Chapter 20 Approach to Paroxysmal Supraventricular Tachycardias
Clinical Considerations
A “supraventricular” origin of a tachycardia implies the obligatory involvement of one or more cardiac structures above the bifurcation of the His bundle (HB), including the atrial myocardium, the atrioventricular node (AVN), the proximal HB, the coronary sinus (CS), the pulmonary veins (PVs), the venae cavae, or abnormal atrioventricular (AV) connections other than the HB (i.e., bypass tracts, BTs).1
Epidemiology
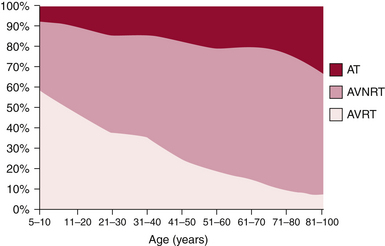
The mechanism of paroxysmal SVT is significantly influenced by both age and gender. In a large cohort of patients with symptomatic paroxysmal SVT referred for ablation, as patients grew older there was a significant and progressive decline in the number of patients presenting with AVRT, which was the predominant mechanism in the first decade, and a striking increase in AVNRT and AT (Fig. 20-1). These trends were similar in both genders, although AVNRT replaced AVRT as the predominant mechanism much earlier in women.2 The early predominance of AVRT is consistent with the congenital nature of the substrate and with the fact that symptom onset occurs earlier in patients with AVRT than AVNRT, most commonly in the first two decades of life. However, a minority of patients have relatively late onset of symptoms associated with AVRT and thus continue to account for a small proportion of ablations in older patients. Men account for a higher proportion of AVRT at all ages.
AVNRT is the predominant mechanism overall in patients undergoing ablation and after the age of 20 years accounts for the largest number of ablations in each age group. AVNRT is unusual in children under 5 years of age, and typically initially manifests in early life, often in the teens. AVRT presents earlier, with an average of more than 10 years separating the time of clinical presentation of AVRT versus AVNRT. There is a striking 2:1 predominance of women in the AVNRT group, which remains without clear physiological or anatomical explanation. Female sex and older age, that is, teens versus early childhood years, favor the diagnosis of AVNRT over AVRT.3
Clinical Presentation
Patients often learn to use certain maneuvers such as carotid sinus massage or the Valsalva maneuver to terminate the arrhythmia, although many require pharmacological treatment to achieve this. In patients without structural heart disease, the physical examination is usually remarkable only for a rapid, regular heart rate. At times, because of the simultaneous contraction of atria and ventricles, cannon A waves can be seen in the jugular venous waveform (described as the “frog” sign). This clinical feature has been reported to distinguish paroxysmal SVT resulting from AVNRT from that caused by orthodromic AVRT. Although the atrial contraction during AVRT will occur against closed AV valves, the longer VA interval results in separate ventricular and then atrial contraction and a relatively lower right atrial (RA) and venous pressure; therefore, the presence of palpations in the neck is experienced less commonly (up to 17%) in patients with AVRT.3 In patients with an AT exhibiting AV block, usually of the Wenckebach type, the ventricular rate is irregular.
Principles of Management
Acute Management
The AVN action potential is calcium channel–dependent, and the non–dihydropyridine calcium channel blockers verapamil and diltiazem are effective for terminating AVN-dependent paroxysmal SVT. The recommended dosage of verapamil is 5 mg intravenously over 2 minutes, followed in 5 to 10 minutes by a second 5- to 7.5-mg dose. The recommended dose of diltiazem is 20 mg intravenously followed, if necessary, by a second dose of 25 to 35 mg. Paroxysmal SVT termination should occur within 5 minutes of the end of the infusion, and over 90% of patients with AVN-dependent paroxysmal SVT respond. As with adenosine, transient arrhythmias, including atrial and ventricular ectopy, AF, and bradycardia, can be seen after paroxysmal SVT termination with calcium channel blockers. Hypotension can occur with calcium channel blockers, particularly if the paroxysmal SVT does not terminate. Adenosine and verapamil have been reported to have a similar high efficacy in terminating paroxysmal SVT, with a rate of success ranging from 59% to 100% for adenosine and from 73% to 98.8% for verapamil, according to the dose and mode of administration. However, data also suggest that the efficacy of adenosine and verapamil is affected by the arrhythmia rate. Increasing SVT rates are significantly associated with higher percentages of sinus rhythm restoration following treatment with adenosine. In contrast, the efficacy of verapamil in restoring sinus rhythm was inversely related to the rate of paroxysmal SVT.4
AVN-dependent paroxysmal SVT can present with a wide QRS complex in patients with fixed or functional aberration, or if a BT is used for anterograde conduction. Most wide complex tachycardias, however, are caused by mechanisms that can worsen after intravenous administration of adenosine and calcium channel blockers. Unless there is strong evidence that a wide QRS tachycardia is AVN-dependent, adenosine, verapamil, and diltiazem should not be used.
Electrocardiographic Features
Assessment of Regularity of the Supraventricular Tachycardia
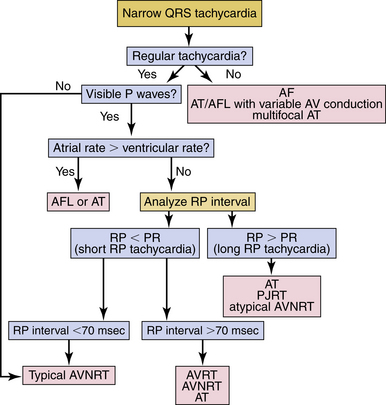
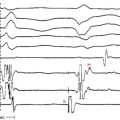
Most SVTs are associated with a regular ventricular rate. If the rhythm is irregular, the ECG should be scrutinized for discrete atrial activity and for any evidence of a pattern to the irregularity (e.g., grouped beating typical of Wenckebach periodicity). If the rhythm is irregularly irregular (i.e., no pattern can be detected), the mechanism of the arrhythmia is either multifocal AT or AF (Fig. 20-2). Multifocal AT is an irregularly irregular atrial rhythm characterized by more than three different P wave morphologies, with the P waves separated by isoelectric intervals and associated with varying P-P, R-R, and PR intervals (see Fig. 11-1). On the other hand, AF is characterized by rapid and irregular atrial fibrillatory activity and, in the presence of normal AVN conduction, by an irregularly irregular ventricular response. P waves cannot be detected in AF, although coarse fibrillatory waves and prominent U waves can sometimes give the appearance of P waves. At times, the fibrillatory activity is so fine as to be undetectable.
Atrial Activity
Identification
If the patient’s rhythm is regular or has a clearly discernible pattern, the ECG should next be assessed for P waves (atrial activity).5 The P waves may be easily discernible; however, frequently, comparison with a normal baseline ECG is needed and can reveal a slight alteration in the QRS, ST segment, or T waves, suggesting the presence of the P wave. If the P waves cannot be clearly identified, carotid sinus massage or the administration of intravenous adenosine may help clarify the diagnosis. These maneuvers may also terminate the SVT.
Carotid Sinus Massage
Carotid sinus massage can result in one of four possible effects: (1) temporary decrease in the atrial rate in patients with sinus tachycardia or automatic AT; (2) slowing of AVN conduction and AVN block, which can unmask atrial electrical activity—that is, reveal P waves or flutter waves in patients with AT or AFL by decreasing the number of QRS complexes that obscure the electrical baseline; (3) with some SVTs that require AVN conduction, especially AVNRT and AVRT, the transient slowing of AVN conduction can terminate the arrhythmia by interrupting the reentry circuit; less commonly, carotid sinus massage can cause some ATs to slow and terminate; or (4) in some cases, no effect is observed.
Termination of the Arrhythmia
Carotid sinus massage or adenosine can terminate the SVT, especially if the rhythm is AVNRT or AVRT. A continuous ECG tracing should be recorded during these maneuvers, because the response can aid in the diagnosis.5 Termination of the tachycardia with a P wave after the last QRS complex is most common in AVRT and typical AVNRT and is rarely seen with AT (see Fig. 18-22), whereas termination of the tachycardia with a QRS complex is more common with AT, atypical AVNRT, and permanent junctional reciprocating tachycardia (PJRT; see Fig. 18-19). If the tachycardia continues despite development of AV block, the rhythm is almost certainly AT or AFL; AVRT is excluded and AVNRT is very unlikely.
Characterization
P Wave Morphology
A P wave morphology identical to sinus P wave suggests sinus tachycardia, inappropriate sinus tachycardia, sinoatrial nodal reentrant tachycardia, or AT arising close to the region of the sinus node. An abnormal P wave morphology can be observed during AVNRT (P wave is concentric; see Fig. 17-3), AVRT (P wave can be eccentric or concentric; see Fig. 18-9), AT (P wave can be eccentric or concentric), and AFL (lack of distinct isoelectric baselines between atrial deflections is suggestive of AFL, but can also be seen occasionally in AT; see Fig. 12-2).5
Characterization of the P/Qrs Relationship
RP/PR Intervals
SVTs are classified as short or long RP interval SVTs (see Fig. 20-2). During short RP SVTs, the ECG will show P waves inscribed within the ST-T wave with an RP interval that is less than half the tachycardia R-R interval. Such SVTs include typical AVNRT (most common), orthodromic AVRT, AT with prolonged AV conduction, and slow-slow AVNRT. A very short RP interval (<70 milliseconds) excludes AVRT.5,6
In typical AVNRT, the P wave is usually not visible because of the simultaneous atrial and ventricular activation. The P wave may distort the initial portion of the QRS (mimicking a q wave in inferior leads) or lie just within the QRS (inapparent) or distort the terminal portion of the QRS (mimicking an s wave in inferior leads or r′ in V1; see Fig. 17-3).
Atrial-Ventricular Relationship
SVTs with an A/V ratio of 1 (i.e., equal number of atrial and ventricular events) include AVNRT, AVRT, and AT. On the other hand, an A/V ratio during the SVT of greater than 1 indicates the presence of AV block and that the ventricles are not required for the SVT circuit, thereby excluding AVRT and suggesting either AT (most common; see Fig. 11-2) or AVNRT (rare; see Fig. 17-4). AV dissociation (i.e., complete AV block) can be observed during AT (most common) or AVNRT (rare).
Electrophysiological Testing
Programmed Electrical Stimulation during Normal Sinus Rhythm
The programmed stimulation protocol should include (1) ventricular burst pacing from the right ventricular (RV) apex (down to pacing CL at which VA block develops); (2) single and double ventricular extrastimuli (VESs, down to the ventricular effective refractory period, ERP) at multiple CLs (600 to 400 milliseconds) from the RV apex; (3) atrial burst pacing from the high right atrium (RA) and coronary sinus (CS; down to the pacing CL at which 2:1 atrial capture occurs); (4) single and double atrial extrastimuli (AESs, down to the atrial ERP) at multiple CLs (600 to 400 milliseconds) from the high RA and CS; and (5) administration of isoproterenol infusion (0.5 to 4 µg/min) as needed to facilitate tachycardia induction.
Atrial Extrastimulation and Atrial Pacing During Normal Sinus Rhythm
Dual Atrioventricular Nodal Physiology
Although the demonstration of dual AVN physiology during programmed atrial stimulation favors AVNRT as the mechanism of SVT, it is not an uncommon finding in patients with other types of SVTs. Furthermore, failure to demonstrate dual AVN physiology does not exclude the possibility of AVNRT, and might be related to similar fast and slow AVN pathway ERPs. Dissociation of refractoriness of the fast and slow AVN pathways can then be necessary (see Chap. 17).
Ventricular Preexcitation
Atrial stimulation can help unmask preexcitation if it is not manifest during NSR because of fast AVN conduction, slow BT conduction, or both. AES and atrial pacing from any atrial site result in slowing of AVN conduction and, consequently, unmask or increase the degree of preexcitation over the AV BT (see Fig. 18-4). Moreover, atrial stimulation close to the AV BT insertion site results in maximal preexcitation and the shortest P-delta interval because of the ability to advance the activation of the AV BT down to its ERP from pacing at this site caused by the lack of intervening atrial tissue, whose conduction time and refractoriness can otherwise limit the ability of the AES to stimulate the BT prematurely (see Fig. 18-14).
Extra Atrial Beats
Atrioventricular Nodal Echo Beats
These beats occur in the presence of anterograde dual AVN physiology (see Fig. 4-23). Such beats require anterograde block of the atrial stimulus in the fast AVN pathway, anterograde conduction down the slow pathway, and then retrograde conduction up the fast pathway. AVN echo beats have several features: they appear reproducibly after a critical AH interval; the atrial activation sequence is consistent with retrograde conduction over the fast pathway, with the earliest atrial activation site in the HB; and the VA interval is very short, but it can be longer if the atrial stimulus causes anterograde concealment (and not just block) in the fast pathway.
Atrioventricular Echo Beats
AV echo beats occur secondary to anterograde conduction of the atrial stimulus over the AVN-HPS and retrograde conduction over an AV BT (concealed or bidirectional BT). If preexcitation is manifest during atrial stimulation, the last atrial impulse inducing the echo beat will demonstrate loss of preexcitation because of anterograde block in the AV BT, and atrial activation sequence and P wave morphology of the echo beat will depend on the location of the BT (see Fig. 3-10). These beats have a relatively short VA interval, but always longer than 70 milliseconds. Moreover, the VA interval of the AV echo beat remains constant, regardless of the varying coupling interval of the AES triggering the echo beat (VA linking). Alternatively, AV echo beats can occur secondary to anterograde conduction of the atrial stimulus over a manifest AV BT and retrograde conduction over an AVN, in which setting the last paced beat is associated with anterograde block in the AVN and fully preexcited QRS complex.
Ventricular Extrastimulation and Ventricular Pacing During Normal Sinus Rhythm
Retrograde Dual Atrioventricular Nodal Physiology
Demonstration of retrograde dual AVN physiology during programmed ventricular stimulation suggests AVNRT (occurring most commonly during atypical AVNRT), but it can also be observed with other SVTs.7 Importantly, failure to demonstrate retrograde dual AVN physiology in patients with AVNRT can be the result of similar fast and slow AVN pathway ERPs, in which setting dissociation of refractoriness of the fast and slow AVN pathways is required (see Chap. 17).
Retrograde Atrial Activation Sequence
VA conduction over the AVN produces a classic concentric atrial activation sequence starting in the anteroseptal or posteroseptal region of the RA because of retrograde conduction over either the fast or the slow AVN pathways, respectively. In the presence of a retrogradely conducting AV BT, atrial activation can result from conduction over the AV BT, over the AVN, or a fusion of both (see Fig. 18-16). An eccentric atrial activation sequence in response to ventricular stimulation suggests the presence of an AV BT mediating VA conduction (see Fig. 18-16). The presence of a concentric retrograde atrial activation sequence, however, does not exclude the presence of a retrogradely conducting BT that could be septal in location or located far from the pacing site, allowing for preferential VA conduction over the AVN.
Extra Ventricular Beats
Bundle Branch Reentrant Beats
During RV stimulation at close coupling intervals, progressive retrograde conduction delay and block occur in the right bundle branch (RB), so that retrograde HB activation occurs via the left bundle branch (LB). At this point, the His potential usually follows the local ventricular electrogram. Further decrease in the coupling interval produces an increase in retrograde HPS conduction delay. When a critical degree of HPS delay (S2-H2) is attained, the impulse can return down the initially blocked RB and result in a QRS of similar morphology to the paced QRS at the RV apex—specifically, it will look like a typical left bundle branch block (LBBB) pattern with left axis deviation because ventricular activation originates from conduction over the RB. The His bundle–ventricular (HV) interval of the bundle branch reentrant (BBR) beat is usually longer than or equal to the HV interval during NSR. Retrograde atrial activation, if present, follows the His potential (see Fig. 4-27).
Atrioventricular Node Echo Beats
These beats are caused by reentry in the AVN in patients with retrograde dual AVN physiology (see Fig. 17-11). The last paced beat conducts retrogradely up the slow AVN pathway and then anterogradely down the fast pathway to produce the echo beat. AVN echoes appear reproducibly after a critical H2-A2 interval (or V2-A2 interval, when the His potential cannot be seen), and manifest as extra beats with a normal anterograde QRS morphology and atrial activity preceding the His potential before the echo beat. This phenomenon can occur at long or short coupling intervals and depends only on the degree of retrograde AVN conduction delay. In most cases, this delay is achieved before the appearance of a retrograde His potential beyond the local ventricular electrogram (i.e., before retrograde block in the RB).
Atrioventricular Echo Beats
These beats occur secondary to retrograde block in the HPS-AVN and VA conduction over an AV BT, followed by anterograde conduction over the AVN, or secondary to retrograde block in the AV BT and VA conduction over the AVN-HPS, followed by anterograde conduction over the AV-BT. In the latter setting, the echo beat is fully preexcited (see Fig. 18-16).
Right Bundle Branch Block during Ventricular Extrastimulation
In the absence of a BT, the AVN can be activated in a retrograde fashion only after retrograde activation of the HB; as a consequence, VA activation will necessarily be delayed with retrograde RBBB, and the increase in the VA interval will be at least as much as the increase in the VH interval. In contrast, when retrograde conduction is via a BT, there will be no expected increase in the VA interval when retrograde RBBB is induced. Thus, the increase in the VA interval is minimal and always less than the increase in the VH interval.8
Induction of Tachycardia
Initiation by Atrial Extrastimulation or Atrial Pacing
Inducibility
All types of paroxysmal SVTs can be inducible with atrial stimulation (except automatic AT). SVT initiation that is reproducibly dependent on a critical AH interval is classic for typical AVNRT (see Fig. 17-8). Atypical AVNRT is usually initiated with modest prolongation of the AH interval along the fast pathway with anterograde block in the slow pathway, followed by retrograde slow conduction over the slow pathway. Therefore, a critical AH interval delay is not obvious (see Fig. 17-10). AT initiation also can be associated with AV delay, but that is not a prerequisite for initiation. Orthodromic AVRT usually requires some AV delay for initiation; however, the delay can occur anywhere along the AVN-HPS axis. In patients with baseline manifest preexcitation, initiation of orthodromic AVRT is usually associated with anterograde block in the AV BT and loss of preexcitation following the initiating atrial stimulus, which would then allow that BT to conduct retrogradely during the SVT.9 Initiation may require catecholamines (isoproterenol) with any type of SVT, and this observation does not help for differential diagnosis.
Initiation by Ventricular Extrastimuli or Ventricular Pacing
Inducibility
Ventricular stimulation commonly induces AVRT, typical and atypical AVNRT, and less frequently AT.
His Bundle–Atrial Interval
During induction of the SVT by ventricular pacing at a CL similar to the tachycardia CL or by a VES that advances the His potential by a coupling interval (i.e., H1-H2) similar to the H-H interval during the SVT (i.e., similar to the tachycardia CL), the His bundle–atrial (HA) or VA interval following the initiating ventricular stimulus then is compared with that during the SVT. During AVNRT, the HA interval of the ventricular stimulus initiating the SVT is longer than that during the SVT, because both the HB and atrium are activated in sequence during ventricular stimulation and in parallel during AVNRT. This is even exaggerated by the fact that the AVN usually exhibits greater decremental conduction with repetitive engagement of impulses than to a single impulse at a similar coupling interval. Therefore, the more prolonged the HA interval with the initiating ventricular stimulus, the more likely the SVT is AVNRT. On the other hand, if the SVT uses an AV BT for retrograde conduction, the HA interval during the initiating ventricular stimulus (at a coupling interval comparable to the tachycardia CL) should approximate that during the SVT, because the atrium and ventricle are activated in sequence in both scenarios (see Fig. 18-16).9
Tachycardia Features
Atrial Activation Sequence
During typical AVNRT, the initial site of atrial activation is usually recorded in the HB catheter at the apex of the triangle of Koch.10 In contrast, the initial site of atrial activation during atypical AVNRT is usually recorded at the base of the triangle of Koch or coronary sinus ostium (CS os) (see Fig. 17-5).10 On the other hand, in orthodromic AVRT, the initial site of atrial activation depends on the location of the AV BT, but is always near the AV groove, without multiple breakthrough points. It is comparable to that during ventricular pacing when VA conduction occurs exclusively over the AV BT. The atrial activation sequence during AT depends on the origin of the AT, and can simulate that of other types of SVTs. In summary, eccentric atrial activation during SVT excludes typical and atypical AVNRT, except for the left variant of AVNRT, during which the earliest atrial activation occurs in the proximal or mid-CS. Moreover, an eccentric atrial activation sequence that originates away from the AV rings is diagnostic of AT and excludes both AVNRT and AVRT.9
Atrial-Ventricular Relationship
PR/RP
During AT, the PR interval is appropriate for the AT rate and is usually longer than that during NSR. The faster the AT rate, the longer the PR interval. Thus, the PR interval can be shorter, longer, or equal to the RP interval. The PR interval can also be equal to the R-R and the P wave can then fall within the preceding QRS, mimicking typical AVNRT. During typical AVNRT, the RP interval is very short (–40 to 75 milliseconds); in contrast, during atypical AVNRT, the RP interval is longer than the PR interval. On the other hand, during orthodromic AVRT, the RP interval is short but longer than that in typical AVNRT (>70 milliseconds), because the wavefront has to activate the ventricle before it reaches the AV BT and subsequently conduct retrogradely to the atrium. Thus, the ventricle and atrium are activated in sequence, in contrast to AVNRT, during which the ventricle and atrium are activated in parallel, resulting in abbreviation of the VA interval.6
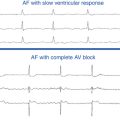
Atrioventricular Block
The presence of AV block during SVT excludes AVRT, is uncommon during AVNRT, and strongly favors AT (Fig. 20-3). AV block occurs commonly during AT, with either Wenckebach periodicity or fixed-ratio block. AV block may also occur during AVNRT because of block below the reentry circuit, usually below the HB and infrequently in the lower common pathway, which can occur especially at the onset of the SVT, during acceleration of the SVT, and following a PVC or a VES (see Fig. 17-4).
Ventriculoatrial Block
VA block during SVT is a rare phenomenon, and may occasionally be observed in infraatrial SVTs, including junctional ectopic tachycardia, orthodromic AVRT using a nodofascicular or nodoventricular BT for retrograde conduction, as well as infra-atrial AVNRT. VA block can rarely occur during AVNRT because of block in an upper common pathway (see Fig. 17-13). Intra-Hisian reentry is another potential mechanism, but it is a theoretical entity whose clinical occurrence has not been convincingly demonstrated.1,11–13
Variation of the P/QRS Relationship
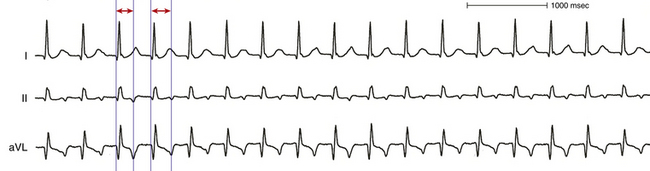
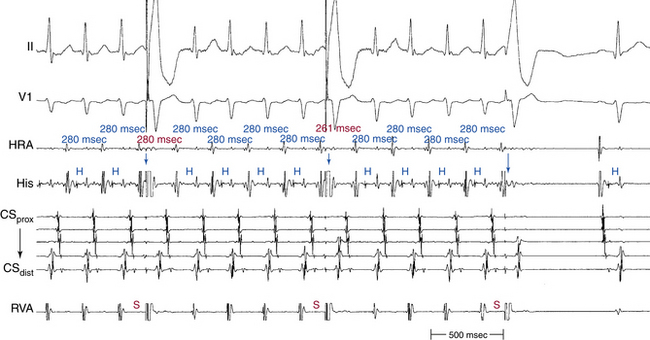
Spontaneous changes in the PR and RP intervals with fixed A-A interval favor AT and exclude orthodromic AVRT (Fig. 20-4, see Fig. 11-13). On the other hand, spontaneous changes in tachycardia CL accompanied by constant VA interval suggest orthodromic AVRT (see Fig. 18-22). During orthodromic AVRT, the RP interval remains fixed, regardless of oscillations in tachycardia CL from whatever cause or changes in the PR (AH) interval. Thus, the RP/PR ratio may change, and the tachycardia CL is most closely associated with the PR interval (i.e., anterograde slow conduction). Variation of the P/QRS relationship (with changes in the AH interval, HA interval, and AH/HA ratio), with or without block, can occur during AVNRT, especially in atypical or slow-slow AVNRT. This phenomenon usually occurs when the conduction system, the reentry circuit, or both are unstable during initiation or termination of the tachycardia or in cases of nonsustained tachycardias. The ECG manifestation of P/QRS variations, with or without AV block during tachycardia, should not be misdiagnosed as AT; they can be atypical or, rarely, typical forms of AVNRT. Moreover, the variations could be of such magnitude that a long RP tachycardia can masquerade for brief periods of time as short RP tachycardia.
Oscillation in Tachycardia Cycle Length
Analysis of tachycardia CL variability can provide useful diagnostic information that is available even when episodes of SVT are nonsustained. SVT CL variability of at least 15 milliseconds in magnitude was found to occur in up to 73% of paroxysmal SVTs and was equally prevalent in AT, AVNRT, and orthodromic AVRT. Changes in atrial CL preceding similar changes in subsequent ventricular CL strongly favor AT or atypical AVNRT (see Fig. 17-14). In contrast, when the change in atrial CL is predicted by the change in preceding ventricular CL, typical AVNRT or orthodromic AVRT is the most likely mechanism (see Fig. 18-22).
Effects of Bundle Branch Block
The development of BBB during SVT that neither influences the tachycardia CL (A-A or H-H interval) nor the VA interval is consistent with AT, AVNRT (see Fig. 17-4), and orthodromic AVRT using a BT in the ventricle contralateral to the BBB (see Fig. 18-21), but excludes orthodromic AVRT using a BT ipsilateral to the BBB.
BBB ipsilateral to the AV BT mediating orthodromic AVRT results in prolongation of the surface VA interval because of the extra time required for the impulse to travel from the AVN down the HB and contralateral bundle branch, and transseptally to the ipsilateral ventricle to reach the AV BT and then activate the atrium (see Fig. 18-23). However, the local VA interval (measured at the site of BT insertion) remains constant. Additionally, the tachycardia CL usually increases in concordance with the increase in the surface VA interval as a result of ipsilateral BBB, because of the now-larger tachycardia circuit; however, because the time the wavefront spends outside the AVN is now longer, AVN conduction may improve, resulting in shortening in the AH interval (PR interval), which can be of a magnitude sufficient to overcome the prolongation of the VA interval. This can consequently result in shortening in the tachycardia CL. Thus, the surface VA interval and not the tachycardia CL should be used to assess the effects of BBB on the SVT (see Fig. 18-9).
Prolongation of the VA interval during SVT in response to BBB by more than 35 milliseconds indicates that an ipsilateral free wall AV BT is present and is participating in the SVT (i.e., diagnostic of orthodromic AVRT). On the other hand, prolongation of the surface VA by more than 25 milliseconds suggests a septal AV BT (posteroseptal AV BT in association with LBBB, and superoparaseptal AV BT in association with RBBB; see Fig. 18-24). In contrast, BBB contralateral to the AV BT does not influence the VA interval or tachycardia CL because the contralateral ventricle is not part of the reentrant circuit (see Figs. 18-21 and 18-23).
Since the occurrence of BBB during SVT is much more common in orthodromic AVRT than AVNRT or AT (90% of SVTs with sustained LBBB are orthodromic AVRTs), the mere presence of LBBB aberrancy during SVT is suggestive of orthodromic AVRT, but can still occur in other types of SVTs.6,9
Termination and Response to Physiological and Pharmacological Maneuvers
Spontaneous Termination
Spontaneous termination of orthodromic AVRT usually occurs because of anterograde gradual slowing and then block in the AVN, sometimes causing initial oscillation in the tachycardia CL, with alternate complexes demonstrating a Wenckebach periodicity before block. However, termination with retrograde block in the AV BT can occur without any perturbations of the tachycardia CL during very rapid orthodromic AVRT or with sudden shortening of the tachycardia CL. Spontaneous termination of AVNRT occurs because of block in the fast or slow pathway. However, the better the retrograde fast pathway conduction, the less likely that it is the site of block. Spontaneous termination of AT is usually accompanied by progressive prolongation of the A-A interval, with or without changes in AV conduction. During AT with 1:1 AV conduction, the last beat of AT is conducted to the ventricle. Spontaneous termination of SVT with a P wave not followed by a QRS practically excludes AT, except coincidentally or in the case of a nonconducted PAC terminating the AT (neither of which will be reproducible).9
Termination with Adenosine
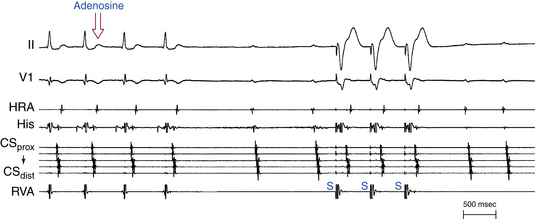
The mere termination of SVT in response to adenosine is usually not helpful in differentiating SVTs. However, the pattern of SVT termination can be helpful in two situations: First, reproducible termination of the SVT with a QRS not followed by a P wave excludes orthodromic AVRT using a rapidly conducting AV BT as the retrograde limb (adenosine blocks the AVN and not the BT), is unusual in typical AVNRT (adenosine blocks the slow pathway but does not affect the fast pathway), and is consistent with AT, PJRT, or atypical AVNRT. Second, reproducible termination of the SVT with a P wave not followed by a QRS excludes AT, because it occurs in AT only if adenosine terminates the AT at the same moment it causes AV block, which is an unlikely coincidence (Fig. 20-5). Most ATs (50% to 80%) can be terminated by adenosine, typically (80%) prior to the onset of AV block. Response to adenosine does not help differentiate between atypical AVNRT and PJRT.
Termination with Vagal Maneuvers
Carotid sinus massage and vagal maneuvers reproducibly slow or terminate up to 25% of ATs. Orthodromic AVRT usually terminates with gradual slowing and then block in the AVN. Typical AVNRT usually terminates with gradual anterograde slowing and then block in the slow pathway; block in the fast pathway is uncommon. Additionally, carotid sinus massage and vagal maneuvers terminate atypical AVNRT by gradual slowing and then block in the retrograde slow pathway.9
Diagnostic Maneuvers during Tachycardia
Atrial Extrastimulation During Supraventricular Tachycardia
Resetting
Additionally, the resetting curve can help differentiate the different subtypes of SVT. Reentrant AT, AVNRT, and orthodromic AVRT are characterized by an increasing or mixed response curve, whereas triggered activity AT exhibits a decreasing resetting response curve and automatic AT exhibits an increasing resetting response curve. It is difficult for an AES not to affect orthodromic AVRT because of the large size of the reentrant circuit, and an AES over a wide range of coupling intervals can reset the SVT via conduction down the AVN-HPS. However, for AVNRT, more premature AESs are needed to demonstrate resetting.9
Atrial Pacing During Supraventricular Tachycardia
Entrainment
Overdrive atrial pacing can entrain reentrant AT, AVNRT, and orthodromic AVRT but not triggered activity or automatic ATs. Entrainment with manifest fusion, on the other hand, can be seen only in AVRT and macroreentrant AT, but not with AVNRT or focal AT (see Fig. 18-27). However, it is important to understand that overdrive pacing of focal AT and AVNRT can result in a certain degree of fusion, especially when the pacing CL is only slightly shorter than the tachycardia CL. Such fusion, however, is unstable during the same pacing drive at a constant CL, because the pacing stimuli fall on a progressively earlier portion of the tachycardia cycle, producing progressively less fusion and more fully paced morphology. Such phenomena should be distinguished from entrainment, and sometimes this requires pacing for long intervals to demonstrate variable degrees of fusion.
During entrainment at a pacing CL close to the tachycardia CL, the AH interval during entrainment is longer than that during AVNRT because the atrium and HB are activated in parallel during AVNRT and in sequence during atrial pacing entraining the AVNRT (because of the presence of an upper common pathway). In contrast, in the setting of AT and orthodromic AVRT, the AH interval is comparable during SVT and entrainment with atrial pacing.9
Acceleration
Overdrive pacing during triggered activity AT generally produces acceleration of the tachycardia CL.
Overdrive Suppression
Automatic AT cannot be entrained by atrial pacing; however, rapid atrial pacing results in overdrive suppression of the AT rate, with the return CL following the pacing train prolonging with increasing the duration and/or rate of overdrive pacing, in contrast to constant return cycles following entrainment of reentrant circuits, regardless of the length of the pacing drive. The AT resumes following cessation of atrial pacing but at a slower rate, gradually speeding up (warming up) back to pre-pacing tachycardia CL.9
Ventriculoatrial Linking
On cessation of overdrive atrial pacing, if the VA interval following the last entrained QRS is reproducibly constant (<10 milliseconds in variation), despite pacing at different CLs or for different durations (“VA linking”) and is similar to that during SVT, AT is unlikely. If no VA linking is demonstrable, AT is more likely than other types of SVTs (see Fig. 18-27).6 VA linking occurs in the setting of typical AVNRT and orthodromic AVRT because the timing of atrial activation is dependent on the preceding ventricular activation and is the result of retrograde VA conduction over the AVN fast pathway (during typical AVNRT) or the AV BT (during orthodromic AVRT), which is relatively fixed and constant. Contrariwise, following cessation of overdrive pacing (with 1:1 AV conduction) during focal AT, the VA interval can vary significantly from the VA interval during AT, because the timing of the tachycardia atrial return cycle is not related to the preceding QRS.2,6
Differential-Site Atrial Pacing
As discussed in Chapter 11, differential site atrial pacing can help distinguish AT from other mechanisms of SVT. When the ΔVA interval (i.e., the maximal difference in the post-pacing VA intervals following cessation of pacing from the high RA and proximal CS) is more than 14 milliseconds, AT is suggested. On the other hand, in orthodromic AVRT and AVNRT, the initial atrial complex following cessation of atrial pacing entraining the SVT is linked to, and cannot be dissociated from, the last captured ventricular complex; hence, the ΔVA interval is typically less than 14 milliseconds.14
Ventricular Extrastimulation During Supraventricular Tachycardia
Resetting
During orthodromic AVRT, a VES can usually reset the SVT. However, the ability of the VES to affect the SVT depends on the distance between the site of ventricular stimulation to the ventricular insertion site of the BT and on the VES coupling interval. Because only parts of the ventricle ipsilateral to the BT are requisite components of the orthodromic AVRT circuit, a VES delivered in the contralateral ventricle may not affect the circuit (see Fig. 18-29). On the other hand, the inability of early single or double VESs to reset the SVT despite advancement of all ventricular electrograms (including the local electrogram in the electrode recording the earliest atrial activation during the SVT, which would be close to the potential BT ventricular insertion site) by more than 30 milliseconds excludes orthodromic AVRT. Several other findings can help confirm the presence of BT function and whether it is participating in the SVT or is only a bystander (see Tables 18-4 and 18-5).
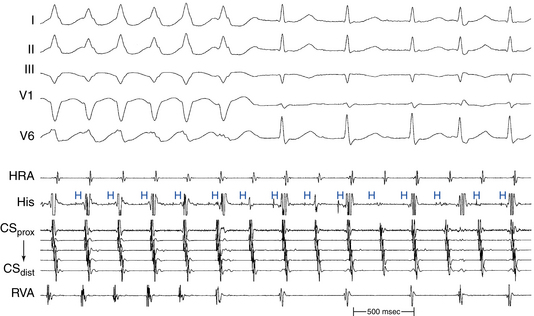
Although resetting the SVT by a VES is not diagnostic by itself of a specific type of SVT, it can be helpful in certain situations. First, the ability of a late VES delivered while the HB is refractory (i.e., when the anterograde His potential is already manifest or within 35 to 55 milliseconds before the time of the expected His potential) to affect (reset or terminate) the SVT excludes AVNRT, because such a VES would not have been able to penetrate the AVNRT circuit (Fig. 20-6; and see Fig. 18-29). It also excludes AT, except for cases of AT associated with the presence of innocent bystander BT, in which case the presence of such a BT is usually easy to exclude with ventricular pacing during NSR. Second, the ability of a VES to delay the next atrial activation excludes AT (see Fig. 18-26). Third, the ability of a VES to reset the SVT without atrial activation (i.e., the VES advances the subsequent His potential and QRS and blocks in the upper common pathway) excludes AT and orthodromic AVRT, because it proves that the atrium is not part of the SVT circuit. Fourth, failure of early single or double VESs to reset the SVT, despite advancement of ventricular electrograms in the electrode recording the earliest atrial activation by more than 30 milliseconds, excludes orthodromic AVRT. Fifth, resetting with manifest QRS fusion can be observed during orthodromic AVRT, especially during pacing at a site closer to the BT ventricular insertion site than the entrance of the reentrant circuit to ventricular tissue (i.e., the HPS). Such a phenomenon, on the other hand, cannot occur during AVNRT or focal AT because of the lack of spatial separation of the entrance and exit to the tachycardia circuit. Sixth, retrograde atrial activation sequence following a VES that resets the tachycardia is usually similar to that during SVT in the setting of AVNRT and orthodromic AVRT, because it should conduct over the tachycardia retrograde limb (except in the presence of a bystander retrogradely conducting AV BT). In contrast, a retrograde atrial activation sequence during the VES is usually different from that during AT, except for ATs originating close to the AV junction.
Termination
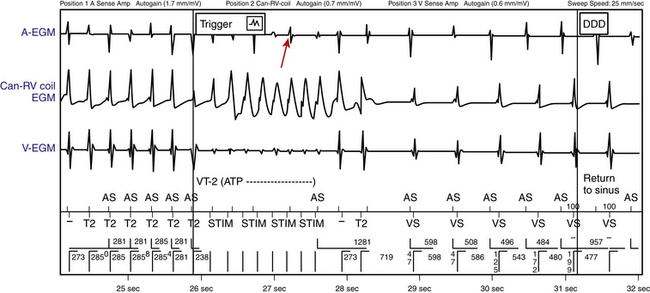
Termination of AVNRT with a single VES is difficult and occurs rarely when the tachycardia CL is less than 350 milliseconds; such termination favors the diagnosis of orthodromic AVRT, which can usually be readily terminated by single or double VESs. Termination of the SVT with a VES delivered when the HB is refractory excludes AVNRT (see Fig. 20-6). It also excludes AT, except for cases of AT associated with the presence of an innocent bystander BT mediating VA conduction. Reproducible termination of the SVT with a VES not followed by atrial activation excludes AT (see Fig. 18-29) and, if this occurs with a VES delivered while the HB is refractory, it excludes both AT and AVNRT (see Fig. 20-6).
Ventricular Pacing During SUPRAVENTRICULAR TACHYCARDIA
Ventricular pacing is performed at a CL 10 to 30 milliseconds shorter than the tachycardia CL; the pacing CL is then progressively reduced by 10 to 20 milliseconds in a stepwise fashion with discontinuation of ventricular pacing after each pacing CL to verify continuation versus termination of the SVT. The presence of ventricular capture during ventricular pacing should be verified. Additionally, the presence of 1:1 VA conduction and acceleration of the atrial rate to the pacing CL should be carefully examined (Fig. 20-7). It is also important to verify the continuation of the SVT following cessation of ventricular pacing and whether SVT termination, with or without reinduction of the SVT, has occurred during ventricular pacing.
Ventriculoatrial Dissociation
When overdrive ventricular pacing during SVT fails to accelerate the atrial CL to the pacing CL (i.e., the ventricles are dissociated from the tachycardia), AVRT is excluded, AT is the most likely diagnosis, but AVNRT is still possible.
Atrial Activation Sequence
As noted, the retrograde atrial activation sequence during ventricular pacing during the SVT is usually similar to that during the SVT in the setting of AVNRT and orthodromic AVRT, because it should conduct over the tachycardia retrograde limb. On the other hand, the retrograde atrial activation sequence during ventricular pacing is usually different from that during AT, except for ATs originating close to the AV junction. A pitfall of this criterion is the presence of a bystander AV BT, which can provide another retrograde route capable of mediating retrograde conduction during ventricular pacing without being part of the SVT circuit. In this setting, ventricular pacing can result in a retrograde atrial activation sequence different from that of AVNRT or orthodromic AVRT. The presence of such an AV BT, however, is generally easy to verify with ventricular stimulation during NSR.
Entrainment
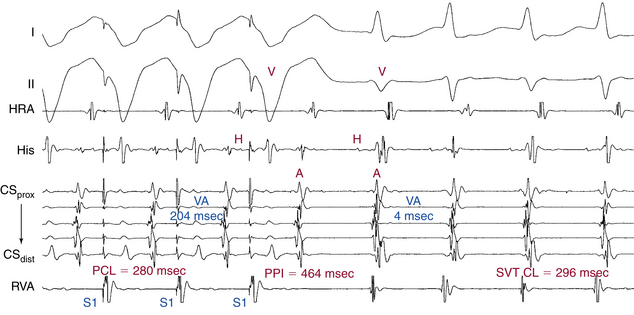
Ventricular pacing is almost always able to entrain AVNRT and orthodromic AVRT and, if 1:1 VA conduction is maintained, reentrant AT. Entrainment of the SVT by RV pacing can help differentiate orthodromic AVRT from AVNRT by evaluating the VA interval during SVT versus that during pacing, and also by evaluating the post-pacing interval (PPI). The ventricle and atrium are activated in sequence during orthodromic AVRT and during ventricular pacing, but in parallel during AVNRT. Therefore, the VA interval during orthodromic AVRT approximates that during ventricular pacing (see Fig. 18-30). In contrast, the VA interval during AVNRT would be much shorter than that during ventricular pacing (see Fig. 17-18). In general, a difference in the VA interval (ΔVA [VApacing – VASVT]) greater than 85 milliseconds is consistent with AVNRT, whereas a ΔVA of less than 85 milliseconds is consistent with orthodromic AVRT (Fig. 20-8).
Additionally, the PPI after entrainment of AVNRT from the RV apex is significantly longer than the tachycardia CL (the [PPI – SVT CL] difference is usually >115 milliseconds), because the reentrant circuit in AVNRT is above the ventricle and far from the pacing site (see Fig. 17-18). In AVNRT, the PPI reflects the conduction time from the pacing site through the RV muscle and HPS, once around the reentry circuit and back to the pacing site. Therefore, the difference between the PPI and the SVT CL [PPI – SVT CL] reflects twice the sum of the conduction time through the RV muscle, the HPS, and any lower common pathway (see Fig. 20-8). In orthodromic AVRT using a septal BT, the PPI reflects the conduction time through the RV to the septum, once around the reentry circuit and back. In other words, the [PPI – SVT CL] reflects twice the conduction time from the pacing catheter through the ventricular myocardium to the reentry circuit. Therefore, the PPI more closely approximates the SVT CL in orthodromic AVRT using a septal BT, compared with AVNRT (see Fig. 18-30). This maneuver was studied specifically for differentiation between atypical AVNRT and orthodromic AVRT, but the principle also applies to typical AVNRT. In general, a [PPI – SVT CL] difference greater than 115 milliseconds is consistent with AVNRT, whereas a [PPI – SVT CL] difference greater than 115 milliseconds is consistent with orthodromic AVRT. For borderline values, ventricular pacing at the RV base can help exaggerate the difference between the PPI and tachycardia CL in the setting of AVNRT, but without significant changes in the setting of orthodromic AVRT, because the site of pacing at the RV base is farther from the AVNRT circuit than the RV apex (because the paced wavefront has to travel first to the RV apex before engaging the HPS and conducting retrogradely to the AVN), but is still close to an AVRT circuit using a septal BT (and, in fact, it is closer to the ventricular insertion of the BT).15,16
Another limitation is that the [PPI – SVT CL] difference does not account for potential decremental anterograde AVN conduction that may be induced by overdrive pacing, which can significantly affect diagnostic interpretations of the PPI. The prolonged AH interval on the last entrained beat will contribute to prolongation of the PPI that is not reflective of the distance of the pacing site from the circuit. Thus, [PPI – SVT CL] differences obtained after entrainment of orthodromic AVRT employing a septal BT can actually overlap with those observed after entrainment of AVNRT. Correction of the [PPI – SVT CL] difference for the degree of decrement in the AVN (by subtracting the difference in AH [or AV] interval during tachycardia and on the return cycle from the [PPI – SVT CL]) has been shown to improve the accuracy of this criterion for distinction between AVNRT and orthodromic AVRT. In a study of patients with both typical and atypical forms of AVNRT, as well as orthodromic using septal and free wall BTs, a corrected [PPI – SVT CL] difference of less than 110 milliseconds was found more accurate in identifying orthodromic AVRT from AVNRT than the uncorrected [PPI – SVT CL] difference. The use of change in VA interval is of course not influenced by prolongation of the AV interval during pacing and does not require correction.15,17,18
Differential Right Ventricular Entrainment
Differential-site RV entrainment (from RV apex versus RV base) can help distinguish AVNRT from orthodromic AVRT. As discussed in Chapter 17, after entrainment of AVNRT, the PPI following entrainment from the RV base is longer than that post entrainment from the RV apex. Conversely, in orthodromic AVRT, the PPI tends to be similar, irrespective of the site of RV pacing.19 To avoid potential errors introduced by decremental conduction within the AVN during RV pacing, it is preferable to perform correction of the PPI (by subtracting any increase in the AV interval of the return cycle beat, as compared with the AV interval during SVT). A differential corrected [PPI – SVT CL] of more than 30 milliseconds after transient entrainment (i.e., corrected PPI following pacing from the RV base being consistently ≥30 milliseconds longer than that following pacing from the RV apex) is consistent with AVNRT, whereas a differential corrected [PPI – SVT CL] of less than 30 milliseconds is consistent with orthodromic AVRT. Additionally, a differential VA interval (ventricular stimulus-to-atrial interval during entrainment from RV base versus RV septum) of more than 20 milliseconds is consistent with AVNRT, whereas a differential VA interval of less than 20 milliseconds is consistent with orthodromic AVRT.19
Entrainment with Manifest Ventricular Fusion
Manifest ventricular fusion during entrainment of SVT indicates that the circuit includes ventricular tissue (i.e., AVRT), excluding both AVNRT and AT (see Fig. 18-30).
Length of Pacing Drive Required for Entrainment
As discussed in Chapter 17, assessing the timing and type of response of SVT to RV pacing can help differentiate orthodromic AVRT from AVNRT. Because the RV pacing site is closer to the reentrant circuit in AVRT than to the AVNRT circuit, RV pacing resets the tachycardia in the setting of AVRT once ventricular capture is achieved, whereas the resetting response is delayed in the setting of AVNRT. Consequently, when resetting of the SVT occurs after a single paced QRS complex, orthodromic AVRT is suggested and AVNRT is generally excluded. On the contrary, if resetting occurs only after at least two beats AVNRT is suggested (Fig. 20-9).20
Atrial Resetting during the Transition Zone
In patients with AVNRT or AT, acceleration of the timing of atrial activation cannot occur via the AVN during the transition zone on initiation of RV pacing (during which the pacing train fuses with anterograde ventricular activation), because the HB is expected to be refractory, as indicated by ventricular activation still occurring via anterograde conduction over the HPS. If perturbation (≥15 milliseconds) of atrial timing occurs during the transition zone, it indicates the presence of a retrogradely conducting BT, which can be an integral part of the SVT circuit (i.e., orthodromic AVRT) or a bystander (as discussed in Chap. 17).21
Atrial and Ventricular Electrogram Sequence Following Cessation of Ventricular Pacing
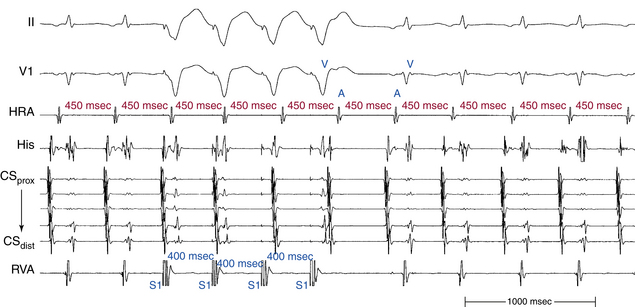
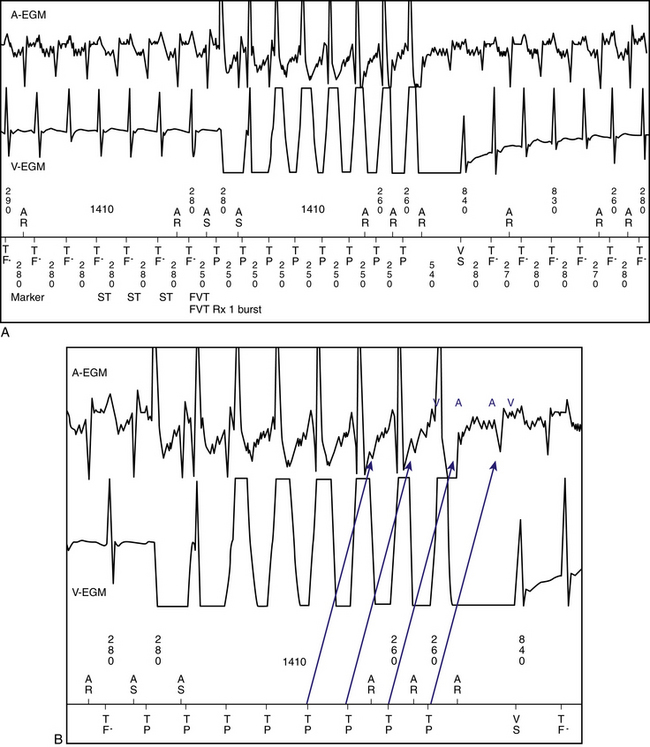
As discussed in Chapter 11, the sequence of atrial and ventricular electrograms following cessation of overdrive ventricular pacing during SVT (without tachycardia termination) can help distinguish between the different mechanisms of SVT. It is necessary to verify the presence of 1:1 VA conduction during ventricular pacing before analyzing electrogram sequence. In the setting of AVNRT and orthodromic AVRT, the electrogram sequence immediately after the last paced QRS complex is atrial-ventricular (i.e., “A-V response”; see Fig. 11-14). In contrast, in the setting of AT, cessation of overdrive ventricular pacing is followed by an atrial-atrial-ventricular electrogram sequence (i.e., an “A-A-V response”; see Fig. 11-14).22
Importantly, a pseudo–A-A-V response can occur: (1) during atypical AVNRT, (2) when 1:1 VA conduction is absent during overdrive ventricular pacing, (3) during typical AVNRT with long His bundle–ventricular (HV) or short HA intervals whereby atrial activation may precede ventricular activation, and (4) in patients with a bystander BT (Fig. 20-10). Replacing ventricular activation with HB activation (i.e., characterizing the response as A-A-H or A-H instead of A-A-V or A-V, respectively) can be more accurate and can help eliminate the pseudo–A-A-V response in patients with AVNRT and long HV intervals, short HA intervals, or both (see Fig. 20-8). On the other hand, a pseudo–A-V response can occur with automatic AT when the maneuver is performed during isoproterenol infusion, and can theoretically occur when AT coexists with retrograde dual AVN pathways or bystander BT (see Chap. 11).22
Diagnostic Maneuvers during Normal Sinus Rhythm after Tachycardia Termination
Ventricular Pacing at the Tachycardia Cycle Length
His Bundle–Atrial Interval
Ventricular pacing during NSR at a CL similar to the tachycardia results in HA and VA intervals that are shorter than those during orthodromic AVRT (see Fig. 18-30). Contrariwise, in the setting of AVNRT, ventricular pacing at the tachycardia CL results in HA and VA intervals that are equal or longer during pacing than those during the SVT (see Fig. 17-20). To help distinguish between orthodromic AVRT and AVNRT, the HA interval is measured from the end of the His potential (where the impulse leaves the HB to enter the AVN) to the atrial electrogram in the high RA recording and the ΔHA interval (HApacing – HASVT) is calculated. In the setting of orthodromic AVRT, the ΔHA interval is typically less than –10 milliseconds; whereas, in the setting of AVNRT, the ΔHA interval is more than –10 milliseconds. When the retrograde His potential is not visualized, using the ΔVA interval instead of the ΔHA interval is not as accurate in discriminating orthodromic AVRT from AVNRT (see Chap. 17).23
Differential Right Ventricular Pacing
The response to differential-site RV pacing can be evaluated by comparing the VA interval and atrial activation sequence during pacing at the RV base versus the RV apex (see Chap. 18).
Ventriculoatrial Interval
When the VA interval during RV apical pacing is shorter than that during RV basal pacing, a retrogradely conducting septal BT is excluded. However, this maneuver may not exclude the presence of a free wall or a slowly conducting BT. On the other hand, when the VA interval during RV apical pacing is longer than that during RV basal pacing, a retrogradely conducting AV BT is diagnosed (see Fig. 18-33).
Para-Hisian Pacing
The response to para-Hisian pacing can be determined by comparing the following four variables between HB-RB capture and noncapture while maintaining local ventricular capture and no atrial capture: (1) atrial activation sequence, (2) S-A interval, (3) local VA interval, and (4) HA interval (see Figs. 18-31 and 18-32).24 Seven patterns of response to para-Hisian pacing can be observed (see Table 18-3). Please refer to Chapter 18 for a more detailed discussion of para-Hisian pacing.
Atrial Activation Sequence
An identical retrograde atrial activation sequence, with and without HB capture, indicates that retrograde conduction is occurring over the same system during HB-RB capture and noncapture (either the BT or the AVN) and does not help prove or exclude the presence of a BT. On the other hand, a retrograde atrial activation sequence that is different depending on whether the HB is captured indicates the presence of a BT.
His Bundle–Atrial and Ventriculoatrial Intervals
The HA and S-A (VA) intervals are recorded at multiple sites, including close to the site of earliest atrial activation during SVT. An S-A (VA) interval that is constant regardless of whether the HB-RB is being captured indicates the presence of a BT, whereas prolongation of the S-A (VA) interval on loss of HB capture, compared with that during HB capture, excludes the presence of a retrogradely conducting BT, except for slowly conducting and far free wall BTs.24
Dual-Chamber Sequential Extrastimulation
Dual-chamber sequential extrastimulation is a useful maneuver for identifying concealed slowly conducting BTs not revealed with standard pacing maneuvers. This maneuver involves the delivery of an eight-beat drive train of simultaneous atrial and RV pacing at a CL of 600 milliseconds, followed by an AES (A2) delivered at a coupling interval equal to the predetermined AVN ERP, followed by a VES (V2) delivered at a coupling interval equal to the drive train CL (600 milliseconds). Repeat drives are then performed with decrements of 10 milliseconds for V2 until VA block is observed.25
The critically timed A2 prolongs the AVN refractory period via concealed anterograde conduction, causing the V2 to block retrogradely in the AVN when it would have conducted had the A2 not been delivered (analogous to delivering a VES during SVT while the HB is refractory). If a BT is present, the V2 conducts back to the atrium while the AVN remains refractory, resulting in a retrograde atrial activation pattern consistent with exclusive BT conduction. Please refer to Chapter 18 for a more detailed discussion on dual-chamber sequential extrastimulation.
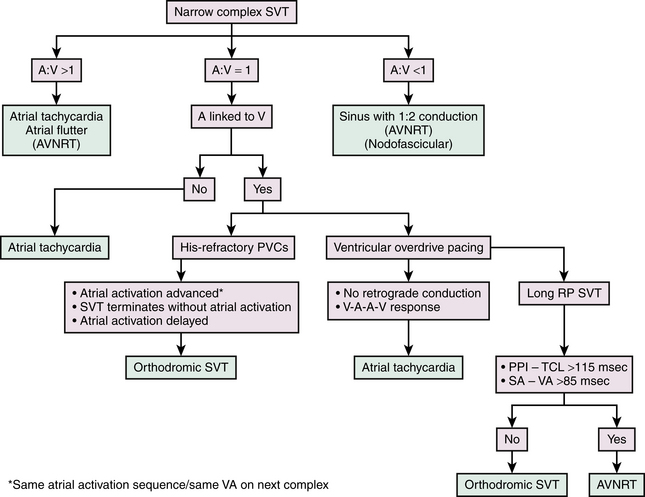
Practical Approach to Electrophysiological Diagnosis of Supraventricular Tachycardia
Tables 20-1, 20-2, and 20-3 and Figure 20-11 outline some of the proposed strategies for the EP diagnosis of narrow complex SVTs. Baseline tachycardia features, atrial and ventricular programmed stimulation during the tachycardia and then during sinus rhythm after tachycardia termination, provide a diagnosis of the mechanism of SVT in the vast majority of cases.
TABLE 20-1 Diagnostic Strategy for Narrow QRS Supraventricular Tachycardia: Tachycardia Features
| Atrial activation sequence |
• BBB does not affect the tachycardia CL or VA interval in AT, AVNRT, or orthodromic AVRT using a BT contralateral to the BBB.
• BBB that prolongs the VA interval, with or without affecting the tachycardia CL, is diagnostic of orthodromic AVRT using an AV BT ipsilateral to the BBB and excludes AT and AVNRT.
• Spontaneous changes in PR and RP intervals with a fixed A-A interval is consistent with AT, and excludes orthodromic AVRT.
• Spontaneous changes in the tachycardia CL accompanied by a constant VA interval favor orthodromic AVRT.
• Changes in atrial CL preceding similar changes in subsequent ventricular CL strongly favor AT or atypical AVNRT.
• Changes in ventricular CL preceded by similar changes in the atrial CL favor typical AVNRT or orthodromic AVRT.
• Spontaneous termination of SVT with a P wave not followed by a QRS practically excludes AT, except in the case of a nonconducted PAC terminating the AT.
• Reproducible SVT termination in response to adenosine with a QRS not followed by a P wave excludes orthodromic AVRT using a rapidly conducting AV BT as the retrograde limb, is unusual in typical AVNRT, and is consistent with AT, PJRT, or atypical AVNRT.
A-A = atrial-atrial; AT = atrial tachycardia; AV = atrioventricular; AVNRT = atrioventricular nodal reentrant tachycardia; AVRT = atrioventricular reentrant tachycardia; BBB = bundle branch block; BT = bypass tract; CL = cycle length; PAC = premature atrial complex; PJRT = permanent junctional reciprocating tachycardia; RA = right atrium; SVT = supraventricular tachycardia; VA = ventriculoatrial.
TABLE 20-2 Diagnostic Strategy for Narrow QRS Supraventricular Tachycardia: Programmed Electrical Stimulation during Tachycardia
A-A = atrial-atrial; A-A-V = atrial-atrial-ventricular; AH = atrial-His bundle interval; AT = atrial tachycardia; AV = atrioventricular; AVNRT = atrioventricular nodal reentrant tachycardia; AVRT = atrioventricular reentrant tachycardia; BT = bypass tract; CL = cycle length; CS = coronary sinus; HB = His bundle; PPI = post-pacing interval; RA = right atrium; RV = right ventricle; SVT = supraventricular tachycardia; VA = ventriculoatrial; VES = ventricular extrastimulus.
TABLE 20-3 Diagnostic Strategy for Narrow QRS Supraventricular Tachycardia: Programmed Electrical Stimulation during Sinus Rhythm
AH = atrial-His bundle interval; AT = atrial tachycardia; AV = atrioventricular; AVN = atrioventricular node; AVNRT = atrioventricular nodal reentrant tachycardia; AVRT = atrioventricular reentrant tachycardia; BT = bypass tract; CL = cycle length; HA = His bundle–atrial interval; RB = right bundle branch; RV = right ventricle; S-A = stimulus-atrial interval; SVT = supraventricular tachycardia; VA = ventriculoatrial.
When sustained SVT is inducible, overdrive pacing from the RV apex and base is performed at a CL 10 to 30 milliseconds shorter than the tachycardia CL; the pacing CL is then progressively reduced by 10 to 20 milliseconds in a stepwise fashion with discontinuation of ventricular pacing after each pacing CL to verify continuation versus termination of the SVT. When acceleration of the atrial CL to the pacing CL during ventricular pacing (with 1:1 VA conduction) is verified, several diagnostic criteria can be applied (see Table 20-2). Overdrive ventricular pacing during the SVT represents the single most important diagnostic maneuver that can provide several clues to the diagnosis of most SVTs. Therefore, it is preferable to employ this maneuver as an initial step in the diagnostic approach.26
Subsequently, a VES is delivered when the HB is refractory and then at progressively shorter VES coupling intervals (approximately 10-millisecond stepwise shortening of the VES coupling interval) so as to scan all of diastole. First, ventricular capture of the VES should be verified, and then the effect of the VES on the following atrial activation (advancement, delay, termination, or no effect) should be evaluated, as well as the timing of the VES in relation to the expected His potential during the SVT. Furthermore, conduction of the VES to the atrium and sequence of atrial activation following the VES should be carefully examined (see Fig. 20-6).
Atrial pacing is then performed at a CL 10 to 20 milliseconds shorter than the tachycardia CL. The pacing CL is then progressively reduced by 10 to 20 milliseconds in a stepwise fashion, with discontinuation of atrial pacing after each pacing CL to ensure continuation versus termination of the SVT. The presence of entrainment, atrial fusion, AH interval, and VA linking should be evaluated (see Table 20-2).
After tachycardia termination, ventricular and atrial pacing at the tachycardia CL, differential site RV pacing (RV apex versus base), and para-Hisian pacing maneuvers are performed (see Table 20-3).
1. Lau E.W. Infraatrial supraventricular tachycardias: mechanisms, diagnosis, and management. Pacing Clin Electrophysiol. 2008;31:490-498.
2. Porter M.J., Morton J.B., Denman R., et al. Influence of age and gender on the mechanism of supraventricular tachycardia. Heart Rhythm. 2004;1:393-396.
3. Gonzalez-Torrecilla E., Almendral J., Arenal A., et al. Combined evaluation of bedside clinical variables and the electrocardiogram for the differential diagnosis of paroxysmal atrioventricular reciprocating tachycardias in patients without pre-excitation. J Am Coll Cardiol. 2009;53:2353-2358.
4. Ballo P., Bernabo D., Faraguti S.A. Heart rate is a predictor of success in the treatment of adults with symptomatic paroxysmal supraventricular tachycardia. Eur Heart J. 2004;25:1310-1317.
5. Blomstrom-Lundqvist C., Scheinman M.M., Aliot E.M., et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Supraventricular Arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol. 2003;42:1493-1531.
6. Knight B.P., Ebinger M., Oral H., et al. Diagnostic value of tachycardia features and pacing maneuvers during paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2000;36:574-582.
7. Kertesz N.J., Fogel R.I., Prystowsky E.N. Mechanism of induction of atrioventricular node reentry by simultaneous anterograde conduction over the fast and slow pathways. J Cardiovasc Electrophysiol. 2005;16:251-255.
8. Kapa S., Henz B.D., Dib C., et al. Utilization of retrograde right bundle branch block to differentiate atrioventricular nodal from accessory pathway conduction. J Cardiovasc Electrophysiol. 2009;20:751-758.
9. Josephson M.E. Supraventricular tachycardias. In: Josephson M.E., editor. Clinical cardiac electrophysiology. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2008:175-284.
10. Lockwood D., Nakagawa H., Jackman W. Electrophysiological characteristics of atrioventricular nodal reentrant tachycardia: implications for the reentrant circuit. In: Zipes D.P., Jalife J., editors. Cardiac electrophysiology: from cell to bedside. ed 5. Philadelphia: WB Saunders; 2009:615-646.
11. Issa Z.F. Mechanism of paroxysmal supraventricular tachycardia with ventriculoatrial conduction block. Europace. 2009;11:1235-1237.
12. Morihisa K., Yamabe H., Uemura T., et al. Analysis of atrioventricular nodal reentrant tachycardia with variable ventriculoatrial block: characteristics of the upper common pathway. Pacing Clin Electrophysiol. 2009;32:484-493.
13. Srivathsan K., Gami A.S., Barrett R., et al. Differentiating atrioventricular nodal reentrant tachycardia from junctional tachycardia: novel application of the delta H-A interval. J Cardiovasc Electrophysiol. 2008;19:1-6.
14. Maruyama M., Kobayashi Y., Miyauchi Y., et al. The VA relationship after differential atrial overdrive pacing: a novel tool for the diagnosis of atrial tachycardia in the electrophysiologic laboratory. J Cardiovasc Electrophysiol. 2007;18:1127-1133.
15. Kannankeril P.J., Bonney W.J., Dzurik M.V., Fish F.A. Entrainment to distinguish orthodromic reciprocating tachycardia from atrioventricular nodal reentry tachycardia in children. Pacing Clin Electrophysiol. 2010;33:469-474.
16. Platonov M., Schroeder K., Veenhuyzen G.D. Differential entrainment: beware from where you pace. Heart Rhythm. 2007;4:1097-1099.
17. Gonzalez-Torrecilla E., Arenal A., Atienza F., et al. First postpacing interval after tachycardia entrainment with correction for atrioventricular node delay: a simple maneuver for differential diagnosis of atrioventricular nodal reentrant tachycardias versus orthodromic reciprocating tachycardias. Heart Rhythm. 2006;3:674-679.
18. Veenhuyzen G.D., Stuglin C., Zimola K.G., Mitchell L.B. A tale of two post pacing intervals. J Cardiovasc Electrophysiol. 2006;17:687-689.
19. Segal O.R., Gula L.J., Skanes A.C., et al. Differential ventricular entrainment: a maneuver to differentiate AV node reentrant tachycardia from orthodromic reciprocating tachycardia. Heart Rhythm. 2009;6:493-500.
20. Dandamudi G., Mokabberi R., Assal C., et al. A novel approach to differentiating orthodromic reciprocating tachycardia from atrioventricular nodal reentrant tachycardia. Heart Rhythm. 2010;7:1326-1329.
21. AlMahameed S.T., Buxton A.E., Michaud G.F. New criteria during right ventricular pacing to determine the mechanism of supraventricular tachycardia. Circ Arrhythm Electrophysiol. 2010;3:578-584.
22. Vijayaraman P., Lee B.P., Kalahasty G., Wood M.A., Ellenbogen K.A. Reanalysis of the “pseudo A-A-V” response to ventricular entrainment of supraventricular tachycardia: importance of His-bundle timing. J Cardiovasc Electrophysiol. 2006;17:25-28.
23. Miller J.M., Rosenthal M.E., Gottlieb C.D., et al. Usefulness of the delta HA interval to accurately distinguish atrioventricular nodal reentry from orthodromic septal bypass tract tachycardias. Am J Cardiol. 1991;68:1037-1044.
24. Nakagawa H., Jackman W.M. Para-Hisian pacing: useful clinical technique to differentiate retrograde conduction between accessory atrioventricular pathways and atrioventricular nodal pathways. Heart Rhythm. 2005;2:667-672.
25. Sauer W.H., Lowery C.M., Cooper J.M., Lewkowiez L. Sequential dual chamber extrastimulation: a novel pacing maneuver to identify the presence of a slowly conducting concealed accessory pathway. Heart Rhythm. 2008;5:248-252.
26. Veenhuyzen G.D., Coverett K., Quinn F.R., et al. Single diagnostic pacing maneuver for supraventricular tachycardia. Heart Rhythm. 2008;5:1152-1158.