23 Basic Principles of Neural Blockade
Local or regional anesthesia is indicated for a diversity of clinical circumstances (Table 23-1). It is easy to provide when one understands the regional anatomy, block technique, and pharmacology of the agents injected. These nerve blocks can provide anesthesia for procedures, as well as rapid diagnostic, prognostic, and therapeutic data when applied in the appropriate clinical setting. The result, either temporary or permanent, allows for pain relief, increased functionality, and independence, especially within the context of a well-designed, multidisciplinary pain treatment program. However, these positive outcomes may only occur when provided to the appropriate patient. The clinician must understand when providing a regional anesthetic may be contraindicated (Table 23-2).1
Table 23-1 Indications for Percutaneous Nerve Blocks
| With Local Anesthetics |
| Provides anesthesia for procedures |
| Differentiates pain problems and helps better understand nociceptive pathways |
| Serves as a treatment for inflammatory compression neuropathies in combination with corticosteroids |
| Provides treatment for sympathetic mediated pain syndromes |
| Differentiates spasticity from joint contractures |
| Helps predict the effect of a neurolytic procedure |
| Allows selective recording in nerve conduction studies7 |
| Promotes functional activities in an occupational or physical therapy program |
| Assists in serial or inhibitory casting |
| With Normal Saline |
| Provides placebo response |
| With Neurolytic Agents (Chemical Neurolysis) |
| Facilitates functional goals in the spastic patient: positioning, ambulation, bracing, transfers |
| Improves caregiver tasks (such as hygiene) in the spastic patient: perineal, axillary, elbow, or hand regions |
| Improves self-image of the spastic patient by reducing joint deformities and improving cosmesis |
| May improve residual voluntary muscle control by eliminating unwanted hypertonia in the spastic patient |
| Reduces pain caused by hypertonia |
| Provides treatment for specific, intractable pain disorders |
| Prevents nerve compression injuries in hyperflexed joints (i.e., median nerve at the wrist from wrist flexor spasticity) |
| Prevents skin breakdown by promoting proper seating and positioning |
Table 23-2 Relative and Absolute Contraindications for Regional Anesthesia1
| Patient Selection Factors | Relative Contraindication | Absolute Contraindication |
|---|---|---|
| Patient cooperation | Psychiatric disorder (e.g., needle phobia, anxiety) | Patient refusal |
| Movement disorder (e.g., essential tremor, tics) | ||
| Language barrier, pediatric patient | ||
| Acutely intoxicated | ||
| Anatomic and physiologic | Anatomic abnormalities | |
| factors | Technical challenges (e.g., obesity, arthritis) | |
| Anesthetic considerations | ||
| Coexisting diseases | Neurological disease (e.g., multiple sclerosis) | Infection at injection site |
| Comatose state | Allergy to anesthetic | |
| Sepsis | Coagulopathy/systemic anticoagulation | |
| Coagulopathy (e.g., hemophilia) | ||
| Trauma (especially neurological trauma) | ||
| Perioperative issues | Surgical duration to outlast regional anesthetic | Block will hinder the procedure |
| Surgical positioning discomfort | ||
| Prolonged surgical time |
Adapted from Tsui, BCH, Finucane, BT: Managing adverse outcomes during regional anesthesia. In Longnecker DE, Brown DL, Newman MF, Zapol WM (eds): Anesthesiology. New York, McGraw Hill, 2008, p 1054.
The American Society of Anesthesiologists (ASA) sets standards for the safe practice of anesthesia, including that for neural blockade.2 This includes appropriate monitoring of the patient and immediate access to supplemental oxygen and resuscitation equipment, in the rare occurrence of a catastrophic event. However, the ASA standards were written for perioperative patients, and not those presenting to the pain clinic. There is no official standard for monitoring within the realm of pain medicine. However, a recent survey of various pain centers within the United States demonstrated that for peripheral nerve blocks, 56% place a noninvasive blood pressure cuff and 52% place a pulse oximeter during the procedure.3 This is despite the fact that 72% of pain clinics had treated an average of 7.3 vasovagal reactions within the 12-month study period.3 Periprocedure nothing by mouth (NPO) status is another area in which the ASA has clear guidelines, yet these too are lacking for patients undergoing office-based neural blockade.
The Joint Commission on the Accreditation of Healthcare Organizations (JCAHO) expects that universal protocols, such as preprocedure patient and site verification, as well as procedural time-outs, occur, before an anesthetic or invasive intervention is instituted. These standards also apply for neural blockade.3a
Neurovascular Bundle Anatomy
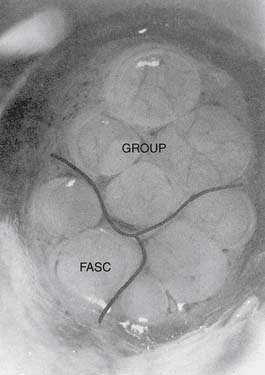
The neurovascular bundle consists of peripheral nerve fibers wrapped in connective tissue, intermingled by a capillary plexus (Fig. 23-1). Three types of connective tissue are present within the peripheral nerve: endoneurium, perineurium, and epineurium. The endoneurium is a delicate, supporting structure located adjacent to individual axons within a fascicle. This layer covers the entire individual nerve fiber. Individual fascicles are bound by the perineurium.4,5 The perineurial barrier is formed by adjacent perineurial cells via tight junctions, which help manage the axonal microenvironment, in addition to the blood-nerve barrier and nerve-cerebrospinal fluid (CSF) barrier.6 The fascicles are bound in groups by the outermost layer, the epineurium (Fig. 23-2), which encloses the nerve as a whole. This layer contains the vasa nervorum, which divides into arterioles that penetrate the perineurium (Fig. 23-3). Ultimately, a network of capillaries reaches each fascicle to supply individual axons. More specifically, the vasa nervorum forms the endoneurial capillaries. The endoneurial capillary endothelium contains tight junctional connections, which create the blood-nerve barrier. Cells that compose the distal layer of the arachnoid membrane are connected by tight junctions as well, which form the boundary of the nerve-CSF barrier. As nerve roots leave the subarachnoid space, the perineurium fuses with the cells of the distal layer of the arachnoid membrane. Anterior and posterior nerve roots, which are motor and sensory, respectively, initially leave the spinal cord separately, but merge via the connective tissue architecture, to become mixed sensorimotor nerves exiting the spinal canal.6
Local Anesthetic Pharmacology
Local anesthetic agents are categorized by their chemical composition—esters and amides (Table 23-3). Ester and amide anesthetics are weak bases—their pKa is near physiologic pH. Each is comprised of a lipophilic group, such as a benzene ring, and a hydrophilic group, such as a tertiary amine. These groups are either connected by an ester or amide linkage. This linkage is what imparts their categorization as an ester or amide. Esters are readily metabolized by plasma cholinesterase, thus their half-life is very short, on the order of minutes. Para-aminobenzoic acid (PABA) is one of the break-down products of this reaction. Amides undergo hepatic metabolism through N-dealkylation and hydrolysis. This is a slower process, imparting a longer half-life (2 to 3 hours), assuming the patient has normal liver function. Some patients with local anesthetic allergy may be sensitive to PABA, and a detailed history and chart review may be necessary to delineate if a ester local anesthetic was really the causative agent responsible for an earlier allergic reaction. Some local anesthetics, esters and amides alike, are stored in multi-use vials with the preservative methylparaben. Methylparaben may also cause an allergic reaction in patients with a PABA allergy.8
| Esters | Amides |
| Procaine | Lidocaine |
| Cocaine | Mepivacaine |
| Chloroprocaine | Bupivacaine |
| Tetracaine | Etidocaine |
| Ropivacaine |
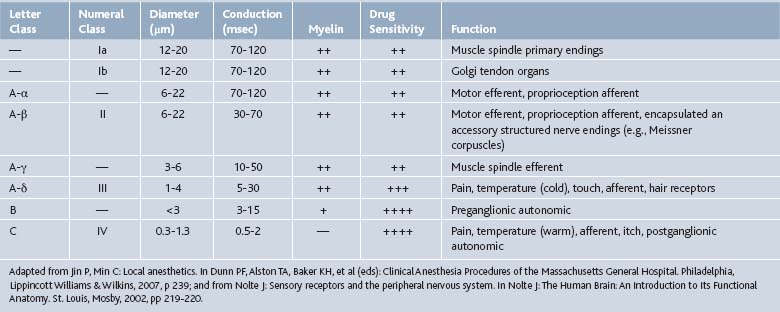
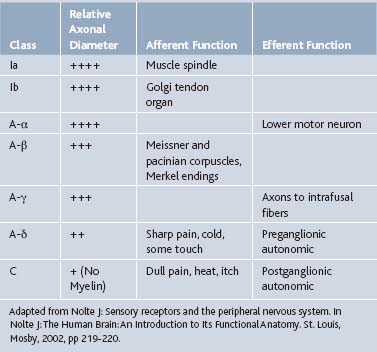
Local anesthetics act on neuronal axons. These agents, when uncharged, passively diffuse to the sodium channels of axons. These sodium channels allow Na+ to enter the axon, depolarize, and propagate an action potential to allow for communication between neurons. Local anesthetics inhibit this process by binding to these sodium channels, ceasing depolarization as well as action potential propagation, and thus neuronal signaling and transmission of pain signals (Tables 23-4 and 23-5).
Local anesthetics block in a sequential order, which is related to the diameter of the axon (see Tables 23-4 and Table 23-5). C-fibers, which carry pain and temperature information, are blocked first, as their diameter is small. A-α and A-β fibers have the largest axonal diameter, and are the last to become blocked. These fibers are primarily motor and proprioceptive. However, nerves with myelin may only require pharmacologic sodium channel blockade at the nodes of Ranvier, leaving these nerves susceptible to local anesthetic action.8
Mechanical or chemical vasoconstriction is sometimes beneficial for local anesthesia. It allows for a block of longer duration and stronger intensity by decreasing systemic uptake. This also imparts protection against systemic local anesthetic toxicity. Dilute epinephrine is the most common agent added to local anesthetics for this purpose, although phenylephrine and norepinephrine may also suffice, with lesser results.9 Epinephrine dilutions of 1:200,000 (5 mcg/mL) and 1:400,000 (2.5 mcg/mL) are typically prepared with the local anesthetic. An added benefit of adding epinephrine is that it may serve as an early indicator of unintentional vascular injection. This is sometimes referred to as a “test dose”, whereby a small dose of local anesthetic with epinephrine is injected while the patient’s heart rate is monitored. An increase in heart rate above 20% of the baseline heart rate would confer a positive test dose, indicating an intravascular injection may have occurred. There are potential contraindications to the addition of a chemical vasoconstrictor to local anesthetics, although the evidence for these may be weak10 (Table 23-6). Furthermore, chemical tourniquets should not be used when anesthetizing digits, ears, noses, genitals, or other areas with a poor collateral blood supply because this could lead to tissue necrosis. However, several recent large trials and literature reviews failed to demonstrate consistent tissue injury from the use of chemical vasoconstriction in some of these areas.12–14
Table 23-6 Relative Contradictions to the Addition of Chemical Vasoconstrictors to Local Anesthetics
What is the maximum allowable dose of local anesthetic that can be administered to the patient? This is a common question asked of clinicians, and it can be a source of confusion. The answer varies, and can depend on factors such as the local anesthetic used, to even the country in which the clinician is practicing. First, it is recommended that the package insert for the local anesthetics used be reviewed as a basis for dosing guidelines. However, a recent review of the maximum allowable dose of common local anesthetics found grade C evidence for dose adjustments depending on several factors (Table 23-7).15 Within the United States, 300 mg of lidocaine is considered the maximum allowable dose. Interestingly, the source for this cannot be found in the scientific literature.15
Table 23-7 Grade C Evidence for Adjusting the Maximum Allowable Dose of Local Anesthetics
Adapted from Rosenberg PH, Veering BT, Urmey WF: Maximum recommended doses of local anesthetics: A multifactorial concept. Reg Anesth Pain Med. 29(6):564-575, 2004.
Neural Block Technique: General Considerations
Needles used for neural blockade are often stiff; to allow for deep penetration, 23- and 22-gauge needles ranging from 25 to 150 mm in length are commonly used, depending on the type of block and procedure to be carried out. Needle trauma (discussed subsequently) is always a concern during neural blockade, and several needle bevel types are available. Sprotte and Whitacre tips, as well as short-bevel needles, are associated with less nerve trauma when compared with standard A-bevel needles.16
The two common approaches used to block nerves are the paresthesia technique (PT) and nonparesthesia technique (NPT), also known as a field block. PT attempts to purposefully provoke paresthesias of the nerve before injection. These paresthesias indicate that the needle is in contact with the nerve and serve as a warning of potential nerve injury. This technique assumes that the patient’s sensory pathways are intact and that the patient is able to cooperate with the procedure. However, this method can be uncomfortable, and can potentially damage the nerve of interest.17–19 To help avoid neural injury, the clinician should make every effort to stop, reposition, and refrain from injecting whenever a serious or persistent paresthesia occurs. The second approach, NPT, avoids this deliberate probing for paresthesias and relies on anatomic landmarks, but generally requires greater volumes of local anesthetic. NPT is best suited for superficial nerves, and often helps supplement a prior regional anesthetic which may be inadequate over certain areas. NPT uses dilute, high volumes of local anesthetic, with the addition of epinephrine, to reduce the risk of local anesthetic toxicity.20 With NPT, the needle tip may not approximate the nerve as closely as with PT. However, to provide the safest regional anesthetic, PT should be avoided when possible.
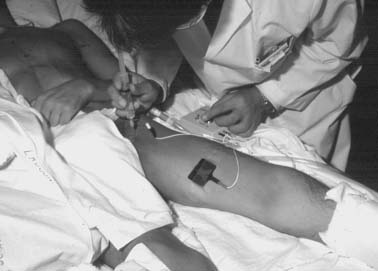
Electrical stimulation (Fig. 23-4) can be used to locate and block peripheral nerves. These stimulators are available commercially, although office electrodiagnostic equipment often will suffice. An electrical impulse generated by the stimulator and controlled by a rheostat is transmitted through the needle. A ground electrode is placed on the patient and connected to the positive lead, while the negative lead is connected to the needle. Insulated needles are available, many of which are covered with materials such as polytetrafluoroethylene (Teflon). In general, insulated needles are preferred over noninsulated needles. As the needle approaches the nerve, either a motor, sensory, or mixed response can be elicited, depending on the fiber types contained within the nerve. Typically, the clinician looks for a twitch in the expected muscle group associated with the nerve. This twitching is not typically uncomfortable for the patient; however, if it is painful in anyway, the needle should be redirected. The neural response should increase as the needle tip approaches the nerve. It is desirable to obtain a strong neural response with a low stimulus output, thereby ensuring needle placement is sufficiently distant from the nerve to provide anesthesia, yet prevent an intraneural injection. This technique is preferred when injecting neurolytic agents for the treatment of spasticity. One can quickly identify selective motor responses and quickly determine the effect of chemical neurolysis after injection.
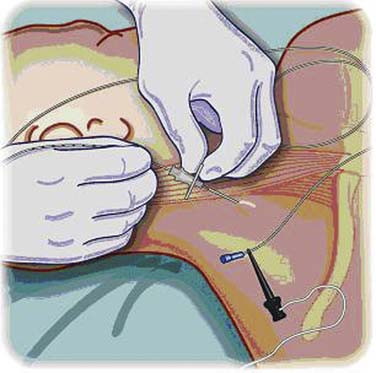
Continuous neural blockade can be achieved through the use of perineural infusion indwelling catheters (Fig. 23-5). These catheters are most commonly placed perioperatively for postoperative pain from ambulatory orthopedic surgery. These catheters can be used in the hospital or at home by the patient in recovery for several days by way of infusion pumps. There are even case reports of portable pumps, which allow the patient to ambulate and regain independence.21 Both continuous infusions, as well as patient-controlled regional anesthesia systems have been described.22 These catheters must be placed under strict sterile conditions because these are indwelling devices that remain in the patient for days. Potential complications include nerve injury and transient neurologic damage, infection, associated unwanted nerve blockade, and local anesthetic side effects and toxicity.22
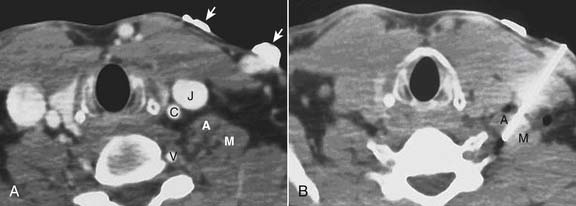
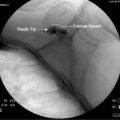
Computed tomography (CT) guidance for peripheral nerve blockade has also been described as an effective means to provide regional anesthesia, especially in patients where the relevant anatomy is difficult to identify (Fig. 23-6).23 This form of image guidance for neural blockade allows for excellent visualization of the anatomy and appropriate spread of local anesthetic. CT-guided nerve blocks are not commonly used because they require expensive equipment and trained radiology technicians. Radiation exposure to the patient and care providers is a concern in performing CT-guided blocks.
Regardless of the technique, the needle tip should always remain in the epineural space during an injection (Fig. 23-7). Intraneural injections have a high association with fascicular damage.24,25 If paresthesias are elicited, the needle should be repositioned before local anesthetic solution is injected. Forceful injection is never recommended because it may be a sign of injecting into a fixed space, such as tendon or adjacent to bone. Repeat anesthetic injections close to the same nerve in a single patient encounter should be avoided because warning paresthesias may be suppressed and occult iatrogenic nerve injury may occur.
Nerve Injuries
Trauma, toxicity, and ischemia are the major causes of nerve injury during neural blockade. Nerve injection injuries are almost always due to one or more of these factors.18 The incidence of neural injury during block procedures is about 1% to 2%, most of which are minor in nature.26
Trauma to the nerve during neural blockade occurs with aggressive probing during needle insertion. This can directly injure the nerve fascicles. Additives, such as epinephrine, increase the toxicity of the anesthetics when injected intrafascicularly.26a,27,28 Selander and colleagues showed a reduction in the incidence of nerve trauma and fascicle injury when short, beveled needles were used. They also demonstrated that nerve fascicles rolled or slid with needle contact.18 Most traumatic nerve injury symptoms self-resolve in 4 to 6 weeks, and essentially all reach resolution within 12 months.27
Intraneural injections of solution not only directly injure nerve fibers, but also cause damage to the blood-nerve barrier function.24,25 The spread of anesthesia with intraneural injections has been demonstrated and, when near the spine, may cause unexpected spinal anesthesia.29 Neural compression or stretching also may result from large volumes of solution injected into a fixed space, thereby traumatizing the nerve.
Neurotoxicity can be caused by direct injection of anesthetics into nervous tissue—intrafascicular injections can cause profound or permanent nerve damage. The degree of damage depends on the type and amount of drug injected, as well as the kind of preservative used for the anesthetic medication.24,25 Ester anesthetics generally demonstrate greater toxicity than amides when injected into the intrafascicular space.30,31 Extrafascicular injections of anesthetics in routine concentrations rarely cause significant histologic nerve damage or disruption of the blood-nerve barrier, but can alter the permeability of the perineurium, leading to endoneurial edema.30,31 The epineurium provides some neuroprotective function during injection, thereby reducing toxicity from anesthetic medication.
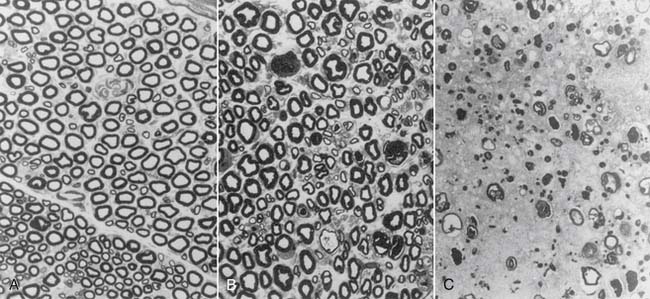
Mackinnon and colleagues found that corticosteroid injections into the epineural tissue did not cause nerve damage.32 When injected into the intrafascicular space, corticosteroids exerted a direct neurotoxic effect with disruption of the blood-nerve barrier, similar to anesthetics. Severe nerve fiber damage was noted with hydrocortisone (Solu-Cortef) and triamcinolone hexacetonide (Aristospan), moderate damage with triamcinolone acetonide (Kenalog) and methylprednisolone (Depo-Medrol), and the least damage with dexamethasone (Decadron) (Fig. 23-8).
Ischemia is typically thought to be the result of the application of a tourniquet for a prolonged time period, or improper positioning of the patient. However, a nerve block itself, if the injection is given into the intrafascicular space, may also cause ischemic damage. The ischemia results from increased intraneural compartment pressure by the injectate volume, which reduces neural perfusion. This intraneural pressure has been shown experimentally to remain above the blood perfusion pressure for 15 minutes without damage.29 A rare potential cause of ischemic nerve injury is the result of a local hematoma formed during the block procedure from inadvertent arterial puncture.
To diagnose a neural injury, a combination of radiologic imaging and electrophysiologic tests can be performed in conjunction with the patient’s history and physical examination. Obtaining input from other specialty consultants, such as neurologists and radiologists, often hastens appropriate testing and diagnosis of a potential nerve injury. Magnetic resonance imaging (MRI) can be useful in revealing nerve compressions secondary to fluid accumulation from blood, edema, or local anesthetic injectate.26 Conduction tests can be used to assess the amount of damage to large diameter sensory and motor axons. Electromyography (EMG) can evaluate and quantify damage to smaller motor units26 as well as help with locating the site of neuronal injury.
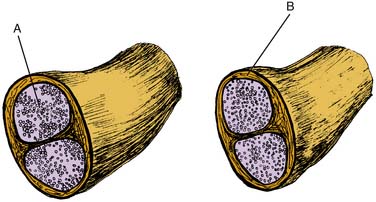
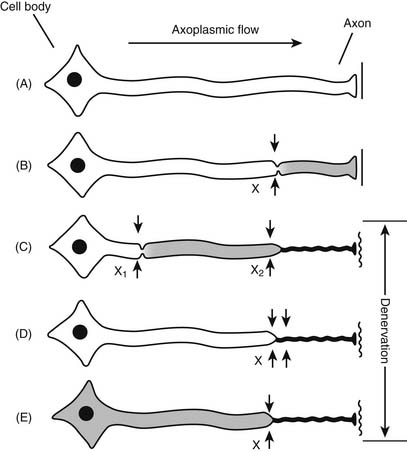
Patients with an underlying peripheral neuropathy, either known or subclinical, may be more susceptible to nerve injury during neural blockade, a phenomenon known as the “double crush” syndrome (Fig. 23-9). The double crush syndrome hypothesis claims that damaged axons may be more prone to damage at a more distal location.33 Furthermore, two relatively small lesions along a single axon are worse than a single larger lesion—the damage of two axonal stressors “far exceeds the expected additive damage caused by each isolated compression”.33 For example, a case report in 2001 of a young female undergoing chemotherapy with cisplatin, an agent known to cause peripheral neuropathies, developed a brachial plexopathy after an interscalene block.33 Bearing this in mind, patients with a potential initial axonal insult from any cause, may be susceptible to nerve damage from subsequent neural blockade.
General Principles of Neural Blockade
Sensory Blocks
Anesthetic peripheral nerve blocks are most commonly used to facilitate surgical procedures by blocking sensory pathways. In an office practice setting, anesthetic blocks can be used to assist in the diagnosis of difficult pain problems.33a When sensory components to nerves are interrupted, expected dermatomal sensory changes can be evaluated and compared with actual blocked changes, thereby diagnosing nociceptive pain pathways previously unappreciated or undiagnosed. For example, a radial sensory neuropathy masked by de Quervain tenosynovitis may be diagnosed after a radial sensory nerve block.33b Other compressive neuropathies, such as carpal tunnel syndrome, may later require corticosteroid injections.
Patients who are malingering, or who magnify symptoms, also may be evaluated using blocks. Response to injections with various concentrations of anesthetic versus placebo can be analyzed to evaluate the psychological component of the pain disorder.
Another way to use sensory blocks is to “reset” the pain generators, especially in cases of sympathetic mediated pain disorders with serial injections.34 When a response to painful stimuli is attenuated, the patient can participate in a therapy program that emphasizes functional tasks, joint range of motion, stretching, and skin desensitization. The goal is to gradually eliminate the painful stimulus over time.
Neurolytic Blocks
Spasticity
The prognosis for motor return and stage of recovery should be established. The physician must determine if the hypertonicity is generalized or focal. Generalized hypertonicity is usually not responsive to nerve blocks unless it is for the purposes of hygiene. Consistent patterns of hypertonicity need to be established and treatment should initially be directed toward proximal tone. Any block should affect the most proximal nerve capable of denervating the maximum number of spastic myotomes. All residual voluntary motor function of the affected limb is to be preserved. Phenol, a common neurolytic agent, generally results in alleviation of spasticity with little decrease of voluntary contraction.35
Education, Training, and Simulation
Teaching clinical fellows, residents and medical students not only the informational and academic aspects of neural blockade, but also the technical procedures and associated ergonomics is important. However, it is not always possible to expose trainees to enough procedures to allow them to perform these blocks individually in a safe and efficient fashion. High-fidelity simulators have become increasingly popular for trainees to gain these valuable skills. Likewise, simulation can allow trainees, and the practicing clinician alike, to manage rare complications and crisis situations as they relate to neural blockade (e.g., anaphylaxis). A recent case report describes how the use of simulation facilitated the management of a patient in cardiac arrest from bupivacaine toxicity.36 High-fidelity simulation for educating trainees to perform a variety procedures and managing critical events will likely become an essential component of medical education.
1. Tsui B.C.H., Finucane B.T. Managing Adverse Outcomes during Regional Anesthesia. In: Longnecker D.E., Brown D., Newman M., Zapol W., editors. Anesthesiology. New York: McGraw Hill; 2008:1054.
2. American Society of Anesthesiologists. Standards for Basic Anesthetic Monitoring, 2005. http://www.asahq.org/For-Healthcare-Professionals/~/media/For%2520Members/documents/Standards%2520Guidelines%2520Stmts/Basic%2520Anesthetic%2520Monitoring%25202005.ashx
3. Ahmed S.U., Tonidandel W., Trella J., et al. Peri-procedural protocols for interventional pain management techniques: a survey of US pain centers. Pain Physician. 2005;8:181-185.
3a. Kirvela O., Nieminen S. Treatment of painful neuromas with neurolytic blockade. Pain. 1990;41:161-165.
4. Ross M.H., Reith E.J. Perineurium: Evidence for contractile elements. Science. 1969;165:604-606.
5. Thomas P.K., Olsson Y. Microscopic anatomy and function of connective tissue components of peripheral nerve. Dyck P.J., Thomas P.K., Lambert E.H., Bunge R., editors. Peripheral Neuropathy, vol. 1. Philadelphia: WB Saunders, 1984. 97-120
6. Anthony D.C., Frosch M.P., Girolami U.D. Peripheral nerve and skeletal muscle. In: Kumar V., Abbas A.K., Fausto N., editors. Pathologic Basis of Disease. Philadelphia: Elsevier; 2005:1327.
7. Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice, 2nd ed. Philadelphia, FA: Davis; 1989.
8. Jin P., Min J.C., et al. Local anesthetics. In: Dunn P.F., Alston T.A., Baker K.H., editors. Clinical Anesthesia Procedures of the Massachusetts General Hospital. 7th ed. Philadelphia, Lippincott: Williams and Wilkins, 2007. 244
9. Heavner J.E. Pharmacology of local anesthetics. In: Longnecker D.E., Brown D., Newman M., Zapol W., editors. Anesthesiology. New York: McGraw Hill, 2008. 964
10. Brown R.S., Rhodus N.L. Epinephrine and local anesthesia revisited. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(4):401-408.
11. Nolte J. Sensory receptors and the peripheral nervous system. In: Nolte J., The Human Brain. An Introduction to Its Functional Anatomy. St. Louis: Mosby, 2002. 219–220
12. Hafner H.M., Rocken M., Breuninger H. Epinephrine-supplemented local anesthetics for ear and nose surgery: Clinical use without complications in more than 10,000 surgical procedures. J Dtsch Dermatol Ges. 2005;3(3):195-199.
13. Lalonde D., Bell M., Benoit P., et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: The Dalhousie Project clinical phase. J Hand Surg Am. 2005;30(5):1061-1067.
14. Thomson C.J., Lalonde D.H., Denkler K.A., Feicht A.J. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Rosenberg P.H., Veering B.T., Urmey W.F. Maximum recommended doses of local anesthetics: A multifactorial concept. Reg Anesth Pain Med. 2004;29(6):564-575.
16. Pasvankas G.W., Sidhu D.S., et al. Regional Anesthesia. In: Dunn P.F., Alston T.A., Baker K.H., editors. Clinical Anesthesia Procedures of the Massachusetts General Hospital. 7th ed. Philadelphia, Lippincott: Williams and Wilkins, 2007. 273
17. Plevak D.J., Linstromberg J.W., Danielson D.R. Paresthesia vs. nonparesthesia: The axillary block. ASA Abstracts, Anesthesiology. 59, 1983.
18. Selander D. Paresthesias or no paresthesias? Nerve complications after neural blockades. Acta Anaesthesiol Belg. 1988;39:173-174.
19. Selander D., Edshage S., Wolff T. Paresthesiae or no paresthesiae? Nerve lesions after axillary blocks. Acta Anaesthesiol Scand. 1979;23:27-33.
20. Morgan G.E., Mikhail M.S., Murray M.J. Peripheral nerve blocks. In Mikhail MS, Murray MJ: Clinical Anesthesiology, 4th ed. New York: Lange/McGraw Hill; 2006. 328
21. Ilfeld B.M., Enneking F.K. A portable mechanical pump providing over four days of patient-controlled analgesia by perineural infusion at home. Reg Anesth Pain Med. 2002;27(1):100-104.
22. Boezaart A.P. Perineural infusion of local anesthetics. Anesthesiology. 2006;104(4):872-880.
23. Mukherji S.K., Wagle A., Armao D.M., Dogra S. Brachial plexus nerve block with CT guidance for regional pain management: Initial results. Radiology. 2000;216:886-890.
24. Gentili F., Hudson A., Kline D.G., Hunter D. Peripheral nerve injection injury: An experimental study. Neurosurgery. 1979;4:244-253.
25. Hudson A.R. Nerve injection injuries. Clin Plast Surg. 1984;11:27-30.
26. Tsui B.C.H., Finucane B.T. Managing adverse outcomes during regional anesthesia. In: Longnecker D.E., Brown D., Newman M., Zapol W., editors. Anesthesiology. 1st ed. New York: McGraw Hill, 2008. 1060-1061
26a. Selander D., Dhuner K.G., Lundborg G. Peripheral nerve injury due to injection needles used for regional anesthesia. An experimental study of the acute effects of needle point trauma. Acta Anaesthesiol Scand. 1977;21:182-188.
27. Covino B.G. Potential neurotoxicity of local anesthetic agents. Can Anaesth Soc J. 1983;30:111-116.
28. Selander D., Brattsand R., Lundborg G., et al. Local anesthetics: Importance of mode of application, concentration and adrenaline for the appearance of nerve lesions. An experimental study of axonal degeneration and barrier damage after intrafascicular injection or topical application of bupivacaine (Marcain). Acta Anesthesiol Scand. 1979;23:127-136.
29. Selander D., Sjostrand J. Longitudinal spread of intraneurally injected local anesthetics. An experimental study of the initial neural distribution following intraneural injections. Acta Anaesthesiol Scand. 1978;22:622-634.
30. Gentili F., Hudson A.R., Hunter D., Kline D.G. Nerve injection injury with local anesthetic agents: A light and electron microscopic, fluorescent microscopic and horseradish peroxidase study. Neurosurgery. 1980;6:263-272.
31. Myers R.R., Kalichman M.W., Reisner L.S., Powell H.C. Neurotoxicity of local anesthetics: Altered perineurial permeability, edema, and nerve fiber injury. Anesthesiology. 1986;64:29-35.
32. Mackinnon S.E., Hudson A.R., Gentili F., et al. Peripheral nerve injection injury with steroid agents. Plast Reconstr Surg. 1982;69:482-490.
33. Hebl J.R., Horlocker T.T., Pritchard D.J. Diffuse brachial plexopathy after interscalene blockade in a patient receiving cisplatin chemotherapy: The pharmacologic double crush syndrome. Anesth Analg. 2001;92:249-251.
33a. Choi Y.K., Liu J. The use of 5% lidocaine for prolonged analgesia in chronic pain patients. A new technique. Reg Anesth Pain Med. 1998;23:96-100.
33b. Fouch R.A., Abram S.E., Hogan Q.H. Neural blockade for upper extremity pain. Hand Clin. 1996;12:791-800.
34. Brown D.L. Somatic or sympathetic block for reflex sympathetic dystrophy. Which is indicated?. Hand Clin, 13. 1997:485-497.
35. Moritz U. Phenol block of peripheral nerves. Scand J Rehabil Med. 1973;5:160-163.
36. Smith H.M., Jacob A.K., Segura L.G., et al. Simulation education in anesthesia training: A case report of successful resuscitation of bupivacaine-induced cardiac arrest link to recent simulation training. Anesth Analg. 2008;106(5):1581-1584.