Chapter 18 Atmospheric pollution
Effects on the environment
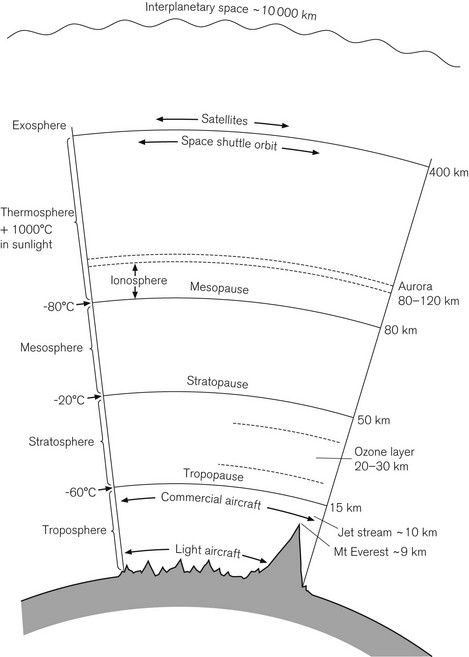
The stratosphere is the region of the atmosphere from about 10 to 50 km above the Earth’s surface, where ozone plays a vital role absorbing harmful short wavelength ultraviolet radiation from the sun and protecting the earth (Fig. 18.1). Stratospheric ozone is depleted by human-made chemicals including hydrochlorofluorocarbons (e.g. halothane, enflurane, isoflurane) and nitrous oxide.
The contribution of these agents to ozone depletion is a function of their lifetimes in the atmosphere, and these lifetimes depend on the reaction of the drugs with hydroxyl radicals in the troposphere1 (Table 18.1). The relatively short lifetimes of these agents along with their minimal production means they have been seen as relatively ‘ozone friendly’. However, with the reduction of chlorofluorocarbons globally, the influence on ozone depletion by volatile anaesthetics is potentially of increasing importance.4
Table 18.1 The effects of anaesthetic gasses on the ozone layer and greenhouse warming
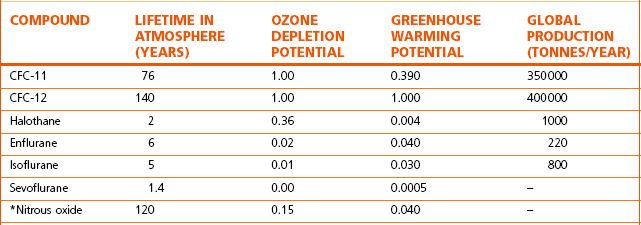
The potential ozone depletion efficacy and greenhouse warming effect are normalized to the principle CFC-12. (Halsey 1996, with permission of The Medicine Group (Education) Ltd2 (based on original data from 1989)1.)
* Represents data from the World Meteorological Organization.3
Nitrous oxide, however, has a much longer lifetime (similar to the traditional chlorofluorocarbons, well known for their ozone depleting effects, and which have been successfully reduced since the 1987 Montreal Treaty) and has now been shown to be the single most important ozone-depleting emission. This is expected to remain throughout the 21st century.5
Nitrous oxide is an exceptionally potent greenhouse gas with approximately 290 times greater global warming potential than carbon dioxide. Although the amount of nitrous oxide generated from medical sources compared with the total global production is small, its contribution in the light of environmental issues and pressures is still significant and difficult to ignore. It would, therefore, seem prudent to reduce the use of nitrous oxide when there are alternative agents and techniques available, including the more potent volatile agents, and regional and intravenous anaesthetic techniques.
Effects on individuals
Chronic exposure to low concentrations of anaesthetic gasses has been associated with adverse health effects. There have been studies and case reports of these effects since the 1960s, although the evidence is sometimes conflicting. Some animal and human studies6–11 have suggested that as a result of chronic exposure to inhalational agents amongst theatre personnel there is a demonstrable increase in:
• minor congenital abnormalities
• subjective complaints (e.g. headaches, fatigue and nervousness)
• problems with balance control
• cancer (leukaemia and lymphoma)
• effects on immune system (secondary to neutrophil apoptosis)
In other studies, long-term exposure to nitrous oxide has been shown to result in:
• reduced fertility and increased miscarriage rate in female dental assistants
• litters that are reduced in number and size compared with control animals (rats)
• neurological symptoms indistinguishable from those caused by vitamin B12 deficiency.
The Health and Safety Commission’s Advisory Committee on Toxic Substances reviewed the literature on the toxic effects of anaesthetic agents in the workplace in 1996.12 They made the following conclusions based on the data available:
• There was no evidence in humans that exposure to nitrous oxide or volatile agents (halothane, isoflurane and enflurane) caused developmental defects in the foetus or other reproductive health effects.
• Animals (rats) continuously exposed to high concentrations of nitrous oxide (1000 ppm for over 8 h) demonstrated developmental toxicity to the embryo/foetus, possibly by the inhibition of cell production by nitrous oxide. However, no adverse effects were seen when animals were exposed to nitrous oxide at lower concentrations (500 ppm).
• Pregnant animals exposed to high concentrations of halothane and isoflurane (1000 ppm) showed effects on the development of the foetus. However, there was no convincing evidence when the concentrations of repeated exposure were lower (100 ppm for halothane and 600 ppm for isoflurane).
• There was no evidence from animal studies that suggested enflurane had any adverse effect on the foetus. However, liver damage was demonstrated in mice when exposed to enflurane continuously (>700 ppm).
Legislation
Various organizations in different parts of the developed world have introduced recommendations for maximum acceptable levels of pollution to protect staff working in these areas. Due to the rather inconclusive evidence on adverse effects of volatile agents, these limits vary in different countries.
In the UK, it is a legal requirement that employers control industrial and medical pollution. The legislation takes the form of a government approved code of practice entitled ‘Control of Substances Hazardous to Health’ (COSHH).13 This was first introduced in 1988, updated in 1994 and amended annually until 2002. There was a further new edition in 2005 (reprinted in 2008) and further amendments made in accordance with the European Commission’s new limits. The HSE’s Advisory Committee on Toxic Substances has drawn up this code of practice under Section 16 of the Health and Safety at Work Act (1974), for the purpose of providing practical guidance on the control of substances hazardous to health in the workplace.
It was in 1996 that COSHH defined the safe maximum exposure limits for a wide variety of substances, including anaesthetic gasses and vapours (EH40/96).14 Since 2005 ‘workplace exposure limits’ (WELs) have been the defined limits used to protect workers, replacing the previously used ‘maximum exposure limits’ (MELs) and ‘occupational exposure standards’ (OES). WELs are defined at concentrations of hazardous substance in the air, averaged over a specified period of time referred to as a time-weighted average (TWA). An 8 h time period is used.
COSHH recommends that: ‘Exposure should be controlled to a level to which nearly all the population can be exposed day after day without adverse effect on health’. Recommended exposure limits for anaesthetic gasses and vapours in some countries are set out in Table 18.2. In the UK in 2010 no WELs are available yet for sevoflurane and desflurane. Both OSHA and NIOSH recommend a global ceiling limit (concentrations that must never be exceeded during any part of the day) of 2 ppm for all volatile agents, though they have no regulatory authority. As a rough guide, substances with exposure limits below 100 ppm are considered highly toxic by inhalation, those substances with exposure limits of 100–500 ppm are considered moderately toxic by inhalation and those substances with exposure limits greater than 500 ppm are slightly toxic by inhalation.
Table 18.2 Exposure limits to anaesthetic gasses and vapours in parts per million (ppm) or as milligrams per cubic metre (mg m−3) and expressed as 8-hour time-weighted averages (8-h TWA)
There are eight principles of good practice for the control of exposure to substances hazardous to health, published by the Health and Safety Executive in 2005.15 They are as follows:
1. Design and operate processes and activities to minimize emission, release and spread of substances hazardous to health.
2. Take into account all relevant routes of exposure – inhalation, skin absorption and ingestion – when developing control measures.
3. Control exposure using measures that are proportionate to the health risk.
4. Choose the most effective and reliable control options which minimize the escape and spread of substances hazardous to health.
5. Where adequate control of exposure cannot be achieved by other means, provide, in combination with other control measures, suitable personal protective equipment.
6. Check and review regularly all elements of control measures for their continuing effectiveness.
7. Inform and train all employees on the hazards and risks from the substances with which they work and the use of control measures developed to minimize the risks.
8. Ensure that the introduction of control measures does not increase the overall risk to health and safety.
Control of pollution
When no steps are taken to avoid pollution, the exposure limits may be exceeded. One study from a 20 hospital survey reported that the levels of halothane varied between 0.1 and 60 ppm (mean of 2.8 ppm) and for nitrous oxide between 10 and 3000 ppm (mean of 388.5 ppm) when scavenging systems were not used.16 In the same study the installation of an active scavenging system in one particular hospital reduced the anaesthetist’s exposure to nitrous oxide (and halothane) from a mean value of 411 ppm (and 1.9 ppm) to a mean value of 24.5 ppm (and <0.1 ppm).
The control of pollution should be tackled using the guidelines recommended in the COSHH in the UK and NIOSH in the USA,17 namely:
• instilling awareness in personnel working in the potentially affected environment
• installation of effective scavenging equipment (see below)
• ensuring good working practices by:
 flushing out the breathing system (including the reservoir bag) through the scavenging device provided, at the end of an anaesthetic
flushing out the breathing system (including the reservoir bag) through the scavenging device provided, at the end of an anaesthetic considering capping off the breathing system at the end of an anaesthetic so as to prevent anaesthetic vapours that have impregnated the breathing hoses from polluting the environment
considering capping off the breathing system at the end of an anaesthetic so as to prevent anaesthetic vapours that have impregnated the breathing hoses from polluting the environment amending workplace practice by reviewing rotas so that the same personnel are not always working in those areas of highest pollution
amending workplace practice by reviewing rotas so that the same personnel are not always working in those areas of highest pollution• maintaining efficient room air-conditioning so as to remove any pollutant that may have inadvertently escaped (a minimum of 15 changes per hour with a balanced supply and extraction process)
The extent of pollution
1. the quantity of anaesthetic gasses and vapours employed
2. the employment of a scavenging system and its efficiency
3. the amount of leakage from the anaesthetic equipment
4. the efficiency of the air-conditioning and ventilation system in an operating theatre or anaesthetic room
5. the size and layout of the operating theatre and any other place where anaesthetic vapours are used.
The employment of a scavenging system and its efficiency
Surplus anaesthetic gasses and vapours are vented from a breathing system or ventilator, via an expiratory valve and, if allowed to escape at this point, would pollute the immediate environment. The valve is normally adapted to discharge into a scavenging system, which collects the escaping gas and vents it to the atmosphere remote from populated areas. The efficiency of the scavenging system depends on its rate of extraction and the gas-tight fit of its components. The former must be greater than the discharge of pollutant gasses, in order to be effective. These systems are discussed in greater detail later in the chapter.
Measurement of pollution
Operating theatres
With the introduction of low-cost non-dispersive, portable infrared analyzers, trace quantities of anaesthetic agents can be measured continuously. A direct reading analyzer (Fig. 18.2) enables spot measurements to be taken at different sites, allowing the background level of nitrous oxide in a room to be assessed. Instant results of nitrous oxide levels (in the range of 0–1000 ppm with a resolution of 5 ppm) are displayed in real-time or as an 8 h TWA. An alarm protects personnel against excessive levels of exposure.
Theatre personnel
Individuals can be issued with sampling tubes for nitrous oxide (Fig. 18.2) and sampling badges for volatile anaesthetics (Fig. 18.3) that are worn for approximately 8 h. They are placed at shoulder height and the pollutants are adsorbed onto the material in the sampler in proportion to their concentration in the ambient atmosphere. For volatile agents, the material is based on activated charcoal, whereas for nitrous oxide, a molecular sieve is used. At the end of the passive sampling period, the samplers are sent to a specialist laboratory where the pollutants are measured using a gas chromatograph linked to an infrared detector.
Biological monitoring of post-volatile anaesthetic exposure, using urine samples analyzed by gas chromatography-mass spectrometry coupled with static headspace sampling, has been shown to be another useful tool to monitor the extent of exposure.18
Scavenging systems
• a collecting system, which conveys waste gasses from the breathing system to a transfer system
• a transfer system, which consists of a section of flexible wide-bore hose linking the collecting system to the receiving system
• a receiving system, which behaves as a reservoir to store surges in the flow of waste gas. From here, these gasses have to pass via disposal tubing to a disposal system
• a disposal system. This then transports the waste gasses to a site on the outside of a building away from populated areas.
Two or more of these items may be embodied in a single item of equipment.
Waste gas normally passes through the collecting and receiving system to the disposal system, using only the power generated in exhalation by the elastic recoil of a patient’s lungs. At this stage there is little difference between the various systems employed. It may then pass through the disposal system using this same power (passive scavenging). However, it may be assisted by some form of gas or electrically powered apparatus, which generates a sub-atmospheric pressure (active scavenging). Only systems that employ active scavenging are able to deal with the wide range of expiratory flow rates (30–120 L min−1) seen in anaesthetic practice, especially when certain ventilator systems are used. Active systems are, therefore, the only ones that can be recommended – provided that they also meet certain specification and performance criteria (BS EN 740:1999).19
The collecting system
1. A 30 mm male conical connector (labelled M in Fig. 18.4) that is fitted either to the expiratory port of a ventilator, the demand valve (for Entonox) or to the APL valve of a breathing system. The version that fits the APL valve shrouds all the exit apertures on the body of the valve enclosing them in a gas-tight fit.
2. A 30 mm female conical connector (labelled F in Fig. 18.4) that fits over the male connector to form a gas-tight fit and is attached to the patient end of the transfer system.
The collecting system may also house an overpressure relief valve, which is normally set to blow off at 1 kPa (10 cm H2O). This device (Fig. 18.5) prevents excessive pressure building up in the breathing system if the scavenging system becomes obstructed; for instance due to crushing or kinking of the transfer tubing.
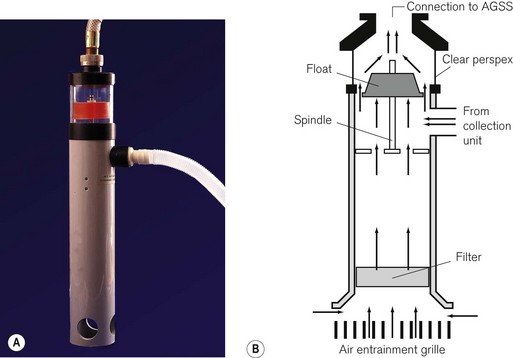
The receiving system
The receiving system (Fig. 18.6) consists of:
• a reservoir (normally a rigid material cylinder) for the expired and driving gasses, from which they are passed to the disposal system. This may also temporarily store this gas if the extraction rate falls below the necessary level
• an air break. The reservoir is open-ended at its base to allow entrainment of air when there is insufficient expired gas. This prevents the transmission of sub-atmospheric pressure from the scavenging system to the patient. It also provides an emergency escape route for the gas should the scavenging system fail
• a flow indicator to show that the unit is working when connected to an active disposal system. There is normally a clear Perspex window sited near the top of the reservoir in which a coloured float appears when the extraction rate is normal (i.e. 120 L min−1). When the flow drops below 80 L min−1 this disappears from view
• a filter sited in the base of the unit to prevent debris entering and blocking the system
• an entry port on the side and an exit port on the top of the container.
The receiving system is connected to the disposal system via a wide-bore hose that is sufficiently strong to prevent collapse from the sub-atmospheric pressure within it. The hose terminates in a probe, which houses a screw-fit connection to the disposal system socket (terminal unit). The latter has a valve that is normally closed, but opens when the male probe from the receiving unit is connected to it and screwed in (Fig. 18.7). The terminal unit may be sited on a wall or pendant.
The disposal system
Active disposal systems
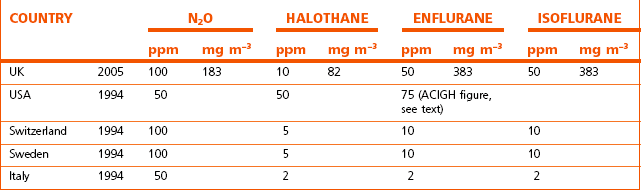
The sub-atmospheric pressure required to power the disposal system is usually provided by an exhauster unit (Fig. 18.8). This works in a similar fashion to a fan and requires a low level of maintenance and no lubrication. The size of the unit depends on the number of scavenging sites to be supplied. Large exhauster units can provide waste gas flow rates of up to 2400 L min−1, servicing 20 sites. Large sites often have a ‘duty’ and a ‘standby’ unit, which are linked. The standby unit operates automatically if the duty unit fails, as well as during periods of high demand. Although the exhauster unit is sited outside the operating theatre suite (sometimes a considerable distance away), the operating control switch is sometimes located within the theatre suite.
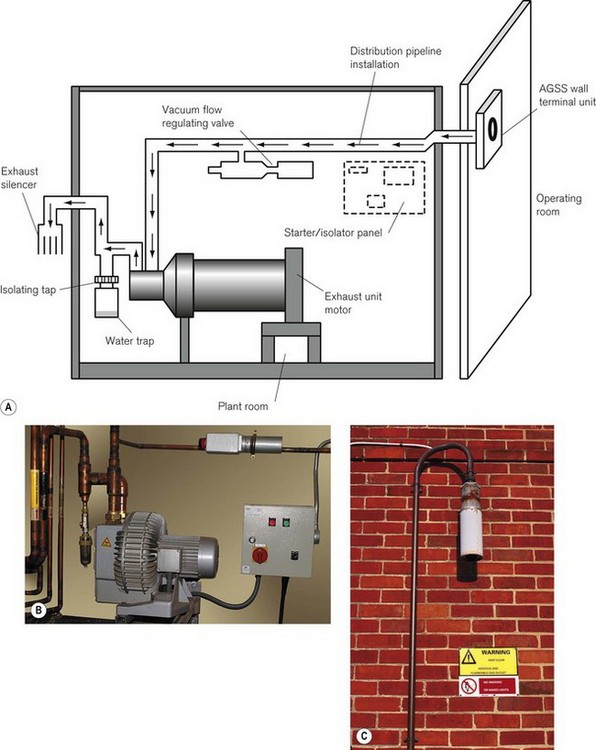
Figure 18.8 Active gas scavenging. A. Schematic diagram of plant room. B. The disposal unit in a plant room. C. Exhaust silencer.
Pressure fluctuations within the disposal system are controlled within precise limits by a vacuum/flow-regulating valve. It consists of an adjustable spring-loaded plate covering the valve aperture and behaves as an air entrainment valve should the vacuum exceed a predetermined level. This level is set, by adjusting the spring tension, during commissioning of the system, to provide the correct flow rates. Several valves may be fitted to large scavenging systems, so as to protect and control specific areas.
• Scavenging requires a high-flow system with a small pressure gradient between the terminal unit and the exhaust unit, whereas the piped vacuum in a hospital uses a lower flow with a larger pressure gradient between the vacuum pump and the terminal unit.
• The extra demand upon the medical vacuum for this purpose may result in other users being deprived of an adequate medical vacuum in an emergency.
• The displacement (flow rate) of the vacuum line may be inadequate to cope with the high flow rate and the pulsating nature of the output of some ventilators.
• The outlet of the vacuum system may be so located that the expired gasses would pollute areas where other personnel are working.
• More importantly, as vacuum lines do not contain safety valves (vacuum/flow-regulating valves, see above), there is a danger that an excessive ‘vacuum’ may be applied to a patient.
Collecting systems for scavenging in paediatric breathing systems are discussed in Chapter 12.
Passive disposal systems
In a passive system (Fig. 18.9), the receiver may house a 2 L neoprene reservoir bag, an inlet and outlet and two relief valves. One of these valves opens at 1 kPa (10 cm H2O) to prevent pressure build-up resulting from an obstruction in the scavenging system. The other valve can be opened if there is a sub-atmospheric pressure within the system greater than 50 Pa (0.5 cmH2O) caused by excessive suction from the ventile (see below).
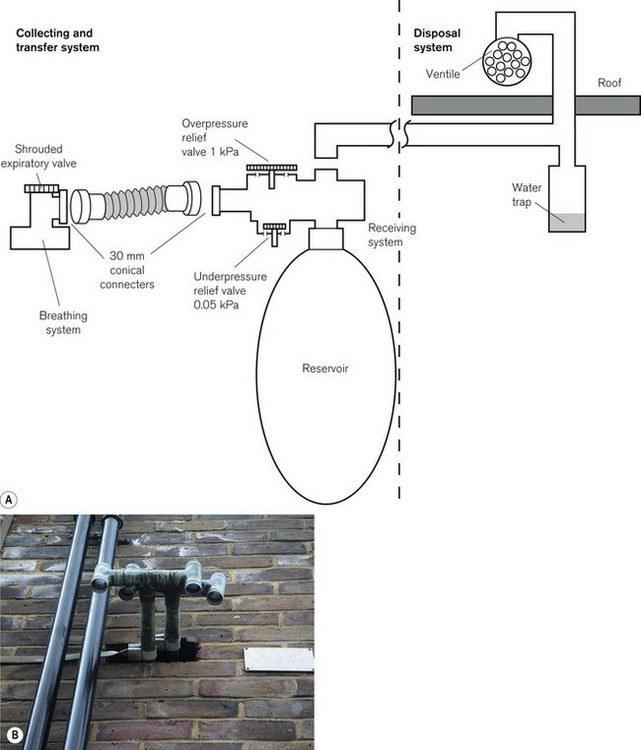
Figure 18.9 A. A passive scavenging system. B. A ventile for waste gas disposal emerging from the wall of an operating theatre suite.
The outlet from the receiver is connected to a wide-bore tube, which passes through one of the walls or the roof of the building and terminates in a ventile. A ventile is a device that uses the wind to entrain the exhaust gasses or air. Unfortunately, the passive system can be relied upon to operate satisfactorily only when the outlet is installed in a suitable position and when the wind is blowing from the desired quarter. It may be affected by the proximity of other buildings. Under adverse conditions, the flow may even be in the opposite direction. To prevent cross-contamination from one operating theatre to another, each point must have its own individual ventile. Cooling of the rising gas causes condensation and pooling, hence a water trap is essential. Alternatively, the wide-bore tubing from the receiver can be connected directly to a ‘hole in the wall’ (Fig. 18.10), although this is no longer advocated.
Absorption systems
Although such systems (Fig. 18.11) can remove the vapours of volatile anaesthetic agents from waste gasses, they do not absorb nitrous oxide and, therefore, cannot be recommended as a scavenging system that meets current standards. There are still, however, occasions where they may be appropriately used: for example with a low-flow breathing system where nitrous oxide is not employed and where scavenging is unavailable, as in developing countries and field hospitals (Fig. 27.10B).
Other devices
All the devices described are intended for use with adult breathing systems. Paediatric scavenging devices are described in Chapter 12. However, there are other situations where gaseous anaesthetic pollution can occur, notably in recovery rooms. Here, patients may continue to exhale anaesthetic agents postoperatively in close proximity to recovery room staff. The collecting systems described above cannot scavenge gas from many of the oxygen delivery devices often used on patients in these sites. Devices such as that shown in Fig. 18.12 are more suitable. A funnel attached to a wide-bore hose (which is then attached to a special active gas scavenging system) can be sited close to a patient’s face to remove pollutants. The funnel is supported by a series of levers. For efficacy, the device requires a calm patient lying under the optimal extraction zone of the device. In practice, an efficient non-recirculating air-conditioning system in these areas would be more appropriate.
1 Brown AC, Canosa-Mas CE, Parr AD, Pierce JM, Wayne RP. Tropospheric lifetimes of halogenated anaesthetics. Nature. 1989;341:635–637.
2 Halsey MJ. Occuaptional exposure to anaesthetics. Anaesthesia Rounds. Abingdon: The Medicine Group (Education) Ltd; 1996.
3 Albritton DL, Auchamp PJ, Mergie G, Watson T. Scientific assessment of ozone depletion: 1998, World Meteorological Organization Global Ozone Research and Monitoring Project Report No. 44. Geneva: WMO; 1999.
4 Langbein T, Sonntag H, Trapp D, Hoffmann A, Malms W, Röth EP, et al. Volatile anaesthetics and the atmosphere: atmospheric lifetimes and atmospheric effects of halothane, enflurane, isoflurane, desflurane and sevoflurane. Br J Anaesth. 1999;82:66–73.
5 Ravishankara AR, Daniel JS, Portmann RW. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science. 2009;326:123–125.
6 Vaisman AI. Working conditions in surgery and their effect on health of anesthesiologists. Eksperimental’naia Khirurgiia i Anesteziologiia. 1967;3:325–330.
7 Bruce DL, Bach MI. Effects of trace anaesthetic gasses on behavioural performance of volunteers. Br J Anaesth. 1976;48:871–876.
8 Guirguis SS, Pelmear PL, Roy ML, Wong L. Health effects associated with exposure to anaesthetic gasses in Ontario hospital personnel. Br J Ind Med. 1990;47:490–497.
9 Vouriot A, Gauchard GC, Chau N, Nadif R, Mur JM, Perrin PP. Chronic exposure to anesthetic gasses affects balance control in operating room personnel. Neurotoxicology. 2005;26:193–198.
10 Rowland AS, Baird DD, Weinberg CR, Shore DL, Shy CM, Wilcox AJ. Reduced fertility amongst women employed as dental assistants exposed to high levels of nitrous oxide. NEJM. 1992;327:993–997.
11 Deacon R, Perry J, Lumb M, Chanarin I, Minty B, Halsey MJ, et al. Selective inactivation of vitamin B12 in rats by nitrous oxide. Lancet. 1978;2:1023–1024.
12 Health and Safety Commission. Anaesthetic agents: controlling exposure under COSHH. Bristol: Health Services Advisory Committee; 1996.
13 Health and Safety Executive. Control of substances hazardous to health. 5th ed. The Control of Substances Hazardous to Health Regulations 2002 (as amended) Approved Code of Practice and Guidance. London: HSE Books; 2005.
14 Health and Safety Executive. Occupational Exposure Limits. Guidance Note EH40/96, 1996.
15 Health and Safety Executive. Workplace exposure limits. EH4O/2005, 2005.
16 Davenport HT, Halsey MJ, Wardley-Smith B. Bateman PE Occupational exposure to anaesthetics in 20 hospitals. Anaesthesia. 1980;35:354–359.
17 National Institute for Occupational Safety and Health. Criteria for a Recommended Standard: Occupational Exposure to Waste Gasses and Vapours, DHEW Publication No. (NIOSH) 77–140 Cincinnati. Ohio, USA: NIOSH; 1977.
18 Accorsi A, Barbieri A, Raffi GB, Violante FS. Biomonitoring of exposure to nitrous oxide, sevoflurane, isoflurane and halothane by automated GC/MS headspace urinalysis. Int Arch Occup Environ Health. 2001;74:541–548.
19 British Standards. Anaesthetic workstations and their modules. Particular requirements. BS EN 740:1999. London: British Standards Institution; 1999.