Basic Sciences
I. Histologic Features of Bone
III. Conditions of Bone Mineralization, Bone Mineral Density, and Bone Viability
SECTION 3 NEUROMUSCULAR AND CONNECTIVE TISSUES
SECTION 4 CELLULAR AND MOLECULAR BIOLOGY, IMMUNOLOGY, AND GENETICS OF ORTHOPAEDICS
SECTION 5 ORTHOPAEDIC INFECTIONS AND MICROBIOLOGY
SECTION 6 PERIOPERATIVE PROBLEMS
SECTION 7 IMAGING AND SPECIAL STUDIES
section 1 Bone
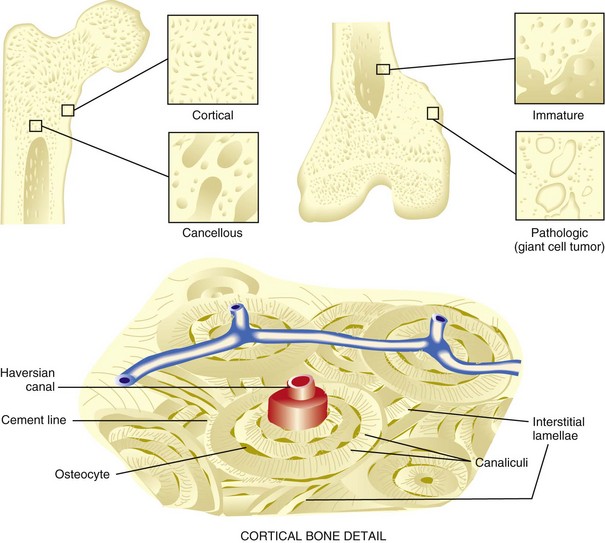
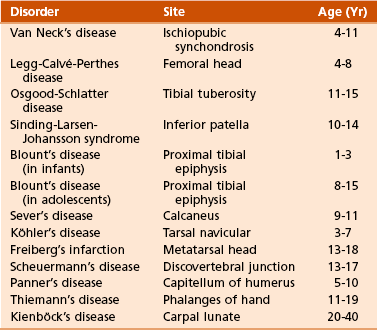
A Types (Figure 1-1; Table 1-1)
Table 1-1

Modified from Brinker MR, Miller MD: Fundamentals of orthopaedics, Philadelphia, 1999, WB Saunders, p 1.
1. Normal bone: lamellar, either cortical or cancellous
2. Immature and pathologic bone: woven; more random, more osteocytes, increased turnover, weaker
 Constitutes 80% of the skeleton
Constitutes 80% of the skeleton
 Consists of tightly packed osteons or haversian systems
Consists of tightly packed osteons or haversian systems
 Connected by Haversian (or Volkmann’s) canals
Connected by Haversian (or Volkmann’s) canals
 Contains arterioles, venules, capillaries, nerves, possibly lymphatic channels
Contains arterioles, venules, capillaries, nerves, possibly lymphatic channels
 Interstitial lamellae: between osteons
Interstitial lamellae: between osteons
 Nutrition provided by intraosseous circulation
Nutrition provided by intraosseous circulation
 Characterized by slow turnover rate, higher Young’s modulus of elasticity, more stiffness
Characterized by slow turnover rate, higher Young’s modulus of elasticity, more stiffness
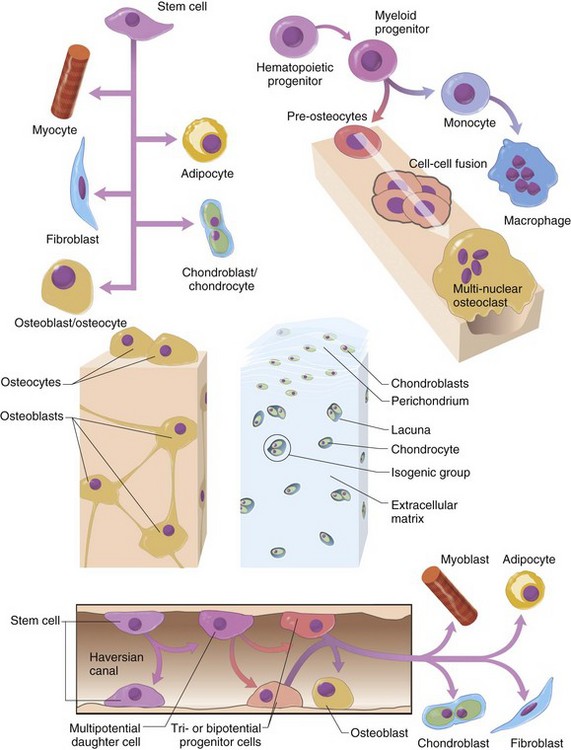
B Cellular biology (Figure 1-2)
 Form bone by generating organic, nonmineralized matrix
Form bone by generating organic, nonmineralized matrix
 Derived from undifferentiated mesenchymal stem cells
Derived from undifferentiated mesenchymal stem cells
 Have more endoplasmic reticulum, Golgi apparatus, and mitochondria than do other cells (for synthesis and secretion of matrix)
Have more endoplasmic reticulum, Golgi apparatus, and mitochondria than do other cells (for synthesis and secretion of matrix)
 RUNX2 is a multifunctional transcription factor that directs mesenchymal cells to the osteoblast lineage.
RUNX2 is a multifunctional transcription factor that directs mesenchymal cells to the osteoblast lineage.
 Bone surfaces lined by more differentiated, metabolically active cells
Bone surfaces lined by more differentiated, metabolically active cells
 “Entrapped cells”: less active cells in “resting regions”; maintain the ionic milieu of bone
“Entrapped cells”: less active cells in “resting regions”; maintain the ionic milieu of bone
 Osteoblast differentiation in vivo effected by the following:
Osteoblast differentiation in vivo effected by the following:
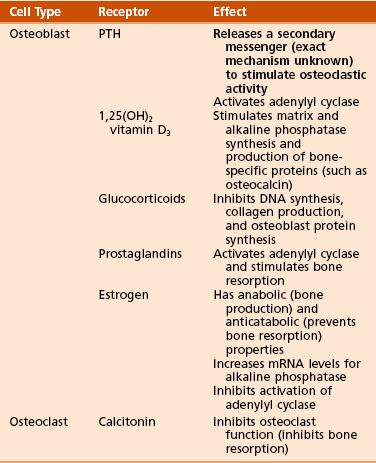
 Receptor-effector interactions in osteoblasts (Table 1-2)
Receptor-effector interactions in osteoblasts (Table 1-2)
 Osteoblasts produce the following:
Osteoblasts produce the following:
 Osteoblast activity stimulated by intermittent (pulsatile) exposure to parathyroid hormone (PTH)
Osteoblast activity stimulated by intermittent (pulsatile) exposure to parathyroid hormone (PTH)
 Osteoblast activity inhibited by tumor necrosis factor-α (TNF-α)
Osteoblast activity inhibited by tumor necrosis factor-α (TNF-α)
2. Osteocytes (see Figure 1-1)
 Constitute 90% of the cells in the mature skeleton
Constitute 90% of the cells in the mature skeleton
 Long interconnecting cytoplasmic processes projecting through the canaliculi
Long interconnecting cytoplasmic processes projecting through the canaliculi
 Less active in matrix production than are osteoblasts
Less active in matrix production than are osteoblasts
 Important for control of extracellular calcium and phosphorus concentration
Important for control of extracellular calcium and phosphorus concentration
 This activity occurs both normally and in certain conditions, including multiple myeloma and metastatic bone disease.
This activity occurs both normally and in certain conditions, including multiple myeloma and metastatic bone disease.
 Multinucleated, irregular giant cells
Multinucleated, irregular giant cells
 Possess a ruffled (“brush”) border and surrounding clear zone
Possess a ruffled (“brush”) border and surrounding clear zone
 Bone resorption occurs in depressions: Howship’s lacunae
Bone resorption occurs in depressions: Howship’s lacunae
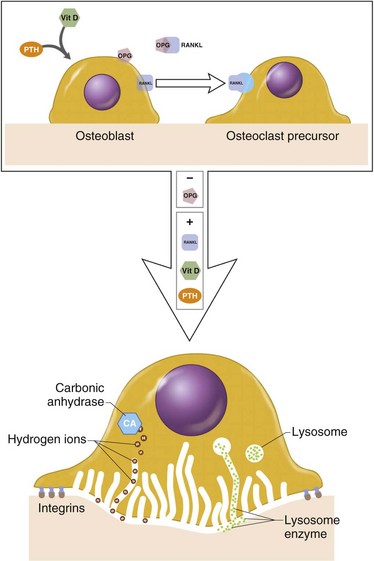
 Osteoblasts (and tumor cells) express RANKL (Figure 1-3), which acts as follows:
Osteoblasts (and tumor cells) express RANKL (Figure 1-3), which acts as follows:
 Synthesize tartrate-resistant acid phosphate
Synthesize tartrate-resistant acid phosphate
 Bind to bone surfaces through cell attachment (anchoring) proteins
Bind to bone surfaces through cell attachment (anchoring) proteins
 Produce hydrogen ions through carbonic anhydrase
Produce hydrogen ions through carbonic anhydrase
 Increase solubility of hydroxyapatite crystals
Increase solubility of hydroxyapatite crystals
 Organic matrix then removed by proteolytic digestion through activity of the lysosomal enzyme cathepsin K
Organic matrix then removed by proteolytic digestion through activity of the lysosomal enzyme cathepsin K
 Have specific receptors for calcitonin
Have specific receptors for calcitonin
 Potent stimulator of osteoclast differentiation and bone resorption
Potent stimulator of osteoclast differentiation and bone resorption
 Inhibit osteoclastic bone resorption.
Inhibit osteoclastic bone resorption.
 Categorized into two classes on the basis of presence or absence of a nitrogen side group
Categorized into two classes on the basis of presence or absence of a nitrogen side group
 Nitrogen-containing bisphosphonates are up to 1000-fold more potent in their antiresorptive activity.
Nitrogen-containing bisphosphonates are up to 1000-fold more potent in their antiresorptive activity.
 Nitrogen-containing bisphosphonates
Nitrogen-containing bisphosphonates
 Zoledronic acid (Zometa) and alendronate (Fosamax) are examples.
Zoledronic acid (Zometa) and alendronate (Fosamax) are examples.
 They inhibit protein prenylation within the mevalonate pathway, blocking farnesyl pyrophosphate synthase.
They inhibit protein prenylation within the mevalonate pathway, blocking farnesyl pyrophosphate synthase.
 This results in a loss of guanosine triphosphatase (GTPase) formation, which is needed for ruffled border formation and cell survival.
This results in a loss of guanosine triphosphatase (GTPase) formation, which is needed for ruffled border formation and cell survival.
 Non–nitrogen-containing bisphosphonates
Non–nitrogen-containing bisphosphonates
 Decreases skeletal events in multiple myeloma
Decreases skeletal events in multiple myeloma
 Associated with osteonecrosis of the jaw
Associated with osteonecrosis of the jaw
Table 1-3
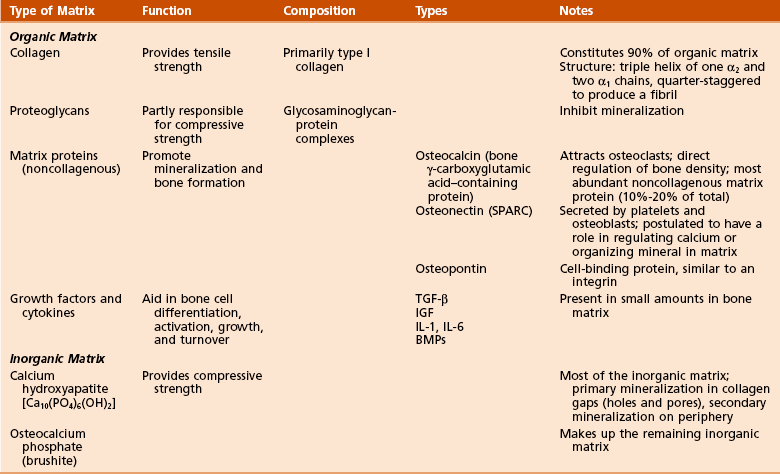
1. Organic components: 40% of the dry weight of bone
 Collagen (90% of organic component)
Collagen (90% of organic component)
 Collagen is primarily type I (mnemonic: “bone” contains the word “one”).
Collagen is primarily type I (mnemonic: “bone” contains the word “one”).
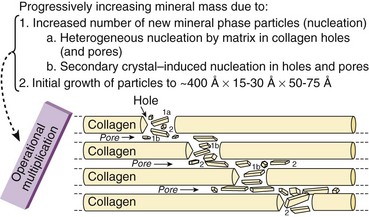
 Hole zones (gaps) exist within the collagen fibril between the ends of molecules.
Hole zones (gaps) exist within the collagen fibril between the ends of molecules.
 Pores exist between the sides of parallel molecules.
Pores exist between the sides of parallel molecules.
 Mineral deposition (calcification) occurs within the hole zones and pores (Figure 1-4).
Mineral deposition (calcification) occurs within the hole zones and pores (Figure 1-4).
 Cross-linking decreases collagen solubility and increases its tensile strength.
Cross-linking decreases collagen solubility and increases its tensile strength.
 Matrix proteins (noncollagenous)
Matrix proteins (noncollagenous)
2. Inorganic (mineral) components: 60% of the dry weight of bone
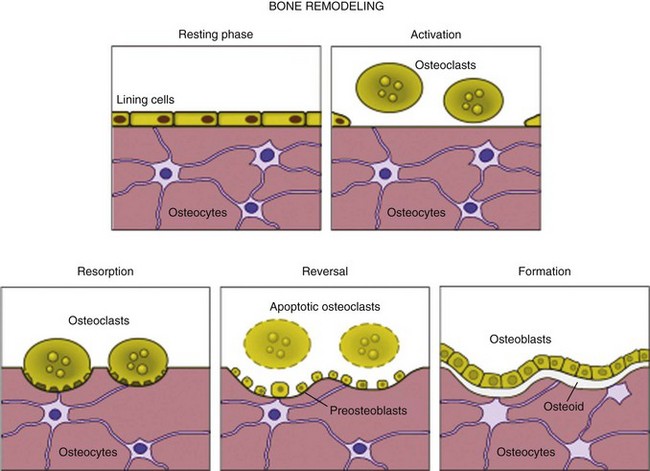
 Cortical and cancellous bone is continuously remodeled throughout life by osteoclastic and osteoblastic activity (Figure 1-5).
Cortical and cancellous bone is continuously remodeled throughout life by osteoclastic and osteoblastic activity (Figure 1-5).
 Wolff’s law: Remodeling occurs in response to mechanical stress.
Wolff’s law: Remodeling occurs in response to mechanical stress.
 Increasing mechanical stress increases bone gain.
Increasing mechanical stress increases bone gain.
 Removing external mechanical stress increases bone loss, which is reversible (to varying degrees) on remobilization.
Removing external mechanical stress increases bone loss, which is reversible (to varying degrees) on remobilization.
 Piezoelectric remodeling occurs in response to electrical charge.
Piezoelectric remodeling occurs in response to electrical charge.
 The compression side of bone is electronegative, stimulating osteoblasts (formation).
The compression side of bone is electronegative, stimulating osteoblasts (formation).
 The tension side of bone is electropositive, stimulating osteoclasts (resorption).
The tension side of bone is electropositive, stimulating osteoclasts (resorption).
 Hueter-Volkmann law: Remodeling occurs in small packets of cells known as basic multicellular units (BMUs).
Hueter-Volkmann law: Remodeling occurs in small packets of cells known as basic multicellular units (BMUs).
 Bone receives 5% to 10% of the cardiac output.
Bone receives 5% to 10% of the cardiac output.
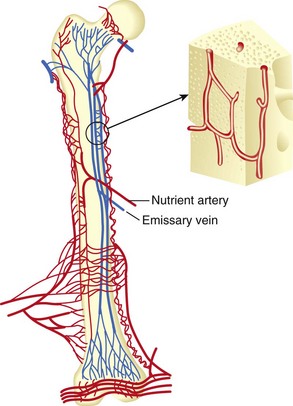
 Long bones receive blood from three sources (systems):
Long bones receive blood from three sources (systems):
 Nutrient arteries branch from systemic arteries, enter the diaphyseal cortex through the nutrient foramen, enter the medullary canal, and branch into ascending and descending arteries (Figure 1-7).
Nutrient arteries branch from systemic arteries, enter the diaphyseal cortex through the nutrient foramen, enter the medullary canal, and branch into ascending and descending arteries (Figure 1-7).
 Further branching into arterioles in the endosteal cortex enables blood supply to at least the inner two thirds of the mature diaphyseal cortex via the Haversian system (Figures 1-8 and 1-9).
Further branching into arterioles in the endosteal cortex enables blood supply to at least the inner two thirds of the mature diaphyseal cortex via the Haversian system (Figures 1-8 and 1-9).
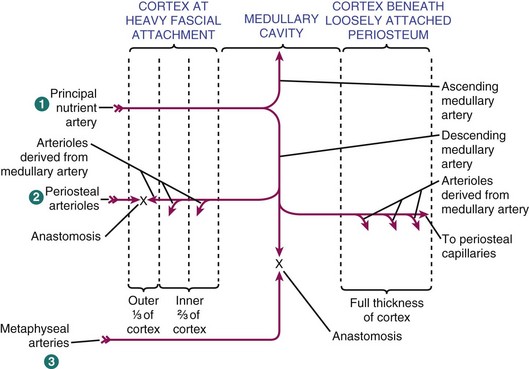
 Direction of flow (Figure 1-10)
Direction of flow (Figure 1-10)
 Arterial flow in mature bone is centrifugal (inside to outside), which is the net effect of the high-pressure nutrient artery system and the low-pressure periosteal system.
Arterial flow in mature bone is centrifugal (inside to outside), which is the net effect of the high-pressure nutrient artery system and the low-pressure periosteal system.
 When fracture disrupts the nutrient artery system, the periosteal system pressure predominates, and blood flow is centripetal (outside to inside).
When fracture disrupts the nutrient artery system, the periosteal system pressure predominates, and blood flow is centripetal (outside to inside).
 Flow in immature, developing bone is centripetal because the highly vascularized periosteal system is the predominant component.
Flow in immature, developing bone is centripetal because the highly vascularized periosteal system is the predominant component.
 Bone blood flow is the major determinant of how well a fracture heals.
Bone blood flow is the major determinant of how well a fracture heals.
 Initial response is a decrease in bone blood flow after vascular disruption at the fracture site.
Initial response is a decrease in bone blood flow after vascular disruption at the fracture site.
 Within hours to days, bone blood flow increases (as part of the regional acceleratory phenomenon), peaks at approximately 2 weeks, and returns to normal in 3 to 5 months.
Within hours to days, bone blood flow increases (as part of the regional acceleratory phenomenon), peaks at approximately 2 weeks, and returns to normal in 3 to 5 months.
 Unreamed intramedullary nails preserve endosteal blood supply.
Unreamed intramedullary nails preserve endosteal blood supply.
 This connective tissue membrane covers bone.
This connective tissue membrane covers bone.
 It is more highly developed in children.
It is more highly developed in children.
 The inner periosteum, or cambium, is loose and vascular and contains cells capable of becoming osteoblasts.
The inner periosteum, or cambium, is loose and vascular and contains cells capable of becoming osteoblasts.
 These cells enlarge the diameter of bone during growth and form periosteal callus during fracture healing.
These cells enlarge the diameter of bone during growth and form periosteal callus during fracture healing.
 The outer (fibrous) periosteum is less cellular and is contiguous with joint capsules.
The outer (fibrous) periosteum is less cellular and is contiguous with joint capsules.
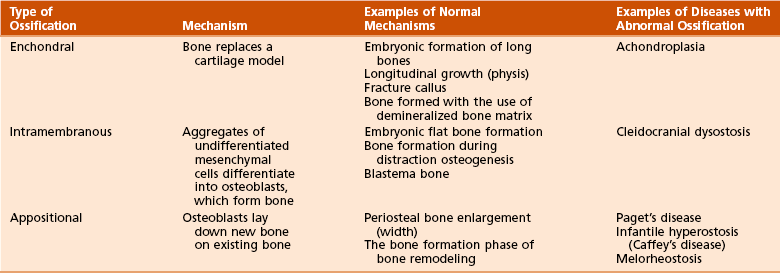
G Types of bone formation (Table 1-4)
1. Enchondral bone formation and mineralization
 Undifferentiated cells secrete the cartilaginous matrix and differentiate into chondrocytes.
Undifferentiated cells secrete the cartilaginous matrix and differentiate into chondrocytes.
 The matrix mineralizes and is invaded by vascular buds that bring osteoprogenitor cells.
The matrix mineralizes and is invaded by vascular buds that bring osteoprogenitor cells.
 Osteoclasts resorb calcified cartilage, and osteoblasts form bone.
Osteoclasts resorb calcified cartilage, and osteoblasts form bone.
 Bone replaces the cartilage model; cartilage is not converted to bone.
Bone replaces the cartilage model; cartilage is not converted to bone.
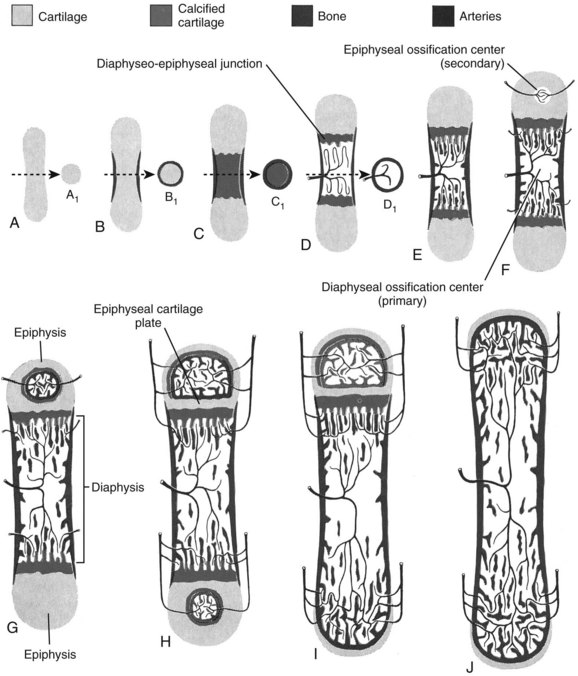
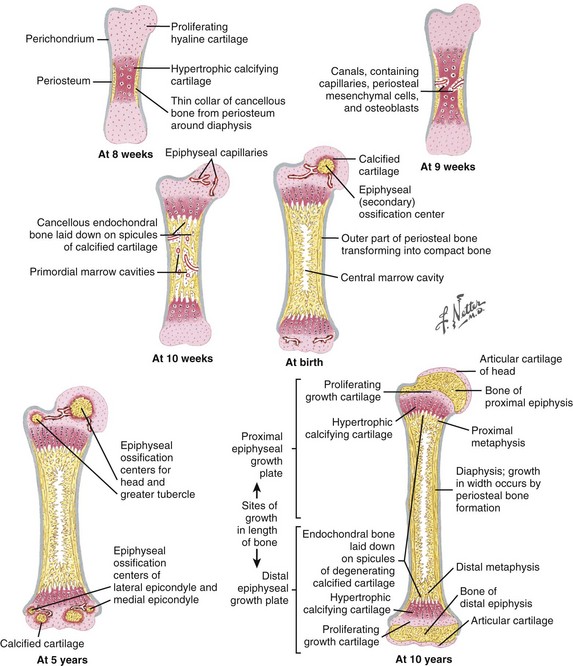
 Embryonic formation of long bones (Figures 1-11 and 1-12)
Embryonic formation of long bones (Figures 1-11 and 1-12)
 These bones are formed from the mesenchymal anlage, at 6 weeks of gestation.
These bones are formed from the mesenchymal anlage, at 6 weeks of gestation.
 Vascular buds invade the mesenchymal model, bringing osteoprogenitor cells that differentiate into osteoblasts and form the primary ossification centers at 8 weeks.
Vascular buds invade the mesenchymal model, bringing osteoprogenitor cells that differentiate into osteoblasts and form the primary ossification centers at 8 weeks.
 The cartilage model increases in size through appositional (width) and interstitial (length) growth.
The cartilage model increases in size through appositional (width) and interstitial (length) growth.
 The marrow forms by resorption of the central cartilage anlage by invasion of myeloid precursor cells that are brought in by the capillary buds.
The marrow forms by resorption of the central cartilage anlage by invasion of myeloid precursor cells that are brought in by the capillary buds.
 Secondary ossification centers develop at the bone ends, forming the epiphyseal centers (growth plates) responsible for longitudinal growth.
Secondary ossification centers develop at the bone ends, forming the epiphyseal centers (growth plates) responsible for longitudinal growth.
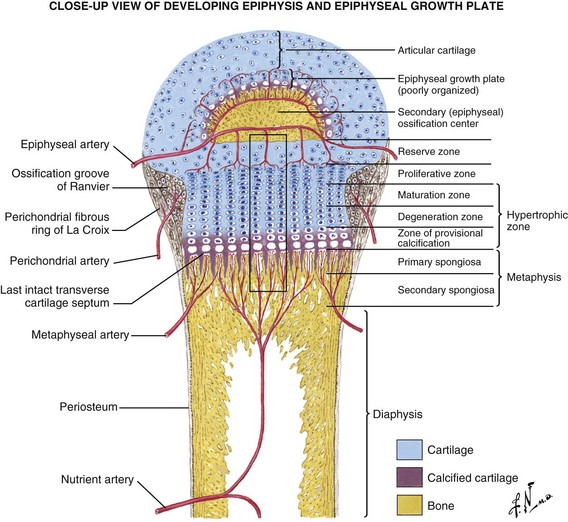
 Arterial supply is rich during development, with an epiphyseal artery (terminates in the proliferative zone), metaphyseal arteries, nutrient arteries, and perichondrial arteries (Figure 1-13).
Arterial supply is rich during development, with an epiphyseal artery (terminates in the proliferative zone), metaphyseal arteries, nutrient arteries, and perichondrial arteries (Figure 1-13).
 Two growth plates exist in immature long bones: (1) horizontal (the physis) and (2) spherical (growth of the epiphysis).
Two growth plates exist in immature long bones: (1) horizontal (the physis) and (2) spherical (growth of the epiphysis).
 The perichondrial artery is the major source of nutrition of the growth plate.
The perichondrial artery is the major source of nutrition of the growth plate.
 Acromegaly and spondyloepiphyseal dysplasia affect the physis; multiple epiphyseal dysplasia affects the epiphysis.
Acromegaly and spondyloepiphyseal dysplasia affect the physis; multiple epiphyseal dysplasia affects the epiphysis.
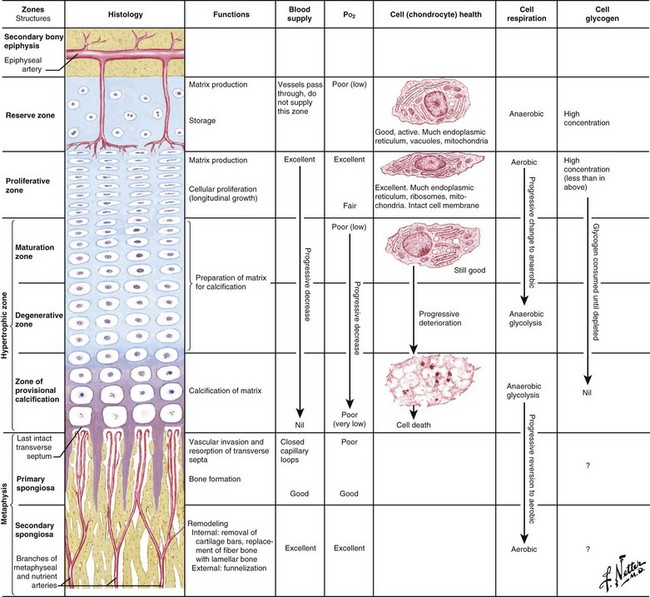
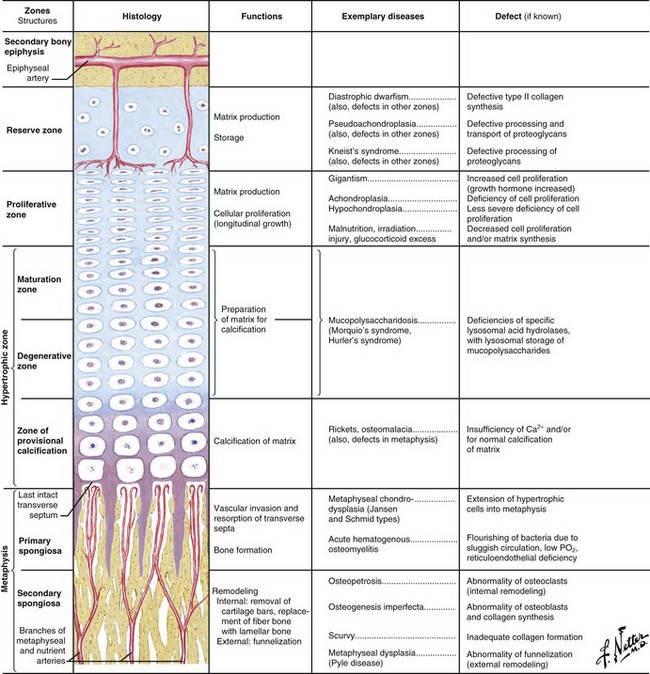
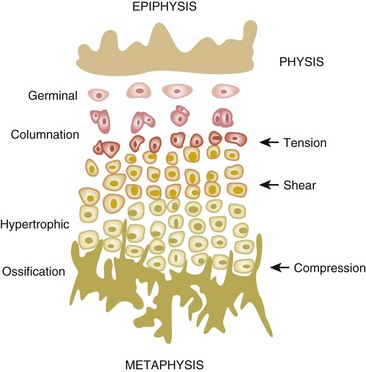
 Delineation of physeal cartilage zones is based on growth (see Figure 1-13) and function (Figures 1-14 and 1-15).
Delineation of physeal cartilage zones is based on growth (see Figure 1-13) and function (Figures 1-14 and 1-15).
 Reserve zone: Cells store lipids, glycogen, and proteoglycan aggregates; decreased oxygen tension occurs in this zone.
Reserve zone: Cells store lipids, glycogen, and proteoglycan aggregates; decreased oxygen tension occurs in this zone.
 Proliferative zone: Growth is longitudinal, with stacking of chondrocytes (the top cell is the dividing “mother” cell), cellular proliferation, and matrix production; increased oxygen tension and increased proteoglycans inhibit calcification.
Proliferative zone: Growth is longitudinal, with stacking of chondrocytes (the top cell is the dividing “mother” cell), cellular proliferation, and matrix production; increased oxygen tension and increased proteoglycans inhibit calcification.
 Hypertrophic zone: This area is sometimes divided into three zones: maturation, degeneration, and provisional calcification.
Hypertrophic zone: This area is sometimes divided into three zones: maturation, degeneration, and provisional calcification.
 Normal matrix mineralization occurs in the lower hypertrophic zone: chondrocytes increase five times in size, accumulate calcium in their mitochondria, die, and release calcium from matrix vesicles.
Normal matrix mineralization occurs in the lower hypertrophic zone: chondrocytes increase five times in size, accumulate calcium in their mitochondria, die, and release calcium from matrix vesicles.
 Chondrocyte maturation is regulated by systemic hormones and local growth factors (PTH-related peptide inhibits chondrocyte maturation; Indian hedgehog is produced by chondrocytes and regulates the expression of PTH-related peptide).
Chondrocyte maturation is regulated by systemic hormones and local growth factors (PTH-related peptide inhibits chondrocyte maturation; Indian hedgehog is produced by chondrocytes and regulates the expression of PTH-related peptide).
 Osteoblasts migrate from sinusoidal vessels and use cartilage as a scaffolding for bone formation.
Osteoblasts migrate from sinusoidal vessels and use cartilage as a scaffolding for bone formation.
 This zone widens in rickets (see Figure 1-15), with little or no provisional calcification.
This zone widens in rickets (see Figure 1-15), with little or no provisional calcification.
 Mucopolysaccharide diseases (see Figure 1-15) affect this zone, leading to chondrocyte degeneration.
Mucopolysaccharide diseases (see Figure 1-15) affect this zone, leading to chondrocyte degeneration.
 Physeal fractures probably traverse several zones, depending on the type of loading (Figure 1-16).
Physeal fractures probably traverse several zones, depending on the type of loading (Figure 1-16).
 Slipped capital femoral epiphysis (SCFE) believed to occur here (through metaphyseal spongiosa with renal failure).
Slipped capital femoral epiphysis (SCFE) believed to occur here (through metaphyseal spongiosa with renal failure).
 This is adjacent to the physis and expands with skeletal growth.
This is adjacent to the physis and expands with skeletal growth.
 Osteoblasts from osteoprogenitor cells align on cartilage bars produced by physeal expansion.
Osteoblasts from osteoprogenitor cells align on cartilage bars produced by physeal expansion.
 Primary spongiosa (calcified cartilage bars) mineralizes to form woven bone and remodels to form secondary spongiosa and a “cutback zone” at the metaphysis.
Primary spongiosa (calcified cartilage bars) mineralizes to form woven bone and remodels to form secondary spongiosa and a “cutback zone” at the metaphysis.
 Cortical bone forms as physeal (enchondral), and intramembranous bone remodels in response to stress along the periphery of the growing long bone.
Cortical bone forms as physeal (enchondral), and intramembranous bone remodels in response to stress along the periphery of the growing long bone.
 Groove of Ranvier: supplies chondrocytes to the periphery for lateral growth (width)
Groove of Ranvier: supplies chondrocytes to the periphery for lateral growth (width)
 Perichondrial ring of La Croix: dense fibrous tissue, primary membrane anchoring the periphery of the physis
Perichondrial ring of La Croix: dense fibrous tissue, primary membrane anchoring the periphery of the physis
 Collagen hole zones are seeded with calcium hydroxyapatite crystals through branching and accretion (crystal growth).
Collagen hole zones are seeded with calcium hydroxyapatite crystals through branching and accretion (crystal growth).
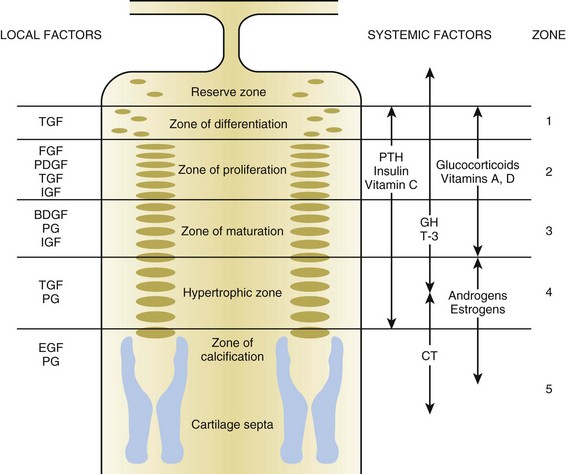
 Hormones and growth factors (Figure 1-17; Table 1-5)
Hormones and growth factors (Figure 1-17; Table 1-5)
Table 1-5
Effects of Hormones and Growth Factors on the Growth Plate
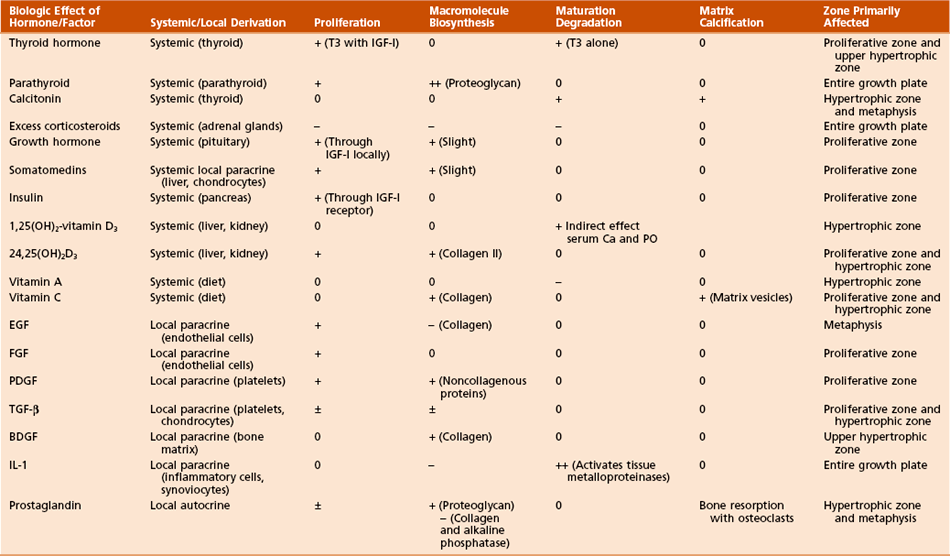
From Simon SR, editor: Orthopaedic basic science, ed 2, Rosemont, Ill, 1994, American Academy of Orthopaedic Surgeons, p 196.
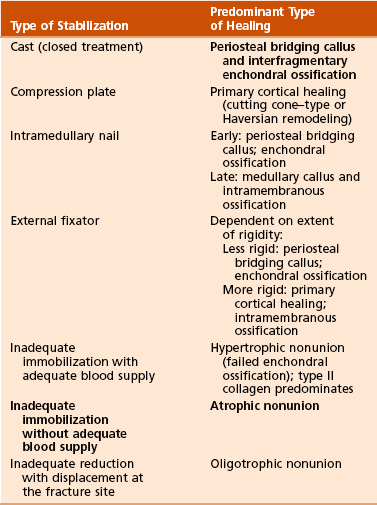
1. A Continuum: inflammation to repair (soft callus followed by hard callus) ending in remodeling
2. Blood supply (bone blood flow): the most important factor
 Bleeding creates a hematoma, which provides hematopoietic cells capable of secreting growth factors.
Bleeding creates a hematoma, which provides hematopoietic cells capable of secreting growth factors.
 Subsequently, fibroblasts, mesenchymal cells, and osteoprogenitor cells form granulation tissue around the fracture ends.
Subsequently, fibroblasts, mesenchymal cells, and osteoprogenitor cells form granulation tissue around the fracture ends.
 Osteoblasts from surrounding osteogenic precursor cells and fibroblasts proliferate.
Osteoblasts from surrounding osteogenic precursor cells and fibroblasts proliferate.
 Primary callus response within 2 weeks.
Primary callus response within 2 weeks.
 For bone ends not in continuity, bridging (soft) callus occurs.
For bone ends not in continuity, bridging (soft) callus occurs.
 Soft callus is later replaced through enchondral ossification by woven bone (hard callus).
Soft callus is later replaced through enchondral ossification by woven bone (hard callus).
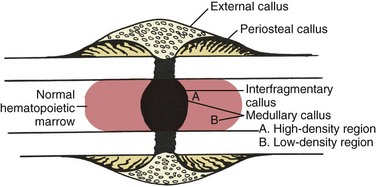
 Medullary callus supplements the bridging callus, forming more slowly and later (Figure 1-18).
Medullary callus supplements the bridging callus, forming more slowly and later (Figure 1-18).
 Remodeling begins in middle of repair phase and continues long after clinically healing (up to 7 years).
Remodeling begins in middle of repair phase and continues long after clinically healing (up to 7 years).
 This process allows the bone to assume its normal configuration and shape according to stress exposure (Wolff’s law).
This process allows the bone to assume its normal configuration and shape according to stress exposure (Wolff’s law).
 Throughout, woven bone is replaced with lamellar bone.
Throughout, woven bone is replaced with lamellar bone.
 Fracture healing is complete when the marrow space is repopulated.
Fracture healing is complete when the marrow space is repopulated.
4. Biochemistry of fracture healing (Table 1-8)
Table 1-8
Biochemical Steps of Fracture Healing
| Step | Collagen Type |
| Mesenchymal | I, II, III, V |
| Chondroid | II, IX |
| Chondroid-osteoid | I, II, X |
| Osteogenic | I |
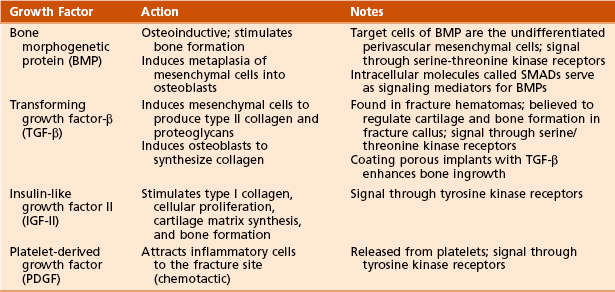
5. Growth factors of bone (Table 1-9)
6. Endocrine effects on fracture healing (Table 1-10)
Table 1-10
Endocrine Effects on Fracture Healing
| Hormone | Effect | Mechanism |
| Cortisone | − | Decreased callus proliferation |
| Calcitonin | +? | Unknown |
| TH, PTH | + | Bone remodeling |
| Growth hormone | + | Increased callus volume |
 Increases time to fracture healing
Increases time to fracture healing
 Increases nonunion risk (particularly in the tibia)
Increases nonunion risk (particularly in the tibia)
 Decreases fracture callus strength
Decreases fracture callus strength
 Increases pseudarthrosis risk after lumbar fusion up to 500%
Increases pseudarthrosis risk after lumbar fusion up to 500%
9. Nonsteroidal anti-inflammatory drugs (NSAIDs)
 These drugs have adverse effects on fracture healing and healing of lumbar spinal fusions.
These drugs have adverse effects on fracture healing and healing of lumbar spinal fusions.
 Cyclooxygenase-2 (COX-2) activity is required for normal enchondral ossification during fracture healing.
Cyclooxygenase-2 (COX-2) activity is required for normal enchondral ossification during fracture healing.
11. Ultrasonography and fracture healing
 Low-intensity pulsed ultrasonography accelerates fracture healing and increases the mechanical strength of callus.
Low-intensity pulsed ultrasonography accelerates fracture healing and increases the mechanical strength of callus.
 A cellular response to the mechanical energy of ultrasonography has been postulated.
A cellular response to the mechanical energy of ultrasonography has been postulated.
12. Effect of radiation on bone
 High-dose irradiation causes long-term changes within the haversian system and decreases cellularity.
High-dose irradiation causes long-term changes within the haversian system and decreases cellularity.
 Protein malnutrition results in negative effects in fracture healing:
Protein malnutrition results in negative effects in fracture healing:
 In experimental models, oral supplementation with essential amino acids improves bone mineral density in fracture callus.
In experimental models, oral supplementation with essential amino acids improves bone mineral density in fracture callus.
14. Electricity and fracture healing
 Types of electrical stimulation
Types of electrical stimulation
 Direct current (DC): stimulates an inflammatory-like response, resulting in decreased oxygen concentrations and increase in tissue pH (stage I)
Direct current (DC): stimulates an inflammatory-like response, resulting in decreased oxygen concentrations and increase in tissue pH (stage I)
 Alternating current (AC): “capacity coupled generators”; affects cyclic adenosine monophosphate (cAMP) synthesis, collagen synthesis, and calcification during the repair stage
Alternating current (AC): “capacity coupled generators”; affects cyclic adenosine monophosphate (cAMP) synthesis, collagen synthesis, and calcification during the repair stage
 Pulsed electromagnetic fields (PEMFs): initiate calcification of fibrocartilage (but not fibrous tissue)
Pulsed electromagnetic fields (PEMFs): initiate calcification of fibrocartilage (but not fibrous tissue)
Table 1-11
Types of Bone Grafts and Bone Graft Properties
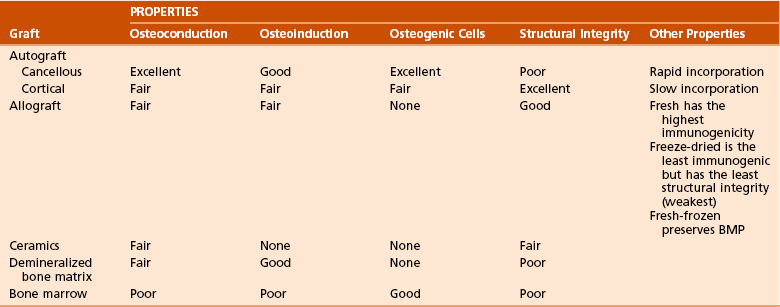
BMP, bone morphogenetic protein.
Modified from Brinker MR, Miller MD: Fundamentals of orthopaedics, Philadelphia, 1999, WB Saunders, p 7.
 Osteoconductive matrix: acts as a scaffold or framework for bone growth
Osteoconductive matrix: acts as a scaffold or framework for bone growth
 Osteoinductive factors: growth factors (BMP) that stimulate bone formation
Osteoinductive factors: growth factors (BMP) that stimulate bone formation
 Osteogenic cells: primitive mesenchymal cells, osteoblasts, and osteocytes
Osteogenic cells: primitive mesenchymal cells, osteoblasts, and osteocytes
 Autografts (from same person) or allografts (from another person)
Autografts (from same person) or allografts (from another person)
 Cancellous bone: for grafting nonunions or cavitary defects; remodels quickly and incorporates through the laying down of new bone on old trabeculae (“creeping substitution”)
Cancellous bone: for grafting nonunions or cavitary defects; remodels quickly and incorporates through the laying down of new bone on old trabeculae (“creeping substitution”)
 Cortical bone: slower to turn over; used for structural defects
Cortical bone: slower to turn over; used for structural defects
 Osteoarticular (osteochondral) allograft used for tumor surgery
Osteoarticular (osteochondral) allograft used for tumor surgery
 Immunogenic (cartilage is vulnerable to inflammatory mediators of immune response)
Immunogenic (cartilage is vulnerable to inflammatory mediators of immune response)
 Articular cartilage preserved with glycerol or dimethyl sulfoxide (DMSO)
Articular cartilage preserved with glycerol or dimethyl sulfoxide (DMSO)
 Cryogenically preserved grafts (leave few viable chondrocytes)
Cryogenically preserved grafts (leave few viable chondrocytes)
 Tissue-matched (syngeneic) osteochondral grafts (produce minimal immunogenic effects and incorporate well)
Tissue-matched (syngeneic) osteochondral grafts (produce minimal immunogenic effects and incorporate well)
 Fresh: increased immunogenicity
Fresh: increased immunogenicity
 Fresh-frozen: less immunogenic than fresh; BMP preserved
Fresh-frozen: less immunogenic than fresh; BMP preserved
 Freeze-dried (lyophilized “croutons”): loses structural integrity and depletes BMP, is least immunogenic, is purely osteoconductive, has lowest risk of viral transmission
Freeze-dried (lyophilized “croutons”): loses structural integrity and depletes BMP, is least immunogenic, is purely osteoconductive, has lowest risk of viral transmission
 Bone matrix gelatin (a digested source of BMP): demineralized bone matrix is osteoconductive and osteoinductive
Bone matrix gelatin (a digested source of BMP): demineralized bone matrix is osteoconductive and osteoinductive
 Allograft bone possesses a spectrum of potential antigens, primarily from cell surface glycoproteins.
Allograft bone possesses a spectrum of potential antigens, primarily from cell surface glycoproteins.
 Classes I and II cellular antigens in allograft are recognized by T lymphocytes in the host.
Classes I and II cellular antigens in allograft are recognized by T lymphocytes in the host.
 Primary mechanism of rejection is cellular, as opposed to humoral.
Primary mechanism of rejection is cellular, as opposed to humoral.
 Cellular components that contribute to antigenicity are marrow origin, endothelium, and retinacular activating cells.
Cellular components that contribute to antigenicity are marrow origin, endothelium, and retinacular activating cells.
 Extracellular matrix components that contribute to antigenicity are as follows:
Extracellular matrix components that contribute to antigenicity are as follows:
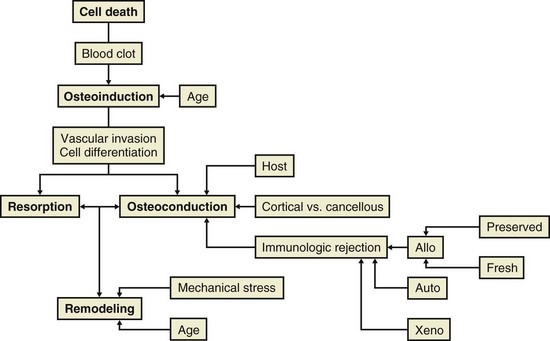
4. Five Stages of graft healing (Urist) (Table 1-12)
Table 1-12
| Stage | Activity |
| 1: Inflammation | Chemotaxis stimulated by necrotic debris |
| 2: Osteoblast differentiation | From precursors |
| 3: Osteoinduction | Osteoblast and osteoclast function |
| 4: Osteoconduction | New bone forming over scaffold |
| 5: Remodeling | Process continues for years |
 Slower incorporation: remodels existing haversian systems through resorption (weakens the graft) and then deposits new bone (restores strength)
Slower incorporation: remodels existing haversian systems through resorption (weakens the graft) and then deposits new bone (restores strength)
 Resorption confined to osteon borders; interstitial lamellae are preserved
Resorption confined to osteon borders; interstitial lamellae are preserved
 Of massive grafts, 25% eventually sustain insufficiency fracture
Of massive grafts, 25% eventually sustain insufficiency fracture
 These grafts revascularize and incorporate quickly.
These grafts revascularize and incorporate quickly.
 Osteoblasts lay down new bone on old trabeculae, which are later remodeled (“creeping substitution”).
Osteoblasts lay down new bone on old trabeculae, which are later remodeled (“creeping substitution”).
 Synthetic bone grafts: calcium, silicon, or aluminum
Synthetic bone grafts: calcium, silicon, or aluminum
 Calcium phosphate–based grafts: capable of osseoconduction and osseointegration
Calcium phosphate–based grafts: capable of osseoconduction and osseointegration
 Highest compressive strength of any graft material
Highest compressive strength of any graft material
 Many prepared as ceramics (heated apatite crystals fuse into crystals [sintered])
Many prepared as ceramics (heated apatite crystals fuse into crystals [sintered])
 Calcium sulfate: osteoconductive
Calcium sulfate: osteoconductive
 Calcium carbonate (chemically unaltered marine coral): resorbed and replaced by bone (osteoconductive)
Calcium carbonate (chemically unaltered marine coral): resorbed and replaced by bone (osteoconductive)
 Coralline hydroxyapatite: calcium carbonate skeleton is converted to calcium phosphate through a thermoexchange process
Coralline hydroxyapatite: calcium carbonate skeleton is converted to calcium phosphate through a thermoexchange process
 Silicate-based incorporate silicon as silicate (silicon dioxide); bioactive glasses and glass-ionomer cement
Silicate-based incorporate silicon as silicate (silicon dioxide); bioactive glasses and glass-ionomer cement
 Aluminum oxide: alumina ceramic bonds to bone in response to stress and strain between implant and bone
Aluminum oxide: alumina ceramic bonds to bone in response to stress and strain between implant and bone
C Distraction osteogenesis (Figure 1-20)
1. Definition: distraction-stimulated formation of bone
 Under optimal stability, intramembranous ossification occurs.
Under optimal stability, intramembranous ossification occurs.
 Under instability, bone forms through enchondral ossification.
Under instability, bone forms through enchondral ossification.
4. Optimal conditions during distraction osteogenesis:
 Low-energy corticotomy/osteotomy
Low-energy corticotomy/osteotomy
 Minimal soft tissue stripping at the corticotomy site (preserves blood supply)
Minimal soft tissue stripping at the corticotomy site (preserves blood supply)
 Stable external fixation and elimination of torsion, shear, and bending moments
Stable external fixation and elimination of torsion, shear, and bending moments
 Latency period (no lengthening) 5 to 7 days
Latency period (no lengthening) 5 to 7 days
 Distraction: 0.25 mm three or four times per day (0.75 to 1.0 mm per day)
Distraction: 0.25 mm three or four times per day (0.75 to 1.0 mm per day)
 Neutral fixation interval (no distraction) during consolidation
Neutral fixation interval (no distraction) during consolidation
 Normal physiologic use of the extremity, including weight bearing
Normal physiologic use of the extremity, including weight bearing
1. Ectopic bone forms in soft tissues.
2. Traumatic brain injury increases risk of heterotopic ossification.
 Recurrence after resection is likely if neurologic compromise is severe.
Recurrence after resection is likely if neurologic compromise is severe.
 The timing of surgery for heterotopic ossification after traumatic brain injury is important:
The timing of surgery for heterotopic ossification after traumatic brain injury is important:
3. Heterotopic ossification may be resected after total hip arthroplasty (THA).
 Resection should be delayed for 6 months or longer after THA.
Resection should be delayed for 6 months or longer after THA.
 Adjuvant radiation therapy may prevent recurrence of heterotopic ossification.
Adjuvant radiation therapy may prevent recurrence of heterotopic ossification.
4. Preoperative radiation (600 to 800 rad) may be used in treatment.
 Helps prevent heterotopic ossification after THA in patients at high risk for this development.
Helps prevent heterotopic ossification after THA in patients at high risk for this development.
 Incidence of heterotopic ossification after THA among patients with Paget’s disease is approximately 50%.
Incidence of heterotopic ossification after THA among patients with Paget’s disease is approximately 50%.
5. When oral bisphosphonate therapy is discontinued, heterotopic ossification may occur.
III CONDITIONS OF BONE MINERALIZATION, BONE MINERAL DENSITY, AND BONE VIABILITY
 Important in muscle and nerve function, clotting, and many other areas
Important in muscle and nerve function, clotting, and many other areas
 >99% of the body’s calcium is stored in bones.
>99% of the body’s calcium is stored in bones.
 Plasma calcium is about equally free and bound (usually to albumin).
Plasma calcium is about equally free and bound (usually to albumin).
 Approximately 400 mg of calcium is released from bone daily.
Approximately 400 mg of calcium is released from bone daily.
 Absorbed in the duodenum by active transport
Absorbed in the duodenum by active transport
 Absorbed in the jejunum by passive diffusion
Absorbed in the jejunum by passive diffusion
 The kidney reabsorbs 98% of calcium (60% in the proximal tubule).
The kidney reabsorbs 98% of calcium (60% in the proximal tubule).
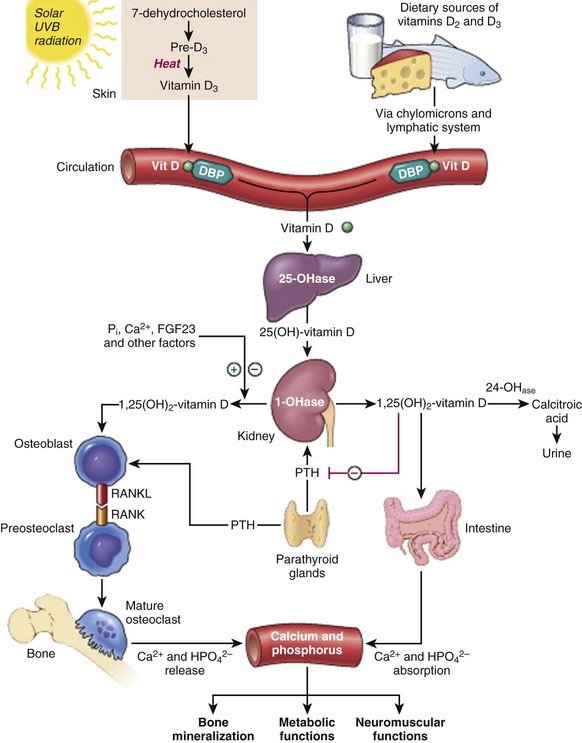
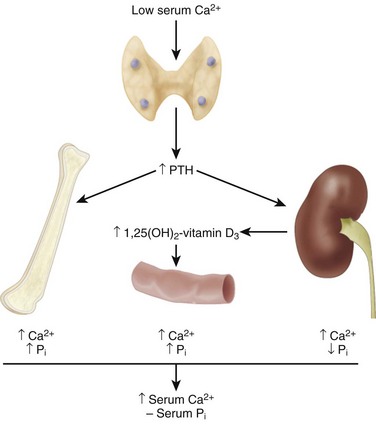
 The primary homeostatic regulators of serum calcium are PTH and 1,25(OH)2-vitamin D3
The primary homeostatic regulators of serum calcium are PTH and 1,25(OH)2-vitamin D3
 Dietary requirement of elemental calcium:
Dietary requirement of elemental calcium:
 Approximately 600 mg/day for children
Approximately 600 mg/day for children
 Approximately 1300 mg/day for adolescents and young adults (ages 10 to 25 years)
Approximately 1300 mg/day for adolescents and young adults (ages 10 to 25 years)
 750 mg/day for adults (ages 25 to 65 years)
750 mg/day for adults (ages 25 to 65 years)
 1500 mg/day for pregnant women
1500 mg/day for pregnant women
 2000 mg/day for lactating women
2000 mg/day for lactating women
 1500 mg/day for postmenopausal women and for patients with a healing fracture in a long bone
1500 mg/day for postmenopausal women and for patients with a healing fracture in a long bone
 Calcium balance is usually positive in the first three decades of life and negative after the fourth decade
Calcium balance is usually positive in the first three decades of life and negative after the fourth decade
 A key component of bone mineral
A key component of bone mineral
 Also important in enzyme systems and molecular interactions
Also important in enzyme systems and molecular interactions
 PTH is an 84–amino acid peptide.
PTH is an 84–amino acid peptide.
 It is synthesized in and secreted from the chief cells of the (four) parathyroid glands.
It is synthesized in and secreted from the chief cells of the (four) parathyroid glands.
 The N-terminal fragment 1-34 is the active portion.
The N-terminal fragment 1-34 is the active portion.
 Teriparatide, the synthetic form of recombinant human PTH, contains this active sequence.
Teriparatide, the synthetic form of recombinant human PTH, contains this active sequence.
 The effect of PTH is mediated by the cAMP second-messenger mechanism.
The effect of PTH is mediated by the cAMP second-messenger mechanism.
 PTH helps regulate plasma calcium.
PTH helps regulate plasma calcium.
 Decreased calcium levels in the extracellular fluid stimulate β2 receptors to release PTH, which acts at the intestines, kidneys, and bones (Table 1-13).
Decreased calcium levels in the extracellular fluid stimulate β2 receptors to release PTH, which acts at the intestines, kidneys, and bones (Table 1-13).
Table 1-13
Regulation of Calcium and Phosphate Metabolism
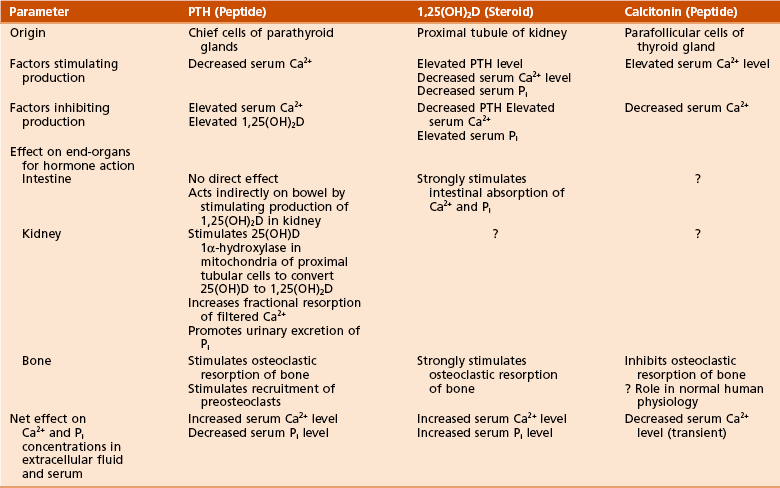
Adapted from Netter FH: CIBA collection of medical illustrations, vol 8: Musculoskeletal system, part I: Anatomy, physiology and developmental disorders, Basel, Switzerland, 1987, CIBA, p 179.
 PTH directly activates osteoblasts.
PTH directly activates osteoblasts.
 PTH modulates renal phosphate filtration.
PTH modulates renal phosphate filtration.
 PTH may accentuate bone loss in elderly persons.
PTH may accentuate bone loss in elderly persons.
 PTH-related protein and its receptor have been implicated in metaphyseal dysplasia.
PTH-related protein and its receptor have been implicated in metaphyseal dysplasia.
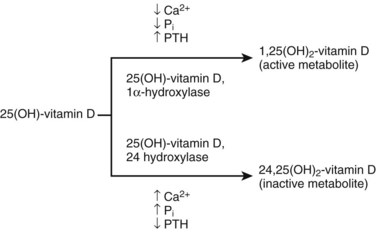
 Hydroxylated to 25(OH)-vitamin D3 in the liver and hydroxylated a second time in the kidney to one of the following:
Hydroxylated to 25(OH)-vitamin D3 in the liver and hydroxylated a second time in the kidney to one of the following:
 1,25(OH)2-vitamin D3 works at the intestines, kidneys, and bones (see Table 1-13).
1,25(OH)2-vitamin D3 works at the intestines, kidneys, and bones (see Table 1-13).
 A 32–amino acid peptide hormone
A 32–amino acid peptide hormone
 Has a limited role in calcium regulation (see Table 1-13)
Has a limited role in calcium regulation (see Table 1-13)
 Increased extracellular calcium levels cause secretion of calcitonin
Increased extracellular calcium levels cause secretion of calcitonin
 Inhibits osteoclastic bone resorption
Inhibits osteoclastic bone resorption
 May also have a role in fracture healing and in reducing vertebral compression fractures in high-turnover osteoporosis
May also have a role in fracture healing and in reducing vertebral compression fractures in high-turnover osteoporosis
6. Other hormones affecting bone metabolism
 Estrogen prevents bone loss by inhibiting bone resorption.
Estrogen prevents bone loss by inhibiting bone resorption.
 Because bone formation and resorption are coupled, estrogen therapy also decreases bone formation.
Because bone formation and resorption are coupled, estrogen therapy also decreases bone formation.
 Supplementation is helpful in postmenopausal women only if started within 5 to 10 years after onset of menopause.
Supplementation is helpful in postmenopausal women only if started within 5 to 10 years after onset of menopause.
 Risk of endometrial cancer is reduced when estrogen therapy is combined with cyclic progestin therapy.
Risk of endometrial cancer is reduced when estrogen therapy is combined with cyclic progestin therapy.
 Certain regimens of hormone replacement therapy may increase risk of heart disease and breast cancer.
Certain regimens of hormone replacement therapy may increase risk of heart disease and breast cancer.
 Calcium and phosphate metabolism
Calcium and phosphate metabolism
 Feedback mechanisms: important in the regulation of plasma levels of calcium and phosphate
Feedback mechanisms: important in the regulation of plasma levels of calcium and phosphate
 After peak, bone loss occurs at a rate of 0.3% to 0.5% per year
After peak, bone loss occurs at a rate of 0.3% to 0.5% per year
 Rate of bone loss is 2% to 3% per year in untreated women during the sixth through tenth years after menopause.
Rate of bone loss is 2% to 3% per year in untreated women during the sixth through tenth years after menopause.
 Occurs at the onset of menopause, when both bone formation and resorption are accelerated
Occurs at the onset of menopause, when both bone formation and resorption are accelerated
 A net negative change in calcium balance: menopause decreases intestinal absorption and increases urinary excretion of calcium
A net negative change in calcium balance: menopause decreases intestinal absorption and increases urinary excretion of calcium
 Both urinary hydroxyproline and pyridinoline cross-links are elevated when bone resorption occurs.
Both urinary hydroxyproline and pyridinoline cross-links are elevated when bone resorption occurs.
 Serum alkaline phosphatase level is elevated when bone formation is increased.
Serum alkaline phosphatase level is elevated when bone formation is increased.
 Estrogen therapy is recommended for patients at high risk for osteoporosis.
Estrogen therapy is recommended for patients at high risk for osteoporosis.
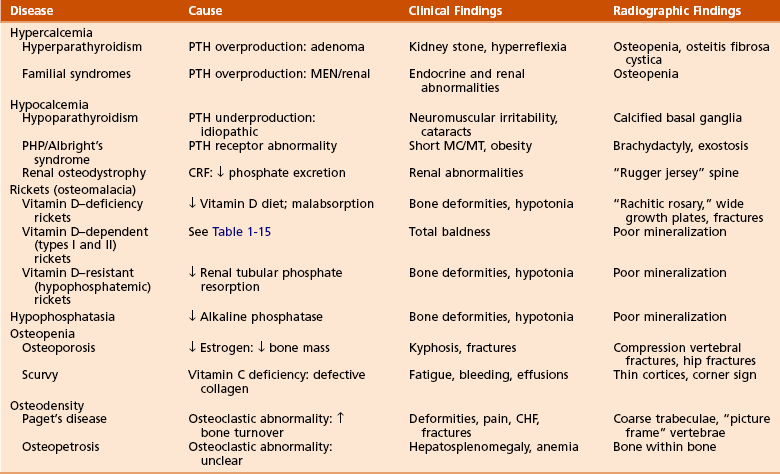
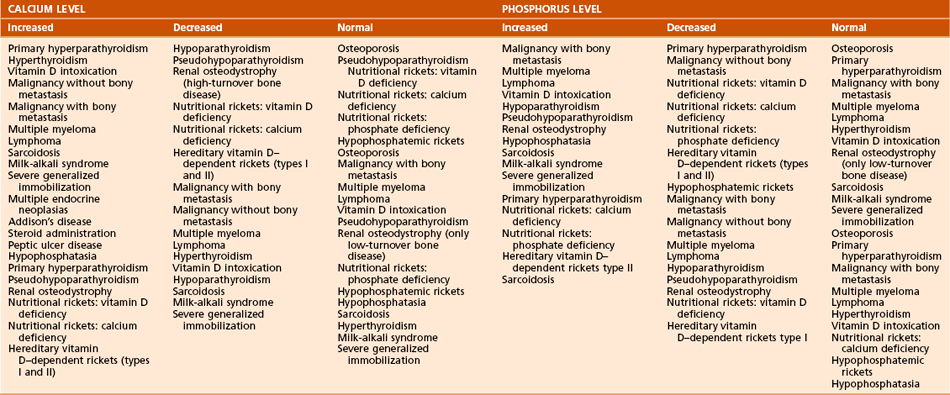
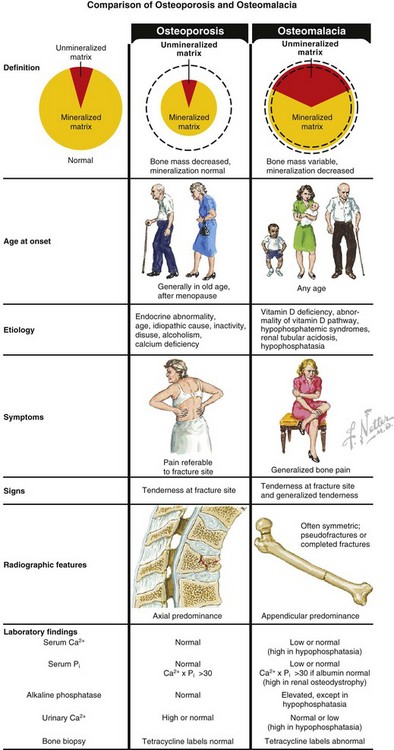
B Conditions of bone mineralization (Tables 1-14 through 1-16)
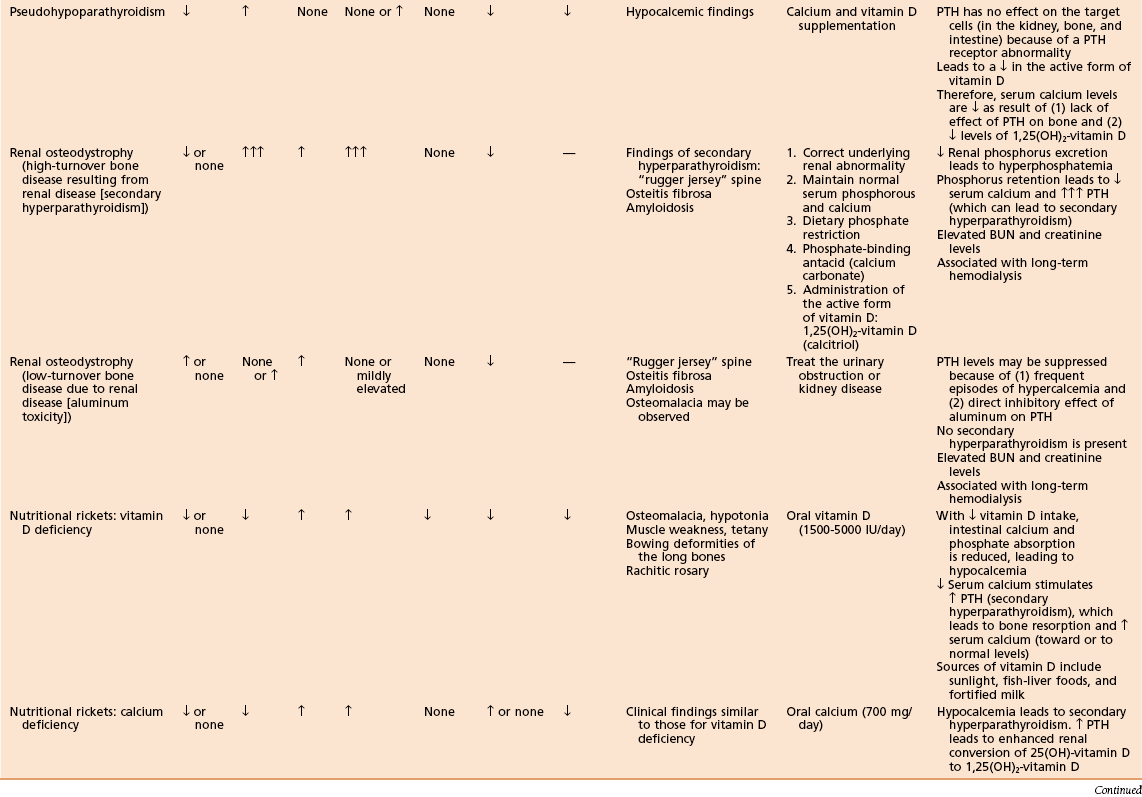
Table 1-15
Laboratory Findings and Clinical Data Regarding Patients with Various Metabolic Bone Diseases
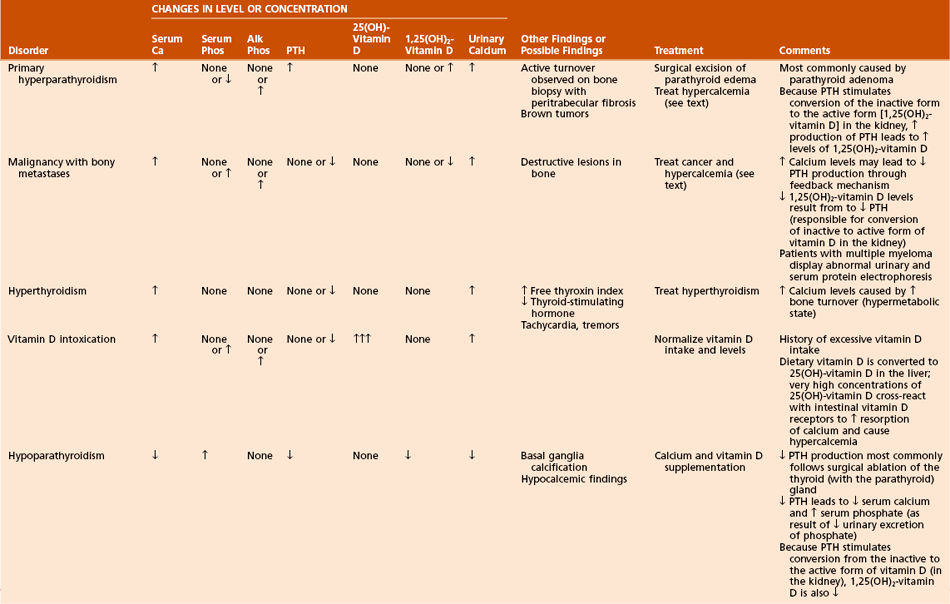
 Can manifest in a number of ways:
Can manifest in a number of ways:
 Excessive bony resorption with or without fibrotic tissue replacement (osteitis fibrosa cystica)
Excessive bony resorption with or without fibrotic tissue replacement (osteitis fibrosa cystica)
 Central nervous system (CNS) effects (confusion, stupor, weakness)
Central nervous system (CNS) effects (confusion, stupor, weakness)
 Can also cause anorexia, nausea, vomiting, dehydration, and muscle weakness
Can also cause anorexia, nausea, vomiting, dehydration, and muscle weakness
 Overproduction of PTH, usually a result of a parathyroid adenoma
Overproduction of PTH, usually a result of a parathyroid adenoma
 Reflected in a net increase in plasma calcium and a decrease in plasma phosphate (as a result of enhanced urinary excretion)
Reflected in a net increase in plasma calcium and a decrease in plasma phosphate (as a result of enhanced urinary excretion)
 Increased osteoclastic resorption and failure of repair attempts (poor mineralization as a result of low phosphate level)
Increased osteoclastic resorption and failure of repair attempts (poor mineralization as a result of low phosphate level)
 Osteitis fibrosa cystica (fibrous replacement of marrow)
Osteitis fibrosa cystica (fibrous replacement of marrow)
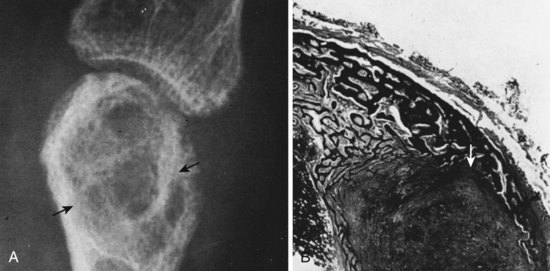
 “Brown tumors” (Figure 1-23): increased giant cells, extravasation of RBCs, hemosiderin staining, fibrous tissue hemosiderin
“Brown tumors” (Figure 1-23): increased giant cells, extravasation of RBCs, hemosiderin staining, fibrous tissue hemosiderin
 Can be life-threatening, commonly associated with muscle weakness
Can be life-threatening, commonly associated with muscle weakness
 Initial treatment should include hydration with normal saline (reverses dehydration).
Initial treatment should include hydration with normal saline (reverses dehydration).
 Can occur in the absence of extensive bone metastasis
Can occur in the absence of extensive bone metastasis
 Most commonly results from the release of systemic growth factors and cytokines that stimulate osteoclastic bone resorption at bony sites not involved in the tumor process
Most commonly results from the release of systemic growth factors and cytokines that stimulate osteoclastic bone resorption at bony sites not involved in the tumor process
 PTH-related protein secretion (lung carcinoma)
PTH-related protein secretion (lung carcinoma)
 Lytic bone metastases and lesions (such as multiple myeloma)
Lytic bone metastases and lesions (such as multiple myeloma)
 Results from low levels of PTH or vitamin D3
Results from low levels of PTH or vitamin D3
 Neuromuscular irritability (tetany, seizures, Chvostek’s sign), cataracts, fungal nail infections, electrocardiographic (ECG) changes (prolonged QT interval), and other signs and symptoms
Neuromuscular irritability (tetany, seizures, Chvostek’s sign), cataracts, fungal nail infections, electrocardiographic (ECG) changes (prolonged QT interval), and other signs and symptoms
 Decreased PTH level causes decrease in plasma calcium level and increase in plasma phosphate level.
Decreased PTH level causes decrease in plasma calcium level and increase in plasma phosphate level.
 Skull radiographs may show basal ganglia calcification.
Skull radiographs may show basal ganglia calcification.
 Iatrogenic hypoparathyroidism most commonly follows thyroidectomy.
Iatrogenic hypoparathyroidism most commonly follows thyroidectomy.
 Pseudohypoparathyroidism (PHP)
Pseudohypoparathyroidism (PHP)
 PTH action is blocked by an abnormality at the receptor, by the cAMP system, or by a lack of required cofactors (e.g., Mg2+).
PTH action is blocked by an abnormality at the receptor, by the cAMP system, or by a lack of required cofactors (e.g., Mg2+).
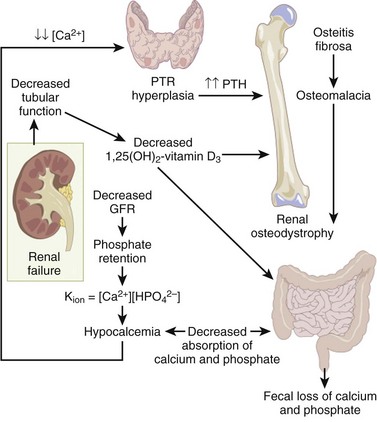
 Renal osteodystrophy (Figure 1-25)
Renal osteodystrophy (Figure 1-25)
 A spectrum of bone mineral metabolism disorders in chronic renal disease
A spectrum of bone mineral metabolism disorders in chronic renal disease
 Renal disease impairs excretion and compromises mineral homeostasis.
Renal disease impairs excretion and compromises mineral homeostasis.
 This process leads to abnormalities in bone mineral metabolism.
This process leads to abnormalities in bone mineral metabolism.
 High-turnover renal bone disease
High-turnover renal bone disease
 Chronically elevated serum PTH level leads to secondary hyperparathyroidism (hyperplasia of the chief cells of the parathyroid gland).
Chronically elevated serum PTH level leads to secondary hyperparathyroidism (hyperplasia of the chief cells of the parathyroid gland).
 Factors contributing to sustained, increased PTH, and secondary hyperparathyroidism include the following:
Factors contributing to sustained, increased PTH, and secondary hyperparathyroidism include the following:
 Diminished renal phosphorus excretion; phosphorus retention promotes PTH secretion by three mechanisms:
Diminished renal phosphorus excretion; phosphorus retention promotes PTH secretion by three mechanisms:
 Hyperphosphatemia lowers serum calcium, stimulating PTH.
Hyperphosphatemia lowers serum calcium, stimulating PTH.
 Phosphorus impairs renal 1α-hydroxylase activity, impairing production of 1,25(OH)2-vitamin D3.
Phosphorus impairs renal 1α-hydroxylase activity, impairing production of 1,25(OH)2-vitamin D3.
 Phosphorus retention may directly increase the synthesis of PTH.
Phosphorus retention may directly increase the synthesis of PTH.
 Impaired renal calcitriol [1,25(OH)2-vitamin D3]
Impaired renal calcitriol [1,25(OH)2-vitamin D3]
 Alterations in the control of PTH gene transcription secretion
Alterations in the control of PTH gene transcription secretion
 Low-turnover renal bone disease (adynamic lesion of bone and osteomalacia)
Low-turnover renal bone disease (adynamic lesion of bone and osteomalacia)
 Secondary hyperparathyroidism is not characteristic with this condition.
Secondary hyperparathyroidism is not characteristic with this condition.
 Bone formation and turnover are reduced.
Bone formation and turnover are reduced.
 Excess deposition of aluminum into bone (aluminum toxicity) negatively affects bone mineral metabolism.
Excess deposition of aluminum into bone (aluminum toxicity) negatively affects bone mineral metabolism.
 Impairs differentiation of precursor cells to osteoblasts
Impairs differentiation of precursor cells to osteoblasts
 Impairs proliferation of osteoblasts
Impairs proliferation of osteoblasts
 Impairs PTH release from the parathyroid gland
Impairs PTH release from the parathyroid gland
 Disrupts the mineralization process
Disrupts the mineralization process
 Adynamic lesion: accounts for the majority of cases of low-turnover bone disease in patients with chronic renal failure
Adynamic lesion: accounts for the majority of cases of low-turnover bone disease in patients with chronic renal failure
 Osteomalacia: defects in mineralization of newly formed bone
Osteomalacia: defects in mineralization of newly formed bone
 Radiographs may demonstrate a “rugger jersey” spine (vertebral bodies appear to have increased density in the upper and lower zones, in a striated appearance), like that in childhood osteopetrosis, and soft tissue calcification
Radiographs may demonstrate a “rugger jersey” spine (vertebral bodies appear to have increased density in the upper and lower zones, in a striated appearance), like that in childhood osteopetrosis, and soft tissue calcification
 β2-microglobulin may accumulate with chronic dialysis, leading to amyloidosis
β2-microglobulin may accumulate with chronic dialysis, leading to amyloidosis
 Amyloidosis may be associated with carpal tunnel syndrome, arthropathy, and pathologic fractures.
Amyloidosis may be associated with carpal tunnel syndrome, arthropathy, and pathologic fractures.
 In amyloidosis, Congo red stain causes material to turn pink.
In amyloidosis, Congo red stain causes material to turn pink.
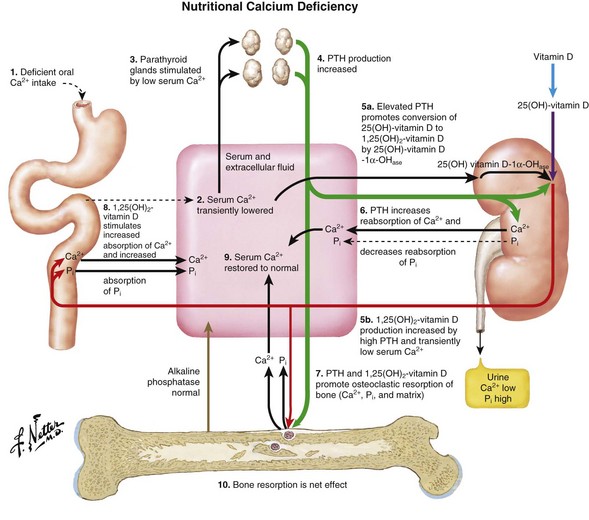
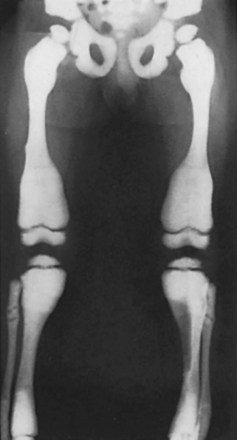
 Rickets (osteomalacia in adults; Box 1-1)
Rickets (osteomalacia in adults; Box 1-1)
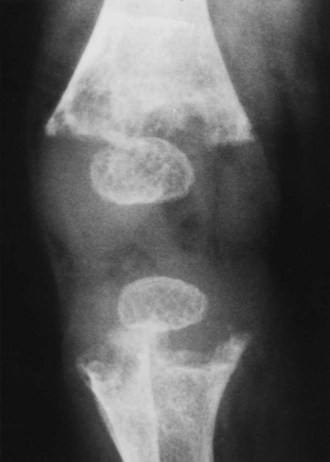
 Failure of mineralization, leading to changes in the physis in the zone of provisional calcification (increased width and disorientation) and bone (cortical thinning, bowing)
Failure of mineralization, leading to changes in the physis in the zone of provisional calcification (increased width and disorientation) and bone (cortical thinning, bowing)
 Nutritional rickets (see Table 1-15)
Nutritional rickets (see Table 1-15)
 Rare after addition of vitamin D to milk, except in the following populations:
Rare after addition of vitamin D to milk, except in the following populations:
 Decreased intestinal absorption of calcium and phosphate leads to secondary hyperparathyroidism
Decreased intestinal absorption of calcium and phosphate leads to secondary hyperparathyroidism
 Low-normal calcium level (maintained by high PTH level)
Low-normal calcium level (maintained by high PTH level)
 Low phosphate level (excreted because of the effect of PTH)
Low phosphate level (excreted because of the effect of PTH)
 Enlargement of the costochondral junction (“rachitic rosary”)
Enlargement of the costochondral junction (“rachitic rosary”)
 Pathologic fractures (Looser’s zones: pseudofracture on the compression side of bone)
Pathologic fractures (Looser’s zones: pseudofracture on the compression side of bone)
 In affected children, height is commonly below the fifth percentile for age.
In affected children, height is commonly below the fifth percentile for age.
 Treatment with vitamin D (5000 IU daily) resolves most deformities.
Treatment with vitamin D (5000 IU daily) resolves most deformities.
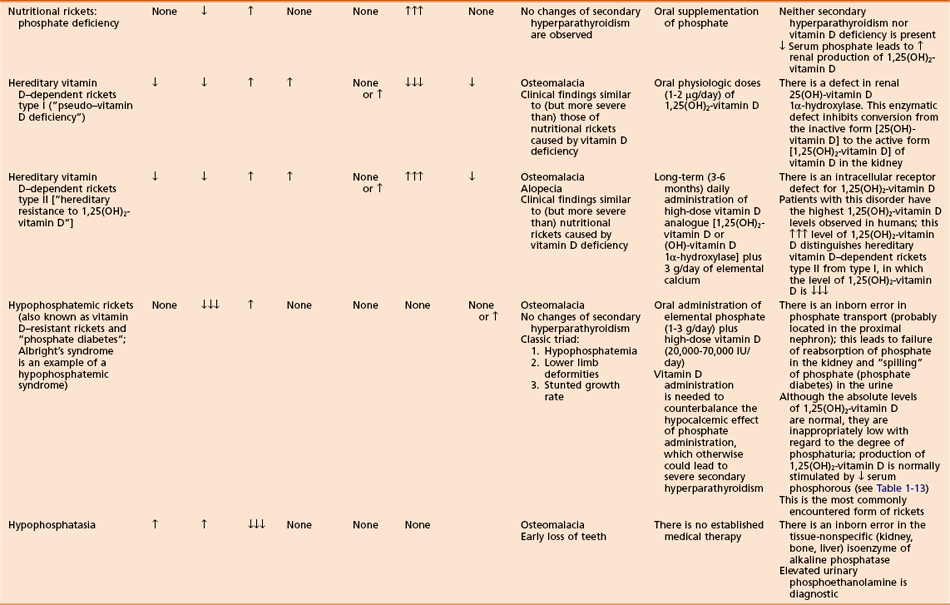
 Hereditary vitamin D–dependent rickets
Hereditary vitamin D–dependent rickets
 Rare disorders with features similar to vitamin D deficiency (nutritional) rickets, except that symptoms may be worse and patients may have total baldness
Rare disorders with features similar to vitamin D deficiency (nutritional) rickets, except that symptoms may be worse and patients may have total baldness
 Familial hypophosphatemic rickets (vitamin D–resistant rickets or “phosphate diabetes”)
Familial hypophosphatemic rickets (vitamin D–resistant rickets or “phosphate diabetes”)
3. Conditions of bone mineral density
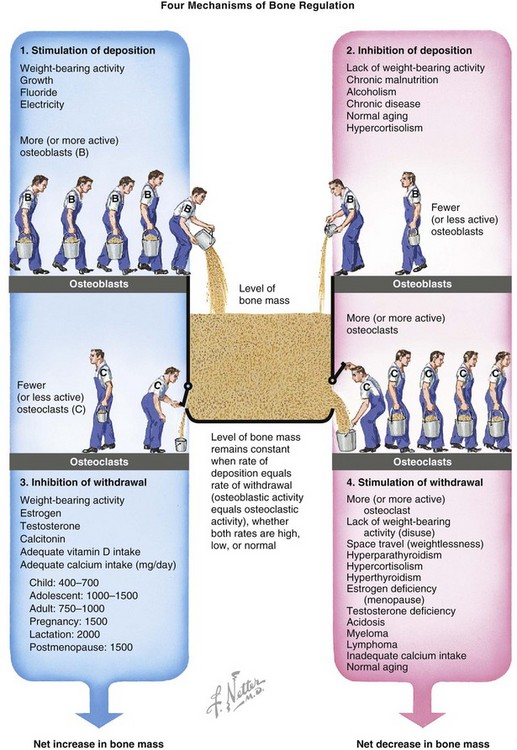
 Bone mass is regulated by relative rates of deposition and withdrawal (Figure 1-29).
Bone mass is regulated by relative rates of deposition and withdrawal (Figure 1-29).
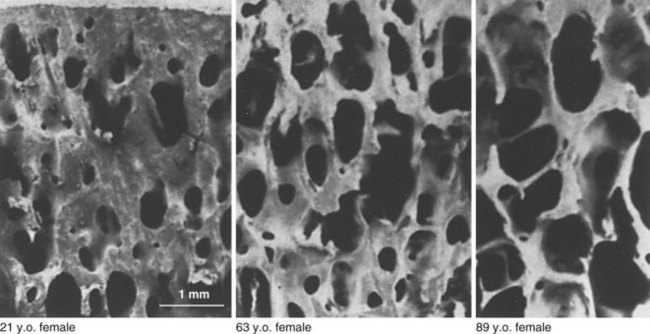
 Age-related decrease in bone mass
Age-related decrease in bone mass
 A quantitative, not qualitative, defect
A quantitative, not qualitative, defect
 World Health Organization’s definition
World Health Organization’s definition
 Lumbar (L2 to L4) density is 2.5 or more standard deviations less than mean peak bone mass of a healthy 25-year-old (T-score).
Lumbar (L2 to L4) density is 2.5 or more standard deviations less than mean peak bone mass of a healthy 25-year-old (T-score).
 Osteopenia: Bone density is 1.0 to 2.5 standard deviations less than the mean peak bone mass of a healthy 25-year-old.
Osteopenia: Bone density is 1.0 to 2.5 standard deviations less than the mean peak bone mass of a healthy 25-year-old.
 Responsible for more than 1 million fractures/year
Responsible for more than 1 million fractures/year
 Fractures of the vertebral body are most common.
Fractures of the vertebral body are most common.
 History of osteoporotic vertebral compression fractures are strongly predictive of subsequent vertebral fracture.
History of osteoporotic vertebral compression fractures are strongly predictive of subsequent vertebral fracture.
 Vertebral compression fracture is associated with increased mortality rate.
Vertebral compression fracture is associated with increased mortality rate.
 Lifetime risk of fracture in white women after 50 years of age: 75%
Lifetime risk of fracture in white women after 50 years of age: 75%
 Cancellous bone is most affected.
Cancellous bone is most affected.
 Type I osteoporosis (postmenopausal)
Type I osteoporosis (postmenopausal)
 Type II osteoporosis (age-related)
Type II osteoporosis (age-related)
 In patients older than 75 years
In patients older than 75 years
 Affects both trabecular and cortical bone
Affects both trabecular and cortical bone
 Obtained to rule out secondary causes of low bone mass
Obtained to rule out secondary causes of low bone mass
 Vitamin D deficiency, hyperthyroidism, hyperparathyroidism, Cushing’s syndrome, hematologic disorders, malignancy
Vitamin D deficiency, hyperthyroidism, hyperparathyroidism, Cushing’s syndrome, hematologic disorders, malignancy
 Complete blood cell count; measurements of serum calcium, phosphorus, 25(OH)-vitamin D, alkaline phosphatase, liver enzymes, creatinine, and total protein and albumin levels; and measurement of 24-hour urinary calcium excretion
Complete blood cell count; measurements of serum calcium, phosphorus, 25(OH)-vitamin D, alkaline phosphatase, liver enzymes, creatinine, and total protein and albumin levels; and measurement of 24-hour urinary calcium excretion
 Results of these studies are usually unremarkable in osteoporosis.
Results of these studies are usually unremarkable in osteoporosis.
 Plain radiographs not helpful unless bone loss exceeds 30%
Plain radiographs not helpful unless bone loss exceeds 30%
 Single-photon (appendicular) absorptiometry
Single-photon (appendicular) absorptiometry
 Double-photon (axial) absorptiometry
Double-photon (axial) absorptiometry
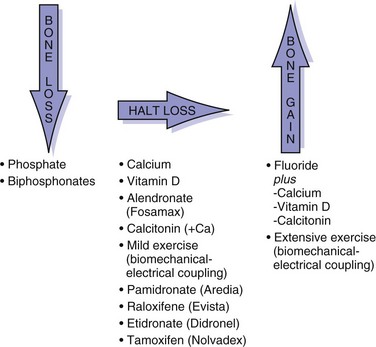
 Bisphosphonates: bind to bone resorption surfaces and inhibit osteoclastic membrane ruffling without destroying the cells
Bisphosphonates: bind to bone resorption surfaces and inhibit osteoclastic membrane ruffling without destroying the cells
 Other drugs, such as intramuscular calcitonin, may be helpful.
Other drugs, such as intramuscular calcitonin, may be helpful.
 Efficacy of bone augmentation with PTH, growth factors, prostaglandin inhibitors, and other therapies remains to be determined.
Efficacy of bone augmentation with PTH, growth factors, prostaglandin inhibitors, and other therapies remains to be determined.
 Prophylaxis for patients at risk for osteoporosis:
Prophylaxis for patients at risk for osteoporosis:
 Diet with adequate calcium intake
Diet with adequate calcium intake
 Weight-bearing exercise program
Weight-bearing exercise program
 Estrogen therapy evaluation at menopause
Estrogen therapy evaluation at menopause
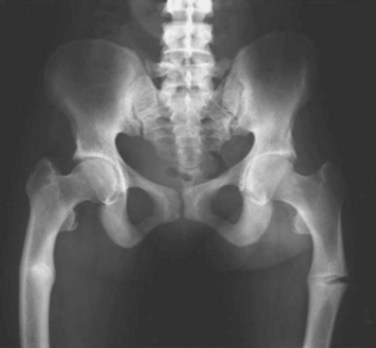
 Idiopathic transient osteoporosis of the hip
Idiopathic transient osteoporosis of the hip
 Uncommon; diagnosis of exclusion
Uncommon; diagnosis of exclusion
 Most common during the third trimester of pregnancy in women but can occur in men
Most common during the third trimester of pregnancy in women but can occur in men
 Groin pain, limited range of motion (ROM), and localized osteopenia without a history of trauma
Groin pain, limited range of motion (ROM), and localized osteopenia without a history of trauma
 Treatment: analgesics and limited weight bearing
Treatment: analgesics and limited weight bearing
 Generally self-limiting and tends to resolve spontaneously after 6 to 8 months
Generally self-limiting and tends to resolve spontaneously after 6 to 8 months
 Biopsy (transiliac); required for diagnosis
Biopsy (transiliac); required for diagnosis
 Femoral neck fractures are common
Femoral neck fractures are common
 Vitamin C (ascorbic acid) deficiency
Vitamin C (ascorbic acid) deficiency
 Produces a decrease in chondroitin sulfate synthesis
Produces a decrease in chondroitin sulfate synthesis
 Leads to defective collagen growth and repair
Leads to defective collagen growth and repair
 Also leads to impaired intracellular hydroxylation of collagen peptides
Also leads to impaired intracellular hydroxylation of collagen peptides
 Osteopetrosis (marble bone disease)
Osteopetrosis (marble bone disease)
 Osteopetrosis is the term for a group of bone disorders.
Osteopetrosis is the term for a group of bone disorders.
 It is characterized by increased sclerosis and obliteration of the medullary canal as a result of decreased osteoclast (and chondroclast) function: failure of bone resorption. Osteoclast numbers may be increased, decreased, or normal.
It is characterized by increased sclerosis and obliteration of the medullary canal as a result of decreased osteoclast (and chondroclast) function: failure of bone resorption. Osteoclast numbers may be increased, decreased, or normal.
 It may result from an abnormality of the immune system (thymic defect).
It may result from an abnormality of the immune system (thymic defect).
 Osteoclasts lack the normal ruffled border and clear zone.
Osteoclasts lack the normal ruffled border and clear zone.
 Marrow spaces fill with necrotic calcified cartilage.
Marrow spaces fill with necrotic calcified cartilage.
 The cartilage may be trapped within the osteoid.
The cartilage may be trapped within the osteoid.
 Empty lacunae and plugging of haversian canals are also observed.
Empty lacunae and plugging of haversian canals are also observed.
 One of these disorders is infantile autosomal recessive (“malignant”) osteopetrosis.
One of these disorders is infantile autosomal recessive (“malignant”) osteopetrosis.
 “Bone within a bone” appearance on radiographs
“Bone within a bone” appearance on radiographs
 Can lead to death during infancy
Can lead to death during infancy
 Bone marrow transplantation (e.g., osteoclast precursors) can be lifesaving during childhood
Bone marrow transplantation (e.g., osteoclast precursors) can be lifesaving during childhood
 High doses of calcitriol with or without steroids may also be helpful
High doses of calcitriol with or without steroids may also be helpful
 Another disorder is autosomal dominant “tarda” (benign) osteopetrosis (Albers-Schönberg disease).
Another disorder is autosomal dominant “tarda” (benign) osteopetrosis (Albers-Schönberg disease).
D Conditions of bone viability
 Death of bony tissue from causes other than infection
Death of bony tissue from causes other than infection
 Caused by loss of blood supply as a result of trauma or another event (e.g., slipped capital femoral epiphysis)
Caused by loss of blood supply as a result of trauma or another event (e.g., slipped capital femoral epiphysis)
 Idiopathic osteonecrosis of the femoral head and Legg-Calvé-Perthes disease may occur in patients with coagulation abnormalities
Idiopathic osteonecrosis of the femoral head and Legg-Calvé-Perthes disease may occur in patients with coagulation abnormalities
 Commonly affects the hip joint
Commonly affects the hip joint
 Associated with the following conditions:
Associated with the following conditions:
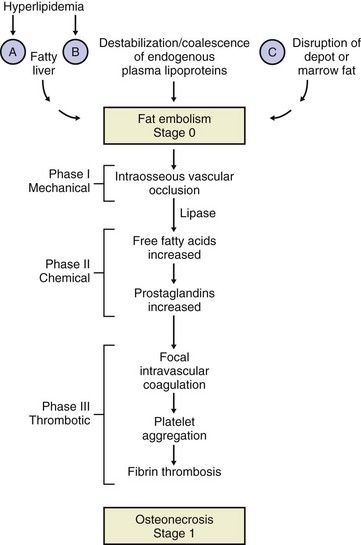
 Osteonecrosis may be related to enlargement of space-occupying marrow fat cells, which lead to ischemia of adjacent tissues
Osteonecrosis may be related to enlargement of space-occupying marrow fat cells, which lead to ischemia of adjacent tissues
 Vascular insults and other factors may also be significant.
Vascular insults and other factors may also be significant.
 Idiopathic (or spontaneous) osteonecrosis is diagnosed when no other cause can be identified.
Idiopathic (or spontaneous) osteonecrosis is diagnosed when no other cause can be identified.
 Chandler’s disease: osteonecrosis of the femoral head in adults
Chandler’s disease: osteonecrosis of the femoral head in adults
 Medial femoral condyle osteonecrosis: most common in women older than 60 years
Medial femoral condyle osteonecrosis: most common in women older than 60 years
 Idiopathic, alcohol, and dysbaric forms of osteonecrosis are associated with multiple insults.
Idiopathic, alcohol, and dysbaric forms of osteonecrosis are associated with multiple insults.
 These may be secondary to a hemoglobinopathy (e.g., sickle cell disease) or marrow disorder (e.g., hemochromatosis).
These may be secondary to a hemoglobinopathy (e.g., sickle cell disease) or marrow disorder (e.g., hemochromatosis).
 Cyclosporine has reduced the incidence of osteonecrosis of the femoral head among renal transplant recipients.
Cyclosporine has reduced the incidence of osteonecrosis of the femoral head among renal transplant recipients.
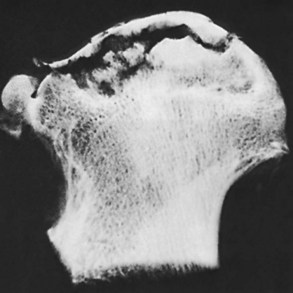
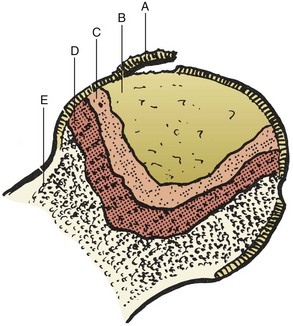
 Grossly necrotic bone, fibrous tissue, and subchondral collapse may be observed (Figures 1-36 and 1-37).
Grossly necrotic bone, fibrous tissue, and subchondral collapse may be observed (Figures 1-36 and 1-37).
 Early changes (14 to 21 days) involve autolysis of osteocytes and necrotic marrow.
Early changes (14 to 21 days) involve autolysis of osteocytes and necrotic marrow.
 These are followed by inflammation with invasion of buds of primitive mesenchymal tissue and capillaries.
These are followed by inflammation with invasion of buds of primitive mesenchymal tissue and capillaries.
 Later, newly woven bone is laid down on top of dead trabecular bone.
Later, newly woven bone is laid down on top of dead trabecular bone.
 This is followed by resorption of dead trabeculae and remodeling through “creeping substitution.”
This is followed by resorption of dead trabeculae and remodeling through “creeping substitution.”
 A careful history (to discern risk factors) and physical examination (e.g., to discern decreased ROM, limp) should precede additional studies.
A careful history (to discern risk factors) and physical examination (e.g., to discern decreased ROM, limp) should precede additional studies.
 Other joints (especially the contralateral hip) should be evaluated to identify the disease process early.
Other joints (especially the contralateral hip) should be evaluated to identify the disease process early.
 The process is bilateral in the hip in 50% of cases of idiopathic osteonecrosis and up to 80% of cases of steroid-induced osteonecrosis.
The process is bilateral in the hip in 50% of cases of idiopathic osteonecrosis and up to 80% of cases of steroid-induced osteonecrosis.