CHAPTER 22 Avoidance of Complications in Neurosurgery
Avoidance of complications in neurosurgery begins with the correct selection of patients who are likely to benefit from the surgical intervention planned. When possible, patients with nonmedical issues that have a known association with poor outcomes, such as workers’ compensation claims or pending lawsuits, should be investigated further to determine the patient’s motivation for recovery.1–7 Taking the time to explain the probable risks and benefits of the procedure allows the patient to make an informed decision and protects the surgeon in the event of an adverse outcome from claims of inadequate consent. The remainder of this chapter focuses on prevention of complications once the patient has arrived in the operating room. Intraoperative complications may be related to anesthetic issues, positioning of the patient, or technical or anatomic aspects of the specific surgery selected.
Before induction of anesthesia, the surgeon and anesthesiologist must discuss the case in detail and review what is likely to happen and the possible risks. Ideally, an experienced neuroanesthesiologist should be available for neurosurgical procedures. Adequate venous access, placement of a single- or double-lumen tube as required by the surgical approach, and insertion of an intracardiac central venous pressure line to potentially remove air emboli must be planned in advance. The presence of blood products in proximity to the operating room and notification of the blood bank that more may be required depend on the scope of the surgical procedure. Antibiotics should be administered within 1 hour before incisions to ensure therapeutic blood levels.8
Complications Related to Patient Positioning
After the anesthesiologist has determined that the airway has been adequately secured and that all lines and monitoring equipment are in place, the patient is ready to be positioned. Several common positioning errors can lead to complications,9–21 but most can be prevented with meticulous positioning protocols.
Prone Positioning
Nerve palsies and compression injuries are the most frequent complications seen and the most easily preventable. Radial and ulnar neuropathies can occur as a result of positioning the patient in the prone position with the arms extended if padding is inadequate or an inappropriate position is used. Keeping the arms in a mildly flexed position prevents excessive traction in either direction. Padding may be in the form of sheets or blankets placed under the elbows and forearms, or egg-crate foam padding can be used. Brachial plexus injuries can occur with rostral or caudal traction on the shoulders22 and is frequently seen in the prone position when the arms are extended in the cruciate position or too far above the head. Downward traction, such as when the shoulders need to be pulled down for x-ray localization in the low cervical or cervicothoracic junction, can also cause brachial plexus injury. If possible, any tension placed on the patient’s shoulders during radiography should be removed after the x-ray film has been obtained. Neurophysiologic monitoring of the ulnar nerve with somatosensory evoked potentials during spinal procedures has been shown to be effective in correcting and preventing position-related stretch injuries to the brachial plexus.23,24 Another common peripheral neuropathy associated with the prone position is inadequate padding of the anterior superior iliac crest, which can lead to pain or numbness in the distribution of the lateral femoral cutaneous nerve.25 A rare complication is obstruction of the external iliac artery or femoral artery from prolonged compression in the inguinal region.26,27
Another difficulty with positioning for spine surgery is the difference between the ideal position for a decompressive procedure, with the spine and hips flexed, and that for spinal fusion, with the spine in a more lordotic position and the hips and spine in neutral positions. Many patients have been subjected to iatrogenic flat-back syndrome because of improper position during a fusion procedure.28
Surgeons must be aware of the potential for unilateral or bilateral blindness after prolonged prone surgery. Causes have been hypothesized to be occlusion of the retinal artery or vein, direct trauma, orbital compartment syndrome, and ischemic optic neuropathy. Although rare, devastating complications have been described even when no direct trauma occurred, and therefore patients’ eyes should be checked frequently during the procedure. Minimizing blood loss and hypotensive episodes and maintaining a slightly elevated head of the bed may reduce the chance for this complication. If orbital compartment syndrome is suspected, emergency orbital decompression is the best chance for recovery.29–33
Lateral Positioning
The lateral or three-quarters lateral decubitus position carries with it specific risks for peripheral nerve injuries. Stretch on the brachial plexus can be prevented by placement of an axillary roll slightly thicker than the diameter of the upper part of the arm. This roll should be placed approximately four fingerbreadths below the armpit to prevent compression of the long thoracic nerve. Failure to place an adequately sized roll may lead to excessive stretch of the brachial plexus, with the greatest effects on the C5 and C6 nerve roots. The upper extremities need to be supported in relatively neutral positions to prevent ulnar neuropathies. Horner’s syndrome can occur when the head is inadequately padded and allowed to hang laterally in such a manner that excessive tension is placed on the superior cervical ganglion.34 Excessive traction on the lateral femoral cutaneous nerve can be caused by undue extension of the upper part of the leg at the hip while bending the dependent leg. Compression of the common peroneal nerve can occur as a result of inadequate padding laterally under the knee.
Intraoperative Monitoring
Various electrophysiologic modalities can be used to detect subtle signs of neurological compromise before they become fixed deficits. The use of intraoperative monitoring can reduce the likelihood of significant neurological deficits in the appropriate circumstances. Some positioning complications can be avoided with the concomitant use of intraoperative monitoring.9,35–39 At our institution, we use motor evoked potentials or somatosensory evoked potentials before and after positioning that may result in injury to the cervical cord. We have found excellent correlation between the lack of changes in evoked potentials and patient outcome. Monitoring is not necessary or indicated in all cases because it is time-consuming, can cause inappropriate movement of the patient, results in bleeding, and has the potential for needlestick injury to the operating room staff. However, in procedures with a potential for significant risk to the cord or neural structures, neurological monitoring is a helpful adjunct to the surgeon. Electrophysiologic neurological monitoring can consist of somatosensory evoked potentials, motor evoked potentials, intraoperative electromyographic responses, nerve action potential monitoring, direct spinal cord stimulation, and other methods.36,37,40–44 The information gleaned from these modalities can be used to determine whether manipulation of the neural elements is compromising conduction. Numerous authors have published series in which the surgeon has changed some portion of the procedure as a reaction to changes in electrophysiologic monitoring.9,35–39,43–48 Changes in ulnar nerve somatosensory evoked potentials can also indicate traction injury to the brachial plexus and is increasingly being used to monitor positioning, even with lumbar and thoracic procedures.23,24
Although not appropriate to monitor for position-related changes, direct epidural electrode motor evoked potential monitoring provides real-time evaluation of the spinal motor tracts and allows quantification of the measured output. This technique may be used during intramedullary spinal cord tumor resection and has been suggested to be helpful in minimizing injury during intramedullary resection.49
Cranial Fixation Complications
Pin site complications include lacerations,50 skull fractures, associated intracranial hemorrhage (i.e., epidural, subdural, or subarachnoid hemorrhages), and infections that can lead to osteomyelitis.51–56 Lacerations can be prevented by making sure that the two-pin arm swivels freely so that the force is evenly distributed between the two pins without one pin being shielded from tension, which can potentially result in pivoting on the other pin. If the pins are placed into muscle, it is wise to recheck tension on the single pin to make sure that the muscle has not settled and reduced the pressure. The three pins should be placed slightly below the center of gravity of the head when it is in final position to prevent gravity or personnel from pulling the head down and out of the pins. Ideally, the pins should not be placed directly into the coronal suture or temporal squamosal bone because these bones are most prone to fracture.57–59 Pins should be tightened to 60 to 80 lb in adults and 40 to 60 lb in children younger than 15 years. Pins are generally avoided in children younger than 2 years; however, some skull clamp systems do exist for these patients for procedures in which they are required.60
Other forms of head support include the horseshoe headrest and the four-cup headrest. Because the horseshoe headrest is not a rigid form of fixation, the head may shift during the procedure, and thus it is imperative that the anesthesiologist continuously observe for any signs of movement. The four-cup headrest is an excellent alternative to the horseshoe, although blindness, skin and scalp compression, and abnormal cervical motion are possible with either support. Alopecia has been reported as a result of scalp compression.61–67
Catastrophic Medical Complications
Venous Air Embolism
In positioning patients for neurosurgical procedures, the anesthesia team and the surgeons must be aware of the gradient between the patient’s head and the right atrium. Venous air embolism (VAE) is most often encountered with the patient in the seated position for posterior fossa surgery or cervical spine surgery.68–73 It has also been described in patients who have undergone procedures in the prone, supine, and lateral positions.68,71–77 Dehydration or blood loss leading to decreased central venous pressure may potentiate the risk for VAE. Patients with a patent foramen ovale or a known right-to-left shunt should be given special consideration before the seated position is used because the risk for paradoxical air embolism after VAE appears to be higher.
Given the dangers of VAE, early detection of the embolus is paramount in reducing the severity of this complication. Monitors used to detect emboli include precordial Doppler ultrasonography, capnography or mass spectrometry, transesophageal echocardiography, transcutaneous oxygen, esophageal stethoscope, and right heart catheter.68,69,71–74,77 The most sensitive are transesophageal echocardiography and Doppler, followed by expired nitrogen and end-tidal carbon dioxide. Electrocardiographic changes, hypotension, and heart murmurs are late signs. Because no single monitor is completely reliable, two or more should be used simultaneously. In awake patients, the presence of a cough may be the earliest sign of VAE, and it can be treated before the VAE becomes hemodynamically significant.78 Detection of VAE has increased over the past several decades, but serious morbidity and mortality have decreased. Its incidence varies from 1.2% to 60%, with morbidity and mortality rates of less than 3% in most series.
Deep Venous Thrombosis and Pulmonary Embolism
Deep venous thrombosis (DVT) and pulmonary embolism are major contributors to morbidity and mortality in postoperative neurosurgical patients. The incidence of DVT, as measured by the labeled fibrinogen technique, ranges from 29% to 43%.79–91 Most DVTs are asymptomatic and never come to medical attention. Pulmonary embolism, however, is thought to subsequently occur in 15% of such patients.80,84,89,92,93 Significant thrombi are thought to arise from the popliteal and iliofemoral veins. Risk factors include prolonged surgery and immobilization, previous DVT, malignancy, direct lower extremity trauma, limb weakness, use of oral contraceptives, gram-negative sepsis, advanced age, hypercoagulability, pregnancy, and congestive heart failure.79,81,83–89,92–100
Because of the often-fatal result of pulmonary embolism, prophylaxis against DVT is of major importance in neurosurgery. Many studies have confirmed the utility of sequential pneumatic leg compression devices in preventing DVT.80,81,83,84,89,90,101,102 These devices are placed on the patient preoperatively and should be continued until the patient is ambulatory. Early mobilization of postoperative patients is important in preventing thrombus formation. The prophylactic use of low-dose (minidose) subcutaneous heparin (e.g., 5000 IU twice daily) has been well studied over the past 25 years and has been demonstrated to be efficacious in preventing DVT.86,102–107 However, some studies have shown an increase in the rate of postoperative intracranial bleeding with minidose administration of heparin.102,103 Low-molecular-weight heparin (LMWH) has more recently been used for DVT prophylaxis in surgical patients. Several meta-analyses have been conducted, but it remains unclear whether unfractioned heparin or LMWH is superior for DVT prophylaxis in neurosurgical patients or whether increased efficacy correlates with increased hemorrhagic complications.108–111
Despite the use of such prophylactic methods, thrombi inevitably develop in one or both lower extremities in some patients. Management options include full-dose heparinization or inferior vena cava interruption. In the immediate and early postoperative period, many neurosurgeons believe that neurosurgical patients with documented DVT should undergo transvenous Greenfield filter placement.80,81,83,84,88,89,98,102 There appears to be a general consensus that full anticoagulation is acceptable 1 to 3 weeks after surgery; our institution uses the 1-week rule. Treatment with intravenous heparin (target partial thromboplastin time of 45 to 60 seconds) is followed by oral warfarin sulfate (target international normalized ratio of 2) when not contraindicated. Anticoagulation should be continued for 6 weeks to 3 months in uncomplicated cases. Gastrointestinal bleeding is the most common serious complication encountered.
Patients experiencing pulmonary embolism complain of pleuritic chest pain, hemoptysis, and dyspnea. Jugular venous distention, fever, rales, tachypnea, hypotension, and altered mental status may be found on physical examination. Arterial blood gas determination reveals a PO2 of less than 80 mm Hg in 85% of patients, accompanied by a widened alveolar-arterial gradient. The level of fibrin degradation products is elevated in most cases. In patients with massive embolism, right axis deviation, right ventricular strain, or right bundle branch block may be identified on electrocardiography. Chest radiography demonstrates an effusion or infiltrate in 90% of cases. A nuclear medicine ventilation-perfusion scan is sensitive in detecting pulmonary embolism but is not specific. The entire clinical scenario, including patient examination, laboratory results, and radiographic evaluation, leads to the diagnosis.80,84,89,93,112–116 Spiral computed tomography (CT) has become the preferred diagnostic study for pulmonary embolism.117 However, pulmonary angiography is the “gold standard” and may be necessary to confirm the diagnosis in as many as half of patients.
Wound Complications
Several potential problems related to the wound area and wound closure can be anticipated and prevented. The first category is postoperative blood collections, or hematomas. Ideally, postoperative hematomas can be prevented by meticulous hemostasis during the procedure, but such is not always the case. The use of postoperative drainage devices (e.g., Hemovac, Jackson-Pratt drain) in wounds for which hemostasis was difficult to achieve before closure can reduce the incidence of postoperative hematoma. Postoperative drainage may also be advantageous in patients in whom postoperative anticoagulation may be required because some of these patients have slightly delayed hematoma formation.118 An obese patient undergoing spine surgery may have significant serous exudation that can continue for up to 5 days or longer postoperatively. It is best to keep a drain in the submuscular space during this time to prevent a postoperative seroma that can become infected.
Risk Factors Related to Anatomy or Technique in Specific Surgeries
Cranial Surgery
Postoperative Seizures
The risk for postoperative seizures within the first week after supratentorial procedures has been well described in the literature.119–132 The underlying cause of these seizures may be metabolic derangements, cerebral hypoxia, preoperative structural defects, stroke and vascular abnormalities, or congenital seizure disorder. Manipulation of brain tissue, postoperative edema, and hematoma formation are common causes of surgically induced seizures. The overall incidence of immediate and early seizures after craniotomy is 4% to 19%.
It is important to identify any risk factors that may contribute to the development of seizures postoperatively. Lesions of the supratentorial intracranial compartment are responsible for seizures after craniotomy in most situations; seizures after infratentorial procedures are attributed to the resultant retraction or movement of supratentorial structures.119,120,124–129,131,133–136 Brain abscesses, hematomas, intra-axial and extra-axial tumors, aneurysms, arteriovenous malformations, and shunts have been reported to be epileptogenic.128,136–149 Patients with a preoperative history of epilepsy are at a higher risk for seizures postoperatively. Patients with subtherapeutic levels of prophylactic agents are also at a higher risk for immediate and early postoperative seizures.126,129,134,150–153
Preventing a seizure is preferable to treating one that has already begun. Adequate preoperative loading of parenteral or oral phenytoin has definitively been shown to decrease the incidence of postoperative seizures.154–156 In patients unable to tolerate phenytoin, phenobarbital or carbamazepine may be substituted. It follows that therapeutic preoperative levels should be measured in patients undergoing supratentorial procedures whenever possible. Administration of the anticonvulsant should continue through the acute and early postoperative period. Electrolyte abnormalities should be corrected immediately in the postoperative period to further reduce the chance for a seizure.
Reports have called into question the routine practice of phenytoin prophylaxis for patients without a history of seizures.126,152,157 How this will affect practices remains to be seen because a full medicoeconomic and medicolegal analysis is not yet available.
Postoperative Edema and Increased Intracranial Pressure
The neurological deficits caused by brain swelling may be permanent or transient, and the severity of the deficit depends on the patient. Edema usually begins within 5 hours after the procedure and reaches its maximum approximately 48 to 72 hours later.158–166 Altered mental status, cranial nerve deficits, and motor or sensory dysfunction can all occur. The diagnosis may be confirmed with non–contrast-enhanced CT, and hemorrhage, hydrocephalus, and pneumocephalus may be ruled out. Cerebral hypodensity, sulcal effacement, midline shift, loss of the gray-white matter interface, and small lateral ventricles are the hallmarks of postoperative edema. If impaired venous drainage secondary to the incompetence of venous sinuses is suspected, conventional venous-phase angiography or magnetic resonance venography may be helpful in diagnosing the location and severity of the occlusion. Appropriate surgical and medical measures may then be instituted.
The goal of treatment of increased ICP is to maintain cerebral perfusion pressure (CPP) at greater than 55 to 60 mm Hg while reducing the amount of cerebral edema.166–173 This entails measuring arterial blood pressure and ICP continuously. Induction of arterial hypertension with vasopressors may be necessary to achieve the desired CPP. Short-term hyperventilation to a PCO2 of 30 mm Hg can reduce ICP effectively. High-dose dexamethasone should be given to patients with vasogenic edema to alleviate tumor-related swelling. Increasing the head of the bed to 30 to 45 degrees can assist in venous return, and maintaining a neutral midline head position and administering diuretics such as furosemide and mannitol can further reduce ICP. When using diuretics, it is important that serum chemistries and osmolalities be monitored to ensure that the patient does not become severely dehydrated. Hypertonic saline solutions are now increasingly being used with success for the treatment of vasogenic edema.174,175 In refractory cases, sedation may be used to suppress cerebral metabolism and paralysis induced to reduce ICP by limiting agitation and muscle exertion. As a final resort, barbiturate coma with mild hypothermia or temporal lobectomy may be used to control ICP and maintain CPP.
Specific Cranial Disorders
Supratentorial Craniotomy
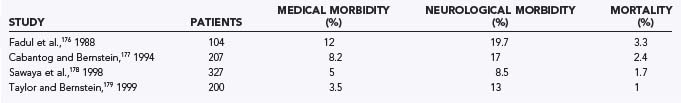
Numerous lesions may be approached via supratentorial craniotomy. In low-grade gliomas, long-term control and cure are possible. Because high-grade gliomas are not curable by surgery, surgery represents a palliative treatment aimed at reducing tumor bulk and maximizing quality of life. Patients with metastatic brain lesions can have a significant improvement in their survival by removal of brain metastases. It is therefore incumbent on neurosurgeons to minimize complications when patients are in the early stages of their disease and their clinical condition is best. The decision about whether surgery is warranted involves carefully weighing the possible surgical complications against the potential benefits. Studies have shown that craniotomies for intraparenchymal lesions typically result in mortality rates of 2.2% and morbidity rates of 15% (Table 22-1).176–179
Tumors located in eloquent or deep brain areas are more difficult to surgically debulk and carry a higher risk for neurological morbidity. Surgery on gliomas typically results in more morbidity and mortality than does surgery on brain metastases.178 Surgical outcome is closely tied to the patient’s age and preoperative neurological status as measured by the Karnofsky Performance Status score.176–178 Patients are at risk for general complications of craniotomies, including complications related to positioning, anesthesia, infection, seizures, hemorrhage, and neurological compromise. Neurological compromise may result from resection or retraction of normal functional brain tissue or compromise of the vascular supply. Neurological morbidities usually consist of motor or sensory deficits or aphasias (Table 22-2). Occasionally, visual field deficits can occur.
TABLE 22-2 Neurological Complications in Intraparenchymal Tumor Surgery
| COMPLICATION | RATE (%) |
|---|---|
| Motor or sensory deficit | 7.5 |
| Aphasia | 0.5 |
| Visual field deficit | 0.5 |
From Sawaya R, Hammoud M, Schoppa D. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044-1055.
Computer-assisted stereotactic systems enhance the ability of the surgeon to delineate between normal brain and tumor. Stereotactic systems also facilitate targeting of tumors that cannot be visualized at the brain’s surface. Intraoperative functional mapping helps identify and avoid injury to eloquent cortex. Craniotomy performed while the patient is awake is particularly helpful in resecting lesions surrounding the speech centers. Using an awake craniotomy technique, Taylor and Bernstein reported an overall complication rate of 16.5% and a mortality rate of 1%.179 New postoperative neurological deficits were seen in 13% of patients, but they were permanent in just 4.5%. Increasingly, functional imaging is being applied intraoperatively, with evidence suggesting that it allows more complete resection while minimizing the risk for deficits. Functional MRI and diffusion-weighted imaging can be integrated with most neuronavigation systems to allow identification and protection of motor tracts.180–183
Hemorrhage into the postoperative tumor bed represents a serious complication that may require reoperation for evacuation of hematoma. Prevention begins with checking preoperative coagulation studies and ensuring that the patient has not been taking an aspirin-containing product. Intraoperatively, meticulous hemostasis must be achieved with a variety of hemostatic agents and bipolar electrocautery. The tumor cavity may be lined with hemostatic agents such as Surgicel. Tight blood pressure control during extubation and in the postoperative period is important. Rarely, distal intracerebral or intracerebellar hemorrhages can occur, although their cause is unexplained.184
All patients undergoing surgery have a risk for thromboembolic events, but those with malignant gliomas are at significantly increased risk. Prophylaxis with low-dose heparin and external pneumatic leg muscle compression must be initiated promptly. The neurosurgical staff must maintain a high index of suspicion for phlebitis and pulmonary embolism so that treatment can be initiated early. Inferior vena cava filters may be placed to prevent the occurrence of pulmonary embolism. Full anticoagulation is preferable in patients seen more than 3 weeks after surgery.90,185
Wound infection and wound dehiscence occur rarely with craniotomies and develop in less than 1% of patients.178 The routine use of steroids in patients with intraparenchymal tumors apparently has little effect on overall wound healing.
Surgery for gliomas is rarely curative, and many patients with recurrences are subject to reoperation. However, studies have demonstrated that reoperation does not necessarily predispose patients to a greater complication rate.176,178,186
Meningiomas differ from parenchymal tumors in that they are often associated with venous sinuses, and thus venous infarction or injury to the sinuses is an additional risk. These tumors can invade the wall of sinuses and eventually narrow and obliterate the sinus lumen. When meningiomas are located in proximity to a sinus, preoperative venous angiography, magnetic resonance angiography, or magnetic resonance venography is essential to avoid complications. Entering a patent sinus can result in difficult bleeding that may require surgical reconstruction or bypass of the sinus. Sacrifice of a major venous sinus should be avoided. Complications associated with sacrificing a major venous sinus include increased ICP as a result of of brain edema and venous hemorrhagic infarction. Obtundation and seizures can develop in such patients (Table 22-3).187–189 Aggressive ICP management is essential in controlling this complication. Prudent surgical management may necessitate leaving a portion of the tumor adherent to the sinus and using adjuvant therapy or observation with surveillance MRI.190 Postoperative seizures are seen with convexity and parasagittal meningiomas.188 Sacrifice of a significant vein can result in venous infarction and an increased risk for seizures. The mortality rate for craniotomies performed for convexity and parasagittal meningiomas is 3.7% to 13%.188,191–198
Posterior Fossa Craniotomy
Leakage of CSF is seen frequently after a posterior fossa craniotomy and occurs in 3% to 15% of patients.199–201 Leakage can occur from the wound or be manifested as rhinorrhea or otorrhea. Openings into the mastoid air cells and air cells in the vicinity of the meatus can lead to otorrhea. Fluid can drain into the nasopharynx through the eustachian tube. Packing the mastoid air cells with bone wax can prevent CSF leakage. Aggressive drilling of the porus acusticus and larger tumor size have been associated with an increasing risk for CSF leaks.202 To minimize the risk for postoperative rhinorrhea, we apply bone wax aggressively to all mastoid air cells exposed during the craniectomy, as well as fibrin glue before closure. However, unroofing of air cells within the internal auditory canal can lead to persistent leakage, and we routinely apply a muscle plug, Gelfoam, and fibrin glue in this region to minimize the risk for leakage. In addition, routine prophylactic high-volume lumbar puncture may be performed daily for 3 days postoperatively to minimize the risk for leakage. When leakage occurs, management typically involves placement of a spinal drain. Operative repair may be necessary in patients who fail a trial of spinal drainage. Early recognition plus treatment of CSF leaks is imperative because CSF leakage places patients at risk for meningitis.201 Meningitis occurs in about 1% of patients, and early treatment with appropriate antibiotics is essential. Aseptic meningitis also occurs infrequently after surgery. Patients may have some elements of ataxia postoperatively, but these symptoms are usually limited and resolve within a few days. Significant headaches occur in half of patients postoperatively, and 25% complain of headaches persisting for more than a year after surgery.203
Mortality from infratentorial surgery is generally higher than that seen with supratentorial procedures. Compromise of the anterior inferior cerebellar artery and the resultant lateral pontine infarction are implicated in a third of postoperative deaths.204 The second biggest contributor to postoperative mortality is aspiration pneumonia resulting from lower cranial nerve deficits. Patients may require placement of a feeding tube and a tracheostomy to prevent aspiration pneumonia. Cerebellar contusions or hematomas can occur as a result of overaggressive retraction. Distant supratentorial hemorrhages occasionally occur for unclear reasons.205 Surgeons must be prepared to place an occipital ventricular catheter intraoperatively on an emergency basis if acute hydrocephalus results.
Transsphenoidal Surgery
Transsphenoidal surgery is commonly used to reach tumors in the sellar region. This procedure can be performed with extremely low mortality and low morbidity. Deaths have occurred in 0% to 1.75% of patients (Table 22-4). Laws206 reported 7 deaths in 786 procedures (0.9%), and Wilson207 reported 2 deaths (0.2%) in a series of 1000 patients. At our institution, 2 deaths occurred in 1800 procedures. Morbidities associated with the transsphenoidal approach are distinct from general neurosurgical complications because the approach is quite different from most transcranial approaches.
TABLE 22-4 Common Complication Rates in Transsphenoidal Surgery
| COMPLICATION | RATE (%) |
|---|---|
| Mortality | 0-1.75 |
| Nasal septum perforation | 1-3 |
| Sinusitis | 1-4 |
| Epistaxis | 2-4 |
| Visual disturbances | 0.6-1.6 |
| Transient diabetes insipidus | 10-60 |
| Permanent diabetes insipidus | 0.5-5 |
| Anterior pituitary insufficiency | 1-10 |
| Cerebrospinal fluid leakage | 1-4 |
| Meningitis | 0-1.75 |
Several complications can arise as a consequence of the transsphenoidal approach. If a sublabial incision is used, anesthesia of the upper lip and anterior maxillary teeth can occur, although this condition is usually transient.208 Removal of the superior cartilaginous septum may result in a saddle nose deformity.209 Perforation of the nasal septum occurs in 1% to 3% of patients and is more likely with reoperations.206 Postoperative sinusitis can occur in 1% to 4% of patients and may be reduced by postoperative antibiotics.210 Opening the speculum can result in diastasis of the maxilla or fracture of the medial orbital wall.206,211 Damage to the optic nerve or the carotid arteries can occur if the speculum is advanced too far. Inadequate removal of mucosa in the sphenoid sinus can lead to the postoperative formation of a mucocele.212
Vascular injuries represent serious morbidities and can lead to death. Intraoperative mucosal bleeding and delayed postoperative bleeding from the mucosal branch of the sphenopalatine artery can occur. If postoperative epistaxis persists, embolization of the internal maxillary artery may be necessary.213 Damage to the carotid arteries can occur in the sphenoid sinus or in the sella. Maintaining a midline trajectory is vital to avoid the carotid artery, and preoperative radiologic studies are essential in localizing the carotids. There are significant variations in the carotid’s parasellar course, and the distance between the two arteries may be as little as 4 mm.208 Frameless stereotaxis can be used to maintain a midline approach and may be especially useful in reoperations.214 Endoscopy is increasingly being used to minimize tissue trauma and to obtain more expansive views than those provided by microscopic visualization alone.215,216 Excessive arterial bleeding signals intraoperative injury to the carotid artery, and the only treatment involves packing the operative field.213 Other maneuvers are limited by the exposure, although if packing fails, ligation of the carotid may be required. Carotid artery injury can result in subarachnoid hemorrhage, vasospasm, false aneurysms, and carotid cavernous fistulas. A postoperative cerebral angiogram is essential to identify any of these complications.217 About 25% of deaths occurring during transsphenoidal operations are attributable to vascular injuries.21,213 Visual disturbances are also possible because of the close association of the chiasm, optic nerve, and pituitary. Damage can occur as a result of direct trauma, traction injury, or vascular compromise. Visual disturbances are more likely after reoperations because of adhesion formation between the chiasm and sella. Adhesions predispose the chiasm, optic nerve, and hypothalamus to traction injuries. In general, visual disturbances occur in 0.6% to 1.6% of patients.218 Postoperative visual loss can also signal the formation of a hematoma in the tumor bed. Such hematomas can be prevented by meticulous hemostasis. They can occur in 0.3% to 1.2% of cases.206,210,212 Injuries to the hypothalamus can also take place and potentially result in death.213 These patients are comatose and exhibit hyperthermia. Hypothalamic injury is the most common cause of death in patients undergoing transsphenoidal operations.213
Several complications can be anticipated in the postoperative period, and early recognition and appropriate treatment can circumvent catastrophic results. Patients should be closely monitored for diabetes insipidus (DI) with frequent serum sodium evaluations and careful accounting of patients’ fluid intake and urine output. An elevated serum sodium level or urine output may indicate DI. Temporary postoperative DI can occur in 10% to 60% of patients.210,219 Permanent DI is much less common and occurs in just 0.5% to 5% of patients.210 Delayed onset of the syndrome of inappropriate antidiuretic hormone secretion can also occur about a week postoperatively.213
Postoperative anterior pituitary insufficiency is one of the most commonly seen postoperative complications. Its incidence varies from 1% to 10%.206,207 Postoperative steroid therapy should be used in all postoperative patients until a thorough endocrine evaluation is complete. Adrenal insufficiency is a potentially serious complication if adequate steroid replacement therapy is not initiated.
CSF rhinorrhea is another commonly encountered complication of the transsphenoidal approach and occurs in 1% to 4% of patients.206,213 Intraoperatively, penetration of the arachnoid membrane can result in a gush of CSF into the operative field and the potential for postoperative CSF rhinorrhea. Packing the sella intraoperatively with an autologous fat graft and bone from the removed vomer can help prevent CSF leakage. Care must be taken to not overpack the sella, which may lead to compression of the chiasm.212 Patients in whom CSF rhinorrhea develops are first treated by spinal drainage for several days. Failure to close a CSF fistula with spinal drainage may indicate the need for reoperation and repacking of the sella. Early recognition and treatment of CSF rhinorrhea are important because a CSF leak can lead to meningitis. The incidence of meningitis in patients undergoing transsphenoidal surgery has been reported to be 0% to 1.75%.210,212,220 Patients with diabetes mellitus are at greater risk for the development of meningitis.
Cranial Base Surgery
The surgical approaches often call for brain retraction to adequately expose the lesion. Overly aggressive retraction can lead to tissue damage and infarction, with postoperative swelling resulting in increased ICP. Several maneuvers, including adequate bone removal, CSF drainage, and diuretics, can aid in achieving adequate exposure without excessive brain retraction. Resection of noneloquent brain tissue may be required to prevent contusions and possible postoperative herniation occurring from retraction injuries. Retraction can also compromise or injure venous outflow and result in venous stasis and hemorrhagic infarctions. This is especially important in regard to the vein of Labbé. Excessive retraction of the posterior temporal lobe can lead to tearing of the vein of Labbé and severe hemorrhagic temporal lobe edema.221
CSF leakage is one of the most common postoperative complications in cranial base surgery. The surgery often creates a communication between the CSF space and the facial sinuses. The sphenoid sinus is most commonly involved because of its association with the clivus and cavernous sinus.221 CSF leaks occur in about 8% of patients undergoing cranial base operations.221 A persistent CSF fistula may develop. Leaks generally occur in the immediate postoperative period, or they rarely develop months after surgery.221 CSF leaks are clinically manifested as clear spinal fluid draining from the nose, ear, or wound. The fluid can be collected on a pledget, and the presence of β2-transferrin confirms the discharge as CSF. Confirmatory radiographic examinations can be performed. Radioisotopic cisternography with cotton pledgets in the nasal cavity can corroborate the presence of a CSF leak. CT cisternography with intrathecal metrizamide or magnetic resonance cisternography can be used to localize the leak.222
Pneumocephalus is another postoperative complication frequently encountered in cranial base surgery. Air may be found in the extradural or intradural spaces. Intracranial air can produce alterations in a patient’s mental status that result in lethargy or agitation. Some degree of pneumocephalus is commonly found on postoperative CT, and the air is usually reabsorbed quickly. Patients operated on in the sitting position have a higher incidence of pneumocephalus.223 Increasing amounts of intracranial air signal the presence of a communication between the subarachnoid space and air sinuses and implies an undetected CSF leak. Having patients lie flat in bed and discontinuing external spinal drainage can facilitate the absorption of intracranial air. Passing a spinal needle through the bur-hole site into the air pocket can decompress the subdural air in the event of a tension pneumocephalus.221
Skull base lesions often involve the cranial blood vessels. Tumors can encase or displace these vessels, and adequate tumor removal may require sacrifice of vessels. The neurosurgeon must know the consequences of sacrificing cranial base blood vessels to minimize morbidity. Sacrifice of vessels can result in ischemic neurological deficits and infarctions in a vascular territory or watershed distribution. Preoperatively, balloon occlusion testing and xenon-enhanced CT cerebral blood flow testing can determine whether patients can tolerate sacrificing a blood vessel. Patients in whom neurological deficits develop with the balloon occlusion test or who have cerebral blood flow of less than 35 mL/100 g per minute cannot tolerate vessel sacrifice and may require a bypass graft.224
Cranial nerve morbidity is commonly encountered with cranial base surgery, and the dysfunction may be temporary or permanent. Accurate preoperative cranial nerve examination is important because postoperative dysfunction is more likely in patients with preoperative deficits. Neurophysiologic monitoring is an important adjuvant for localizing cranial nerves and preventing injury. Cranial nerve VII may be monitored via continuous facial electromyographic responses. A nerve stimulator can help locate the facial nerve. Cranial nerve XI can be localized with a nerve stimulator and observation of shoulder twitching.198
Cranial nerve injury can occur as a result of nerve retraction or direct injury during tumor dissection. Cranial nerves may also be injured by compromise of the nerves’ blood supply during surgical dissection distant from the nerves. Damage to the cranial nerves is especially significant during surgery in the cavernous sinus. Optic nerve damage occurs in 0% to 6% of patients.197,225 Permanent damage involving extraocular nerve function (i.e., cranial nerves III, IV, and VI) occurs in 20% to 30% of patients.196,197 The incidence of V1 neuropathy is 8% to 20%.196,197,225
Certain cranial nerves are more susceptible to injury than others. Cranial nerves I, II, and VIII are very sensitive to injury. Minimal manipulation can result in profound deficits, and the loss of function is often irreversible. Cranial nerves III, IV, and VI are less sensitive to manipulation, and some recovery typically occurs postoperatively if the nerve’s continuity is maintained. Injury to these nerves results in diplopia. Loss of cranial nerve IV function can be corrected by tilting the head or the use of prism glasses. Oculoplastic procedures may be necessary to correct persistent diplopia caused by injury to cranial nerve III or VI. Cranial nerve V damage is generally well tolerated, with the exception of damage to the V1 segments, which mediate the corneal reflex. Damage to the V1 division results in corneal sensory dysfunction, and patients must have meticulous eye care to prevent corneal abrasions and loss of vision in the desensitized eye.221
Unilateral injury to cranial nerve XII is generally well tolerated. When combined with other cranial nerve injuries, such as injury to cranial nerves VII, IX, or X, significant dysarthria can occur. Bilateral cranial nerve XII injury results in severe functional limitation and ultimately requires a tracheostomy and placement of a feeding tube.221
Cranial base surgery can cause morbidity from TMJ manipulation. Dislocation of the TMJ can result in postoperative trismus. Resection of the mandibular condyle may be preferred because it avoids retraction of the mandible and associated postoperative trismus. Resection of the condyle leads to a contralateral jaw deviation but no functional loss.221
Complications of Stereotactic Brain Surgery
A stereotactic frame is applied to the patient, and CT or MRI is performed. The most commonly used frames are the Leksell (Elektra Instruments, Atlanta, GA) and the Brown-Roberts-Wells (Radionics, Burlington, MA) systems.226 The fiducial markers on the frame are registered into the system and allow accurate three-dimensional navigation and localization in reference to the neuroimaging. Proper application of the stereotactic frame and precise registration are essential to achieve accurate results. Frameless systems that use cutaneous fiducial markers are available, as well as some that use surface landmarks alone, with no need to place cutaneous fiducial markers.
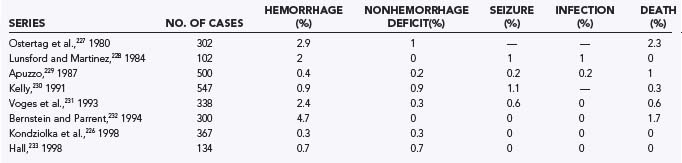
One of the most commonly performed stereotactic procedures is brain biopsy. Brain biopsies are safe and effective procedures. The procedure can usually be performed under monitored anesthesia care and can avoid the complications associated with general anesthesia. CT or MRI is used to stereotactically guide biopsy of a lesion through a small bur hole. Possible complications include hemorrhage, neurological deficits, seizures, and infections.226 The mortality rate in several large series has been less than 1%, and complication rates vary from 0% to 7% (Table 22-5).226–233 Seizures and infections are rare during brain biopsy. The most serious complication usually involves postoperative hematoma formation. Properly performed brain biopsies are more than 90% effective in establishing a tissue diagnosis in patients with radiographic lesions.226
Preventing complications related to brain biopsy requires adequate preoperative planning. Only patients in whom the results of brain biopsy may change medical management should undergo biopsy. Because thrombocytopenia or coagulopathies predispose patients to intracranial hemorrhage, all candidates should have normal coagulation profiles and platelet counts. Preoperative radiographic imaging is essential to rule out vascular lesions that may result in serious hemorrhage when biopsied. The planned trajectory must avoid vessels and important structures. Intraoperative hypertension may predispose patients to hemorrhage.226
When bleeding is discovered during a brain biopsy, allowing the blood to drain out of the needle may prevent the formation of a hematoma.226 Craniotomy may be required to control persistent hemorrhage. Instillation of thrombin through the biopsy canula has been used to control hemorrhage.234 Routine postoperative CT can be performed to rule out hematoma formation, and asymptomatic hematomas are often discovered postoperatively. Neurological deficits develop in about 10% of patients with asymptomatic postoperative hematomas.235 Most postoperative hematomas are managed by observation and serial CT.
Brain biopsies are increasingly being performed on patients infected with human immunodeficiency virus (HIV), who may be subject to several central nervous system infections or neoplasms. Biopsies in patients with acquired immunodeficiency syndrome have higher complication rates. Skolasky and coworkers reviewed 435 HIV-positive patients undergoing biopsy and determined that the morbidity rate was 8.4% and the mortality rate was 2.9%.236 Complications were associated with preoperative poor functional status and thrombocytopenia. It is not clear whether the presence of HIV infection predisposes to higher complication rates.
Stereotactic Radiosurgery
Stereotactic radiosurgery is a safe and effective treatment modality for vascular malformations, brain tumors, and in some cases, functional surgery. Stereotactically applied radiation provides precise delivery of high-dose radiation to a well-defined target. Complications in radiosurgery are related to the effects of radiation on the brain and structures in proximity to the lesion. Significant early complications rarely occur but can include seizures or worsening neurological deficits. Approximately a third of patients experience mild transitory symptoms, including headaches, nausea, and dizziness.237 Late complications develop 6 to 9 months after the procedure and can include facial palsy, trigeminal neuropathy, and visual symptoms.226 Exposure of the optic nerve to more than 8 to 10 Gy of radiation leads to visual deterioration and optic neuropathy.238,239 Patients may become symptomatic from radiation necrosis or local brain edema. In a review of 1600 patients undergoing radiosurgery with at least a 3-year follow-up, the rate of significant morbidity was 1.9%.226 The risk for carcinogenesis secondary to radiosurgery is estimated to be less than 1 in 1000.240
Gamma Knife radiosurgery has been applied effectively to the treatment of acoustic neuromas. The complications associated with acoustic neuroma radiosurgery are related to exposure of cranial nerves to radiation. The rate of facial nerve paresis after 5-year follow-up has been 21%, and the rate of trigeminal dysfunction has been 27%. Hearing was preserved in 51% of patients undergoing radiosurgery for acoustic neuromas.241,242 Peritumoral edema after radiosurgery has occasionally led to hydrocephalus.243 There is a significant increase in mass effect and tumor size, approximately 43%, after high-dose Gamma Knife radiosurgery for vestibular schwannomas that correlates with deterioration of facial and trigeminal function. The effect is much smaller at lower doses.244 Because tumor control is greater with larger doses of radiation, fractionated stereotactic radiosurgery is usually performed to allow increased control of growth while minimizing risk to the facial, cochlear, and trigeminal nerves.245 Intracanalicular tumors may be associated with higher cranial nerve morbidity when treated with radiosurgery.246 Improvements in target imaging and reduction in doses have led to lower cranial nerve morbidity.241,243
Radiosurgery has also been applied to cranial base meningiomas. The morbidity rate is about 5% to 8%.238,239,247,248 Most complications involve transient cranial nerve palsies and occur 3 to 31 months after surgery. High radiation doses applied to Meckel’s cave increase risk for the development of trigeminal neuropathy.239 Radiosurgery is also used to treat gliomas and brain metastasis. Preliminary reports indicate a morbidity rate of about 10% and a mortality rate of 1%.249 Early complications can involve increased ICP, which may lead to death.250 Radiotherapy for brain parenchymal lesions can result in seizure complications. Patients with lesions in the motor cortex are especially susceptible to seizures after radiosurgery.251 Gamma Knife radiosurgery for trigeminal neuralgia is generally well tolerated and associated with minimal morbidity. Loss of facial sensation has been reported infrequently.252
Spine Surgery
Cerebrospinal Fluid Leak or Pseudomeningocele Formation
Prevention of CSF leakage is critical for optimizing wound healing, for preventing neural elements from herniating through the defect in the dura and leading to pain syndromes or neurological deficits, and for eliminating positional headaches. It is generally accepted that reduction of intraspinal CSF pressure facilitates healing of a dural defect. This can be achieved by maintenance of strict bed rest or by placement of a CSF diversion drain, such as a lumbar drain. The use of spinal subarachnoid drains after a CSF leak is supported as an adjunct.253–257 One treatment element that seems to be accepted almost uniformly as being beneficial is the use of fibrin glue sealants.258–263 The sealant can be prepared autologously in the operating room, from cryoprecipitate obtained from the blood bank, or from commercial kits made from donated blood products. Regardless of the cause, fibrin glue sealants, when applied in the area of the dural repair, dramatically increase the rate of healing. The use of dural replacements is more controversial. Repair with fascia, AlloDerm, Duragen, or other techniques is more a matter of choice than evidence-based medicine.
Primary repair of a dural violation, when possible, is clearly indicated. Multiple surgeons have documented increased infection rates and decreased fusion rates associated with CSF leaks.255,262–265 In addition to CSF leaking from the durotomy, nerve roots have been known to herniate into the durotomy and result in painful syndromes.266
Instrumentation-Related Risks
Instrumentation has increased the incidence of complications in all series that have compared the results of instrumented with noninstrumented fusions.267–270 This finding is not surprising in that instrumentation adds time, complexity, and an implanted foreign body to the operative procedure. Fusion rates are uniformly higher in instrumented cases, and most experienced spine surgeons believe that the risks are outweighed by the benefits of rigid segmental fixation. However, each surgeon must feel confident and comfortable with any technique because morbidity rates vary from surgeon to surgeon.268,271–289
The use of intraoperative imaging has grown dramatically. Ultrasonography as an intraoperative localizing device can help verify the correct level and locate hidden, deep lesions within the spinal cord.290–293 More medical centers are using portable and dedicated MRI and CT scanners for determination of the adequacy of procedures for resection of tumor or osteophytes, placement of instrumentation, or other needs of the surgeon. Stand-alone MRI scanners have been developed that function in an operating room or even as an operating room.294 Some of these modalities require specialized equipment that is compatible with the modality (e.g., nonmagnetic instruments for intraoperative MRI). Each has its advantages and limitations, and the use of these devices depends on the needs of the surgeon and the institution. Intraoperative CT scanners are available, as are fluouroscopy-based systems that create three-dimensional reconstructions resembling CT scans. These modalities can be useful in confirming the adequacy of decompression or screw placement before leaving the operating room.
Stereotactic navigational adjuncts have increasingly been used in spine surgery.294–298 The accuracy of stereotaxis depends on the quality of the scan used, the position of the patient intraoperatively and in the scanner, performance of the stereotactic portion of the procedure before any resection or opening that would distort the landmarks used for calibration, and user-dependent variables. Currently, numerous intraoperative navigation techniques are available that rely on preoperative CT, intraoperative three-dimensional reconstruction from planar fluoroscopy, or three-dimensional reconstruction using intraoperative isocentric circumferential fluoroscopy. All appear to provide accuracy with respect to screw placement.299–303 Although each system has its pros and cons, there is no evidence that one system is clearly superior to another.301 Miniature robotic systems are also being developed to improve the accuracy of targeting and screw placement.302,303 Navigational techniques are increasing being applied to spinal arthroplasty procedures, as well as fusion procedures.304
Complications of Bracing and Halo Use
No intervention is without risk for complications, and the use of external orthoses is no exception. Problems are related to improper placement, to proper placement but brace limitations, and to the brace itself. Improper placement of cervical collars can result in skin and spinal cord injuries. The skin can be abraded if the chin falls inside the jaw support. Use of a properly fitted collar and instruction to the patient that the chin is not supposed to slide under the chin support can significantly reduce this risk. Spinal cord injury can occur when an unstable spine is moved as a result of placement of a brace. A brace should be applied in such a way that the spine is not moved, and this includes not reducing a deformity. One common situation is a patient with ankylosing spondylitis and a fixed kyphotic deformity who suffers a transdiskal fracture.305–314 A well-intentioned first responder may place this patient in neutral alignment and cause a spinal cord injury. It is critical to obtain a history from the patient or family before reduction, if possible, and to keep the patient in the baseline position, not just what “looks right.” Many spinal cord injuries occur after the patient has been placed in a collar. Injury may also result because no external orthotic device limits movement completely.315–317 The range of motion in a given device varies but is easily quantifiable. Wearing a brace of any kind can trap moisture and impede dressing changes, thereby leading to wound maceration and cellulitis. A brace that does not contour the patient’s anatomy can cause pressure, pain, necrosis, and wound breakdown.
Use of a halo vest orthotic, which has less range of motion than nonfixed devices, is complicated by several factors, including local pin site complications, problems with the vest device, movement despite the halo, and issues related to the size, bulk, and location of the device.51–56,288,314,318–327
Local complications range from the mundane, such as cellulitis at the skin insertion site, to deeper complications related to the point at which the pin enters the skull.52,53,56,328,329 Pin-related complications also include the development of epidural hematoma or epidural/subdural abscess at the placement site. These complications are insidious because they cannot be seen directly. Loosening of the pin in the outer table may result in a catastrophic loss of tension, which leads to loss of fixation, scalp laceration, and in rare instances, oculofacial trauma. Fracture of the outer table can also lead to fracture of the inner table and intracranial injury.321,326,328 The halo is large, unwieldy, and for many frail or slender patients, heavy. It raises the center of gravity for the patient and challenges the coordination skills of many patients, especially those already neurologically impaired.
Anterior Cervical Approach
The transoral approach, because of passage through the oral cavity, is associated with a significant incidence of wound infection and healing problems.330–332 They can be diminished by judicious minimization of steroids, care on opening to not destroy tissue planes and the mucosa, and the perioperative use of antibiotics. Unfortunately, many patients requiring a transoral approach are metabolically or nutritionally challenged to begin with, and they may not heal well. Palate injury is also a significant potential problem. The palate (soft and hard) may need to be split for adequate exposure, and it does not always heal well afterward. The assistance of an ear, nose, and throat surgeon for the approach and closure can help a surgeon who is not familiar with the management of these tissues. The potential neurological morbidities related to the transoral approach to the dens and anterior rostral spinal cord are related to the approach, the use of rongeurs instead of drilling, and the adequacy of exposure.
Esophageal injury can result from the dissection or from manipulation during the procedure after the retractors are in place. Migration of the retractors may tear the esophagus directly, or the esophagus may creep into the surgical field and then be injured by a wayward instrument. Injury can be prevented by the surgeon remaining constantly aware of the position of the retractors and the esophagus. After the procedure but before closure, the entire length of the exposed esophagus should be inspected for tears because an unnoticed tear can allow spillage of contents into the surgical bed and lead to infection, pseudarthrosis, or osteomyelitis. The esophagus can be repaired directly with a muscle patch from the sternocleidomastoid (as a vascularized pedicle of the manubrial head or as a free segment) or with a direct external drain and an esophagostomy.333–337 If the surgeon does not have experience with such a repair, an ear, nose, and throat surgeon should be called in to perform the restoration. Reoperations are frequently associated with problems related to scarring of the esophagus at the old surgical site, especially with instrumentation. If there is a question about difficult planes of dissection, an ear, nose, and throat surgeon should obtain exposure. The incidence of acute or subacute esophageal tears ranges from 0% to 1.9% and averages less than 1% in most series.338 Delayed perforation has been described and may occur a decade after the surgery. Whether this represents an injury at the time of surgery or a delayed injury caused by erosion from the anterior plate or screws is unclear. Every attempt should be made to place the anterior plate as flush along the spine as possible.339 Esophageal perforations appear to occur most commonly at C5-6 because the wall of the esophagus is thinnest at this level.339 Some surgeons prefer to place a nasogastric tube at the beginning of the procedure to serve as a palpable landmark for the esophagus in an effort to avoid injuring it.
Dysphagia without direct esophageal perforation is far more common in patients after anterior cervical spine surgery. Reports range from rates of 10% to 60%. When carefully studied, there appears to be a 13.6% rate of dysphagia in patients 2 years after surgery.340 Dysphagia was more common in women, after revision surgery, and in patients undergoing multilevel surgeries. Minimizing retraction and retraction time and avoiding injury to the upper pharyngeal nerves are recommended.
Recurrent laryngeal nerve (RLN) injury is a well-described risk with this anterior cervical approach. It leads to hoarseness and other changes in voice quality. The incidence is generally reported to be 2% to 3%.341 RLN injury appears to be less likely when the spine is approached through a left-sided exposure because of anatomic differences in the right and left RLNs. There does not appear to be a clear benefit from endotracheal tube cuff deflation.342 When considering the choice of approach for a revision anterior cervical procedure, preoperative laryngoscopy should be performed to look for evidence of existing unilateral RLN palsy.343 If identified, the approach should be through the ipsilateral side to prevent bilateral RLN palsy and the need for emergency tracheostomy. Continuous RLN electromyographic monitoring during surgery is practiced by some surgeons in an attempt to mimize the risk for injury.344,345
One of the most feared complications in the anterior cervical approach is injury to the vertebral artery. The incidence of this injury during anterior cervical approaches is less than 0.2%.346,347 When such an injury occurs, packing of the vessel to obtain hemostasis should be followed by angiography and consideration of endovascular vessel occlusion. The risk for vertebral artery injury can be minimized by an understanding of the anatomy of the transverse foramen to the vertebral bodies and careful evaluation of preoperative CT and MRI studies.348
Postoperative formation of a hematoma in the operative field can have devastating consequences.349–353 It may lead to a retropharyngeal hematoma or an epidural hematoma. It can initially be manifested as dysphagia or pain but may result in stridor and airway obstruction. Immediate surgical evacuation and reestablishment of hemostasis must be instituted if there is any chance of significant size of the hematoma. It may be able to be prevented with the use of a drain leading from the vertebral surface (bone edges are often the source of the bleeding), although removal of the drain sometimes promotes bleeding.
Complications related specifically to corpectomies rather than diskectomies include C5 traction injuries, collapse of the fusion segment, dislodgement of the implant, and a higher incidence of CSF leaks as a result of the more extensive involvement, especially in patients with ossification of the posterior longitudinal ligament.354–362 The C5 nerve root is especially at risk because of the short length of the root and its tendency to be injured when overdistraction takes place.356,361,363 By limiting the distraction and width of the decompression, this risk can be minimized.
The approach for placing an odontoid screw is similar to that for anterior cervical diskectomy and fusion. This method has all the risks of complication as the other anterior cervical approaches, with additional risks related to capture of the odontoid tip. Risks include failure to maintain the correct lateral angle and missing the tip of the dens and the potential for spinal cord injury from migration of the dens or a poorly placed drill or screw.275,364–366 Risks can be minimized by wide exposure of the C2-3 interspace to demonstrate the uncovertebral joints bilaterally and determine the midline more accurately. Patients should be selected in whom the dens is aligned with the C2 body and not significantly displaced. Screw fracture, because of the long moment arm and high torque on the odontoid screw, can be prevented by using a tapered thread (i.e., the screw is thicker at the end), which strengthens the screw at the point where the force is greatest. Dens capture is easier with threaded lag screws because they reduce the likelihood of the screw pushing the fragment instead of threading into it.
Posterior Cervical Approach
Posterior cervical surgical procedures carry risks different from those of anterior procedures. The prototypical procedure is cervical laminectomy, which is performed for numerous indications, from Chiari decompression to cervical stenosis to intramedullary tumor exposure. The primary risks associated with cervical laminectomy are similar to those of laminectomy at other levels and include cord injury, dural injury, and nerve root injury. The simplest way to minimize injury to these elements is to judiciously and minimally use monopolar cautery when down to the lamina and dura, use cottonoids to retract the dura away from the ligamentum flavum and lamina, and take care in preventing overly aggressive use of rongeurs, which can result in fragments being twisted into the dura or nerve roots. Even with no evidence of direct trauma to the roots, transient C5 palsy can be seen in approximately 5% to 15% of patients undergoing posterior cervical decompression, with or without instrumentation.367,368 This injury is manifested as a deltoid muscle weakness. Although some authors recommend intraoperative monitoring with motor evoked potentials and deltoid electromyographic recording, C5 root injury may occur in the absence of intraoperative findings.367,369
The risk for injury to vascular elements is primarily limited to the vertebral artery, which runs laterally in the vertebral canal until its exit from the C2 body. At this point, the artery becomes most vulnerable to injury because the vessel turns from a lateral course and moves dorsally before entering the dura adjacent to the C1 lamina. Frequently, injury to the venous plexus is initially confused with injury to the vertebral artery, but the consequences are not nearly as significant. As with most venous bleeding, it can be controlled easily by tamponade with Gelfoam or Surgicel and a cottonoid. Injury to the vertebral artery may require opening the dura and ligating or performing a bypass or end-to-end anastomosis, depending on the nature of the injury and its location. Injury to the vertebral artery during posterior cervical procedures occurs more frequently than during anterior procedures, with a rate of up to 1.9%.347
Thoracic Spinal Procedures
Thoracic spinal procedures, because of the surrounding organs, carry risks different from those of cervical spine procedures. Anterior approaches, such as the transthoracic, endoscopic, and retropleural approaches, put major arteries, veins, and organs such as the heart, lungs, and diaphragm at risk for injury.273,370–380 Posterior approaches, such as laminectomy, costotransversectomy, and transpedicular approaches, have fewer risks but can still injure the ventral organs and vessels if reaching too far forward.80,273,381–384 All approaches can result in complications involving neural elements, CSF leakage, and infection. Some complications are related to the exposure, whereas others are related to the procedure being performed.
Thoracic laminectomy has long been performed for many procedures, including repair of intramedullary, intradural, and epidural lesions. Risks are similar to those for the subaxial cervical spine, and it is important to keep the lateral exposure to the minimum that can provide the necessary exposure. Too wide an exposure risks taking down the costotransverse ligaments and even risks pneumothorax. For tumor patients in whom the wound has been or will be irradiated, a curvilinear incision with a myocutaneous flap should be used to maintain vascular supply to the skin and reduce the risk for infection and tissue breakdown.385,386
Thoracic pedicle screw instrumentation can be performed safely by experienced surgeons using freehand techniques.387–389 However, many of the intraoperative navigation systems previously discussed were designed specifically to increase safety in thoracic instrumentation.
Thoracoscopic procedures need smaller incisions to approach the spine and thus reduce the likelihood of significant wound breakdown and postoperative incisional pain, but because of the multiple ports used and the limited sight angles, the potential for injury to structures such as vessels and organs remains significant.370,373,390–392 Conversion to open thoracotomy should be performed if there is a significant problem because trying to fix a large injury through a small opening will probably provide greater challenges.
Anterior Lumbar Procedures
The transperitoneal open procedure is performed through a laparotomy, usually through a midline incision, although a Pfannenstiel bathing suit line incision can also be performed. The procedure calls for mobilization of the abdominal viscera with a midline anterior approach to the spine after mobilization of the various branches of the aorta, inferior vena cava, or iliac vessels. This approach has a higher risk than the retroperitoneal approach for postoperative complications such as injury to the major vessels, adhesions, and adynamic ileus.393–398 Injury to other structures, including the ureter and pelvic contents, is rare but of significant consequence.
Anterior endoscopic procedures are performed through multiple small incisions and with the use of multiple ports.399,400 The smaller incisions are thought to heal better than a single, large incision of the same total length. The approach is essentially the same as an anterior transperitoneal approach in terms of mobilization of the abdominal viscera and major vessels, although because of the port size and the use of endoscopic techniques, mobilization is more difficult. The assistance of a general surgeon with significant endoscopic experience in performing the exposures is recommended for neurosurgeons who are not comfortable with the management of injury to the structures being mobilized. Other possible complications include hypercapnia if carbon dioxide insufflation is used and delay in converting to an open procedure if bleeding or another major complication occurs. Lost time in gaining control of a difficult situation can lead to greater morbidity from blood loss.
The retroperitoneal approach can be used in two ways. It can be performed with a wide exposure to allow extensive instrumentation,397,401,402 or it can be used with a short incision for placement of an interbody fusion construct (e.g., mini–anterior lumbar interbody fusion [mini-ALIF]). The main risks are vascular, although entry into the peritoneum or sigmoid colon is possible. Previous surgery in this area distorts the anatomy and leads to scarring. The primary risk with this approach is tearing segmental arteries and veins that may be under tension and difficult to visualize as retraction for the exposure proceeds. This exposure may be extended up to the diaphragm, with further mobilization of the kidney and, if necessary, the spleen and liver. The approach is usually done from the left-hand side because of the smaller size of the liver on the left. Because of the retroperitoneal exposure, the ureter is less subject to injury in the lower levels than with a transperitoneal approach. The location of the ureter should be anticipated to reduce the chance for injury.
One significant risk related to the anterior approach is retrograde ejaculation in male patients undergoing L5-S1 fusion.403 The incidence of this complication was initially reported to be about 5%, but the later literature has reported an incidence as high as 20%. There is a 10-fold higher incidence of retrograde ejaculation with a transperitoneal approach than with a retroperitoneal approach to L4-5 and L5-S1.404 This is thought to be due to the fact that the superior hypogastric sympathetic plexus lies midline over the disk spaces at L4-5 and L5-S1. When approaching from a retroperitoneal trajectory, the plexus is mobilized off the disk spaces along with the posterior peritoneum to protect it from injury. When the approach is via a midline transperitoneal route, the plexus itself is directly injured. This may play a role in the choice of approach in men. Minimal use of electrocautery in this region is also recommended. If a transperitoneal approach is required, dissecting the plexus off the right-sided iliac vessels and mobilizing the fascia toward the left may protect the plexus and prevent this complication.405
Posterior Lumbar Procedures
Prevention of postoperative epidural scarring after dorsal procedures is a challenge that does not have a simple answer. Several techniques are available, such as placement of a fat graft, Gelfoam sponge, or artificial adhesion barrier.406–409 None is without complications or is universally effective.410–413
Postoperative reherniation of disk fragments occurs in approximately 10% of cases.414–422 Differentiating reherniation from scar requires a contrast-enhanced scan (unless it is in the first week or two after surgery); the scar enhances, and the disk usually enhances only in the periphery of a fragment.254,407,423–426
Cauda equina syndrome as an immediate or delayed result of lumbar diskectomy is a catastrophic neurological complication. It can occur as a result of injury to the nerve roots from epidural hematoma after closure, from infection of the arachnoid or epidural space, from retraction of neural elements against a calcified herniated fragment, or from extrusion of disk or end-plate fragments postoperatively.427–433 The mechanism usually determines the time frame for the onset of symptoms.
Catastrophic injury to the organs or vessels of the abdomen and pelvis can result from diskectomy.393,434–441 Injury can occur from placement of any sharp instrument into the disk space that passes through the annulus and anterior longitudinal ligament. Bleeding, which may or may not well up into the surgical field, is not responsive to attempts to arrest it. The patient may become tachycardic or hypotensive. The onset of symptoms may be more insidious and not appear until the patient is in recovery, or in the case of bowel injury, symptoms can develop after discharge. Management of life-threatening vascular injury requires termination of the neurosurgical procedure, turning the patient over, and performing an exploratory laparotomy and vascular repair of some kind. Ignoring the problem, failing to obtain a vascular surgical consultation, or simply transfusing the patient can result in catastrophic blood loss and perhaps death.
Minimally invasive techniques for the treatment of lumbar disease include chemonucleosis, thermal or laser coagulation, and automated percutaneous diskectomy.442–459 These procedures are performed under local anesthesia with fluoroscopic guidance, and their aim is internal decompression of the disk and the affected nerve roots. One benefit of the absence of regional or global anesthesia is that any irritation or compression of the nerve root can be felt, and the surgeon is able to change whatever it was that triggered the response. The entry point is from the side of the disk, and it may be difficult to enter the L5-S1 space directly because of the position of the iliac crest relative to the disk space. Up to 10% of patients are unable to have percutaneous instruments placed into this disk space. Causalgia, injury to the thecal sac or nerve roots, injury to the end plate, fracture of an instrument, injury to a hollow viscus, injury to a vessel, and hematoma of the psoas muscle are all acute complications of percutaneous diskectomy.443–447,450,457,460–462 Delayed complications include diskitis and progression of the degenerative processes.463,464 Success rates for percutaneous treatment are in the range of 60% to 80%,442–447,460,464,465 much lower than those for microdiskectomy but without the attendant risks associated with general or regional anesthesia.
The risks related to posterior lumbar interbody fusion (PLIF) are similar to those for posterior decompression but are amplified by the additional manipulation required to distract the two end plates, retract the neural elements, and implant bone wedges or segments.267,276,466–477 Overdistraction can lead to neurapraxia of one of the nerve roots and may tear adherent dura. Blood loss is greater with PLIF than with laminectomy or diskectomy because of the extra removal of the end plate and osteophytic ridges and the extensive epidural exposure. Graft migration is a significant risk with uninstrumented PLIF because there is nothing to maintain the alignment and prevent relaxation of the tension that keeps the graft in place. Actions that can reduce the complications associated with PLIF include having the appropriate instruments for distraction and implantation. When performing PLIF for spondylolisthesis, the nerve roots exiting through the same foramen (e.g., the L5 root for L5-S1 PLIF) may be under significant compression and tension because of the anterolisthesis and pseudodisk. The path of the nerve root takes it directly over the desired entry point into the interspace, and the plane of the disk space causes distractors to go through the region of the axilla of this root. One way to avoid the problem is to use a drill or osteotome to remove the dorsal osteophyte lateral to the lower root and medial to the exiting root. This allows a flatter trajectory into the disk space and avoids unnecessary manipulation of an already tenuous root.
Pedicle Screw Fixation
The use of pedicle screw fixation has significantly increased fusion rates over those with noninstrumented fusions.82,285,478–480 Use of the pedicle screw fixation technique, which has undergone significant medicolegal scrutiny, has been vindicated, and it is applied to lumbar, thoracic, and cervical spinal cases. The major risks are related to misplacement of the screws, fracture of the neural elements being stabilized, injury to neural and vascular structures, and infection or poor wound healing.267,272,285–287,471,478,479,481–494 Reduction of risk is undertaken on several fronts. Understanding the biomechanical parameters and indications can reduce the risk for surgical misadventure. Pedicle screws can be placed by relying only on anatomic parameters to determine the entry point and angulation, but for surgeons who wish to have confirmatory assistance, several imaging and image-guided techniques are available, as discussed previously.298,483,485
Facet Screw Fixation
Two types of facet screws can be used for segmental fixation: the Boucher technique of facet screw fixation495,496 and the Magerl translaminar facet screw fixation.281,497–503 The translaminar screw fixation technique is as stiff as pedicle screw fixation except in extension, in which it is less stiff than pedicle screws.504 Fusion rates are reportedly comparable to those with pedicle screws, but the lower perioperative morbidity rates of translaminar screws make them an acceptable option for some surgeons and patients. The practice at our institution is to not place them at L5-S1 because of stress concentration or when significant spondylolisthesis is present (i.e., grade II or greater after reduction).
, Baumann SB, Welch WC, Bloom MJ. Intraoperative SSEP detection of ulnar nerve compression or ischemia in an obese patient: a unique complication associated with a specialized spinal retraction system. Arch Phys Med Rehabil. 2000;81:130-132.
, Bednarik J, Kadanka Z, Vohanka S, et al. The value of somatosensory and motor-evoked potentials in predicting and monitoring the effect of therapy in spondylotic cervical myelopathy: prospective randomized study. Spine. 1999;24:1593-1598.
, Bekar A, Tureyen K, Aksoy K. Unilateral blindness due to patient positioning during cervical syringomyelia surgery: unilateral blindness after prone position. J Neurosurg Anesthesiol. 1996;8:227-229.
, Bergeson RK, Schwend RM, DeLucia T, et al. How accurately do novice surgeons place thoracic pedicle screws with the free hand technique? Spine. 2008;33:E501-E507.
, Browd SR, Ragel BT, Davis GE, et al. Prophylaxis for deep venous thrombosis in neurosurgery: a review of the literature. Neurosurg Focus. 2004;17(4):E1.
, Brown CA, Lenke LG, Bridwell KH, et al. Complications of pediatric thoracolumbar and lumbar pedicle screws. Spine. 1998;23:1566-1571.
, Dickinson LD, Miller LD, Patel CP, et al. Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep venous thrombosis prophylaxis in patients with brain tumors. Neurosurgery. 1998;43:1074-1081.
, Forbes SS, Stephen WJ, Harper WL, et al. Implementation of evidence-based practices for surgical site infection prophylaxis: results of a pre- and postintervention study. J Am Coll Surg. 2008;207:336-341.
, Geisler FH, Laich DT, Goldflies M, et al. Anterior tibial compartment syndrome as a positioning complication of the prone-sitting position for lumbar surgery. Neurosurgery. 1993;33:1117.
, Grossman MG, Ducey SA, Nadler SS, et al. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9:336-344.
, Jaffe TB, McLeskey CH. Position-induced Horner’s syndrome. Anesthesiology. 1982;56:49-50.
, Kondziolka D, Firlik A, Lunsford L. Complications of stereotactic brain surgery. Neurol Clin. 1998;16:35-54.
, Kothbauer KF, Deletis V, Epstein FJ. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus. 1998;4(5):e1.
, McAfee PC, Regan JR, Zdeblick T, et al. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery. A prospective multicenter study comprising the first 100 consecutive cases. Spine. 1995;20:1624-1632.
, Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol. 2008;145:604-610.
, Paniello RC, Martin-Bredahl KJ, Henkener LJ, et al. Preoperative laryngeal nerve screening for revision anterior cervical spine procedures. Ann Otol Rhinol Laryngol. 2008;117:594-597.
, Roth S, Tung A, Ksiazek S. Visual loss in a prone-positioned spine surgery patient with the head on a foam headrest and goggles covering the eyes: an old complication with a new mechanism. Anesth Analg. 2007;104:1185-1187.
Schierhout Schierhout G, Roberts I. Anti-epileptic drugs for preventing seizures following acute traumatic brain injury. Cochrane Database Syst Rev. 2000;2:CD000173.
, Schwartz DM, Drummond DS, Hahn M, et al. Prevention of positional brachial plexopathy during surgical correction of scoliosis. J Spinal Disord. 2000;13:178-182.
, Vossler DG, Stonecipher T, Millen MD. Femoral artery ischemia during spinal scoliosis surgery detected by posterior tibial nerve somatosensory-evoked potential monitoring. Spine. 2000;25:1457-1459.
1 Guyer RD, Siddiqui S, Zigler JE, et al. Lumbar spinal arthroplasty: analysis of one center’s twenty best and twenty worst clinical outcomes. Spine. 2008;33:2566-2569.
2 Rohan MXJr, Ohnmeiss DD, Guyer RD, et al. Relationship between the length of time off work preoperatively and clinical outcome at 24-month follow-up in patients undergoing total disc replacement or fusion. Spine J. 2008.
3 Steinmetz MP, Patel R, Traynelis V, et al. Cervical disc arthroplasty compared with fusion in a workers’ compensation population. Neurosurgery. 2008;63:741-747.
4 Rasmussen C, Leboeuf-Yde C, Hestbaek L, et al. Poor outcome in patients with spine-related leg or arm pain who are involved in compensation claims: a prospective study of patients in the secondary care sector. Scand J Rheumatol. 2008;37:462-468.
5 Atlas SJ, Chang Y, Keller RB, et al. The impact of disability compensation on long-term treatment outcomes of patients with sciatica due to a lumbar disc herniation. Spine (Phila Pa 1976). 2006;31:3061-3069.
6 Maghout Juratli S, Franklin GM, Mirza SK, et al. Lumbar fusion outcomes in Washington State workers’ compensation. Spine (Phila Pa 1976). 2006;31:2715-2723.
7 Anderson PA, Schwaegler PE, Cizek D, et al. Work status as a predictor of surgical outcome of discogenic low back pain. Spine (Phila Pa 1976). 2006;31:2510-2515.
8 Forbes SS, Stephen WJ, Harper WL, et al. Implementation of evidence-based practices for surgical site infection prophylaxis: results of a pre- and postintervention study. J Am Coll Surg. 2008;207:336-341.
9 Baumann SB, Welch WC, Bloom MJ. Intraoperative SSEP detection of ulnar nerve compression or ischemia in an obese patient: a unique complication associated with a specialized spinal retraction system. Arch Phys Med Rehabil. 2000;81:130-132.
10 Bekar A, Tureyen K, Aksoy K. Unilateral blindness due to patient positioning during cervical syringomyelia surgery: unilateral blindness after prone position. J Neurosurg Anesthesiol. 1996;8:227-229.
11 Bertalanffy H, Eggert HR. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta Neurochir (Wien). 1989;99:41-50.
12 Geisler FH, Laich DT, Goldflies M, et al. Anterior tibial compartment syndrome as a positioning complication of the prone-sitting position for lumbar surgery. Neurosurgery. 1993;33:1117.
13 Grossman MG, Ducey SA, Nadler SS, et al. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9:336-344.
14 Holekamp NM, Meredith TA, Landers MB, et al. Ulnar neuropathy as a complication of macular hole surgery. Arch Ophthalmol. 1999;117:1607-1610.
15 Hoski JJ, Eismont FJ, Green BA. Blindness as a complication of intraoperative positioning: a case report. J Bone Joint Surg Am. 1993;75:1231-1232.
16 Huber JF, Grob D. Bilateral cortical blindness after lumbar spine surgery: a case report. Spine. 1998;23:1807-1809.
17 Manfredini M, Ferrante R, Gildone A, et al. Unilateral blindness as a complication of intraoperative positioning for cervical spinal surgery. J Spinal Disord. 2000;13:271-272.
18 Merle H, Delattre O, Trode M, et al. [Central retinal artery occlusion in surgery of the cervical vertebrae.]. J Fr Ophthalmol. 1994;17:603-607.
19 Miura Y, Mimatsu K, Iwata H. Massive tongue swelling as a complication after spinal surgery. J Spinal Disord. 1996;9:339-341.
20 Slone RM, MacMillan M, Montgomery WJ. Spinal fixation. Part 3. Complications of spinal instrumentation. Radiographics. 1993;13:797-816.
21 Wolfe SW, Lospinuso MF, Burke SW. Unilateral blindness as a complication of patient positioning for spinal surgery: a case report. Spine. 1992;17:600-605.
22 Schwartz DM, Drummond DS, Hahn M, et al. Prevention of positional brachial plexopathy during surgical correction of scoliosis. J Spinal Disord. 2000;13:178-182.
23 Schwartz DM, Sestokas AK, Hilibrand AS, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20:437-444.
24 Labrom RD, Hoskins M, Reilly CW, et al. Clinical usefulness of somatosensory evoked potentials for detection of brachial plexopathy secondary to malpositioning in scoliosis surgery. Spine. 2005;30:2089-2093.
25 Grossman MG, Ducey SA, Nadler SS, et al. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9:336-344.
26 Akagi S, Yoshida Y, Kato I, et al. External iliac artery occlusion in posterior spinal surgery. Spine. 1999;24:823-825.
27 Vossler DG, Stonecipher T, Millen MD. Femoral artery ischemia during spinal scoliosis surgery detected by posterior tibial nerve somatosensory-evoked potential monitoring. Spine. 2000;25:1457-1459.
28 Kostuik JP, Maurais GR, Richardson WJ, et al. Combined single stage anterior and posterior osteotomy for correction of iatrogenic lumbar kyphosis. Spine. 1988;13:257-266.
29 Yu YH, Chen WJ, Chen LH, et al. Ischemic orbital compartment syndrome after posterior spinal surgery. Spine. 2008;33(16):E569-E572.
30 Heitz JW, Audu PB. Asymmetric postoperative visual loss after spine surgery in the lateral decubitus position. Br J Anaesth. 2008;101:380-382.
31 Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol. 2008;145:604-610.
32 Roth S, Tung A, Ksiazek S. Visual loss in a prone-positioned spine surgery patient with the head on a foam headrest and goggles covering the eyes: an old complication with a new mechanism. Anesth Analg. 2007;104:1185-1187.
33 Stambough JL, Dolan D, Werner R, et al. Ophthalmologic complications associated with prone positioning in spine surgery. J Am Acad Orthop Surg. 2007;15:156-165.
34 Jaffe TB, McLeskey CH. Position-induced Horner’s syndrome. Anesthesiology. 1982;56:49-50.
35 Baba H, Maezawa Y, Imura S, et al. Spinal cord evoked potential monitoring for cervical and thoracic compressive myelopathy. Paraplegia. 1996;34:100-106.
36 Bednarik J, Kadanka Z, Vohanka S, et al. The value of somatosensory and motor-evoked potentials in predicting and monitoring the effect of therapy in spondylotic cervical myelopathy: prospective randomized study. Spine. 1999;24:1593-1598.
37 Dennis GC, Dehkordi O, Millis RM, et al. Somatosensory evoked potential, neurological examination and magnetic resonance imaging for assessment of cervical spinal cord decompression. Life Sci. 2000;66:389-397.
38 Padberg AM, Bridwell KH. Spinal cord monitoring: current state of the art. Orthop Clin North Am. 1999;30:407-433. viii
39 Sloan TB. Evoked potential monitoring. Int Anesthesiol Clin. 1996;34:109-136.
40 Balzer JR, Rose RD, Welch WC, et al. Simultaneous somatosensory evoked potential and electromyographic recordings during lumbosacral decompression and instrumentation. Neurosurgery. 1998;42:1318-1324.
41 Deutsch H, Arginteanu M, Manhart K, et al. Somatosensory evoked potential monitoring in anterior thoracic vertebrectomy. J Neurosurg. 2000;92(suppl):155-161.
42 Fujimoto Y, Baba I, Sumida T, et al. Microsurgical transdural discectomy with laminoplasty: new treatment for paracentral and paracentroforaminal cervical disc herniation associated with spinal canal stenosis. Spine. 2002;27:715-721.
43 Fujioka H, Shimoji K, Tomita M, et al. Spinal cord potential recordings from the extradural space during scoliosis surgery. Br J Anaesth. 1994;73:350-356.
44 Gokaslan ZL. Spine surgery for cancer. Curr Opin Oncol. 1996;8:178-181.
45 Kitagawa H, Itoh T, Takano H, et al. Motor evoked potential monitoring during upper cervical spine surgery. Spine. 1989;14:1078-1083.
46 Lang EW, Chesnut RM, Beutler AS, et al. The utility of motor evoked potential monitoring during intramedullary surgery. Anesth Analg. 1996;83:1337-1341.
47 Manninen PH. Monitoring evoked potentials during spinal surgery in one institution. Can J Anaesth. 1998;45:460-465.
48 Mineiro J, Weinstein SL. Delayed postoperative paraparesis in scoliosis surgery: a case report. Spine. 1997;22:1668-1672.
49 Kothbauer KF, Deletis V, Epstein FJ. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus. 1998;4(5):e1.
50 Nimityongskul P, Bose WJ, Hurley DPJr, et al. Superficial temporal artery laceration: a complication of skull tong traction. Orthop Rev. 1992;21:761. 764-765
51 Copley LA, Pepe MD, Tan V, et al. A comparison of various angles of halo pin insertion in an immature skull model. Spine. 1999;24:1777-1780.
52 Kameyama O, Ogawa K, Suga T, et al. Asymptomatic brain abscess as a complication of halo orthosis: report of a case and review of the literature. J Orthop Sci. 1999;4:39-41.
53 Muller EJ, Wick M, Muhr G. [Subdural abscess as a complication of halo fixator.]. Unfallchirurg. 1998;101:655-657.
54 Nottmeier EW, Bondurant CP. Delayed onset of generalized tonic-clonic seizures as a complication of halo orthosis: case report. J Neurosurg. 2000;92(suppl):233-235.
55 Voor MJ, Anderson RC, Hart RT. Stress analysis of halo pin insertion by non-linear finite element modeling. J Biomech. 1997;30:903-909.
56 Williams FH, Nelms DK, McGaharan KM. Brain abscess: a rare complication of halo usage. Arch Phys Med Rehabil. 1992;73:490-492.
57 Lee M, Rezai AR, Chou J. Depressed skull fractures in children secondary to skull clamp fixation devices. Pediatr Neurosurg. 1994;21:174-177.
58 Grinberg F, Slaughter TF, McGrath BJ. Probable venous air embolism associated with removal of the Mayfield skull clamp. Anesth Analg. 1995;80:1049-1050.
59 Yan HJ. Epidural hematoma following use of a three-point skull clamp. J Clin Neurosci. 2007;14:691-693.
60 Sgouros S, Grainger MC, McCallin S. Adaptation of skull clamp for use in image-guided surgery of children in the first 2 years of life. Childs Nerv Syst. 2005;21:148-149.
61 Berger GS, Peterson B. Pressure alopecia after microsurgical anastomosis. Am J Obstet Gynecol. 1978;131:704.
62 Boyer JD, Vidmar DA. Postoperative alopecia: a case report and literature review. Cutis. 1994;54:321-322.
63 Bruce IA, Simmons MA, Hampal S. “Horseshoe-shaped” postoperative alopecia following lengthy head and neck surgery. J Laryngol Otol. 2002;116:230-232.
64 Gormley TP, Sokoll MD. Permanent alopecia from pressure of a head strap. JAMA. 1967;199:747-748.
65 Lwason NW, Mills NL, Ochsner JL. Occipital alopecia following cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1976;71:342-347.
66 Neal PR, Merk PF, Norins AL. Halo scalp ring: a form of localized scalp injury associated with caput succedaneum. Pediatr Dermatol. 1984;2:52-54.
67 Wiles JC, Hansen RC. Postoperative (pressure) alopecia. J Am Acad Dermatol. 1985;12:195-198.
68 Black S, Muzzi DA, Nishimura RA, et al. Preoperative and intraoperative echocardiography to detect right-to-left shunt in patients undergoing neurosurgical procedures in the sitting position. Anesthesiology. 1990;72:436-438.
69 Cabezudo JM, Gilsanz F, Vaquero J, et al. Air embolism from wounds from a pin-type head-holder as a complication of posterior fossa surgery in the sitting position: case report. J Neurosurg. 1981;55:147-148.
70 du Toit HJ, Rose-Innes AP, le Roux D, et al. Quadriplegia following venous air embolism during posterior fossa exploration: a case report. S Afr Med J. 1983;63:378-379.
71 McCarthy RE, Lonstein JE, Mertz JD, et al. Air embolism in spinal surgery. J Spinal Disord. 1990;3:1-5.
72 Palmon SC, Moore LE, Lundberg J, et al. Venous air embolism: a review. J Clin Anesth. 1997;9:251-257.
73 Roe BB. Air embolism prevention. Ann Thorac Surg. 1987;44:212-213.
74 Kytta J, Randell T, Tanskanen P, et al. Monitoring lung compliance and end-tidal oxygen content for the detection of venous air embolism. Br J Anaesth. 1995;75:447-451.
75 Nehls DG, Carter LP. Air embolism through a ventriculoatrial shunt during posterior fossa operation: case report. Neurosurgery. 1985;16:83-84.
76 Sutherland RW, Winter RJ. Two cases of fatal air embolism in children undergoing scoliosis surgery. Acta Anaesthesiol Scand. 1997;41:1073-1076.
77 von Gosseln HH, Samii M, Suhr D, et al. The lounging position for posterior fossa surgery: anesthesiological considerations regarding air embolism. Childs Nerv Syst. 1991;7:368-374.
78 Hooper AK, Okun MS, Foote KD, et al. Venous air embolism in deep brain stimulation. Stereotact Funct Neurosurg. 2008;87:25-30.
79 Bucek RA, Koca N, Reiter M, et al. Algorithms for the diagnosis of deep-vein thrombosis in patients with low clinical pretest probability. Thromb Res. 2002;105:43-47.
80 Dearborn JT, Hu SS, Tribus CB, et al. Thromboembolic complications after major thoracolumbar spine surgery. Spine. 1999;24:1471-1476.
81 Ferree BA, Stern PJ, Jolson RS, et al. Deep venous thrombosis after spinal surgery. Spine. 1993;18:315-319.
82 Gertzbein SD, Betz R, Clements D, et al. Semirigid instrumentation in the management of lumbar spinal conditions combined with circumferential fusion: a multicenter study. Spine. 1996;21:1918-1925.
83 Lee HM, Suk KS, Moon SH, et al. Deep vein thrombosis after major spinal surgery: incidence in an East Asian population. Spine. 2000;25:1827-1830.
84 Levi AD, Wallace MC, Bernstein M, et al. Venous thromboembolism after brain tumor surgery: a retrospective review. Neurosurgery. 1991;28:859-863.
85 Marras LC, Geerts WH, Perry JR. The risk of venous thromboembolism is increased throughout the course of malignant glioma: an evidence-based review. Cancer. 2000;89:640-646.
86 Melamed AJ, Suarez J. Detection and prevention of deep venous thrombosis. Drug Intell Clin Pharm. 1988;22:107-114.
87 Mol WE, Egberts TC. Prophylaxis for venous thromboembolism in hip fracture surgery: total costs and cost effectiveness in The Netherlands. Pharmacoeconomics. 1994;5:48-55.
88 Morgan MK, Sundt TMJr. The case against staged operative resection of cerebral arteriovenous malformations. Neurosurgery. 1989;25:429-435.
89 Oda T, Fuji T, Kato Y, et al. Deep venous thrombosis after posterior spinal surgery. Spine. 2000;25:2962-2967.
90 Swann K, Black P, Baker M. Management of symptomatic deep venous thrombosis and pulmonary embolism on the neurosurgical service. J Neurosurg. 1986;64:563-567.
91 Weis JC, Betz RR, Clements DH3rd, et al. Prevalence of perioperative complications after anterior spinal fusion for patients with idiopathic scoliosis. J Spinal Disord. 1997;10:371-375.
92 Greer IA, Thomson AJ. Management of venous thromboembolism in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2001;15:583-603.
93 Johnson MS. Current strategies for the diagnosis of pulmonary embolus. J Vasc Interv Radiol. 2002;13:13-23.
94 Dryjski M, O’Brien-Irr MS, Harris LM, et al. Evaluation of a screening protocol to exclude the diagnosis of deep venous thrombosis among emergency department patients. J Vasc Surg. 2001;34:1010-1015.
95 Fraser DG, Moody AR, Morgan PS, et al. Diagnosis of lower limb deep venous thrombosis: a prospective blinded study of magnetic resonance direct thrombus imaging. Ann Intern Med. 2002;136:89-98.
96 Owings JT, Gosselin RC, Anderson JT, et al. Practical utility of the D-dimer assay for excluding thromboembolism in severely injured trauma patients. J Trauma. 2001;51:425-429.
97 Petrakis IE, Sciacca V, Katsamouris AN. Upper extremities deep venous thrombosis: comparison of light reflection rheography and colour duplex ultrasonography for diagnosis and followup. Panminerva Med. 2001;43:69-75.
98 Ricciotti HA, Wong GP, Ludmir J. Deep venous thrombosis treated with an inferior vena cava filter in a pregnant woman after recent neurosurgery: a case report. J Reprod Med. 1995;40:404-406.
99 Rosen CL, Tracy JA. The diagnosis of lower extremity deep venous thrombosis. Emerg Med Clin North Am. 2001;19:895-912. vi
100 Savage KJ, Wells PS, Schulz V, et al. Outpatient use of low molecular weight heparin (Dalteparin) for the treatment of deep vein thrombosis of the upper extremity. Thromb Haemost. 1999;82:1008-1010.
101 Schraibman IG, Lewis B, Parmar JR. Clinical comparison of elastic supports for venous diseases of the lower limb and thrombosis prevention. Phlebologie. 1982;35:61-71.
102 Wen DY, Hall WA. Complications of subcutaneous low-dose heparin therapy in neurosurgical patients. Surg Neurol. 1998;50:521-525.
103 Dickinson LD, Miller LD, Patel CP, et al. Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep venous thrombosis prophylaxis in patients with brain tumors. Neurosurgery. 1998;43:1074-1081.
104 Estrada CA, Mansfield CJ, Heudebert GR. Cost-effectiveness of low-molecular-weight heparin in the treatment of proximal deep vein thrombosis. J Gen Intern Med. 2000;15:108-115.
105 Heaton D, Pearce M. Low molecular weight versus unfractionated heparin: a clinical and economic appraisal. Pharmacoeconomics. 1995;8:91-99.
106 Kakkar VV. Cost-effectiveness of the low molecular weight heparin reviparin sodium in thromboprophylaxis. Thromb Res. 1996;81(suppl):S75-S77.
107 Matzsch T. Thromboprophylaxis with low-molecular-weight heparin: economic considerations. Haemostasis. 2000;30(suppl 2):141-145.
108 Collen JF, Jackson JL, Shorr AF, et al. Prevention of venous thromboembolism in neurosurgery: a metaanalysis. Chest. 2008;134:237-249.
109 Chibbaro S, Tacconi L. Safety of deep venous thrombosis prophylaxis with low-molecular-weight heparin in brain surgery. Prospective study on 746 patients. Surg Neurol. 2008;70:117-121.
110 Danish SF, Burnett MG, Ong JG, et al. Prophylaxis for deep venous thrombosis in craniotomy patients: a decision analysis. Neurosurgery. 2005;56:1286-1292.
111 Browd SR, Ragel BT, Davis GE, et al. Prophylaxis for deep venous thrombosis in neurosurgery: a review of the literature. Neurosurg Focus. 2004;17(4):E1.
112 Cain JEJr, Major MR, Lauerman WC, et al. The morbidity of heparin therapy after development of pulmonary embolus in patients undergoing thoracolumbar or lumbar spinal fusion. Spine. 1995;20:1600-1603.
113 Gossage JR. Early intervention in massive pulmonary embolism: a guide to diagnosis and triage for the critical first hour. Postgrad Med. 2002;111:27-28. 33-34, 39-40
114 Lee LC, Shah K. Clinical manifestation of pulmonary embolism. Emerg Med Clin North Am. 2001;19:925-942.
115 Luckraz H, Dunning J. Pulmonary thromboendarterectomy. Ann R Coll Surg Engl. 2001;83:427-430.
116 Weiner SG, Burstein JL. Nonspecific tests for pulmonary embolism. Emerg Med Clin North Am. 2001;19:943-955.
117 Schoepf UJ, Schneider AC, Das M, et al. Pulmonary embolism: computer-aided detection at multidetector row spiral computed tomography. J Thorac Imaging. 2007;22:319-323.
118 Spanier DE, Stambough JL. Delayed postoperative epidural hematoma formation after heparinization in lumbar spinal surgery. J Spinal Disord. 2000;13:46-49.
119 Chozick B, Reinert S, Greenblatt S. Incidence of seizures after surgery for supratentorial meningiomas: a modern analysis. J Neurosurg. 1996;84:382-386.
120 Clinchot DM, Kaplan P, Murray DM, et al. Cerebral aneurysms and arteriovenous malformations: implications for rehabilitation. Arch Phys Med Rehabil. 1994;75:1342-1351.
121 Faccenda KA, Finucane BT. Complications of regional anaesthesia: incidence and prevention. Drug Saf. 2001;24:413-442.
122 Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional analysis of complication outcomes after arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys. 1999;44:67-74.
123 Foy P, Copeland G, Sawy M. The incidence of postoperative seizures. Acta Neurochir (Wien). 1981;55:253-264.
124 Fukamachi A, Koizumi H, Nukui H. Immediate postoperative seizures: incidence and computed tomographic findings. Surg Neurol. 1985;24:671-676.
125 Gerstle de Pasquet E, Pietra M, Iniquez RA. Epileptic seizures as an early complication of neurosurgery. Acta Neurol Latinoam. 1976;22:144-151.
126 Hayashi T, Hadeishi H, Kawamura S, et al. Postoperative anticonvulsant prophylaxis for patients treated for cerebral aneurysms. Neurol Med Chir (Tokyo). 1999;39:828-833.
127 Kvam DA, Loftus CM, Copeland B, Quest DO. Seizures during the immediate postoperative period. Neurosurgery. 1983;12:14-17.
128 Lee ST, Lui TN, Chang CN, et al. Early postoperative seizures after posterior fossa surgery. J Neurosurg. 1990;73:541-544.
129 North JB, Penhall RK, Hanieh A, et al. Phenytoin and postoperative epilepsy: a double-blind study. J Neurosurg. 1983;58:672-677.
130 Salanova V, Markand O, Worth R. Temporal lobe epilepsy surgery: outcome, complications, and late mortality rate in 215 patients. Epilepsia. 2002;43:170-174.
131 Telfeian AE, Philips MF, Crino PB, et al. Postoperative epilepsy in patients undergoing craniotomy for glioblastoma multiforme. J Exp Clin Cancer Res. 2001;20:5-10.
132 Wang EC, Geyer JR, Berger MS. Incidence of postoperative epilepsy in children following subfrontal craniotomy for tumor. Pediatr Neurosurg. 1994;21:165-172.
133 Ebel H, Kuchta J, Balogh A, et al. Operative treatment of tentorial herniation in herpes encephalitis. Childs Nerv Syst. 1999;15:84-86.
134 Kieburtz K, Ricotta JJ, Moxley RT3rd. Seizures following carotid endarterectomy. Arch Neurol. 1990;47:568-570.
135 Whittle IR, Beaumont A. Seizures in patients with supratentorial oligodendroglial tumours: clinicopathological features and management considerations. Acta Neurochir (Wien). 1995;135:19-24.
136 Yeh HS, Yeh HS, Blisard K, et al. Surgical management of epilepsy associated with cerebral arteriovenous malformations. J Neurosurg. 1990;72:216-223.
137 Cowie R, Williams B. Late seizures and morbidity after subdural empyema. J Neurosurg. 1983;58:569-573.
138 Kaplan SL, Woods CR. Neurologic complications of bacterial meningitis in children. Curr Clin Top Infect Dis. 1992;12:37-55.
139 Rosenfeld EA, Rowley AH. Infectious intracranial complications of sinusitis, other than meningitis, in children: 12-year review. Clin Infect Dis. 1994;18:750-754.
140 Smith HP, Hendrick EB. Subdural empyema and epidural abscess in children. J Neurosurg. 1983;58:392-397.
141 Yen PT, Chan ST, Huang TS. Brain abscess: with special reference to otolaryngologic sources of infection. Otolaryngol Head Neck Surg. 1995;113:15-22.
142 Buitrago M, Edwards B, Rosner F. Neurocysticercosis: report of fifteen cases. Mt Sinai J Med. 1995;62:439-444.
143 Chadduck W, Adametz J. Incidence of seizures in patients with myelomeningocele: a multifactorial analysis. Surg Neurol. 1988;30:281-285.
144 Johnson DL, Conry J, O’Donnell R. Epileptic seizure as a sign of cerebrospinal fluid shunt malfunction. Pediatr Neurosurg. 1996;24:223-227.
145 Klepper J, Busse M, Strassburg HM, et al. Epilepsy in shunt-treated hydrocephalus. Dev Med Child Neurol. 1998;40:731-736.
146 Loftus CM, Copeland BR, Carmel PW. Cystic supratentorial gliomas: natural history and evaluation of modes of surgical therapy. Neurosurgery. 1985;17:19-24.
147 Patir R, Banerji AK. Complications related to pre-craniotomy shunts in posterior fossa tumours. Br J Neurosurg. 1990;4:387-390.
148 Stellman GR, Bannister CM, Hillier V. The incidence of seizure disorder in children with acquired and congenital hydrocephalus. Z Kinderchir.. 1986;41(suppl 1):38-41.
149 Suri A, Mahapatra AK, Bithal P. Seizures following posterior fossa surgery. Br J Neurosurg. 1998;12:41-44.
150 Schierhout G, Roberts I. Anti-epileptic drugs for preventing seizures following acute traumatic brain injury. Cochrane Database Syst Rev. 2001;4:CD000173.
151 Schierhout G, Roberts I. Anti-epileptic drugs for preventing seizures following acute traumatic brain injury. Cochrane Database Syst Rev. 2000;2:CD000173.
152 Temkin NR. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia. 2001;42:515-524.
153 Tsolaki M, Yannacou-Peftoulidou M, Grammaticos P, et al. Phenytoin plasma levels after intraoperative administration, for the prevention of post-craniotomy seizures. Acta Neurochir (Wien). 1987;84:36-38.
154 Samii M, Cordula M. Management of 1000 vestibular schwannomas: hearing function in 1000 tumor resections. Neurosurgery. 1997;40:248-259.
155 Post K, Eisenberg M, Catalano P. Hearing preservation in vestibular schwannoma surgery: what factors influence outcome? J Neurosurg. 1995;83:191-196.
156 Wade P, House W. Hearing preservation in patients with acoustic neuromas via the middle fossa approach. Otolaryngol Head Neck Surg. 1984;92:184-193.
157 De Santis A, Villani R, Sinisi M, et al. Add-on phenytoin fails to prevent early seizures after surgery for supratentorial brain tumors: a randomized controlled study. Epilepsia. 2002;43:175-182.
158 Auer LM, Mokry M. Disturbed cerebrospinal fluid circulation after subarachnoid hemorrhage and acute aneurysm surgery. Neurosurgery. 1990;26:804-808.
159 Coplin WM. Intracranial pressure and surgical decompression for traumatic brain injury: biological rationale and protocol for a randomized clinical trial. Neurol Res. 2001;23:277-290.
160 Csokay A, Nagy L, Novoth B. Avoidance of vascular compression in decompressive surgery for brain edema caused by trauma and tumor ablation. Neurosurg Rev. 2001;24:209-213.
161 Gaab MR, Rittierodt M, Lorenz M, et al. Traumatic brain swelling and operative decompression: a prospective investigation. Acta Neurochir Suppl. 1990;51:326-328.
162 Lafuente JV, Pouschman E, Cervos-Navarro J, et al. Dynamics of tracer distribution in radiation induced brain oedema in rats. Acta Neurochir Suppl. 1990;51:375-377.
163 Lownie S, Wu X, Karlik S, et al. Brain retractor edema during induced hypotension: the effect of the rate of return of blood pressure. Neurosurgery. 1990;27:901-905.
164 Meixensberger J, Dings J, Kuhnigk H, et al. Studies of tissue PO2 in normal and pathological human brain cortex. Acta Neurochir Suppl. 1993;59:58-63.
165 Rinaldi A, Mangiola A, Anile C, et al. Hemodynamic effects of decompressive craniectomy in cold induced brain oedema. Acta Neurochir Suppl. 1990;51:394-396.
166 Umezawa H, Shima K, Chigasaki H, et al. Local cerebral blood flow and glucose metabolism in hydrostatic brain oedema. Acta Neurochir Suppl. 1990;51:351-353.
167 The Brain Trauma Foundation, The American Association of Neurological Surgeons, The Joint Section on Neurotrauma and Critical Care. Guidelines for cerebral perfusion pressure. J Neurotrauma. 2000;17:507-511.
168 Allen CH, Ward JD. An evidence-based approach to management of increased intracranial pressure. Crit Care Clin. 1998;14:485-495.
169 Kunze E, Meixensberger J, Janka M, et al. Decompressive craniectomy in patients with uncontrollable intracranial hypertension. Acta Neurochir Suppl. 1998;71:16-18.
170 Morgan MK, Johnston IH, Sundt TMJr. Normal perfusion pressure breakthrough complicating surgery for the vein of Galen malformation: report of three cases. Neurosurgery. 1989;24:406-410.
171 Palmer J. Management of raised intracranial pressure in children. Intensive Crit Care Nurs. 2000;16:319-327.
172 Suarez JI, Qureshi AI, Bhardwaj A, et al. Treatment of refractory intracranial hypertension with 23.4% saline. Crit Care Med. 1998;26:1118-1122.
173 Young WL, Kader A, Ornstein E, et al. Cerebral hyperemia after arteriovenous malformation resection is related to “breakthrough” complications but not to feeding artery pressure. The Columbia University Arteriovenous Malformation Study Project. Neurosurgery. 1996;38:1085-1093.
174 Koenig MA, Bryan M, Lewin JL3rd, et al. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70:1023-1029.
175 Himmelseher S. Hypertonic saline solutions for treatment of intracranial hypertension. Curr Opin Anaesthesiol. 2007;20:414-426.
176 Fadul C, Wood J, Thaler H. Morbidity and mortality of craniotomy for excision of supratentorial gliomas. Neurology. 1988;38:1374-1379.
177 Cabantog A, Bernstein M. Complications of first craniotomy for intra-axial brain tumour. Can J Neurol Sci. 1994;21:213-218.
178 Sawaya R, Hammoud M, Schoppa D. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044-1055.
179 Taylor M, Bernstein M. Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: A prospective trial of 200 cases. J Neurosurg. 1999;90:35-41.
180 Wu JS, Zhou LF, Tang WJ, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61:935-948.
181 Nilsson D, Rutka JT, Snead OC3rd, et al. Preserved structural integrity of white matter adjacent to low-grade tumors. Childs Nerv Syst. 2008;24:313-320.
182 Nimsky C, Grummich P, Sorensen AG, et al. Visualization of the pyramidal tract in glioma surgery by integrating diffusion tensor imaging in functional neuronavigation. Zentralbl Neurochir. 2005;66:133-141.
183 Nimsky C, Ganslandt O, Hastreiter P, et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005;56:130-137.
184 Brisman MH, Bederson JB, Sen CN, et al. Intracerebral hemorrhage occurring remote from the craniotomy site. Neurosurgery. 1996;39:1114-1121.
185 Choucair A, Silver P, Levin V. Risk of intracranial hemorrhage in glioma patients receiving anticoagulant therapy. J Neurosurg. 1987;66:357-358.
186 Mandl ES, Dirven CM, Buis DR, et al. Repeated surgery for glioblastoma multiforme: only in combination with other salvage therapy. Surg Neurol. 2008;69:506-509.
187 Ramamurthi B, Ravi R, Ramachandran V. Convulsions with meningiomas: incidence and significance. Surg Neurol. 1980;14:415-416.
188 Chan R, Thompson G. Morbidity, mortality, and quality of life following surgery for intracranial meningiomas. J Neurosurg. 1984;60:52-60.
189 Pertuiset B, Farah S, Clayes L. Operability of intracranial meningiomas, personal series of 353 cases. Acta Neurochir (Wien). 1985;76:2-11.
190 Sa M, Quest D. Complications in the surgery of meningiomas. In: Post K, Friedman E, McCormick P, editors. Postoperative Complications in Intracranial Neurosurgery. New York: Thieme, 1993.
191 Awad I, Kalfas I, Hahn J. Intracranial meningiomas in the aged. Surgical outcome in the era of computed tomography. Neurosurgery. 1989;24:557-560.
192 Djindjian R, Caron J, Athayde A. Intracranial meningiomas in the elderly (over 70 years old). Acta Neurochir (Wien). 1988;90:121-123.
193 Beks W, deWindt H. The recurrence of supratentorial meningiomas after surgery. Acta Neurochir (Wien). 1988;95:3-5.
194 Giombini S, Solero C, Lasio G. Immediate and late outcome of operations for parasagittal and falx meningiomas. Surg Neurol. 1984;21:427-435.
195 Giombini S, Solero S, Morello G. Late outcome of operations for supratentorial convexity meningiomas. Surg Neurol. 1984;22:588-594.
196 DeMonte F, Smith H, Al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245-251.
197 O’Sullivan M, Loveren HV, Tew J. The surgical resectability of meningiomas of the cavernous sinus. Neurosurgery. 1997;40:238-242.
198 Tator C. Petrous meningiomas. In: Apuzzo M, editor. Brain Surgery. New York: Churchill Livingstone; 1993:1727-1739.
199 Harner S, Beatty C, Ebersold M. Retrosigmoid removal of acoustic neuroma: experience 1978-1988. Otolaryngol Head Neck Surg. 1990;103:40-45.
200 Gormley W, Sekhar LN, Wright DC, et al. Acoustic neuromas: results of current surgical management. Neurosurgery. 1997;41:50-58.
201 Samii M, Matthies C. Management of 1000 vestibular schwannomas: surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40:1-23.
202 DiTullio M, Malkasian D, Rand R. A critical comparison of neurosurgical and otolaryngological approaches to acoustic neuromas. J Neurosurg. 1978;48:1-12.
203 Hanson M, Glasscock ME3rd, Brandes JL, et al. Medical treatment of headache after suboccipital acoustic tumor removal. Laryngoscope. 1998;108:1111-1114.
204 Reidal C, Post K. Complications in the treatment of acoustic neuromas. In: Post K, Friedman E, McCormick P, editors. Postoperative Complications in Intracranial Neurosurgery. New York: Thieme; 1993:91-110.
205 Seiler R, Zurbrugg H. Supratentorial intracerebral hemorrhage after posterior fossa operation. Neurosurgery. 1986;18:472-474.
206 Laws E. Transsphenoidal approach to lesions in and about the sella turcica. In: Schmidek H, Sweet W, editors. Operative Neurosurgical Techniques: Indications, Methods, and Results. Philadelphia: WB Saunders; 1998:309-319.
207 Wilson C. A decade of pituitary microsurgery. The Herbert Olivecrona lecture. J Neurosurg. 1984;48:13-22.
208 Lee K. The sublabial transseptal transsphenoidal approach to the hypophysis. Laryngoscope. 1978;88:1-65.
209 Kennedy D, Cohn E, Papel I. Transsphenoidal approach to the sella: The Johns Hopkins experience. Laryngoscope. 1984;94:1066-1074.
210 Black P, Zervas N, Candia G. Incidence and management of complications of transsphenoidal operation for pituitary adenomas. Neurosurgery. 1987;20:920-924.
211 Newmark H3rd, Kant N, Duerksen R, et al. Orbital floor fracture: an unusual complication of trans-septal transsphenoidal hypophysectomy. Neurosurgery. 1983;12:555-556.
212 Onesti S, Post K. Complications of transsphenoidal microsurgery. In: Post K, Friedman E, McCormick P, editors. Postoperative Complications in Intracranial Neurosurgery. New York: Thieme, 1993.
213 Ciric I, Ragin A, Baumgartner C, et al. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225-230.
214 Greenfield JP, Howard BM, Huang C, et al. Endoscopic endonasal transsphenoidal surgery using a skull reference array and laser surface scanning. Minim Invasive Neurosurg. 2008;51:244-246.
215 Koc K, Anik I, Ozdamar D, et al. The learning curve in endoscopic pituitary surgery and our experience. Neurosurg Rev. 2006;29:298-305.
216 Cinalli G, Cappabianca P, de Falco R, et al. Current state and future development of intracranial neuroendoscopic surgery. Expert Rev Med Devices. 2005;2:351-373.
217 Ahuja A, Guerman L, Hopkins L. Carotid cavernous fistula and false aneurysms of the cavernous carotid artery: complications of transsphenoidal surgery. Neurosurgery. 1992;31:774-779.
218 Barrow D, Tindall G. Loss of vision after transsphenoidal surgery. Neurosurgery. 1990;27:60-68.
219 Nakane T, Kuwayama A, Watanabe M. Transsphenoidal approach to pituitary adenomas with suprasellar extension. Surg Neurol. 1981;16:225-229.
220 Romanowski B, Tyrrell D, Bryce K. Meningitis complicating transsphenoidal hypophysectomy. Can Med Assoc J. 1981;124:1172-1175.
221 Sen C, Sekhar L. Complications of cranial base surgery. In: Post K, Friedman E, McCormick P, editors. Postoperative Complications in Intracranial Neurosurgery. New York: Thieme, 1993.
222 Lloyd KM, DelGaudio JM, Hudgins PA. Imaging of skull base cerebrospinal fluid leaks in adults. Radiology. 2008;248:725-736.
223 Toung T, McPherson R, Donham R. Pneumocephalus: effects of patient position on the incidence and location of aerocele after posterior fossa and upper cervical cord surgery. Anesth Analg. 1986;65:65-70.
224 Erba S, Horton J, Latchaw R. Balloon test occlusion of the internal carotid artery with stable xenon/CT cerebral blood flow imaging. AJNR Am J Neuroradiol. 1988;9:533-538.
225 Hirsch W, Sekhar LN, Lanzino G, et al. Meningiomas involving the cavernous sinus: value of imaging for predicting surgical complications. AJR Am J Roentgenol. 1993;160:1083-1088.
226 Kondziolka D, Firlik A, Lunsford L. Complications of stereotactic brain surgery. Neurol Clin. 1998;16:35-54.
227 Ostertag C, Mennel H, Kiessling M. Stereotactic biopsy of brain tumors. Surg Neurol. 1980;14:275-283.
228 Lunsford L, Martinez A. Stereotactic exploration of the brain in the era of computed tomography. Surg Neurol. 1984;22:222-230.
229 Appuzzo M. Computed imaging stereotaxy: experience and perspective related to 500 procedures applied to brain masses. Neurosurgery. 1987;20:930-937.
230 Kelly P. Stereotaxis. Philadelphia: WB Saunders; 1991.
231 Voges J, Schroder R, Treuer H. CT-guided and computer-assisted stereotactic biopsy. Acta Neurochir (Wien). 1993;125:142-149.
232 Bernstein M, Parrent A. Complications of CT-guided stereotactic biopsy of intra-axial brain lesions. J Neurosurg. 1994;81:165-168.
233 Hall W. The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer. 1998;82:1749-1755.
234 Chimowitz M, Barnett G, Palmer J. Treatment of intractable arterial hemorrhage during stereotactic brain biopsy with thrombin. J Neurosurg. 1991;74:301-303.
235 Kulkarni A, Guha A, Lozano A, et al. Incidence of silent hemorrhage and delayed deterioration after stereotactic brain biopsy. J Neurosurg. 1998;89:31-35.
236 Skolasky R, Dal Pan GJ, Olivi A, et al. HIV-associated primary CNS morbidity and utility of brain biopsy. J Neurol Sci. 1999;163:32-38.
237 Werner-Wasik M, Rudoler S, Preston P. Immediate side effects of stereotactic radiotherapy and radiosurgery. Int J Radiat Oncol Biol Phys. 1999;43:299-304.
238 Girkin C, Comey CH, Lunsford LD, et al. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology. 1997;104:1634-1643.
239 Morita A, Coffey RJ, Foote RL, et al. Risk of injury to cranial nerves after Gamma Knife radiosurgery for skull base meningiomas: experience in 88 patients. J Neurosurg. 1999;90:42-49.
240 Muracciole X, Regis J. Radiosurgery and carcinogenesis risk. Prog Neurol Surg. 2008;21:207-213.
241 Kondziolka D, Lunsford LD, McLaughlin MR, et al. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339:1426-1433.
242 Thomassin J, Epron JP, Regis J, et al. Preservation of hearing in acoustic neuromas treated by Gamma Knife surgery. Stereotact Funct Neurosurg. 1998;80:74-79.
243 Noren G. Long-term complications following gamma knife radiosurgery of vestibular schwannomas. Stereotact Funct Neurosurg. 1998;70:65-73.
244 Nagano O, Higuchi Y, Serizawa T, et al. Transient expansion of vestibular schwannoma following stereotactic radiosurgery. J Neurosurg. 2008;109:811-816.
245 Abram S, Rosenblatt P, Holcomb S. Stereotactic radiation techniques in the treatment of acoustic schwannomas. 2007. Neurosurg Clin N Am. 2008;19:367-377. vii-viii
246 Vermeulen S, Young R, Posewitz A, et al. Stereotactic radiosurgery toxicity in the treatment of intracanalicular acoustic neuromas: the Seattle Northwest Gamma Knife experience. Stereotact Funct Neurosurg. 1998;70:80-87.
247 Kondziolka D, Levy EI, Niranjan A, et al. Long-term outcomes after meningioma radiosurgery: physician and patient perspective. J Neurosurg. 1999;91:44-50.
248 Subach B, Lunsford LD, Kondziolka D, et al. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery. 1998;42:437-443.
249 Muacevic A, Kreth FW, Horstmann GA, et al. Surgery and radiotherapy compared with Gamma Knife radiosurgery in the treatment of solitary cerebral metastases of small diameter. J Neurosurg. 1999;91:35-43.
250 Chamberlain M, Barba D, Kormanik P, Shea WM. Stereotactic radiosurgery for recurrent glioma. Cancer. 1994;74:1342-1347.
251 Gelblum D, Lee H, Bilsky M, et al. Radiographic findings and morbidity in patients treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 1998;42:391-395.
252 Young R, Vermeulen SS, Grimm P, et al. Gamma knife radiosurgery for treatment of trigeminal neuralgia: idiopathic and tumor related. Neurology. 1997;48:608-614.
253 Agrillo U, Simonetti G, Martino V. Postoperative CSF problems after spinal and lumbar surgery: general review. J Neurosurg Sci. 1991;35:93-95.
254 Jinkins JR, Van Goethem JW. The postsurgical lumbosacral spine. Magnetic resonance imaging evaluation following intervertebral disk surgery, surgical decompression, intervertebral bony fusion, and spinal instrumentation: early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Radiol Clin North Am. 2001;39:1-29.
255 McCormack BM, Taylor SL, Heath S, et al. Pseudomeningocele/CSF fistula in a patient with lumbar spinal implants treated with epidural blood patch and a brief course of closed subarachnoid drainage: a case report. Spine. 1996;21:2273-2276.
256 Stambough JL, Templin CR, Collins J. Subarachnoid drainage of an established or chronic pseudomeningocele. J Spinal Disord. 2000;13:39-41.
257 Waisman M, Schweppe Y. Postoperative cerebrospinal fluid leakage after lumbar spine operations: conservative treatment. Spine. 1991;16:52-53.
258 Al-Yamany M, del Maestro RF. Prevention of subdural fluid collections following transcortical intraventricular and/or para-ventricular procedures by using fibrin adhesive. J Neurosurg. 2000;92:406-412.
259 Dunn CJ, Goa KL. Fibrin sealant: a review of its use in surgery and endoscopy. Drugs. 1999;58:863-886.
260 Knoringer P. [Percutaneous fibrin gluing in subcutaneous cerebrospinal fluid fistulas following operations on the brain and spinal cord.]. Zentralbl Neurochir. 1985;46:256-262.
261 Pomeranz S, Constantini S, Umansky F. The use of fibrin sealant in cerebrospinal fluid leakage. Neurochirurgia (Stuttg). 1991;34:166-169.
262 Siedentop KH, O’Grady K, Park JJ, et al. Fibrin sealant for treatment of cerebrospinal fluid leaks. Am J Otol. 1999;20:777-780.
263 Yoshimoto T, Sawamura Y, Houkin K, et al. Effectiveness of fibrin glue for preventing postoperative extradural fluid leakage. Neurol Med Chir (Tokyo). 1997;37:886-889.
264 Shapiro SA, Scully T. Closed continuous drainage of cerebrospinal fluid via a lumbar subarachnoid catheter for treatment or prevention of cranial/spinal cerebrospinal fluid fistula. Neurosurgery. 1992;30:241-245.
265 Violon P, Patay Z, Braeckeveldt J, et al. An atypical infectious complication of anterior cervical surgery. Neuroradiology. 1997;39:278-281.
266 Toppich HG, Feldmann H, Sandvoss G, et al. Intervertebral space nerve root entrapment after lumbar disc surgery: two cases. Spine. 1994;19:249-250.
267 Elias WJ, Simmons NE, Kaptain GJ, et al. Complications of posterior lumbar interbody fusion when using a titanium threaded cage device. J Neurosurg. 2000;93(suppl):45-52.
268 Hadjipavlou A, Enker P, Dupuis P, et al. The causes of failure of lumbar transpedicular spinal instrumentation and fusion: a prospective study. Int Orthop. 1996;20:35-42.
269 McAfee PC. Complications of anterior approaches to the thoracolumbar spine: emphasis on Kaneda instrumentation. Clin Orthop Relat Res. 1994;306:110-119.
270 Wilber RG, Thompson GH, Shaffer JW, et al. Postoperative neurological deficits in segmental spinal instrumentation: a study using spinal cord monitoring. J Bone Joint Surg Am. 1984;66:1178-1187.
271 Albert TJ, Pinto M, Denis F. Management of symptomatic lumbar pseudarthrosis with anteroposterior fusion: a functional and radiographic outcome study. Spine. 2000;25:123-129.
272 Bernhardt M, Swartz DE, Clothiaux PL, et al. Posterolateral lumbar and lumbosacral fusion with and without pedicle screw internal fixation. Clin Orthop Relat Res. 1992;284:109-115.
273 Betz RR, Harms J, Clements DH3rd, et al. Comparison of anterior and posterior instrumentation for correction of adolescent thoracic idiopathic scoliosis. Spine. 1999;24:225-239.
274 Brara HS, Fessler RG. The role of anterior lumbar interbody allograft bone dowel fusion as an adjunct to posterior segmental lumbar fixation. Clin Neurosurg. 2000;47:528-533.
275 Dickman CA, Sonntag VK. Surgical management of atlantoaxial nonunions. J Neurosurg. 1995;83:248-253.
276 Enker P, Steffee AD. Interbody fusion and instrumentation. Clin Orthop Relat Res. 1994;300:90-101.
277 Faraj AA, Webb JK. Early complications of spinal pedicle screw. Eur Spine J. 1997;6:324-326.
278 Fehlings MG, Cooper PR, Errico TJ. Posterior plates in the management of cervical instability: long-term results in 44 patients. J Neurosurg. 1994;81:341-349.
279 Gill K, Blumenthal SL. Posterior lumbar interbody fusion: a 2-year follow-up of 238 patients. Acta Orthop Scand Suppl. 1993;251:108-110.
280 Guven O, Esemenli T, Yalcin S, et al. Transpedicular fixation in the treatment of various spinal disorders. Acta Chir Belg. 1993;93:188-192.
281 Kornblatt MD, Casey MP, Jacobs RR. Internal fixation in lumbosacral spine fusion: a biomechanical and clinical study. Clin Orthop Relat Res. 1986;203:141-150.
282 Lauerman WC, Bradford DS, Transfeldt EE, et al. Management of pseudarthrosis after arthrodesis of the spine for idiopathic scoliosis. J Bone Joint Surg Am. 1991;73:222-236.
283 Lipton GE, Miller F, Dabney KW, et al. Factors predicting postoperative complications following spinal fusions in children with cerebral palsy. J Spinal Disord. 1999;12:197-205.
284 Heidecke V, Rainov NG, Burkert W. Anterior cervical fusion with the Orion locking plate system. Spine. 1998;23:1796-1802.
285 Masferrer R, Gomez CH, Karahalios DG, et al. Efficacy of pedicle screw fixation in the treatment of spinal instability and failed back surgery: a 5-year review. J Neurosurg. 1998;89:371-377.
286 Razak M, Mahmud MM, Hyzan MY, et al. Short segment posterior instrumentation, reduction and fusion of unstable thoracolumbar burst fractures—a review of 26 cases. Med J Malaysia. 2000;55(suppl C):9-13.
287 Schwab FJ, Nazarian DG, Mahmud F, et al. Effects of spinal instrumentation on fusion of the lumbosacral spine. Spine. 1995;20:2023-2028.
288 Seybold EA, Bayley JC. Functional outcome of surgically and conservatively managed dens fractures. Spine. 1998;23:1837-1845.
289 Troyanovich SJ, Stroink AR, Kattner KA, et al. Does anterior plating maintain cervical lordosis versus conventional fusion techniques? A retrospective analysis of patients receiving single-level fusions. J Spinal Disord Tech. 2002;15:69-74.
290 Bains RS, Althausen PL, Gitlin GN, et al. The role of acute decompression and restoration of spinal alignment in the prevention of post-traumatic syringomyelia: case report and review of recent literature. Spine. 2001;26:E399-E402.
291 Degreif J, Wenda K. Ultrasound-guided spinal fracture repositioning. Surg Endosc. 1998;12:164-169.
292 Maiuri F, Iaconetta G, Gallicchio B, et al. Intraoperative sonography for spinal tumors. Correlations with MR findings and surgery. J Neurosurg Sci. 2000;44:115-122.
293 Raynor RB. Intraoperative ultrasound for immediate evaluation of anterior cervical decompression and discectomy. Spine. 1997;22:389-395.
294 Woodard EJ, Leon SP, Moriarty TM, et al. Initial experience with intraoperative magnetic resonance imaging in spine surgery. Spine. 2001;26:410-417.
295 Nolte LP, Slomczykowski MA, Berlemann U, et al. A new approach to computer-aided spine surgery: fluoroscopy-based surgical navigation. Eur Spine J. 2000;9(suppl 1):S78-S88.
296 Roessler K, Ungersboeck K, Dietrich W, et al. Frameless stereotactic guided neurosurgery: clinical experience with an infrared-based pointer device navigation system. Acta Neurochir (Wien). 1997;139:551-559.
297 Tessman C. Exploring frameless stereotactic image guided surgery. AORN J. 1999;69:498-500. 503-505, 508-510
298 Youkilis AS, Quint DJ, McGillicuddy JE, et al. Stereotactic navigation for placement of pedicle screws in the thoracic spine. Neurosurgery. 2001;48:771-778.
299 Nowitzke A, Wood M, Cooney K. Improving accuracy and reducing errors in spinal surgery—a new technique for thoracolumbar-level localization using computer-assisted image guidance. Spine J. 2008;8:597-604.
300 Nottmeier EW, Foy AB. Placement of C2 laminar screws using three-dimensional fluoroscopy-based image guidance. Eur Spine J. 2008;17:610-615.
301 Lekovic GP, Potts EA, Karahalios DG, et al. A comparison of two techniques in image-guided thoracic pedicle screw placement: a retrospective study of 37 patients and 277 pedicle screws. J Neurosurg Spine. 2007;7:393-398.
302 Barzilay Y, Liebergall M, Fridlander A, et al. Miniature robotic guidance for spine surgery—introduction of a novel system and analysis of challenges encountered during the clinical development phase at two spine centres. Int J Med Robot. 2006;2:146-153.
303 Shoham M, Lieberman IH, Benzel EC, et al. Robotic assisted spinal surgery—from concept to clinical practice. Comput Aided Surg. 2007;12:105-115.
304 Marshman LA, Friesem T, Rampersaud YR, et al. Significantly improved lumbar arthroplasty placement using image guidance: technical note. Spine. 2007;32:2027-2030.
305 Alaranta H, Luoto S, Konttinen YT. Traumatic spinal cord injury as a complication to ankylosing spondylitis: an extended report. Clin Exp Rheumatol. 2002;20:66-68.
306 Apple DFJr, Anson C. Spinal cord injury occurring in patients with ankylosing spondylitis: a multicenter study. Orthopedics. 1995;18:1005-1011.
307 Broom MJ, Raycroft JF. Complications of fractures of the cervical spine in ankylosing spondylitis. Spine. 1988;13:763-766.
308 Farmer J, Vaccaro A, Albert TJ, et al. Neurologic deterioration after cervical spinal cord injury. J Spinal Disord. 1998;11:192-196.
309 Graham B, Van Peteghem PK. Fractures of the spine in ankylosing spondylitis: diagnosis, treatment, and complications. Spine. 1989;14:803-807.
310 Harun S. Delayed spinal cord compression in ankylosing spondylitis. J Accid Emerg Med. 1998;15:336.
311 Rowed DW. Management of cervical spinal cord injury in ankylosing spondylitis: the intervertebral disc as a cause of cord compression. J Neurosurg. 1992;77:241-246.
312 Taggard DA, Traynelis VC. Management of cervical spinal fractures in ankylosing spondylitis with posterior fixation. Spine. 2000;25:2035-2039.
313 Tico N, Ramon S, Garcia-Ortun F, et al. Traumatic spinal cord injury complicating ankylosing spondylitis. Spinal Cord. 1998;36:349-352.
314 Weinstein PR, Karpman RR, Gall EP, et al. Spinal cord injury, spinal fracture, and spinal stenosis in ankylosing spondylitis. J Neurosurg. 1982;57:609-616.
315 Chandler DR, Nemejc C, Adkins RH, et al. Emergency cervical-spine immobilization. Ann Emerg Med. 1992;21:1185-1188.
316 Richter D, Latta LL, Milne EL, et al. The stabilizing effects of different orthoses in the intact and unstable upper cervical spine: a cadaver study. J Trauma. 2001;50:848-854.
317 Schultz KDJr, Petronio J, Haid RW, et al. Pediatric occipitocervical arthrodesis. A review of current options and early evaluation of rigid internal fixation techniques. Pediatr Neurosurg. 2000;33:169-181.
318 Baum JA, Hanley ENJr, Pullekines J. Comparison of halo complications in adults and children. Spine. 1989;14:251-252.
319 Dorfmuller G, Hollerhage HG. Severe intracranial injury from a fall in the halo external fixator. J Orthop Trauma. 1992;6:366-369.
320 Ersmark H, Dalen N, Kalen R. Cervical spine injuries: a followup of 332 patients. Paraplegia. 1990;28:25-40.
321 Garfin SR, Botte MJ, Waters RL, et al. Complications in the use of the halo fixation device. J Bone Joint Surg Am. 1986;68:320-325.
322 Hann J, de Bakker HM. Brain abscess as a complication of halo traction: role of CT in diagnosing penetration of the skull. AJNR Am J Neuroradiol. 1989;10:446.
323 Kaplan SL, Rocco TP, Tun CG, et al. Acute pulmonary edema following removal of a spinal orthosis: an unusual complication of a halo vest. Arch Phys Med Rehabil. 1990;71:255-257.
324 McCarron RF, Robertson WW. Brooks fusion for atlantoaxial instability in rheumatoid arthritis. South Med J. 1988;81:474-476.
325 Muller EJ, Wick M, Russe O, et al. Management of odontoid fractures in the elderly. Eur Spine J. 1999;8:360-365.
326 Rizzolo SJ, Piazza MR, Cotler JM, et al. The effect of torque pressure on halo pin complication rates: a randomized prospective study. Spine. 1993;18:2163-2166.
327 Rosenblum D, Ehrlich V. Brain abscess and psychosis as a complication of a halo orthosis. Arch Phys Med Rehabil. 1995;76:865-867.
328 Papagelopoulos PJ, Sapkas GS, Kateros KT, et al. Halo pin intracranial penetration and epidural abscess in a patient with a previous cranioplasty: case report and review of the literature. Spine. 2001;26:E463-467.
329 Tomonaga T, Krag MH, Novotny JE. Clinical, radiographic, and kinematic results from an adjustable four-pad halo vest. Spine. 1997;22:1199-1208.
330 Jones DC, Hayter JP, Vaughan ED, et al. Oropharyngeal morbidity following transoral approaches to the upper cervical spine. Int J Oral Maxillofac Surg. 1998;27:295-298.
331 Klockner C, Kern O, Zierski J, et al. [Microsurgical transoral decompression in diseases of and injuries to the cranio-cervical junction.]. Orthopade. 1998;27:477-481.
332 Menezes AH, VanGilder JC, Clark CR, et al. Odontoid upward migration in rheumatoid arthritis: an analysis of 45 patients with “cranial settling.”. J Neurosurg. 1985;63:500-509.
333 Elies W. Esophageal complications following ventral cervical disc surgery [author’s translation.]. HNO. 1979;27:380-381.
334 Iannettoni MD, Vlessis AA, Whyte RI, et al. Functional outcome after surgical treatment of esophageal perforation. Ann Thorac Surg. 1997;64:1606-1609.
335 Kelly MF, Spiegel J, Rizzo KA, et al. Delayed pharyngoesophageal perforation: a complication of anterior spine surgery. Ann Otol Rhinol Laryngol. 1991;100:201-205.
336 Lambert JR, Tepperman PS, Jimenez J, et al. Cervical spine disease and dysphagia. Four new cases and a review of the literature. Am J Gastroenterol. 1981;76:35-40.
337 White RK, Morris DM. Diagnosis and management of esophageal perforations. Am Surg. 1992;58:112-119.
338 Patel NP, Wolcott WP, Johnson JP, et al. Esophageal injury associated with anterior cervical spine surgery. Surg Neurol. 2008;69:20-24.
339 Jones WG2nd, Ginsberg RJ. Esophageal perforation: a continuing challenge. Ann Thorac Surg. 1992;53:534-543.
340 Lee MJ, Bazaz R, Furey CG, et al. Risk factors for dysphagia after anterior cervical spine surgery: a two-year prospective cohort study. Spine J. 2007;7:141-147.
341 Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine. 2007;32:2310-2317.
342 Audu P, Artz G, Scheid S, et al. Recurrent laryngeal nerve palsy after anterior cervical spine surgery: the impact of endotracheal tube cuff deflation, reinflation, and pressure adjustment. Anesthesiology. 2006;105:898-901.
343 Paniello RC, Martin-Bredahl KJ, Henkener LJ, et al. Preoperative laryngeal nerve screening for revision anterior cervical spine procedures. Ann Otol Rhinol Laryngol. 2008;117:594-597.
344 Johnson S, Goldenberg D. Intraoperative monitoring of the recurrent laryngeal nerve during revision thyroid surgery. Otolaryngol Clin North Am. 2008;41:1147-1154.
345 Chiang FY, Lu IC, Kuo WR, et al. The mechanism of recurrent laryngeal nerve injury during thyroid surgery—the application of intraoperative neuromonitoring. Surgery. 2008;143:743-749.
346 Peng CW, Chou BT, Bendo JA, et al. Vertebral artery injury in cervical spine surgery: anatomical considerations, management, and preventive measures. Spine J. 2009;9:70-76.
347 Neo M, Fujibayashi S, Miyata M, et al. Vertebral artery injury during cervical spine surgery: a survey of more than 5600 operations. Spine. 2008;33:779-785.
348 Hong JT, Park DK, Lee MJ, et al. Anatomical variations of the vertebral artery segment in the lower cervical spine: analysis by three-dimensional computed tomography angiography. Spine. 2008;33:2422-2426.
349 Jain KK. Anterior approach to the cervical spine. Can Med Assoc J. 1974;111:49-50.
350 Mazzon D, Zanatta P, Curtolo S, et al. Upper airway obstruction by retropharyngeal hematoma after cervical spine trauma: report of a case treated with percutaneous dilational tracheostomy. J Neurosurg Anesthesiol. 1998;10:237-240.
351 Ross JS. Magnetic resonance imaging of the postoperative spine. Semin Musculoskelet Radiol. 2000;4:281-291.
352 U HS, Wilson CB. Postoperative epidural hematoma as a complication of anterior cervical discectomy. Report of three cases. J Neurosurg. 1978;49:288-291.
353 Yonenobu K, Hosono N, Iwasaki M, et al. Neurologic complications of surgery for cervical compression myelopathy. Spine. 1991;16:1277-1282.
354 Eleraky MA, Llanos C, Sonntag VK. Cervical corpectomy: report of 185 cases and review of the literature. J Neurosurg. 1999;90(suppl):35-41.
355 ElSaghir H, Bohm H. Anterior versus posterior plating in cervical corpectomy. Arch Orthop Trauma Surg. 2000;120:549-554.
356 Epstein N. Anterior approaches to cervical spondylosis and ossification of the posterior longitudinal ligament: review of operative technique and assessment of 65 multilevel circumferential procedures. Surg Neurol. 2001;55:313-324.
357 Fessler RG, Steck JC, Giovanini MA. Anterior cervical corpectomy for cervical spondylotic myelopathy. Neurosurgery. 1998;43:257-265.
358 Majd ME, Vadhva M, Holt RT. Anterior cervical reconstruction using titanium cages with anterior plating. Spine. 1999;24:1604-1610.
359 Mayr MT, Subach BR, Comey CH, et al. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(suppl):10-16.
360 Saunders RL, Bernini PM, Shirreffs TGJr, et al. Central corpectomy for cervical spondylotic myelopathy: a consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74:163-170.
361 Wada E, Suzuki S, Kanazawa A, et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term follow-up study over 10 years. Spine. 2001;26:1443-1447.
362 Zeidman SM, Ducker TB, Raycroft J. Trends and complications in cervical spine surgery: 1989-1993. J Spinal Disord. 1997;10:523-526.
363 Sridhar K, Ramamurthi R, Vasudevan MC, et al. Surgery for ossification of the posterior longitudinal ligament of the cervical spine. Neurol India. 2001;49:116-123.
364 Etebar S, Cahill DW. Failure of transodontoid screw fixation: case report. J Neurosurg. 1998;88:158-160.
365 Laus M, Zappoli FA, Alfonso C, et al. Anterior surgery in trauma of the cervical spine. Chir Organi Mov. 1997;82:97-104.
366 Subach BR, Morone MA, Haid RWJr, et al. Management of acute odontoid fractures with single-screw anterior fixation. Neurosurgery. 1999;45:812-819.
367 Tanaka N, Nakanishi K, Fujiwara Y, et al. Postoperative segmental C5 palsy after cervical laminoplasty may occur without intraoperative nerve injury: a prospective study with transcranial electric motor-evoked potentials. Spine. 2006;31:3013-3017.
368 Takemitsu M, Cheung KM, Wong YW, et al. C5 nerve root palsy after cervical laminoplasty and posterior fusion with instrumentation. J Spinal Disord Tech. 2008;21:267-272.
369 Fan D, Schwartz DM, Vaccaro AR, et al. Intraoperative neurophysiologic detection of iatrogenic C5 nerve root injury during laminectomy for cervical compression myelopathy. Spine. 2002;27:2499-2502.
370 Baulot E, Trouilloud P, Ragois P, et al. [Anterior spinal fusion by thoracoscopy. A non-traumatic technique.]. Rev Chir Orthop Reparatrice Appar Mot. 1997;83:203-209.
371 Faciszewski T, Winter RB, Lonstein JE, et al. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults: a review of 1223 procedures. Spine. 1995;20:1592-1599.
372 Verhoeven W, Low CO, See HF, et al. Massive chylothorax after anterior fusion of the thoracic spine. Ann Acad Med Singapore. 1996;25:286-288.
373 Visocchi M, Masferrer R, Sonntag VK, et al. Thoracoscopic approaches to the thoracic spine. Acta Neurochir (Wien). 1998;140:737-743.
374 Bhat AL, Lowery GL. Chylous injury following anterior spinal surgery: case reports. Eur Spine J. 1997;6:270-272.
375 Floch NR, Harvey JC, Beattie EJJr. Aortoesophageal fistula after reconstruction of the thoracic spine. Ann Thorac Surg. 1995;60:191-192.
376 McAfee PC, Regan JR, Zdeblick T, et al. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery. A prospective multicenter study comprising the first 100 consecutive cases. Spine. 1995;20:1624-1632.
377 McDonnell MF, Glassman SD, Dimar JR2nd, et al. Perioperative complications of anterior procedures on the spine. J Bone Joint Surg Am. 1996;78:839-847.
378 Nagai H, Shimizu K, Shikata J, et al. Chylous leakage after circumferential thoracolumbar fusion for correction of kyphosis resulting from fracture: report of three cases. Spine. 1997;22:2766-2769.
379 Ohnishi T, Neo M, Matsushita M, et al. Delayed aortic rupture caused by an implanted anterior spinal device: case report. J Neurosurg. 2001;95(suppl):253-256.
380 Propst-Proctor SL, Rinsky LA, Bleck EE. The cisterna chyli in orthopaedic surgery. Spine. 1983;8:787-792.
381 Ahlgren BD, Herkowitz HN. A modified posterolateral approach to the thoracic spine. J Spinal Disord. 1995;8:69-75.
382 Arlet V, Marchesi D, Aebi M. Correction of adolescent idiopathic thoracic scoliosis with a new type of offset apical instrumentation: preliminary results. J Spinal Disord. 1998;11:404-409.
383 Bauer HC. Posterior decompression and stabilization for spinal metastases: analysis of sixty-seven consecutive patients. J Bone Joint Surg Am. 1997;79:514-522.
384 Foley MJ, Calenoff L, Hendrix RW, et al. Thoracic and lumbar spine fusion: postoperative radiologic evaluation. AJR Am J Roentgenol. 1983;141:373-380.
385 Clark MA, Plank LD, Hill GL. Wound healing associated with severe surgical illness. World J Surg. 2000;24:648-654.
386 Disa JJ, Smith AW, Bilsky MH. Management of radiated reoperative wounds of the cervicothoracic spine: the role of the trapezius turnover flap. Ann Plast Surg. 2001;47:394-397.
387 Modi HN, Suh SW, Fernandez H, et al. Accuracy and safety of pedicle screw placement in neuromuscular scoliosis with free-hand technique. Eur Spine J. 2008;17:1686-1696.
388 Bergeson RK, Schwend RM, DeLucia T, et al. How accurately do novice surgeons place thoracic pedicle screws with the free hand technique? Spine. 2008;33:E501-E507.
389 Kim YJ, Lenke LG, Bridwell KH, et al. Free hand pedicle screw placement in the thoracic spine: is it safe? Spine. 2004;29:333-342.
390 Horowitz MB, Moossy JJ, Julian T, et al. Thoracic discectomy using video-assisted thoracoscopy. Spine. 1994;19:1082-1086.
391 Lischke V, Westphal K, Behne M, et al. Thoracoscopic microsurgical technique for vertebral surgery—anesthetic considerations. Acta Anaesthesiol Scand. 1998;42:1199-1204.
392 Regan JJ, Ben-Yishay A, Mack MJ. Video-assisted thoracoscopic excision of herniated thoracic disc: description of technique and preliminary experience in the first 29 cases. J Spinal Disord. 1998;11:183-191.
393 Baker JK, Reardon PR, Reardon MJ, et al. Vascular injury in anterior lumbar surgery. Spine. 1993;18:2227-2230.
394 Dewald CJ, Millikan KW, Hammerberg KW, et al. An open, minimally invasive approach to the lumbar spine. Am Surg. 1999;65:61-68.
395 Hackenberg L, Liljenqvist U, Halm H, et al. Occlusion of the left common iliac artery and consecutive thromboembolism of the left popliteal artery following anterior lumbar interbody fusion. J Spinal Disord. 2001;14:365-368.
396 Marsicano J, Mirovsky Y, Remer S, et al. Thrombotic occlusion of the left common iliac artery after an anterior retroperitoneal approach to the lumbar spine. Spine. 1994;19:357-359.
397 Mayer HM. The ALIF concept. Eur Spine J. 2000;9(suppl 1):S35-S43.
398 Olinger A, Hildebrandt U, Vollmar B, et al. [Laparoscopic-transperitoneal and lumboscopic-retroperitoneal surgery of the spine: developments from animal experiments for use in clinical practice.]. Zentralbl Chir. 1999;124:311-317.
399 Regan JJ, Aronoff RJ, Ohnmeiss DD, et al. Laparoscopic approach to L4-L5 for interbody fusion using BAK cages: experience in the first 58 cases. Spine. 1999;24:2171-2174.
400 Zdeblick TA, David SM. A prospective comparison of surgical approach for anterior L4-L5 fusion: laparoscopic versus mini anterior lumbar interbody fusion. Spine. 2000;25:2682-2687.
401 de Peretti F, Hovorka I, Fabiani P, et al. New possibilities in L2-L5 lumbar arthrodesis using a lateral retroperitoneal approach assisted by laparoscopy: preliminary results. Eur Spine J. 1996;5:210-216.
402 McAfee PC, Regan JJ, Geis WP, et al. Minimally invasive anterior retroperitoneal approach to the lumbar spine: emphasis on the lateral BAK. Spine. 1998;23:1476-1484.
403 Flynn JC, Price CT. Sexual complications of anterior fusion of the lumbar spine. Spine. 1984;9:489-492.
404 Sasso RC, Burkus JK, LeHuec JC. Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine. 2003;28:1023-1026.
405 Birch N, Shaw M. Retrograde ejaculation after anterior lumbar interbody fusion. Spine. 2004;29:106-107.
406 Coskun E, Suzer T, Topuz O, et al. Relationships between epidural fibrosis, pain, disability, and psychological factors after lumbar disc surgery. Eur Spine J. 2000;9:218-223.
407 Dina TS, Boden SD, Davis DO. Lumbar spine after surgery for herniated disk: imaging findings in the early postoperative period. AJR Am J Roentgenol. 1995;164:665-671.
408 Israel Z, Constantini S. Compressive epidural autologous free fat graft in a patient with failed back syndrome: case report. J Spinal Disord. 1995;8:240-242.
409 Pospiech J, Pajonk F, Stolke D. Epidural scar tissue formation after spinal surgery: an experimental study. Eur Spine J. 1995;4:213-219.
410 Cobanoglu S, Imer M, Ozylmaz F, et al. Complication of epidural fat graft in lumbar spine disc surgery: case report. Surg Neurol. 1995;44:479-481.
411 Le AX, Rogers DE, Dawson EG, et al. Unrecognized durotomy after lumbar discectomy: a report of four cases associated with the use of ADCON-L. Spine. 2001;26:115-117.
412 Robertson JT, Maier K, Anderson RW, et al. Prevention of epidural fibrosis with ADCON-L in presence of a durotomy during lumbar disc surgery: experiences with a pre-clinical model. Neurol Res. 1999;21(suppl 1):S61-S66.
413 Rogers LA. Experience with limited versus extensive disc removal in patients undergoing microsurgical operations for ruptured lumbar discs. Neurosurgery. 1988;22:82-85.
414 Balderston RA, Gilyard GG, Jones AA, et al. The treatment of lumbar disc herniation: simple fragment excision versus disc space curettage. J Spinal Disord. 1991;4:22-25.
415 Davis RA. A long-term outcome analysis of 984 surgically treated herniated lumbar discs. J Neurosurg. 1994;80:415-421.
416 Frymoyer JW, Matteri RE, Hanley EN, et al. Failed lumbar disc surgery requiring second operation: a long-term followup study. Spine. 1978;3:7-11.
417 Law JD, Lehman RA, Kirsch WM. Reoperation after lumbar intervertebral disc surgery. J Neurosurg. 1978;48:259-263.
418 Mattmann E. [The problem of recurrence after surgery of herniated lumbar disks.]. Schweiz Arch Neurol Neurochir Psychiatr. 1971;108:39-44.
419 Paus B, Skalpe IO. The recurrence of pain following operation for herniated lumbar disc: fresh herniation or extradural scar tissue? Int Orthop. 1979;3:133-136.
420 Rish BL. A critique of the surgical management of lumbar disc disease in a private neurosurgical practice. Spine. 1984;9:500-504.
421 Weber H. Lumbar disc herniation: a controlled, prospective study with ten years of observation. Spine. 1983;8:131-140.
422 Williams RW. Microlumbar discectomy: a conservative surgical approach to the virgin herniated lumbar disc. Spine. 1978;3:175-182.
423 Firooznia H, Kricheff II, Rafii M, et al. Lumbar spine after surgery: examination with intravenous contrast-enhanced CT. Radiology. 1987;163:221-226.
424 Grane P. The postoperative lumbar spine. A radiological investigation of the lumbar spine after discectomy using MR imaging and CT. Acta Radiol Suppl. 1998;414:1-23.
425 Hochhauser L, Kieffer SA, Cacayorin ED, et al. Recurrent postdiskectomy low back pain: MR-surgical correlation. AJR Am J Roentgenol. 1988;151:755-760.
426 Ross JS, Zepp R, Modic MT. The postoperative lumbar spine: enhanced MR evaluation of the intervertebral disk. AJNR Am J Neuroradiol. 1996;17:323-331.
427 Friedman J, Whitecloud TS3rd. Lumbar cauda equina syndrome associated with the use of Gelfoam: case report. Spine. 2001;26:E485-E487.
428 Gehri R, Zanetti M, Boos N. Subacute subdural haematoma complicating lumbar microdiscectomy. J Bone Joint Surg Br. 2000;82:1042-1045.
429 Henriques T, Olerud C, Petren-Mallmin M, et al. Cauda equina syndrome as a postoperative complication in five patients operated for lumbar disc herniation. Spine. 2001;26:293-297.
430 Kardaun JW, White LR, Shaffer WO. Acute complications in patients with surgical treatment of lumbar herniated disc. J Spinal Disord. 1990;3:30-38.
431 McLaren AC, Bailey SI. Cauda equina syndrome: a complication of lumbar discectomy. Clin Orthop Relat Res. 1986;204:143-149.
432 Onik G, Maroon JC, Jackson R. Cauda equina syndrome secondary to an improperly placed nucleotome probe. Neurosurgery. 1992;30:412-414.
433 Prusick VR, Lint DS, Bruder WJ. Cauda equina syndrome as a complication of free epidural fat-grafting: a report of two cases and a review of the literature. J Bone Joint Surg Am. 1988;70:1256-1258.
434 Brewster DC, May AR, Darling RC, et al. Variable manifestations of vascular injury during lumbar disk surgery. Arch Surg. 1979;114:1026-1030.
435 Fruhwirth J, Koch G, Amann W, et al. Vascular complications of lumbar disc surgery. Acta Neurochir (Wien). 1996;138:912-916.
436 Goodkin R, Laska LL. Vascular and visceral injuries associated with lumbar disc surgery: medicolegal implications. Surg Neurol. 1998;49:358-370.
437 Gower DJ, Culp P, Ball M. Lateral lumbar spine roentgenograms: potential role in complications of lumbar disc surgery. Surg Neurol. 1987;27:316-318.
438 Smith EB, DeBord JR, Hanigan WC. Intestinal injury after lumbar discectomy. Surg Gynecol Obstet. 1991;173:22-24.
439 Stern MB. Early experience with percutaneous lateral discectomy. Clin Orthop Relat Res. 1989;238:50-55.
440 Tsai YD, Yu PC, Lee TC, et al. Superior rectal artery injury following lumbar disc surgery: case report. J Neurosurg. 2001;95(suppl):108-110.
441 Villano M, Cantatore G, Narciso N, et al. Vascular injury related to lumbar disk surgery. Neurochirurgia (Stuttg). 1992;35:57-59.
442 Castro WH, Jerosch J, Schilgen M, et al. Automated percutaneous nucleotomy: restricted indications based on CT scan appearance. Neurosurg Clin N Am. 1996;7:43-47.
443 Choy DS. Percutaneous laser disc decompression (PLDD): twelve years’ experience with 752 procedures in 518 patients. J Clin Laser Med Surg. 1998;16:325-331.
444 Choy DS. Clinical experience and results with 389 PLDD procedures with the Nd:YAG laser 1986 to 1995. J Clin Laser Med Surg. 1995;13:209-213.
445 Ditsworth DA. Endoscopic transforaminal lumbar discectomy and reconfiguration: a postero-lateral approach into the spinal canal. Surg Neurol. 1998;49:588-597.
446 Dullerud R, Amundsen T, Lie H, et al. Clinical results after percutaneous automated lumbar nucleotomy: a follow-up study. Acta Radiol. 1995;36:418-424.
447 Grevitt MP, McLaren A, Shackleford IM, et al. Automated percutaneous lumbar discectomy: an outcome study. J Bone Joint Surg Br. 1995;77:626-629.
448 Jacobson S. Back pain sufferers see quick recovery with laser disc surgery. Clin Laser Monit. 1993;11:137-138.
449 Kambin P, Savitz MH. Arthroscopic microdiscectomy: an alternative to open disc surgery. Mt Sinai J Med. 2000;67:283-287.
450 Leu HF, Schreiber A, Elsig JP, et al. Percutaneous disc surgery at Balgrist since 1979—from discotomy to interbody fusion. Bull Hosp Jt Dis. 1996;54:190-197.
451 Mochida J, Arima T. Percutaneous nucleotomy in lumbar disc herniation: a prospective study. Spine. 1993;18:2063-2068.
452 Mochida J, Nishimura K, Okuma M, et al. Percutaneous nucleotomy in elite athletes. J Spinal Disord. 2001;14:159-164.
453 Mochida J, Toh E, Nishimura K, et al. Percutaneous nucleotomy in lumbar disc herniation: patient selection and role in various treatments. Spine. 1993;18:2212-2217.
454 Nordby EJ, Wright PH. Efficacy of chymopapain in chemonucleolysis: a review. Spine. 1994;19:2578-2583.
455 Quigley MR. Percutaneous laser discectomy. Neurosurg Clin N Am. 1996;7:37-42.
456 Quigley MR, Maroon JC. Automated percutaneous discectomy. Neurosurg Clin N Am. 1996;7:29-35.
457 Shapiro S. Long-term follow up of 57 patients undergoing automated percutaneous discectomy. J Neurosurg. 1995;83:31-33.
458 Teng GJ, Jeffery RF, Guo JH, et al. Automated percutaneous lumbar discectomy: a prospective multi-institutional study. J Vasc Interv Radiol. 1997;8:457-463.
459 Wittenberg RH, Oppel S, Rubenthaler FA, et al. Five-year results from chemonucleolysis with chymopapain or collagenase: a prospective randomized study. Spine. 2001;26:1835-1841.
460 Mochida J, Toh E, Nomura T, et al. The risks and benefits of percutaneous nucleotomy for lumbar disc herniation: a 10-year longitudinal study. J Bone Joint Surg Br. 2001;83:501-505.
461 Plancarte R, Calvillo O. Complex regional pain syndrome type 2 (causalgia) after automated laser discectomy: a case report. Spine. 1997;22:459-461.
462 Ramberg N, Sahlstrand T. Early course and long-term followup after automated percutaneous lumbar discectomy. J Spinal Disord. 2001;14:511-516.
463 Farrar MJ, Walker A, Cowling P. Possible salmonella osteomyelitis of spine following laser disc decompression. Eur Spine J. 1998;7:509-511.
464 Kleinpeter G, Markowitsch MM, Bock F. Percutaneous endoscopic lumbar discectomy: minimally invasive, but perhaps only minimally useful? Surg Neurol. 1995;43:534-549.
465 Haaker RG, Senkal M, Kielich T, Kramer J. Percutaneous lumbar discectomy in the treatment of lumbar discitis. Eur Spine J. 1997;6:98-101.
466 Agazzi S, Reverdin A, May D. Posterior lumbar interbody fusion with cages: an independent review of 71 cases. J Neurosurg. 1999;91(suppl):186-192.
467 Barnes B, Rodts GE, McLaughlin MR, et al. Threaded cortical bone dowels for lumbar interbody fusion: over 1-year mean follow up in 28 patients. J Neurosurg. 2001;95(suppl):1-4.
468 Chitnavis B, Barbagallo G, Selway R, et al. Posterior lumbar interbody fusion for revision disc surgery: review of 50 cases in which carbon fiber cages were implanted. J Neurosurg. 2001;95(suppl):190-195.
469 Diedrich O, Perlick L, Schmitt O, Kraft CN. Radiographic characteristics on conventional radiographs after posterior lumbar interbody fusion: comparative study between radiotranslucent and radiopaque cages. J Spinal Disord. 2001;14:522-532.
470 Diedrich O, Perlick L, Schmitt O, et al. Radiographic spinal profile changes induced by cage design after posterior lumbar interbody fusion: preliminary report of a study with wedged implants. Spine. 2001;26:E274-E280.
471 Freeman BJ, Licina P, Mehdian SH. Posterior lumbar interbody fusion combined with instrumented postero-lateral fusion: 5-year results in 60 patients. Eur Spine J. 2000;9:42-46.
472 Hashimoto T, Shigenobu K, Kanayama M, et al. Clinical results of single-level posterior lumbar interbody fusion using the Brantigan I/F carbon cage filled with a mixture of local morselized bone and bioactive ceramic granules. Spine. 2002;27:258-262.
473 Janssen ME, Lam C, Beckham R. Outcomes of allogenic cages in anterior and posterior lumbar interbody fusion. Eur Spine J. 2001;10(suppl 2):S158-S168.
474 Matge G, Leclercq TA. Rationale for interbody fusion with threaded titanium cages at cervical and lumbar levels. Results on 357 cases. Acta Neurochir (Wien). 2000;142:425-433.
475 Miyakoshi N, Abe E, Shimada Y, et al. Outcome of one-level posterior lumbar interbody fusion for spondylolisthesis and postoperative intervertebral disc degeneration adjacent to the fusion. Spine. 2000;25:1837-1842.
476 Nakai S, Yoshizawa H, Kobayashi S. Long-term follow-up study of posterior lumbar interbody fusion. J Spinal Disord. 1999;12:293-299.
477 Walker C, Kahanovitz N. Recurrent superior mesenteric artery syndrome complicating staged reconstructive spinal surgery: alternative methods of conservative treatment. J Pediatr Orthop. 1983;3:77-80.
478 Boos N, Webb JK. Pedicle screw fixation in spinal disorders: a European view. Eur Spine J. 1997;6:2-18.
479 Van Brussel K, Vander Sloten J, Van Audekercke R, et al. Internal fixation of the spine in traumatic and scoliotic cases: the potential of pedicle screws. Technol Health Care. 1996;4:365-384.
480 West JL3rd, Bradford DS, Ogilvie JW. Results of spinal arthrodesis with pedicle screw-plate fixation. J Bone Joint Surg Am. 1991;73:1179-1184.
481 Brantigan JW. Pseudarthrosis rate after allograft posterior lumbar interbody fusion with pedicle screw and plate fixation. Spine. 1994;19:1271-1279.
482 Brown CA, Lenke LG, Bridwell KH, et al. Complications of pediatric thoracolumbar and lumbar pedicle screws. Spine. 1998;23:1566-1571.
483 Glossop ND, Hu RW, Randle JA. Computer-aided pedicle screw placement using frameless stereotaxis. Spine. 1996;21:2026-2034.
484 Heini P, Scholl E, Wyler D, et al. Fatal cardiac tamponade associated with posterior spinal instrumentation: a case report. Spine. 1998;23:2226-2230.
485 Laine T, Lund T, Ylikoski M, et al. Accuracy of pedicle screw insertion with and without computer assistance: a randomised controlled clinical study in 100 consecutive patients. Eur Spine J. 2000;9:235-240.
486 Lee TC. Pedicle fixation: an adjuvant for the treatment of thoracolumbar metastases. Ann Acad Med Singapore. 1993;22(suppl):418-421.
487 Lonstein JE, Denis F, Perra JH, et al. Complications associated with pedicle screws. J Bone Joint Surg Am. 1999;81:1519-1528.
488 Macdessi SJ, Leong AK, Bentivoglio JE. Pedicle fracture after instrumented posterolateral lumbar fusion: a case report. Spine. 2001;26:580-582.
489 Matsuzaki H, Tokuhashi Y, Matsumoto F, et al. Problems and solutions of pedicle screw plate fixation of lumbar spine. Spine. 1990;15:1159-1165.
490 Odgers CJ4th, Vaccaro AR, Pollack ME, et al. Accuracy of pedicle screw placement with the assistance of lateral plain radiography. J Spinal Disord. 1996;9:334-338.
491 Sapkas GS, Papadakis SA, Stathakopoulos DP, et al. Evaluation of pedicle screw position in thoracic and lumbar spine fixation using plain radiographs and computed tomography: a prospective study of 35 patients. Spine. 1999;24:1926-1929.
492 Shapiro SA, Snyder W. Spinal instrumentation with a low complication rate. Surg Neurol. 1997;48:566-574.
493 Suk KS, Jeon CH, Park MS, et al. Comparison between posterolateral fusion with pedicle screw fixation and anterior interbody fusion with pedicle screw fixation in adult spondylolytic spondylolisthesis. Yonsei Med J. 2001;42:316-323.
494 Vanichkachorn JS, Vaccaro AR, Cohen MJ, et al. Potential large vessel injury during thoracolumbar pedicle screw removal: a case report. Spine. 1997;22:110-113.
495 Graham CE. Lumbosacral fusion using internal fixation with a spinous process for the graft: a review of 50 patients with a five-year maximum follow-up. Clin Orthop Relat Res. 1979;140:72-77.
496 Margulies JY, Seimon LP. Clinical efficacy of lumbar and lumbosacral fusion using the Boucher facet screw fixation technique. Bull Hosp Jt Dis. 2000;59:33-39.
497 Benini A, Magerl F. Selective decompression and translaminar articular facet screw fixation for lumbar canal stenosis and disc protrusion. Br J Neurosurg. 1993;7:413-418.
498 Grob D, Humke T. Translaminar screw fixation in the lumbar spine: technique, indications, results. Eur Spine J. 1998;7:178-186.
499 Grob D, Rubeli M, Scheier HJ, Dvorak J. Translaminar screw fixation of the lumbar spine. Int Orthop. 1992;16:223-226.
500 Heggeness MH, Esses SI. Translaminar facet joint screw fixation for lumbar and lumbosacral fusion: a clinical and biomechanical study. Spine. 1991;16(suppl):S266-S269.
501 Humke T, Grob D, Dvorak J, et al. Translaminar screw fixation of the lumbar and lumbosacral spine: a 5-year follow-up. Spine. 1998;23:1180-1184.
502 Jacobs RR, Montesano PX, Jackson RP. Enhancement of lumbar spine fusion by use of translaminar facet joint screws. Spine. 1989;14:12-15.
503 Lu J, Ebraheim NA, Yeasting RA. Translaminar facet screw placement: an anatomic study. Am J Orthop. 1998;27:550-555.
504 Deguchi M, Cheng BC, Sato K, et al. Biomechanical evaluation of translaminar facet joint fixation: a comparative study of poly-L-lactide pins, screws, and pedicle fixation. Spine. 1998;23:1307-1312. discussion 1313