Autotransfusion
Introduction
Autologous blood transfusion, or autotransfusion, is the collection and reinfusion of a patient’s own blood for volume replacement.1 Preoperative blood banking and intraoperative cell salvage techniques have increased in a multitude of surgical specialties.2–7 Autotransfusion in the emergency department (ED) is usually limited to patients with severe, traumatic hemothorax and clinically significant blood loss.
Autotransfusion is usually performed in trauma centers or in EDs with high trauma volume. Though not a uniform standard of care, it is applicable to any ED. Since the procedure requires familiarity with the equipment, continuing education, and quality control, it may be counterproductive to institute it in a hospital that has a low trauma census or in a setting in which it will be used infrequently enough that staff education issues are problematic. Clinicians practicing in more austere environments with a paucity of clinical resources, such as combat and disaster zones, may find that the procedure’s benefits outweigh its risks.8–10
Background
Reports of autotransfusion can be found as early as 1818 when Blundell, an English practitioner, reinfused shed blood after witnessing a woman exsanguinate from uterine hemorrhage.11 In 1886, Duncan published the first known human account of autotransfusion in which he reinfused shed blood in a patient with a traumatic amputation without any notable ill effects.12 In 1917, Elmendorf published a description of the first case of autotransfusion in a patient with traumatic hemothorax.13
The discovery of ABO blood typing at the turn of the century and the institution of blood banks in the 1930s led to the almost exclusive use of allogeneic (homologous) blood up to and following World War II. During the 1960s and 1970s, cardiopulmonary bypass surgery and combat trauma experience during the Vietnam War generated extensive data regarding intraoperative retrieval of large quantities of blood for reinfusion.14–17 This revitalized interest, coupled with increased experience in surgical, trauma, and combat situations, has thus initiated a new era in autotransfusion.
Anatomy
Hemothorax refers to a collection of blood within the pleural space. Severe hemorrhage is more often associated with laceration of vessels on the inside of the chest wall, such as the internal mammary and intercostal arteries. Blunt trauma and penetrating trauma are by far the most common causes of hemothorax. Spontaneous hemothorax can occur secondary to intrathoracic malignancy,18 pulmonary infarction, bullous emphysema, virulent pulmonary or mediastinal infection, vascular malformation, and endometriosis of the pleura (catamenial).19
Pathophysiology
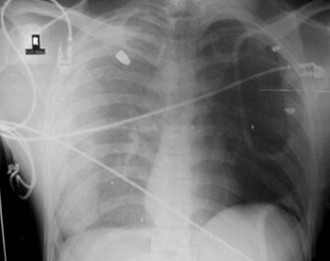
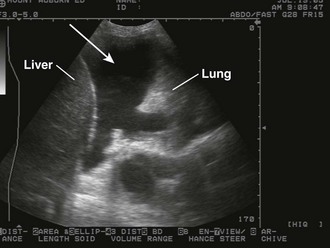
The pathophysiologic sequelae of hemothorax include both hemodynamic instability and respiratory compromise. The pleural cavity can accommodate more than 50% of the total blood volume, so clinically significant intrathoracic blood loss can occur with minimal external signs of bleeding. The clinician should suspect hemothorax in the setting of chest trauma with clinical signs of hypovolemia. Physical examination may demonstrate decreased breath sounds and reduced tactile fremitus. Imaging during the initial resuscitation period is usually limited to supine chest radiographs, which may show haziness in the affected lung field as blood layers in the posterior pleural space (Fig. 27-1). Ultrasound is also useful in the initial evaluation of hemothorax (Fig. 27-2).20 Tube thoracostomy is the treatment of choice for acute hemothorax, with thoracotomy and video-assisted thoracic surgery performed as indicated for severe or ongoing hemorrhage.
Advantages
Shed blood from traumatic hemothorax is immediately available for rapid transfusion. The blood is normothermic and compatible, which avoids the risk of allergic reaction or infection from transfusion transmissible diseases. There are numerous transfusion transmissible diseases, including viruses such as human immunodeficiency virus and hepatitis, bacteria, parasites, and most recently reported, variant Creutzfeldt-Jakob disease.21,22 Although the risk for transfusion transmissible diseases has decreased dramatically in developed countries, it is still very problematic worldwide.23 Immunologic transfusion reactions and posttransfusion sepsis continue to be risks as well.24 In trauma patients, allogeneic transfusions have been shown to be an independent risk factor for infection,25–28 which may be dose dependent and independently associated with increased morbidity and mortality.28–30 In patients whose religious convictions (e.g., Jehovah’s Witness) prohibit transfusions with homologous blood, reinfusion of autologous blood that does not involve blood storage may be an acceptable alternative.31
Autologous blood provides societal benefits by preserving the limited stores of banked blood and reducing the cost of medical care.32–34 Adias and colleagues compared the direct cost of banked blood with the cost of autologous blood transfusion and found substantial savings with autotransfusion.35 Box 27-1 summarizes the advantages of autotransfusion.
Indications
In general, all victims of severe trauma, whether blunt or penetrating, should be considered potential candidates for autotransfusion. Several categories of patients for whom emergency autotransfusion is suitable have been described and are summarized in Review Box 27-1. Reul and associates described the ideal autotransfusion candidate as a blunt or penetrating trauma victim with hemothorax consisting of 1500 mL or more of blood.41 Other patients who may benefit include those with an immediate need for transfusion for whom insufficient homologous blood is available (because of a shortage or a difficult crossmatch) and those whose religious convictions prohibit homologous transfusion.36,39,40
Contraindications
In some situations emergency autotransfusion may pose more risk than benefit. Coagulopathy and disseminated intravascular coagulation (DIC) are important contraindications.8,42 Others include active infection, gross contamination, and the possibility of malignant cells in the salvaged blood.43 With direct autotransfusion of pleural blood through a microfilter, the risk of reinfusing tumor cells is unknown. However, recent work using perioperative cell salvage suggests that the risk for dissemination of malignant disease is minimal.44 Concerns regarding gross contamination from concomitant gastrointestinal tract injury have also been disputed.45 An early study of autotransfusion before cell washing was implemented found that despite reinfusion of massively contaminated blood, 17 of 25 patients survived without evidence of septic complications.46
Recent work on contamination involving cell salvage systems incorporates a cell-washing step.47,48 Several investigators believe that reinfusion of limited amounts of contaminated blood from the peritoneal cavity may now be accomplished with acceptable risk. Nonetheless, the current consensus is that exsanguinating hemorrhage without available homologous blood is the only acceptable indication for autotransfusion when there is recognized intestinal contamination.49–51 Concurrent use of systemic antibiotics is advised.8,9,47,48,51,52
Autotransfusion should be performed within 4 to 6 hours from the time of injury,37,53 although it has been performed after this period in combat situations without significant complications.8,48
Equipment and Material
In-line filtration is used routinely during reinfusion of blood products to reduce the danger of microembolization and resultant pulmonary insufficiency.1,37,53 The relationship between the presence of microaggregates and the development of acute respiratory distress syndrome is controversial.1 However, most investigators advise the use of a micropore filter with pore size recommendations ranging from 20 to 170 µm.54–56 The majority of investigators believe that a pore size of 40 µm minimizes the risk for microembolization without undue elevation in filtration pressure.41,57,58 The manufacturers of the commercial devices discussed in this chapter also recommend at least a 40-µm filter size.
Vacuum Suction
The level of vacuum suction should be limited to minimize hemolysis of red blood cells.58 The precise level at which clinically significant hemolysis occurs is uncertain, and there is wide variation (5 to 100 mm Hg) in recommendations.14,41,55,59–61 Several commercial products recommend a starting vacuum pressure of 20 mm Hg.62
Anticoagulation
Blood retrieved from the pleural and abdominal cavities frequently will not clot because it is devoid of fibrinogen.63 This is believed to be due to the fact that moderate rates of bleeding allow time for defibrination by contact with the serosal surfaces and by mechanical agitation from respiratory and cardiac movement. For this reason, some recommend simple reinfusion through a filter without any anticoagulant.17,54,60,64 However, wounds of the great vessels may bleed at a rate that allows coagulable blood to enter the collection reservoir and clot the entire system.52,60,61 In the ED setting where autotransfusion is indicated for rapid blood loss, anticoagulation is recommended.
The use of heparin as an anticoagulant in trauma patients is not recommended by the manufacturers of autotransfusion devices.62 Heparin anticoagulation is possible; however, local heparinization of the tubing and reservoir may lead to the formation of platelet microaggregates on the filter and in the line, as well as increase the risk for systemic heparinization.41,65
Citrate compounds, an alternative to heparin, are frequently recommended for autotransfusion.42 Citrates bind with the calcium ion and prevent conversion of protein fibrinogen into insoluble fibrin, which would cause the blood to clot. Because citrate binds only with calcium, it anticoagulates just the blood in which it is dissolved. Once the anticoagulated blood is infused, citrate is rapidly metabolized by the liver. Early studies used acid citrate dextrose (ACD).37 More recently, citrate phosphate dextrose (CPD) has been used because it necessitates less volume as an anticoagulant and results in less acidosis than ACD.41,66,67 Rarely, excessive use of CPD can cause cardiac dysrhythmias.41 Moreover, use of insufficient or outdated CPD may result in clotting of collected blood. Baldan and colleagues described the use of simple autotransfusion in more than 200 patients with penetrating chest injuries and recommended using half-dose citrate phosphate dextrose adenine (CPDA) solution.9 Although anticoagulant therapy and dosage recommendations are at the discretion of the physician, commercially available devices do offer some general guidelines (Table 27-1).
TABLE 27-1
ACD-A and CPD Dosage Recommendations
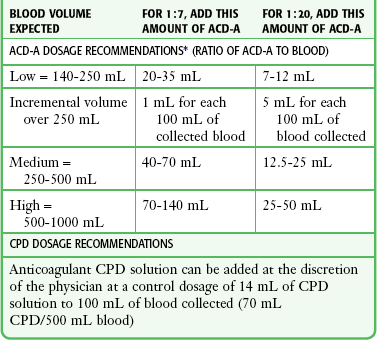
ACD-A, Acid Citrate Dextrose anticoagulant; CPD, citrate phosphate dextrose.
*Dosage ratios are approximate. Note that 35 mL of ACD-A provides a 1:7 ratio of ACD-A to blood for low volume and a 1:20 ratio for high-volume blood and may be a good starting point if it is unclear how much blood will accumulate.
From A Personal Guide to Managing Chest Drainage Autotransfusion. Atrium Medical Corporation, pp 26, 27; available at http://www.atriummed.com/PDF/Red%20Handbook.pdf.
Historical Techniques Using Standard ED Equipment
Before purpose-built systems were available, autotransfusion was possible by using common items available in most EDs. These techniques have not been as widely tested and are more appropriate for use in dire situations, such as may occur in a disaster or on the battlefield.9
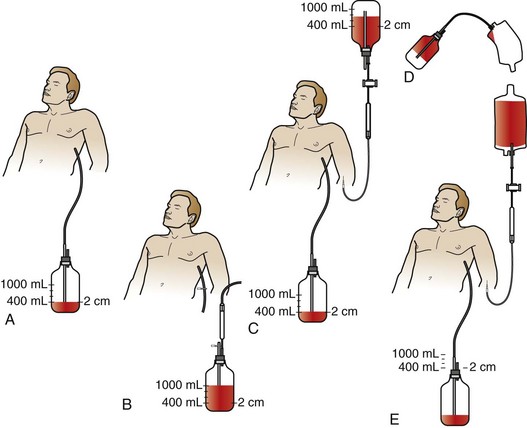
Symbas described a simplified collecting system involving a standard chest tube bottle.68 More than 400 patients were autotransfused via this method with no adverse effects attributable to the procedure. After insertion of a chest tube, blood was collected into a standard bottle containing 400 mL of normal saline, with suction maintained at 12 to 16 mm Hg. The blood collected in the chest bottle can be reinfused in one of two possible ways as shown in Figure 27-3. Improvements on his historical technique, such as the addition of anticoagulation and blood filters, should be considered.
Autotransfusion Units
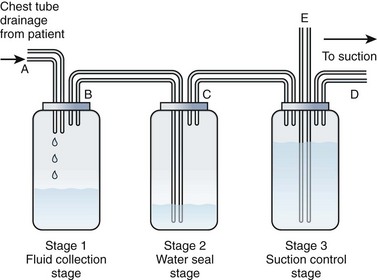
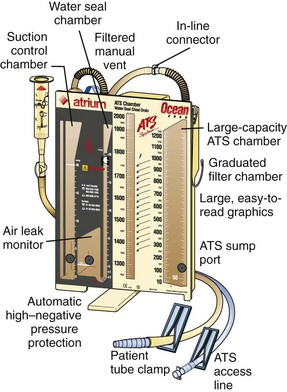
All the commercially available autotransfusion systems (ATSs) are based on the same three-stage system (called the “three-bottle system”) for collection of pleural fluid (Fig. 27-4). Currently used devices incorporate the three-bottle system into one unit. Stage 1 is the collection of pleural fluid (blood). Stage 2 is the water seal stage, in which water acts as a one-way valve (water seal) that allows air to be sucked out of the pleural space but not leak back in. Stage 3 is the suction control stage, which is essentially a safety stage so that if the degree of suction suddenly increases beyond the set point, the system will pull air from the atmosphere instead of inappropriately increasing suction in the pleural cavity. Historically, water was (and still frequently is) used in the suction control stage.
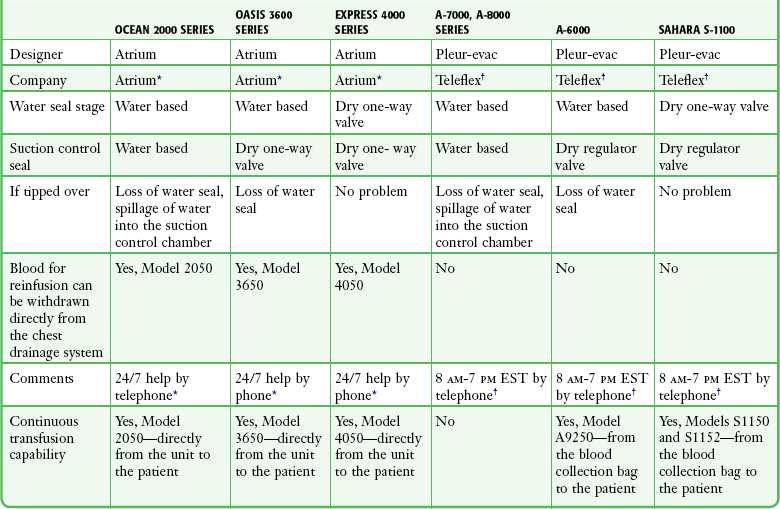
The disadvantage of using water in stages 2 or 3 is that if the device is knocked over, loss of the water seal and spillage of water from the suction control stage can occur. Newer designs have solved this problem, and two systems currently available do not use water (Atrium Express and Pleur-evac Sahara). These newer devices use a one-way valve instead of a water seal in stage 2 and a pressure regulator instead of water in the suction control stage (stage 3). Table 27-2 describes features of several of the more commonly used devices.
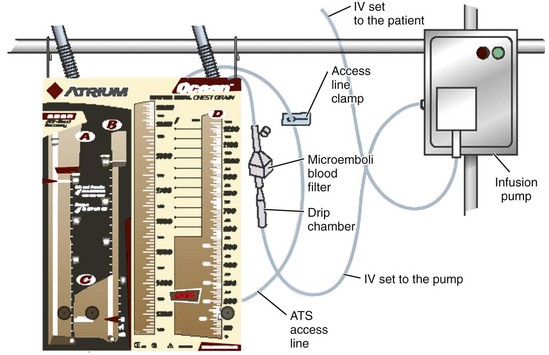
Some models allow these chest drainage systems to be connected directly to the patient to provide continuous autotransfusion. In this setup, a blood filter with intravenous (IV) blood tubing is hooked up to an IV infusion pump. The blood can then be continuously reinfused as it collects in the chest drainage system; it goes from the blood collection chamber out the ATS access line and is pumped into the patient with an IV infusion pump in a closed system (Fig. 27-5). Other models offer continuous infusion directly from a blood collection bag and not the chest drainage device. The blood simply goes from the chest tube into the blood collection bag, and a port on the bag is accessed to return the blood back to the patient.
Procedure for Autotransfusion
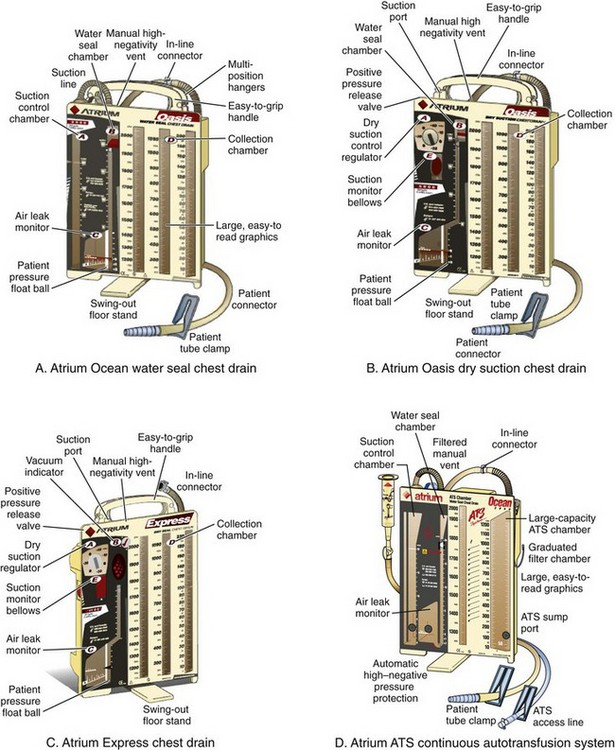
Whether in-line, self-filling, or continuous techniques are chosen depends on the chest tube drainage system used. Some systems allow more than one technique to collect blood for autotransfusion. The specific port locations and connections may vary slightly among the different designs, but the general concepts are relatively universal. See Figures 27-6 and 27-7 for a description of several different systems from Atrium and Pleur-evac.
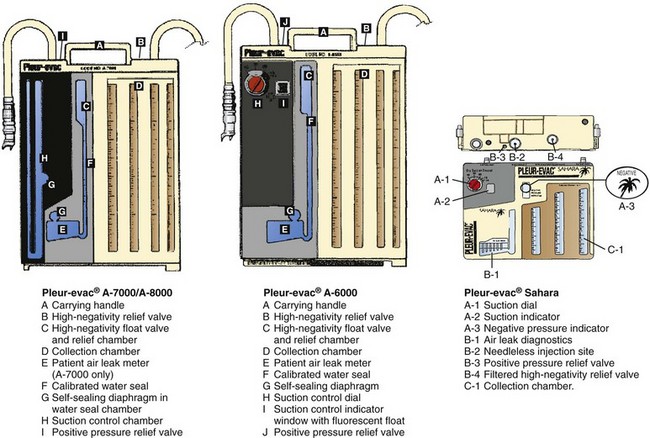
Figure 27-6 Pleur-evac chest drainage systems. (Courtesy of Teleflex Medical, Research Triangle Park, NC.)
Atrium Chest Drainage Devices
In-Line Autotransfusion Blood Collection and Infusion Procedure (Fig. 27-8)
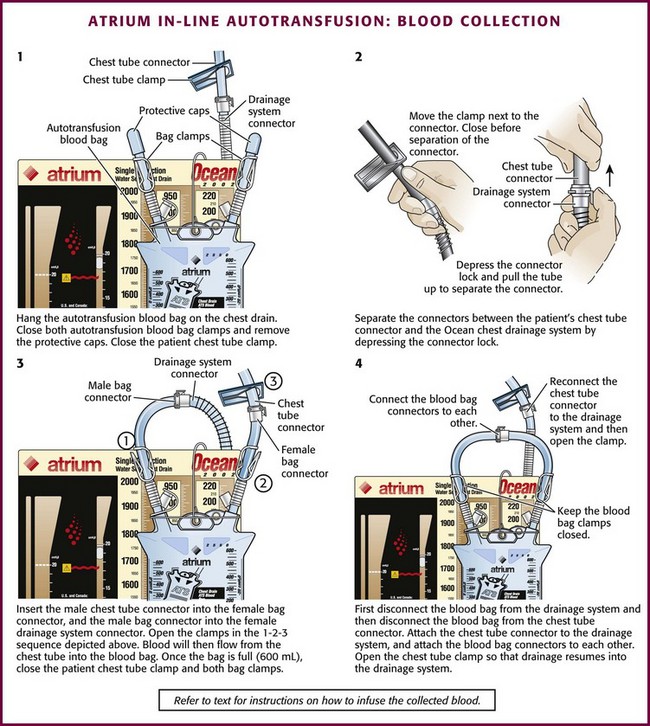
Figure 27-8 Atrium in-line autotransfusion: blood collection. (Courtesy of Atrium Medical Corporation, Hudson, NH.)
1. Place the autotransfusion blood bag onto the chest drain via the attached flexible hanger.
2. Close both autotransfusion blood bag clamps and remove the protective caps over the autotransfusion bag connectors.
3. Close the patient’s chest tube clamp.
4. Separate the connectors between the patient’s chest tube and the Ocean chest drain by depressing the connector lock.
5. Insert the male patient chest tube connector into the female autotransfusion bag connector.
6. Insert the male autotransfusion bag connector into the female Ocean chest drain connector.
7. Open the clamps in the following order:
a. Open the autotransfusion bag clamp going to the Ocean chest drain.
b. Open the autotransfusion bag clamp going to the patient’s chest tube.
c. Open the patient’s chest tube clamp to resume drainage. (Blood will then flow from the patient’s chest tube into the autotransfusion blood bag.)
8. When the blood bag is filled (600 mL maximum), close the patient’s chest tube clamp and both autotransfusion blood bag clamps.
9. Disconnect the autotransfusion blood bag connection with the Ocean chest drain connection first.
10. Disconnect the autotransfusion blood bag from the patient’s chest tube connector.
11. Insert the male patient chest tube connector into the female Ocean chest drain connector.
12. Open the patient’s chest tube clamp so that drainage may resume into the Ocean chest drain device.
13. Connect the male and female autotransfusion bag connectors to each other.
14. Connect and pre-prime the blood filter and the IV blood tubing with sterile saline (spike the blood tubing into the filter).
15. Invert the autotransfusion bag so that the spike port at the bottom points upward.
16. Remove the tethered cap with sterile technique.
17. Insert the saline-primed filter spike into the autotransfusion bag spike port with a firm twisting motion.
18. Return the autotransfusion bag to an upright position and place it on a standard IV pole.
19. Open the filtered air vent located on top of the autotransfusion bag first, and then open the IV tubing clamp.
20. Evacuate all the remaining air from the IV line and attach it to the patient to begin the infusion.
21. For gravity infusion, leave the air vent at the top of the autotransfusion bag open.
22. For pressure infuser application, the filtered air vent must remain closed (the maximum ATS bag infuser pressure is 150 mm Hg).
Self-Filling Autotransfusion Blood Collection and Infusion Procedure
1. Identify the chest drain ATS access line (at the bottom of the collection chamber of the Ocean chest drain), and close the ATS access line clamp (Fig. 27-9).
2. Remove the spike port cap of the ATS access line and insert the autotransfusion bag spike into the Ocean ATS access line spike port with a firm twisting motion.
3. Position the autotransfusion bag at least 2 to 4 inches below the base of the Ocean chest drain.
4. Open the clamps to the autotransfusion blood bag (on the ATS access line and on the autotransfusion blood bag).
5. Activate the autotransfusion bag spring-generated vacuum by gently bending the autotransfusion bag upward where indicated to initiate transfer of blood. A “pop” will be heard and the bag will expand when properly activated. The self-filling autotransfusion bag will begin to fill and expand as blood enters from the chest drain. Do not activate the autotransfusion bag before connecting it to the chest drain (Fig. 27-10).
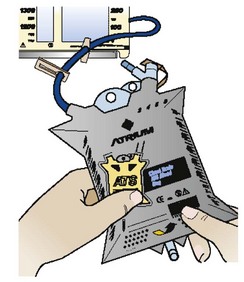
Figure 27-10 Activation of the self-filling Atrium autotransfusion bag. (Courtesy of Atrium Medical Corporation, Hudson, NH.)
6. Displace any air in the autotransfusion bag by gently squeezing the autotransfusion bag as necessary.
7. When the autotransfusion blood bag is filled (700-mL capacity), close both the ATS access line clamp and the autotransfusion blood bag clamp and cap the end of the ATS access line.
8. Remove the autotransfusion blood bag spike from the ATS access line spike port and place the spike back into the original bag port. Position the ATS access line in the holder on top of the Ocean chest drain. Keep the ATS access line clamp fully closed at all times when not in use.
9. Connect the filter to the IV blood infusion set (spike the filter with the blood tubing spike) and pre-prime the blood filter and IV blood tubing with sterile saline.
10. Invert the autotransfusion bag so that the spike port at the bottom of the autotransfusion bag points upward.
11. Remove the tethered cap via sterile technique.
12. Insert the saline-primed filter spike into the autotransfusion bag spike port with a firm twisting motion.
13. Return the autotransfusion bag to an upright position and place it on a standard IV pole.
14. Open the filtered air vent located at the top of the autotransfusion bag first, and then open the IV line clamp.
15. Evacuate all the remaining air from the IV line and attach it to the patient to begin infusion.
16. For gravity infusion, leave the air vent at the top of the ATS bag open.
17. For pressure infuser application, the filtered air vent must remain closed (the maximum ATS bag infuser pressure is 150 mm Hg).
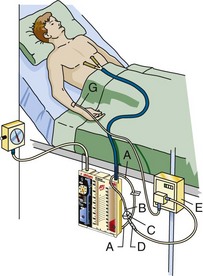
Continuous Autotransfusion (Fig. 27-11)
1. Spike the blood IV tubing set into the filter and prime it with sterile saline.
2. Identify the chest drain ATS access line at the bottom of the Ocean chest drain and close the ATS access line clamp (see Fig. 27-9).
3. Drape the ATS access line around the hanger or the patient’s line so that the terminal end of the ATS access line is facing the floor.
4. Remove the spike port cap on the ATS access line and insert the blood filter spike into the ATS access line.
5. Turn the filter to a “spike down” position.
6. Unclamp the ATS access line and IV tubing.
7. Use a 60-mL syringe to aspirate blood through the filter and into the drip chamber of the IV tubing set.
8. When the drip chamber is  full, turn the filter to the “spike up” position and continue purging air from the line.
full, turn the filter to the “spike up” position and continue purging air from the line.
9. When purging is complete, insert the IV cassette into the pump.
10. Purge all air before connection to the patient.
11. Set the pump to the desired volume and rate (mL/hr) to be infused.
12. Watch the infusion carefully and make sure that the infusion pump is programmed to stop before all the blood in the Ocean chest drain is empty to prevent air embolism.
Pleur-evac Chest Drainage Devices
All Pleur-evac models have similar designs. The method described here is for the Sahara design.69
In-Line Blood Collection and Autotransfusion Procedure
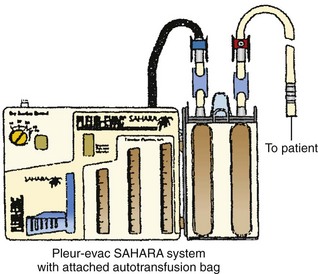
1. Close the two clamps on the top of the autotransfusion bag (Fig 27-12).
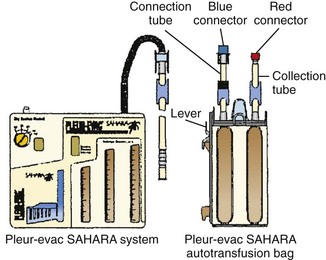
Figure 27-12 Pleur-evac Sahara in-line autotransfusion setup. (Courtesy of Teleflex Medical, Research Triangle Park, NC.)
2. Attach the autotransfusion bag to the side of the chest drain device by aligning the bottom leg and the lever of the adapter with their mating receptacles on the chest drain system. Insert with a downward motion until the lever “clicks” into position. The autotransfusion bag should be firmly attached to the chest drain device (Fig. 27-13).
3. Close the clamp on the patient’s chest tube and disconnect the red and blue connectors separating the patient’s chest tube from the chest drainage device.
4. Remove the red protective cap from the autotransfusion bag tube and connect this end to the patient’s chest tube.
5. Remove the blue protective cap from the autotransfusion collection bag tube and connect this end to the chest drainage device (red connected to red and blue connected to blue).
6. Open all the clamps. The autotransfusion blood bag will begin to fill.
7. To discontinue autotransfusion, first reduce excessive negative pressure with the high-negativity relief valve on the Sahara chest drain.
8. Close the clamp on the patient’s chest tube, and then close both clamps on the autotransfusion bag.
9. Separate the red connectors going to the patient’s chest tube and the blue connectors going to the Sahara chest drain.
10. Connect the red and blue connectors on the autotransfusion bag.
11. Join the patient’s chest tube red connector to the Sahara chest drain blue connector.
12. Open the clamp on the patient’s chest tube so that drainage from the patient to the Sahara chest drain may resume.
13. Remove the autotransfusion bag from the Sahara chest drain by depressing the lever and lifting the autotransfusion bag up.
14. Remove the adaptor bracket from the wire frame of the autotransfusion bag by twisting it slightly to disengage the bottom and then unhook the top.
15. Slide the autotransfusion bag off the wire support frame. Make sure that the red and blue connectors on the top of the autotransfusion bag are secure and that the clamps on the autotransfusion bag are closed.
16. Connect the filter and IV blood tubing (spike the filter with the IV blood tubing spike).
17. Invert the autotransfusion bag so that the spike port points upward and then remove the protective cap.
18. Insert the filter (use the spike end of the filter) into the spike port on the autotransfusion bag.
19. Evacuate residual air from the autotransfusion bag by opening the IV infusion set clamp, keep the autotransfusion bag inverted, and carefully squeeze all the air from the autotransfusion bag through the filter and IV line.
20. Continue to squeeze the autotransfusion bag to allow blood to slowly prime the filter. Continue squeezing until the filter is saturated with blood and the drip chamber on the IV tubing is half full.
22. Invert the autotransfusion bag and suspend it from an IV pole.
23. Open the IV infusion line clamp to carefully flush all the blood tubing of any remaining air.
24. Attach the distal end of the infusion set to the patient’s IV line and begin infusion.
Continuous Infusion
1. Set up a blood-compatible IV pump.
2. Connect the filter to the IV blood tubing (spike the blood tubing into the filter), and then connect it to a bag of saline (spike the filter into the saline bag). Prime the filter, drip chamber, and IV blood tubing with the sterile saline bag. Remove the bag of saline used to prime it.
3. Remove the blue protective cap from the autotransfusion bag and spike the autotransfusion bag with the blood filter spike.
4. Use an IV pump to prime the filter, drip chamber, and infusion line with blood.
5. If needed, depress the high-negativity relief valve on the top of the Sahara chest drain to relieve excessive negative pressure.
6. Make sure that the infusion line is filled with blood and contains no air, and then attach it to the patient’s IV catheter.
7. Make sure that all the connections are secure.
8. Set the infusion rate on the IV pump.
9. Attach the Sahara drain and the autotransfusion bag to the bed rail with hangers.
Complications
Hematologic Complications
The degree to which autotransfusion contributes to the development or exacerbation of coagulopathy in an actively bleeding patient has still not been conclusively determined.70
Broadie and coworkers found that blood collected from trauma victims during thoracotomy or laparotomy had markedly elevated prothrombin, partial thromboplastin, and thrombin times, as well as absent fibrinogen.63 The dilemma is that severely injured trauma patients who are candidates for autotransfusion are more likely to have other independent risk factors for coagulopathy, such as hypothermia and acidosis. Horst and colleagues studied 154 trauma patients who underwent intraoperative autotransfusion and found that patients who received more than 15 units of autologous blood or more than 50 units of combined autologous and banked blood were coagulopathic but also more severely injured, hypothermic, and acidotic.71 Although coagulation test abnormalities following the infusion of salvaged blood have been interpreted as evidence of DIC, these changes are probably the result of infusion of fibrin degradation products and do not represent consumptive coagulopathy.72 In general, even though coagulopathy should be anticipated, it has not proved to be clinically important when volumes of autotransfused blood remain below 1500 to 2000 mL in adult patients.73,74 When reinfused volumes exceed 3500 mL, laboratory evidence of dilutional coagulopathy may become evident.56 When volumes of autotransfused blood are greater that the patient’s total blood volume, animal studies suggest that the risk for a true consumptive coagulopathy is increased.38
Recommendations regarding the volume of autologous blood that should trigger the infusion of plasma or platelets range from 25% of the total blood volume (about 1250 mL in a 70-kg adult) to 3500 mL56,75 (Box 27-2). Others advise reliance on laboratory tests and clinical findings rather than a volume-based protocol.38 Prudent clinical judgment dictates application of the more liberal guidelines for replacement therapy in patients with extensive hepatic injury, intractable shock, or ongoing loss requiring immediate surgical intervention.
Hemolysis occurs with autotransfusion, in part because of prolonged exposure of cells to the serosa of the traumatized body cavities.76 Hemolysis also results from mechanical damage during collection and reinfusion or from excessive exposure to air-fluid interfaces.41 The hematocrit falls in direct proportion to the quantity of blood transfused, with the average decline being 10% to 20%.41,50,68 However, nontraumatized red blood cell survival has been reported to be normal in all cases studied.17,56,77
Nonhematologic Complications
The theoretical risk for sepsis after the administration of potentially contaminated blood always exists within the nonsterile environment of the typical ED. Experience has shown this risk to be minimal after autotransfusion from an isolated hemothorax, and there is no evidence suggesting that routine prophylaxis with systemic antibiotics is beneficial.15,37 Collected blood is generally transfused as soon as the collection bag is full. The American Association of Blood Banks guidelines allow a 6-hour interval between collection and reinfusion.53 The age of collected blood, however, should be calculated from the time of injury (see Box 27-2). Blood reinfused after this 6-hour period should be considered potentially hazardous.
The risk for microemboli secondary to platelet aggregation and fat emboli has largely been eliminated by the use of micropore filters.50,56,59 During reinfusion of collected blood there is usually a mild increase in screen filtration pressure, indicative of the formation of microemboli trapped by the filter.59 There has been no clinical evidence of pulmonary insufficiency or elevation of the alveolar-arterial oxygen gradient that might be attributed to the passage of microemboli beyond micropore filter systems.41
As with all IV infusions, improper technique, such as applying pressure to air-containing systems, may lead to air embolism. This uncommon but potentially fatal complication has been associated with autotransfusion systems that use automated roller pump units in which the aspirate reservoir was inadvertently allowed to run dry.37,78–80 Air embolism with gravity or with a manually assisted technique is rare.
Infusion of large quantities of unwashed blood that contains hemolyzed red blood cells may contribute to renal failure, particularly in patients with preexisting renal insufficiency.43 Elevated plasma free hemoglobin is a consistent finding in patients who have undergone autotransfusion.41,65,68 In the past it was believed that elevated levels of free hemoglobin following hemolytic transfusion reactions caused renal failure by its precipitation and obstruction of renal tubules. However, more recent evidence suggests that the mechanism in this setting is independent of free hemoglobin but rather is the result of an antigen-antibody–induced intravascular coagulation that when compounded by vasoconstriction and hypotension, leads to renal ischemia.81 Even though renal failure as a direct consequence of autotransfusion has not been reported, transient elevations in serum creatinine do occur. In the presence of shock and systemic acidosis, acute tubular necrosis remains a potential complication.28,36,82 The clinician must weigh the urgency of blood transfusion against the timely availability of an alternative source.
Resources
Brief educational videos are available online for Atrium products at http://www.atriummed.com/EN/Chest_Drainage/edu-files/ATS-video1.asp (accessed July 2012) and show in detail the step-by-step processes to initiate autotransfusion with their devices. They also have a 24-hour help line to assist with any questions during the setup or use of their devices (800-528-7486). Pleur-evac has some limited information available online (www.teleflexmedical.com) and can be contacted during regular business hours (919-544-8000, 8 am to 7 pm EST) to answer questions.
References
1. Schaff, HV, Hauer, JM, Brawley, RK. Autotransfusion in cardiac surgical patients after operation. Surgery. 1978;84:713–718.
2. Yoshiba, F. [Autologous transfusion for patients with increased risk of massive and/or critical bleeding—indication, efficacy and limitations.]. Masui. 2011;60:40–46.
3. Tavare, AN, Parvizi, N. Does use of intraoperative cell-salvage delay recovery in patients undergoing elective abdominal aortic surgery? Interact Cardiovasc Thorac Surg. 2011;12:1028–1032.
4. Shantikumar, S, Patel, S, Handa, A. The role of cell salvage autotransfusion in abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg. 2011;42:577–584.
5. Ralph, CJ, Sullivan, I, Faulds, J. Intraoperative cell salvaged blood as part of a blood conservation strategy in caesarean section: is fetal red cell contamination important? Br J Anaesth. 2011;107:404–408.
6. Konstantinou, EA, Brady, JM, Soultati, A, et al. Intraoperative use of cell saver on patients undergoing open abdominal aortic aneurysm surgical repair: a Greek hospital experience. J Perianesth Nurs. 2011;26:225–230.
7. Hansen, E, Seyfried, T. [Cell salvage.]. Anaesthesist. 2011;60:381–389. [quiz 390].
8. Ahmed, AM, Sabrie, MH, Baldan, M. Autotransfusion in penetrating chest war trauma with haemothorax: the Key-saney Hospital experience. East Cent Afr J Surg. 2003;8:51–54.
9. Baldan, M, Glannou, CP, Rizzardi, G, et al. Autotransfusion from haemothorax after penetrating chest trauma: a simple, life-saving procedure. Trop Doct. 2006;36:21–22.
10. Jevtic, M, Petrovic, M, Ignjatovic, D, et al. Treatment of wounded in the combat zone. J Trauma. 1996;40(3 suppl):S173–S176.
11. Blundell, J. Experiments on the transfusion of blood by the syringe. Med Chir Trans. 1818;9:56–92.
12. Duncan, J. On re-infusion of blood in primary and other amputations. Br Med J. 1886;1:192–193.
13. Elmendorf, A. Weiderinfusion nach Punktion eines frischen Hamatothorax. Munch Med Wochenschr. 1917;64:36.
14. Dyer, RH, Jr., Alexander, JT, Brighton, CT. Atraumatic aspiration of whole blood for intraoperative autotransfusion. Am J Surg. 1972;123:510–514.
15. Klebanoff, G, Phillips, J, Evans, W. Use of a disposable autotransfusion unit under varying conditions of contamination. Preliminary report. Am J Surg. 1970;120:351–354.
16. Klebanoff, G. Early clinical experience with a disposable unit for the intraoperative salvage and reinfusion of blood loss (intraoperative autotransfusion). Am J Surg. 1970;120:718–722.
17. Symbas, PN, Levin, JM, Ferrier, FL, et al. A study on autotransfusion from hemothorax. South Med J. 1969;62:671–674.
18. Chetcuti, K, Barnard, J, Loggos, S, et al. Massive hemothorax secondary to metastatic renal carcinoma. Ann Thorac Surg. 2010;89:2014–2016.
19. Black, H, Sigal, D, Barnes, D, et al. A 25-year-old patient with spontaneous hemothorax. Chest. 2005;128:3080–3083.
20. McEwan, K, Thompson, P. Ultrasound to detect haemothorax after chest injury. Emerg Med J. 2007;24:581–582.
21. Llewelyn, CA, Hewitt, PE, Knight, RS, et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421.
22. Peden, AH, Head, MW, Ritchie, DL, et al. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet. 2004;364:527–579.
23. Moore, A, Herrera, G, Nyamongo, J, et al. Estimated risk of HIV transmission by blood transfusion in Kenya. Lancet. 2001;358:657–660.
24. Madjdpour, C, Heindl, V, Spahn, DR. Risks, benefits, alternatives and indications of allogenic blood transfusions. Minerva Anestesiol. 2006;72:283–298.
25. Beale, E, Chu, J, Chan, L, et al. Blood transfusion in critically injured patients: a prospective study. Injury. 2006;37:455–465.
26. Claridge, JA, Sawyer, RG, Schulman, AM, et al. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner. Am Surg. 2002;68:566–572.
27. Hill, GE, Frawley, WH, Griffin, KE, et al. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54:908–914.
28. Dunne, JR, Malone, DL, Tracy, JK, et al. Allogenic blood transfusion in the first 24 hours after trauma is associated with increased systemic inflammatory response syndrome (SIRS) and death. Surg Infect (Larchmt). 2004;5:395–404.
29. Charles, A, Shaikh, AA, Walters, M, et al. Blood transfusion is an independent predictor of mortality after blunt trauma. Am Surg. 2007;73:1–5.
30. Malone, DL, Dunne, J, Tracy, JK, et al. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54:898–905. [discussion 905-907].
31. Dixon, JL, Smalley, MG. Jehovah’s Witnesses. The surgical/ethical challenge. JAMA. 1981;246:2471–2472.
32. Autologous blood transfusions. Council on Scientific Affairs. JAMA. 1986;256:2378–2380.
33. Davies, L, Brown, TJ, Haynes, S, et al. Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess. 2006;10(44):iii–iv. [ix-x, 1-210].
34. Brown, CV, Foulkrod, KH, Sudler, HT, et al. Autologous Blood Transfusion during emergency trauma operations. Arch Surg. 2010;145:690–694.
35. Adias, TC, Jeremiah, Z, Uko, E, et al. Autologous blood transfusion—a review. S Afr J Surg. 2006;44(3):114–116. [118].
36. O’Riordan, WD. Autotransfusion in the emergency department of a community hospital. JACEP. 1977;6:233–237.
37. Mattox, KL, Walker, LE, Beall, AC, et al. Blood availability for the trauma patient—autotransfusion. J Trauma. 1975;15:663–669.
38. Silva, R, Moore, EE, Bar-Or, D, et al. The risk:benefit of autotransfusion—comparison to banked blood in a canine model. J Trauma. 1984;24:557–564.
39. Hughes, DB, Ullery, BW, Barie, PS. The contemporary approach to the care of Jehovah’s Witnesses. J Trauma. 2008;65:237–247.
40. Victorino, G, Wisner, DH. Jehovah’s Witnesses: unique problems in a unique trauma population. J Am Coll Surg. 1997;184:458–468.
41. Reul, GJ, Jr., Solis, RT, Greenberg, SD, et al. Experience with autotransfusion in the surgical management of trauma. Surgery. 1974;76:546–555.
42. Atrium Medical Corporation. Available at http://www.atriummed.com/PDF/Red%20Handbook.pdf.
43. The use of autologous blood. The National Blood Resource Education Program Expert Panel. JAMA. 1990;263:414–417.
44. Kruskall, MS. Autologous blood transfusion and related alternatives to allogenic transfusion. In: Hillyer CD, Silberstein LE, Ness PM, et al, eds. Blood Banking and Transfusion Medicine. Philadelphia: Churchill Livingstone, 2003.
45. Roostar, L. Clinical pictures of penetrating chest injuries: infusion therapy and haemotransfusion. In: Gunshot Chest Injuries. Tartu, Estonia: Tartu University Press; 1996:91.
46. Griswold, RA, Ortner, AB. The use of autotransfusion in surgery of the serous cavities. Surg Gynecol Obstet. 1943;77:167.
47. Bowley, DM, Barker, P, Boffard, KD. Intraoperative blood salvage in penetrating abdominal trauma: a randomised, controlled trial. World J Surg. 2006;30:1074–1080.
48. Özmen, V, McSwain, NE, Jr., Nichols, RL, et al. Autotransfusion of potentially culture-positive blood (CPB) in abdominal trauma: preliminary data from a prospective study. J Trauma. 1992;32:36–39.
49. Jurkovich, GJ, Moore, EE, Medina, G. Autotransfusion in trauma. A pragmatic analysis. Am J Surg. 1984;148:782–785.
50. Smith, RN, Yaw, PB, Glover, JL. Autotransfusion of contaminated intraperitoneal blood: an experimental study. J Trauma. 1978;18:341–344.
51. Timberlake, GA, McSwain, NE, Jr. Autotransfusion of blood contaminated by enteric contents: a potentially life-saving measure in the massively hemorrhaging trauma patient? J Trauma. 1988;28:855–857.
52. Upton, DA, Proehl, JA. General principles of autotransfusion. In Proehl JA, ed.: Emergency Nursing Procedures, 3rd ed, St. Louis: Saunders, 2004.
53. Burch, JM. Blood transfusion, microfiltration, and the adult respiratory distress syndrome. Curr Concepts Trauma Care. 1983;6:16.
54. Barriot, P, Riou, B, Viars, P. Prehospital autotransfusion in life-threatening hemothorax. Chest. 1988;93:522–526.
55. Noon, GP. Intraoperative autotransfusion. Surgery. 1978;84:719–721.
56. Raines, J, Buth, J, Brewster, DC, et al. Intraoperative autotransfusion: equipment, protocols, and guidelines. J Trauma. 1976;16:616–623.
57. Mattox, KL, Espada, T, Beall, AC. Performing thoracotomy in the emergency center. JACEP. 1974;3:13.
58. Wright, CB, Solis, RT. Microaggregation in canine autotransfusion. Am J Surg. 1973;126:25–29.
59. Brewster, DC, Ambrosino, JJ, Darling, RC, et al. Intraoperative autotransfusion in major vascular surgery. Am J Surg. 1979;137:507–513.
60. Davidson, SJ. Emergency unit autotransfusion. Surgery. 1978;84:703–707.
61. Von Koch, L, Wilson Defore, W, Mattox, KL. A practical method of autotransfusion in the emergency center. Am J Surg. 1977;133:770–772.
62. Atrium Medical Corporation, http://www.atriummed.com/Products/Chest_drains/edu-ats.asp.
63. Broadie, TA, Glover, JL, Bang, N, et al. Clotting competence of intracavitary blood in trauma victims. Ann Emerg Med. 1981;10:127–130.
64. Bell, W. The hematology of autotransfusion. Surgery. 1978;84:695–699.
65. Mattox, KL, Beall, AC, Jr. Autotransfusion: use in penetrating trauma. Tex Med. 1975;71(12):69–77.
66. Jacobs, LM, Hsieh, JW. A clinical review of autotransfusion and its role in trauma. JAMA. 1984;251:3283–3287.
67. Mollison, PL. Some clinical consequences of red cell incompatibility: the Bradshaw lecture 1978. J R Coll Physicians Lond. 1979;13:15–20.
68. Symbas, PN. Extraoperative autotransfusion from hemothorax. Surgery. 1978;84:722–727.
69. Upton, DA, Proehl, JA. Autotransfusion devices: Pleur-evac. In Proehl JA, ed.: Emergency Nursing Procedures, 3rd ed, St. Louis: Saunders, 2004.
70. Özmen, VE, McSwain, NE, Nichols, RL, et al, Autotransfusion induced coagulopathy: a clinical problem?. Presented at the Eastern Association for the Surgery of Trauma, Fifth Scientific Assembly, Bermuda, January 16-18, 1992.
71. Horst, HM, Dlugos, S, Fath, SS, et al. Coagulopathy and intraoperative blood salvage (IBS). J Trauma. 1992;32:646–652. [discussion 652-653].
72. National Heart, Lung and Blood Institute, http://www.nhlbi.nih.gov/health/prof/blood/transfusion/logo.htm#periop.
73. Boffard, KD, Joseph, C. Autotransfusion. In: Hahn RG, Prough DS, Svensen CH, eds. Perioperative Fluid Therapy. New York: Informa Healthcare USA, 2007. [ed., C.].
74. Thurer, RL, Hauer, JM. Autotransfusion and blood conservation. Curr Probl Surg. 1982;19:97–156.
75. Napoli, VM, Symbas, PJ, Vroon, DH, et al. Autotransfusion from experimental hemothorax: levels of coagulation factors. J Trauma. 1987;27:296–300.
76. Stillman, RM, Wrezlewicz, WW, Stanczewski, B, et al. The haematological hazards of autotransfusion. Br J Surg. 1976;63:651–654.
77. Schweitzer, EJ, Hauer, JM, Swan, KG, et al. Use of the Heimlich valve in a compact autotransfusion device. J Trauma. 1987;27:537–542.
78. Bretton, P, Reines, HD, Sade, RM. Air embolization during autotransfusion for abdominal trauma. J Trauma. 1985;25:165–166.
79. Duncan, SE, Klebanoff, G, Rogers, W. A clinical experience with intraoperative autotransfusion. Ann Surg. 1974;180:296–304.
80. Noon, GP, Solis, RT, Natelson, EA. A simple method of intraoperative autotransfusion. Surg Gynecol Obstet. 1976;143:65–70.
81. Galel, SA, Nguyen, DD, Fontaine, MJ, Goodnough, LT, et al, Transfusion medicine. Wintrobe’s Clinical Hematology, 12th ed. Greer, JP, Foerster, J, Rodgers, GM, Paraskevas, F, et al, eds. Wintrobe’s Clinical Hematology, Philadelphia, Lippincott, Williams & Wilkins, 2009;Vol 1.
82. Barbanel, C. Hemoglobinuria and myoglobinuria. In: Hamburger J, Crosnier J, Grunfeld JP, eds. Nephrology. New York: John Wiley & Sons, 1979.