Chapter 18 Atrioventricular Reentrant Tachycardia
Types of Bypass Tracts
Bypass tracts (BTs) are remnants of the atrioventricular (AV) connections caused by incomplete embryological development of the AV annuli and failure of the fibrous separation between the atria and ventricles. There are several types of BTs. Atrioventricular BTs are strands of working myocardial cells connecting atrial and ventricular myocardium across the electrically insulating fibrofatty tissues of the AV junction bypassing the atrioventricular node–His-Purkinje system (AVN-HPS). In the older literature, these BTs were called Kent bundles, although incorrectly (Kent described AVN-like tissue in the right atrial [RA] free wall that did not connect to the ventricle). Thus, the use of the term bundle of Kent should be discouraged.1 Atrionodal BTs connect the atrium to the distal or compact AVN. They have been called James fibers and are of uncertain physiological significance. Atrio-Hisian BTs connect the atrium to the His bundle (HB); these BTs are rare.2,3 Atypical BTs include various types of Hisian-fascicular BTs, which connect the atrium (atriofascicular pathways), AVN (nodofascicular pathways), or HB (fasciculoventricular) to distal Purkinje fibers or ventricular myocardium, in addition to slowly conducting short atrioventricular BTs and long atrioventricular BTs. These atypical BTs are sometimes collectively referred to as Mahaim fibers, a term to be discouraged because it is more illuminating to name the precise BT according to its connections.
Types of Preexcitation Syndromes
In the Wolff-Parkinson-White (WPW) syndrome, AV conduction occurs, partially or entirely, through an AV BT, which results in earlier activation (preexcitation) of the ventricles than if the impulse had traveled through the AVN.1 In the setting of Lown-Ganong-Levine (LGL) syndrome, preexcitation purportedly occurs via atrio-Hisian BTs or, alternatively, no BT is present and enhanced AVN conduction accounts for the electrocardiographic (ECG) findings. The net effect is a short PR interval without delta wave or QRS prolongation. It is important to stress, however, that LGL is not a recognized syndrome with an anatomical basis, but only an ECG description, and the use of the term should be discouraged.2,3 The so-called Mahaim variant of preexcitation does not typically result in a delta wave, because these pathways, which usually terminate in the conducting system or in the ventricular myocardium close to the conducting system, conduct slowly, and the AVN-HPS has adequate time to activate most of the ventricular muscle mass. Concealed AV BTs refer to AV BTs that do not manifest anterograde conduction and therefore do not result in ventricular preexcitation. Because they do not result in alteration of the QRS complex in the ECG, they cannot be detected by inspection of the surface ECG; they are called concealed. However, the concealed BT can conduct in a retrograde fashion, thereby creating a reentrant circuit with impulses traveling from the atrium to the AVN, HPS, ventricle, and then back to the atrium via the BT.
Pathophysiology
Wolff-Parkinson-White Syndrome
WPW pattern refers to the constellation of ECG abnormalities related to the presence of an AV BT (short PR interval, delta wave) in asymptomatic patients.1 WPW syndrome refers to a WPW ECG pattern associated with tachyarrhythmias.
Because the AV BT typically conducts faster than the AVN, the onset of ventricular activation is earlier than if depolarization occurred only via the AVN, resulting in a shortened PR (P-delta) interval. Additionally, because the BT exhibits practically nondecremental conduction, the early activation (and P-delta interval) remains almost constant at all heart rates. Preexcited intraventricular conduction in WPW propagates from the insertion point of the AV BT in the ventricular myocardium via direct muscle-to-muscle conduction. This process is inherently slower than ventricular depolarization resulting from rapid HPS conduction. Thus, although the initial excitation of the ventricles (via the BT) occurs earlier, it is followed by slower activation of the ventricular myocardium than occurs normally. The net effect is that the QRS complex consists of fusion between the early ventricular activation caused by preexcitation with the later ventricular activation resulting from impulse propagation through the AVN and HPS to the ventricles. The initial part of ventricular activation resulting in the upstroke of the QRS complex is slurred because of slow muscle-to-muscle conduction; this is termed a delta wave. The more rapid the conduction along the BT in relation to the AVN, the greater the amount of myocardium depolarized via the BT, resulting in a more prominent or wider delta wave and increasing prolongation of the QRS complex duration.
Atrioventricular Bypass Tracts
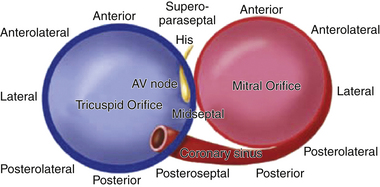
AV BTs are aberrant muscle bundles that connect the atria to the ventricles outside of the normal AV conduction system. AV BTs are found most often in the parietal AV junctional areas, including the paraseptal areas. They breach the insulation provided by the fibrofatty tissues of the AV groove (sulcus tissue) and the hinge lines (fibrous annulus) of the valves. They are rarely found in the area of fibrous continuity between the aortic and mitral valves because in this area, there is usually a wide gap between the atrial myocardium and ventricular myocardium to accommodate the aortic outflow tract.4,5 The remainder of the AV groove may be divided into quadrants consisting of the left free wall, right free wall, and posteroseptal and anteroseptal spaces. The distribution of BTs within these regions is not homogeneous—46% to 60% of BTs are found within the left free wall space; 25% are within the posteroseptal space; 13% to 21% of BTs are within the right free wall space; and 2% are within the right superoparaseptal (formerly called anteroseptal) space (Fig. 18-1).
BTs are usually very thin muscular strands (rarely thicker than 1 to 2 mm) but can occasionally exist as broad bands of tissue. The AV BT can run in an oblique course rather than perpendicular to the transverse plane of the AV groove. As a result, the fibers can have an atrial insertion point that is transversely from less than one to several centimeters removed from the point of ventricular attachment.6 Some posteroseptal pathways insert into coronary sinus (CS) musculature rather than atrial myocardium and can be associated with the coronary venous system or diverticula from a CS branch vein.
Although the majority (approximately 60%) of AV BTs conduct both anterogradely and retrogradely (i.e., bidirectionally), some AV BTs are capable of propagating impulses in only one direction. BTs that conduct only in the anterograde direction are uncommon (<5%), often cross the right AV groove, and frequently possess decremental conduction properties.3 On the other hand, BTs that conduct only in the retrograde direction occur more frequently, accounting for 17% to 37% of all BTs. When the BT is capable of anterograde conduction, ventricular preexcitation is usually evident during normal sinus rhythm (NSR), and the BT is referred to as manifest. BTs capable of retrograde-only conduction are referred to as concealed.
Atrioventricular Reentry
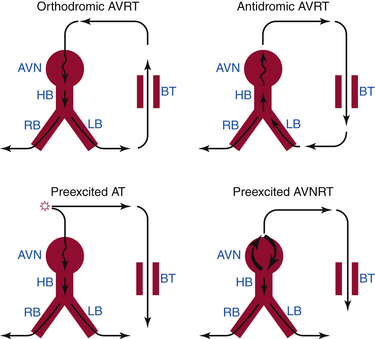
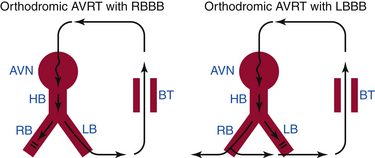
AVRT is a macroreentrant tachycardia with an anatomically defined circuit that consists of two distinct pathways, the normal AV conduction system and an AV BT, linked by common proximal (atrial) and distal (ventricular) tissues. If sufficient differences in conduction time and refractoriness exist between the normal conduction system and the BT, a properly timed premature impulse of atrial or ventricular origin can initiate reentry. AVRTs are the most common (80%) tachycardias associated with the WPW syndrome. AVRT is divided into orthodromic and antidromic according to the direction of conduction in the AVN-HPS (Fig. 18-2). Orthodromic indicates normal direction (anterograde) of conduction over AVN-HPS during the AVRT.
Orthodromic Atrioventricular Reentrant Tachycardia
In orthodromic AVRT, the AVN-HPS serves as the anterograde limb of the reentrant circuit (i.e., the pathway that conducts the impulse from the atria to the ventricles), whereas an AV BT serves as the retrograde limb (see Fig. 18-2). Approximately 50% of BTs participating in orthodromic AVRT are manifest (able to conduct bidirectionally) and 50% are concealed (able to conduct retrogradely only). Therefore, a WPW pattern may or may not be present on the surface ECG during NSR. When preexcitation is present, the delta wave seen during NSR is lost during orthodromic AVRT, because anterograde conduction during the tachycardia is not via the BT (i.e., the ventricle is not preexcited) but over the normal AV conduction system. Orthodromic AVRT accounts for approximately 95% of AVRTs and 35% of all paroxysmal supraventricular tachycardias (SVTs).
Antidromic Atrioventricular Reentrant Tachycardia
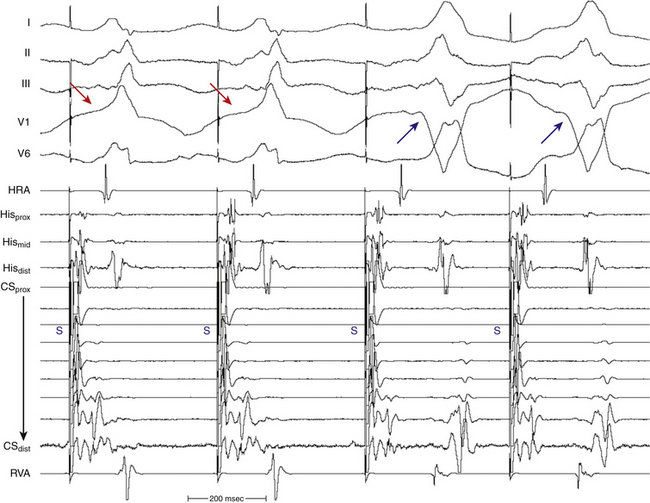
In antidromic AVRT, an AV BT serves as the anterograde limb of the reentrant circuit (see Fig. 18-2). Consequently, the QRS complex during antidromic AVRT is fully preexcited (i.e., the ventricles are activated totally by the BT with no contribution from the normal conduction system). The BT involved in the antidromic AVRT circuit must be capable of anterograde conduction and, therefore, preexcitation is typically observed during NSR. During classic antidromic AVRT, retrograde VA conduction occurs over the AVN-HPS. Other less frequent, nonclassic forms of antidromic AVRT can use a second BT as the retrograde limb of the reentrant circuit or a combination of one BT plus the AVN-HPS in either direction (Fig. 18-3). Antidromic AVRT occurs in 5% to 10% of patients with WPW syndrome. Susceptibility to antidromic AVRT appears to be facilitated by a distance of at least 4 cm between the BT and the normal AV conduction system. Consequently, most antidromic AVRTs use a lateral (right or left) BT as the anterograde route for conduction. Because posteroseptal BTs are in close proximity to the AVN, those BTs are less commonly part of antidromic AVRT if the other limb is the AVN and not a second free wall BT.3 Up to 50% to 75% of patients with spontaneous antidromic AVRT have multiple BTs (manifest or concealed), which may or may not be used as the retrograde limb during the tachycardia. Antidromic SVT is thus a subset of preexcited tachycardias.
Other Arrhythmias Associated with Wolff-Parkinson-White Syndrome
Atrioventricular Nodal Reentrant Tachycardia and Atrial Tachycardia
Physiology consistent with dual AVN physiology has been reported in 8% to 40% of patients with WPW syndrome, although spontaneous sustained AVNRT is less frequent. Both AVNRT and AT can use the bystander BT to transmit impulses to the ventricle (see Fig. 18-2). When AVNRT occurs in the WPW syndrome, the arrhythmia can be difficult to distinguish from AVRT without electrophysiological (EP) testing.
Atrial Fibrillation
Paroxysmal AF occurs in 50% of patients with WPW, and is the presenting arrhythmia in 20%. Chronic AF, however, is rare in these patients.3 Spontaneous AF is most common in patients with anterograde conduction through the BT. Patients with antidromic AVRT, multiple BTs, and BTs that have a short anterograde effective refractory period (ERP) are more liable to develop AF.3 In individuals with WPW, AF is often preceded by AVRT that degenerates into AF.
Atrial Flutter
AFL, like AF, can conduct anterogradely via a BT causing a preexcited tachycardia. Depending on the various refractory periods of the normal and pathological AV conduction pathways, AFL potentially can conduct 1:1 to the ventricles during a preexcited tachycardia, making the arrhythmia difficult to distinguish from ventricular tachycardia (VT; see Fig. 12-6).
Ventricular Fibrillation and Sudden Cardiac Death
The mechanism of sudden cardiac death (SCD) in most patients with WPW is likely the occurrence of AF with a very rapid ventricular rate that leads to VF. Although the frequency with which AF having rapid AV conduction via a BT degenerates into VF is unknown, the incidence of SCD in patients with WPW syndrome is rather low, ranging from 0% to 0.39% annually in several large case series. The trigger for AF in this population of patients (who usually are otherwise healthy individuals and are expected to have a low rate of AF) is generally an episode of SVT. In fact, most patients who have been resuscitated from VF secondary to preexcitation have previous history of AVRT, AF, or both (although in some patients, SCD may be the presenting symptom), and induction of SVT during EP testing is also predictive of clinical symptoms in some asymptomatic individuals.7–11
Several factors can help identify the patient with WPW who is at increased risk for VF, including symptomatic SVT, septal location of the BT, presence of multiple BTs, and male gender.8 Nonetheless, it is clear that the most important factor for the occurrence of VF in these patients is the ability of the BT to conduct rapidly to the ventricles. This is best measured by determining the shortest and average preexcited intervals during AF or alternatively by measuring the anterograde ERP of the BT. If the BT has a very short anterograde effective refractory period (ERP <250 milliseconds), a rapid ventricular response can occur with degeneration of the rhythm to VF. A short preexcited R-R interval during AF (≤220 milliseconds) appears to be a sensitive clinical marker for identifying patients at risk for SCD in children, although its positive predictive value in adults is only 19% to 38%.9,10,12
Clinical Considerations
Epidemiology of Wolff-Parkinson-White Syndrome
Wolff-Parkinson-White Pattern
The prevalence of WPW pattern on the surface ECG is 0.15% to 0.25% in the general population. The prevalence is increased to 0.55% among first-degree relatives of affected patients, suggesting a familial component. The yearly incidence of newly diagnosed cases of preexcitation in the general population was substantially lower, 0.004% in a diverse population of residents from Olmsted County, Minnesota, 50% of whom were asymptomatic.12 The incidence in men is twice that in women and highest in the first year of life, with a secondary peak in young adulthood.
The WPW pattern on the ECG can be intermittent and can even permanently disappear (in up to 40% of cases) over time. Intermittent and/or persistent loss of preexcitation may indicate that the BT has a relatively longer baseline ERP, which makes it more susceptible to age-related degenerative changes and variations in autonomic tone. Consistent with this hypothesis is the observation that, compared with patients with a persistent WPW pattern, those in whom anterograde conduction via the BT disappeared were older (50 versus 39 years) and had a longer ERP of the BT at initial EP study (414 versus 295 milliseconds). The lifetime risk of mortality related to this in asymptomatic individuals can never be accurately known but has been estimated at 0.1% annual risk, with the majority of patients identified between the ages of approximately 10 and 40 years.
In a recent report, about 90% of 293 adults who were asymptomatic at the time of diagnosis of WPW ECG pattern had no arrhythmic events, remaining totally asymptomatic over a median follow-up of 67 months, and 30% of them had disappearance of preexcitation. Only a minority of young adult patients (10%) developed a first arrhythmic event, which was potentially life-threatening in approximately 5%, but no one died. Compared with patients who experienced potentially life-threatening events, those who did not showed a characteristic EP profile (older age, lower tachyarrhythmia inducibility, longer anterograde refractory ERP of BTs, and low likelihood of baseline retrograde BT conduction or multiple BTs).10
In a similar report in 184 children (8 to 12 years of age) with WPW ECG pattern who were totally asymptomatic at the time of diagnosis (which was made incidentally in the majority of cases either at a routine medical examination or on a screening ECG before admission to sports), during a median follow-up of 57 months, no child lost preexcitation, more than 70% had no arrhythmic events, and about 30% developed a first arrhythmic event, which was potentially life-threatening in 10%. Compared with children who experienced potentially life-threatening tachyarrhythmias, those who did not showed a characteristic electrophysiological profile (lower tachyarrhythmia inducibility, longer anterograde refractory period of BTs, and low likelihood of baseline retrograde AP conduction or multiple BTs).9
Wolff-Parkinson-White Syndrome
The prevalence of the WPW syndrome is substantially lower than that of the WPW ECG pattern. In a review of 22,500 healthy aviation personnel, the WPW pattern on surface ECG was seen in 0.25%; only 1.8% of these patients had documented arrhythmias. In another report of 228 subjects with WPW ECG pattern followed for 22 years, the overall incidence of arrhythmia was 1% per patient-year. The occurrence of arrhythmias is related to the age at the time preexcitation was discovered. In the Olmsted County population, one-third of asymptomatic individuals younger than 40 years at the time of diagnosis of WPW eventually had symptoms, compared with none of those who were older than 40 years at diagnosis. In a recent report, of 293 asymptomatic adults with the WPW ECG pattern who underwent EP testing without ablative intervention, almost 90% remained asymptomatic over a median follow-up of 67 months, whereas 31 patients had an arrhythmic event, and 17 had a “potentially” life-threatening event (AF with mean rate of 250 beats/min or faster).10 In another report, of 188 asymptomatic children (between 8 and 12 years of age) with the WPW ECG pattern who underwent EP testing without ablative intervention, 72% remained asymptomatic over a median follow-up of 57 months, whereas 31 patients had an arrhythmic event.9 Symptomatic arrhythmias developed more commonly in initially asymptomatic patients with a WPW ECG pattern who had inducible SVTs in the EP laboratory compared with those with no inducible tachycardias. In one report, less than 4% of patients developed clinical SVT over a 37.7-month follow-up period, compared with 67% of those with inducible SVT on EP testing.
A recent report demonstrated that women more commonly had right-sided BTs compared with men and that Asians had right free wall BTs substantially more frequently than other races. These relationships may suggest a potential inherited component of development of the AV annuli.13
Familial Wolff-Parkinson-White Syndrome
Among patients with the WPW syndrome, 3.4% have first-degree relatives with a preexcitation syndrome. A familial form of WPW has infrequently been reported and is usually inherited as an autosomal dominant trait. The genetic cause of a rare form of familial WPW syndrome has been described.14,15 The clinical phenotype is characterized by the presence of preexcitation on the ECG; frequent SVTs, including AF; progressive conduction system disease; and cardiac hypertrophy. Patients typically present in late adolescence or the third decade with syncope or palpitations. Premature SCD occurred in 10% of patients. Paradoxically, by the fourth decade of life, progression to advanced sinus node dysfunction or AV block (with the loss of preexcitation) requiring pacemaker implantation was common. Approximately 80% of the patients older than 50 years had chronic AF. Causative mutations in the PRKAG2 gene were identified in these families. The PRKAG2 gene encodes the gamma-2 regulatory subunit of the adenosine monophosphate (AMP)–activated protein kinase, which is a key regulator of metabolic pathways, including glucose metabolism. The penetrance of the disease for WPW syndrome was complete, but the expression was variable. The described phenotype of this syndrome is similar to the autosomal recessive glycogen storage disease, Pompe disease. Given the function of the AMP-activated protein kinase and this similarity, the PRKAG2 syndrome is likely a cardiac-specific glycogenosis syndrome. This syndrome thus belongs to the group of genetic metabolic cardiomyopathies, rather than to the congenital primary arrhythmia syndromes.
Clinical Presentation
An AVRT that in general is well tolerated by the patient when additional heart disease is absent can deteriorate into AF. AF can be a life-threatening arrhythmia in the WPW syndrome if the BT has a short anterograde ERP, resulting in very fast ventricular rates, with possible deterioration into VF and SCD.
Initial Evaluation
EP testing can help risk-stratify asymptomatic patients with WPW pattern for developing symptoms and SCD secondary to preexcitation. As noted, inducibility of AVRT, a shorter BT ERP (<250 milliseconds), a shorter preexcited R-R interval during induced AF (<220 milliseconds), the presence of multiple pathways, and septal and right-sided pathway locations appear to identify a higher risk group.9–11 However, a strategy to perform an EP study for all asymptomatic patients with the WPW ECG pattern for the purpose of risk stratification is still controversial and not widely accepted.16
Methods for Evaluation of Bypass Tract Refractory Period
Assessing Ability of Antiarrhythmic Agents to Produce Anterograde Block in Bypass Tracts
When, during NSR, the intravenous injection of ajmaline (1 mg/kg over 3 minutes) or procainamide (10 mg/kg over 5 minutes) results in complete block of the BT, a long anterograde ERP (>270 milliseconds) of the BT is likely. The shorter the BT ERP, the less likely it would be blocked by these drugs. Furthermore, the amount of ajmaline required to block conduction over the BT correlates with the duration of the anterograde ERP of the BT. However, the incidence of BT block in response to these drugs is low and, although the occurrence of block predicts a long ERP of the BT, failure to produce block does not necessarily suggest a short ERP. Moreover, pharmacological testing is carried out at rest and therefore does not indicate what effect the drug will have on the BT ERP during sympathetic stimulation, such as exercise, emotion, anxiety, and recreational drug use.8,17,18
Principles of Management
Management of Asymptomatic Patients with Preexcitation
The role of EP testing and catheter ablation in asymptomatic patients with preexcitation is still controversial. Guidelines of the American College of Cardiology and European Society of Cardiology on the management of asymptomatic WPW patients suggest restricting catheter ablation of BTs to those in high-risk occupations (e.g., school bus drivers, police, and pilots) and professional athletes.19 Catheter ablation in asymptomatic preexcitation was classified as a class IIA indication with a B level of evidence. This means essentially that it is “reasonable” to offer ablation in selected patients but is not mandated in all patients. According to the North American Society of Pacing and Electrophysiology (NASPE; now the Heart Rhythm Society [HRS]) Expert Consensus Conference, asymptomatic WPW pattern on the ECG without recognized tachycardia is a class IIB indication for catheter ablation in children older than 5 years and a class III indication in younger children.20
Some studies, however, have questioned the approach discussed above.8,17,18 Those studies have shown that in the asymptomatic WPW population, a negative EP study with no AVRT or AF inducibility identifies subjects at very low risk for the development of spontaneous arrhythmias. Inducibility of sustained preexcited AF with a fast ventricular response, particularly in the presence of multiple BTs, may help select asymptomatic WPW subjects at definite risk for dying suddenly, and catheter ablation of the BT(s) appears to be required to prevent SCD. Because extensive studies have reported extremely rare complications from EP testing and radiofrequency (RF) ablation in experienced centers, it has been suggested that all asymptomatic patients with WPW pattern should undergo EP testing for risk stratification, and those with inducible AVRT or AF or who have a short BT ERP should be considered for catheter ablation of the BT, whereas patients who are noninducible and have a long BT ERP may be followed without treatment.8–1021 This argument is further supported by the fact that assessment of the future VF risk in an asymptomatic patient with WPW is not easy. Noninvasive markers of lower risk such as intermittent loss of preexcitation, sudden loss of BT conduction on exercise stress testing, and loss of BT conduction after treatment with antiarrhythmic drugs are limited by inadequate sensitivity or specificity and the low incidence of future adverse events. With this approach, prophylactic ablation of BTs in high-risk subjects may be justified but is not an acceptable option for low-risk individuals. The physician involved needs to use special care and discretion in making the decision to proceed to ablation and must especially consider his or her own success and complication rates for ablation of the specific location of the BT identified.
In summary, the potential value of EP testing in identifying high-risk patients who may benefit from catheter ablation must be balanced against the approximately 2% risk of a major complication associated with catheter ablation.22 If RF catheter ablation were a totally risk-free procedure, one would logically advise such a procedure to the asymptomatic WPW patient with a short anterograde ERP. However, complications such as AV block, stroke, tamponade, and even death have been reported. It is not difficult to envision a small potential mortality benefit, if present, erased or eclipsed by a small complication rate if thousands of patients with BTs in various locations undergo ablation.16 Although complications of a diagnostic EP study are minor and non–life-threatening and are less common than those of catheter ablation, if routine EP testing of all asymptomatic WPW patients were considered, many patients would proceed immediately to RF ablation and, in others, there would be a strong temptation to ablate when catheters are in place (regardless of predicted SCD risk), especially given the fact that the criteria for ablation usually will not be black or white. This greatly increases the risk to the patient. Furthermore, invasive EP assessment has drawbacks, because no single factor has both a high sensitivity and specificity for identifying at-risk individuals. For example, a shortest preexcited R-R interval of less than 250 milliseconds during sustained induced AF is a very sensitive but not specific marker of the risk of VF in WPW patients, because approximately one-third of patients will have a shortest R-R interval of less than 250 milliseconds during induced AF. In fact, the addition of isoproterenol to the baseline study may shorten the ERP of the accessory pathway to levels of potential concern in the majority of individuals with WPW. In view of those considerations, prudence dictates that noninvasive testing with a Holter monitor and (if the Holter monitor does not show intermittent preexcitation) exercise testing should be considered before the EP study to identify the low-risk patient because of a long anterograde ERP of the BT.21,22
For the low-risk patient, it may be appropriate to pursue a strategy of follow-up with ECGs and reevaluation at selected intervals with a high degree of suspicion for new arrhythmia symptoms. This strategy is supported by recent prospective studies of asymptomatic patients with WPW pattern; although “potentially” life-threatening arrhythmias were observed during follow-up, no patients had actually died. This observation implies that patient education about the potential risks associated with preexcitation and about the symptoms of arrhythmias that should prompt them to seek attention can prove very important at reducing the risk of mortality without necessarily exposing the patient to the risks of catheter ablation.10,16 It is also advisable to give the patient a copy of his or her ECG and a short note about the fact that the WPW pattern is present to prevent the misdiagnosis of myocardial infarction (MI) and to explain the basis of cardiac arrhythmias in case they develop later. Patients should also be encouraged to seek medical expertise whenever arrhythmia-related symptoms occur.
In patients not showing block in their BT during noninvasive studies, esophageal pacing may be performed to determine the anterograde ERP of the BT and the ability to induce sustained arrhythmias. This procedure is neither pleasant for patients nor often definitive, but if arrhythmias can be induced, the benefits and risk of an invasive investigation and catheter ablation should be based on individual considerations such as age, gender, occupation, and athletic involvement. This should be discussed with the patient or, in the case of a child, with the parents. Because knowledge about the success and complication rate at the EP center plays a major role in decision making, that information should be made available so that the appropriate place for invasive diagnosis and treatment can be selected. If an EP study is performed for risk stratification, the combination of inducible AVRT and a shortest preexcited R-R interval during AF of less than 250 milliseconds provides the most compelling indications for ablation. The key is a clear understanding by the patient of the relative merits of each strategy. The well-informed patient needs to choose between a very small risk of potentially life-threatening arrhythmia over a long period of time and a one-time small procedural risk associated with EP testing and catheter ablation. Certain patients such as athletes and those in higher risk occupations will generally choose ablation. Others, especially patients older than 30 years, may prefer the small risk of a conservative strategy.10,16,21,22
Management of Symptomatic Patients
Acute Management
Patients with AVRT are treated in a similar fashion as those with paroxysmal SVT. In patients with orthodromic and antidromic AVRT, drug treatment can be directed at the BT (ibutilide, procainamide, flecainide) or at the AVN (beta blockers, diltiazem, verapamil) because both are critical components of the tachycardia circuit. Adenosine should be used with caution because it can induce AF with a rapid ventricular rate in patients with preexcited tachycardias.19
AVN blocking drugs are ineffective in patients with antidromic AVRT who have anterograde conduction over one BT and retrograde conduction over a separate BT because the AVN is not involved in the circuit.19 Additionally, caution is advised against AVN blocking agents for the treatment of preexcited tachycardias occurring in patients with AT, AFL, or AF with a bystander BT. Antiarrhythmic drugs such as ibutilide, procainamide, or flecainide, which prevent rapid conduction through the bystander pathway, are preferable, even if they may not convert the atrial arrhythmia. When drug therapy fails or hemodynamic instability is present, electrical cardioversion should be considered.
Chronic Management
The NASPE policy statement on catheter ablation states that catheter ablation is considered first-line therapy (class I) and the treatment of choice for patients with the WPW syndrome—that is, patients with manifest preexcitation along with symptoms.20 It is curative in more than 95% of patients and has a low complication rate. It also obviates the unwanted side effects of antiarrhythmic agents. For patients with preexcitation who are not candidates for ablation, antiarrhythmic drugs to block BT conduction should be used, such as sodium or potassium channel blockers.23 However, there have been no controlled trials of pharmacological therapy in patients with AVRT, but small nonrandomized trials have reported the safety and efficacy of drug therapy. Despite the absence of data from clinical trials, chronic oral beta blocker therapy can be used for the treatment of patients with WPW syndrome, particularly if their BT has been demonstrated during EP testing to be incapable of rapid anterograde conduction. Verapamil, diltiazem, and digoxin, on the other hand, generally should not be used as the sole long-term therapy for patients with BT that might be capable of rapid conduction during AF.
Catheter ablation is also considered first-line therapy (class I) for patients with paroxysmal SVT involving a concealed BT. However, because concealed BTs are not associated with an increased risk of SCD in these patients, catheter ablation can be presented as one of a number of potential therapeutic approaches, including pharmacological therapy and clinical follow-up alone.19 When pharmacological therapy is selected for patients with concealed BTs, it is reasonable to consider a trial of beta blocker therapy, calcium channel blockers, or a class IC antiarrhythmic agent.
Electrocardiographic Features
Electrocardiography of Preexcitation
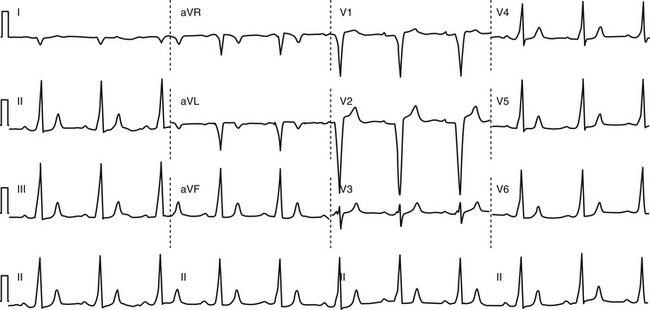
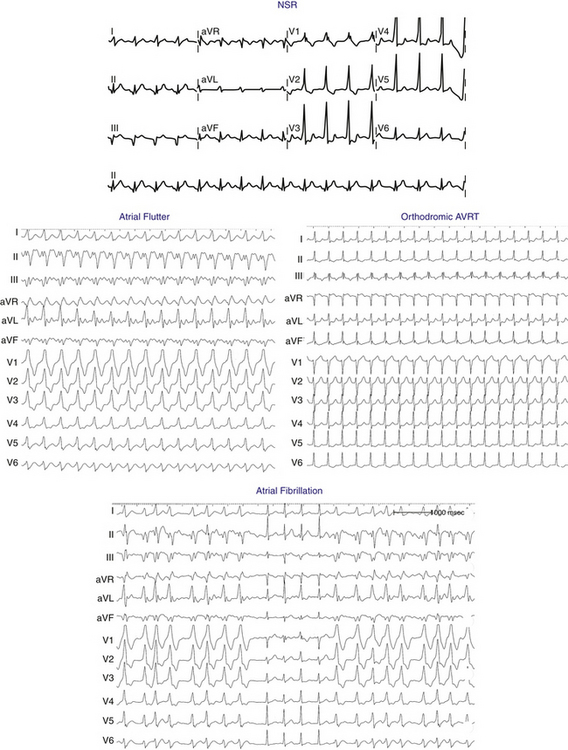
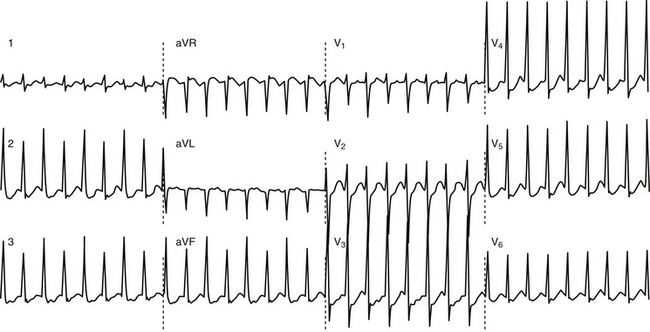
Anterogradely conducting AV BTs produce the classic WPW ECG pattern characterized by a fusion between conduction via the BT and the normal AVN-HPS: (1) short PR (P-delta) interval (<120 milliseconds); (2) slurred upstroke of the QRS (delta wave); and (3) wide QRS (>120 milliseconds) (Fig. 18-4).
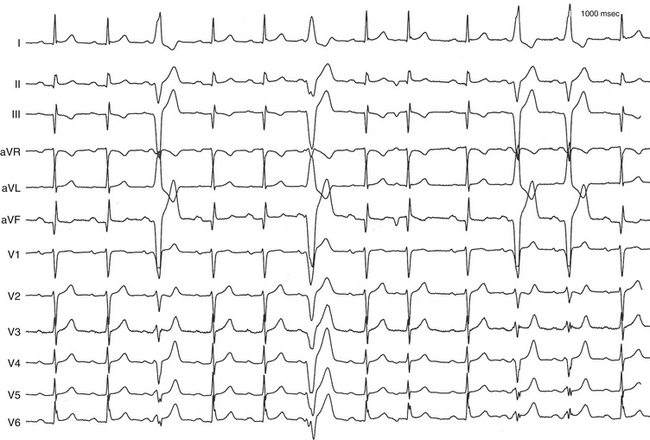
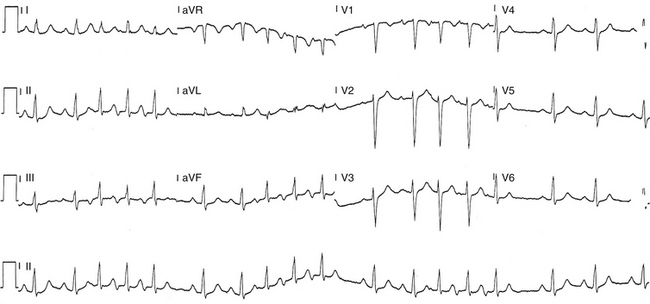
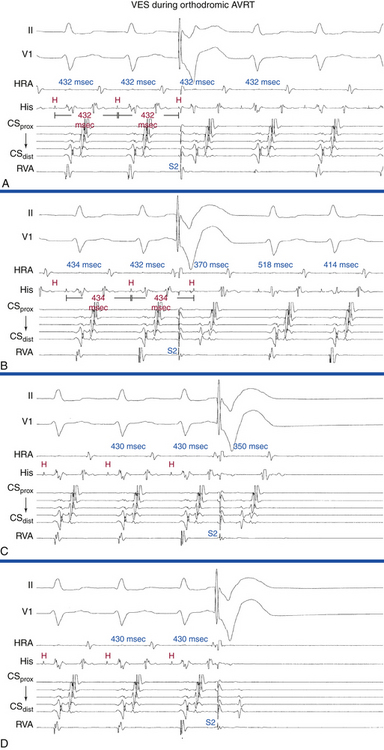
The ECG pattern displayed by some patients with the WPW syndrome can simulate the pattern found in other cardiac conditions and can alter the pattern seen in the presence of other cardiac disease. A negative delta wave (presenting as a Q wave) can mimic an MI pattern. Conversely, a positive delta wave can mask the presence of a previous MI. Intermittent WPW can also be mistaken for frequent premature ventricular complexes (PVCs; Fig. 18-5). If the WPW pattern persists for several beats, the rhythm can be misdiagnosed as an accelerated idioventricular rhythm. The WPW pattern is occasionally seen on alternate beats and may suggest ventricular bigeminy. An alternating WPW and normal pattern can occasionally suggest electrical alternans. On the other hand, late-coupled PVCs (Fig. 18-6) and ventricular pacing (Fig. 18-7) with inapparent pacing artifacts can occasionally mimic ventricular preexcitation.
Inapparent Versus Intermittent Preexcitation
Inapparent Preexcitation
With inapparent preexcitation, preexcitation is absent on the surface ECG despite the presence of an anterogradely conducting AV BT, because conduction over the AVN-HPS reaches the ventricle faster than that over the BT. In this setting, the PR interval is shorter than what the P-delta interval would be if preexcitation were present. Therefore, when preexcitation becomes inapparent, the PR interval becomes shorter, reflecting the now better AVN-HPS conduction.3
Intermittent Preexcitation
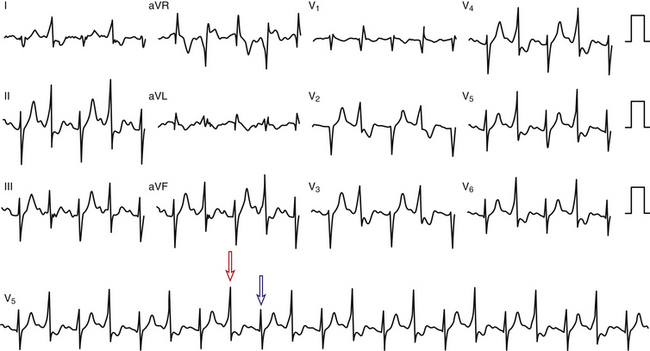
Intermittent preexcitation is defined as the presence and absence of preexcitation on the same tracing (Fig. 18-8). True intermittent preexcitation is characterized by an abrupt loss of the delta wave (independently of how fast or slow is AVN conduction), with prolongation (normalization) of the PR interval (reflecting the loss of the faster BT conduction, and the subsequent conduction over the slower AVN-HPS), and normalization of the QRS in the absence of any significant change in heart rate.
Intermittent preexcitation is usually caused by (1) phase 3 (i.e., tachycardia-dependent) or phase 4 (i.e., bradycardia-dependent) block in the BT (see Chap. 10); (2) anterograde or retrograde concealed conduction produced by PVCs, premature atrial complexes (PACs), or atrial arrhythmias; (3) BTs with long ERP and the gap phenomenon in response to PACs; and/or (4) BTs with long ERP and supernormal conduction.
Preexcitation alternans is a form of intermittent preexcitation in which a QRS complex manifesting a delta wave alternates with a normal QRS complex (see Fig. 18-5). Concertina preexcitation is another form of intermittent preexcitation in which the PR intervals and QRS complex durations show a cyclic pattern; that is, preexcitation becomes progressively more prominent over a number of QRS complex cycles followed by a gradual diminution in the degree of preexcitation over several QRS cycles, despite a fairly constant heart rate.
Differentiation between intermittent preexcitation and inapparent preexcitation on an ECG showing QRS complexes with and without preexcitation can be achieved by comparing the P-delta interval during preexcitation and the PR interval when preexcitation is absent. Loss of preexcitation associated with a PR interval longer than the P-delta interval is consistent with intermittent preexcitation (see Fig. 18-8), whereas loss of preexcitation associated with a PR interval shorter than the P-delta interval is consistent with inapparent preexcitation.
Supraventricular Tachycardias Associated with Wolff-Parkinson-White Syndrome
Orthodromic Atrioventricular Reentrant Tachycardia
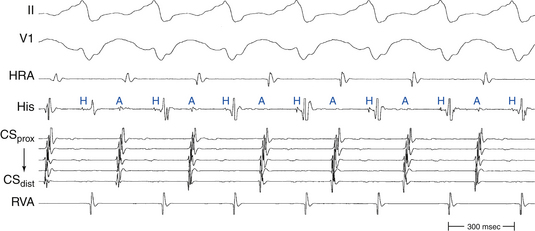
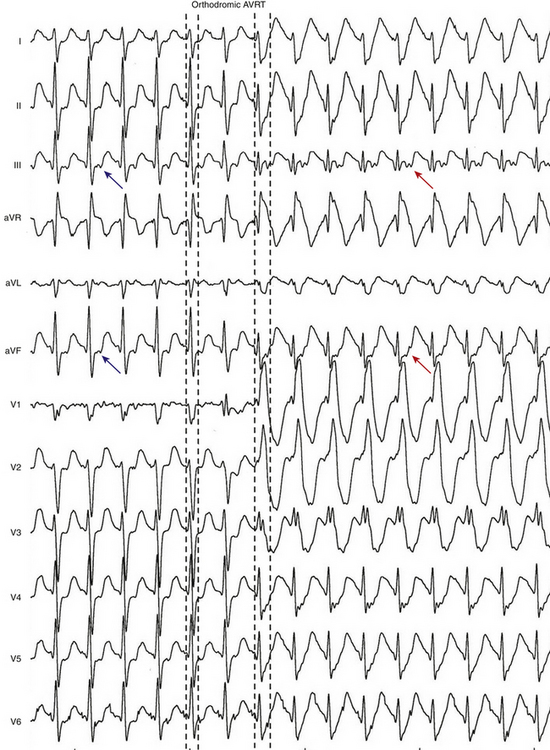
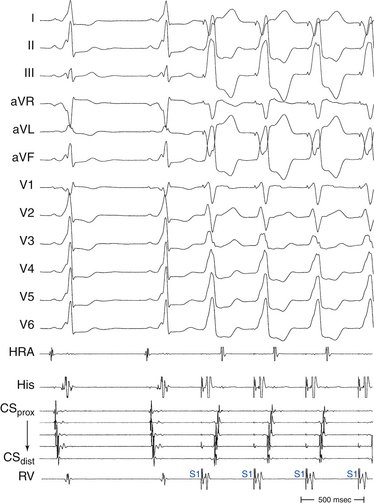
The ECG during orthodromic AVRT shows P waves inscribed within the ST-T wave segment with an RP interval that is usually less than half of the tachycardia R-R interval (i.e., RP interval < PR interval) (Fig. 18-9). The RP interval remains constant, regardless of the tachycardia CL, because it reflects the nondecremental conduction over the BT. QRS morphology during orthodromic AVRT is generally normal and not preexcited, even when preexcitation is present during NSR (Fig. 18-10). Functional bundle branch block (BBB) can be observed frequently during orthodromic AVRT (see Fig. 18-9). The presence of BBB during SVT in a young person (<40 years) should raise the suspicion of orthodromic AVRT incorporating a BT ipsilateral to the blocked bundle, because the longer conduction time through the involved ventricle engendered by the BBB facilitates orthodromic reentry by enabling all portions of the circuit enough time to recover excitability from the prior cycle. This is particularly true with left bundle branch block (LBBB), which is very uncommon in younger patients.
Permanent Junctional Reciprocating Tachycardia
PJRT tends to be incessant, stopping and starting spontaneously every few beats without initiating PACs or PVCs. The heart rate is usually between 120 and 200 beats/min and the QRS duration is generally normal. Slow retrograde conduction over the BT causes the RP interval during PJRT to be long, usually more than half of the tachycardia R-R interval (Fig. 18-11). The P waves resulting from retrograde conduction are easily seen on the ECG and are inverted in leads II, III, aVF, and V3 to V6.
Atrial Fibrillation
There are several characteristic findings on the ECG in patients with AF conducting over a BT, so-called preexcited AF (see Fig. 18-10). The rhythm is irregularly irregular, and can be associated with very rapid ventricular response caused by the nondecremental anterograde AV conduction over the BT. However, a sustained rapid ventricular rate of more than 180 to 200 beats/min will often create pseudo-regularized R-R intervals when the ECG is recorded at 25 mm/sec. Although the QRS complexes are conducted aberrantly, resembling those during preexcited NSR, their duration can be variable and they can become normalized. This is not related to the R-R interval (i.e., it is not a rate-related phenomenon), but rather is related to the variable relationship between conduction over the BT and AVN-HPS. Preexcited and normal QRS complexes often appear “clumped” (see Fig. 18-10). This can result from concealed retrograde conduction into the BT or the AVN.
Atrial Flutter
AFL, like AF, can conduct anterogradely via a BT resulting in a preexcited tachycardia (see Fig. 18-10). Depending on the various refractory periods of the normal and pathological AV conduction pathways, AFL potentially can conduct 1:1 to the ventricles during a preexcited tachycardia, making the arrhythmia difficult to distinguish from VT (see Fig. 12-6).
Electrocardiographic Localization of the Bypass Tract
Localization Using the Delta Wave
Careful analysis of the preexcitation pattern during sinus rhythm can potentially allow an accurate approximation of the location of the BT. This provides the electrophysiologist with important information that can guide patient counseling with regard to risks and benefits of ablation, in particular, providing some guidance about the proximity of the BT to the normal conduction system and the subsequent risk of AV block associated with an ablation attempt, as well as the need for left heart catheterization and atrial septal puncture and their potential complications. Additionally, it can also allow planning the subsequent catheter ablation, such as the use of cryoablation for septal BTs or the need for special equipment for atrial septal puncture for left-sided BTs.24
The delta wave vector is helpful in approximating BT location, especially when maximal preexcitation is present. However, during NSR, only partial ventricular preexcitation is usually present, which limits the accuracy of ECG localization of the BT. Additionally, the degree of preexcitation may vary in an individual, depending on heart rate, autonomic tone, and AVN function, as well as BT location and EP characteristics. Therefore, it is important first to assess the degree of preexcitation visible throughout the entire ECG and then use only the delta wave polarity for localization (the first 40 milliseconds of the QRS in most cases, unless fully preexcited) rather than the overall QRS polarity, which may vary from each other.25–29
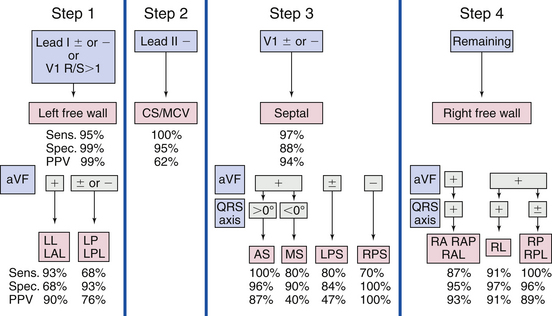
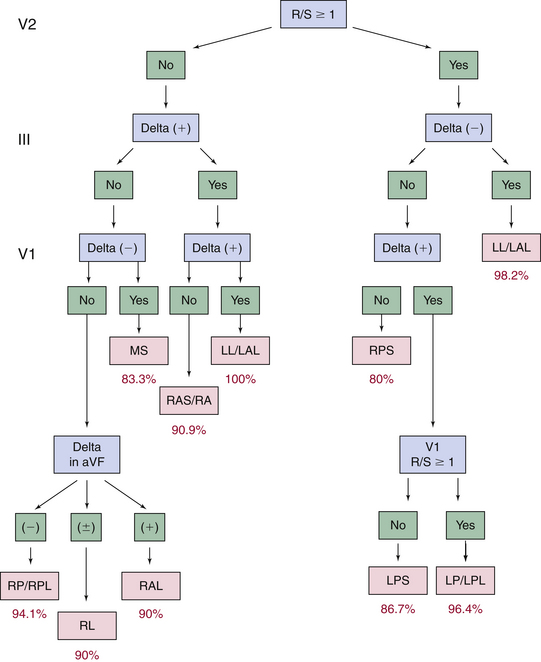
Several algorithms have been developed to precisely locate a BT to a specific anatomic location, using the delta wave polarity (Table 18-1; Figs. 18-12 and 18-13).25,26 Although these algorithms facilitate prediction of the location of a BT, they are inherently limited by biological variability in anatomy (e.g., rotation of the heart within the thorax), variable degree of preexcitation and QRS fusion, the presence of more than one manifest BT, intrinsic ECG abnormalities (such as prior myocardial infarction and ventricular hypertrophy), as well as technical variability in ECG acquisition and electrode positioning. These factors can subject all algorithms to exceptions and inaccuracies.24
TABLE 18-1 Delta Wave Characteristics during Preexcitation According to Bypass Tract Location*
| Left Lateral/Left Anterolateral BTs |
A. R/S ≥1 in V1 and V2, no positive delta in III and positive delta in V1
B. R/S transition ≤V1, no ≥2 positive delta in the inferior leads, no S > R in aVL, in lead I R <(S 0.8 mV), and the sum of the inferior delta is not negative
C. Positive QRS in aVL and an equiphasic QRS or positive in V1 and no negative QRS in III
A. R/S ≥1 in V2, no positive delta in III, and positive delta and R/S <1 in V1
B. R/S transition ≤V1, no ≥2 positive delta in the inferior leads, no S > R in aVL, in lead I R <(S 0.8 mV), and the sum of the inferior delta is negative
C. Negative QRS in III, V1, and aVF, tallest precordial R wave in V2-V4 and R wave width in V1 >0.06 msec
A. R/S <1 in V2, no positive delta in III, and negative delta in V1
B. R/S transition between V2 and V3 or between V3 and V4 but the delta amplitude in lead II ≥1.0 mV (septal location), and the sum of delta polarities in inferior leads is −1, 0, or +1 mV
C. Negative QRS in leads III, V1, and aVF, tallest precordial R in leads V2-V4 and R wave width in V1 <0.06 msec
A. R/S <1 in V2, no positive delta in III and no negative delta in V1, and negative delta polarity in aVF
B. R/S transition between V3 and V4 with delta amplitude in lead II <1.0 mV or≥V4 and the delta axis is <0 degrees and the R in lead III is ≤0 mV
C. Positive QRS in III, negative in V1, and RS morphology in aVL
BT = bypass tract; R/S = R-S wave ratio.
* A, algorithm of Chiang and colleagues27; B, algorithm of Fitzpatrick and colleagues25; C, algorithm of Xie and colleagues.28
From Katsouras CS, Greakas GF, Goudevenos JA, et al: Localization of accessory pathways by the electrogram, Pacing Clin Electrophysiol 27:189, 2004.
The mere presence and the degree of preexcitation can sometimes help in predicting the location of the BT. In fact, an apparently normal ECG does not fully rule out anterograde conducting left lateral BTs or rate-dependent or decremental BTs. Posteroseptal and right-sided BTs tend to be associated with a prominent degree of preexcitation because of the proximity to the sinus node, whereas left lateral BTs are often associated with subtle preexcitation. Nevertheless, when preexcitation is prominent, the accuracy of surface ECG localization of manifest BTs tends to be higher for the diagnosis of left free wall BTs than for BTs in other locations. The presence of multiple manifest BTs may result in fusion of preexcitation patterns or predominance of right-sided conduction.24
Localization Using Polarity of the Retrograde P Wave During Orthodromic Atrioventricular Reentrant Tachycardia
The polarity of the retrograde P waves during orthodromic AVRT is dependent on the location of the atrial insertion of the BT, and is helpful to localize the BT. However, the P wave is usually inscribed within the ST segment and its morphology may not be easily determined.3,30,31
In general, P wave morphology in leads I and V1 and in the inferior leads is most helpful. Negative P wave vector in lead I is highly suggestive of left free wall BTs, whereas a positive P wave is suggestive of right free wall BTs. On the other hand, a negative P wave in lead V1 is highly suggestive of right-sided BTs. P wave polarity in the inferior leads, positive or negative, suggests superior or inferior location of the BT, respectively.3,30,31
Posteroseptal BTs have positive P waves in leads V1, aVR, and aVL and negative P waves in the inferior leads. Left posterior BTs also have negative P waves in the inferior leads, but the P waves are more negative in lead II than in lead III and are more positive in lead aVR than in lead aVL, which is often isoelectric. Left lateral BTs have negative P waves in leads I and aVL, with a tendency for more positive P waves in lead III than in leads II and aVF as the location of the BT moves more superiorly.3,30,31
Electrophysiological Testing
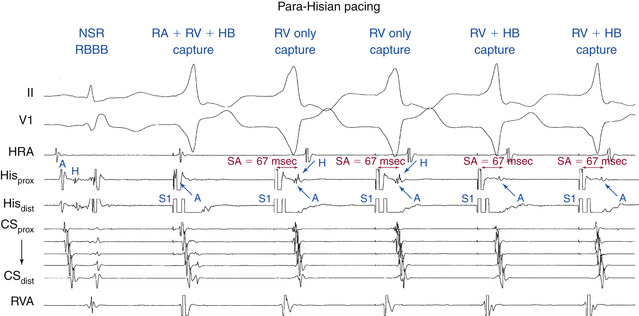
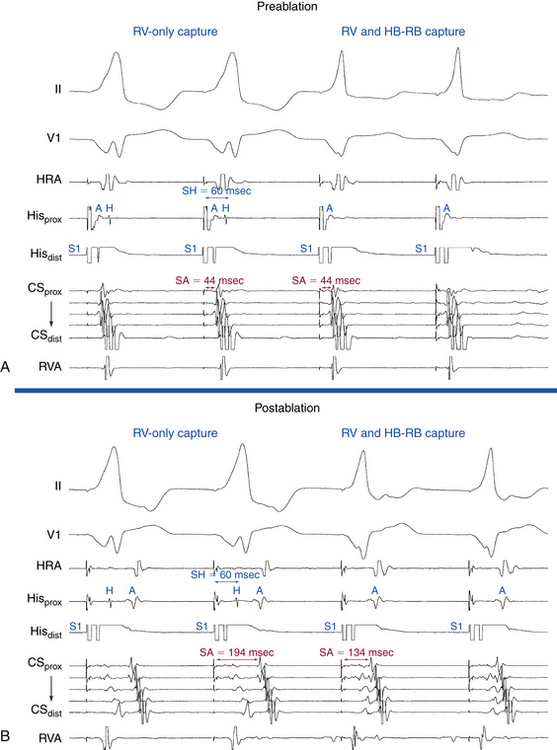
EP testing is used to study the features, location, and number of BTs and the tachycardias, if any, associated with them (Table 18-2). Typically, three quadripolar catheters are positioned in the high RA, right ventricle (RV) apex or septum, and HB region, and a decapolar catheter is positioned in the CS (see Fig. 4-7). If a right-sided BT is suspected, a duo-decapolar (Halo; Biosense Webster, Diamond Bar, Calif.) catheter along the tricuspid annulus can be helpful.
TABLE 18-2 Goals of Electrophysiological Evaluation in Patients with Wolff-Parkinson-White Syndrome
Baseline Observations during Normal Sinus Rhythm
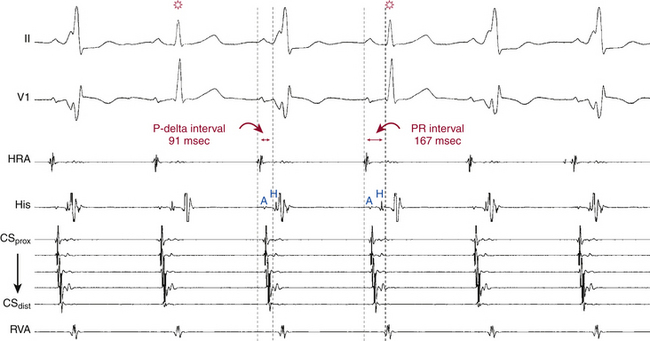
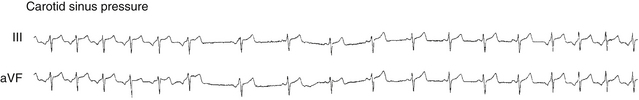
Preexcitation is associated with a short His bundle–ventricular (HV) or H-delta interval during NSR. The HV interval can even be negative or the His potential can be buried in the local ventricular electrogram (see Fig. 18-4). The QRS is a fusion between conduction over the BT and that over the AVN-HPS. The site of earliest ventricular activation is near the ventricular insertion site of the BT (i.e., near the tricuspid annulus or mitral annulus at the base of the heart). Slowing of conduction in the AVN by carotid sinus massage, AVN blockers, or rapid atrial pacing unmasks and increases the degree of preexcitation, because these maneuvers do not affect the conduction over the BT. Dual AVN pathways are present in 8% to 40% of patients.
Atrial Pacing and Atrial Extrastimulation During Normal Sinus Rhythm
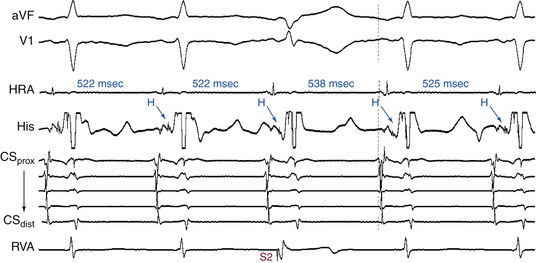
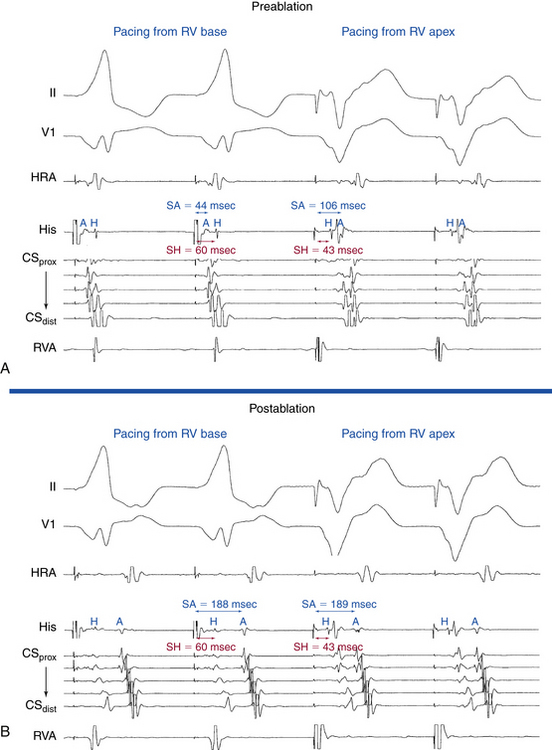
In the presence of a manifest AV BT, atrial stimulation from any atrial site can help unmask preexcitation if it is not manifest during NSR because of fast AVN conduction. Incremental rate atrial pacing and progressively premature AES produce decremental conduction over the AVN (but not over the BT), increasing the degree of preexcitation and shortening the HV interval, until the His potential is inscribed within the QRS. The His potential is still activated anterogradely over the AVN until anterograde block in the AVN occurs; the QRS then becomes fully preexcited, and the His potential becomes retrogradely activated (see Fig. 18-4).
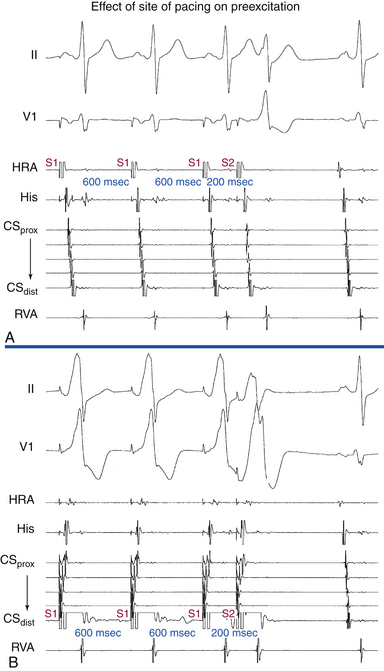
Atrial stimulation close to or at the AV BT insertion site results in maximal preexcitation and the shortest P-delta interval because of the lack of intervening atrial tissue whose refractoriness may otherwise limit the ability of atrial stimulation to activate the AV BT as early (Fig. 18-14). The failure of atrial stimulation to increase the amount of preexcitation can be caused by markedly enhanced AVN conduction, the presence of another AV BT, a pacing-induced block in the AV BT because of a long ERP of the BT (longer than that of the AVN), total preexcitation already present at the basal state caused by prolonged or absent AVN-HPS conduction, and/or decremental conduction in the BT. Atrial pacing can reveal the presence of multiple BTs (Fig. 18-15). Rare cases of catecholamine-dependent BTs have been reported that require isoproterenol infusion to manifest preexcitation that is absent at baseline.
Ventricular Pacing and Ventricular Extrastimulation During Normal Sinus Rhythm
In the presence of a retrogradely conducting AV BT (whether manifest or concealed), ventricular extrastimulation (VES) during NSR can result in VA conduction over the BT, AVN, both, or neither (Fig. 18-16). Conduction over the BT alone is the most common pattern at short pacing CLs or short VES coupling intervals. In this setting, the VA conduction time is fairly constant over a wide range of pacing CLs and VES coupling intervals, in the absence of intraventricular conduction abnormalities or additional BTs. On the other hand, retrograde conduction over the BT and HPS-AVN is especially common when RV pacing is performed in the presence of a left-sided BT at long pacing CLs or long VES coupling intervals. This occurs because it is easier to engage the right bundle branch (RB) and conduct retrogradely through the AVN than it is to reach a distant left-sided BT. In this setting, the atrial activation pattern depends on the refractoriness and conduction times over both pathways and usually exhibits a variable degree of fusion. In addition, VA conduction can proceed over the HPS-AVN alone, resulting in a normal pattern of VA conduction, or can be absent because of block in both the HPS-AVN and BT, which is especially common with short pacing CLs and very early VES.
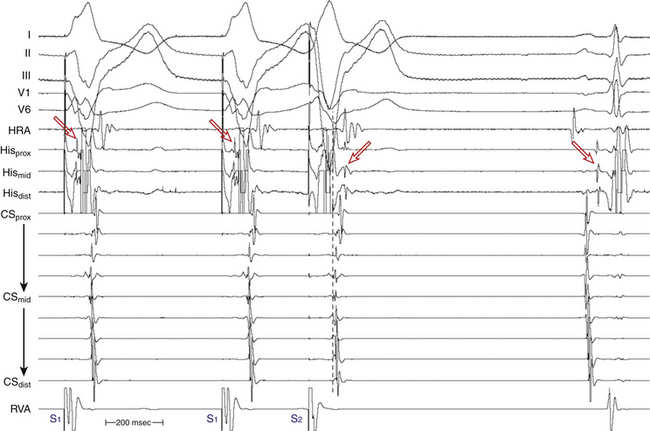
Ventricular stimulation during NSR also helps prove the presence of a retrogradely conducting AV BT. Ventricular stimulation resulting in retrograde VA conduction not consistent with normal conduction over the AVN (i.e., eccentric atrial activation sequence; see Fig. 18-16) and a VES delivered when the HB is refractory that results in atrial activation are indicators of the presence of a retrogradely conducting AV BT. However, if a VES delivered when the HB is refractory does not result in atrial activation, this does not necessarily exclude the presence of a retrogradely conducting AV BT, because such a VES can be associated with retrograde block in the BT itself (see Fig. 18-16). Additionally, the lack of such a response does not exclude the presence of unidirectional (anterograde-only) AV BTs. A VES that conducts to the HB and results in an atrial activation that either precedes HB activation (Fig. 18-17) or is associated with an apparent His bundle–atrial (HA) interval shorter than that during drive complexes indicates the presence of a BT. Ventricular pacing can also reveal the presence of multiple BTs (Fig. 18-18).
During the delivery of progressively premature single VESs, an abrupt increase in the VA conduction interval is often observed. This may be due to a variety of reasons including a change in activation from a BT block to the AVN or a change from fast to slow pathway conduction; or it may be the result of an abrupt change when the refractory period of the RB has been reached. Retrograde right bundle branch block (RBBB) occurs frequently during VES testing, and can be diagnosed by observing the retrograde His potential during the drive train and its abrupt delay following the VES. Often, however, it is difficult to visualize the retrograde His potential during the pacing train; nevertheless, the sudden appearance of an easily distinguished retrograde His potential, separate from the ventricular electrogram following the VES, may be sufficient to recognize retrograde RBBB. Prolongation of the V-H interval is observed on development of retrograde RBBB, because conduction must traverse the interventricular septum (which requires approximately 60 to 70 milliseconds in normal hearts), enter retrograde via the left bundle branch (LB), and ascend to reach the HB. Although an increase in the V-H interval necessarily occurs with retrograde RBBB, whether a similar increase occurs in the VA interval depends on the nature of VA conduction. Measurement of the effect of retrograde V-H and VA intervals on the development of retrograde RBBB during VES can help the distinction between retrograde AVN and BT conduction. In the absence of a BT, the AVN can be activated in a retrograde fashion only after retrograde activation of the HB; as a consequence, VA activation will necessarily be delayed with retrograde RBBB, and the increase in the VA interval will be at least as much as the increase in the V-H interval. On the other hand, when retrograde conduction is via a BT, there will be no expected increase in the VA interval when retrograde RBBB is induced. Thus, the increase in the VA interval is minimal and always less than the increase in the V-H interval.32
Induction of Tachycardia
Initiation by Atrial Extrastimulation or Atrial Pacing
Orthodromic Atrioventricular Reentrant Tachycardia: Manifest Atrioventricular Bypass Tract
In the presence of a manifest AV BT, initiation of orthodromic AVRT with an AES requires the following: (1) anterograde block in the AV BT; (2) anterograde conduction over the AVN-HPS; and (3) slow conduction over the AVN-HPS, with adequate delay to allow for the recovery of the atrium and AV BT and subsequent retrograde conduction over the BT (see Fig. 3-10).3 The reason this occurs is that, whereas the BT conducts more rapidly than the AVN, it has a longer ERP, so the early atrial impulse blocks anterogradely in the BT but conducts over the AVN. The site of AV delay is less important; it is most commonly in the AVN, but it can occur also in the HB, bundle branches, or ventricular myocardium. Because the coupling intervals of the AES required to achieve anterograde block in the BT are usually short, sufficient AVN delay is usually present so that orthodromic AVRT is initiated once anterograde BT block occurs. The presence of dual AVN physiology can facilitate the initiation of orthodromic AVRT by providing adequate AV delay by mediating anterograde conduction over the slow AVN pathway. BBB ipsilateral to the AV BT provides an additional AV delay that can facilitate tachycardia initiation.
Orthodromic Atrioventricular Reentrant Tachycardia: Slowly Conducting Concealed Atrioventricular Bypass Tract (Permanent Junctional Reciprocating Tachycardia)
PJRT is usually incessant and is initiated by spontaneous shortening of the sinus CL, without a triggering PAC or PVC. The tachycardia can be transiently terminated by PACs or PVCs but usually resumes after a few sinus beats (Fig. 18-19). This phenomenon has three potential mechanisms: a rate-related decrease in the retrograde ERP of the BT, a rate-related decrease in atrial refractoriness that allows the impulse to reactivate the atrium retrogradely over the BT, or a concealed Wenckebach block with block at the atrial-BT junction terminating the Wenckebach cycle, relieving any anterograde concealed conduction that may have prevented retrograde conduction up the BT. The latter is the most likely mechanism, because such slow BTs actually demonstrate decremental conduction at rapid rates and, in most cases, the atrial ERP at the atrial-BT junction is shorter than the RP (VA) interval. Thus, some sort of anterograde concealment during NSR in the BT must be operative, preventing tachycardia from always occurring.3 Late-coupled AESs can also readily initiate PJRT.
Antidromic Atrioventricular Reentrant Tachycardia
The initiation of classic antidromic AVRT by an AES requires the following: (1) intact anterograde conduction over the BT; (2) anterograde block in the AVN or HPS; and (3) intact retrograde conduction over the HPS-AVN once the AVN resumes excitability following partial anterograde penetration (see Fig. 18-4). The latter is usually the limiting factor for the initiation of antidromic AVRT. A delay of more than 150 milliseconds between atrial insertion of the BT and HB is probably required for the initiation of antidromic AVRT.3
Several mechanisms of antidromic AVRT initiation can be operative.3 The AES may block in the AVN with anterograde conduction down the BT and subsequent retrograde conduction over the HPS-AVN. In this setting, ventricular–His bundle (V-H) delay is required to allow recovery of the AVN. Because antidromic AVRTs have relatively short VA intervals, this mechanism of initiation is probably uncommon, except with left-sided BTs, which would potentially provide sufficient V-H delay to allow retrograde conduction. Tachycardia initiation can be facilitated by a short retrograde AVN ERP, a common finding in patients with these SVTs. Alternatively, the AES may block in the AVN, with anterograde conduction down the BT and subsequent retrograde conduction over a different BT. Subsequent complexes can conduct retrogradely over the AVN-HPS or the second BT. Changing tachycardia CL (and VA interval) may relate to whether retrograde conduction proceeds over the AVN-HPS or the second BT. A third potential mechanism for the initiation of antidromic AVRT involves AES conduction over the BT and simultaneously over the slow pathway of a dual AVN pathway situation, with anterograde block in the fast AVN pathway. Conduction beyond the HB to the ventricle is not possible because of ventricular refractoriness, yet an AVN echo to the atrium may occur, which in turn may conduct anterogradely over the BT, when the ventricle would have recovered excitability, and subsequently back up the now-recovered AVN, initiating antidromic AVRT. AVN reentry may not persist or may be preempted by retrograde conduction up the fast AVN pathway because of the premature ventricular activation over the BT. In this scenario, the location of the His potential will depend on whether or not it was anterograde or retrograde.
Initiation by Ventricular Extrastimulation or Ventricular Pacing
Orthodromic Atrioventricular Reentrant Tachycardia: Manifest or Concealed Atrioventricular Bypass Tract
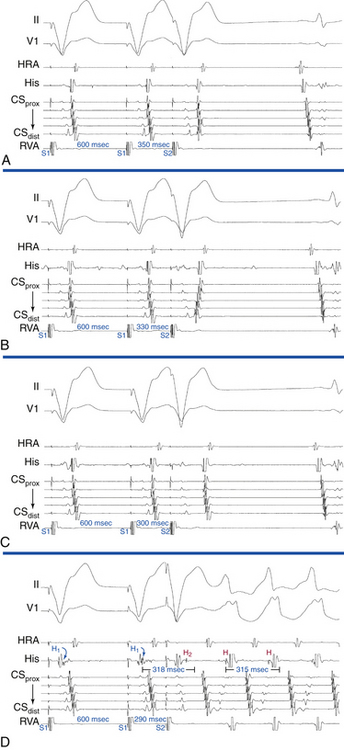
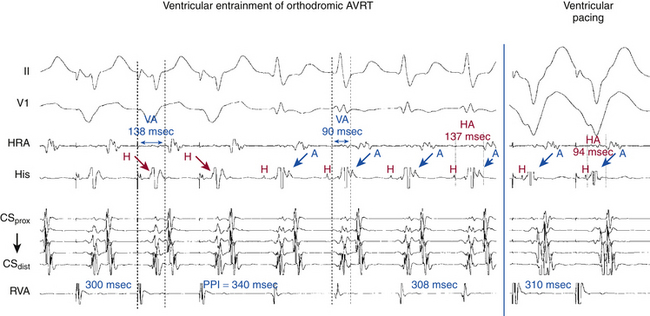
Ventricular stimulation is usually able to induce orthodromic AVRT (inducibility rate of 60% with VES and 80% with ventricular pacing), and is similar in patients with manifest or concealed BTs (Fig. 18-20). Initiation of orthodromic AVRT by ventricular stimulation requires the following: (1) retrograde block of the ventricular impulse in the HPS-AVN; (2) retrograde conduction only over the AV BT; and (3) adequate VA conduction delay to allow for recovery of the AVN-HPS from any concealment produced by ventricular stimulation, so it can support anterograde conduction of the reentrant impulse. Because the BT retrograde ERP is usually very short, the prime determinant of orthodromic AVRT initiation is the extent of retrograde conduction and/or concealment in the HPS-AVN.3
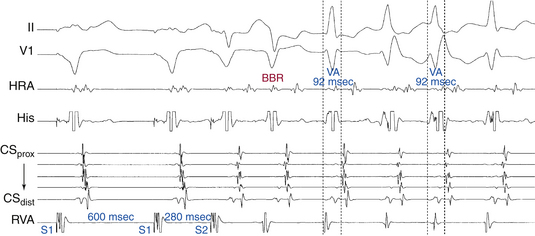
Multiple modes of initiation of orthodromic AVRT can be present, depending on the pacing CL or VES coupling interval, conduction velocities, and refractoriness of the HPS-AVN and BT, as well as the site of ventricular stimulation.3 Ventricular pacing at a CL or a VES with a coupling interval shorter than the ERP of the AVN but longer than that of the HPS and AV BT would block retrogradely in the AVN and conduct over the AV BT to initiate orthodromic AVRT. Block in the AVN, which is more likely to occur with rapid ventricular pacing or VES delivered after a short pacing drive CL, can cause concealment and subsequent delay in anterograde conduction of the first SVT impulse over the AVN, resulting in longer AH and PR intervals of the first SVT beat compared with subsequent beats (see Fig. 18-20). On the other hand, a VES with a coupling interval or ventricular pacing at a CL shorter than the ERP of the HPS, but longer than that of the AV BT, would block retrogradely in the HPS and conduct over the AV BT to initiate orthodromic AVRT. When block occurs in the HPS, which is more likely to occur with a VES delivered during NSR or after a long pacing drive CL, the first SVT beat will approach a fully recovered AVN and conduct with short AH and PR intervals equal to subsequent SVT beats. In this case, adequate prolongation of the HV interval may be required to allow for the recovery of ventricular refractoriness for the ventricle to be activated and support reentry, because AVN delay may have not been sufficient. When HV interval prolongation is required to initiate orthodromic AVRT, it is almost invariably associated with LBBB. A short-coupled VES, especially following a pacing drive with a long CL, that blocks retrogradely in both the AV BT and RB and conducts transseptally and then retrogradely over the LB, can result in a bundle branch reentrant (BBR) beat that conducts to the ventricle down the RB, and then retrogradely to the atrium over the AV BT, mediating the initiation of orthodromic AVRT. The long HV interval often associated with BBR beats, plus the LBBB pattern, facilitate the induction of orthodromic AVRT using a left-sided BT (Fig. 18-21).
Orthodromic Atrioventricular Reentrant Tachycardia: Slowly Conducting Concealed Atrioventricular Bypass Tract (Permanent Junctional Reciprocating Tachycardia)
Antidromic Atrioventricular Reentrant Tachycardia
Initiation of classic antidromic AVRT by ventricular pacing and VES requires the following: (1) retrograde block in the BT; (2) retrograde conduction over the AVN or HPS; and (3) adequate VA delay to allow for recovery of the atrium and BT so it can support subsequent anterograde conduction (see Fig. 18-16).3
When induction of the SVT is achieved by ventricular pacing at a CL similar to the tachycardia CL or by a VES that activates the HB at a coupling interval (i.e., H1-H2 interval) similar to the H-H interval during the SVT, the HA interval following the initiating ventricular stimulus is always equal to or shorter than that during the antidromic AVRT. This is because the His potential and atrium are activated in sequence during antidromic AVRT and in parallel during ventricular stimulation. Therefore, an HA interval of the initiating ventricular stimulus longer than the HA interval during SVT favors AVNRT and excludes antidromic AVRT (see Fig. 18-16). Moreover, because the AVN usually exhibits greater decremental conduction with repetitive engagement of impulses than to a single impulse at a similar coupling interval, the more prolonged the HA interval with the initiating ventricular stimulus, the more likely that the SVT is AVNRT.
Tachycardia Features
Orthodromic Atrioventricular Reentrant Tachycardia: Manifest or Concealed Atrioventricular Bypass Tract
Atrial Activation Sequence
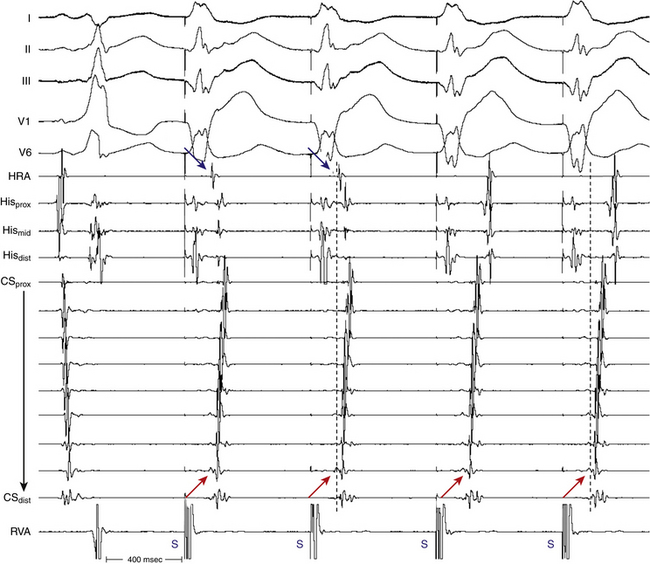
The initial site of atrial activation during orthodromic AVRT depends on the location of the AV BT, but is always near the AV groove and without multiple breakthrough points. The atrial activation sequence during orthodromic AVRT should be identical to that during ventricular pacing at comparable CLs when VA conduction occurs exclusively over the AV BT. However, retrograde conduction during ventricular pacing may proceed over the AVN or over both the BT and AVN, resulting in fusion of atrial activation, depending on the site of ventricular stimulation relative to the BT and HPS, and on retrograde conduction and refractoriness of the HPS-AVN.
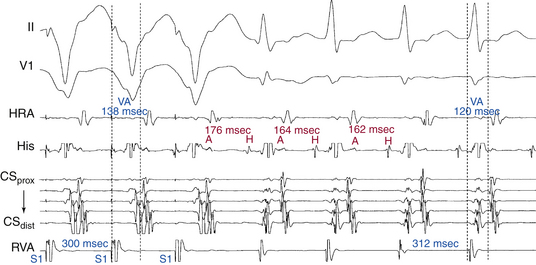
Atrial–Ventricular Relationship
Conduction time over the classic (fast) BTs is approximately 30 to 120 milliseconds. Therefore, the RP interval during orthodromic AVRT is short, but longer than that during typical AVNRT, because in the setting of orthodromic AVRT the wavefront has to activate the ventricle before it reaches the AV BT ventricular insertion site at the AV groove and subsequently conduct to the atrium. Consequently, a very short VA interval (<70 milliseconds) or V–high RA interval (<95 milliseconds) largely excludes orthodromic AVRT, and is consistent with typical AVNRT.33 The RP and VA intervals remain constant during orthodromic AVRT regardless of oscillations in tachycardia CL from whatever cause or changes in the PR interval (AH interval); as a consequence, the tachycardia CL is most closely associated with the PR interval (i.e., anterograde slow conduction) and the RP/PR ratio may vary (Fig. 18-22).
Effects of Bundle Branch Block
The presence of BBB during SVT is much more common in orthodromic AVRT than AVNRT or AT (90% of SVTs with sustained LBBB are orthodromic AVRTs). Two reasons have been proposed to explain why prolonged aberration occurs less commonly during AVNRT than orthodromic AVRT. First, the induction of AVNRT requires significant AVN delay, which makes the H1-H2 interval longer and makes aberration unlikely, whereas in orthodromic AVRT, AVN conduction need not be slow, resulting in a shorter AH interval and an impulse encroaching on HPS refractoriness, in turn resulting in BBB. Second, LBBB facilitates the induction of orthodromic AVRT when a left-sided AV BT is present.34
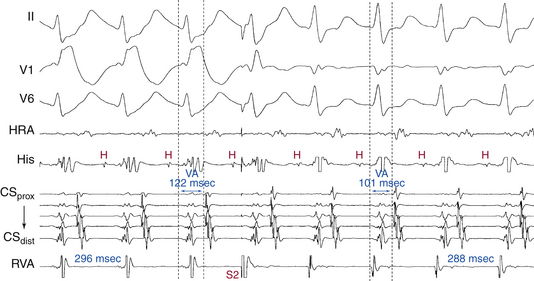
BBB ipsilateral to the AV BT results in prolongation of the surface VA interval because more time is needed for the impulse to travel from the AVN down the HB and contralateral bundle branch, and transseptally to the ipsilateral ventricle to reach the AV BT and then activate the atrium (Fig. 18-23). However, the local VA interval (measured at the site of BT insertion) remains constant. On the other hand, the tachycardia CL usually increases in concordance with the increase in the surface VA interval as a result of ipsilateral BBB, because of the now larger tachycardia circuit; however, because the time the wavefront spends outside the AVN is now longer, AVN conduction may improve, resulting in shortening of the AH interval (PR interval), which can be sufficient to overcome the prolongation of the VA interval. This can consequently result in shortening of the tachycardia CL. Thus, the surface VA interval and not the tachycardia CL should be used to assess the effects of BBB on the SVT (Fig. 18-24; and see Fig. 18-23).
Prolongation of the surface VA interval during SVT in response to BBB by more than 35 milliseconds compared to that with normal QRS or contralateral BBB indicates that an ipsilateral free wall AV BT is present and is participating in the SVT (i.e., diagnostic of orthodromic AVRT). On the other hand, prolongation of the surface VA by more than 25 milliseconds suggests a septal AV BT (posteroseptal AV BT in association with LBBB, and superoparaseptal AV BT in association with RBBB; see Fig. 18-24). In contrast, BBB contralateral to the AV BT does not influence the VA interval or tachycardia CL (because the contralateral ventricle is not part of the reentrant circuit; see Figs. 18-21 and 18-23). Prolongation of the VA interval by more than 45 milliseconds in response to RV pacing entraining the orthodromic AVRT is also diagnostic of a left-sided BT, whereby RV pacing results in effects analogous to those created by LBBB and, as a consequence, VA interval prolongation.
Oscillations in the Tachycardia Cycle Length
Oscillation of the tachycardia CL during orthodromic AVRT can occur and generally is caused by changes in the anterograde conduction over the AVN (see Fig. 18-22). Because retrograde conduction through the BT is much less variable, the changes in ventricular CL that result from variability in the anterograde AVN conduction precede the subsequent changes in atrial CL, and changes in atrial CL do not predict changes in subsequent ventricular CL (similar to observations during typical AVNRT). Contrariwise, changes in atrial CL predict the changes in subsequent ventricular CL during atypical AVNRT and AT.35
QRS Alternans
Alternans of the QRS complex amplitude during relatively slow SVTs is almost always indicative of orthodromic AVRT (Fig. 18-25). On the other hand, although QRS alternans during fast SVTs is most commonly seen in orthodromic AVRT, it can also be seen with other types of SVTs.
Termination and Response to Physiological and Pharmacological Maneuvers
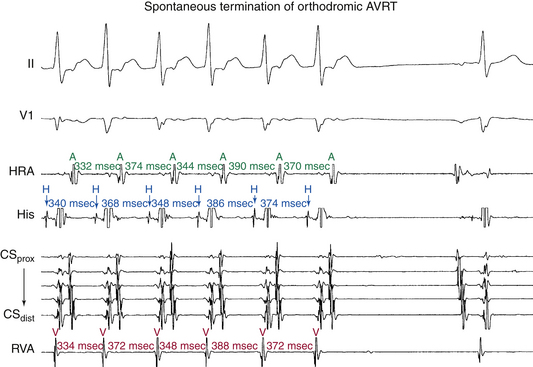
Spontaneous termination of orthodromic AVRT is usually caused by gradual slowing and then block in the AVN (see Fig. 18-22), sometimes causing initial oscillation in the tachycardia CL, with alternate complexes demonstrating a Wenckebach periodicity before block. However, termination with block in the AV BT without any perturbations of the tachycardia CL can occur during very rapid AVRT or following a sudden shortening of the tachycardia CL.
Orthodromic Atrioventricular Reentrant Tachycardia: Slowly Conducting Concealed Atrioventricular Bypass Tract (Permanent Junctional Reciprocating Tachycardia)
Atrial Activation Sequence
The initial site of atrial activation is most often in the posteroseptal part of the triangle of Koch near the CS os, similar to that in atypical AVNRT (Fig. 18-26).
Atrial–Ventricular Relationship
Because the retrograde limb of the reentry circuit is the slow BT (which conducts more slowly than the AVN), the RP interval is longer than the PR interval, similar to atypical AVNRT (see Figs. 18-11 and 18-26). In contrast to the classic fast BTs, the RP interval during PJRT is not fixed, because the BT serving as the retrograde limb of the reentrant circuit has decremental properties. Similar to all types of AVRTs, a 1:1 A-V relationship is a prerequisite to sustenance of the tachycardia.
Effects of Bundle Branch Block
BBB affects PJRT in a manner analogous to that described for orthodromic AVRT.
Termination and Response to Physiological and Pharmacological Maneuvers
Carotid sinus massage and AVN blockers (adenosine, digoxin, calcium channel blockers, and beta blockers) usually terminate PJRT by block in the AVN (two-thirds) or in the BT (one-third) (see Fig. 18-19). Although the mode of tachycardia termination by adenosine has been suggested to distinguish between PJRT and atypical AVNRT, one report has shown that termination of atypical AVNRT with adenosine can also result from block in the fast AVN pathway and therefore its value in distinguishing between atypical AVNRT and PJRT is questionable.
Antidromic Atrioventricular Reentrant Tachycardia
Atrial Activation Sequence
The initial site of atrial activation in classic antidromic AVRT is consistent with retrograde conduction over the AVN. If the antidromic AVRT is using a second BT for retrograde conduction, then the atrial activation sequence will depend on the location of that BT (see Fig. 18-3). Additionally, ventricular activation precedes HB activation during classic antidromic AVRT. Therefore, during preexcited SVT, a positive HV interval or a V-H interval not more than 10 milliseconds, especially when the HA interval is not more than 50 milliseconds, favors preexcited AVNRT over antidromic AVRT.
Oscillations in the Tachycardia Cycle Length
The tachycardia CL tends to be shorter during classic antidromic AVRT than orthodromic AVRT when these arrhythmias occur in the same patient. This may be explained by the fact that antidromic AVRT uses the fast AVN pathway (of a dual AVN physiology) retrogradely, whereas orthodromic AVRT uses the slow pathway anterogradely or, in the absence of dual AVN physiology, this may be merely supportive evidence that retrograde conduction during antidromic AVRT uses another fast BT instead of the slower AVN. On the other hand, antidromic AVRTs using two or more BTs may have longer tachycardia CLs than orthodromic AVRT or classic antidromic AVRT because the two BTs are typically in opposite chambers and are incorporated in a larger reentrant circuit than one involving a midline AVN.3
Effects of Bundle Branch Block
Retrograde BBB affects antidromic AVRT in a manner analogous to that described for orthodromic AVRT.
Diagnostic Maneuvers during Tachycardia
Atrial Extrastimulation and Atrial Pacing During Supraventricular Tachycardia
Orthodromic Atrioventricular Reentrant Tachycardia
Atrial pacing at a CL slightly shorter than the tachycardia CL generally can entrain orthodromic AVRT (Fig. 18-27). If the P waves on the surface ECG can be seen, which usually is not the case, they may appear to be fusion beats resulting from intraatrial collision of the impulse propagating from the paced site with the one emerging from the BT. In general, when pacing is initiated orthodromically to the zone of slow conduction (the AVN in this case), conduction time within the area of slow conduction is long enough to allow a wide atrial antidromic wavefront to generate surface ECG fusion (see Fig. 18-27).
The initial atrial complex following cessation of atrial pacing entraining orthodromic AVRT is linked to, and cannot be dissociated from, the last captured ventricular complex. As a consequence, the VA intervals of the return cycle after cessation of atrial pacing are fixed and similar to those during tachycardia (with <10 milliseconds of variation) after different attempts at SVT entrainment (see Fig. 18-27). The post-pacing VA intervals typically remain constant regardless of the site, duration, or CL of the entraining atrial pacing drive because retrograde VA conduction of the last entrained QRS is mediated by the BT, which is fixed and constant. VA linking can also be observed in typical AVNRT but not AT.33,36
Antidromic Atrioventricular Reentrant Tachycardia
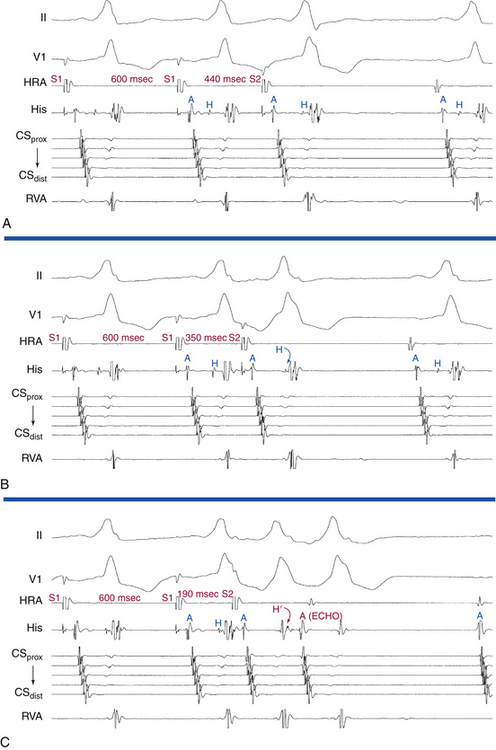
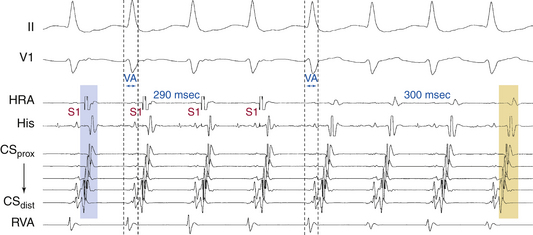
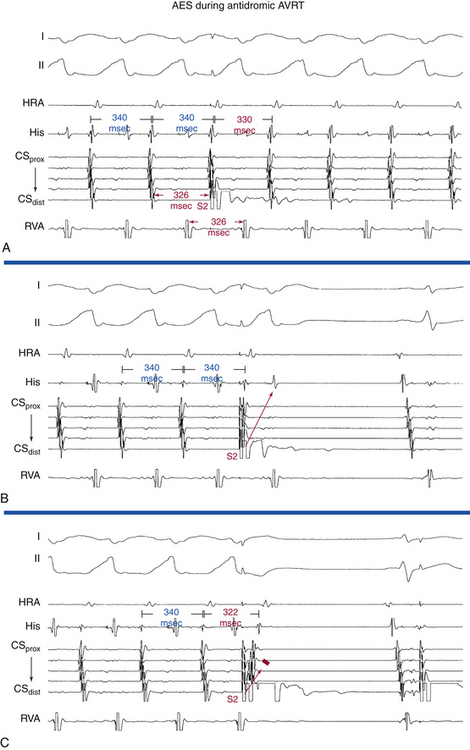
AES is of value in distinguishing antidromic AVRT from preexcited AVNRT. A late-coupled AES, delivered close to the BT atrial insertion site during SVT when the AV junctional atrium is refractory (i.e., when the atrial electrogram is already manifest in the HB recording at the time of AES delivery) that advances (accelerates) the timing of both the next ventricular activation as well as the subsequent atrial activation, proves that the SVT is an antidromic AVRT using an AV BT anterogradely, and excludes preexcited AVNRT (Fig. 18-28A). Because the AV junctional atrium is refractory at the time of the AES, the AES cannot penetrate the AVN, and resetting of the SVT by such an AES is therefore incompatible with AVNRT.
Also, an AES delivered during the SVT that advances ventricular activation and does not influence the VA interval excludes preexcited AVNRT and is diagnostic of antidromic AVRT (see Fig. 18-28A). The VA interval should change in the setting of preexcited AVNRT because the AES penetrates the AVN, producing slower conduction down the AVN before resumption of the tachycardia, which, in the presence of a fixed AV interval (caused by conduction down the BT) in response to the AES, would lead to a longer VA interval. In addition, the advanced QRS could invade and capture the HB retrogradely and conduct up the fast AVN pathway and reset the AVN circuit. Then the V-H interval of the advanced QRS plus the HA interval in response to this QRS should add up to the same VA interval on an undisturbed AVNRT, which is clearly unlikely.
Exact atrial and ventricular capture by an AES delivered when the AV junction is depolarized excludes AVNRT (see Fig. 18-28A). An AES that captures the ventricle at the same coupling interval as that of the AES indicates that the atrial stimulation site is inside the reentrant circuit, because if there were intervening atrial tissue between the stimulation site and the tachycardia circuit (as is the case during AVNRT), the AV interval would increase, and consequently, the V-V interval would exceed the AES coupling interval.3
An early-coupled AES can terminate the SVT by retrograde block in the AVN-HPS (the SVT terminates with an AES followed by a QRS; i.e., VA block; Fig. 18-28B) or by anterograde block in the BT (the SVT terminates with an AES not followed by a QRS; i.e., AV block; Fig. 18-28C).
Ventricular Extrastimulation and Ventricular Pacing During Supraventricular Tachycardia
Orthodromic Atrioventricular Reentrant Tachycardia: Manifest or Concealed Atrioventricular Bypass Tract
VES and ventricular pacing can easily reset, entrain, and may terminate orthodromic AVRT (Fig. 18-29).33 However, the ability of the VES to affect the SVT depends on the distance between the site of ventricular stimulation to the ventricular insertion site of the BT and on the VES coupling interval. Because only parts of the ventricle ipsilateral to the BT are requisite components of the orthodromic AVRT circuit, a VES delivered in the contralateral ventricle may not affect the circuit.
Resetting and/or Entrainment with Manifest Ventricular Fusion
The relative proximity (conduction time) of the pacing site, the site of entrance to a reentrant circuit, and the site of exit from the circuit to the paced chamber is critical for the occurrence of fusion during resetting and/or entrainment. A requirement for the presence of fusion, independent of the pacing site, is spatial separation between the sites of entrance to and exit from the reentrant circuit. In orthodromic AVRT, the entrance and exit of the reentrant circuit (to and from ventricular tissue) are separated from each other, the entrance being from the HPS and the exit being at the ventricular insertion site of the BT. Therefore, pacing at a site closer to the BT ventricular insertion site (e.g., left ventricular [LV] pacing in the setting of left free wall BTs, and RV pacing in the setting of right-sided or septal BTs) than the entrance of the reentrant circuit to ventricular tissue (i.e., the HPS) would result in fusion of QRS morphology between baseline morphology during orthodromic AVRT and that of fully paced QRS (Fig. 18-30). Manifest ventricular fusion during entrainment is proof that the ventricle is a part of the SVT circuit because fusion is due to collision of the antidromic stimulated wavefront with the orthodromic wavefront from the preceding beat occurring within ventricular myocardium. On the other hand, such phenomena cannot occur during AVNRT because of the lack of spatial separation of the entrance and exit to the AVNRT circuit and because the ventricles are not an obligatory part of the AVNRT circuit.
Entrainment by Right Ventricular Apical Pacing
This technique can help differentiate orthodromic AVRT with a right lateral or septal BT from AVNRT. The VA interval during ventricular pacing is compared with that during SVT. The ventricle and atrium are activated in sequence during orthodromic AVRT and during ventricular pacing, whereas during AVNRT the ventricle and atrium are activated in parallel. Therefore, the VA interval during orthodromic AVRT approximates that during ventricular pacing. On the other hand, the VA interval during AVNRT is much shorter than that during ventricular pacing (see Fig. 18-30). Therefore, a ΔVA interval (VA interval during ventricular pacing minus VA interval during SVT) of more than 85 milliseconds is consistent with AVNRT, whereas a ΔVA interval of less than 85 milliseconds is consistent with orthodromic AVRT. In addition, evaluation of the post-pacing interval (PPI) versus the tachycardia CL is of value. In AVNRT (typical or atypical), the PPI reflects conduction time from the RV pacing site through the RV muscle and HPS, once around the reentry circuit and back. Therefore, the [PPI – tachycardia CL] difference represents twice the sum of the conduction time through the RV muscle and HPS. In orthodromic AVRT using a septal BT, the PPI reflects the conduction time through the RV to the septum, once around the reentry circuit and back. Hence, the PPI more closely approximates the tachycardia CL in orthodromic AVRT using a septal BT compared with AVNRT (see Fig. 18-30). Therefore, a [PPI – tachycardia CL] difference of more than 115 milliseconds is consistent with AVNRT, whereas a [PPI – tachycardia CL] difference of less than 115 milliseconds is consistent with orthodromic AVRT. For borderline values, ventricular pacing at the RV base can help exaggerate the difference between the PPI and tachycardia CL in the setting of AVNRT, but without significant changes in the setting of orthodromic AVRT, because the site of pacing at the RV base is farther from the AVNRT circuit than the RV apex, but is still close to an AVRT circuit using a septal BT (and in fact is closer to the ventricular insertion of the BT).37
A relatively common phenomenon encountered during entrainment of orthodromic AVRT by ventricular pacing is the prolongation of the AH interval because of either decremental conduction properties of the AVN or (in the presence of dual AVN physiology) a jump of anterograde conduction from the fast pathway to the slow pathway. The prolonged AH interval on the last entrained beat will contribute to prolongation of the PPI that is not reflective of the distance of the pacing site from the circuit. Thus, the [PPI – SVT CL] differences obtained after entrainment of orthodromic AVRT employing a septal BT can actually overlap with those observed after entrainment of AVNRT. Subtracting the increment in AVN conduction time in the first PPI (post-pacing AH interval minus pre-pacing AH interval) from the [PPI – SVT CL] difference (“corrected” [PPI – SVT CL]) has been found to improve the accuracy of this criterion. The difference between AV intervals (post-pacing AV interval minus pre-pacing AV interval) can be taken for the latter adjustment when a His deflection is not clearly visible (assuming the HV interval remains constant). In a study of patients with both typical and atypical forms of AVNRT, as well as orthodromic using septal and free wall BTs, a corrected [PPI – SVT CL] difference of less than 110 milliseconds was found more accurate in identifying orthodromic AVRT from AVNRT than the uncorrected [PPI – SVT CL] difference. The use of change in the VA interval is of course not influenced by prolongation of the AV interval during pacing and does not require correction.38–40
Differential Right Ventricular Entrainment
As discussed in Chapter 17, differential-site RV entrainment (from RV apex versus RV base) can help distinguish AVNRT from orthodromic AVRT. In orthodromic AVRT, in which the ventricles are an obligatory part of the circuit, the basal pacing site relative to the RV apex is variably related to the circuit, being closer than the RV apex with septal BTs and equidistant with free wall BTs, but the paced wavefront from either RV apex or RV base tends to have, on average, approximately equal access and proximity (electrophysiologically) to the reentrant circuit involved in orthodromic AVRT and, therefore, the time taken to reach the circuit (and hence the PPI) tends to be similar, irrespective of the location of the BT. Conversely, the base of the RV is electrically more distant than the RV apex (where the His-Purkinje network directly inserts) from the AVNRT circuit. Hence, following ventricular entrainment of AVNRT, the difference in PPI from RV base versus RV apex will largely be composed of the extra time required to reach the circuit from the base versus the apex (approximately 30 milliseconds).
Correction of the PPI (to avoid potential error introduced by decremental conduction within the AVN during ventricular pacing) increases the accuracy of this method. The “corrected PPI” is obtained by subtracting any increase in the AV interval of the return cycle beat (as compared with the AV interval during SVT). A differential corrected [PPI – SVT CL] difference of more than 30 milliseconds after transient entrainment was found to be consistent with AVNRT (i.e., corrected [PPI – SVT CL] difference following pacing from the RV base was consistently at least 30 milliseconds longer than that following pacing from the RV apex), and a corrected [PPI – SVT CL] difference of less than 30 milliseconds was observed in all cases of orthodromic AVRT. Additionally, a differential VA interval (ventricular stimulus-to-atrial interval during entrainment from RV base versus RV septum) of more than 20 milliseconds was consistent with AVNRT, whereas a differential VA interval of less than 20 milliseconds was consistent with orthodromic AVRT.41
Length of Pacing Drive Required for Entrainment
As discussed in Chapter 17, assessing timing and type of response of SVT to RV pacing also can help differentiate orthodromic AVRT from AVNRT with high positive and negative predictive values. In the setting of orthodromic AVRT, once ventricular capture is achieved during RV pacing, the paced wavefront propagates to the ventricular insertion site of the BT quickly and resets the tachycardia. In the setting of AVNRT, on the other hand, where the pacing wavefront has to penetrate the HPS followed by AVN tissue prior to resetting the tachycardia, resetting of the tachycardia is delayed as compared with orthodromic AVRT. When resetting of the SVT occurs after a single paced beat orthodromic AVRT is suggested and AVNRT is generally excluded. On the contrary, if resetting occurs only after at least two beats AVNRT is suggested.42
Atrial Resetting During the Transition Zone
Similar to the concept discussed earlier for entrainment with manifest QRS fusion, perturbation (at least 15 milliseconds) of atrial timing during “transition zone” on initiation of RV pacing (the zone during which the pacing train fuses with anterograde ventricular activation) indicates the presence of a retrogradely conducting BT, and cannot occur in AT or AVNRT unless a bystander retrogradely conducting BT is present (see Chap. 17).43
Termination of orthodromic AVRT by VES can occur secondary to block of the VES retrogradely in the AV BT, conduction of the VES retrogradely over the AVN-HPS with or without conduction up the BT, or retrograde conduction of the VES up the BT and preexcitation of the atrium and subsequent anterograde block in the AVN-HPS (the most common mechanism) (see Fig. 18-29). Termination of SVT with a single VES strongly suggests orthodromic AVRT as the mechanism of SVT in three settings: when the VES is late-coupled (>80% of tachycardia CL), when the tachycardia CL is less than 300 milliseconds, and when the VES is delivered during HB refractoriness and is associated with no atrial activation.
Maneuvers to Prove Presence of an Atrioventricular Bypass Tract
When VES or ventricular pacing results in eccentric atrial activation sequence, the presence of a BT is strongly suggested. A VES delivered when the HB is refractory (i.e., when the His potential is already manifest or within 35 to 55 milliseconds before the time of the expected His potential) that advances (accelerates) the next atrial activation is diagnostic of the presence of a retrogradely conducting BT. Such a VES has to conduct and advance atrial activation via an AV BT because the HPS-AVN is already refractory and cannot mediate retrograde conduction of the VES to the atrium (see Fig. 18-29). Although such an observation excludes AVNRT, it does not exclude AT or prove orthodromic AVRT, and the preexcited atrial activation can reset or even terminate an AT, whereby the AV BT is an innocent bystander. However, if this VES advances atrial activation with an activation sequence identical to that during the SVT, this suggests that the SVT is orthodromic AVRT and the AV BT is participating in the SVT, although it does not exclude the rare case of an AT originating at a site close to the atrial insertion site of a bystander AV BT. Furthermore, a VES delivered when the HB is refractory may not affect the next atrial activation if the ventricular stimulation site is far from the BT. Conduction from the ventricular stimulation site to the BT, local ventricular refractoriness, and the tachycardia CL all determine the ability of a VES to reach the reentrant circuit before ventricular activation over the normal AVN-HPS.33
One maneuver is a VES delivered when the HB is refractory that delays the next atrial activation. Although such a VES can advance atrial activation during AT through fast retrograde conduction over a bystander BT, it should not be able to delay an AT beat by conduction over the AV BT. Such delay indicates that the VES was conducted with some delay over the AV BT and that the next atrial activation was dependent on this slower conduction; thus, the AV BT is participating in the SVT, proving orthodromic AVRT as the mechanism of the tachycardia. Similarly, a VES delivered when the HB is refractory that terminates the SVT without atrial activation is diagnostic of AVRT. Furthermore, entrainment of the SVT by ventricular pacing that results in prolongation in the surface VA interval indicates that the SVT is orthodromic AVRT mediated by an AV BT in the ventricle contralateral to the site of ventricular pacing; this is analogous to the influence of ipsilateral BBB on the VA interval during orthodromic AVRT (see Fig. 18-30). Exact and paradoxical capture phenomena are also diagnostic of AVRT. VES that captures the atrium at the same coupling interval as that of the VES (exact capture phenomenon) indicates that the ventricular stimulation site is inside the reentrant circuit, because if there were intervening tissue involved, the VA interval would increase and, subsequently, the A-A interval would exceed the VES coupling interval. Similarly, a VES that captures the atrium at a shorter coupling interval than that of the VES (paradoxical capture phenomenon) indicates that the ventricular stimulation site is not only inside the reentrant circuit but also closer to the ventricular insertion site of the AV BT than the initial site of ventricular activation over the AVN-HPS during the SVT, so that the VA interval following the VES is shorter than that during the SVT. This is easier to demonstrate with RV apical pacing during orthodromic AVRT mediated by a right-sided BT.3
Orthodromic Atrioventricular Reentrant Tachycardia: Slowly Conducting Concealed Atrioventricular Bypass Tract (Permanent Junctional Reciprocating Tachycardia)
A VES delivered during PJRT can produce decremental conduction in the BT and prolongation of the VA interval, resulting in possible delay of the next atrial activation (i.e., postexcitation or delay of excitation; see Fig. 18-26). Such a response excludes AT, and when this occurs in response to a VES delivered when the HB is refractory, it is diagnostic of orthodromic AVRT, and excludes both AT and AVNRT. Additionally, a late-coupled VES introduced when the HB is refractory frequently blocks retrogradely in the BT and reproducibly terminates the tachycardia without reaching the atrium, again excluding both AT and AVNRT.
Diagnostic Maneuvers during Normal Sinus Rhythm after Tachycardia Termination
Ventricular Pacing at the Tachycardia Cycle Length
Ventricular pacing during NSR at a CL similar to the tachycardia results in HA and VA intervals that are shorter than those during orthodromic AVRT, because the HB and atrium are activated sequentially during orthodromic AVRT but in parallel during ventricular pacing (see Fig. 18-30). To help distinguish between orthodromic AVRT and AVNRT, the HA interval is measured from the end of the His potential (where the impulse leaves the HB to enter the AVN) to the atrial electrogram in the high RA recording and the ΔHA interval (HA interval during ventricular pacing minus HA interval during SVT) is calculated. In the setting of orthodromic AVRT the ΔHA interval is typically less than –10 milliseconds. In contrast, in the setting of AVNRT the ΔHA interval is more than –10 milliseconds. This criterion has 100% specificity and sensitivity and positive predictive accuracy for differentiation between AVNRT and orthodromic AVRT. The main limitation of the ΔHA interval criterion is the ability to record the retrograde His potential during ventricular pacing. Retrograde His potential generally appears before the local ventricular electrogram in the HB tracing, and can be verified by the introduction of a VES that causes the His potential to occur after the local ventricular electrogram. Moreover, pacing from different sites (e.g., midseptum) may allow earlier penetration into the HPS and facilitate observation of a retrograde His potential. When the retrograde His potential is not visualized, using the ΔVA interval instead of the ΔHA interval is not as accurate in discriminating orthodromic AVRT from AVNRT. Another limitation is that VA conduction during ventricular pacing may not occur over the BT but propagates preferentially over the HPS-AVN, leading to earlier atrial activation over this pathway than over the BT. If this were the case, the HA interval during ventricular pacing would be shorter than that observed if the atrium were activated via the BT. This would yield a more negative ΔHA interval.
Para-Hisian Pacing During Normal Sinus Rhythm
Technique
Ideally, two quadripolar catheters (one for pacing and one for recording) or a single octapolar catheter (for both pacing and recording) is placed at the distal His bundle–right bundle branch (HB-RB) region. Alternatively, a single quadripolar HB catheter (which is typically used during a diagnostic EP study) is used, taking into account that such an approach would limit the ability to record the retrograde His potential and HA interval.44
Overdrive ventricular pacing is performed (from the pair of electrodes on the HB catheter that records activation of the distal HB-RB) at a long pacing CL (>500 milliseconds) and high output. During pacing, direct HB-RB capture is indicated by narrowing of the paced QRS width. The pacing output and pulse width are then decreased until the paced QRS widens, which is associated with a delay in the timing of the retrograde HB potential, indicating loss of HB-RB capture. The pacing output is increased and decreased to gain and lose HB-RB capture, respectively, while local ventricular capture is maintained. Occasionally, the HB can be captured uniquely (without myocardial capture), resulting in a QRS identical to the patient’s normally conducted QRS.44
Concept of Para-Hisian Pacing
Para-Hisian pacing can result in capture of the ventricle (indicated by a wide paced QRS), the atrium (indicated by atrial activation in the HB region immediately following the pacing artifact), the HB (indicated by narrow paced QRS), or any combination of these (Fig. 18-31).44 Careful attention must be given to minimize the atrial signal seen on the recording from the pacing electrode pair to ensure that local atrial capture does not occur during pacing.
Response to Para-Hisian Pacing
Seven patterns of response to para-Hisian pacing can be observed (Table 18-3 and Fig. 18-32; and see Fig. 18-31). In patients with retrogradely conducing AV BTs, in whom retrograde conduction occurs over both the AVN and BT during para-Hisian pacing, the amount of atria activated by each of the two pathways (atrial fusion) is dependent on four variables: (1) the magnitude of delay in retrograde activation of the HB (i.e., S-H interval); (2) retrograde conduction time over the AVN (HA interval); (3) intraventricular conduction time from the para-Hisian pacing site to the ventricular end of the BT (S-VBT); and (4) retrograde conduction time over the BT (V-ABT). The first two variables (S-H plus HA) form the S-A interval resulting from retrograde VA conduction over the AVN, and the latter two variables (S-VBT plus V-ABT) form the S-A interval resulting from retrograde VA conduction over the BT. The amount of the atria activated by the AVN is greater during HB-RB capture, secondary to a minimal S-H interval (i.e., S-A interval = HA interval). Loss of HB-RB capture results in prolongation of the S-H interval and, therefore, an increase in the amount of atria activated by the BT, resulting in a change in the retrograde atrial activation sequence. Consequently, a change in the retrograde atrial activation sequence with loss of HB-RB capture always indicates the presence of retrograde conduction over both the BT and AVN. There are four such patterns (patterns 4 through 7). In patterns 4 and 5, HB-RB capture is associated with activation of the atria exclusively by retrograde conduction over the AVN. In patterns 6 and 7, HB-RB capture results in atrial activation over both the AVN and the BT.44
• Retrograde conduction occurs exclusively over a single BT.
• The S-A interval is identical during HB-RB capture and noncapture, indicating that retrograde conduction is dependent on local ventricular activation and not on HB activation.
• This pattern does not exclude the presence of retrograde conduction over the AVN with longer conduction time or a second BT with longer conduction time or located far from the pacing site.
• Retrograde conduction occurs exclusively over a BT.
• Loss of HB-RB capture is associated with a delay in the timing of ventricular activation close to the BT. This results in an increase in the S-A interval in all electrograms, with no change in the atrial activation sequence. The local VA interval, recorded close to the BT, remains approximately the same. The increase in S-A interval is less than the increase in the S-H interval. Therefore, the HA interval is shortened with loss of HB-RB capture, indicating that retrograde conduction cannot be occurring over the AVN. Two mechanisms have been identified accounting for the delay in timing of ventricular activation close to the BT.
• Activation of the HPS results in earlier ventricular activation near some BTs located far from the para-Hisian pacing site, such as left lateral or anterolateral BTs.
• Decreasing the pacing output to lose HB-RB capture occasionally results in a small delay in ventricular activation close to the pacing site.
• Pattern 3 is referred to as the BT-BTL pattern, where BTL refers to a lengthening of the S-A interval with loss of HB-RB capture.
• Loss of HB-RB capture is associated with atrial activation exclusively over the BT.
• Loss of HB-RB capture results in an increase in S-A and local VA intervals in all electrograms, with the least increase occurring in the electrogram closest to the BT.
• The HA interval shortens, indicating that the atrium near the AVN is activated by the BT before retrograde conduction over the AVN is complete.
• Loss of HB-RB capture results in activation of part of the atria by the AVN and part by the BT.
• Loss of HB-RB capture is associated with an increase in S-A and local VA intervals in all electrograms.
• The HA interval remains constant, indicating that part of the atria was still activated by the AVN.
• Loss of HB-RB capture results in atrial activation exclusively over the BT.
• Loss of HB-RB capture is associated with no change in the S-A or local VA intervals recorded near the BT.
• In the HB electrogram, the S-A interval increases, but not as much as the S-H interval, leading to a decrease in the HA interval. This indicates that the atrial myocardium in that region is no longer activated by the AVN.
• The atria continue to be activated by both the AVN and the BT during loss of HB-RB capture, with more of the atria activated by the BT than during HB-RB capture.
• Like pattern 6, loss of HB-RB capture is associated with minimal change in the S-A or local VA intervals recorded close to the BT; however, the HA interval remains essentially the same, indicating that part of the atria is still activated by the AVN.
AVN = atrioventricular node; AVNRT = atrioventricular nodal reentrant tachycardia; BT = bypass tract; HA = His bundle–atrial; HB-RB = His bundle–right bundle branch; HPS = His-Purkinje system; PJRT = permanent junctional reciprocating tachycardia; S-A = stimulus-atrial; S-H = stimulus–His bundle; VA = ventriculoatrial.
Interpretation of Results of Para-Hisian Pacing
The response to para-Hisian pacing can be determined by comparing the following four variables between HB-RB capture and noncapture while maintaining local ventricular capture and no atrial capture: (1) atrial activation sequence, (2) S-A interval, (3) local VA interval, and (4) HA interval (see Figs. 18-31 and 18-32).
The HA interval is recorded in the HB electrogram; however, this measurement can be obtained only if two catheters are placed in the HB position (one for pacing and one for recording) or if an octapolar catheter is used for pacing and sensing around the HB. The use of a single quadripolar HB catheter, which is typically used during a diagnostic EP study, negates the ability to record the retrograde His potential and HA interval during pacing. However, the combination of the S-A and local VA intervals is sufficient to identify the presence of retrograde BT.
An identical retrograde atrial activation sequence during HB-RB capture and noncapture indicates that retrograde conduction is occurring over the same system (either the BT or AVN) and does not help prove or exclude the presence of a BT (especially a septal BT; see Fig. 18-32). A change in retrograde atrial activation sequence with loss of HB-RB capture, however, indicates the presence of retrograde conduction over both a BT and the AVN. Morphological change in the atrial electrogram recorded at the AV junction without overlapping the ventricular electrogram also seems to have diagnostic significance, indicating the presence of both BT and AVN conduction.
Limitations of Para-Hisian Pacing
The location of the BT, as well as retrograde conduction time over the BT, must be taken into account when interpreting the results of para-Hisian pacing. For superoparaseptal BTs, the S-VBT interval is short. For BTs located progressively farther from the para-Hisian pacing site, the S-VBT increases progressively. This is not a significant factor for midseptal, posteroseptal, or most right free wall BTs. However, for left free wall BTs, which are located far from the pacing site, the S-VBT interval can be sufficiently long to have the entire atria activated by the AVN, even during loss of HB-RB capture. In this setting, para-Hisian pacing can produce an AVN retrograde conduction pattern, regardless of whether or not the HB-RB is captured (pattern 1: AVN-AVN), failing to identify the presence of retrograde BT conduction (because of the long S-VBT). However, a left lateral BT should not be a diagnostic challenge because of the obvious eccentric retrograde atrial activation sequence during orthodromic AVRT, and para-Hisian pacing is performed mainly to investigate the presence of a septal BT. Additionally, for BTs located far from the para-Hisian pacing site, it is important to record atrial activation close to the suspected site of the BT. Otherwise, without recording electrograms near the BT, the change in atrial activation sequence may not be identified, incorrectly suggesting that retrograde conduction is occurring over just the AVN. This is most likely to occur in patients with short retrograde AVN conduction (short HA interval) and a BT located far from the pacing site.
In patients with very proximal retrograde RBBB, RB capture may fail to produce early retrograde activation of the HB, limiting the use of para-Hisian pacing in these patients. This observation suggests that HB-RB capture actually represents capture of the proximal RB and not HB capture. This is supported by the observation that, during HB-RB capture, the HB potential is often recorded 10 to 20 milliseconds after the pacing stimulus. Importantly, para-Hisian pacing has been performed successfully in many patients with more distal RBBB (see Fig. 18-31).
Para-Hisian Pacing During Supraventricular Tachycardia (Para-Hisian Entrainment or Resetting)
Technique
Entrainment of the tachycardia is performed by pacing at the para-Hisian region using the HB catheter, as described previously, at a pacing CL 10 to 30 milliseconds shorter than the tachycardia CL. Entrainment is confirmed when the atrial CL accelerates to the pacing CL, without a change in the atrial activation sequence, and the tachycardia continues after pacing is discontinued.45
Para-Hisian entrainment is performed by alternately pacing at high-energy output for HB-RB capture or lower energy output for HB-RB noncapture. Entrainment with HB-RB capture is recorded separately from that without HB-RB capture. The S-A and local VA intervals during HB-RB capture and noncapture are then examined.45
Interpretation of Results of Para-Hisian Entrainment or Resetting
In AVNRT (typical or atypical), the AVN-AVN pattern is observed in response to para-Hisian entrainment/resetting. Both the S-A and the local VA intervals increase during HB-RB noncapture compared with HB-RB capture.45
In orthodromic AVRT, the BT-BT pattern or BT-BTL pattern is observed. In the setting of a BT-BT pattern, the S-A and local VA intervals are usually not significantly different between HB-RB capture and noncapture. Conversely, in the case of a BT-BTL pattern, the S-A interval increases on HB-RB noncapture, but without significant change in the local VA interval.45
An AVN-AVN or fusion pattern during para-Hisian entrainment or resetting has not been observed in patients with AVNRT, a potential advantage over para-Hisian pacing during NSR in identifying the presence of a BT. Because retrograde VA conduction can only proceed over a single route during entrainment of the SVT (assuming that a complex scenario such as multiple BTs is not present), the various forms of retrograde fusion that might be seen during para-Hisian pacing during NSR cannot occur during para-Hisian entrainment or resetting.45
Differential Right Ventricular Pacing
The response to differential-site RV pacing can be evaluated by comparing the VA interval and atrial activation sequence during pacing at the RV base versus the RV apex (Fig. 18-33). The RV apex, although anatomically more distant from the atrium than the RV base, is nonetheless electrically closer because of the proximity of the distal RB to the pacing site. Consequently, in the absence of a retrogradely conducting septal AV BT, pacing at the RV apex allows entry into the rapidly conducting HPS and results in a shorter stimulus-to-atrial (S-A) interval during pacing from the apex than from the base. Pacing from the RV base requires the paced wavefront to travel a longer distance by muscle-to-muscle conduction to reach the RV apex and then propagate retrogradely through the RB and HB. In other words, the V-H interval is shorter with pacing at the RV apex versus the RV base. In the presence of a septal AV BT, pacing at the RV base allows the wavefront to access the AV BT rapidly and activate the atrium with a shorter S-A interval than during pacing at the RV apex, which is distant from the ventricular insertion site of AV BT (i.e., because the V-BT interval is shorter with pacing at the RV base versus the RV apex).
In the absence of a retrogradely conducting AV BT, atrial activation sequence will be similar during pacing both at the RV apex and at the RV base because the atrium is activated over the AVN in both settings. On the other hand, if a septal AV BT is present, atrial activation results from VA conduction over the AV BT during pacing at the RV base, and over either the AVN, the AV BT, or a fusion of both during pacing at the RV apex. Therefore, a variable retrograde atrial activation sequence in response to differential RV pacing (RV base versus RV apex) is indicative of the presence of an AV BT, but a constant atrial activation sequence is not helpful in excluding the presence of an AV BT.
The occurrence of RBBB (but not LBBB) also can alter the significance of the VA interval criterion, especially when VA conduction propagates over the HPS-AVN. In the presence of retrograde RBBB, VA conduction occurs over the LB-HB; therefore, the VA interval depends on the distance between the pacing site and the LB rather than the RB, and access of the paced wavefront to the LB can be faster for RV basilar or septal pacing compared with pacing from the RV apex (see Fig. 17-21).
Dual-Chamber Sequential Extrastimulation
Although the various ventricular pacing maneuvers described previously can expose an eccentric or nondecremental atrial activation pattern that suggests retrograde conduction over a BT rather than the AVN, in certain circumstances, these maneuvers may not be adequate to confirm the presence or absence of BT conduction, especially when the BT has an ERP, retrograde atrial activation pattern, and conduction time similar to the AVN. In particular, identification, mapping, and verification of success of ablation of BT function can be challenging in the setting of septal BTs with a retrograde activation pattern similar to retrograde AVN conduction, slowly conducting BTs, as well as BTs with decremental properties.46
Dual-chamber sequential extrastimulation is a useful maneuver for identifying concealed slowly conducting BTs not revealed with standard pacing maneuvers. This maneuver relies on concealed AVN conduction during a critically timed AES to cause transient retrograde AVN blockade at the time a VES is delivered, thereby allowing the BT to become manifest with the VES (analogous to delivering a VES during SVT while the HB is refractory).46
The dual-chamber sequential extrastimulation maneuver consists of an eight-beat drive train of simultaneous atrial and RV pacing at 600 milliseconds, followed by an AES (A2) delivered at a coupling interval equal to the AVN ERP, followed by a VES (V2) delivered at a coupling interval equal to the drive train CL (600 milliseconds). Repeat drives are then performed with decrements of 10 milliseconds for V2 until VA block is observed.46
The critically timed A2 prolongs the AVN refractory period via concealed anterograde conduction, causing V2 to block in the AVN when it would have conducted had A2 not been delivered. If a BT is present, V2 conducts back to the atrium while the AVN remains refractory, resulting in a retrograde atrial activation pattern consistent with exclusive BT conduction. Although there can also be some degree of concealed anterograde conduction into the BT during A2 stimulation, the more pronounced decremental properties of AVN tissue should prolong AVN refractoriness to a greater degree than that of the BT, allowing exclusive retrograde conduction over the BT to remain intact during V2 stimulation.46
This maneuver has several potential limitations. First, atrial ERP may exceed anterograde AVN ERP. Additionally, local atrial ERP at the site of BT insertion can render the atrium refractory to the wavefront traveling retrogradely over the BT. Therefore, atrial pacing during this maneuver ideally should be performed at a site in close proximity to the atrial insertion of the BT if possible. Furthermore, the AES may cause anterograde concealed conduction in the BT, potentially resulting in BT conduction block during delivery of V2. The success of this pacing maneuver relies on the differential effects of concealed conduction into the AVN and BT, with greater extension of refractoriness in the former than the latter.46
Exclusion of Other Arrhythmia Mechanisms
AVNRT and AT arising near the AV groove can mimic orthodromic AVRT and, in the presence of a manifest BT, those tachycardias can be associated with ventricular preexcitation mimicking antidromic AVRT, whereby the BT is functioning as an innocent bystander. Therefore, EP testing is required, not just to identify the presence of a BT, but also to define its role in any clinical or inducible arrhythmia. Tables 18-4 and 18-5 summarize the EP findings indicative of the presence of a BT and its potential participation in an inducible SVT. Exclusion of other SVT mechanisms is necessary, because the mere presence of a BT is not adequate to make a diagnosis and a treatment strategy (Tables 18-6, 18-7, and 18-8).
TABLE 18-4 Electrophysiological Findings Indicating Presence of Retrograde Atrioventricular Bypass Tract Function
HB = His bundle; RV = right ventricle; SVT = supraventricular tachycardia; VA = ventriculoatrial; VES = ventricular extrastimulus.
TABLE 18-5 Electrophysiological Findings Indicating Presence and Participation of Atrioventricular Bypass Tract in Supraventricular Tachycardia
BBB = bundle branch block; CL = cycle length; HB = His bundle; SVT = supraventricular tachycardia; VA = ventriculoatrial; VES = ventricular extrastimulus.
TABLE 18-6 Exclusion of Atrial Tachycardia
AT = atrial tachycardia; AVRT = atrioventricular reentrant tachycardia; BBB = bundle branch block; CL = cycle length; NSR = normal sinus rhythm; SVT = supraventricular tachycardia; VA = ventriculoatrial; VES = ventricular extrastimulus.
TABLE 18-7 Exclusion of Atrioventricular Nodal Reentrant Tachycardia
AH = atrial–His bundle; AVNRT = atrioventricular nodal reentrant tachycardia; AVRT = atrioventricular reentrant tachycardia; BBB = bundle branch block; CL = cycle length; HA = His bundle–atrial interval; HB = His bundle; NSR = normal sinus rhythm; PPI = post-pacing interval; RV = right ventricle; SVT = supraventricular tachycardia; VA = ventriculoatrial; VES = ventricular extrastimulus.
TABLE 18-8 Differentiation Between Antidromic AVRT and Preexcited AVNRT
AES = atrial extrastimulus; AV = atrioventricular; AVNRT = atrioventricular nodal reentrant tachycardia; AVRT = atrioventricular reentrant tachycardia; BBB = bundle branch block; BT = bypass tract; CL = cycle length; HA = His bundle–atrial; HV = His bundle–ventricular; NSR = normal sinus rhythm; RV = right ventricle; SVT = supraventricular tachycardia; VA = ventriculoatrial; VES = ventricular extrastimulus; V-H = ventricular–His bundle.
Furthermore, the presence of multiple BTs is not infrequent, and careful EP testing is required to evaluate this possibility. Several clinical and EP findings are indicative of the presence of multiple BTs (Table 18-9 and Fig. 18-34; and see Fig. 18-3). However, despite these various methods, many BTs are not identified until after catheter ablation of the first BT. Failure to detect the presence of multiple BTs during EP testing has been reported in as many as 5% to 15% of patients. This may be explained by the fact that changes in the preexcitation pattern can be subtle in shifting from one BT to another. Furthermore, one BT can preferentially conduct during atrial pacing or participate in preexcited tachycardias while another BT can be responsible for the retrograde limb during orthodromic AVRT or ventricular pacing. Additionally, there may be fusion of BT conduction, anterograde or retrograde. Repetitive concealed conduction into the BT during AVRT also may preclude identification of that BT before ablation of the first BT.
TABLE 18-9 Electrophysiological Findings Indicating Presence of Multiple Atrioventricular Bypass Tracts
AF = atrial fibrillation; AVN = atrioventricular node; AVRT = atrioventricular reentrant tachycardia; BBB = bundle branch block; BT = bypass tract; CL = cycle length; CS = coronary sinus; HPS = His-Purkinje system; LA = left atrium; NSR = normal sinus rhythm; PAC = premature atrial complex; RA = right atrium; VA = ventriculoatrial.