Chapter 133 Arachnoiditis and Syringomyelia
Syringomyelia can develop secondary to many pathologic processes such as Chiari malformations, spine tumors, cysts, traumatic events, spinal deformities, and arachnoiditis. All these conditions likely share a common pathophysiologic disruption of cerebrospinal fluid (CSF) flow regulation.1,2 This chapter focuses solely on syringomyelia derived from spinal arachnoiditis, excluding traumatic etiologies. Spinal adhesive arachnoiditis has been associated most commonly with postsurgical inflammation, myelography, and infection. However, rare cases of idiopathic arachnoiditis, arachnoiditis after spinal subarachnoid hemorrhage, and familial arachnoiditis have also been reported.3–5
The first clinical presentation of syringomyelia was described by Portal in 1804.6 However, the first case of cervical spinal cord cavitation secondary to arachnoiditis was probably described in 1861 by Vulpian from a necropsy.7 The first treatment was performed by Abbe and Coley in 1892 as drainage of a postmeningitic syrinx.8 Subsequently, more clinical cases attributed to postsyphilitic inflammation were published.9 The first association between degenerative disc disease and arachnoiditis was largely attributed to French in 1946.10 Although theories about the pathologic process and causative agents have evolved, treatment of arachnoiditis has improved little over the years.
Epidemiology and Definition
Spinal adhesive arachnoiditis (SAA) describes the scarring of pia and arachnoid membranes within the thecal sac. Although the pathophysiology remains elusive, thickening and adhesion of pia and arachnoid membranes are thought to arise secondarily from chronic inflammation. SAA can range from mild focal adhesions to severe scarring as is seen with arachnoid ossificans. SAA seems to have a predilection for the lumbar spine (up to 86% in some series), although this may simply reflect a predominance of postmyelographic arachnoiditis in the literature.11 The diagnosis of arachnoiditis is confirmed radiographically by using MRI or myelography. The disease spectrum varies considerably from focal scarring or slight nerve root sheath changes to dense scarring throughout the spinal axis. Variations in defining the severity of arachnoiditis likely influence the reported incidence.
Risk factors are largely inherent in the varying underlying pathologic processes that cause inflammation (Box 133-1). Infection, subarachnoid hemorrhage, degenerative lumbar disease, history of myelography, prior lumbar anesthesia, prior spine surgery, transverse myelitis, prior baclofen pump insertion, and even intrathecal steroid injection have all been postulated as causative triggers leading to the development of spinal adhesive arachnoiditis.12–18 Infectious etiologies include tuberculous meningitis, pyogenic infections, cysticercosis, candida tropicalis, chromoblastomycosis, cryptococcosis, listeriosis, and syphilis.9,19–23 Some studies have suggested that the addition of blood products to contrast agents in myelography contributed to the increased risk of arachnoiditis. No study has evaluated whether durotomies or synthetic sealants used to reinforce durotomy closures influence the risk of developing arachnoiditis.
Box 133-1 Etiologies of Arachnoiditis
Several series have estimated the incidence of lumbar postmyelography arachnoiditis and symptomatic fibrosis to be near 1%.24 However, published data on postmyelography arachnoiditis are limited by the use of varying contrast agents and a variable time to presentation. For example, one study reported the development of syringomyelia 44 years after myelography.25 Some series have found asymptomatic but radiographic signs of arachnoiditis in 16.5% to 35% of patients after myelography.26 However, other studies suggest a lower incidence in the absence of surgical intervention with myelography alone. Laitt et al. reported that 62.4% (68 of 109) of their patients had some degree of arachnoiditis after Myodil myelography, but 50 of their patients had prior lumbar surgery.27 The incidence was only 3% in patients with myelography alone, whereas arachnoiditis was present in 88% of people who had prior lumbar surgery and myelography.
Failed back syndrome is an entity that is familiar to most surgeons. Some series have estimated that 6% to 16% of patients who have had prior spine surgery develop some degree of arachnoiditis contributing to persistent pain. Fitt and Stevens found 20% (26 of 129) of patients with a history of lumbar surgery to have some degree of arachnoiditis.28 Many studies have grouped epidural scarring and intradural pathology such as arachnoiditis together in their evaluations; therefore, the incidence of arachnoiditis alone is likely overreported in the literature.29 Burton reported that 11% of their failed back surgeries were due to arachnoiditis. Several studies have clearly associated an increased risk of arachnoiditis developing in patients who have undergone prior spine surgeries.27,30 Certainly, arachnoiditis should be considered in managing postoperative patients with persistent or recurrent symptoms.
Pathophysiology of Arachnoiditis-Associated Syringomyelia
Although no definitive pathologic mechanism of arachnoiditis-associated syringomyelia has been elucidated thus far, several different theories have been postulated. One of these theories speculates that the blood products from myelography act as a catalyst coating oil drops in fibrin, thus emulsifying them and contributing to the development of severe arachnoiditis.31 Several other authors propose that an initial minor trauma or local irritants such as contrast-myelography could serve as causative agents that trigger an inflammatory response.17,25 McLean et al. hypothesize that tethering of the spinal cord from arachnoiditis causes repeated compression along the spinal cord with normal physiologic movements leading to pathologic injury.32,33
Another theory postulates that syringomyelia associated with arachnoiditis is secondary to formation of microcysts that develop within the spinal cord from ischemic injury. The impaired CSF flow from the arachnoiditis contributes to intramedullary cystic degeneration from ischemia due to circulatory disturbance in the pia-arachnoid, which would coalesce into a syrinx.34,35
Williams and Bentley created a syringomyelia model in dogs by injecting kaolin in the cisterna magna and causing significant arachnoiditis.36 They noted that typical findings associated with ischemia were absent in their pathologic specimens. However, whether their model truly mimics the pathology that is seen in human arachnoiditis remains unclear, as all their specimens had a patent fourth ventricular communication with the syrinx.
Alternatively, other researchers argue that the increased CSF pressure differential from an obstruction leads to direct communication of CSF via Virchow-Robin spaces into the spinal cord that forms a syrinx.37,38 Alternative drainage pathways that have been hypothesized include the dorsal root entry zone and perivascular channels.39,40
A more recent hypothesis that has growing support from various studies is that extracellular fluid from interstitial edema contributes to the formation of a syrinx secondary to the scarring of the pia-arachnoid. The scarring of the pia-arachnoid is thought to impair some degree of CSF absorption while increasing venous stasis, thus increasing the amount of interstitial fluid.41 This theory has been supported by animal and mathematical models that obstruct the CSF space, leading to development of edema and possibly a “pre-syrinx” state.42,43 Yet another hypothesis incorporates pressure dissociation above and below the obstruction with disruption along the blood–spinal cord barrier leading to ultrafiltration of protein-poor fluid into a syrinx.44
Hypotheses explaining the development of syringomyelia have ranged from hydrodynamic theories to pressure dissociation theories involving accumulation of extracellular fluid.35,44,45 However, we still have an incomplete understanding of the pathophysiologic process that leads to syringomyelia formation.
Radiographic Findings of Arachnoiditis
With the advent of myelography, lumbar arachnoiditis has been described as nerve root clumping, contraction of the thecal sac, an appearance of an empty or thickened thecal sac, short/blunted perineural sheathes, or obliteration of nerve root sheathes.46 Wilkinson further classified these findings into grades of severity47:
1. Unilateral defect on the nerve root exit pouch adjacent to the disc
2. Circumferential or anular defect with bilateral notch and filiform passage of contrast medium
3. Complete transverse obstruction with “stalagmites,” candle-guttering, or paintbrush defects
4. Infundibulum cul-de-sac, loss of radicular striation, cutting off of root sleeves
However, with the advent of MRI, less invasive imaging studies were available. Ross and Delamarter summarized their MRI findings into a classification with 92% sensitivity, 100% specificity, and 99% accuracy (Table 133-1). Classification of MRI findings associated with lumbar arachnoiditis48,49:
1. Conglomeration of nerve roots centrally located, no enhancement
2. Nerve roots attached laterally/clumped with meningeal thickening
3. “Empty thecal sac” appearance (Fig. 133-1)
4. Soft tissue mass within the canal obliterating subarachnoid space
Table 133-1 MRI Findings Associated with Lumbar Arachnoiditis
| Grade | MRI Findings |
|---|---|
| 1 | Conglomeration of nerve roots centrally located, no enhancement |
| 2 | Nerve roots attached laterally, clumped with meningeal thickening, “empty thecal sac” appearance |
| 3 | Soft tissue mass within canal obliteration of subarachnoid space |
Ross and Delamarter’s definitions are commonly used, although other authors have further simplified their findings into even fewer categories. Jorgensen et al. divided MRI findings into two categories50:
1. Adhesion of roots inside meninges, “sleeveless” appearance (Ross and Delamarter types 1 and 2)
2. Thecal sac changes, filling defects/narrowing/shortened/occlusion (Ross and Delamarter type 3)
Usually, MRI is adequate to confirm the diagnosis of arachnoiditis,28,51 but some cases are quite subtle and might not be as evident on MRI. Brodbelt and Stoodley described three cases in which a mild, single arachnoid web was sufficient to cause CSF flow impairment.14 Some authors have advocated that constructive interface steady state images are better suited to visualize dural thickening, syringomyelia, microcystic lesions, and deformity of the cord, but less adequate to identify “pre-syrinx” findings.52 Myelography can be invaluable to identify small areas of CSF flow obstruction that are responsible for symptoms.
It is important to note that lumbar spinal stenosis in itself is often associated with radiographic findings that resemble Delamarter type 1 clumping of nerve roots and should be distinguished from arachnoiditis based on the clinical history.53 These findings are likely due to mechanical pressures from the stenosis as opposed to arachnoid/pial fibrosis as seen in arachnoiditis.
Clinical Presentation and Clinical Course
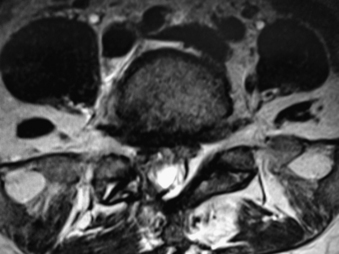
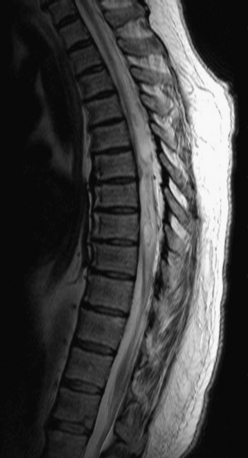
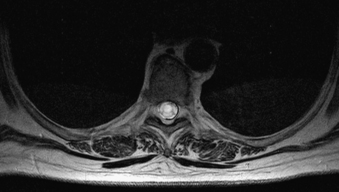
Patients typically present with slowly progressive, chronic symptoms. The symptomatology is largely defined by the anatomic location of the pathology. Given that lumbar arachnoiditis is more prevalent, a greater percentage of patients initially present with lumbar radiculopathy, sensory deficits, and back pain.54,55 Eventually, patients develop more objective findings of decreased reflexes and motor deficits progressing to bowel and bladder dysfunction, essentially a cauda equina syndrome.55 These patients often have a history of an invasive lumbar procedure, either myelography, surgery, or even a percutaneous injection for pain control. Alternatively, patients who develop cervicothoracic arachnoiditis present with myelopathy secondary to spinal cord compression (Figs. 133-2 to 133-5). Thoracic myelopathy is more commonly encountered, but cervical cases have been described.
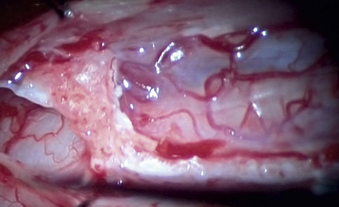
FIGURE 133-4 An intraoperative image of calcification/adhesions/syrinx.
(Courtesy of Dr. Julian Wu.)
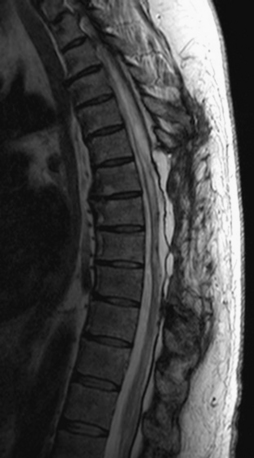
FIGURE 133-5 Sagittal T2-weighted follow-up MRI after decompression and duraplasty.
(Courtesy of Dr. Julian Wu.)
In the absence of syringomyelia, many authors have stated that arachnoiditis is relatively stable with little clinical deterioration over time.11,16 However, once the patient is symptomatic, the majority of cases progress clinically. Nevertheless, occasional cases of clinical stabilization for several years prior to deteriorating symptoms have been published.56 The onset of symptoms can be quite variable and may range from 14 days to 44 years after an assumed cause such as myelography or infection.11,25 The early onset of symptoms is atypical and most likely related to the initial causative event, such as vascular thrombosis associated with an infection. Most symptoms develop years after the inciting event.
Klekamp and Samii introduced a grading system to standardize the clinical examination and progression of patients with syringomyelia. Patients were divided into six categories (0–5) based on sensory, motor, gait ataxia, bladder function, and bowel function.57
Treatment and Outcomes
Nonsurgical Treatment
The clinical results of treating arachnoiditis have remained dismal. Generally, once symptoms appear, the course of the disease is progressive. Some authors have reported treating symptomatic syringomyelia from arachnoiditis conservatively with oral steroids.58 This may transiently alleviate symptoms, but the clinical picture typically worsens over time. Trials of intrathecal steroids were conducted, but several reports of arachnoiditis developing from intrathecal steroid administration have tempered this approach.13 Some mild success has been achieved by administering intrathecal hyaluronidase in attempts to minimize fibrin deposition, but this has not gained widespread practice.59
Surgical Management of Lumbar Arachnoiditis
Primary lumbar arachnoiditis rarely extends beyond the lumbar spine, but treatment of this condition should be differentiated from treatment of cervicothoracic arachnoiditis leading to syringomyelia. Most commonly, treatment for lumbar arachnoiditis targets symptomatic pain relief. Unfortunately, outcomes for lumbar arachnoiditis have been equally disappointing. Roca et al. had “good” results (pain relief and only occasional medications) in 8 of 23 patients, fair outcomes in 9, and poor results in 6.54 Intuitively, they had better results in patients with milder arachnoiditis (Wilkinson grade 1 and 2 as compared to grade 3 and 4), although most cases were reexplored for epidural fibrosis. Jorgensen et al. reported that 50% of patients in their series remained unemployed after repeat surgery, 20% worked full-time with intermittent symptoms, and only 10% to 15% were symptom free, but their study included extradural scarring as well.50
Johnston and Metheny compared 28 patients, of whom half were treated surgically with chymotrypsin intradurally as well as a postoperative oral regimen, and found long-term results comparable between their surgical and observational cohorts.60 Patients did improve transiently, but the researchers concluded that until a method of limiting adhesive scar formation existed, surgery was not recommended for lumbar arachnoiditis. Wilkinson and Schuman treated 17 patients with arachnoidolysis and noted that 76% had initial improvement, which reduced to 50% after 1 year. They found that 71% of patients with neurologic deficits improved, but the study had only 1 year of follow-up.61 Other authors have resorted to spinal cord stimulation, rhizotomies, or even cordotomies to treat unilateral pain, with mixed results.16
Surgical Management of Syringomyelia
Once symptomatic syringomyelia has developed, surgical intervention revolves between drainage such as syringocavitary, syringosubarachnoid, or lumboperitoneal shunting and direct adhesion lysis and duraplasty. Some authors favor syringoperitoneal shunting, while others prefer syringosubarachnoid shunts.4,62 Many authors state that surgical lysis of adhesions is mandatory with consideration of duraplasty.63–65
Koyanagi et al. reported that 60% of their 15 patients treated surgically improved over a mean of 2.7 years of follow-up, but 8 patients required multiple procedures.41 While all patients had decreased syrinx size on follow-up imaging, only 31% improved with a shunting procedure. Despite this low number, the authors concluded that there remains a role for shunting. Sgouros et al. placed 56 syringopleural shunts for varying etiologies and noted that initially 64.2% improved, but only 53.5% had sustained improvement with at least 1 year of follow-up.65 Furthermore, shunting carries significant risks; 26.7% of their patients suffered clinical deterioration after placement of a shunt. In the subgroup of spinal adhesive arachnoiditis, 8 of 11 patients deteriorated even after multiple reoperations (two improved initially but then worsened), while three remained stable.
Batzdorf noted that 50% of their 42 patients with syringocavitary shunts experienced a complication or failure. In the subgroup of patients with postinflammatory syringomyelia, the rate was 36%.66 This subset, however, had the greatest number of patients with postoperative deterioration. Sgouros and Williams have also advocated leaving the dura unopposed to create a meningocele, but others argue that this technique may promote more blood product accumulation and scarring.67 Other complications of shunt placement include neurologic deficits from the myelotomy, shunt failure, shunting of a compartment within the syrinx, tethering of cord via the shunt, overdrainage of subarachnoid CSF, infection, CSF leak, aseptic meningitis, pneumothorax, and brachial plexus palsy.66,68 A hemilaminectomy and placement of a syringosubarachnoid shunt have been advocated by some to minimize selective risks.69
Other researchers have also advocated that the primary goal of surgery should be to release adhesions and perform a duraplasty.64,65 Shikata et al. reported good results using release of adhesions and duraplasty with or without spine fusion in 58.3% of patients. They encountered fair results in 19.4% of patients and deterioration in 22.2% of their sample. More recently four fifths of their patients with spine fusions had improved results.55 Klekamp et al. reported that 67 patients treated for progressive symptoms all had scarring near the syrinx. Ninety-seven percent of patients who underwent shunting recurred symptomatically, whereas 78% of patients who underwent arachnoid release and duraplasty remained stable.68 Klekamp and Samii reported far inferior results with either treatment in 154 patients; 11% of patients improved with shunting, while 31% had amelioration with arachnoidolysis. Complications were comparable between the two groups: 18% in the shunted group and 17% with arachnoidolysis.70
Klekamp et al. previously reported treating syringomyelic patients primarily with shunts but have subsequently adapted their algorithm to initially treat patients with arachnoidlysis and decompression. They do caution against overly aggressive arachnoidlysis with severe scarring that may contribute to further neurologic deterioration, and the most severe scarring tends to develop with postinflammatory arachnoiditis. Only 17% of patients with more severe scarring stabilized, whereas 83% of patients with mild arachnoiditis stabilized neurologically in their series. They found that the following correlated with a higher recurrence of symptoms: syrinx shunting, severe preoperative scarring, multiple prior surgeries, postoperative scarring, increase in syrinx size postoperatively, and severe neurologic deficits preoperatively.68
Parker et al. had equally tepid outcomes. In 12 patients with postinfectious etiologies, only 2 improved, while 4 worsened. In 11 patients with syringomyelia from cryptogenic, postoperative, or spinal subarachnoid hemorrhage etiologies, 4 patients improved and 3 worsened. Overall, drainage of the syrinx led to clinical deterioration in 60% of patients, while 40% remained stable or improved. With arachnoidolysis, 66% of patients remained stable, while 33% had poor outcomes. With infectious etiology, the outcomes were worse: 46.5% worsened clinically, 46.5% remained stable, and only 7% improved.71
In deciding on surgical approaches, consideration should be given to the extent of scarring. Certainly, focal areas can be treated with better results by arachnoidolysis and duraplasty. Extensive scarring may favor syringocavitary shunting provided that the syrinx is not loculated. Adjuvant treatment should also include management for spasticity and neuropathic pain.72 Patients should be well informed of the risks and the often limited benefits.
Interpretation of published results needs to be tempered by the limited amount of long-term follow-up. Most series show initial success, but longer follow-up supports the concern that clinical deterioration is often encountered. In treating lumbar arachnoiditis, Wilkinson showed 75% initial improvement with 18% of patients worsening acutely. However, after 1 year, only 50% of patients had maintained that initial improvement.73 Furthermore, most of the larger series with longer follow-up had worse outcomes, which may simply reflect the natural course of the disease even after intervention. Consideration should be given to weighing the considerable risk of iatrogenic clinical deterioration in these series against short-term gains. With our limited understanding of the pathophysiologic processes involved and our inability to reduce recurrent arachnoiditis after surgical intervention, surgical intervention should be recommended cautiously and only in patients with ongoing neurologic deterioration. If arachnoidolysis strategies are employed, the operative goal should be the establishment and maintenance of a patent subarachnoid space from rostral to caudal, spanning the entirety of the pathology. This often requires that the procedure include a duraplasty. Some surgeons have utilized the off-label use of DuraGen (Integra LifeSciences, Plainsboro, NJ) for this application. The senior author has applied DuraGen under a fascial graft (duraplasty) after arachnoidolysis. The DuraGen was used to create a DuraGen bridge from the rostral to caudal subarachnoid spaces.
Summary
Spinal adhesive arachnoiditis has been associated with varying inflammation-inducing factors that can lead to lumbar arachnoiditis or cervicothoracic syringomyelia. The clinical presentation of arachnoiditis is largely dependent on the location and anatomic structures that are affected, varying from radiculitis to myelopathy. Evaluation of arachnoiditis should include an entire spinal axis MRI and consideration of a CT myelogram if necessary. Unfortunately, both conservative and surgical treatment options have had limited results. Perhaps the most important concept in addressing arachnoiditis is prevention through careful surgical technique.
Batzdorf U., Klekamp J., Johnson J.P. A critical appraisal of syrinx cavity shunting procedures. J Neurosurg. 1998;89:382-388.
Delamarter R.B., Ross J.S., Masaryk T.J., et al. Diagnosis of lumbar arachnoiditis by magnetic resonance imaging. Spine (Phila Pa 1976). 1990;15:304-310.
Klekamp J., Batzdorf U., Samii M., Bothe H.W. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997;86:233-240.
Klekamp J., Samii M. Introduction of a score system for the clinical evaluation of patients with spinal processes. Acta Neurochir (Wien). 1993;123:221-223.
Milhorat T.H., Capocelli A.L.Jr., Anzil A.P., et al. Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J Neurosurg. 1995;82:802-812.
Sgouros S., Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995;82:1-10.
Shikata J., Yamamuro T., Iida H., Sugimoto M. Surgical treatment for symptomatic spinal adhesive arachnoiditis. Spine (Phila Pa 1976). 1989;14(8):870-875.
1. Milhorat T.H. Classification of syringomyelia. Neurosurg Focus. 8(3), 2000.
2. Milhorat T.H., Capocelli A.L.Jr., Anzil A.P., et al. Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J Neurosurg. 1995;82:802-812.
3. Skalpe I.O., Sortland O. Adhesive arachnoiditis in patients with spinal block. Neuroradiology. 1982;22:243-254.
4. Kahler R.J., Knuckey N.W., Davis S. Arachnoiditis ossificans and syringomyelia: a unique case report. J Clin Neurosci. 2000;7(1):66-68.
5. Duke R.J., Hashimoto S.A. Familial spinal arachnoiditis. Arch Neurol. 1974;30:300-303.
6. Portal A. Cours d’anatomie medicale. Paris: Bourdoin, 1804;vol. 4.
7. Caplan L.R., Norohna A.B., Amico L.L. Syringomyelia and arachnoiditis. J Neurol Neurosurg Psychiatry. 1990;53:106-113.
8. Abbe R., Coley W.B. Syringomyelia; operation, exploration of the cord; withdrawal of fluid. J Nerv Ment Dis. 1892;19:512-520.
9. Schwarz E. Praparate von einem falle syphilitischer Myelomeningitis mit Holenbildung im Ruchenmarke und besonderen degenerativen Veranderungen der Neurologlia. Wien Klin Wochenschr. 1897;10:177.
10. French J.D. Clinical manifestations of lumbar arachnoiditis. Surgery. 1946;20:718-729.
11. Mooij J.J. Spinal arachnoiditis: disease or coincidence? Acta Neurochir (Wien). 1980;53:151-160.
12. Matsui H., Tsuji H., Kanamori M., et al. Laminectomy-induced arachnoradiculitis: a postoperative serial MRI study. Neuroradiology. 1995;37:660-666.
13. Roche J. Steroid-induced arachnoiditis. Med J Aust. 1984;140:281-284.
14. Brodbelt A.R., Stoodley M.A. Syringomyelia and the arachnoid web. Acta Neurochir (Wien). 2003;145:707-711.
15. Benner B., Ehni S. Spinal arachnoiditis. The postoperative variety in particular. Spine (Phila Pa 1976). 1975;3:40-41.
16. Guyer D.W., Wiltse L.L., Eskay M.L., Guyer B.H. The long-range prognosis of arachnoiditis. Spine (Phila Pa 1976). 1989;14(12):1332-1341.
17. Quiles M., Marchisello P.J., Tsaris P. Lumbar adhesive arachnoiditis. Spine (Phila Pa 1976). 1978;3:45-50.
18. Smolik E.A., Nash F.P. Lumbar spinal arachnoiditis: a complication of the intervertebral disc operation. Ann Surg. 1951;133:490-495.
19. Leite C.C., Jinkins J.R., Escobar B.E., et al. MR imaging of intramedullary and intradural-extramedullary spinal cysticercosis. AJR Am J Roentgenol. 1997;169:1713-1717.
20. Phanthumchinda K., Kaoropthum S. Syringomyelia associated with post-meningitic spinal arachnoiditis due to Candida tropicalis. Postgrad Med J. 1991;67:767-769.
21. Takahashi H., Sasaki A., Arai T., et al. Chromoblastomycosis in the cisterna magna and the spinal arachnoid space. J Neurosurg. 1963;38:506-509.
22. Davison S. Cryptococcal spinal arachnoiditis. J Neurol Neurosurg Psychiatry. 1968;31:76-80.
23. Nadone R., Alessandrini F., Tezzon F. Syringomyelia following Listeria meningoencephalitis: report of a case. Neurol Sci. 2003;24:40-43.
24. Shaw M.D.M., Russell J.A., Grossart K.W. The changing pattern of spinal arachnoiditis. J Neurol Neurosurg Psychiatry. 1978;41:97-107.
25. Kubota M., Shin M., Taniguchi M., et al. Syringomyelia caused by intrathecal remnants of oil-based contrast medium. J Neurosurg Spine. 2008;8(2):169-173.
26. Skalpe I.O. Adhesive arachnoiditis following lumbar myelography. Spine (Phila Pa 1976). 1978;3(1):61-64.
27. Laitt R., Jackson A., Isherwood I. Patterns of chronic adhesive arachnoiditis following Myodil myelography: the significance of spinal canal stenosis and previous surgery. Br J Radiol. 1996;69:693-698.
28. Fitt O.J., Stevens J.M. Postoperative arachnoiditis diagnosed by high-resolution fast spin-echo MRI of the lumbar spine. Neuroradiology. 1995;37:139-145.
29. Burton C.V. Causes of failure of surgery on the lumbar spine: ten-year follow-up. Mt Sinai J Med. 1991;58(2):183-187.
30. Irstam L., Sundstrom R., Sigstedt B. Lumbar myelography and adhesive arachnoiditis. Acta Radiol. 1974;15(1):356-368.
31. Howland W.J., Curry J.L. Experimental studies of Pantopaque arachnoiditis. Radiology. 1965;87:253-261.
32. McLean D.R., Miller J.D.R., Allen P.B.R. Posttraumatic syringomyelia. J Neurosurg. 1973;39:485-492.
33. Savoiardo M. Syringomyelia associated with postmeningitic spinal arachnoiditis. Neurology. 1976;26:551-554.
34. Barnett H. Syringomyelia associated with spinal arachnoiditis. In: Barnett H., Foster J., Hudgson P., editors. Syringomyelia. London: Saunders, 1973.
35. Klekamp J. The pathophysiology of syringomyelia: a historical overview and current concept. Acta Neurochir (Wien). 2002;144:649-664.
36. Williams B., Bentley J. Experimental communicating syringomyelia in dogs after cisternal kaolin injection. J Neurol Sci. 1980;48:93-107.
37. Ball M.J., Dayan A.P. Pathogenesis of syringomyelia. Lancet. 1972;2:799-801.
38. Stoodley M.A., Jones N.R., Yang L., Brown C.J. Mechanisms underlying the formation and enlargement of noncommunicating syringomyelia: experimental studies. Neurosurg Focus. 8(3), 2000.
39. Aboulker J. La syringomyelie et les liquids intra-rachidiens. Neurochirurgie. 1979;25:1-144.
40. Oldfield E.H., Muraszko K., Shawker T.H., Patronas N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. J Neurosurg. 1994;80:3-15.
41. Koyanagi I., Iwasaki Y., Hida K., Houkin K. Clinical features and pathomechanisms of syringomyelia associated with spinal arachnoiditis. Surg Neurol. 2005;63:350-356.
42. Josephson A., Greitz D., Klason T., et al. A spinal thecal sac constriction model supports the theory that induced pressure gradients in the cord cause edema and cyst formation. Neurosurgery. 2001;48(3):636-646.
43. Chang H.S., Nakagawa H. Theoretical analysis of the pathophysiology of syringomyelia associated with adhesive arachnoiditis. J Neurol Neurosurg Psychiatry. 2004;75:754-757.
44. Levine D.N. The pathogenesis of syringomyelia associated with lesions at the foramen magnum: a critical review of existing theories and proposal of a new hypothesis. J Neurol Sci. 2004;220:3-21.
45. Greitz D. Unraveling the riddle of syringomyelia. Neurosurg Rev. 2006;29:251-264.
46. Autio E., Suolanen J., Norrback S., Slatis P. Adhesive arachnoiditis after lumbar myelography with meglumine iothalamate (Conray). Acta Radiol Diagn (Stockh). 1972;12:17-24.
47. Wilkinson H.A. The failed back syndrome. Philadelphia: Harper & Row; 1983.
48. Delamarter R.B., Ross J.S., Masaryk T.J., et al. Diagnosis of lumbar arachnoiditis by magnetic resonance imaging. Spine (Phila Pa 1976). 1990;15:304-310.
49. Ross J.S., Masaryk T.J., Modic M.T., et al. MR imaging of lumbar arachnoiditis. AJR Am J Roentgenol. 1987;149:1025-1032.
50. Jorgensen J., Hansen P.H., Steenskov V., Ovesen N. A clinical and radiological study of chronic lower spinal arachnoiditis. Neuroradiology. 1975;9:139-144.
51. Frizzell B., Kaplan P., Dussault R., Sevick R. Arachnoiditis ossificans: MR imaging features in five patients. AJR Am J Roentgenol. 2001;177:461-464.
52. Ikushima I., Korogi Y., Hirai T., Yamashita Y. High-resolution constructive interference in a steady state imaging of cervicothoracic adhesive arachnoiditis. J Comput Assist Tomogr. 2007;31(1):143-147.
53. Jackson A., Isherwood I. Does degenerative disease of the lumbar spine cause arachnoiditis? A magnetic resonance study and review of the literature. Br J Radiol. 1994;67:840-847.
54. Roca J., Moreta D., Ubierna M.T., et al. The results of surgical treatment of lumbar arachnoiditis. Int Orthop. 1993;17:77-81.
55. Shikata J., Yamamuro T., Iida H., Sugimoto M. Surgical treatment for symptomatic spinal adhesive arachnoiditis. Spine (Phila Pa 1976). 1989;14(8):870-875.
56. de Mattos J.P., André C., Couto B.A. Recurrent spinal adhesive arachnoiditis. Arq Neuropsiquiatr. 1988;46(1):65-68.
57. Klekamp J., Samii M. Introduction of a score system for the clinical evaluation of patients with spinal processes. Acta Neurochir (Wien). 1993;123:221-223.
58. Hui A.C.F., Chan Y.L., Kay R. Syrinx and tuberculoma formation in tuberculous arachnoiditis. Can J Neurol Sci. 2001;28:148-149.
59. Gouri-Devi M., Padmini R., Satish P. Use of intrathecal hyaluronidase in spinal arachnoiditis complicating tuberculous meningitis. Indian J Med Res. 1980;71:581-593.
60. Johnston J.D., Matheny J.B. Microscopic lysis of lumbar adhesive arachnoiditis. Spine (Phila Pa 1976). 1978;3(1):36-39.
61. Wilkinson H.A., Schuman N. Results of surgical lysis of lumbar adhesive arachnoiditis. Neurosurgery. 1979;4(5):401-409.
62. Kakar A., Madan V.S., Prakash V. Syringomyelia: a complication of meningitis: case report. Spinal Cord. 1997;35:629-631.
63. Dolan R.A. Spinal adhesive arachnoiditis. Surg Neurol. 1993;39:479-484.
64. Ohata K., Gotoh T., Matsusaka Y., et al. Surgical management of syringomyelia associated with spinal adhesive arachnoiditis. J Clin Neurosci. 2001;8(1):40-42.
65. Sgouros S., Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995;82:1-10.
66. Batzdorf U., Klekamp J., Johnson J.P. A critical appraisal of syrinx cavity shunting procedures. J Neurosurg. 1998;89:382-388.
67. Sgouros S., Williams B.W. Management and outcome of posttraumatic syringomyelia. J Neurosurg. 1996;85:197-205.
68. Klekamp J., Batzdorf U., Samii M., Bothe H.W. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997;86:233-240.
69. Iwasaki Y., Koyanagi I., Ida K., Abe H. Syringo-subarachnoid shunt for syringomyelia using partial hemilaminectomy. Br J Neurosurg. 1999;13:41-45.
70. Klekamp J., Samii M. Syringomyelia: diagnosis and management. Berlin: Springer; 2002.
71. Parker F., Aghakhani N., Tadie M. Arachnoidite non-traumatique et syringomyelie: a propos d’une serie de 32 cas. Neurochirurgie. 1999;45(Suppl 1):67-83.
72. Batzdorf U. Primary spinal syringomyelia. J Neurosurg Spine. 2005;3:429-435.
73. Wilkinson H.A. Alternative therapies for the failed back syndrome. In: Frymoyer J.W., editor. The adult spine: principles and practice. New York: Raven Press, 1991.