Chapter 6 Approaches to Intellectual and Memory Impairments
Francis Crick (1994), who with James Watson won the Nobel Prize for the discovery of the structure of DNA, expressed the “astonishing hypothesis” that “you, your joys and your sorrows, your sense of personal identity and free will, are in fact no more than the behavior of a vast assembly of nerve cells and their associated molecules” (p. 3). This chapter considers our knowledge of intellect and memory, mind and brain, from the perspective of the clinical neurologist who must assess disorders of the higher functions.
Neural Basis of Cognition
Cerebral Cortex
The cognitive operations discussed in this chapter take place among a large network of cortical cells and connections, the neural switchboard that gives rise to conscious thinking. The cortical mantle of the human brain is very large compared with animal brains, containing more than 14 billion neurons. The information stored in the human cerebral cortex rivals that found in large libraries. Within the cortical mantle, the areas that have expanded the most from animal to human are the association cortices, cortical zones that do not carry out primary motor or sensory functions but rather interrelate the functions of the primary motor and sensory areas. According to Nauta and Feirtag’s 1986 text, 70% of neurons in the human central nervous system reside in the cerebral cortex, and 75% of those are in the association cortex. Higher cortical functions, with few exceptions, take place in the association cortex.
The second heteromodal association cortex involves the lateral prefrontal region (Goldman-Rakic, 1996). This region is thought to be involved with attention or “working memory” and with sequential processes such as storage of temporally ordered stimuli and the planning of motor activities. This temporal sequencing of information and motor planning is referred to by neuropsychologists as the executive function of the brain—the decisions we make every instant regarding which of the myriad of sensory stimuli reaching the sensory cortices merit attention, which require a motor response, and in what sequence and timing these motor responses will occur.
Another frontal cortical area, the orbitofrontal portion of the prefrontal cortex, is thought to be involved in emotional states, appetites, and drives, or in the integration of internal bodily states with sensations from the external world. The orbitofrontal cortex is known as the supramodal cortex (Benson, 1996) because it relates the functions of the heteromodal cortex regarding attention and sequencing of responses with interoceptive inputs from the internal milieu of the body. The orbitofrontal area has close connections with the limbic system and autonomic, visceral, and emotional processes. In studying brain evolution from primitive reptiles to humans, the neurobiologist Paul MacLean hypothesized that the internal and emotional parts of the brain, the limbic system, must be tied into the newer neocortical areas responsible for intellectual function, and that the linking of these two systems must underlie the phenomenon of consciousness. In a review of neuronal mechanisms of consciousness, Ortinski and Meador (2004) defined conscious awareness as “the state in which external and internal stimuli are perceived and can be intentionally acted on” (p. 1017). Benson and Ardila (1996), in reviewing clinical data from individuals with frontal lobe damage, state that the supramodal cortex is the brain system that “anticipates, conjectures, ruminates, plans for the future, and fantasizes.” In other words, this part of the brain brings specific cognitive processes to conscious awareness and may be responsible for the phenomena of consciousness and self-awareness themselves.
Consciousness
Crick and Koch (1995) hypothesized that activation of the frontal cortex is necessary for visual percepts to enter consciousness, although subconscious awareness in the form of blindsight may exist at the level of the occipital cortex. Conscious visual perception involves interactions between the visual parts of the brain and the prefrontal systems for attention and working memory (Ungerleider, Courtney, and Haxby, 1998). The orbitofrontal cortex contains neurons that integrate interoceptive stimuli related to changes in the internal milieu with exteroceptive sensory inputs such as vision. Ortinski and Meader (2004) also point out the varying latencies of perception of specific sensory stimuli, such as color versus identification of a visual object. A synchronization of inputs through the thalamus to the cortex may be necessary before the perception becomes conscious. As stated earlier, the interaction between attention to external stimuli and internal stimuli underlies conscious awareness.
In the visual system, Goodale and Milner (1992; Milner and Goodale, 2008; see also McIntosh and Schenk, 2009) have divided the visual system, after processing in the occipital cortex, into a ventral and a dorsal stream. The ventral stream, involved in perception of objects, is usually subject to conscious awareness and involves an occipital-temporal pathway, whereas the dorsal stream, involved in spatial localization of perceived objects to plan action, is usually less conscious.
There are many clinical examples of “unconscious” mental processing, and a number of these involve vision. Patients with cortical blindness sometimes show knowledge of items they cannot see, a phenomenon called blindsight. Patients with right hemisphere lesions who extinguish objects in the left visual field when presented with bilateral stimuli nonetheless show activation of the right visual cortex by functional magnetic resonance imaging (MRI), indicating that the objects are perceived, although not with conscious awareness (Rees et al., 2000). Libet (1999) demonstrated experimentally that visual and other sensory stimuli have to persist at least 500 milliseconds to reach conscious awareness, yet stimuli of shorter duration can elicit reactions. An experimental example of unconscious visual processing comes from Gur and Snodderly (1997), who tested color vision in monkeys. When two colors were projected at a frequency of greater than 10 Hz, the monkey perceived a fused color, yet cellular recordings clearly demonstrated coding of information about the two separate colors in the monkey’s visual cortex. Motor responses to sensory stimuli can occur before conscious awareness, as in the ability to pull one’s hand away from a hot stove before feeling the heat. Racers begin running before they are aware of having heard the starting gun (Crick and Koch, 1998). A familiar example of unconscious visual processing is the drive home from work; most individuals can remember very little they see on the trip, yet they avoid oncoming vehicles and obstacles, stop for red lights, and drive without accidents. Crick and Koch (1998) refer to the unconscious visual processing as an “online” visual system. We shall discuss unconscious or “implicit” memories later in this chapter. In language syndromes, patients can match spoken to written words without knowledge of their meaning, suggesting that there are unconscious rules of language. Brust (2000) has called all of these unconscious mental processes the “non-Freudian unconscious.”
Recent research has linked the right frontal cortex to the sense of self. Keenan and colleagues (2001) studied patients undergoing the Wada test, in which a barbiturate is injected into the carotid artery to determine cortical language dominance. They presented subjects with a self-photograph and a photograph of a famous person, followed by a “morphed” photograph of a famous person and the patient. When the left hemisphere was anesthetized, the subjects said that the morphed photograph represented the subject himself, whereas with right hemisphere anesthesia, the subject selected the famous face. Patients with frontotemporal dementia also indicate a relationship between the right frontal lobe and self-concept. In the series by Miller and colleagues (2001), six of the seven patients who developed a major change in self-concept during their illness had predominant atrophy in the nondominant frontal lobe. A last example of the sense of self is the so-called Theory of Mind, which alludes to the understanding of another person as a conscious human being. Keenan and colleagues (2005) cite evidence that the right hemisphere frontotemporal cortex is dominant for both the sense of self and the recognition of other people.
Clinical neurology provides important information about how lesions in the brain impair consciousness. The functioning of the awake mind requires the ascending inputs referred to as the reticular activating system, with its way stations in the brainstem and thalamus, as well as an intact cerebral cortex. Bilateral lesions of the brainstem or thalamus produce coma. Very diffuse lesions of the hemispheres produce an “awake” patient who shows no responsiveness to the environment, a state sometimes called coma vigil or persistent vegetative state, as in the well-known Terri Schiavo case (Bernat, 2006; Perry, Churchill, and Kirshner, 2005). Patients with very slight responses to environmental stimuli are said to be in a minimally conscious state (Wijdicks and Cranford, 2005). Recently, functional brain imaging studies have suggested that at least in a few patients labeled as having persistent vegetative state or minimally conscious state after traumatic brain injury, patients can think of playing tennis or standing in their home and seeing the other rooms, and the brain areas activated are similar to those of normal subjects. These same subjects, a small minority of patients with chronically impaired consciousness secondary to traumatic brain injury, showed evidence of conscious modulation of brain activity to indicate “yes” or “no” responses (Monti et al., 2010). This report has engendered controversy over our ability to determine when a patient truly lacks consciousness. In an accompanying editorial, Ropper noted that activation on brain imaging studies does not equal conscious awareness, and the concept that “I have brain activation, therefore I am…would seriously put Descartes before the horse” (Ropper, 2010).
Still less severe diffuse abnormalities of the association cortex produce encephalopathy, delirium, or dementia. These topics involve very common syndromes of clinical neurology. Stupor and coma are discussed in Chapter 5, and encephalopathy, or delirium, is covered in Chapter 4.
Focal lesions of the cerebral cortex generally produce deficits in specific cognitive systems. A detailed listing of such disorders would include much of the subject matter of behavioral neurology. Examples include Broca aphasia from a left frontal lesion, Wernicke aphasia from a left temporal lesion, Gerstmann syndrome (acalculia, left-right confusion, finger agnosia, and agraphia) from a left parietal lesion, visual agnosia or failure to recognize visual objects (usually from bilateral posterior lesions), apraxia from a left parietal lesion, and constructional impairment from a right parietal lesion. Multiple focal lesions can affect cognitive function in a more global fashion, as in the dementias (Chapter 66). Some authorities separate “cortical” dementias such as Alzheimer disease, in which combinations of cortical deficits are common, from “subcortical” dementias, in which mental slowing is the most prominent feature.
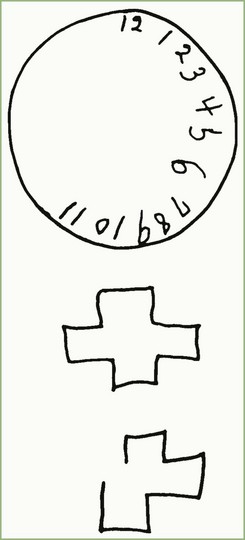
The frontal lobes are heavily involved in integration of the functions provided by other areas of cortex, and lesions there may affect personality and behavior in the absence of easily discernible deficits of specific cognitive, language, or memory function. In severe form, extensive lesions of the orbitofrontal cortex may leave the individual awake but staring, unable to respond to the environment, a state called akinetic mutism. With lesser lesions, patients with frontal lobe lesions may lose their ability to form mature judgments, reacting impulsively to incoming stimuli in a manner reminiscent of animal behavior. Such patients may be inappropriately frank or disinhibited. A familiar example is the famous case of Phineas Gage, a worker who sustained a severe injury to the frontal lobes. Gage became irritable, impulsive, and so changed in personality that coworkers said he was “no longer Gage.” Bedside neurological testing and even standard neuropsychological tests of patients with frontal lobe damage may reveal normal intelligence except for concrete or idiosyncratic interpretation of proverbs and similarities. Experimentally, subjects with frontal lobe lesions can be shown to have difficulty with sequential processes or shifting of cognitive sets, as tested by the Wisconsin Card Sorting Test or the Category Test of the Halstead-Reitan battery. Luria introduced a simple bedside test of sequential shapes (Fig. 6.1). In contrast to the subtlety of these deficits to the examiner, the patient’s family may state that there is a dramatic change in the patient’s personality.

Fig. 6.1 Luria’s test of alternating sequences.
(Adapted from Luria, A.R., 1969. Frontal lobe syndromes, In: Vynken P., Bruyn, G.W. (Eds.), Handbook of Clinical Neurology, vol. 2, Elsevier, New York. Reprinted with permission from Kirshner, H.S., 2002. Behavioral Neurology: Practical Science of Mind and Brain, second ed. Butterworth Heinemann, Boston.)
Another clinical window into the phenomena of consciousness comes from surgery to separate the hemispheres by cutting the corpus callosum. In split-brain or commissurotomized patients, each hemisphere seems to have a separate consciousness. The left hemisphere, which has the capacity for speech and language, can express this consciousness in words. For example, a split-brain patient can report words or pictures that appear in the right visual field. The right hemisphere cannot produce verbal accounts of items seen in the left visual field, but the subject can choose the correct item by pointing with the left hand; at the same time, the subject claims to have no conscious knowledge of the item. In terms of the speaking left hemisphere, the right hemisphere has “unconscious” visual knowledge, or blindsight. At times, the left hand of the patient may seem to operate under a different agenda from the right hand. A split-brain patient may select a dress from a rack with the right hand while the left hand puts it back or selects a more daring fashion. This rivalry of the left hand with the right is called the alien hand syndrome, a striking example of the separate consciousnesses of the two divided hemispheres (Gazzaniga, 1998). Callosal syndromes, including the alien hand syndrome, have also been described in patients with strokes involving the corpus callosum (Chan and Ross, 1997).
Memory
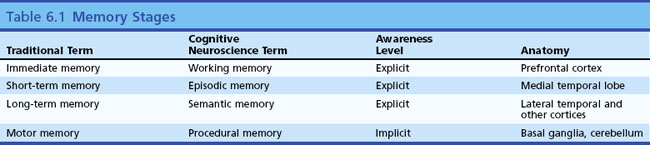
Memory Stages
Memory refers to the ability of the brain to store and retrieve information, the necessary prerequisite for all learning. Some memories are so vivid they seem like a reliving of a prior experience, as in Marcel Proust’s sudden recollections of his youth on biting into a madeleine pastry. Other memories are more vague or bring up a series of facts rather than a perceptual experience. Memory has been divided into several types and stages, leading to a confusing set of terms and concepts. Clinical neurologists have historically divided memory into three temporal stages. The first stage, called immediate memory span, corresponds to Baddeley’s concept of working memory (Baddeley, 2010). Immediate memory refers to the amount of information a subject can keep in conscious awareness without active memorization. The normal human being can retain seven digits in active memory span. Perhaps by coincidence, seven digits comprise a local telephone number. Most normal people can hear a telephone number, walk across the room, and dial the number without active memorization. Numbers of more than seven digits, called supraspan numbers, do require active memory processing, as do unnatural tasks such as reverse digit span. Disorders of attention affect digit span and very focal lesions of the superior frontal neocortex affecting Brodmann areas 8 and 9 may have profound effects on immediate memory (Goldman-Rakic, 1996). Many patients with aphasia secondary to left frontal lesions have impaired immediate memory. The items in immediate memory are normally forgotten as soon as the subject’s attention switches to another topic (telephone numbers we look up and call once tend to be completely forgotten quickly unless we actively try to “memorize” them).
The second stage of memory, referred to by clinicians as short-term or recent memory, involves the ability to register and recall specific items such as words or events after a delay of minutes or hours. Synonyms for this type of memory include declarative and episodic memory (Squire and Zola, 1996; Dickerson and Eichenbaum, 2010). Some memory investigators and cognitive neuroscientists refer to immediate memory span as short-term and recent memory as long-term. This differs from clinical usage and causes confusion; for this reason, it is better to use the less ambiguous term, episodic memory. This second stage of memory requires the function of the hippocampus and parahippocampal areas of the medial temporal lobe for both storage and retrieval. The amygdala, a structure adjacent to the medial temporal cortex, is not essential for episodic memory but seems crucial for recall of emotional contexts of specific events and the reactions of fear or pleasure associated with those events. The familiar bedside test of recalling three unrelated memory items at 5 minutes demonstrates episodic (short-term) memory, as do questions about this morning’s breakfast.
Long-term memory, also called remote memory, refers to long-known information such as where one grew up, who was one’s first grade teacher, or the names of grandparents. In current parlance, the factual knowledge we recall consciously is called semantic memory (Budson and Price, 2005). Recall of famous figures or events, such as presidents or wars, and knowledge of semantic information, such as the definitions of words and the differences between words, are used to test semantic memory. Semantic memory differs from personal long-term memory in that the subject can continuously replenish such knowledge by reading and conversation.
Semantic memory is thought to reside in multiple cortical regions such as the visual association cortex for visual memories, the temporal cortex for auditory memories, and so on. This concept of multiple localizations of semantic memory is supported by functional brain imaging research (Cappa, 2008). Specific semantic knowledge of word meanings is thought to reside in the left lateral temporal cortex. Remote memory, as we shall see later, resists the effects of medial temporal damage; once memory is well stored in the neocortex, it can be retrieved without use of the hippocampal system.
Other categories of memory exist that do not fit into this temporal classification, such as motor and procedural memories. These will be discussed later in this chapter. Table 6.1 is a classification of memory stages.
Amnestic Syndrome
The amnestic syndrome (Box 6.1) refers to profound loss of the second stage of memory, episodic, recent, or short-term memory. These patients, most of whom have bilateral hippocampal damage, have normal immediate memory span and largely normal ability to recall remote memories such as their childhood upbringing and education. Other cognitive or higher cortical functions may be completely intact, which distinguishes these patients from those with dementias such as Alzheimer disease. Motor memories (see Other Types of Memory) tend to be preserved in patients with amnestic syndrome, who may be taught to perform a new motor skill such as mirror writing. When asked to perform it again, the patient will typically not recall knowing how to do it, but once he or she gets started, the action comes back (the memory is in the hands). Other more variable features of the amnestic syndrome include disorientation to time and sometimes place and confabulation, or making up information the memory system does not supply. Amnestic patients live in an eternal present in which they can interact, speak intelligently, and reason appropriately, but they do not remember anything about the interaction a few minutes after it ends. An amnestic patient may complete an IQ test with high scores but not recall taking the examination minutes later. These patients are condemned to repeat the same experiences without learning from them.
The neuroanatomy of the amnestic syndrome is one of the best-studied areas of cognitive neuropsychology. In animal models, bilateral lesions of the hippocampus, parahippocampal gyrus, and entorhinal cortex produce profound amnesia (Squire and Zola, 1996). Human patients undergoing temporal lobectomy for epilepsy have shown very similar syndromes. In the early period of this surgery, a few patients were deliberately subjected to bilateral medial temporal ablations, with disastrous results for memory, as seen in the famous patient, H.M. (Corkin, 2002; Squire, 2009). In other cases, unilateral temporal lobectomy caused severe amnesia. In one such case, an autopsy many years later showed preexisting damage to the contralateral hippocampus. Patients currently receive extensive evaluation such as the Wada test (intracarotid barbiturate infusion) to ensure that ablation of one hippocampus will not result in the amnestic syndrome, although partial memory deficits still occur. Other common causes of the amnestic syndrome involving bilateral medial temporal lesions include bilateral strokes in the posterior cerebral artery territory, involving the hippocampus, and herpes simplex encephalitis, which has a predilection for the orbitofrontal and medial temporal cortices. Gold and Zola (2006) described three new cases of the amnestic syndrome with detailed neurobehavioral testing in life and neuropathology at autopsy. One had bilateral hippocampal damage, one had Wernicke-Korsakoff syndrome with damage in the mammillary bodies and dorsomedial thalamus, and one had bilateral thalamic infarctions; we will return to these other anatomic substrates of memory later.
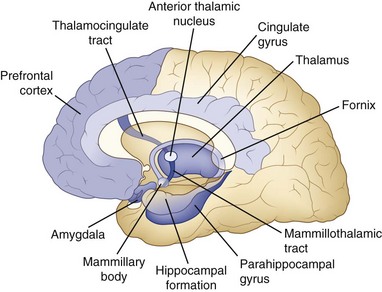
Although the anatomy of memory storage and retrieval has been known for many years, numerous recent refinements have been made. Fig. 6.2 shows a simplified diagram of the memory system in the human brain. The hippocampus on each side projects via the fornix to the septal areas, then to the mammillary bodies, which in turn project to the anterior nucleus of the thalamus and on to the cingulate gyrus of the frontal lobe, which projects back to the hippocampus. This circuit (Papez circuit) is critical for short-term memory registration and retrieval. Disease processes that affect extrahippocampal parts of this circuit also cause amnesia. One well-studied example is the Wernicke-Korsakoff syndrome induced by thiamine deficiency, usually in the setting of alcoholism, with damage to the mammillary bodies and dorsomedial thalamic nuclei (Gold and Squire, 2006). A second clinical example is that of patients with ruptured aneurysms of the anterior communicating artery, which are associated with damage to the deep medial frontal areas such as the septal nuclei. These two amnestic syndromes are commonly associated with confabulation. The anterior communicating artery aneurysm syndrome also involves frontal executive dysfunction (Diamond, DeLuca, and Kelley, 1997). Traumatic brain injuries commonly produce memory loss, probably because the most common sites of damage are in the frontal and temporal lobes, but other deficits besides memory frequently occur. Of course, memory loss can be seen in several other neurological conditions, including brain tumors of the thalamus or temporal lobes, white matter diseases such as multiple sclerosis, and dementing diseases such as Alzheimer disease, which has a predilection for the hippocampus, basal frontal nuclei, and neocortex. In these other disorders, memory loss is usually not as isolated a deficit as in the amnestic syndrome.
Recently, use of functional brain imaging in awake patients has contributed to knowledge of the anatomy of memory function. According to an early positron emission tomography (PET) study, several brain regions show consistent activation in normal subjects during memory testing. These brain regions include (1) the prefrontal cortex, especially on the right; (2) the hippocampal and adjacent medial temporal regions; (3) the anterior cingulate cortex; (4) the posterior midline regions of the cingulate, precuneate, and cuneate gyri; (5) the inferior parietal cortex, especially on the right; and (6) the cerebellum, particularly on the left (Cabeza et al., 1997). A model for the functions of these areas in memory are as follows: the prefrontal cortex appears to relate to retrieval activation and attention, the hippocampi to conscious recollection, the cingulate cortex to the activation of memory and selection of a specific response, the posterior midline regions to visual imagery, the parietal cortex to spatial awareness, and the cerebellum to voluntary self-initiated retrieval (Cabeza et al., 1997; Wagner et al., 1998; Dickerson and Eichenbaum, 2010). In subjects asked to recognize previously presented pairs of associated words, the right prefrontal cortex, anterior cingulate cortex, and inferior parietal region were the most activated. When the subject had to recall the words, the basal ganglia and left cerebellum also became active. In similar studies using functional MRI, Wagner and colleagues (1998) found that the left prefrontal region was predominantly involved when words were semantically encoded in memory; the right frontal activations seen in the previous study reflected nonverbal memory stimuli. Even in the hippocampus, words elicited activation of the left hippocampus, objects evoked activation in both hippocampi, and faces mainly activated the right hippocampus (Fliessbach et al., 2010; Rosazza et al., 2009). In studies of the recognition of visual designs, Petersson and colleagues (1997) found that the medial temporal cortex activates more during new learning tasks than during previously trained and practiced memory tasks. Other areas activated during the new learning task included the prefrontal and anterior cingulate areas, more on the right side, and the parieto-occipital lobes bilaterally. Trained tasks activated the hippocampi much less but did activate the right infero-occipitotemporal region. This finding correlates with human studies indicating that overlearned memories gradually become less dependent on the hippocampus. Rugg and colleagues (1997) also found greater activation of the left medial temporal cortex in tasks in which the subject remembered words by “deep encoding” of their meaning compared to simpler “shallow” encoding of the specific word. Other studies have shown that the deeper the encoding of a word’s meaning, the better the subject remembers it (Schacter, 1996). Finally, the amygdala appears necessary for affective aspects of memory items, such as recall of fear associated with a specific stimulus (Knight, Waters, and Bandettini, 2009).
Budson and Price (2005) provide a simple analogy for remembering the anatomical organization of episodic memory. By this analogy, the frontal lobes are the “filing clerk” of the memory system, deciding what memories to file or retrieve. The medial temporal lobes are the “recent memory filing cabinet,” where recent memories are consolidated. More remote memories are stored in the “remote memory filing cabinet,” thought to be located more diffusely in other cortical regions.
Basic research on animals has begun to unravel the fundamental biochemical processes involved in memory. Bailey and colleagues (1996) have studied memory formation in the giant snail, Aplysia. Development of long-term facilitation, a primitive form of memory, requires activation of a gene called CREB (cyclic adenosine monophosphate response element-binding protein) in sensory neurons. In this system and also in similar studies on the fruit fly, Drosophila, gene activation and protein synthesis are necessary for memory formation. Injection of protein-synthesis inhibitors into the hippocampus can prevent consolidation of memories (McGaugh, 2000). Although similar studies have not been performed in humans, it is likely that similar gene activation and protein synthesis, perhaps beginning in the hippocampi but proceeding through its neocortical connections, is necessary for the transition from immediate working memory to longer-term storage of memory (Bear, 1997). This field of research may hold promise for the development of drugs to enhance memory storage.
Transient Amnesia
Transient amnesia is a temporary version of amnestic syndrome. The most striking example of transient amnesia is the syndrome of transient global amnesia, lasting from several to 24 hours. In this syndrome, an otherwise cognitively intact individual suddenly loses memory for recent events, asks repetitive questions about his or her environment, and sometimes confabulates. During the episode, the patient has both anterograde and retrograde amnesia, as in the permanent amnestic syndrome. As recovery occurs, however, the retrograde portion “shrinks” to a short period, leaving a permanent gap in memory of the brief retrograde amnesia before the episode and the period of no learning during the episode. The syndrome is of unknown cause but can be closely imitated by disorders of known etiology such as partial complex seizures, migraine, and possibly transient ischemia of the hippocampus on one or both sides. Strupp and colleagues (1998) reported that 7 of 10 patients imaged during episodes of transient global amnesia showed abnormal diffusion MRI signal in the left hippocampus; 3 of these had bilateral hippocampal abnormalities. Permanent infarctions were not found. Yang and colleagues (2008) reported similar hippocampal lesions in the lateral or CA1 region in 17 of 20 cases of TGA. Other investigators have found frontal lobe abnormalities by diffusion-weighted MRI or PET. Gonzalez-Martinez and colleagues (2010) recently reported a case in which a small left thalamic infarction found by diffusion-weighted MRI was associated with hypometabolism in the left thalamic region, seen on FDG-PET. These studies do not prove an ischemic etiology for transient global amnesia; rather, they indicate transient dysfunction in the hippocampus or its connections. The last several patients with transient global amnesia observed at our hospital have had normal diffusion-weighted MRI studies, except for two patients who had incomplete recovery; these patients both had left medial temporal infarctions. Confusional migraine, partial epilepsy (Bilo et al., 2009), drug intoxication, alcoholic “blackouts,” and minor head injuries can also produce transient amnesia.
Other Types of Memory (Nondeclarative or Implicit Memory)
A confusing array of memory classifications and terminology has arisen, as shown in Table 6.2. Several aspects of memory do not involve the conscious recall involved in the three temporal memory stages. A simple example is motor memory, such as the ability to ride a bicycle, which is remarkably resistant to hippocampal damage. Such motor memories probably reside in the basal ganglia and cerebellum. In Squire and Zola’s (1996) classification, motor memories of this type are called procedural or implicit nondeclarative memories; note that all three of the temporal stages of memory—working (immediate) memory, episodic (short-term) memory, and semantic (long-term) memory—are declarative, explicit.
| Types of Recent Memory | Localization |
|---|---|
| DECLARATIVE (EXPLICIT) | |
| Facts, events | Medial temporal lobe |
| NONDECLARATIVE (IMPLICIT) | |
| Procedural skills | Basal ganglia, frontal lobes |
| Classical conditioning | Cerebellum (+ amygdala) |
| Probabilistic classification learning | Basal ganglia |
| Priming | Neocortex |
Another term for the class of memories for which subjects have no conscious awareness is implicit or nondeclarative memory (in contrast to the explicit declarative memory of episodic events). Implicit memories have in common storage and retrieval mechanisms that do not involve the hippocampal system; perhaps for this reason, the subject has no conscious knowledge of them. These procedural memories involve “knowing how” rather than “knowing that.” Amnestic patients can learn new motor memories such as mirror drawing, which they can perform once started, although they have no recollection of knowing the task. Motor learning likely involves the supplementary motor cortex, basal ganglia, and cerebellum. Strokes in the territory of the recurrent artery of Heubner (affecting the caudate nucleus) can affect procedural memory (Mizuta and Motomura, 2006). Another type of memory localized to the cerebellum is classical conditioning, in which an unconditioned stimulus becomes associated with a reward or punishment given when the conditioned stimulus is presented (Thompson and Kim, 1996; Clark, Manns, and Squire, 2002). The conditioning itself clearly involves the cerebellum, but the emotional aspect of the reward or punishment stimulus may reside in the amygdala. Classical conditioning can continue to function after bilateral hippocampal damage. Squire and Zola (1996) outlined other types of nondeclarative memory that take place independent of the hippocampal system. Probabilistic classification learning (e.g., predicting the weather from a combination of cues that are regularly associated with sunny or rainy weather) is unaffected by hippocampal damage but impaired in diseases of the basal ganglia such as Huntington and Parkinson diseases (Thompson and Kim, 1996; Gluck, Myers, and Shohamy, 2002). Learning artificial grammar can also take place in the presence of amnestic syndrome, with functional imaging showing activation in the left parietal and occipital lobes (Skosnik et al., 2002). In all these memory experiments, the subject has no awareness of how he or she is able to answer the questions. The last form of nondeclarative memory is called priming, the presentation of a stimulus associated with the word or idea to be remembered, which then aids in retrieval of the item (e.g., recalling the word doctor when nurse appears on a priming list). Priming appears to involve the neocortex (Thompson and Kim, 1996; Levy et al., 2004). Schacter and Buckner (1998) have shown that deliberate use of priming can help amnestic patients compensate for their memory loss in everyday life.
Bedside Tests of Memory and Cognitive Function
One answer to the dilemma of mental status testing is to use the MMSE as a screening test and then supplement it with more focused tests. Box 6.2 lists the key elements of a mental status examination, whether the examiner chooses to adopt the MMSE, one of the other bedside cognitive instruments, or to create an individual test battery. Several texts provide further detail on such a battery. Although the mental status examination is the most neglected area of the neurological examination, it generally requires only a few minutes, and its cost-effectiveness compares well with brain imaging studies such as MRI or PET.
The formal mental status examination should always include explicit testing of orientation including the date, place, and situation. Memory testing should include an immediate attention test, of which the most popular are forward digit span, serial-7 subtractions from 100, or the MMSE test “spell world backward.” Short-term memory should include recall of three unrelated words at 5 minutes. The subject should always be asked to say them back after presentation to make sure the three items have registered. At times, nonverbal short-term memory, such as recalling the locations of three hidden coins or reproducing drawings, can be useful to test. Remote memory can be tested by having the subject name children or siblings. Fund of information can be tested with recent presidents or other political figures. For patients who do not pay attention to politics, use of athletic stars or television celebrities may be more appropriate. Language testing should include spontaneous speech, naming, repetition, auditory comprehension, reading, and writing (the bedside language test is described in more detail in Chapter 12). In our practice, we like to show subjects more difficult naming items such as the drawings from the NIH Stroke Scale or body parts such as the thumb or the palm of the hand. Praxis testing should include the use of both imaginary and real (e.g., saw, hammer, pencil) objects. Both hands should be tested separately. Calculation tasks include the serial-7 subtraction test and simple change-making problems. Visual-spatial-constructional tasks can include line bisection, copying a cube or other design, and drawing a clock or a house (Fig. 6.3). The MMSE contains only one constructional task, the copying of intersecting pentagons. Many neurologists supplement this with the clock-drawing test. Insight and judgment are probably best tested by assessing the patient’s understanding of his own illness. Artificial tests include interpretation of proverbs (e.g., “Those who live in glass houses should not throw stones”) or stating why an apple and an orange are similar. An artificial test sometimes used to test frontal lobe processing is the copying and continuation of Luria’s test of alternating sequences (sequential squares and triangles; see Fig. 6.1). With these tests, preliminary localization can be made in the deep memory structures of the medial temporal lobes, the frontal lobes (insight and judgment, proverbs, similarities, Luria’s sequence test), the left hemisphere language cortex in the frontal and temporal lobes, the left parietal region (calculations), and the right parietal lobe (visual-constructional tasks).
Baddeley A. Working memory. Curr Biol. 2010;20:136-140.
Bailey C.H., Bartsch D., Kandel E.R. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA. 1996;93:13445-13452.
Bear M.F. How do memories leave their mark? Nature. 1997;385:481-482.
Benson DF, 1996. Approaches to intellectual memory impairments, in Neurology in Clinical Practice. In: Bradley WG. 2nd ed.
Benson D.F., Ardila A. Aphasia: a Clinical Perspective. New York: Oxford University Press; 1996.
Bernat J.L. Chronic disorders of consciousness. Lancet. 2006;367:1181-1192.
Bilo L., Meo R., Ruosi P., et al. Transient epileptic amnesia: an emerging late-onset epileptic syndrome. Epilepsia. 2009;50(Suppl 5):58-61.
Brust J.C.M. The non-Freudian unconscious. Neurologist. 2000;6:224-231.
Budson A.E., Price B.H. Memory dysfunction. N Engl J Med. 2005;352:692-699.
Cabeza R., Mangels J., Nyberg L., et al. Brain regions differentially involved in remembering what and when: a PET study. Neuron. 1997;19:863-870.
Cappa S.F. Imaging studies of semantic memory. Curr Opin Neurol. 2008;21:669-675.
Chan J.-L., Ross E.D. Alien hand syndrome: influence of neglect on the clinical presentation of frontal and callosal variants. Cortex. 1997;33:287-299.
Clark R.E., Manns J.R., Squire L.R. Classical conditioning, awareness, and brain systems. Trends Cogn Sci. 2002;6:524-531.
Corkin C. What’s new with the amnesic patient H.M.? Nat. Rev Neurosci. 2002;3:153-160.
Crick F. The astonishing hypothesis. The scientific search for the soul. New York: Scribner; 1994.
Crick F., Koch C. Are we aware of neural activity in primary visual cortex? Nature. 1995;375:121-123.
Crick F., Koch C. Consciousness and neuroscience. Cereb Cortex. 1998;8:97-107.
Diamond B.J., DeLuca J., Kelley S.M. Memory and executive functions in amnesic and non-amnesic patients with aneurysms of the anterior communicating artery. Brain. 1997;120:1015-1025.
Dickerson B.C., Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86-104.
Fliessbach K., Buerger C., Trautner P., et al. Differential effects of semantic processing on memory encoding. Hum Brain Mapp. 2010;31:1653-1664.
Gazzaniga M.S. The split brain revisited. Sci Am. 1998;279:51-55.
Gluck M.A., Shohamy D., Myers C. How do people solve the “weather prediction” task: individual variability in strategies for probabilistic category learning. Learn Mem. 2002;9:408-418.
Gold J.J., Squire L.S. The anatomy of amnesia: a neurohistological analysis of three new cases. Learn Mem. 2006;13:699-710.
Goldman-Rakic P.S. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445-1453.
Gonzalez-Martinez V., Comte F., de Verbizier D., et al. Transient global amnesia: concordant hippocampal abnormalities on positron emission tomography and magnetic resonance imaging. Arch Neurol. 2010;67:510-511.
Goodale M.A., Milner A.D. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20-25.
Gur M., Snodderly D.M. A dissociation between brain activity and perception: chromatically opponent cortical neurons signal chromatic flicker that is not perceived. Vision Res. 1997;37:377-382.
Keenan J.P., Nelson A., O’Connor M., et al. Self-recognition and the right hemisphere. Nature. 2001;409:305-306.
Keenan J.P., Rubio J., Racioppi C., et al. The right hemisphere and the dark side of consciousness. Cortex. 2005;41:705-707.
Kirshner H.S. Behavioral Neurology: Practical Science of Mind and Brain, second ed. Boston: Butterworth Heinemann; 2002.
Knight D.C., Waters N.S., Bandettini P.A. Neural substrates of explicit and implicit fear memory. Neuroimage. 2009;45:208-214.
Levy D.A., Stark C.E., Squire L.R. What conceptual primary is the absence of declarative memory. Psychol Sci. 2004;15:680-686.
Libet B. How does conscious experience arise? The neural time factor. Brain Res Bull. 1999;50:339-340.
McGaugh J.L. Memory—a century of consolidation. Science. 2000;287:248-251.
McIntosh R.D., Schenk T. Two visual streams for perception and action: current trends. Neuropsychologia. 2009;47:1391-1396.
Miller B.L., Seeley W.W., Mychack P., et al. Neuroanatomy of the self. Evidence from patients with frontotemporal dementia. Neurology. 2001;57:817-821.
Milner A.D., Goodale M.A. Two visual systems re-viewed. Neuropsychologia. 2008;46:774-785.
Mizuta H., Motomura N. Memory dysfunction in caudate infarction caused by Heubner’s recurring artery occlusion. Brain Cogn. 2006;61:133-138.
Ortinski P., Meador K.J. Neuronal mechanisms of conscious awareness. Arch Neurol. 2004;61:1017-1020.
Monti M.M., Vanhaudenhuyse A., Coleman M.R., et al. Willful modulation of brain activity in disorders of consciousness. New Engl. J Med. 2010;362:579-589.
Navta W.J.H., Feirtag M. Fundamental neuroanatomy. Freeman. 1986:1-340.
Perry J., Churchill L., Kirshner H.S. The Terri Schiavo case: legal, ethical, and medical perspectives. Ann Intern Med. 2005;143:744-748.
Petersson K.M., Elfgren C., Ingvar M. A dynamic role of the medial temporal lobe during retrieval of declarative memory in man. Neuroimage. 1997;6:1-11.
Rees G., Wojciulik E., Clarke K., et al. Unconscious activation of visual cortex in the damaged right hemisphere of a parietal patient with extinction. Brain. 2000;123:1624-1633.
Ropper A.H. Cogito Ergo Sum by MRI. N Engl J Med. 2010;362:648-649.
Rosazza C., Minati L., Ghielmetti F., et al. Engagement of the medial temporal lobe in verbal and nonverbal memory: assessment with functional MR imaging in healthy subjects. Am J Neuroradiol. 2009;30:1134-1141.
Rugg M.D., Fletcher P.C., Frith C.D., et al. Brain regions supporting intentional and incidental memory: a PET study. Neuroreport. 1997;8:1283-1287.
Schacter D.L. Searching for Memory. New York: The Brain, the Mind, and the Past, Basic Books; 1996.
Schacter D.L., Buckner R.L. Priming and the brain. Neuron. 1998;20:185-195.
Skosnik P.D., Mirza F., Gitelman D.R., et al. Neural correlates of artificial grammar learning. Neuroimage. 2002;17:1306-1314.
Squire L.R., Zola S.M. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci USA. 1996;93:13515-13522.
Squire L.R. The legacy of patient HM for neuroscience. Neuron. 2009;61:6-9.
Strupp M., Bruning R., Wu R.H., et al. Diffusion-weighted MRI in transient global amnesia: elevated signal intensity in the left mesial temporal lobe in 7 of 10 patients. Ann Neurol. 1998;43:164-170.
Thompson R.F., Kim J.J. Memory systems in the brain and localization of a memory. Proc Natl Acad Sci USA. 1996;93:13438-13444.
Ungerleider L.G., Courtney S.M., Haxby J.V. A neural system for human visual working memory. Proc Natl Acad Sci USA. 1998;95:883-890.
Wagner A.D., Schacter D.L., Rotte M., et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188-1191.
Wijdicks E.F., Cranford R.E. Clinical diagnosis of prolonged states of impaired consciousness in adults. Mayo Clin Proc. 2005;80:1411-1413.
Yang Y., Kim S., Kim J.H. Ischemic evidence of transient global amnesia: location of the lesion in the hippocampus. J Clin Neurol. 2008;4:59-66.