgical management. Am J Surg. 1983;146:225-227.
52 Ramadan F, Rutledge R, Oller D, et al. Carotid artery trauma: a review of contemporary trauma center experiences. J Vasc Surg. 1995;21:46-55.
53 Cohen JE, Gomori JM, Umansky F. Endovascular management of symptomatic vertebral artery dissection achieved using stent angioplasty and emboli protection device. Neurol Res. 2003;25:418-422.
54 Fateri F, Groebli Y, Rufenacht DA. Intraarterial thrombolysis and stent placement in the acute phase of blunt internal carotid artery trauma with subocclusive dissection and thromboembolic complication: case report and review of the literature. Ann Vasc Surg. 2005;19:434-437.
55 Biffl WL, Moore EE, Ray C, Elliott JP. Emergent stenting of acute blunt carotid artery injuries: a cautionary note. [comment]. J Trauma Injury Infect Crit Care. 2001;50;:969-971.
56 Cothren CC, Moore EE, Ray CEJr, et al. Carotid artery stents for blunt cerebrovascular injury: risks exceed benefits. [see comment]. Arch Surg. 2005;140;:480-485.
57 Kerby JD, May AK, Gomez CR, Rue LW3rd. Treatment of bilateral blunt carotid injury using percutaneous angioplasty and stenting: case report and review of the literature. [see comment]. J Trauma Injury Infect Crit Care. 2000;49;:784-787.
58 Morgan MK, Sekhon LH. Extracranial-intracranial saphenous vein bypass for carotid or vertebral artery dissections: a report of six cases. J Neurosurg. 1994;80:237-246.
59 Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330:393-397.
60 Biffl WL, Moore EE, Elliott JP, et al. The devastating potential of blunt vertebral arterial injuries. Ann Surg. 2000;231:672-681.
61 Louw JA, Mafoyane NA, Small B, Neser CP. Occlusion of the vertebral artery in cervical spine dislocations. J Bone Joint Surg Br. 1990;72:679-681.
62 Schellinger PD, Schwab S, Krieger D, et al. Masking of vertebral artery dissection by severe trauma to the cervical spine. Spine. 2001;26:314-319.
63 Schwarz N, Buchinger W, Gaudernak T, et al. Injuries to the cervical spine causing vertebral artery trauma: Case reports. J Trauma. 1991;31:127-133.
64 Hatzitheofilou C, Demetriades D, Melissas J, et al. Surgical approaches to vertebral artery injuries. Br J Surg. 1988;75:234-237.
65 Landreneau RJ, Weigelt JA, Meier DE, et al. The anterior operative approach to the cervical vertebral artery. J Am Coll Surg. 1995;180:475-480.
66 Meier DE, Brink BE, Fry WJ. Vertebral artery trauma: acute recognition and treatment. Arch Surg. 1981;116:236-239.
67 Sankaran S. Penetrating wounds of the neck: principles and some controversies. Surg Clin North Am. 1977;57:139-149.
68 Stapleford RG, Gruenberg JC, Wolford DG, Kerchner JB. Vertebral artery injury: case report and review of operative approaches. Henry Ford Hosp Med J. 1981;29:148-152.
69 Heuer GG, Gabel B, Bhowmick DB, et al. Symptomatic high flow arteriovenous fistula after a C2 fracture: Case report. J Neurosurg Spine. 2008;8:381-384.
70 Higashida RT, Halbach VV, Tsai FY, et al. Interventional neurovascular treatment of traumatic carotid and vertebral artery lesions: results in 234 cases. AJR Am J Roentgenol. 1989;153:577-582.
71 Islamoglu F, Posacioglu H, Memis A, Durmaz I. Endovascular transcatheter occlusion for traumatic vertebral artery pseudoaneurysm. Eur J Vasc Endovasc Surg. 2000;20:576-578.
72 Ahn JY, Han IB, Kim TG, et al. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. ANJR Am J Neuroradiol. 2006;27:1514-1520.
73 Cohen JE, Gomori JM, Umansky F. Endovascular management of spontaneous bilateral symptomatic vertebral artery dissections. AJNR Am J Neuroradiol. 2003;24:2052-2056.
74 Halbach VV, Higashida RT, Dowd CF, et al. Endovascular treatment of vertebral artery dissections and pseudoaneurysms. J Neurosurg. 1993;79:183-191.
75 Lee Y-J, Ahn JY, Han IB, et al. Therapeutic endovascular treatments for traumatic vertebral artery injuries. J Trauma Injury Infect Crit Care. 2007;62:886-891.
76 Cloward RB. Complications of anterior cervical disc operation and their treatment. Surgery. 1971;69:175-182.
77 Cosgrove GR, Theron J. Vertebral arteriovenous fistula following anterior cervical spine surgery. J Neurosurg. 1987;66:297-299.
78 Daentzer D, Deinsberger W, Boker D-K. Vertebral artery complications in anterior approaches to the cervical spine: report of two cases and review of literature. Surg Neurol. 2003;59:300-309.
79 de los Reyes RA, Moser FG, Sachs DP, Boehm FH. Direct repair of an extracranial vertebral artery pseudoaneurysm: case report and review of the literature. Neurosurgery. 1990;26:528-533.
80 Katsaridis V, Papagiannaki C, Violaris C. Treatment of an iatrogenic vertebral artery laceration with the Symbiot self expandable covered stent. Clin Neurol Neurosurg. 2007;109:512-515.
81 Pfeifer BA, Freidberg SR, Jewell ER. Repair of injured vertebral artery in anterior cervical procedures. Spine. 1994;19:1471-1474.
82 Weinberg PE, Flom RA. Traumatic vertebral arteriovenous fistula. Surg Neurol. 1973;1:162-167.
83 Biffl WL, Ray CEJr, Moore EE, et al. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg. 2002;235:699-706.
84 Miller PR, Fabian TC, Croce MA, et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236:386-393.