147 Antidepressants and Antipsychotics
• Central nervous system depression is the most common sign of antidepressant or antipsychotic overdose.
• Tachycardia, hypotension, seizures, and ventricular dysrhythmias can also occur, especially after tricyclic antidepressant (TCA) overdose.
• Airway intervention, benzodiazepines, intravenous fluids, and cooling measures (especially for serotonin syndrome and/or neuroleptic malignant syndrome) are the mainstays of supportive care and treatment.
• Specific treatment options include sodium bicarbonate for TCA toxicity and crystalloid fluids or hemodialysis for lithium poisoning.
• Controversial treatment modalities include dantrolene for toxin-induced hyperthermia, cyproheptadine for serotonin syndrome, bromocriptine for neuroleptic malignant syndrome, and prophylactic magnesium for long QTc intervals without evidence of hypomagnesemia or torsades de pointes.
Epidemiology
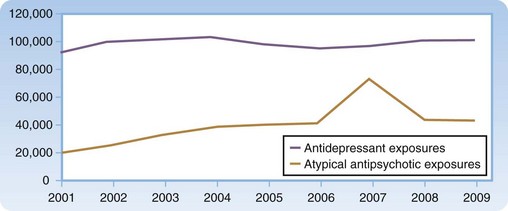
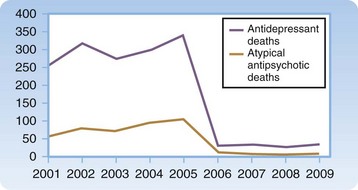
Data reported from United States poison control centers reveal that toxic exposures from antidepressants and antipsychotic agents continue to remain significant (Figs. 147.1 and 147.2). Tricyclic antidepressant (TCA) and monoamine oxidase inhibitor (MAOI) overdoses have historically resulted in the most significant morbidity and mortality. Currently, however, these agents are prescribed much less frequently than serotonin reuptake inhibitors (SRIs), atypical antipsychotics, and lithium. Atypical antipsychotic agents have largely replaced the older typical agents because these newer agents effectively reduce hallucinations, restructure thinking, and control agitation while assisting with the negative effects of psychotic disorders (flattened affect, avolition, social withdrawal). In addition, movement disorders such as dystonia, akathisia, tardive dyskinesia, and neuroleptic malignant syndrome occur less often with atypical antipsychotics than with the typical agents.
Pathophysiology
Tricyclic Antidepressants
TCAs have similar ring structures and, with only a few exceptions, result in a related toxicity. Examples are amitriptyline (Elavil), imipramine (Tofranil), and doxepin (Sinequan). The five major pharmacologic effects of TCAs are listed in Table 147.1.
Table 147.1 Five Major Pharmacologic Effects of Tricyclic Antidepressants
| PHARMACOLOGIC EFFECT | SYMPTOMS |
|---|---|
| Blockade of sodium conductance through fast channels in the myocardium | Prolonged phase 0 of the cardiac action potential that results in a widened QRS complex on an electrocardiogram |
| Blockade of potassium efflux | Prolonged phase 3 of the cardiac action potential resulting in an increased QTc interval that lends itself to the development of torsades de pointes |
| Peripheral α1-receptor blockade | Vasodilation, decreased perfusion, and hypotension |
| Serotonin and norepinephrine reuptake inhibition | Agitation, delirium, or seizure activity |
| Anticholinergic activity | Range of physical findings (coma, delirium, urinary retention, mydriasis, seizures, tachycardia, flushing hyperthermia, dry skin) |
| “Hot as a hare, dry as a bone, red as a beet, blind as a bat, mad as a hatter, fast as a cat, full as a tick” |
Monoamine Oxidase Inhibitors
Phenelzine (Nardil) and tranylcypromine (Parnate) are the two most commonly prescribed MAOIs in the United States, and they account for the majority of toxicity seen with this class of agents. The pharmacologic effects of MAOIs are listed in Box 147.1. These effects in overdose may result in a sympathomimetic toxidrome followed by profound hypotension. A therapeutic dose of an MAOI can interact with certain foods, drinks, and other pharmacologic agents and cause serious toxicity.
selective Serotonin Reuptake Inhibitors
Selective SRI antidepressants commonly prescribed are sertraline (Zoloft), paroxetine (Paxil), fluoxetine (Prozac), citalopram (Celexa), and escitalopram (Lexapro). The clinically beneficial central nervous system (CNS) effects of SRIs are thought to result from blockade of presynaptic reuptake of serotonin at 5-hydroxytryptamine type 1 (5-HT1) receptors. This blockade leads to higher synaptic serotonin levels and hence has positive effects on mood. Overdoses of these agents are much safer than overdoses of TCAs or MAOIs, although significant morbidity and mortality may occur with significant overdose or, more commonly, with ingestion of an SRI in combination with ingestion of agents possessing proserotonergic activity.1
Atypical Antipsychotics
The pharmacologic mechanism of action of atypical agents includes blockade at dopamine (D2) receptors and serotonin (5-HT2A) receptors.2 These agents can also cause repolarization abnormalities by blocking potassium efflux in the myocardium and thereby increasing the risk of torsades de pointes (Box 147.2).
Presenting Signs and Symptoms
Tricyclic Antidepressants
Serious toxicity is usually seen within 6 hours of ingestion. Signs and symptoms include obtundation, seizures, hypertension (early), hypotension (late), tachycardia (supraventricular or ventricular), and respiratory depression. Patients can deteriorate rapidly and usually do so within an hour of ingestion of the drug.3 Seizures and cardiovascular collapse can occur.4–6 In addition, profound hemodynamic instability follows seizure activity in some patients who have been poisoned with TCAs. Seizures result in further acidemia, which contributes to cardiovascular poisoning.
Monoamine Oxidase Inhibitors
Overdose of an MAOI results in sympathomimetic overdrive. Several phases have been described,7,8 and they can be classified as follows:
MAOI interactions with foods or beverages (aged cheeses, fava beans, ales, wines) produce early onset of signs and symptoms within minutes to hours.8 Because of tyramine’s short-lived action on the adrenal medulla (to increase endogenous amines), these interactions last only several hours. Interactions of MAOIs with other drugs (sympathomimetics, methylxanthines, SRIs, meperidine) also lead to elevated sympathetic tone. This effect manifests within minutes to hours and can last several hours to days.
Serotonin Reuptake Inhibitors
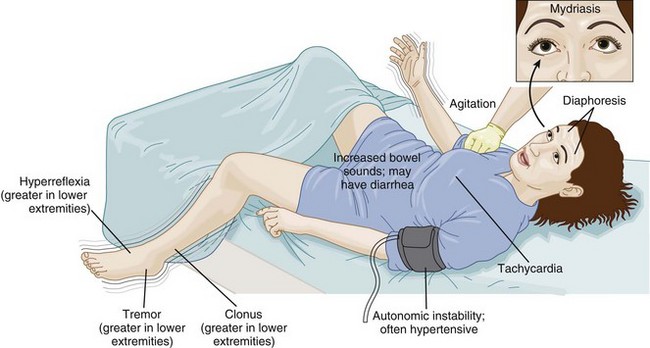
Overdose of SRIs causes CNS abnormalities (sedation, agitation, delirium), peripheral alterations (tremor, hyperreflexia, rigidity), cardiovascular changes (tachycardia, bradycardia), nausea or vomiting, and lightheadedness.9–11 The patient with citalopram or escitalopram overdose should be observed for seizures and QTc and/or QRS interval lengthening. Although isolated SRI ingestions frequently result in only mild toxicity, severe overdose or concomitant ingestion of proserotonergic medications can lead to serotonin excess and serotonin syndrome (Fig. 147.3). A history of ingestion of serotonergic agents, altered mental status, autonomic instability, and peripheral signs of rigidity or hyperreflexia are usually present.
Atypical Antipsychotics
The most significant extrapyramidal effect is neuroleptic malignant syndrome (NMS).12 NMS results when dopamine-blocking agents yield “dopamine-depleted” activity at D2 receptors in the CNS. Although NMS can occur after an intentional overdose, it usually arises after an increase in dose or after the addition of agents with similar activity (e.g., lithium inhibition of dopamine secretion). Manifestations of NMS include CNS abnormalities (sedation, agitation, delirium), peripheral alterations (tremor, hyperreflexia, rigidity), and cardiovascular changes with autonomic instability (tachycardia, bradycardia, hyperthermia) much like those seen in serotonin syndrome. Unlike serotonin syndrome, in which onset of symptoms is normally rather quick, NMS occurs insidiously. Historical information and medication lists are often required to differentiate between the two conditions (Table 147.2).
Table 147.2 Comparison of the Manifestations of Serotonin Syndrome and Neuroleptic Malignant Syndrome
| FEATURE | SEROTONIN SYNDROME | NEUROLEPTIC MALIGNANT SYNDROME |
|---|---|---|
| History | Drug(s) with serotonergic activity | Dopamine-blocking agents |
| Time of onset | Hours | Days |
| Mental status | Agitation to coma | Agitation to coma |
| Tone | Rigidity, greater in lower than in upper extremities | “Lead-pipe” rigidity |
| Vital signs | Hypertension, tachycardia, and hyperthermia | Hypertension, tachycardia, and hyperthermia |
Differential Diagnosis and Medical Decision Making
Any sedating agent (e.g., opioids, ethanol, benzodiazepines) should be considered in the differential diagnosis of most antidepressant and antipsychotic overdoses. Serotonin syndrome (e.g., SRIs, ecstasy, meperidine, lithium, dextromethorphan, L-tryptophan), neuroleptic malignant syndrome (e.g., antipsychotics such as phenothiazines), malignant hyperthermia (e.g., volatile anesthetic agent use), sympathomimetic overdrive (e.g., cocaine, amphetamines), and MAOI overdose or drug-food interaction should also be considered (Box 147.3).
Tricyclic Antidepressants
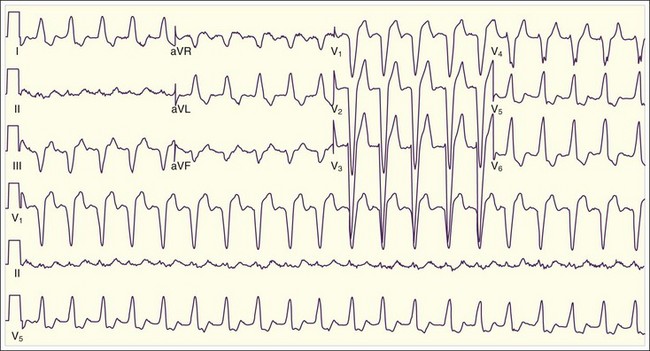
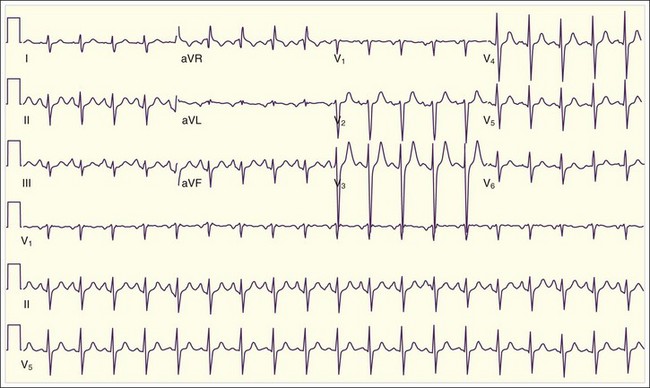
The differential diagnosis of TCA overdose should be broader and should include anticholinergic and antihistamine products (e.g., diphenhydramine) and agents that can poison fast sodium channels, thereby lengthening the QRS interval (e.g., type I antidysrhythmics, cocaine, diphenhydramine, propoxyphene, carbamazepine, cyclobenzaprine, phenothiazines). Life-threatening features are hyperthermia associated with mental status changes, autonomic instability, and tremors, clonus, and rigidity. Life-threatening toxicity should be anticipated in an adult who has ingested TCA doses of 10 mg/kg or greater. Qualitative urine screens for TCAs are of no diagnostic benefit. Although quantitative serum levels of TCAs greater than 1000 ng/mL (therapeutic, 50 to 300 ng/mL) have been correlated with severe toxicity, quantitative testing may not be available in a timely fashion. In addition, depending on the time from ingestion, the type of TCA taken, and the chronicity of dosing, patients may be very ill with serum levels much lower than 1000 ng/mL. An electrocardiogram (ECG) is the diagnostic test of choice.13 Normal ECG findings do not fully exclude TCA poisoning, but QRS prolongation greater than 120 msec should be a threshold for treatment (Fig. 147.4).14 Cardiac monitoring helps discern the severity of toxicity. Maximal limb-lead QRS interval duration is a sensitive indicator of illness.15 Generally, QRS intervals longer than 100 msec are associated with a 33% incidence of seizure activity, and QRS intervals longer than 160 msec are associated with a 50% incidence of ventricular dysrhythmia. One study showed that the sensitivity of a QRS interval longer than 100 msec can be matched by two other parameters: the terminal 40 msec of lead aVR measuring longer than 3 mm (R wave in aVR > 3 mm), and a ratio of R-wave to S-wave amplitude in the aVR lead greater than 0.714 (Fig. 147.5).
Serotonin Reuptake Inhibitors
The diagnosis of SRI toxicity is purely clinical. The criteria for serotonin syndrome are met through focused elicitation of historical information and the finding of signs and symptoms consistent with the disorder (Table 147.3).16
Table 147.3 Criteria to Determine Serotonin Syndrome and Toxicity
| Sternbach’s diagnostic criteria for serotonin syndrome |
Treatment
Tricyclic Antidepressants
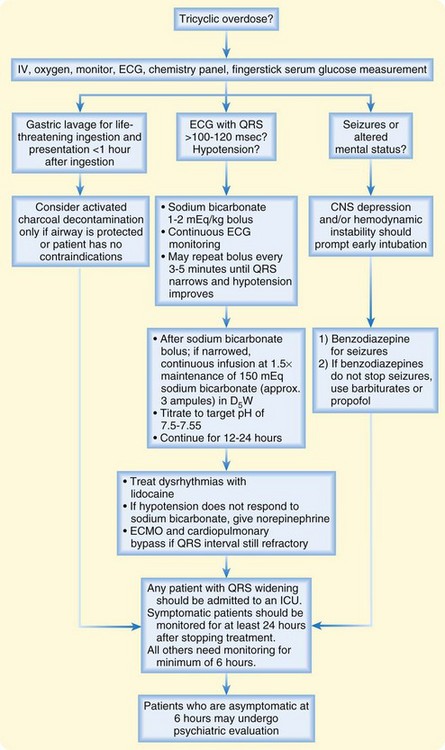
The treatment of TCA overdose depends on symptoms and is most effectively judged from the ECG (Fig. 147.6). Decontamination is best done early after the overdose. Gastric lavage can be considered after life-threatening ingestion and early presentation (<1 hour). The mainstay of decontamination is activated charcoal. The risks of each technique should be weighed against the potential benefits. Unruly behavior, seizure activity, decreased mental status, and loss of airway reflexes are poor predictors of success and thus raise the risk of aspiration.
Seizure activity should be managed with sedatives such as benzodiazepines and barbiturates. If muscle paralysis is necessary, continuous electroencephalographic techniques should be used to measure occult seizure activity. Lidocaine is an alternative to NaHCO3 therapy for dysrhythmias. Class IA, IC, and III antidysrhythmics, beta-antagonists, and calcium channel blockers are contraindicated in the patient with TCA overdose. Flumazenil and physostigmine are also poor treatment strategies because they have been reported to cause seizure activity4 and asystolic arrest,17 respectively.
Atypical Antipsychotics
Acute overdose of atypical antipsychotic agents is managed with supportive care because no specific antidote exists. These agents bind to activated charcoal, use of which should be the standard mode of decontamination. Correction of electrolyte (potassium, magnesium, and calcium) disturbances helps prevent widening or further lengthening of the QTc interval.13,18 The prophylactic use of magnesium to prevent a widened QTc interval from degenerating into torsades de pointes has no proven benefit when magnesium concentrations are normal. Treatment of NMS is for the most part identical to that of serotonin syndrome (aggressive cooling, benzodiazepines for agitation, fluids, and ventilatory support as warranted). Bromocriptine, an antihistamine with dopamine agonist activity, has been used without consistent benefit. Dantrolene, which works peripherally to inhibit release of calcium from the sarcoplasmic reticulum, has never been proven to be of benefit in NMS but should be considered for the patient with significant rigidity and life-threatening hyperthermia.19
Lithium
Enhancing elimination through hemodialysis is a controversial topic in the setting of lithium poisoning.16,20,21 The patient likely to benefit is the one with acute ingestion, mental status abnormalities, and/or significant renal dysfunction or pulmonary edema. Many patients experience lithium “redistribution” and an asymptomatic period after hemodialysis. This result often leads to further hemodialysis sessions, but whether this approach has an ultimate beneficial outcome remains controversial. The more common approach, barring any of the preceding abnormalities, is fluid hydration. Lithium’s clearance depends on the glomerular filtration rate, which, in turn, depends on volume status. Dehydrated patients continue to reabsorb, rather than eliminate, lithium because of its physical characteristics. Crystalloids given at two times maintenance doses should suffice. Forced diuresis or diuretic therapy has no role in this situation.
Table 147.4 summarizes treatment of overdoses involving antidepressant and antipsychotic agents other than TCAs.
Table 147.4 Treatment of Non–Tricyclic Antidepressant Overdoses
| DRUG | GASTROINTESTINAL DECONTAMINATION | TREATMENT |
|---|---|---|
| Lithium | Consider whole-bowel irrigation for sustained-release formulations (acute ingestions) |
For significant ingestions, lavage before charcoal
IV, intravenous.
Follow-Up, Next Steps in Care, and Patient Education
![]() Documentation
Documentation
Patient Instructions
• Document the discussion with the patient regarding diagnosis, warning signs, what to do, follow-up, and when to return.
• With pediatric accidental ingestions, document poison prevention counseling and assessment of the home situation.
• The regional poison control center can be contacted by telephone at 1-800-222-1222. All poison emergencies should be reported by the patient to the local poison control center.
• A dose of medication other than what was prescribed by the patient’s physician should never be taken or given unless it has been approved by the ordering physician.
Tips and Tricks
• Although normal electrocardiographic findings do not fully exclude tricyclic antidepressant (TCA) poisoning, QRS prolongation to more than 100 to 120 msec should be a threshold for treatment.
• Generally, QRS duration longer than 100 msec is associated with a 33% incidence of seizure activity, and a QRS greater than 160 msec is associated with a 50% incidence of ventricular dysrhythmia. Cardiac monitoring is a valid way of discerning the severity of toxicity in TCA poisoning.
• Devastating hemodynamic instability follows seizure activity in 13% of patients with TCA poisoning.
• Rightward deviation of the terminal 40-msec QRS axis (R wave in aVR > 3 mm) should be a cause for concern in a patient with TCA overdose.
• Significant, late monoamine oxidase inhibitor poisoning results in asystole from depletion of catecholamines, so affected patients should be monitored for 24 hours.
• Atypical antipsychotic toxicity can be associated with sedation or coma and miosis and therefore can be confused with opiate intoxication.
• Lithium toxicity is often precipitated by dehydration or worsening renal function.
• A serum lithium value obtained less than 6 hours after the last dose may result in excessively elevated concentrations (therapeutic, 0.6-1.2 ng/mL).
Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;17:1112–1120.
Burns MJ. The pharmacology and toxicology of atypical antipsychotic agents. J Toxicol Clin Toxicol. 2001;39:1–14.
Ellison DW, Pentel PR. Clinical features and consequences of seizures due to cyclic antidepressant overdoses. Am J Emerg Med. 1989;7:5–10.
McDaniel K. Clinical pharmacology of monoamine oxidase inhibitors. Clin Neuropharmacol. 1986;9:207–234.
Rusyniak DE, Sprague JE. Toxin-induced hyperthermic syndromes. Med Clin North Am. 2005;89:1277–1296.
1 Bryant SM, Kolodchak J. Serotonin syndrome resulting from an herbal detox cocktail. Am J Emerg Med. 2004;22:625–626.
2 Burns MJ. The pharmacology and toxicology of atypical antipsychotic agents. J Toxicol Clin Toxicol. 2001;39:1–14.
3 Bryant SM, Mycyk MB. Human exposure to pet prescription medication. Vet Hum Toxicol. 2002;44:218–219.
4 Ellison DW, Pentel PR. Clinical features and consequences of seizures due to cyclic antidepressant overdoses. Am J Emerg Med. 1989;7:5–10.
5 Shannon M, Merola J, Lovejoy FH. Hypotension in severe tricyclic antidepressant overdose. Am J Emerg Med. 1988;6:439–442.
6 Callaham M, Kassel D. Epidemiology of fatal tricyclic antidepressant ingestion: implications for management. Ann Emerg Med. 1985;14:1–9.
7 McDaniel K. Clinical pharmacology of monoamine oxidase inhibitors. Clin Neuropharmacol. 1986;9:207–234.
8 Shulman K, Walker S, MacKenzie S, et al. Dietary restrictions, tyramine, and the use of monoamine oxidase inhibitors. J Clin Pharmacol. 1989;9:397-402.
9 Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705–713.
10 Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635–642.
11 Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;17:1112–1120.
12 Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77:185–202.
13 Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation, torsades de pointes and sudden death. Drugs. 2002;62:1649–1671.
14 Liebelt EL, Francis PD, Wolf AD. ECG lead aVR versus QRS interval in predicting seizures and arrhythmias in acute tricyclic antidepressant toxicity. Ann Emerg Med. 1995;26:195–201.
15 Boehnert M, Lovejoy FH. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313:474–479.
16 Jaeger A, Sauder P, Kopferschmitt J, et al. When should dialysis be performed in lithium poisoning? A kinetic study in 14 cases of lithium poisoning. Clin Toxicol. 1993;31:429–447.
17 Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9:588–590.
18 Bryant SM, Zilberstein J, Cumpston KL, et al. A case series of ziprasidone overdoses. Vet Hum Toxicol. 2003;45:81–82.
19 Rusyniak DE, Sprague JE. Toxin-induced hyperthermic syndromes. Med Clin North Am. 2005;89:1277–1296.
20 Eyer F, Pfab R, Felgenhauer N, et al. Lithium poisoning: pharmacokinetics and clearance during different therapeutic measures. J Clin Psychopharmacol. 2006;26:325–330.
21 Bailey B, McGuigan M. Comparison of patients hemodialyzed for lithium poisoning and those for whom dialysis was recommended by PCC but not done: what lessons can we learn? Clin Nephrol. 2000;4:388–392.