Anesthetic considerations for weight loss surgery
Surgical procedures for weight loss
Current WLS involves restrictive or restrictive-malabsorption components. Restrictive procedures (adjustable gastric banding, gastric sleeve resection) create a small stomach but do not alter how food is digested. The most common restrictive procedure at this time is the laparoscopic adjustable gastric banding procedure. The U.S. Food and Drug Administration approved the adjustable gastric band for clinical use in 2001, and its application has increased annually since its introduction. In this procedure, a limited dissection of connective tissue is performed at the top of the stomach, and an inflatable band is passed that encircles the upper stomach (Figure 164-1, A). The band can be adjusted via a port attached to the body wall by adding or withdrawing saline. The surgical risk is considered to be very low. In select patients, this is being performed as an outpatient procedure.
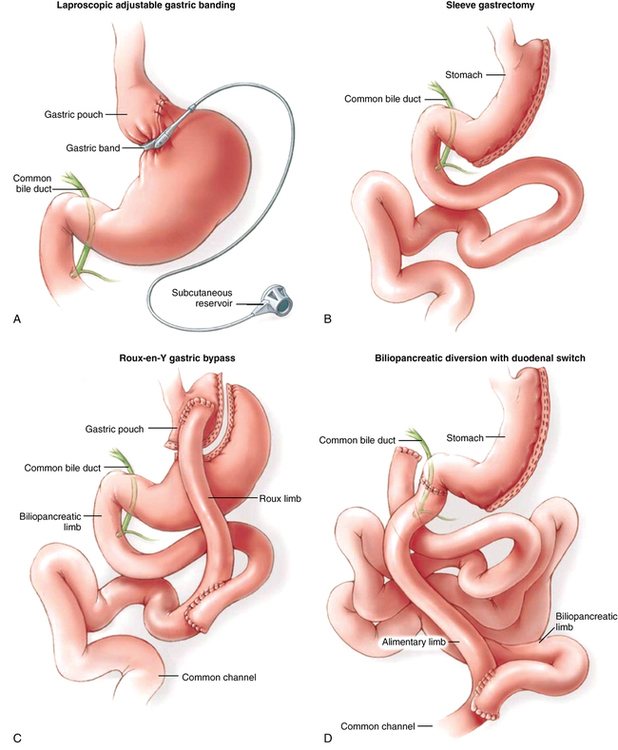
The gastric sleeve resection is typically performed laparoscopically and reduces stomach volume to approximately 100 mL by externally stapling the stomach to exclude the fundus and greater curvature to form a narrow tube along the lesser curvature of the stomach (Figure 164-1, B). Both laparoscopic adjustable gastric banding and gastric sleeve resection procedures result in significant weight loss, providing that the patients receiving these interventions comply with proper eating habits, particularly those who avoid high-caloric content (e.g., milkshakes, candy, ice cream). Blood loss is minimal. Surgical duration is relatively brief. Complications are infrequent.
The laparoscopic Roux-en-Y gastric bypass is currently the most commonly performed WLS in the United States. This procedure creates a small gastric pouch (30 mL) that empties into a limb of bowel that excludes a large portion of the small intestine (restrictive and maldigestive interventions) (Figure 164-1, C). As a result, satiety is achieved at relatively low volumes (restriction), and the surface area of small bowel that can absorb calories and nutrients is bypassed (maldigestion), resulting in reliable, sustained weight loss. Blood loss is minimal.
The biliopancreatic diversion/duodenal switch procedure is a more complex restrictive-malabsorptive procedure normally reserved for use in patients with a BMI in excess of 50 kg/m2. With this procedure, a gastric sleeve resection is performed, the stomach is separated from the duodenum, and the small bowel is divided at a point proximal (approximately 100 cm) to the terminal ileum. The stomach is reanastomosed to the distal limb of small bowel, creating an “alimentary channel” where food enters but is not digested. Biliary and pancreatic fluids drain into the duodenum (now separated from the stomach) and contact food where this “biliopancreatic limb” of proximal small bowel is anastomosed to the limb of the alimentary channel (Figure 164-1, D). Digestive enzymes contact food late in the process, and the short segment of small bowel that is available to absorb nutrients and calories is significantly restricted. Compared with the Roux-en-Y gastric bypass, the biliopancreatic diversion/duodenal switch procedure requires considerably longer time, but blood loss and fluid shifts are not significantly different.




