Chapter 38 Assisted Reproductive Technology: Clinical Aspects
INTRODUCTION
In vitro fertilization (IVF) is a remarkable scientific approach to the common clinical problem of infertility. The initial development of IVF in humans can be attributed directly to a team of two investigators, Drs. Patrick Steptoe and Robert Edwards. It was in 1969 that Dr. Edwards first announced, “Human oocytes have been matured and fertilized by spermatozoa in vitro. There may be certain clinical and scientific uses for human eggs fertilized by this procedure.”1 This understated conclusion marked the first successful attempt to fertilize human eggs in a laboratory.
Currently, more than 100,000 cycles of human IVF and similar techniques are performed each year in the United States, resulting in the birth of more than 40,000 babies. IVF, together with the much less commonly used techniques of gamete intrafallopian transfer (GIFT) and zygote intrafallopian transfer (ZIFT), are collectively referred to as assisted reproductive technologies (ART). Today, ART procedures are responsible for approximately 1% of all children born in the United States annually.2
Assisted Reproductive Technology Techniques
In Vitro Fertilization
In 1891, the first successful transfer of an embryo from one animal to another that resulted in birth was reported, using rabbits of two different strains. However, these embryos were obtained from eggs fertilized in vivo. Further progress toward the goal of IVF was slowed because of limited understanding of the maturation of eggs and sperm required to achieve fertilization and embryo development. In 1959, successful IVF was reported using rabbits.3
The first human birth to result from IVF was achieved in England in 1978.4 John and Lesley Brown had 9 years of infertility secondary to bilateral fallopian tube obstruction. Dr. Patrick Steptoe surgically retrieved a single mature oocyte from one of Lesley’s ovaries during a natural cycle. Dr. Robert G. Edwards combined John’s sperm with the oocyte in the laboratory and the resulting embryo was placed into Lesley’s uterus a few days later. On July 25, 1978, Louise Joy Brown was delivered by cesarean section at approximately 37 weeks’ gestation, and weighed 5 pounds 12 ounces.
Gamete Intrafallopian Transfer
Although early reports of GIFT indicated higher success rates than with IVF, improvements in the formulation of culture media and other laboratory techniques have resulted in equivalent success rates for GIFT and IVF.5,6 A disadvantage of GIFT is that it can only be performed in patients with normal fallopian tubes. Another disadvantage of GIFT compared to IVF is that it generally requires laparoscopy, with all the risks and expenses associated with this outpatient surgery. Currently, GIFT procedures account for less than 0.2% of ART treatments in the United States.
Zygote Intrafallopian Transfer
Although some studies comparing ZIFT to conventional IVF have found ZIFT to be superior, other studies have shown little difference.7,8 This uncertainty and the need for two surgical procedures for ZIFT (i.e., transvaginal egg retrieval and laparoscopic embryo transfer) have limited the popularity of this technique. However, ZIFT has found a place in the treatment of infertile women with congenital or acquired cervical abnormalities and in patients who fail to achieve pregnancy after repeated IVF cycles.9,10 Currently, ZIFT procedures account for approximately 0.5% of ART treatments in the United States.
INDICATIONS FOR ASSISTED REPRODUCTION
Tubal Factor Infertility
Tubal Adhesions
Tubal adhesions account for 30% to 40% of cases of female infertility. Tubal damage or adnexal adhesion typically arise from salpingitis, appendicitis, endometriosis, or previous pelvic surgery. Laparoscopic surgery to remove adhesions and open tubes can yield pregnancy rates of more than 50% in women with relatively mild disease.11 In women with severe tubal damage or extensive dense adhesions, surgery is unlikely to result in pregnancy. IVF is indicated in these patients and in women who have failed to conceive after infertility surgery.
Hydrosalpinges
Tubal surgery is also indicated in women with hydrosalpinges who are contemplating IVF. Hydrosalpinges are associated with decreased pregnancy and live birth rates after IVF.12 Although the pathophysiology of this relationship is not completely understood, bilateral salpingectomy improves the success of subsequent IVF, especially in women with bilateral hydrosalpinges.
Tubal Ligation
Up to 25% of women who have undergone bilateral tubal sterilization will come to regret their decision and wish to have more children. Risk factors for tubal ligation regret include young age at time of sterilization, relationship with a new partner, loss of a child, and having been sterilized immediately postpartum or postabortion. Up to 5% will seek tubal anastomosis, which is a highly effective treatment option in properly selected patients.13 Success rates depend on the site of the anastomosis, the length of residual tube, the age of the patient, and the presence of other infertility causes.
Endometriosis
Endometriosis is a common cause of both infertility and pain (see Chapter 49). The effects of endometriosis on fertility can be decreased but not completely circumvented by the combination of gonadotropin stimulation and intrauterine insemination.14 The decrease in monthly fecundibility roughly correlates with the severity of disease, although there is a poor correlation between endometriosis stage and the chance of pregnancy after surgical treatment.15
In vitro fertilization is an effective treatment for infertile women with endometriosis who fail to conceive with less aggressive treatment. Some, but not all, studies suggest that endometriosis affects IVF success. Endometriosis has been implicated in poor ovarian reserve, poor quality of oocytes and embryos, and poor implantation.16–21 Prolonged hormonal suppression using gonadotropin-releasing hormone (GnRH) analogues appears to improve IVF success in women with endometriosis.22
Male Factor Infertility
At least 40% of men who are members of infertile couples have abnormal semen analyses.23 Achieving pregnancy via conventional IVF in oligospermic men met with disappointing results largely due to fertilization failure.24 The treatment of male infertility dramatically improved with the development of intracytoplasmic sperm injection (ICSI).25 Injection of a single sperm into the egg appears to solve fertilization failure for the majority of male infertility problems. Currently, ICSI is used in about half of all ART treatment cycles. This technique is discussed in more detail in Chapter 39.
Antisperm Antibodies
Antisperm antibodies are a relatively uncommon and difficult to treat cause of infertility. IVF with ICSI has been found to be an effective treatment for women with antisperm antibodies, even in patients with a higher density of such antibodies.26 In one study, patients with antisperm antibodies had a 32% clinical pregnancy rate after IVF with ICSI.27 Because antisperm antibodies are relatively uncommon, it does not appear that routine screening for antisperm antibodies before IVF is cost-effective.28
Unexplained Infertility
Up to 30% of infertile couples will have unexplained infertility.29 Treatment options for unexplained infertility include ovarian stimulation with clomiphene citrate or gonadotropins, plus intrauterine insemination. IVF is an effective treatment for couples with unexplained infertility who fail to conceive with these approaches. The success of IVF in couples with unexplained infertility appears to be comparable to that achieved in cases of tubal damage or endometriosis.24
Failure to Conceive After Ovulation Induction
Anovulatory women who fail to conceive with ovulation induction are good candidates for IVF. Unfortunately, the pregnancy rates after IVF are lower and the complication rates higher for patients with polycystic ovary syndrome (PCOS). In one study of 110 women with PCOS who underwent IVF/ICSI, women with a body mass index (BMI) greater than 29 kg/m2 had a lower pregnancy rate per oocyte retrieval and a higher occurrence of ovarian hyperstimulation syndrome as compared to PCOS patients with a BMI of 29 kg/m2 or lower.30 Treatment with metformin to lower insulin levels in patients with PCOS undergoing IVF has been reported to improve success rates.31
Diethylstilbestrol Exposure
Women exposed in utero to diethylstilbestrol (DES) are known to have an increased risk of infertility.32 These women are also at increased risk of pregnancy complications as a result of reproductive tract abnormalities such as a T-shaped or hypoplastic cavity, a septate uterus, or uterine synechiae.33 IVF outcomes in DES-exposed women are comparable with respect to ovarian response and embryo quality, but delivery rates are lower, possibly due to uterine abnormalities.33
PATIENT SELECTION—PREDICTORS OF SUCCESS FOR IVF
In theory, only three things are needed to accomplish a successful IVF cycle: eggs, sperm, and a uterus into which the embryos are transferred. Although fertility testing is covered in Chapter 34 and Chapter 35, certain aspects of the evaluation specific to ART treatment follow.
Evaluation of the Uterus
Endometrial Polyps
Endometrial polyps, benign localized overgrowths of endometrial tissue of uncertain etiology, are present in up to 10% of asymptomatic premenopausal women over age 30.34 It is assumed that endometrial polyps decrease fertility. For this reason, all large polyps are removed prior to IVF.
For smaller polyps that first appear during ovarian stimulation for IVF, optimal management remains to be determined. The problem is that removal requires interruption of the cycle and delay of IVF. A series of 49 women with endometrial polyps less than 2 cm in diameter who underwent IVF without removing the polyp found pregnancy and miscarriage rates were comparable to the general pregnancy rate of their IVF clinic population, although there was a trend toward a higher miscarriage rate.35 At the present time, the decision to continue despite the polyp or stop the cycle and remove the polyp must be made on a case-by-case basis.
Hydrosalpinges
The presence of hydrosalpinges is well documented to decrease the success rate for IVF.36 Whether pregnancy rates can be improved by salpingectomy remains less certain. A recent meta-analysis of three randomized, controlled trials indicated that the chance of live birth with IVF was doubled by pretreatment salpingectomy.12 This effect seems to be most apparent in women with bilateral hydrosalpinges and with hydrosalpinges that are sonographically visible.37 Drainage of hydrosalpinges at the time of egg retrieval can be performed, but it is uncertain if this improves IVF pregnancy rates. At present, many IVF programs offer patients with a sonographically visible hydrosalpinx the option of pretreatment salpingectomy.
Evaluation of the Ovaries
Age
It has long been known that reproductive capacity declines with increasing age.38 The age-dependent decline in female fertility can be partly attributed to the fact that women have a finite and nonreplenishable number of germ cells. The peak number of germ cells occurs at midgestation during fetal life and declines continuously thereafter, with an accelerated loss of oocytes between ages 37 and 38.39 Diminished ovarian reserve is a term used to indicate decline in reproductive capacity associated with ovarian follicular depletion and diminished oocyte quality.
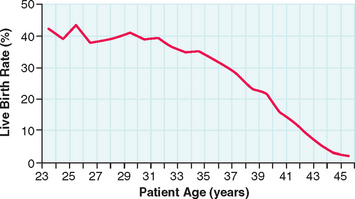
The success of IVF declines with age in a similar fashion (Fig. 38-1). For women undergoing IVF, diminished ovarian reserve is associated with poor ovarian response to gonadotropins, cycle cancellation, and lower chances of conception. Despite the known correlation with chronologic age and ovarian reserve, there exists a tremendous amount of variability in patients. As a result, multiple markers of ovarian reserve have been sought to supplement age as a predictor of ovarian response to stimulation in IVF. Timely recognition of reduced ovarian reserve is important for counseling patients prior to IVF.
Basal FSH
A direct correlation of the basal FSH and IVF outcome measurements was found in a study of 441 patients undergoing 758 consecutive IVF cycles.40 Patients with basal FSH levels greater than 25 mIU/mL had only a 3.6% ongoing pregnancy rate, whereas those with basal FSH levels less than 15 mIU/mL had a 17% pregnancy rate. They also noted that fewer follicles were aspirated, fewer oocytes were obtained, and fewer embryos were available for transfer in the high FSH group compared to the low FSH group.
Day 3 Estradiol
Serum estradiol levels on day 3 of the menstrual cycle have also been found to be predictive of subsequent IVF pregnancy rates. One study found that the ongoing pregnancy rates for patients with day 3 estradiol levels less than 30 pg/mL were significantly higher than for patients with estradiol levels between 31 and 75 pg/mL.41 No pregnancies occurred in patients with day 3 estradiol levels greater than 75 pg/mL. Another study found that the 97.5th percentile predictive value for day 3 estradiol for pregnancy was 56 pg/mL.
Basal elevations of estradiol are an independent marker of poor ovarian response to stimulation even in cycles without a rise in FSH. This is presumably because high circulating levels of serum estradiol suppress FSH levels. This hypothesis was confirmed in a study of 225 patients, where no pregnancies occurred after IVF with day 3 estradiol greater than 100 pg/mL, despite FSH levels less than 15 mIU/mL in all patients.42 In a study of 2476 IVF patients who had normal day 3 FSH levels, patients with day 3 estradiol levels either less than 20 pg/mL or greater than 80 pg/mL had an increased cancellation rate.43 However, estradiol levels are no longer predictive of IVF pregnancy rates once the patients had more than three maturing follicles.
Inhibin B
Elevation of FSH occurs in part as a result of diminished inhibin secretion by developing follicles. It follows that inhibin levels may also predict IVF success. Inhibin is a heterodimer that is secreted in two forms: inhibin A and inhibin B. In one IVF study, women with a day 3 serum inhibin B concentration less than 45 pg/mL had lower estradiol levels, fewer oocytes retrieved, a higher cycle cancellation rate, and a lower clinical pregnancy rate than women whose inhibin B concentration was at least 45 pg/mL.44 However, another study of 120 women undergoing IVF found that inhibin B levels did not improve the prediction of IVF success over using patient age and basal FSH level.
Antimüllerian Hormone
Antimüllerian hormone is produced by developing granulosa cells. A recent study of 130 women undergoing their first IVF cycle found that low antimüullerian hormone levels were associated with fewer eggs retrieved and increased chance of cycle cancellation.45 According to another study, basal serum antimüllerian hormone level may be a better predictor of IVF outcome than FSH, estradiol, or inhibin levels.46
Antral Follicle Count
The number of small follicles visible by transvaginal ultrasonography early in the follicular phase is another means used to predict ovarian response in IVF. A prospective IVF study of 130 women younger than age 45 indicated that the number of antral follicles on cycle day 3 provided better prognostic information regarding poor ovarian response during hormone stimulation for IVF than age or basal FSH, estradiol, or inhibin B levels. Another IVF study of 120 women also found that a single antral follicle count was predictive of poor ovarian response, although the predictive accuracy could be increased by repeating the count on a subsequent cycle and using the higher of the two counts.47 The exact number of antral follicles that predicts outcome is still uncertain.
Clomiphene Citrate Challenge Test
The CCCT decribed by Navot and colleagues is a method to dynamically elucidate the ovarian response.48 The test is performed by measuring basal FSH on day 3 (day 2 is acceptable), administering clomiphene citrate (100 mg, days 5 to 9), and then remeasuring a FSH on day 10 (days 9 and 11 are also acceptable). An abnormal test was originally defined as an FSH level after clomiphene citrate more than 2 standard deviations from the basal level. Many clinicians define an abnormal CCCT as an FSH value greater than 12 mIU/mL on either cycle day 3 or 10.
The CCCT has been shown to have a sensitivity of 43% and a specificity of 76%, using IVF cycle cancellation as an endpoint in a study of 198 women.49 Positive and negative predictive values were 37% and 80%, respectively. The estradiol levels during ovarian stimulation, the number of retrieved oocytes, and the rate of transfer cycles were significantly lower in patients with an abnormal CCCT. Forty-three percent of the abnormal test results were abnormal only on their elevation of day 10 or 11 FSH and not on their basal FSH level. However, the rate of pregnancies per started cycle did not show a statistically significant difference, which was attributed to the low numbers of patients.
A recent meta-analysis of a total of 1352 patients from 12 studies on basal FSH and 7 studies on CCCT found that basal FSH had a sensitivity of 6.6% and a specificity of 99.6% for identifying inability to achieve pregnancy in an IVF cycle, whereas CCCT sensitivity was 25.9% and specificity was 98.1%.50 This study suggests that basal FSH and CCCT are similar in the ability to predict a clinical pregnancy and that, although a normal test is not helpful, an abnormal test is highly predictive that pregnancy will not occur with IVF. Based on this, the authors recommended that basal FSH be used rather than CCCT because of its simplicity and lower cost.
Combining Screening Markers
It is apparent that there are clear limitations to each of the proposed markers for ovarian response to stimulation. Therefore, attempts have been made to combine some of these markers to improve the predictive ability compared to a single marker alone. When basal FSH and estradiol are combined for patients undergoing IVF cycles, one study reported no pregnancies in patients with basal FSH levels greater than 17 mIU/mL and basal estradiol level greater than 45 pg/mL.41 Another study of 74 IVF patients with basal FSH levels less than 15 mIU/mL examined the ratio of basal FSH to LH.51 This study found that an elevated FSH-to-LH ratio greater than 3.6 correlated with a lower day 8 estradiol, lower peak estradiol, and fewer follicles greater than 15 mm in size
A randomized study of 110 patients evaluated multiple markers for ovarian reserve, including the exogenous FSH ovarian reserve test. This test involves the administration of 300 IU of rFSH (Gonal-F) on cycle day 3 with serum measurements of FSH, estradiol, and inhibin B before and 24 hours after administration. They concluded that the exogenous FSH ovarian reserve test was better than the CCCT at predicting the number of large follicles resulting from controlled ovarian hyperstimulation.52
OVARIAN STIMULATION FOR IVF
Clomiphene Citrate
Wood and colleagues were the first to report that administration of clomiphene citrate followed by human chorionic gonadotropin (hCG) to complete oocyte maturation both increased IVF success rates and improved scheduling efficiency for egg retrieval.53 Clomiphene citrate was commonly used by early IVF programs in the United States until higher success rates were reported using human menopausal gonadotropins.
Clomiphene citrate is used at an oral dose of 50 to 150 mg/day for 5 days beginning on day 2 or 3 of the menstrual cycle, and hCG (5,000 to 10,000 units intramuscular) is given when the lead follicle reaches 18 mm in diameter. Although gonadotropins remain the standard ovarian stimulants for IVF, the convenience and low cost of clomiphene has caused it to be reconsidered as an option for good-prognosis IVF patients.54–56
Gonadotropins
The use of the injectable gonadotropin FSH, with or without LH, circumvents the natural decline of FSH that occurs with development of the dominant follicle. In effect this rescues oocytes that would be physiologically lost to atresia in a natural cycle, which selects only one dominant follicle. Initial reports in the United States using human menopausal gonadotropins (a combination of FSH and LH) for IVF were considered spectacular, with pregnancies achieved in 5 of 24 laparoscopic egg retrievals (21%), at a time when IVF pregnancy rates after other stimulation protocols were less than 10%.57 Although initial success likely was due in part to improvements in laboratory techniques and patient selection, gonadotropin injections soon became the standard treatment to prepare women for egg retrieval.
Human chorionic gonadotropin 5,000 to 10,000 units is typically used to mimic the LH surge and complete oocyte maturation in gonadotropin cycles. Egg retrieval is performed 34 to 36 hours after the hCG injection. Urinary and recombinant hCG products appear to give equivalent results.58
There has been much investigation and discussion of the relative merits of different gonadotropin preparations.59,60 Most programs in the United States have gravitated to some combination of FSH and LH for ovarian stimulation, along with a GnRH analogue. Recombinant and urinary FSH products seem to give equivalent pregnancy rates.61 Adjunctive stimulation with clomiphene in gonadotropin cycles, while lowering total gonadotropin doses required, has fallen out of favor because of the risk of spontaneous ovulation.
Gonadotropin-Releasing Hormone Agonists
In the early years of IVF, more than one quarter of stimulation cycles did not reach the stage of egg retrieval, primarily due to a premature LH surge.62 Long-term administration of GnRH agonists initially stimulates LH and FSH release, referred to as a flare or agonist phase. This is followed within 2 weeks by suppression of gonadotropin levels. This effect has been exploited for more than 20 years in IVF cycles.63 Initially this drug was reserved for patients who demonstrated a premature LH surge, but its popularity surged when high pregnancy rates were documented with routine use.64
In the United States, the most popular GnRH agonist is leuprolide acetate, given subcutaneously at doses of 0.25–1.0 mg/day. The routine use of GnRH agonists improves IVF success by reducing the rate of cycle cancellation, but the chance of pregnancy per embryo transfer is also increased, probably because more eggs are obtained, hence giving a larger selection of embryos for transfer.65
Flare Protocol
Alternatively, the agonist can be given in the early follicular phase along with or just before initiation of gonadotropins. Use of a GnRH agonist flare protocol reduces the gonadotropin dose required to achieve follicular development, and this feature is particularly appealing for patients who have a poor response to gonadotropins. However, pregnancy rates in poor responders using the long and short GnRH agonist protocols seem to be equivalent.66 One study found that routine use of the GnRH agonist flare protocol resulted in lower pregnancy rates compared to the long protocol, possibly because stimulation of LH during the follicular phase may impair normal oocyte maturation.67
Gonadotropin-Releasing Hormone Antagonists
Antagonists of GnRH have recently become commercially available for clinical use. Earlier antagonists were associated with severe histamine release with local and systemic side effects. Histamine release does not seem to be a problem with the newer antagonists, ganirelix and cetrorelix. The advantage of these drugs is the absence of a gonadotropin flare when the drugs are started in the follicular phase. A recent multicenter IVF trial compared the GnRH antagonist ganirelix acetate with a long protocol using a GnRH agonist, leuprolide.68 In this study, GnRH analogue administration was required for only 4 days on average using the GnRH antagonist compared to 19 days with the GnRH agonist. However, fewer eggs were obtained in the GnRH antagonist group.
The dose of GnRH antagonist administered affects IVF success. Excessive or insufficient suppression of LH and progesterone levels with GnRH antagonist decreases clinical pregnancy rates.69 In poor-responder IVF patients, a protocol using GnRH antagonist is associated with lower pregnancy rates than the GnRH agonist flare protocol.70 In a meta-analysis of five randomized, controlled IVF trials of GnRH antagonist versus GnRH agonist protocols, antagonist protocols were associated with a lower clinical pregnancy rate.71 It is possible that the lack of experience with the antagonists may explain this difference. Given the potential advantages of GnRH antagonists over agonists for IVF, protocols using these drugs will continue to be investigated.
Natural-Cycle IVF
IVF only became clinically useful after the development of methods to obtain mature eggs that could be reliably fertilized in vitro. Nevertheless, the complications and expense of ovarian stimulation are often treatment barriers for many women, especially for those with poor or exaggerated responses and those desiring egg retrieval to preserve fertility before cancer treatment. Although IVF programs have periodically reassessed the feasibility of natural-cycle IVF, the vast majority of IVF cycles continue to be performed in stimulated cycles because of higher efficiency and success rates.72–74 Recently, the possibility of harvesting multiple immature eggs in a spontaneous menstrual cycle has been proposed to overcome some of the limitations of natural-cycle IVF.
In Vitro Maturation
Mammalian oocytes are maintained in meiotic arrest throughout most of follicular development; the resumption of meiosis I is induced by the preovulatory LH surge, which is emulated during an IVF cycle by intramuscular administration of hCG. Although the precise mechanisms that regulate the control of oocyte maturation remain obscure, it has been recognized for more than 70 years that immature oocytes liberated from antral follicles undergo spontaneous maturation in culture, termed in vitro maturation, without the need for hormonal stimulation.75
Immature oocytes are typically obtained in the mid- to late follicular phase of the menstrual cycle. Administration of hCG 36 hours before egg collection appears to facilitate oocyte maturation.76 Because oocytes that have undergone in vitro maturation have a decreased IVF rate, ICSI is routinely performed on these oocytes. If embryos are transferred back to the patient in the same cycle, endometrial preparation with estradiol and progesterone is required.
To date, in vitro maturation has been most successfully employed in young women with multiple antral follicles, who typically have a high chance of pregnancy with conventional IVF. Despite this selection bias, IVF pregnancy rates are substantially lower than in stimulated cycles.77 However, in cancer patients, where the time and hormonal milieu associated with the traditional IVF cycle may adversely affect the patient’s survival, there may be some advantage to the in vitro maturation technique. Likewise, patients with PCOS who undergo ovarian hyperstimulation with ovulation induction agents may be candidates for in vitro maturation.
MONITORING OVARIAN STIMULATION
The goal of ovarian stimulation for IVF is to stimulate the development of multiple follicles containing mature oocytes. Improved timing for hCG administration and subsequent oocyte retrieval has been an important factor in the improvement of IVF pregnancy rates. Initially, timing for IVF was determined by measuring estradiol levels in urine or serum. Unfortunately, estradiol monitoring alone cannot distinguish between the development of a single preovulatory follicle or multiple immature follicles. Fortunately, follicle size does correlate with oocyte maturity. When ultrasonography, first abdominally and then vaginally, was used to monitor ovulation induction, IVF pregnancy rates improved. By the time IVF became widely available clinically, the use of vaginal sonography as an adjunct to gonadotropin stimulation was well established.
Endometrial Monitoring
Endometrial Thickness
Periovulatory endometrial thickness, determined by measuring both the anterior and posterior endometrium, has been shown to be greater in IVF cycles that resulted in conception.78 Studies suggest that pregnancy after IVF appears to be most likely in patients with an endometrial thickness of 9 to 12 mm on the day of hCG administration and may be reduced with endometrial thickness greater than this.
Endometrial Pattern
Smith and colleagues79 described several ultrasonic endometrial patterns developing during ovarian stimulation for IVF. Two periovulatory endometrial patterns can be consistently recognized by high-resolution sonography: (1) a hypoechoic pattern, usually with a trilaminar appearance, and (2) a homogeneous, hyperechoic pattern.79 Some studies have reported that the pregnancy rates of IVF patients with hyperechoic endometrium are lower than that obtained when a trilaminar or homogeneous hypoechoic pattern is observed, although other studies have failed to confirm this observation.
It has been speculated that the endometrial patterns seen by ultrasound are a reflection of changes in uterine blood flow. In a color flow Doppler study of 96 IVF cycles, 24% of women with hyperechoic endometrium had no subendometrial blood flow, compared to only 4% of women with a trilaminar endometrial pattern.80 In this study, none of eight patients with undetectable endometrial blood flow conceived. In a study of subendometrial blood flow index just prior to ovarian stimulation for IVF, Doppler sonography of the spiral or uterine arteries did not predict subsequent IVF cycle outcome.81
Treatment of Abnormal Endometrial Vascularity
Aspirin has been proposed as an adjunct to ovarian stimulation for IVF, with one report of improved ovarian and uterine blood flow and increased pregnancy rates with its administration during gonadotropin treatment.82 However, two large trials failed to confirm any of these beneficial effects.83,84
It has been hypothesized that uterine artery blood flow could be increased by the administration of sildenafil (Viagra), a type 5-specific phosphodiesterase inhibitor that relaxes vascular smooth muscle. Although a small IVF series suggested that the chances of pregnancy could be dramatically increased by administration of vaginal sildenafil before and during ovarian stimulation, a subsequent randomized, controlled trial failed to show any effect of sildenafil on endometrial thickness or uterine blood flow.85
Estradiol Monitoring
Estradiol monitoring during ovarian stimulation for IVF has not been found to be useful for timing of the hCG injection, but does appear useful in adjusting the dose of gonadotropin and for predicting the risk of ovarian hyperstimulation. Part of the art of IVF is knowing when to adjust gonadotropin levels up or down or cancel cycles based on number of follicles and estradiol levels. Although exact guidelines vary widely from center to center, many patients will cancel an IVF cycle if the estradiol levels do not reach 300 pg/mL by day 8 of stimulation. After this point, the dose of gonadotropin is usually not changed if the estradiol levels rise between 50% and 100% every 48 hours. The risk of severe hyperstimulation increases if the estradiol levels are greater than 3800 pg/mL at the time of hCG injection in women with polycystic ovaries or greater than 2400 pg/mL for women with hypothalamic amenorrhea.
OOCYTE RETRIEVAL
Retrieval Techniques
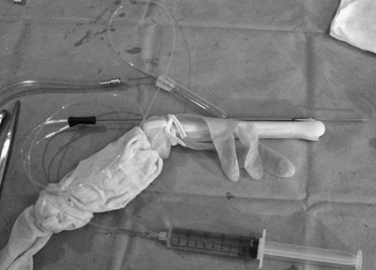
Transvaginal egg retrieval employs a needle guide mounted atop a high-frequency endovaginal ultrasound probe (Fig. 38-2). The room setup for a typical IVF oocyte retrieval procedure is quite minimal (Fig. 38-3). The patient is placed in the dorsal lithotomy position, and the perineum and vagina are irrigated with sterile saline solution. Some programs precede this with an antiseptic cleanser, although IVF outcomes may be compromised by antiseptic contamination.86 A broad-spectrum antibiotic is frequently given IV or by mouth before the egg retrieval; for example, oral doxycycline 100 mg daily for four days starting on the day of the retrieval. Pelvic infections are uncommon, but patients with endometriomas may be at higher risk despite the use of perioperative antibiotics.87
Anesthesia
A variety of anesthetic techniques have been reported for transvaginal egg retrievals, including local, epidural, spinal, or general anesthetics, but many programs use IV sedation/analgesia.88 Typically, a short-acting analgesic such as fentanyl is used in conjunction with a benzodiazepine or propofol.89 Supplemental oxygen may be administered by mask or nasal cannula as needed. No clear relationship has been confirmed between IVF outcome and the choice of anesthestic technique for transvaginal egg retrieval, although general anesthesia has been reported to decrease IVF pregnancy rates.88
EMBRYO TRANSFER
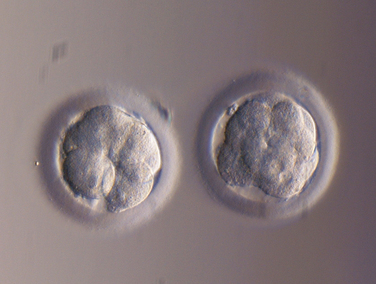
The critical part between oocyte retrieval and embryo transfer occurs in an embryology laboratory (see Chapter 39). The patient is called the day after the retrieval and told how many oocytes fertilized (Fig. 38-4). Embryos may be transferred into the uterus anytime during preimplantation development. The first successful IVF pregnancy occurred after transfer of a single blastocyst, but clinicians unable to duplicate this success turned to the transfer of embryos on day 2 or 3 after retrieval to overcome the limitations of their culture systems. At present, most IVF programs in the United States transfer embryos on day 3 after oocyte retrieval, but embryo transfers done on day 2 appear to give comparable results2,90 (Fig. 38-5). Pregnancy can occur when the fertilized egg is transferred to the uterus 1 day after fertilization.
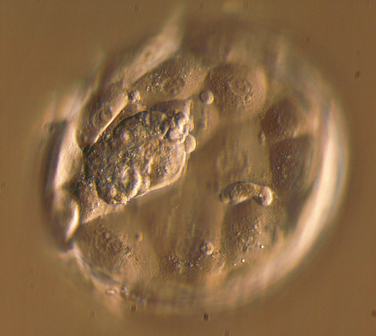
With the increasing availability of high-quality commercially produced IVF culture media, the transfer of one or two embryos on day 5 or 6 after fertilization (at the blastocyst stage) has been proposed as a way of minimizing the risk of high-order multiple pregnancy while maintaining satisfactory pregnancy rates. Blastocyst transfer has several potential advantages: (1) delaying the embryo transfer to day 5 or 6 after fertilization allows for more detailed examination of embryo morphology (Fig. 38-6); (2) the embryonic genome is activated after about 2 days of development, and prolonged culture facilitates selection of the most robust embryos; (3) preimplantation embryos do not reach the uterine cavity until day 4 or 5 in vivo.91 The uterus provides a different nutritional milieu from the oviduct, and it has been postulated that the transfer of day 2 or 3 embryos to the uterine cavity may reduce their potential for implantation.92
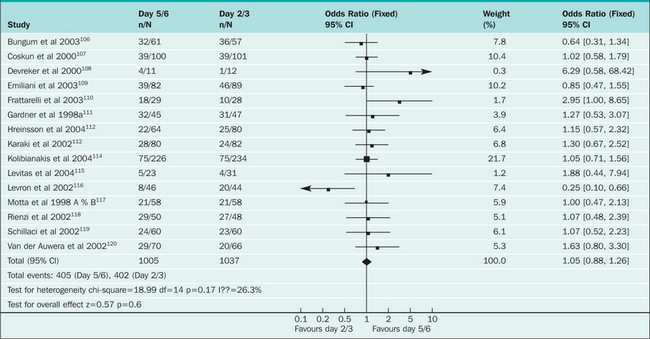
However, prolonged culture of embryos may decrease their capacity to develop and implant. Depending on embryo quality and culture conditions, in some IVF cycles few or no embryos may reach the blastocyst stage, resulting in few or no embryos to transfer or cryopreserve.93 A recent meta-analysis of 16 randomized, controlled trials of day 2 or 3 versus day 5 or 6 embryo transfer found no significant differences in the rates of pregnancy, birth, multiple gestation, or high-order multiple gestation94 (Table 38-1). Rates of embryo freezing per couple were significantly higher in day 2 or 3 transfers, and patients randomized to day 5 or 6 transfer were three times more likely to have no embryos to transfer. The results were similar in studies in which only patients with a favorable prognosis were enrolled. In studies where cumulative pregnancy rates for both fresh and frozen embryos were specified, pregnancies were more likely to occur in the day 2 or 3 group.
Table 38-1 Meta-analysis Results of Cleavage Stage Versus Blastocyst Transfer: Effect on Clinical Pregnancy Rates (Blake 2005)94
 |
Balancing the risks and benefits of multiple-embryo transfer remains one of the most vexing problems of ART. The transfer of more than one embryo increases the chance of pregnancy but also increases the risk of multiple gestations. The Society for Assisted Reproductive Technology recommends that no more than two embryos be transferred to women under age 35 who have a favorable prognosis for pregnancy.95 A recent randomized, controlled trial of elective single-embryo versus double-embryo transfer suggested that comparable pregnancy rates could be achieved and multiple pregnancies avoided in young women with good embryo quality if embryo cryopreservation was routinely used for the second embryo.96
Embryo Transfer Technique
Embryo transfers are performed in the dorsal lithotomy position. A speculum is inserted into the vagina, and the cervix is cleansed with culture medium or sterile saline solution. A trial transfer using an empty catheter is performed if not previously done, and the embryos are then transferred in a small volume (5 to 20 μL) of culture medium. A variety of embryo transfer catheter types are available, including rigid or soft, side- or end-loading, with or without an introducer. The chance of pregnancy with IVF is maximized if embryos are accurately and atraumatically placed within the uterus.
Ultrasound guidance for embryo transfer was first reported by Strickler and colleagues in 1985.97 Since then, a number of retrospective studies have shown favorable effects on pregnancy rates when ultrasound was used to facilitate placement of the transfer in the uterine cavity. Recently, a randomized, controlled trial of ultrasound-assisted embryo transfer found that 50% of patients conceived in the ultrasound-guidance group, compared with only 34% of controls.98
LUTEAL PHASE MANAGEMENT
The ability to achieve pregnancy in ART cycles is impaired unless hormone supplementation is administered. Aspiration of granulosa cells during oocyte retrieval impairs corpus luteum function, as does the use of GnRH agonists, which limit the secretion of LH in the luteal phase. The result can be an iatrogenic luteal phase defect.99
Luteal phase support with hCG or progesterone after IVF results in an increased pregnancy rate. The use of hCG has not been shown to be better than intramuscular progesterone, but is associated with a greater risk of ovarian hyperstimulation syndrome. Progesterone can be given orally, intravaginally, or intramuscularly. The optimal route of progesterone administration has not yet been established, but pregnancy rates may be higher with intramuscular administration.100
Many IVF programs still administer intramuscular progesterone in oil 50 mg/day, beginning on the day of the egg retrieval. In patients who conceive, progesterone supplementation continues until a fetal heartbeat is seen on ultrasound. This might not be necessary based on a randomized, controlled trial of more than 300 IVF cycles that did not show a difference in the rate of successful pregnancies between women who continued progesterone in early pregnancy compared with those who stopped treatment after the first positive pregnancy test.101
SUCCESS OF ASSISTED REPRODUCTIVE PROCEDURES
Choosing the denominator for calculating success rates is also problematic. Most commonly, all initiated cycles are included. However, statistics are sometimes calculated using only cycles that have progressed to retrieval or those that have resulted in embryo transfer. The most meaningful calculations are made using the total number of initiated cycles, but using only cycles in which oocytes are retrieveds or embryos are transferred is more likely to reflect the quality of the IVF laboratory.
Less than 30% of all “fresh” IVF cycles (i.e., cycles using the patient’s own nonfrozen embryos) result in a live birth. If a woman does not conceive in her first IVF attempt but has a normal response to ovarian stimulation, her chance of subsequent IVF success decreases by 2% to 5% in each subsequent cycle.2,102,103
The success rates for IVF are highly dependent on the age of the woman, with birth rates declining from about 40% per initiated cycle in women younger than age 23 to about 15% at age 40.2 This decline in success is almost entirely due to increasing age of the eggs, because IVF success using donor oocytes is independent of the recipient’s age.
About 30% of ART births are twins, and 3% are triplets or higher-order multiples. The incidence of high-order multiple births from IVF has declined significantly over the past decade.2 The success of alternative ART procedures, such as GIFT or ZIFT, is about the same as for IVF, but these procedures currently account for less than 5% of ART cycles in the United States.
OVUM DONATION
The Recipient
The recipient’s uterus must be primed and the endometrium prepared to receive the fertilized embryos, which requires significant coordination. Most centers will take their recipients through a “mock” cycle before the donor cycle to ensure that adequate endometrial thickness can be achieved. These cycles often include endometrial biopsies to demonstrate a histologic response to exogenous hormones.104
Success Rates
The use of donor oocytes for IVF consistently results in high pregnancy rates when young healthy fertile women donated their oocytes, with pregnancy rates from 51% to 58% per IVF cycle.105 The pregnancy rates did not differ significantly according to the number of previous donated cycles or the interval between donation cycles.
1 Edwards RG, Bavister BD, Steptoe PC. Early stages of fertilization in vitro of human oocytes matured in vitro. Nature (London). 1969;221:632-635.
2 Centers for Disease Control and Prevention. 2003 Assisted Reproductive Technology Success Rates. Available at http://www.cdc.gov/reproductivehealth/ART/index.htm. Accessed 17 September 2005.
3 Chang MC. Fertilisation of rabbit ova in vitro. Nature (London). 1952;179:466-467.
4 Australian Broadcasting Corporation. Interview with Roberts Edwards. The Health Report. Available at http://www.abc.net.au/rn/talks/8.30/helthrpt/stories/s1349685.htm, 25 April 2005. Accessed on 18 September 2005.
5 Sayama M, Araki S, Motoyama M, et al. The clinical efficacy of gamete intrafallopian transfer by minilaparotomy versus in vitro fertilization and embryo transfer. J Obstet Gynaecol Res. 1996;22:409-416.
6 Pandian Z, Bhattacharya S, Nikolaou D, et al. The effectiveness of IVF in unexplained infertility: A systematic Cochrane review. Hum Reprod. 2003;18:2001-2007.
7 Van Voorhis BJ, Syrop CH, Vincent RD, et al. Tubal versus uterine transfer of cryopreserved embryos: A prospective randomized trial. Fertil Steril. 1995;63:578-583.
8 Levran D, Mashiach S, Dor J, et al. Zygote intrafallopian transfer may improve pregnancy rate in patients with repeated failure of implantation. Fertil Steril. 1998;69:26-30.
9 Fluker MR, Bebbington MW, Munro MG. Successful pregnancy following zygote intrafallopian transfer for congenital cervical hypoplasia. Obstet Gynecol. 1994;84:659-661.
10 Levran D, Farhi J, Nahum H, et al. Prospective evaluation of blastocyst stage transfer versus zygote intrafallopian tube transfer in patients with repeated implantation failure. Fertil Steril. 2002;77:971-977.
11 Valle RF. Tubal cannulation. Obstet Gynecol Clin North Am. 1995;22:519-540.
12 Johnson NP, Mak W, Sowter MC. Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. Cochrane Database Syst Rev. 2004. CD002125.pub2.
13 Hanafi MM. Factors affecting the pregnancy rate after microsurgical reversal of tubal ligation. Fertil Steril. 2003;80:434-440.
14 Hughes EG. The effectiveness of ovulation induction and intrauterine insemination in the treatment of persistent infertility: A meta-analysis. Hum Reprod. 1997;12:1865-1872.
15 Guzick DS, Silliman NP, Adamson GD, et al. Prediction of pregnancy in infertile women based on the American Society for Reproductive Medicine’s revised classification of endometriosis. Fertil Steril. 1997;67:822-829.
16 Olivennes F, Feldberg D, Liu HC, et al. Endometriosis: A stage by stage analysis— role of in vitro fertilization. Fertil Steril. 1995;64:392-398.
17 Pellicer A, Oliveira N, Ruiz A, et al. Exploring the mechanism(s) of endometriosis related infertility: An analysis of embryo development and implantation in assisted reproduction. Hum Reprod. 1995;10:91-97.
18 Brizek CL, Schlaff S, Pellegrini VA, et al. Increased incidence of aberrant morphological phenotypes in human embryogenesis: An association with endometriosis. J Assist Reprod Genet. 1995;12:106-112.
19 Tinkanen H, Kujansuu E. In vitro fertilization in patients with ovarian endometriomas. Acta Obstet Gynecol Scand. 2000;79:119-122.
20 Diaz I, Navarro J, Blasco L, et al. Impact of stage III—IV endometriosis on recipients of sibling oocytes: Matched case control study. Fertil Steril. 2000;74:31-34.
21 Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77:1148-1155.
22 Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility. Fertil Steril. 2004;82(Suppl 1):S40-S45.
23 Schlegel PN, Girardi SK. Clinical review 87: In vitro fertilization for male factor infertility. J Clin Endocrinol Metab. 1997;82:709-716.
24 Guzick DS, Wilkes C, Jones HWJr. Cumulative pregnancy rates for in vitro fertilization. Fertil Steril. 1986;46:663-667.
25 Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:7-8.
26 Nagy ZP, Verheyen G, Liu J, et al. Results of 55 intracytoplasmic sperm injection cycles in the treatment of male-immunological infertility. Hum Reprod. 1995;10:1775-1780.
27 Lahteenmaki A, Rasanen I, Hovatta O. Low dose prednisolone does not improve the outcome of in vitro fertilization in male immunologic infertility. Hum Reprod. 1995;10:3124-3129.
28 Culligan PJ, Crane MM, Boone WR, et al. Validity and cost-effectiveness of antisperm antibody testing before in vitro fertilization. Fertil Steril. 1998;69:894-898.
29 Crosignani PG, Collins J, Cooke ID, et al. Recommendations of the ESHRE workshop “Unexplained Infertility.”. Hum Reprod. 1993;8:977-980.
30 Kolibiankis E, Zikopoulos K, Albano C, et al. Reproductive outcome of polycystic ovarian syndrome patients treated with GnRH antagonists and recombinant FSH for IVF/ICSI. Reprod Biomed Online. 2003;7:313-318.
31 Stadtmauer LA, Toma SK, Riehl RM, Talbert LM. Impact of metformin therapy on ovarian stimulation and outcome in “coasted” patients with polycystic ovary syndrome undergoing in vitro fertilization. Reprod Biomed Online. 2002;5:112-116.
32 Palmer JR, Hatch EE, Rao RS, et al. Infertility among women exposed prenatally to diethylstilbestrol. Am J Epidemiol. 2001;154:316-321.
33 Kerjean A, Poirot C, Epelboin S, Jouannet P. Effect of in utero diethylstilboestrol exposure on human oocyte quality and fertilization in a programme of in vitro fertilization. Hum Reprod. 1999;14:1578-1581.
34 Clevenger-Hoeft M, Syrop CH, Stovall DW, Van Voorhis BJ. Sonohysterography in premenopausal women with and without abnormal bleeding. Obstet Gynecol. 1999;94:516-520.
35 Lass A, Williams G, Abusheikha N, Brinsden P. The effect of endometrial polyps on outcomes of in vitro fertilization (IVF) cycles. J Assisted Reprod Genet. 1999;16:410-415.
36 The influence of hydrosalpinx on IVF and embryo transfer. A review. Hum Reprod Update. 2000;6:387-395.
37 Strandell A, Lindhard A, Waldenstrom U, Thorburn J. Hydrosalpinx and IVF outcome: Cumulative results after salpingectomy in a randomized controlled trial. Hum Reprod. 2001;16:2403-2410.
38 Tietze C. Reproductive span and rate of reproduction among Hutterite women. Fertil Steril. 1957;8:89-97.
39 Faddy MJ, Gosden RG, Gougeon A, et al. Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum Reprod. 1992;7:1342-1346.
40 Scott RT, Toner JP, Muasher SJ, et al. Follicle-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651-654.
41 Licciardi FL, Liu H-C, Rosenwaks Z. Day 3 estradiol serum concentrations as prognosticators of stimulation response and pregnancy outcome in patients undergoing in vitro fertilization. Fertil Steril. 1995;64:991-994.
42 Smotrich D, Widra E, Gindoff P, et al. Prognostic value of day 3 estradiol on in vitro fertilization outcome. Fertil Steril. 1995;64:1136-1140.
43 Frattarelli JL, Bergh PA, Drews MR, et al. Evaluation of basal estradiol levels in assisted reproductive technology cycles. Fertil Steril. 2000;74:518-524.
44 Seifer DB, Lambert-Masserlian B, Hogan JW, et al. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67:110-114.
45 van Rooij IA, Broekmans FJ, te Velde ER, et al. Serum anti-Müllerian hormone levels: A novel measure of ovarian reserve. Hum Reprod. 2002;17:3065-3071.
46 Hazout A, Bouchard P, Seifer DB, et al. Serum antimüllerian hormone/müllerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004;82:1323-1329.
47 Bancsi LF, Broekmans FJ, Looman CW, et al. Impact of repeated antral follicle counts on the prediction of poor ovarian response in women undergoing in vitro fertilization. Fertil Steril. 2004;81:35-72.
48 Navot D, Rosenwaks Z, Margalioth EJ. Prognostic assessment of female fecundity. Lancet. 1987;1:645-647.
49 Kahraman S, Vicdan K, Isik A, et al. Clomiphene citrate challenge test in the assessment of ovarian reserve before controlled ovarian hyperstimulation for intracytoplasmic sperm injection. Eur J Obstet Gynecol Reprod Biol. 1997;73:177-182.
50 Jain T, Soules M, Collins J. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 2004;82:180-185.
51 Mukherjee T, Cooperman AB, Sandler B, et al. An elevated day three follicle-stimulating hormone:luteinizing hormone ratio (FSH:LH) in the presence of a normal day 3 FSH predicts a poor response to controlled ovarian hyperstimulation. Fertil Steril. 1996;65:588-593.
52 Kwee J, Elting MW, Schats R, et al. Comparison of endocrine tests with respect to their predictive value on the outcome of ovarian hyperstimulation in IVF treatment: Results of a prospective randomized study. Hum Reprod. 2003;18:1422-1427.
53 Wood C, Trounson A, Leeton J, et al. A clinical assessment of nine pregnancies obtained by in vitro fertilization and embryo transfer. Fertil Steril. 1981;35:502-508.
54 Steinkampf MP, Kretzer PA, McElroy E, Conway-Myers BA. A simplified approach to in vitro fertilization. J Reprod Med. 1992;37:199-204.
55 Corfman RS, Milad MP, Bellavance TL, et al. A novel ovarian stimulation protocol for use with the assisted reproductive technologies. Fertil Steril. 1993;60:864-870.
56 Hurd WW, Randolph JFJr, Christman GM, et al. Luteal support with both estradiol and progesterone after clomiphene citrate stimulation for in vitro fertilization. Fertil Steril. 1996;66:587-592.
57 Jones HWJr, Jones GS, Andrews MC, et al. The program for in vitro fertilization at Norfolk. Fertil Steril. 1982;38:14-21.
58 Al-Inany HG, Aboulghar M, Mansour R, Proctor M. Recombinant versus urinary human chorionic gonadotrophin for ovulation induction in assisted conception. Cochrane Database Syst Rev. 2005. CD003719.
59 Gleicher N, Vietzke M, Vidali A. Bye-bye urinary gonadotrophins? Recombinant FSH: A real progress in ovulation induction and IVF? Hum Reprod. 2003;18:476-482.
60 Lunenfeld B. Historical perspectives in gonadotrophin therapy. Hum Reprod Update. 2004;10:453-467.
61 Al-Inany H, Aboulghar M, Mansour R, Serour G. Meta-analysis of recombinant versus urinary-derived FSH: An update. Hum Reprod. 2003;18:305-313.
62 In vitro fertilization/embryo transfer in the United States. 1987 results from the National IVF-ET Registry. Fertil Steril. 1989;51:13-19.
63 Porter RN, Smith W, Craft IL, et al. Induction of ovulation for in-vitro fertilisation using buserelin and gonadotropins. Lancet. 1984;2:284-285.
64 Meldrum DR, Wisot A, Hamilton F, et al. Routine pituitary suppression with leuprolide before ovarian stimulation for oocyte retrieval. Fertil Steril l. 1989;51:455-459.
65 Hughes EG, Fedorkow DM, Daya S, et al. The routine use of gonadotropin-releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: A meta-analysis of randomized controlled trials. Fertil Steril. 1992;58:888-896.
66 Confino E, Zhang X, Kazer RR. GnRHa flare and IVF pregnancy rates. Int J Gynaecol Obstet. 2004;85:36-39.
67 Cramer DW, Powers DR, Oskowitz SP, et al. Gonadotropin-releasing hormone agonist use in assisted reproduction cycles: The influence of long and short regimens on pregnancy rates. Fertil Steril. 1999;72:83-89.
68 Barmat LI, Chantilis SJ, Hurst BS, Dickey RP. A randomized prospective trial comparing gonadotropin-releasing hormone (GnRH) antagonist/recombinant follicle-stimulating hormone (rFSH) versus GnRH-agonist/rFSH in women pretreated with oral contraceptives before in vitro fertilization. Fertil Steril. 2005;83:321-330.
69 Huirne JA, van Loenen AC, Schats R, et al. Dose-finding study of daily GnRH antagonist for the prevention of premature LH surges in IVF/ICSI patients: Optimal changes in LH and progesterone for clinical pregnancy. Hum Reprod. 2005;20:359-367.
70 Mohamed KA, Davies WA, Allsopp J, Lashen H. Agonist “flare-up” versus antagonist in the management of poor responders undergoing in vitro fertilization treatment. Fertil Steril. 2005;83:331-335.
71 Al-Inany H, Aboulghar M. GnRH antagonist in assisted reproduction: A Cochrane review. Hum Reprod. 2002;17:874-885.
72 Paulson RJ, Sauer MV, Francis MM, et al. In vitro fertilization in unstimulated cycles: The University of Southern California experience. Fertil Steril. 1992;57:290-293.
73 Taymor ML, Ranoux CF, Gross GL. Natural oocyte retrieval with intravaginal fertilization: A simplified approach to in vitro fertilization. Obstet Gynecol. 1992;80:888-891.
74 Elizur SE, Aslan D, Shulman A, et al. Modified natural cycle using GnRH antagonist can be an optional treatment in poor responders undergoing IVF. J Assisted Reprod Genet. 2005;22:75-79.
75 Pincus G, Enzmann EV. The comparative behaviour of mammalian egg in vivo and in vitro. J Exper Med. 1935;62:655-675.
76 Chian RC, Gulekli B, Buckett WM, Tan SL. Priming with human chorionic gonadotropin before retrieval of immature oocytes in women with infertility due to the polycystic ovary syndrome. NEJM. 1999;341:1624-1626.
77 Mikkelsen AL. Strategies in human in vitro maturation and their clinical outcome. Reprod Biomed Online. 2005;10:593-599.
78 Glissant A, de Mouzon J, Frydman R. Ultrasound study of the endometrium during in vitro fertilization cycles. Fertil Steril. 1985;44:786-790.
79 Smith B, Porter R, Ahuja K, Craft I. Ultrasonic assessment of endometrial changes in stimulated cycles in an in vitro fertilization and embryo transfer. J In Vitro Fertil Embryo Transfer. 1984;1:233-239.
80 Zaidi J, Campbell S, Pittrof R, Tan SL. Endometrial thickness, morphology, vascular penetration and velocimetry in predicting implantation in an in vitro fertilization program. Ultrasound Obstet Gynecol. 1995;6:191-198.
81 Schild RL, Holthaus S, d’Alquen J, et al. Quantitative assessment of subendometrial blood flow by three-dimensional-ultrasound is an important predictive factor of implantation in an in-vitro fertilization programme. Hum Reprod. 2000;15:89-94.
82 Rubinstein M, Marazzi A, Polak de Fried E. Low-dose aspirin treatment improves ovarian responsiveness, uterine and ovarian blood flow velocity, implantation, and pregnancy rates in patients undergoing in vitro fertilization: A prospective, randomized, double-blind placebo-controlled assay. Fertil Steril. 1999;71:825-829.
83 Lok IH, Yip SK, Cheung LP, et al. Adjuvant low-dose aspirin therapy in poor responders undergoing in vitro fertilization: A prospective, randomized, double-blind, placebo-controlled trial. Fertil Steril. 2004;81:556-561.
84 Pakkila M, Rasanen J, Heinonen S, et al. Low-dose aspirin does not improve ovarian responsiveness or pregnancy rate in IVF and ICSI patients: A randomized, placebo-controlled double-blind study. Hum Reprod. 2005;20:2211-2214.
85 Check JH, Graziano V, Lee G, et al. Neither sildenafil nor vaginal estradiol improves endometrial thickness in women with thin endometria after taking oral estradiol in graduating dosages. Clin Exper Obstet Gynecol. 2004;31:99-102.
86 van Os HC, Roozenburg BJ, Janssen-Caspers HA, et al. Vaginal disinfection with povidone iodine and the outcome of in vitro fertilization. Hum Reprod. 1992;7:349-350.
87 Younis JS, Ezra Y, Laufer N, Ohel G. Late manifestation of pelvic abscess following oocyte retrieval, for in vitro fertilization, in patients with severe endometriosis and ovarian endometriomata. J Assist Reprod Genet. 1997;14:343-346.
88 Ditkoff EC, Plumb J, Selick A, Sauer MV. Anesthesia practices in the United States common to in vitro fertilization (IVF) centers. J Assisted Reprod Genet. 1997;14:145-147.
89 Hadimioglu N, Aydogdu Titiz T, Dosemeci L, Erman M. Comparison of various sedation regimens for transvaginal oocyte retrieval. Fertil Steril. 2002;78:648-649.
90 Oatway C, Gunby J, Daya S. Day three versus day two embryo transfer following in vitro fertilization or intracytoplasmic sperm injection. Cochrane Database Syst Rev. 2004. CD004378.
91 Croxatto HB, Fuentaealba B, Diaz S, et al. A simple nonsurgical technique to obtain unimplanted eggs from human uteri. Am J Obstet Gynecol. 1972;112:662-668.
92 Gardner DK, Lane M, Calderon I, Leeton J. Environment of the preimplantation human embryo in vivo: Metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cell. Fertil Steril. 1996;65:349-353.
93 Tsirgotis M. Blastocyst stage transfer: Pitfalls and benefits—too soon to abandon practice? Hum Reprod. 1998;13:3285-3295.
94 Blake D, Proctor M, Johnson N, Olive D. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev. 2005. CD002118.pub2.
95 The Practice Committee of the Society for Assisted Reproductive Technology, the American Society for Reproductive Medicine. Guidelines on the number of embryos transferred. Fertil Steril. 2004;82:773-774.
96 Thurin A, Hausken J, Hillensjo T, et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. NEJM. 2004;351:2392-2402.
97 Strickler RC, Christianson C, Crane JP, et al. Ultrasound guidance for human embryo transfer. Fertil Steril. 1985;43:54-61.
98 Coroleu B, Carreras O, Veiga A, et al. Embryo transfer under ultrasound guidance improves pregnancy rates after in vitro fertilization. Hum Reprod. 2000;15:616-620.
99 Macklon NS, Fauser BCJM. Impact of ovarian hyperstimulation on the luteal phase. J Reprod Fertil. 2000;55(Suppl):101-108.
100 Daya S, Gunby J. Luteal phase support in assisted reproduction cycles. Cochrane Database Syst Rev. 2004. CD004830.
101 Nyboe Andersen A, Popovic-Todorovic B, Schmidt KT, et al. Progesterone supplementation during early gestations after IVF or ICSI has no effect on the delivery rates: A randomized controlled trial. Hum Reprod. 2002;17:357-361.
102 Meldrum DR, Silverberg KM, Bustillo M, Stokes S. Success rate with repeated cycles of in vitro fertilization–embryo transfer. Fertil Steril. 1998;69:1005-1009.
103 Silberstein T, Trimarchi JR, Gonzalez L, et al. Pregnancy outcome in in vitro fertilization decreases to a plateau with repeated cycles. Fertil Steril. 2005;84:1043-1045.
104 Sauer MV, Paulson RJ, Moyer DL. Assessing the importance of endometrial biopsy prior to oocyte donation. J Assist Reprod Genet. 1997;14:125.
105 Opsahl MS, Blauer KL, Black SH, et al. Pregnancy rates in sequential in vitro fertilization cycles by oocyte donors. Obstet Gynecol. 2001;97:201.
106 Bungum M, Bungum L, Humaidan P, et al. Day 3 versus day 5 embryo transfer: A prospective randomized study. Reprod BioMed Online. April 2003;7:98-104.
107 Coskum S, Hollanders J, Al-Hassan S, et al. Day 5 versus day 3 embryo transfer: A controlled randomized trial. Hum Reprod. 2000;15:1947-1952.
108 Devreker F, Delbaere A, Emiliani S, et al: Prospective and randomized comparison between transfer on day 2 or day 5 for patients with more than four IVF attempts. ESHRE P135, 2000.
109 Emiliani S, Delbaere A, Vannin A, et al. Similar delivery rates in a selected group of patients, for day 2 and day 5 embryos both cultured in sequential medium: A randomized study. Hum Reprod. 2003;18:2145-2150.
110 Frattarelli JL, Leondires MP, McKeeby JL, et al. Blastocyst transfer decreases multiple pregnancy rates in vitro fertilization cycles: A randomized controlled trial. Fertil Steril. 2003;79:228-230.
111 Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod. 1998;13:3434-3440.
112 Hreeinsson J, Rosenlund B, Fridstrom M, et al. Embryo transfer is equally effective at cleavage stage and blastocyst stage: A randomized prospective study. Eur J Obstet Gyn Reprod Biol. 2004;117:194-200.
113 Karaki RZ, Samarraie SS, Younis NA, et al. Blastocyst culture and transfer: A step toward improved in vitro fertilization outcome. Fertil Steril. 2002;77:114-118.
114 Kolibianakis EM, Zilopoulos K, Verpoest W, et al. Should we advise patients undergoing in vitro fertilization to start a cycle leading to a day 3 or day 5 transfer? Hum Reprod. 2004;19:2550-2554.
115 Levitas E, Lunenfeld E, Har-Vardi I, et al. Blastocyst-stage embryo transfer in patients who failed to conceive in three or more day 2-3 embryo transfer cycles: A prospective, randomized study. Fertil Steril. 2004;81:567-571.
116 Levron J, Shulman A, Bider D, et al. A prospective randomized study comparing day 3 with blastocyst-stage embryo transfer. Fertil Steril. 2002;77:1300-1301.
117 Motta LA, Alegretti JR, Pico M, et al. Blastocyst vs. cleaving embryo transfer: A prospective randomized trial. Fertil Steril. 1998;70(Suppl 1):S17.
118 Rienzi I, Ubaldi F, Iacobelli M, et al. Day 3 embryo transfer with combined evaluation at the pronuclear and cleavage stages compares favourably with day 5 blastocyst transfer. Hum Reprod. 2002;17:1852-1855.
119 Schillaci R, Castelli A, Vassiliadis A, et al: Blastocyst stage versus day 2 embryo transfer in IVF cycles. Abstracts of the 18th Annual Meeting of ESHRE. Vienna, 2002, p 418.
120 Van der Auwera I, Debrock S, Spiessens C, et al. A prospective randomized study: Day 2 versus day 5 embryo transfer. Hum Reprod. 2002;17:1507-1512.