Chapter 21 Arrhythmias
Disorders of cardiac rhythm are common in the intensive care unit (ICU). Life-threatening arrhythmias such as ventricular tachycardia and complete heart block require immediate intervention. Even arrhythmias such as atrial fibrillation can cause marked hemodynamic instability in cardiac surgery patients. Arrhythmias are commonly a manifestation of underlying cardiac pathology; ventricular fibrillation may be due to acute myocardial ischemia, whereas atrial fibrillation may reflect raised atrial pressure due to ventricular dysfunction. Thus, it is essential that treatment be directed to the underlying cause as well as to the suppression of the arrhythmia.
In this chapter the pathophysiology, diagnosis, and treatment of cardiac arrhythmias, including the use of temporary pacing for the treatment of bradyarrhythmias, are reviewed. The physiology of cardiac conduction and the pharmacology of antiarrhythmic agents are discussed in Chapters 1 and Chapter 3, respectively. The interpretation of an electrocardiogram (ECG) is reviewed in Chapter 8.
PATHOPHYSIOLOGY OF TACHYARRHYTHMIAS
Enhanced and Abnormal Automaticity
Cells of the sinoatrial (SA) node and around the atrioventricular (AV) node normally undergo spontaneous depolarization during phase 4 of the action potential. Any factor that increases the slope of phase 4 (such as β1-adrenoreceptor stimulation) causes the threshold potential to be reached earlier, increasing the rate of action-potential generation (see Fig. 1-2). This is called normal automaticity. If the firing rate of a subsidiary pacemaker is abnormally increased such that sinus node dominance is usurped, it is termed enhanced automaticity, and accelerated junctional rhythm is an example of this. When cells that are normally quiescent become partially depolarized, and resting membrane potential rises to −60 or −50 mV, pathologic pacemaker activity may occur. This is called abnormal automaticity, and it is the basis of some atrial tachycardias.
Reentry
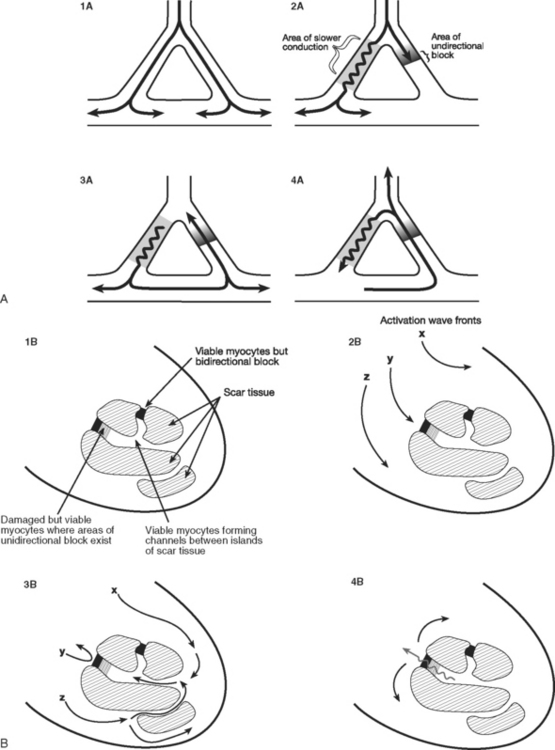
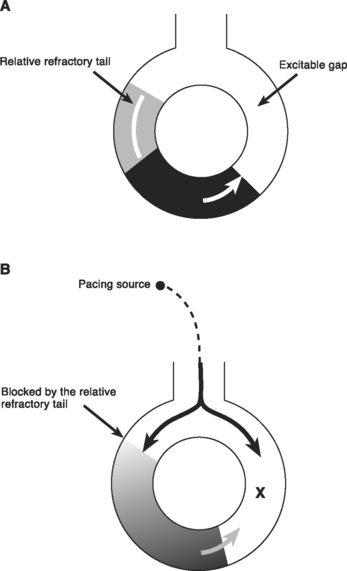
Reentry is the basis of most arrhythmias encountered clinically. Reentry is continuous circulating electricity in which an impulse reenters and repetitively excites a region of the heart. Three requirements are necessary for reentry to occur: (1) an abnormal electrical circuit; (2) slow conduction; and (3) unidirectional block (Fig. 21-1). These conditions may arise because of a fixed anatomic structure such as that which occurs in the Wolff-Parkinson-White syndrome, but more commonly, the conditions for reentry arise due to abnormal physiologic states. For instance, dynamic circuits with unidirectional block may develop due to variability in recovery times (dispersion of refractoriness) among various regions of myocardium. Slow conduction may occur because of partial membrane depolarization or loss of gap junctions.
Proarrhythmic Factors
Hypokalemia
Hypokalemia decreases the resting membrane potential (i.e., it becomes less negative), which would be expected to increase the likelihood of spontaneous depolarization. However, a more important effect is that hypokalemia decreases the permeability of the membrane to potassium, which prolongs repolarization and increases the dispersion of recovery times, thereby increasing the likelihood that a reentry circuit will occur. Hypokalemia is associated with a wide range of arrhythmias, including atrial and ventricular extrasystoles, atrial fibrillation, and torsades de pointes ventricular tachycardia. The ECG signs of hypokalemia are described in Chapter 32.
Myocardial Infarction
The mechanism by which ventricular tachycardia is propagated late after myocardial infarction is quite different from the mechanism responsible for its generation immediately after the onset of ischemia. Within the first 10 minutes of acute ischemia, ventricular tachycardia and fibrillation may occur due to reentry allowed by slowed conduction and unidirectional block in ischemic tissue. In the days that follow myocardial infarction, infarcted myocardium is gradually replaced by scar tissue. Filaments of surviving myocardium encased in this scar tissue show slowed conduction, partly as a consequence of altered membrane potential and partly due to altered architecture and increased intracellular resistance. If unidirectional entrance block occurs at one site on the scar border, with subsequent delayed emergence of a different wave front, ventricular tachycardia may ensue when adjacent, fully repolarized normal myocardium is then immediately reactivated (or depolarized; see Fig. 21-1). Removal of this scar-related circuit is the basis of the “endocardial peel” operation.
NARROW-COMPLEX TACHYCARDIAS
Narrow-complex tachycardia may be defined as a heart rate greater than 100 per minute and a QRS duration less than 0.12 seconds (3 small squares on the ECG). Narrow-complex tachycardias activate the ventricles in a normal manner unless bundle branch block is present (see later material).
Approach to the Patient With Narrow-Complex Tachycardia
A careful review of the 12-lead ECG provides important clues to the diagnosis:
Atrial Electrocardiogram
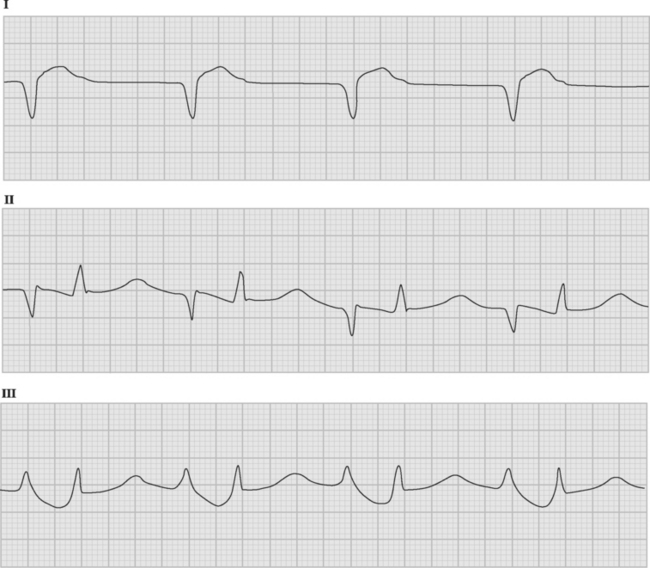
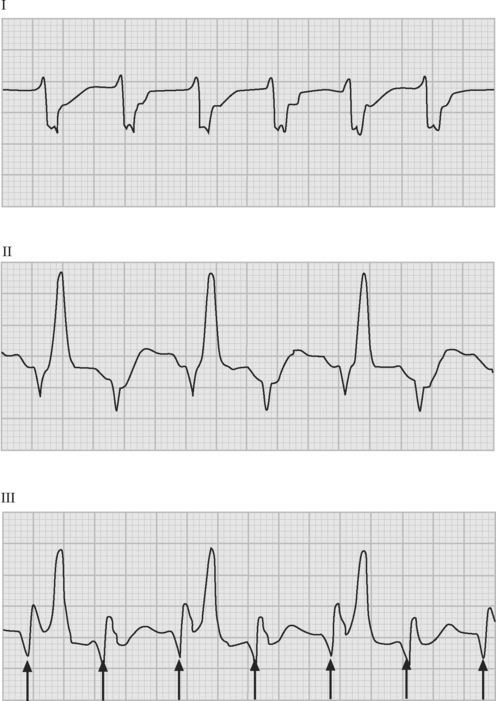
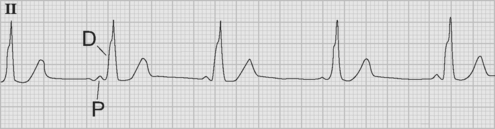
If temporary epicardial pacing wires are in place, an atrial ECG can be performed to clarify the relationship between the P wave and the QRS. One method involves connecting the left and right arm ECG cables to the atrial pacing wires. The left and right lower limb cables are connected normally, and leads I, II, and III are recorded at 50 mm/sec (Fig. 21-2). P waves appear as the dominant complexes in lead I (the bipolar atrial lead), whereas the QRS is usually dominant in leads II and III (the unipolar atrial leads). Alternatively, a single upper limb lead can be connected to an atrial pacing wire and a unipolar atrial lead examined on the monitor: if the left arm cable is used, lead I or III is examined; if the right arm cable is used, lead I or II may be examined.
Atrial Fibrillation
Atrial fibrillation following cardiac surgery is very common, with most series reporting an incidence of between 30% and 60%.1,2 The peak incidence is on the second postoperative day. Postoperative atrial fibrillation is not benign; it prolongs ICU and hospital stay, increases costs, and is associated with an increased incidence of stroke.2
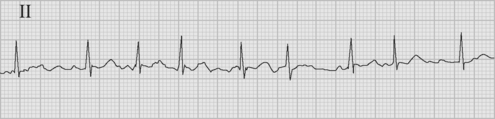
The arrhythmia is characterized by the abrupt onset of an irregularly irregular narrow-complex tachycardia (Fig. 21-3), often with varying R wave amplitude. Heart rate varies widely, depending on adrenergic tone, but is typically between 120 and 160 beats per minute. In cardiac surgery patients, the onset of atrial fibrillation may cause marked hypotension.
Pathophysiology
Atrial fibrillation was regarded for a long time as a classic example of random reentry due to multiple wandering wavelets.1 However, it is now recognized that in many patients the atria are only passively involved and that the initiating trigger is rapid focal discharges from cells located in the muscle sleeves of the pulmonary veins.3
Sustained atrial fibrillation leads to mechanical and electrical remodeling of the atria, with loss of contractility (atrial stunning) and shortening of refractory periods. Atrial stunning persists for several weeks after reestablishment of sinus rhythm, increasing the risk for atrial thrombus formation, especially in the left atrial appendage. The shortening of atrial refractory periods results in reinforcement of the arrhythmia the longer it remains untreated.4 Furthermore, patients are very vulnerable to reinduction of atrial fibrillation immediately after reversion to sinus rhythm. For these reasons, after successful cardioversion of atrial fibrillation of more than 48 hours’ duration, anticoagulation and antiarrhythmic therapy should continue for at least 30 days.5
Risk Factors
The principal risk factor for atrial fibrillation after cardiac surgery is increased age.2 Previous atrial fibrillation, mitral and aortic surgery, atrial enlargement, and withdrawal of β blockers are also important risk factors. Other factors associated with the development of atrial fibrillation include the requirement for inotropes or vasopressors, hypomagnesemia, prolonged cardiopulmonary bypass, and impaired ventricular function. Acute atrial stretch due to impaired ventricular function or tamponade may precipitate atrial fibrillation. Thus, atrial fibrillation may be both a cause and a consequence of hemodynamic instability.
Prophylaxis
β Blockers (metoprolol, carvedilol, and sotalol) and amiodarone reduce the incidence of atrial fibrillation by as much as 50%.6–9 The combination of amiodarone and a β blocker appears to be more effective than either agent alone.10 Other drugs such as magnesium and diltiazem may also be useful for prophylaxis but data are inconclusive.9 Standard (right atrial) pacing may have a small protective effect against atrial fibrillation, but the benefit is greater if simultaneous left and right atrial pacing is used.11,12 Digoxin is not indicated for prophylaxis of postoperative atrial fibrillation.7,9
Prophylaxis against atrial fibrillation should include avoiding β-blocker withdrawal in patients chronically treated with them. For patients at increased risk for atrial fibrillation, routine prophylaxis with a β blocker or amiodarone is appropriate.9 Prophylactic biatrial pacing should also be considered in high-risk patients.12
The preoperative administration of 600 mg per day of amiodarone for 1 week prior to surgery has been shown to be effective8 but may not be feasible. A practical alternative is intraoperative dosing of 5 mg/kg intravenously followed by oral treatment of 400 mg three times daily for 2 days, 200 mg twice daily for 2 days, and then 200 mg daily until hospital discharge. Amiodarone should be withheld in the presence of sick sinus syndrome, bradycardia (heart rate <50 beats per minute), or marked first-degree AV block (PR interval >0.30 seconds). In patients with paroxysmal atrial fibrillation who are chronically treated with amiodarone, low-dose oral sotalol (40 to 80 mg twice daily) may be commenced postoperatively, with careful monitoring for excessive bradycardia or QT prolongation. When atrial fibrillation is associated with planned mitral valve surgery, a surgical maze procedure should be considered (see Chapter 10).
Treatment
Atrial fibrillation that is associated with hemodynamic compromise should be treated aggressively. The success rate for primary cardioversion is low—less than 20% in one study.13 The success rate of electrical cardioversion may be increased by the use of biphasic energy (with an energy level of 200 joules) and the prior correction of hypokalemia and hypomagnesemia. If atrial pressures are elevated, an echocardiogram should be considered to rule out ventricular dysfunction and pericardial tamponade. If cardioversion is unsuccessful, it may be repeated following intravenous amiodarone (a bolus dose of 5 mg/kg over 30 minutes or an infusion of 2 mg/min for 4 hours). Amiodarone provides acute control of heart rate through its β-blocker actions and may itself result in pharmacologic cardioversion. Following successful cardioversion, an infusion of amiodarone (1 mg/min) should be administered until the patient is able to take oral amiodarone or a β blocker. Atrial pacing (AAI mode at 80 to 90 beats per minute for 24 to 48 hours) should be instituted to minimize the chance of recurrence. Intravenous sotalol or ibutilide may be used as an alternative in patients with good left ventricular function.14 If, despite these measures, the patient remains in rapid atrial fibrillation, pharmacologic control of heart rate may be achieved by an intravenous β blocker or diltiazem.15 In patients with unstable hemodynamics, intravenous digoxin may be tried, but it has limited efficacy in the setting of heightened adrenergic tone.15
Pharmacologic treatment for atrial fibrillation should be continued for 4 to 6 weeks following surgery, even if the patient reverts to sinus rhythm.15 If atrial fibrillation persists at 6 weeks following surgery, a choice should be made between repeat cardioversion and permanent rate control and anticoagulation. The latter strategy is favored by the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study, which did not show a better outcome for older patients with attempted maintenance of sinus rhythm by antiarrhythmic drugs.16
Cardioversion Guided by Transesophageal Echocardiography
Cardioversion should not be performed without the guidance of transesophageal echocardiography in patients who have experienced atrial fibrillation for more than 48 hours and are not undergoing therapeutic anticoagulation. This is so because of the risk for systemic embolization of intracardiac thrombi.17 If left atrial thrombus is present on transesophageal echocardiography (TEE) examination, the patient should be given anticoagulants with heparin and warfarin, and cardioversion should be performed 4 weeks later if repeat (TEE) shows resolution of the thrombus. If thrombus is not present, cardioversion may proceed immediately, after which the patient should undergo anticoagulation with warfarin for 4 to 6 weeks (see subsequent material).
Anticoagulation for Atrial Fibrillation
If there are no contraindications, patients with sustained or paroxysmal atrial fibrillation for more than 24 to 48 hours after cardiac surgery should be given an anticoagulant with warfarin, aiming for an International Normalised Ratio (INR, see Chapter 30) between 2 and 3. Warfarin should be continued for 4 to 6 weeks following surgery, at which time, if the patient is in sinus rhythm, it may be stopped.5 However, if there are factors predictive of recurrence, such as a history of paroxysmal atrial fibrillation, a left atrial diameter greater than 5 cm, or mitral valve surgery, warfarin should be continued.
Patients at increased risk for thromboembolic complications, notably those with documented atrial thrombus, a history of systemic embolization, or a prosthetic mitral or aortic valve, should also be given anticoagulants with unfractionated heparin, which should be continued until the patient has a therapeutic INR.5 Compared with warfarin, aspirin is less effective in reducing stroke frequency in patients with atrial fibrillation,18,19 and it is therefore not usually an acceptable alternative.
Nonpharmacologic Treatment of Atrial Fibrillation
A number of catheter-based techniques involving isolation of the triggering foci around the pulmonary veins and ablation of fractionated atrial potentials have been developed for the treatment of chronic atrial fibrillation.20,21 In older patients, radiofrequency ablation of the bundle of His with permanent pacemaker implantation provides good symptom relief, although the need for anticoagulation remains. These techniques have a minimal role in the management of postoperative atrial fibrillation.
Atrial Flutter
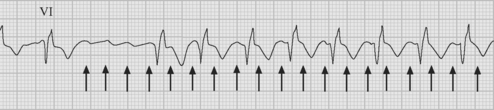
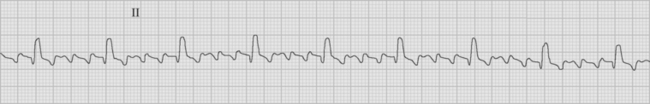
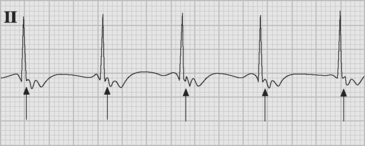
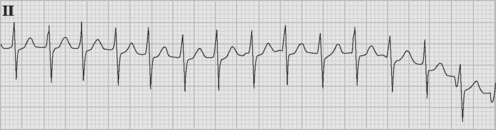
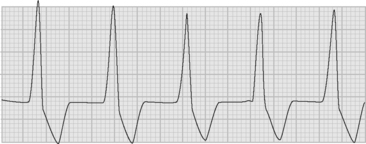
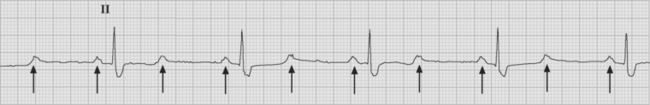
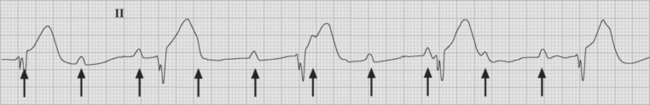
Atrial flutter is usually caused by a reentry circuit around the tricuspid valve with an area of slow conduction near the orifice of the coronary sinus, which results in abnormal flutter waves on the ECG that have a characteristic sawtooth pattern at a rate of between 250 and 330 per minute. Because of the long refractory period of the AV node, usually only a proportion of the flutter waves are conducted to the ventricles; typically in a ratio of 2:1 (Fig. 21-4), resulting in a heart rate of about 150. Higher ratios of AV block (e.g., 3:1 or 4:1) resulting in slower heart rates are also seen (Fig. 21-5). Postoperative atrial flutter typically has 2:1 conduction. If antiarrhythmic drugs have been given as part of routine prophylaxis, the ventricular rate with 2:1 block may be less (125 to 150 beats per minute). Atrial flutter with 2:1 AV block can be difficult to distinguish from other, regular narrow-complex tachycardias because one of the flutter waves may be concealed within the QRS complex; the rhythm may be mistaken for sinus tachycardia with first-degree AV block. Negative flutter waves on the inferior ECG leads and a fixed heart rate of 140 to 150 per minute usually confirm the diagnosis, but if doubt persists, an atrial ECG should be performed if possible (Fig. 21-6). Alternatively, adenosine (6 or 12 mg) as a rapid intravenous bolus will cause temporary AV block, exposing the characteristic flutter waves.
Treatment
The risks for thromboembolism with atrial flutter are no different from those with atrial fibrillation, and the same indications for anticoagulation and cardioversion guided by TEE apply. Typical atrial flutter can be permanently prevented at the time of cardiac surgery by creating a surgical or radiofrequency scar line between the tricuspid annulus and the orifice of the inferior vena cava, extending from endocardium to epicardium (see Fig. 10-8).22
Technique of Overdrive Pacing for Atrial Flutter
Within a reentry circuit, at any point in time a portion of the circuit is not being activated. This is the distance between the advancing head of the circulating wave front and the tail of depolarized cells; it is called the excitable gap. Pacing impulses can invade the circuit via this gap and, by colliding with the arrhythmia wave front, eliminate the arrhythmia (Fig. 21-7). This technique is known as overdrive pacing and is useful for certain reentry arrhythmias, including ventricular tachycardia and AV nodal reentry tachycardia, but it is particularly useful for atrial flutter.
Accelerated Junctional Rhythm
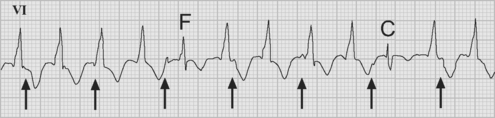
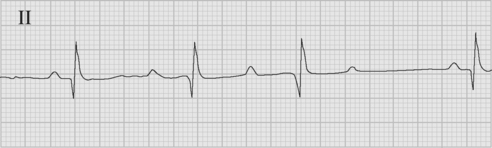
Accelerated junctional rhythm (nodal tachycardia) is a regular narrow-complex tachycardia in which no P wave can be seen preceding the QRS complex. The tachycardia typically develops gradually (warm up), slowly increasing up to a heart rate of 110 to 150 beats per minute. Retrograde-conducted P waves that follow the QRS are visible on an atrial ECG and may be seen on a standard rhythm strip (Fig. 21-8). Depending on the ventriculoatrial interval, regular cannon A waves may be visible on the central venous pressure (CVP) trace (see Chapter 8). Occasionally, the atrial ECG demonstrates AV dissociation, in which case irregular cannon waves are seen instead.
Paroxysmal Supraventricular Tachycardia
AV Node Reentry Tachycardia
Reentry within the AV node (“supraventricular tachycardia”) occurs as a consequence of abnormal nodal architecture, in which there are two routes across the AV node from the atria to the His bundle, a fast and a slow pathway.23 A reentry circuit is created by antegrade block across the fast pathway, activation across the slow pathway that continues on to the ventricles, and retrograde activation back to the atria via the fast pathway.
It presents with the abrupt onset of a regular narrow-complex tachycardia with a typical rate of 150 to 180 beats per minute (Fig. 21-9). On the atrial ECG, retrograde-conducted P waves may be seen within the QRS and, depending on the ventriculoatrial interval, regular cannon A waves may be seen on the CVP trace.
Accessory Pathways
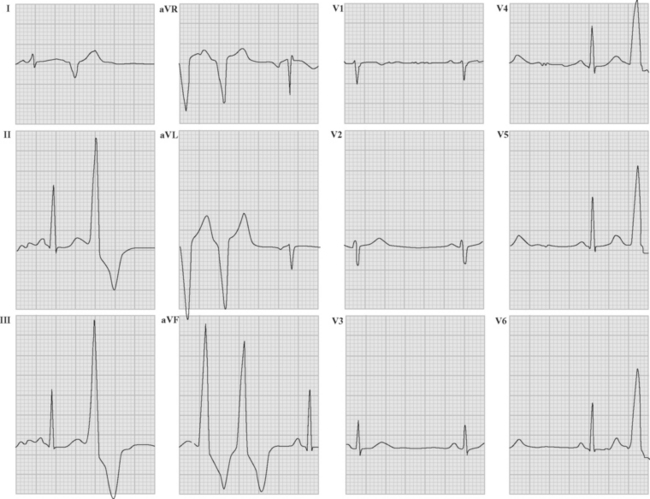
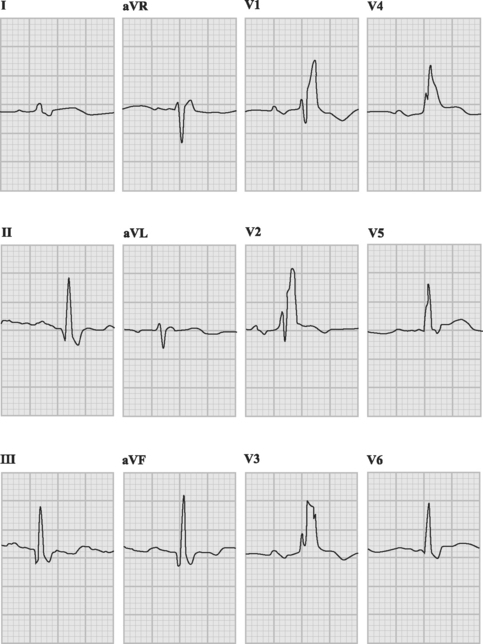
If conduction via the accessory pathway is faster than through the AV node, early (pre-) excitation of the ventricles occurs, resulting in a short PR interval and a delta wave on the resting ECG (Fig. 21-10). When conduction from atria to ventricles across the accessory pathway does not occur (so-called concealed accessory pathway), the resting ECG does not demonstrate preexcitation.
Patients with accessory pathways are at risk for paroxysmal supraventricular tachycardias. The most common arrhythmia associated with the Wolff-Parkinson-White syndrome is orthodromic tachycardia, in which antegrade conduction occurs via the AV node and retrograde conduction back to the atria occurs across the accessory pathway. This results in a narrow-complex regular tachycardia similar to AV node reentry tachycardia—and it responds to similar treatment. Less commonly, antidromic tachycardia occurs; in this case antegrade conduction occurs across the accessory pathway and retrograde conduction progresses up the normal conducting system. This results in a broad-complex tachycardia that is indistinguishable from ventricular tachycardia.24 This is best treated by electrical cardioversion. Atrial fibrillation may be conducted across the accessory pathway at very fast rates, resulting in a broad-complex tachycardia that resembles ventricular tachycardia but is usually irregular.
BROAD-COMPLEX TACHYCARDIAS
Ventricular Tachycardia Versus Supraventricular Tachycardia
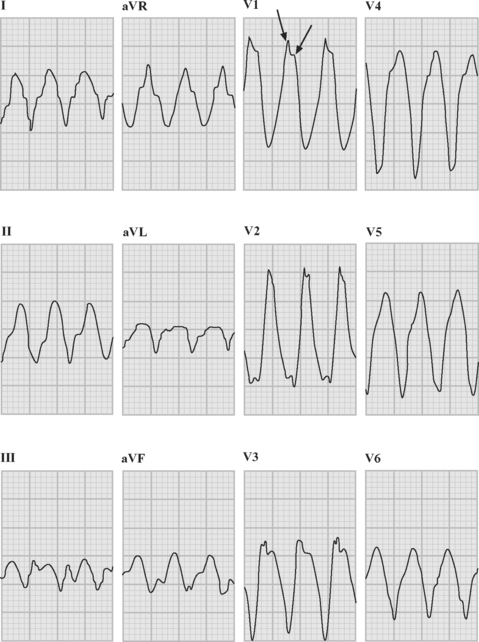
If a previous ECG demonstrates bundle branch block (see subsequent material) with the same morphology as the tachycardia, supraventricular tachycardia is highly likely—unfortunately, aberrant conduction is often present only during tachycardia, so the absence of a bundle branch pattern on a previous ECG does not rule out a supraventricular origin. If a previous ECG shows ventricular extrasystoles that have the same morphology (lead for lead) as the tachycardia, ventricular tachycardia is diagnosed (Fig. 21-11). If a delta wave is present on a previous ECG, and the initial QRS vectors during tachycardia are an exact match, supraventricular conduction over an accessory pathway is probable; irregular RR intervals strongly suggest atrial fibrillation. Additional supporting information that is suggestive of ventricular tachycardia includes:
Ventricular Tachycardia
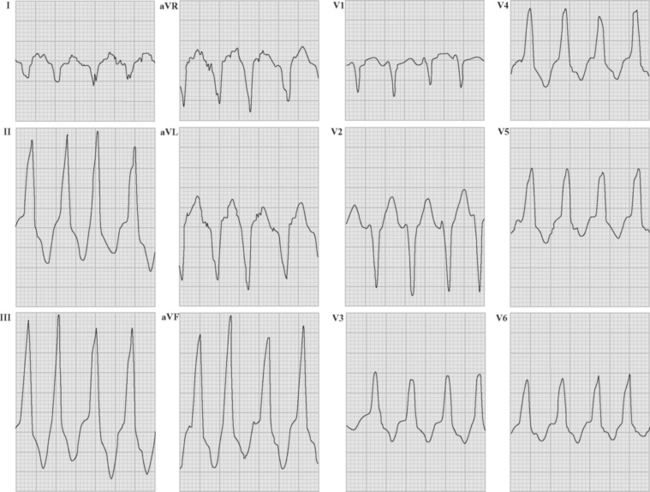
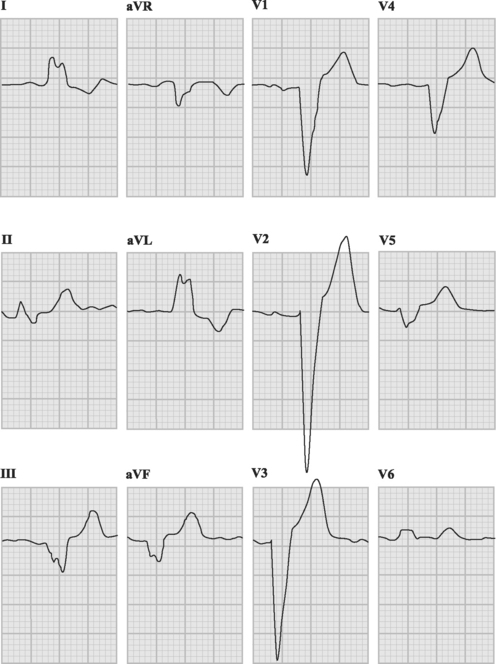
Ventricular tachycardia may be monomorphic (see Figs. 21-11 and 21-13), in which all the QRS complexes are of similar amplitude, or polymorphic, in which the QRS complexes are of variable amplitude (Fig. 21-14).
Polymorphic Ventricular Tachycardia
Polymorphic ventricular tachycardia is a rapid ventricular tachycardia with a characteristic variable morphology and sinusoidal variation in amplitude. Polymorphic ventricular tachycardia with an alternating QRS morphology is often associated with prolongation of the QT interval during sinus rhythm, in which case it is known as torsades de pointes. Torsades de pointes is thought to be initiated by delayed after-depolarizations and maintained by reentry. On the ECG rhythm strip, a ventricular premature beat followed by a long pause followed by a conducted QRS complex and a ventricular premature beat, which initiates the arrhythmia, is commonly seen (the so-called long-short initiation; see Fig. 21-14). Torsades de pointes is commonly recurrent. The ECG appearances of polymorphic ventricular tachycardia may be difficult to distinguish from “coarse” ventricular fibrillation (Fig. 21-15). Factors that worsen QT prolongation increase the risk for developing torsades de pointes; namely, bradycardia and AV block, hypokalemia, and antiarrhythmic drugs with class III activity. Polymorphic ventricular tachycardia may also occur in the absence of QT prolongation, most commonly in the setting of acute myocardial ischemia or infarction.
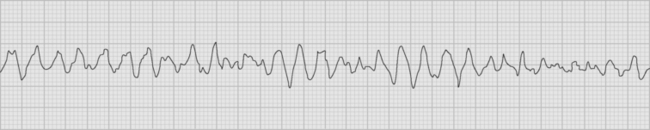
Figure 21.15 Rhythm strip showing coarse ventricular fibrillation. No discrete QRS complexes are visible.
Polymorphic ventricular tachycardia deriving from any cause is typically poorly tolerated and may degenerate into ventricular fibrillation. Immediate electrical cardioversion is usually necessary. In addition to cardioversion, treatment of torsades de pointes includes intravenous magnesium sulfate (1 to 2 g slowly over 1 to 2 minutes),25 pacing to a rate of at least 90 beats per minute, and correction of hypokalemia (aiming for a potassium concentration >4.5 mmol/l). Class III antiarrhythmic drugs such as sotalol and amiodarone are contraindicated, and agents such as lidocaine and β blockers are ineffective.
Recurrent Ventricular Tachycardia
Magnesium should be given empirically regardless of the QRS morphology because it is safe and sometimes highly effective. If torsades de pointes is excluded, amiodarone may be given as a bolus dose of 5 mg/kg followed by an infusion of 1 mg/min (see Table 3-6). The serum potassium should be increased to more than 5 mmol/l. If ventricular tachycardia persists, the addition of an intravenous β blocker may be highly effective. Occasionally, the addition of lidocaine to amiodarone may also be effective: the effect of two boluses of 75 mg 5 minutes apart, with the subsequent infusion of 2 mg/min can be tried; it can be discarded if not rapidly effective.
Measurement of the QT Interval
It is often hard to be certain where the T wave merges with the baseline. Consequently, measuring the QT interval is imprecise and is variable among different observers.26 Lead II is a good choice for measurement. It may be helpful to draw a line along the downslope of the T wave to the point where it intersects the baseline taken as the end of the QT interval (see Fig. 8-4). The interval varies according to heart rate. As a consequence it is normalized as the QTc, most commonly by dividing the measured QT interval by the square root of the preceding RR interval. Conventional normal values are less than or equal to 0.44 seconds for males and less than or equal to 0.46 seconds for females. With torsades de pointes, the QTc is commonly more than 0.55 seconds. When the QT interval is prolonged, the following U wave is usually prominent too and often is fused with the T wave. If there is no clear separation, the combined QTU interval should be measured.
Follow-up Management of Ventricular Tachycardia
If ventricular tachycardia occurs solely in the first 24 hours after heart surgery in the context of myocardial dysfunction, there is usually no long-term predisposition to recurrence. For ventricular tachycardia occurring later than that, a strategy for future protection of the patient is required. This most often will be implantation of a cardioverter-defibrillator. In patients with ejection fractions greater than 40% and hemodynamically tolerated ventricular tachycardia, long-term sotalol or amiodarone is an acceptable alternative. In patients with LBBB morphology ventricular tachycardia, especially if left ventricular function appears relatively normal, imaging of the right ventricle to confirm or exclude arrhythmogenic right ventricular cardiomyopathy is indicated.27
Nonsustained Ventricular Tachycardia
Nonsustained ventricular tachycardia is a common arrhythmia and although of prognostic importance when linked to heart disease, management is empiric rather than of proven advantage. As a rule, it reflects transient electrolyte disturbance, especially hypokalemia, in the immediate postoperative period. When nonsustained ventricular tachycardia persists after potassium repletion, patients should be examined for evidence of ventricular dysfunction, myocardial infarction, and acute ischemia. Persistent arrhythmias in patients with ejection fractions less than 40% and coronary artery disease are indications for an electrophysiologic study, looking for inducible sustained ventricular tachycardia.28 Patients with inducible sustained ventricular tachycardia are candidates for implantable cardioverter-defibrillators.
Ventricular Fibrillation
Ventricular fibrillation is a macro reentrant arrhythmia in which there are no clearly formed QRS or T waves. The rhythm has a gradually undulating baseline and looks the same when viewed upside down. Initially, the appearances are similar to those of polymorphic ventricular tachycardia, with relatively coarse fibrillation waves (see Fig. 21-15), but over a period of minutes the amplitude of the waves becomes progressively smaller (fine ventricular fibrillation), until eventually asystole develops. Ventricular fibrillation is not associated with any cardiac output and thus defines a state of cardiac arrest. Treatment of ventricular fibrillation is outlined in Chapter 20. In any patient who develops recurrent ventricular fibrillation (or ventricular tachycardia that deteriorates into ventricular fibrillation), the presence of acute myocardial ischemia should be a considered. In this situation, emergency coronary revascularization may be a lifesaving treatment. If recurrent ventricular fibrillation is persistently initiated by the same premature ventricular contraction (constant morphology), an urgent electrophysiology consultation should be sought with a view to emergency ablation of the triggering focus.
Ventricular Premature Beats
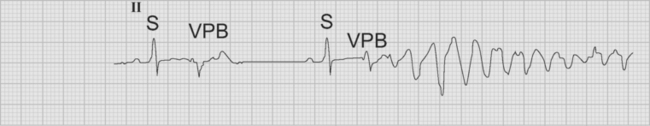
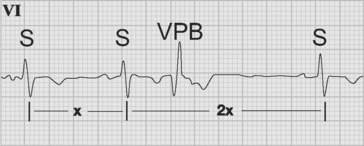
Ventricular premature beats are ectopic beats that originate in the ventricles. A typical ventricular premature beat occurs early—on or shortly after the T wave of the preceding sinus beat—and is associated with a wide QRS complex (Fig. 21-16). Ventricular premature beats are usually associated with a full compensatory pause, meaning that the interval between the preceding and following sinus beats is equal to two complete cycles (compare this with atrial premature beats, described earlier). If a ventricular premature beat follows every sinus beat, ventricular bigeminy is said to exist. When ventricular premature beats have different QRS morphologies they are assumed to arise from different foci and are commonly labeled multifocal (strictly speaking, they are multimorphic because different exits from a similar focus are also possible). When ventricular premature beats occur very early—with the R wave of the ectopic beat occurring on the T wave of the preceding beat—they can induce ventricular fibrillation. This is most likely to occur in the setting of myocardial infarction, hypokalemia, or QT prolongation. Despite the increased incidence of ventricular fibrillation associated with multifocal ventricular premature beats following myocardial infarction, no data show improved outcomes as a result of their suppression by antiarrhythmic drugs.
Accelerated Ventricular Rhythm
Accelerated ventricular rhythm (accelerated idioventricular rhythm, or slow ventricular tachycardia) is a broad-complex arrhythmia that arises due to enhanced automaticity within the bundle branches or fascicles of the Purkinje system (Fig. 21-17). It is associated with a heart rate of between 50 and 100 beats per minute and is usually self-terminating. Accelerated ventricular rhythm most commonly occurs in the early period following myocardial infarction or as a consequence of digoxin toxicity. There is AV dissociation, with the atrial and ventricular rates typically being relatively similar.
ELECTRICAL CARDIOVERSION
Cardioversion is a safe and highly effective therapy. Recently, the development of machines that deliver biphasic shocks has further increased the efficacy of cardioversion, compared with traditional direct-current monophasic machines. There are different types of biphasic waveforms, but it is not clear that any one of them is superior in terms of efficacy or reduced myocardial damage.
With rapid heart rates, low-energy synchronized shocks may cause ventricular fibrillation. Shocking with high energies (above the upper limit of vulnerability29) eliminates this risk. Thus, a general recommendation is that all elective cardioversions use energies of 200 joules monophasic or biphasic. The technique for cardioversion is described in Chapter 40.
ATRIOVENTRICULAR BLOCK
AV block is divided into first, second, and third (or “complete”) degrees.
First-Degree AV Block
First-degree AV block is diagnosed when the PR interval exceeds 0.2 seconds (5 small squares on the ECG; Fig. 21-18). It occurs as a consequence of disease in the AV node and is common in older patients. In the context of an aortic root abscess, first-degree AV block with a rapidly lengthening PR interval (over days) may signal the imminent development of complete AV block. In general, no treatment is required for first-degree AV block unless prolongation of the PR interval is extreme (>400 ms) or rapidly evolving, in which case pacing is indicated. Prophylactic antiarrhythmic drug therapy is best avoided in patients with marked first-degree AV block.
Second-Degree AV Block
Wenckebach Second-Degree Block (Mobitz Type I)
Wenckebach AV block is reasonably common in older patients with heart disease. There is progressive prolongation of the PR interval with eventual failure to conduct a P wave, followed by resumption of conduction (Fig. 21-19). The PR interval after the blocked beat is always shorter than the PR interval immediately before it. Wenckebach block usually reflects AV nodal disease but is not an automatic indication for pacing. When second-degree block has appeared for the first time following cardiac surgery and is associated with bradycardia, permanent pacing should be considered.
2:1 AV Block and High-Grade AV Block
2:1 AV block describes bradycardia in which every second P wave fails to conduct to the ventricles (Fig. 21-20). Block may be either at the level of the AV node or in the His-Purkinje system. With the latter, there is nearly always associated bundle branch block. Persistent block requires permanent pacing to restore normal heart rate. Transient 2:1 AV block may occur during sleep or because of a surge of vagal tone (e.g., during vomiting).
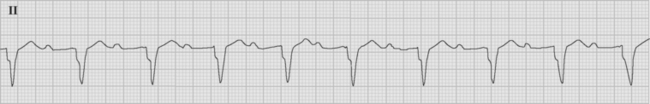
Complete, or Third-Degree, AV Block
With complete heart block there is complete failure of conduction through the AV node and dissociation between the atria and the ventricles (Fig. 21-21). P waves can be seen marching through the QRS complex. The width and frequency (i.e., heart rate) of the QRS complex depends upon the level within the conducting system at which the escape rhythm originates. If the escape rhythm is near the AV node, the heart rate is typically about 50 beats per minute and the QRS relatively normal. An escape rhythm within the His bundle results in a heart rate of 30 to 40 beats per minute, and the QRS complex may be normal or widened. An escape rhythm that originates below the His bundle is very wide and very slow. Intermittent cannon A waves may be seen on the CVP trace.
The treatment for complete heart block is pacing. In the absence of pacing wires, a bolus dose of atropine (0.6 mg, repeated up to 0.04 mg/kg) combined with an infusion of epinephrine may be used; isoproterenol may also be used but commonly causes hypotension (see Chapter 3). Transcutaneous pacing (see subsequent material) may be necessary until more definitive treatment (e.g., transvenous or permanent pacing) is available.
Bundle Branch Block and Fascicular Block
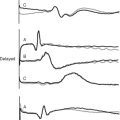
The His bundle gives rise to the left and right bundle branches, with the left bundle further dividing into anterior and posterior fascicles (see Chapter 1). Thus, there are three terminal branches (or fascicles), one on the right and two on the left. Block of any one branch is called unifascicular block and is not a risk factor for complete AV block. Block of any two branches—either complete LBBB or RBBB with associated block of the anterior (common) or posterior (rare) fascicle—is termed bifascicular block. Block of the anterior fascicle is termed left anterior hemiblock, and block of the posterior fascicle is termed left posterior hemiblock. ECGs showing LBB and RBBB are shown in Figure 21-22 and Figure 21-23, respectively; the ECG criteria for bundle branch and fascicular block are listed in Table 21-1.
Table 21-1 ECG Criteria for Bundle Branch and Fascicular Block
| Unifascicular Block | ||
| Anterior fascicle of the left bundle branch: left anterior hemiblock | Normal QRS duration | |
| Left axis deviation | ||
| Small Q in leads I and aVL | ||
| Small R in leads II, III, and aVF | ||
| Posterior fascicle of the left bundle branch: left posterior hemiblock | Normal QRS duration | |
| Right axis deviation | ||
| Small R in leads I and aVL | ||
| Small Q in leads II, III, and aVF | ||
| No evidence of right ventricular hypertrophy (see Chapter 8 and Fig 24.1) | ||
| Right bundle branch block (see Fig. 21-23) | QRS | >0.12 sec |
| Lead V1 | Late secondary R wave (rSR) | |
| Lead V6 | Late slurred S wave | |
| Lead I | Late slurred S wave | |
| Bifascicular Block | ||
| Left bundle branch block (see Fig. 21-22) | QRS | >0.12 sec |
| Lead V1 | Wide slurred S wave or QS complex | |
| Lead V6 | No Q wave | |
| Broad, monophasic or notched R wave | ||
| Lead 1 | Broad, monophasic or notched R wave | |
| Right bundle branch block with left anterior hemiblock (common) | QRS >0.12 sec | |
| Left axis deviation | ||
| Appearances of right bundle branch block in lead V1 | ||
| Initial R wave and prominent S wave in leads II, III, and aVF | ||
| Right bundle branch block with left posterior hemiblock (uncommon) | QRS > 0.12 sec | |
| Appearances of right bundle branch block in lead V1 | ||
| Right axis deviation | ||
| Initial R wave and prominent S wave in leads I and aVL | ||
LBBB is usually indicative of structural heart disease, particularly coronary artery disease, whereas RBBB may be a normal variant. Occasionally, RBBB develops during insertion of a pulmonary artery catheter. Thus, LBBB is a relative contraindication to pulmonary artery catheterization (see Chapter 8).
Atrioventricular Block Following Cardiac Surgery
Sick Sinus Syndrome
Sick sinus syndrome is a term that includes inappropriate sinus bradycardia, sinus pauses, sinus node exit block, and bradycardia-tachycardia syndrome. In sinus node exit block, pauses of the same duration, or multiples of the sinus rate, without preceding P waves are seen. In bradycardia-tachycardia syndrome, bouts of tachycardia (atrial fibrillation, atrial flutter, AV node reentry) are interspersed with bradycardia. Sick sinus syndrome is the most common indication for permanent pacing.
TEMPORARY EPICARDIAL PACING
Commonly, following cardiac surgery, four pacing wires are sutured to the heart, two each to the right atrium and right ventricle. The leads are then attached to the positive and negative terminals of the atrial and ventricular outputs of the temporary pacing unit. However, only one lead, conventionally connected to the negative terminal of the pacemaker, must be actually attached to the chamber being paced. The positive terminal may be attached to any conducting surface in the body such as a subcutaneous pacing skin suture or another cardiac chamber. Although it may be possible to pace using the positive terminal attached to the heart (anodal pacing), the thresholds will be higher (particularly with a skin electrode) and capture is less likely. Indications for conversion to a permanent pacemaker are described earlier.
Commonly Used Pacing Modes
Atrial Pacing
Atrial pacing (AOO and AAI) maintains the benefits of atrial systole but requires normal AV conduction. In the early postoperative period, AV conduction may be generally intact but there may be marked PR prolongation. In this situation, intermittent second- and third-degree AV block can occur, and atrial pacing is not recommended.
Dual-Chamber Pacing
DOO is asynchronous dual-chamber pacing; like VOO, it carries the risk for inadvertent ventricular fibrillation. In DDD mode, both chambers are sensed and paced. The pacemaker senses native atrial activity, and if the rate is greater than the set pacemaker rate, the pacemaker is inhibited. If the atrial rate is less than the set rate, the atrium is paced. If AV conduction is intact (i.e., if it takes less time than the AV interval that is set on the pacemaker), ventricular pacing is inhibited. If AV conduction is absent or is delayed for longer than the set AV interval, ventricular pacing is triggered. In DDD mode, native sinus rhythm above the set rate, in the presence of normal AV conduction, completely inhibits the pacemaker.
Pacemaker Settings in Cardiac Surgery Patients
When setting up temporary epicardial pacing, the following parameters must be set.
Mode
Only three pacing modes are commonly used in the ICU:
Testing for the Presence of an Escape Rhythm
When pacing is begun for AV block, it is essential to check periodically for resumption of conduction or the presence of an escape pacemaker. Because of overdrive suppression, if temporary pacing is stopped abruptly without prior rate reduction, asystole commonly occurs, and it may be incorrectly interpreted as pacemaker dependence. To test for an escape rhythm, the pacing rate should be decreased from a rate of 60 beats per minute by 10 beats per minute every 30 seconds to about 30 beats per minute to look for the emergence of normal rhythm or an escape rhythm. If there is still only paced rhythm, output can be briefly inhibited for 3 to 4 seconds to see if there is intrinsic activity; then pacing at a normal rate is resumed.
Troubleshooting Temporary Epicardial Pacing
Failure to Capture
Failure to capture may arise because of a problem with the epicardial wires, which may become disconnected from the heart or, over time, become fibrosed and ineffective. In one study, loss of capture occurred in more than 50% of patients by postoperative day 5.30 In the first instance, the outputs of the pacemaker should be increased to their maximum values (20 to 25 mA). Reversing the polarity of the leads may help, or connecting each epicardial lead in turn to the negative pole of the pacemaker and using a subcutaneous pacing suture for the positive lead. Correction of electrolyte and acid-base disturbances may help. In DDD or AAI mode, failure to capture sometimes occurs because the pacemaker is over sensitive (sensitivity value too low) and inappropriate inhibition occurs; this problem can be rectified by increasing the sensitivity (see earlier discussion). Failure to capture the atrium in DDD mode is occasionally improved by changing to DVI mode, in which the atrial component is asynchronous. If pacing is essential and these interventions are ineffective, placement of a permanent pacemaker or a temporary transvenous pacing wire may be necessary.
Other Types of Pacing
Implantable Cardioverter-Defibrillators
The superiority of implantable cardioverter-defibrillators over antiarrhythmic drug therapy for survivors of cardiac arrest is well established.31 Prophylactic insertion of implantable cardioverter-defibrillators also confers a survival benefit in patients with coronary artery disease and severe left ventricular impairment or congestive cardiac failure.32 Implantation is a low-risk procedure (mortality rate <1%) and patients have a good quality of life. Therapy is flexible, with initial antitachycardia pacing successful in upwards of 80% of cases of ventricular tachycardia, followed by low-energy cardioversion and high-energy defibrillation shocks. Dual-chamber devices that allow full flexibility in programming are especially useful when there is coexistent atrial flutter or fibrillation. A small subset of cardiac surgery patients with impaired ventricular function and persistent arrhythmias are candidates for early implantation.
1 Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720-1729.
2 Hogue CWJr, Creswell LL, Gutterman DD, et al. Epidemiology, mechanisms, and risks: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:9S-16S.
3 Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:66-659.
4 Wijffels MC, Kirchhof CJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation. 1995;92:1954-1968.
5 Epstein AE, Alexander JC, Gutterman DD, et al. Anticoagulation: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:24S-27S.
6 Crystal E, Garfinkle MS, Connolly SS, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database of Systematic Reviews. 2004:CD 003611.
7 Kowey PR, Taylor JE, Rials SJ, et al. Meta-analysis of the effectiveness of prophylactic drug therapy in preventing supraventricular arrhythmia early after coronary artery bypass grafting. Am J Cardiol. 1992;69:963-965.
8 Daoud EG, Strickberger SA, Man KC, et al. Preoperative amiodarone as prophylaxis against atrial fibrillation after heart surgery. N Engl J Med. 1997;337:1785-1791.
9 Bradley D, Creswell LL, Hogue CWJr, et al. Pharmacologic prophylaxis: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:39S-47S.
10 Auer J, Weber T, Berent R, et al. A comparison between oral antiarrhythmic drugs in the prevention of atrial fibrillation after cardiac surgery: the pilot study of prevention of postoperative atrial fibrillation (SPPAF), a randomized, placebo-controlled trial. Am Heart J. 2004;147:636-643.
11 Crystal E, Connolly SJ, Sleik K, et al. Interventions on prevention of postoperative atrial fibrillation in patients undergoing heart surgery: a meta-analysis. Circulation. 2002;106:75-80.
12 Maisel WH, Epstein AE. The role of cardiac pacing: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:36S-38S.
13 Mayr A, Ritsch N, Knotzer H, et al. Effectiveness of direct-current cardioversion for treatment of supraventricular tachyarrhythmias, in particular atrial fibrillation, in surgical intensive care patients. Crit Care Med. 2003;31:401-405.
14 Martinez EA, Bass EB, Zimetbaum P. Pharmacologic control of rhythm: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:48S-55S.
15 Martinez EA, Epstein AE, Bass EB. Pharmacologic control of ventricular rate: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:56S-60S.
16 Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
17 Klein AL, Grimm RA, Murray RD, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344:1411-1420.
18 van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288:2441-2448.
19 Hart RG, Halperin JL, Pearce LA, et al. Lessons from the Stroke Prevention in Atrial Fibrillation trials. Ann Intern Med. 2003;138:831-838.
20 Pappone C, Oreto G, Rosanio S, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001;104:2539-2544.
21 Khaykin Y, Nassir F, Marrouche NF, et al. Pulmonary vein antrum isolation for treatment of atrial fibrillation in patients with valvular heart disease or prior open heart surgery. Heart Rhythm. 2004;1:33-39.
22 Atiga WL, Worley SJ, Hummel J, et al. Prospective randomized comparison of cooled radiofrequency versus standard radiofrequency energy for ablation of typical atrial flutter. Pacing Clin Electrophysiol. 2002;25:1172-1178.
23 Mazgalev TN, Ho SY, Anderson RH. Anatomic-electrophysiological correlations concerning the pathways for atrioventricular conduction. Circulation. 2001;103:2660-2667.
24 Bardy GH, Packer DL, German LD, et al. Preexcited reciprocating tachycardia in patients with Wolff-Parkinson-White syndrome: incidence and mechanisms. Circulation. 1984;70:377-391.
25 Tzivoni D, Banai S, Schuger C, et al. Treatment of torsades de pointes with magnesium sulfate. Circulation. 1988;77:392-397.
26 Dmitrienko A, Smith BP. Analysis of the QT interval in clinical trials. Drug Info J. 2002;36:269-279.
27 Corrado D, Basso C, Thiene G. Arrhythmogenic right ventricular cardiomyopathy: diagnosis, prognosis, and treatment. Heart. 2000;83:588-595.
28 Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease: Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882-1890.
29 Trayanova N. Defibrillation of the heart: insights into mechanisms from modelling studies. Exp Physiol. 2006;91:323-337.
30 Daoud EG, Dabir R, Archambeau M, et al. Randomized, double-blind trial of simultaneous right and left atrial epicardial pacing for prevention of post-open heart surgery atrial fibrillation. Circulation. 2000;102:761-765.
31 Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576-1583.
32 Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933-1940.