Chapter 2 Basic Pathology Concepts
![]() Additional videos for this topic are available online at www.expertconsult.com.
Additional videos for this topic are available online at www.expertconsult.com.
The full text of this chapter can be accessed online at www.expertconsult.com.
Muscle and Tendon Injury
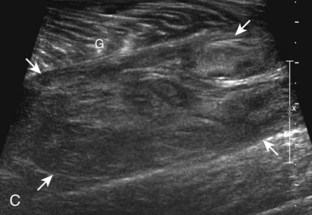
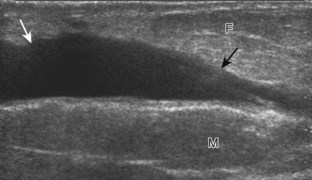
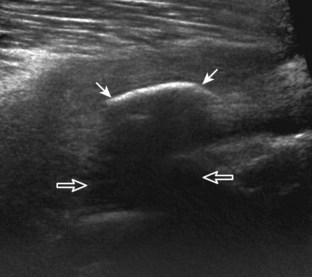
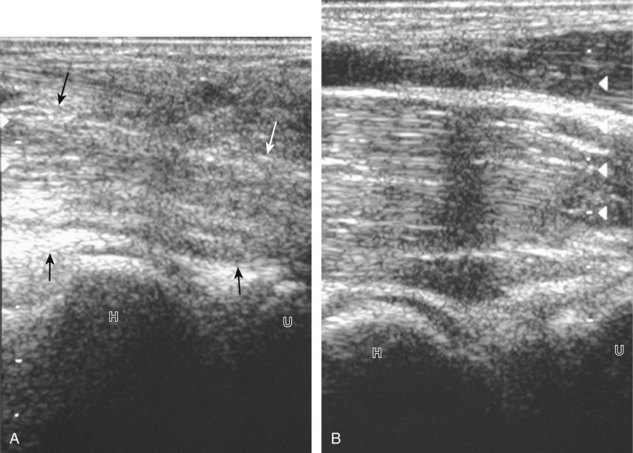
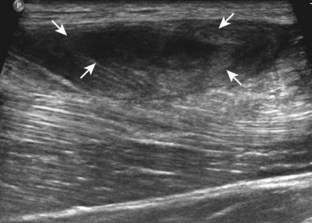
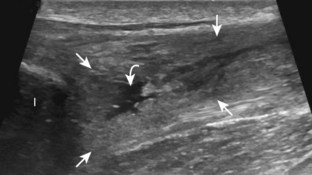
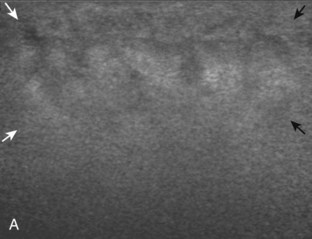
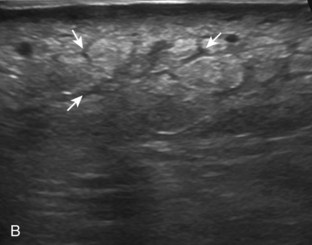
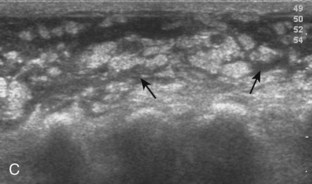
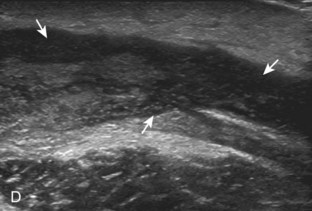
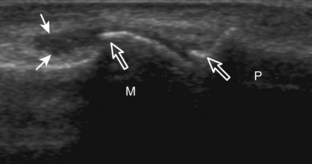
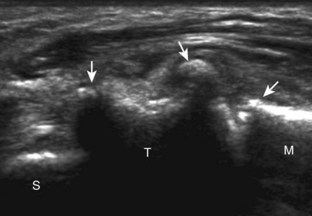
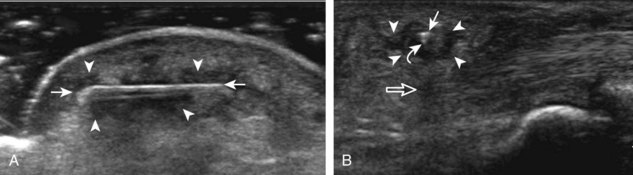
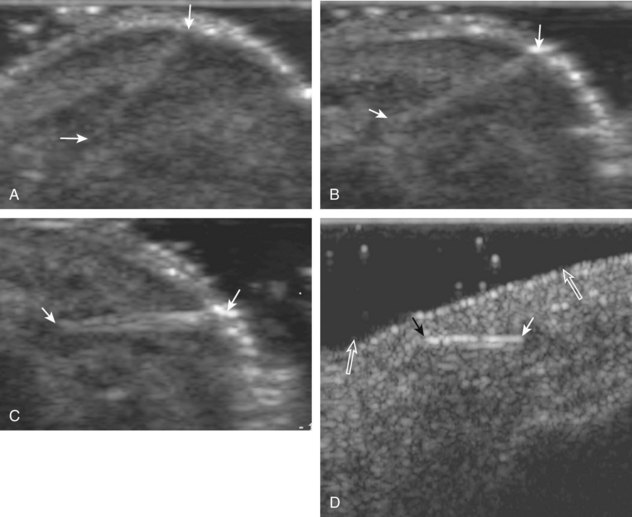
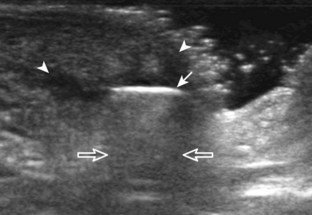
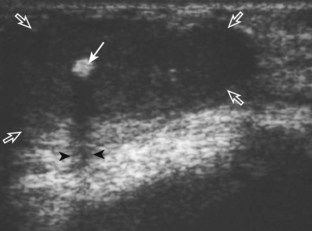
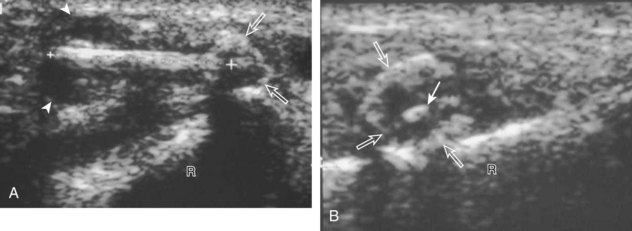
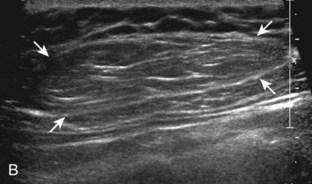
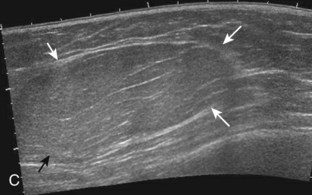
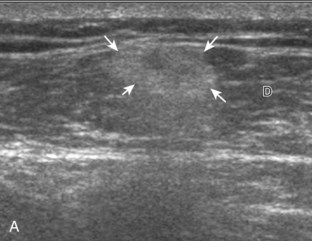
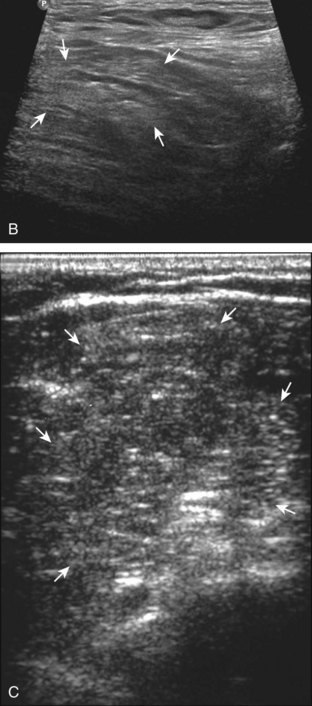
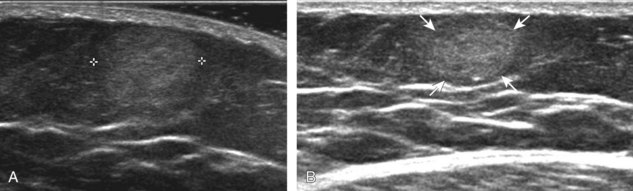
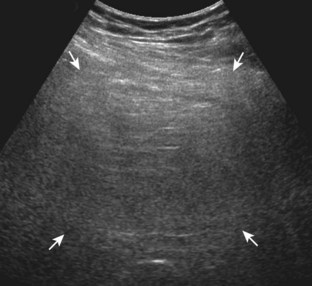
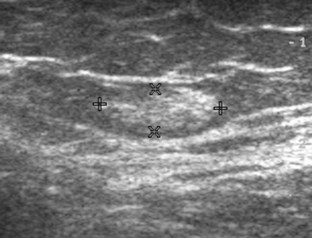
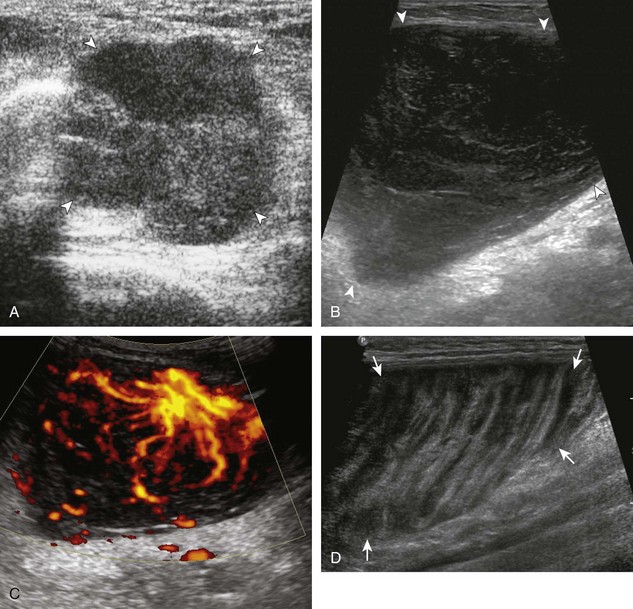
Muscle and tendon injuries may be categorized as acute and chronic. Acute injuries tend to take the form of direct impact injury, stretch injury during contraction (strain), or penetrating injury. Acute muscle injury can be clinically categorized as grade 1 (no appreciable fiber disruption), grade 2 (partial tear or moderate fiber disruption with compromised strength), and grade 3 (complete fiber disruption).1 At sonography, muscle contusion and hemorrhage acutely appear hyperechoic (Fig. 2-1).2,3 Excessive and intense muscle activity may produce diffuse muscle hyperechogenicity if imaged acutely from transient muscle edema (Fig. 2-2).4 Partial fiber disruption indicates partial-thickness tear, whereas complete fiber disruption indicates full-thickness tear. One hallmark of full-thickness tear is muscle or tendon retraction, which is made more obvious with passive movement or active muscle contraction. Hemorrhage will later appear more hypoechoic (Fig. 2-3), although a heterogeneous appearance with mixed echogenicity is common (Fig. 2-4). As soft tissue hemorrhage resorbs, a hematoma will become smaller and more echogenic, beginning at the periphery (Fig. 2-5). A residual anechoic fluid collection or seroma may remain (Fig. 2-6). Hemorrhage located between the subcutaneous fat and the adjacent hip musculature can occur with trauma as a degloving-type injury, called the Morel-Lavallée lesion (Fig. 2-7).5 Residual scar formation appears hyperechoic (Fig. 2-8). Heterotopic ossification may remain and is hyperechoic with posterior acoustic shadowing (Fig. 2-9). An area of damaged muscle may ossify, termed myositis ossificans (Fig. 2-10), and ultrasound can show early mineralization before visualization on radiography.6 Often, computed tomography (CT) is needed to demonstrate the peripheral rim of mineralization characteristic of myositis ossificans, given shadowing seen on ultrasound. Prior trauma to muscle or its nerve supply can result in muscle atrophy, which causes increased echogenicity and decreased size of the muscle (Fig. 2-11).
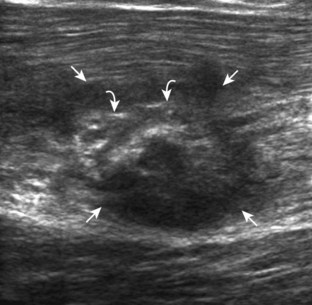
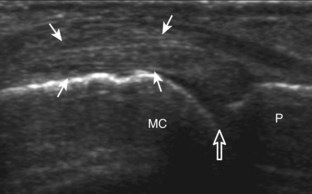
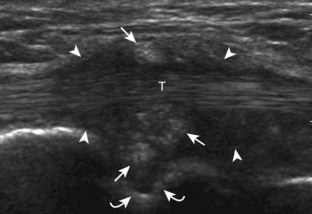
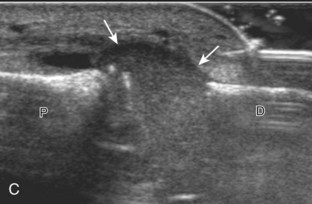
FIGURE 2-10 Myositis ossificans.
Ultrasound image shows hypoechoic hemorrhage (arrows) with echogenic mineralization (curved arrows).
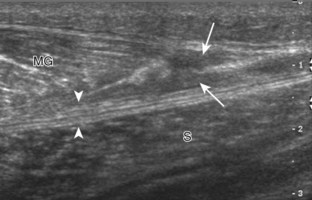
With direct impact injury, the belly of a muscle is typically involved with hematoma and variable fiber disruption (Fig. 2-12). In contrast, stretching of a contracting muscle typically results in injury at the musculotendinous junction and is more common with muscles that span two joints, such as the hamstring muscles of the thigh (Fig. 2-13) and the medial head of the gastrocnemius (Fig. 2-14). It is important to consider the architecture of the muscle imaged in evaluation for myotendinous injury. For example, a muscle with a unipennate architecture (e.g., the medial head of the gastrocnemius) shows injury at the myotendinous junction located at its periphery (see Fig. 2-14).7 A muscle with circumpennate or bipennate architecture (e.g., the indirect head of the rectus femoris) may show injury at its distal musculotendinous junction or within the muscle belly as a central aponeurosis tear (see Fig. 6-59A in Chapter 6).8 Musculotendinous injury also demonstrates variable echogenicity from hemorrhage and fluid, depending on the age of the injury and the degree of fiber disruption. Passive joint movement or active muscle contraction can demonstrate retraction at the site of the injury that indicates full-thickness tear. Particularly in children, these types of acute tendon injuries may be associated with bone fragment avulsion at the tendon attachments, which appear hyperechoic with possible shadowing.
With penetrating injury or laceration, acute muscle and tendon injury may occur at any site (see Fig. 7-47 in Chapter 7). The obvious physical examination findings usually guide the ultrasound evaluation. Muscle and tendon injuries are again classified as partial-thickness or full-thickness tears. Dynamic imaging is helpful in this distinction because it makes retraction related to full-thickness tears more conspicuous. Gas introduced during the penetrating injury can make evaluation extremely difficult; air appears hyperechoic with heterogeneous posterior shadowing. It is also important to recognize adjacent soft tissue or osseous injuries outside the muscle because lacerations may cause peripheral nerve injury as well (see Fig. 6-80B in Chapter 6).
Chronic muscle and tendon injuries are usually the result of overuse, with tendon degeneration and possible tear. It has been shown that such involved tendons show eosinophilic, fibrillar, and mucoid degeneration but do not contain acute inflammatory cells; therefore, the term tendinosis is used rather than tendinitis.9–11 At sonography, tendinosis appears as hypoechoic swelling of the involved tendon, but without tendon fiber disruption (see later chapters). Several tendons may commonly show increased blood flow on color or power Doppler imaging in the setting of tendinosis, such as the patellar tendon, Achilles tendon, and common extensor tendon of the elbow. This increase in blood flow is not due to inflammation but rather represents neovascularity. Tendinosis may progress to partial-thickness and full-thickness tendon tear. Chronic muscle and tendon injuries that result in tear can be associated with atrophy of the muscle, which appears hyperechoic and decreased in size. After surgery, misplaced hardware or screw-tip penetration beyond the bone cortex may cause excessive wear of an adjacent tendon (Fig. 2-15). Ultrasound is helpful in this diagnosis because artifact from metal hardware does not obscure overlying soft tissues. In addition, dynamic imaging with joint movement or muscle contraction can determine whether a tendon is in contact with metal hardware with specific positions (Video 2-1).![]()
Bone Injury
The normal osseous surfaces are smooth and echogenic with posterior shadowing and possibly reverberation when imaged perpendicular to the sound beam. The hallmark of an acute fracture is discontinuity of the bone cortex with possible step-off deformity (Fig. 2-16).12 Adjacent mixed echogenicity hemorrhage may also be present. A stress fracture, for example involving a metatarsal, may initially appear as a focal hypoechoic area adjacent to bone, which may progress to fracture step-off deformity or hyperechoic callus formation (see Fig. 8-146 in Chapter 8).13 This is typically associated with point tenderness induced by pressure from the transducer. A patient also commonly indicates focal pain in the area. It is important at the completion of any ultrasound examination to ask the patient about such focal symptoms because they are often clues to underlying pathology that may not be otherwise evaluated.
Other types of bone injuries involve avulsion at tendon and ligament attachments. In these situations, a small fragment of bone with variable shadowing is seen attached to the involved tendon or ligament (see Fig. 8-143 in Chapter 8). Asymmetrical widening and irregularity of an open growth plate with point tenderness can indicate a physeal injury (Fig. 2-17).14 It is important to differentiate the findings of bone injury at ultrasound from bone irregularity resulting from osteophytes. This differentiation is possible because osteophytes occur at margins of synovial joints usually without point tenderness, whereas a fracture shows a cortical step-off deformity. Correlation with radiography may also be considered to assist with this differentiation.
In many situations in which a fracture is identified at ultrasound, the fracture is unsuspected. The indication for the examination is often to evaluate a soft tissue or joint abnormality after a “negative” radiograph. This is not uncommon in the foot and ankle, where multiple overlapping osseous structures may make radiographic diagnosis of fracture difficult. The other situation is the greater tuberosity fracture of the proximal humerus, which can be overlooked at radiography because of suboptimal patient positioning or suboptimal radiographic technique (see Fig. 3-103 in Chapter 3).15 It has been shown that ultrasound is more effective than radiography in the diagnosis of rib fracture (see Fig. 2-16A).16,17 As a fracture begins to heal, early hypoechoic callus becomes hyperechoic hard callus, which can eventually bridge the fracture gap or step-off deformity.12 This can also be applied to limb-lengthening procedures, in which ultrasound can detect new bone before radiography. Ultrasound has also been shown to be effective in diagnosis of tibial fracture nonunion with static interlocked nail placement; ultrasound can detect healing before radiography, whereas visualization of the hyperechoic nail indicates no overlying callus formation (Fig. 2-18).12 Another advantage of ultrasound is in the evaluation of nonossified structures, such as the distal humeral epiphyses in children and the anterior costocartilage.18,19
Infection
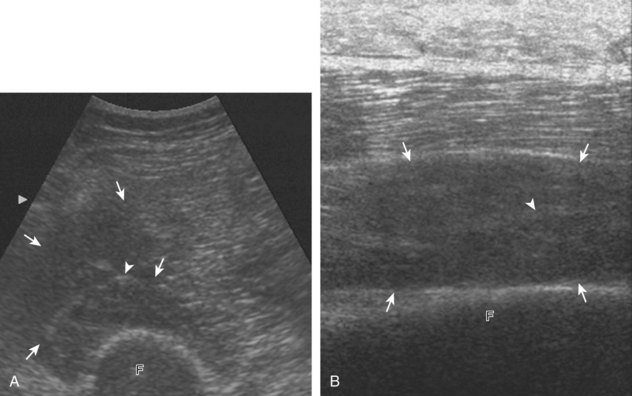
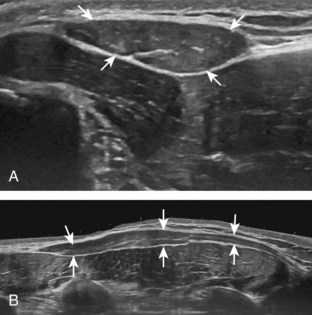
The imaging appearances of soft tissue infection are largely predicted by the route of infection spread. For example, in adults, infection commonly occurs through a puncture wound or skin ulcer. This produces infection of the soft tissues or cellulitis, which may have several appearances (Fig. 2-19). Acutely, cellulitis appears as hyperechoic and thickened subcutaneous tissue.20,21 Later, hypoechoic or anechoic branching channels are visualized, with distortion of the soft tissues and possibly increased flow on color or power Doppler imaging.20 Such branching channels can coalesce as purulent fluid and can progress to frank abscess, where ultrasound-guided aspiration may be of benefit.20 However, ultrasound-guided aspiration may be less effective in the setting of methicillin-resistant Staphylococcus aureus infection.22 When evaluating for cellulitis, the findings of anechoic perifascial fluid and gas (appearing as hyperechoic foci with comet-tail artifact or dirty shadowing) at the deep fascia can indicate necrotizing fasciitis.23 The differential diagnosis for ultrasound findings of hyperechoic subcutaneous fat, as seen with acute cellulitis, includes fat necrosis (Fig. 2-20); however, the latter condition is usually more focal, may be multiple, and is without physical examination findings of infection.24
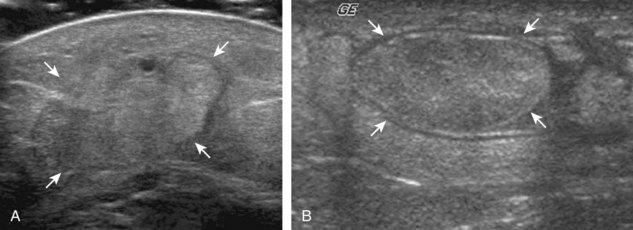
FIGURE 2-20 Fat necrosis.
(From Walsh M, Jacobson JA, Kim SM, et al: Sonography of fat necrosis involving the extremity and torso with magnetic resonance imaging and histologic correlation. J Ultrasound Med 27:1751–1757, 2008. Reproduced with permission from the American Institute of Ultrasound in Medicine.)
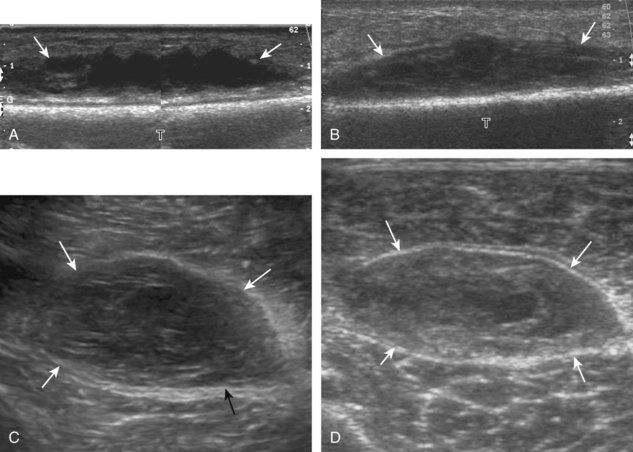
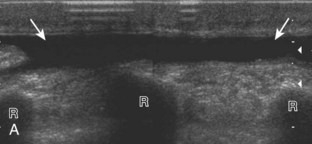
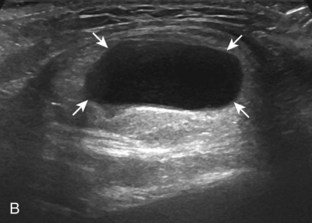
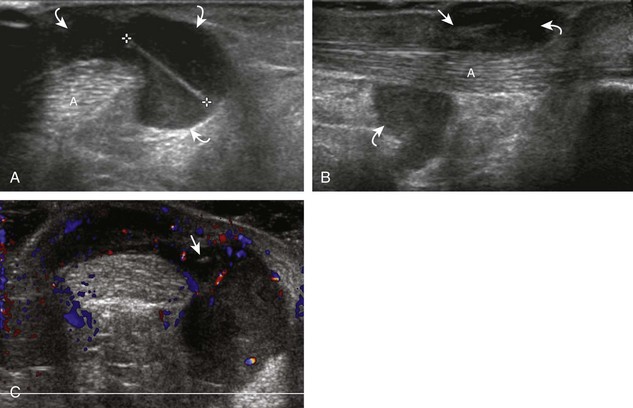
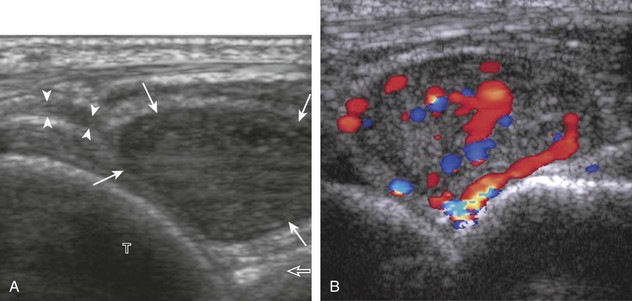
The ultrasound appearance of abscess is variable but predominantly appears as well-defined hypoechoic heterogeneous fluid collection with posterior through-transmission and hyperemia on color or power Doppler imaging (Fig. 2-21).25 A thick hyperechoic and hyperemic wall may also be seen, as may soft tissue gas.26 Uncommonly, an abscess may be isoechoic or hyperechoic relative to the adjacent soft tissues (see Fig. 2-21D). In this situation, in which it may be difficult to identify an abscess, increased through-transmission and swirling of echoes within the abscess with transducer pressure are helpful features to indicate the presence of a fluid component (Videos 2-2 and 2-3).27 ![]() Increasing the depth and field of view around a possible abscess often causes the increased through-transmission to become more conspicuous relative to the surrounding tissues.
Increasing the depth and field of view around a possible abscess often causes the increased through-transmission to become more conspicuous relative to the surrounding tissues.
Some infections occur after surgery and may be located immediately adjacent to metal hardware (see Fig. 2-21E). Ultrasound is ideal for evaluation in this situation because the reverberation artifact from the hardware occurs deep to the metal and does not obscure the overlying soft tissues.28 Soft tissue infection may also involve a bursa, which can produce complex fluid and synovitis, and possibly gas, which appears hyperechoic with comet-tail artifact (Fig. 2-22) (Video 2-4).![]() Unlike a nonspecific abscess, a bursal fluid collection tends to be more defined and, more importantly, occurs in an area of a known bursa. If an area of soft tissue infection is identified adjacent to bone, then osteomyelitis should be considered (Fig. 2-23). In the presence of cortical irregularity resulting from erosions or destruction, osteomyelitis is likely, although confirmation with magnetic resonance imaging (MRI) is often needed to assess the extent of infection fully.
Unlike a nonspecific abscess, a bursal fluid collection tends to be more defined and, more importantly, occurs in an area of a known bursa. If an area of soft tissue infection is identified adjacent to bone, then osteomyelitis should be considered (Fig. 2-23). In the presence of cortical irregularity resulting from erosions or destruction, osteomyelitis is likely, although confirmation with magnetic resonance imaging (MRI) is often needed to assess the extent of infection fully.
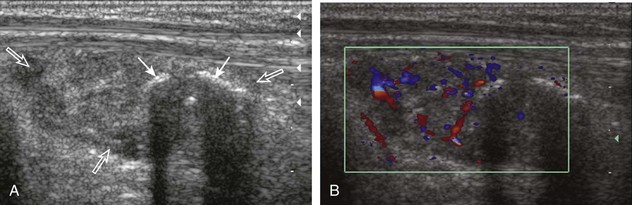
Another route of infection is hematogenous, which may manifest as a muscle abscess, as septic arthritis, or as osteomyelitis. This mode of infection is more common in children, intravenous drug abusers, or patients with sepsis. In the correct clinical scenario, septic arthritis is suspected when there is fluid distention of a joint recess, which may range from anechoic to hyperechoic, with possible hypoechoic or isoechoic synovial hypertrophy (see later). The echogenicity of fluid or the presence of flow on color or power Doppler imaging cannot predict the presence of infection, and therefore ultrasound-guided percutaneous fluid aspiration should be considered. When distention of a joint recess is not anechoic, the possibility of complex fluid versus synovial hypertrophy must be considered. To help in this distinction, compressibility of the recess, redistribution of the contents with joint positions, and lack of internal flow on color Doppler imaging suggest complex fluid rather than synovial hypertrophy. When synovial hypertrophy related to a septic joint is present, discontinuity or irregularity of the adjacent bone cortex suggests erosions and possible osteomyelitis (Fig. 2-24). Joint inflammation and synovitis from infection are indistinguishable from other inflammatory conditions, such as rheumatoid arthritis. In children, hematogenous spread of infection may also directly infect the bone. In this situation, a subperiosteal abscess may be identified because the periosteum is loosely adherent in children when compared with adults (Fig. 2-25).
Arthritis
The foregoing descriptions relate to infection of soft tissues and bone. However, inflammation may have noninfective causes. Other inflammatory conditions, such as rheumatoid arthritis, can produce joint findings (effusion, synovial hypertrophy, and erosions), which can resemble infection.29 Often, the distribution of the abnormalities and the clinical history assist with the differential diagnosis. Infection more commonly causes abnormalities at one site, and this diagnosis must be excluded before considering single-site involvement of a systemic inflammatory arthritis. The following represents general concepts of some inflammatory conditions with additional examples and text found in later chapters.
Rheumatoid Arthritis
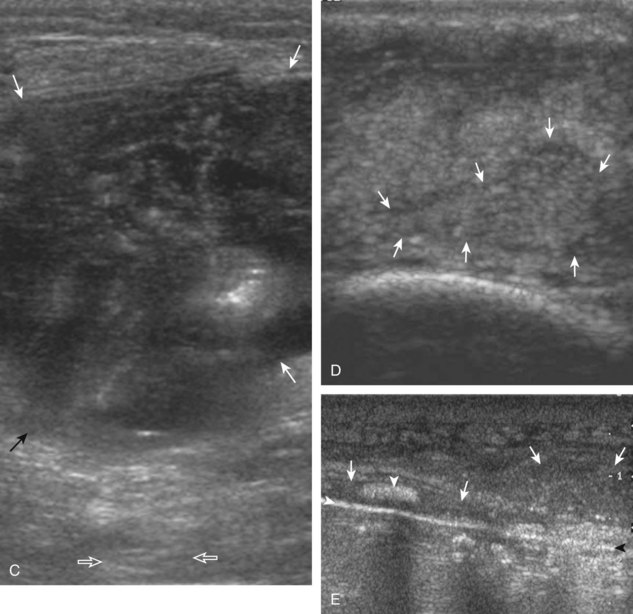
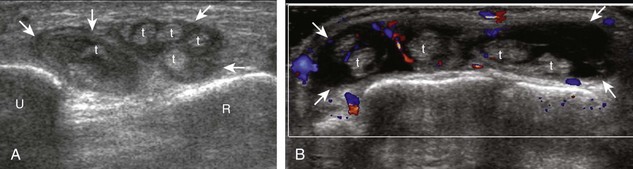
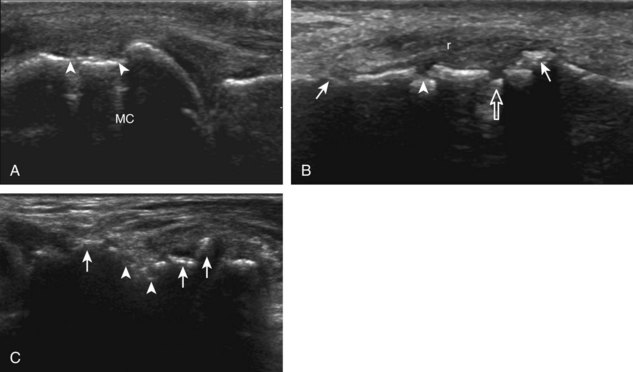
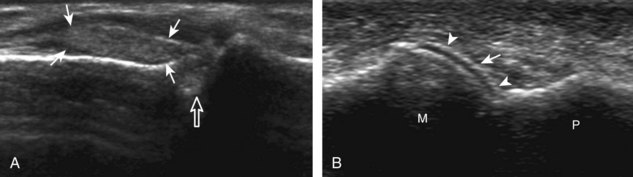
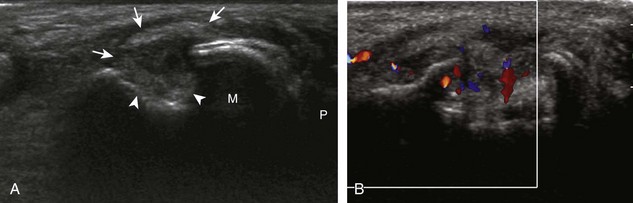
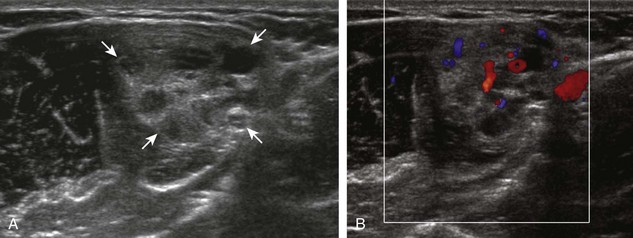
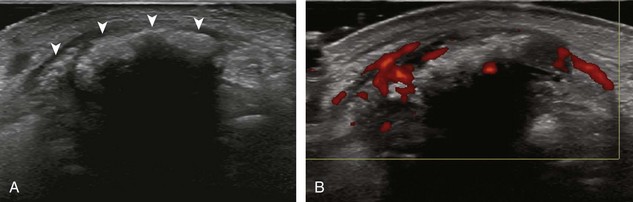
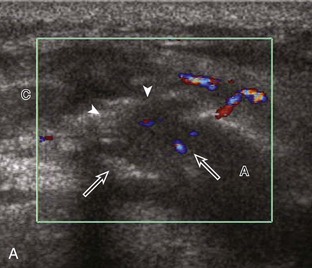
The characteristic features of rheumatoid arthritis include synovial hypertrophy and erosions. Ultrasound can be used from early diagnosis to assessment of response to therapy and can guide injections or aspirations. Synovial hypertrophy appears as hypoechoic (Fig. 2-26) or, less commonly, isoechoic (Fig. 2-27) or hyperechoic relative to subdermal fat, poorly compressible tissue within a joint or a joint recess.30 Synovial hypertrophy may also involve other synovial spaces, such as a bursa or tendon sheath (Fig. 2-28). Flow may be seen on color or power Doppler imaging, depending on the inflammatory activity of the synovitis. When assessing for hyperemia of synovial hypertrophy, it is important to minimize transducer pressure to avoid occluding or dampening flow (Fig. 2-29) (Video 2-5).![]() Joint synovial hypertrophy may be seen in the dorsal recesses of the wrist, the volar and dorsal recesses of the metacarpophalangeal and interphalangeal joints of the hand, and the metatarsophalangeal joints of the feet. 31,32 Erosions appear as discontinuity of the bone cortex seen in two orthogonal planes (Fig. 2-30). Such erosions begin in the marginal regions of a joint, where the bone cortex is not covered with hyaline cartilage and is directly exposed to joint inflammation. Ultrasound is sensitive to bone cortex abnormalities but is not specific for erosions, with a reported false-positive rate of 29% for diagnosis of erosions.33 The finding of synovial hypertrophy directly over a cortical irregularity also increases the likelihood that an erosion is present. Correlation with radiographic and clinical findings remains important, in addition to the distribution of imaging findings. For example, rheumatoid arthritis commonly involves the metacarpophalangeal joints of the hands (especially the second), the metatarsophalangeal joints of the feet (usually at least the fifth), and the wrist joints. A rheumatoid nodule typically appears as a hypoechoic nodule at ultrasound (Fig. 2-31).34
Joint synovial hypertrophy may be seen in the dorsal recesses of the wrist, the volar and dorsal recesses of the metacarpophalangeal and interphalangeal joints of the hand, and the metatarsophalangeal joints of the feet. 31,32 Erosions appear as discontinuity of the bone cortex seen in two orthogonal planes (Fig. 2-30). Such erosions begin in the marginal regions of a joint, where the bone cortex is not covered with hyaline cartilage and is directly exposed to joint inflammation. Ultrasound is sensitive to bone cortex abnormalities but is not specific for erosions, with a reported false-positive rate of 29% for diagnosis of erosions.33 The finding of synovial hypertrophy directly over a cortical irregularity also increases the likelihood that an erosion is present. Correlation with radiographic and clinical findings remains important, in addition to the distribution of imaging findings. For example, rheumatoid arthritis commonly involves the metacarpophalangeal joints of the hands (especially the second), the metatarsophalangeal joints of the feet (usually at least the fifth), and the wrist joints. A rheumatoid nodule typically appears as a hypoechoic nodule at ultrasound (Fig. 2-31).34
Psoriatic Arthritis
Psoriatic arthritis also involves synovial articulations, which can cause joint effusion, synovial hypertrophy, and erosions (Fig. 2-32A). One distinguishing feature of psoriatic arthritis, similar to other seronegative spondyloarthropathies, is the presence of bone proliferation at tendon and ligament attachments (see Fig. 2-32B and C).35 It is therefore important to assess such sites during evaluation for psoriatic arthritis, such as the collateral ligaments of the digits. Because bone proliferation of psoriatic arthritis may at times appear similar to other forms of bone proliferation, such as osteophytes with osteoarthritis, it is critical to correlate with radiography to assist in this distinction. The presence of hyperemia, often seen in psoriatic arthritis, is another feature. Similarly, it is important to differentiate a degenerative enthesophyte from true inflammatory enthesopathy at a tendon attachment, the latter showing hyperemia and adjacent tendon abnormality with ultrasound and indistinct margins on radiography. The soft tissues over a joint or tendon may also show abnormal swelling and hyperemia.36
Gout
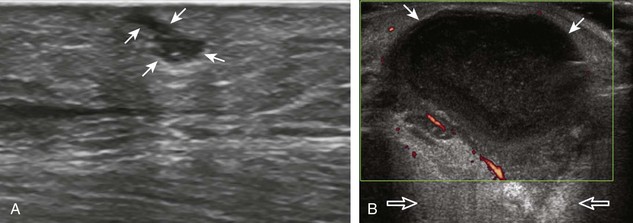
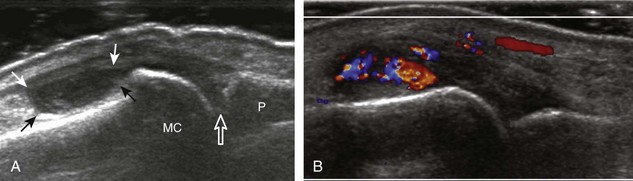
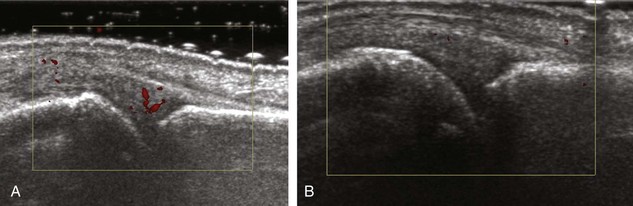
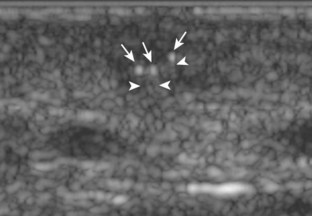
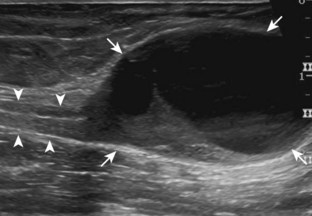
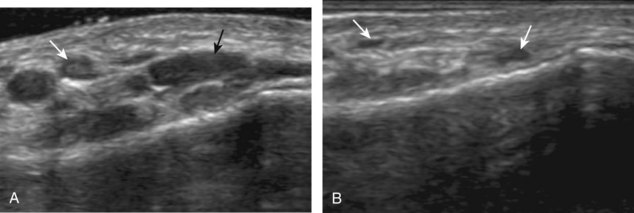
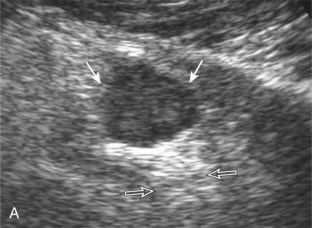
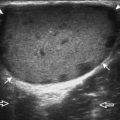
The ultrasound findings of gout include joint effusion (with possible visualization of crystals), erosions, and tophi.37 Joint distention may range from anechoic to heterogeneous, especially in the presence of crystals, tophi, and synovial hypertrophy (Fig. 2-33A). In addition, crystal deposition on the surface of the cartilage (urate icing) will appear hyperechoic, also called the double contour sign (see Fig. 2-32B). This finding is differentiated from the normal hyperechoic cartilage interface in that the latter is only seen when the sound beam is perpendicular to the cartilage surface and is uniform. The double contour sign is also different from chondrocalcinosis, in which reflective echoes are located within the cartilage rather than on the surface, as seen with calcium pyrophosphate deposition disease.38 Monosodium urate tophi characteristically appear as an amorphous but fairly well-defined echogenic area surrounded by a hypoechoic inflammatory halo (Fig. 2-34). A tophus may be associated with adjacent cortical erosion, especially at the medial aspect of the distal first metatarsal (Fig. 2-35). Tendon sheath involvement is also possible (Fig. 2-36). Other common sites for tophi include the olecranon region at the elbow (see Fig. 4-31 in Chapter 4), the patellar tendon (see Fig. 7-54 in Chapter 7), and the popliteus tendon (see Fig. 7-55 in Chapter 7) at the knee.
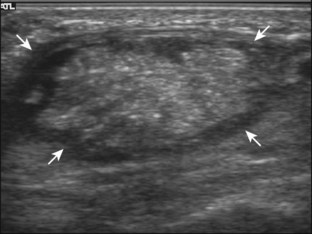
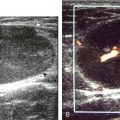
FIGURE 2-34 Gout: tophus.
Ultrasound image shows hyperechoic soft tissue tophus with hypoechoic rim (arrows).
Osteoarthritis
The hallmark of osteoarthritis is cartilage loss and osteophyte formation, typically in a predictable distribution related primarily to wear-and-tear of a joint. Synovial hypertrophy is often secondary and relatively mild without hyperemia compared with other conditions, such as rheumatoid arthritis.39 Ultrasound can detect change of osteoarthritis, especially in peripheral joints where image resolution is optimum.40 Osteophytes appear as a well-defined bone excrescence at a margin of an involved joint. Joint effusion may also be present. Common sites of involvement include the first metatarsophalangeal joint (Fig. 2-37), the interphalangeal and first carpometacarpal joints of the hand and wrist (Fig. 2-38), and the acromioclavicular joint.41 First metatarsophalangeal joint fluid and acromioclavicular joint involvement are commonly asymptomatic with preclinical osteoarthritis. Synovial hypertrophy may also be seen as hypoechoic, minimally compressible tissue distending a joint recess, although such minimal findings are also commonly seen in asymptomatic joints such as the interphalangeal joints of the hand.41 In addition, increased flow on color or power Doppler imaging is uncommon, and the presence of synovial hypertrophy does not necessarily correlate with patient symptoms.39
Myositis and Diabetic Muscle Infarction
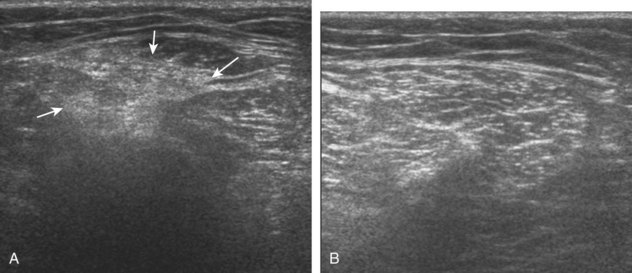
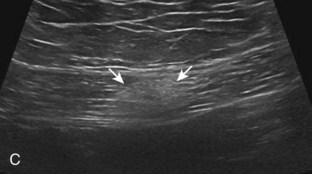
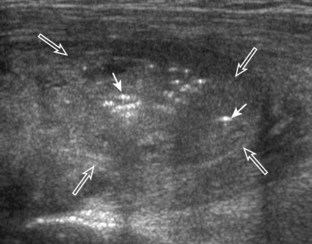
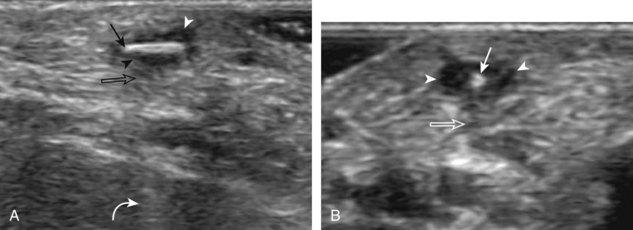
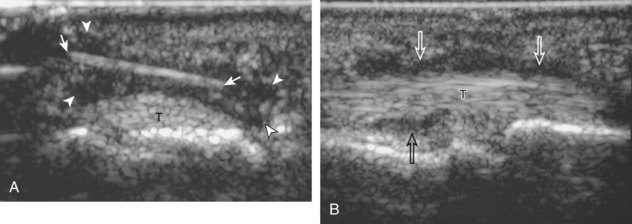
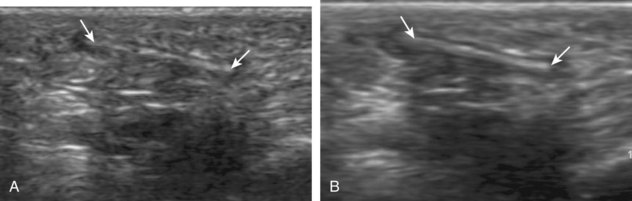
Inflammatory myositis, such as polymyositis, appears hyperechoic with possible increased flow on color or power Doppler imaging (Fig. 2-39).4 In later stages, increased muscle echogenicity and diminished volume are characteristic of muscle atrophy. Sarcoidosis may also involve muscle, where the nodular type of sarcoidosis produces hypoechoic masses or nodules.42
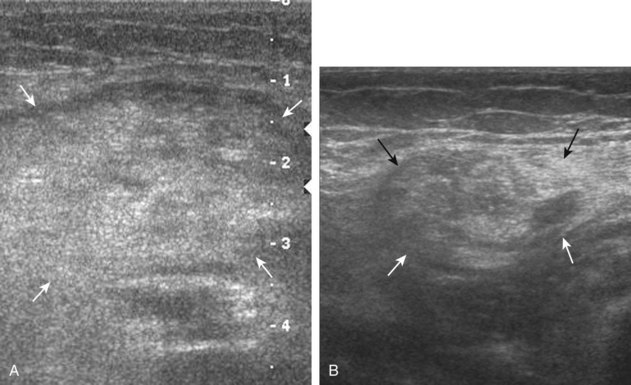
FIGURE 2-39 Myositis.
Ultrasound images show (A) increased echogenicity and size of the sartorius (arrows) representing inflammatory myositis and (B) increased echogenicity of the rectus femoris (arrows) representing postchemotherapy and radiation recall myositis.
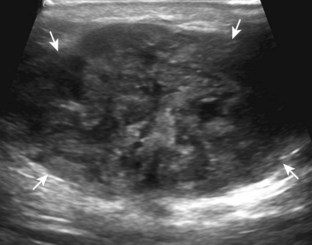
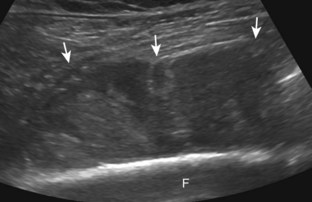
In the evaluation of inflammation or infection around the thigh or calf, one condition in the differential diagnosis is diabetic muscle infarction. In this condition, the involved thigh musculature is hypoechoic and swollen, although the hyperechoic fibroadipose septum or epimysium are still identified throughout, a feature that helps to exclude soft tissue abscess (Fig. 2-40).43 Subfascial fluid may also be seen. Diabetic muscle infarction most commonly involves the thigh or calf musculature, it may be bilateral, and it occurs in patients with longstanding diabetes.
Soft Tissue Foreign Bodies
Another cause of soft tissue infection is a soft tissue foreign body. At sonography, all foreign bodies are initially hyperechoic (Fig. 2-41), although organic or plant material may become less echogenic over time.44 The surface of the foreign body is more echogenic and conspicuous when the sound beam is perpendicular to the surface of the foreign body (Fig. 2-42). It is therefore important not only to image directly over the entry site, but also to interrogate the involved soft tissues from various angles in order to have the sound beam perpendicular to the surface of the foreign body. It is often helpful to use a thick layer of gel to float the transducer above the skin surface so as not to overlook any superficial foreign bodies and to optimize the sound beam angulation (see Fig. 2-42D).
Conspicuity of a soft tissue foreign body is additionally enhanced by the soft tissue reaction around the foreign body and the foreign body artifact if present.45 A hypoechoic halo with possible hyperemia may be present, and it represents hemorrhage, granulation tissue, and abscess. This produces a halo appearance as the hypoechoic reaction surrounds the hyperechoic foreign body (Fig. 2-43). Some foreign bodies, such as metal, may have little if any foreign body response (Fig. 2-44).
Foreign body artifact depends on the surface attributes of the foreign body more than on its internal composition.44 For example, a foreign body with a smooth and flat surface, such as glass, produces posterior reverberation artifact (Fig. 2-45). A foreign body with an irregular surface and small radius of curvature usually shows posterior shadowing (Fig. 2-46). Many foreign bodies show both shadowing and reverberation artifact (see Figs. 2-41 and 2-44). It is important that the field of view around the foreign body include soft tissues deep to the foreign body to help the viewer recognize any posterior artifact.
Although ultrasound can accurately identify and localize all soft tissue foreign bodies, it is most important in evaluation of foreign bodies that are not radiopaque on radiography, such as those composed of wood or plastic (Fig. 2-47).44 All glass is opaque on radiographs if it is large enough to be resolved, if it is projected off any adjacent osseous structures, and if it is imaged with proper radiographic technique.44 One potential pitfall in sonography of soft tissue of foreign bodies is the presence of soft tissue gas, usually the result of prior attempted removal or, less likely, infection, which in itself may simulate a foreign body and alter the sonographic characteristics of a foreign body (Fig. 2-48) (Video 2-6).46 ![]()
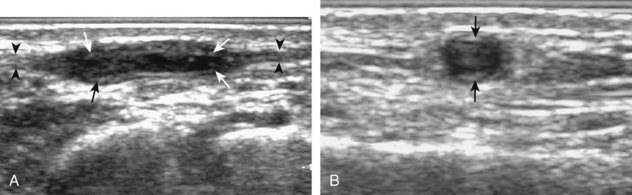
FIGURE 2-47 Plastic catheter foreign body.
(From Fessell DP, Jamadar DA, Jacobson JA, et al: Sonography of dorsal ankle and foot abnormalities. AJR Am J Roentgenol 181:1571–1573, 2003.)
Ultrasound can also assess for related complications, such as adjacent tenosynovitis (Fig. 2-49), periostitis (Fig. 2-50), and abscess (Fig. 2-51).45 Ultrasound can aid removal by accurately marking the skin surface over the foreign body before removal, by guiding a localization wire, or by directly guiding percutaneous removal.47,48 A chronic foreign body reaction may simulate a soft tissue mass. The use of spatial compound sonography may smooth out the image and affects the appearance of the foreign body and associated artifacts (Fig. 2-52).
Peripheral Nerve Entrapment
There are specific anatomic sites where a peripheral nerve may be entrapped, typically when a nerve traverses a confined space as a result of osseous, ligamentous, or fibrous constraints.49–51 Examples in the upper extremity include the median nerve in the carpal tunnel (carpal tunnel syndrome) (see Fig. 5-61 in Chapter 5), the ulnar nerve in the Guyon canal (ulnar canal syndrome) (see Fig. 5-70 in Chapter 5), the ulnar nerve in the cubital tunnel of the elbow (cubital tunnel syndrome) (see Fig. 4-54 in Chapter 4), and the deep branch of the radial nerve at the level of the supinator muscle (posterior interosseous nerve or supinator syndrome) (see Fig. 4-66 in Chapter 4). Examples in the lower extremity include the tibial nerve at the ankle (tarsal tunnel syndrome) (see Fig. 8-153 in Chapter 8) and the common plantar digital nerve in the distal foot (Morton neuroma) (see Fig. 8-152 in Chapter 8). Common sonographic features of each of these conditions are hypoechoic swelling of the involved nerve at the entrapment site and possible compression distally. Many times, transducer pressure on the nerve elicits symptoms. Evaluation for denervation and muscle atrophy is also a clue to peripheral nerve entrapment and chronicity, where ultrasound shows increased echogenicity of the involved muscle. Knowledge of peripheral nerve anatomy and of sites prone to nerve compression is vital for an accurate diagnosis.
Soft Tissue Masses
Although the etiology of some soft tissue tumors may be suggested based on anatomic location, physical examination findings, and the patient’s history and age, many masses remain nonspecific by ultrasound. The primary roles of ultrasound in this situation are to differentiate cyst versus solid mass and to guide biopsy for definitive histologic diagnosis. Ultrasound does play an important role in the assessment of benign subcutaneous masses by improving diagnostic accuracy.52 In the following chapters, soft tissue masses that are specific or common to each anatomic region are discussed. Some masses occur throughout the body and have similar sonographic features regardless, and several of these are discussed here.
Lipoma
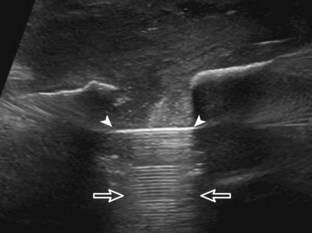
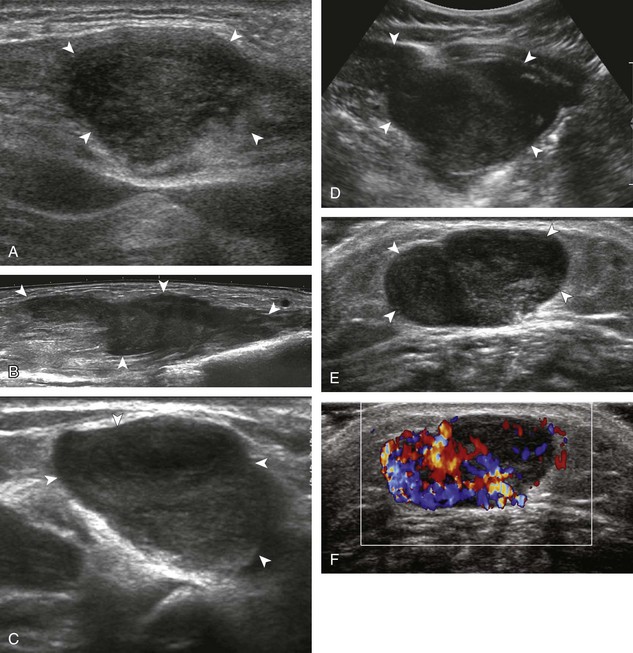
Soft tissue lipomas can occur anywhere in the body and may be multiple, although many lipomas involve the shoulder region, upper extremity, trunk, and back. Soft tissue lipomas may be located within the subcutaneous fat, within muscle, or within tissue planes. When present in the subcutaneous tissues, the findings of a homogeneous, oval, isoechoic to minimally hyperechoic mass, with little or no flow on color or power Doppler imaging, that is soft and pliable with transducer pressure are compatible with lipoma (Fig. 2-53) (Video 2-7).![]() When a lipoma is located in an intramuscular location, the appearance is somewhat nonspecific but often is relatively hyperechoic (Fig. 2-54).53 Because an intramuscular lipoma and its margins are more difficult to define and a lipomatous tumor is more likely to be malignant when in a deep compared with a superficial location, MRI is typically indicated to confirm a suspected intramuscular lipoma.
When a lipoma is located in an intramuscular location, the appearance is somewhat nonspecific but often is relatively hyperechoic (Fig. 2-54).53 Because an intramuscular lipoma and its margins are more difficult to define and a lipomatous tumor is more likely to be malignant when in a deep compared with a superficial location, MRI is typically indicated to confirm a suspected intramuscular lipoma.
The variable echogenicity of a lipoma is related to the amount of fat and connective tissue in the tumor as well as to the surrounding tissue echogenicity. For example, a homogeneous fatty mass is hypoechoic; as the amount of fibrous tissue within the lipoma increases, the lipoma will appear more hyperechoic owing to the reflective soft tissue interfaces (Fig. 2-55).53 In addition, a lipoma that is isoechoic to the surrounding subcutaneous fat appears relatively hyperechoic when it is located in muscle. Subcutaneous lipomas that are isoechoic to the surrounding tissues may not be immediately apparent on ultrasound. It is important to correlate directly with physical examination findings, with direct palpation of the mass under ultrasound visualization (Video 2-8),![]() or by placing an opened paperclip or other similar marker over the edge of the palpable mass and then scanning the region.47
or by placing an opened paperclip or other similar marker over the edge of the palpable mass and then scanning the region.47
The sensitivity and specificity of ultrasound in the diagnosis of a subcutaneous lipoma are 88% and 99%, respectively.52 In the correct clinical setting, sonography may determine that a soft tissue mass is compatible with a lipoma; however, a mass that is enlarging or a painful mass requires MRI or histologic evaluation for confirmation. A low-grade well-differentiated liposarcoma has a variable appearance but is often hyperechoic, related to the amount of soft tissue stranding or nodules within the predominantly fatty tumor (Fig. 2-56). A high-grade or poorly differentiated liposarcoma is heterogeneous but predominantly hypoechoic, similar to other sarcomas (see later section in this chapter). Any fatty mass that is not isolated to the subcutaneous tissue should undergo MRI for confirmation.
If a small hyperechoic mass is seen in the subcutaneous tissues, additional diagnoses should be considered. With this appearance, one possibility would be an angiolipoma, which is considered a vascular variant of a lipoma or hamartoma; it is multiple and painful in about 50% of patients (Fig. 2-57). Subcutaneous fat necrosis (as part of panniculitis or after trauma) has a variable appearance but may look like a focal hyperechoic mass or nodule (see Fig. 2-20B).24 Dermatofibrosarcoma protuberans may appear as either a hypoechoic (discussed later in Malignant Soft Tissue Tumors) or hyperechoic subcutaneous mass; the latter appearance is different from a lipoma, given a wide base contact with the skin with possible ill-defined borders and hyperemia.54
Peripheral Nerve Sheath Tumors
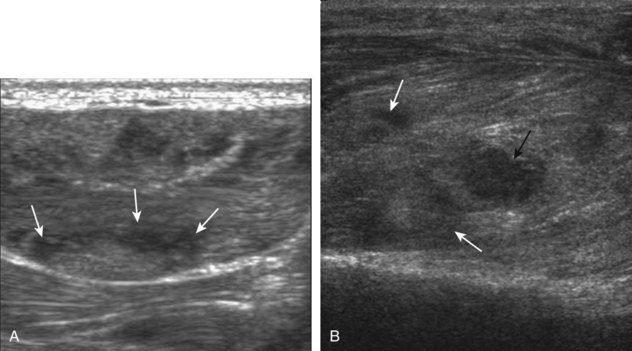
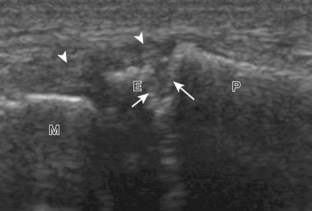
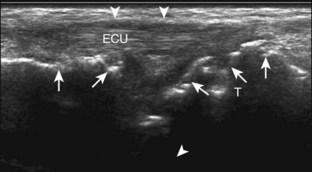
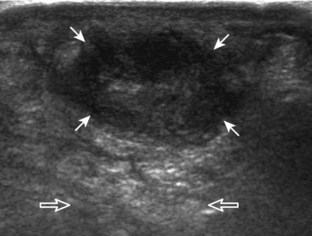
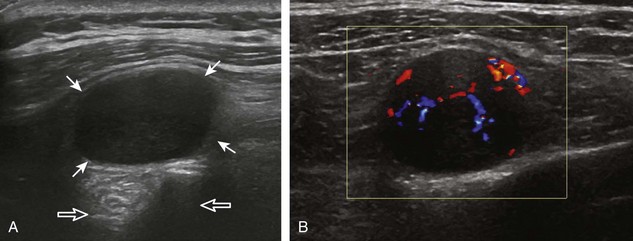
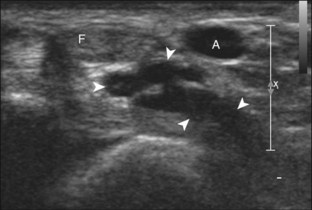
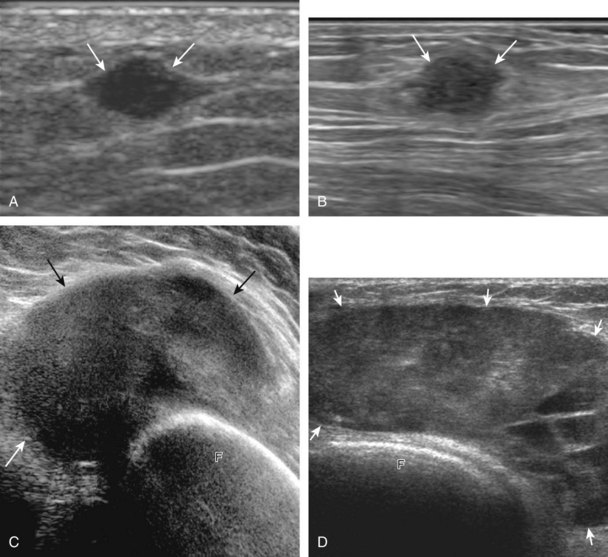
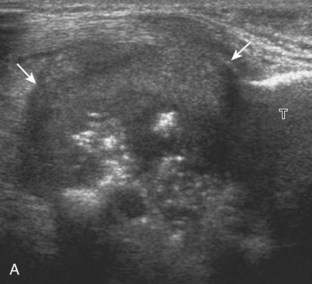
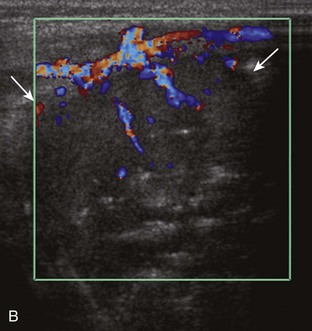
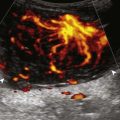
A solid soft tissue mass that is in continuity with a peripheral nerve is diagnostic for a peripheral nerve sheath tumor. Ultrasound is often used to demonstrate peripheral nerve continuity given its high resolution. At ultrasound, a peripheral nerve sheath tumor is hypoechoic with a low level of homogeneous internal echoes, round or oval, and appears well defined (Fig. 2-58). Increased through-transmission is usually seen deep to the mass, which may cause the hypoechoic mass to be mistaken for a complex cyst; however, the presence of flow on color or power Doppler imaging confirms the solid nature of the mass (Fig. 2-59) (Video 2-9).55![]() Transducer pressure over a peripheral nerve sheath tumor usually elicits symptoms.
Transducer pressure over a peripheral nerve sheath tumor usually elicits symptoms.
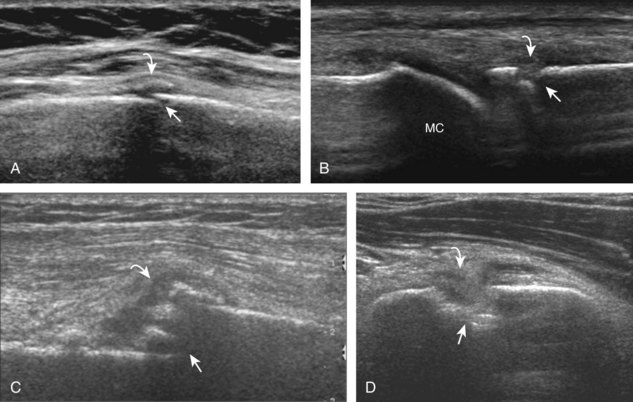
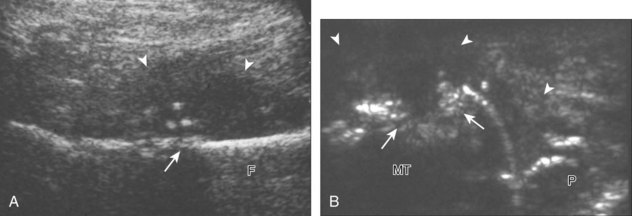
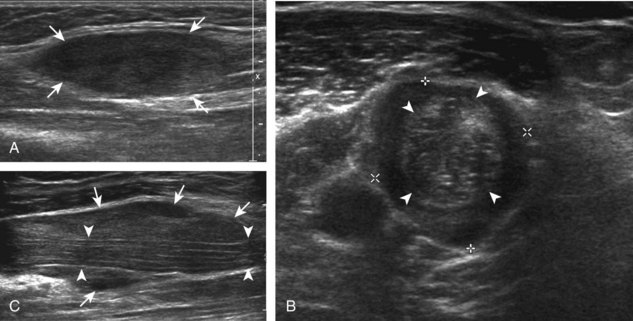
A solitary peripheral nerve sheath tumor that is eccentric to the peripheral nerve is characteristic of a schwannoma (or neurilemmoma) (see Fig. 8-154 in Chapter 8), whereas neurofibromas tend to be central relative to the nerve, although differentiation between the two is often not possible with ultrasound.56 A target appearance has also been described in neurofibromas, which appears as an echogenic fibrous center surrounded by a hypoechoic myxoid periphery, reported as a possible indicator of a benign peripheral nerve sheath tumor (Fig. 2-60A).57 Neurofibromas may have three different forms: localized (see Fig. 2-60A), plexiform, and diffuse.58 Plexiform neurofibroma is described as a “bag of worms” appearance (see Fig. 2-60B), whereas the diffuse form appears as diffuse echogenic subcutaneous tissues with hypoechoic tubules (see Fig. 2-60C), most commonly involving the head and neck region. Peripheral nerve sheath tumors may have internal cystic areas (Fig. 2-61) and calcification (such as in a longstanding or ancient schwannoma). Ultrasound cannot accurately differentiate benign from malignant peripheral nerve sheath tumors; the latter often appear similar to other soft tissue malignancies (Fig. 2-62).
Vascular Anomalies
Based on clinical and histologic findings, soft tissue vascular anomalies can be categorized into vascular tumors and vascular malformations.59,60 A common childhood vascular tumor is an infantile hemangioma, which undergoes spontaneous involution in most cases. Vascular malformations are subcategorized as low flow (capillary, venous, lymphatic, or a combination of each) and high flow (arteriovenous fistula and arteriovenous malformation).59 Although this is one described classification system, focal and well-defined intramuscular vascular lesions commonly presenting in an adult may also be called hemangiomas, subdivided by their dominant vascularity.61
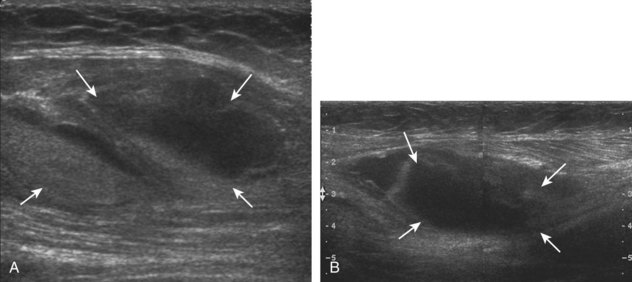
At ultrasound, an infantile hemangioma is characterized by a mixed hyperechoic and hypoechoic mass with few or no visible vessels but with increased flow on color or power Doppler imaging.59 Intramuscular vascular malformations have a heterogeneous appearance, with a variable echogenicity, ranging from hypoechoic to isoechoic to hyperechoic, which often infiltrates the involved soft tissue (Figs. 2-63 and 2-64).59,62 Anechoic or hypoechoic channels that demonstrate flow on color or power Doppler imaging are typical, although flow may be very slow and difficult to identify without augmenting flow with manual compression. The hyperechoic areas represent the interfaces with the vascular structures, associated fatty tissue, and adjacent soft tissues. Focal hyperechoic and shadowing phleboliths, which represent dystrophic calcification in an organizing thrombus, may also be seen. When evaluating a vascular anomaly with ultrasound, the presence of an area of abnormal vascular channels without an associated soft tissue mass suggests the diagnosis of a vascular malformation, such as an arteriovenous malformation having the appearance of a tangle of vessels (Fig. 2-65).59,63 Both infantile hemangiomas and arteriovenous malformations tend to have a greater vessel density than other vascular malformations.63 It is important to distinguish the foregoing features of vascular anomalies from more nonspecific neovascularity and possible dystrophic calcification of a malignant soft tissue neoplasm. Demonstration of the characteristic features of phleboliths on radiography is helpful; however, percutaneous biopsy may be required.
Ganglion Cysts
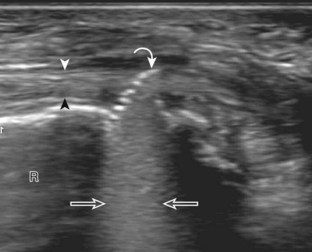
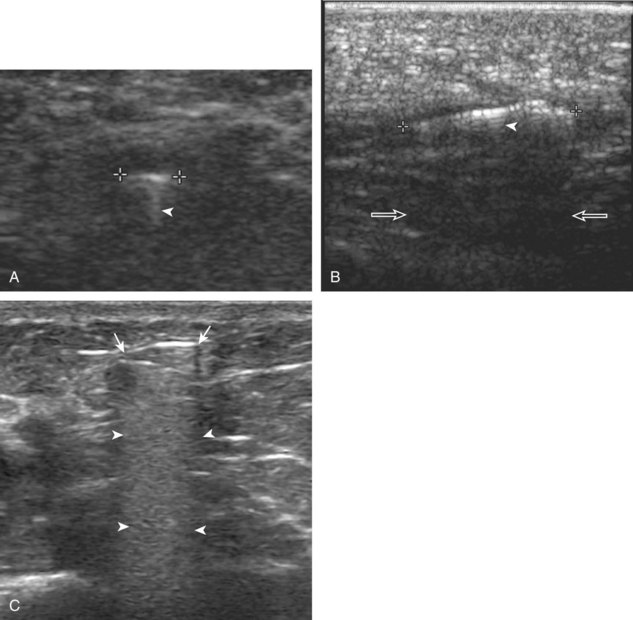
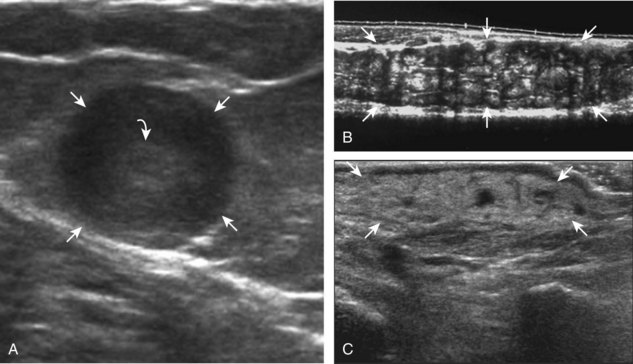
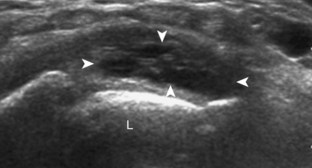
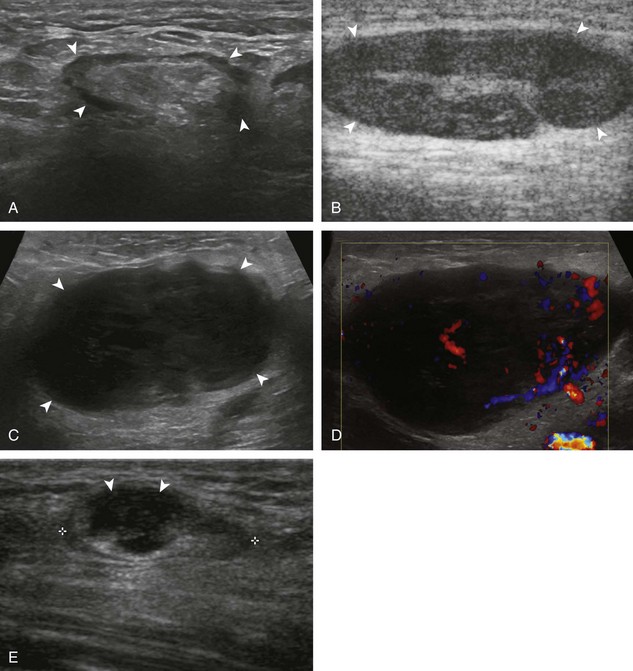
Ganglion cysts have several appearances at ultrasound. The most common appearance is that of a hypoechoic or anechoic, multilocular or multilobular, noncompressible cyst that may look complex.64,65 Smaller ganglion cysts are more likely hypoechoic and may show only limited increased through-transmission.65 The multilocular appearance of a cyst is specific to both ganglion cysts and fibrocartilage cysts (parameniscal and paralabral); the location of the multilocular cyst assists in this diagnosis. If in contact with fibrocartilage, then parameniscal or paralabral cyst is likely. If located superficial to the scapholunate ligament (Fig. 2-66), near the radial artery at the wrist (a very common site) (Fig. 2-67), at the sinus tarsi of the ankle (see Fig. 8-159 in Chapter 8), or within the Hoffa infrapatellar fat pad or at the gastrocnemius tendon origin at the knee (see Figs. 7-73 and 7-74 in Chapter 7), ganglion cyst is likely. The other appearance of a ganglion cyst is one of a more unilocular fluid collection, which can be associated with wrist, hand, ankle, and foot tendons.64 Unlike a bursal fluid collection, such unilocular ganglion cysts are usually not compressible and not in a location of an expected bursa. Aspiration should only be attempted with a larger diameter needle (such as a 16- or 18-gauge needle), given the high viscosity of the gel-like fluid.
Lymph Nodes
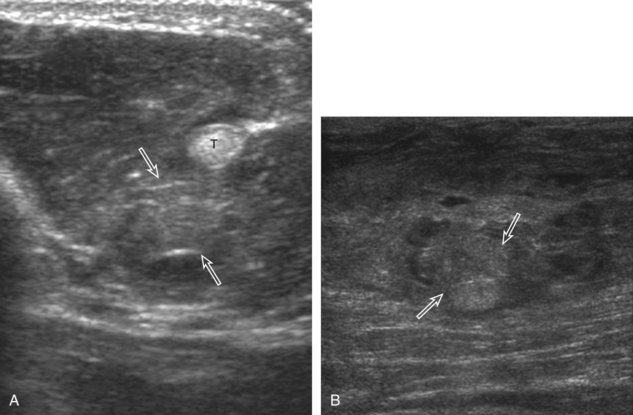
A normal lymph node will appear oval, with a central hyperechoic hilum and a variable-thickness hypoechoic peripheral cortex rim (Fig. 2-68A).66 The central echogenicity is not from fat but rather interfaces with sinuses and lymphatic cords.66 The peripheral hypoechoic cortex will be of variable thickness but should be uniform. Flow on color or power Doppler imaging, if present, should have a hilar pattern. With age and after repeated inflammation, the outer cortex of the node will thin, whereas the central aspect becomes more hyperechoic but may decrease or increase in size. A hyperplastic lymph node will be enlarged but maintain the essential sonographic features of a lymph node as described earlier (see Fig. 2-68B) (Video 2-10).![]() When a lymph node is malignant (primary or metastatic), the echogenic hilum will narrow and could disappear, whereas the outer hypoechoic cortex will enlarge, and the lymph node will lose its oval shape and become round (see Fig. 2-68C). Flow on color or power Doppler imaging will become heterogeneous, mixed, and peripheral (see Fig. 2-68D) Although size criteria are used throughout the body to determine when a lymph node has enlarged, it is critical not to rely solely on size criteria but rather to evaluate the sonographic characteristics for early malignancy, taking into account patient history (see Fig. 2-68E). Increased posterior through-transmission is usually present with abnormal lymph nodes.
When a lymph node is malignant (primary or metastatic), the echogenic hilum will narrow and could disappear, whereas the outer hypoechoic cortex will enlarge, and the lymph node will lose its oval shape and become round (see Fig. 2-68C). Flow on color or power Doppler imaging will become heterogeneous, mixed, and peripheral (see Fig. 2-68D) Although size criteria are used throughout the body to determine when a lymph node has enlarged, it is critical not to rely solely on size criteria but rather to evaluate the sonographic characteristics for early malignancy, taking into account patient history (see Fig. 2-68E). Increased posterior through-transmission is usually present with abnormal lymph nodes.
Malignant Soft Tissue Tumors
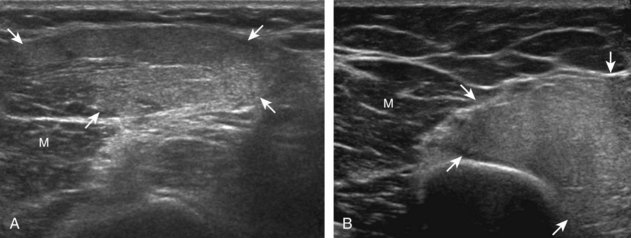
The precise diagnosis of a malignant soft tissue tumor typically cannot be made with ultrasound; however, a large soft tissue mass that does not originate from a joint or synovial space (bursa or tendon sheath) and that is hypoechoic with hypervascularity suggests a possible malignant origin, although biopsy is required for confirmation. Soft tissue sarcomas are predominantly hypoechoic (Fig. 2-69), with possible heterogeneous hyperechoic and hypervascular regions and anechoic necrotic regions as they enlarge, especially when high grade. Increased posterior through-transmission is usually present, as with most solid soft tissue masses. An important teaching point is that a mass that originates within a joint or synovial space is related to a synovial process (proliferation or inflammation) and rarely malignancy; synovial sarcoma is similar to other sarcomas and appears as a hypoechoic mass near but outside of a joint (see Fig. 2-69C). Granulocytic or myeloid sarcoma (also called chloroma), as a complication of myelogenous leukemia, may also appear as a hypoechoic mass (Fig. 2-70).67 Lymphoma also presents as a hypoechoic mass with increased through-transmission or an infiltrating hypoechoic mass (Fig. 2-71).68 A soft tissue tumor that is calcified or ossified will require further evaluation with MRI or CT because shadowing may obscure much of the mass (Fig. 2-72).
Common diagnoses can be suggested based on the patient’s age and the location of the tumor, but percutaneous biopsy with use of ultrasound guidance is usually needed.69 With ultrasound guidance, a needle can be accurately placed into the soft tissue component of the tumor, while avoiding the necrotic center and adjacent neurovascular structures and thus increasing diagnostic yield. Soft tissue metastases are commonly hypoechoic with possible hypervascularity (Fig. 2-73).70 Ultrasound is also effective in evaluation for recurrence of soft tissue malignancy after treatment (Fig. 2-74).71 With melanoma, ultrasound can detect soft tissue recurrence or metastasis before findings at clinical examination (see Fig. 2-74A).72 It has been shown that ultrasound is as effective as MRI in evaluation for soft tissue sarcoma recurrence after treatment (see Fig. 2-74B and C).73
Bone Masses
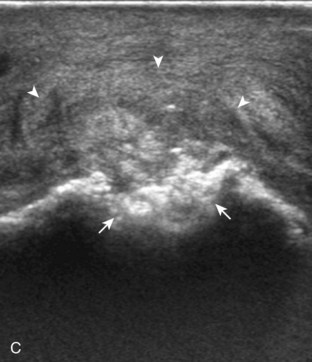
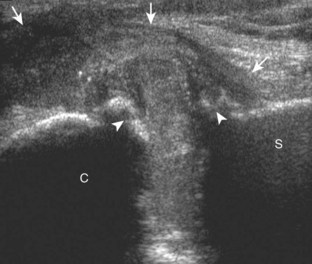
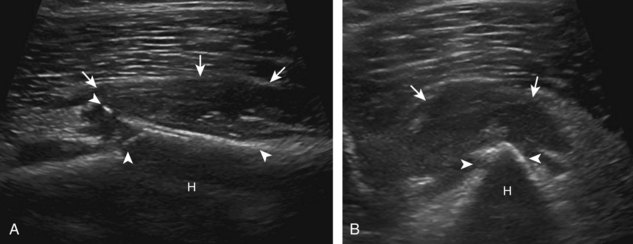
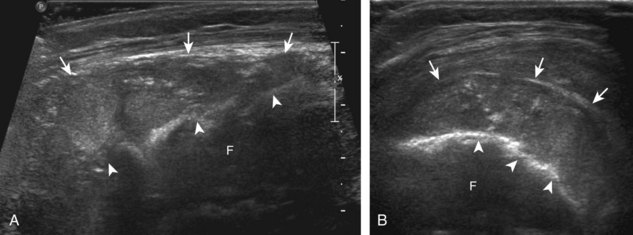
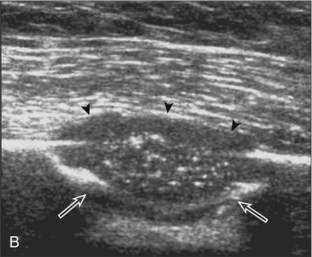
One primary benign bone abnormality that may be visible at ultrasound is an osteochondroma (or exostosis) (Fig. 2-75) (Video 2-11), ![]() which appears as a well-demarcated osseous excrescence that typically points away from the adjacent joint. Correlation with radiography is essential to identify both cortical and medullary continuity with the underlying bone to ensure the correct diagnosis. Ultrasound can also identify complications related to an enchondroma, such as fracture, bursa formation (Fig. 2-76), pseudoaneurysm, and malignant degeneration to chondrosarcoma. Other benign bone lesions that may be visible at ultrasound include aneurysmal bone cysts (Fig. 2-77).
which appears as a well-demarcated osseous excrescence that typically points away from the adjacent joint. Correlation with radiography is essential to identify both cortical and medullary continuity with the underlying bone to ensure the correct diagnosis. Ultrasound can also identify complications related to an enchondroma, such as fracture, bursa formation (Fig. 2-76), pseudoaneurysm, and malignant degeneration to chondrosarcoma. Other benign bone lesions that may be visible at ultrasound include aneurysmal bone cysts (Fig. 2-77).
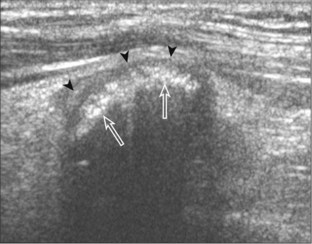
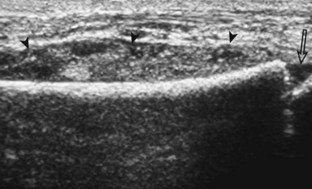
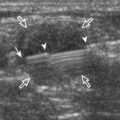
FIGURE 2-75 Osteochondroma (exostosis).
Ultrasound image shows a hyperechoic ossified surface (open arrows) and an overlying hypoechoic cartilage cap (arrowheads) of osteochondroma.
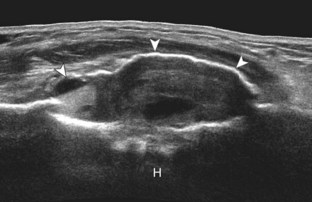
FIGURE 2-77 Aneurysmal bone cyst.
Ultrasound images show expansile nature of aneurysmal bone cyst (arrowheads). H, humerus.
When there is destruction of the bone cortex, an aggressive process is present, and considerations include both primary and secondary bone malignancy. Correlation with patient age, history, radiography, and distribution of pathology can suggest primary versus secondary processes. Considerations for primary bone tumor include osteosarcoma (Fig. 2-78), malignant fibrous histiocytoma (Fig. 2-79), chondrosarcoma, lymphoma, and Ewing sarcoma (Fig. 2-80). Osseous metastasis may also produce bone destruction (Fig. 2-81) (Video 2-12).74![]() A cortically based destructive process suggests lung cancer metastasis (see Fig. 2-81B), whereas an expansile hyperemic process could indicate a vascular metastasis, such as from renal cell or thyroid carcinoma.
A cortically based destructive process suggests lung cancer metastasis (see Fig. 2-81B), whereas an expansile hyperemic process could indicate a vascular metastasis, such as from renal cell or thyroid carcinoma.
1 Speer KP, Lohnes J, Garrett WE, Jr. Radiographic imaging of muscle strain injury. Am J Sports Med. 1993;21:89–95. discussion 6
2 Blankenbaker DG, Tuite MJ. Temporal changes of muscle injury. Semin Musculoskelet Radiol. 2010;14:176–193.
3 Lee JC, Healy J. Sonography of lower limb muscle injury. AJR Am J Roentgenol. 2004;182:341–351.
4 Adler RS, Garofalo G. Ultrasound in the evaluation of the inflammatory myopathies. Curr Rheumatol Rep. 2009;11:302–308.
5 Neal C, Jacobson JA, Brandon C, et al. Sonography of Morel-Lavallée lesions. J Ultrasound Med. 2008;27:1077–1081.
6 Tyler P, Saifuddin A. The imaging of myositis ossificans. Semin Musculoskelet Radiol. 2010;14:201–216.
7 Peetrons P. Ultrasound of muscles. Eur Radiol. 2002;12:35–43.
8 Bianchi S, Martinoli C, Waser NP, et al. Central aponeurosis tears of the rectus femoris: sonographic findings. Skeletal Radiol. 2002;31:581–586.
9 Khan KM, Bonar F, Desmond PM, et al. Patellar tendinosis (jumper’s knee): findings at histopathologic examination, US, and MR imaging. Victorian Institute of Sport Tendon Study Group. Radiology. 1996;200:821–827.
10 Kjellin I, Ho CP, Cervilla V, et al. Alterations in the supraspinatus tendon at MR imaging: correlation with histopathologic findings in cadavers. Radiology. 1991;181:837–841.
11 Potter HG, Hannafin JA, Morwessel RM, et al. Lateral epicondylitis: correlation of MR imaging, surgical, and histopathologic findings. Radiology. 1995;196:43–46.
12 Craig JG, Jacobson JA, Moed BR. Ultrasound of fracture and bone healing. Radiol Clin North Am. 1999;37:737–751. ix
13 Drakonaki EE, Garbi A. Metatarsal stress fracture diagnosed with high-resolution sonography. J Ultrasound Med. 2010;29:473–476.
14 Farley FA, Kuhns L, Jacobson JA, et al. Ultrasound examination of ankle injuries in children. J Pediatr Orthop. 2001;21:604–607.
15 Patten RM, Mack LA, Wang KY, et al. Nondisplaced fractures of the greater tuberosity of the humerus: sonographic detection. Radiology. 1992;182:201–204.
16 Griffith JF, Rainer TH, Ching AS, et al. Sonography compared with radiography in revealing acute rib fracture. AJR Am J Roentgenol. 1999;173:1603–1609.
17 Turk F, Kurt AB, Saglam S. Evaluation by ultrasound of traumatic rib fractures missed by radiography. Emerg Radiol. 2010;17:473–477.
18 Malghem J, Vande Berg B, Lecouvet F, et al. Costal cartilage fractures as revealed on CT and sonography. AJR Am J Roentgenol. 2001;176:429–432.
19 Ziv N, Litwin A, Katz K, et al. Definitive diagnosis of fracture-separation of the distal humeral epiphysis in neonates by ultrasonography. Pediatr Radiol. 1996;26:493–496.
20 Chao HC, Lin SJ, Huang YC, et al. Sonographic evaluation of cellulitis in children. J Ultrasound Med. 2000;19:743–749.
21 Huang MN, Chang YC, Wu CH, et al. The prognostic values of soft tissue sonography for adult cellulitis without pus or abscess formation. Intern Med J. 2009;39:841–844.
22 Gaspari RJ, Resop D, Mendoza M, et al. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2011;57:483–491. e1
23 Wronski M, Slodkowski M, Cebulski W, et al. Necrotizing fasciitis: early sonographic diagnosis. J Clin Ultrasound. 2011;39:236–239.
24 Walsh M, Jacobson JA, Kim SM, et al. Sonography of fat necrosis involving the extremity and torso with magnetic resonance imaging and histologic correlation. J Ultrasound Med. 2008;27:1751–1757.
25 Bureau NJ, Chhem RK, Cardinal E. Musculoskeletal infections: US manifestations. Radiographics. 1999;19:1585–1592.
26 Turecki MB, Taljanovic MS, Stubbs AY, et al. Imaging of musculoskeletal soft tissue infections. Skeletal Radiol. 2010;39:957–971.
27 Loyer EM, DuBrow RA, David CL, et al. Imaging of superficial soft-tissue infections: sonographic findings in cases of cellulitis and abscess. AJR Am J Roentgenol. 1996;166:149–152.
28 Weiss DB, Jacobson JA, Karunakar MA. The use of ultrasound in evaluating orthopaedic trauma patients. J Am Acad Orthop Surg. 2005;13:525–533.
29 Lund PJ, Heikal A, Maricic MJ, et al. Ultrasonographic imaging of the hand and wrist in rheumatoid arthritis. Skeletal Radiol. 1995;24:591–596.
30 Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487.
31 Backhaus M, Burmester GR, Gerber T, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60:641–649.
32 Vlad V, Berghea F, Libianu S, et al. Ultrasound in rheumatoid arthritis—volar versus dorsal synovitis evaluation and scoring. BMC Musculoskelet Disord. 2011;12:124.
33 Finzel S, Ohrndorf S, Englbrecht M, et al. A detailed comparative study of high-resolution ultrasound and micro-computed tomography for detection of arthritic bone erosions. Arthritis Rheum. 2011;63:1231–1236.
34 Nalbant S, Corominas H, Hsu B, et al. Ultrasonography for assessment of subcutaneous nodules. J Rheumatol. 2003;30:1191–1195.
35 Gutierrez M, Filippucci E, De Angelis R, et al. A sonographic spectrum of psoriatic arthritis: “the five targets.”. Clin Rheumatol. 2010;29:133–142.
36 Gutierrez M, Filippucci E, Salaffi F, et al. Differential diagnosis between rheumatoid arthritis and psoriatic arthritis: the value of ultrasound findings at metacarpophalangeal joints level. Ann Rheum Dis. 2011;70:1111–1114.
37 Thiele RG. Role of ultrasound and other advanced imaging in the diagnosis and management of gout. Curr Rheumatol Rep. 2011;13:146–153.
38 Filippucci E, Riveros MG, Georgescu D, et al. Hyaline cartilage involvement in patients with gout and calcium pyrophosphate deposition disease: an ultrasound study. Osteoarthritis Cartilage. 2009;17:178–181.
39 Hayashi D, Roemer FW, Katur A, et al. Imaging of synovitis in osteoarthritis: current status and outlook. Semin Arthritis Rheum. 2011;41:116–130.
40 Keen HI, Conaghan PG. Ultrasonography in osteoarthritis. Radiol Clin North Am. 2009;47:581–594.
41 Arrestier S, Rosenberg C, Etchepare F, et al. Ultrasound features of nonstructural lesions of the proximal and distal interphalangeal joints of the hands in patients with finger osteoarthritis. Joint Bone Spine. 2011;78:65–69.
42 Tohme-Noun C, Le Breton C, Sobotka A, et al. Imaging findings in three cases of the nodular type of muscular sarcoidosis. AJR Am J Roentgenol. 2004;183:995–999.
43 Delaney-Sathy LO, Fessell DP, Jacobson JA, et al. Sonography of diabetic muscle infarction with MR imaging, CT, and pathologic correlation. AJR Am J Roentgenol. 2000;174:165–169.
44 Horton LK, Jacobson JA, Powell A, et al. Sonography and radiography of soft-tissue foreign bodies. AJR Am J Roentgenol. 2001;176:1155–1159.
45 Boyse TD, Fessell DP, Jacobson JA, et al. US of soft-tissue foreign bodies and associated complications with surgical correlation. Radiographics. 2001;21:1251–1256.
46 Lyon M, Brannam L, Johnson D, et al. Detection of soft tissue foreign bodies in the presence of soft tissue gas. J Ultrasound Med. 2004;23:677–681.
47 Creel SA, Girish G, Jamadar DA, et al. Sonographic surface localization of subcutaneous foreign bodies and masses. J Clin Ultrasound. 2009;37:158–160.
48 Shiels WE, 2nd., Babcock DS, Wilson JL, et al. Localization and guided removal of soft-tissue foreign bodies with sonography. AJR Am J Roentgenol. 1990;155:1277–1281.
49 Jacobson JA, Fessell DP, Lobo Lda G, et al. Entrapment neuropathies I: upper limb (carpal tunnel excluded). Semin Musculoskelet Radiol. 2010;14:473–486.
50 Klauser AS, Faschingbauer R, Bauer T, et al. Entrapment neuropathies II: carpal tunnel syndrome. Semin Musculoskelet Radiol. 2010;14:487–500.
51 Martinoli C, Bianchi S, Gandolfo N, et al. US of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. Radiographics. 2000;20:S199–S213. discussion S-7
52 Kuwano Y, Ishizaki K, Watanabe R, et al. Efficacy of diagnostic ultrasonography of lipomas, epidermal cysts, and ganglions. Arch Dermatol. 2009;145:761–764.
53 Inampudi P, Jacobson JA, Fessell DP, et al. Soft-tissue lipomas: accuracy of sonography in diagnosis with pathologic correlation. Radiology. 2004;233:763–767.
54 Shin YR, Kim JY, Sung MS, et al. Sonographic findings of dermatofibrosarcoma protuberans with pathologic correlation. J Ultrasound Med. 2008;27:269–274.
55 Reynolds DL, Jr., Jacobson JA, Inampudi P, et al. Sonographic characteristics of peripheral nerve sheath tumors. AJR Am J Roentgenol. 2004;182:741–744.
56 Tsai WC, Chiou HJ, Chou YH, et al. Differentiation between schwannomas and neurofibromas in the extremities and superficial body: the role of high-resolution and color Doppler ultrasonography. J Ultrasound Med. 2008;27:161–166. quiz 8–9
57 Lin J, Jacobson JA, Hayes CW. Sonographic target sign in neurofibromas. J Ultrasound Med. 1999;18:513–517.
58 Chen W, Jia JW, Wang JR. Soft tissue diffuse neurofibromas: sonographic findings. J Ultrasound Med. 2007;26:513–518.
59 Dubois J, Alison M. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol. 2010;40:895–905.
60 Hein KD, Mulliken JB, Kozakewich HP, et al. Venous malformations of skeletal muscle. Plast Reconstr Surg. 2002;110:1625–1635.
61 Kransdorf MJ, Murphey MD, Fanburg-Smith JC. Classification of benign vascular lesions: history, current nomenclature, and suggestions for imagers. AJR Am J Roentgenol. 2011;197:8–11.
62 Derchi LE, Balconi G, De Flaviis L, et al. Sonographic appearances of hemangiomas of skeletal muscle. J Ultrasound Med. 1989;8:263–267.
63 Paltiel HJ, Burrows PE, Kozakewich HP, et al. Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology. 2000;214:747–754.
64 Teefey SA, Dahiya N, Middleton WD, et al. Ganglia of the hand and wrist: a sonographic analysis. AJR Am J Roentgenol. 2008;191:716–720.
65 Wang G, Jacobson JA, Feng FY, et al. Sonography of wrist ganglion cysts: variable and noncystic appearances. J Ultrasound Med. 2007;26:1323–1328. quiz 30–31
66 Esen G. Ultrasound of superficial lymph nodes. Eur J Radiol. 2006;58:345–359.
67 Pui MH, Fletcher BD, Langston JW. Granulocytic sarcoma in childhood leukemia: imaging features. Radiology. 1994;190:698–702.
68 ter Braak BP, Guit GL, Bloem JL. Case 111: Soft-tissue lymphoma. Radiology. 2007;243:293–296.
69 Kransdorf MJ. Malignant soft-tissue tumors in a large referral population: distribution of diagnoses by age, sex, and location. AJR Am J Roentgenol. 1995;164:129–134.
70 Giovagnorio F, Valentini C, Paonessa A. High-resolution and color Doppler sonography in the evaluation of skin metastases. J Ultrasound Med. 2003;22:1017–1022. quiz 23–25
71 Arya S, Nagarkatti DG, Dudhat SB, et al. Soft tissue sarcomas: ultrasonographic evaluation of local recurrences. Clin Radiol. 2000;55:193–197.
72 Fornage BD, Lorigan JG. Sonographic detection and fine-needle aspiration biopsy of nonpalpable recurrent or metastatic melanoma in subcutaneous tissues. J Ultrasound Med. 1989;8:421–424.
73 Choi H, Varma DG, Fornage BD, et al. Soft-tissue sarcoma: MR imaging vs sonography for detection of local recurrence after surgery. AJR Am J Roentgenol. 1991;157:353–358.
74 Cho KH, Lee YH, Lee SM, et al. Sonography of bone and bone-related diseases of the extremities. J Clin Ultrasound. 2004;32:511–521.