Chapter 170 Blood Loss Management
Preoperative Evaluation
Medication History
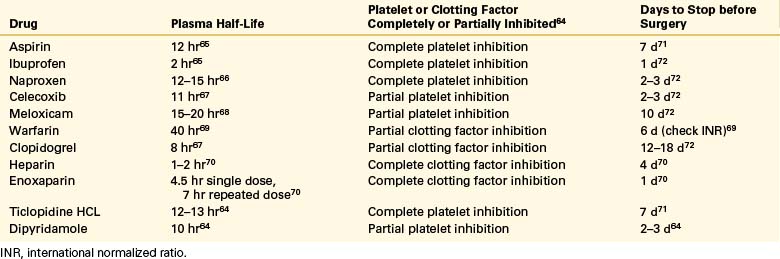
Patients with a history of transient ischemic attack, stroke, deep venous thrombosis (DVT), heavy bleeding after minor trauma, alcoholism, hepatic dysfunction, neuromuscular scoliosis, cerebral palsy,1 and cardiac disease have an increased risk of bleeding. Many patients who undergo spinal surgery take nonsteroidal anti-inflammatory drugs (NSAIDs). NSAIDs inhibit platelet function and may increase bleeding times. The bleeding time often returns to normal within 24 hours of cessation of some NSAIDs, whereas others require a full 18 days for the drug to be eliminated and normal platelet function restored. Antiplatelet drugs, such as ticlopidine hydrochloride (Ticlid), clopidogrel (Plavix), dipyridamole (Persantine), and aspirin may prolong the bleeding time and must be discontinued before surgery.2 Patients who take warfarin (Coumadin) may require cessation or reversal of the anticoagulation (Table 170-1). Those rare patients with congenital coagulation deficits may undergo surgery after factor replacement and consultation with a hematologist.3
Patients treated with the anticonvulsant valproic acid (Depakote) appear to have increased blood loss during spinal surgery. This is thought to be related to an inhibition of platelet function. If possible, another antiepileptic drug should be used in the immediate preoperative and postoperative periods for such patients.4
Preoperative Estimation of Blood Loss
Factors associated with increased blood loss with spinal surgery include tumor, low preoperative hemoglobin level, numbers of levels fused, history of pulmonary disease, suboptimal autologous blood availability, the use of operating tables other than dedicated spine surgery tables, and antiplatelet drugs.5–7 When surgery is contemplated for reconstruction in cases of spinal metastasis from renal cell carcinoma, preoperative embolization has been shown to significantly reduce intraoperative blood loss. Therefore, embolization should at least be considered as a preoperative adjunct in all vascular spinal tumors.8
The blood loss for a particular operation should be estimated and plans generated to ensure that such can be replaced if needed. An estimate of 200 mL of blood lost per fused segment can be made before surgery to plan the amount of blood that will be required for replacement.8 Patients can usually tolerate a loss of 15% of the blood volume before transfusion.9 In a 70-kg patient, estimating 70 mL blood/kg body weight, a volume of 735 mL would reflect a 15% blood loss.
Autologous Blood Use
Autologous blood donation can be instituted if the estimated blood loss exceeds 15% of the blood volume. Autologous blood can be obtained in three ways: preoperative donation, intraoperative salvage, and postoperative salvage. Although each requires expensive specialized equipment, a reduction in the potential for the development of chronic liver disease and infection transmission may be well worth the cost.10 One unit of blood can be donated per week if the hematocrit remains above 34%.11 Other guidelines for autologous donation include a patient age range of 12 to 70 years11 and a hemoglobin of at least 11 mg/dL.12 Full units can be taken from patients weighing more than 50 kg and half units from those between 25 and 50 kg. Supplemental iron should be administered when appropriate. In some instances, recombinant erythropoietin can be administered to facilitate increased blood volume for autologous donation.13–16 This has been shown to increase blood production significantly both in animal and clinical studies.13,17
Consideration should be given as to whether autologous packed red blood cells or whole blood should be transfused. The patient donates whole blood, and in autologous donation it may be best to return whole blood to the patient. Whole blood contains platelets, plasma, and cryoprecipitate, all of which are lacking in packed cells, and these components may help lessen bleeding.13 Using autologous blood in a community hospital, 95% of the transfusion needs were met for 1600 patients who underwent major orthopaedic procedures.18 Other studies have shown that autologous blood can supply approximately 70% to 80% of the transfusion needs.10,12 Autologous blood may be cryopreserved or preserved with a storage solution.19 Cryopreserved blood has a longer shelf life, and the in vitro survival of red blood cells is equivalent to that of fresh erythrocytes. Also, no preoperative effect on the hemodynamic status of the patient exists.20 Oga et al.19 used cryopreserved cells in patients who underwent scoliosis surgery and solution-stored blood for patients who underwent other spine procedures. More blood was collected for the scoliosis patients, and the less costly storage method was used for other patients.19 There are risks to autologous blood transfusion, which include septicemia from bacterial contamination of the unit, nonimmune hemolytic transfusion reactions, febrile reactions, volume overload, and the possible risk of clerical error resulting in the administration of the wrong unit of blood.21
The criteria for returning autologous blood may vary. Albert et al.22 found that the transfusion of blood during surgery was beneficial for the early postoperative hemoglobin and postoperative patient mobilization. There are different indications for homologous versus autologous blood return. Hemoglobin of less than 7 g/dL for homologous transfusions and less than 10 g/dL for autologous transfusions is a common indicator.12,22
Indications for transfusion of homologous blood include a hemoglobin of less than 7 g/dL in a medically healthy but symptomatic patient, a hemoglobin of less than 10 mg/dL in a critically ill patient, and a patient with medical risk factors such as cardiovascular disease, cerebrovascular disease, or active hemorrhage. Symptoms indicating hypovolemia, such as tachycardia, tachypnea, or low venous oxygenation, also indicate the need for transfusion.12,22,23 Other transfusion requirements have been published. They include hemoglobin less than 8 g/dL in an otherwise healthy patient, hemoglobin less than 11 g/dL in patients with increased risk of ischemia, acute blood loss with 15% of blood volume lost, diastolic pressure less than 60 mm Hg, systolic blood pressure decrease of greater than 30 mm Hg, tachycardia, oliguria, symptomatic anemia with tachycardia, mental status changes, cardiac ischemia, and dyspnea. General transfusion requirements for coagulation products have been provided. Platelet transfusion for platelet dysfunction or thrombocytopenia is an effective strategy in such cases. Fresh-frozen plasma may be administered for evidence of coagulation factor deficiencies with prothrombin time or activated partial thromboplastin time greater than 1.5 times the upper limit of normal. Cryoprecipitate is administered for suspected specific factor deficiencies or fibrinogen less than 100 mg/dL.5
Homologous Blood Use
Since the early 1980s, there has been an increasing concern about the risks of blood transfusions. Although transmission of HIV has been the primary concern, allergic reactions, isosensitization, and the transmission of hepatitis are far more serious in terms of the number of patients affected. Hepatitis following transfusion has been reported to occur in as many as 10% of patients after homologous transfusion, with 3.3% developing chronic liver disease.10
The risks of allogeneic blood transfusion have decreased over the past several decades. Currently, transfusion-related acute lung injury (TRALI), hemolytic transfusion reactions (HTRs), and transfusion-associated sepsis are the leading causes of allogeneic blood transfusion–related fatalities. TRALI appears clinically similar to acute respiratory distress syndrome, but in TRALI the infiltrates typically clear in 96 hours, and the case fatality rate is only 5%. Fresh-frozen plasma is the most frequently implicated transfused component. White blood cell antibodies in the transfused component appear to be the most frequent trigger for TRALI. HTR occurred at a rate of 1 per 76,000 units transfused with a case fatality rate of 2% in a recent 10-year New York state study. These cases of HTR are largely attributable to clerical error or undetected non-ABO antibodies.24 Since the introduction of tests in 1990 for the detection of hepatitis C, the incidence of transfusion-acquired hepatitis has significantly decreased. The predominant risk is from donors in the “window period” of their infection who have donated blood before they have developed antibodies to the virus, rendering it impossible to detect the infection.
The current risk of hepatitis C transmission is 1 in one million units transfused, and the current risk of hepatitis B transmission is 1 in 100,000 units transfused. The current risk of HIV transmission in tested blood varies with the region of the United States, but is approximately 1 in one million units transfused.24 This estimate of risk assumes a safe, plentiful, well-regulated, and tested blood supply; unfortunately, this is not universally the case in developing countries.25
Anesthetic Techniques for Lessening Intraoperative Blood Loss
Randomized, controlled trials have shown no difference in the amount of blood loss in patients who received desmopressin compared with those who did not.26 Other studies have shown desmopressin to reduce blood loss in spine surgery by 32.5%.23 It is administered immediately before surgery at a dose of 10 μg/m2 of body surface area, up to a maximum dose of 20 μg. It is prepared by diluting it to a concentration of 0.5 μg/mL in normal saline and infusing over 20 minutes. Desmopressin does lessen bleeding in some subsets of patients, such as those with acquired platelet dysfunction from aspirin administration, von Willebrand disease, and uremia.5,27 Desmopressin has been found to decrease the partial thromboplastin time, whereas factor VIII coagulant activity and von Willebrand antigen concentrations increased the partial thromboplastin time. A Cochrane review found no convincing evidence that desmopressin minimizes perioperative allogeneic red blood cell transfusion in patients who do not have congenital bleeding disorders.28 Potential postoperative problems include oliguria, which usually responds to furosemide, and hyponatremia due to its potent antidiuretic hormone activity.29
Randomized, controlled trials of aprotinin (Trasylol) in dorsal spinal fusion reveal significant reductions in autologous but not homologous blood transfusion. Aprotinin has been shown to reduce blood transfusion requirements in cardiac surgery, liver resection, and some orthopaedic surgical procedures.30 Aprotinin has been found to increase mortality in cardiac surgery patients and was relabeled by the U.S. Food and Drug Administration (FDA) for use only in high-risk cardiac patients. It was removed from the U.S. market in November 2007.31
ε-Aminocaproic acid (Amicar) is an antifibrinolytic agent that has been shown to reduce blood loss in cardiac surgery.32 It has been tested in lumbar spinal fusion and has been shown to reduce autologous blood transfusion in a prospective, nonrandomized study by approximately 50%. No complications were noted, including thromboembolism or DVT. ε-Aminocaproic acid was given in an initial dose of 100 mg/kg, not to exceed 5 g over 15 minutes, followed by a continuous infusion of 10 mg/kg/hr over the remainder of the case, with the infusion terminating at skin closure.33
Tranexamic acid is a synthetic antifibrinolytic agent that has been studied as a means to reduce blood requirements in scoliosis surgery. In a double-blinded, prospective, placebo-controlled study, tranexamic acid, given in an initial dose of 10 mg/kg at the time of patient positioning and in a maintenance infusion of 1 mg/kg/hr, reduced blood transfusion requirements by 28%.34 A recent study of tranexamic acid using a loading dose of 2 g, followed by a continuous infusion of 100 mg/hr in adults and 1 mg/kg/hr in children, resulted in a 49% reduction of blood loss.35 Because tranexamic acid is an antifibrinolytic agent, it does not change the blood’s intrinsic clotting ability but rather slows the breakdown of preexisting clots. Tranexamic acid has also been studied for use in reducing blood loss in metastatic spine tumor surgery and unfortunately did not significantly reduce blood loss in a retrospective study.36
Recombinant coagulation factor VIIa has shown promise in reducing blood loss in spinal surgery. Factor VIIa has been used in trauma management, craniotomy, and to reverse the effects of warfarin. Factor VIIa is approved by the FDA for use in patients with hemophilia with inhibitors to replacement factor VIII or IX. Use for hemostasis in other conditions is considered off-label at this time. The drug has a short half-life and is given as hourly bolus doses or by continuous infusion. Until more data are available, the drug should be used only as an agent of last resort in the event of bleeding when all other measures have failed. The drug is available in 1200-μg vials, and doses have been given in a wide range, from 16 μg/kg to 212 μg/kg, with no reported incidence of untoward events.17,24,25,29,37,38 One multicenter, randomized, controlled trial of factor VIIa used in spine surgery demonstrated no adverse events and significantly reduced blood loss.39
Acute hemodilutional autotransfusion is another technique used to reduce the need for homologous blood transfusion. After the induction of anesthesia, a venesection is performed and 15% to 25% of the patient’s blood volume is withdrawn into a sterile bag containing the anticoagulant citrate. The blood volume withdrawn is then replaced with colloid, on a milliliter-for-milliliter basis, or crystalloid, on a three-to-one basis. At the conclusion of surgery, the autologous blood is returned to the patient. The patient is then diuresed of excess fluid.35,40 Patients with type O blood have lower concentrations of factor VIII and von Willebrand factor than patients with other blood types. Type O patients are at increased risk for development of disseminated intravascular coagulation (DIC) when blood is replaced with the colloid 6% hydroxyethyl starch.41
Hypotensive anesthesia has been evaluated in patients undergoing scoliosis surgery and fusion for degenerative disease.15 Hypotension was induced with 25 mg of nitroprusside and 125 mg of trimethaphan in 500 mL of 5% dextrose, which was infused to maintain a systolic blood pressure between 60 and 70 mm Hg.11,15 Enflurane can also be used as a supplement to the general anesthetic agents to induce hypotension. Both methods have been effective in decreasing blood loss in these patients with no adverse sequelae.42
A significant concern associated with patients with spinal cord injuries is whether the hypotension can cause neurologic deterioration. Ullrich et al.43 performed a retrospective study comparing hypotensive and normotensive techniques in patients who underwent fixation of thoracolumbar fractures. There was a significant decrease in blood loss with hypotensive anesthesia, and there was no documented neurologic injury in either group. It is emphasized, however, that hypotensive anesthetic technique, in the presence of a compressive spinal cord lesion, poses at least the potential for further ischemia and adverse neurologic sequelae.
Somatosensory evoked potentials have been used as a monitoring tool in normotensive and hypotensive patients undergoing spinal instrumentation.44 In the hypotensive group, the systolic blood pressure was maintained between 80 and 90 mm Hg with nitroprusside alone, or in combination with trimethaphan or propranolol. There was a significant difference in blood loss between the two groups. Intraoperative evoked potentials were altered in 5 of 24 patients, 2 in the normotensive and 3 in the hypotensive group. Wake-up testing revealed satisfactory motor function in all patients, and all 24 patients were neurologically intact postoperatively.
Enderby45 reported a series of 9107 anesthesia sessions with an overall death rate of 0.1%. However, of the nine patients who made up the 0.1%, it was believed that only one death was due to the hypotensive technique.
Hypotensive anesthesia has the potential for neurologic or systemic complications. With careful cardiac, renal, and pulmonary monitoring and the maintenance of systolic blood pressure greater than 70 mm Hg, however, many of the complications can be avoided.25,38 Somatosensory evoked potentials in the normal or incompletely injured patient also offer the ability to monitor physiologic changes during the hypotension and to take corrective measures if indicated.44 It is also emphasized that the efficacy of such monitoring techniques is not proven and that the meaningfulness of the information provided is often in question. This can add to the already confusing nature of the decision-making process.
Surgical Techniques for Reducing Blood Loss
Appropriate intraoperative technique can reduce blood loss. Less blood loss is expected if meticulous attention is paid to subperiosteal dissection, hemostasis is applied at each stage of the operation by cautery and packing, decortication is performed at the termination of the operation, and consideration is given to performing fusion with spinous processes instead of iliac crest bone graft. Infiltrating the skin with a dilute solution of epinephrine (1:500,000) and packing with sponges soaked in dilute epinephrine has been reported to diminish bleeding.40
Operative time is important, not only for minimizing blood loss but for diminution of the nonhematologic complication rate.46 Bostman et al.47 evaluated blood loss, operating time, and positioning of the patient. In discectomy patients with a shorter operative time, there was less bleeding. Although the lower blood loss was ascribed to patient positioning, there was a definite correlation between operative speed and blood loss.
Ventral approaches to the lumbar spine have increased in popularity for fusion procedures and disc arthroplasty. The ventral approach to the lumbar spine carries a reported vascular injury rate of 1.9% to 15.6%. The vast majority of these vascular injuries are venous, and hemorrhage may be massive. Teaming with an experienced vascular surgeon for this approach, depending on the experience of the spine surgeon, may be invaluable in lessening this complication and in repairing vascular injuries as they occur.48
Keene and McKinley49 used spinous processes instead of iliac crest for fusion grafts in patients with thoracolumbar fractures. This eliminated donor site bleeding and decreased operative time, with a resultant decrease in blood loss.
Local Agents for the Reduction of Blood Loss
Bleeding during spine surgery may arise from several sources, including bone, muscle, and epidural veins. The topical agents bone wax, gelatin foam (Gelfoam), oxidized cellulose, and microcrystalline collagen can be very useful in controlling intraoperative bleeding.25
Microcrystalline collagen provides a surface for platelet aggregation and allows the platelets to undergo their release reaction, resulting in hemostasis. Because functioning platelets are required for hemostasis, microcrystalline collagen is not effective in cases of thrombocytopenia. Microcrystalline collagen works best when applied dry, and any excess should be removed because the material can absorb fluid and swell, which may cause neural compression. Some evidence suggests that the microcrystalline collagen causes less epidural scarring than some of the other topical agents.50 Microcrystalline collagen does not appear to inhibit bone healing or fusion.37,51
Fibrin Glue
Fibrin glue may be useful in reducing blood loss from decorticated bone. Fibrin glue is formed by combining cryoprecipitate with thrombin and calcium. Thirty milliliters of cryoprecipitate is obtained from the blood bank and is placed in a syringe. In a separate container, 20,000 U of topical thrombin and one ampule of calcium chloride are mixed together. This is then aspirated into a separate syringe. Antibiotics are often added to the syringe containing the thrombin/calcium chloride solution to diminish the risk of postoperative infection. Equal volumes of the mixture are injected onto the decorticated bone.52 This fibrin glue recipe can also be used in the treatment of cerebrospinal fluid leak. There is a potential risk of infection transmission because the fibrin is derived from donor cryoprecipitate. Fibrin sealants are commercially available, have been shown to diminish postoperative drainage by as much as 50%, and may have particular use in preventing postoperative hematoma in anterior cervical surgery.53
Positioning
Positioning for spine surgery, especially for lumbar surgery, ideally decreases intra-abdominal pressure, and subsequently venous pressure. A lower pressure in the inferior vena cava decreases the volume of blood in the Batson plexus. This in turn decreases blood loss from epidural veins. Multiple operative frames have been designed to decrease the pressure on the abdomen while the patient is in the prone position.7,11,47,54 Although the kneeling position has been strongly advocated by some authors to decrease abdominal pressure and blood loss, others have shown no relationship between blood loss and vena caval pressure.55 More recent studies have demonstrated a correlation of the use of the Jackson table with decreased blood loss during spine surgery.5,7
Intraoperative and Postoperative Blood Salvage
Intraoperative autologous transfusion (IAT) has been shown to be a safe and effective method to reduce the need for homologous blood use.11,25,56,57 IAT blood may even have the potential to deliver more oxygen to the tissues than previously stored blood, and may contain more platelets and clotting factors than bank blood.11,45 IAT requires a system that suctions the wound, separates the red blood cells from other blood products and debris, washes the red blood cells, and returns them to the patient.11,45,57 Flynn et al.9,58 estimated that approximately 54% of red blood cells were returned to the patient with this technique. To improve efficiency, they recommended an open-tip suction wand with suction pressures below 100 mm Hg, the avoidance of blood pooling and clotting, the use of fewer sponges, and rinsing of the sponges to collect the red blood cells.9,59,60
There are some limitations to IAT. Approximately half of the red blood cells are salvaged by standard autotransfusion techniques. IAT may be contraindicated in patients with systemic infections, gross wound infections, or malignancy because of the potential for hematogenous spread of the disease.60 It also potentially depletes the coagulation factors if they are not appropriately replaced. For every 1000 mL of autologous red blood cells returned to the patient, one unit of fresh-frozen plasma should be administered. The platelet count should be maintained at greater than 85,000 µL.9,40
Pulmonary complications due to tissue debris accumulation and reinfusion are rare.9 Hemoglobinuria may occur in as many as 5% of patients. With adequate hydration and urine output (>100 mL/hr), these complications should be transient, with minimal or no clinical sequelae.9,54
Just as intraoperative blood can be retransfused, so can postoperative blood.9 It is collected, filtered, anticoagulated, washed, and returned to the patient. Unwashed blood, however, appears to be associated with few complications. This technique has been reported to be safe and may decrease blood bank requirements significantly.61 Newer postoperative drainage systems have been used in orthopaedic surgery and are used to reinfuse blood without filtration directly from the drain into the patient. These systems only return blood lost during the first 8 hours after surgery. These, however, are safe and effective.
Postoperative Drugs and Blood Loss
Deep venous thrombosis occurs after spinal surgery in about 2.2% of cases. However, in the case of spinal cord injury, DVT may occur in up to 80% of cases. DVT prophylaxis with heparin, enoxaparin (Lovenox), or warfarin increases postoperative bleeding. The risk of prophylaxis should be balanced between the risk of DVT and other risks, such as neurologic deterioration from epidural hematoma. Patients with the following risk factors are at increased risk for development of DVT: age greater than 60 years, body mass index of 30 or more, genetic thrombophilia, history of prior DVT, and those undergoing a ventral and ventral/dorsal combined procedure.50 Of note, ketorolac (Toradol) has been used after microlumbar discectomy with good control of pain and no reported increase in bleeding-related complications.45
Treatment and Diagnosis of Diffuse Intraoperative and Postoperative Bleeding
Significant intraoperative hemorrhage rarely occurs. When it does, the causative factors include platelet washout, acute thrombocytopenia, platelet dysfunction, heparin administration, DIC, impaired hepatic function, an undiagnosed hereditary coagulation disorder, or laboratory error failing to detect a coagulopathy. The source(s) of bleeding should be carefully ascertained (e.g., solely from the wound site, from intravenous cannula wounds and other puncture sites, and from occult or distant sites). Isolated bleeding from the surgical site may indicate a coagulation disorder or simply poor surgical hemostasis. Bleeding from multiple sites strongly suggests dysfunctional clotting mechanisms, in which case prothrombin time, partial thromboplastin time, fibrinogen, d-dimer, complete blood count, platelet count, fibrin split products, and bleeding time should be assessed.
DIC is a syndrome characterized by the generation of thrombin in the peripheral blood. The principal target protein of thrombin is fibrinogen. A delicate balance exists between the synthesis and catabolism of fibrinogen. In DIC, this balance is upset and greatly shifted toward catabolism. DIC can be caused by tissue damaged in spine surgery. The diagnosis can be confirmed by the observation of an elevated prothrombin time, an elevated partial thromboplastin time, a low fibrinogen, and a positive d-dimer test. Treatment is directed toward clotting factor replacement.62,63
Brenn B.R., Theroux M.C., Dabney K.W., et al. Clotting parameters and thromboelastography in children with neuromuscular and idiopathic scoliosis undergoing posterior spinal fusion. Spine (Phila Pa 1976). 2004;29:E310-E314.
Chambers H.G., Weinstein C.H., Mubarak S.J., et al. The effect of valproic acid on blood loss in patients with cerebral palsy. J Pediatr Orthop. 1999;19:792-800.
Kolban M., Balachowska-Kosciolek I., Chmielnicki M. Recombinant coagulation factor VIIa: a novel haemostatic agent in scoliosis surgery? Eur Spine J. 2006;15:944-952.
Vamvakas E., Blajchman M.A. Transfusion related mortality: the ongoing risks of allogenic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406-3417.
1. Brenn B.R., Theroux M.C., Dabney K.W., et al. Clotting parameters and thromboelastography in children with neuromuscular and idiopathic scoliosis undergoing posterior spinal fusion. Spine (Phila Pa 1976). 2004;29:E310-E314.
2. Robinson C.M., Christie J., Malcolm-Smith N. Nonsteroidal antiinflammatory drugs perioperative blood loss, and transfusion requirements in elective hip arthroplasty. J Arthroplasty. 1993;8:607-610.
3. Green W.B., McMillan C.W. Surgery for scoliosis in congenital factor VII deficiency. Am J Dis Child. 1982;136:411-413.
4. Chambers H.G., Weinstein C.H., Mubarak S.J., et al. The effect of valproic acid on blood loss in patients with cerebral palsy. J Pediatr Orthop. 1999;19:792-800.
5. Nitu-Whalley I.C., Griffioen A., Harrington C. Retrospective review of the management of elective surgery with desmopressin and clotting factor concentrates in patients with von Willebrand disease. Am J Hematol. 2000;66:280-284.
6. Nuttall G.A., Horlocker T.T., Santrach P.J. Predictors of blood transfusions in spinal instrumentation and fusion surgery. Spine (Phila Pa 1976). 2000;25:596-601.
7. Relton J.E., Hall J.E. An operation frame for spinal fusion: a new apparatus designed to reduce haemorrhage during operation. J Bone Joint Surg [Br]. 1967;49:327-332.
8. Murray D.J., Forbes R.B., Titone M.B., et al. Transfusion management in pediatric and adolescent scoliosis surgery: efficacy of autologous blood. Spine (Phila Pa 1976). 1997;22:2735-2740.
9. Flynn J.C., Metzgar C.R., Csencsitz T.A. Intraoperative autotransfusion (IAT) in spinal surgery. Spine (Phila Pa 1976). 1982;7:432-435.
10. Thomson J.D., Callaghan J.J., Savory C.G., et al. Prior deposition of autologous blood in elective orthopaedic surgery. J Bone Joint Surg [Am]. 1987;69:320-324.
11. Tate D.E.Jr., Friedman R.J. Blood conservation in spinal surgery: review of current techniques, review. Spine (Phila Pa 1976). 1992;17:1450-1456.
12. Bailey T.E.Jr., Mahoney O.M. The use of banked autologous blood in patients undergoing surgery for spinal deformity. J Bone Joint Surg [Am]. 1987;69:329-332.
13. Goodnough L.T., Rudnick S., Price T.H., et al. Increased preoperative collection of autologous blood with recombinant human erythropoietin therapy [see comments]. N Engl J Med. 1989;321:1163-1168.
14. Kickler T., Spivak J. Effect of repeated whole blood transfusions on serum immunoreactive erythropoietin levels in autologous donors. Spine (Phila Pa 1976). 1989;14:358-362.
15. Malcolm-Smith N., McMaster M. The use of induced hypotension to control bleeding during posterior spinal fusion for scoliosis. J Bone Joint Surg [Br]. 1983;65:255-258.
16. Rothstein P., Roye D., Verdisco L., et al. Preoperative use of erythropoietin in an adolescent Jehovah’s Witness. Anesthesiology. 1990;73:568-570. [published erratum appears in Anesthesiology 74:206, 1991]
17. Levine E., Gould S., Rosen A., et al. Perioperative recombinant human erythropoietin. Surgery. 1989;106:432-438.
18. Haugen R., Hill G. A large scale autologous blood program in a community hospital. JAMA. 1987;257:1211-1214.
19. Oga M., Ikuta H., Sugioka Y. The use of autologous blood in the surgical treatment of spinal disorders. Spine (Phila Pa 1976). 1992;17:1381-1385.
20. Kolban M., Balachowska-Kosciolek I., Chmielnicki M. Recombinant coagulation factor VIIa: a novel haemostatic agent in scoliosis surgery? Eur Spine J. 2006;15:944-952.
21. Janatpour K., Holland P. Blood support for pediatric surgery: review. Indian J Pediatr. 2001;68:159-165.
22. Albert T.J., Desai D., McIntosh T., et al. Early versus late replacement of autotransfused blood in elective spinal surgery: a prospective randomized study. Spine (Phila Pa 1976). 1993;18:1071-1078.
23. James S.E., Smith M.A. Autologous blood transfusion in elective orthopaedic surgery. J R Soc Med. 1987;80:284-285.
24. Vamvakas E., Blajchman M.A. Transfusion related mortality: the ongoing risks of allogenic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406-3417.
25. Lindop M.J. Complications and morbidity of controlled hypotension. Br J Anaesth. 1975;47:799-803.
26. Theroux M.C., Corddry D.H., Tietz A.E. A study of desmopressin and blood loss during spinal fusion for neuromuscular scoliosis: a randomized, controlled, double-blinded study. Anesthesiology. 1997;87:260-267.
27. Letts M., Pang E., D’Astous J., et al. The influence of desmopressin on blood loss during spinal fusion surgery in neuromuscular patients. Spine (Phila Pa 1976). 1998;23:475-478.
28. Henry D.A., Moxey A.J., Carless P.A., et al. Desmopressin for minimizing perioperative allogenic blood transfusion. Cochrane Database Syst Rev. 2001;2:CD001884.
29. Kobrinsky N.L., Letts R.M., Patel L.R., et al. l-Desamino-8-d-arginine vasopressin (desmopressin) decreases operative blood loss in patients having Harrington rod spinal fusion surgery: a randomized, double-blinded, controlled trial. Ann Intern Med. 1987;107:446-450.
30. Lentschener C., Cottin P., Bouaziz H., et al. Reduction of blood loss and transfusion requirement by aprotinin in posterior lumbar spine fusion. Anesth Analg. 1999;89:590-597.
31. Thompson G.H., Florentino-Pineda I., Poe-Kochert C., et al. Role of Amicar in surgery for neuromuscular scoliosis. Spine (Phila Pa 1976). 2008;33:2623-2629.
32. Fremes S.E., Wong B.I., Lee E., et al. Metanalysis of prophylactic drug treatment in the prevention of postoperative bleeding. Ann Thorac Surg. 1994;58:1580-1588.
33. Florentino-Pineda I., Blakemore L.C., Thompson G.H., et al. The effect of epsilon-aminocaproic acid on perioperative blood loss in patients with scoliosis undergoing posterior spinal fusion: a preliminary prospective study. Spine (Phila Pa 1976). 2001;26:1147-1151.
34. Neilipovitz D.T. Tranexamic acid for major spinal surgery. Eur Spine J. 2004;13(Suppl 1):S62-S65.
35. Du Toit G., Relton J.E., Gillespie R. Acute haemodilutional autotransfusion in the surgical management of scoliosis. J Bone Joint Surg [Br]. 1978;60:178-180.
36. Bednar D.A., Bednar V.A., Chaudhary A., et al. Tranexamic acid for hemostasis in the surgical treatment of metastatic tumors of the spine. Spine (Phila Pa 1976). 2006;31:954-957.
37. Schonauer C., Tessitore E., Barbagallo G., et al. The use of local agents: bone wax, gelatin, collagen, oxidized cellulose. Eur Spine J. 2004;13(Suppl 1):S89-S96.
38. Strumin L. Ogran perfusion during controlled hypotension. Br J Anaesth. 1975;47:173-178.
39. Sachs B., Delacy D., Green J., et al. Recombinant activated factor VII in spinal surgery: a multicenter, randomized, double-blind, placebo-controlled, dose-escalation trial. Spine (Phila Pa 1976). 2007;32:2285-2293.
40. Phillips W.A., Hensinger R.N. Control of blood loss during scoliosis surgery: review. Clin Orthop Relat Res. 1988;229:88-93.
41. Kang J.G., Ahn H.J., Kim G.S., et al. The hemostatic profiles of patients with type O and non-O blood after acute normovolemic hemodilution with 6% hydroxyethyl starch (130/0.4). Anesth Analg. 2006;103:1543-1548.
42. Patel N.J., Patel B.S., Paskin S., et al. Induced moderate hypotensive anesthesia for spinal fusion and Harrington-rod instrumentation. J Bone Joint Surg [Am]. 1985;67:1384-1387.
43. Ullrich P.F.Jr., Keene J.S., Hogan K.J., et al. Results of hypotensive anesthesia in operative treatment of thoracolumbar fractures. J Spinal Disord. 1990;3:329-333.
44. Grundy B., Nash C., Brown R. Deliberate hypotension for spinal fusion: prospective randomized study with evoked potential monitoring. Can Anaesth Soc J. 1982;29:453-462.
45. Enderby G.E.H. A report on morbidity and mortality following 9,107 hypotensive anaesthetics. Br J Anaesth. 1961;33:109-113.
46. Stolke D., Sollmann W.P., Seifert V. Intra- and postoperative complications in lumbar disc surgery. Spine (Phila Pa 1976). 1989;14:56-59.
47. Bostman O., Hyrkas J., Hirvensalo E., et al. Blood loss, operating time, and positioning of the patient in lumbar disc surgery. Spine (Phila Pa 1976). 1990;15:360-363.
48. Hamdan A.D., Malek J.Y., Schermerhorn M.L., et al. Vascular injury during anterior exposure of the spine. J Vasc Surg. 2008;48:650-654.
49. Keene J.S., McKinley N.E. Iliac crest versus spinous process grafts in posttraumatic spinal fusions. Spine (Phila Pa 1976). 1992;17:790-794.
50. Jacobs R.R., McClain O., Neff J. Control of postlaminectomy scar formation: an experimental and clinical study. Spine (Phila Pa 1976). 1980;5:223-229.
51. Gabay M. Absorbable hemostatic agents. Am J Health Syst Pharm. 2006;63:1244-1253.
52. Tredwell S.J., Sawatzky B. The use of fibrin sealant to reduce blood loss during Cotrel-Dubousset instrumentation for idiopathic scoliosis. Spine (Phila Pa 1976). 1990;15:913-915.
53. Yeom J.S., Buchowski J.M., Shen H.X., et al. Effect of fibrin sealant on drain output and duration of hospitalization after multilevel anterior cervical fusion: a retrospective matched pair analysis. Spine (Phila Pa 1976). 2008;33:E543-E547.
54. Hastings D.E. A simple frame for operations on the lumbar spine. Can J Surg. 1969;12:251-253.
55. McNulty S.E., Weiss J., Azad S.S., et al. The effect of the prone position on venous pressure and blood loss during lumbar laminectomy. J Clin Anesth. 1992;4:220-225.
56. Blevins F.T., Shaw B., Valeri C.R., et al. Reinfusion of shed blood after orthopaedic procedures in children and adolescents. J Bone Joint Surg [Am]. 1993;75:363-371.
57. Ray J.M., Flynn J.C., Bierman A.H. Erythrocyte survival following intraoperative autotransfusion in spinal surgery: an in vivo comparative study and 5-year update. Spine (Phila Pa 1976). 1986;11:879-882.
58. Flynn J.C., Price C.T., Zink W.P. The third step of total autologous blood transfusion in scoliosis surgery: harvesting blood from the postoperative wound. Spine (Phila Pa 1976). 1991;16(Suppl 8):S328-S329.
59. Mann D.C., William M.R., Brower E.M., et al. Decreasing homologous blood transfusion in spinal surgery by use of the cell saver and predeposited blood. Spine (Phila Pa 1976). 1989;14:1296-1300.
60. Osborn J.J., Cohn K., Hait M. Hemolysis during perfusion: sources and means of reduction. J Thorac Cardiovasc Surg. 1961;43:459-464.
61. Brown M.D., Seltzer D.G. Perioperative care in lumbar spine surgery: review. Orthop Clin North Am. 1991;22:353-358.
62. Isselbacher K.J., Harrison T.R. Harrison’s principles of internal medicine, ed 13. New York: McGraw-Hill; 1995.
63. Raphael B.G., Lackner H., Engler G.L. Disseminated intravascular coagulation during surgery for scoliosis. Clin Orthop Relat Res. 1982;162:41-46.
64. Physicians’ desk reference, ed 59. Montvale, NJ: Thompson; 2005.
65. Malanga G.A., Nadler S.F., Lipetz J.S. Pharmacologic treatment of low back pain. Phys Med Rehabil STAR. 1999;13:531-549.
66. American Medical Association. AMA drug evaluations annual. Chicago: American Medical Association; 1991. p 113
67. Schiff M., Hochberg M., Oldenhof J., et al. Platelet inhibitory effects of OTC doses of naproxen sodium compared with prescription dose naproxen sodium and low-dose aspirin. Curr Med Res Opin. 2009;25:2471-2477.
68. Boehringer Ingelheim. Mobic (meloxicam) tablets prescribing information. Ridgefield, CT: Boehringer Ingelheim; July 10, 2006.
69. Osol A. Remington’s pharmaceutical sciences, ed 16. Easton, PA: Mack Publishing; 1980. p 768
70. Kearon C., Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336:1505-1507.
71. Korinth M., Gilsbach J., Weinzierl M. Low-dose aspirin before spinal surgery: results of a survey among neurosurgeons in Germany. Eur Spine J. 2007;16:365-372.
72. American College of Chest Physicians (ACCP). Evidence-based clinical practice guidelines for antithrombotic and thrombolytic therapy: 8th edition. Chest. 2008;133(Suppl 6):67S-968S.