Chapter 80 Bacterial Infections of the Nervous System
The brain is normally a sterile site that is protected from infection by specialized barriers, including the bony skull and the blood–brain barrier. Consequently, infections of the central nervous system (CNS) are comparatively rare. In the United States, the incidence of bacterial meningitis, the most frequent bacterial infection of the CNS, was 3–5 cases per 100,000 population in 1995 [Schuchat et al., 1997], in stark contrast to the incidence of sepsis, which was more than 200 cases per 100,000 in the same year [Martin et al., 2003].
Acute Bacterial Meningitis
Bacterial meningitis is an inflammation of the leptomeninges, triggered by bacteria present in the subarachnoid space. Children still die or suffer permanent neurologic sequelae as a result of meningitis [De Louvois et al., 2007]. Prevention or prompt diagnosis and aggressive management are the goals [Schaad, 1997]. Before the discovery of antibiotics, therapy consisted of withdrawal of cerebrospinal fluid (CSF) through repeated lumbar puncture and intrathecal or intravenous administration of antimeningococcal antiserum raised in horses. More recent milestones include the introduction of new, potent, bactericidal antibiotics, active vaccination, and anti-inflammatory therapy [Tunkel et al., 2004].
Epidemiology
Bacterial meningitis has a unique epidemiologic profile. The disease has a worldwide distribution, the typical host is a previously healthy patient, the typical symptoms usually develop rapidly, there are numerous and severe sequelae, and the disease is increasingly preventable [Schuchat et al., 1997]. Bacterial meningitis occurs in people of all ages, but there are striking age-related differences in the predominant etiologic agents. Differences relate to immunologic maturation, social or behavioral influences, and the coexistence of other chronic or acute clinical conditions.
Neonatal meningitis typically follows obstetric complications, and the predominant bacteria are group B streptococcus, Listeria monocytogenes, and Gram-negative enteric bacteria. Older infants have substantial risk of meningitis caused by Haemophilus influenzae type b and Streptococcus pneumoniae, although where H. influenzae conjugate vaccines are in use, H. influenzae type b meningitis has been virtually eliminated. School-age children are at greatest risk of meningococcal disease, which occurs in outbreaks throughout the world and causes major epidemics in sub-Saharan Africa and parts of Asia. S. pneumoniae is the leading cause of bacterial meningitis in adults, and the emergence of antimicrobial-resistant pneumococci has complicated pediatric meningitis worldwide [Schuchat et al., 1997].
Bacterial meningitis occurs with increased frequency in certain population groups. Native Americans and aboriginal populations are at particular risk for H. influenzae type b disease [Coulehan et al., 1984; Ward et al., 1986]. Risk of group B streptococcal disease is substantially higher among African Americans than other racial or ethnic groups in the United States [Zangwill et al., 1992].
Institutional settings, such as daycare centers, provide opportunities for enhanced transmission of respiratory pathogens, including H. influenzae type b, meningococcus, and pneumococcus. Transmission of antimicrobial-resistant infections among daycare center attendees is an emerging problem [Reichler et al., 1992]. Meningococcal meningitis clusters have been described in schools, on college campuses, and in refugee settings.
Invasive pneumococcal disease, including pneumococcal meningitis, has declined among children and adults since the introduction of the pediatric heptavalent pneumococcal conjugate vaccine (PCV7) (USA in 2000, Europe in 2001–2004) [Tsai et al., 2008]. Although the overall effect of the vaccine remains substantial, a recent increase in meningitis caused by non-PCV7 serotypes, including strains nonsusceptible to antibiotics, is a concern [Hsu et al., 2009]. Pneumococcal serotype 19A has emerged as most frequent cause of invasive pneumococcal disease in children in various regions [Pelton et al., 2007]. In children with pneumococcal meningitis, infections with non-PCV7 serogroups seem less likely to result in death. Among survivors, there is preliminary evidence of parity in neurologic sequelae between PCV7 and non-PCV7 serogroups [Rajasingham et al., 2008].
Pathogenesis
From the nasopharyngeal surface, encapsulated organisms cross the epithelial cell layer and invade the small subepithelial blood vessels. In the bloodstream, pathogens are protected from complement-mediated bactericidal mechanisms and neutrophil phagocytosis by their capsule. Impairment of the alternative complement pathway, sickle cell disease, and lack of a functional spleen predispose to the development of pneumococcal meningitis. Similarly, functional deficiencies of components involved in activation and function of complement-mediated defenses (i.e., mannose binding lectin, properdin, lack of terminal complement components) increase the susceptibility for invasive meningococcal infections [Van Deuren et al., 2000]. Neonates are at an increased risk of bacterial meningitis because of impaired nonspecific immunity. This impaired immunity includes defects in leukocyte chemotaxis, phagocytosis, bactericidal activity, and low levels of immunoglobulin M (IgM) antibodies and complement, which are not transferred across the placenta but are important in defense against Gram-negative organisms [Mariscalco et al., 2002].
Studies in models of bacterial meningitis indicate that cells of the choroid plexus and cerebral capillaries possess receptors for meningeal pathogens. The interaction between these host cell receptors (e.g., 37kd laminin receptor protein, platelet-activating factor receptor) and bacterial ligands, such as outer membrane proteins or pili, are necessary for the bacterial invasion of the CSF space [Orihuela et al., 2009; Kim, 2010]. The interaction between host-cell receptor and bacterial ligand triggers intracellular pathways, upon which pathogens can traverse the blood–brain barrier either transcellularly or paracellularly. Some pathogens, such as L. monocytogenes, may also be transported into the CSF by macrophages [Kim et al., 2005; Kim, 2010]. Not all bacterial infections of the CNS are the result of bacteremia. Nonhematogenous invasion of the CSF by bacteria occurs in situations of compromised integrity of the barriers surrounding the brain (e.g., in otitis media, mastoiditis, sinusitis). Direct communication between the subarachnoid space and the skin or mucosal surfaces as a result of malformation or trauma gives rise to meningeal infection. Bacteria also can reach the CSF as a complication of neurosurgery, spinal anesthesia, or ventriculostomy placement [Alleyne et al., 2000]. Infections of CSF shunts are another cause of meningitis. Most cases occur shortly after implantation of the shunt and are attributed to intraoperative inoculation, but late shunt infections can occur several years after implantation [Vinchon et al., 2002]. Staphylococci, particularly coagulase-negative staphylococci, are the major pathogens in shunt infections [Filka et al., 1999]. These organisms have unique capabilities to colonize the surface of foreign bodies because of specialized surface proteins (e.g., clumping factor, fibronectin binding protein) that allow the organism to adhere to the catheters – in particular, after they are coated with host-derived proteins, such as fibrin and fibronectin [Ziebuhr et al., 2000]. Adhering organisms rapidly develop into a biofilm, characterized by bacteria embedded in an extracellular matrix. Elimination of bacterial biofilms from the surface of foreign bodies by antibiotics or host defenses is notoriously difficult [Ziebuhr et al., 2000]. Colonization of CSF shunts with coagulase-negative staphylococci leads to clinically apparent meningitis or ventriculitis in only approximately 30 percent [Odio et al., 1984; Vinchon et al., 2002].
Pathogens reaching the CSF are likely to survive because of a paucity of resident macrophages and deficient opsonization caused by low concentrations of capsule-specific immunoglobulins and complement in the CSF. Lack of opsonization greatly reduces the effectiveness of incoming granulocytes and allows largely unrestricted multiplication of the meningeal pathogens. Bacterial multiplication is associated with the release of bacterial products (fragments of cell wall, lipopolysaccharide) that trigger the inflammatory response in the subarachnoid space by inducing the production and release of inflammatory cytokines and chemokines. Cytokines, including tumor necrosis factor-α, interleukin-1, and interleukin-6; chemokines, such as interleukin-8, growth-related protein-α, and monocyte chemotactic protein-1; and lipid inflammatory mediators, such as platelet activation factor, upregulate adhesion molecules on brain vascular endothelial cells and promote the recruitment of granulocytes into the CSF. Matrix metalloproteases contribute to the inflammatory process by mediating breakdown of the blood–brain barrier and activating cytokines [Kieseier et al., 1999]. This granulocytic inflammation is primarily responsible for the complex pathophysiologic CNS alterations and clinical manifestations of bacterial meningitis [Meli et al., 2002].
Diagnosis
Prompt diagnosis is crucial for a life-threatening and treatable illness such as bacterial meningitis. Routine early diagnosis requires educated suspicion of meningitis and immediate performance of lumbar puncture and adequate tests when the CSF has been obtained [Schaad, 1995].
Clinical Features
The sudden onset of a stiff neck with fever in a child who appears ill usually indicates the presence of meningitis (Box 80-1). In these cases, a lumbar puncture is a crucial part of the initial evaluation.
Meningismus
Meningismus reflects tense neck and back muscles as a reflex to avoid painful extension of inflamed meninges. Clinical findings of meningeal irritation include Kernig’s sign (passive extension of the knee from the flexed thigh position elicits pain in the back), Brudzinski’s sign (passive flexion of the neck elicits spontaneous flexion of the lower extremities), tripod sign (while sitting, the patient holds the back erect by placing both extended arms behind the buttock), and knee-kissing sign (the patient is unable to kiss his or her own knees). Nuchal rigidity is absent throughout the course of meningitis in only 1–2 percent of infants and children; generally, the younger the patient, the less prominent nuchal rigidity [Geiseler and Nelson, 1982].
Initial presentation
Fever (94 percent), vomiting (82 percent), and nuchal rigidity (77 percent) are the most common presenting symptoms of meningitis in children 1–4 years old. Many children also experience lethargy, irritability, anorexia, and photophobia [Ashwal et al., 1993]. Seizures, bulging fontanel, and coma occur less commonly and usually are seen later in the course of the illness. Older patients report headache and neck pain.
Two modes of presentation
The first pattern develops over several days, with fever and nonspecific manifestations suggesting viral infection without or with localization (e.g., upper respiratory or gastrointestinal tract). In these patients, it is difficult to pinpoint the exact onset of meningitis. The second pattern is acute, sometimes fulminant, and the symptoms and signs of sepsis (e.g., cardiovascular instability; cutaneous manifestations, such as an erythematous, maculopapular, or petechial rash) and meningitis evolve rapidly over a few hours [Thompson et al., 2006].
Neurologic findings
Seizures occur before hospital admission or within the first 2 days of hospitalization in 20–30 percent of pediatric patients with bacterial meningitis [Feigin et al., 1992]. Generalized seizures usually are not a predictor of poor outcome. In contrast, focal neurologic signs, such as paresis, cranial nerve palsy, and focal seizure activity, often precede more adverse outcomes of irreversible brain damage. Severely depressed consciousness at the time of hospital admission usually signals a poor prognosis because of the likelihood of extensive encephalitic involvement or massively increased intracranial pressure in these patients [Tunkel and Scheld, 1995].
Arthritis and other systemic manifestations
Arthritis can precede or follow H. influenzae and Neisseria meningitidis meningitis, and confuses the neurologic examination by producing the superficial appearance of a monoparesis. Knees and elbows are common sites for arthritis. Of patients with H. influenzae type b septic arthritis, approximately 30 percent have concurrent meningitis [Rotbart and Glode, 1985]. Periorbital cellulitis occurs in H. influenzae meningitis.
Differential diagnosis
The differential diagnosis of bacterial meningitis includes aseptic meningitis, encephalitis, brain abscess, febrile seizure, head trauma, intracranial hemorrhage, and neoplastic meningitis. In addition, unusual infectious agents, such as fungus, rickettsia, or tuberculosis, must be considered. A fever with a simple seizure is unlikely to be bacterial meningitis [Green et al., 1993].
Neonatal meningitis
The traditional clinical signs of meningitis are usually lacking in neonates. Nuchal rigidity rarely occurs. Fever and a full fontanel may be absent. An associated sepsis is common. Bacterial meningitis should be considered at any age (so-called early and late onset) in any newborn who fails to thrive and has irritability, apnea, seizures, a tendency to opisthotonos, poor feeding, emesis, hypothermia or hyperthermia, hypotonia or hypertonia, a gray appearance, jaundice, or other evidence of sepsis. Apnea may be the only sign of meningitis in a neonate [Heath et al., 2003; Schaad, 1992].
Shunt infections
Infections of CNS shunt occur in approximately 5–10 percent of patients, usually within 1–2 months of surgical insertion [McGirt et al., 2003; Ronan et al., 1995]. Most of these infections are due to coagulase-negative Staphylococcus or Staphylococcus aureus; however, a variety of other organisms, including Gram-negative bacteria and other skin flora, can be causative.
Diagnosis of CNS shunt infection versus CNS shunt malfunction often represents a dilemma in children who present with constitutional signs and symptoms [McClinton et al., 2001]. Clinical manifestations of CNS shunt infection usually include low-grade fever, vomiting, irritability or lethargy, and other signs of increased intracranial pressure. The onset is often insidious but can be more acute, particularly if shunt malfunction also is present. Neck stiffness is uncommon. Shunt infection should be suspected in any child with an indwelling CNS drainage system and fever.
Meningitis after cochlear implants
Cochlear implant is the standard treatment for patients with severe to profound hearing loss, in which well-fit hearing aids fail to permit effective oral communication. The implant is a neural stimulator whose electrode array is placed surgically in the lumen of the cochlea, near the auditory nerve. An external microphone picks up speech signals, and the signal processor transforms these signals into digital impulses that a radiofrequency carrier transmits percutaneously into the internal receiver-stimulator and electrode array. The auditory cortex is stimulated by the implant, permitting the perception of the digitally processed information as speech [Gates and Miyamoto, 2003].
Complications from cochlear implantation are unusual. Reports of postoperative meningitis have called attention to a previously unrecognized risk [Arnold et al., 2002; Reefhuis et al., 2003]. Most cases of meningitis occur in children with a typical presentation. Available information indicates that the mode for infection is a time-limited or, rarely, persistent CSF leak into the middle ear. Bacteria from the middle ear – usually causing otitis media – may enter the meninges. The most common offending pathogens are S. pneumoniae and H. influenzae.
Lumbar Puncture
The diagnosis of bacterial meningitis is based on examination of CSF [Feigin et al., 1992]. Minimizing sequelae of bacterial meningitis depends on the prompt initiation of effective antibiotic therapy. A lumbar puncture should therefore be performed whenever there is even slight clinical evidence of meningitis. Rarely, other sites may be used to obtain CSF, including ventricles or shunts installed to divert CSF.
Lumbar puncture is a safe procedure for most patients. Minor discomfort may occur, but serious consequences are rare. Headache 1–6 hours after lumbar puncture is a troublesome but benign complication. When present, the headache is frontal and is exaggerated by movement from a lying to a sitting or standing position. Headache after lumbar puncture seems to be caused by continued leak of CSF from the site of the lumbar puncture, with resulting low CSF pressure and traction on pain-sensitive structures. The problem can be mitigated by removal of a minimal amount of fluid and use of a small-bore needle [Lynch et al., 1992; Lavi et al., 2010]. Pain in the low back or legs occurs occasionally after lumbar puncture; this pain is of uncertain etiology and resolves spontaneously in several days.
Herniation of the brainstem and cerebellar tonsils into the foramen magnum is extremely rare in children [Marton and Gean, 1986]. A risk of herniation is clearly related to substantially and focally increased intracranial pressure [Richards and Towu-Aghantse, 1986]. The risk of producing meningitis by performing a lumbar puncture in a child with septicemia is insignificant and is not a contraindication to the test [Shapiro et al., 1986].
With the widespread introduction of highly effective bacterial conjugate vaccines against H. influenzae type b and S. pneumoniae, there has been a significant decrease in the incidence of bacterial meningitis in children. This has reduced the probability that a child with CSF pleocytosis will have bacterial meningitis. Several studies were able to validate a previously developed clinical prediction rule, the so-called Bacterial Meningitis Score, for identifying children with CSF pleocytosis at very low risk of bacterial meningitis [Nigrovic et al., 2007]. Patients are classified as very low risk if they lack all of the following criteria: positive CSF Gram stain, CSF absolute neutrophil count of at least 1000 cells/μL, CSF protein of at least 80 mg/dL, peripheral blood absolute neutrophil count of at least 10,000 cells/μL, and a history of seizure before or at the time of presentation.
Laboratory tests
The initial diagnosis of meningitis is based on examination of CSF. Bacterial infection is suggested by increased opening and closing pressure; a cloudy (i.e., not clear) CSF; or a CSF pleocytosis with polymorphonuclear predominance, increased protein and lactate concentration, or decreased glucose concentration. Details and normal values have been described previously (Table 80-1) [Ahmed et al., 1996; Kim, 2010].
Confirmation of the diagnosis requires the detection in the CSF of a specific bacterial pathogen by microscopy or a positive culture, polymerase chain reaction, or rapid antigen-detection test of CSF. Smears of CSF should be examined by Gram stain (acid-fast stain if tuberculosis is suspected). Gram-stained smears of the CSF sediment reveal bacteria in 70–90 percent of untreated cases of meningitis caused by S. pneumoniae or N. meningitidis, but only in one-third of cases caused by L. monocytogenes [Kim, 2010]. The CSF should be cultured on a blood agar plate, on a chocolate agar plate (or on Fildes or Leventhal medium), and in broth. Special cultures for mycobacteria, fungi, or other microorganisms should be undertaken if clinically indicated. In an area of increasing antimicrobial resistance, adequate in vitro susceptibility testing must be routine for any cultured neonatal pathogen. Various antigen-detection tests are available (e.g., countercurrent immunoelectrophoresis, latex particle agglutination, enzyme-linked immunosorbent assay) and may be helpful in establishing an etiologic diagnosis rapidly.
Polymerase chain reaction and other sequence-based microbial detection methods are being used increasingly in many clinical microbiology laboratories. These tests improve detection of pathogens, particularly in patients pretreated with antibiotics [Fredricks and Relman, 1999; Chiba et al., 2009]. Careful standardization and validation of these tests is necessary.
Nonspecific parameters have been studied in the CSF to establish or exclude a bacterial etiology rapidly, including various indices of inflammation (e.g., cytokines, C-reactive protein, and lactate) and measurement of various enzyme activities [Gray et al., 1986; Lutsar et al., 1994]. With the exception of lactate, these parameters are not routinely used to assess patients with meningitis.
Contraindications
Four contraindications to lumbar punctures are as follows:
Other Tests
Most pediatric patients with bacterial meningitis are initially bacteremic, as might be expected based on the hematogenous pathogenesis of the disease. Blood cultures taken at hospital admission are valuable and are positive in 80–90 percent of cases of bacterial meningitis [Feigin et al., 1992].
Among new markers, serum procalcitonin level seems to be one of the most sensitive and specific predictors for discriminating between bacterial and aseptic infections. Preliminary experiences indicate that a serum procalcitonin level greater than 0.5 ng/mL predicts bacterial rather than aseptic meningitis in children [Dubos et al., 2008].
Brain Imaging
Neuroimaging studies, such as CT, MRI, and cranial sonography (in young infants with access through open fontanel), should be used in patients with signs of increased intracranial pressure (e.g., depressed consciousness, sluggishly reactive or dilated pupils, ophthalmoplegia, retinal changes, abnormal respiratory patterns, and cardiovascular instability). Increased intracranial pressure usually is caused by cerebral edema or other intracranial complications, such as subdural collection, thrombosis, infarction, brain abscess, and hydrocephalus [Ashwal, 1995; Tunkel and Scheld, 1995].
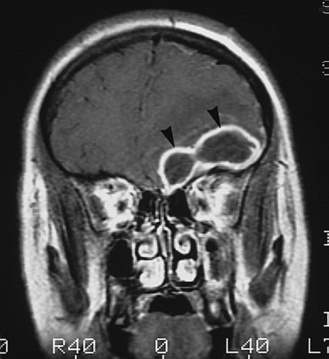
MRI has improved resolution compared with CT, provides additional insights into the pathogenesis of neurologic complications, and eventually may replace CT in most situations [Smith and Caldemeyer, 1999]. Cortical enhancement may be identified and is presumed to indicate cerebritis. Repeat imaging is useful in following the evolution of abnormalities [Bodino et al., 1982] and in determining the need for subsequent intervention. Abnormalities on imaging are associated with an unfavorable outcome [Packer et al., 1982]. Contrast MRI, although not usually considered a diagnostic test for meningitis, can show meningeal enhancement (Figure 80-1) [Kioumehr et al., 1995; Runge et al., 1995].
Complications
Pathophysiologic Changes
Blood–brain barrier disruption
The permeability of the blood–brain barrier increases in meningitis as a result of disrupted intercellular tight junctions and increased transendothelial pinocytosis [Quagliarello et al., 1986]. Inflammatory components, including granulocytes and matrix metalloproteinases, contribute to the increased permeability of the blood–brain barrier [Leppert et al., 2001]. This increased permeability results in increased extravasation of serum components and contributes to vasogenic brain edema, but also allows for approximately fivefold higher concentrations of antibiotics in the CSF during therapy compared with the uninfected state.
Brain edema
Brain edema during meningitis has vasogenic, cytotoxic, and interstitial components. Vasogenic cerebral edema is primarily a consequence of increased blood–brain barrier permeability (see earlier). Cytotoxic edema results from an increase in intracellular water after alterations of the cell membrane and loss of cellular homeostasis with influx of sodium and calcium into the cell. Cytotoxic mechanisms include ischemia and the effect of excitatory amino acids [Derugin et al., 2000]. Interstitial edema occurs as a result of an increase in CSF volume, through either increased CSF production (increased blood flow in the choroid plexus) or decreased resorption secondary to increased CSF outflow resistance across the arachnoid villi system of the sagittal sinus [Scheld et al., 1980]. The major dangers of extensive brain edema during meningitis are herniation of brain tissue and compression of the brainstem secondary to increased intracranial pressure, which can cause complete cessation of cerebral circulation (Table 80-2). Brain edema contributes substantially to the acute fatal outcome of bacterial meningitis [Winkler et al., 2002].
Table 80-2 Common Neurologic Sequelae after Bacterial Meningitis
| Deficit | Approximate Frequency (%) |
|---|---|
| Hearing loss | 15–30 |
| Parenchymal damage | 5–30 |
| Cerebral palsy | 5–10 |
| Learning disabilities | 5–20 |
| Seizure disorders | <5 |
| Cortical blindness | <5 |
| Cerebral herniation | 3–20 |
| Hydrocephalus | 2–3 |
Hyponatremia, dehydration, and inappropriate secretion of antidiuretic hormone
Patients with acute meningitis often present with hypovolemia secondary to dehydration, and some develop hyponatremia. Based on clinical signs, it is impossible to distinguish between hyponatremia secondary to dehydration and the syndrome of inappropriate antidiuretic hormone secretion (SIADH) in patients with meningitis. It also is unclear how frequently the hyponatremia is related to SIADH with secondary fluid retention [Moller et al., 2001]. SIADH results in hypotonicity of extracellular fluid, which in meningitis may contribute to cytotoxic edema [Kaplan and Feigin, 1978]. The overall risk of worsened clinical outcome owing to SIADH seems small, however, and does not justify routine fluid restrictions in patients with meningitis and hyponatremia because fluid restriction may have harmful effects on systemic blood pressure and cerebral perfusion. In cases of severe or persistent hyponatremia, SIADH should be excluded with appropriate laboratory tests.
Intracranial hypertension
Increased intracranial pressure is present early in most patients with bacterial meningitis. In infants with open fontanels and symptoms compatible with meningitis, the full fontanel may lead to the correct diagnosis. Early in the course of meningitis, cerebral hyperemia may be the most important mechanism of intracranial pressure elevation. In more advanced stages, brain edema is probably the most important factor contributing to increased intracranial pressure, but obstructive hydrocephalus, cerebritis, cerebral infarction, cerebral venous thrombosis, status epilepticus, and SIADH also contribute to increases in intracranial pressure [Brown and Feigin, 1994].
Cerebral blood flow changes
In the early phase of bacterial meningitis, an increase in cerebral blood flow is observed, which seems to be mediated by nitric oxide and oxidative radicals resulting from the inflammation in the CSF space. As the disease progresses, cerebral blood flow reductions are observed globally and focally [Pfister et al., 1992]. Global cerebral blood flow reduction is the result of reduced cerebral perfusion pressure because of increased intracranial pressure, systemic hypotension, or both, in the setting of impaired cerebral blood flow autoregulation. Focal ischemia is the result of vascular involvement of cerebral arteries and veins by the subarachnoid space inflammation [Pfister et al., 1992]. Reactive oxygen radicals and endothelins, a family of vasoactive peptides released into the CSF during bacterial meningitis, contribute to reductions of cerebral blood flow [Koedel et al., 1997; Pfister et al., 2000]. Several clinical studies have found an association between severe cerebral blood flow reduction and adverse outcome in children and adults with meningitis, making ischemia an important mediator of brain damage in meningitis [Ashwal et al., 1992; Förderreuther et al., 1992; Odio et al., 1991].
Seizures
Seizures complicate 20–30 percent of cases of childhood bacterial meningitis. Patients with severe illness are more likely to have seizures. They occur most frequently 2–3 days into the illness and cease 1–3 days later. Seizures may be focal or generalized, and the electroencephalogram (EEG) may be normal or may show generalized or focal epileptiform abnormalities, focal slowing, or only background slowing. The functional and structural alterations of the brain resulting from the meningitic process that may induce seizures include vasculitis and infarction, fever, electrolyte imbalances, and metabolic changes. Chronic seizures after recovery from meningitis are approximately 3-fold more common in patients than in age-matched control children [Bedford et al., 2001].
Deafness and Cranial Nerve Damage
Deafness is the most frequent neurologic sequela of bacterial meningitis. Of patients with bacterial meningitis, 1–30 percent develop hearing loss (see Table 80-2), and the risk is highest when meningitis is caused by S. pneumoniae. Low CSF glucose concentrations are another risk factor for hearing loss [Eisenhut et al., 2003]. Hearing impairment after meningitis can improve for several months, but many children have severe, permanent, and bilateral hearing loss [Wellman et al., 2003]. Typically, sensorineural hearing loss in both ears is found; rarely, a conductive hearing loss associated with a middle ear infection may be present. Hearing should be assessed in children with bacterial meningitis before discharge. Audiometry is an appropriate test in older children. In young infants, brainstem auditory-evoked responses or transient otoacoustic emissions can be used to detect hearing loss early [Cohen et al., 1988; Francois et al., 1997]. When hearing loss occurs, early intervention by a team that is knowledgeable in the management of this problem in infants and children should be encouraged. Cochlear implants are a consideration in the most severe cases.
Experimental studies suggest that bacteria reach the cochlea primarily via the cochlear aqueduct [Bhatt et al., 1993]. The deafness is caused by infection and inflammation in the cochlea [Klein et al., 2003]. Nitric oxide, other inflammatory mediators, and bacterial products, specifically the pneumococcal toxin pneumolysin, contribute to the loss of hairy cells in the cochlea [Amaee et al., 1995].
Neuronal Damage
The neurologic sequelae after meningitis, in addition to hearing loss (see previously), include focal sensorimotor deficits, mental retardation, seizure disorders, and cortical blindness (see Table 80-2). Detailed studies of the neurologic outcome after bacterial meningitis have led to an appreciation of the significant long-term functional impairment resulting from the disease [Grimwood et al., 1995; Bedford et al., 2001; Oostenbrink et al., 2002]. In a cohort of 5-year-old children who had survived bacterial meningitis during their first year of life, there was a 10-fold increase in the risk of moderate to severe disabilities, with 7.5 percent demonstrating learning difficulties; 8.1 percent, neuromotor disabilities; 7.3 percent, seizure disorders; 25.8 percent, hearing problems; and 11.9 percent, behavioral problems [Bedford et al., 2001]. Neurologic sequelae of childhood meningitis can persist at least into young adulthood, impairing learning during the entire time that children go to school [Grimwood et al., 2000]. S. pneumoniae consistently was associated with higher rates of disabilities than infection with H. influenzae or N. meningitidis [Grimwood et al., 1996].
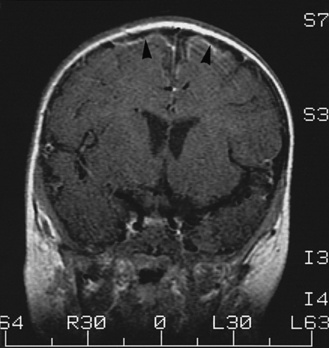
Meningitis causes brain damage of cortical and subcortical structures (Figure 80-2). For much of the brain damage, particularly in the cortex, ischemia represents an important mechanism of injury (Figure 80-3) [Pfister et al., 2000]. In keeping with this notion, inhibition of excitatory amino acids and preservation of cerebral blood flow through inhibition of reactive oxygen radicals or endothelins are protective in experimental meningitis [Meli et al., 2002]. Matrix metalloproteinases, in addition to their effect on the blood–brain barrier and on cytokine release, also may contribute directly to ischemic changes in the brain.
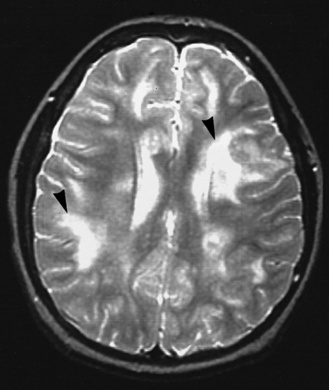
The meningitis is complicated by multiple areas of cerebritis (arrowheads and other locations).
(Courtesy of Dr. Blaine Hart.)
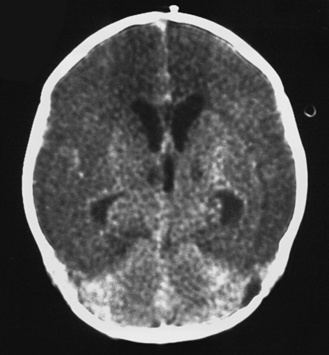
Fig. 80-3 Computed tomography scan of a 16-day-old neonate with group B streptococcal meningitis.
There is massive infarction of the anterior circulation.
(Courtesy of Dr. Blaine Hart.)
A structure frequently damaged during meningitis is the dentate gyrus of the hippocampus. This structure is important for learning and memory acquisition, and represents one of the few areas of the brain where neurogenesis is on-going throughout life. Within this structure, pathology studies in humans dying from meningitis and animal models of meningitis consistently have documented damage to neurons [Gianinazzi et al., 2003; Nau et al., 1999]. Two forms of injury seem to affect the dentate gyrus neurons [Bifrare et al., 2003]. One is mediated by activation of caspase-3 and corresponds to classic apoptosis. This form of injury preferentially affects immature, postmitotic neurons in the subgranular zone, is associated with learning deficits in experimental animals surviving meningitis, and may represent an anatomic substrate for cognitive impairment and learning disabilities after meningitis [Bifrare et al., 2003; Leib et al., 2003]. The other form of dentate gyrus injury exhibits no obvious selectivity for a subset of neuronal cells and is associated with translocation of apoptosis-inducing factor. For both forms of dentate gyrus injury, the exact molecular triggers are unknown, but ischemia does not seem to be important. Potential mediators include pneumococcal products (pneumolysin and hydrogen peroxide), inflammatory mediators (e.g., tumor necrosis factor-α), matrix metalloproteinases, excitatory amino acids, reactive oxygen species, and others [Leib et al., 1996; Meli et al., 2002].
Hydrocephalus
Meningitis leads to disturbances of CSF homeostasis, with increased CSF production and reduced CSF absorption across the sagittal sinus – arachnoid villi system [Scheld et al., 1980]. In the acute phase, these disturbances can lead to ventriculomegaly associated with intracranial hypertension. This form of ventriculomegaly is frequently transient. Chronic hydrocephalus occurs as a long-term sequela of bacterial meningitis in infants and children (see Table 80-2). Its pathogenesis is either obstructive, secondary to inflammatory changes of CSF drainage pathways, or ex vacuo, secondary to loss of brain parenchyma. Chronic internal shunting with internal drainage is necessary in patients with persistent obstructive hydrocephalus.
Septic Shock and Disseminated Intravascular Coagulation
Bacterial meningitis can be complicated by sepsis and septic shock, independent of the infecting organism. All patients with bacterial meningitis need to undergo close cardiovascular monitoring until they are clinically stabilized with antimicrobial and supportive therapy. The association of sepsis and extensive disseminated intravascular coagulation suggests infection caused by N. meningitidis [Yung and McDonald, 2003]. A similarly fulminant and devastating course can be seen with S. pneumoniae in patients without a functional spleen. These patients present with rapidly progressive signs of severe illness, rigors, severe muscular pain, and skin lesions that progress from an early maculopapular rash to petechiae to enlarging purpuric lesions [Yung and McDonald, 2003]. The organism sometimes can be visualized on Gram stain of scrapings from purpuric lesions. Signs and symptoms of meningitis often are not prominent in patients with disseminated meningococcal sepsis. Laboratory abnormalities include thrombocytopenia, hypofibrinogenemia, and massively elevated D-dimers. Overt adrenal insufficiency seems to be relatively rare in children with meningococcal disease [Riordan et al., 1999]. The most fulminant form of sepsis and disseminated intravascular coagulation is the Waterhouse–Friderichsen syndrome, which leads to sudden cardiovascular collapse associated with adrenal hemorrhage.
Extra-Axial Fluid Collections
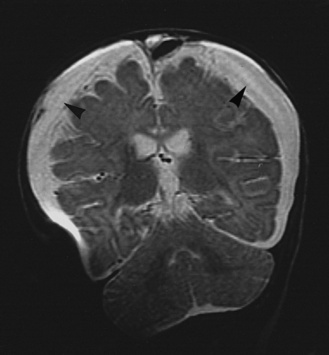
The subdural space is a potential intracranial space situated between the arachnoid and dura. Fluid can collect in the subdural space and in the subarachnoid space. The collection in the subarachnoid space may represent loculated CSF, widening of the subarachnoid space in response to inflammation, or loss of brain parenchyma. Because imaging often cannot distinguish between a fluid collection and a widened subarachnoid space, the fluid collection as seen on imaging is referred to as an extra-axial fluid collection. Extra-axial fluid collections are found in 50 percent of children with bacterial meningitis who have cranial CT performed [Syrogiannopoulos et al., 1986].
Extra-axial fluid collections occur more commonly in bacterial meningitis during the first year of life and often are recognized during the first week of illness. The extra-axial fluid is preferentially situated over the convexities, may accumulate bilaterally, is serosanguineous with granulocytes and high protein concentrations, and is usually sterile (Figure 80-4) [Snedeker et al., 1990]. Extra-axial fluid collections often are detected on imaging studies performed because of persistent fever, clinical signs of meningitis, seizures, or focal neurologic deficits. Often, they represent a coincidental finding, but they may also be the cause of persistent increase in intracranial pressure or focal neurologic deficits. Extra-axial fluid collections, as discussed here, must be distinguished from subdural empyema, which frequently is a complication of sinusitis or otitis media and needs emergent treatment. Subdural empyema will be discussed later in this chapter.
Pathology
Subarachnoid space inflammation appears as a grayish yellow to green exudate covering the base and convexities of the brain. Histologic examination indicates that the exudate in acute bacterial meningitis consists predominantly of granulocytes; there is a mixture of lymphocytes, macrophages, and granulocytes in subacute to chronic forms of meningitis. Cerebral arteries and veins have focal infiltration of the vessel walls by the inflammatory cells. The inflammation of the vessel wall may be associated with thrombosis and vascular occlusion [Cairns and Russell, 1946]. In fatal cases of neonatal meningitis, inflammatory vasculitis is uniformly present, possibly indicating that the cerebral vasculature of the neonate is particularly susceptible to inflammatory damage [Berman and Banker, 1966]. Some of the severe structural damage in the neonatal brain after meningitis may be related to this susceptibility of the vasculature to inflammatory damage.
Damage to the brain parenchyma is manifested by signs of brain edema and frequently cerebral herniation (see later), and by areas of cerebral infarction resulting from ischemia [Arseni and Nereantiu, 1972; Berman and Banker, 1966]. Throughout the brain, there is loss of neurons, usually in a focal pattern, most extensively in patients who survived the acute meningitis for several days before succumbing to the disease. Neuronal loss is associated with a marked reaction of astrocytes and microglia.
In the dentate gyrus of the hippocampus, evidence of cell death of neurons seems common in meningitis based on limited pathologic studies in humans and extensive work in animal models [Nau et al., 1999]. Apoptotic neuronal cell death can be detected primarily in the subgranular zone of the dentate gyrus and is characterized morphologically by condensed, fragmented nuclei. The apoptotic nature of this form of cell death has been confirmed by the detection of fragmented DNA (i.e., TUNEL-stain) and activation of caspase-3 [Gianinazzi et al., 2003].
Treatment
Antibiotics
Effective eradication of the infecting pathogen requires that antibiotics display bactericidal activity in the CSF. Experimental studies have documented the fact that CSF concentrations that exceed the in vitro minimal inhibitory concentration severalfold (>10-fold for most antibiotics) are required to achieve maximal bacterial killing rates. CSF concentrations of antibiotics, even with inflamed meninges, are only a fraction of simultaneous serum concentration (usually between 5 and 25 percent), and antibiotics must be dosed higher for the treatment of meningitis than for most other infections [Täuber and Sande, 1990].
Empiric therapy should be instituted rapidly in all children with suspected bacterial meningitis. The progressive pathophysiologic alterations during meningitis, which rapidly lead to brain damage and death in most untreated patients, strongly suggest that any delay in antibiotic treatment may be harmful and should be avoided [Aronin et al., 1998, Koster-Rasmussen et al., 2008]. In practice, the appropriate sequence of obtaining CSF and blood for cultures and other tests, instituting antibiotics, and obtaining imaging studies to exclude mass lesions and other pathologies needs to be adapted to the individual patient. The sicker the patient and the more rapidly progressive the disease, the more urgent is the start of antibiotic therapy. Antibiotic therapy should not be delayed by more than approximately 15 minutes in critically ill patients for an attempt to obtain CSF or blood, despite the reduced yield of bacterial cultures if the samples are obtained after initiation of antibiotics. A CT scan is required before the lumbar puncture in all patients in whom there is suspicion of important CNS alterations, particularly if a mass lesion is suspected, and the patient has signs of increased intracranial pressure. In adults, criteria have been established to identify patients with suspected meningitis in whom a lumbar puncture can be performed safely [Hasbun et al., 2001]. Whether these criteria are valid in children has not been established. The contraindications for lumbar puncture were discussed earlier.
The choice of empiric antibiotics depends on the suspected pathogens and their antibiotic susceptibilities, which may display regional differences. All pathogens commonly encountered in a particular patient population should be covered empirically, and rare pathogens should be empirically targeted in patients with corresponding risk factors (Table 80-3 and Table 80-4). Recommended empiric treatment regimens adequate for patients without additional risk factors are shown in Table 80-5. When the causative pathogen has been isolated, and antibiotic sensitivities are known, therapy should be adjusted to cover the respective pathogen optimally (see later).
Table 80-3 Common Causes of Acute Bacterial Meningitis According to Age
| Age | Pathogen |
|---|---|
| 0–4 wk | Streptococcus agalactiae Escherichia coli Listeria monocytogenes Streptococcus pneumoniae |
| 1–3 mo | E. coli L. monocytogenes Neisseria meningitidis S. agalactiae S. pneumoniae Haemophilus influenzae |
| 3 mo to18 yr | N. meningitidis S. pneumoniae H. influenzae |
Table 80-4 Rare Causes of Bacterial Meningitis in Infants and Children
| Pathogen | Predisposing Factors |
|---|---|
| Staphylococcus aureus | Trauma, neurosurgery, shunt |
| Staphylococcus epidermidis | Trauma, neurosurgery, shunt |
| Enterococcus spp. | Neonatal period |
| Group A streptococcus | Pharyngitis, sepsis |
| Viridans streptococci | Endocarditis, anatomic defect |
| Moraxella | Otitis media |
| Escherichia coli* | Urinary tract infection, immunosuppression |
| Klebsiella pneumoniae | Immunosuppression |
| Salmonella | Sickle cell disease, immunosuppression |
| Proteus | Neonatal period, immunosuppression |
| Citrobacter | Neonatal period, immunosuppression |
| Other Enterobacteriaceae | Immunosuppression |
| Pseudomonas aeruginosa | Immunosuppression |
| Pasteurella multocida | Animal bites |
| Francisella tularensis | Wild animal contacts |
| Propionibacterium acnes | Shunt |
| Nocardia | Chronic pulmonary disease |
* Rare past the neonatal period.
Table 80-5 Recommendations for Empiric Antibiotic Therapy of Bacterial Meningitis
| Patients and Special Modifying Circumstances | Antibiotic | Dosage (IV) |
|---|---|---|
| Neonate/infant <3 mo | Ampicillin plus | 50–100 mg/kg every 6–8 hr |
| Cefotaxime or | 100 mg/kg every 8–12 hr | |
| Gentamicin | 2.5 mg/kg every 8 hr | |
| Neonate preterm, low birth weight | Vancomycin plus | 15 mg/kg every 8–24 hr |
| Ceftazidime | 100 mg/kg every 8–12 hr | |
| >3 mo to <50 yr | Ceftriaxone or | 100 mg/kg every 24 hr (max. 4 g every 24 hr) |
| Cefotaxime | 100 mg/kg every 8 hr (max. 12 g per day) | |
| Drug-resistant Streptococcus pneumoniae | Ceftriaxone plus | 100 mg/kg every 24 hr (max. 4 g every 24 hr) |
| Rifampicin or | 10–20 mg/kg every 24 hr (max. 600 mg every 24 hr) | |
| Vancomycin | 15 mg/kg every 6 hr (max. 500 mg every 6 hr) | |
| Neurosurgery, cerebrospinal fluid shunt, or head trauma | Ceftazidime plus | 100 mg/kg every 8 hr (max. 12 g per day) |
| Nafcillin or Flucloxacillin | 50 mg/kg every 6 hr (max. 12 g per day) | |
| (or Vancomycin plus | 15 mg/kg every 6 hr (max. 500 mg every 6 hr) | |
| Aminoglycoside) | 2.0–2.5 mg/kg every 8 hr |
The duration of therapy is not strictly defined and should take into account the clinical response to treatment. Meningitis caused by N. meningitidis can be treated for 4 days [Crosswell et al., 2006], and for meningitis caused by S. pneumoniae or H. influenzae, 4–7 days treatment were not inferior to 7–14 days in a recent meta-analysis [Karageorgopoulos et al., 2009]. Meningitis caused by Enterobacteriaceae, L. monocytogenes, other unusual pathogens, or organisms with reduced antibiotic sensitivity may require longer treatment courses. Antibiotic therapy should not be prolonged past the recommended durations because of a persistent fever in a child who has otherwise displayed a favorable clinical response. Other causes of fever, such as drug fever, intravenous line infections, other undiagnosed infections, and extra-axial fluid collections, should be considered.
Streptococcus pneumoniae
For sensitive S. pneumoniae, penicillin G remains the drug of choice, and third-generation cephalosporins (ceftriaxone or cefotaxime) are effective alternatives. The prevalence of pneumococci resistant to β-lactam antibiotics and the average minimal inhibitory concentration for these antibiotics have been increasing worldwide over the last decades [Jones et al., 2004]. Penicillin and chloramphenicol are ineffective against strains displaying any degree of resistance, whereas third-generation cephalosporins may fail in highly penicillin-resistant strains. A combination of a third-generation cephalosporin with vancomycin is most commonly recommended for these strains [Bradley and Scheld, 1997]. Dexamethasone reduces CSF concentrations of vancomycin in adults, but apparently not in children. Rifampicin can be used instead of vancomycin, if the strain is sensitive. There is currently limited clinical experience with newer quinolones [Saez-Llorens et al., 2002], which look promising in experimental meningitis. In these models, the newer quinolones display strong synergism with β-lactams or vancomycin, and these combinations may provide a treatment option in cases caused by high-level resistant pneumococci [Cottagnoud and Täuber, 2004].
Haemophilus influenzae
Ampicillin is the drug of choice for sensitive H. influenzae, but 5–30 percent of strains worldwide are resistant to ampicillin owing to a β-lactamase. These strains are best treated with a third-generation cephalosporin. Rapid spread of non-β-lactamase-producing, ampicillin-resistant H. influenzae type b strains has been reported from Japan [Sakata et al., 2009]. These strains exhibit mutations in their penicillin-binding proteins and increased minimal inhibitory concentration to cephalosporins. Optimal treatment of these strains may require the combination of high-dose third-generation cephalosporins with meropenem or possibly a fluoroquinolone [Hasegawa et al., 2004].
Neisseria meningitidis
Until recently, most strains of N. meningitidis were sensitive to penicillin G. An increase in penicillin minimal inhibitory concentration is reported increasingly from various parts of the world, in particular in low-income countries [du Plessis et al., 2008]. In areas with penicillin-intermediate or highly resistant N. meningitidis, third-generation cephalosporins represent the most reliable choice for empiric therapy of meningitis [Canica et al., 2004].
Listeria monocytogenes
The best treatment for L. monocytogenes meningitis is an aminopenicillin. Based on data in rabbits, which indicated increased bactericidal activity of the combination of penicillin with gentamicin [Scheld et al., 1979], an aminoglycoside usually is added to penicillin. Trimethoprim-sulfamethoxazole is used in patients who cannot tolerate penicillin.
Corticosteroids
In studies of experimental meningitis, corticosteroids reduce brain edema, reduce intracranial pressure, and stabilize the blood–brain barrier [Täuber et al., 1985]. The last effect is associated with a reduction of antibiotic concentrations in the CSF to a degree that does not, with the possible exception of vancomycin in adults, jeopardize antibacterial effectiveness. Corticosteroids, when given at the time of the first antibiotic dose, reduce the increase in inflammation in the CSF that follows the release of proinflammatory bacterial products induced by the antibiotics [Saez-Llorens et al., 1991]. As a potential risk, dexamethasone increased the number of apoptotic cells in the dentate gyrus of animals with experimental meningitis, and was associated with impaired learning in infant rats 3 weeks after pneumococcal meningitis [Leib et al., 2003]. The relevance of this observation for humans is currently unknown.
Dexamethasone has been tested in several controlled trials in patients with bacterial meningitis. Although some studies failed to prove a significant benefit, most studies found some beneficial effect on outcome, with little toxicity and no adverse effect on clearing of bacteria from the CSF [Van de Beek et al., 2007]. Based on the most recent meta-analysis from 2007, dexamethasone reduces hearing loss in children with H. influenzae and possibly with non-Haemophilus meningitis, and showed a favorable trend for short-term neurologic sequelae. These beneficial effects were limited to children from high-income countries and were absent in children from low-income countries [Van de Beek et al., 2007]. A controlled study in adults found improved survival and overall outcome in patients with pneumococcal meningitis [De Gans et al., 2002].
Based on the available data, we favor the administration of dexamethasone to patients with strong clinical evidence of bacterial meningitis in high-income countries. Because the drug mitigates the inflammatory response after institution of antibiotics, it is best given shortly before or simultaneously with the first antibiotic dose [Odio et al., 1991]. The recommended dose is 0.15 mg/kg every 6 hours for a maximum of 4 days; 2 days is equally effective [Schaad et al., 1993]. The course of nonbacterial meningitis is not adversely affected by dexamethasone [Waagner et al., 1990], but the drug temporarily reduces symptoms, even if the empiric antimicrobial therapy is ineffective. Little experience exists with dexamethasone in the neonatal period, and the drug should not be used routinely in this patient population.
Approach to Seriously Ill Patients
Shock and disseminated intravascular coagulation
Standard support for all vital systems in an intensive care setting must be provided to critically ill patients who have signs of sepsis, organ failure, and disseminated intravascular coagulopathy. A detailed discussion of these complex therapies is beyond the scope of this chapter. Attempts have been made to develop specific therapies for sepsis that are based on interfering with microbial mediators, host defense pathways, or coagulation pathways involved in the pathogenesis of sepsis. Although most of these attempts have failed in clinical trials, activated protein C (drotrecogin alfa) was found to improve organ failure, including disseminated intravascular coagulation, and survival in a large controlled trial [Bernard et al., 2001]. A randomized controlled trial in children with sepsis, including some with meningitis, showed no benefit of drotrecogin alfa, and an increased risk for CNS haemorrhage, particularly in children below 60 days of age [Nadel et al., 2007]. The drug is not recommended in children.
Fluid balance and electrolytes
In bacterial meningitis, a balanced fluid status should be established and maintained. Dehydration is potentially harmful because of hypotension and intravascular sludging and thrombosis, all of which can adversely affect cerebral perfusion and increase ischemia [Moller et al., 2001; Tureen et al., 1992]. A review of controlled trials found some adverse neurological effects of fluid restriction in severely ill children [Maconochi et al., 2008]. Many meningitis patients are dehydrated at diagnosis. Their fluid deficit should be corrected promptly, and appropriate maintenance fluid should be administered subsequently [Powell et al., 1990]. Usually, the fluid is given as one-quarter to one-third normal saline in 5 percent dextrose. Substantial overhydration should be avoided, but its potential to exacerbate brain edema probably has been overestimated in the past [Täuber et al., 1993].
Intracranial hypertension
Invasive monitoring of intracranial pressure may be helpful in patients who have signs of severe meningitis – in particular, patients with markedly reduced Glasgow Coma Scale score and very high opening pressure on initial lumbar puncture. These patients usually must be mechanically ventilated. The PCO2 should be maintained at the lower range of normal, but pronounced hyperventilation must be avoided because it can worsen cerebral ischemia [Ashwal et al., 1990]. Sedation or administration of a neuromuscular blocking agent may be necessary to enhance controlled ventilation. A paradigm for the management of seriously ill patients with bacterial meningitis, using the results of intracranial pressure measurements and neuroimaging, has been published [Ashwal, 1995].
In patients with cerebral edema on CT or MRI, administration of dexamethasone is indicated, given its beneficial effects on brain edema and intracranial hypertension [Odio et al., 1991; Täuber et al., 1985]. Mannitol, 0.25–1 g/kg, can be administered intravenously over 20–30 minutes. This dose can be repeated every 2–4 hours, but the effect of subsequent doses progressively diminishes. Use of diuretics, such as furosemide (Lasix), 0.5–1 mg/kg, should be restricted to patients with evidence of fluid overload to avoid the adverse effects of fluid depletion and hypotension.
Although randomized controlled trials have not been performed, the published case series, which included children, documented pronounced effects on intracranial pressure and outcome, including the reversal of impending cerebral herniation, in most patients [Grande et al., 2002; Lindvall et al., 2004].
Prognosis
Knowledge about factors associated with a poor prognosis is important in selecting patients for more intensive surveillance and treatment, and in identifying candidates for new preventive or therapeutic strategies. Various studies have reported different and partially conflicting risk factors [Algren et al., 1993; Baraff et al., 1993; Kaaresen and Flaegstad, 1995; Kornelisse et al., 1995]. Box 80-2 summarizes the major prognostic factors.
Box 80-2 Risk Factors Associated with Poor Prognosis in Bacterial Meningitis
Bacterial etiology has a substantial impact on outcome. A meta-analysis found a 4-fold higher mortality from S. pneumoniae (15.3 percent) compared with H. influenzae (3.8 percent), and a corresponding 2- to 3-fold higher rate of neurologic sequelae (S. pneumoniae 15–30 percent versus H. influenzae 5–20 percent) [Kaaresen and Flaegstad, 1995; Kornelisse et al., 1995]. Although the mortality from N. meningitidis meningitis (7.5 percent) is midway between the other two principal etiologies of bacterial meningitis, sequelae from this infection are much less frequent (2–5 percent). This discrepancy is explained by the relatively frequent presence of sepsis with diffuse coagulopathy in patients with meningococcal meningitis [Algren et al., 1993]. The quantity of antigen in the initial CSF specimen and the number of bacteria present (bacteriorrhachia) also are correlated significantly with sequelae of meningitis [Feldman et al., 1982].
Bacterial meningitis in newborns is associated with high mortality (15–30 percent), and 20–40 percent of survivors have permanent neurologic sequelae. Factors contributing to this poor prognosis are specific etiologies (e.g., Gram-negative enteric bacilli), immaturity, delayed diagnosis, and concomitant disease [Schaad, 1992; Stevens et al., 2003].
Other indicators of poor outcome are focal neurologic findings (e.g., seizures), coma, cardiovascular compromise (e.g., peripheral vasoconstriction, shock), absence of fever, and fewer than 1000 × 106/L leukocytes in CSF at the time of admission. There is no clear association between duration of symptoms before diagnosis and outcome [Kaaresen and Flaegstad, 1995; Kilpi et al., 1993]. Relevant for the prognosis after diagnosis are prompt management (e.g., intensive care, antibacterial and anti-inflammatory therapies); time to CSF sterilization; and number and severity of complications, such as seizures, cranial nerve palsies, coma, subdural effusion, and cerebral herniation (see Table 80-2) [Schaad, 1997]. A recently published post hoc analysis of 654 children with bacterial meningitis, treated in South and Central America, showed that the level of consciousness was the most important predictor of death or neurological sequelae, more than was etiology per se [Roine et al., 2008].
Prevention
Immunoprophylaxis
The available conjugate vaccines against H. influenzae type b are dramatically effective and extremely safe [Feigin et al., 1992]. The vaccine prevents all invasive diseases resulting from bacteremia, including meningitis and epiglottitis. Concerted efforts to vaccinate all infants in all countries must continue.
Meningococcal disease remains a substantial problem worldwide, with approximately 1.2 million cases per year [Balmer and Miller, 2002]. Vaccines comprising the polysaccharide capsule of serogroups A, C, W-135, and Y have been available for approximately 30 years.
These polysaccharide vaccines are not immunogenic in young children, however, and induce only short-lived protection. To improve the immunogenicity of the meningococcal serogroup C polysaccharide vaccine, a carrier protein was covalently attached, converting the vaccine to a T cell-dependent antigen, which can stimulate long-term immunity. This vaccine has proved to be successful in reducing the incidence of serogroup C meningococcal disease in various countries, including the United Kingdom [Miller et al., 2001], Spain [Salleras et al., 2001], and Canada [De Wals et al., 2001].
A comprehensive vaccine conferring protection against all disease-associated serogroups remains elusive. The major challenge in the prevention of meningococcal disease by vaccination is the development of an effective serogroup B meningococcal vaccine. Serogroup B disease is endemic in most industrialized countries, with the burden of disease occurring mainly in young children. The serogroup B capsular polysaccharide is poorly immunogenic, possibly because it shares homology with glycopeptides of neural cell adhesion molecules [Pon et al., 1997]. Advances in bacterial genomics and proteomics have provided new and promising approaches in the identification of novel vaccine candidates [Rappuoli, 2001; Pollard and Levin, 2000].
As is true for all meningeal pathogens, the polysaccharide capsule of S. pneumoniae is a critical virulence factor, helping the organism to avoid phagocytosis. Over 90 different capsular serotypes have been identified. Vaccine development throughout most of the 20th century focused on using combinations of polysaccharides as the key vaccine components [Whitney, 2002].
As mentioned before, vaccines composed of polysaccharide antigens alone produce weak and short-lived immune responses in infants and toddlers. Conjugating pneumococcal polysaccharide to a carrier protein induces a T cell-dependent response that can occur even in early infancy. The first protein-polysaccharide conjugate pneumococcal vaccine that is safe and effective in infants and toddlers was approved in February 2000 in the United States and is now licensed for use in many other countries. The vaccine includes polysaccharide antigens for the seven most common serotypes occurring in children in the United States and in many other countries [Pelton and Klein, 2002]. Clinical trials indicate that conjugate vaccines are highly efficacious against invasive pneumococcal disease, including meningitis, and modestly efficacious against otitis media and pneumonia [Giebink, 2001]. The seven-valent pneumococcal conjugate vaccine is now part of routine infant immunization in the United States and some other countries. As stated under “Epidemiology” (above), nonvaccine serotypes have assumed a greater importance in populations vaccinated with the 7-valent conjugate vaccine [Hsu et al., 2009].
Chemoprophylaxis
Many studies have documented an increased risk of disease in close contacts of index patients with invasive disease caused by N. meningitidis and H. influenzae type b [Feigin et al., 1992]. The risk is age-dependent, and disease among unvaccinated household contacts younger than 4 years old is increased. The spread of such strains is a particular concern in daycare centers.
Numerous studies have found that maternal intrapartum chemoprophylaxis can prevent maternal febrile morbidity and early-onset neonatal group B streptococcal disease, including meningitis [Krohn et al., 1999]. Guidelines recommend the use of risk-based, screening-based, or combined strategies for the administration of intrapartum prophylaxis with amoxicillin [Schrag et al., 2000; Boyer and Gotoff, 1998]. However, screening does not prevent all cases of neonatal group B streptococcal disease, and children with signs and risk factors need be evaluated, even if their mothers were screened [Puopolo et al., 2005].
Recurrent Acute Bacterial Meningitis
Recurrent bacterial meningitis is rare and must prompt a careful evaluation for either an anatomic defect facilitating access of bacteria to the CSF space, or an immunologic deficit. Anatomic defects can be acquired or developmental [Schick et al., 1997], and typically lead to meningitis caused by S. pneumoniae or, rarely, H. influenzae. Defects may involve the temporal bone or the anterior skull base and are not always associated with CSF rhinorrhea or otorrhea. Developmental defects include meningomyelocele, dermal sinus, neurenteric cyst, and malformations of the inner ear. Contrast-enhanced high-resolution CT, MRI cisternography, and search for CSF leakage into the nose or middle ear can help identify an anatomic defect [Drummond et al., 1999; Meco and Oberascher, 2004]. If such a defect is found, surgical correction is indicated.
Among the immunologic conditions predisposing to recurrent meningitis, deficiencies of the latter components of the complement pathway (C5 through C9) or properdin (alternative pathway) have been associated with recurrent infections caused by Neisseria [Overturf, 2003]. Functional or anatomic asplenia, agammaglobulinemia, and deficiencies of the early components of complement (C1 through C3) have been associated with recurrent episodes of meningitis caused by S. pneumoniae, H. influenzae, and N. meningitidis [Overturf, 2003]. Commonly, children with congenital immunodeficiencies have a history of frequent infections other than meningitis. After a second episode of bacterial meningitis, immunoglobulin levels, total complement activity (CH50), and possibly individual complement components should be determined. Splenic function should be assessed, if there is a risk for splenic dysfunction (e.g., sickle cell anemia) or congenital asplenia (e.g., congenital heart disease). Identified deficiencies should be corrected where possible, and chronic antibiotic prophylaxis must be considered in children at high risk for recurrent, severe infections (e.g., in children with asplenia).
Conditions other than bacterial meningitis are frequently the cause of recurrent meningitis, but these are rare in children. They include Behçet’s syndrome, sarcoidosis, and Mollaret’s meningitis associated with recurrent herpes simplex infection [Ginsberg, 2004]. Acute meningitis indistinguishable clinically from bacterial meningitis can result from an allergic reaction to drugs (e.g., trimethoprim-sulfamethoxazole, nonsteroidal anti-inflammatory drugs, and intravenous immunoglobulins).
Chronic Bacterial Meningitis
Tuberculous Meningitis
Epidemiology and Pathogenesis
Tuberculosis remains one of the most prevalent infections worldwide. Mycobacterium tuberculosis is transmitted by respiratory aerosols and frequently infects infants and children, particularly in developing countries. CNS involvement is a life-threatening extrapulmonary manifestation of tuberculosis, and 1–2 percent of children with untreated tuberculosis develop meningitis [Starke, 1999]. Tuberculous meningitis is rare before 3 months of age but increases during the first 5 years of life. A history of close contact with a known case of tuberculosis is often found in children with CNS tuberculosis.
Clinical Characteristics
Tuberculous meningitis usually evolves over several days to a few weeks, although an acute onset is found in about half of the affected children. Initial symptoms are vague and consist of generally poor health, irritability, and apathy (stage I). In young infants, fever, cough, altered consciousness, bulging anterior fontanel, and generalized tonic-clonic seizures are presenting symptoms [Tung et al., 2002]. In older children, low-grade fever, nausea, vomiting, headache, and abdominal pain occur [Farinha et al., 2000]. Nuchal rigidity is not prominent. In stage II, unilateral or bilateral cranial nerve deficits result from the basilar meningitis. Neuro-ophthalmologic changes, including retrobulbar neuritis, gaze palsies, and lesions of the chorioretina, are common [Amitava et al., 2001]. As the disease progresses to stage III, the patient develops depression of consciousness, convulsions, possibly papilledema, and major neurologic deficits. Tuberculosis can involve the spinal cord directly, by pressure from vertebral abscess, and by the production of arachnoiditis [Hernandez-Albujar et al., 2000]. Many patients present with hyponatremia, either on the basis of SIADH or, less commonly, as a cerebral salt-wasting syndrome [Farinha et al., 2000].
Diagnosis
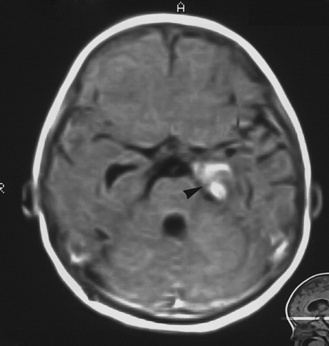
Head CT and MRI document findings similar to those of bacterial meningitis, especially around the base of the brain, and may reveal parenchymal lesions, infarction, and tuberculomas (Figure 80-5). Hydrocephalus is found in most patients [Farinha et al., 2000]. Intracranial tuberculomas, which continue to be found frequently in countries with a high prevalence of tuberculosis, present with symptoms of a space-occupying lesion, with headache, seizures, and other focal neurologic symptoms. Imaging is essential in the diagnosis of spinal involvement [Bernaerts et al., 2003]. CT or MRI of the spine is indicated in a child with suspected tuberculosis and neurologic signs of cord involvement. Chest radiographs are commonly abnormal, with lymphadenopathy or pulmonary infiltrates, but can be normal [Yaramis et al., 1998]. Fifty percent of patients with tuberculous meningitis have a negative tuberculin skin test [Starke, 1999].
The CSF opening pressure is often elevated. The CSF seldom contains more than 500 cells/mm3, most of which are lymphocytes. Protein content is elevated but rarely greater than 500 mg/dL. Glucose concentration usually is decreased to varying degrees [Farinha et al., 2000]. Detection of the infecting organism in CSF is notoriously difficult. Large quantities of CSF (10 mL, if possible) should be collected and examined for acid-fast bacilli. Culture and detection of the M. tuberculosis genome may yield the diagnosis, but in a substantial proportion of cases, all tests remain negative [Farinha et al., 2000]. Repeated CSF studies may allow the diagnosis to be established.
Treatment
The appearance of organisms resistant to antituberculous drugs is an increasing problem in several areas of the world. Isoniazid, rifampicin, ethambutol, pyrazinamide, and streptomycin remain the first-line drugs for the treatment of tuberculous meningitis caused by sensitive organisms. Newer fluoroquinolones also are highly active against tuberculosis, and although their role in the treatment of tuberculous meningitis has not been clearly delineated, they may represent a back-up option in difficult-to-treat cases. Initial therapy is started with four drugs for 2 months (isoniazid, rifampicin, pyrazinamide, and ethambutol or streptomycin), particularly when strains resistant to first-line drugs are a possibility. After 2 months, treatment is reduced to two active drugs, based on sensitivity testing, with rifampicin and isoniazid as first choices [Shingadia and Novelli, 2003]. Pyridoxine is recommended in malnourished children to prevent isoniazid-induced peripheral neuropathy [Shingadia and Novelli, 2003]. Response to antituberculous treatment usually is evident within 2 weeks. Therapy should be continued for at least 9–12 months, and in patients with slow responses, extensive disease, or resistant organisms necessitating treatment with second-line drugs, for 18–24 months. Treatment of pulmonary tuberculosis in children reduces the incidence of CNS infection. Surgical shunting procedures may be necessary for hydrocephalus, particularly in cases of noncommunicating hydrocephalus [Schoeman et al., 2002].
Dexamethasone reduced the mortality and adverse events in adolescents and adults in a large study in Vietnam [Thwaites et al., 2004]. In an earlier controlled study in children, prednisone had beneficial effects on the resolution of tuberculomas and basal inflammation, and improved survival and intellectual outcome [Schoeman et al., 1997]. The use of corticosteroids is advocated, particularly in patients with a decreased level of consciousness, papilledema, focal deficits, or tuberculomas. A course of prednisone or dexamethasone for 6 weeks, with subsequent tapering over several weeks, is the most common regimen recommended.
The mortality of tuberculous meningitis is 10–20 percent. Major sequelae occur and are most common in children in stage III. Visual and auditory impairments are common, as are hemiparesis, mental retardation, and seizures [Schoeman et al., 2002]. Involvement of the hypothalamus and basal cisterns leads to endocrinopathies, such as diabetes insipidus, growth retardation, sexual precocity, and obesity.
Syphilis
Epidemiology and Pathogenesis
Syphilis, which is caused by the spirochete Treponema pallidum, remains an important and prevalent sexually transmitted disease worldwide. The introduction of penicillin led to a decline of the disease, but its prevalence has increased in more recent years. In developed countries, syphilis in children is rare, but it is disproportionately frequent in underprivileged segments of the population and in the setting of drug abuse [Woods, 2005]. In countries with few resources, congenital syphilis remains an important health issue. Transmission occurs across the placenta after the fifth month of gestation or, rarely, at birth by contact with infectious maternal lesions. Congenital syphilis can be prevented by screening and treating pregnant women [Walker and Walker, 2002]. When older children acquire syphilis, this is the result of either sexual abuse or precocious sexual activity. T. pallidum disseminates widely in the body in congenital and acquired syphilis, and can cause disease in various organs, including the CNS. Pathologic lesions involve endothelial cell proliferation and perivascular inflammation with lymphocytes and plasma cells.
Clinical Characteristics
Congenital syphilis is associated with stillbirth or perinatal death in half of the cases [Darville, 1999]. Early congenital syphilis, which includes all manifestations in the first 2 years of life, manifests at birth or within the first weeks of life. Early signs include prematurity and low birth weight, skin and mucous membrane lesions, hepatosplenomegaly, and skeletal abnormalities (osteochondritis), which can be painful and prevent the child from moving affected limbs. Snuffles consist of a thick nasal discharge rich with spirochetes. The eyes (chorioretinitis, uveitis) and CNS (meningitis, meningovascular syphilis, hydrocephalus) are commonly involved, but clinical signs of neurosyphilis are rare in neonates [Parish, 2000; Woods, 2005].
In late congenital syphilis, clinical findings do not appear until several years of age. Interstitial keratitis occurs typically in the second decade. Usually, both eyes are involved. Bone lesions result in destruction of the palate and nasal septum, with depression of the nose (saddle nose). Scars appear about the mouth from earlier fissures (rhagades). The tibia may become bowed (saber shins), and the knee joint can be affected with hydrarthrosis (Clutton’s joints). Permanent dentition is abnormal, especially the upper central incisors, which are dwarfed and notched (Hutchinson’s teeth), and the first lower molars, which have poorly developed cusps (mulberry molars). CNS involvement can manifest with multiple defects. Meningovascular syphilis leads to findings of chronic meningoencephalitis, such as intellectual decline (juvenile paresis), which may begin by 4–5 years of age, headache, seizures, blindness, cranial nerve VIII deafness, other cranial nerve involvement, hemiparesis, and hydrocephalus. Hutchinson’s triad consists of Hutchinson’s teeth, interstitial keratitis, and nerve deafness [Woods, 2005].
Diagnosis
The diagnosis of syphilis relies on clinical suspicion, serologic testing, and visualization of the organism from infected superficial lesions. Tissue biopsy specimens and attempts to culture the organism do not play a role in clinical practice. Serology relies on nontreponemal tests (Venereal Disease Research Laboratory [VDRL]), which are useful for screening and monitoring of treatment, and treponemal tests, such as the microhemagglutination-T. pallidum (MHA-TP) and fluorescent treponemal antibody absorption (FTA-ABS) tests, which are used to confirm the diagnosis. Suspected infections should be investigated by serologic testing of the mother and child. The tests can be negative in a newborn if the mother acquired the disease late in pregnancy, whereas passive transfer of maternal antibodies can result in a false-positive test in an unaffected newborn. Microscopic examination by darkfield microscopy or direct fluorescence antibodies can identify organisms in mucocutaneous lesions. If acquired syphilis is diagnosed in a child, the possibility of sexual abuse must be investigated [Connors et al., 1998].
Treatment
Penicillin remains the treatment of choice for all clinical forms of syphilis. For early stages of acquired syphilis, one injection of benzathine penicillin is sufficient. For neurosyphilis and congenital syphilis, high-dose treatment with intravenous penicillin G is recommended [Workowski and Berman, 2002]. In allergic patients, desensitization should be attempted because penicillin is more effective than alternative treatments that can be given in children.
Lyme Borreliosis
Clinical Characteristics
Neurologic manifestations of Lyme disease are observed in 10 percent of patients, and include lymphocytic meningitis, radiculitis and neuritis, encephalomyelitis, peripheral neuropathy, and subtle encephalopathic syndromes with memory and cognitive dysfunctions [Gerber et al., 1996]. Neurologic symptoms develop within weeks after onset of erythema chronicum migrans and tend to be more frequent and severe in Europe than in the United States, likely as a result of the increased neurotropism of Borrelia garinii, which is found only in Europe. In children, fatigue, peripheral palsy of cranial nerve VII, loss of appetite, and fever are common manifestations of CNS Lyme disease [Christen, 1996; Skogman et al., 2008]. Rare manifestations of neuroborreliosis in children include cerebrovascular disease with sensorimotor deficits, optic nerve impairment caused by inflammation, and increased intracranial pressure. Chronic neuroborreliosis, which is very rare in children, is manifested as a failure to thrive, headaches, and pseudotumor cerebri [Rothermel et al., 2001; Wilke et al., 2000].
Treatment and Outcome
In early stages of the disease, doxycycline or amoxicillin is recommended. The latter is preferred in children younger than 8 years old. Doxycycline and ceftriaxone are equally effective for CNS disease in adults [Ljostad et al., 2008] and are both used in children with CNS symptoms, with preference for ceftriaxone in children less than 8 years old [Skogman et al., 2008]. The prognosis is generally good for children with CNS Lyme disease who are treated with appropriate antimicrobial agents, but in approximately 10 percent with facial palsy, the palsy has not fully recovered after 6 months [Skogman et al., 2008].
Leptospirosis
Leptospirosis, caused by spirochetes of the species Leptospira, is acquired through the skin in water infested by rats but also can be transmitted to children by dogs. After an incubation period of 1–2 weeks, the acute, septicemic illness manifests itself by high fevers and constitutional symptoms, headaches, gastrointestinal symptoms, muscle pain, and conjunctivitis. After a period of apparent recovery, a second, immunologically mediated phase occurs. During this second phase, the liver, kidney, and nervous system may be affected. Nervous system involvement takes the form of meningitis, encephalitis, or neuritis. Meningitis is documented in approximately 10–20 percent of cases [Rajajee et al., 2002]. Meningitis and encephalitis rarely may become chronic. Neuritis tends to involve the brachial plexus or cranial nerves.
Pleocytosis is found in the CSF, which, in the initial phase, is polymorphonuclear, whereas lymphocytes are found in the second phase. The protein is elevated, with normal glucose concentrations. The diagnosis usually is established by documenting rising antibody titers in serum, but the organism also can be visualized by dark-field microscopy in blood, urine, and occasionally CSF (in the early phase), and can be cultured using Fletcher medium. Polymerase chain reaction facilitates early diagnosis [Gerke and Rump, 2003]. Penicillin, cefotaxime, and doxycycline are among the antibiotics effective for the treatment of leptospirosis [Suputtamongkol et al., 2004]. Patients with encephalitis may be left with sequelae, even when treated with antibiotics.
Brain Abscess
Brain abscess is rare in children, with a relative peak in the neonatal period and in children between 4 and 7 years. Since brain abscess leads to substantial morbidity and mortality, a high level of suspicion is necessary in order to diagnose the condition early. CT and MRI are sensitive tools to detect brain abscesses, and treatment must involve a multidisciplinary approach [Frazier et al., 2008].
Clinical Characteristics
Brain abscess should be suspected in children with fever, headaches, new-onset seizures, vomiting, focal neurologic deficits, decreased consciousness and signs of increased intracranial pressure (Box 80-3). Nuchal rigidity may occur in a few patients. Multiple brain abscesses do not have a unique presentation [Sheehan et al., 2008].
Diagnosis
MRI or CT should be used in patients with suspected brain abscess. Early cerebritis will be suggested by hypodense areas on CT and focal edema on MRI. With a more mature abscess, classic ring-enhancing lesions with necrotic center and perifocal edema can be documented. Diffusion-weighted imaging may help differentiate the nature of cerebral masses [Reddy et al., 2006].
Lumbar Puncture
Lumbar puncture must not be performed in patients with suspected brain abscess because of the risk of herniation secondary to elevated intracranial pressure, and because CSF is rarely helpful for the diagnosis of brain abscess. Identification of the causative organisms is best attempted in abscess material obtained by aspiration or extirpation [Frazier et al., 2008].
Pathogenesis and Pathology
Either hematogenous or direct spread of the organism can lead to a brain abscess. Brain abscesses occur in children most commonly in association with cyanotic congenital heart disease and infections of the sinuses or middle ear [Goodkin et al., 2004]. Other conditions associated with brain abscess are neurosurgical procedures, penetrating head trauma, immunosuppression, and chronic pulmonary disease. Brain abscess is rare as a complication of bacterial meningitis, except in neonates (typically caused by Citrobacter and other Gram-negative organisms) [Renier et al., 1988; Schaad, 1992].
The infection of the brain parenchyma leads initially to a focal cerebritis with surrounding edema. Progressing from the cerebritis phase, a capsule of inflammatory granulation tissue develops over a period of a few weeks around the necrotic infected area. Abscess location depends on the pathogenesis of the abscess, with hematogenous abscess typically reflecting the vascular supply, often of the middle cerebral artery. Infections of sinuses or the oral cavity tend to cause frontal lobe abscesses, while otitis media often leads to abscesses in the temporal lobe or cerebellum [Sheehan et al., 2008].
Treatment
Surgical drainage by stereotactic needle aspiration or endoscopy or excision of the abscess and antibiotics constitutes appropriate treatment [Frazier et al., 2008]. Surgery provides an opportunity to culture the organisms and hastens the resolution of abscesses.
Empiric antibiotic therapy usually consists of vancomycin, ceftriaxone, and metronidazole for 4–8 weeks. Brain abscesses have been cured by antibiotics without surgical drainage [Aebi et al., 1991; Wong et al., 1989]. Medical management, with or without needle aspiration, should be considered when abscess formation is still in the cerebritis stage, when there are multiple abscesses, or when the abscess is located in a critical area [Aebi et al., 1991; Brook, 1995]. Serial CT or MRI should be used to determine when medical therapy is failing. Corticosteroids should only be used to control symptomatically increased intracranial pressure. Other methods, such as controlled hyperventilation or osmotic agents (e.g., mannitol), are rarely necessary.
Prognosis
Despite earlier diagnosis of brain abscess and the ability to follow treatment with regular imaging, the disease is still associated with important morbidity and mortality. Currently, mortality rates are between 4 and 12 percent [Frazier et al., 2008]. The outlook is poor when multiple abscesses are present, in immunocompromised children, and when age is less than 1 year [Tekkok and Erbengi, 1992]. Chronic seizures are common after brain abscess; they often become manifest several years after the brain abscess occurred [Frazier et al., 2008].
Cranial Empyema and Spinal Abscess
Cranial Subdural Empyema
The virtual space between the dura and the arachnoid membrane covering the brain can occasionally be the site of pus collection in response to a bacterial infection. In infants, subdural empyema is a rare complication of meningitis and must be distinguished from extra-axial fluid collections, which are typically sterile (see earlier in this chapter) [Dill et al., 1995]. In older children, the most common cause of subdural empyema in developed countries is sinusitis [Farah et al., 2009], while otitis appears more common in the developing world [Pathak et al., 1990]. Osteomyelitis of the skull, trauma, and intracerebral shunts are other rare causes of subdural empyema. Most subdural empyemas in children occur supratentorially, while infratentorial and spinal locations are exceedingly rare.
Symptoms of subdural empyemas include fever, headache, new-onset seizures, nausea and vomiting, hemiparesis, decreased mental status, meningismus, and other signs of intracranial mass lesions [Dill et al., 1995; Quraishi and Zevallos, 2006]. In infants with meningitis, subdural empyema must be excluded if signs and symptoms worsen after initial improvement under antibiotic therapy. CT and MRI are the most sensitive tests to diagnose subdural empyemas and imaging should be performed promptly in patients when a subdural empyema or other suppurative CNS infection is suspected (Figure 80-6). Lumbar puncture should be avoided in patients with subdural empyemas because of the risk of herniation and the lack of diagnostic utility.
The most common organisms isolated from subdural empyemas are streptococci, frequently of the microaerophilic Streptococcus anginosus (S. milleri) group [Dill et al., 1995; Farah et al., 2009]. Other organisms causing subdural empyema include anaerobic bacteria from the oropharynx, S. aureus, Haemophilus spp., and Gram-negative rods.
Treatment of subdural empyema includes early evacuation of the empyema by craniotomy or multiple burr holes and intravenous antibiotic treatment (see treatment of bacterial meningitis for choice of antibiotics) [Nathoo et al., 2001]. Since empyemas can reaccumulate, confirmation of therapeutic success by repeat imaging studies is recommended [Farah et al., 2009].
Cranial Epidural Abscess
Suppurative infections in the epidural space are usually a complication of trauma, neurosurgery, or spread of infection from contiguous structures. The condition is rare in childhood [Auletta and John, 2001]. S. aureus, S. epidermidis, Streptococcus spp., and B. fragilis are some of the organisms found. The abscess acts as an enlarging mass. Symptoms may be subtle and include fever, headache, stiff neck, focal seizures, and focal neurologic deficits, such as hemiparesis. Lumbar puncture usually is contraindicated.
Imaging of cranial epidural abscess typically reveals a lenticular collection of fluid between the bone and CNS of variable appearance and enhancement characteristics. MRI is preferred to CT [Weingarten et al., 1989]. Treatment consists of antibiotics (see discussion on treatment of meningitis for appropriate choices) and surgical drainage [Auletta and John, 2001]. Delay in diagnosis and treatment is associated with an unfavorable outcome.
Spinal Epidural Abscess
Spinal epidural abscess is usually secondary to bacteremia or direct extension from a focal infection of the spine. The condition is rare as a complication of lumbar puncture or epidural analgesia [Strafford et al., 1995]. S. aureus is the most common organism recovered, although M. tuberculosis is always a consideration. Presentation is a “painful, febrile, spinal syndrome” [Hancock, 1973]. Progression can be rapid, with motor and sphincter involvement and a clinical picture resembling acute transverse myelitis. Children often have no clear-cut presentation.
With a suspicion of spinal epidural abscess, immediate MRI with gadolinium is indicated, and lumbar puncture usually is contraindicated. Surgical treatment with appropriate antibiotic coverage is the mainstay of therapy [Hlavin et al., 1990; Rosenfeld and Rowley, 1994]. Antibiotics alone may be appropriate in selected cases without neurologic deficits [Slade and Lonano, 1990].
Other Bacterial Infections of the Nervous System
Bartonella
B. henselae causes CNS involvement in the form of encephalitis, cerebral vasculitis, or radiculitis. It is estimated that up to 2 percent of cases of cat-scratch disease are complicated by encephalopathy [Wheeler et al., 1997]. Typical symptoms include generalized tonic-clonic seizures, peripheral facial nerve palsy, inflammatory polyneuropathy, and other deficits attributable to encephalitis [Carithers and Margileth, 1991]. Onset of the disease is subacute over days to weeks, unless acute seizures or status epilepticus are the primary manifestation. Imaging studies usually are normal, and CSF indicates no signs of inflammation [Lewis and Tucker, 1986]. More recently, cases of encephalitis or focal cerebral lesions caused by B. quintana have been described [Parrott et al., 1997; Mantadakis et al., 2007]. The diagnosis is based on serology, which does not discriminate reliably between the two species (cross-reactivity), and polymerase chain reaction, which can differentiate between them [Parrott et al., 1997]. The fastidious pleomorphic rods are difficult to culture, but can be detected in tissue by Warthin–Starry silver stain.
Uncomplicated cat-scratch disease is self-limiting and does not require antimicrobial therapy. In patients with encephalitis or focal brain lesions associated with Bartonella infection, antibiotics may be prescribed, although their benefit for this indication is uncertain. Macrolides, doxycycline with or without aminoglycosides, ceftriaxone, trimethoprim-sulfamethoxazole, and rifampicin are potentially active drugs [Rolain et al., 2004].
Mycoplasma pneumoniae
Although Mycoplasma pneumoniae, a small bacterium without a cell wall, is primarily a respiratory tract pathogen causing pneumonia, it is prone to induce immune-mediated secondary clinical manifestations, such as hemolytic anemia, and has been implicated in diseases affecting the CNS. Of cases of encephalitis in children, 10 percent have been attributed to M. pneumoniae [Bitnun et al., 2001]. After a prodromal respiratory illness in about two-thirds of patients, a variety of neurologic syndromes, such as meningitis, acute disseminated encephalomyelitis, hemorrhagic encephalitis, cerebral ataxia, cranial nerve neuropathies, transverse myelitis, polyradiculitis, and an ascending paralysis similar to Guillain–Barré syndrome, have been described [Lin et al., 2002; Guleria et al., 2005]. In 11 children with probable CNS involvement secondary to M. pneumoniae, the most common neurologic manifestations were seizures in 7 children, and focal motor deficits and ataxia in 4 each. Half of the children had CSF abnormalities, primarily a mononuclear pleocytosis, and pathologic imaging studies, whereas 80 percent had abnormal findings on EEG [Bitnun et al., 2001].
The diagnosis of CNS involvement secondary to M. pneumoniae is difficult. Polymerase chain reaction in brain tissue or CSF provides the most compelling evidence for CNS invasion by the organism [Bitnun et al., 2001]. Serology (IgM) was the only evidence for the infection in a majority of 84 pediatric patients with M. pneumoniae-associated encephalitis, and only one of these patients had a positive CSF polymerase chain reaction [Christie et al., 2007]. The mechanisms by which M. pneumoniae causes damage to nervous tissue have not been clearly delineated. Direct invasion of nervous tissue and immune-mediated mechanisms may play a role.
In suspected cases, antimicrobial therapy with drugs that penetrate into the CNS and have activity against M. pneumoniae (e.g., doxycycline, quinolones) should be instituted, despite the lack of firm evidence of a beneficial effect [Bitnun et al., 2003]. The benefit of immune modulation (corticosteroids, immunoglobulins, plasmapheresis) is also uncertain [Sakoulas, 2001]. Outcome is guarded, with a case fatality rate of 9 percent, and minor to major sequelae in 34 percent among 58 pediatric patients with Mycoplasma encephalitis [Daxboeck et al., 2004].
Leprosy
Despite increasing efforts to control leprosy (Hansen’s disease), it is still prevalent in some countries of Asia, Africa, and South America. The disease should be considered in patients with persistent skin lesions and peripheral neuropathy who have lived in countries with warm climates and limited resources. Children comprise only a small proportion of patients presenting with leprosy (2 percent in India, the country with the highest prevalence currently) [Britton and Lockwood, 2004].
M. leprae binds to a specific epitope of laminin in the basal lamina of Schwann cells of peripheral nerves and then triggers intracellular signaling cascades that initiate the processes leading to myelin damage and subsequent nerve dysfunction [Soares de Lima et al., 2005; Tapinos et al., 2006]. The disease affects peripheral nerve trunks in fibro-osseous tunnels (e.g., ulnar, median, lateral popliteal, and posterior tibial nerves) and small dermal nerves. The symptoms are sensory and motor loss, hypesthesia, and anhydrosis. The diagnosis is made clinically, based on the presence of one or more of the following signs: hypopigmented or reddish patches with reduced sensation, thickening of peripheral nerves, or acid-fast bacilli on skin smears or biopsy material. Polymerase chain reaction methods have been developed and appear sensitive and specific [Bang et al., 2009].
Treatment depends on the form of disease and uses multidrug approaches, consisting of dapsone, clofazimine, rifampicin, ofloxacin, and minocycline [Britton and Lockwood, 2004]. Guidelines issued by the World Health Organization should be consulted. Neuritis (painful peripheral nerves, new anesthesia, or motor loss) and inflamed skin lesions should be treated with corticosteroids in addition to antimicrobial therapy [Britton, 1998]. However, prophylactic steroids have not led to sustained beneficial effects on nerve dysfunction [Smith et al., 2004].
References
![]() The complete list of references for this chapter is available online at www.expertconsult.com.
The complete list of references for this chapter is available online at www.expertconsult.com.
Aebi C., Kaufmann F., Schaad U.B. Brain abscess in childhood – long-term experiences. Eur J Pediatr. 1991;150:282.
Ahmed A., Hickey S.M., Ehret S., et al. Cerebrospinal fluid values in the term neonate. Pediatr Infect Dis J. 1996;15:298.
Algren J.T., Lal S., Cutliff S.A., et al. Predictors of outcome in acute meningococcal infection in children. Crit Care Med. 1993;21:447.
Alleyne C.H., Hassan M., Zabramski J.M. The efficacy and cost of prophylactic and perioprocedural antibiotics in patients with external ventricular drains. Neurosurgery. 2000;47:1124.
Amaee F.R., Comis S.D., Osborne M.P. NG-methyl-L-arginine protects the guinea pig cochlea from the cytotoxic effects of pneumolysin. Acta Otolaryngol. 1995;115:386.
Amitava A.K., Alarm S., Hussain R. Neuro-ophthalmic features in pediatric tubercular meningoencephalitis. J Pediatr Ophthalmol Strabismus. 2001;38:229.
Arnold W., Bredberg G., Gstöttner W., et al. Meningitis following cochlear implantation: Pathomechanisms, clinical symptoms, conservative and surgical treatments. Otorhinolaryngology. 2002;64:382.
Aronin S.I., Peduzzi P., Quagliarello V.J. Community-acquired bacterial meningitis: Risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129:862.
Arseni C., Nereantiu F. Multiple vascular thrombosis with vasculogenic lesions in the course of meningoencephalitis. Confin Neurol. 1972;34:339.
Ashwal S. Neurologic evaluation of the patient with acute bacterial meningitis. Neurol Clin. 1995;13:549.
Ashwal S., Perkin R.M., Thompson J.R., et al. Bacterial meningitis in children: Current concepts of neurologic management. Adv Pediatr. 1993;40:185.
Ashwal S., Stringer W., Tomasi L., et al. Cerebral blood flow and carbon dioxide reactivity in children with bacterial meningitis. J Pediatr. 1990;117:523.
Ashwal S., Tomasi L., Schneider S., et al. Bacterial meningitis in children: Pathophysiology and treatment. Neurology. 1992;42:739.
Auletta J.J., John C.C. Spinal epidural abcesses in children: A 15-year experience and review of the literature. Clin Infect Dis. 2001;32:9.
Balmer P., Miller E. Meningococcal disease: How to prevent and how to manage. Curr Opin Infect Dis. 2002;15:275.
Bang P.D., Suzuki K., Phuong L.T., et al. Evaluation of polymerase chain reaction-based detection of Mycobacterium leprae for the diagnosis of leprosy. J Dermatol. 2009;36:269.
Baraff L.J., Lee S.I., Schriger D.L. Outcomes of bacterial meningitis in children: A meta-analysis. Pediatr Infect Dis J. 1993;12:389.
Bedford H., De Louvois J., Halket S., et al. Meningitis in infancy in England and Wales: Follow up at age 5 years. BMJ. 2001;323:533.
Berman P.H., Banker B.Q. Neonatal meningitis: A clinical and pathological study of 29 cases. Pediatrics. 1966;38:6.
Bernaerts A., Vanhoenacker F.M., Parizel P.M., et al. Tuberculosis of the central nervous system: Overview of neuroradiological findings. Eur Radiol. 2003;13:1876.
Bernard G.R., Vincent J.L., Laterre P.F., et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699.
Bhatt S.M., Lauretano A., Cabellos C., et al. Progression of hearing loss in experimental pneumococcal meningitis: Correlation with cerebrospinal fluid cytochemistry. J Infect Dis. 1993;167:675.
Bifrare Y.D., Gianinazzi C., Imboden H., et al. Bacterial meningitis causes two distinct forms of cellular damage in the hippocampal dentate gyrus in infant rats. Hippocampus. 2003;13:481.
Bitnun A., Ford-Jones E., Blaser S., et al. Mycoplasma pneumoniae encephalitis. Semin Pediatr Infect Dis. 2003;14:96.
Bitnun A., Ford-Jones E.L., Petric M., et al. Acute childhood encephalitis and Mycoplasma pneumoniae. Clin Infect Dis. 2001;32:1674.
Bodino J., Lylyk P., de Valle M., et al. Computed tomography in purulent meningitis. Am J Dis Child. 1982;136:495.
Boyer K.M., Gotoff S.P. Alternative algorithms for prevention of perinatal group B streptococcal infections. Pediatr Infect Dis J. 1998;17:973.
Bradley J.S., Scheld W.M. The challenge of penicillin-resistant Streptococcus pneumoniae meningitis: Current antibiotic therapy in the 1990s. Clin Infect Dis. 1997;24(Suppl 2):S213.
Britton W.J. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225.
Britton W.J., Lockwood D.N. Leprosy. Lancet. 2004;363:1209.
Brook I. Brain abscess in children: Microbiology and management. J Child Neurol. 1995;10:283.
Brown L.W., Feigin R.D. Bacterial meningitis: Fluid balance and therapy. Pediatr Ann. 1994;23:93.
Cairns H., Russell D.S. Cerebral arteritis and phlebitis in pneumococcal meningitis. J Pathol Bacteriol. 1946;58:649.
Canica M., Dias R., Ferreira E. Neisseria meningitidis C:2b:P1.2,5 with intermediate resistance to penicillin, Portugal. Emerg Infect Dis. 2004;10:526.
Carithers H.A., Margileth A.M. Cat-scratch disease: Acute encephalopathy and other neurologic manifestations. Am J Dis Child. 1991;145:98.
Chiba N., Murayama S.Y., Morozumi M., et al. Rapid detection of eight causative pathogens for the diagnosis of bacterial meningitis by real-time PCR. J Infect Chemother. 2009;15:92.
Christen H.J. Lyme neuroborreliosis in children. Ann Med. 1996;28:235.
Christie L.J., Honarmand S., Talkington F., et al. Pediatric encephalitis: what is the role of Mycoplasma pneumoniae? Pediatrics. 2007;120:305.
Cohen B.A., Schenk V.A., Sweeney D.B. Meningitis-related hearing loss evaluated with evoked potentials. Pediatr Neurol. 1988;4:18.
Connors J.M., Schubert C., Shapiro R. Syphilis or abuse: Making the diagnosis and understanding the implications. Pediatr Emerg Care. 1998;14:139.
Cottagnoud P.H., Täuber M.G. New therapies for pneumococcal meningitis. Expert Opin Investig Drugs. 2004;13:393.
Coulehan J.L., Michaels R.H., Hallowell C., et al. Epidemiology of Haemophilus influenzae type b disease among Navajo Indians. Public Health Rep. 1984;99:404.
Crosswell J.M., Nicholson W.R., Lennon D.R. Rapid sterilisation of cerebrospinal fluid in meningococcal meningitis: Implications for treatement duration. J Paediatr Child Health. 2006;42:829.
Darville T. Syphilis. Pediatr Rev. 1999;20:160.
Daxboeck F., Blacky A., Seidl R., et al. Diagnosis, treatment and prognosis of Mycoplasma pneumoniae childhood encephalitis; systematic review of 58 cases. J Child Neurol. 2004;19:865.
for theDe Gans J., Van de Beek D., European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549.
De Louvois J., Halket S., Harvey D. Effect of meningitis in infancy on school-leaving examination results. Arch Dis Child. 2007;92:959.
Derugin N., Wendland M., Muramatsu K., et al. Evolution of brain injury after transient middle cerebral artery occlusion in neonatal rats. Stroke. 2000;31:1752.
De Wals P., de Serres G., Niyonsenga T. Effectiveness of a mass immunization campaign against serogroup C meningococcal disease in Quebec. JAMA. 2001;285:177.
Dill S.R., Cobbs C.G., McDonald C.K. Subdural empyema: Analysis of 32 cases and review. Clin Infect Dis. 1995;20:327.
Drummond D.S., de Jong A.L., Giannoni C., et al. Recurrent meningitis in the pediatric patient – the otolaryngologist’s role. Int J Pediatr Otorhinolaryngol. 1999;48:199.
Dubos F., Korczowski B., Aygun D.A., et al. Serum procalcitonin level and other biological markers to distinguish between bacterial and aseptic meningitis in children. A European multicenter case cohort study. Arch Pediatr Adolesc Med. 2008;162:1157.
Du Plessis M., von Gottberg A., Cohon C., et al. Neisseria meningitidis immediately resistant to penicillin and causing invasive disease in South Africa in 2001 to 2005. J Clin Microbiol. 2008;46:3208.
Eisenhut M., Meehan T., Batchelor L. Cerebrospinal fluid glucose levels and sensorineural hearing loss in bacterial meningitis. Infection. 2003;31:247.
Farah J.O., Kandasamy J., May P., et al. Subdural empyema secondary to sinus infection in children. Childs Nerv Syst. 2009;25:199.
Farinha N.J., Razali K.A., Holzel H., et al. Tuberculosis of the central nervous system in children: A 20-year survey. J Infect. 2000;41:61.
Feigin R.D., McCracken G.H.Jr, Klein J.O. Diagnosis and management of meningitis. Pediatr Infect Dis J. 1992;11:785.
Feldman W.E., Ginsburg C.M., McCracken G.H.Jr, et al. Relation of concentrations of Haemophilus type b in cerebrospinal fluid to late sequelae of patients with meningitis. J Pediatr. 1982;100:209.
Filka J., Huttova M., Tuharsky J., et al. Nosocomial meningitis in children after ventriculoperitoneal shunt insertion. Acta Paediatr. 1999;88:576.
Förderreuther S., Tatsch K., Einhäupl K.M., et al. Abnormalities of cerebral blood flow in the acute phase of bacterial meningitis in adults. J Neurol. 1992;239:431.
Francois M., Laccourreye L., Huy E.T., et al. Hearing impairment in infants after meningitis: Detection by transient evoked otoacoustic emissions. J Pediatr. 1997;130:712.
Frazier J.L., Ahn E.S., Jallo G.I. Management of brain abscesses in children. Neurosurg Focus. 2008;24:E8.
Fredricks D.N., Relman D.A. Application of polymerase chain reaction to the diagnosis of infectious diseases. Clin Infect Dis. 1999;29:475.
Gates G.A., Miyamoto T. Cochlear implants. N Engl J Med. 2003;349:421.
Geiseler P.J., Nelson K.E. Bacterial meningitis without clinical signs of meningeal irritation. South Med J. 1982;75:448.
Gerber M.A., Shapiro E.D., Burke G.S., et al. Lyme disease in children in southeastern Connecticut. Pediatric Lyme Disease Study Group. N Engl J Med. 1996;335:1270.
Gerke P., Rump L.C. Leptospirosis – 3 cases and a review. Clin Nephrol. 2003;60:42.
Gianinazzi C., Grandgirard D., Imboden H., et al. Caspase-3 mediates hippocampal apoptosis in pneumococcal meningitis. Acta Neuropathol (Berl). 2003;105:499.
Giebink G.S. The prevention of pneumococcal disease in children. N Engl J Med. 2001;345:1177.
Ginsberg L. Difficult and recurrent meningitis. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 1):i16.
Goodkin H.P., Harper M.B., Pomeroy S.L. Intracerebral abscess in children: Historical trends at Children’s Hospital Boston. Pediatrics. 2004;113:1765.
Grande P.O., Myhre E.B., Nordstrom C.H., et al. Treatment of intracranial hypertension and aspects on lumbar dural puncture in severe bacterial meningitis. Acta Anaesthesiol Scand. 2002;46:264.
Gray B.M., Simmons D.R., Mason H., et al. Quantitative levels of C-reactive protein in cerebrospinal fluid in patients with bacterial meningitis and other conditions. J Pediatr. 1986;108:665.
Green S.M., Rothrock S.G., Clem K.J., et al. Can seizures be the sole manifestation of meningitis in febrile children? Pediatrics. 1993;92:527.
Grimwood K., Anderson P., Anderson V., et al. Twelve year outcomes following bacterial meningitis: Further evidence for persisting effects. Arch Dis Child. 2000;83:111.
Grimwood K., Anderson V.A., Bond L., et al. Adverse outcomes of bacterial meningitis in school-age survivors. Pediatrics. 1995;95:646.
Grimwood K., Nolan T.M., Bond L., et al. Risk factors for adverse outcomes of bacterial meningitis. J Paediatr Child Health. 1996;32:457.
Guleria R., Nisar N., Chawla T.C., et al. Mycoplasma and central nervous system complications: a review. J Lab Clin Med. 2005;146:55.
Hancock D.O. A study of 49 patients with acute spinal extradural abscess. Paraplegia. 1973;10:285.
Hasbun R., Abrahams J., Jekel J., et al. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001;345:1727.
Hasegawa K., Chiba N., Kobayashi R., et al. Rapidly increasing prevalence of beta-lactamase-nonproducing, ampicillin-resistant Haemophilus influenzae type b in patients with meningitis. Antimicrob Agents Chemother. 2004;48:1509.
Heath P.T., Nik N.K., Baker C.J. Neonatal meningitis. Arch Dis Child Fetal Neonatal Ed. 2003;88:F173.
Hernandez-Albujar S., Arribas J.R., Royo A., et al. Tuberculous radiculomyelitis complicating tuberculous meningitis: Case report and review. Clin Infect Dis. 2000;30:915.
Hlavin M.L., Kaminski H.J., Ross J.S., et al. Spinal epidural abscess: A ten-year perspective. Neurosurgery. 1990;27:177.
Hsu H.E., Shutt K.A., Moore M.R., et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009;360:244.
Jones M.E., Draghi D.C., Karlowsky J.A., et al. Prevalence of antimicrobial resistance in bacteria isolated from central nervous system specimens as reported by U.S. hospital laboratories from 2000 to 2002. Ann Clin Microbiol Antimicrob. 2004;3:3.
Kaaresen P.I., Flaegstad T. Prognostic factors in childhood bacterial meningitis. Acta Pediatr. 1995;84:873.
Kaplan S.L., Feigin R.D. The syndrome of inappropriate secretion of antidiuretic hormone in children with bacterial meningitis. J Pediatr. 1978;92:758.
Karageorgopoulos D.E., Valkimadi P.E., Kapaskelis A., et al. Short vs. long duration of antibiotic therapy for bacterial meningitis; a meta-analysis of randomised controlled trials in children. Arch Dis Child. 2009;94:607.
Kieseier B.C., Paul R., Koedel U., et al. Differential expression of matrix metalloproteinases in bacterial meningitis. Brain. 1999;122:1579.
Kilpi T., Anttila M., Kallio M.J.T., et al. Length of prediagnostic history related to the course and sequelae of childhood bacterial meningitis. Pediatr Infect Dis. 1993;12:184.
Kim B.Y., Kang J., Kim K.S. Invasion process of pathogenic Escherichia coli. Int J Med Microbiol. 2005;295:463.
Kim K.S. Acute bacterial meningitis in infants and children. Lancet Infect Dis. 2010;10:32.
Kioumehr F., Dadsetan M.R., Feldman N., et al. Postcontrast MRI of cranial meninges: Leptomeningitis versus pachymeningitis. J Comput Assist Tomogr. 1995;19:713.
Klein M., Koedel U., Pfister H.W., et al. Morphological correlates of acute and permanent hearing loss during experimental pneumococcal meningitis. Brain Pathol. 2003;13:123.
Koedel U., Gorriz C., Lorenzl S., et al. Increased endothelin levels in cerebrospinal fluid samples from adults with bacterial meningitis. Clin Infect Dis. 1997;25:329.
Kornelisse R.F., Westerbeek C.M.L., Spoor A.B., et al. Pneumococcal meningitis in children: Prognostic indicators and outcome. Clin Infect Dis. 1995;21:1390.
Koster-Rasmussen R., Korshin A., Meyer C.N. Antibiotic treatment delay and outcome in bacterial meningitis. J Infect. 2008;75:449.
Krohn M.A., Hillier S.L., Baker C.J. Maternal peripartum complications associated with vaginal group B streptococci colonization. J Infect Dis. 1999;179:1410.
Lavi R., Rowe J.M., Avivi I. Lumbar Puncture: It Is Time to Change the Needle. Eur Neurol. 2010;64:108.
Leib S.L., Heimgartner C., Bifrare Y.D., et al. Dexamethasone aggravates hippocampal apoptosis and learning deficiency in pneumococcal meningitis in infant rats. Pediatr Res. 2003;4:4.
Leib S.L., Kim Y.S., Chow L.L., et al. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Invest. 1996;98:2632.
Leppert D., Lindberg R.L., Kappos L., et al. Matrix metalloproteinases: Multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res Brain Res Rev. 2001;36:249.
Lewis D.W., Tucker S.H. Central nervous system involvement in cat scratch disease. Pediatrics. 1986;77:714.
Lin W.C., Lee P.I., Lu C.Y., et al. Mycoplasma pneumoniae encephalitis in childhood. J Microbiol Immunol Infect. 2002;35:173.
Lindvall P., Ahlm C., Ericsson M., et al. Reducing intracranial pressure may increase survival among patients with bacterial meningitis. Clin Infect Dis. 2004;38:384.
Ljostad U., Skogvoll E., Eikeland R., et al. Oral doxycycline versus intravenous ceftriaxone for European Lyme neuroborreliosis: A multicentre, non-inferiority trial. bouble-blind, randomised trial. Lancet Neurol. 2008;7:690.
Lutsar I., Haldre S., Topman M., et al. Enzymatic changes in the cerebrospinal fluid in patients with infections of the central nervous system. Acta Pediatr. 1994;83:1146.
Lynch J., Arhelger S., Krings-Ernst I. Post-dural puncture headache in young orthopaedic in-patients: Comparison of a 0.33 mm (29-gauche) Quincke-type with a 0.7 mm (22-gauche) Whitacre spinal needle in 200 patients. Acta Anaesthesiol Scand. 1992;36:58.
Maconochie I.K., Baumer J.H., Stewart M.. Fluid therapy for acute bacterial meningitis. Cochrane Database Syst Rev. 2008(1):CD004786.
Mantadakis E., Spanaki A.-M., Psaroulaki A., et al. Encephalopathy complicated by Guillain-Barré syndrome and hydrocephalus and associated with acute Bartonella quintana infection. Pediatr Infect Dis J. 2007;26:860.
Mariscalco M.M., Vergara W., Mei J., et al. Mechanisms of decreased leukocyte localization in the developing host. Am J Physiol Heart Circ Physiol. 2002;282:H636.
Martin G.S., Mannino D.M., Eaton S., et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546.
Marton K.I., Gean A.D. The diagnostic spinal tap. Ann Intern Med. 1986;104:880.
McClinton D., Carraccio C., Englander R. Predictors of ventriculoperitoneal shunt pathology. Pediatr Infect Dis J. 2001;20:593.
McGirt M.J., Zaas A., Fuchs H.E., et al. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis. 2003;36:858.
Meco C., Oberascher G. Comprehensive algorithm for skull base dural lesion and cerebrospinal fluid fistula diagnosis. Laryngoscope. 2004;114:991.
Meli D.N., Christen S., Leib S.L., et al. Current concepts in the pathogenesis of meningitis caused by Streptococcus pneumoniae. Curr Opin Infect Dis. 2002;15:253.
Miller E., Salisbury D., Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: A success story. Vaccine. 2001;20(Suppl 1):S58.
Moller K., Larsen F.S., Bie P., et al. The syndrome of inappropriate secretion of antidiuretic hormone and fluid restriction in meningitis – how strong is the evidence? Scand J Infect Dis. 2001;33:13.
Nadel S., Goldstein B., Williams M.D., et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicenter phase III randomized controlled trial. Lancet. 2007;369:836.
Nathoo N., Nadvi S.S., Gouws E., et al. Craniotomy improves outcomes for cranial subdural empyemas: computed tomography-era experience with 699 patients. Neurosurg. 2001;49:872.
Nau R., Soto A., Bruck W. Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J Neuropathol Exp Neurol. 1999;58:265.
Nigrovic L.E., Kuppermann N., Macias C.G., et al. Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis. JAMA. 2007;297:52.
Odio C., McCracken G.H.Jr, Nelson J.D. CSF shunt infections in pediatrics: A seven-year experience. Am J Dis Child. 1984;138:1103.
Odio C.M., Faingezicht I., Paris M., et al. The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. N Engl J Med. 1991;324:1525.
Oostenbrink R., Maas M., Moons K.G., et al. Sequelae after bacterial meningitis in childhood. Scand J Infect Dis. 2002;34:379.
Orihuela C.J., Mahdavi J., Thornton J., et al. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest. 2009;119:1638-1646.
Overturf G.D. Indications for the immunological evaluation of patients with meningitis. Clin Infect Dis. 2003;36:189.
Packer R.J., Bilaniuk L.T., Zimmerman R.A. CT parenchymal abnormalities in bacterial meningitis: Clinical significance. J Comput Assist Tomogr. 1982;6:1064.
Parish J.L. Treponemal infections in the pediatric population. Clin Dermatol. 2000;18:687.
Parrott J.H., Dure L., Sullender W., et al. Central nervous system infection associated with Bartonella quintana: A report of two cases. Pediatrics. 1997;100:403.
Pathak A., Sharma B.S., Mathuriya S.N., et al. Controversies in the management of subdural empyema. A study of 41 cases with review of literature. Acta Neurochir (Wien). 1990;102:25.
Pelton S.I., Huot H., Finkelstein J.A., et al. Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468.
Pelton S.I., Klein J.O. The future of pneumococcal conjugate vaccines for prevention of pneumococcal diseases in infants and children. Pediatrics. 2002;110:805.
Pfister H.W., Borasio G.D., Dirnagl U., et al. Cerebrovascular complications of bacterial meningitis in adults. Neurology. 1992;42:1497.
Pfister L.A., Tureen J.H., Shaw S., et al. Endothelin inhibition improves cerebral blood flow and is neuroprotective in pneumococcal meningitis. Ann Neurol. 2000;47:329.
Pollard A.J., Levin M. Vaccines for prevention of meningococcal disease. Pediatr Infect Dis J. 2000;19:333.
Pon R.A., Lussier M., Yang Q.L., et al. N-propionylated group B meningococcal polysaccharide mimics a unique bactericidal capsular epitope in group B Neisseria meningitidis. J Exp Med. 1997;185:1929.
Powell K.R., Sugarman L.I., Eskenazi A.E., et al. Normalization of plasma arginine vasopressin concentrations when children with meningitis are given maintenance plus replacement fluid therapy. J Pediatr. 1990;117:515.
Puopolo K.M., Madoff L.C., Eichenwald E.C. Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics. 2005;115:1240.
Quagliarello V.J., Long W.J., Scheld W.M. Morphologic alterations of the blood-brain barrier with experimental meningitis in the rat: Temporal sequence and role of encapsulation. J Clin Invest. 1986;77:1084.
Quraishi H., Zevallos J.P. Subdural empyema as a complication of sinusitis in the pediatric population. Intern J Pediatr Otolarng. 2006;70:1581.
Rajajee S., Shankar J., Dhattatri L. Pediatric presentations of leptospirosis. Indian J Pediatr. 2002;69:851.
Rajasingham C.R., Bonsu B.K., Chapman J.I., et al. Serious neurologic sequelae in cases of meningitis arising from infection by conjugate vaccine-related and nonvaccine-related serogroups of Streptococcus pneumoniae. Pediatr Infect Dis J. 2008;27:771.
Rappuoli R. Reverse vaccinology, a genome-based approach to vaccine development. Vaccine. 2001;19:2688.
Reddy J.S., Mishra A.M., Behari S., et al. The role of diffusion-weighted imaging in the differential diagnosis of intracranial cystic mass lesions: a report of 147 lesions. Surg Neurol. 2006;66:246.
Reefhuis J., Honein M.A., Whitney C.G., et al. Risk of bacterial meningitis in children with cochlear implants. N Engl J Med. 2003;349:435.
Reichler M.R., Allphin A.A., Breimann R.F., et al. The spread of multiply-resistant Streptococcus pneumoniae at a day care center in Ohio. J Infect Dis. 1992;166:1346.
Renier D., Flandin C., Hirsch E., et al. Brain abscess in neonates: A study of 30 cases. J Neurosurg. 1988;69:877.
Richards P.G., Towu-Aghantse E. Dangers of lumbar puncture. BMJ. 1986;292:605.
Riordan F.F., Thomson A.P., Ratcliffe J.M., et al. Admission cortisol and adrenocorticotrophic hormone levels in children with meningococcal disease: Evidence of adrenal insufficiency? Crit Care Med. 1999;27:2257.
Roine I., Peltola H., Fernández J., et al. Influence of admission findings on death and neurological outcome from childhood bacterial meningitis. Clin Infect Dis. 2008;46:1245.
Rolain J.M., Brouqui P., Koehler J.E., et al. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother. 2004;48:1921.
Ronan A., Hogg G.G., Klug G.L. Cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J. 1995;14:782.
Rosenfeld E.A., Rowley A.H. Infectious intracranial complications of sinusitis, other than meningitis, in children: 12-Year review. Clin Infect Dis. 1994;18:750.
Rotbart H.A., Glode M.P. Haemophilus influenzae type b septic arthritis in children: Report of 23 cases. Pediatrics. 1985;75:75.
Rothermel H., Hedges T.R.3rd, Steere A.C. Optic neuropathy in children with Lyme disease. Pediatrics. 2001;108:477.
Runge V.M., Wells J.W., Williams N.M., et al. Detectability of early brain meningitis with magnetic resonance imaging. Invest Radiol. 1995;30:484.
Saez-Llorens X., Jafari H.S., Severien C., et al. Enhanced attenuation of meningeal inflammation and brain edema by concomitant administration of anti-CD18 monoclonal antibodies and dexamethasone in experimental Haemophilus meningitis. J Clin Invest. 1991;88:2003.
Saez-Llorens X., Mccoig C., Feris J.M., et al. Quinolone treatment for pediatric bacterial meningitis: A comparative study of trovafloxacin and ceftriaxone with or without vancomycin. Pediatr Infect Dis J. 2002;21:14.
Sakata H., Toyonaga Y., Sato Y., et al. Nationwide survey of the development of drug-resistance in the pediatric field: drug sensitivity of Haemophilus influenzae in Japan. J Infect Chemother. 2009;15:402.
Sakoulas G. Brainstem and striatal encephalitis complicating Mycoplasma pneumoniae pneumonia: Possible benefit of intravenous immunoglobulin. Pediatr Infect Dis J. 2001;20:543.
Salleras L., Dominguez A., Prats G., et al. Dramatic decline of serogroup C meningococcal disease incidence in Catalonia (Spain) 24 months after a mass vaccination programme of children and young people. J Epidemiol Community Health. 2001;55:283.
Schaad U.B. Current concepts of bacterial meningitis. Eur J Pediatr. 1995;154:S20.
Schaad U.B. Etiology and management of neonatal bacterial meningitis. Antibiot Chemother. 1992;45:192.
Schaad U.B. Management of bacterial meningitis in childhood. Rev Med Microbiol. 1997;8:171.
Schaad U.B., Lips U., Gnehm H.E., et al. Dexamethasone therapy for bacterial meningitis in children. Swiss Meningitis Study Group. Lancet. 1993;342:457.
Scheld W.M., Dacey R.G., Winn H.R., et al. Cerebrospinal fluid outflow resistance in rabbits with experimental meningitis: Alterations with penicillin and methylprednisolone. J Clin Invest. 1980;66:243.
Scheld W.M., Fletcher D.D., Fink F.N., et al. Response to therapy in an experimental rabbit model of meningitis due to Listeria monocytogenes. J Infect Dis. 1979;140:287.
Schick B., Draf W., Kahle G., et al. Occult malformations of the skull base. Arch Otolaryngol Head Neck Surg. 1997;123:77.
Schoeman J.F., Van Zyl L.E., Laubscher J.A., et al. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics. 1997;99:226.
Schoeman J., Wait J., Burger M., et al. Long-term follow up of childhood tuberculous meningitis. Dev Med Child Neurol. 2002;44:522.
Schrag S.J., Zywicki S., Farley M.M., et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342:15.
Schuchat A., Robinson K., Wenger J.D., et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970.
Shapiro E.D., Aaron N.H., Wald E.R., et al. Risk factors for development of bacterial meningitis among children with occult bacteremia. J Pediatr. 1986;109:15.
Sheehan J.P., Jane J.A.Jr, Ray D.K., et al. Brain abscess in children. Neurosurg Focus. 2008;24:E6.
Shingadia D., Novelli V. Diagnosis and treatment of tuberculosis in children. Lancet Infect Dis. 2003;3:624.
Skogman B.H., Croner S., Nordwall M., et al. Lyme neuroborreliosis in chidren – a prospective study of clinical features, prognosis and outcome. Pediatr Infect Dis J. 2008;27:1089.
Slade W.R., Lonano F. Acute spinal epidural abscess. J Natl Med Assoc. 1990;82:713.
Smith R.R., Caldemeyer K.S. Neuroradiologic review of intracranial infection. Curr Probl Diagn Radiol. 1999;28:1.
Smith W.C.S., Anderson A.M., Withingtoon S., et al. Steroid prophylaxis for the prevention of nerve function impairment in leprosy: randomized, placebo controlled trial (TRIPOD 1). BMJ. 2004. doi:10.1136/bmj.38107.645926.AE(published 24 May 2004)
Snedeker J.D., Kaplan S.L., Dodge P.R., et al. Subdural effusion and its relationship with neurologic sequelae of bacterial meningitis in infancy: A prospective study. Pediatrics. 1990;86:163.
Soares de Lima C., Zulianello L., de Melo Marques MÂ, et al. Mapping the laminin-binding and adhesive domain of the cell surface-associated Hlp/LBP protein from Mycobacterium leprae. Microbes Infect. 2005;7:1097.
Starke J.R. Tuberculosis of the central nervous system in children. Semin Pediatr Neurol. 1999;6:318.
Stevens J.P., Eames M., Kent A., et al. Long term outcome of neonatal meningitis. Arch Dis Child Fetal Neonatal Ed. 2003;88:F179.
Strafford M.A., Wilde R.T., Berde M.C. The risk of infection from epidural analgesia in children: A review of 1620 cases. Anaesth Analg. 1995;80:234.
Suputtamongkol Y., Niwattayakul K., Suttinont C., et al. An open, randomized controlled trial of penicillin, doxycycline and cefotaxime for patients with severe leptospirosis. Clin Infect Dis. 2004;39:1417.
Syrogiannopoulos G.A., Nelson J.D., McCracken G.H.Jr. Subdural collections of fluid in acute bacterial meningitis: A review of 136 cases. Pediatr Infect Dis. 1986;5:343.
Tapinos N., Ohnishi M., Rambukkana A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat Med. 2006;12:961.
Täuber M.G., Khayam-Bashi H., Sande M.A. Effects of ampicillin and corticosteroids on brain water content, cerebrospinal fluid pressure, and cerebrospinal fluid lactate levels in experimental pneumococcal meningitis. J Infect Dis. 1985;151:528.
Täuber M.G., Sande E., Fournier M.A., et al. Fluid administration, brain edema, and cerebrospinal fluid lactate and glucose concentrations in experimental Escherichia coli meningitis. J Infect Dis. 1993;168:473.
Täuber M.G., Sande M.A. General principles of therapy of pyogenic meningitis. Infect Dis Clin North Am. 1990;4:661.
Tekkok I.H., Erbengi A. Management of brain abscess in children: Review of 130 cases over a period of 21 years. Childs Nerv Syst. 1992;8:411.
Thwaites G.E., Nguyen D.B., Nguyen H.D., et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741-1751.
Thompson M.J., Ninis N., Perera R., et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367:397.
Tsai C.J., Griffin M.R., Nuorti J.P., et al. Changing epidemiology of pneumococcal meningitis after the introduction of conjugate pneumococcal vaccine in the United States. Clin Infect Dis. 2008;46:1664-1672.
Tung Y.R., Lai M.C., Lui C.C., et al. Tuberculous meningitis in infancy. Pediatr Neurol. 2002;27:262.
Tunkel A.R., Hartmann B.J., Kaplan S.L., et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267.
Tunkel A.R., Scheld W.M. Acute bacterial meningitis. Lancet. 1995;346:1675.
Tureen J.H., Täuber M.G., Sande M.A. Effect of hydration status on cerebral blood flow and cerebrospinal fluid lactic acidosis in rabbits with experimental meningitis. J Clin Invest. 1992;89:947.
Van de Beek D., de Gans J., McIntyre P., et al. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. (1):2007. Art No.: CD004405. DOI:10.1002/14651858.CD004405.pub2
Van Deuren M., Brandtzaeg P., van der Meer J.W. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev. 2000;13:144.
Vinchon M., Lemaitre M.P., Vallee L., et al. Late shunt infection: Incidence, pathogenesis, and therapeutic implications. Neuropediatrics. 2002;33:169.
Waagner D.C., Kennedy W.A., Hoyt M.J., et al. Lack of adverse effects of dexamethasone therapy in aseptic meningitis. Pediatr Infect Dis J. 1990;9:922.
Walker D.G., Walker G.J. Forgotten but not gone: The continuing scourge of congenital syphilis. Lancet Infect Dis. 2002;2:432.
Ward J.I., Lum M.K., Hall D.B., et al. Invasive Haemophilus influenzae type b disease in Alaska: Background epidemiology for a vaccine efficacy trial. J Infect Dis. 1986;153:17.
Weingarten K., Zimmerman R.D., Becker R.D., et al. Subdural and epidural empyemas: MR imaging. Am J Roentgenol. 1989;152:615.
Wellman M.B., Sommer D.D., Mckenna J. Sensorineural hearing loss in postmeningitic children. Otol Neurotol. 2003;24:907.
Wheeler S.W., Wolf S.M., Seinberg E.A. Cat-Schrach encephalopathy. Neurology. 1997;49:876.
Whitney C.G. The potential of pneumococcal conjugate vaccines for children. Pediatr Infect Dis J. 2002;21:961.
Wilke M., Eiffert H., Christen H.J., et al. Primarily chronic and cerebrovascular course of Lyme neuroborreliosis: Case reports and literature review. Arch Dis Child. 2000;83:67.
Winkler F., Kastenbauer S., Yousry T.A., et al. Discrepancies between brain CT imaging and severely raised intracranial pressure proven by ventriculostomy in adults with pneumococcal meningitis. J Neurol. 2002;249:1292.
Wong T., Lee L., Wang H., et al. Brain abscesses in children – a cooperative study of 83 cases. Childs Nerv Syst. 1989;5:19.
Woods C.R. Syphilis in children: Congenital and acquired. Semin Pediatr Infect Dis. 2005;16:245.
Workowski K.A., Berman S.M. CDC sexually transmitted diseases treatment guidelines. Clin Infect Dis. 2002;35:S135.
Yaramis A., Gurkan F., Elevli M., et al. Central nervous system tuberculosis in children: A review of 214 cases. Pediatrics. 1998;102:E49.
Yung A.P., McDonald M.I. Early clinical clues to meningococcaemia. Med J Aust. 2003;178:134.
Zangwill K.M., Schuchat A., Wenger J.D. Group B streptococcal disease in the United States, 1990: Report from a multistate active surveillance system. MMWR CDC Surveill Summ. 1992;41:25.
Ziebuhr W., Dietrich K., Trautmann M., et al. Chromosomal rearrangements affecting biofilm production and antibiotic resistance in a Staphylococcus epidermidis strain causing shunt-associated ventriculitis. Int J Med Microbiol. 2000;290:115.