2
Bedside Diagnostics
Potassium Hydroxide (KOH) Preparation of Scales
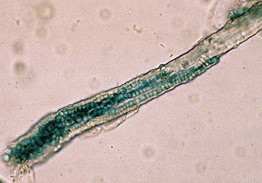
• Microscopic examination of scale (stratum corneum), obtained via scraping with a metal blade or glass slide and mounted in KOH, is commonly performed to confirm superficial cutaneous fungal infections (Fig. 2.1).
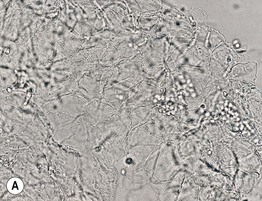
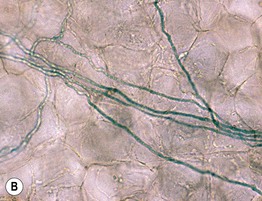
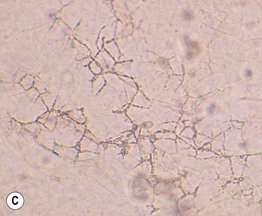

Fig. 2.1 Microscopic examination of potassium hydroxide (KOH) preparations of scale. A Tinea (pityriasis) versicolor due to Malassezia spp. with short mycelial forms and clusters of yeast forms. B Tinea corporis due to a dermatophyte with hyphae that cross over more than one cell (squame) and are branching. Chorazol black has been added to the KOH and the stained hyphae are easier to detect. C Branching mosaic pattern that represents the junctures of normal epidermal cells; this is a cause of false-positive KOH exams. D Cutaneous candidiasis with yeast forms and pseudohyphae. Pseudohyphae can sometimes be difficult to distinguish from hyphae. A, Courtesy, Ronald Rapini, MD; C, Courtesy, Louis Fragola, MD; D, Courtesy, Frank Samarin, MD.
• These fungal infections include tinea (pityriasis) versicolor, tinea corporis/faciei/manuum/cruris/pedis, and cutaneous candidiasis (see Chapter 64 on fungal diseases).
• Addition of chlorazol black to the KOH can improve detection (see Fig. 2.1B).
• For onychomycosis, both nail plates and subungual debris are examined; in addition, nail plates can be fixed in formalin and stained with periodic acid–Schiff (PAS) or Gomori methenamine silver stain (see Chapter 64).
Potassium Hydroxide (KOH) Preparation of Hair Shafts
• Tinea capitis is divided into two major forms: (1) endothrix – conidia occur within the hair shaft; and (2) ectothrix – while the fungus grows inside the hair shaft, conidia form on its surface (Figs. 2.2 and 2.3; see Chapter 64).
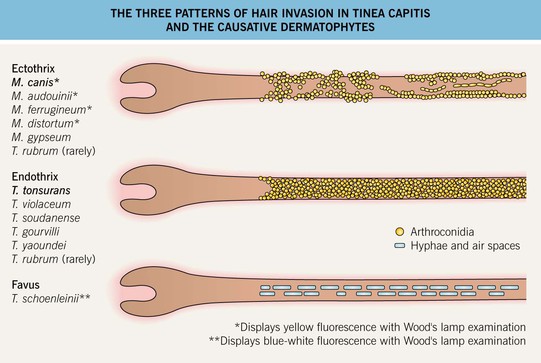
Fig. 2.2 The three patterns of hair invasion in tinea capitis and the causative dermatophytes. See Chapter 64 for additional details.
Mineral Oil Scraping for Suspected Scabies
• Place 2–3 drops of mineral oil on a glass slide. Dip the metal blade into the oil and then scrape suspicious lesions, e.g. burrows, inflammatory papules. Next place skin scrapings on a glass slide. Several skin lesions should be scraped. Dermoscopy can be used to better identify burrows and an adult female mite at the end of the burrow prior to scraping (see Chapter 71). Sometimes, KOH is used rather than mineral oil. Firm application of transparent adhesive tape to suspicious lesions followed by rapid removal and transfer to a glass slide is another technique that can be used, providing easier transport to a laboratory.
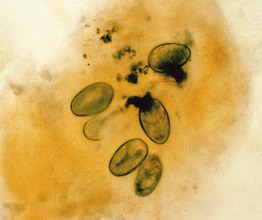
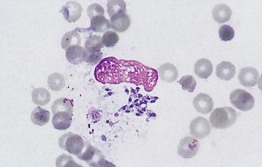
• In addition to adult mites, eggs and feces (scybala) can be seen when scrapings are examined microscopically (Figs. 2.4 and 2.5).
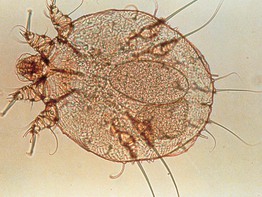
Fig. 2.4 Microscopic examination of scrapings from a patient with scabies. Female Sarcoptes scabiei var. hominus mite with eggs. There is a flattened, oval body with wrinkle-like corrugations and eight legs. With permission from Taplin D, Meinking TL. Infestations. In: Schachner LA, Hansen RC (Eds.), Pediatric Dermatology, 4th edn. Edinburgh, UK: Mosby, 2011:1141–1180.
Tzanck Smear
• The roof is retracted and scraping of the base and angles of the vesicle should be performed in order to obtain virally infected keratinocytes, which are thinly spread onto a glass slide, allowed to dry, and then stained with Giemsa stain (Figs. 2.6 and 2.7).
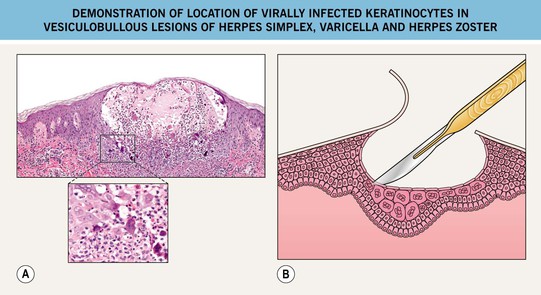
Fig. 2.6 Demonstration of location of virally infected keratinocytes in vesiculobullous lesions of herpes simplex, varicella, and herpes zoster. A Histologically, viral changes (e.g. multinucleated giant cells) are seen at the base of the vesicle; note their absence on the roof. B Scraping of the base of the vesicle is performed after the roof of the blister is reflected. A, Courtesy, Lorenzo Cerroni, MD.
Microscopic Examination of Molluscum Bodies
• The center of the papulonodule, which is often paler in color, is gently curetted to remove a core composed of the viral particles, and the contents are then thinly spread onto a glass slide; saline or KOH can be added prior to placing the coverslip (Fig. 2.8).
Gram Stain
• Performed when pustular material is available and identifies both gram-positive and gram-negative bacteria (Fig. 2.9).
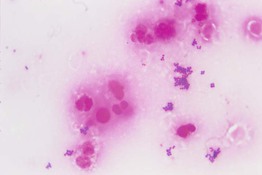
Fig. 2.9 Gram stain of pus demonstrating polymorphonuclear leukocytes (neutrophils) and Gram-positive cocci in clusters (Staphylococcus aureus). From Ekkelenkamp, MB, Rooijakkers, SHM, Bonten, MJM. In: Cohen J, Powderly W, Opal S (Eds.), Infectious Diseases, 3rd edn. Edinburgh, UK: Mosby, 2010.
• In addition, fungal organisms (e.g. Candida) can be identified.
Dermal Scrapings and Touch Preps
• When there is suspicion of a septic embolus or a primary infection involving the dermis and/or subcutis (bacterial, fungal, parasitic), then in addition to a sterile skin biopsy (Fig. 2.10), a touch prep or a dermal scraping can be performed. Often, the patient is immunocompromised. If there is pustular drainage, then a Gram stain and KOH preparation is performed first.
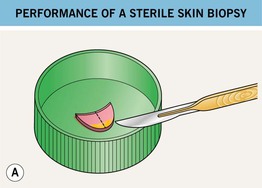
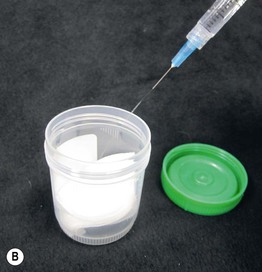
Fig. 2.10 Performance of a sterile skin biopsy. This is done when there is a suspicion of a septic embolus or primary skin infection of the dermis and/or subcutis. The equipment required includes a chemical antiseptic (e.g. chlorhexidine), alcohol pads, local anesthetic, punch biopsy instrument or scalpel, sterile scissors and forceps, sterile urine cup containing sterile gauze dampened with nonbacteriostatic saline, and glass slides. Following cleansing of the skin (antiseptic followed by alcohol), the skin is injected with local anesthesia, and a biopsy of dermis plus subcutaneous fat is performed (see Chapter 1). A The specimen is cut into two pieces on the sterile side of the urine container lid, while avoiding compression of the tissue. B One piece is placed in a sterile urine cup with moistened gauze, and the bottom of the second piece is tapped several times against a glass slide before being placed in formalin. The sealed sterile urine cup is hand carried to the microbiology laboratory.
• In a touch prep, the base of a skin biopsy which includes dermis ± subcutis is tapped multiple times against a glass slide. After drying for several minutes, the glass slide is stained (e.g. Gram stain, Giemsa stain; Fig. 2.11).
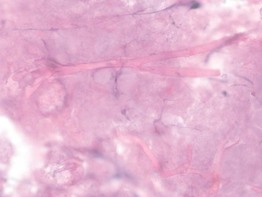
Fig. 2.11 Microscopic examination of a ‘touch prep’ from an incisional biopsy performed of a necrotic lesion on the chest of an immunocompromised patient. Note the branching, ribbon-like, nonseptate hyphae characteristic of Rhizopus. Courtesy, Jean L. Bolognia, MD.
• In a dermal scraping, the epidermis (if present) is reflected back after injection of local anesthesia, and a curette is used to scrape dermal tissue onto a slide.
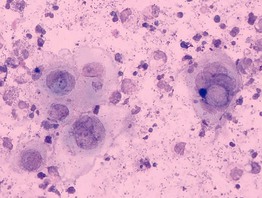
Giemsa Stain for Eosinophils or Amastigotes
• A skin slit and scraping of cutaneous lesions of leishmaniasis demonstrate amastigotes in macrophages when stained with a Giemsa stain (Fig. 2.12).
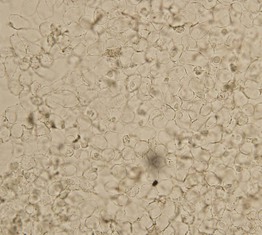
Acid-Fast Stain for Leprosy
• When leprosy is suspected, a fold of skin is firmly squeezed and a small incision is made with a scalpel, with the liquid expressed smeared onto a slide, allowed to dry, and stained with a Fite or Ziehl–Neelsen stain (Fig. 2.13).
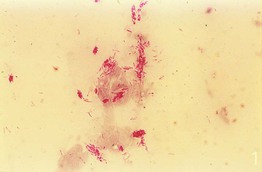
Fig. 2.13 Smear stained with a Ziehl–Neelsen stain demonstrating acid-fast bacilli in a patient with leprosy. From Peters W, Pasvol G. Tropical Medicine and Parasitology, 6th edn. London: Mosby, 2007.
• While acid-fast Mycobacterium leprae organisms are found in ≤5% of patients with tuberculoid leprosy, they are seen in 100% of patients with lepromatous leprosy (see Chapter 62 on mycobacteria).
Evaluation of Folliculitis
Dark Field Microscopy for Treponemal Infections
• Dark field microscopy is utilized for the examination of unstained live organisms and in dermatology, primarily for the diagnosis of primary syphilis and less often secondary cutaneous syphilis (see Chapter 69).
• The Treponema spirochetes have a characteristic morphology and movement pattern (Fig. 2.15).
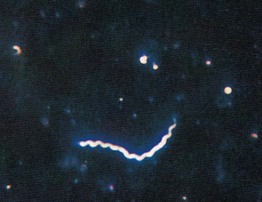
Fig. 2.15 Dark field microscopic examination of a spirochete. Treponemes are recognized by their characteristic corkscrew shape and deliberate forward and backward movement with rotation about the longitudinal axis. From Morse et al. Atlas of Sexually Transmitted Diseases and AIDS, 3rd edn. London: Mosby; 2003.
Hair Shaft Examination
• Assessment of hair thinning, which may be due to miniaturization, shedding, or breakage, most commonly includes a gentle hair pull and a hair shaft examination of cut, rather than pulled, hairs; some clinicians also do a vigorous hair pluck referred to as a trichogram (20–40 scalp hairs grasped by a hemostat with rubber-covered jaws).
• In general, telogen hairs are observed with a gentle hair pull, but in disorders such as loose anagen syndrome, anagen hairs may be seen (see Chapter 56 on alopecia).
• In a trichogram, the ratio of anagen:telogen hairs is determined by microscopic examination of the hair bulbs (Fig. 2.16). The normal anagen-to-telogen ratio is 9 : 1, but in telogen effluvium, it can be 7 : 3 or less.
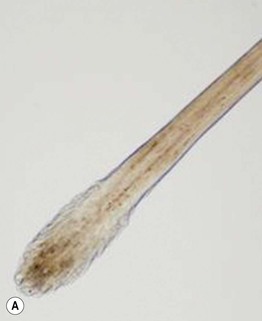
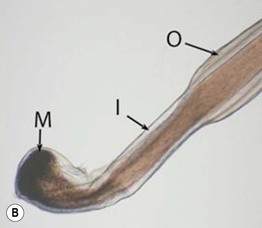
Fig. 2.16 Comparison of a telogen versus anagen hair shaft. A A telogen hair shaft has a club-shaped bulb. B An anagen hair has attached root sheaths as well as a pigmented and distorted bulb, sometimes resembling a hockey stick. M, matrix; I, inner root sheath; O, outer root sheath. B, Courtesy, Leonard Sperling, MD.
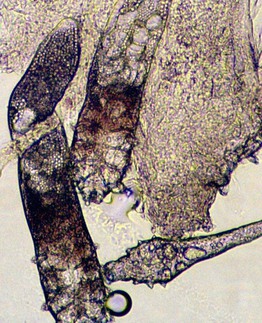
• Hair shaft examination can also detect bacterial and fungal infections (e.g. trichomycosis axillaris, white piedra, black piedra), hair casts, nits due to head lice infestation, and hair shaft abnormalities (e.g. trichorrhexis nodosa) (Fig. 2.17) (see Chapters 56, 64, and 71).



Fig. 2.17 Hair shaft examination as a diagnostic tool. A Nodule composed of numerous conidia of Trichosporon inkin in a patient with white piedra. B Two head louse egg casings (nits) are seen attached to hair shafts. C In trichorrhexis nodosa, the two ends of the hair shaft resemble opposing broomsticks. B, With permission from Taplin D, Meinking TL. Infestations. In: Schachner LA, Hansen RC (Eds.), Pediatric Dermatology, 4th edn. Edinburgh, UK: Mosby, 2011:1141–1180; C, Courtesy, Christine Ko, MD.
• Another bedside diagnostic procedure is the identification of lice, insects (e.g. bedbugs), and arachnids (e.g. ticks). Chapters 71 and 72 review their identification via macroscopic and microscopic findings.