Chapter 26 Benign Brain Tumors
Meningiomas and Vestibular Schwannomas
Michael Chan and C. Leland Rogers
Harvey Cushing first used the term meningioma to describe tumors originating predominately from the meningeal coverings of the brain and spinal cord. Although the term does not disclose a cell of origin, it does aptly describe a range of clinically comparable histologic patterns and has endured. Though meningiomas are often approached as benign tumors, studies with long-term follow-up reveal that they are prone to infiltrate locally and to recur.1 This chapter will consider the available data on intracranial meningiomas in a succinct fashion. For a more detailed review and lengthier bibliography, readers can consult the online version of this chapter.![]()
Etiology and Epidemiology
Based upon surgical series, approximately 8000 meningiomas are diagnosed each year in the United States. Radiographic and autopsy studies suggest an even greater number of patients with clinically occult tumors.2 A recent epidemiologic study revealed that meningiomas are the most frequently reported primary intracranial neoplasm, constituting approximately 30% of all brain primary tumors.3
The likelihood of developing a meningioma is proportional to age. Pediatric meningiomas are rare but are more likely to exhibit an aggressive clinical course.4 Meningiomas are most often diagnosed during the sixth to seventh decades of life. However, the age-specific incidence continues to rise thereafter, even beyond 85 years of age.3
Although there are known associations with certain genetic, environmental, and hormonal risk factors, most meningiomas arise without discernible causation. Genetic factors will be discussed in an ensuing section on Biologic Characteristics and Molecular Biology. Radiation exposure—stemming largely from studies of atomic bomb fallout but also from studies of cranial and scalp irradiation for tinea capitis—is a well-described environmental etiologic factor for meningiomas. Indeed, radiation-induced meningiomas are the most commonly reported secondary neoplasm.2
A role for sex hormones in meningioma induction is supported by several findings. Meningiomas occur more frequently in females, at a ratio of 2 or 3:1.2 The incidence appears to increase with hormone replacement therapy or long-acting oral contraceptive use and in obese individuals. Moreover, tumor size or symptoms may worsen during the luteal phase of menses or pregnancy. In spite of these observations, the precise role of hormones is unclear. In both sexes, more than 70% of meningiomas express progesterone receptors, and up to 40% estrogen receptors, but nearly 40% express androgen receptors as well.2,5
Biologic Characteristics and Molecular Biology
Meningiomas occur more frequently in certain rare genetic conditions, such as type 2 neurofibromatosis (NF2).4 Mutation in the NF2 gene on chromosome 22q12 is the most common cytogenetic alteration. Nearly all NF2 meningiomas have mutations of the NF2 gene, and most susceptible families have alterations of the NF2 locus.6 Genetic losses of chromosomes 1p, 10, and 14q have been linked with malignant progression or recurrence but have not yet been validated as independent prognostic markers.2,6 Markers of tumor aggressiveness have been studied. MIB-1 expression has been correlated with time to recurrence in some series7 but not in others.8
Pathology and Pathways of Spread
The World Health Organization (WHO) recently published updated grading criteria.5 This system describes 3 grades and 13 histologic subtypes of meningioma (Table 26-1). Strong associations between grade, relapse-free survival (RFS), and overall survival (OS) have been confirmed,5 but even with improved criteria there remain disparities, particularly regarding the proportion of patients with grade II (atypical) histology. A recent large analysis reported that 5% of meningiomas were atypical.3 However, Perry and associates5 found that over 20% of meningiomas were WHO grade II tumors.3 Willis and colleagues9 re-graded patients using modern WHO guidelines and reported that 20.4% were atypical. Another recent analysis found that, whereas 4.4% of meningiomas were categorized as atypical from 1994 to 1999, this percentage has steadily increased to 32.7% to 35.5% since 2004.10
TABLE 26-1 The 2007 World Health Organization (WHO) Criteria for Meningioma Grading
| Grade I (Benign Tumor) | Grade II (Atypical Tumor) | Grade III (Anaplastic or Malignant Tumor) |
|---|---|---|
hpf, high-power field.
Modified from Perry A, Louis DN, Scheithauer BW, et al: Meningeal tumours. In Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds): WHO Classification of Tumours of the Central Nervous System. Lyon, IARC 2007. Modified with the kind assistance of Arie Perry.
The WHO grade is a dominant prognostic factor. Compared with a grade I meningioma, a grade II tumor carries a sevenfold to eightfold increased recurrence risk at 3 to 5 years. Grade III meningiomas are even more aggressive, with a 5-year OS of 32% to 64%.11,12,13,14
Clinical Manifestations, Patient Evaluation, and Staging
Most data regarding the diagnosis and treatment of meningiomas are based on surgical series. As such, there is an inherent bias toward symptomatic tumors. Symptoms depend largely on the location of the lesion but can be influenced considerably by the presence of edema. Skull base meningiomas can present with cranial nerve palsies or neuropathies. Sphenoid wing meningiomas may present with seizures.15
With the increasing use of contrast-enhanced CT and MRI scanning for head trauma, headaches, and so on, the number of incidentally discovered meningiomas has risen. Incidentally discovered meningiomas are often smaller and may show little growth over time.16Although the clinical behavior of incidental meningiomas is not uniformly predictable, younger age and larger size at detection portend increased progression risk.16
Contrast-enhanced MRI is the imaging modality of choice for meningiomas. Tumors at the skull base may also be imaged with CT to evaluate bony invasion, involvement of skull base foramina, or hyperostosis. Additionally, CT may identify calcifications, a finding predictive of more indolent growth.2 MRI is generally superior for visualization of the contrast-enhanced lesion, and MRI T2 signal changes may presage more aggressive behavior.2
Primary Therapy
Tumors with Benign Histologic Findings (WHO Grade I Tumors)
Surgery
Surgery remains the standard of care, and the extent of surgical resection has been correlated with rates of tumor recurrence. In 1957 Simpson reported a series of 265 patients treated surgically, giving rise to the standard system used to describe the extent of resection.1 The Simpson grade of resection and the associated crude recurrence rates are depicted in Table 26-2. Modern series employing current surgical and imaging techniques have validated this association.17
TABLE 26-2 Simpson’s Definitions of “Five Distinct Grades of Operation,” with Respective Recurrence Risk*
| Grade | Definition of Resection Extent | Recurrence |
|---|---|---|
| I | Macroscopically complete removal of tumor, with excision of its dural attachments and any abnormal bone | 9% |
| II | Macroscopically complete removal of tumor, with coagulation of its dural attachments | 19% |
| III | Macroscopically complete removal of tumor, without resection or coagulation of dural attachments or of extradural extensions (e.g., invaded sinus or hyperostotic bone) | 29% |
| IV | Partial removal, leaving tumor in situ | 44% |
| V | Simple decompression or biopsy | N/A |
N/A, not applicable.
* Recurrences were identified “in a purely clinical sense to imply the reappearance of symptoms.” Dr. Simpson calculated recurrence risk in a crude fashion, often excluding patients who had had surgery within the prior 5 years.
Modified from Simpson D: J Neurol Neurosurg Psychiatry 20:22-39, 1957.
Postoperative Radiotherapy
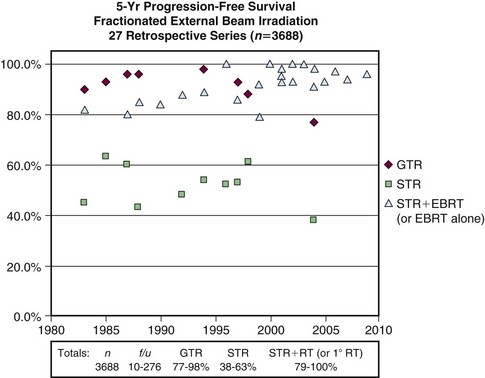
Adjuvant radiotherapy is not recommended following gross total resection of a newly diagnosed grade I meningioma. Radiotherapy has often been used after subtotal resection, although considering the absence of randomized trials, differences in clinical practice are to be expected. Many patients are observed after subtotal resection. However, a multitude of retrospective series have reported that radiotherapy improves local control after subtotal resection. Figure 26-1 illustrates progression-free survival (PFS) outcomes from 27 series with long-term results following gross total resection alone, subtotal resection alone, and subtotal resection with external beam irradiation (EBRT).2,18 Some of the series, including many of the recent reports, include patients treated with primary radiotherapy.
Goldsmith and colleagues11 reported a series of 140 patients who received radiotherapy following subtotal resection and identified a dose response. Benign subtotally resected tumors had significantly improved rates of PFS (93% vs. 65% at 10 years) when a dose greater than 52 Gy was delivered. Moreover, the researchers found significantly improved outcome with CT- or MRI-based treatment planning11 (Fig. 26-2). Other studies evaluating radiotherapy after subtotal resection have exhibited superior cause-specific and possibly even OS.19,20
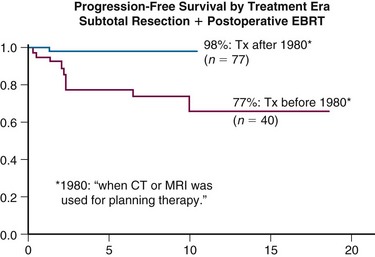
Figure 26-2 Progression-free survival rates of meningioma patients treated with external beam radiotherapy based on treatment era. Treatment planning techniques changed after 1980 with the advent of CT and MRI. Local control has likely improved secondary to better targeting of the tumor.5
Recurrent meningiomas, whether benign or atypical, display a considerably higher rate of recurrence after surgery alone than newly diagnosed tumors.19,20,21 In this setting, postoperative radiotherapy decreases the rate of tumor progression.20,21 In a series by Taylor and associates,20 the local control benefit of postoperative radiotherapy at the time of first recurrence (88% vs. 30% at 5 years) translated into an OS benefit (90% vs. 45% at 5 years).20
Definitive External Beam Radiation Therapy
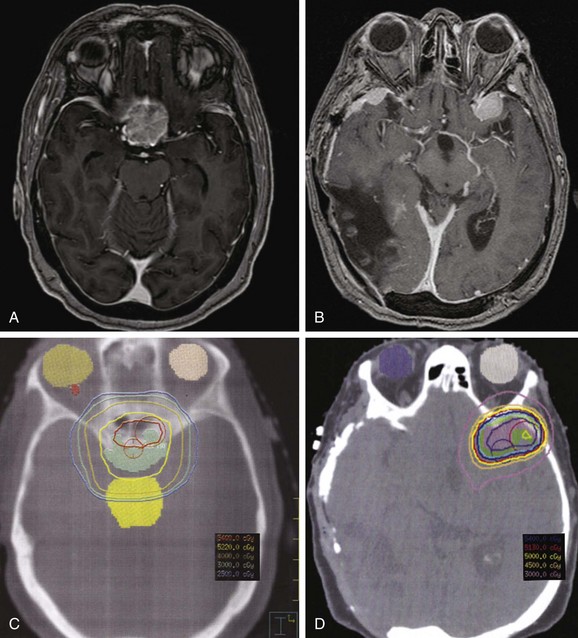
Early reports of primary radiotherapy revealed inferior results, with local control rates on the order of 47%.22 However, these reports included patients treated in the 1960s and 1970s, before the advent of current imaging and treatment planning paradigms. Many recent series using conformal techniques have corroborated excellent results from definitive radiotherapy with local control in excess of 90% at 5 to 10 years.2,18,23,24 Total doses have ranged from 45 Gy18 to 57.6 Gy,25 typically in 1.8- to 2-Gy fractions. The higher cumulative doses were predominately for higher-grade, large, or recurrent meningiomas. For most patients, modern imaging-based planning and treatment with doses in the range of 50 to 54 Gy with standard fractionation have produced excellent results. Figure 26-3 depicts two examples of tumors appropriate for definitive EBRT.
Optic Nerve Sheath Meningioma
Optic nerve sheath meningiomas represent only 1% to 2% of meningiomas2 but pose considerable clinical challenges owing to their intimate association with the optic nerve and its vasculature. Historically, treatments included resection or observation, both leaving patients with poor functional outcomes. On this account, fractionated radiation therapy is being increasingly used.
Turbin and colleagues26 reported 64 patients managed with surgery alone, surgery plus radiotherapy, radiotherapy alone, or observation. They found that radiotherapy alone resulted in excellent tumor control and was the only modality not leading to worsening of visual acuity. Other series have confirmed excellent outcomes, with stabilization or improvement in visual acuity in up to 90% of patients, with local control exceeding 90%.2,21 Doses of 40 to 54 Gy with fractions of 1.6 to 1.8 Gy have been standard and have produced results more favorable than observation, surgery alone, or surgery plus irradiation.2,26,27
Stereotactic Radiosurgery
Over the past two decades, radiosurgery has become an acceptable and frequently used modality and is generally considered suitable for meningiomas of less than 3 to 4 cm in maximum diameter, with distinct margins, with little or no surrounding edema, and situated at a sufficient distance from critical normal tissues to permit appropriate dose restrictions.17,28 Long-term local control has exceeded 85% in the majority of studies.2
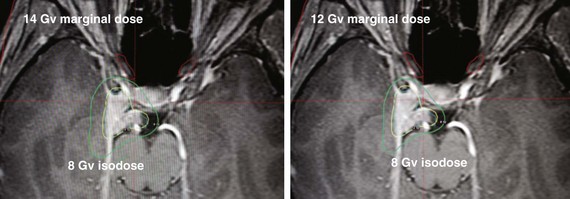
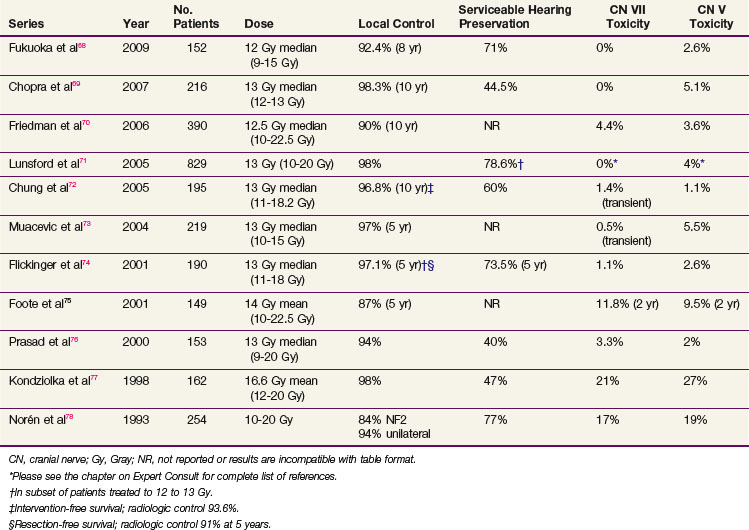
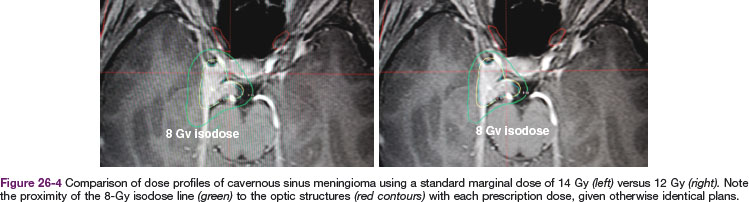
Table 26-3 compares the outcomes for radiosurgery with those of definitive EBRT; this is not a direct comparison but is a compilation of retrospective series reported in the literature and is therefore inherently biased in terms of patient selection. The University of Pittsburgh’s initial publication of long-term outcomes used a median marginal dose of 16 Gy.28 The rate of new neurologic toxicity was 5%. Since this report, it has become evident that lower single doses may be sufficient. In the University of Pittsburgh’s recent update29 of 972 patients, the median marginal dose was 14 Gy. Other reports have shown good local control with doses of 12 Gy. Figure 26-4 illustrates the potential dosimetric advantages of lower marginal doses.
TABLE 26-3 Comparative Analysis of Results with SRS and EBRT by Grade of Meningioma in Multiple Retrospective Series*
Tumor volume has also been shown to be a predictor for success with radiosurgery. DiBiase and colleagues30 reported 5-year disease-free intervals of 91.9% for patients with tumors of 10 cc or smaller versus 68% for larger tumors. Kondziolka and associates28 similarly cited a decreased control rate with larger tumors.
Tumors in Higher-Risk Patients (WHO Grades II and III Tumors)
Tumors with Atypical Histology (WHO Grade II Tumors)
Considerable controversy exists regarding the proper treatment paradigms for grade II meningiomas. These tumors carry considerably higher recurrence risk.19 Although grade II tumors are often treated with combined surgery and postoperative irradiation, this has not been universally adopted, particularly following gross total resection.13
After an incomplete resection, postoperative radiotherapy is typically recommended. This can be either fractionated EBRT or radiosurgery. A relatively higher dose, as compared with the dose for grade I tumors, may be advisable. One study demonstrated improved local control with doses exceeding 53 Gy.11 A recent analysis of combined photon and proton therapy for atypical and anaplastic meningiomas found improved cause-specific (CSS) and (OS) with a total dose exceeding 60 CGE (cobalt Gy equivalent).31
Radiosurgery may also be considered for atypical tumors.32,33 Hakim and colleagues,33 using a median marginal dose of 15 Gy, achieved a 4-year local control rate of 83%. However, caution is to be recommended. Although control of disease within the radiosurgical volume has been acceptable, marginal failures remain problematic.32 Radiosurgery may best be reserved for less aggressive meningiomas or for salvage.
Analyzing 108 atypical meningioma patients after Simpson grade I resection, Aghi and colleagues34 reported a 5-year recurrence rate of 41% following gross total resection alone. Recurrences led to numerous reoperations and to worsened survival rates. Eight of these patients received postoperative fractionated radiotherapy, to a mean dose of 60.2 Gy to the resection bed with a 1-cm margin. Among this small cohort of patients there were no recurrences.34
Tumors with Malignant Histology (WHO Grade III Tumors)
Five-year recurrence rates in two large surgical series ranged from 72% to 78%.14,35 Overall survival rates at 5 years ranged from 32% to 64%. Failure of local tumor control is often a cause of death in patients with these tumors.12,13
Patients with WHO grade III tumors are frequently treated with postoperative radiotherapy; however, given the relative rarity of grade III tumors, this has not been explicitly tested in a clinical trial. A recent series from the Cleveland Clinic reported 13 patients over 23 years, 3 of whom received postoperative irradiation. The report suggested that postoperative radiotherapy improved median survival (5.4 vs. 3.4 years, p = .13) over surgery alone.36 In a publication by Dziuk and associates,37 19 of 38 patients received initial postoperative radiation therapy and experienced significant improvement in 5-year disease-free survival (DFS) rates (80% vs. 15% for surgery alone; p = .002).37 As with grade II tumors, there appears to be a dose response with grade III meningiomas.12,31,38 Boskos and associates31 reported improved OS with doses exceeding 60 Gy and a trend toward further improvement beyond 65 Gy. Nevertheless, caution must be used with aggressive dose escalation. One report of accelerated hyperfractionation revealed a 55% rate of grade 3 to 5 toxicities.
Systemic Therapy
Systemic agents have not had a significant impact on the general management of meningioma to date. Several classes of agents have been evaluated, including hormonal therapy agents, chemotherapy agents, immune modulators, and targeted agents. Hydroxyurea has been used for several years, but radiographic responses are uncommon, and rates of progression for atypical and malignant histologic types have remained high.39
Because a majority of meningiomas express progesterone receptors, a randomized trial of the antiprogestin mifepristone versus placebo was conducted. Mifepristone resulted in increased toxicity but no improvement in response.40 Several recent trials employing novel growth factor agents have been disappointing as well.41 There is a critical need for new approaches to patients who have anaplastic tumors, have failed multiple local therapies, or have diffuse and progressive meningiomatosis. This remains a challenge for future investigation.
Irradiation Techniques and Tolerance
Several methods of target delineation have been described, and there is controversy concerning the optimal method to account for microscopic spread along dura and bone and into the brain. With EBRT, margins as large as 2 cm beyond the gross tumor volume (GTV) have been described using modern imaging.19 However, margins as small as 1 to 2 mm in fractionated stereotactic radiotherapy series have produced good long-term outcome (although the follow-up in such series is shorter, and the patients selected may be inherently different).23,24 Such tight margins may not be safe for meningiomas beyond grade I. Recent reports of radiosurgery for atypical meningiomas have found that increased conformality predicted for a higher rate of local failure.31
Targeting of the dural tail within the clinical target volume (CTV) is also controversial. Surgical series have found that the dural tail is composed almost entirely of hypervascular dura, and is typically an imaging finding, not routinely visualized at surgery.2 The dural tail is not included in the Simpson resection grading scale, and the results of surgery alone have largely been obtained without specific attention to it. Furthermore, radiosurgical series have generally not targeted the dural tail.2,28,42
Inclusion of hyperostotic bone within the CTV is also worthy of discussion. A Simpson grade 1 resection includes gross total resection of tumor, its dural attachments, and any abnormal bone.1 Biopsy series have demonstrated a significant rate of bone involvement by meningioma in cases of hyperostosis,43 and this is associated with a higher rate of recurrence.44 This question needs to be addressed more thoroughly, but it is clearly justifiable to target hyperostotic bone within the treatment volume when this can be accomplished safely.
Toxicities of EBRT
The toxicities associated with EBRT are commonly related to the location. Many patients experience fatigue and some develop alopecia. Late toxicities include cataract formation, cranial nerve palsy or neuropathy, and panhypopituitarism. Rarely, patients experience symptomatic radionecrosis and may require long-term steroid therapy or surgical intervention.11 Debus and colleagues23 reported a series of 189 patients who received radiotherapy with modern fractionation schedules and treatment planning and delivery techniques. The rate of grade 3 toxicities was 2.2%; it was even lower in patients with no preexisting deficit.
Cognitive decline remains a possible late effect. Like most other sequelae, it has site-specific dose-volume relationships. Temporal lobe dose predicts for cognitive decline,45 and large treatment volumes are more likely to lead to symptomatic decline.46 The likelihood of radiation-induced cognitive loss rises over time after brain radiotherapy. Cavernous sinus meningiomas, located just medial to the hippocampus, and sphenoid wing meningiomas, lateral to the hippocampus, may carry a higher risk of cognitive decline.
Toxicities of SRS
The nature of the toxicities of SRS is also dependent on tumor size and location, and most toxicities are related to damage to nerves or to radiation-induced edema. Sensory nerves, such as the optic nerve, are more susceptible to injury than motor nerves. Fraction sizes of 10 Gy or less to the anterior visual pathway carry a 1% to 2% rate of optic neuropathy, but rates rise quickly at doses higher than 10 Gy.42
Edema is a more common complication with single-fraction radiosurgery than with standard fractionation.2,47 Several risk factors have been identified for radiosurgery-induced edema, including higher marginal dose, tumor size greater than 4 cm, presence of pretreatment edema, and periventricular or parasagittal location.2,28,48 A recent series found that parasagittal meningiomas had a greater than fourfold increased risk of peritumoral edema after radiosurgery.49
Treatment Algorithm, Controversies, and Clinical Trials
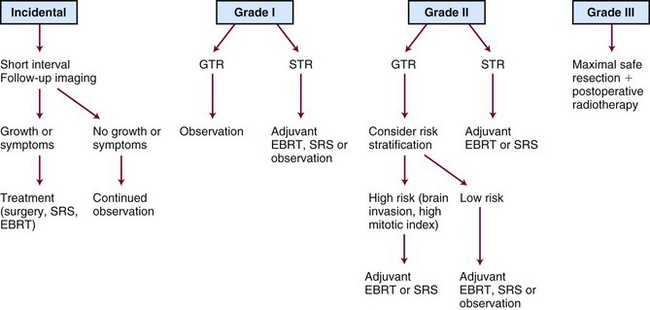
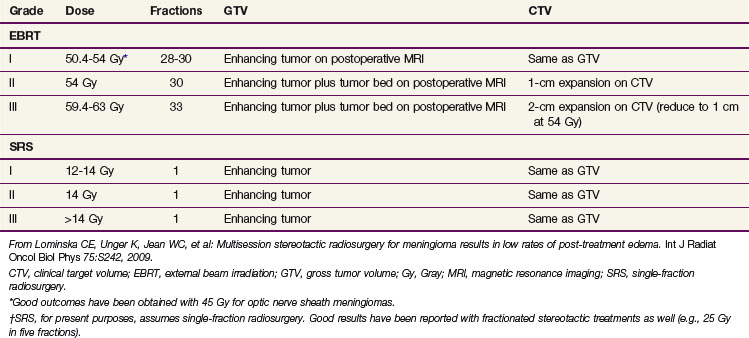
A treatment algorithm is presented in Figure 26-5. Corresponding dose and treatment volume guidelines are contained in Table 26-4. These depict a literature-based approach defined by risk strata. However, many controversies persist. There is substantial debate concerning which patients to observe, which to treat with primary fractionated radiotherapy or radiosurgery rather than surgery, and which to select for postoperative radiotherapy. Gross total resection of a benign (WHO grade I) meningioma is considered definitive therapy, but there is less agreement about this following subtotal resection of a WHO grade I tumor or after gross total resection of a grade II meningioma. Subtotally resected grade II meningiomas are frequently managed with irradiation, and all grade III meningiomas demand aggressive treatment, irrespective of resection extent. Despite aggressive management, however, current approaches are insufficient for many high-risk patients. New systemic and biologic treatments are needed.
Contemporary Trials
Bethany M. Anderson and Deepak Khuntia
Etiology and Epidemiology
The incidence of vestibular schwannoma has been estimated at 0.6 to 0.8 per 100,000 person-years, and is increasing, at least in part because of the more widespread use of intracranial imaging with CT and MRI and a controversially claimed association with cellular telephone usage, although there is no convincing evidence for this.50 Roughly 85% to 90% of cerebellopontine angle tumors are vestibular schwannomas. Vestibular schwannomas are identified on MRI in 0.2% of asymptomatic patients.51 Some cases can be attributed to genetic syndromes such as NF2, which pathognomonically presents with bilateral vestibular schwannomas. Over 90% of cases, however, are sporadic and unilateral. Case-control series suggest a potential correlation between vestibular schwannoma and cell phone usage—although these data remain highly controversial52—or occupational exposure to loud noise,53 also an association mired in controversy. The median age of patients at diagnosis is 50 years; however, patients with NF2 typically manifest symptoms by age 20 to 30 years.
Biologic Characteristics
Biallelic inactivating mutations of the tumor suppressor gene NF2, located on chromosome 22q12, are commonly found in both sporadic and NF2-associated vestibular schwannomas.54 NF2 encodes the cytoskeletal protein merlin, which functions in embryonic development, cell adherence, and cell proliferation. Merlin as a growth suppressor is activated when it is dephosphorylated by MYPT-1, which in turn can be inactivated by CPI-17, an up-regulated gene in humans. Multiple pathways, including Ras, Raf, MEK, and ERK, can be blocked via merlin as opposed to normally being activated by growth factors such as insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF). Merlin is also able to inhibit ErbB2 by binding to CD44, a known promoter of tumorigenesis. As a result, merlin may become a novel target for therapy.55
Pathology and Pathways of Spread
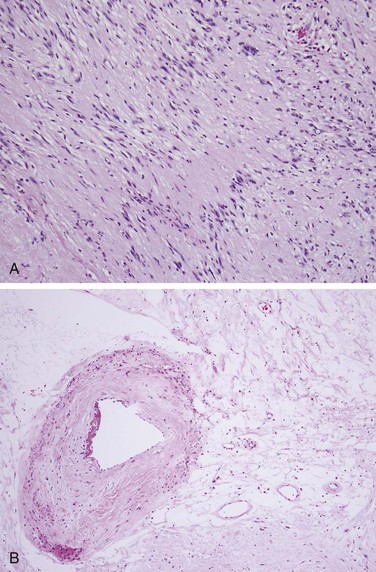
Vestibular schwannomas are histologically similar to schwannomas arising from other cranial and peripheral nerves. Their histologic appearance is characterized by alternating zones of dense and sparse cellularity, known as Antoni A and B areas (Fig. 26-6).
The natural history of vestibular schwannoma is typically characterized by gradually progressive growth with gradual sensorineural hearing loss and potentially other cranial nerve deficits. A subset of patients can present with sudden hearing loss. Less than 5% of tumors may regress slightly with surveillance.56 The average growth rate has been estimated at 1 to 2 mm per year for sporadic lesions56,57 and 3 mm per year for patients with NF2-associated lesions.58 Tumor size and growth rate do not consistently correlate with hearing loss, however. Cystic schwannoma is a well-recognized tumor subtype that tends to behave more aggressively, invading and splaying adjacent cranial nerves. It is rare for malignant degeneration to occur.
Clinical Manifestations, Patient Evaluation, and Staging
Vestibular schwannoma is increasingly detected as an incidental finding on MRI or CT performed for other reasons. MRI is the imaging method of choice for visualizing vestibular schwannomas, which typically appear as contrast-enhancing lesions originating within the internal auditory canal on T1-weighted MRI images (Fig. 26-7A and B). The extent of tumor may be described using the Koos grading system (Table 26-5).
| Stage I | Small intracanalicular tumor |
| Stage II | Small intracanalicular tumor with extension into cerebellopontine angle |
| Stage III | Larger tumor occupying cerebellopontine cistern without brainstem displacement |
| Stage IV | Extremely large tumor with marked displacement of brainstem and cranial nerves |
Adapted from Koos WT, Day JD, Matula C, Levy DI: Neurotopographic considerations in the microsurgical treatment of small acoustic neuromas, J Neurosurg 88(3):506-512, 1998.
Symptoms associated with vestibular schwannoma are related to the tumor’s size and location with respect to nearby critical structures. When tumors are symptomatic, patients may complain of hearing loss (95% objective, 66% subjective), tinnitus (63%), imbalance or vertigo (61%), facial numbness or pain (17%), facial paresis or taste disturbance (6%), other cranial nerve deficits, or cerebellar dysfunction.59
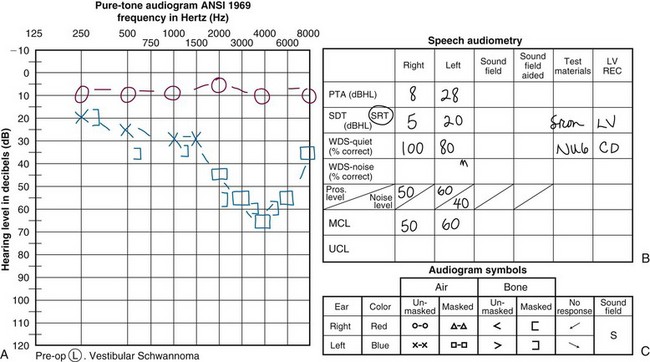
Sensorineural hearing loss classically affects high frequencies preferentially and may therefore be mistaken for noise- or age-related hearing loss. An example of a typical patient’s audiogram is shown in Figure 26-8, along with the two mainstream classification systems for audiogram findings specific to patients with vestibular schwannoma, namely the Gardner-Robertson60 and American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS)61 methods. As a rule of thumb, patients whose speech discrimination score is 50% or less at 50 dB or more may not benefit from an approach focused on the preservation of hearing.62 Each case must be considered individually, however, particularly in patients with bilateral vestibular schwannomas or other causes of contralateral hearing compromise.
Facial nerve function is characterized clinically by the House-Brackmann grading scale (Table 26-6). When clinically indicated, additional studies may be performed, such as facial nerve electromyography, caloric testing, or electronystagmography (Table 26-7).
TABLE 26-6 House-Brackmann Facial Nerve Grading Scale
| Grade | Description | Characteristics |
|---|---|---|
| I | Normal | Normal facial function in all areas |
| II | Mild dysfunction |
From House JW, Brackmann DE, Facial nerve grading system. Otolaryngol Head Neck Surg 93(2):146-147, 1985.
TABLE 26-7 Approach to Diagnosis and Surveillance of Vestibular Schwannoma
| Initial Evaluation |
| Follow-up/Surveillance |
Primary Therapy
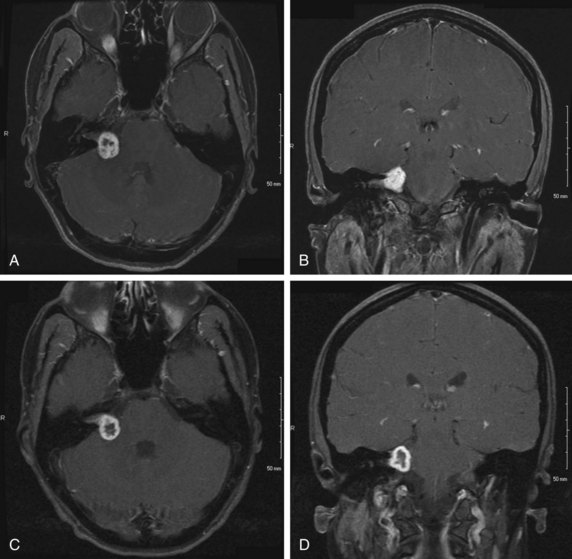
There have been no true randomized, controlled trials published about patients with vestibular schwannoma, and interpretation of single-institution series is limited by differences in factors such as patient selection, treatment techniques, and methods of assessing and reporting tumor control and treatment or tumor-related neurologic deficits. For example, local control may be determined radiographically as a lack of tumor growth, a sign of progressive tumor growth, or a sign of need for further intervention. The natural history of vestibular schwannoma following radiation therapy may be characterized by a transient increase in size,63 often with a necrotic-appearing center (Fig. 26-7C and D), followed by stability or regression. This type of MRI finding may confound the results of some published SRS series, particularly in early years before this phenomenon was recognized. Hearing deficits are also analyzed in multiple different fashions, including subjectively (typically by querying patients about their ability to use the telephone with the affected ear) or using objective audiometric data. Facial function is typically scored using the House-Brackmann system (see Table 26-6), but investigators differ in their method of defining “significant” facial and other cranial nerve toxicity. Outcomes may also be reported as crude or actuarial rates, adding to the heterogeneity of published case series.
Single-Modality Therapy
Surgery
Resection of vestibular schwannoma has been performed since 1894; it can be accomplished through a suboccipital (retrosigmoid), middle fossa, or translabyrinthine approach (Table 26-8). The suboccipital approach is feasible for tumors of any size but poses risk for craniospinal fluid leak and headache. The middle fossa approach is most suitable for tumors measuring less than 1.5 to 2 cm in size and has the risk for damage to the seventh cranial nerve. Hearing preservation can be attempted with either of these two approaches, although the fundus of the internal auditory canal is incompletely visualized, which can increase the likelihood for incomplete resection. The translabyrinthine approach can be performed for tumors of any size and eliminates this risk but results in inevitable sacrifice of hearing. Recurrence after gross total resection is uncommon.
| Surgical Approach* | Indications | Advantages and Disadvantages |
|---|---|---|
| Suboccipital (retrosigmoid) | Tumor of any size with attempted hearing preservation |
* The three major approaches are listed along with their general indications, advantages, and disadvantages.
With suboccipital and middle fossa approaches, published hearing preservation rates for vestibular schwannoma range from 20% to 71%.64 Factors associated with increased preservation of functional hearing include better preoperative hearing, shorter intra-aural and absolute wave V latency on preoperative auditory brainstem response testing, and superior vestibular nerve origin.64 Better preoperative hearing measured by audiogram and smaller tumor size predict for hearing preservation in some studies but not in others.64,65,66
Other potential side effects of surgery include craniospinal fluid leakage, facial nerve injury, headache, and meningitis.62 Preservation of facial nerve function may be more likely for smaller tumors (i.e., <2 to 3 cm) and with increasing surgical experience.67
Stereotactic Radiosurgery
Several large, single-institution experiences with SRS for vestibular schwannoma have been reported (Table 26-9). Using modern techniques and doses, as detailed below, local control rates with SRS are generally greater than 90% and significant cranial nerve toxicity rates are less than 10%.
Published hearing preservation rates are variable; 57% overall in a recent systemic review of patients treated with an average dose of 16 Gy.79 Proposed mechanisms of hearing loss following SRS (in the absence of progressive tumor growth) include transient conductive hearing loss resulting from serous otitis media and delayed sensorineural hearing loss because of direct radiation injury to the vestibulocochlear nerve or cochlea, compression of the vestibulocochlear nerve or internal auditory artery from tumor edema, or thrombosis of the internal auditory artery. Patient-related factors that may correlate with increased likelihood of hearing preservation after SRS include age younger than 60 years, tumor volume less than 0.75 cm3, intracanalicular tumor location, and better pretreatment hearing (class I Gardner-Robertson, speech discrimination score ≥80%, pure tone average <20 dB).80
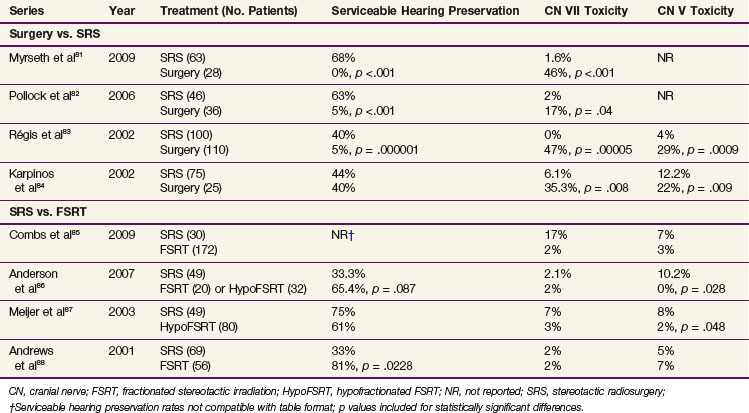
Several retrospective reports have compared outcomes of patients treated with surgery versus SRS81–84 (Table 26-10), demonstrating that both approaches achieve comparable local control rates of 91% to 100%,82,84 but SRS produces equivalent or superior functional outcomes. A prospective analysis of 82 patients with small tumors (≤3 cm) eligible for either surgery or SRS at the Mayo Clinic found that patients who chose SRS subsequently had better preservation of normal facial movement and serviceable hearing.82 A prospective analysis of 91 patients with small vestibular schwannomas (≤2.5 cm) treated with surgery (n = 28) or SRS (n = 28) at Haukeland University Hospital also showed better facial nerve function and hearing preservation in patients who underwent radiosurgery.81 A comparison of patients treated with surgery (n = 110) or SRS (n = 100) published by Régis and colleagues83 found better preservation of serviceable hearing and facial and trigeminal function with SRS. Finally, a retrospective analysis of 96 patients performed by Karpinos and associates84 showed better preservation of measurable (but not serviceable) hearing and lower rates of facial and trigeminal neuropathy in patients treated with SRS. Tumor size was larger in the surgical group, however.
Fractionated Stereotactic Radiation Therapy
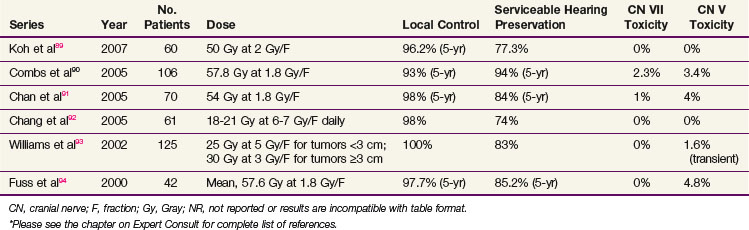
Published single-institution outcomes of FSRT are comparable with those achieved with current, moderate-dose SRS, with local control rates greater than 90% and cranial nerve toxicity rates less than 10% (Table 26-11). Serviceable hearing preservation rates may be higher than those achieved with SRS. It will be important to monitor local control rates with increasing follow-up to ensure that they remain high.
Four (one prospective and three retrospective) single-institution analyses have compared outcomes of SRS with those of FSRT (see Table 26-10), all of which show equal rates of local control.85,87,88 Meijer and colleagues87 conducted a prospective study of 129 patients with vestibular schwannomas who were allocated to SRS versus hypofractionated FSRT on the basis of dentition, with dentate patients (n = 80) receiving FSRT and edentulous patients (n = 49) receiving SRS. Toxicity rates were low for both groups, but trigeminal nerve preservation was slightly higher in the FSRT group (98% vs. 92% at 5 years, p = .048). A review of 125 patients treated with SRS (n = 69) or conventional FSRT (n = 56) at Thomas Jefferson University also showed comparable outcomes, with the exception of functional hearing preservation, which was better for patients treated with FSRT.88 A recent analysis of 202 vestibular schwannomas treated with SRS (n = 30) versus conventional FSRT (n = 172) at the University of Heidelberg found equivalent outcomes between the two groups, with the exception of patients treated with SRS doses of more than 13 Gy, who had lower rates of hearing preservation.85 Finally, in a recent analysis from the University of Wisconsin, patients treated with SRS (n = 49), conventional FSRT (n = 32), and hypofractionated FSRT (n = 32) were found to have equivalent 5-year PFS of 92.3% overall, whereas FSRT produced lower actuarial rates of late trigeminal nerve toxicity (0% vs. 10.2%; p = .028) and a trend toward better serviceable hearing preservation (65.4% vs. 33.3%; p = .087).86
Surveillance
A systematic review of 903 patients observed by MRI and/or CT over a mean period of 3.1 years found that 51% of tumors grew and 20% required intervention after a mean time of 2.1 years, primarily because of tumor growth with progressive symptoms.56 Audiologic data from a subset of 60 patients with serviceable hearing at presentation revealed loss of serviceable hearing in 37%. Recently, analysis of nearly 1000 patients managed expectantly demonstrated an overall hearing preservation rate of 54%.95 Hearing preservation was more likely in tumors with average annual growth rates of 2.5 mm per year or less (75% vs. 32% for faster-growing tumors). The facial nerve preservation rate was higher than 97%.
Targeted Therapy
Very recent data indicate that antiangiogenic agents such as bevacizumab can induce tumor regression and also, in some patients, restore hearing.96 Larger trials with this agent are therefore planned in NF2-associated vestibular schwannoma. Other systemic agents currently under investigation include sorafenib, trastuzumab, lapatinib, protein kinase inhibitors, and p21-activated kinase inhibitors.97
Management of Vestibular Schwannoma in the Patient with Neurofibromatosis 2
Bilateral vestibular schwannomas, as mentioned above, occur commonly in patients with type 2 neurofibromatosis, a disorder with an estimated prevalence of 1 in 100,000.98 NF2 patients also have a predisposition to develop other tumors of the central nervous system (meningiomas, ependymomas, astrocytomas, neurofibromas), peripheral neuropathy, ocular findings (retinal hamartomas, epiretinal membranes, cataracts), and skin lesions (plaques and subcutaneous tumors), as recently reviewed by Asthagiri and colleagues.97 Pretreatment evaluation should therefore include MRI studies of the entire craniospinal axis.
In comparison with sporadic tumors, vestibular schwannomas in patients with NF2 tend to grow more quickly and to more aggressively surround or infiltrate the adjacent cochlear and facial nerves. Functional outcomes after treatment tend to be poorer in this population of patients. For example, in the experience of Combs and colleagues,90 a diagnosis of NF2 was associated with significantly reduced likelihood of serviceable hearing preservation in patients treated with FSRT (64% for NF2 vs. 98% for sporadic tumors).
Mathieu and associates99 recently published the University of Pittsburgh’s experience treating 74 vestibular schwannomas in 62 NF2 patients with SRS, with a mean marginal dose of 14 Gy (range, 11 to 20 Gy). The actuarial local control rate was 81% at 10 to 15 years. The margin dose was significantly related to serviceable hearing preservation on multivariate analysis, and the actuarial serviceable hearing preservation rate was 48% at 5 years in patients treated with a marginal dose of 14 Gy or less. Tumor volume of more than 5 cm3 and maximum dose of more than 28 Gy predict for the development of other complications, such as facial weakness, trigeminal neuropathy or neuralgia, and vestibular dysfunction. The authors recommend offering treatment only for large symptomatic tumors or tumors manifesting with progressive growth or hearing loss in order to maximize preservation of function and quality of life.99 One advantage of earlier intervention may be the potential for sparing function of the cochlear nerve, thereby allowing for future cochlear implantation.
Irradiation Techniques and Tolerance
Stereotactic Radiosurgery
High-resolution MRI images should be used during treatment planning, either as the primary dataset or through fusion with a treatment planning CT scan. Care should be taken when relying solely on MRI, however, as spatial distortion may result in targeting errors with resulting compromise in local control.100 The tumor is best visualized with contrast-enhanced T1 images, whereas the cochlea and semicircular canals are best seen with fast spin echo T2 sequences.
The ideal SRS prescription (marginal) dose is typically 12 to 13 Gy; local control appears to be compromised with doses below this range74,77 and cranial nerve toxicity increases with higher doses.76,85,101,102 For example, a recent retrospective analysis by Foote and colleagues77 found the 2-year incidence of any cranial neuropathy to be 2% with prescription doses of 12.5 Gy or less versus 24% with doses of more than 12.5 Gy (p <.0003).
Facial, trigeminal, and auditory toxicities have also been shown to correlate with the length of nerve irradiated (represented by the transverse tumor diameter).101 For example, in the early experience with higher-dose SRS, trigeminal and facial neuropathy rates were, respectively, higher in patients with an extracanalicular tumor dimension of more than 1 cm and a sum of extracanalicular and intracanalicular diameter of more than 2 cm.103
Other factors that correlate with increased facial nerve preservation rates include age of 60 years or less, tumor volume of 1.5 cm3 or less, and marginal dose of 13 Gy or less.104 Most cranial neuropathies develop within 2 years of SRS, but hearing loss can occur much later.
MRI treatment planning techniques have been found to correlate with reduced facial and trigeminal neuropathy101 and increased hearing preservation,77,101 although inter-era comparisons may be confounded by a tendency to prescribe lower marginal doses in more recent years. Other SRS parameters correlated with improved hearing preservation rates include prescribing a marginal dose of 13 Gy or less,105 limiting the cochlear dose to less than 4.2 to 4.75 Gy,106 and limiting the dose to the cochlear nucleus to less than 10 Gy.107 Kondziolka and associates108 recommend prescribing 12 Gy for tumors of more than 2 cm in extracanalicular diameter, 12.5 Gy for smaller tumors, 13 Gy for patients without serviceable hearing, and potentially up to 14 Gy for patients with hearing loss and facial weakness.
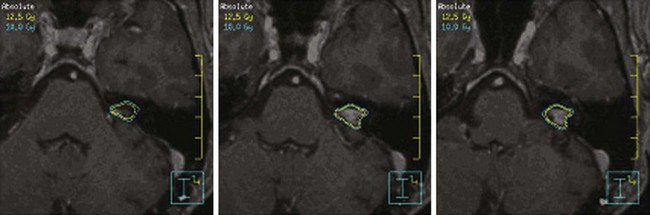
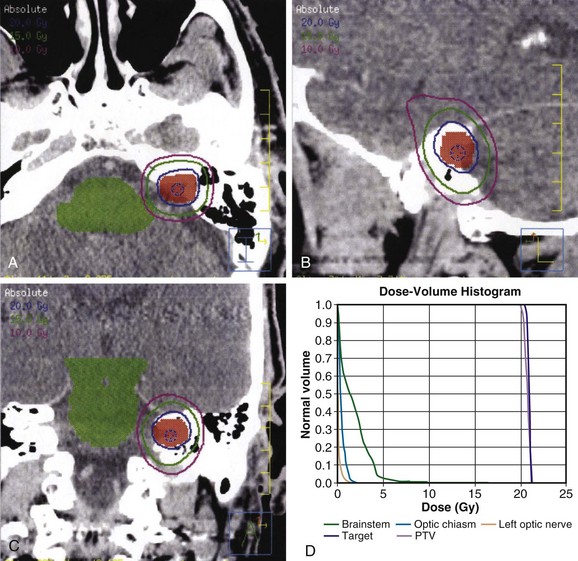
The radiation dose to the brainstem must also be carefully monitored. In 1994, Kihlstrom and colleagues46 recommended limiting the brainstem dose to 14 Gy or less with SRS, based on the development of adverse radiation imaging effects (ARIE) in 6 of 7 patients who received 14 to 35 Gy for low-grade gliomas of the tectal midbrain.109 In 2008, Sharma and colleagues110 demonstrated that ARIE and new neurologic deficits could develop after exposing 0.1 cm or more of brainstem to doses of more than 12 Gy. An example of an SRS treatment plan is shown in Figure 26-9.
Fractionated Stereotactic Radiation Therapy
At the University of Wisconsin, FSRT is typically delivered by a linear accelerator with the patient immobilized in an aquaplast head mask (Fig. 26-10). To ensure treatment delivery accuracy, pretreatment and intratreatment continuous, real-time patient localization is carried out using optical guidance. For this purpose, a patient-specific maxillary bite block is manufactured before CT simulation, to which a fiducial array with passive optical markers is attached. Before the planning CT scan and before each treatment fraction, the fiducial array bite block complex is inserted, and setup is continuously monitored with optical guidance. If the patient moves out of the predetermined error tolerance band of 0.3 mm and/or 0.3 degrees of rotation, treatment is interrupted until realignment is achieved.111
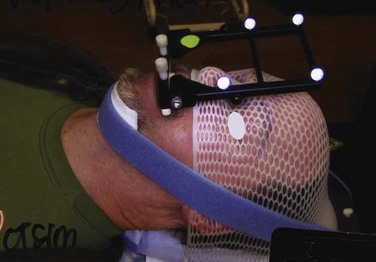
Figure 26-10 Patient immobilization and localization system for FSRT at the University of Wisconsin.
Common regimens prescribed are on the order of 45 to 50 Gy at 1.8 to 2 Gy per fraction, or 20 to 25 Gy at 4 to 5 Gy per fraction. A recent retrospective analysis of 89 patients treated with FSRT to 46.8 Gy versus 50.4 Gy at 1.8 Gy per fraction found that patients treated to 46.8 Gy were more likely to have preservation of serviceable hearing; however, there was no statistically significant relationship between cochlear dose and serviceable hearing preservation.112 In contrast, a prospective analysis of 34 patients treated with FSRT to 45 Gy at 1.8 Gy per fraction prescribed to the 90% isodose line found that the dose to the cochlea was the only significant prognostic factor for hearing deterioration.113 In their experience, the median hearing loss was 10 dB for patients with less than 73.3% of the cochlea exposed to 90% or more of the prescription dose (45 Gy), as opposed to 25 dB for patients with a volume getting 90% of the prescription dose (V90) of 73.3% or more. An example of an FSRT plan is shown in Figure 26-11.
Treatment Algorithms, Challenges, and Future Directions
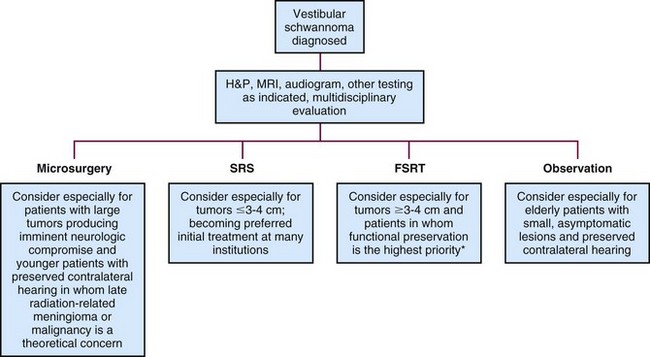
Patients with newly diagnosed vestibular schwannomas should be evaluated by a multidisciplinary team with expertise in neurosurgery, SRS, and FSRT for discussion of the risks and benefits of treatment, as well as observation (see the treatment algorithm in Fig. 26-12). Resection may be performed for virtually any tumor and especially for large tumors causing brainstem compression or hydrocephalus that cannot effectively be managed nonsurgically. Hearing preservation rates may be lower with surgery than with radiation therapy; however, this has never been tested in a prospective randomized fashion. The ideal candidate for SRS is a patient with a small intracanalicular tumor not approaching the brainstem, with marginal doses of 12 to 13 Gy. Conventional or hypofractionated FSRT may be a safer choice for tumors measuring 3 to 4 cm or larger and may result in higher hearing preservation rates. Randomized, controlled trials comparing various treatment options would be very valuable.
The cost-effectiveness of various forms of treatment is another important issue to consider. Banerjee and colleagues114 recently performed a comparative analysis of the costs incurred through microsurgery versus SRS for vestibular schwannoma. They determined that the mean cost of microsurgery is higher than the mean cost of SRS ($23,788 vs. $16,143), but the follow-up costs after SRS tend to be higher because of factors such as increased use of MRI and audiograms, although this reflects practice patterns more than clinical necessity. The authors conclude that SRS is likely to be less expensive than microsurgery from a societal perspective, provided that recurrence rates remain low over prolonged follow-up times.114
1 Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
2 Rogers CL. Radiation therapy for Intracranial meningiomas. In: Mehta MP, editor. Principles and Practice of Neuro-oncology: A Multi-disciplinary Approach. New York: Demos Medical; 2011:820-841.
5 Perry A, Louis DN, Scheithauer BW, Budka H, editors. WHO Classification of Tumours of the Central Nervous System. Lyon: IARC, 2007.
10 Pearson BE, Markert JM, Fisher WS, et al. Hitting a moving target. Evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24:E3.
11 Goldsmith BJ, Wara WM, Wilson CB, Larson DA. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80:195-201.
13 Palma L, Celli P, Franco C, et al. Long-term prognosis for atypical and malignant meningiomas. A study of 71 surgical cases. J Neurosurg. 1997;86:793-800.
14 Perry A, Scheithauer BW, Stafford SL, et al. “Malignancy” in meningiomas. A clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85:2046-2056.
19 Condra KS, Buatti JM, Mendenhall WM, et al. Benign meningiomas. Primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39:427-436.
20 Taylor BWJr, Marcus RBJr, Friedman WA, et al. The meningioma controversy. Postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:299-304.
22 Glaholm J, Bloom HJ, Crow JH. The role of radiotherapy in the management of intracranial meningiomas. The Royal Marsden Hospital experience with 186 patients. Int J Radiat Oncol Biol Phys. 1990;18:755-761.
23 Debus J, Wuendrich M, Pirzkall A, et al. High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas. Long-term results. J Clin Oncol. 2001;19:3547-3553.
26 Turbin RE, Thompson CR, Kennerdell JS, et al. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology. 2002;109:890-900.
28 Kondziolka D, Flickinger JC, Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas. Outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery. 1998;43:405-414.
30 DiBiase SJ, Kwok Y, Yovino S, et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004;60:1515-1519.
31 Boskos C, Feuvret L, Noel G, et al. Combined proton and photon conformal radiotherapy for intracranial atypical and malignant meningioma. Int J Radiat Oncol Biol Phys. 2009;75:399-406.
32 Attia A, Chan M, Seif D, et al. Treatment of atypical meningiomas with gamma knife radiosurgery. The role of conformality index and margin dose. Int J Radiat Oncol Biol Phys. 2009;75:S226-S227.
33 Hakim R, Alexander EIII, Loeffler JS, et al. Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery. 1998;42:446-454.
34 Aghi MK, Carter BS, Cosgrove GR, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64:56-60.
37 Dziuk TW, Woo S, Butler EB, et al. Malignant meningioma. An indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol. 1998;37:177-188.
39 Mason WP, Gentili F, Macdonald DR, et al. Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningioma. J Neurosurg. 2002;97:341-346.
40 Chargari C, Vedrine L, Bauduceau O, et al. Reapprasial of the role of endocrine therapy in meningioma management. Endocr Relat Cancer. 2008;15:931-941.
42 Stafford SL, Pollock BE, Foote RL, et al. Meningioma radiosurgery. Tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029-1038.
44 Bikmaz K, Mrak R, Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107:905-912.
48 Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62:53-60.
49 Patil CG, Hoang S, Borchers DJ3rd, et al. Predictors of peritumoral edema after stereotactic radiosurgery of supratentorial meningiomas. Neurosurgery. 2008;63:435-442.
56 Yamakami I, Uchino Y, Kobayashi E, Yamaura A. Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neurinomas. A systematic review of outcome and risk of three therapeutic options. Neurol Res. 2003;25:682-690.
59 Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery. 1997;40:1-10.
64 Brackmann DE, Owens RM, Friedman RA, et al. Prognostic factors for hearing preservation in vestibular schwannoma surgery. Am J Otolaryngol. 2000;21:417-424.
75 Foote KD, Friedman WA, Buatti JM, et al. Analysis of risk factors associated with radiosurgery for vestibular schwannoma. J Neurosurg. 2001;95:440-449.
79 Yang I, Aranda D, Han SJ, et al. Hearing preservation after stereotactic radiosurgery for vestibular schwannoma. A systematic review. J Clin Neurosci. 2009;16:742-747.
80 Kano H, Kondziolka D, Khan A, et al. Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuroma. J Neurosurg. 2009;111:863-873.
81 Myrseth E, Moller P, Pedersen PH, Lund-Johansen M. Vestibular schwannoma. Surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. 2009;64:654-663.
82 Pollock BE, Driscoll CL, Foote RL, et al. Patient outcomes after vestibular schwannoma management. A prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59:77-85.
83 Régis J, Pellet W, Delsanti C, et al. Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg. 2002;97:1091-1100.
84 Karpinos M, Teh BS, Zeck O, et al. Treatment of acoustic neuroma. Stereotactic radiosurgery vs. microsurgery. Int J Radiat Oncol Biol Phys. 2002;54:1410-1421.
85 Combs SE, Welzel T, Schulz-Ertner D, et al. Differences in clinical results after LINAC-based single-dose radiosurgery versus fractionated stereotactic radiotherapy for patients with vestibular schwannomas. Int J Radiat Oncol Biol Phys. 2010;76:193-200.
86 Anderson BM, Khuntia D, Toma WA, et al. Single institution experience treating 100 vestibular schwannomas with fractionated stereotactic radiation therapy or stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2007;69:S121.
87 Meijer OW, Vandertop WP, Baayen JC, Slotman BJ. Single-fraction vs. fractionated linac-based stereotactic radiosurgery for vestibular schwannoma. A single-institution study. Int J Radiat Oncol Biol Phys. 2003;56:1390-1396.
88 Andrews DW, Suarez O, Goldman HW, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas. Comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001;50:1265-1278.
90 Combs SE, Volk S, Schulz-Ertner D, et al. Management of acoustic neuromas with fractionated stereotactic radiotherapy (FSRT). Long-term results in 106 patients treated in a single institution. Int J Radiat Oncol Biol Phys. 2005;63:75-81.
95 Yang I, Sughrue ME, Han SJ, et al. A comprehensive analysis of hearing preservation after radiosurgery for vestibular schwannoma. J Neurosurg. 2010;112:851-859.
97 Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet. 2009;373:1974-1986.
99 Mathieu D, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2. An analysis of tumor control, complications, and hearing preservation rates. Neurosurgery. 2007;60:460-470.
105 Linskey ME, Flickinger JC, Lunsford LD. Cranial nerve length predicts the risk of delayed facial and trigeminal neuropathies after acoustic tumor stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 1993;25:227-233.
108 Kondziolka D, Flickinger JC, Lunsford LD. The principles of skull base radiosurgery. Neurosurg Focus. 2008;24:E11.
109 Kihlstrom L, Lindquist C, Lindquist M, Karlsson B. Stereotactic radiosurgery for tectal low-grade gliomas. Acta Neurochir. 1994;62(Suppl):55-57.
110 Sharma MS, Kondziolka D, Khan A, et al. Radiation tolerance limits of the brainstem. Neurosurgery. 2008;63:728-733.
112 Andrews DW, Werner-Wasik M, Den RB, et al. Toward dose optimization for fractionated stereotactic radiotherapy for acoustic neuromas. Comparison of two dose cohorts. Int J Radiat Oncol Biol Phys. 2009;74:419-426.
113 Thomas C, Di Maio S, Ma R, et al. Hearing preservation following fractionated stereotactic radiotherapy for vestibular schwannomas. Prognostic implications of cochlear dose. J Neurosurg. 2007;107:917-926.
114 Banerjee R, Moriarty JP, Foote RL, Pollock BE. Comparison of the surgical and follow-up costs associated with microsurgical resection and stereotactic radiosurgery for vestibular schwannoma. J Neurosurg. 2008;108:1220-1224.
1 Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
2 Rogers CL. Radiation therapy for Intracranial meningiomas. In: Mehta MP, editor. Principles and practice of neuro-oncology: A multi-disciplinary approach. New York: Demos Medical; 2011:820-841.
3 Claus EB, Bondy ML, Schildkraut JM, et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088-1095.
4 Perry A, Dehner LP. Meningeal tumors of childhood and infancy. An update and literature review. Brain Pathol. 2003;13:386-408.
5 Perry A, Louis DN, Scheithauer BW, Budka H, editors. WHO Classification of Tumours of the Central Nervous System. Lyon: IARC, 2007.
6 Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5:1045-1054.
7 Pfisterer WK, Coons SW, Aboul-Enein F, et al. Implicating chromosomal aberrations with meningioma growth and recurrence: results from FISH and MIB-I analysis of grades I and II meningioma tissue. J Neurooncol. 2008;87:43-50.
8 Moller ML, Braendstrup O. No prediction of recurrence of meningiomas by PCNA and Ki-67 immunohistochemistry. J Neurooncol. 1997;34:241-246.
9 Willis J, Smith C, Ironside JW, et al. The accuracy of meningioma grading. A 10-year retrospective audit. Neuropathol Appl Neurobiol. 2005;31:141-149.
10 Pearson BE, Markert JM, Fisher WS, et al. Hitting a moving target. Evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24:E3.
11 Goldsmith BJ, Wara WM, Wilson CB, Larson DA. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80:195-201.
12 Hug EB, Devries A, Thornton AF, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48:151-160.
13 Palma L, Celli P, Franco C, et al. Long-term prognosis for atypical and malignant meningiomas. A study of 71 surgical cases. J Neurosurg. 1997;86:793-800.
14 Perry A, Scheithauer BW, Stafford SL, et al. “Malignancy” in meningiomas. A clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85:2046-2056.
15 Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. 2004;363:1535-1543.
16 Yoneoka Y, Fujii Y, Tanaka R. Growth of incidental meningiomas. Acta Neurochir (Wien). 2000;142:507-511.
17 Pollock BE, Stafford SL, Utter A, et al. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003;55:1000-1005.
18 Litre CF, Colin P, Noudel R, et al. Fractionated stereotactic radiotherapy treatment of cavernous sinus meningiomas. A study of 100 cases. Int J Radiat Oncol Biol Phys. 2009;74:1012-1017.
19 Condra KS, Buatti JM, Mendenhall WM, et al. Benign meningiomas. Primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39:427-436.
20 Taylor BWJr, Marcus RBJr, Friedman WA, et al. The meningioma controversy. Postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:299-304.
21 Miralbell R, Linggood RM, de la Monte S, et al. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurooncol. 1992;13:157-164.
22 Glaholm J, Bloom HJ, Crow JH. The role of radiotherapy in the management of intracranial meningiomas. The Royal Marsden Hospital experience with 186 patients. Int J Radiat Oncol Biol Phys. 1990;18:755-761.
23 Debus J, Wuendrich M, Pirzkall A, et al. High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas. Long-term results. J Clin Oncol. 2001;19:3547-3553.
24 Henzel M, Gross MW, Hamm K, et al. Stereotactic radiotherapy of meningiomas. Symptomatology, acute and late toxicity. Strahlenther Onkol. 2006;182:382-388.
25 Milker-Zabel S, Zabel-du Bois A, Huber P, et al. Intensity-modulated radiotherapy for complex-shaped meningioma of the skull base: long-term experience of a single institution. Int J Radiat Oncol Biol Phys. 2007;68:858-863.
26 Turbin RE, Thompson CR, Kennerdell JS, et al. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology. 2002;109:890-899.
27 Narayan S, Cornblath WT, Sandler HM, et al. Preliminary visual outcomes after three-dimensional conformal radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2003;56:537-543.
28 Kondziolka D, Flickinger JC, Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas. Outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery. 1998;43:405-413.
29 Kollova A, Liscak R, Novotny JJr, et al. Gamma Knife surgery for benign meningioma. J Neurosurg. 2007;107:325-336.
30 DiBiase SJ, Kwok Y, Yovino S, et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004;60:1515-1519.
31 Boskos C, Feuvret L, Noel G, et al. Combined proton and photon conformal radiotherapy for intracranial atypical and malignant meningioma. Int J Radiat Oncol Biol Phys. 2009;75:399-406.
32 Attia A, Chan M, Seif D, et al. Treatment of atypical meningiomas with Gamma Knife radiosurgery. The role of conformality index and margin dose. Int J Radiat Oncol Biol Phys. 2009;75:S226-S227.
33 Hakim R, Alexander EIII, Loeffler JS, et al. Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery. 1998;42:446-453.
34 Aghi MK, Carter BS, Cosgrove GR, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64:56-60.
35 Jaaskelainen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26:461-469.
36 Rosenberg LA, Prayson RA, Lee J, et al. Long-term experience with World Health Organization grade III (malignant) meningiomas at a single institution. Int J Radiat Oncol Biol Phys. 2009;74:427-432.
37 Dziuk TW, Woo S, Butler EB, et al. Malignant meningioma. An indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol. 1998;37:177-188.
38 DeVries A, Munzenrider JE, Hedley-Whyte T, Hug EB. [The role of radiotherapy in the treatment of malignant meningiomas]. Strahlenther Onkol. 1999;175:62-67.
39 Mason WP, Gentili F, Macdonald DR, et al. Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningioma. J Neurosurg. 2002;97:341-346.
40 Chargari C, Vedrine L, Bauduceau O, et al. Reapprasial of the role of endocrine therapy in meningioma management. Endocr Relat Cancer. 2008;15:931-941.
41 Norden AD, Raizer JJ, Abrey LE, et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. 2010;96(2):211-217.
42 Stafford SL, Pollock BE, Foote RL, et al. Meningioma radiosurgery. Tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029-1038.
43 Pieper DR, Al-Mefty O, Hanada Y, Buechner D. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44:742-747.
44 Bikmaz K, Mrak R, Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107:905-912.
45 Lee PW, Hung BK, Woo EK, et al. Effects of radiation therapy on neuropsychological functioning in patients with nasopharyngeal carcinoma. J Neurol Neurosurg Psychiatry. 1989;52:488-492.
46 Torres IJ, Mundt AJ, Sweeney PJ, et al. A longitudinal neuropsychological study of partial brain radiation in adults with brain tumors. Neurology. 2003;60:1113-1118.
47 Arvold ND, Lessell S, Bussiere M, et al. Visual outcome and tumor control after conformal radiotherapy for patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2009;75(4):1166-1172.
48 Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62:53-60.
49 Patil CG, Hoang S, Borchers DJIII, et al. Predictors of peritumoral edema after stereotactic radiosurgery of supratentorial meningiomas. Neurosurgery. 2008;63:435-442.
50 Propp JM, McCarthy BJ, Davis FG, Preston-Martin S. Descriptive epidemiology of vestibular schwannomas. Neuro Oncol. 2006;8(1):1-11.
51 Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821-1828.
52 Ahlbom A, Feychting M, Green A, et al. Epidemiologic evidence on mobile phones and tumor risk. A review. Epidemiology. 2009;20(5):639-652.
53 Hours M, Bernard M, Arslan M, et al. Can loud noise cause acoustic neuroma? Analysis of the INTERPHONE study in France. Occup Environ Med. 2009;66(7):480-486.
54 Antinheimo J, Sallinen SL, Sallinen P, et al. Genetic aberrations in sporadic and neurofibromatosis 2 (NF2)-associated schwannomas studied by comparative genomic hybridization (CGH). Acta Neurochir (Wien). 2000;142(10):1099-1105.
55 Welling DB, Packer MD, Chang LS. Molecular studies of vestibular schwannomas. A review. Curr Opin Otolaryngol Head Neck Surg. 2007;15(5):341-346.
56 Yamakami I, Uchino Y, Kobayashi E, Yamaura A. Conservative management, Gamma Knife radiosurgery, and microsurgery for acoustic neurinomas. A systematic review of outcome and risk of three therapeutic options. Neurol Res. 2003;25:682-690.
57 Rosenberg SI. Natural history of acoustic neuromas. Laryngoscope. 2000;110(4):497-508.
58 Abaza MM, Makariou E, Armstrong M, Lalwani AK. Growth rate characteristics of acoustic neuromas associated with neurofibromatosis type 2. Laryngoscope. 1996;106(6):694-699.
59 Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas). Clinical presentation. Neurosurgery. 1997;40:1-10.
60 Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97(1):55-66.
61 Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol Head Neck Surg. 1995;113(3):179-180.
62 Wackym PA. Stereotactic radiosurgery, microsurgery, and expectant management of acoustic neuroma: basis for informed consent. Otolaryngol Clin North Am. 2005;38(4):653-670.
63 Meijer OW, Weijmans EJ, Knol DL, et al. Tumor-volume changes after radiosurgery for vestibular schwannoma: implications for follow-up MR imaging protocol. AJNR Am J Neuroradiol. 2008;29(5):906-910.
64 Brackmann DE, Owens RM, Friedman RA, et al. Prognostic factors for hearing preservation in vestibular schwannoma surgery. Am J Otolaryngol. 2000;21:417-424.
65 Betchen SA, Walsh J, Post KD. Long-term hearing preservation after surgery for vestibular schwannoma. J Neurosurg. 2005;102(1):6-9.
66 Khrais T, Sanna M. Hearing preservation surgery in vestibular schwannoma. J Laryngol Otol. 2006;120(5):366-370.
67 Wiet RJ, Mamikoglu B, Odom L, Hoistad DL. Long-term results of the first 500 cases of acoustic neuroma surgery. Otolaryngol Head Neck Surg. 2001;124(6):645-651.
68 Fukuoka S, Takanashi M, Hojyo A, et al. Gamma knife radiosurgery for vestibular schwannomas. Prog Neurol Surg. 2009;22:45-62.
69 Chopra R, Kondziolka D, Niranjan A, et al. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2007;68(3):845-851.
70 Friedman WA, Bradshaw P, Myers A, Bova FJ. Linear accelerator radiosurgery for vestibular schwannomas. J Neurosurg. 2006;105(5):657-661.
71 Lunsford LD, Niranjan A, Flickinger JC, et al. Radiosurgery of vestibular schwannomas. Summary of experience in 829 cases. J Neurosurg. 2005;102(Suppl):195-199.
72 Chung WY, Liu KD, Shiau CY, et al. Gamma Knife surgery for vestibular schwannoma. 10-year experience of 195 cases. J Neurosurg. 2005;102(Suppl):87-96.
73 Muacevic A, Jess-Hempen A, Tonn JC, Wowra B. Results of outpatient Gamma Knife radiosurgery for primary therapy of acoustic neuromas. Acta Neurochir. 2004;91(Suppl):75-78.
74 Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD, et al. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg. 2001;94(1):1-6.
75 Foote KD, Friedman WA, Buatti JM, et al. Analysis of risk factors associated with radiosurgery for vestibular schwannoma. J Neurosurg. 2001;95:440-449.
76 Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000;92(5):745-759.
77 Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339(20):1426-1433.
78 Norén G, Greitz D, Hirsch A, Lax I. Gamma Knife surgery in acoustic tumours. Acta Neurochir Suppl (Wien). 1993;58:104-107.
79 Yang I, Aranda D, Han SJ, et al. Hearing preservation after stereotactic radiosurgery for vestibular schwannoma. A systematic review. J Clin Neurosci. 2009;16:742-747.
80 Kano H, Kondziolka D, Khan A, et al. Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuroma. J Neurosurg. 2009;111:863-873.
81 Myrseth E, Moller P, Pedersen PH, Lund-Johansen M. Vestibular schwannoma. Surgery or Gamma Knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. 2009;64:654-663.
82 Pollock BE, Driscoll CL, Foote RL, et al. Patient outcomes after vestibular schwannoma management. A prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59:77-85.
83 Regis J, Pellet W, Delsanti C, et al. Functional outcome after Gamma Knife surgery or microsurgery for vestibular schwannomas. J Neurosurg. 2002;97:1091-1100.
84 Karpinos M, Teh BS, Zeck O, et al. Treatment of acoustic neuroma. Stereotactic radiosurgery vs. microsurgery. Int J Radiat Oncol Biol Phys. 2002;54:1410-1421.
85 Combs SE, Welzel T, Schulz-Ertner D, et al. Differences in clinical results after LINAC-based single-dose radiosurgery versus fractionated stereotactic radiotherapy for patients with vestibular schwannomas. Int J Radiat Oncol Biol Phys. 2010;76:193-200.
86 Anderson BM, Khuntia D, Toma WA, et al. Single institution experience treating 100 vestibular schwannomas with fractionated stereotactic radiation therapy or stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2007;69:S121.
87 Meijer OW, Vandertop WP, Baayen JC, Slotman BJ. Single-fraction vs. fractionated linac-based stereotactic radiosurgery for vestibular schwannoma. A single-institution study. Int J Radiat Oncol Biol Phys. 2003;56:1390-1396.
88 Andrews DW, Suarez O, Goldman HW, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas. Comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001;50:1265-1278.
89 Koh ES, et al. Fractionated stereotactic radiotherapy for acoustic neuroma: single-institution experience at The Princess Margaret Hospital. Cancer. 2007;109(6):1203-1210.
90 Combs SE, Volk S, Schulz-Ertner D, et al. Management of acoustic neuromas with fractionated stereotactic radiotherapy (FSRT). Long-term results in 106 patients treated in a single institution. Int J Radiat Oncol Biol Phys. 2005;63:75-81.
91 Chan AW, et al. Stereotactic radiotherapy for vestibular schwannomas: favorable outcome with minimal toxicity. Neurosurgery. 2005;57(1):60-70. discussion 60-70
92 Chang SD, et al. Staged stereotactic irradiation for acoustic neuroma. Neurosurgery. 2005;56(6):1254-1261. discussion 1261-3
93 Williams JA. Fractionated stereotactic radiotherapy for acoustic neuromas. Int J Radiat Oncol Biol Phys. 2002;54(2):500-504.
94 Fuss M, et al. Conventionally fractionated stereotactic radiotherapy (FSRT) for acoustic neuromas. Int J Radiat Oncol Biol Phys. 2000;48(5):1381-1387.
95 Yang I, Sughrue ME, Han SJ, et al. A comprehensive analysis of hearing preservation after radiosurgery for vestibular schwannoma. J Neurosurg. 2010;112:851-859.
96 Plotkin SR, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361(4):358-367.
97 Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet. 2009;373:1974-1986.
98 Evans DG, et al. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26(1):93-97.
99 Mathieu D, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2. An analysis of tumor control, complications, and hearing preservation rates. Neurosurgery. 2007;60:460-468. 2007; discussion Neurosurgery 60:468-470
100 Pollock BE, Link MJ, Foote RL. Failure rate of contemporary low-dose radiosurgical technique for vestibular schwannoma. J Neurosurg. 2009.
101 Flickinger JC, et al. Evolution in technique for vestibular schwannoma radiosurgery and effect on outcome. Int J Radiat Oncol Biol Phys. 1996;36(2):275-280.
102 Chihara Y, et al. Neurological complications after acoustic neurinoma radiosurgery: revised risk factors based on long-term follow-up. Acta Otolaryngol Suppl. 2007;559:65-70.
103 Linskey ME, Flickinger JC, Lunsford LD. Cranial nerve length predicts the risk of delayed facial and trigeminal neuropathies after acoustic tumor stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 1993;25(2):227-233.
104 Yang I, et al. Facial nerve preservation after vestibular schwannoma Gamma Knife radiosurgery. J Neurooncol. 2009;93(1):41-48.
105 Linskey ME, Flickinger JC, Lunsford LD. Cranial nerve length predicts the risk of delayed facial and trigeminal neuropathies after acoustic tumor stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 1993;25:227-233.
106 Timmer FC, et al. Gamma knife radiosurgery for vestibular schwannomas: results of hearing preservation in relation to the cochlear radiation dose. Laryngoscope. 2009;119(6):1076-1081.
107 Paek SH, et al. Hearing preservation after gamma knife stereotactic radiosurgery of vestibular schwannoma. Cancer. 2005;104(3):580-590.
108 Kondziolka D, Flickinger JC, Lunsford LD. The principles of skull base radiosurgery. Neurosurg Focus. 2008;24:E11.
109 Kihlstrom L, Lindquist C, Lindquist M, Karlsson B. Stereotactic radiosurgery for tectal low-grade gliomas. Acta Neurochir Suppl. 1994;62:55-57.
110 Sharma MS, Kondziolka D, Khan A, et al. Radiation tolerance limits of the brainstem. Neurosurgery. 2008;63:728-732. 2008; discussion Neurosurgery 63:732-733
111 Tome WA, et al. A high-precision system for conformal intracranial radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47(4):1137-1143.
112 Andrews DW, Werner-Wasik M, Den RB, et al. Toward dose optimization for fractionated stereotactic radiotherapy for acoustic neuromas. Comparison of two dose cohorts. Int J Radiat Oncol Biol Phys. 2009;74:419-426.
113 Thomas C, Di Maio S, Ma R, et al. Hearing preservation following fractionated stereotactic radiotherapy for vestibular schwannomas. Prognostic implications of cochlear dose. J Neurosurg. 2007;107:917-926.
114 Banerjee R, Moriarty JP, Foote RL, Pollock BE. Comparison of the surgical and follow-up costs associated with microsurgical resection and stereotactic radiosurgery for vestibular schwannoma. J Neurosurg. 2008;108:1220-1224.