CHAPTER 337 Blast-Induced Neurotrauma
Blast Injury is a Frequent Cause of Traumatic Brain Injury in the Modern Combat Theater
Blast injury is a common mechanism of traumatic brain injury (TBI) among soldiers serving in Iraq and Afghanistan. One study found that approximately two thirds of war zone casualties evacuated to Walter Reed Army Medical Center had injuries attributable to blast mechanisms.1 Another study found that 88% of military personnel treated at an echelon II medical unit in Iraq had been injured by improvised explosive devices (IEDs) or mortars, 47% of which involved head injuries.2 A third study reported that 97% of the injuries to marines in one unit in Iraq were due to explosions (65% IEDs, 32% mines). The majority of them (53%) involved the head or neck.3 Finally, between July and November 2003, the Defense and Veterans Brain Injury Center (DVBIC) screened 155 patients who had returned from Iraq and were deemed as being at risk for brain injury. Of the 88 victims of blast injuries included in the total number screened, 61% were identified as having sustained a brain injury.4 The DVBIC screening tool that has been used to screen soldiers returning from deployment for TBI is listed in Figure 337-1. Although penetrating TBI is typically identified and treated immediately, mild TBI may be missed, particularly in the presence of other more obvious injuries. Currently, however, the diagnosis of a blast concussive injury is based primarily on the characteristics of the injury event and not by the severity of symptoms after the trauma.
Blast Injury May Induce Changes in the Brain Not Seen With Non–Blast-Related Mechanisms
Blast-induced neurotrauma is the term given to describe an injury to the brain that occurs after exposure to a blast.5 Blast injury can be classified into five types:
* Blast injuries can be classified into one of five predominant treatment classes based on the acute events after the blast. Frequently, these injuries are characterized by more than one type of blast injury.
The blast is produced by a transient pressure wave in air or water after denotation of an explosive device. Rapidly expanding gas transfers mechanical, thermal, and electromagnetic energy into the surrounding medium. A typical blast wave is depicted in Figure 337-2. The amount of force exerted on the victim is not only determined by the peak value of the overpressure but is also a function of duration. Survival in the vicinity of a blast explosion is a function of the peak overpressure and range (Figure 337-3). Explosions in a contained space can increase the force of the blast waves as they are reflected off the walls. This creates a complex wave field in which overpressure waves can be additive.
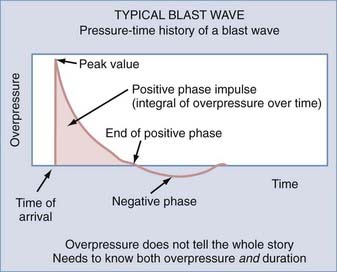
FIGURE 337-2 Typical blast wave that encompasses both overpressure and underpressure over a given duration.
(Courtesy of Colonel John B. Holcomb, U.S. Army Institute of Surgical Research.)
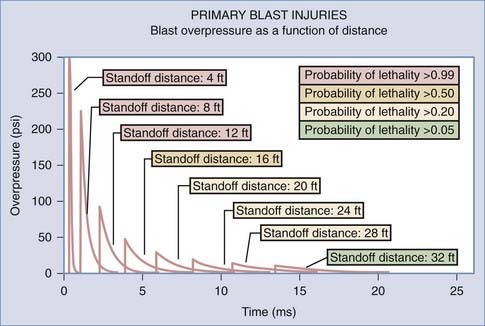
FIGURE 337-3 The magnitude of the primary blast injury inversely correlates with the distance from the blast.
(Courtesy of Colonel John B. Holcomb, U.S. Army Institute of Surgical Research.)
The precise mechanism of primary blast-induced neurotrauma remains unknown. Combat body armor provides soldiers with considerable protection against penetrating ballistic injury, yet it is unlikely to afford significant protection from the effects of blast overpressure. Clinical experience in the combat theater has shown that blast lung injury is less frequent than blast-induced neurotrauma. This is somewhat surprising because the lung is an air-filled organ and was therefore historically thought to be more susceptible to blast injury.6 The bowel, another air-filled organ, is also rarely injured after exposure to blast trauma. Therefore, the brain represents an organ that is uniquely vulnerable to blast-induced injury. The mechanism of blast-induced neurotrauma remains an area of active research.
Animal models are currently being developed to better understand the pathophysiology of blast injury.7,8 Gene expression profiling of animal models of blast injury to the brain shows that proteins involved in regulation of the cell cycle are differentially expressed after TBI.9 Astrocytes and neurons may enter the cell cycle again after TBI; scar formation is due to proliferating astrocytes, whereas neurons die on re-entering the cell cycle because of caspase-mediated apoptosis.10 The cell cycle inhibitor flavopiridol was found to decrease scar formation and promote cognitive recovery after fluid percussion injury in rats.11 Although blast-induced neurotrauma shares many similarities with axonal damage sustained by non–blast-involved mechanisms, there are also important differences.12 Blast-induced neurotrauma may be more likely to result in axonal swelling and edema and less likely to produce hemorrhage. The frequency of hemorrhage detected on head computed tomography (CT) among all civilian mild TBI cohorts typically ranges from 3% to 10%. Ongoing studies of mild TBI in Iraq and Afghanistan suggest that the percentage of positive CT scans is even lower than in the civilian setting (G. Grant, personal communication). This underscores the unique pathophysiology of blast injury to the brain.
primary Blast injury Biomarkers
Because the symptoms of primary blast injury are often not manifested until some time after the injury has occurred and the findings on imaging are usually normal, there has been a search for an objective test to verify the presence of primary blast-induced neurotrauma in the acute setting, such as a biomarker or neurocognitive screen.13 S100β has been the biomarker studied best in the setting of civilian TBI. One study showed that S100β levels correlate with the Marshall and colleagues’ CT classification of the severity of TBI.14,15 Other studies have likewise shown a relationship between the severity of head injury and levels of S100β.16–18 However, S100β was also found to be a poor predictor of outcome in mild TBI19 and has been shown to increase even in patients with long-bone fractures and no head injury. Its expression is not limited to the central nervous system (CNS); it is also expressed in the Schwann cells of peripheral nerves. Therefore, the usefulness of S100β as a biomarker to determine the outcome of blast-induced neurotrauma remains questionable, especially in the military setting, where polytrauma is much more common. Glial fibrillary acidic protein (GFAP) is expressed only in the CNS and therefore has recently received attention as a possible biomarker. Indeed, GFAP was found to be a predictor of mortality in TBI.18,20 Thus, the clinical applicability of GFAP to determine the outcome of primary blast-induced neurotrauma remains to be determined. Neuron-specific enolase is not used anymore as an acute biomarker of brain injury because of its expression in non-neuronal tissue, such as red blood cells and platelets, and its long half-life of 20 hours.21 A number of other proteins are currently under investigation as suitable biomarkers for TBI, such as cleaved-tau (c-tau), αII-spectrin breakdown products, N-methyl-D-aspartate receptor fragments, and neuroinflammatory cytokine markers. C-tau, however, has been shown to be a poor predictor of 3-month outcome after mild TBI,19 whereas the other proteins are currently being investigated for their suitability to measure outcome after mild TBI. In summary, no biomarker has proved reliable thus far in measuring the presence of mild TBI. Further research is needed to identify other marker proteins that can be used to predict the presence of mild TBI.
The ear is the organ that is most vulnerable to damage by blast overpressure. Rupture of the tympanic membrane (TM) is a sentinel finding of blast exposure and occurs at a relatively low pressure differential. In a study of 210 U.S. soldiers, the overall incidence of TM perforation and loss of consciousness was evaluated.22 There was a significant association between TM perforation and loss of consciousness (relative risk, 2.76; 95% confidence interval [CI], 1.91 to 3.97). The authors concluded that physicians treating blast-injured soldiers with TM perforation need to have a high index of suspicion for concomitant neurological injury. On the contrary, in a separate study of 167 soldiers who suffered a blast injury, TM perforation had low sensitivity (50%; 95% CI, 22% to 78%) and a specificity of 87% (95% CI, 81% to 92%) in detecting serious or occult primary blast injury and was therefore not a good biomarker. Furthermore, on the basis of the findings of this study, the absence of TM perforation does not appear to exclude other serious primary blast injury.23
Cognitive Sequelae of primary Blast-Induced Neurotrauma
Primary blast-induced neurotrauma results in impairment in the performance of neuropsychological tests such as the Trail-Making Test; the Wechsler Adult Intelligence Scale, third edition; the brief Visuospatial Memory Test—Revised; and the California Verbal Learning Test, second edition. Cognitive sequelae after the injury, however, were found to be determined by the severity of the injury and not by the mechanism of the injury.24 Thus, TBI from blast-induced neurotrauma does not seem to carry a unique signature of cognitive deficits when compared with other forms of TBI, such as blunt TBI from a motor vehicle accident. These detailed neuropsychological tests are not practical for acute evaluation in the combat zone. Therefore, decision making regarding return to combat becomes very complex for soldiers in the acute setting.
Differentiation between Posttraumatic Stress Disorder and Mild Traumatic Brain Injury
The military operations in Afghanistan and Iraq have shown that there is a significant overlap between posttraumatic stress disorder (PTSD) and mild TBI.25 Historically, these two clinical manifestations have not been linked because they were treated by different groups of health care professionals: PTSD by mental health professionals and TBI by neurosurgeons, neurologists, and physical therapy specialists. Mild TBI is characterized by an alteration in the level of consciousness or loss of consciousness for up to 30 minutes.26 PTSD is not diagnosed until at least 3 to 6 months later and is characterized by repeated, disturbing memories, thoughts, or images of a stressful experience, avoidance of certain activities or situations that are reminiscent of the stressful experience, and a general feeling of irritability.27 PTSD in reference to a specific event can be quantified with a 17-measure checklist, the PCL-M.28 Recent studies have confirmed a link between PTSD and mild TBI. Forty-four percent of U.S. soldiers returning from the Iraq War with mild TBI met the criteria for PTSD in one study29; mild TBI doubled the risk for PTSD in another study.30 Eighty-five percent of 94 combat veterans who tested positive for mild TBI by the Veterans Affairs TBI screening tool were found to have PTSD.31 Service members with a higher IQ at baseline (determined by the Armed Forces Qualifying Test) were found to be at less risk for the development of PTSD after exposure to trauma than were soldiers with lower cognitive ability.32 Functional neuroimaging studies show overactivity of the amygdala and insular cortex in patients with PTSD.33 In contrast, the orbital prefrontal cortex and subcortical white matter are particularly vulnerable in TBI. Although there are certain areas of overlap between TBI and PTSD, with common comorbid conditions such as depression, insomnia, irritability, trouble concentrating, fatigue, hyperarousal, and avoidance, both clinical conditions are distinct. The overlap in symptoms between the disorders, however, has made distinguishing them clinically challenging.
Current Neurosurgical Experience in Operation Iraqi Freedom
Within the combat theater, military neurosurgeons have learned to become very aggressive in neurosurgical resuscitation of victims of blast-induced neurotrauma. A summary of the initial management is given in the Guidelines for the Field Management of Combat-Related Head Trauma.34 Combat medics first protect the patient against further harm. Airway, breathing, and circulation are examined and treated if necessary. Once the patient has been stabilized, attention is turned to the patient’s Glasgow Coma Scale (GCS) score. If the GCS score is 13 or less, expeditious evacuation of the patient to higher echelons of care where a neurosurgeon is deployed is recommended.35
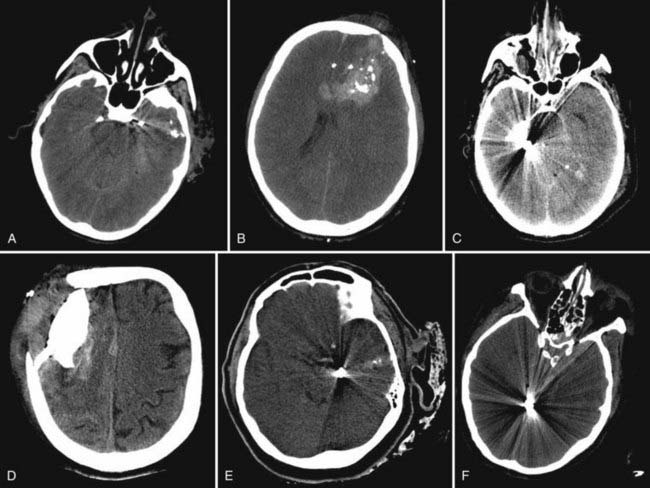
After the patient has been transferred to the hospital, a more detailed neurological examination should be performed. Extremely rapid aerial transport to a facility with neurosurgical capability may result in examination by a neurosurgeon within 1 to 2 hours of exposure to this high-energy trauma. The extremely high force of injury from a powerful blast creates a situation in which the patient may be rendered unconscious for a prolonged period. Pupillary examination is often difficult because of ocular trauma. In fact, in some instances both globes may be disrupted. Examples of CT scans in patients after secondary blast injuries from IEDs and gunshot wounds are depicted in Figure 337-4. Many of the predictors of outcome after penetrating civilian TBI such as transventricular injury and GCS score do not hold up in patients in a combat zone after secondary blast injury. The reason for this variance is multifactorial and includes the following factors: (1) inability to perform a reliable neurological assessment because of the sedative and paralytic medications used for helicopter transport, (2) lower velocity of IED fragments (secondary blast injury) than occurs with penetrating civilian gunshot wounds, or (3) some other yet undefined aspect of the pathophysiology of blast injury. Large craniectomies, intracranial pressure (ICP) monitoring during flight by critical care teams on sophisticated intensive care unit–equipped aircraft, and reassessment at each stop along the international evacuation route have provided the best possible care during a medical evacuation traversing a third of the globe.
Decompressive craniectomies have more commonly been performed in the military conflicts in Iraq and Afghanistan than in the civilian arena.36–38 In the military setting, wide decompression carries the benefit of reducing malignant elevations in ICP in the first several hours after the TBI. Head CT is frequently not indicative of the severity of blast injury to the brain and is not predictive of the degree of intracranial hypertension and brain edema. Decompressive craniectomies are also superior to barbiturate coma in the military setting because pharmacologic coma is not as feasible in the deployed environment and requires the use of continuous electroencephalographic monitoring and additional systemic support that is unavailable in the deployed setting. Increased ICP from blast injuries can also develop after the injury and may even occur in delayed fashion as late as 14 to 21 days after blast-induced TBI, a phenomenon that has been attributed to the delayed onset of vasospasm, which may respond to angioplasty.39 Acute elevations in ICP can be treated with 23% NaCl and continuous infusions of 3% NaCl to increase serum osmolarity,40 but mannitol is rarely used in view of the high incidence of polytrauma and risk for hypotension. Factor VIIa is also commonly used for intracranial hemorrhage in the combat arena after a blast because of the high risk for disseminated intravascular coagulation (DIC) resulting in consumptive coagulopathy in the setting of polytrauma.41,42 Mild hypothermia has not proved to be beneficial in the treatment of blunt head injury in the adult population and is not feasible in the combat zone.43
Long-term outcome studies of this patient group are pending, but our early impression is that in this subset of trauma patients, a low GCS score on initial evaluation may not predict an unsatisfactory outcome in a significant percentage of cases.44
Bazarian JJ, Zemlan FP, Mookerjee S, et al. Serum S-100β and cleaved-tau are poor predictors of long-term outcome after mild traumatic brain injury. Brain Inj. 2006;20:759-765.
Cernak I. Animal models of head trauma. NeuroRx. 2005;2:410-422.
Cernak I. Blast (explosion)-induced neurotrauma: a myth becomes reality. Restor Neurol Neurosci. 2005;23:139-140.
Cernak I, Stoica B, Byrnes KR, et al. Role of the cell cycle in the pathobiology of central nervous system trauma. Cell Cycle. 2005;4:1286-1293.
Cernak I, Wang Z, Jiang J, et al. Ultrastructural and functional characteristics of blast injury–induced neurotrauma. J Trauma. 2001;50:695-706.
Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556-563.
Di Giovanni S, Movsesyan V, Ahmed F, et al. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci U S A. 2005;102:8333-8338.
Harrison CD, Bebarta VS, Grant GA. Tympanic membrane perforation after combat blast exposure in Iraq: a poor biomarker of primary blast injury. J Trauma. 2009;67:210-211.
Hill JJ3rd, Mobo BHJr, Cullen MR. Separating deployment-related traumatic brain injury and posttraumatic stress disorder in veterans: preliminary findings from the Veterans Affairs Traumatic Brain Injury Screening Program. Am J Phys Med Rehabil. 2009;88:605-614.
Hoge CW, McGurk D, Thomas JL, et al. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358:453-463.
Knuth T, Letarte PB, Ling GSF, et al. Guidelines for the Field Management of Combat-Related Head Trauma. New York: Brain Trauma Foundation; 2005.
Ling G, Bandak F, Armonda R, et al. Explosive blast neurotrauma. J Neurotrauma. 2009;26:815-825.
Ling GS, Marshall SA. Management of traumatic brain injury in the intensive care unit. Neurol Clin. 2008;26:409-426. viii
Natale JE, Ahmed F, Cernak I, et al. Gene expression profile changes are commonly modulated across models and species after traumatic brain injury. J Neurotrauma. 2003;20:907-927.
Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043-2047.
Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol. 2008;167:1446-1452.
Stein MB, McAllister TW. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am J Psychiatry. 2009;166:768-776.
Svetlov SI, Larner SF, Kirk DR, et al. Biomarkers of blast-induced neurotrauma: profiling molecular and cellular mechanisms of blast brain injury. J Neurotrauma. 2009;26:913-921.
Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398-402.
Warden DL, French L. Traumatic brain injury in the war zone. N Engl J Med. 2005;353:633-634.
Xydakis MS, Bebarta VS, Harrison CD, et al. Tympanic-membrane perforation as a marker of concussive brain injury in Iraq. N Engl J Med. 2007;357:830-831.
1 Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398-402.
2 Murray CK, Reynolds JC, Schroeder JM, et al. Spectrum of care provided at an echelon II medical unit during operation Iraqi freedom. Mil Med. 2005;170:516-520.
3 Gondusky JS, Reiter MP. Protecting military convoys in Iraq: an examination of battle injuries sustained by a mechanized battalion during operation Iraqi freedom II. Mil Med. 2005;170:546-549.
4 Defense and Veterans Brain Injury Center. Available at http://www.Dvbic.Org/cms.Php?P=blast_injury Accessed 2009
5 Cernak I. Blast (explosion)-induced neurotrauma: a myth becomes reality. Restor Neurol Neurosci. 2005;23:139-140.
6 Bowen IG, Fletcher ER, Richmond DR, et al. Biophysical mechanisms and scaling procedures applicable in assessing responses of the thorax energized by air-blast overpressures or by nonpenetrating missiles. Ann N Y Acad Sci. 1968;152:122-146.
7 Cernak I. Animal models of head trauma. NeuroRx. 2005;2:410-422.
8 Cernak I, Wang Z, Jiang J, et al. Ultrastructural and functional characteristics of blast injury–induced neurotrauma. J Trauma. 2001;50:695-706.
9 Natale JE, Ahmed F, Cernak I, et al. Gene expression profile changes are commonly modulated across models and species after traumatic brain injury. J Neurotrauma. 2003;20:907-927.
10 Cernak I, Stoica B, Byrnes KR, et al. Role of the cell cycle in the pathobiology of central nervous system trauma. Cell Cycle. 2005;4:1286-1293.
11 Di Giovanni S, Movsesyan V, Ahmed F, et al. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci U S A. 2005;102:8333-8338.
12 Cernak I, Savic J, Malicevic Z, et al. Involvement of the central nervous system in the general response to pulmonary blast injury. J Trauma. 1996;40:S100-S104.
13 Svetlov SI, Larner SF, Kirk DR, et al. Biomarkers of blast-induced neurotrauma: profiling molecular and cellular mechanisms of blast brain injury. J Neurotrauma. 2009;26:913-921.
14 Marshall LF, Marshall SB, Klauber MR, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287-S292.
15 Korfias S, Stranjalis G, Boviatsis E, et al. Serum S-100B protein monitoring in patients with severe traumatic brain injury. Intensive Care Med. 2007;33:255-260.
16 Berger RP, Beers SR, Richichi R, et al. Serum biomarker concentrations and outcome after pediatric traumatic brain injury. J Neurotrauma. 2007;24:1793-1801.
17 Berger RP, Adelson PD, Pierce MC, et al. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J Neurosurg. 2005;103:61-68.
18 Pelinka LE, Kroepfl A, Leixnering M, et al. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J Neurotrauma. 2004;21:1553-1561.
19 Bazarian JJ, Zemlan FP, Mookerjee S, et al. Serum S-100B and cleaved-tau are poor predictors of long-term outcome after mild traumatic brain injury. Brain Inj. 2006;20:759-765.
20 Nylen K, Ost M, Csajbok LZ, et al. Increased serum-GFAP in patients with severe traumatic brain injury is related to outcome. J Neurol Sci. 2006;240:85-91.
21 Johnson P, Blomquist S, Luhrs C, et al. Neuron-specific enolase increases in plasma during and immediately after extracorporeal circulation. Ann Thorac Surg. 2000;69:750-754.
22 Xydakis MS, Bebarta VS, Harrison CD, et al. Tympanic-membrane perforation as a marker of concussive brain injury in Iraq. N Engl J Med. 2007;357:830-831.
23 Harrison CD, Bebarta VS, Grant GA. Tympanic membrane perforation after combat blast exposure in Iraq: a poor biomarker of primary blast injury. J Trauma. 2009;67:210-211.
24 Belanger HG, Curtiss G, Demery JA, et al. Factors moderating neuropsychological outcomes following mild traumatic brain injury: a meta-analysis. J Int Neuropsychol Soc. 2005;11:215-227.
25 Stein MB, McAllister TW. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am J Psychiatry. 2009;166:768-776.
26 Warden DL, French L. Traumatic brain injury in the war zone. N Engl J Med. 2005;353:633-634.
27 Boals A, Schuettler D. PTSD symptoms in response to traumatic and non-traumatic events: the role of respondent perception and A2 criterion. J Anxiety Disord. 2009;23:458-462.
28 Blanchard EB, Jones-Alexander J, Buckley TC, et al. Psychometric properties of the PTSD checklist (PCL). Behav Res Ther. 1996;34:669-673.
29 Hoge CW, McGurk D, Thomas JL, et al. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358:453-463.
30 Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol. 2008;167:1446-1452.
31 Hill JJ3rd, Mobo BHJr, Cullen MR. Separating deployment-related traumatic brain injury and posttraumatic stress disorder in veterans: preliminary findings from the Veterans Affairs Traumatic Brain Injury Screening Program. Am J Phys Med Rehabil. 2009;88:605-614.
32 Kremen WS, Koenen KC, Boake C, et al. Pretrauma cognitive ability and risk for posttraumatic stress disorder: a twin study. Arch Gen Psychiatry. 2007;64:361-368.
33 Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry. 2006;60:376-382.
34 Knuth T, Letarte PB, Ling GSF, et al. Guidelines for the Field Management of Combat-Related Head Trauma. New York: Brain Trauma Foundation; 2005.
35 Ling G, Bandak F, Armonda R, et al. Explosive blast neurotrauma. J Neurotrauma. 2009;26:815-825.
36 Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043-2047.
37 Ling GS, Marshall SA. Management of traumatic brain injury in the intensive care unit. Neurol Clin. 2008;26:409-426. viii
38 Schlifka B. Lessons learned from OIF: a neurosurgical perspective. J Trauma. 2007;62:S103-S104.
39 Armonda RA, Bell RS, Vo AH, et al. Wartime traumatic cerebral vasospasm: recent review of combat casualties. Neurosurgery. 2006;59:1215-1225.
40 Qureshi AI, Suarez JI. Use of hypertonic saline solutions in treatment of cerebral edema and intracranial hypertension. Crit Care Med. 2000;28:3301-3313.
41 Holcomb JB. Use of recombinant activated factor VII to treat the acquired coagulopathy of trauma. J Trauma. 2005;58:1298-1303.
42 Holcomb JB, Hoots K, Moore FA. Treatment of an acquired coagulopathy with recombinant activated factor VII in a damage-control patient. Mil Med. 2005;170:287-290.
43 Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556-563.
44 Huang J, Johannigman, JA, Clatterbuck, RE. Blast-induced traumatic brain injury: a review of current neurosurgical experience in operation Iraqi freedom. Presented at the 2008 Annual Meeting of the Congress of Neurological Surgeons, Orlando, FL, 2008.