16 Aortic Stenosis
I. CASE
A. Fetal echocardiography findings
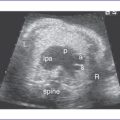
1. Fetal echocardiography reveals situs solitus of the atria, levocardia, left aortic arch, and normal cardiothoracic ratio (0.3). The heart rate is 140 bpm.
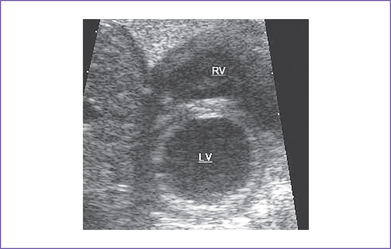
2. The left ventricle (LV) is hypoplastic (80% of the right ventricle [RV] length), with a mildly hypoplastic mitral valve annulus.
3. The LV is significantly echogenic, suggesting scarring from elevated LV pressure (endocardial fibroelastosis of the LV), with decreased function.
4. The aortic valve is bicuspid, with a mildly hypoplastic annulus (4 mm) and a gradient of 25 to 30 mm Hg and mild poststenotic dilation of the ascending aorta.
5. There is no mitral or aortic valve regurgitation.
6. There is poststenotic dilation of the ascending aortic arch, with mild isthmus hypoplasia (2.5 mm).
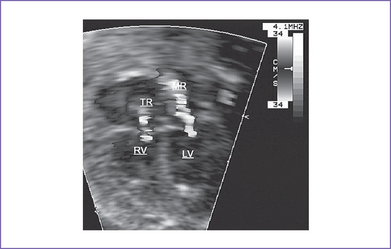
7. The RV is significantly dilated, with a trace of holosystolic tricuspid regurgitation and normal pulmonary artery flow.
8. The mitral valve has echogenic papillary muscles and there is an inflow Doppler with a single peak of short duration.
9. The LV is markedly dilated and the Tei index (myocardial performance index) is mildly increased at 0.6 (Fig. 16-1). The RV Tei index is normal.
10. There are no signs of hydrops fetalis.
11. Flow in the aortic arch is retrograde, with normal ductal flow (pulmonary to aorta) and a nonrestricted reversed flow (left to right) shunt across the foramen ovale.
D. Fetal management and counseling
1. Amniocentesis was done before referral and was reported to be normal, including fluorescent in situ hybridization (FISH) analysis for chromosome 22 microdeletion.
F. Neonatal management
a. Following delivery, prostaglandin E1 (PGE1) may be initiated if the stenosis is clearly more severe or if there is an additional important left heart obstructive lesion such as critical coarctation.
b. A baby with a prenatal diagnosis of probable critical aortic stenosis can develop congestive heart failure with a low cardiac output following delivery.
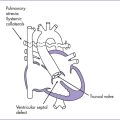
c. Use of prostaglandins followed by neonatal intervention is indicated if prenatally there is retrograde arch flow near term. The heart murmur (ejection systolic) may be absent, and the peripheral pulses may be poor due to the low cardiac output state. Additional anticongestive medication and inotropic support may be necessary (Fig. 16-3).
d. Critically ill newborns with severe aortic stenosis should be treated urgently because the mortality rate is high. The treatment strategy depends on:
e. Options include transcatheter valvuloplasty or closed surgical aortic valvotomy (not under cardiopulmonary bypass). Additional pathology such as aortic coarctation might need to be addressed surgically as well.
a. Postnatal treatment of aortic stenosis has been associated with significant mortality. However, recent advances in the palliation of infants with critical aortic stenosis with or without LV hypoplasia have improved the outlook for these patients. Criteria that influence the decision include the size of the LV (ratio to the RV and whether apex forming or not) and the size of the mitral and aortic valve orifices.
b. The treatment strategy depends on:
G. Follow-up
1. Reexamination is initially scheduled monthly until the degree of residual aortic stenosis has reached a plateau.
a. At times, with rapid growth of the baby in general in the first 6 months, the aortic stenosis progresses and requires an additional intervention.
b. Once serial evaluation suggests the degree of aortic stenosis is relatively stable or only progressing slowly, subsequent clinical evaluations may occur every 6 to 12 months.
2. Most patients require further intervention for residual obstruction.
3. Subacute bacterial endocarditis (SBE) prophylaxis should be continued on indications because of the frequently associated bicuspid aortic valve, residual aortic stenosis, or aortic regurgitation.
4. Follow-up should include surveillance for systemic hypertension.
5. The baby should be monitored for development of subaortic aortic stenosis or mitral stenosis.
6. Patients with moderate residual aortic stenosis should not participate in competitive sports.
H. Risk of recurrence
Because the baby’s karyotype is normal, the risk of recurrence is 1% to 2% in future pregnancies.
I. Outcome of this case
1. The baby was delivered at term weighing 3.3 kg. Apgar scores were good: 8 at 1 minute and 9 at 5 minutes.
2. Pulse oximeter reading was 87% in room air but with no metabolic acidosis.
3. PGE1 infusion was started at 0.05 μg /kg per minute.
4. The aortic valve was bicuspid and thickened. The aortic valve annulus (4 mm) had antegrade flow, and LV size (LV: RV, 0.8:0.9) was borderline but apex forming.
5. Transcatheter balloon valvuloplasty was done. Gradient dropped from 50 mm Hg at rest to 20 mm Hg at rest.
6. There was gradual improvement in the baby’s clinical condition, including normalization of respiratory rate weaning off PGE1. However, at 2 weeks of age the patient was unstable.
7. Transthoracic echocardiogram showed a gradient of 50 mm Hg across the aortic valve.
8. A second successful balloon dilation was done. There was a mild residual gradient across the transverse arch (18 mm Hg), but his was not addressed.
9. The baby was discharged home on PO digoxin, furosemide, and spironolactone, which he was weaned off within 2 months.
10. At 6 months of age, the infant was thriving, and follow-up echo revealed a mild gradient across the aortic valve (20 mm Hg) with mild aortic regurgitation.
II. YOUR HANDY REFERENCE
A. Prevalence
1. Aortic valve stenosis accounts for 2% to 3% of congenital heart disease manifesting in the first year of life.
2. Most cases of aortic stenosis are isolated; the lesion is usually part of the spectrum of left heart obstruction, such as mitral valve abnormalities.
3. The coexistence of endocardial fibroelastosis is well described and is associated with poor outcome.
4. In the fetal series of Allan and colleagues, aortic stenosis accounted for about 2.9% of structural heart disease.
a. A total of 67 fetuses had aortic stenosis; five cases were not critical.
b. Pregnancy was terminated in 32 of the 62 remaining cases.
c. Of the remaining 30 fetuses, three died in utero, 20 died in the neonatal period or infancy, and only five survived.
5. Other series reported that critical aortic stenosis or atresia affects 4% to 5% of fetuses with congenital heart disease.
B. Outcome
1. Aortic atresia, stenosis or regurgitation, or an aorta-to-LV tunnel are all lesions that can affect the aortic valve.
2. Aortic stenosis tends to be critical when diagnosed prenatally, but other, less-severe forms have been reported.
3. Aortic atresia in association with mitral atresia or severe stenosis (spectrum of the hypoplastic left heart syndrome) is the second most common form of heart disease seen in the fetus. Isolated aortic atresia is rare.
C. Associated syndromes and extracardiac anomalies
1. Isolated valvar aortic stenosis in female fetuses may be associated with Turner’s syndrome.
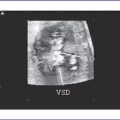
2. Extracardiac structural pathology is not very common in aortic stenosis. However, when associated with other forms of left heart obstruction, such as mitral atresia, aortic valve lesions may be associated with chromosomal abnormalities including trisomy 18 and deletion of chromosome 22q11, especially in the presence of a malalignment VSD.
D. Clues to fetal sonographic diagnosis
The appearance of the fetal heart depends on the degree of obstruction at the aortic valve.
1. Moderate to severe valvar aortic stenosis.
a. LV might appear normal or only mildly hypertrophied.
2. Critical aortic stenosis typically shows:
a. Aortic root smaller than normal for gestation.
b. Minimal antegrade flow across the aortic valve orifice or even increased outflow tract velocity due to poor ventricular function. The Doppler outflow tract velocity may be:
c. Retrograde filling of the ascending aorta via the duct (reverse arch flow) in the transverse arch, with reversal of flow across the foramen ovale (left to right).
d. Dilated and poorly contractile LV with evidence of endocardial fibroelastosis (increased echogenicity of the ventricular walls and the papillary muscles of the mitral valve).
e. Associated mitral regurgitation, giving the first clue to the diagnosis of moderate valvar aortic stenosis.
f. Restricted mitral valve opening due to either increased LV diastolic pressure or associated mitral stenosis.
a. Abnormal four-chamber view: RV bigger than LV after 34 weeks’ gestation.
b. Abnormal size and function of the LV (especially if associated with mitral atresia).
c. Ascending aorta: Usually hypoplastic (variable degree of hypoplasia).
d. Absent forward flow across the aortic valve on pulsed Doppler or color flow mapping (critical aortic stenosis).
e. Retrograde filling of the ascending aorta via the duct (reverse arch flow) in the transverse arch.
g. In critical aortic stenosis with VSD, the color flow mapping shows a left-to-right shunt, in contrast to bidirectional shunting in isolated VSD.
h. As seen in hypoplastic left heart syndrome (HLHS):
4. Evaluation for postnatal surgery.
a. The RV should be carefully assessed for the suitability of the Norwood procedure or otherwise listing for heart transplant after delivery.
E. Progression in utero
1. Sequential fetal studies have shown a reduced rate of growth of left heart structures (aortic valve annulus, LV, and mitral valve) in cases of severe left heart outflow tract obstruction when diagnosed in the second trimester; the same observation has been made in animal fetal models.
2. Aortic valve stenosis can progress to the spectrum of HLHS by term secondary to the failure of growth of the LV.
3. At times, even in the presence of antegrade flow through the narrowed aortic valve, as pregnancy advances the apex of the heart is gradually taken over by the RV, thus precluding a biventricular repair of the left heart obstruction.
4. If the aortic stenosis only evolves late in pregnancy (second to third trimester) or very slowly, growth of the LV could be more normal, although the transvalvar gradient in utero might not reflect the true gradient at birth (stenosis is usually more severe at birth).
5. In all forms of left heart obstruction (mild or severe), the size of transverse arch, isthmus, and LV (including mitral and aortic valve annuli) must be evaluated sequentially for progression of the disease.
F. Pathophysiology
1. Hastreiter and colleagues (1963) divided the various entities into three main groups.
a. Group 1: Patients with isolated aortic stenosis characterized by relatively well formed valves with an adequate aortic annulus and ascending aorta and without associated cardiovascular malformation.
b. Group 2: Patients with primary endocardial fibroelastosis with secondary involvement of the aortic valve.
c. Group 3: Patients with marked deformity of the aortic valve.
2. The newborn with valvar aortic stenosis usually appears healthy at birth, and the timing of presentation depends on the severity of valve obstruction.
a. Patients with mild or moderate stenosis usually present with asymptomatic murmur or ejection click on routine pediatric checkup. The diagnosis will be confirmed by echocardiography. Echocardiography provides information about:
b. In more severe cases, the infant presents with heart failure or, if the obstruction is critical, circulatory collapse in the first few days of life as the duct closes (duct-dependent circulation). This is a medical and surgical emergency.
3. The majority of cases diagnosed in fetal life are likely to represent a more severe spectrum of the disease.
1. Suggested criteria for selection for fetal aortic valve balloon dilation (Fig. 16-4).
a. Large LV with persistent patency of the aortic valve.
b. Normal-appearing mitral valve.
c. Reversed flow in the aortic arch.
d. Left-to-right shunt at the atrial level.
2. Antenatal intervention has been performed for aortic valve stenosis and atresia and for defects associated with restriction or closure of the interatrial septum.
a. Valves considered to be, or likely to become, critical or likely to progress to atresia are considered for treatment.
b. To date, the number of fetuses suitable for intervention is not large.
3. The postnatal outlook for children born today with valvar stenosis has improved, but the quality of surgical programs varies and reports may be biased by patient selection, the particular interest of the surgeon, and the publication of short-term 30-day survival data.
4. Reasons for fetal intervention.
a. The early experience of cardiac interventions was poor, with few survivors. More recent experience has thus far been more encouraging and is advancing.
b. The aims in fetal intervention in either aortic or pulmonary stenosis are:
c. Fetal intervention promotes cardiac growth.
d. Fetal intervention can minimize secondary damage. There are three possible mechanisms of ventricular damage in utero.
e. A biventricular circulation provides a better quality of life than a univentricular circulation.
f. Possibility of optimizing the pulmonary vascular bed (ongoing research).
5. Timing of fetal intervention.
a. The approach at most centers is to offer an intervention soon after diagnosis in cases that fulfill the criteria, provided that adequate time has been given for counseling and parental decision making.
b. Elevated ventricular pressure and abnormal hemodynamics lead to fibrosis and calcification in the fetal ventricle. This can be documented on ultrasound and is important in the early timing of procedures intended to relieve pressure loading.
c. The largest reported series in fetal intervention for aortic valvuloplasty is from Children’s Hospital in Boston, where 33 procedures have been attempted. Twenty-six were successful and three fetuses died during the procedure. Five of the 26 successful cases have a biventricular circulation.
6. Maternal and fetal morbidity.
7. Biventricular repair in congenital aortic stenosis (Boxes 16-1 and 16-2).
a. Decisions regarding surgical strategy in patients with multiple left heart obstructive or hypoplastic lesions often must be made in the newborn period and are seldom reversible.
b. Predictors of outcome of biventricular repair have not been well defined in this heterogeneous group of patients, and risk factors described for critical aortic valve stenosis have been shown to be inapplicable to patients with other left heart obstructive lesions.
c. In 1991, Rhodes stated that it was possible to predict outcome after classic valvotomy (two-ventricle-type repair) with 95% accuracy based on the following criteria:
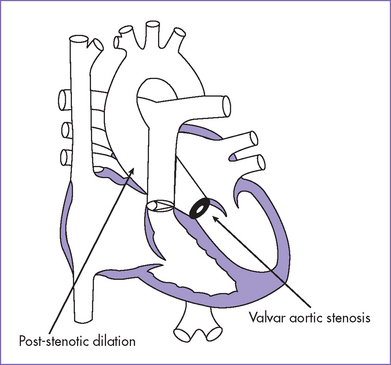
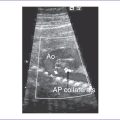
Fig. 16-4 Aortic valve stenosis with post-stenotic dilation.
(Modified from Mullins CE, Mayer DC: Congenital Heart Disease: A Diagrammatic Atlas. New York, Liss, 1988.)
BOX 16-1 SUGGESTED CONTRAINDICATIONS TO BIVENTRICULAR REPAIR IN CONGENITAL AORTIC STENOSIS
Small left ventricle, <20 mL/m2 body surface area
Narrow aortic valve ring <5 mm, although there are survivors with biventricular repairs in whom the aortic valve was smaller than this cut-off value
Adapted from McCrindle BW, Blackstone EH, Williams WG, et al: Are outcomes of surgical versus transcatheter balloon valvotomy equivalent in neonatal critical aortic stenosis? Circulation 104(12 Suppl 1):I152-I158, 2001.
BOX 16-2 AORTIC VALVE STENOSIS ASSOCIATED WITH NEED FOR SINGLE VENTRICLE PALLIATION
Mitral valve area <4.75 cm2/m2 body surface area
Small LV: Ratio of apex-to-base dimension of LV to that of RV <0.8
LV, left ventricle; RV, right ventricle.
Adapted from McCrindle BW, Blackstone EH, Williams WG, et al: Are outcomes of surgical versus transcatheter balloon valvotomy equivalent in neonatal critical aortic stenosis? Circulation 104(12 Suppl 1):I152-I158, 2001.
III. TAKE-HOME MESSAGE
A. Diagnosis
1. Fetal aortic coarctation and critical aortic stenosis both can overlap the spectrum of left heart syndromes.
2. Serial Doppler measurements that are indicators of increased LA pressure in the setting of critical aortic stenosis or raised LA pressure secondary to a restrictive oval foramen include:
a. High pulsatility index of the branch pulmonary arteries.
b. Significant reversal of pulmonary venous flow with atrial contraction.
B. Treatment
1. Management of fetuses with aortic stenosis depends on the severity of the lesion and gestational age at which the diagnosis is made.
2. Successful prenatal fetal intervention for aortic valvuloplasty depends on the selection of cases (e.g., those with reverse flow through the arch with minimal antegrade flow). Fetal aortic valvuloplasty has an increasing role in improving LV growth and function.
3. Surgical aortic valvotomy and balloon aortic valvuloplasty for neonatal critical aortic stenosis have similar outcomes, although most centers offer valvuloplasty if there is no other indication for surgery.
4. Persistent pulmonary hypertension late after neonatal aortic valvotomy is a previously unreported late complication of “successful” biventricular repair for neonatal critical aortic stenosis. This is an important consideration in determining the initial management.
5. Studies have shown that the methods established to determine the suitability for biventricular repair in cases with critical aortic stenosis (e.g., Rhodes criteria or LV cavity <20 mL /m2) cannot be applied to other diagnoses with LV hypoplasia.
C. Prognosis
1. As pregnancy advances, the LV might fail to grow at a normal rate. Thus, critical aortic stenosis diagnosed in the second trimester can progress to HLHS by term due to failure of growth of the LV and the development of complete aortic atresia. This fact and its implications have to be shared with the parents, particularly with regard to the possibility of precluding a biventricular circulation after birth.
2. Fetuses with aortic stenosis diagnosed in the second trimester with LV dilation and global dysfunction are likely to develop HLHS at term. In contrast, fetuses who have critical aortic stenosis diagnosed in the second trimester but who have a normal systolic function have a better chance of biventricular repair after delivery.
Allan LD, Sharland GK, Milburn A, et al. Prospective diagnosis of 1,006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol. 1994;23(6):1452-1458.
Blaufox AD, Lai WW, Lopez L, et al. Survival in neonatal biventricular repair of left-sided cardiac obstructive lesions associated with hypoplastic left ventricle. Am J Cardiol. 1998;82(9):1138-1140.
Hammon JWJr, Lupinetti FM, Maples MD, et al. Predictors of operative mortality in critical valvular aortic stenosis presenting in infancy. Ann Thorac Surg. 1988;45(5):537-540.
Hastreiter AR, Oshima M, Miller RA, et al. Congenital aortic stenosis syndrome in infancy. Circulation. 1963;28:1084-1095.
Hein S, Arnon E, Kostin S, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: Structural deterioration and compensatory mechanisms. Circulation. 2003;107(7):984-991.
Hornberger LK, Sahn DJ, Kleinman CS, et al. Antenatal diagnosis of coarctation of the aorta: A multicenter experience. J Am Coll Cardiol. 1994;23(2):417-423.
Hornberger LK, Weintraub RG, Pesonen E, et al. Echocardiographic study of the morphology and growth of the aortic arch in the human fetus. Observations related to the prenatal diagnosis of coarctation. Circulation. 1992;86(3):741-747.
Huhta J, Quintero RA, Suh E, Bader RS. Advances in fetal cardiac intervention. Curr Opin Paediatr. 2004;16:487-493.
McCrindle BW, Blackstone EH, Williams WG, et al. Are outcomes of surgical versus transcatheter balloon valvotomy equivalent in neonatal critical aortic stenosis? Circulation. 2001;104(12 Suppl 1):I152-I158.
Ovaert C, Qureshi SA, Rosenthal E, et al. Growth of the right ventricle after successful transcatheter pulmonary valvotomy in neonates and infants with pulmonary atresia and intact ventricular septum. J Thorac Cardiovasc Surg. 1998;115:1055-1062.
Rasanen J, Huhta JC, Weiner S, et al. Fetal branch pulmonary arterial vascular impedance during the second half of pregnancy. Am J Obstet Gynecol. 1996;174:1441-1449.
Rhodes LA, Colan SD, Perry SB, et al. Predictors of survival in neonates with critical aortic stenosis. Circulation. 1991;84(6):2325-2335. Erratum in Circulation 92(7):2005, 1995.
Serraf A, Piot JD, Bonnet N, et al. Biventricular repair approach in ducto-dependent neonates with hypoplastic but morphologically normal left ventricle. J Am Coll Cardiol. 1999;33(3):827-834.
Tulzer G, Arzt W, Franklin RC, et al. Fetal pulmonary valvoplasty for critical pulmonary stenosis with intact septum. Lancet. 2002;360(9345):1567-1568.
Tworetzky W, Wilkins-Haug L, Jennings RW, et al. Balloon dilation of severe aortic stenosis in the fetus: Potential for prevention of hypoplastic left heart syndrome: Candidate selection, technique, and results of successful intervention. Circulation. 2004;110:2125-2131.
Van Son JA, Falk V, Schneider P, et al. Repair of coarctation of the aorta in neonates and young infants. J Card Surg. 1997;12(3):139-146.
Ziemer G, Jonas RA, Perry SB, et al. Surgery for coarctation of the aorta in the neonate. Circulation. 1986;74(3 Pt 2):I25-131.

 G.
G.