Chapter 196 Arachnoiditis
Spinal arachnoiditis is a nonspecific inflammatory process of the arachnoid layer of the spinal cord or cauda equina. Arachnoiditis was first described by Victor Horsley in 1909.1 Since Horsley, numerous authors have described this condition with a variety of terms, including chronic spinal arachnoiditis, adhesive spinal arachnoiditis, meningitis serosa circumscripta spinalis, chronic spinal meningitis, spinal meningitides with radiculomyelopathy, lumbar adhesive arachnoiditis, spinal arachnoiditis, spinal fibrosis, and lumbosacral adhesive arachnoiditis. Furthermore, on the basis of the specific radiographic or pathologic findings, arachnoiditis can be termed arachnoiditis ossificans, calcific arachnoiditis, or pachymeningitis.1
Anatomy
The arachnoid mater is an avascular membrane that lies between two vascularized membranes: the pia mater and the dura mater. The arachnoid is attached to the underlying pia by numerous arachnoid trabeculae, which create a space between the arachnoid and the pia.2 This space, or potential space in some instances, transmits arterioles and is referred to as the subarachnoid space. The arachnoid is composed of layers of squamous cells held together by a network of connective tissue. The arachnoid contains intercellular pores that allow for the passage of molecules.3
Pathogenesis
A chronic infection or irritation can cause the arachnoid membrane to become thickened and adherent to both the overlying dura mater and the subjacent pia mater.4 The pia-arachnoid carries the blood vessels to the spinal cord, and this layer contains mesenchymal cells. In 1951, Smolik and Nash recognized that when the outer arachnoid layer is injured, both the blood vessels and mesenchymal cells lend themselves to extensive proliferation. The ensuing reaction between the pia-arachnoid and the dura mater leads to obliterative arachnoiditis.5
When the arachnoid membrane is exposed to an insult, an inflammatory response ensues, which is characterized by fibrinous exudates, neovascularization, and a relative paucity of inflammatory cellular exudates.6,7 Vascular occlusive changes can occur, which can lead to spinal cord ischemia.4,8–11 The small perforating blood vessels that supply the portions of the white matter may be obliterated, with resultant necrosis and cavitation of the spinal cord parenchyma.8,9,11 In addition to ischemia, blockage of venous return from the spinal cord or occlusion of cerebrospinal fluid (CSF) pathways may occur.8
Burton described the stages of progressive inflammation of the arachnoid that occur in lumbosacral arachnoiditis. The initial stage, radiculitis, consists of an inflamed pia-arachnoid with associated hyperemia and swelling of the nerve roots. The second stage, arachnoiditis, is characterized by fibroblast proliferation and collagen deposition. During this stage, nerve root swelling decreases, and the nerve roots adhere to each other and to the pia-arachnoid. The final stage, adhesive arachnoiditis, is the resolution of the inflammatory process and is characterized by dense collagen deposition. There is marked proliferation of the pia-arachnoid as well as complete nerve root encapsulation, hypoxemia, and progressive atrophy.12 For reasons that are not fully understood, the adhesions occur preferentially on the dorsal segments.1 The exact time course of these three phases has not been elucidated. Furthermore, it is not known how the specific causative insult for the development of arachnoiditis might affect the time course of each of the three phases.
Yamagami et al. postulated that the pathologic changes in arachnoiditis may be secondary to diminished nutritional supply. They found that in an experimental rat model, the development of arachnoiditis and neural degeneration directly corresponded to the magnitude of extradural inflammation and wound-healing processes that occurred after laminectomy, with or without foreign bodies. Furthermore, adhesions of the arachnoid cause the nerve roots to lump together, and in the process, these nerve roots are isolated from contact with the CSF, with resultant nutritional compromise.13
Etiology
In the first half of the 20th century, arachnoiditis was most often attributed to infectious causes.8 Furthermore, arachnoiditis had been described mainly in the cervical and thoracic regions.1 Since the 1950s, there has been a trend toward a higher incidence of arachnoiditis of noninfectious origin affecting the lumbar region.1,8 The precise causes of spinal arachnoiditis are not clear; likewise, the incidence and prevalence of spinal arachnoiditis in the general population are unknown8 (Table 196-1).
| Infectious | Noninfectious |
|---|---|
| Tuberculosis | Trauma |
| Bacterial infections | Postsurgery |
| SyphilisParasitic diseasesViral meningitis | Myelographic contrast media (oil-based > water-soluble; water-soluble ionic > water-soluble nonionic) |
| Intrathecal medications | |
| Steroids | |
| Anesthesia | |
| Epidural injections | |
| Neoplasms | |
| Arthritis (especially ankylosing spondylitis) | |
| Spinal stenosis | |
| Herniated intervertebral disc | |
| Intrathecal hemorrhage | |
| Foreign materials |
As was stated previously, arachnoiditis was mainly of infectious origin in the first half of the 20th century. Syphilis, tuberculosis, and gonorrhea were the most prevalent causes.1,14 Less common infectious causes include parasitic diseases and viral meningitis.15,16 These infectious causes are important to differentiate from noninfectious causes of arachnoiditis because, in most cases, effective treatment is available for arachnoiditis of infectious origin. However, despite adequate treatment of the causative agent, scarring of the arachnoid membrane may lead to permanent damage.
Arachnoiditis has a number of important noninfectious etiologies. In the 1940s, blood in the CSF following subarachnoid hemorrhage or surgery became the most prevalent cause of arachnoiditis.1 Spinal arachnoiditis following subarachnoid hemorrhage continues to be common and is usually treated in a conservative fashion.17 The breakdown products of hemoglobin form free radicals, and it has been postulated that these cause damage to nerves.18,19 In experiments on dogs, it has been shown that injecting blood breakdown products into the subarachnoid space causes more meningeal inflammation than does the injection of fresh blood.18 Cases of patients who have received epidural blood patches have given controversial results. Digiovanni et al. described that the placement of an autologous blood patch into the epidural space produced no more inflammation than a standard lumbar puncture.20 Other authors, though, have described cases in which an epidural blood patch had allegedly been responsible for arachnoiditis.21 Abouleish et al. described 118 cases of epidural blood patches over a 2-year period. This group found 19 cases of axial back pain, 2 cases of radiculopathy, and no cases of arachnoiditis.22
Oil-based contrast media have been an historically important cause of arachnoiditis. Iophendylate (Myodil, Pantopaque) is an oil-based contrast medium used in diagnostic myelograms. It was first used in the United States in 1944, and its usage continued for 40 years. In Sweden, iophendylate was banned from clinical use in 1948 because of animal studies that identified it as a causative agent for arachnoiditis.23 The incidence of arachnoiditis after the use of iophendylate is dose dependent and is quoted as 1%.24 Iophendylate has a very long half–life, so it is usually removed from the thecal space by aspiration at the conclusion of the myelogram.8 Often, this removal process is not entirely successful; in fact, incomplete removal of the contrast dye may produce further trauma and cause bleeding into the CSF.25
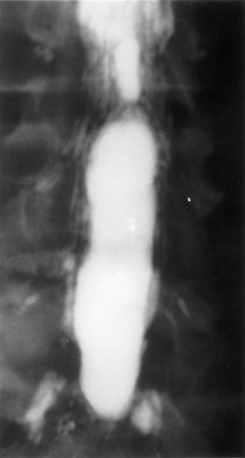
Guyer et al. listed the following factors as influencing the development of arachnoiditis after myelography: the type of contrast agent used (the risk is greater with oil-based than with water-soluble media and greater with ionic than with nonionic media), the dosage of contrast medium, and the observation time after myelography25 (Fig. 196-1).
The use of intrathecal medications, either steroids or anesthetic agents, has been implicated as a cause of arachnoiditis. Intrathecal injection of corticosteroids was previously used for multiple sclerosis.8 Epidural injection of corticosteroids for back pain is a common practice. One of the most commonly used agents is methylprednisolone acetate (MPA), which has been reported to cause arachnoiditis.26–28 MPA is suspended in polyethylene glycol, which can cause arachnoiditis.26–28 Furthermore, MPA is known to easily cross the intrathecal space, thus causing arachnoiditis.28 However, animal studies have not shown MPA to cause significant meningeal inflammation after epidural injections.29–31
The use of intrathecal bupivacaine, with or without epinephrine, has also been reported to cause arachnoiditis. Boiardi et al. described several cases of arachnoiditis after administration of bupivacaine with epinephrine.32 Gemma et al. described a case of arachnoiditis after intrathecal administration of bupivacaine without epinephrine.33 It is unclear in these cases whether the arachnoiditis was triggered by the bupivacaine or other preservatives. Furthermore, it is unclear whether epinephrine plays a role in the pathogenesis of arachnoiditis.
A history of spine surgery is a risk factor for arachnoiditis.8 In particular, some investigators have specifically stated that surgery for a herniated intervertebral disc may lead to arachnoiditis.5,7,25 Carroll and Wiesel showed that a postoperative pain-free interval lasting between 1 and 6 months, followed by the gradual onset of leg pain, increases the likelihood that some scar tissue is responsible for the symptoms.34 Smolik and Nash showed that simple dural retraction for the visualization of a ruptured intervertebral disc may trigger arachnoiditis.5 Haughton et al. showed that in monkeys, the nucleus pulposus of an intervertebral disc was able to cause focal arachnoiditis.35
Clinical Features
The diagnosis of arachnoiditis requires a detailed medical history and physical examination as well as a review of confirmatory radiographic imaging studies. In obtaining a medical history from a patient with arachnoiditis, the clinician should seek three major characteristics of the pain. Pain of arachnoiditis is typically described as a burning pain that is constant and worsened by activity.12 The pain of arachnoiditis may be located in the back, the lower limbs, or both. The symptoms of arachnoiditis can vary from nonspecific back pain to radiculopathy and myelopathy.36 Intractable pain that occurs secondary to arachnoiditis has a diffuse, poorly localized pain pattern. In many patients, arachnoiditis is asymptomatic and is discovered as an incidental radiographic finding.37 The pain symptoms of chronic arachnoiditis may be similar to those of other chronic pain syndromes, such as complex regional pain syndrome. The exact relationship of these pain syndromes has not been fully elucidated.
The physical examination findings in patients with arachnoiditis have been reviewed in two large clinical series. Burton followed 100 patients with arachnoiditis and found little motor weakness to be present. These patients were commonly found to have a positive straight-leg raise sign, a tender sciatic notch, limited range of motion of the trunk, and paravertebral muscle spasms.12 Guyer et al. followed 51 patients over more than 10 years and found that a decreased range of motion of the trunk was the most common finding on physical examination.25 In cases of chronic arachnoiditis with resultant syrinx formation, physical examination findings of syringomyelia are present. These include dissociative sensory loss and variable long tract signs.8
Radiographic Features
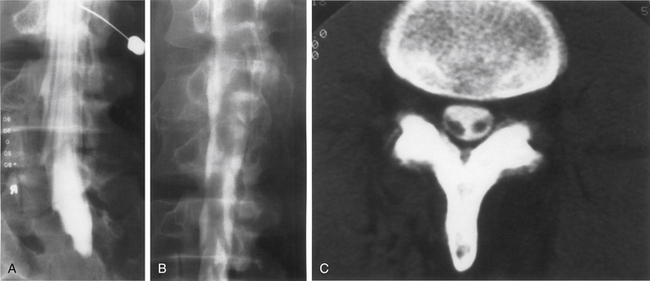
On myelography, two distinct patterns of radiographic arachnoiditis can be differentiated. In type I arachnoiditis, there is pure adhesion of the nerve roots to the meninges with a homogeneous contrast pattern. No nerve root shadows are seen, and there is a rounded shortening of the nerve root pocket. In type II arachnoiditis, some proliferation is added inside the dural sac that may be localized or diffuse.38 The filling defects, narrowing, shortening, or occlusion of the spinal canal are also seen in this type of arachnoiditis. In early arachnoiditis, there is central nerve root clumping and thickening. As the arachnoiditis progresses, the nerve roots become adherent peripherally to the thecal sac and the terminal thecal sac appears “sleeveless,” when the nerve roots do not fill it out in the normal pattern.8 This finding can cause the thecal sac to appear empty.
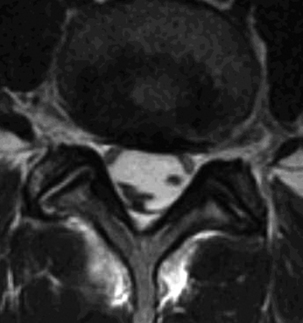
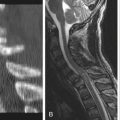
On MRI, one of three patterns is commonly found.39 The first pattern is characterized by conglomerations of nerve roots, which are located centrally within the thecal sac. The second pattern is characterized by nerve roots that are clumped and attached peripherally to the meninges (Fig. 196-2). This appearance is similar to the empty sac appearance of myelography. The third pattern demonstrates increased soft tissue signal within the thecal sac with central obliteration of the subarachnoid space (Fig. 196-3).
There are times when CT or MRI reveals calcification or ossification of the spinal arachnoid in an entity called arachnoiditis ossificans. There are several subtypes of spinal arachnoiditis ossificans based on imaging characteristics. Type I has a semicircular arrangement, type II is circular, and type III demonstrates englobing of the caudal fibers.40–42
Spinal Epidural Fibrosis
Spinal epidural fibrosis is an entity observed after spine surgery that contributes to up to 14% of cases of failed back syndrome. Spinal epidural fibrosis is caused when fibroblasts from damaged paraspinal muscles enter the vertebral canal and proliferate, forming extensive epidural scarring.43 This entity has been most typically described after cases of discectomy, whether open or percutaneous, as well as cases of implantation of spine-stimulating electrodes.43,44
Treatment
In a rat model of spinal epidural fibrosis, the administration of tissue plasminogen activator helped to prevent postlaminectomy epidural fibrosis. The presence of arachnoiditis was also less in the treatment group (P = .01).45 Lee et al. showed that in a rat model, the administration of 0.1 mg/mL of mitomycin C reduced epidural fibrosis after lumbar laminectomy. This group made macroscopic, histologic, and MRI evaluations of the animals.46 Epidural scarring was significantly reduced and dural adhesions were absent, while wound healing was not affected.
In a dog postlaminectomy model, it has been shown that a single fraction of 700-cGy external-beam radiation helped to prevent epidural fibrosis as well as arachnoiditis. The authors demonstrated statistically significant reductions in the extent of fibrosis and density of fibroblasts. MRI confirmation of the efficaciousness of the therapy was also demonstrated.47
A recent study, in humans, aimed to evaluate the role of epidural steroids in preventing epidural fibrosis. Eighty-five of 178 patients received epidural steroids following discectomy. Patients were followed for 1 year and were assessed by questionnaire containing the pain scale. Application of epidural steroids resulted in less pain on the first and third days after surgery and resulted in shorter hospital stays but did not prevent failed back syndrome or prevent epidural scar formation.48
The role of surgery in the treatment of arachnoiditis and epidural fibrosis is controversial. Surgical procedures that have been used to treat arachnoiditis include spine fusion procedures, decompressive spine procedures without fusion, neuroablative procedures, and implantation of spinal cord stimulators.8
A substantial body of literature exists that suggests that open surgical procedures are not useful in the treatment of arachnoiditis. Carroll and Wiesel found that no open surgical technique could eliminate the pathologic scar or significantly reduce the pain of arachnoiditis.34 Grahame et al. also found that open surgical procedures had little or no effect on the long-term course of arachnoiditis.37
Some groups argue for aggressive open surgical intervention for arachnoiditis and spinal epidural fibrosis. Shikata et al. compared microlysis for arachnoiditis with and without spine fusion.49 They found significant improvement in the clinical results when fusion was performed.
Spinal cord stimulation has been shown to have some benefit in patients with arachnoiditis. North et al. have shown that with proper patient selection, spinal cord stimulation can be a successful therapy. North et al. used temporary percutaneous electrodes as a screening technique before implantation of a permanent stimulator. A minimum of 50% pain relief with temporary electrodes over a 2- to 3-day course, as well as evidence of improved activity level and stable or decreased use of analgesics, was deemed satisfactory pain relief.50–54
Recent work has focused on minimally invasive techniques to treat arachnoiditis and spinal epidural fibrosis. A number of endoscopic techniques for adhesiolysis and promotion of CSF flow pathways have been developed, with promising results.55,56 Manchikanti et al. demonstrated, in a recent randomized controlled trial of spinal endoscopic adhesiolysis in chronic, refractory, low back pain and lower-extremity pain, that adhesiolysis with targeted delivery of local anesthesia and steroids is a successful technique in the treatment of arachnoiditis. This study demonstrated significant improvement in pain in 48% of subjects at 1 year follow-up.57
Burton C.V. Lumbosacral arachnoiditis. Spine (Phila Pa 1976). 1978;3(1):24-30.
Delamarter R.B., Ross J.S., Masaryk T.J., et al. Diagnosis of lumbar arachnoiditis by magnetic resonance imaging. Spine (Phila Pa 1976). 1990;5(4):304-310.
Manchikanti L., Boswell M.V., Rivera J.J., et al. A randomized, controlled trial of spinal endoscopic adhesiolysis in chronic refractory low back and lower extremity pain. BMC Anesthesiol. 2005;5:10.
North R.B., Kidd D.H., Piantadosi S. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a prospective, randomized study design. Acta Neurochir Suppl. 1995;64:106-108.
Smolik E.A., Nash F.P. Lumbar spinal arachnoiditis: a complication of the intervertebral disc operation. Ann Surg. 1951;133(4):490-495.
1. Rice I., Wee M.Y., Thompson K. Obstetric epidurals and chronic adhesive arachnoiditis. Br J Anaesth. 2004;92(1):109-120.
2. April E.W. Clinical anatomy, ed 3. Philadelphia: Lippincott Williams & Wilkins; 1997. 149–151
3. Shantha T.R., Evans J.A. The relationship of epidural anesthesia to neural membranes and arachnoid villi. Anesthesiology. 1972;37:543-557.
4. Ransford A.O., Harries B.J. Localised arachnoiditis complicating lumbar disc lesions. J Bone Joint Surg [Br]. 1972;54(4):656-665.
5. Smolik E.A., Nash F.P. Lumbar spinal arachnoiditis: a complication of the intervertebral disc operation. Ann Surg. 1951;133(4):490-495.
6. Delamarter R.B., Ross J.S., Masaryk T.J., et al. Diagnosis of lumbar arachnoiditis by magnetic resonance imaging. Spine (Phila Pa 1976). 1990;5(4):304-310.
7. Reigel D.H., Bazmi G., Shih S-R., et al. A pilot investigation of poloxamer 407 for the prevention of leptomeningeal adhesions in the rabbit. Pediatr Neurosurg. 1993;19(5):250-255.
8. Heary R.F., Northrup B.E., Barolat G. Arachnoiditis. In: Benzel E.C., editor. Spine surgery. ed 2. Philadelphia: Elsevier; 2005:2004-2012.
9. Mackay R.P. Chronic adhesive spinal arachnoiditis. JAMA. 1939;112:802.
10. McLaurin R.L., Bailey O.T., Schurr P.H., et al. Myelomalacia and multiple cavitations of spinal cord secondary to adhesive arachnoiditis. Arch Pathol. 1954;57(2):138-146.
11. Sklar E.M., Quencer R.M., Green B.A., et al. Complications of epidural anesthesia: MR appearance of abnormalities. Radiology. 1991;181(2):549-554.
12. Burton C.V. Lumbosacral arachnoiditis. Spine (Phila Pa 1976). 1978;3(1):24-30.
13. Yamagami T., Matsui H., Tsuji H., et al. Effects of laminectomy and retained extradural foreign body on cauda equina adhesions. Spine (Phila Pa 1976). 1993;18(13):1774-1781.
14. Poon T.L., Ho W.S., Pang K.Y., et al. Tuberculous meningitis with spinal tuberculous arachnoiditis. Hong Kong Med J. 2003;9(1):59-61.
15. Hoffman G.S. Spinal arachnoiditis: what is the clinical spectrum? Spine (Phila Pa 1976). 1993;8(5):538-540.
16. Jackson A., Isherwood I. Does degenerative disease of the lumbar spine cause arachnoiditis? A magnetic resonance study and review of the literature. Br J Radiol. 1994;67(801):840-847.
17. Kok A.J., Verhagen W.I., Bartels R.H., et al. Spinal arachnoiditis following subarachnoid haemorrhage: report of two cases and review of the literature. Acta Neurochir. 2000;142(7):795-799.
18. Jackson I.J. Aseptic hemogenic meningitis. Arch Neurol Psych. 1949;62:572-589.
19. Renk H. Neurological complications of central nerve blocks. Acta Anaesthesiol Scand. 1995;39:859-868.
20. Digiovanni A.J., Galbert M.W., Wahle W.M. Epidural injection of autologous blood for postlumbar-puncture headache: Part II. Additional clinical experiences and laboratory investigation. Anesth Analg. 1972;51:226-232.
21. Aldrete J.A., Brown T. Intrathecal hematoma and arachnoiditis after prophylactic blood patch through a catheter. Anesth Analg. 1997;84:228-236.
22. Abouleish E., De la Vega S., Bledinger I., et al. Long-term follow-up of epidural blood patch. Anesth Analg. 1975;54:459-463.
23. Burton C.V. Adhesive arachnoiditis. In: Youmans J.R., editor. Neurological surgery. ed 3. Philadelphia: WB Saunders; 1990:2856-2863.
24. Shaw M., Russell J.A., Grossart K.W. The changing pattern of spinal arachnoiditis. J Neurol Neurosurg Psych. 1978;41:97-107.
25. Guyer D.W., Wiltse L.L., Eskay M.L., et al. The long range prognosis of arachnoiditis. Spine (Phila Pa 1976). 1989;12(12):1332-1341.
26. Berg G., Hammar M., Moller-Nielsen J., et al. Low back pain during pregnancy. Obstet Gynecol. 1988;71:71-75.
27. Bernat J.L. Intraspinal steroid therapy. Neurology. 1981;31:168-170.
28. Nelson D.A. Dangers from methylprednisolone acetate therapy by intraspinal injection. Arch Neurol. 1988;45:804-806.
29. Abram S., Marasala M., Yaksh T. Analgesic and neurotoxic effects of intrathecal corticosteroids in rats. Anesthesiology. 1994;81:149-162.
30. Cicala R.S., Turner R., Moran E., et al. Methylprednisolone acetate does not cause inflammatory changes in the epidural space. Anesthesiology. 1990;72:556-558.
31. Delany T.J., Rowlingson J.C., Carron H., et al. Epidural steroid effects on nerves and meninges. Anesth Analg. 1980;59:610-614.
32. Boiardi A., Sghirlanzoni A., La Mantia L., et al. Diffuse arachnoiditis following epidural analgesia. J Neurol. 1983;230:253-257.
33. Gemma M., Bricchi M., Grisoli M., et al. Neurologic symptoms after epidural anesthesia. Report of three cases. Acta Anaesthesiol Scand. 1994;38:742-743.
34. Carroll S.E., Wiesel S.W. Neurologic complications and lumbar laminectomy: a standardized approach to the multiply-operated lumbar spine. Clin Orthop Relat Res. 1992;284:14-23.
35. Haughton V.M., Nguyen C.M., Ho K- C. The etiology of focal spinal arachnoiditis: an experimental study. Spine (Phila Pa 1976). 1993;18(9):1193-1198.
36. Smith A.S., Blaser S.I. Infectious and inflammatory processes of the spine. Radiol Clin North Am. 1991;29(4):809-827.
37. Grahame R., Clark B., Watson M., et al. Toward a rational therapeutic strategy for arachnoiditis: a possible role for d-penicillamine. Spine (Phila Pa 1976). 1991;16(2):172-175.
38. Jorgensen J., Hansen P.H., Steenskov V., et al. A clinical and radiological study of chronic lower spinal arachnoiditis. Neuroradiology. 1975;9(3):139-144.
39. Ross J.S., Masaryk T.J., Modic M.T., et al. MR imaging of lumbar arachnoiditis. Am J Roentgenol. 1987;149(5):1025-1032.
40. Chan C.C., Lau P.Y., Sun L.K., et al. Arachnoiditis ossificans. Hong Kong Med J. 2009;15:146-148.
41. Domenicucci M., Ramieri A., Passacantilli E., et al. Spinal arachnoiditis ossificans: report of three cases. Neurosurgery. 2004;55(4):985.
42. Papavlasopoulos F., Stranjalis G., Kouyialis A.T., et al. Arachnoiditis ossificans with progressive syringomyelia and spinal arachnoid cyst. J Clin Neurosci. 2007;14(6):572-576.
43. Smuck M., Benny B., Han A., et al. Epidural fibrosis following percutaneous disc decompression with coblation technology. Pain Physician. 2007;10:691-696.
44. Reynolds A.F., Shetter A.G. Scarring around cervical stimulating electrode. Neurosurgery. 1983;13(1):63-65.
45. Kemaloglu S., Ozkan U., Yilmaz F., et al. Pention of spinal epidural fibrosis by recombinant tissue plasminogen activator in rats. Spinal Cord. 2003;41:427-431.
46. Lee J.Y., Stenzel W., Impekoven P., et al. The effect of mitomycin C in reducing epidural fibrosis after lumbar laminectomy in rats. J Neurosurg Spine. 2006;5(1):53-60.
47. Gerszten P.C., Moossy J.J., Flickinger J.C., et al. Inhibition of peridural fibrosis after laminectomy using low-dose external beam radiation in a dog model. Neurosurgery. 2000;46(6):1478-1485.
48. Hackel M., Masopust V., Bojar M., et al. The epidural steroids in the prevention of epidural fibrosis: MRI and clinical findings. Neuro Endocrinol Lett. 2009;30(1):51-55.
49. Shikata J., Yamamuro T., Iida H., et al. Surgical treatment for symptomatic spinal adhesive arachnoiditis. Spine (Phila Pa 1976). 1989;14(8):870-875.
50. North R.B., Campbell J.N., James C.S., et al. Failed back surgery syndrome: 5-year follow up in 102 patients undergoing repeated operation. Neurosurgery. 1991;28(5):685-690.
51. North R.B., Ewend M.G., Lawton M.T., et al. Failed back surgery syndrome: 5 year follow-up after spinal cord stimulator implantation. Neurosurgery. 1991;28(5):692-699.
52. North R.B., Kidd D.H., Piantadosi S. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a prospective, randomized study design. Acta Neurochir Suppl. 1995;64:106-108.
53. North R.B., Kidd D.H., Shipley J., et al. Spinal cord stimulation versus reoperation for failed back syndrome: a cost effectiveness and cost utility analysis based on a randomized controlled trial. Neurosurgery. 2007;61(2):361-368.
54. North R.B., Kidd D.H., Zahurak M., et al. Spinal cord stimulation for chronic, intractable pain: experience over two decades. Neurosurgery. 1993;32(3):384-394.
55. Warnke J.P., Mourgela S. Adhesive lumbar arachnoiditis: endoscopic subarachnoepidurostomy as a new treatment. Nervenarzt. 2007;78(10):1182-1187.
56. Warnke J.P., Mourgela S. Endoscopic treatment of lumbar arachnoiditis. Minim Invasive Neurosurg. 2007;50(1):1-6.
57. Manchikanti L., Boswell M.V., Rivera J.J., et al. A randomized, controlled trial of spinal endoscopic adhesiolysis in chronic refractory low back and lower extremity pain. BMC Anesthesiol. 2005;5:10.