CHAPTER 368 Anterior Communicating Artery Aneurysms
Surgical Management of Anterior Communicating Artery and Proximal Anterior Cerebral Artery Aneurysms
Landmark clinical studies have revealed the anterior communicating artery (ACoA) region as the most common site for intracranial aneurysms. In the original Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage (1958 to 1965), 30.3% of 2349 aneurysms were located in the ACoA region.1 Also in this study, the incidence of anterior cerebral artery (ACA) aneurysms proximal to the ACoA junction (on the A1 segment) was 1.5%, and the incidence of aneurysms distal to the ACoA junction was 2.8%. Therefore, the total incidence of A1 segment–ACoA–distal ACA aneurysms in the original Cooperative Study was 34.6%. This incidence has remained fairly constant over the years. In the more recent International Cooperative Study on the Timing of Aneurysm Surgery (1980 to 1983), the incidence of ACoA-ACA aneurysms was 39%.2
Embryology of the Anterior Communicating Artery Region
Basic understanding of the embryologic development of the ACoA region allows comprehension of its most common congenital anomalies. Our current understanding of the developmental anatomy of the intracranial arteries is based on Hager Paget’s 1948 article on this subject.3 At 35 days (12- to 14-mm stage), the primitive anterior division of the internal carotid artery (ICA) develops a distinct distal branch that is the stem of the ACA. By 40 days (16- to 18-mm stage), the stem of the ACA elongates medially toward its counterpart. It is at this stage that a midline cluster of plexiform anastomoses begins to form between the adjacent and elongating ACAs. At 44 days (20- to 24-mm stage), the channels of the midline cluster of plexiform anastomoses coalesce and form one or more ACoAs. In addition, the coalescing channels of the midline cluster of plexiform anastomoses give rise to a median ACA that originates from the ACoA.
Given this description, we can predict the most common congenital anomalies of this region, which are (1) multiple or fenestrated ACoAs, (2) triplicate A2 segments, and (3) the azygous A2 segment. Perlmutter and Rhoton4 found two or three ACoAs in 30% and 10%, respectively, of their 50 cadaver brain dissections. In addition, they confirmed that absence of the ACoA is exceedingly rare.5 Hager Paget calculated that the ACoA is absent in only 0.2% of cases (3 of 1803).6 Persistence of the median artery of the corpus callosum creates three A2 segments. Baptista identified triplicate ACAs in 13.1% of his specimens (50 out of 381),7 but Perlmutter and Rhoton found triplicate ACAs in only 2% of their specimens (1 out of 50).5 An azygous or solitary A2 segment arises when the paired ACAs regress after formation and enlargement of the median artery of the corpus callosum.3 An azygous A2 has been identified in only 0.26% of general autopsies7 and in only 0.22% of unselected angiograms.8 The higher incidence of azygous A2 segments in aneurysm series results from the fact that 41.1% of azygous A2 segments have a terminal aneurysm.8
Microsurgical Anatomy of the A1 Segment–Anterior Communicating Artery–A2 Segment Region
The anatomy of this region is reviewed in detail elsewhere in this book. Therefore, here we discuss only a few definitions and anatomic details that will serve as a background for our subsequent surgical discussion. The ACA is divided into five anatomic segments, A1 to A5.4 The A1 segment starts at the ICA termination and ends at ACoA junction. The A2 segment starts at the ACoA junction, follows the course of the rostrum of the corpus callosum, and terminates at the junction of the rostrum and genu of the corpus callosum. It is commonly referred to as the pericallosal artery. The A3 segment follows the curve of the genu of the corpus callosum and terminates where the ACA turns posteriorly above the genu. The A4 and A5 segments run over the body of the corpus callosum; the transition from A4 to A5 is arbitrarily set at the level of the plane defined by the coronal suture.4
A1 Segment
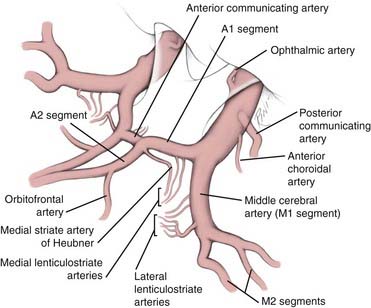
The average diameter of the A1 segment is 2.6 mm (range, 0.9 to 4 mm),5 about that of the middle cerebral artery (MCA) at the ICA bifurcation and about half that of the supraclinoid ICA at its origin (Fig. 368-1). Although absence of the A1 segment is extremely rare, hypoplasia of the A1 segment is recognized in about 10% of cases.5 Perlmutter and Rhoton chose a diameter of 1.5 mm as the threshold for labeling the A1 segment as hypoplastic, but it should be recognized that there is no agreed threshold below which an artery is labeled “hypoplastic.” They found that 10% of their specimens had A1 segments that were 1.5 mm or less in diameter and that 2% had A1 segments 1 mm or less in diameter.5 Another rare but surgically important anatomic variant is the duplication of the A1 segment (2%), which occurs only unilaterally.5
The paired A1 segments are of equal diameter in only half of cases. In 50% of cases, there is a difference of 0.5 mm or more between the diameters of the A1 segments. In 12% of cases, the difference is of 1 mm or more.5 This discrepancy in diameter between the paired A1 segments is even more prevalent when one considers cases with an ACoA aneurysm. In the presence of an ACoA aneurysm, the paired A1 segments are of unequal diameter in as many as 85% of cases.9 Typically, the base of the aneurysm arises on the side of the larger A1, and the dome points toward the side of the hypoplastic A1 segment.
Anterior Communicating Artery
The diameter of the ACoA is on average about half that of the A1 segment (see Fig. 368-1). The average diameter of the A1 segment is 2.6 mm, whereas that of the ACoA is 1.5 mm (range, 0.2 to 3.4 mm).5 There is a constant relationship between the diameter of the ACoA and the difference in the diameters of the A1 segments: the larger the difference between the A1 segments, the larger the ACoA.5 Because unequal A1 segments are typically found in the presence of an aneurysm and unequal A1 segments are typically associated with a larger ACoA, one can expect to always find a patent ACoA when exploring an aneurysm in this region, even when it is not demonstrated angiographically. The ACoA is rarely oriented in a strictly transverse plane, as depicted in most non-neurosurgical textbooks. At the level of the ACoA junction, the left ACA courses anterior to the right in 48% of cases, the right ACA courses anterior to the left in 34% of cases, and only in 18% of cases do they enter the interhemispheric fissure side by side.5 Because of the course of the ACAs, the ACoA is usually oriented in an oblique or sagittal plane. Absence of the ACoA is exceedingly rare (only 0.2% of cases).6
A2 Segment
Although most neurosurgeons refer to the A2 segment as the pericallosal artery and consider the callosomarginal artery a branch of the pericallosal artery, some use this term to define one of the two branches at the bifurcation of the distal A2 segment, the other branch being the callosomarginal artery. The main problem with this alternative nomenclature is that because the callosomarginal artery is absent in 18% of cases, it is difficult to define where the pericallosal artery starts.4 The callosomarginal artery originates most frequently (60% of cases) from the A3 segment, an average of 43 mm (range, 12 to 47 mm) from the ACoA junction, which places it beyond the junction of the rostrum and genu of the corpus callosum. It originates more proximally (from the A2 segment) in 10% of cases and more distally (from the A4 segment) in 12% of cases.4
Perforators of the A1 Segment, Anterior Communicating Artery, and A2 Segment
The A1 segment gives rise to an average of eight perforators (range, 2 to 15), 41% of which terminate in the anterior perforated substance.5 These perforators are sometimes identified as the medial lenticulostriate arteries to distinguish them from the lateral lenticulostriate arteries, which originate from the M1 segment and also terminate in the anterior perforated substance.10,11 Typically, the proximal half of A1 gives rise to twice as many perforators as the distal half. Another way of stating this is that of all the perforators of the A1 segment, two thirds arise from the proximal half and one third arise from the distal half. The proximal half gives rise to an average of 5.3 perforators (range, 1 to 11), whereas the distal half gives rise to an average of 2.5 perforators (range, 0 to 6).5 Most (86%) of the A1 perforators arise from the superior (54%) and posterior (32%) surfaces of this segment. Only rarely (14%) do they arise from the inferior (9%) and anterior (5%) surfaces of A1.5 Of all the A1 perforators, 41% terminate in the anterior perforated substance. These perforators branch into as many as 49 vessels as they reach the anterior perforated substance.12 Most enter the medial portion of the anterior perforated substance and typically course posterior to the branches of the medial striate artery of Heubner.12 The remaining A1 perforators terminate in the dorsal optic chiasm or suprachiasmatic hypothalamus (29%), inferior frontal lobe (10%), optic tract (11%), sylvian fissure (5%), dorsal optic nerve (2%), or interhemispheric fissure (2%).5
There have been conflicting anatomic reports concerning the number of ACoA perforators. Perlmutter and Rhoton,5 in their study of 50 brains, found that the ACoA may have no perforators or as many as four.5 By contrast, Dunker and Harris13 and Crowell and Morawetz14 examined 20 and 10 brains, respectively, and both concluded that the ACoA always has at least three perforators.
In a pattern similar to that seen on the A1 segment, most (90%) of the ACoA perforators arise from its superior (54%) and posterior (36%) surfaces, and only rarely (10%) from its anterior (7%) and inferior (3%) surfaces.5 Therefore, most ACoA perforators are hidden from the surgeon’s view. Of all the ACoA perforators, 51% terminate in the suprachiasmatic region, 21% terminate on the dorsal optic chiasm, and 15% reach the anterior perforated substance.5
Medial Striate Artery (Recurrent Artery of Heubner)
The most important perforator from the proximal A2 segment is the medial striate artery, better known as the recurrent artery of Heubner (see Fig. 368-1). We have reviewed elsewhere the anatomy of this vessel and the life and accomplishments of its discoverer, Johann Otto Leonhardt Heubner (1843-1926), a German pediatrician who described it in an article published in 1872.15 The medial striate artery of Heubner arises from the A2 segment in 78% of cases, from the A1 segment in 14% of cases, and at the level of the ACoA in 8% of cases.5 Perlmutter and Rhoton found that it originates within 4 mm of the ACoA junction (either proximal or distal to it) in 95% of cases.5 They also found this artery to be absent (only on one side) in 2% of cases and duplicated (also only on one side) in 2% of cases.5 By contrast, Gomes and colleagues found this artery to be absent in 3% of cases but duplicated in 12% of cases.16
The medial striate artery of Heubner courses anterior to the A1 segment in 60% of cases and superior to the A1 segment in 40% of cases.5 Therefore, this artery will be encountered before the A1 segment on initial retraction of the frontal lobe during surgery in most cases (60%). The length of the medial striate artery of Heubner is on average twice that of the A1 segment. Whereas the average length of the A1 segment is 12.7 mm,5 that of the medial striate artery is 23.4 mm (range, 12 to 38 mm).16 Its length therefore increases its exposure to injury during surgery.
The medial striate artery of Heubner should not be confused during surgery with the orbitofrontal artery, which is typically the second major branch of the A2 segment (see Fig. 368-1). The medial striate artery of Heubner is usually the first branch of the A2 segment immediately after the ACoA junction. It is also typically the largest vessel arising from the A2 segment. The orbitofrontal artery originates on average 5 mm (range, 0 to 15 mm) from the ACoA junction and has an average diameter of 0.9 mm (range, 0.4 to 2 mm).4 Based on diameter alone, the orbitofrontal artery can be mistaken for the medial striate artery of Heubner, which has an average diameter of 1 mm (range, 0.2 to 2.9 mm).5 Their courses, however, are very different. The medial striate artery of Heubner follows the course of the A1 segment, whereas the orbitofrontal artery courses perpendicularly over the gyrus rectus and across the olfactory tract11 (the subfrontal gyral and sulcal anatomy is depicted in Fig. 368-2). Another important anatomic distinction of the orbitofrontal artery is that it typically demarcates the boundary of the lamina terminalis cistern (where it originates) and the beginning of the callosal cistern.11
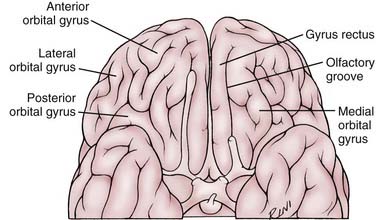
FIGURE 368-2 Anatomy of the subfrontal gyri and sulci in relation to the course of the olfactory nerve.
After the orbitofrontal artery, the third major branch of the A2 segment is the frontopolar artery. The frontopolar artery is a cortical branch that originates on average 14 mm (range, 2 to 30 mm) from the ACoA junction and has an average diameter of 1.3 mm (range, 0.6 to 1.8 mm).4 It courses anteriorly along the medial surface of the frontal lobe and crosses the subfrontal sulcus (see Fig. 368-2).4
The medial striate artery of Heubner supplies the anterior striatum (caudate nucleus and putamen), a portion of the outer segment of the globus pallidus, and the anterior limb of the internal capsule.5 Injury to this vessel typically results in a moderate paresis of the contralateral upper extremity and mild paresis of the contralateral face. It also causes dysfunction of the tongue and palate, which can only be documented during a careful swallowing evaluation. If the dominant hemisphere is involved, an expressive aphasia may be evident. In most patients, these deficits tend to resolve completely in a matter of months.
In addition to the medial striate artery of Heubner, the orbitofrontal artery, and the frontopolar artery, the proximal A2 segment gives rise to an average of 4.8 (range, 0 to 10) basal perforating branches. These branches supply the optic chiasm, anterior hypothalamus, medial portion of the anterior commissure, pillars of the fornix, and anterior-inferior portion of the striatum (caudate nucleus and putamen).4
Arachnoid Cisterns of the A1 Segment–Anterior Communicating Artery–A2 Segment Region
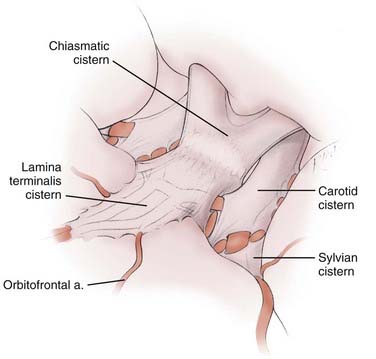
The sequential recognition and opening of three arachnoid cisterns (carotid, chiasmatic, and lamina terminalis) leads to the ACoA junction (Fig. 368-3). The A1 segment originates within the confines of the carotid cistern. It then courses within the lamina terminalis cistern over either the optic chiasm (70% of the time) or, less frequently, over the optic nerve (30% of the time).5 It enters into the interhemispheric fissure still within the confines of the lamina terminalis cistern.11 In addition to the origin of the A1 segment, the carotid cistern contains the supraclinoid ICA and the origins of its branches. The carotid cistern shares its medial wall with the chiasmatic cistern, which contains the optic nerves, optic chiasm, and infundibulum, but does not contain any major arteries. The lamina terminalis cistern is a midline structure that contains the paired A1 and proximal A2 segments, the ACoA, and the medial striate arteries of Heubner.11
Because the lamina terminalis cistern contains the A1-ACoA-A2 complex, ACoA aneurysms by definition arise within the confines of this cistern (see Fig. 368-3). A clear understanding of the boundaries of this cistern is therefore important microsurgically. Inferiorly, the lamina terminalis cistern stretches over the surface of the optic chiasm, where it apposes the chiasmatic cistern. Posteriorly, it is bounded by the lamina terminalis. Superiorly, it stretches into the interhemispheric fissure, where it is bounded by the rostrum of the corpus callosum. Its anterior surface stretches free over the A1-ACoA-A2 complex. Laterally, the lamina terminalis cistern surrounds the entire A1 segment after it emerges from the carotid cistern. Yaşargil points out that the lateral boundary of the lamina terminalis cistern is a thickened band of arachnoid fibers that stretch between the area immediately medial to the olfactory nerve and the optic nerve. The A1 segment enters the lamina terminalis cistern below this thickened band of arachnoid fibers. The medial striate artery of Heubner and the orbitofrontal artery originate within the confines of the lamina terminalis cistern.11
For the purpose of the arachnoid dissection during the approach to an ACoA aneurysm, it is important to remember that the optic nerves and chiasm are found in a separate cistern, namely the chiasmatic cistern. This means that if the aneurysm dome is oriented superiorly or posteriorly, one can dissect along the anterior edge of the optic chiasm and the optic nerves without entering the lamina terminalis cistern and potentially disturbing the aneurysm (Fig. 368-4).
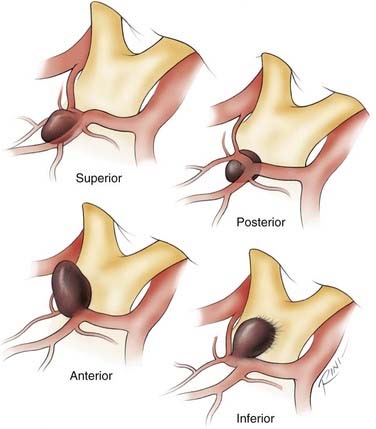
In the sagittal plane, ACoA aneurysms may project superiorly, anteriorly, inferiorly, or posteriorly. Although most ACoA aneurysms do not fall neatly into one of these four categories, the recognition of the predominant sagittal projection of these aneurysms is useful in anticipating the complications associated with each projection. This helpful classification scheme for ACoA aneurysms was formulated by Yaşargil.40 It should be noted that our anterior/posterior and superior/inferior nomenclature refers to the standard anatomic orientation, not the surgical orientation of the lesion, as originally described by Yaşargil.
Most ACoA aneurysms (71.2%) project into the interhemispheric fissure, and only a minority (12.8%) project inferiorly into the chiasmatic cistern (16% of all ACoA aneurysms have complex, multilobulated projections).40 Whereas superior- and posterior-pointing aneurysms are found entirely within the interhemispheric fissure, anterior-pointing aneurysms are typically found only partially inside the interhemispheric fissure. Inferior-pointing aneurysms, the most treacherous of ACoA aneurysms if ruptured, are almost entirely outside the interhemispheric fissure and are typically adherent to the optic chiasm, the optic nerves, or the dura of the interoptic space. In terms of the initial subfrontal exposure of ACoA aneurysms, superior- and posterior-pointing aneurysms are the most favorable because they rarely rupture at this stage. By contrast, inferior-pointing aneurysms are the worst in this respect because elevation of the frontal lobes may avulse the dome from the optic chiasm, optic nerves, or the dura of the interoptic space early in the subarachnoid dissection.
History of Surgical Approaches for ACoA Aneurysms
The history of surgical treatment of aneurysms is reviewed in detail elsewhere in this book. Table 368-1 provides a chronology of the landmark surgical contributions pertinent to microsurgery of ACoA aneurysms. The modern frontosphenotemporal or “pterional” craniotomy as described by Yaşargil and Fox17,18 is the culmination of a 40-year neurosurgical refinement of a safe and efficient craniotomy to approach ACoA aneurysms. It is based on Dandy’s lateral subfrontal approach through a frontotemporal craniotomy,19–21 it incorporates elements of Kempe’s sphenoid wing removal,22 and it acknowledges the need to resect a portion of the frontal lobe as advocated by Norlén and Barnum,23 but only in a limited fashion (gyrus rectus resection) as advocated by Kempe and VanderArk.22,24 The pterional craniotomy is currently favored by most neurosurgeons around the world. A few neurosurgeons, however, still advocate a frontal interhemispheric approach as originally advocated by Tönnis25 and Pool26,27 through a frontal parasagittal craniotomy for some ACoA aneurysms.28–30
TABLE 368-1 Chronology of Landmark Surgical Contributions Pertinent to Microsurgery of Anterior Communicating Artery Aneurysms
| DATE | SURGEONS | CONTRIBUTION |
|---|---|---|
| 1918 | George J. Heuer | First description of frontotemporal craniotomy for lateral subfrontal approach to the circle of Willis19 |
| Walter E. Dandy | ||
| 1931 | Norman M. Dott | First direct surgical attack of an aneurysm (internal carotid artery bifurcation)60 |
| 1935 | W. Tönnis | Anterior interhemispheric approach first used for anterior communicating artery aneurysms25 |
| 1937 | Walter E. Dandy | First clipping of an aneurysm (posterior communicating artery)20 |
| 1941 | Walter E. Dandy | Frontotemporal approach first used for anterior communicating artery aneurysms21 |
| 1953 | Gösta Norlén | Transfrontal approach (partial frontal lobectomy) first used for anterior communicating artery aneurysms23 |
| Alec S. Barnum | ||
| 1956 | Valentine Logue | Proximal ligation of A1 segment44 |
| 1961 | Lawrence Pool | Bilateral anterior subfrontal/interhemispheric approach for large series of anterior communicating artery aneurysms26,27 |
| 1962 | Lyle French | Transfrontal approach (partial frontal lobectomy) for large series of anterior communicating artery aneurysms49 |
| 1968 | Ludwig Kempe | Sphenoid extension of frontotemporal craniotomy and gyrus rectus resection22 |
| 1971 | Ludwig Kempe, G. D. VanderArk | Gyrus rectus approach first used for anterior communicating artery aneurysms24 |
| 1975 | M. G. Yaşargil, John L. Fox | Frontolateral, spheno-orbital, or pterional craniotomy for aneurysms17,18 |
Clinical and Radiographic Presentation of Anterior Communicating Artery Aneurysms
The clinical presentation of aneurysmal subarachnoid hemorrhage (SAH) is reviewed in another chapter and elsewhere.31 Because the clinical presentation of ruptured ACoA aneurysms is in general not different from that of aneurysms in other locations, we will not discuss this issue in this chapter.
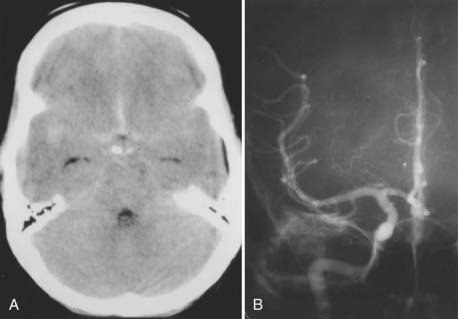
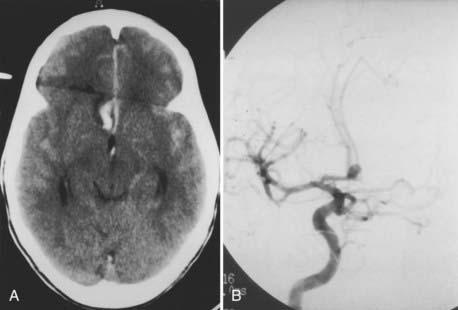
The radiographic evaluation of ACoA aneurysms, however, bears further discussion because there are two radiographic issues that are unique to ACoA aneurysms. The first is that not infrequently the diagnosis of an ACoA aneurysm can be made on the basis of the computed tomography (CT) scan alone because the CT scan may reveal either subarachnoid blood only in the interhemispheric fissure or a thicker clot in the interhemispheric fissure (Fig. 368-5A). Similarly, an intraparenchymal hemorrhage in the region of the gyrus rectus is indicative of an ACoA aneurysm (Fig. 368-6A).
The second radiographic issue is that angiography of ACoA aneurysms has the highest false-negative rate of angiography of any intracranial aneurysm. Iwanaga and colleagues performed repeat angiograms in 38 of 469 patients with SAH whose initial angiograms did not show an aneurysm: of the 38 studies, 8 (21%) revealed an aneurysm, and of the 8 positive repeat studies, 7 were ACoA aneurysms.32 Van Rooij and coworkers found that in 18 of 23 patients with three-dimensional rotational angiography demonstrating an aneurysm after an initial negative digital subtraction angiogram, 11 (61%) aneurysms were located on the ACoA.33 The reason for the higher false-negative rate of ACoA aneurysms by angiography is probably the balanced flow into the ACoA from the paired A1 segments, which may prevent filling of the aneurysm by the dye. It is therefore critical that a cross compression study be carried out routinely during angiography for SAH to completely visualize the ACoA region.
Operative Technique for ACoA and Proximal ACA Aneurysms
ACoA aneurysms remain surgically challenging lesions mainly because of three anatomic features: (1) their bilateral anterograde arterial supply; (2) their deep, midline location; and (3) their intimate relationship to 11 critical arteries. Unlike most other intracranial aneurysms, which typically have only one parent artery, ACoA aneurysms are supplied anterogradely by two arteries, namely the paired A1 segments. Therefore, during surgery for ACoA aneurysms, both A1 segments have to be exposed early in the dissection to obtain proximal control. Furthermore, although ACoA aneurysms are midline structures, they are nevertheless best approached through a lateral craniotomy. Although the lateral subfrontal approach is the shortest route to the ACoA region, it has the disadvantage of requiring early retraction of the ipsilateral frontal lobe, which may decompress the aneurysm dome before identification of the paired A1 segments. Finally, there is an increased risk for ischemic injury during surgery of ACoA aneurysms because these lesions are intimately associated with 11 vessels and their perforators, namely the paired A1 segments, the paired A2 segments, the two medial striate arteries of Heubner, the two orbitofrontal arteries, the two frontopolar arteries, and the ACoA. There is no other aneurysm intimately associated with as many vessels. In this section, we expand our previous description of our microsurgical approach to midline and contralateral aneurysms,34 which is ideal for ACoA aneurysms.
Surgical Adjuncts
Currently at our institution, we routinely use (1) mild hypothermia, (2) intraoperative electroencephalography (EEG) and somatosensory evoked potential (SSEP) monitoring, (3) temporary clipping, (4) mild hypertension, (5) EEG burst-suppression, and (6) intraoperative angiography during surgery of most aneurysms. Brain relaxation is obtained with a mannitol infusion, mild hyperventilation, drainage of the arachnoid cisterns, and fenestration of the lamina terminalis. In the case of some ACoA aneurysms, opening of the lamina terminalis may have to be delayed after the aneurysm is clipped because an inferior-pointing aneurysm may obstruct access to the lamina terminalis, or the dome of a posterior-pointing aneurysm may be prematurely decompressed with this maneuver. Opening the lamina terminalis may have the additional benefit of reducing the incidence of post-SAH chronic hydrocephalus.35 Cerebrospinal fluid (CSF) drainage with an intraoperative intraventricular catheter (IVC) is sometimes necessary. The best insertion point for an intraoperative IVC is the one described by Paine, Batjer, and Samson36: the apex of an isosceles right-angle triangle with a 3.54-cm base that starts at the sphenoid ridge and overlies the sylvian fissure, with 2.5-cm sides. A perpendicular pass of the IVC through this point enters the frontal horn just anterior to the foramen of Monro.
Choice of the Side of the Craniotomy
We approach the aneurysm from the side of the dominant A1 segment. The paired A1 segments are of unequal diameter in as many as 85% of cases of ACoA aneurysms.9 The neck of the aneurysm typically originates on the side of the larger A1 segment, and the dome is more closely associated with the side of the smaller A1 segment. Approaching the aneurysm from the side of the dominant A1 segment has no significant benefit in terms of proximal control, but it is advantageous in the sense that the aneurysm neck is exposed before its dome. If the A1 segments are of equal diameter or the aneurysm dome is truly midline, we prefer a right (nondominant) craniotomy. Some surgeons advocate approaching all ACoA aneurysms from the nondominant side.
Incision
The incision is shown in Figure 368-7 and described in the legend. This incision is more aesthetic than one placed anteriorly along the edge of the hairline.
Dissection of the Temporalis Muscle
The superficial fascia of the temporalis muscle is incised vertically about 1 cm anterior to the ascending limb of the skin incision and horizontally about 1 cm inferior to the linea temporalis and separated from the underlying temporalis fibers as described by Yaşargil.37 This protects the frontal branch of the facial nerve, which runs along the surface of this superficial temporalis fascia.38 To reflect the temporalis muscle, it is divided superiorly with a monopolar cautery leaving a 1.5-cm cuff attached to the skull.39
Frontosphenotemporal (Pterional) Craniotomy
Up to five bur holes are made as shown in Figure 368-7 and described in the legend.
Drilling the Greater and Lesser Sphenoid Wings
Drilling the lateral aspects of the greater and lesser wings of the sphenoid bone is one of the key features and crucial technical steps of this craniotomy, as shown in Figure 368-7 and described in the legend.
Dural Opening
The dura is opened as shown on Figure 368-7 and described in the legend. Sutures are then placed over the region of the sphenoid wing, the anterior fossa ledge, and the middle fossa ledge to retract the base of the dural flap, which otherwise may obstruct the view along the skull base.
Sylvian Fissure Dissection
This initial approach entirely through the sylvian fissure is particularly important in the case of an inferior-pointing ACoA aneurysm, which may have its dome attached to the optic chiasm, optic nerve, or dura of the interoptic space (see Fig. 368-4). With this type of ACoA aneurysm, the standard initial maneuver of retracting the frontal lobe until the olfactory nerve is visualized may result in avulsion of the dome and a catastrophic hemorrhage too early in the exposure.
Exposure of the Ipsilateral and Contralateral A1 Segments
Reaching the ipsilateral A1 segment is the first step in accomplishing proximal control of the aneurysm (Fig. 368-8). Only when both A1 segments are exposed, however, will proximal control be accomplished because the ACoA is almost always present (99.8% of cases6), and the aneurysm can be filled by either A1 segment. It is also important to recognize that there is usually retrograde flow through the A2 segments when the A1 segments are occluded.
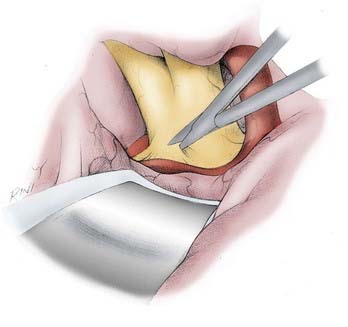
FIGURE 368-8 Initial microsurgical approach to the anterior communicating artery (ACoA) region. After dissecting the ipsilateral A1 segment and preparing its midportion for temporary clipping, the contralateral A1 segment is exposed. The ipsilateral opening of the chiasmatic cistern is extended over the anterior edge of the optic chiasm and over the distal contralateral optic nerve by following the anterior curve of the optic chiasm. This maneuver usually does not disturb most ACoA aneurysms, most of which (71.2%) project either superiorly, anteriorly, or posteriorly into the interhemispheric fissure.40 The contralateral optic nerve is then dissected posteriorly until the A1 segment is seen draping over it. The contralateral A1 segment is then dissected distally toward the interhemispheric fissure.
The dissection of the A1 segment is then continued distally beyond where most of the medial lenticulostriate perforators arise (see Figs. 368-1 and 368-8). The midpoint of the A1 segment is therefore a good place for a temporary clip because placement of a temporary clip on the A1 segment too close to the ACoA region may compromise placement of the permanent clip. Particular care must be taken to avoid catching the medial striate artery of Heubner with the temporary clip.
After dissecting the ipsilateral A1 segment and preparing its midportion for temporary clipping, the contralateral A1 segment is exposed as follows. The ipsilateral opening of the chiasmatic cistern is extended over the anterior edge of the optic chiasm and over the distal contralateral optic nerve (see Fig. 368-8). This is done by following the anterior curve of the optic chiasm. This maneuver usually does not disturb most (71.2%) ACoA aneurysms, which project either superiorly, anteriorly, or posteriorly into the interhemispheric fissure.40 Furthermore, most ACoA aneurysms are found within the confines of the lamina terminalis cistern, which is separate from the chiasmatic cistern. At this point, if the aneurysm orientation permits, it is usually helpful to fenestrate the lamina terminalis, which makes the remainder of the contralateral dissection easier. Fenestration of the lamina terminalis at this stage is obviously not possible with an inferior-pointing aneurysm and may be risky with a posterior-pointing aneurysm.
The contralateral optic nerve is then dissected posteriorly until the A1 segment is seen draped over it (see Fig. 368-8). The contralateral A1 segment is then dissected distally toward the interhemispheric fissure. The midportion of the A1 segment is cleared, and a temporary clip is applied. This temporary clip application eliminates temporarily the second ACoA aneurysm feeder and leaves the ipsilateral A1 segment as the only feeder of the aneurysm. Because the ipsilateral A1 segment is usually dominant and the ACoA is almost always present, temporary clipping of the contralateral A1 segment rarely results in significant SSEP changes.
Gyrus Rectus Resection
Resection of the gyrus rectus is essential for adequate exposure of most ACoA aneurysms (Fig. 368-9). To resect the gyrus rectus, the retractor is repositioned over the medial orbital gyrus just lateral to the olfactory nerve aiming toward the estimated level of the ACoA junction in the interhemispheric fissure. The pia of the gyrus rectus, immediately medial and parallel to the olfactory nerve, is cauterized. At this point, the mean arterial pressure is raised about 10% above baseline, burst-suppression is initiated, and, if appropriate, a temporary clip is applied to the ipsilateral A1 segment. An incision is made longitudinally along the lateral aspect of the gyrus rectus. Using the suction and the bipolar cautery simultaneously, the gyrus rectus resection is rapidly accomplished until the medial pia arachnoid of the gyrus rectus is recognized draped over the aneurysm and the ipsilateral A1 and A2 segments (see Fig. 368-9). This pial-arachnoidal remnant is resected, thus entering into the interhemispheric fissure and fully exposing the A1-ACoA-A2 complex.
Identification of the A1-ACoA-A2 Complex Vessels
The dissection is then continued along the lateral aspect of the ipsilateral A1 segment to identify the ipsilateral A2 segment (Fig. 368-10). The ipsilateral medial striate artery of Heubner and the orbitofrontal artery are identified. From this point on, the dissection will vary depending on the orientation of the aneurysm (see Fig. 368-4). In the case of superior-pointing aneurysms, the contralateral A1 segment can be exposed and the distal course of the contralateral medial striate artery of Heubner identified, but the contralateral A2 segment is usually hidden. In the case of inferior-pointing aneurysms, the contralateral A2 segment and origin of the contralateral medial striate artery of Heubner can be exposed, but the contralateral A1 segment is usually hidden and may have to be traced backward, following the course of the A2 segment from distal to proximal. Posterior-pointing aneurysms may or may not obstruct the view of the contralateral A2 segment, depending on the course of this vessel and the size of the aneurysm. Anterior-pointing aneurysms may partially obstruct the view of the contralateral A2 segment or the contralateral A1 segment. In any case, further sharp dissection of the neck and either displacement or mobilization of the aneurysm body are usually required to visualize the vessels initially hidden from view. Not infrequently, the clip has to be applied without complete visualization of the hidden vessel and repositioned after decompression of the sac and further dissection.
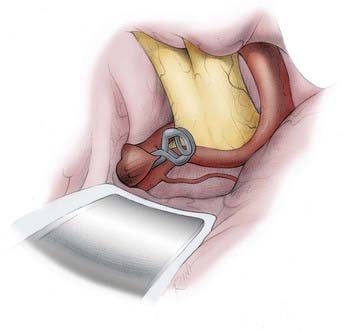
FIGURE 368-10 Clipping of a superior-pointing anterior communicating artery (ACoA) aneurysm. After identifying the entering A1 segments, the exiting A2 segments, the ACoA, the medial striate arteries of Heubner, the orbitofrontal arteries, and the frontopolar arteries, the critical perforators of the ACoA and A2 segments must be identified and cleared out of the path of the clip blades. Most ACoA aneurysms project superiorly (34.4% in Yaşargil’s series of 375 ACoA aneurysms40). Few ACoA aneurysms have necks that are ready to be clipped upon initial exposure. With ACoA aneurysms, it is usually the case that an extended analysis of the neck, the lobes of the aneurysm, and their relationship to the critical vessels is necessary to figure out a safe clipping strategy. It is also not unusual that after application of the initial clip, the body and fundus of the aneurysm have to be reshaped with the bipolar forceps (aneurysmoplasty), and even part of the dome has to be resected to then fully inspect the complex. This inspection often reveals the need to either reposition the clip or to apply a second or third clip. Superior-pointing aneurysms grow between the A2 segments. The posterior wall of their neck is usually intimately associated with the infundibular and hypothalamic perforators, which must be cleared and displaced below the path of the clip blade. Not infrequently, superior-pointing aneurysms have an additional complicating feature, which is that either one or both A2 segments may be densely adherent to the body of the aneurysm. When the ipsilateral A2 segment is not easily separated from the aneurysm neck, it is prudent to use a fenestrated clip around the ipsilateral A2 origin. Otherwise, if the neck can be separated from the adjacent A2 segments, a straight clip almost perpendicular to the ACoA may be adequate, as shown.
After identifying the entering A1 segments, exiting A2 segments, ACoA, medial striate arteries of Heubner, orbitofrontal arteries, and even frontopolar arteries, the critical perforators of the ACoA and A2 segments must be identified and cleared out of the path of the clip blades (see Fig. 368-10). Inadvertent occlusion or injury to these perforators can result in dramatic neurologic, endocrine, or cognitive deficits. Small orbitofrontal and frontopolar arteries may be sacrificed, but preservation of the medial striate arteries of Heubner is crucial. Meticulous preservation of all these vessels is the supreme challenge of clipping ACoA aneurysms.
Dissection of the Aneurysm Neck
The early dissection of superior- and posterior-pointing aneurysms differs from that of the inferior- and anterior-pointing aneurysms. Most ACoA aneurysms project either superiorly, anteriorly, or posteriorly into the interhemispheric fissure, and a minority project inferiorly out of the interhemispheric fissure and into the chiasmatic cistern.18 In Yaşargil’s series of 375 ACoA aneurysms, 34.4% projected superiorly, 22.7% projected anteriorly, 14.1% projected posteriorly, 12.8% projected inferiorly, and 16% had complex, multilobulated projections.40
Superior- and posterior-pointing aneurysms have the advantage of being buried in the interhemispheric fissure, but have the disadvantage of being more intimately associated with the hypothalamic and infundibular perforators (see Fig. 368-4). Because they are contained within the interhemispheric fissure, they usually do not rupture when the retractor is placed across the interhemispheric fissure during the exposure of the contralateral A1 segment. By contrast, inferior-pointing aneurysms have the disadvantage of being usually adherent to the optic chiasm, optic nerves, or interoptic space, but have the advantage of a more favorable relation to the hypothalamic and infundibular perforators. Similarly, the dome of anterior-pointing aneurysms may be adherent to the gyrus rectus and may rupture during early subfrontal retraction.
Once exposed, anterior- and inferior-pointing aneurysms are easier to clip than superior- and posterior-pointing aneurysms because of their more favorable relationship to the infundibular and hypothalamic perforators (see Fig. 368-4). Anterior-pointing aneurysms have the most favorable anatomy in this respect because the critical infundibular and hypothalamic perforators course in a direction opposite the aneurysm. A straight clip placed parallel to the ACoA typically spares these perforators. A complicating feature of anterior-pointing aneurysms, however, is that either the orbitofrontal or a proximal frontopolar artery is often adherent to the wall of the aneurysm and may even have to be sacrificed during the clipping. A complicating feature of inferior-pointing aneurysms is that they often have infundibular and hypothalamic perforators adherent to the posterior aneurysmal wall. In the case of inferior-pointing aneurysms, the posterior wall should always be displaced anteriorly to visualize and separate the perforators before application of a straight clip. The orientation of the ACoA can be helpful with inferior- and also posterior-pointing aneurysms because a straight clip oriented exactly parallel to the course of the ACoA is usually ideal for these two types of aneurysms.
Superior-pointing aneurysms, the most common type of ACoA aneurysm, grow between the A2 segments. The posterior wall of their neck is usually intimately associated with the infundibular and hypothalamic perforators, which must be cleared and displaced below the path of the clip blade. Not infrequently, superior-pointing aneurysms have an additional complicating feature, that is, that either one or both A2 segments may be densely adherent to the body of the aneurysm. When the ipsilateral A2 segment is not easily separated from the aneurysm neck, it is prudent to use a fenestrated clip encircling the ipsilateral A2. Otherwise, if the neck can be separated from the adjacent A2 segments, a straight clip almost perpendicular to the ACoA may be adequate (see Fig. 368-10).
Complications of Anterior Communicating Artery Aneurysms
The general postoperative complications of aneurysm surgery are reviewed in another chapter and elsewhere31 and therefore will not be discussed here in detail. There are, however, two complications that are more common in patients with ACoA aneurysms that bear further discussion here: electrolyte abnormalities and cognitive dysfunction.
Patients with ruptured ACoA aneurysms have a high incidence of electrolyte abnormalities. The most common electrolyte abnormality in these patients is hyponatremia. In Yaşargil’s series of 371 ruptured ACoA aneurysms, hyponatremia was present in 18.1% of patients preoperatively and in 40.5% postoperatively.18 It lasted 1 to 5 days in most patients (about 84%) and was more common in the higher-grade patients. By contrast, hypernatremia was present in this series in only 1.6% of patients preoperatively and in 6.7% postoperatively. Although originally thought to be uniformly a manifestation of the syndrome of inappropriate antidiuretic hormone secretion (SIADH),41 post-SAH hyponatremia may be more commonly due to cerebral salt wasting (CSW).42 CSW was first described in 195043 and consists of the inability of the kidneys to retain sodium in the presence of central nervous system pathology, which results in hyponatremia and obligatory water loss during natriuresis.
Another common complication of patients with ruptured ACoA aneurysms is cognitive dysfunction. Logue and colleagues were the first to document in a large series (79 patients) the cognitive and emotional sequelae associated specifically with ruptured ACoA aneurysms.44 In a later study of cognitive impairment in aneurysmal SAH patients without neurological deficits, Ljunggren and colleagues also documented an association between ACoA aneurysms and marked cognitive dysfunction: only 14% of patients with no or mild cognitive dysfunction had ACoA aneurysms, whereas 43% of patients with marked cognitive dysfunction had aneurysms in this location.45 A similar study by Bornstein and colleagues reached the same conclusion.46 In this study, 28% of patients in the good cognitive outcome group had ACoA aneurysms, and 57% of patients in the poor outcome group had aneurysms in this location. Numerous studies have confirmed the association of ACoA aneurysm rupture or repair with cognitive dysfunction. This association has been occasionally referred to as the ACoA syndrome.47 The characteristic deficits of this syndrome are impaired memory, personality changes, and confabulation. It is generally assumed that these deficits are the result of a focal lesion in the basal forebrain.47
Outcomes after Anterior Communicating Artery Aneurysms
Before the 1970s, the outcome of patients with ruptured ACoA aneurysms was dismal. Between 1958 and 1963, McKissock and colleagues conducted a prospective randomized trial of 300 patients with ruptured ACoA aneurysms assigned to either medical (153 patients) or surgical (147 patients) treatment, with 6-months’ follow-up.48 It is important to note that the overall mortality for this group of patients was even higher than that documented for either treatment group because the exclusion criteria for the trial included, among others, irreversible neurological damage, coma, inoperable lesion, death before angiography, and obligatory surgery for a life-threatening hematoma. Surgical treatment consisted of common carotid artery ligation in 21% of cases, anterior cerebral artery ligation in 40%, wrapping of the aneurysm in 25%, and clipping of the aneurysmal neck in only 14% of cases. Medical treatment was associated with a 40% mortality rate and surgical treatment with a 44% mortality rate. The mortality rate in patients who underwent clipping of the aneurysmal neck was 37%. The proportions of patients who returned to full work were 41% in the medical group and 37% in the surgical group. These results reported by McKissock and colleagues were typical for the period, as evidenced by the fact that similar operative mortality figures for the same period were reported in the original Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage (1958 to 1965), in which the mortality rate associated with surgery for ACoA aneurysms was 36%.1
A few early neurosurgeons, however, reported excellent surgical results in small series of selected patients with ruptured ACoA aneurysms. For instance, French and colleagues reported a series of 25 patients with a 4% mortality rate,49 Höök and Norlén reported 67 patients with a 7% mortality rate,50 and Pool reported 56 patients with a 7% mortality rate.51 These encouraging results indicated that better surgical techniques might produce better outcomes.
The surgical series of ACoA aneurysms reported by Yaşargil and colleagues in 1975 is the first modern series for the treatment of these lesions.17 They reported 203 cases of ruptured ACoA aneurysms (grades I to IV) operated on by Yaşargil between 1967 and 1974. All patients were operated on using Yaşargil’s microsurgical pterional approach. In 1984, Yaşargil published a detailed analysis of this extended ruptured ACoA aneurysm series, which at that point consisted of 371 cases with a total surgical mortality rate of 5.9%.40 Although these outstanding results established the standard against which surgical outcomes have since been measured, it should be noted that this series included only patients selected for surgery and therefore does not provide an overall management mortality for each grade. This selection bias, which is clearly described by the authors, is apparent in the relatively high proportion of good-grade patients in this series (grades I and II, 298 of 374 patients, or 80%) as compared with that reported in the International Cooperative Study on the Timing of Aneurysm Surgery (1980 to 1983), which is the largest, most recent hospital-based series (fully alert, 1723 of 3521 patients, or 48.9%).2 A review of our own published series of ruptured aneurysms and that of other centers showed that between 49.5% and 59.3% (average, 54.4%) of patients were either grade I or II (Hunt-Hess scale) on admission.52
The most representative current figures on the specific surgical outcome and the overall management outcome of patients with ruptured ACoA aneurysms are found in the International Cooperative Study on the Timing of Aneurysm Surgery (1980 to 1983).2,53 Unfortunately, in the Cooperative Study, ACoA and ACA aneurysms were reported together, and therefore specific outcome figures for ACoA aneurysms were not provided. It is reasonable to assume, however, that most of these aneurysms (>95%)1 were ACoA lesions and that the specific mortality statistics for ACoA aneurysms might be only slightly worse than those for the ACoA-ACA group as a whole. In the Cooperative Study, the surgical mortality rate for ACoA-ACA aneurysms was 16.8%, and the overall management mortality rate was 30.1%.2 These mortality figures for ACoA aneurysms were slightly higher than the mortality figures for all aneurysms in this study, which had a 14.3% surgical mortality rate and a 26% overall management mortality rate, respectively.2,53
In the 1990s, these outcome figures were further improved in neurosurgical centers with dedicated cerebrovascular programs. Although there are currently few large published series of exclusively ACoA aneurysms treated in the 1990s,54 it is reasonable to consider that the trend in outcomes of ACoA aneurysms has been similar to the trend in outcomes documented for all aneurysms. It is apparent that the aneurysm treatment outcomes in the 1990s are better than those reported in the International Cooperative Study on the Timing of Aneurysm Surgery, which accrued patients between 1980 and 1983.2,53 For instance, the Johns Hopkins Hospital’s 17.8% overall management mortality rate of ruptured aneurysms treated between 1992 and 199652 represents an improvement over the 26% overall management mortality rate of the International Cooperative Study.2 These improved statistics for ACoA aneurysms are typical for most major centers with dedicated cerebrovascular programs.55
Anatomic and Morphologic Selection Criteria for Endovascular Treatment of Anterior Communicating Artery Aneurysms
ACoA aneurysms pose significant challenges in microsurgical and endovascular treatments, both of which are safe and effective options in properly selected patients. Several variables must be considered in deciding the best treatment plan. Hemodynamic flow patterns across the ACoA are complex and are implicated in the setting of postcoiling recurrence in these aneurysms.56 Moret and colleagues found 25% angiographic recurrence rate at 12-month follow-up arteriography for coil-embolized ACoA aneurysms.57 Although endovascular treatment of intracranial aneurysms is discussed in a separate chapter, certain anatomic and morphologic selection criteria are associated with more successful outcomes after endovascular therapy and are elucidated here.
An absent A1 segment is a reason for concern but not an absolute contraindication to endovascular therapy. Clear visualization of the surrounding vasculature and the neck and dome of the aneurysm must be obtained. The geometry of the aneurysm neck and dome plays a significant role in determining the success or failure of an endovascular approach. In the largest case series of coil embolization of ACoA aneurysms, Gonzalez and colleagues reported on 181 such aneurysms treated between 1991 and 2005.58 Anatomic features associated with complete embolization included small aneurysm size (<10 mm), small neck size (<4 mm), and anterior dome projection. Factors associated with aneurysm recanalization included large size (domes > 10 mm), neck location of the ACoA, posterior dome orientation, and incomplete embolization. In a prospective study comparing treatment of ACoA aneurysms, Proust and coworkers compared 37 patients who received endovascular with 186 patients who underwent microsurgical treatment. The authors found a statistically significant higher rate of complete occlusion with microsurgical clipping. A review of the endovascular results alone showed a higher rate of complete occlusion with smaller aneurysm size and anteriorly directed domes. The only morphologic risk factor associated with microsurgical treatment was aneurysm dome orientation, with higher rates of surgical complication found with posteriorly projecting domes. The authors concluded that microsurgical clip application should be the preferred option for anteriorly directed domes, and endovascular coiling should be selected as the treatment choice for posteriorly directed domes.59
Ammirati M, Spallone A, Ma J, et al. An anatomicosurgical study of the temporal branch of the facial nerve. Neurosurgery. 1993;33:1038-1043.
Gonzalez N, Sedrak M, Martin N, et al. Impact of anatomic features in the endovascular embolization of 181 anterior communicating artery aneurysms. Stroke. 2008;39:2776-2782.
Hager Paget D. The circle of Willis. Its embryology and anatomy. In: Dandy WE, editor. Intracranial Arterial Aneurysms. Ithaca, New York: Comstock Publishing; 1944:67-90.
Hager Paget D. The development of the cranial arteries in the human embryo. Contrib Embryol. 1948;32:205-261.
Hernesniemi J, Dashti R, Lehecka M, et al. Microneurosurgical management of anterior communicating artery aneurysms. Surg Neurol. 2008;70:8-28.
Kassell NF, Torner JC, Haley EC. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18-36.
Kassell NF, Torner JC, Haley EC, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 2: Surgical results. J Neurosurg. 1990;73:37-47.
Lin CL, Kwan AL, Howng SL. Surgical outcome of anterior communicating artery aneurysms. Kaohsiung J Med Sci. 1998;14:561-568.
Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Based on 6368 cases in the cooperative study. J Neurosurg. 1966;25:219-239.
Oshiro EM, Rini DA, Tamargo RJ. Contralateral approaches to bilateral cerebral aneurysms: a microsurgical anatomical study. J Neurosurg. 1997;87:163-169.
Oshiro EM, Walter KA, Piantadosi S, et al. A new subarachnoid hemorrhage grading system based on the Glasgow Coma Scale: a comparison with the Hunt and Hess and World Federation of Neurological Surgeons Scales in a clinical series. Neurosurgery. 1997;41:140-147.
Paine JT, Batjer HH, Samson D. Intraoperative ventricular puncture. Neurosurgery. 1988;22:1107-1109.
Perlmutter D, Rhoton ALJr. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J Neurosurg. 1976;45:259-272.
Perlmutter D, Rhoton ALJr. Microsurgical anatomy of the distal anterior cerebral artery. J Neurosurg. 1978;49:204-228.
Piotin M, Spelle L, Mounayer C, et al. Intracranial aneurysms: treatment with bare platinum coils—aneurysm packing, complex coils, and angiographic recurrence. Radiology. 2007;243:500-508.
Rosner SS, Rhoton AL, Ono M, Rarry M. Microsurgical anatomy of the anterior perforating arteries. J Neurosurg. 1984;61:468-485.
Ujiie H, Tachibana H, Hiramatsu O, et al. Hemodynamic study of the anterior communicating artery. Stroke. 1996;27:2086-2093.
Yaşargil MG, Fox JL. The microsurgical approach to intracranial aneurysms. Surg Neurol. 1975;3:7-14.
Yaşargil MG, Fox JL, Ray MW. The operative approach to aneurysms of the anterior communicating artery. In: Krayenbuhl HA, editor. Advances and Technical Standards in Neurosurgery. New York: Springer-Verlag; 1975:113-170.
Yaşargil MG, Reichman MV, Kubik S. Preservation of the frontotemporal branch of the facial nerve using the interfascial temporalis flap for pterional craniotomy. Technical article. J Neurosurg. 1987;67:463-466.
Yaşargil MG. Microneurosurgery. I. Microsurgical anatomy of the basal cisterns and vessels of the brain, diagnostic studies, general operative techniques and pathological considerations of the intracranial aneurysms. Stuttgart/New York: Georg Thieme Verlag/Thieme Stratton; 1984.
Yaşargil MG. Microneurosurgery. II. Clinical considerations, surgery of intracranial aneurysms and results. Stuttgart/New York: Georg Thieme Verlag/Thieme Stratton; 1984.
1 Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Based on 6368 cases in the cooperative study. J Neurosurg. 1966;25:219-239.
2 Kassell NF, Torner JC, Haley ED, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18-36.
3 Hager Paget D. The development of the cranial arteries in the human embryo. Contrib Embryol. 1948;32:205-261.
4 Perlmutter D, Rhoton ALJr. Microsurgical anatomy of the distal anterior cerebral artery. J Neurosurg. 1978;49:204-228.
5 Perlmutter D, Rhoton ALJr. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J Neurosurg. 1976;45:259-272.
6 Hager Paget D. The circle of Willis. Its embryology and anatomy. In: Dandy WE, editor. Intracranial Arterial Aneurysms. Ithaca, New York: Comstock Publishing; 1944:67-90.
7 Baptista AG. Studies on the arteries of the brain. II. The anterior cerebral artery: some anatomic features and their clinical implications. Neurology. 1963;13:825-835.
8 Huber P, Braun J, Hirschmann D, Agyeman JF. Incidence of berry aneurysms of the unpaired pericallosal artery: angiographic study. Neuroradiology. 1980;19:143-147.
9 Wilson G, Riggs HE, Rupp C. The pathologic anatomy of ruptured cerebral aneurysms. J Neurosurg. 1954;11:128-134.
10 Truex RC, Carpenter MB, editors. Human Neuroanatomy. Baltimore: Williams & Wilkins, 1969.
11 Yaşargil MG. Microneurosurgery. I. Microsurgical anatomy of the basal cisterns and vessels of the brain, diagnostic studies, general operative techniques and pathological considerations of the intracranial aneurysms. Stuttgart/New York: Georg Thieme Verlag/Thieme Stratton; 1984.
12 Rosner SS, Rhoton ALJr, Ono M, Barry M. Microsurgical anatomy of the anterior perforating arteries. J Neurosurg. 1984;61:468-485.
13 Dunker RO, Harris AB. Surgical anatomy of the proximal anterior cerebral artery. J Neurosurg. 1976;44:359-367.
14 Crowell RM, Morawetz RB. The anterior communicating artery has significant branches. Stroke. 1977;8:272-273.
15 Haroun RI, Rigamonti D, Tamargo RJ. Recurrent artery of Heubner: Otto Heubner’s description of the artery and his influence on pediatrics in Germany. J Neurosurg. 2000;93:1084-1088.
16 Gomes F, Dujovny M, Umansky F, et al. Microsurgical anatomy of the recurrent artery of Heubner. J Neurosurg. 1984;60:130-139.
17 Yaşargil MG, Fox JL. The microsurgical approach to intracranial aneurysms. Surg Neurol. 1975;3:7-14.
18 Yaşargil MG, Fox JL, Ray MW. The operative approach to aneurysms of the anterior communicating artery. In: Krayenbuhl HA, editor. Advances and Technical Standards in Neurosurgery. New York: Springer-Verlag; 1975:113-170.
19 Heuer G. A new hypophysis operation. Johns Hopkins Hosp Bulletin. 1918;29:154-155.
20 Dandy WE. Intracranial aneurysm of the internal carotid artery: cured by operation. Ann Surg. 1938;107:654-659.
21 Dandy WE. Intracranial Arterial Aneurysms. Ithaca, New York: Comstock Publishing; 1944.
22 Kempe LG. Operative Neurosurgery. New York: Springer-Verlag; 1968.
23 Norlén G, Barnum AS. Surgical treatment of aneurysms of the anterior communicating artery. J Neurosurg. 1953;10:634-650.
24 Kempe LG, VanderArk GD. Anterior communicating artery aneurysms. Gyrus rectus approach. Neurochirurgia (Stuttg). 1971;14:63-70.
25 Tönnis W. Erfolgreiche Behandlung eines Aneurysma der Art. commun. ant. cerebri. Zentralb Neurochir. 1936;1:39-42.
26 Pool JL. Aneurysms of the anterior communicating artery. Bifrontal craniotomy and routine use of temporary clips. J Neurosurg. 1961;18:98-112.
27 Pool JL. Early treatment of ruptured intracranial aneurysms of the circle of Willis with special clip technique. Bull N Y Acad Med. 1959;35:357-369.
28 Yeh H, Tew JMJr. Anterior interhemispheric approach to aneurysms of the anterior communicating artery. Surg Neurol. 1985;23:98-100.
29 Suzuki J, Mizoi K, Yoshimoto T. Bifrontal interhemispheric approach to aneurysms of the anterior communicating artery. J Neurosurg. 1986;64:183-190.
30 Fukushima T, Miyazaki S, Takusagawa Y, Reichman M. Unilateral interhemispheric keyhole approach for anterior cerebral artery aneurysms. Acta Neurochir Suppl (Wien). 1991;53:42-47.
31 Tamargo RJ, Walter KA, Oshiro EM. Aneurysmal subarachnoid hemorrhage: prognostic features and outcomes. New Horiz. 1997;5:364-375.
32 Iwanaga H, et al. Ruptured cerebral aneurysms missed by initial angiographic study. Neurosurgery. 1990;27:45-51.
33 van Rooij WJ, Peluso JPP, Sluzewski M, Beute GN. Additional value of 3D rotational angiography in angiographically negative aneurysmal subarachnoid hemorrhage: how negative is negative? AJNR Am J Neuroradiol. 2008;29:962-966.
34 Oshiro EM, Rini DA, Tamargo RJ. Contralateral approaches to bilateral cerebral aneurysms: a microsurgical anatomical study. J Neurosurg. 1997;87:163-169.
35 Tomasello F, d’Avella D, de Divitiis O. Does lamina terminalis fenestration reduce the incidence of chronic hydrocephalus after subarachnoid hemorrhage? Neurosurgery. 1999;45:827-831.
36 Paine JT, Batjer HH, Samson D. Intraoperative ventricular puncture. Neurosurgery. 1988;22:1107-1109.
37 Yaşargil MG, Reichman MV, Kubik S. Preservation of the frontotemporal branch of the facial nerve using the interfascial temporalis flap for pterional craniotomy. Technical article. J Neurosurg. 1987;67:463-466.
38 Ammirati M, Spallone A, Ma J, et al. An anatomicosurgical study of the temporal branch of the facial nerve. Neurosurgery. 1993;33:1038-1043.
39 Spetzler RF, Lee KS. Reconstruction of the temporalis muscle for the pterional craniotomy. Technical note. J Neurosurg. 1990;73:636-637.
40 Yaşargil MG. Microneurosurgery. II. Clinical considerations, surgery of intracranial aneurysms and results. Stuttgart/New York: Georg Thieme Verlag/Thieme Stratton; 1984.
41 Doczi T, Bende J, Huzka E, Kiss J. Syndrome of inappropriate secretion of antidiuretic hormone after subarachnoid hemorrhage. Neurosurgery. 1981;9:394-397.
42 Harrigan MR. Cerebral salt wasting syndrome: a review. Neurosurgery. 1996;38:152-160.
43 Peters JP, Welt LG, Sims EAH, et al. A salt-wasting syndrome associated with cerebral disease. Trans Assoc Am Physicians. 1950;63:57-64.
44 Logue V, Durward M, Pratt RTC, et al. The quality of survival after rupture of an anterior cerebral aneurysm. Br J Psychiatry. 1968;114:137-160.
45 Ljunggren B, Sonesson B, Saveland H, Brandt L. Cognitive impairment and adjustment in patients without neurological deficits after aneurysmal SAH and early operation. J Neurosurg. 1985;62:673-679.
46 Bornstein RA, Weir BK, Petruk KC, et al. Neuropsychological function in patients after subarachnoid hemorrhage. Neurosurgery. 1987;21:651-654.
47 DeLuca J. Cognitive dysfunction after aneurysm of the anterior communicating artery. J Clin Exp Neuropsychol. 1992;14:924-934.
48 McKissock W, Richardson A, Walsh L. Anterior communicating aneurysms: a trial of conservative and surgical treatment. Lancet. 1965;1:874-876.
49 French LA, Zarling ME, Schultz EA. Management of aneurysms of the anterior communicating artery. J Neurosurg. 1962;19:870-876.
50 Höök O, Norlén G. Aneurysms of the anterior communicating artery. Acta Neurol Scand. 1964;40:219-240.
51 Pool JL. Bifrontal craniotomy for anterior communicating artery aneurysms. J Neurosurg. 1972;36:212-220.
52 Oshiro EM, Walter KA, Piantadosi S, et al. A new subarachnoid hemorrhage grading system based on the Glasgow Coma Scale: a comparison with the Hunt and Hess and World Federation of Neurological Surgeons Scales in a clinical series. Neurosurgery. 1997;41:140-147.
53 Kassell NF, Torner JC, Haley EC, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 2: Surgical results. J Neurosurg. 1990;73:37-47.
54 Lin CL, Kwan AL, Howng SL. Surgical outcome of anterior communicating artery aneurysms. Kaohsiung J Med Sci. 1998;14:561-568.
55 Hernesniemi J, Dashti R, Lehecka M, et al. Microneurosurgical management of anterior communicating artery aneurysms. Surg Neurol. 2008;70:8-28.
56 Ujiie H, Tachibana H, Hiramatsu O, et al. Hemodynamic study of the anterior communicating artery. Stroke. 1996;27:2086-2093.
57 Piotin M, Spelle L, Mounayer C, et al. Intracranial aneurysms: treatment with bare platinum coils—aneurysm packing, complex coils, and angiographic recurrence. Radiology. 2007;243:500-508.
58 Gonzalez N, Sedrak M, Martin N, et al. Impact of anatomic features in the endovascular embolization of 181 anterior communicating artery aneurysms. Stroke. 2008;39:2776-2782.
59 Proust F, Debono B, Hannequin D, et al. Treatment of anterior communicating artery aneurysms: complementary aspects of microsurgical and endovascular procedures. J Neurosurg. 2003;99:3-14.
60 Dott NM. Intracranial aneurysmal formations. Clin Neurosurg. 1969;16:1-16.

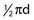 = 1.5d (because π ≈ 3 and 3 ÷ 2 = 1.5). Therefore, a 10-mm neck requires at least a 15-mm clip. In the case of a particularly broad neck, which is often encountered in ACoA aneurysms, the clip should be applied well above the origin of the aneurysm so that the tension on closure does not tear the origin of the aneurysm.
= 1.5d (because π ≈ 3 and 3 ÷ 2 = 1.5). Therefore, a 10-mm neck requires at least a 15-mm clip. In the case of a particularly broad neck, which is often encountered in ACoA aneurysms, the clip should be applied well above the origin of the aneurysm so that the tension on closure does not tear the origin of the aneurysm.





