Chapter 152 Anterior Cervical Foraminotomy (Jho Procedure)
Microscopic or Endoscopic
The optimal surgical treatment of degenerative cervical spine disease that involves radiculopathy and/or myelopathy is the direct surgical removal of compressive pathology while preserving segmental motion. Historically, current cervical spine surgical techniques were introduced more than a half century ago as anterior approach (anterior cervical discectomy) and posterior approach decompression procedures. Although anterior cervical discectomy directly targets the compressive pathology, which usually consists of soft disc herniation or spondylotic stenosis, the approach also involves removing structural elements important for segmental motion—such as the anterior longitudinal ligament and nonherniated portions of the disc—for the sake of surgical exposure. In comparison, some posterior decompression techniques can preserve segmental motion, but in many cases they do not target the compressive pathology directly. To optimize the two surgical goals of directly removing compressive pathology while maximizing the preservation of segmental motion, anterior cervical foraminotomy techniques were developed and previously reported by the senior author as the “Jho procedure.”1,2
The Jho procedure was developed to overcome the deficits of classic anterior and posterior cervical procedures in selected patients to optimize the achievement of surgical goals. Microsurgical anterior cervical foraminotomy was first reported by H.D. Jho in 1996 under the minimally invasive concept of functional spine surgery, in which compressive pathology is directly removed via an anterior approach while the remaining disc and functioning motion unit is preserved without the use of implants or bone fusion.1 The originally reported technique for anterior cervical foraminotomy involved removing the uncovertebral juncture (the most lateral part of the intervertebral disc) to access the compressive pathology. Once the surgical access is made, the soft disc and/or bone spurs that compose the compressive pathology are excised. This surgical approach directly addresses the compressive pathology with access via the lateral portion of the spinal column and effective preservation of the anatomic structures so that segmental motion remains intact and bony fusion is not necessary.
Originally, the Jho procedure was performed under the operating microscope; hence the surgery was also called anterior cervical microforaminotomy.2 Several variations of the surgical technique gradually evolved to achieve surgical goals more efficiently while minimizing surgical impact to the spinal column and functioning motion unit. Because no precise terminology currently prevails to describe the evolution of surgical techniques, the term surgiology was coined to represent the progressive pursuit of scientific or artistic knowledge to improve a particular operative treatment. The loose derivation of this term is from roots defined by the Webster’s dictionary with “surgery” being “a) the treatment of disease, injury, or deformity by manual or instrumental operations, as the removal of diseased parts or tissue by cutting, b) an operation of this kind, c) the branch of medicine dealing with this” and the suffix “-ology” as “the science, doctrine, or theory of” something. Surgiology has historically been an inherent process with the tendency to result in the eventual elimination of ineffective surgeries and the improvement of effective techniques. The Jho procedure involving anterior cervical foraminotomy therefore underwent surgiologic refinement to result in four basic variations and use of the endoscope.
Further variations on this technique evolved from the concept that the trajectory from the skin incision to the surgical target in the sagittal plane of the cervical spine directs where a bone opening should be made to access the target pathology efficiently and effectively. Thus, surgical technique became tailored depending on the trajectory, as determined by the nature of the pathology and cervical anatomy. The surgiologic result was the progressive development of the following variations: 1) transuncal approach; 2) upper-vertebral transcorporeal approach; 3) lower-vertebral transcorporeal approach; and 4) anterior cervical foraminoplasty.3
Surgical Tools and Techniques
Endoscopes
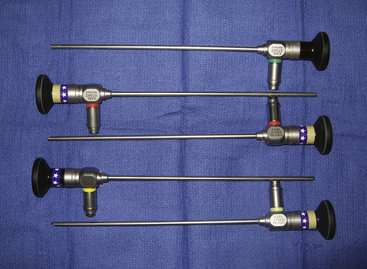
The endoscopes we use are rod-lens endoscopes that are 4 mm in diameter and 18 cm in length. One set consists of five endoscopes: a 0-degree-lens endoscope, 30-degree lens angled toward the light source, 30-degree lens angled away from the light source, 70-degree lens angled toward the light source, and 70-degree lens angled away from the light source (Fig. 152-1) The 0-degree-lens endoscope is the basic working configuration used for most applications. Because the endoscope provides a wide-angle view, the 0-degree-lens endoscope usually provides adequate views for exposure at the nerve root as well as the spinal cord. However, the 30-degree-lens endoscope angled toward the light source can be used when a more-angled view toward the spinal cord is desired, and a 30-degree-lens endoscope angled away from the light source can be used when a more-angled view toward the nerve root at the neural foramen is desired.
Endoscope Lens-Cleaner
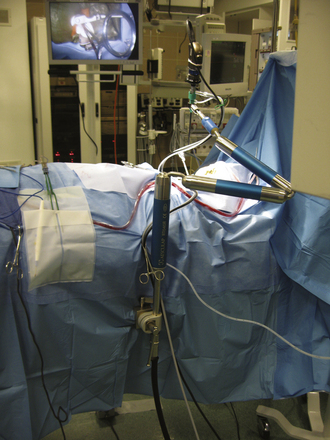
An endoscopic lens-cleansing device is required to keep the lens clear so that the surgeon can continually operate without interruption (Fig. 152-2). The device consists of a disposable irrigation tube that passes through an electric-powered motor. The endoscope is placed through a rigid tubular irrigating sheath, which is connected to the irrigating tube. The irrigation tube is connected to a saline bag, which is hung on a pole. This motor-powered irrigation device is controlled by a foot pedal to flush saline forward. When the foot pedal is released, the motor reverses its rotary direction and draws the saline back from the tip of an endoscope for 1 to 2 seconds. The forward flow of irrigating saline cleans the lens, and the reverse flow clears away water bubbles at the tip of the endoscope. Although this device is not yet ideal, it helps the surgeon significantly in the task of keeping the endoscope lens clean without removing the endoscope from the surgical site.
Endoscope Holder
Two different types of endoscope holders are available, but both are not yet ideal. One is a simple manual holder with multiple joints that can be tightened by hand, and the other is a holder with joints powered by nitrogen gas and controlled with a single button. Manual holders are inconvenient to maneuver with releasing, repositioning, and tightening; they also have limitations in flexibility for reaching certain positions. Nitrogen gas–powered devices are more expedient than manual types but are not as smooth as the operating microscope in releasing and locking at various positions. Currently, we use a Mitaka endoscope holder (distributed by Karl Stortz) for cranial applications and an Aesculap holder for spine applications (Fig. 152-3). The Mitaka holder is relatively bulky at the attachment shaft and has very narrow accessibility at the holding terminal; thus the endoscope holding terminal has to be appropriately positioned at the surgical area before tightening at the shaft joints. Because the holding terminal maneuverability of the Mitaka holder is superior to Aesculap, we like to use the Mitaka holder for cranial endoscopic surgery. The Aesculap holder has longer flexible arms compared to the Mitaka, but its holding terminal has a limited range of motion, even with custom modifications. We prefer to use the Aesculap holder for spine endoscopy, but holding terminals of both types of holders are not yet ideal for endoscopic spine surgery. Sagging of a few millimeters after release of the powerbutton is another suboptimal feature of the nitrogen-powered holders.
Endoscopic Surgical Instruments
Because the region of bone removal in anterior cervical foraminotomy requires high precision, a fine drilling device is required. We use a Midas telescoping tubular drill with a 2-mm diamond bit. The drill bit tip can be progressively extended as the depth of drilling advances. Other commercial drill products are available that have comparable systems. Bipolar forceps are shaped to accommodate the endoscopic surgical environment, and the blades of the bipolar forceps are parallel to each other, similar to a single-bladed instrument, once the blades are approximated. Various surgical curettes and other endoscopic instruments were customized and developed in order to function efficiently within the uniquely curved endoscopic surgical trajectory.
Surgical Technique
Surgical Exposure of the Uncovertebral Juncture
By palpating the transverse tubercles, the extent of the longus colli is identified. The longus colli is split just medial to the transverse tubercles rostral and caudal to the intervertebral disc level, being careful to avoid injury to the sympathetic trunk and fibers located laterally along the longus colli. A cervical retractor system is applied between the split longus colli muscle fibers to maintain exposure of the uncovertebral juncture. The original description mentioned sectioning of the medial part of the longus colli muscle, but this soon evolved to splitting the longus colli muscle, allowing its preservation.
Original Description of Microsurgical Anterior Cervical Foraminotomy
The original technique for microsurgical anterior cervical foraminotomy was reported as a new approach for cervical disc herniation in 1996.1 In the original description, the lateral 5- to 8-mm portion of the uncovertebral juncture was drilled and removed, which eventually evolved into less bone removal. The vertical dimensions of bone removal originally extended from the inferior margin of the rostral vertebra’s medial transverse process to the superior margin of the caudal vertebra’s medial transverse process, usually measuring 7 to 10 mm in total length. Bone removal is performed using a 2-mm cutting bit in a slender high-speed drill. The intervertebral disc is kept largely intact, and the posterior longitudinal ligament (PLL) is sometimes opened to confirm an adequate decompression from the lateral portion of the spinal cord to the nerve exit behind the vertebral artery. It is possible for venous bleeding to be cumbersome while the PLL is open. Once adequate decompression is accomplished, the platysma and subcutaneous tissue are closed with 3-0 absorbable sutures. The surgical incision site is infiltrated with a local anesthetic, and skin is closed with absorbable stitches or adhesive glue.
Surgiologic Evolution of Anterior Cervical Foraminotomy
Transuncal Approach (Jho Procedure Type 1)
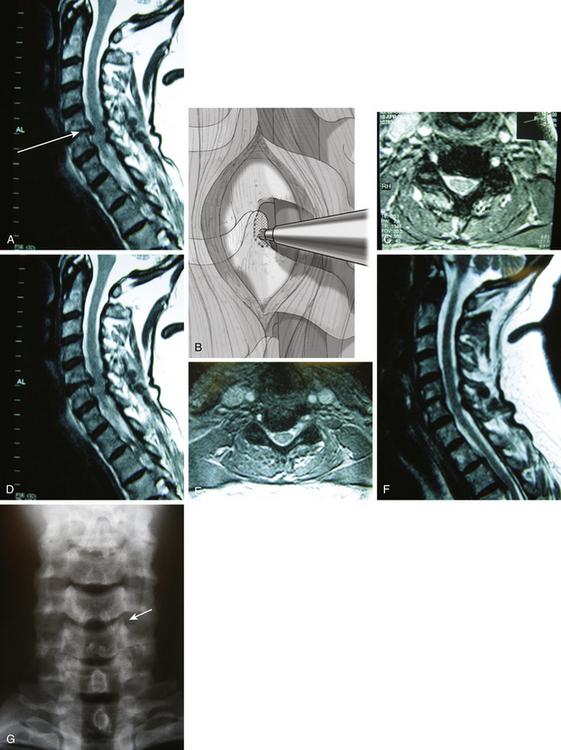
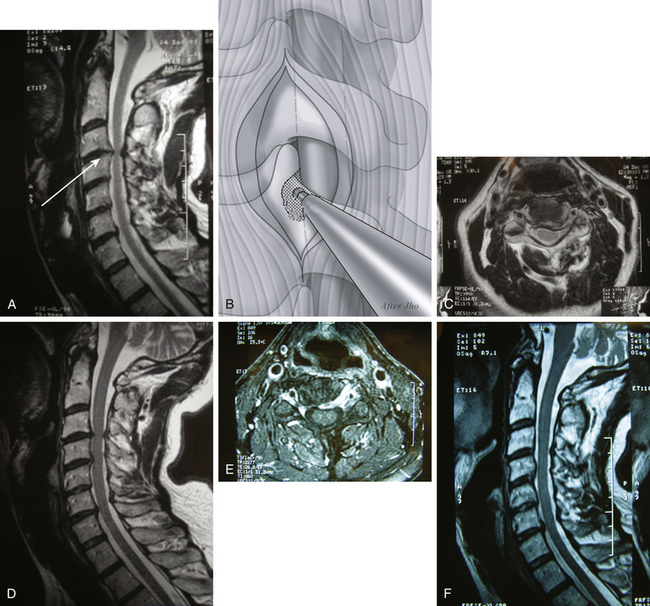
When the surgical trajectory from the skin incision to target pathology is perpendicular to the sagittal plane of the cervical spine, a bone opening at the anterolateral spine should be made along this trajectory line. Particularly for C4–5 or C5–6 operations, a routine skin incision at the upper or mid portion of the neck produces such a perpendicular surgical trajectory. In this case, the uncinate process lies directly along the perpendicular surgical trajectory (Fig. 152-4A). The skin incision to the point of bone exposure is similar to the general description of the anterior cervical foraminotomy approach. The most medial 1- to 2-mm portions of the transverse processes of both upper and lower vertebrae are removed to identify the proximal and distal extent of the vertebral artery in the region.
The vertebral artery is dissected off laterally from the lateral portion of the uncinate process. Often the uncinate process protrudes laterally far beyond the line drawn between the medial margin of upper and lower transverse foramina. Thus, the most lateral extent of the uncinate has to be well defined in relation to the vertebral artery. Then, the most lateral 2- to 3-mm portion of the uncinate is drilled just medial to the vertebral artery toward the PLL (see Fig. 152-4B).
Once the PLL is exposed posteriorly, the thin layer of uncinate cortical bone that was left attached at the nerve root is dissected and removed. Additional compressing pathologies such as herniated soft disc and/or bone spurs are then removed off the nerve root and lateral portion of the spinal cord (see Fig. 152-4C-F). Often the PLL is opened to expose the dura mater at the most lateral portion of the spinal cord and proximal nerve root to detect any hidden migrated disc fragments. When the PLL is opened, the ligament should be opened medially in front of the spinal cord because the epidural vein is located at the junction between the nerve root and the spinal cord. Copious epidural bleeding can occur when the PLL is opened laterally. Herniated disc fragments or bone spurs are removed with variously curved curettes. Awareness is necessary to avoid damaging the bony wall of the medial uncinate to maintain the integrity of the intervertebral disc (see Fig. 152-4G). When spinal cord decompression is required, a specially designed curette system is used to achieve further medial decompression by undercutting the compressive pathology posterior to the rostral and caudal vertebral bodies.
Surgical closure is made using the aforementioned techniques.
Upper-Vertebral Transcorporeal Approach (Jho Procedure Type 2)
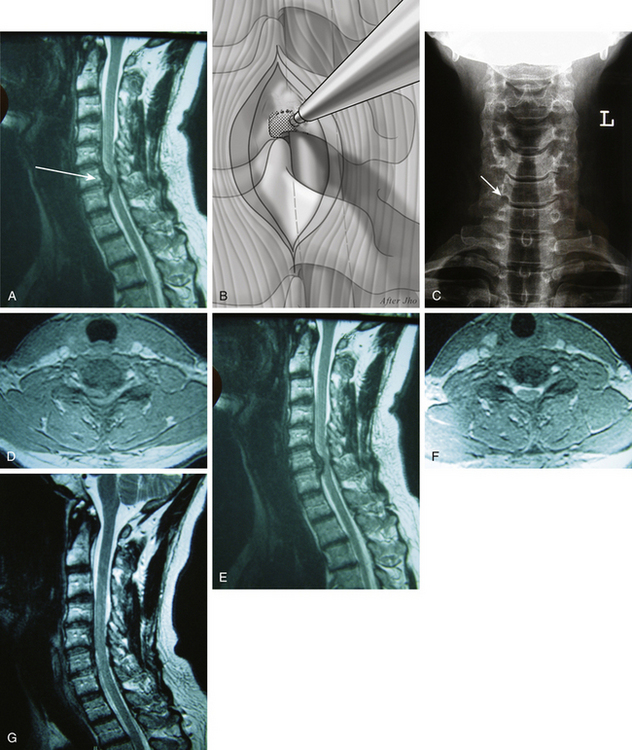
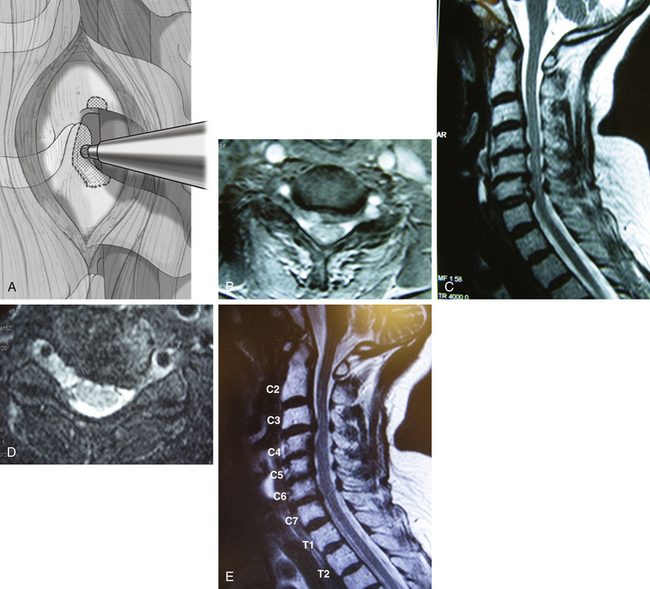
The term upper-vertebral transcorporeal approach refers to the location of the bone opening at the lateral portion of the upper vertebra to the intervertebral disc. This technique involves creating a bone opening at the inferolateral portion of the upper vertebra when the anteroposterior surgical trajectory inclines caudally (Fig. 152-5A and B). This approach is most often used in C6–7 or C7–T1 surgery but is also commonly used with other levels when the skin incision is made purposefully cephalad. When the vertebral artery is shown to enter at the C6 transverse foramen on preoperative MRI scans, an intraoperative x-ray is not necessary for C6–7 or C7–T1 surgery because the vertebral artery entrance into the C6 transverse foramen is visible at the time of surgery. However, an intraoperative x-ray is still confirmatory.
The vertebral artery is exposed and a 2-mm medial portion of the transverse process is removed at the upper vertebra. Bone opening is then made at the inferolateral 2- to 3-mm portion of the upper vertebra with drilling toward the PLL (see Fig 152-5C). The surgical trajectory is directed toward the pathologic target through only the most posterior portion of the intervertebral endplate. Damage to the intervertebral endplate at the anterior two thirds portion of the intervertebral disc must be avoided (see Fig 152-5D to G). The rest of the procedure is the same as described for other approaches.
Lower-Vertebral Transcorporeal Approach (Jho Procedure Type 3)
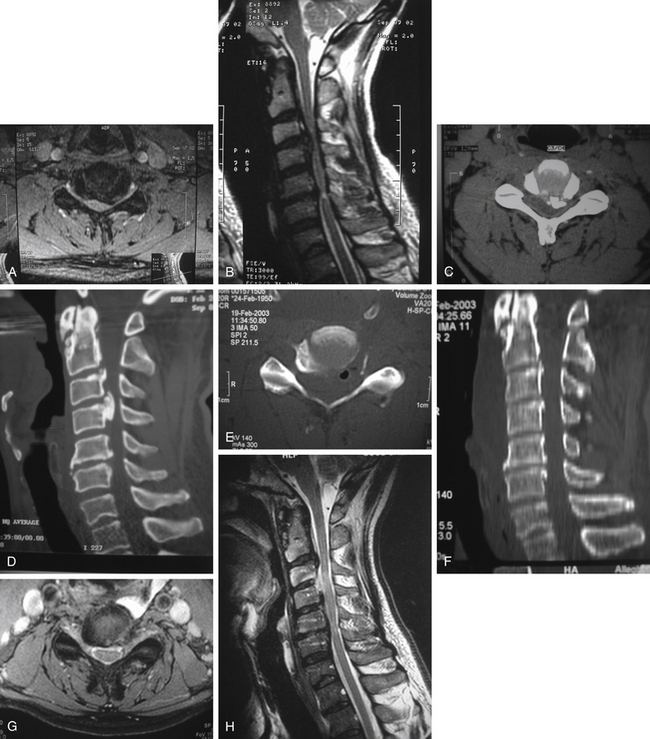
The term lower-vertebral transcorporeal approach refers to the location of the bone opening at the lateral portion of the lower vertebra to the intervertebral disc. For a C3–4 operation or when a skin incision is made inadvertently more caudal than it should be at any cervical disc level, this technique is required (Fig. 152-6A).
The medial portions of the transverse processes at the rostral and caudal vertebrae are identified. The superomedial 1- to 2-mm portion of the transverse process at the lower vertebra is removed, and the vertebral artery is identified. Just medial to the vertebral artery, the superolateral 2- to 3-mm width portion of the lower vertebra is drilled away posteriorly using a 2-mm cutting drill bit (see Fig. 152-6B). The total vertical dimension of bone removal is approximately 5 mm in length. A cephalad-directed surgical trajectory leads the drilling posteriorly toward the target. In other words, a superior-posterior surgical trajectory from a bone opening at the rostral lower vertebral body leads to the compressing pathology at the intervertebral disc while preserving the uncovertebral juncture at the ventral part of the cervical spine. Microdissectors and various up-curved curettes are used to remove compressing herniated soft disc or bone spurs. The nerve root and the most lateral portion of the spinal cord are released from compression.
The amount of bone removal posteriorly must be tailored depending on the extent of pathology. As drilling is advanced posteriorly, the surgical reference points include the endplate of the lower vertebra, followed by the intervertebral disc space, and the endplate of the upper vertebra at the area of target pathology. The PLL is first exposed at the uncompressed portion just caudal to the compressing pathology, then it is exposed rostral to the compressing pathology. Drilling must be done with caution at the lateral portion where the nerve root is located. The thin cortical bone covering the nerve root is dissected and removed, followed by lifting the compressing pathology away from the PLL and removing it. The PLL can be opened medially with a microdissector and excised laterally except when the MRI scans do not suggest soft disc herniation and the PLL fails to show any defect, in which case the PLL should not be removed. Removal of the PLL laterally can cause cumbersome epidural bleeding because the epidural veins run between the two layers of the PLL at the lateral spinal cord canal. When the PLL is opened longitudinally with a microdissector, the white glistening dura mater is visualized. After the spinal cord dura mater is identified, the PLL can be excised. When spinal cord decompression is required, the compressing pathology is removed farther medially along the posterior margin of the rostral and caudal vertebrae (see Fig. 152-6C-F).
Anterior Cervical Foraminoplasty (Jho Procedure Type 4)
The 2-mm medial portion of the transverse process at the vertebral artery foramen is removed at both the upper and lower vertebrae. Then the inferolateral portion of the upper vertebra, superolateral portion of the lower vertebra, and lateral 2-mm portion of the uncinate process are drilled toward the PLL (Fig. 152-7A). Drilling is directed along the nerve passage from pedicle to pedicle in order to have complete decompression in the vertical dimension. After the PLL is exposed, posterior bone spurs are excised in front of the lateral spinal cord. If spinal cord decompression is required, bone spurs anterior to the spinal cord are excised through a foraminoplasty hole (see Fig. 152-7B-E). The PLL is excised, and the dura mater is exposed from pedicle to pedicle. Sometimes it is necessary to shave the superior portion of the pedicle of the caudal vertebra when the vertical dimension of the neural foramen is excessively narrowed, which is relatively common in elderly patients.
Spinal Cord Decompression via an Anterior Cervical Foraminotomy
Compressive pathology at the neural foramen is somewhat cephalad to that on the spinal cord. This has to be kept in mind when the spinal cord decompression is required through one of the anterior foraminotomy techniques. Although the foraminoplasty technique is often used when the spinal cord decompression is the main part of the operation, the aforementioned various anterior foraminotomy techniques can be used depending on the location of the surgical pathology. These techniques can also be used for spinal tumor surgery, such as a meningioma located anterior to the spinal cord or a neurofibroma located intradurally and/or extradurally as a dumbbell-shaped tumor. Multilevel spinal cord decompression for the ossification of the posterior longitudinal ligament (OPLL) or severe spondylotic stenosis is also performed with these anterior foraminotomy techniques (Fig. 152-8). In earlier cases, multilevel surgery was performed at as many levels as pathology extends. However, this approach has been refined to multiple smaller staged operations, generally to address two levels at a time with each stage of surgery.
Postoperative Management
Exercises or taking a shower are allowed the following day. Although light exercises are encouraged right after surgery, contact sports activities and heavy weight-lifting are delayed for 4 to 6 weeks. Return to office-type work is allowed within a few days, but return to physically laborious jobs is delayed for 4 to 6 weeks. Postoperative contrast-enhanced MRI scans and dynamic x-rays are obtained in all patients 6 weeks after surgery as a routine protocol.
Results
We previously reported our series of 104 patients who met the following study criteria: unilateral cervical radiculopathy that had not responded to conservative treatment after at least 6 weeks (or at least 4 weeks if patients exhibited profound motor weakness), imaging studies confirming pathoanatomic features corresponding to the clinical symptoms, no previous cervical spine surgery, and no significant spondylotic stenosis causing spinal cord compression. Forty-five patients were men, and 59 were women. Patient ages ranged from 26 to 74 years, with the median age being 46 years. Compressive pathology was spondylotic spurs in 44 patients (42.3%), soft disc herniation in 54 patients (51.9%), and a combination of the two in 6 patients (5.8%). The duration of symptoms ranged from 4 weeks to 156 months (mean, 17.6 months). Follow-up periods ranged from 12 to 86 months (median, 36 months). In addition to radiculopathy, preoperative symptoms included severe neck pain in 83 patients (79.8%) and significant occipital head pain in 11 patients (10.6%).
Surgical results were graded as follows: “excellent” meant that the patient exhibited complete resolution of all symptoms, “good” indicated that the patient experienced relief of radiculopathy but still experienced occasional minimal or mild residual nonradicular discomfort, “fair” meant that the patient exhibited mild residual radiculopathy with or without mild to moderate residual nonradicular discomfort, and “poor” meant that the patient continued to exhibit significant radicular symptoms with or without nonradicular discomfort (including those unchanged from or worse than at preoperative conditions). Among 104 patients, 83 patients (79.8%) demonstrated excellent results, 20 patients (19.2%) demonstrated good results, and one patient (1%) experienced a fair outcome. No patient had a poor outcome, and there were no results that were unchanged or worse. One patient developed discitis, which resulted in spontaneous fusion at the operated-on level following antibiotic treatment, although his radiculopathy resolved well. One patient developed transient position-related hemiparesis, which resolved in 6 weeks. Two patients developed transient Horner’s syndrome, which resolved 6 weeks postoperatively.4
Discussion
Although variations in surgical trajectories to the cervical spine such as lateral approaches have been reported, the notion of discectomy with bone fusion was retained.5,6 Conventional anterior cervical disc surgery evolved over a half century into complete removal of the intervertebral disc with bone graft fusion and metal implant by adhering to this idea.7 The more-recent methods using arthroplasty with artificial discs attempt to reestablish the motion segment but still rely on complete discectomy. The superiority and validity of an artificial disc Implant over conventional spinal fusion surgery remain to be proved.
The original description of anterior cervical foraminotomy involved not only new surgical techniques using access via an anterolateral route through the uncovertebral juncture but also introduced the novel concept of functional spine surgery.1 The goal of functional spine surgery entails preserving the motion unit while achieving direct removal of the compressive pathology. The original description involved removing the most lateral 5-mm portion of the uncovertebral juncture as a surgical access to the compressive pathology, followed by wide decompression of the nerve root from its origin at the spinal cord to its exit posterior to the vertebral artery. Then the subsequent evolution of our surgical techniques was reported.2,3,4,8–12
Generally, the intervertebral discs of the cervical spine in the sagittal plane incline cephalad from an anterior-to-posterior direction. Therefore, the surgical approach involved in the originally described foraminotomy usually arrives at the superior portion of the pedicle and inferior portion of the surgical target. To compensate for this, the surgical trajectory must be inclined cephalad while proceeding posteriorly. The skin opening also has to line up with the surgical trajectory of this foraminotomy. Thus, the skin incision has to be made more cephalad than in conventional anterior discectomy, and the anterior bone opening is also shifted cephalad to arrive at the surgical target efficiently. The anterior bone opening is made at the most lateral portion of the upper vertebral body to arrive naturally at the surgical target when the foraminotomy hole is advanced posteriorly perpendicular to the longitudinal axis of the spinal column. The posterior one third portion of the anteroposterior surgical trajectory involves the intervertebral juncture, which is the posterior portion of the uncovertebral juncture and usually comprises the actual compressive pathology. This technique consists of bone opening at the upper vertebrae; thus, the technique is termed the upper-vertebral transcorporeal approach. When an anteroposterior surgical trajectory is perpendicular to the longitudinal axis of the spine, the bone opening has to be made at the lateral portion of the uncinate; hence, the variant technique is called a transuncal approach. When the surgical trajectory inclines cephalad, a lower-vertebral transcorporeal approach has to be adopted, with a bone opening made at the lateral lower vertebrae. The medial 2-mm portion of the vertebral artery is exposed to minimize the amount of bone removal at the vertebral body. When a narrowed neural foramen requires reconstruction into a larger normal shape, anterior foraminoplasty is performed, with direct removal of the medial bone spurs along the longitudinal axis of the neural foramen. Since the introduction of these refined surgical techniques, others have also reported their own experiences with anterior cervical foraminotomy.13–21
Biomechanical effects of various techniques of anterior cervical foraminotomy on the cervical spinal column still remain to be fully tested. However, clinical findings sometimes contrast with cadaveric biomechanical studies, which may be explained in part by the fact that live patients undergo healing at the surgical site while cadaver specimens lack this ability and also increase specimen fatigue with repeated testing. Kotani and colleagues.22 reported that the unilateral foraminotomy procedure reduced 30% of spinal stiffness in their cadaver study and raised the question of spinal instability. However, their specific technique of foraminotomy involved an annulotomy and partial discectomy, along with a medial-to-lateral removal of the uncinate process instead of a lateral-to-medial approach.
In live patients, one of the most common preoperative complaints is stiffness and limited range of motion in the cervical spine, particularly head tilt and rotation to the symptomatic side. Most patients experienced a significant improvement of these complaints postoperatively such that their head tilt and rotation to the operated side improves significantly. However, there is sometimes continued limitation in their range of motion opposite to the operative side related to degenerative spondylotic disease. When anterior cervical foraminotomy is performed correctly, there can be improvement of the stiff decreased range of motion associated with cervical spondylotic disease toward normal ranges. Thus, improved range of motion can be a beneficial effect observed in live patients with various techniques of anterior cervical foraminotomy, akin to some other types of operative treatment of arthritic joint disease in various parts of the body.23 However, excessive or inadvertent removal of disc, ligament, or bone beyond the target range can theoretically result in spinal instability as in any type of spine surgery.13,24,25
Spinal instability can occur if bone removal is substantial.13 When patients complain of significant neck pain postoperatively, spinal instability has to be considered. When patients have significant spinal instability, fusion may be necessary. In our experiences, we have not had any single case that required spinal fusion to address spinal instability after this operation.
Jho H.D. Microsurgical anterior cervical foraminotomy: a new approach to cervical disc herniation. J Neurosurg. 1996;84:155-160.
Jho H.D., Ha H.G. Anterior cervical microforaminotomy. Oper Tech Orthop. 1998;8:46-52.
Jho H.D. Editorial. Failed anterior cervical foraminotomy. J Neurosurg (Spine 2). 2003;98:121-125.
Jho H.D., Kim W.K., Kim M.H. Anterior microforaminotomy for treatment of cervical radiculopathy. Part 1: Disc-preserving “functional cervical disc surgery.”. Neurosurgery. 2002;51(suppl 2):46-53.
Hakuba A. Trans-unco-discal approach: A combined anterior and lateral approach to cervical discs. J Neurosurg. 1976;45:284-291.
Verbiest H. A lateral approach to the cervical spine: Technique and indications. J Neurosurg. 1968;28:191-203.
Sampath P., Bendebba M., Davis J.D., Ducker T. Outcome in patients with cervical radiculopathy. Prospective, multicenter study with independent clinical review. Spine. 1999;24(6):591-597.
Jho H.D. Decompression via microsurgical anterior foraminotomy for cervical spondylotic myelopathy. J Neurosurg. 1997;86:121-126.
Jho H.D. Decompression via microsurgical anterior foraminotomy for spondylotic cervical myelopathy: Technical note. Neurosurg Focus. 1996;4:1-11.
Jho H.D. Treatment of spondylotic cervical myelopathy via anterior foraminotomy. Tech Neurosurg. 1999;5:124-132.
Jho H.D., Ha H.G. Anterolateral approach for spinal cord tumors. Minim Invas Neurosurg. 1999;42:1-6.
Jho H.D., Kim M.H., Kim W.K. Anterior cervical microforaminotomy for spondylotic cervical myelopathy: Part 2. Neurosurgery. 2002;51(suppl 2):54-59.
Hacker R.J., Miller C.G. Failed anterior foraminotomy. J Neurosurg (Spine 2). 2003;98:126-130.
Yi S., Lim J.H., Choi K.S., et al. Comparison of anterior cervical foraminotomy vs arthroplasty for unilateral cervical radiculopathy. Surg Neurol. 2009;71:677-680.
Lee J.Y., Lohr M., Impekoven P., et al. Small keyhole transuncal foraminotomy for unilateral cervical radiculopathy. Acta Neurochir (Wien). 2006;148:951-958.
Koc R.K., Menku A., Tucer B., et al. Anterior cervical foraminotomy for unilateral spondylotic radiculopathy. Minim Invas Neurosurg. 2004;47:186-189.
Saringer W.F., Reddy B., Nobauer-Huhmann I., et al. Endoscopic anterior cervical foraminotomy for unilateral radiculopathy: Anatomical morphometric analysis and preliminary clinical experience. J Neurosurg (Spine 2). 2003;98:171-180.
Saringer W., Nobauer I., Reddy M., et al. Microsurgical anterior cervical foraminotomy (uncoforaminotomy) for unilateral radiculopathy: Clinical results of a new technique. Acta Neurochir. 2002;144:685-694.
Tascioglu A.O., Attar A., Tascioglu B. Microsurgical anterior cervical foraminotomy (uncinatectomy) for cervical disc herniation. J Neurosurg (Spine 1). 2001;94:121-125.
Johnson J.P., Filler A.G., McBride D.Q., Batzdorf U. Anterior cervical foraminotomy for unilateral radicular disease. Spine. 2000;25(8):905-909.
Cornelius J.F., Bruneau M., George B. Microsurgical cervical nerve root decompression via an anterolateral approach: Clinical outcome of patients treated for spondylotic radiculopathy. Neurosurgery. 2007;61:972-980.
Kotani Y., McNulty P.S., Abumi K., et al. The role of anteromedial foraminotomy and the uncovertebral joints in the stability of the cervical spine: A biomechanical study. Spine. 1998;23:1559-1565.
Jho H.D., Jho D.H. Editorial. Ventral uncoforaminotomy. J Neurosurg Spine. 2007;7:533-536.
Chen B.H., Natarajan R.N., An H.S., Andersson G.B.J. Comparison of biomechanical response to surgical procedures used for cervical radiculopathy: Posterior keyhole foraminotomy versus anterior foraminotomy and discectomy versus anterior discectomy with fusion. J Spinal Disord. 2001;14:17-20.
Schmieder K., Kettner A., Brenke C., et al. In vitro flexibility of the cervical spine after ventral uncoforaminotomy. Laboratory investigation. J Neurosurg Spine. 2007;7:537-541.
1. Jho H.D. Microsurgical anterior cervical foraminotomy: a new approach to cervical disc herniation. J Neurosurg. 1996;84:155-160.
2. Jho H.D., Ha H.G. Anterior cervical microforaminotomy. Oper Tech Orthop. 1998;8:46-52.
3. Jho H.D. Failed anterior cervical foraminotomy. J Neurosurg (Spine 2). 2003;98:121-125.
4. Jho H.D., Kim W.K., Kim M.H. Anterior microforaminotomy for treatment of cervical radiculopathy. I. Disc-preserving “functional cervical disc surgery.”. Neurosurgery. 2002;51(suppl 2):46-53.
5. Hakuba A. Trans-unco-discal approach: A combined anterior and lateral approach to cervical discs. J Neurosurg. 1976;45:284-291.
6. Verbiest H. A lateral approach to the cervical spine: technique and indications. J Neurosurg. 1968;28:191-203.
7. Sampath P., Bendebba M., Davis J.D., Ducker T. Outcome in patients with cervical radiculopathy: prospective, multicenter study with independent clinical review. Spine. 1999;24(6):591-597.
8. Jho H.D. Decompression via microsurgical anterior foraminotomy for cervical spondylotic myelopathy. J Neurosurg. 1997;86:121-126.
9. Jho H.D. Decompression via microsurgical anterior foraminotomy for spondylotic cervical myelopathy: technical note. Neurosurg Focus. 1996;4:1-11.
10. Jho H.D. Treatment of spondylotic cervical myelopathy via anterior foraminotomy. Tech Neurosurg. 1999;5:124-132.
11. Jho H.D., Ha H.G. Anterolateral approach for spinal cord tumors. Minim Invas Neurosurg. 1999;42:1-6.
12. Jho H.D., Kim M.H., Kim W.K. Anterior cervical microforaminotomy for spondylotic cervical myelopathy. II. Neurosurgery. 2002;51(suppl 2):54-59.
13. Hacker R.J., Miller C.G. Failed anterior foraminotomy. J Neurosurg (Spine 2). 2003;98:126-130.
14. Yi S., Lim J.H., Choi K.S., et al. Comparison of anterior cervical foraminotomy vs arthroplasty for unilateral cervical radiculopathy. Surg Neurol. 2009;71:677-680.
15. Lee J.Y., Lohr M., Impekoven P., et al. Small keyhole transuncal foraminotomy for unilateral cervical radiculopathy. Acta Neurochir (Wien). 2006;148:951-958.
16. Koc R.K., Menku A., Tucer B., et al. Anterior cervical foraminotomy for unilateral spondylotic radiculopathy. Minim Invas Neurosurg. 2004;47:186-189.
17. Saringer W.F., Reddy B., Nobauer-Huhmann I., et al. Endoscopic anterior cervical foraminotomy for unilateral radiculopathy: anatomical morphometric analysis and preliminary clinical experience. J Neurosurg (Spine 2). 2003;98:171-180.
18. Saringer W., Nobauer I., Reddy M., et al. Microsurgical anterior cervical foraminotomy (uncoforaminotomy) for unilateral radiculopathy: clinical results of a new technique. Acta Neurochir. 2002;144:685-694.
19. Tascioglu A.O., Attar A., Tascioglu B. Microsurgical anterior cervical foraminotomy (uncinatectomy) for cervical disc herniation. J Neurosurg (Spine 1). 2001;94:121-125.
20. Johnson J.P., Filler A.G., McBride D.Q., Batzdorf U. Anterior cervical foraminotomy for unilateral radicular disease. Spine. 2000;25(8):905-909.
21. Cornelius J.F., Bruneau M., George B. Microsurgical cervical nerve root decompression via an anterolateral approach: clinical outcome of patients treated for spondylotic radiculopathy. Neurosurgery. 2007;61:972-980.
22. Kotani Y., McNulty P.S., Abumi K., et al. The role of anteromedial foraminotomy and the uncovertebral joints in the stability of the cervical spine: a biomechanical study. Spine. 1998;23:1559-1565.
23. Jho H.D., Jho D.H. Ventral uncoforaminotomy. J Neurosurg Spine. 2007;7:533-536.
24. Chen B.H., Natarajan R.N., An H.S., Andersson G.B.J. Comparison of biomechanical response to surgical procedures used for cervical radiculopathy: posterior keyhole foraminotomy versus anterior foraminotomy and discectomy versus anterior discectomy with fusion. J Spinal Disord. 2001;14:17-20.
25. Schmieder K., Kettner A., Brenke C., et al. In vitro flexibility of the cervical spine after ventral uncoforaminotomy: laboratory investigation. J Neurosurg Spine. 2007;7:537-541.