CHAPTER 22 Assisted reproduction treatments
Introduction
Since the first successful birth following IVF in 1978, there have been considerable advances in the field of ART and more than 3 million babies have been born across the world. ART is now widely available, and in a single year (2004), more than 360,000 treatment cycles were carried out in Europe. In the UK, a total of 44,275 IVF/micromanipulation and frozen embryo replacement (FER) cycles were reported in 2006, resulting in 12,596 livebirth events (Human Fertilisation and Embryology Authority 2008). The number of conventional IVF cycles remained reasonably steady between 1998 and 2008, while the number of ICSI treatment cycles has been increasing steadily since its introduction (Figure 22.1) Human Fertilisation and Embryology Authority 2008).
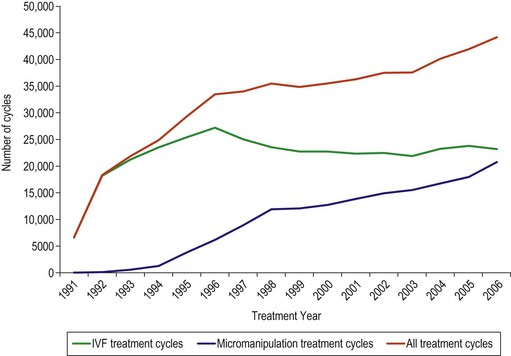
Figure 22.1 Number of treatment cycles 1991–2006.
Source: Human Fertilisation and Embryology Authority (2008).
The Human Fertilisation and Embryology Authority (HFEA) was established in 1991 by the Human Fertilisation and Embryology Act 1990 to regulate and inspect all UK clinics providing ART, and was the first statutory body of its kind in the world. The introduction of guidelines into clinical practice in general and in assisted reproduction in particular has led to an ever-increasing role for an evidence-based approach to the provision of these services. Clinical guidelines for the assessment and treatment of people with fertility problems have been published by the National Institute for Clinical Excellence (2004) to streamline services and avoid unnecessary waste of resources. This chapter will review the current status of conventional IVF, ICSI and other treatments, new developments in ART and the clinical risks associated with these techniques. The obstetric and neonatal consequences of these treatments and, in particular, the impact of the number of embryos transferred on the outcome will be discussed.
Selection and Evaluation of Patients
Although IVF was initially introduced to bypass tubal blockage, the currently available techniques enable the treatment of various causes of infertility. The current indications are shown in Box 22.1.
Evaluation of pelvis
The diagnosis of PCOS is currently based on the definition derived by the Rotterdam Consensus Workshop, which suggested the presence of 12 or more follicles measuring 2–9 mm in diameter and/or an ovarian volume of more than 10 cm3 for the ultrasound diagnosis (Balen et al 2003). Stromal vascularity and blood flow velocities are higher in women with polycystic ovaries (Agrawal et al 1998), and this may account for their tendency towards ovarian hyperstimulation in response to gonadotrophin therapy.
Endometriosis has been shown to have a negative effect on the outcome of ART, with affected women demonstrating a reduced response to ovarian stimulation (Gupta et al 2006); however, reports on its effects on embryo quality and implantation potential are conflicting. Moderate and severe degrees of endometriosis, often characterized by rectovaginal disease and the presence of ovarian endometrioma, can usually be diagnosed by transvaginal ultrasound (Moore et al 2002).
The presence of a hydrosalpinx is associated with poor implantation and pregnancy rates, and early pregnancy loss. The exact mechanism is unknown but substances toxic to the endometrium are thought to be produced that have a negative effect on endometrial receptivity (Strandell 2007). Salpingectomy, ideally performed laparoscopically, prior to IVF has been shown to be beneficial and is recommended. The diagnosis of hydrosalpinx can generally be made using transvaginal ultrasound with a high degree of confidence.
Ultrasound assessment of the uterus is generally restricted to measurement of the endometrial thickness and a description of the locality and size of any fibroids. Conventional ultrasound may also detect uterine anomalies, although these are more readily identified and correctly qualified with three-dimensional ultrasound. The diagnosis of uterine anomalies is important as they are associated with lower implantation rates and increased rates of early miscarriage, preterm labour and malpresentations (Lin 2004). The outcome appears to relate more to the length of the remaining uterine cavity than the degree of septum, and this may be measured reliably with three-dimensional ultrasound (Salim et al 2003).
The diagnosis and classification of fibroids into subserosal, intramural and submucosal is usually straightforward, and this is important as these have a progressively negative effect on pregnancy rates. Whilst intramural fibroids measuring more than 4 cm in diameter and submucous fibroids of any size are thought to have a negative effect on the outcome of fertility treatment and to be associated with lower pregnancy rates, the effect of smaller intramural fibroids on reproductive outcome is unclear (Oliveira et al 2004). There is also doubt about the exact impact of endometrial polyps, the other commonly encountered intrauterine pathology, on treatment outcome and early pregnancy. Some authors suggest conservative management, especially if the polyp is small (Isikoglu et al 2006), but this can be difficult in patients undergoing fertility treatment or with a history of miscarriage when a polyp is identified before treatment begins. Polyps are usually evident with conventional transvaginal imaging, although false-positive diagnoses are common. Saline infusion sonohysterography can reduce this and facilitate appropriate operative planning (Bartkowiak et al 2006). Delineation of the polyp with saline ensures that the size and position of the polyp can be defined accurately, allowing surgeons to modify their approach and resect the larger, more broad-based polyps rather than planning for simple polypectomy. Hysteroscopy is the gold standard technique for uterine cavity assessment, and may be necessary if diagnosis of intrauterine pathologies is in doubt.
Assessment of ovarian reserve
Evaluation of ovarian reserve has become an integral part of the pretreatment assessment of a woman about to undergo ART, and is recommended for all women planning ART (Speroff and Fritz 2005). The aim is to identify women likely to respond poorly, those who have a low chance of success and who are more likely to have their treatment cycle cancelled, and those prone to ovarian hyperstimulation which is associated with significant morbidity and even mortality. Accurate assessment of ovarian reserve and prediction of response therefore facilitates pretreatment counselling of couples of their potential risks, and allows treatment to be tailored to the individual, potentially increasing the number of oocytes retrieved without risking an exaggerated response. Ovarian reserve, defined by the size and quality of the remaining ovarian follicular pool at any given time, reflects the fertility potential of a woman (Broekmans et al 2006).
Although women’s age is an important predictor of ovarian reserve and response, a great deal of interindividual variation exists, even among women of the same age (te Velde and Pearson 2002). This is indicative of a wide variation in the rate of age-related decline in the ovarian follicle population. Several endocrine and ultrasound markers have been reported, with the aim of estimating the number of gonadotrophin-responsive or ‘selectable follicles’ more accurately. The endocrine markers include factors produced by the developing follicles [oestradiol, inhibin B and anti-Müllerian hormone (AMH)] or hormones under the inhibitory control of these factors [follicule-stimulating hormone (FSH)]. The ultrasound markers are antral follicle count, ovarian volume and ovarian vascularity. The sensitivities, specificities and predictive values of these tests have been evaluated in many studies by correlating the test results with the number of oocytes retrieved, poor or exaggerated ovarian response, and pregnancy or livebirth rates during ART (Broekmans et al 2006).
Serum follicle-stimulating hormone
Early-follicular-phase FSH is the most widely used marker of ovarian reserve, and women with raised FSH levels (>10–15 IU/l) are more likely to have a reduced ovarian response and less successful ART outcome (Scott et al 1989, Sharif et al 1998). A meta-analysis of 21 studies looking at the value of basal FSH in the prediction of IVF outcome concluded that the performance of basal FSH as a screening test is limited in predicting poor ovarian response and non-pregnancy. Predictions with a substantial shift from pre-FSH-test probability to post-FSH-test probability are only achieved at extremely high cut-off levels (Bancsi et al 2003). Moreover, the cycle-to-cycle variability of basal FSH levels limits the reliability of a single measurement for assessment of ovarian reserve. However, it is important to note that ovarian response and pregnancy rates during IVF are low in women with high intercycle variability (Scott et al 1990) or who demonstrate abnormal FSH levels in any menstrual cycle (Abdalla and Thum 2006).
Oestradiol
Measurement of basal oestradiol in addition to FSH is recommended to improve predictive accuracy. Premature elevation of the oestradiol level due to advanced follicular development and early selection of a dominant follicle may tend to suppress the FSH level, thus masking a rise that might otherwise reflect a low ovarian reserve (Frattarelli et al 2000). An elevated level of basal oestradiol (≥60 pg/ml), even in the presence of a normal FSH level, is associated with increased risk of cycle cancellation due to poor ovarian response (Evers et al 1998). However, the use of serum oestradiol estimation for ovarian reserve assessment is limited by the fact that some women with a normal ovarian reserve may have an occasional accelerated cycle with FSH levels of mid-follicular range in their true early-follicular phase (Frattarelli et al 2000). Furthermore, elevated oestrogen may simply reflect the presence of functional ovarian cysts.
Inhibin B
Inhibin B is principally produced by the granulosa cells of the developing cohort of antral follicles during the luteal–follicular transition. Its level in the early-follicular phase is considered to be indicative of the quantity or quality of developing follicles (Hall et al 1999). A low level (<45 pg/ml) of early-follicular-phase inhibin B may predict a poor ovarian response (Seifer et al 1997). However, the reliability of its measurement has been questioned because of significant intercycle variability (McIlveen et al 2007).
Anti-Müllerian hormone
Studies have shown that AMH is a promising serum marker of ovarian reserve (van Rooij et al 2002). AMH, a member of the transforming growth factor-β family of growth and differentiation factors, is primarily produced by the preantral and small antral follicles in women of reproductive age. The serum level of AMH is indicative of the growing follicle pool (de Vet et al 2002). While AMH is highly predictive of potential poor or exaggerated responders to ovarian stimulation during ART, its accuracy to predict the occurrence of pregnancy after one cycle of ART is only marginal (Broekmans et al 2008). Recent studies have suggested that AMH levels vary minimally between menstrual cycles and are cycle independent (Fanchin et al 2005, La Marca et al 2006). Therefore, AMH measurement could be undertaken during any cycle and at any time during the cycle for adequate prediction of ovarian response, and this makes it an attractive marker for routine use. However, there are potential limitations to the regular use of AMH as a marker of ovarian reserve because of different cut-off levels reported in different studies. Currently, there is no international assay standard for AMH measurement, which may contribute to the discordance between different studies and make comparison between laboratories quite difficult. Once the available assays are standardized and automated analysis systems are developed, AMH has the potential to become the test of choice for ovarian reserve.
Antral follicle count
Antral follicle count (AFC) is the most significant predictor of ovarian response among the ultrasound markers (Kwee et al 2007, Jayaprakasan et al 2009), although decreased ovarian volume (<3 cm3) and stromal blood flow are also implicated for poor treatment outcome during ART. The number of gonadotrophin-responsive antral follicles measuring 2–10 mm can be measured using two- or three-dimensional ultrasound with an acceptably high level of agreement both between and within observers (Jayaprakasan et al 2008a). A total follicle count of between seven and 22 is generally considered as the optimum cut-off level for the prediction of poor and exaggerated ovarian response, respectively. AFC measurement can be performed either in the early-follicular phase of the cycle before treatment begins or after downregulation with similar predictive accuracy (Jayaprakasan et al 2008b). However, assessment prior to treatment has an additional advantage in that it provides an opportunity for pretreatment evaluation of the pelvis to screen for pathology. AFC and AMH are currently the best two predictors of ovarian reserve among all the endocrine and ultrasound markers, and are equally predictive of poor ovarian response during ART (Jayaprakasan et al 2010). AFC is currently the test of choice for ovarian reserve assessment as it is easy to perform and due to inherent availability of ultrasound machines.
Ovarian reserve tests including AFC and AMH have limited value in the prediction of non-conception despite their adequate capacity to predict ovarian response during ART (Broekmans et al 2006). Pregnancy may occur even at extreme cut-offs for an abnormal test result; therefore, IVF treatment cannot be denied based on these tests, especially in participants who are seeking their first cycle of treatment (Broekmans et al 2007). However, the identification of participants who are likely to respond poorly during IVF treatment is clinically relevant, as a couple can be counselled accordingly. They can be made aware that they have an increased chance of cycle cancellation and a significantly lower chance of success, allowing an informed decision to be made. Such prediction also allows clinicians to formulate individualized treatment protocols to improve or at least maximize ovarian response.
Evaluation of male partner
In order to give adequate counselling to couples and their families, genetic tests should be performed before, during or after ART in cases such as women with Turner’s syndrome, men with 47XXY, men or women with structural chromosomal aberrations, and men with either Yq11 deletion or congenital bilateral absence of the vas deferens (ESHRE Capri Workshop Group 2000).
Clinical Management of the Treatment Cycle
The main components of typical conventional ART include:
Normal folliculogenesis
This involves several important and inter-related steps.
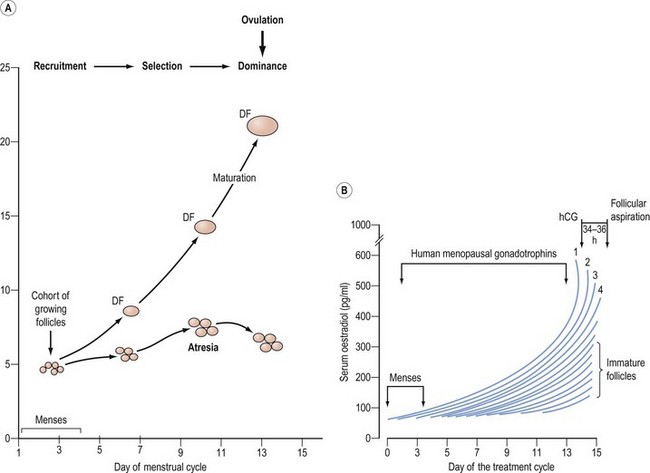
Follicular development beyond the antral stage depends on the concentration of FSH in the circulation. Once a threshold level has been attained, follicular growth beyond 4 mm occurs. The interval during which FSH remains elevated above the threshold level can be regarded as a window through which a follicle must pass to avoid atresia. The width of the window will therefore determine the number of follicles that can be selected for ovulation (Figure 22.2). The preovulatory luteinizing hormone (LH) surge is triggered by the positive feedback of oestradiol from the dominant follicle, as well as other follicular contributory factors. Ovulation occurs 24–36 h after the onset of the LH surge.
Pituitary downregulation
In the conventional ART protocol, it is standard practice to use a continuous and supraphysiological dose of GnRH agonist to obtain control over the hypothalamic–pituitary–ovarian axis. GnRH agonists cause an initial flare response, characterized by increased gonadotrophin secretion, due to upregulation of GnRH receptors. However, prolonged administration of GnRH agonists induces downregulation of GnRH receptors. This prevents a premature surge in LH and subsequent spontaneous ovulation with luteinization, which occurs in up to 23% of cycles when gonadotrophins alone are used and facilitates the timing of oocyte retrieval. The use of GnRH agonists in ART has been shown to increase the number of oocytes retrieved, improve the pregnancy rate and significantly reduce the chance of cycle cancellation (Hughes et al 1992).
Two different GnRH agonist protocols are used in most assisted conception units: long and short protocols. In the long protocol, GnRH agonists (buserelin or nafarelin) are started in the mid-luteal phase (day 21) of the previous menstrual cycle to achieve pituitary downregulation in approximately 8–21 days, after which gonadotrophins are commenced. GnRH agonists are administered for approximately 10–14 days in the short protocol. In both regimens, GnRH agonists are administered daily until the day of hCG or LH injection to trigger oocyte maturation. The short protocol takes advantage of the initial flare effect, which can be utilized to augment the ovarian response to gonadotrophin stimulation. However, a systematic review of 26 randomized-controlled trials found an increased clinical pregnancy rate per cycle and fewer cycle cancellations with the long protocol compared with the short protocol (Daya 2000). Therefore, the long GnRH agonist protocol is generally recommended in most ART cycles.
Use of the long GnRH agonist protocol often prolongs the stimulation phase, resulting in increased requirement for gonadotrophins and increased treatment cost in addition to causing undesirable side-effects of hypo-oestrogenism. It may also be associated with poor response in some patients, as well as introducing a higher risk of ovarian hyperstimulation syndrome (OHSS). Some of the disadvantages can be obviated with the use of GnRH antagonists, such as cetrorelix and ganirelix. GnRH antagonists induce immediate pituitary downregulation by competitively binding to GnRH receptors, and avoid the flare effects or the need for prolonged treatment associated with GnRH agonist protocols. Two different regimes have been described. The multiple-dose protocol involves the administration of 0.25 mg cetrorelix or ganirelix daily from day 6–7 of stimulation, or when the leading follicle is 14–15 mm, until hCG/LH administration. The single-dose protocol involves the single administration of 3 mg cetrorelix on day 7–8 of stimulation. GnRH antagonist protocols are therefore short and simple, but more importantly they reduce the incidence of severe OHSS. However, the clinical pregnancy rates following IVF treatment were significantly lower with GnRH antagonist use compared with the long GnRH agonist protocol [odds ratio (OR) 0.82, 95% confidence interval (CI) 0.68–0.97] (Al-Inany et al 2007), although data from units with experience of the GnRH antagonist protocol are encouraging. More recent trials of GnRH antagonists have reported better pregnancy rates than earlier trials, which used antagonist protocols that would now be recognized as suboptimal.
Controlled ovarian stimulation
Genetic and molecular engineering has led to the production of recombinant human FSH (rFSH) with high purity (>99%), high specific bioactivity (>10,000 IU/mg protein) and absent intrinsic LH activity which is suitable for intramuscular or subcutaneous administration (Mannaerts et al 1991). Two different rFSH preparations are commercially available: follitropin alpha (Gonal F) and follitropin beta (Puregon). These two rFSH preparations differ in that follitropin alpha has a more acidic isoform profile than follitropin beta due to a slight difference in its carbohydrate component. Consequently, they differ in metabolic clearance, half-life and biological activity, but both are equally effective during ART cycles in terms of livebirth rates. A number of well-designed randomized-controlled trials have compared both preparations of rFSH against HMG in their clinical effectiveness during conventional IVF with the long GnRH agonist protocol. While none of them individually showed any statistically significant difference in livebirth rates between the two treatment arms, two recent systematic reviews have demonstrated a significant 4% increase in livebirth rate in the HMG arm (Al-Inany et al 2008, Coomarasamy et al 2008). However, the results of the meta-analysis need to be interpreted with caution because of the clinical heterogeneity of the trials; therefore, a large multicentre randomized double-blind trial is warranted. Moreover, such studies should consider reporting the more clinically relevant cumulative livebirth rates as the primary outcome.
Recombinant DNA technologies have also made it possible to produce LH (rLH). Due to the potential role of LH in follicular development according to the two-cell theory of gonadotrophin system, supplemental rLH has been suggested to improve IVF outcome if rFSH is used for ovarian stimulation. However, a recent systematic review of randomized trials failed to demonstrate any difference in livebirth rates with adding rLH to the rFSH protocol (Kolibianakis et al 2007). Although it is possible that rLH supplementation may be beneficial in a subset of the IVF population, such as poor responders (Mochtar et al 2007), its generalized use is currently limited.
The collective evidence from many studies suggests that 150–225 IU/day gonadotrophin is the appropriate starting dose for most women undergoing conventional IVF treatment. The majority of units use a daily dose according to women’s age and the presence or absence of polycystic ovaries: 150 IU in women under 30 years of age or with polycystic ovaries, 225 IU for those between 30 and 39 years of age, and 300 IU for those aged 40 years or over. The lowest possible dose to achieve the optimum ovarian response should be used to reduce the risks of ovarian hyperstimulation. Although there is no consensus on what constitutes optimum ovarian stimulation, a recent study has reported that the implantation rate is maximum when 10 oocytes are retrieved following conventional IVF using the long downregulation protocol (Verberg et al 2009a).
Recently, mild ovarian stimulation protocols have gained a lot of interest in the field of ART, with the current trend of limiting the number of embryos transferred reducing the need for large numbers of oocytes. The principal aim with this approach is to reduce the number of developing follicles and thereby minimize the adverse effects of ovarian stimulation, particularly OHSS and multiple pregnancies. Three reported mild stimulation protocols include clomiphene citrate alone or in combination with gonadotrophins, delayed commencement (day 5 of the cycle) of gonadotrophins with GnRH antagonist co-medication, and late follicular replacement of FSH with administration of hCG/LH (Verberg et al 2009b). All of these protocols have been shown to reduce the gonadotrophin dosage and have the potential to reduce the cost of treatment and patient distress associated with conventional ART. As expected, the ongoing pregnancy rates/livebirth rates per cycle have been lower compared with the conventional protocol, but similar effectiveness could be achieved with more treatment cycles.
Oocyte Retrieval
It is important to provide effective anaesthesia and analgesia for transvaginal oocyte recovery, as this is painful. No techniques for anaesthesia, analgesia or sedation are free from side-effects. Whichever technique is used, recognized standards of practice should be adhered to and all IVF professionals should follow the safe practice of administering sedative drugs published by the Academy of Medical Royal Colleges (2001). Conscious sedation should be offered to all women having the procedure as it is a safe and acceptable method of providing analgesia.
Technique
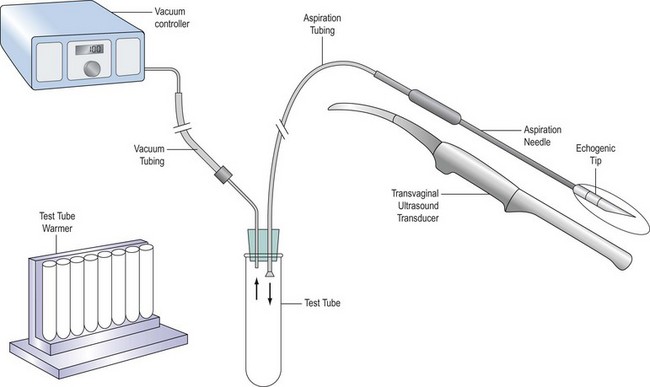
Several types of aspiration needle are available. They may have a single or double lumen to enable aspiration and flushing through different routes. The needle is usually 16-guage and must have a very sharp tip to enable easy puncture of mobile ovaries; the distal 2 cm should be roughened to enhance ultrasound visualization (Figure 22.3). The needle is connected to a test tube by tubing, and suction is applied either from a foot-operated pump (Figure 22.4) or manually. The ultrasound transducer (Figure 22.5) is enclosed in a special sterile condom and plastic sleeve prior to insertion into the vagina, and should be cleaned thoroughly with a damp cloth after each procedure.
Generally, very few technical difficulties are encountered during vaginal egg collection. The main risks of transvaginal oocyte recovery are pelvic infection (0.6%) and bleeding (>100 ml in 0.8% cases) (Bennett et al 1993), which may be serious, sometimes even fatal. Appropriate preoperative vaginal preparation and minimizing the number of repeated vaginal penetrations may serve to lower the risk of infection. While there is no evidence that routine antibiotics reduce the risk of infection, the administration of an intravenous bolus of antibiotic is generally recommended for women with a history of severe pelvic inflammatory disease or if an endometrioma is punctured. Intestinal, vascular, uterine and tubal injuries with the aspiration needle have also been reported. Bleeding and infections may be serious, sometimes even fatal, complications.
Laboratory Techniques
In cases of ICSI, meiotic maturity is assessed after denudation; only the mature oocytes are injected with sperm, following immobilization. Fertilization, as determined by the presence of two pronuclei, is assessed 18–20 h after insemination. On day 2 or 3, based on the cleavage rate, the size, shape, symmetry and cytoplasmic appearance of the blastomeres and the presence or absence of nucleus, embryo quality is graded by embryologists as grade 1, 2, 3 or 4 (Van Royen et al 1999). Grade 1 and 2 embryos are considered to be of high quality. While ET is performed with one or two of the best-available embryo/s, regardless of their grading, only top-quality (grade 1 and 2) embryos are considered eligible for freezing and this option is then offered to the couple.
Embryo Transfer
ET is a critical step in determining the final outcome of the treatment. The convention has been to replace embryos on the second or third day post insemination, when the embryos are usually at the two- to eight-cell stage of cleavage. With improvements in culture media, there is an increasing tendency for delaying ET with the aim of improving implantation rates. While there is no improvement in livebirth rates (OR 1.07, 95% CI 0.84–1.37) with delaying ET from day 2 to day 3 (Oatway et al 2004), transfer of embryos at the blastocyst stage (day 5/6) has shown a significant increase in the livebirth rate (OR 1.35, 95% CI 1.05–1.74) compared with transfers on day 2 or 3 (Blake et al 2007). It is a more physiological approach, allowing synchronization of the embryo with the endometrium, and selection of the viable embryos for transfer will be more efficient. However, the rates of embryo freezing are lower and the treatment cancellation rates are higher with blastocyst transfer. However, in certain couples, blastocyst transfer has the potential to favour single ET without compromising the overall success rates, but can significantly reduce the occurrence of multiple pregnancies. The most favoured couples for blastocyst transfer are those with high numbers of eight-cell embryos on day 3, in whom cycle cancellation is not increased.
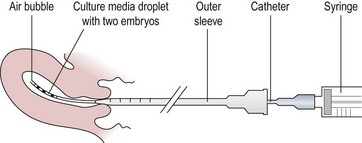
Transcervical embryo replacement into the uterine cavity is a relatively simple procedure, which must be carried out meticulously to ensure appropriate placement of the embryos (Figure 22.6). Variations among clinicians in the technique of ET may influence the pregnancy rate. Avoidance of blood, mucus, bacterial contamination, excessive uterine contractions and trauma to the endometrium is associated with optimal pregnancy and implantation rates after transcervical ET. Transabdominal ultrasonographic guidance to determine the precise depth of embryo placement within the uterus appears to facilitate successful ET and is preferred (Brown et al 2007), although a recent large randomized trial from a single centre did not show any benefit over ‘clinical touch’ transfer (Drakeley et al 2008). The tip of the catheter is placed approximately 1 cm from the uterine fundus so that the embryo/s are expelled gently into the mid-cavity of the uterus. Soft catheters are preferable to rigid catheters as they are less likely to traumatize the cervix or endometrium, or to invoke any uterine contractions. The commonly used soft catheters (Wallace catheters) (Figure 22.7) have a softer inner cannula and a stiffer outer sheath. Occasionally, resistance to pass the soft inner cannula into the uterus, usually at the level of internal os, is encountered; in these cases, the stiffer outer sheath is advanced into the cervical canal to negotiate the resistance, and the inner cannula can be advanced into the uterine cavity. A firmer malleable catheter with a stylet and an outer sheath is another option in difficult cases, where the catheter is advanced up to the level of the internal os. The inner stylet is then removed and the softer cannula loaded with embryos is fed through the outer sheath to advance into the uterine cavity for transfer. The outer sheath or the malleable catheter should never be advanced beyond the internal os.
Outcome Measures and Factors Affecting Success Rate
Pregnancy and livebirth rates per treatment cycle for IVF and ICSI have steadily improved worldwide over the past decade. Figure 22.8 shows the improvement in the livebirth rate per treatment cycle in the UK between 1991 and 2005 (Human Fertilisation and Embryology Authority 2008). It is now widely accepted that when attempting to ascertain whether or not a treatment is required, it is essential that the time-specific or cycle-specific conception rate is used. Crude pregnancy rate per couple is almost meaningless. Pregnancy rate per cycle can also be misleading if limited to the first cycle or two, because the rate may fall in subsequent cycles. Thus, cumulative conception or cumulative livebirth rates have been used increasingly in reporting conventional and ART outcomes. Cumulative conception rates for some of the most common causes of infertility in the untreated population were compared with those in couples following treatment with conventional methods (Hull 1992). The results showed that in some conditions, such as ovulatory dysfunction, or in women being treated with donor insemination, the cumulative conception rate following conventional therapy is almost the same as in normal women. However, in other circumstances, such as salpingostomy for distal tubal occlusion or the use of high-dose glucocorticosteroids for seminal antisperm antibodies, the prognosis is worse and the cumulative conception rates are lower than that which can be expected in a single cycle of IVF treatment.
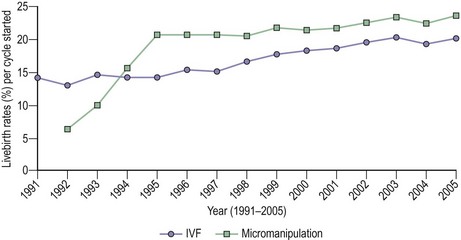
Figure 22.8 Livebirth rates per treatment cycle 1991–2005.
Source: Human Fertilisation and Embryology Authority (2008).
The effectiveness of infertility treatments (IVF, ICSI, egg and sperm donation, embryo donation) is dependent on a reasonable likelihood that embryos can be created in vitro, and then placed in the uterus with a reasonable expectation that implantation will occur. Many factors determine the outcome of treatment, such as patient selection, age, cause and duration of infertility, and the number of attempts that couples undergo. The decision to recommend ART should be based on the likelihood that a pregnancy will occur without treatment, the possibility that a less invasive form of treatment might be effective and the likely outcome of IVF treatment (National Institute for Clinical Excellence 2004). The likelihood of treatment-independent pregnancy depends on the woman’s age, the cause and duration of infertility, and previous pregnancy history. In a retrospective study (Vardon et al 1995), the incidence of spontaneous treatment-independent pregnancy in couples enlisted on an IVF programme was reported to be 11% of couples with low fertility. The main difference from those in whom pregnancy did not occur was a shorter duration of infertility.
Female age
Indeed, when women over 40 years of age had four or more embryos transferred, their pregnancy rate was not significantly different from that of younger women, whether following IVF (Widra et al 1996) or ICSI (Alrayyes et al 1997). Hence, it may be concluded that older women with a good ovarian response, producing three or more embryos suitable for transfer, have similar prospects for establishing a pregnancy as younger patients. However, other data support reduced implantation rates in older women, regardless of the number of embryos transferred (Piette et al 1990). Younger women, even if their ovarian response is poor, have a better implantation rate than women over 40 years of age experiencing a normal ovarian response (van Rooij et al 2003). This indicates that age is the major determinant of the implantation potential of oocytes and/or of endometrial receptivity. However, the reported finding of similar pregnancy rates in older women having treatment using donor eggs compared with younger women indicates that oocyte quality or implantation potential, rather than endometrial receptivity, declines with women’s age (Van Voorhis 2007).
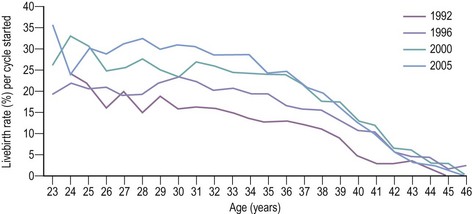
Although livebirth rates per treatment cycle following IVF and ICSI have increased consistently over the past decade, they decline with advancing age of women when using their own eggs (Figure 22.9). A number of investigators have examined different age cut-offs such as 40 years (Widra et al 1996, Sharif et al 1998) or 35 years (Preutthipan et al 1996). Mardesic et al (1994) reported that the cut-off point of effectiveness for an IVF programme was 36–37 years, with a marked decline in pregnancy rate per ET in women over 38 years of age.
In an attempt to identify factors that affect the outcome of treatment, Templeton et al (1996) analysed the HFEA database between 1991 and 1994. They reported that the overall livebirth rate per treatment cycle was 13.9%. The highest livebirth rates were in women aged 25–30 years, with younger women (<25 years) having lower rates, and a sharp decline noted in older women. At all ages over 30 years, the use of donor eggs was associated with significantly higher livebirth rates compared with the use of the women’s own eggs, although there was equally a downward trend in success rates with age. After adjustment for age, increasing duration of infertility was associated with a significant decrease in livebirth rates. The indications for treatment had no significant effect on the outcome, while previous pregnancy and live birth increased treatment success significantly.
Miscarriage rates have been reported to be higher in all women following IVF, and there is a two- to three-fold increase in the rate of spontaneous abortion in women aged 40 years or more (Toner and Flood 1993). Embryo quality is more likely to be the main factor influencing the poor reproductive performance of women with advancing age than a defective response of the uterine vasculature to steroids or uterine ageing. Increasing maternal age correlates with a higher risk of fetal chromosomal aneuploidy, which results in an increased rate of miscarriage (Spandorfer et al 2004).
Obesity
Although the consequences of obesity for women’s fertility are well known, there is controversy regarding the effect of obesity on the outcome of IVF treatment. Overweight [body mass index (BMI) ≥25] and obesity (BMI ≥30) have been associated with the need for higher doses of gonadotrophins, increased cycle cancellation rates and retrieval of fewer oocytes. Lower rates of ET, pregnancy and live birth have also been reported, as have higher miscarriage rates (Fedorcsak et al 2004). Other studies have not found that obesity has a negative impact on ART outcome (Dechaud et al 2006, Sneed et al 2008). However, a recent systematic review of all the reported studies confirmed that obesity has an adverse effect on the success of IVF (Maheshwari et al 2007); therefore, obese women should be advised to lose weight before commencing ART in order to improve their chance of success.
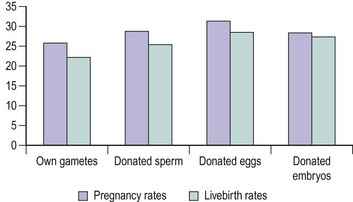
Cause of infertility
Reports have differed in their analysis of the impact of infertility factors on cumulative conception and livebirth rates. While some have found significant differences, with the lowest rates being reported in patients with male infertility or multiple infertility factors (Tan et al 1992), others found no significant effect on outcome (Templeton et al 1996). However, a history of previous pregnancy and live birth increased treatment success significantly. The IVF clinical pregnancy and livebirth rates in the HFEA report of 2000 show similar results for tubal disease, endometriosis and unexplained infertility (Figure 22.10). Reports of higher fertilization rates after ICSI suggest that this technique may be better than the conventional method for all couples seeking IVF. A multicentre randomized-controlled trial comparing clinical outcome after ICSI or conventional IVF in couples with non-male-factor infertility showed higher implantation and pregnancy rates per cycle after IVF, hence supporting the practice of reserving ICSI for severe male factor infertility (Bhattacharya et al 2001).
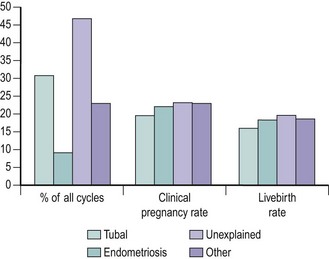
Figure 22.10 Clinical pregnancy and livebirth rates following in-vitro fertilization for female causes of infertility.
Source: Human Fertilisation and Embryology Authority (2000).
All stages of endometriosis are viewed as suitable indications for ART. The timing of treatment is dependent on the severity of the disease, previous therapy and other factors, such as female age and duration of infertility. In women with severe endometriosis with mechanical tubal blockage and where surgery is inappropriate, IVF should be expedited. IVF should also be recommended 1–2 years after previous unsuccessful medical or surgical therapy, while in minimal or mild endometriosis, the balance of choice seems to be in favour of IVF after more than 2 years of expectant management. Initially, poor results were reported in women with severe disease (Matson and Yovich 1986). The introduction of ultrasound-guided techniques for oocyte collection resulted in the retrieval of more oocytes, and hence led to higher pregnancy and implantation rates in advanced-stage disease (Geber et al 1995). The use of GnRH agonists in stimulation protocols or as a pretreatment for women with endometriosis has also resulted in the retrieval and transfer of more preovulatory oocytes, lower cancellation of treatment cycles and a higher pregnancy rate, especially in women with advanced-stage disease. A recent meta-analysis indicated that the presence of ovarian endometrioma does not affect the quality of oocytes, as the pregnancy rate was similar to that of controls, although the ovarian response to gonadotrophins was reduced (Gupta et al 2006). While prospective randomized-controlled trials are lacking, evidence based on case–control studies suggests that surgery for bilateral endometrioma could impair IVF outcome significantly, possibly due to damage to the remaining healthy ovarian follicles (Somigliana et al 2008).
For male factor infertility, ICSI was successfully introduced in 1992 (Palermo et al 1992). Since then, the annual number of treatment cycles has increased steadily (see Figure 22.1). Previous techniques, such as partial zona dissection and subzonal insemination, were very disappointing (Cohen et al 1991), and comparative studies indicated that ICSI was much more efficient (Tarin 1995). It gave men who had previously been diagnosed with severe male factor infertility the chance to have their own genetic children. The sperm may be obtained either by ejaculation, percutaneous aspiration from the epididymis or testis, or testicular extraction, resulting in equally high fertilization, pregnancy and implantation rates, especially in men with borderline or very poor sperm quality. Recognized indications for ICSI include:
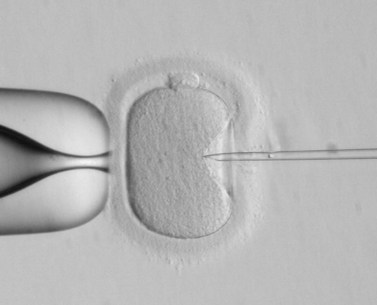
Livebirth rates following ICSI are shown in Figure 22.8. A remarkable change in fertilization rate with a significant increase in the percentage of two pronuclei oocytes occurred when the technique was modified slightly by breaking the sperm’s tail before injection (Fishel et al 1995). The technique requires a high-quality inverted microscope and special equipment, with holding and injection pipettes being used to stabilize and inject the oocyte, respectively. The injecting pipette is pushed almost entirely through the ooplasm before the spermatozoon is deposited inside the oocyte (Figure 22.11).
Duration of infertility
The duration of infertility remains one of the most important variables that influences the outcome of assisted reproduction, with lower pregnancy and livebirth rates associated with a longer period of infertility. Analysis of the HFEA database between 1991 and 1994 showed that, even with adjustment for age, there was a significant decrease in livebirth rate with increasing duration of infertility from 1 to 12 years (Templeton et al 1996).
Endometrial thickness
Endometrial thickness alone is a poor predictor for pregnancy. However, no conception has been recorded for endometrial thickness below 5 mm on the day of transfer. As such, it is recommended that consideration should be given to cryopreserving all embryos and preparing the endometrium with exogenous hormones in a subsequent cycle (Friedler et al 1996).
Number of embryos transferred
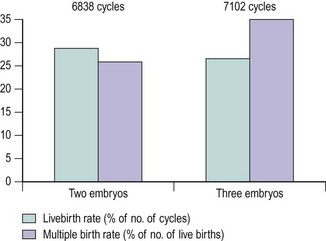
Regarding the optimum number of embryos to be transferred, there is a great deal of debate and a wide variation in practice across the world. Unarguably, ART is the single most important cause for the increase in multiple pregnancies over the last two decades, which is a direct consequence of the number of embryos transferred. Retrospective studies, as early as 1985, argued that multiple pregnancies and births increased with the increase in the number of embryos replaced (Wood et al 1985). Randomized studies comparing double ET with treble ET (Staessen et al 1993) or quadruple ET (Vauthier-Brouzes et al 1994) reported similar pregnancy rates, provided that there were sufficient morphologically regular embryos available for transfer. Analysis of the HFEA database of more than 44,000 cycles (Templeton and Morris 1998) and the HFEA reports (2000) showed that when four or more fertilized eggs were available, the transfer of three embryos did not result in improved pregnancy rates compared with the elective transfer of two embryos. However, the incidence of triplets or higher order multiple births decreased considerably when two embryos were replaced (Figure 22.12).
Despite only transferring two embryos during most IVF cycles, 40% of all IVF babies are twins (Human Fertilisation and Embryology Authority 2008). In order to deal with the unacceptably high multiple birth rates following ART, there is a recent move from the IVF community, particularly in Europe, to limit the number of embryos transferred to one in carefully selected patients. In the UK, the HFEA has set a target of reducing the multiple pregnancy rate to 10% over a period of about 3 years, and has urged the fertility clinics to devise a ‘multiple pregnancy minimization strategy’ to achieve the agreed target. To facilitate this, the British Fertility Society in unison with the Association of Clinical Embryologists in the UK have produced guidelines for practice which focus on the effectiveness of elective single ET (eSET) and patient selection for eSET (Cutting et al 2008). The two most important criteria when choosing a couple for eSET are female age and the quality of embryos available. Twin pregnancy has increased risk for the fetuses and the mother. Given the higher risks of premature delivery (three-fold), perinatal mortality (six-fold), cerebral palsy (four- to six-fold) and pregnancy complications (hypertension, pre-eclampsia, gestational diabetes etc.), and the consequent resource implications for the health service with twin births compared with singleton deliveries, the HFEA in the UK has urged for successful IVF to be redefined as ‘full-term singletons with a normal birth weight’ in order to promote eSET and thereby reduce multiple births (Cutting et al 2008). A Cochrane review comparing the outcome of double ET in a single cycle compared with eSET (combined with transfer of a single frozen–thawed embryo in a subsequent cycle if necessary) identified similar pregnancy rates in both groups (OR 1.19, 95% CI 0.87–1.62) with a substantially higher multiple pregnancy rate following double ET (OR 62.83, 95% CI 8.52–463.57) (Pandian et al 2005). Several European countries have now adopted eSET coupled with effective cryopreservation programmes in patients with a good prognosis, and have reported no significant difference in pregnancy rates but a significant reduction in the rate of twin pregnancies. In carefully selected patients (e.g. women <37 years of age, undergoing their first IVF cycle and with more than one top-quality embryo), eSET plus subsequent frozen embryo replacement can be as effective as double ET.
Number of attempts
The literature appears to be somewhat divided regarding pregnancy and livebirth rates in relation to the number of ART attempts. In one study (Padilla and Garcia 1989), the pregnancy rate per ET was similar for at least seven attempts, while other studies (Tan et al 1992, Templeton et al 1996) reported a decline in pregnancy and livebirth rates with successive treatment cycles. The data from the HFEA 2000 annual report show a steady decline in pregnancy and livebirth rates per cycle after the fifth attempt, being 11.1% and 8.6%, respectively, at the 11th attempt (Figure 22.13).
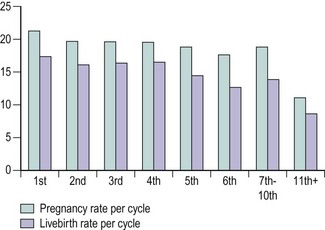
Figure 22.13 Livebirth rates by number of attempts following in-vitro fertilization.
Source: Human Fertilisation and Embryology Authority (2000).
When IVF cumulative pregnancy rates were estimated for a cohort of women, the rates showed a constant rise during the six initial IVF treatments and plateaued subsequently (Dor et al 1996). Similarly, the rates were reported to be as high as 80% after seven cycles, and a history of previous pregnancy improved a couple’s probability of conception significantly (Croucher et al 1998).
Embryo cryopreservation
In 2005, the HFEA annual register reported a clinical pregnancy rate of 18% and a livebirth rate of 15.6% per FER cycle in women using their own gametes (Human Fertilisation and Embryology Authority 2008). Over the past decade, considerable improvement has taken place. The French register (FIVNAT 1996) reported an increase in pregnancy rate per transfer from 11.5% to 16% between 1987 and 1995. It has been estimated that one FER cycle increases the ‘take-home baby’ rate by 5% (Kahn et al 1993), while treatment involving one fresh and two FER cycles achieves a cumulative viable pregnancy rate of 41% (Horne et al 1997). Frozen–thawed embryos can be replaced in natural or hormonally adjusted cycles utilizing GnRH agonist and oestrogen/progestogen preparations with comparable results. The use of GnRH agonist is useful in anovulatory or irregular cycles, and the use of different progestogens for luteal-phase support is equally effective. The cost per delivery for an FER cycle has been estimated to be between 25% and 45% of the cost of a fresh cycle. In view of these advantages, cryopreservation should be accessible and discussed with all couples where surplus good-quality embryos are available.
Oocyte donation
Oocyte donation is an effective treatment for women with premature ovarian failure, Turner’s syndrome, following oophorectomy, chemo- or radiotherapy-related ovarian failure, in certain cases where there is a high risk of transmitting a genetic disorder to the child, and where repeated failure of fertilization is attributed to poor oocyte quality. High pregnancy rates have been reported following oocyte donation for patients with Turner’s syndrome (Khastgir et al 1997). However, Turner’s patients were reported to have significantly higher biochemical pregnancy rates and early miscarriages, and lower clinical pregnancy and delivery rates compared with other women with premature ovarian failure (Yaron et al 1996). An important factor in the establishment of pregnancy is an endometrial thickness of greater than 6.5 mm. Other factors include the number of previous natural conceptions and live births, and the fertilization rate, while increasing female age does not affect the outcome (Burton et al 1992).
Livebirth rates following treatment using oocyte donation are excellent. Pregnancy and livebirth rates per treatment cycle following oocyte donation in comparison with other treatments are depicted in Figure 22.14 where fresh embryos are replaced, and in Figure 22.15 where frozen–thawed embryos are replaced (Human Fertilisation and Embryology Authority 2008). Neither the recipient’s age nor the indication of treatment affects the success of oocyte donation substantially. However, pregnancy resulting from oocyte donation should be considered as high risk, especially in those with ovarian failure, because of an increased incidence of small-for-gestational-age infants. Oocyte recipients should also be counselled regarding their increased risk for pregnancy-induced hypertension and postpartum haemorrhage.
Health Risks Associated with Assisted Reproduction Treatment
Ectopic pregnancy
The first pregnancy following IVF was an ectopic pregnancy. There is a higher rate of ectopic pregnancy after IVF/ICSI treatment, principally due to the high prevalence of tubal factor infertility. The obstetric risks and outcomes of ART have always been the subject of considerable debate. Between 2% and 5% of ART pregnancies have been reported to be ectopic and 0.1–0.3% have been reported to be heterotopic (Clayton et al 2006). For comparison, the prevalence of ectopic pregnancy and heterotopic pregnancy following natural conception is approximately one in 100 and one in 30,000 respectively. The overall ectopic pregnancy rate per treatment cycle was 0.3% of over 467,778 IVF/ICSI cycles performed in the UK from 1991 to 2005 (Human Fertilisation and Embryology Authority 2008).
Ovarian hyperstimulation syndrome
OHSS remains the most serious and potentially lethal complication of ovarian hyperstimulation, correlates positively with conceptual cycles and is almost exclusively related to either exogenous or endogenous hCG stimulation. The pathophysiology of OHSS is characterized by increased capillary permeability, mediated by the vasoactive substances released from the hyperstimulated ovaries. Fluid leaks from the intravascular compartment into the third space, causing intravascular dehydration and, in severe cases, multisystem dysfunction. Young age, presence of PCOS, lean body mass and previous history of OHSS have all been reported to be risk factors for the development of OHSS. The risk increases with excessive ovarian response to gonadotrophins, characterized by the development of a large number of follicles including immature and intermediate follicles and/or high serum oestradiol levels. Mild forms are common, affecting up to 33% of IVF cycles. The overall prevalence of moderate-to-severe OHSS varies from 3% to 8% of IVF/ICSI cycles (Mathur et al 2007). The time of onset of OHSS may determine the prognosis; late-onset OHSS is likely to be more severe and to last longer than early-onset OHSS. Early-onset OHSS is due to the effects of exogenous hCG and presents within 9 days of the ovulatory trigger hCG. Endogenous hCG from an early pregnancy triggers the development of late-onset OHSS, which develops 9 days after the ovulatory hCG.
Prevention and early recognition are the two most important components in the management of OHSS. The key to prevention is proper identification of the population at risk before treatment, and close monitoring of hormone levels as well as follicular response on ultrasound (Figure 22.16). When a high-risk situation is recognized, withholding the ovulatory dose of hCG and cancellation of the treatment cycle will almost certainly prevent OHSS. The couple should be advised to avoid intercourse as spontaneous ovulation may occur up to 11 days after discontinuing gonadotrophin treatment, resulting in conception and development of severe OHSS.
Alternative strategies have been attempted and, when effective, they usually ameliorate the severity of OHSS rather than prevent it absolutely (see Box 22.2).
Box 22.2 Alternative strategies to reduce the risk of OHSS
A diagnosis of OHSS is usually straightforward, with typical symptoms of abdominal distension, abdominal pain, nausea and vomiting, reduced urine output and breathing difficulties. Women with OHSS should have the severity of their condition assessed (RCOG 2006). Treatment of established OHSS depends on its severity, the stage at which the diagnosis is made and whether or not the patient is pregnant. In mild cases and most moderate cases of OHSS (haematocrit <45%), bed rest, simple analgesia, increased fluid intake and close monitoring of electrolyte balance may be sufficient. Women should be encouraged to drink to thirst, rather than to excess. The patient’s progress can be supervised on an outpatient basis. Hospital admission should be recommended to some women with moderate OHSS and all those with severe OHSS (haematocrit ≥45%, massive ascites). Women should be kept under review until resolution of the condition. Inpatient management is mainly aimed towards preventing or alleviating haemoconcentration, renal hypoperfusion and thromboembolic phenomena. Initiation of intravenous fluid is not always necessary unless oral intake cannot maintain renal perfusion and function. Analgesics and antiemetics should be instituted for symptomatic relief, along with subcutaneous heparin for antithrombotic prophylaxis. Diuretics should not be used in women with oliguria secondary to a reduced blood volume and decreased renal perfusion, as they may worsen intravascular dehydration. Paracentesis may be necessary for symptomatic relief or failing renal function as it results in a dramatic improvement in clinical symptoms, diuresis and improved creatinine clearance (Aboulghar et al 1992). Multidisciplinary input including critical care may be required for women who develop severe complications such as severe ascites, liver or renal dysfunction, thromboembolism or adult respiratory distress syndrome.
Risk of genital and breast cancer
The potential risk of genital cancer from follicular stimulation has initiated considerable debate (Brinton 2007). While the results of earlier studies were alarming, subsequent studies with much longer follow-up periods and better designs showed reassuring results. In a cohort study of 12,193 women, Brinton et al (2004) reported no increased risk associated with clomiphene or gonadotrophin use among subjects who had been followed-up for a median of 18.8 years. Subsequent larger studies concentrating on the effect of exposure to drugs used during IVF rather than those prescribed in earlier times also reported reassuring results (Venn et al 1999, Klip et al 2003). However, the average follow-up period in these studies was only 6–7 years. Therefore, long-term follow-up data are needed to fully evaluate the risk of exposure to gonadotrophins during IVF. Moreover, appropriate control groups should be used to determine whether the link is causal, as it is difficult to distinguish the effect of fertility treatment from that of infertility per se on the development of malignancies.
It is well established that endometrial cancer is sensitive to oestrogen, which is produced excessively during ovarian hyperstimulation. Most smaller cohort studies have not found any association between the fertility drugs and endometrial cancer, but the follow-up period in these studies has been less than 10 years (Brinton 2007). In a larger series with an average of over 20 years of follow-up, a significant two-fold increase in endometrial cancer was associated with ovulation induction agents (Modan et al 1998). However, this study evaluated women who had undergone fertility treatment between 1964 and 1974, and therefore these results cannot be extrapolated to modern-day IVF treatment.
Concerns relating to the effect of high oestrogen and progesterone levels on the potential risk for the development of breast cancer have also been expressed. A large cohort study of over 90,000 subjects showed no association between exposure to ART and breast cancer (Gauthier et al 2004).
Risk of congenital anomalies and malignancy in the newborn
Since IVF and ICSI became common fertility treatment modalities, concerns have been raised regarding the risk of major congenital malformations in children born following these treatments. While the majority of studies have shown an increased risk of major anomalies compared with spontaneously conceived pregnancies, some studies have been reassuring. A recent meta-analysis of 25 studies concluded that there is a statistically significant increase (30–40%) in birth defects compared with background risk (Hansen et al 2005). The authors expressed this result in terms of the number needed to harm, which in this case equates to the number of children that need to be conceived by ART for one additional child to be born with a birth defect. Therefore, for an underlying prevalence of birth defects between 1% and 4% of all births, the number needed to harm is between 250 and 62 treatments. ICSI enabled men with severe oligospermia or azoospermia to pass their genes on to their own progeny; an event that may not have been possible a few years ago. This raises certain questions about the genetic constitution of any resulting pregnancies after ICSI. A 1.2-fold increased risk of major birth defects in children born after ICSI (95% CI 0.97–1.28) compared with IVF was noted in a systematic review of the reported studies (Lie et al 2005). Sons of infertile males with Y chromosome microdeletions and born following ICSI treatment may inherit the same abnormality and are likely to be infertile.
The increased risk of congenital malformations in ART children may largely be due to the underlying parental background, rather than the treatment techniques themselves. In a recent comparative study of malformation in children born to fertile (time to pregnancy interval of ≤12 months) and subfertile parents (time to pregnancy interval of >12 months), the overall prevalence increased with increasing time to pregnancy. Compared with children in the fertile group, singletons born to infertile couples had a higher rate of congenital malformations regardless of whether they were conceived naturally (OR 1.20, 95% CI 1.07–1.35) or after infertility treatment (OR 1.39, 95% CI 1.23–1.57) (Zhu et al 2006).
There have been concerns regarding increased risk of rare imprinting disorders such as Beckwith–Wiedemann’s syndrome and Angelman’s syndrome in children following ART. However, the data are inconsistent, with some studies reporting increased frequency in ART children (Sutcliffe et al 2006) and other studies not finding any association (Bowdin et al 2007). Due to the rarity of these conditions, much larger studies are required for reliable detection of such associations.
Risk of malignancy in children
The issue of childhood cancer among children born after ART has been addressed in a few studies. An increased prevalence of retinoblastoma and Langerhan’s histiocytosis among children born after ART in comparison with the general population has been reported in two separate studies (Moll et al 2003, Kallen et al 2005). However, the overall risk of cancer and the individual incidence of all other recorded cancers were no greater than that in the general population (Kallen et al 2005). As childhood cancers are rare, larger studies are required for reliable evaluation of any increase in risk amongst ART children.
In the context of pregnancy outcomes, the aim of ART should be a healthy singleton delivery at term. However, infertile couples may not share this target. They do not appreciate the risks associated with multiple pregnancy; indeed, between 67% and 90% of couples may desire a twin birth, while less than one-third of couples regard a single child as an ideal outcome. Increasing age or duration of infertility has been associated with greater desire for multiple births (Murdoch 1997). As discussed earlier in this chapter, an increased multiple pregnancy rate is a major reason for the increased adverse obstetric and perinatal outcomes associated with ART.
Obstetric and Perinatal Outcomes of Assisted Reproduction Treatment
Antenatal and intrapartum complications following assisted reproduction
Many studies have examined obstetric outcomes after fertility treatment. The risk of spontaneous miscarriage appears to be higher among ART pregnancies than among the general population. This comparison can be misleading as ART pregnancies are commonly under intense surveillance; losses from a very early stage of pregnancy are often carefully documented and reported, whereas miscarriage rates among natural conceptions are extremely difficult to measure and can be easily underestimated. However, a large prospective study with a meticulously chosen control group concluded that the risk of spontaneous abortion may be increased by 20–34% in ART pregnancies compared with natural pregnancies after adjusting for differences in maternal age and previous spontaneous abortion (Wang et al 2004). However, this increased risk may be as a result of infertility itself rather than due to ART, as there was no difference in miscarriage rates between patients who achieved pregnancy following any fertility treatment, including ART, and those who conceived naturally while waiting for fertility treatment (Pezeshki et al 2000).
Comparison of obstetric outcomes of IVF/ICSI pregnancies with matched normally conceived pregnancies showed a significantly increased incidence of premature delivery, intrauterine growth restriction, vaginal bleeding and hypertension requiring hospitalization, pre-eclampsia and caesarean births (Halliday 2007). The increased obstetric risk can be attributed to the high rate of multiple pregnancies in most cases. However, the risk of a more complicated pregnancy was found to be increased even when the ART twin and singleton pregnancies were compared with spontaneously conceived twins and singletons, respectively, in two separate meta-analyses, even after the subjects were matched for maternal age (McDonald et al 2005a,b). A similar conclusion was drawn in another systematic review with similar objectives (Helmerhorst et al 2004).
Pregnancy following ICSI treatment has generated interest due to the additional risks involved with these pregnancies, such as the microinjection technique and the use of testicular or epidydimal sperm. However, no significant difference in obstetric outcomes between IVF and ICSI pregnancies was noted, except for prematurity which was higher in IVF singletons (Ombelet et al 2005). Couples undergoing ICSI for severe male infertility (oligoasthenoteratozoospermia) have slightly reduced fertilization rates, but have similar rates of pregnancy loss and other obstetric outcomes as other couples undergoing ICSI and IVF for non-male-factor infertility (Mercan et al 1998).
Women who conceive following oocyte donation, especially those with a history of ovarian failure, should be considered as high risk. While the majority of oocyte recipients experience a favourable outcome, an increased rate of obstetric complications in these pregnancies has been reported by many investigators (Soderstrom-Anttila 2001). Pregnancy-induced hypertension appears to occur more often than expected, even among young recipients (Wiggins and Main 2005), and the caesarean section rate is high.
Embryo cryopreservation and obstetric outcome
Cryopreservation of embryos has no apparent negative effect on obstetric outcomes. The results were comparable in pregnancies following transfer of frozen embryos derived from IVF or ICSI. However, the frozen ICSI group showed a significantly higher miscarriage rate than the frozen conventional IVF patients in one study (Aytoz et al 1999).
Perinatal outcome of assisted reproduction pregnancies
It is widely accepted that the increased incidence of multiple pregnancies following ART accounts for a disproportionately large share of adverse perinatal outcomes, including the increased incidence of very low birth weight, low birth weight, perinatal and neonatal mortality, and infant death. The increased incidence of death and morbidity in twin pregnancies compared with singleton pregnancies has been attributed mainly to prematurity and to adverse outcomes associated with premature delivery, such as hyaline membrane disease, hypocalcaemia, hypoglycaemia, hyperbilirubinaemia, small for gestational age and low Apgar scores. Slotnick and Ortega (1996) suggested that monoamniotic multiple gestations may be increased in zona-manipulated cycles (ICSI, zona drilling, assisted hatching), and although all resulting monoamniotic pregnancies ended in live births, there was a high incidence of intrauterine discordance in fetal growth. Even when compared with matched control spontaneous twin pregnancies, ART twin pregnancies had low birth weights and needed more neonatal intensive care (Helmerhorst et al 2004). However, twins conceived with IVF/ICSI have comparable perinatal mortality and morbidity rates to spontaneously conceived controls (McDonald et al 2005a).
Elimination of multiple pregnancies will not eliminate the increased risk of adverse perinatal outcomes in ART. While twins born from assisted conception are not significantly disadvantaged compared with spontaneous twin pregnancies, ART singletons have a worse perinatal outcome compared with other singletons (Helmerhorst et al 2004). Singletons born from ART are at risk for lower mean birth weight and small for gestational age. Perinatal mortality appears to be significantly higher, even after matching for maternal age, parity and infant gender. ICSI treatment does not appear to differ from conventional IVF when perinatal outcomes are compared. Retrospective comparison of births resulting from cryopreserved embryos with those after conventional IVF and fresh ET showed no difference in perinatal mortality between the two groups (Wada et al 1994).
Preimplantation Genetic Diagnosis/Screening
Preimplantation genetic diagnosis (PGD) has been developed as an alternative to prenatal diagnosis of chromosomal or sex-linked disorders in embryos formed through ART procedures. Preimplantation genetic screening (PGS) refers to the application of PGD in infertile couples undergoing conventional ART as an alternative to the traditional selection of embryos based on morphological criteria, which do not detect aneuploidy embryos that are non-viable or which could give rise to a viable but disabled child (Anderson and Pickering 2008). Aneuploid embryos most commonly result from fertilization of oocytes that are affected with meiotic non-disjunction, a common reason for age-related decline in oocyte quality. By detecting and avoiding the transfer of aneuploid embryos and only selecting euploid embryos for transfer, the success of ART cycles could potentially be improved. The application of PGD has also been extended to preimplantation human leukocye antigen (HLA) matching to ensure the birth of HLA-identical offspring for stem cell transplantation therapy to siblings.
The enzyme approach has been disregarded, for the few genes studied, as it has been difficult to differentiate between maternal gene expression (mRNA inherited in the egg cytoplasm) and embryonic gene expression in the early embryo (Braude et al 1989). However, the analysis of chromosomes using fluorescent in-situ hybridization and the analysis of specific gene sequences using sensitive polymerase chain reaction for sexing or detection of a specific gene mutation, such as cystic fibrosis, have been used successfully.
Analysis of the first and second polar bodies is an alternative to embryonic biopsy. As the polar bodies contain the genetic information from the oocyte, it is possible to diagnose maternally derived mutations, translocations and aneuploidy. Paternally derived chromosomal abnormalities will be missed by polar body biopsies. In cases of recessive disorders, polar body biopsies are helpful as they can provide information about the maternal contribution to the embryo. It may be difficult to achieve a reliable diagnosis even with embryo biopsy, with which mosaicism and/or uniparental disomies deriving from trisomies originating from female meiotic errors will be missed. With the expanding range of PGD indications, combined testing is required when testing for the causative gene, linked markers, HLA typing and aneuploidy in the same case. Therefore, single, double or even triple biopsies may be required in order to establish an accurate PGD (Kuliev and Verlinsky 2008). Sequential or simultaneous additional biopsy procedures have been shown to have no detrimental effect on embryo development (Cieslak-Janzen et al 2006).
PGS has been proposed to improve the IVF outcome in women of advanced age, and in those with a history of recurrent miscarriage, repeated implantation failure or severe male infertility, who are at risk for having increased proportions of aneuploid embryos. Most commonly, nine chromosomes (13, 14, 15, 16, 18, 21, 22, X and Y) are screened for aneuploidy. Although PGS has no adverse effect on embryo development, the current available evidence does not support its use outside the context of research. A meta-analysis of five prospective randomized-controlled trials evaluating the effect of PGS for the indication of advanced women’s age has reported a significant reduction in ongoing pregnancy rates (OR 0.56, 95% CI 0.42–0.76) compared with controls (Mastenbroek et al 2008). While the studies evaluating the use of PGS for other indications mentioned above are limited, the early evidence suggests that PGS does not improve the outcome, even in patients with a good prognosis (young age) who are undergoing single ET (Staessen et al 2008). In conclusion, the available evidence does not support the use of PGS to improve livebirth rates or reduce miscarriage rates in ART cycles, particularly for women of advanced age.
Looking into the Future
Improving pregnancy and livebirth rates will remain a challenge for the future. Molecular cytogenetic procedures on polar bodies or blastomeres have indicated that more than 50% of embryos derived after ART are aneuploid. Elective transfer of euploid or top-quality embryo(s) increases the chance of a successful outcome. Currently, the embryos are scored based on morphology, but this is notoriously difficult and often subjective. Available evidence does not support the use of more invasive PGS techniques to improve livebirth rates following ART, and therefore more accurate assessment methods are needed. A number of non-invasive tests, such as embryo viability, assessment of metabolomic profile and amino acid turnover in the embryo culture media, are being evaluated in many studies and have the potential to predict which embryos have the highest implantation potential (Nagy et al 2008, Sturmey et al 2008).
Oocyte cryopreservation is possible and requires superovulation treatment to induce multifollicular development followed by oocyte retrieval (Albani et al 2008). Oocyte cryopreservation is considered to be extremely inefficient, with approximately 100 cryopreserved oocytes needed to achieve one pregnancy. The mature oocyte is extremely fragile due to intracellular ice formation during the freezing or thawing process. There is potentially an increased risk of damage to the meiotic spindle apparatus and hardening of the zona pellucida, leading to reduced post-thaw survival, fertilization and pregnancy rates. Improvements in both advanced vitrification and slow-freezing methods have recently resulted in an increase in the efficiency of using human cryopreserved oocytes in assisted reproduction. Although the technique is currently considered experimental, this is likely to become the fastest growing area of fertility therapy in the future. It is reassuring that no increased chromosomal abnormalities, birth defects or developmental delays have been reported in children born from cryopreserved oocytes based on the limited number of pregnancies and deliveries reported to date.
Ovarian cryopreservation has been regarded as a potential method of fertility preservation for more than a decade (Anderson et al 2008). This method has the potential advantages of preservation of a large number of oocytes within primordial follicles, it does not require hormonal stimulation when time is short, and it may be appropriate for the prepubertal. Ovarian tissues can subsequently be autotransplanted, either at orthotopic (at or around the ovarian fossa) or heterotopic locations (subcutaneously at various locations including the forearm and abdominal wall), after the patient has completed treatment. Although few livebirths following either spontaneous or IVF conceptions have been reported, more research is needed in order to enhance the revascularization process, with the goal of reducing the follicular loss that takes place after tissue grafting. These technologies are still experimental, although tremendous progress has been made recently. There are many ethical concerns with these interventions, particularly their experimental nature in emotionally vulnerable patients, who often present to ART units with unrealistic expectations, the possibility of harvesting malignant cells with germ cells, and the potential for continued transmission of germ-line mutations in cancer predisposition genes. All of these highlight the need for further research and well-designed controlled clinical trials.
The technique of in-vitro maturation (IVM) of oocytes has been practised in a few IVF units worldwide (Suikkari 2008). The primary aim of IVM is to make IVF safer and simpler for women with polycystic ovaries and those at high risk of OHSS. Immature oocytes are recovered by puncture during non-stimulated cycles or after a low-dose stimulation protocol in a woman with polycystic ovaries. Priming the follicle with FSH for 2–3 days to stimulate growth to 8–12 mm diameter has been associated with improvements in implantation rates. Additional treatment with 10,000 IU hCG 36 h before oocyte retrieval has also been shown to improve the maturation rate of immature oocytes and accelerate the maturation process. Immature oocytes collected are allowed to mature in culture for approximately 30 h before insemination or ICSI. The clinical outcome has improved substantially in recent years, with pregnancy rates of over 20% in some centres. It is estimated that over 1000 children have been born following IVM worldwide. Postnatal follow-up studies of the children have been reassuring. IVM has not yet become a mainstream fertility treatment, mainly due to its lower success rate compared with conventional IVF. Knowledge regarding the molecular mechanisms of oocyte maturation and improvement in the culture system will improve the efficacy of IVM. While there is definite benefit for the use of IVM in women with polycystic ovaries and those at risk of OHSS, its role in couples with unexplained infertility or poor ovarian reserve remains to be defined.
KEY POINTS
Abdalla H, Thum MY. Repeated testing of basal FSH levels has no predictive value for IVF outcome in women with elevated basal FSH. Human Reproduction. 2006;21:171-174.
Aboulghar MA, Mansour RT, Serour GI, Riad R, Ramzi AM. Autotransfusion of the ascitic fluid in the treatment of severe ovarian hyperstimulation syndrome. Fertility and Sterility. 1992;58:1056-1059.
Academy of Medical Royal Colleges. Implementing and Ensuring Safe Sedation Practice for Healthcare Procedures in Adults. Report of an Intercollegiate Working Party Chaired by the Royal College of Anaesthetists. London: AOMRC, London; 2001.
Agrawal R, Conway G, Sladkevicius P, et al. Serum vascular endothelial growth factor and Doppler blood flow velocities in in vitro fertilization: relevance to ovarian hyperstimulation syndrome and polycystic ovaries. Fertility and Sterility. 1998;70:651-658.
Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception: a Cochrane review. Reproductive Biomedicine Online. 2007;14:640-649.
Al-Inany HG, Abou-Setta AM, Aboulghar MA, Mansour RT, Serour GI. Efficacy and safety of human menopausal gonadotrophins versus recombinant FSH: a meta-analysis. Reproductive Biomedicine Online. 2008;16:81-88.
Albani E, Barbieri J, Novara PV, Smeraldi A, Scaravelli G, Levi Setti PE. Oocyte cryopreservation. Placenta. 2008;29(Suppl B):143-146.
Alrayyes S, Fakih H, Khan I. Effect of age and cycle responsiveness in patients undergoing intracytoplasmic sperm injection. Fertility and Sterility. 1997;68:123-127.
Anderson RA, Pickering S. The current status of preimplantation genetic screening: British Fertility Society policy and practice guidelines. Human Fertility (Cambridge, England). 2008;11:71-75.
Anderson RA, Wallace WH, Baird DT. Ovarian cryopreservation for fertility preservation: indications and outcomes. Reproduction. 2008;136:681-689.
Aytoz A, Van den Abbeel E, Bonduelle M, et al. Obstetric outcome of pregnancies after the transfer of cryopreserved and fresh embryos obtained by conventional in-vitro fertilization and intracytoplasmic sperm injection. Human Reproduction. 1999;14:2619-2624.
Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Human Reproduction Update. 2003;9:505-514.
Bancsi LF, Broekmans FJ, Mol BW, Habbema JD, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertility and Sterility. 2003;79:1091-1100.
Bartkowiak R, Kaminski P, Wielgos M, Bobrowska K. The evaluation of uterine cavity with saline infusion sonohysterography and hysteroscopy in infertile patients. Neuroendocrinology Letters. 2006;27:523-528.
Bennett SJ, Waterstone JJ, Cheng WC, Parsons J. Complications of transvaginal ultrasound-directed follicle aspiration: a review of 2670 consecutive procedures. Journal of Assisted Reproduction and Genetics. 1993;10:72-77.
Bhattacharya S, Hamilton MP, Shaaban M, et al. Conventional in-vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: a randomised controlled trial. The Lancet. 2001;357:2075-2079.
Blake DA, Farquhar CM, Johnson N, Proctor M 2007 Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database of Systematic Reviews: CD002118.
Bowdin S, Allen C, Kirby G, et al. A survey of assisted reproductive technology births and imprinting disorders. Human Reproduction. 2007;22:3237-3240.
Braude PR, Monk M, Pickering SJ, Cant A, Johnson MH. Measurement of HPRT activity in the human unfertilised oocyte and pre-embryo. Prenatal Diagnosis. 1989;9:839-850.
Brinton L. Long-term effects of ovulation-stimulating drugs on cancer risk. Reproductive Biomedicine Online. 2007;15:38-44.
Brinton LA, Lamb EJ, Moghissi KS, Scoccia B, Althuis MD, Mabie JE, Westhoff CL. Ovarian cancer risk after the use of ovulation-stimulating drugs. Obstetrics and Gynecology. 2004;103:1194-1203.
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Human Reproduction Update. 2006;12:685-718.
Broekmans FJ, Knauff EA, te Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: current knowledge and future trends. Trends in Endocrinology and Metabolism. 2007;18:58-65.
Broekmans FJ, Visser JA, Laven JS, Broer SL, Themmen AP, Fauser BC. Anti-Müllerian hormone and ovarian dysfunction. Trends in Endocrinology and Metabolism. 2008;19:340-347.
Brown JA, Buckingham K, Abou-Setta A, Buckett W 2007 Ultrasound versus ‘clinical touch’ for catheter guidance during embryo transfer in women. Cochrane Database of Systematic Reviews: CD006107.
Burton G, Abdalla HI, Kirkland A, Studd JW. The role of oocyte donation in women who are unsuccessful with in-vitro fertilization treatment. Human Reproduction. 1992;7:1103-1105.
Cieslak-Janzen J, Tur-Kaspa I, Ilkevitch Y, Bernal A, Morris R, Verlinsky Y. Multiple micromanipulations for preimplantation genetic diagnosis do not affect embryo development to the blastocyst stage. Fertility and Sterility. 2006;85:1826-1829.
Clayton HB, Schieve LA, Peterson HB, Jamieson DJ, Reynolds MA, Wright VC. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstetrics and Gynecology. 2006;107:595-604.
Cohen J, Alikani M, Malter HE, Adler A, Talansky BE, Rosenwaks Z. Partial zona dissection or subzonal sperm insertion: microsurgical fertilization alternatives based on evaluation of sperm and embryo morphology. Fertility and Sterility. 1991;56:696-706.
Coomarasamy A, Afnan M, Cheema D, van der Veen F, Bossuyt PM, van Wely M. Urinary hMG versus recombinant FSH for controlled ovarian hyperstimulation following an agonist long down-regulation protocol in IVF or ICSI treatment: a systematic review and meta-analysis. Human Reproduction. 2008;23:310-315.
Croucher CA, Lass A, Margara R, Winston RM. Predictive value of the results of a first in-vitro fertilization cycle on the outcome of subsequent cycles. Human Reproduction. 1998;13:403-408.
Cutting R, Morroll D, Roberts SA, Pickering S, Rutherford A. Elective single embryo transfer: guidelines for practice. British Fertility Society and Association of Clinical Embryologists. Human Fertility (Cambridge, England). 2008:1-16.
Daya S 2000 Gonadotropin releasing hormone agonist protocols for pituitary desensitization in in vitro fertilization and gamete intrafallopian transfer cycles. Cochrane Database of Systematic Reviews: CD001299.
de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertility and Sterility. 2002;77:357-362.
Dechaud H, Anahory T, Reyftmann L, Loup V, Hamamah S, Hedon B. Obesity does not adversely affect results in patients who are undergoing in vitro fertilization and embryo transfer. European Journal of Obstetrics, Gynecology and Reproductive Biology. 2006;127:88-93.
Dor J, Seidman DS, Ben-Shlomo I, Levran D, Ben-Rafael Z, Mashiach S. Cumulative pregnancy rate following in-vitro fertilization: the significance of age and infertility aetiology. Human Reproduction. 1996;11:425-428.
Drakeley AJ, Jorgensen A, Sklavounos J, et al. A randomized controlled clinical trial of 2295 ultrasound-guided embryo transfers. Human Reproduction. 2008;23:1101-1106.
ESHRE Capri Workshop Group. Optimal use of infertility diagnostic tests and treatments. Human Reproduction. 2000;15:723-732.
Evers JL, Slaats P, Land JA, Dumoulin JC, Dunselman GA. Elevated levels of basal estradiol-17beta predict poor response in patients with normal basal levels of follicle-stimulating hormone undergoing in vitro fertilization. Fertility and Sterility. 1998;69:1010-1014.
Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Müllerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Human Reproduction. 2005;20:923-927.
Fedorcsak P, Dale PO, Storeng R, et al. Impact of overweight and underweight on assisted reproduction treatment. Human Reproduction. 2004;19:2523-2528.
Fishel S, Lisi F, Rinaldi L, et al. Systematic examination of immobilizing spermatozoa before intracytoplasmic sperm injection in the human. Human Reproduction. 1995;10:497-500.
FIVNAT. Evaluation of frozen embryo transfers from 1987 to 1994. Contraception Fertilite Sexualite. 1996;24:700-705.
Frattarelli JL, Bergh PA, Drews MR, Sharara FI, Scott RT. Evaluation of basal estradiol levels in assisted reproductive technology cycles. Fertility and Sterility. 2000;74:518-524.
Friedler S, Schenker JG, Herman A, Lewin A. The role of ultrasonography in the evaluation of endometrial receptivity following assisted reproductive treatments: a critical review. Human Reproduction Update. 1996;2:323-335.
Gauthier E, Paoletti X, Clavel-Chapelon F. Breast cancer risk associated with being treated for infertility: results from the French E3N cohort study. Human Reproduction. 2004;19:2216-2221.
Geber S, Paraschos T, Atkinson G, Margara R, Winston RM. Results of IVF in patients with endometriosis: the severity of the disease does not affect outcome, or the incidence of miscarriage. Human Reproduction. 1995;10:1507-1511.
Gupta S, Agarwal A, Agarwal R, Loret de Mola JR. Impact of ovarian endometrioma on assisted reproduction outcomes. Reproductive Biomedicine Online. 2006;13:349-360.
Hall JE, Welt CK, Cramer DW. Inhibin A and inhibin B reflect ovarian function in assisted reproduction but are less useful at predicting outcome. Human Reproduction. 1999;14:409-415.
Halliday J. Outcomes of IVF conceptions: are they different? Best Practice and Research Clinical Obstetrics and Gynaecology. 2007;21:67-81.
Hansen M, Bower C, Milne E, de Klerk N, Kurinczuk JJ. Assisted reproductive technologies and the risk of birth defects — a systematic review. Human Reproduction. 2005;20:328-338.
Helmerhorst FM, Perquin DA, Donker D, Keirse MJ. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ (Clinical Research Ed.). 2004;328:261.
Horne G, Critchlow JD, Newman MC, Edozien L, Matson PL, Lieberman BA. A prospective evaluation of cryopreservation strategies in a two-embryo transfer programme. Human Reproduction. 1997;12:542-547.
Hughes EG, Fedorkow DM, Daya S, Sagle MA, Van de Koppel P, Collins JA. The routine use of gonadotropin-releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: a meta-analysis of randomized controlled trials. Fertility and Sterility. 1992;58:888-896.
Hull MG. Infertility treatment: relative effectiveness of conventional and assisted conception methods. Human Reproduction. 1992;7:785-796.
Human Fertilisation and Embryology Authority. Ninth annual report and accounts. http://www.hfea.gov.uk/docs/Annual-Report-9th-2000.pdf, 2000.
Human Fertilisation and Embryology Authority. Facts and figures 2006. http://www.hfea.gov.uk/docs/Facts_and_Figures_2006_fertility_probs_and_treatment_2008-10-08.pdf, 2008.
Isikoglu M, Berkkanoglu M, Senturk Z, Coetzee K, Ozgur K. Endometrial polyps smaller than 1.5 cm do not affect ICSI outcome. Reproductive Biomedicine Online. 2006;12:199-204.
Jayaprakasan K, Campbell BK, Clewes JS, Johnson IR, Raine-Fenning NJ. Three-dimensional ultrasound improves the interobserver reliability of antral follicle counts and facilitates increased clinical work flow. Ultrasound in Obstetrics and Gynecology. 2008;31:439-444.
Jayaprakasan K, Hopkisson JF, Campbell BK, Clewes J, Johnson IR, Raine-Fenning NJ. Quantification of the effect of pituitary down-regulation on 3D ultrasound predictors of ovarian response. Human Reproduction. 2008;23:1538-1544.
Jayaprakasan K, Al-Hasie H, Jayaprakasan R, et al. The three-dimensional ultrasonographic ovarian vascularity of women developing poor ovarian response during assisted reproduction treatment and its predictive value. Fertility and Sterility. 2009;92:1862-1869.
Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Müllerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertility and Sterility. 2010;93:855-864.
Kahn JA, von During V, Sunde A, Sordal T, Molne K. The efficacy and efficiency of an in-vitro fertilization programme including embryo cryopreservation: a cohort study. Human Reproduction. 1993;8:247-252.
Kallen B, Finnstrom O, Nygren KG, Olausson PO. In vitro fertilization in Sweden: child morbidity including cancer risk. Fertility and Sterility. 2005;84:605-610.
Khastgir G, Abdalla H, Thomas A, Korea L, Latarche L, Studd J. Oocyte donation in Turner’s syndrome: an analysis of the factors affecting the outcome. Human Reproduction. 1997;12:279-285.
Klip H, van Leeuwen FE, Schats R, Burger CW. Risk of benign gynaecological diseases and hormonal disorders according to responsiveness to ovarian stimulation in IVF: a follow-up study of 8714 women. Human Reproduction. 2003;18:1951-1958.
Kolibianakis EM, Kalogeropoulou L, Griesinger G, et al. Among patients treated with FSH and GnRH analogues for in vitro fertilization, is the addition of recombinant LH associated with the probability of live birth? A systematic review and meta-analysis. Human Reproduction Update. 2007;13:445-452.
Kuliev A, Verlinsky Y. Preimplantation genetic diagnosis: technological advances to improve accuracy and range of applications. Reproductive Biomedicine Online. 2008;16:532-538.
Kwee JJ, Elting MM, Schats RR, McDonnell JJ, Lambalk NC. Ovarian volume and antral follicle count for the prediction of low and hyper responders with in vitro fertilization. Reproductive Biology and Endocrinology. 2007;5:9.
La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Müllerian hormone throughout the human menstrual cycle. Human Reproduction. 2006;21:3103-3107.
Lie RT, Lyngstadaas A, Orstavik KH, Bakketeig LS, Jacobsen G, Tanbo T. Birth defects in children conceived by ICSI compared with children conceived by other IVF-methods; a meta-analysis. International Journal of Epidemiology. 2005;34:696-701.
Lin PC. Reproductive outcomes in women with uterine anomalies. Journal of Womens Health. 2004;13:33-39.
Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology — a systematic review. Human Reproduction Update. 2007;13:433-444.
Mannaerts B, de Leeuw R, Geelen J, et al. Comparative in vitro and in vivo studies on the biological characteristics of recombinant human follicle stimulating hormone. Endocrinology. 1991;129:2623-2630.
Mardesic T, Muller P, Zetova L, Mikova M. [Factors affecting the results of in vitro fertilization — I. The effect of age]. Ceska Gynekologie. 1994;59:259-261.
Mastenbroek S, Scriven P, Twisk M, Viville S, Van der Veen F, Repping S. What next for preimplantation genetic screening? More randomized controlled trials needed? Human. Reproduction. 2008;23:2626-2628.
Mathur R, Kailasam C, Jenkins J. Review of the evidence base of strategies to prevent ovarian hyperstimulation syndrome. Human Fertility (Cambridge, England). 2007;10:75-85.
Matson PL, Yovich JL. The treatment of infertility associated with endometriosis by in vitro fertilization. Fertility and Sterility. 1986;46:432-434.
McDonald S, Murphy K, Beyene J, Ohlsson A. Perinatal outcomes of in vitro fertilization twins: a systematic review and meta-analyses. American Journal of Obstetrics and Gynecology. 2005;193:141-152.
McDonald SD, Murphy K, Beyene J, Ohlsson A. Perinatal outcomes of singleton pregnancies achieved by in vitro fertilization: a systematic review and meta-analysis. Journal of Obstetrics and Gynaecology Canada. 2005;27:449-459.
McIlveen M, Skull JD, Ledger WL. Evaluation of the utility of multiple endocrine and ultrasound measures of ovarian reserve in the prediction of cycle cancellation in a high-risk IVF population. Human Reproduction. 2007;22:778-785.
Mercan R, Lanzendorf SE, Mayer JJr, Nassar A, Muasher SJ, Oehninger S. The outcome of clinical pregnancies following intracytoplasmic sperm injection is not affected by semen quality. Andrologia. 1998;30:91-95.
Mochtar MH, Van der V, Ziech M, van Wely M 2007 Recombinant luteinizing hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles. Cochrane Database of Systematic Reviews: CD005070.
Modan B, Ron E, Lerner-Geva L, et al. Cancer incidence in a cohort of infertile women. American Journal of Epidemiology. 1998;147:1038-1042.
Moll AC, Imhof SM, Cruysberg JR, Schouten-van Meeteren AY, Boers M, van Leeuwen FE. Incidence of retinoblastoma in children born after in-vitro fertilisation. The Lancet. 2003;361:309-310.
Moore J, Copley S, Morris J, Lindsell D, Golding S, Kennedy S. A systematic review of the accuracy of ultrasound in the diagnosis of endometriosis. Ultrasound in Obstetrics and Gynecology. 2002;20:630-634.
Murdoch A. Triplets and embryo transfer policy. Human Reproduction. 1997;12:88-92.
Nagy ZP, Sakkas D, Behr B. Symposium: innovative techniques in human embryo viability assessment. Non-invasive assessment of embryo viability by metabolomic profiling of culture media (‘metabolomics’). Reproductive Biomedicine Online. 2008;17:502-507.
National Institute for Clinical Excellence. Fertility: Assessment and Treatment for People with Fertility Problems. National Collaborating Centre for Women’s and Children’s Health for the National Institute for Clinical Excellence. London: RCOG Press; 2004. p 33
Oatway C, Gunby J, Daya S 2004 Day three versus day two embryo transfer following in vitro fertilization or intracytoplasmic sperm injection. Cochrane Database of Systematic Reviews: CD004378.
Oliveira FG, Abdelmassih VG, Diamond MP, Dozortsev D, Melo NR, Abdelmassih R. Impact of subserosal and intramural uterine fibroids that do not distort the endometrial cavity on the outcome of in vitro fertilization–intracytoplasmic sperm injection. Fertility and Sterility. 2004;81:582-587.
Ombelet W, Cadron I, Gerris J, et al. Obstetric and perinatal outcome of 1655 ICSI and 3974 IVF singleton and 1102 ICSI and 2901 IVF twin births: a comparative analysis. Reproductive Biomedicine Online. 2005;11:76-85.
Padilla SL, Garcia JE. Effect of maternal age and number of in vitro fertilization procedures on pregnancy outcome. Fertility and Sterility. 1989;52:270-273.
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. The Lancet. 1992;340:17-18.
Pandian Z, Templeton A, Serour G, Bhattacharya S. Number of embryos for transfer after IVF and ICSI: a Cochrane review. Human Reproduction. 2005;20:2681-2687.
Pezeshki K, Feldman J, Stein DE, Lobel SM, Grazi RV. Bleeding and spontaneous abortion after therapy for infertility. Fertility and Sterility. 2000;74:504-508.
Piette C, de Mouzon J, Bachelot A, Spira A. In-vitro fertilization: influence of women’s age on pregnancy rates. Human Reproduction. 1990;5:56-59.
Preutthipan S, Amso N, Curtis P, Shaw RW. The influence of number of embryos transferred on pregnancy outcome in women undergoing in vitro fertilization and embryo transfer (IVF-ET). Journal of the Medical Association of Thailand. 1996;79:613-617.
Royal College of Obstetricians and Gynaecologists. The management of ovarian hyperstimulation syndrome. Greentop Guideline 5. London: RCOG Press; 2006.
Salim R, Woelfer B, Backos M, Regan L, Jurkovic D. Reproducibility of three-dimensional ultrasound diagnosis of congenital uterine anomalies. Ultrasound in Obstetrics and Gynecology. 2003;21:578-582.
Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertility and Sterility. 1989;51:651-654.
Scott RTJr, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertility and Sterility. 1990;54:297-302.
Seifer DB, Lambert-Messerlian G, Hogan JW, Gardiner AC, Blazar AS, Berk CA. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertility and Sterility. 1997;67:110-114.
Sharif K, Elgendy M, Lashen H, Afnan M. Age and basal follicle stimulating hormone as predictors of in vitro fertilisation outcome. British Journal of Obstetrics and Gynaecology. 1998;105:107-112.
Slotnick RN, Ortega JE. Monoamniotic twinning and zona manipulation: a survey of U.S. IVF centers correlating zona manipulation procedures and high-risk twinning frequency. Journal of Assisted Reproduction and Genetics. 1996;13:381-385.
Sneed ML, Uhler ML, Grotjan HE, Rapisarda JJ, Lederer KJ, Beltsos AN. Body mass index: impact on IVF success appears age-related. Human Reproduction. 2008;23:1835-1839.
Soderstrom-Anttila V. Pregnancy and child outcome after oocyte donation. Human Reproduction Update. 2001;7:28-32.
Somigliana E, Arnoldi M, Benaglia L, Iemmello R, Nicolosi AE, Ragni G. IVF–ICSI outcome in women operated on for bilateral endometriomas. Human Reproduction. 2008;23:1526-1530.
Spandorfer SD, Davis OK, Barmat LI, Chung PH, Rosenwaks Z. Relationship between maternal age and aneuploidy in in vitro fertilization pregnancy loss. Fertility and Sterility. 2004;81:1265-1269.
Speroff L, Fritz MA. Assisted reproductive technologies. In: Speroff L, Fritz MA, editors. Clinical Gynecologic Endocrinology and Infertility. Philadelphia: Lippincott Williams and Wilkins; 2005:1215-1274.
Staessen C, Janssenswillen C, van den Abbeel E, Devroey P, van Streitegham AC. Avoidance of triplet pregnancies by elective transfer of two good quality embryos. Human Reproduction. 1993;8:1650-1653.
Staessen C, Verpoest W, Donoso P, et al. Preimplantation genetic screening does not improve delivery rate in women under the age of 36 following single-embryo transfer. Human Reproduction. 2008;23:2818-2825.
Strandell A. Treatment of hydrosalpinx in the patient undergoing assisted reproduction. Current Opinions in Obstetrics and Gynecology. 2007;19:360-365.
Sturmey RG, Brison DR, Leese HJ. Symposium: innovative techniques in human embryo viability assessment. Assessing embryo viability by measurement of amino acid turnover. Reproductive Biomedicine Online. 2008;17:486-496.
Suikkari AM. In-vitro maturation: its role in fertility treatment. Current Opinion in Obstetrics and Gynecology. 2008;20:242-248.
Sutcliffe AG, Peters CJ, Bowdin S, et al. Assisted reproductive therapies and imprinting disorders — a preliminary British survey. Human Reproduction. 2006;21:1009-1011.
Tan SL, Royston P, Campbell S, et al. Cumulative conception and livebirth rates after in-vitro fertilisation. The Lancet. 1992;339:1390-1394.
Tarin JJ. Subzonal insemination, partial zona dissection or intracytoplasmic sperm injection? An easy decision? Human Reproduction. 1995;10:165-170.
te Velde ER, Pearson PL. The variability of female reproductive ageing. Human Reproduction Update. 2002;8:141-154.
Templeton A, Morris JK, Parslow W. Factors that affect outcome of in-vitro fertilisation treatment. The Lancet. 1996;348:1402-1406.
Templeton A, Morris JK. Reducing the risk of multiple births by transfer of two embryos after in vitro fertilization. New England Journal of Medicine. 1998;339:573-577.
Toner JP, Flood JT. Fertility after the age of 40. Obstetrics and Gynecology Clinics of North America. 1993;20:261-272.
van Rooij IA, Bancsi LF, Broekmans FJ, Looman CW, Habbema JD, te Velde ER. Women older than 40 years of age and those with elevated follicle-stimulating hormone levels differ in poor response rate and embryo quality in in vitro fertilization. Fertility and Sterility. 2003;79:482-488.
van Rooij IA, Broekmans FJ, te Velde ER, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Human Reproduction. 2002;17:3065-3071.
Van Royen E, Mangelschots K, De Neubourg D, et al. Characterization of a top quality embryo, a step towards single-embryo transfer. Human Reproduction. 1999;14:2345-2349.
Van Voorhis BJ. Clinical practice. In vitro fertilization. New England Journal of Medicine. 2007;356:379-386.
Vardon D, Burban C, Collomb J, Stolla V, Erny R. [Spontaneous pregnancies in couples after failed or successful in vitro fertilization]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction. 1995;24:811-815.
Vauthier-Brouzes D, Lefebvre G, Lesourd S, Gonzales J, Darbois Y. How many embryos should be transferred in in vitro fertilization? A prospective randomized study. Fertility and Sterility. 1994;62:339-342.
Venn A, Watson L, Bruinsma F, Giles G, Healy D. Risk of cancer after the use of fertility drugs with in-vitro fertilisation. Lancet. 1999;354:1586-1590.
Verberg MF, Eijkemans MJ, Macklon NS, et al. The clinical significance of the retrieval of a low number of oocytes following mild ovarian stimulation for IVF: a meta-analysis. Human Reproduction Update. 2009;15:5-12.
Verberg MF, Macklon NS, Nargund G, et al. Mild ovarian stimulation for IVF. Human Reproduction Update. 2009;15:13-29.
Wada I, Macnamee MC, Wick K, Bradfield JM, Brinsden PR. Birth characteristics and perinatal outcome of babies conceived from cryopreserved embryos. Human Reproduction. 1994;9:543-546.
Wang JX, Norman RJ, Wilcox AJ. Incidence of spontaneous abortion among pregnancies produced by assisted reproductive technology. Human Reproduction. 2004;19:272-277.
Wennerholm WB. Cryopreservation of embryos and oocytes: obstetric outcome and health in children. Hum Reprod. 2000;15(Suppl 5):18-25.
Widra EA, Gindoff PR, Smotrich DB, Stillman RJ. Achieving multiple-order embryo transfer identifies women over 40 years of age with improved in vitro fertilization outcome. Fertility and Sterility. 1996;65:103-108.
Wiggins DA, Main E. Outcomes of pregnancies achieved by donor egg in vitro fertilization — a comparison with standard in vitro fertilization pregnancies. American Journal of Obstetrics and Gynecology. 2005;192:2002-2006. discussion 2006–2008
Wood C, McMaster R, Rennie G, Trounson A, Leeton J. Factors influencing pregnancy rates following in vitro fertilization and embryo transfer. Fertility and Sterility. 1985;43:245-250.
Yaron Y, Ochshorn Y, Amit A, Yovel I, Kogosowki A, Lessing JB. Patients with Turner’s syndrome may have an inherent endometrial abnormality affecting receptivity in oocyte donation. Fertility and Sterility. 1996;65:1249-1252.
Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility, infertility treatment, and congenital malformations: Danish national birth cohort. BMJ (Clinical Research Ed.). 2006;333:679.