Chapter 6 Antibodies and Biological Products
| Abbreviations | |
|---|---|
| APC | Antigen presenting cell |
| CNS | Central nervous system |
| CSF | Colony-stimulating factor |
| CTL | Cytolytic T lymphocyte |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IL | Interleukin |
| IM | Intramuscular |
| IV | Intravenous |
| M-CSF | Macrophage colony-stimulating factor |
| MHC | Major histocompatibility complex |
| MS | Multiple sclerosis |
| NFAT | Nuclear factor for activated T cells |
| SC | Subcutaneous |
| Th | T-helper (cells) |
| TNF | Tumor necrosis factor |
Therapeutic Overview
compounds such as monoclonal antibodies against cytokines, receptors, and specific immune cell antigens, as well as recombinant cytokines and cytokine receptor antagonists have provided greater selectivity and less toxicity. These drugs have made important contributions to the treatment of autoimmune diseases such multiple sclerosis (MS) and rheumatoid arthritis.
The benefits of these agents are shown in the Therapeutic Overview Box.
| Therapeutic Overview |
|---|
| Immunosuppressive/anti-inflammatory drugs |
| Prevent or modulate immune-mediated organ/tissue transplantation rejection |
| Inhibit initiation and/or progression of autoimmune diseases |
| Immunostimulatory drugs |
| Enhance immune responses against infectious disease (viral, bacterial, fungal) |
| Enhance immune responses against neoplastic cells |
| Stimulate development of immunocompetent cells from bone marrow |
Mechanisms of Action
The role of the immune system is to recognize and remove invading microorganisms and tumor cells, while ignoring host cells, through innate and acquired immune responses. When exposed to an invading organism, nonspecific innate immunity, comprising the first line of defense, is immediately called into action. The antigen-specific responses of acquired immunity also develop in tandem to more effectively remove the organism. When re-exposed to the invading organism, antigen-specific cells of the acquired immune response are called back into action for a more rapid and effective response. This memory and the ability to specifically recognize antigens differentiates acquired from innate immunity. Key differences between innate and acquired immune responses are listed in Table 6-1.
TABLE 6–1 Characteristics of Innate and Acquired Immune Responses
| Characteristic | Innate Immunity | Acquired Immunity |
|---|---|---|
| Onset of action | Immediate | Days to weeks |
| Mechanism of antigen recognition | Receptors that recognize common molecules on microbes and viruses | Unique antigen specific receptors (T-cell receptor, B-cell receptor) |
| Cell types/factors | Macrophages, neutrophils, mast cells, natural killer cells, complement, interferon | Antigen presenting cells, T lymphocytes, B lymphocytes |
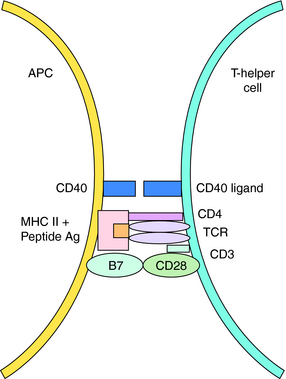
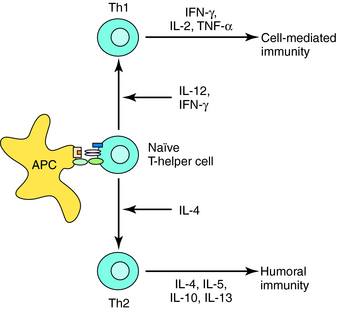
Acquired immunity is commonly divided into humoral and cell-mediated responses. Initiation of acquired immunity initially involves antigen-specific activation of naive T cells (CD4+, T-helper cells). This requires participation of antigen presenting cells (APC) (dendritic cells, macrophages, and B cells) that take up and process antigens into peptide fragments. Peptides bind to major histocompatibility complex (MHC) Class II molecules within the APC and are presented to CD4+ T cells that possess a T-cell receptor specific for the peptide-MHC complex. The naïve T cell requires two signals to be fully activated. The first is provided by the peptide binding to the T-cell receptor, and the second from interaction of costimulatory molecules of the APC and the T cell (Fig. 6-1). Signal One in the absence of Signal Two leads to tolerance, or functional silencing of the T cell. Fully activated T cells proliferate and differentiate into effector T-helper (Th) cells that produce cytokines, such as ILs. In general, there are two types of effector Th cells: Th1 and Th2 cells. The type of cytokines produced by each Th cell determines its function. Cytokines produced by Th1 cells (IFN-γ, IL- 2, tumor necrosis factor [TNF]-α) stimulate generation of cell-mediated immune responses (Fig. 6-2), whereas Th2 cells produce cytokines (IL-4, IL-5, IL-10, IL-13) that drive formation of an antibody response (humoral immunity).
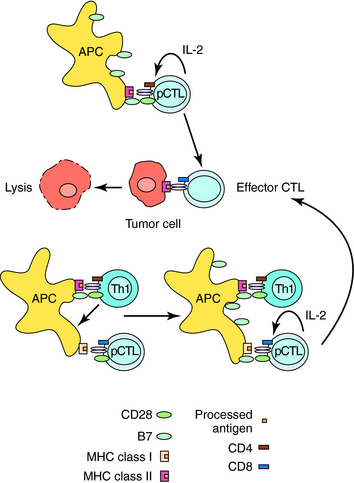
CTLs mediate antigen-specific lysis of tumor cells, virally infected cells, and graft/transplant cells. Generation of CTLs for all three functions generally involves similar mechanisms (Fig. 6-3). Naïve, precursor CTLs (pCTLs) require activation by two signals, as described for Th cells. The first is delivered by binding of peptide antigens associated with MHC Class I molecules on APCs to the T-cell receptor on CD8+ pCTLs. The second is provided by receptor-ligand interaction of costimulatory molecules. Th1 cells produce cytokines that stimulate dendritic cells to up regulate a costimulatory molecule that will activate antigen-stimulated CD8+ cells. Activated CTLs produce IL-2, which stimulates its own proliferation and differentiation. In certain situations, APCs that contain high levels of costimulatory molecules are able to activate CD8+ CTLs without the help of Th1 cells. Antigen recognition and binding of activated CTLs to antigen on cells result in cell lysis.
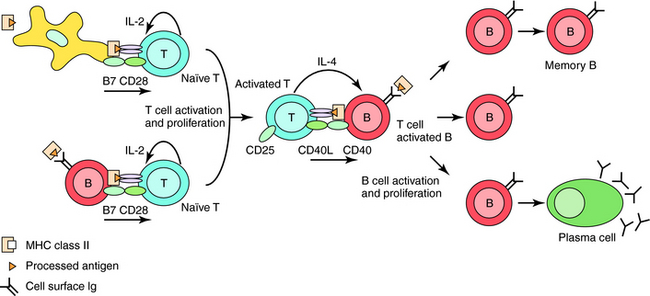
Th2 cells secrete cytokines that stimulate proliferation and differentiation of B cells to antibody-secreting plasma cells or to long-lived memory cells (Fig. 6-4). Specific antibodies can remove harmful foreign antigens (e.g., bacterial toxins) by binding to and neutralizing their effects. Antigen-antibody immune complexes can activate complement to elicit a local inflammatory reaction for further antigen removal by phagocytes. Once bound to foreign protein or bacteria, the Fc region of antibodies can bind to receptors on phagocytic cells, leading to internalization of the invading pathogens.
Pharmacological Immunosuppression
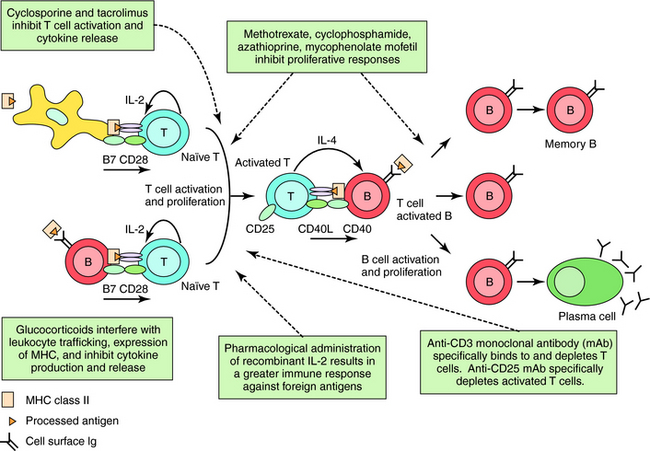
Pharmacological approaches to immunosuppressive therapy may involve selective eradication of immunocompetent cells, similar to the selective killing of tumor cells by antineoplastic drugs (see Chapter 54), or down regulation of the immune response without deleting the target cell. In both cases the goal is to balance the activity and selectivity of the drug to optimize clinical efficacy while preventing adverse effects. The principal drugs used currently to obtain immunosuppression include glucocorticoids, antiproliferative/antimetabolite agents, calcineurin inhibitors, and biologicals. Most of these compounds are highly effective in inhibiting the immune response. However, their usefulness is limited by their severe toxicities. Therefore the different drugs are used in combination at lower doses to obtain a synergistic effect on immune responses while minimizing adverse effects. Immunosuppressive drugs are used primarily to prevent transplant rejection and treat autoimmune diseases.
The actions of the corticosteroids are discussed in Chapter 39. Glucocorticoids are effective in treating autoimmune diseases and preventing graft rejection because of their immunosuppressive and anti-inflammatory effects. When glucocorticoids are administered, the numbers of circulating lymphocytes, basophils, and eosinophils decrease over a 24-hour period, whereas the number of neutrophils increases. In addition, changes in glucocorticoid concentrations during the normal diurnal cycle and in stressful situations correlate with decreases in circulating lymphocytes. Lymphopenia is attributed to migration of cells into extravascular spaces, with more T cells than B cells or monocytes migrating. Most cells migrate to bone marrow. High-dose glucocorticoid therapy is also known to reduce the size of lymphoid organs.
Antiproliferative/Antimetabolite Agents
The structure of cyclophosphamide, its activation to phosphoramide mustard and acrolein, and its antitumor actions are discussed in Chapter 54. The ways in which the active metabolites phosphoramide mustard and acrolein alter the immune response are unclear. The mustard is believed to alkylate DNA and mediate the antiproliferative and immunosuppressive effects. This is consistent with the hypothesis that selective cytotoxic effects on B cells are attributable to a greater proliferation rate. However, the highly reactive, sulfhydryl-binding acrolein may also play an important role in the drug’s action.
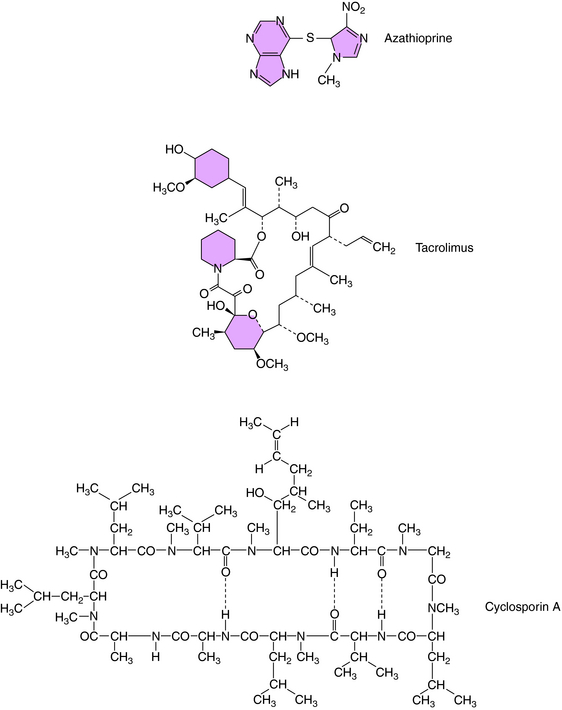
The structure of azathioprine is shown in Figure 6-5. This drug is metabolized to the antiproliferative drug 6-mercaptopurine (see Chapter 54), which is further metabolized to the active antitumor and immunosuppressive thioinosinic acid inhibiting hypoxanthine-guanine phosphoribosyltransferase, which catalyzes the conversion of purines to the corresponding phosphoribosyl-5′ phosphates and the conversion of hypoxanthine to inosinic acid. This leads to the inhibition of cellular proliferation. The immunosuppressive effects of azathioprine stem from its antiproliferative actions.
Methotrexate was originally developed as an anticancer drug (see Chapter 54) but is now being used widely at lower doses in several inflammatory diseases, including rheumatoid arthritis. The immunological and antitumor mechanisms are similar. An antimetabolite, methotrexate binds and inactivates dihydrofolate reductase, leading to inhibition of the synthesis of thymidylate, inosinic acid, and other purine metabolites. Methotrexate also stimulates the release of adenosine, which inhibits stimulated neutrophil function and has potent anti-inflammatory properties.
Calcineurin Inhibitors/Immunophilin Binding Agents
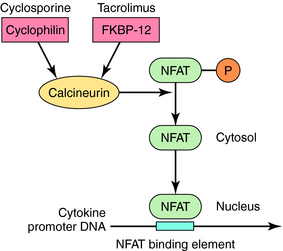
Calcineurin inhibitors down regulate immune responses by inhibiting the production of IL-2 in activated T cells. IL-2 is a key driver of many immune responses and especially important in mediating organ transplant rejection. The two calcineurin inhibitors cyclosporine and tacrolimus bind to cyclophilin and FK binding protein, respectively, and the drug-immunophilin complex binds to calcineurin. This leads to dephosphorylation of nuclear factor for activated T cells (NFAT) and prevention of its translocation to the nucleus, causing down regulation of cytokine transcription (Fig. 6-6).
Cyclosporine is a cyclic endecapeptide purified from fungi (see Fig. 6-5). It primarily affects T-cell–mediated responses, whereas most humoral immune responses not requiring T cells are spared. The effectiveness of cyclosporine stems from its selective inhibition of Th cell activation. Its major effect on Th cells is inhibition of cytokine production. Decreased IL-2 production in turn leads to a decrease in IL-2 receptors and in a lack of responsiveness of CTL precursor cells. Because there is positive feedback through IL-2 production and IL-2 receptors, the decreased IL-2 production of Th cells also leads to decreased IL-2 receptors. Cyclosporine does not, however, affect the proliferative response of activated CTLs to IL-2 or the lytic activity of CTLs. Consistent with this is the observation that cyclosporine is effective only during the very early stages of antigen activation of Th cells. There is also evidence for inhibition of macrophage antigen presentation and IL-1 production by macrophages.
The structure of tacrolimus (formerly known as FK506) is shown in Figure 6-5. Its mechanism of action is similar to that of cyclosporine (see Fig. 6-6) in inhibiting cytokine synthesis. Tacrolimus also binds to a cytosolic receptor known as FK506-binding protein, which is also a peptidylpropyl cis-trans isomerase but is distinct from cyclophilin. Like cyclosporine, the tacrolimus-FK506 binding protein complex also binds and inhibits calcineurin. The major effects of these immunosuppressive actions are summarized in Figure 6-7.
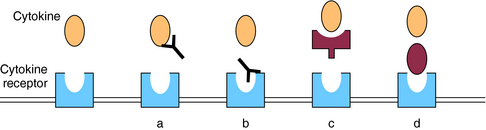
Advances in biotechnology and understanding the mechanisms underlying immunologic diseases have resulted in many novel biological agents. “Biologicals” refers to a diverse group of naturally occurring compounds that are manufactured primarily by advanced biotechnology techniques and includes recombinant vaccines, blood products, cytokines, growth factors, and monoclonal antibodies. Antibodies and cytokine antagonists designed to bind to soluble proteins and surface receptors that mediate inflammatory processes and drive the immune response have demonstrated clinical efficacy in a variety of neoplastic and immune disorders. Monoclonal antibodies exert their effects, depicted in Figure 6-8, through one or more of the following mechanisms:
Biologicals Targeting Lymphocytes
Anti-thymocyte globulin is purified IgG obtained from rabbits immunized with human thymocytes. Intravenous (IV) administration of these polyclonal antibodies leads to rapid and profound depletion of peripheral lymphocytes and is used to prevent graft rejection. Severe side effects limit the use of these antibodies to second-line status behind calcineurin inhibitors. In addition, because of the nature of production, there is poor standardization between batches. Monoclonal antibodies, on the other hand, are well standardized. OKT3 (muromonab) is a monoclonal antibody directed against the CD3 molecule on the membrane of T cells. CD3 is associated with the T-cell antigen receptor complex involved in signal transduction to the T cell after binding of antigen (see Fig. 6-1). Muromonab is approved for treatment of acute rejection of renal transplants, but it has severe side effects and is generally reserved for patients resistant to first-line therapies.
Biologicals Targeting Cytokines
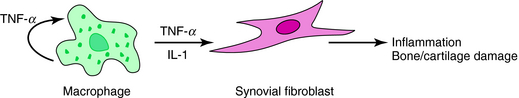
TNFα and IL-1β are pro-inflammatory cytokines implicated in the pathogenesis of inflammatory disorders such as rheumatoid arthritis (Fig. 6-9) and Crohn’s disease (see Chapter 18). They are involved in activation and proliferation of synovial cells, inducing the production of collagenases and other cytokines that lead to continued inflammation and bone resorption. Infliximab and adalimumab are antibodies that bind to soluble TNF-α and lower its level in blood. The former is a murine/human chimeric antibody, and the latter is a recombinant humanized monoclonal antibody. Etanercept is a dimeric fusion protein combining the p75 TNF receptor with the Fc portion of human IgG1.
IL-2 receptor antagonists prevent IL-2 from binding to activated T lymphocytes. Unlike muromonab, which targets both resting and activated lymphocytes, IL-2 receptor antagonists target actively dividing cells by binding to the α chain of the trimolecular IL-2 receptor (CD25), which is transiently expressed only on antigen-activated T cells. There are two currently available monoclonal antibodies directed against IL-2 receptor α, basiliximab and daclizumab. Early clinical studies demonstrate efficacy in combination with calcineurin inhibitors. Although long-term data are lacking, these antibodies appear to be well tolerated.
Biologicals Targeting Hypersensitivity Mediators
Allergen binding and cross-linking of specific-IgE bound to mast cells leads to mast cell degranulation and release of various mediators (histamine, leukotrienes) involved in asthma (see Chapter 16). A monoclonal antibody specific for IgE (omalizumab) is approved for allergic asthma. Omalizumab inhibits binding of IgE to the IgE Fc receptor on mast cells, preventing antigen-induced mediator release.
Pharmacological Immunostimulation
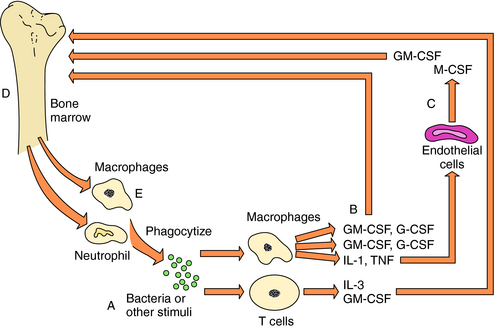
Colony-stimulating factors (CSFs) comprise a group of cytokines named for their ability to induce formation of certain types of colonies from bone marrow cells in soft agar cultures. They affect bone marrow cells at different stages of maturity. Multi-CSF (IL-3) stimulates the primitive progenitor cells that give rise to granulocytes, megakaryocytes, mast cells, macrophages, and erythrocytes. In contrast, more-differentiated progenitor cells are stimulated by G-CSF and M-CSF to proliferate and differentiate into granulocytes and macrophages, respectively. Both of these cell lineages are stimulated by GM-CSF. As cells of certain lineages mature from progenitors to more committed states, however, they become refractory to certain CSFs and sensitive to others. With exposure to a pathogen (e.g., bacteria, virus-infected cells), T cells activate and produce IL-3 and GM-CSF, whereas activated macrophages produce M-CSF, G-CSF, and GM-CSF. Activated macrophages also produce IL-1 and TNF, which stimulate production of GM-CSF, G-CSF, and M-CSF by endothelial and mesenchymal cells. In this manner the host produces more granulocytes and macrophages to combat the invading organism (Fig. 6-10).
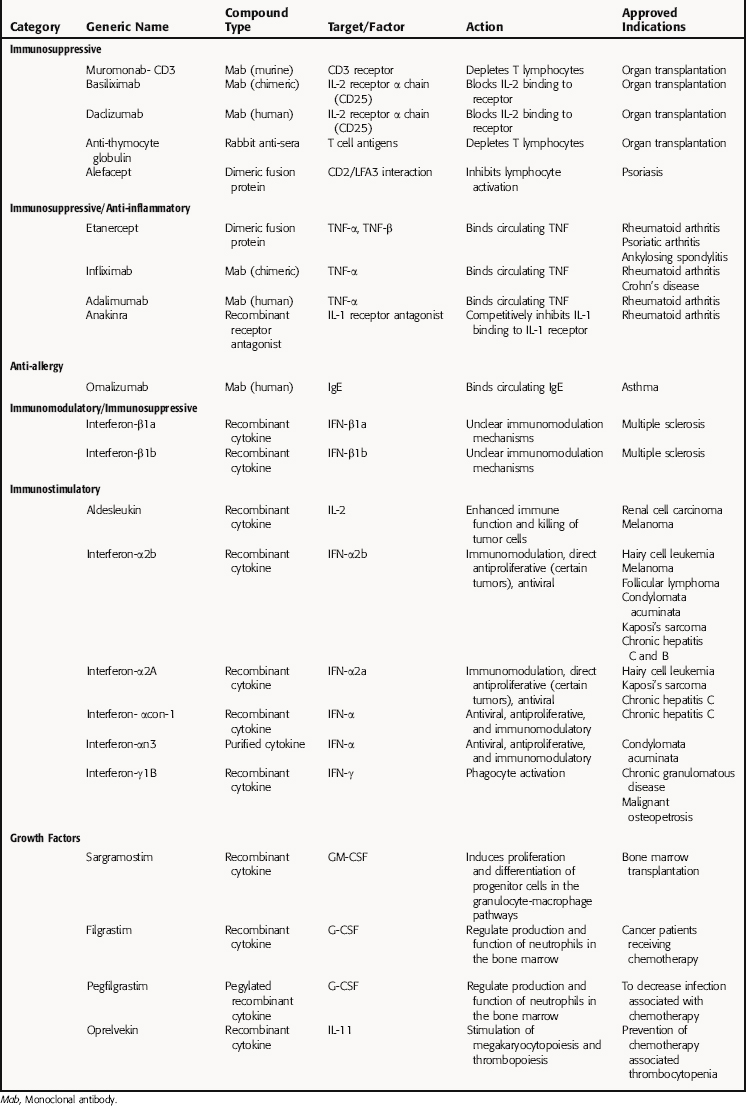
A summary of selected biologicals and their targets, actions, and indications are listed in Table 6-2.
Pharmacokinetics
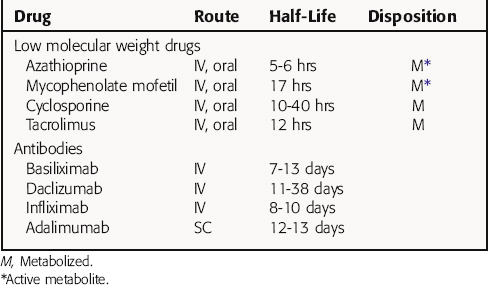
Many immunopharmacological agents have relatively narrow therapeutic indexes and are often used in combination. Therefore the combined toxicity and the effect of drug-drug interactions must be considered in choosing a safe and effective dosing regimen. In addition, biologicals have unique pharmacokinetics that greatly affect their use. Pharmacokinetic parameters for selected drugs are shown in Table 6-3. Because of their unique properties, little information is available about many biologicals.
Glucocorticoids are discussed in Chapter 39, while cyclophosphamide and methotrexate are discussed in Chapter 54. Methotrexate is used at low oral doses to treat chronic autoimmune diseases. Azathioprine is usually given IV as a loading dose on the day of transplantation, with subsequent oral maintenance doses. It is rapidly absorbed and converted to 6-mercaptopurine, which is the active drug. Most metabolites are excreted in urine. One pathway of metabolism of 6-mercaptopurine involves oxidation by xanthine oxidase, which is inhibited by allopurinol. Therefore coadministration of these drugs requires a dose adjustment.
Mycophenolate mofetil is rapidly metabolized to the active metabolite, mycophenolic acid. The mofetil moiety dramatically increases bioavailability. Mycophenolic acid is bound appreciably to serum albumin and is primarily excreted in the kidney as the glucuronide conjugate.
Cyclosporine is poorly absorbed from the small intestine (bioavailability of ~30%) and is dependent on biliary flow for absorption. It is metabolized by cytochrome P4503A, which may cause drug interactions (see Chapter 2). A major constraint in dosing is nephrotoxicity, and as a result of high variability in absorption and metabolism, levels fluctuate. Tacrolimus is 10 to 100 times more potent than cyclosporine and does not rely on bile for absorption. It is also metabolized by cytochrome P4503A, and nephrotoxicity occurs with similar frequencies as with cyclosporine. Blood concentrations for these drugs are monitored to optimize their effects.
To increase the t1/2 of biologicals, polyethylene glycol is added. Conjugation with polyethylene glycol increases the size and modifies the overall charge to decrease glomerular filtration. In addition, it is thought to decrease immunogenicity by masking antigenic sites. IFN-α2b (peginterferon α2b) and G-CSF (pegfilgrastim) have been conjugated to enhance pharmacokinetic properties. For example, the serum t1/2 of IFN-α2b is increased from 7 to 9 hours to 40 hours after conjugation with polyethylene glycol.
Relationship of Mechanisms of Action to Clinical Response
Many synthetic glucocorticoid derivatives (see Chapter 39) are used as immunosuppressive agents. Glucocorticoids are often administered at high doses during acute exacerbations of disease for rapid control, followed by a slow tapering and maintenance on the lowest efficacious dose to minimize toxicity. Glucocorticoids are usually administered with other immunosuppressive agents to treat graft rejection and autoimmune diseases. Many of these combinations result in a synergistic effect. This allows doses of glucocorticoids to be decreased, decreasing the risk of toxicity. Because of their anti-inflammatory actions, glucocorticoids are effective in treatment of immunological problems exacerbated by inflammatory reactions. This is especially evident in the topical use of these agents for treatment of dermatological problems such as contact hypersensitivity to poison ivy and atopic dermatitis and in treatment of asthma (see Chapter 16).
Azathioprine is approved for prevention of acute rejection of kidney transplants. Azathioprine is ineffective alone in solid organ transplantation and is commonly used with glucocorticoids and cyclosporine in triple-combination therapy. It is also often used in treatment of moderate to severe manifestations of systemic lupus erythematosus and is indicated for rheumatoid arthritis, although not as a first-line treatment. The primary targets of azathioprine are cell-mediated immune responses. Inhibition of the in vitro immune response is maximal during initiation of the response. This time-dependent action is consistent with clinical observations that azathioprine is ineffective against ongoing graft rejection. Additional in vitro investigations have revealed that azathioprine primarily affects antigen-stimulated lymphocytes, whereas unstimulated spleen cells are unaffected. Primary immune responses are suppressed by azathioprine, whereas secondary responses are not.
Although methotrexate is a potent immunosuppressive agent, its numerous adverse effects (see Chapter 54) have limited its widespread use in treatment of immune-associated diseases. However, low-dose, weekly administered oral methotrexate is used widely to treat rheumatoid arthritis. It is also used for psoriasis and to reduce required doses of glucocorticoids in chronic vasculitis or conditions that require prolonged periods of immunosuppression.
Recombinant human IFN-α (2a or 2b) is marketed for treatment of hepatitis C, hairy cell leukemia, and AIDS-related Kaposi’s sarcoma. IFN-α2b is also indicated for chronic hepatitis B, malignant melanoma, follicular lymphoma, and condylomata acuminata (genital warts associated with human papilloma virus). Two additional α IFNs are also marketed, IFN-αcon1 (for chronic hepatitis C) and -αn3 (for condylomata acuminata). The antitumor and antiviral effects are mediated through a direct effect on tumor and viral infected cells and through stimulation of immune responses. A polyethylene glycol conjugate of IFN-α2a (peginterferon α2a) was also developed to increase its t1/2.
Pharmacovigilance: Side Effects, Clinical Problems, and Toxicity
Side Effects Common to Immunosuppressive Therapy
The side effects of glucocorticoids are discussed in Chapter 39 and those of cyclophosphamide and methotrexate in Chapter 54.
Side Effects Associated with Biologicals
Local irritation/inflammation at the site of injection (IM or SC) is common for most biologicals.
Dessain SK, Adekar SP, Berry JD. Exploring the native human antibody repertoire to create antiviral therapeutics. Curr Top Microbiol Immunol. 2008;317:155-183.
Liu PM, Handl H, Zou L, Kim B. Immunobiological aspects of therapeutic antibodies and related characterization approaches. Curr Opin Drug Discov Devel. 2007;10(5):515-522.
Loertscher R. The utility of monoclonal antibody therapy in renal transplantation. Transplant Proceed. 2002;34:797-800.
López-Guillermo A, Mercadal S. The clinical use of antibodies in haematological malignancies. Ann Oncol. 2007;18(Suppl 9):51-57.