Bacillus and Similar Organisms
1. Describe the general characteristics of B. anthracis, including colonial morphology and Gram stain appearance.
2. State the location of the organisms in the natural environment, and list the modes of transmission as they relate to human infections.
3. Describe the three forms of B. anthracis infection, including source, route of transmission, signs, and symptoms.
4. Summarize the types of infections associated with B. cereus.
5. Outline the laboratory tests utilized to differentiate B. anthracis from other Bacillus species.
6. State the culture media used to differentiate Bacillus spp., and include the chemical principle and interpretation.
7. Summarize the approach to species differentiation within the genera Bacillus, Brevibacillus, and Paenibacillus.
8. Indicate the appropriate therapy for B. anthracis infection.
General Characteristics
Bacillus Anthracis
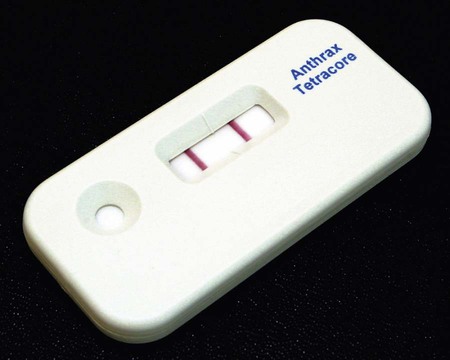
Clinical microbiologists are sentinels for recognition of a bioterrorist event, especially involving microorganisms such as B. anthracis. Even though this organism is rarely found, sentinel laboratory protocols require ruling out the possibility of anthrax before reporting any blood, CSF, or wound cultures in which a large gram-positive aerobic rod is isolated. During the 2001 terrorist attacks on the United States, the index case associated with the anthrax distribution was discovered by an astute clinical microbiologist who identified large gram-positive rods in a patient’s cerebrospinal fluid. B. anthracis should be suspected if typical nonhemolytic “Medusa head” or ground glass colonies are observed on 5% sheep blood agar. The Red Line Alert Test (Tetracore, Inc., Gaithersburg, Maryland) is a Food and Drug Administration (FDA)-cleared immunochromatographic test that presumptively identifies B. anthracis from blood agar (Figure 16-1). The sentinel laboratory anthrax protocol was revised in 2005 and again in 2010 to use FDA-cleared tests in order to rule out nonhemolytic, nonmotile Bacillus spp. as potential isolates of B. anthracis.
Epidemiology
Anthrax remains the most widely recognized bacillus in clinical microbiology laboratories. It is primarily a disease of wild and domestic animals including sheep, goats, horses, and cattle. The decline in animal and human infections is a result of the development of veterinary and human vaccines as well as improvements in industrial applications for handing and importing animal products. The organism is normally found in the soil and primarily causes disease in herbivores. Humans acquire infections when inoculated with the spores, either by traumatic introduction, ingestion, or inhalation during exposure to contaminated animal products, such as hides (Table 16-1). Bacillus anthracis produces endospores, which are highly resistant to heat and desiccation. The spores remain viable in a dormant state until they are deposited in a suitable environment for growth, including moisture, temperature, oxygenation, and nutrient availability. Because of the ability to survive harsh environments, infectiousness, ease of aerosol dissemination, and high mortality rate, the spores may be effectively used as an agent of biologic warfare (see Chapter 80 for additional information).
TABLE 16-1
| Species | Habitat (Reservoir) | Mode of Transmission |
| Bacillus anthracis | Soil: contracted by various herbivores | Direct contact: animal tissue or products such as wool or hair (infecting organisms) Trauma or insect bites: organisms or spores Inhalation: spores; Woolsorters’ disease Ingestion: contaminated meat Person-to-person transmission has not been documented |
| Bacillus cereus, Bacillus circulans, Bacillus licheniformis, Bacillus subtilis, other Bacillus spp., Brevibacillus sp., and Paenibacillus spp. | Vegetative cells and spores ubiquitous in nature; may transiently colonize skin or the gastrointestinal or respiratory tracts | Trauma Associated with immunocompromised patients Ingestion of food (rice) contaminated with B. cereus or toxins formed by this organism |
Pathogenesis and Spectrum of Disease
B. anthracis is the most highly virulent species for humans and is the causative agent of anthrax. The three forms of disease are cutaneous, gastrointestinal (ingestion), and pulmonary (inhalation) or woolsorters’ disease (Table 16-2). The cutaneous form accounts for most human infections and is associated with contact with infected animal products. Infection results from close contact and inoculation of endospores through a break in the skin. Following inoculation and incubation period of approximately 2 to 6 days in most cases, a small papule appears that progresses to a ring of vesicles. The vesicles then develop into an ulceration. The typical presentation is of a black, necrotic lesion known as an eschar. The mortality rate for untreated cutaneous anthrax is low, approximately 1%.
TABLE 16-2
Pathogenesis and Spectrum of Disease
| Species | Virulence Factors | Spectrum of Diseases and Infections |
| Bacillus anthracis | Capsule exotoxins (edema toxin and lethal toxin) swelling and tissue death | Causative agent of anthrax, of which there are three forms: Cutaneous anthrax occurs at site of spore penetration 2 to 5 days after exposure and is manifested by progressive stages from an erythematous papule to ulceration and finally to formation of a black scar (i.e., eschar); may progress to toxemia and death Pulmonary anthrax, also known as woolsorters’ disease, follows inhalation of spores and progresses from malaise with mild fever and nonproductive cough to respiratory distress, massive chest edema, cyanosis, and death Gastrointestinal anthrax may follow ingestion of spores and affects either the oropharyngeal or the abdominal area; most patients die from toxemia and overwhelming sepsis |
| Bacillus cereus | Enterotoxins and pyogenic toxin | Food poisoning of two types: diarrheal type, characterized by abdominal pain and watery diarrhea, and emetic type, which is manifested by profuse vomiting; B. cereus is the most commonly encountered species of Bacillus in opportunistic infections including posttraumatic eye infections, endocarditis, and bacteremia; infections of other sites are rare and usually involve intravenous drug abusers or immunocompromised patients |
| Bacillus circulans, Bacillus licheniformis, Bacillus subtilis, other Bacillus spp., Brevibacillus sp., and Paenibacillus spp. | Virulence factors unknown | Food poisoning has been associated with some species but is uncommon; these organisms may also be involved in opportunistic infections similar to those described for B. cereus |
Laboratory Diagnosis
Specimen Processing
With few exceptions, special processing considerations are not required. The organisms are capable of survival in fresh clinical specimens and standard transport medium. Refer to Table 5-1 for general information on specimen processing.
Direct Detection Methods
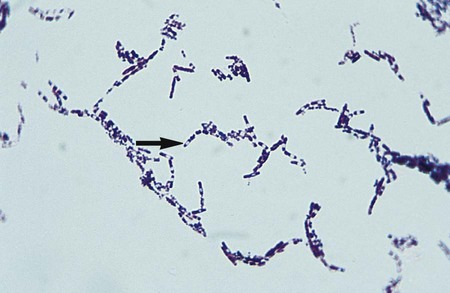
The Gram stain is the only specific procedure for the direct detection of Bacillus spp. in clinical specimens. Microscopically the organisms appear as large gram-positive rods in singles, pairs, or serpentine changes (Figure 16-3).
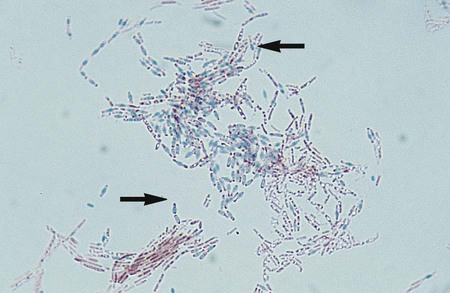
Bacillus spp. are the only clinically relevant aerobic organisms capable of producing endospores in the presence of oxygen. Sporulation is inhibited by high concentrations of CO2. The production of spores may be induced by growth in triple sugar iron (TSI), urea, or nutrient agar containing 5 mg/L manganese sulfate. Spores may appear as intra or extracellular clear oval structures upon Gram staining. Special staining is required in order to visualize endospores. The smear is covered with malachite green, and a piece of filter paper is placed over the stain. The microscope slide is then heated for several minutes to force the dye into the cell walls of the spore. During the heating process, it is important to keep the filter paper moist so that the stain is steamed rather than baked into the endospores. A safranin counterstain follows the primary stain. The endospores stain green and the vegetative cells will appear pink from the secondary stain, safranin (Figure 16-4).
Cultivation
Media of Choice
Colonial Appearance
Table 16-3 describes the colonial appearance on blood agar and other distinguishing characteristics (e.g., hemolysis) of each species of Bacillus or related genera. Colonies of B. anthracis growing on bicarbonate agar appear large and mucoid.
TABLE 16-3
Colonial Appearance and Other Characteristics
| Organism | Appearance on 5% Sheep Blood Agar |
| B. anthracis | Medium-large, gray, flat, irregular with swirling projections (“Medusa head”) or ground glass appearance; nonhemolytic |
| B. cereus and B. thuringiensis | Large, feathery, spreading; beta-hemolytic |
| B. mycoides | Rhizoid colony that resembles a fungus; weakly beta-hemolytic |
| B. megaterium | Large, convex, entire, moist; nonhemolytic |
| B. licheniformis | Large blister colony; becomes opaque with dull to rough surface with age; beta-hemolytic |
| B. pumilus | Large, moist, blister colony; may be beta-hemolytic |
| B. subtilis | Large, flat, dull, with ground-glass appearance; may be pigmented (pink, yellow, orange, or brown); may be beta-hemolytic |
| B. circulans | Large, entire, convex, butyrous; smooth, translucent surface; may be beta-hemolytic |
| B. coagulans | Medium-large, entire, raised, butyrous, creamy-buff; may be beta-hemolytic |
| B. sphaericus | Large, convex, smooth, opaque, butyrous; nonhemolytic |
| Brevibacillus brevis | Medium-large, convex, circular, granular; may be beta-hemolytic |
| Paenibacillus macerans | Large, convex, fine granular surface; nonhemolytic |
| P. alvei | Swarms over agar surface; discrete colonies are large, circular, convex, smooth, glistening, translucent or opaque; may be beta-hemolytic |
| P. polymyxa | Large, moist blister colony with “ameboid spreading” in young cultures; older colonies wrinkled; nonhemolytic |
Approach to Identification
Commercial biochemical identification systems or molecular techniques may be used in clinical laboratories for identification of Bacillus spp. Species differentiation within the genera Bacillus, Brevibacillus, and Paenibacillus is based on the size of the vegetative cell, sporulation resulting in swelling of the vegetative cell, and biochemical analysis (Table 16-4), including the production of the enzyme lecithinase (see Figure 16-2).
TABLE 16-4
Differentiation of Clinically Relevant Bacillus spp., Brevibacillus, and Paenibacillus
| Organism | Bacillary Body Width >1 µm | Wide Zone Lecithinase | Spores Swell Sporangium | Voges Proskauer | Glucose with Gas | Fermentation of: | Citrate | Indole | Motility | Parasporal Crystals | ||
| Mannitol | Xylose | Anaerobic Growth | ||||||||||
| Bacillus anthracis | + | + | – | + | – | – | – | + | v | – | – | – |
| B. cereus | + | + | – | + | – | – | – | + | + | – | + | – |
| B. thuringiensis | + | + | – | + | – | – | – | + | + | – | + | + |
| B. mycoides | + | + | – | + | – | – | – | + | + | – | – | – |
| B. megaterium | + | – | – | – | – | + or (+) | v | – | + | – | + | – |
| B. licheniformis | – | – | – | + | – | + | + | + | + | – | + | – |
| B. pumilus | – | – | – | + | – | + | + | – | + | – | + | – |
| B. subtilis | – | – | – | + | – | + | + | – | + | – | + | – |
| B. circulans | – | – | + | – | – | + | + | v | – | – | + | – |
| B. coagulans | – | – | v | v | – | – | v | + | v | – | + | – |
| Brevibacillus brevis | – | – | + | – | – | + | – | – | v | – | + | v |
| Paenibacillus macerans | – | –* | + | – | + | + | + | + | – | – | + | – |
| P. alvei | – | – | + | + | – | – | – | + | – | + | + | – |
| P. polymyxa | – | –* | + | + | + | v | + | + | – | – | + | – |
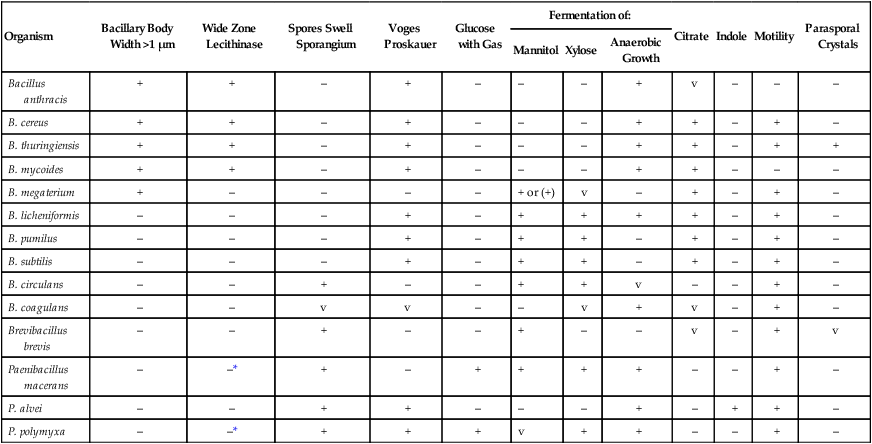
+, 90% or more of species or strains are positive; –, 90% or more of species or strains are negative, v, variable reactions; ( ), reactions may be delayed.
Compiled from Drobniewski FA: Bacillus cereus and related species, Clin Microbiol Rev 6:324, 1993; Logan NA, Turnbull PC: Bacillus and other aerobic endospore-forming bacteria. In Murray PR, Baron EJ, Jorgensen JH, et al, editors: Manual of clinical microbiology, ed 10, Washington, DC, 2011, ASM Press; and Parry JM, Turnbull PC, Gibson JR: A colour atlas of Bacillus species, London, 1983, Wolf Medical Publications.
Antimicrobial Susceptibility Testing and Therapy
Although ciprofloxacin has been established as the preferred therapy for anthrax, the infrequent nature with which other species are encountered limits recommendations concerning therapy (Table 16-5). Nonetheless, the threat of bioterrorism has spawned interest in the development of in vitro testing of antimicrobial agents against B. anthracis. The Clinical and Laboratory Standards Institute (CLSI) document M100 addresses the technical issues required for antimicrobial sensitivity testing for Bacillus spp. Most other Bacillus spp. will grow on the media under the conditions recommended for testing the common organisms encountered in clinical specimens (see Chapter 12 for more information regarding validated testing methods), and technical information regarding the testing of the additional species is provided in CLSI document M45, “Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria.” Careful evaluation of the organism’s clinical significance must be established before extensive antimicrobial susceptibility testing efforts are undertaken.
TABLE 16-5
Antimicrobial Therapy and Susceptibility Testing
| Species | Therapeutic Options | Resistance to Therapeutic Options | Validated Testing Methods* | Comments |
| Bacillus anthracis | Ciprofloxacin or doxycycline plus one or two other antibiotics; other agents with in vitro activity include rifampin, vancomycin, penicillin, ampicillin, chloramphenicol, imipenem, clindamycin, and clarithromycin | Beta-lactamases | See CLSI document M100-S22; performed in approved reference laboratories only | |
| Other Bacillus spp., Brevibacillus sp., Paenibacillus spp. | No definitive guidelines; vancomycin, ciprofloxacin, imipenem, and aminoglycosides may be effective | B. cereus frequently produces beta-lactamase | See CLSI document M45; methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria | Whenever isolated from clinical specimens, the potential for the isolate to be a contaminant must be strongly considered |
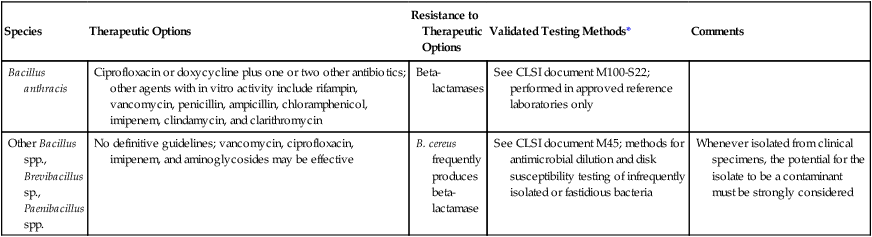
*Validated testing methods include those standard methods recommended by the Clinical Laboratory Science Institute (CLSI) and those commercial methods approved by the Food and Drug Administration (FDA).
1. The virulence factor associated with B. cereus is:
2. Pulmonary anthrax is also known as:
3. A large, aerobic, gram-positive, spore-forming rod is isolated from a blood culture. It can be further confirmed as B. anthracis if it is:
4. A large, aerobic, beta-hemolytic, gram-positive rod is isolated from an eye culture. Subsequent testing reveals it is motile and produces a wide zone on egg yolk agar. The most likely identification of this organism is:
5. The most appropriate therapy for inhalation anthrax is:
_____ Bacillus species are the only organisms to produce spores in the presence of oxygen.
_____ Bacillus species are rarely found to be laboratory contaminants.
_____ B. cereus is resistant to penicillin.
_____ Rapid tests for the presumptive identification of B. anthracis are not available.
_____ Vegetative cell size is used to differentiate species with the genera Bacillus and Paenibacillus.
_____ Bacillus spp. grow on sheep blood agar and phenylethyl alcohol agar.