Chapter 38 Anesthetic and Airway Management of Microlaryngeal Surgery and Upper Airway Endoscopy
I General Considerations
The range and precision of laryngologic surgical procedures have dramatically expanded over the past decade. Patient outcomes have improved due to technologic advances such as the introduction of high-magnification operating microscopes and video endoscopes, instrument miniaturization, new injection materials, and the use of powered instrumentation and optical fiber–based lasers.1–5
Advances and demands of the new surgical techniques and an expanding patient population that was previously considered unsuitable for surgery have created novel challenges for the anesthesiologist. State-of-the-art anesthesia and airway management for laryngeal surgery require the anesthesiologist to be adept with various methods of managing the difficult airway and performing airway exchange, to competently execute intraoperative ventilation strategies, to be proficient with inhalational and total intravenous anesthesia, and to quickly tailor anesthetic techniques to the various durations of the surgical cases. New challenges have developed with the widespread introduction of minimally invasive laryngeal robotic surgery, which demands a fully open surgical field for three-dimensional visualization and superior motor control.4,6,7
Successful anesthetic management of microlaryngeal cases requires a high degree of cooperation with the surgeon, a reciprocal understanding of the potential problems, and adequate preparation on both sides to meet the anticipated challenges that may arise.1,3,8,9 Thorough appreciation by the anesthesiologist of the complexity of the upper airway anatomy, the pathologic process involved, and all steps of the surgical procedure is necessary for devising a rational anesthetic plan and maintaining a good working relationship with the surgeon.1,8,10 The expert ability to safely share the patient’s airway with the surgeon, in conjunction with an intimate knowledge of possible immediate intraoperative and early postoperative complications of laryngeal surgery, greatly contributes to safe patient management in the perioperative period.8
Microlaryngeal surgery encompasses a wide range of laryngeal procedures that can be organized in two broad categories: phonomicrosurgery (i.e., benign and malignant vocal cord lesions, laser laryngeal surgery, and vocal cord augmentation) and laryngeal framework surgery (i.e., vocal cord paralysis and motion disorders, scarring, stenosis of the glottic, subglottic, and tracheal areas, and laryngeal trauma).1 For practical purposes, these may be further categorized as involving endoscopy alone, surgical excision, injection, dilation, or a combination of these approaches.
This chapter focuses on airway management and anesthesia for microlaryngeal surgery, diagnostic direct laryngoscopy, and endoscopy (i.e., bronchoscopy and esophagoscopy). A combination of these three interventions, called panendoscopy, is typically performed as part of the diagnostic work-up for patients with head and neck cancer, and is accompanied by surgical biopsies of the base of the tongue, piriform sinuses, nasopharynx, and other diseased or suspicious areas.11–13 Additional surgical indications for bronchoscopy and esophagoscopy are discussed in the corresponding sections of this chapter, and management of pediatric patients is discussed separately from approaches to adults.
II Patient Preoperative Evaluation and Preparation
Patients presenting for phonomicrosurgery may have a variety of comorbidities contributing to their voice symptoms and affecting anesthetic management. Changes in voice quality can be exaggerated by inadequate airflow production (e.g., chronic obstructive pulmonary disease [COPD]) or vocal fatigue caused by neuromuscular disorders (e.g., myasthenia gravis, muscular dystrophy, Parkinson’s disease).1 Various rheumatologic and musculoskeletal ailments can alter posture, impairing voice quality, and endocrine disorders, such as hypothyroidism, can cause dysphonia as a result of swelling in the Reinke’s space (i.e., superficial lamina propria) of the vocal cords.1
Almost one half of the patients presenting with laryngeal and voice disorders have silent laryngopharyngeal reflux as the primary cause or as a significant etiologic factor.1,14 Coexistent significant glottic insufficiency (e.g., vocal cord paralysis) may place these patients at increased risk for aspiration of gastric contents,13,15 and it can usually be diagnosed during a routine preoperative flexible fiberoptic laryngoscopy or laryngostroboscopy performed by the surgeon. Those presenting for esophagoscopy for evaluation and treatment of esophageal obstructing lesions, achalasia, Zenker’s diverticulum, active gastrointestinal bleeding, or esophageal foreign body removal constitute another category of patients at high risk for aspiration. Even when gastroesophageal reflux is not clinically significant, adequate preoperative pharmacologic control of the symptoms is warranted: the combination of acid exposure and direct trauma from the operating procedure and the endotracheal intubation can lead to laryngeal mucosal injury.16
Many patients presenting for laryngeal surgery and panendoscopy have a long history of heavy smoking and drinking,17 which are directly linked to the development of squamous cell carcinoma of the larynx, the second most common malignancy of the head and neck.18 It is not uncommon for these patients to present with anemia.18 Appropriate laboratory studies should be obtained, and the electrolyte and fluid status of these patients should be optimized preoperatively.
Chronic cigarette smoking and alcohol use can cause induction of the cytochrome P450 multi-enzyme system, leading to increased perioperative requirements for opioids and neuromuscular blockers and generation of higher levels of potentially toxic metabolites of volatile halogenated anesthetic agents.19–22 Patients with chronic alcohol consumption require preoperative evaluation of liver function and coagulation status. For those with advanced liver disease, controlled hypotensive techniques should be avoided, and intraoperative hypotension should be treated aggressively to prevent adverse outcomes associated with prolonged decrease in hepatic circulation and further deterioration of liver function.23
Many patients who present for laryngeal surgery are elderly and have cardiovascular disease. Appropriate diagnostic tests are indicated for them as part of the preoperative work-up. The pulmonary status of COPD patients should be optimized to decrease airway reactivity and the possibility of postoperative pulmonary complications. Patients with significant lung disease and ventilation-perfusion (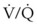 ) mismatch may not be suitable candidates for intraoperative ventilation techniques, such as spontaneous ventilation, apneic intermittent ventilation (AIV), or jet ventilation (JV),22–24 which may be required for microlaryngeal surgery (see “Intraoperative Ventilation Techniques and Strategies for Microlaryngeal Surgery”).
) mismatch may not be suitable candidates for intraoperative ventilation techniques, such as spontaneous ventilation, apneic intermittent ventilation (AIV), or jet ventilation (JV),22–24 which may be required for microlaryngeal surgery (see “Intraoperative Ventilation Techniques and Strategies for Microlaryngeal Surgery”).
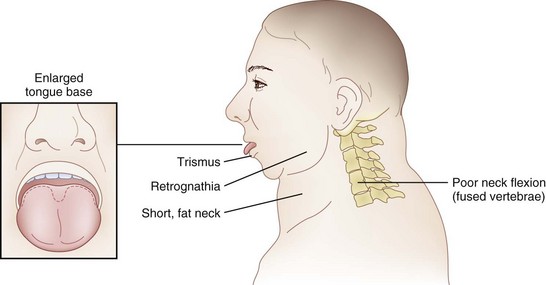
The rate of difficult endotracheal intubation may reach almost 16% among patients presenting for ear, nose, or throat cancer surgery,25 which is on average six times higher than among the general surgical patient population.25–29 Comprehensive preoperative airway assessment is paramount (see Chapter 8); however, standard anesthesia airway assessment tests fail to account for aspiration risk, lower airway problems, and base of the tongue pathology (e.g., epiglottic cancer, epiglottic and vallecula cysts, lingual tonsillar hypertrophy). Pathology of the base of the tongue may be encountered with increased frequency in patients presenting for panendoscopy and microlaryngeal surgery (Fig. 38-1).
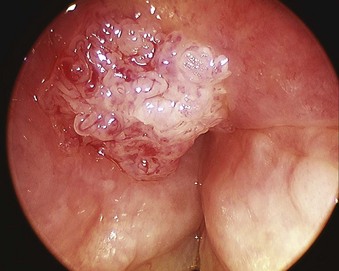
Figure 38-1 Epiglottic carcinoma.
(Courtesy of Edward Damrose, MD, Stanford University Medical Center, Stanford, CA.)
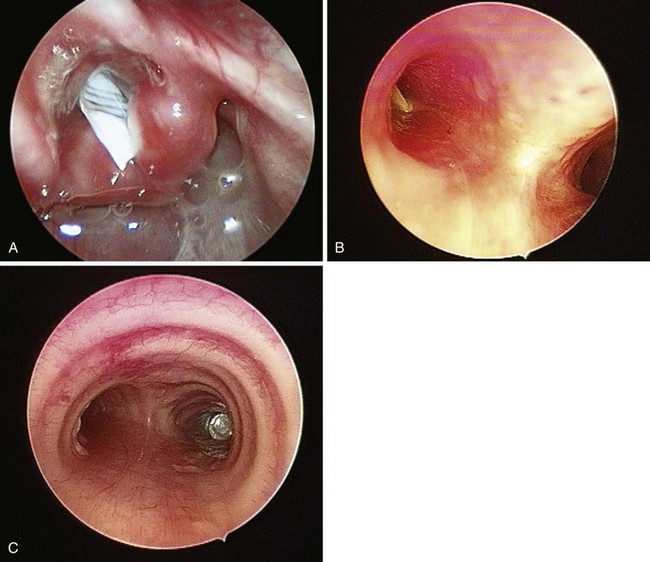
Postradiation changes in the neck and decreased mandibular protrusion are important factors predicting the risk of impossible mask ventilation, difficult mask ventilation, and difficult intubation in patients at risk for these conditions (see Chapter 8),30,31 and these risk factors may occur with increased frequency among patients presenting for microlaryngeal surgery or panendoscopy.12 The pharyngeal space may also be reduced by limited submandibular compliance of the soft tissues (e.g., cancerous involvement, masses, inflammation, previous radiation therapy) (Fig. 38-2), which may result in difficult intubation or failed intubation due to the restriction of the space that accommodates the tongue during direct laryngoscopy.32
Pharyngeal restriction can be further accentuated by a large tongue or intraoral masses that can be exophytic and mobile.32 Drooling, dysphagia, and expiratory snoring are the signs of marked pharyngeal restriction,17,32 but inspiratory stridor at rest represents the most worrisome sign, suggesting a reduction in airway diameter at the supraglottic, periglottic, or glottic level of at least 50%.15,31–33
Airway compromise in these patients may also involve the lower airways. Airway narrowing at the tracheal or tracheobronchial level is typically characterized by expiratory stridor, whereas biphasic inspiratory-expiratory stridor usually points to obstructive subglottic lesions.8 In some cases, preoperative examination of the flow-volume loops may be helpful.34
It is prudent to assess the laryngeal mobility, the degree of tracheal deviation, and the location of the cricothyroid membrane (CTM).18 Significant tracheal deviation, especially in combination with the fixed hemilarynx (Fig. 38-3) and poor or absent visualization of the vocal cords during preoperative nasal endoscopy, can be an ominous sign,33,35 warranting performance of an awake tracheostomy, if technically feasible. Usually, the extent of disease in elective cases has been comprehensively evaluated preoperatively by routine chest radiography, computed tomography (CT), magnetic resonance imaging (MRI), and flexible fiberoptic laryngoscopy, providing the anesthesiologist with valuable information regarding the location, size, spread, and vascularity of the obstructive lesions; the degree of obstruction; the mobility of the vocal cords; and the extent of laryngeal and tracheal deviation or compression.8,18,36 Preoperative discussion of these findings with the surgeon helps to devise safe and rational airway management and anesthetic plans for the patient.18
Other airway considerations for patients presenting for microlaryngeal surgery or panendoscopy include anticipation of the presence of supraglottic and glottic edema due to inflammation, infection, tumors, previous radiation therapy or repeated endoscopies,37 and careful dental assessment. Gentle airway manipulation during direct laryngoscopy is essential. The use of a smaller-diameter endotracheal tube (ETT) is frequently warranted, and the absence of dental trauma should be documented before surgical instrumentation of the patient’s airway commences.
III Operative Laryngoscopy and Microlaryngeal Surgery
A Special Considerations and Anesthesia Objectives
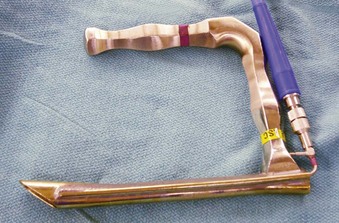
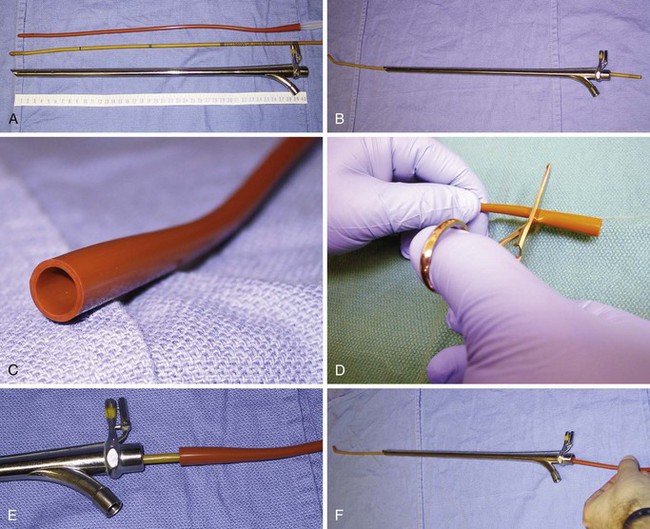
In contrast to direct laryngoscopes used by the anesthesiologists, which are designed only to identify the glottic opening, operating laryngoscopes can provide excellent and wide laryngeal exposure and allow diagnostic examination, biopsy, and operation on structures in the larynx and pharynx, with minimal distortion of the areas of surgical interest.16,38 The handles of these laryngoscopes are integrated with the blades and have a wide proximal aperture that facilitates the passage of instruments during suspension laryngoscopy.38,39 Many types of laryngoscopes exist (Fig. 38-4), each offering certain advantages for its intended application, such as the ability to better expose supraglottic, glottic, or subglottic areas.38 Many laryngoscopes are multipurpose, and selection is frequently dictated by individual or institutional preference.38

Figure 38-4 Commonly used operating laryngoscopes. The posterior apertures of the Kleinsasser (left), Dedo (center), and Holinger (right) operating laryngoscopes are shown. The bigger size and wider channel of the Kleinsasser and Dedo laryngoscopes allows passage of the surgical instruments and binocular microlaryngeal surgery. Notice the metal cannula inserted into the left side port of the Kleinsasser laryngoscope for supraglottic jet ventilation (JV). In the Dedo scope, the JV port is integrated with the lumen, and it can be used for smog evacuation during laser surgery. The Holinger laryngoscope is monocular but has greater maneuverability. It is widely used for diagnostic laryngoscopy and visualization of the anterior commissure. The left side channel of the Holinger laryngoscope is occupied by a light guide. Supraglottic JV through the Holinger laryngoscope requires insertion of a metal cannula inside its lumen (see Fig. 38-15).
With the use of these laryngoscopes, systematic endoscopy of the larynx and pharynx frequently proceeds in three stages, progressing from handheld examination to suspension laryngoscopy (for more detailed evaluation with the straight and angled telescopes) and then to microlaryngoscopy using the operating microscope for image magnification, biopsy, microsurgery, or laser surgery.38 Suspension laryngoscopy (Fig. 38-5) frees the surgeon’s hands for precision bimanual surgery and facilitates maintenance of a stable plane of anesthesia.38,40 The use of a video monitor by the surgeon permits the anesthesiologist to observe the surgical procedure and monitor the patient’s airway.
The essential requirements for precision microlaryngeal surgery and optimal preservation of function include a clear and still surgical field, absence of patient movement, and allocation of sufficient time to carefully complete the procedure in an unhurried manner.9,38,39,41 The patient’s airway must be protected from blood, debris, and irrigation fluid, and ventilation must be adequately controlled.3,8,41 The anesthesiologist must safely share the patient’s airway with the surgeon, and must be prepared to skillfully and confidently switch from one ventilation technique to another during the case if needed or dictated by surgery.
In most surgical procedures, the patient’s airway is shared with the surgeon, and immediate access to the airway is difficult or impossible because the operating room (OR) table is turned 90 or 180 degrees away from the anesthesiologist. The ETT must be secured diligently to prevent accidental extubation under the surgical drapes or withdrawal of the ETT into the larynx, resulting in a sudden air leak or possible compression of the anterior branch of the recurrent laryngeal nerve by the ETT cuff.42,43
Performance of conventional and operative direct laryngoscopy, supraglottic tissue distention, and laryngeal stimulation elicit intense cardiovascular responses, resulting in tachycardia, arterial and pulmonary hypertension, and arrhythmias.44–46 Although these responses are usually short lived, myocardial ischemia and compromise of cerebral circulation may occur in high-risk patients, resulting in adverse outcomes.47–49 Anesthetic technique should ensure a stable plane of anesthesia, nonstimulating emergence from anesthesia, a rapid return of consciousness, and protective airway reflexes, and it should facilitate quick discharge of patients, because most of these surgical procedures are done on an outpatient basis.3,8
Special attention should be directed to adequately protecting the patient’s eyes and arms to prevent accidental injury or compression by heavy surgical instrumentation.9 When rigid endoscopy is planned, a tooth guard should be used routinely.34 It may be prudent to warn the patients in advance of the potential for dental trauma, and any previous dental damage should be carefully documented.34,43
Patients who are vocal performers or use the voice in some professional capacity present unique challenges.16 The anesthesiologist must frequently think outside the box and exercise different advanced airway management options to avert trauma to the patient’s vocal cords and cricoarytenoid joints.
B Airway Management for Microlaryngeal Surgery
1 Conventional and Advanced Airway Management
Video laryngoscopy reliably improves laryngeal exposure by at least one grade,50–54 allows continuous observation of the entire intubation procedure by the entire team, and may therefore be a near-ideal technique for managing difficult airways in patients presenting for microlaryngeal surgery. Choosing the video laryngoscopic device depends on the operator’s preference and must consider the nature and location of the lesions. For example, it may be safer to navigate around tumors at the base of the tongue with the Pentax Airway Scope, whose blade engages under the epiglottis, unlike other devices that typically require the tip of the blade to be placed in the vallecula. Video laryngoscopes that use the steering technique (i.e., styleted ETT), such as the Glidescope video laryngoscope, offer better control of intubation and may facilitate ETT maneuvering around the intraoral masses.55 Although the use of the Glidescope may be less traumatic compared with the Airtraq in patients presenting for microlaryngeal surgery,56 in the largest published series of Glidescope-assisted intubations in more than 2000 patients with difficult airways,57 the strongest predictor of the steering technique failure was altered neck anatomy with presence of a surgical scar, radiation changes, or a mass. These conditions are frequently encountered in patients presenting for diagnostic direct laryngoscopy and microlaryngeal surgery.
Although advanced airway management techniques can be highly successful when direct laryngoscopy fails, the patient’s unfavorable anatomy (Fig. 38-6) may not be modifiable for the surgical exposure, which requires the use of the largest operating laryngoscope and placement of the patient’s head in the Boyce-Jackson position using a combination of cervical flexion and atlantooccipital extension (see Fig. 38-5).1,38,58,59 If suspension laryngoscopy fails or if the location of the lesion is not easily accessible, it can be performed, to the extent microlaryngeal surgery permits, with the help of the flexible fiberoptic bronchoscope (FFB) inserted through the laryngeal mask airway (LMA).60–62 The intubating laryngeal mask airway (iLMA) offers certain advantages, such as a rigid, wide metal tube that can accommodate a large-diameter FFB,61 optimal alignment of the iLMA aperture with the glottic opening,63 diminished hemodynamic responses compared with suspension laryngoscopy,61 and superior ventilation capabilities.63–66
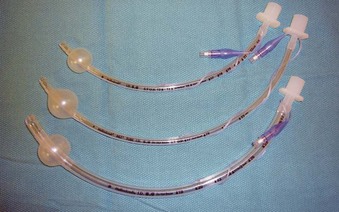
The iLMA is associated with an outstanding success rate for blind endotracheal intubation in patients with difficult airways.63,65 Unfortunately, the manufacturer-supplied iLMA ETTs are too big for most microlaryngoscopic surgery. An ETT with a smaller inner diameter (ID) (e.g., 5.0-mm ID microlaryngeal tracheal [MLT] tube) is typically required to maximize the surgical view (Fig. 38-7). Placement of MLT tubes through the iLMA can be achieved with the help of a small-diameter FFB; however, passage of the ETT through the laryngeal inlet into the trachea is blind. Blind advancement of the ETT may cause inadvertent laryngeal trauma and core out pedunculated supraglottic or glottic tumors, nodules, or cysts.56,67 When the FFB route (with or without the use of a supralaryngeal airway device) is chosen for endotracheal intubation, it is advantageous to closely match the outer diameter (OD) of the scope with the ID of the ETT to minimize the risk of complications associated with blind ETT advancement. Use of optical stylets (e.g., Bonfils, Shikani, Clarus Video System) may also be beneficial in that regard, because the ETT will follow the trajectory of the stylet navigated under direct vision through the vocal cords. However, most of the available adult-size stylets require the use of an ETT with a minimum ID of 5.5 to 6.0 mm.
The decision to proceed with an awake or asleep approach to an anticipated difficult airway should follow the American Society of Anesthesiologists (ASA) difficult airway algorithm,68 with special attention directed to predictors of difficult mask ventilation, impossible mask ventilation, and their association with difficult intubation (see Chapter 8). The anesthesiologist also should review the pertinent preoperative findings identified on flexible fiberoptic laryngoscopy, chest radiography, CT, and MRI and should discuss these findings with the surgeon.
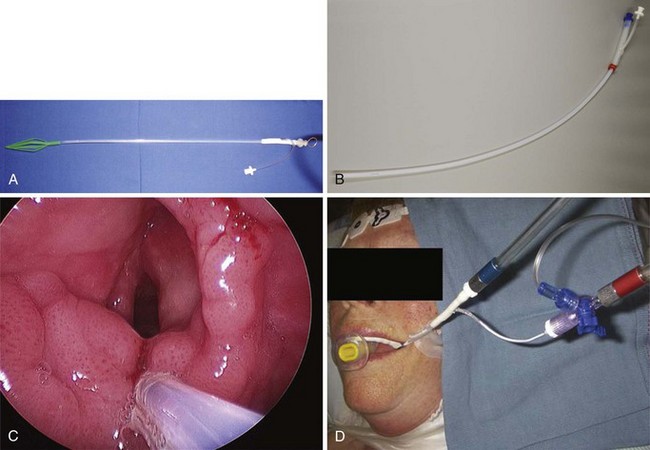
If an asleep approach to the difficult airway is chosen, several preformulated alternative airway management plans must be in place before induction of anesthesia. If the airway is marginal, the patient’s neck should be prepped, and the surgical team should be present on induction, ready to perform an emergent cricothyrotomy or tracheostomy, or to employ rescue techniques such as the use of the surgical anterior commissure scope or a rigid bronchoscope.11,18,69,70 The anterior commissure scopes (e.g., Holinger, Ossoff-Pilling, Benjamin Slimline/Super-Slimline, Jackson) (Fig. 38-8; see Fig. 38-4) have great leverage capabilities, incorporate the recessed lighting and concurrent rigid microsuction, and can be very effective in handling poor laryngeal exposure or glottic obstruction.1,13,38,69 The anterior flare at the distal oval end allows these scopes to be used as a conduit for orotracheal intubation when the bougie introducer or the ETT is passed directly down the lumen (Fig. 38-9).69
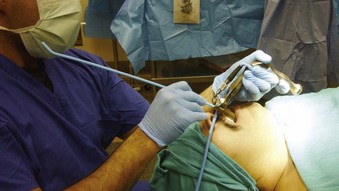
Figure 38-9 Airway rescue is achieved with a bougie introducer and a Holinger laryngoscope in a patient with difficult laryngeal exposure. The endotracheal tube (ETT) cannot be directly advanced down the narrow barrel of the Holinger laryngoscope due to impaired visualization during tube advancement. The bougie introducer is used first, followed by removal of the laryngoscope and railroading the ETT over the bougie into the patient’s trachea. The ETT can be directly advanced through the wider lumen of other laryngoscopes, such as the Lindholm or Dedo (see Fig. 38-14).
In experienced hands, rigid bronchoscopy may be used to rescue failed direct laryngoscopy and failed intubation and to manage a “cannot intubate, cannot ventilate” (CICV) situation.68 It also serves as an indispensable tool for managing acute airway obstruction resulting from foreign bodies, hemoptysis, or tumors.71 After the bronchoscope is placed into the patient’s trachea by the surgeon, manual (Fig. 38-10) or JV can commence in a safe manner through the lumen of the bronchoscope. Subsequent airway exchange to the ETT can be performed using a bougie introducer (Fig. 38-11).72,73 This exchange technique can also be conducted when the rigid bronchoscope is employed first as part of a panendoscopy procedure in patients with abnormal airway.
Patients with an advanced airway obstruction and inspiratory stridor at rest comprise some of the most feared and complicated cases for the anesthesiologist.33 The incidence of difficult mask ventilation and impossible mask ventilation among patients with severe stridor and upper airway obstruction of more than 75% of the lumen reaches 40% and 6%, respectively,74 compared with 1.4% and 0.15% for the general surgical population.30,31,75 These patients frequently present for panendoscopy and microlaryngeal surgery on an emergent or semi-emergent basis, yet they require a systematic and thoughtful approach by the anesthesiologist and the surgeon.38 The nature of the obstructing lesion (e.g., vascular, submucosal, pedunculated, inflammatory) and its location (e.g., supraglottic, glottic, subglottic, midtracheal, lower tracheal, and bronchial [mediastinal]) may require completely different intubation considerations and approaches.17,33,34,36,38,69,71
In the context of laryngeal surgery, the optimal technique of airway management of the stridorous patient with an advanced proximal airway obstruction (i.e., supraglottic, glottic, and subglottic levels) remains a subject of controversy. An awake flexible fiberoptic intubation, inhalational induction, and intravenous induction with muscle relaxants17,33,74,76 have been used successfully, but none should be considered fail-safe. Thorough preoperative discussion of the surgical pathology and formulation of closely coordinated airway management plan with the surgeon are essential for safe management of these patients.
Based on our experience and review of the pertinent literature,17,33,35,38,69,71,74,76–80 current recommendations for management of the critically obstructed airway can be outlined as follows:
1. For patients with severe stridor (e.g., symptoms exaggerated at night, hypoxemia-induced agitation or panic attacks, use of accessory muscles on inspiration, a large tumor, fixed hemilarynx, gross anatomic distortion, a larynx not visible on preoperative nasal endoscopy or flexible fiberoptic laryngoscopy), strongly consider tracheostomy under local anesthesia without sedation.
2. Patients with moderate stridor and a significant lesion seen on nasal endoscopy or flexible fiberoptic laryngoscopy, but who are considered possible to intubate, are best managed with an inhalational induction or an awake fiberoptic intubation. All airway instrumentation should proceed in a careful and gentle manner. Endotracheal intubation should be accomplished rapidly, with a small ETT. The number of attempts should not exceed two, because critical airway obstruction can quickly progress to complete as a result of manipulation of the airway.
3. If an inhalational induction is chosen, a sufficiently deep and stable plane of anesthesia is essential to avoid loss of the airway (e.g., avoidance of cough, laryngospasm). Endotracheal intubation should be performed under direct vision (e.g., direct laryngoscopy, video laryngoscopy, flexible fiberoptic bronchoscopy). Muscle relaxants should be avoided until after the intubation is completed to prevent sudden, complete airway obstruction, especially when the tumor is subglottic. The patient’s neck should be prepared, and the surgical team should be present and ready to attempt an airway rescue with an anterior commissure scope or a ventilating rigid bronchoscope or by emergent cricothyrotomy or tracheostomy.
4. If endotracheal intubation under direct vision (e.g., direct laryngoscopy, video laryngoscopy, flexible fiberoptic bronchoscopy) fails in an anesthetized or awake patient or is deemed problematic, tracheostomy should be performed expeditiously, with the patient breathing spontaneously.
5. An awake fiberoptic intubation should be used with caution, because sudden loss of the airway can be precipitated by one or more of the following factors:
6. Additional fallback strategies may include the following:
7. Patients with inspiratory obstruction due to bilateral vocal cord paralysis or fixation of cricoarytenoid joints typically do not present ventilation or intubation problems.
8. If tracheostomy is avoided, an extubation strategy must be decided on with the surgeon. Extubation should be performed over an airway exchange catheter (AEC), with the necessary reintubation equipment immediately available. Some patients should remain intubated until the airway inflammation and edema subside, and the patient’s airway is then reevaluated.
2 Intraoperative Ventilation Techniques and Strategies for Microlaryngeal Surgery
Surgery can be conducted in an awake patient, frequently under conscious sedation, or with the patient anesthetized (Box 38-1). The ventilation options under general anesthesia consist of “tube” (i.e., endotracheal intubation) and “tubeless” techniques, with the latter represented by the techniques of spontaneous ventilation, AIV, and JV.8,13,38,81,82
Box 38-1 Ventilation Techniques and Strategies for Microlaryngoscopic Surgery
a Awake Airway Surgery with Conscious Sedation
For selected patients, many laryngoscopic procedures can be safely and effectively performed in an office-based setting, including diagnostic endoscopy, laser surgery, panendoscopy for cancer screening and biopsies, and therapeutic vocal cord injections.2–516 The key to success for office-based surgery remains adequate topical and regional anesthesia of the patient’s airway, which is usually performed by the surgeon and typically follows preparation of the patient for awake oral and nasal flexible fiberoptic intubation (see Chapter 19). Although highly motivated patients can undergo office-based laryngoscopic surgery strictly under local anesthesia, most desire sedation and amnesia.3
If presence of the anesthesiologist is requested, the main objectives are to monitor for possible local anesthetic toxicity (see Chapter 19), to supplement local anesthesia with a rapidly titratable and reversible state of sedation, and to treat acute hyperdynamic responses that can occur in up to 20% to 30% of patients, despite seemingly adequate topical anesthesia of the airway.3,83 Judicious use of intravenous opioids or sedatives/hypnotics, or both, is paramount, because a loss of patient cooperation may result in intraoperative injury.3,16,34 Sedation of the patients with obstructive sleep apnea and morbid obesity should be performed with extreme caution.84,85
b Asleep Airway Surgery with General Anesthesia
General anesthesia for microlaryngeal surgery represents a unique example of some of the conflicting intraoperative goals that exist between the surgeon and the anesthesiologist with regard to the patient’s airway control and maintenance. For the surgeon, ideal operating conditions would be completely unobstructed surgical visualization, unimpeded surgical manipulation, and absence of movement in the surgical field. From the anesthesiologist’s perspective, the ideal anesthetic technique would allow adequate protection of the patient’s lower airway from aspiration and the use of stable, controlled mechanical ventilation with the ability to measure the concentration of anesthetic gases, peak inspiratory pressure (PIP), inspired oxygen concentration (FIO2), and end-tidal carbon dioxide level (EtCO2).81 In most cases, these objectives can be balanced by the use of a small MLT tube, maximizing the patient’s safety and the success of surgery.
Endotracheal Intubation with Microlaryngeal Tracheal Tubes
The use of a small (5.0-mm ID) MLT tube with positive-pressure ventilation remains the standard for airway management in most nonlaser microlaryngeal surgery, and it is associated with minimal or no intraoperative complications.86,87 (For anesthetic management of the laser airway surgery see Chapter 40.) Adequate gas exchange can be maintained through small-ID ETTs in most adult patients,88,89 unless the duration of surgery approaches 2 hours (which happens rarely).88 Even then, despite a consistent trend toward progressive hypercapnia and respiratory acidosis, the pH and EtCO2 values remain within physiologic range.89
With most glottic pathology originating in the anterior two thirds of the larynx,90 consistent positioning of a small MLT tube between the arytenoid cartilages in the posterior part of the glottis leaves most of the surgical field unobstructed to the surgical view and manipulations.4,13,16,38,91 Even with many posterior glottic disorders, it may be possible for the surgeon to gently displace the MLT tube anteriorly with the microsurgical cupped forceps or to perform the surgery using the specially designed posterior glottic laryngoscopes.90,92
However, if the posterior glottis is occupied by a significant surgical pathology (e.g., posterior glottic or subglottic stenosis, transglottic tumor) (Fig. 38-12), use of alternative, tubeless ventilation techniques becomes necessary.38 Because of the surgeon’s preference, tubeless ventilation can also be requested as a primary ventilation mode from the outset of the procedure.
Tubeless Techniques
Spontaneous Ventilation
Spontaneous ventilation is rarely used in adult microlaryngeal surgery,93–95 but it is commonly employed in the pediatric patient population, for whom it offers the additional ability to evaluate dynamic airway function and the level of obstruction (see “Anesthesia for Pediatric Airway Endoscopy and Microlaryngeal Surgery”). Anesthetic gases can be delivered (insufflated) through a nasal trumpet connected through an ETT adapter to the anesthesia circuit,96–99 an ETT positioned in the nasopharynx,82,100,101 a metal cannula, a side port of the rigid bronchoscope or operating laryngoscope (Fig. 38-13; see Fig. 38-10),38 or a catheter placed through the vocal cords into the patient’s trachea.9,102,103 Scavenging of anesthetic gases can be facilitated with an open suction tube at the corner of the patient’s mouth.
Although this technique offers free access to the larynx, it does not provide a still surgical field for precision surgery, it affords no protection of the lower airway, and it contaminates the OR environment.34,87,103 Deep planes of anesthesia are usually required to blunt the laryngeal responses and to prevent patient movement, which tends to provoke cardiovascular instability and ventilatory compromise (i.e., hypoxemia, hypercarbia, and short periods of apnea).8,39,104 With careful technique, inhalational agents can be substituted for total intravenous anesthesia (TIVA).82,96,105 However, control of the patient’s movement and a stable plane of anesthesia frequently remains problematic.104 Monitoring an adequate hypnotic state (e.g., processed electroencephalographic activity) may be advisable for these patients.
The protagonists of spontaneous ventilation technique may wish to routinely supplement general anesthesia with topical or local anesthesia of the airway (usually done by the surgeon after deployment of suspension laryngoscopy), which facilitates maintenance of a more stable and lighter plane of anesthesia, promotes hemodynamic and respiratory stability, and decreases the incidence of intraoperative laryngospasm.39,82,96,103,105,106
Apneic Intermittent Ventilation
AIV remains a relatively popular technique for microlaryngeal surgical procedures of short duration in some surgical centers.87 Compared with spontaneous ventilation, it affords more stable and controlled anesthetic conditions, as well as full muscle relaxation. After induction of anesthesia, the patient’s lungs are ventilated by a face mask or an LMA, which is followed by a period of apnea to allow deployment of a suspension laryngoscope by the surgeon. The patient’s trachea is subsequently intubated by the surgeon with a small-diameter, preferably uncuffed ETT that is placed through the lumen of the laryngoscope,87 and the patient’s lungs are hyperventilated with an FIO2 of 1.0 (Fig. 38-14). The ETT is then removed to provide a fully unobstructed and still surgical view of the larynx. The ETT is withdrawn and reinserted as frequently as necessary to maintain an oxygen saturation by pulse oximetry (SpO2) of 90% or greater and EtCO2 between 40 and 60 mm Hg,18,87,107 allowing periods of apnea up to 5 to 10 minutes in healthy adult patients.87,108 Apneic oxygenation through the hypopharyngeal catheter, preceded by an adequate period (10 minutes) of preoxygenation and denitrogenation of the patient’s lungs, can be tried in anesthetized and paralyzed patients.108
TIVA is typically used for maintenance. Monitoring the hypnotic state of anesthesia is advisable during AIV, because the incidence of awareness and recall may reach 4% (30 times higher than in the general surgical population), especially when the inhalational agents are used to supplement intravenous anesthesia.109,110
The disadvantages of AIV include slowing the pace of surgery, disruption of the surgical field, possible trauma to the vocal cords and lower airway due to repeated endotracheal intubation, and a propensity for laryngospasm.87 In a study of more than 350 patients,87 the incidence of intraoperative laryngospasm with AIV was 1.4%. The AIV may not be suitable for patients with significant lung or cardiovascular disease,107 and it leaves the patient’s lower airway unprotected to aspiration.18
Appropriate and successful phonomicrosurgery can rarely be performed using AIV, because the apnea periods are too short to permit unhurried precision surgery.1,111 This technique may be better reserved for short, uncomplicated cases.
Jet Ventilation
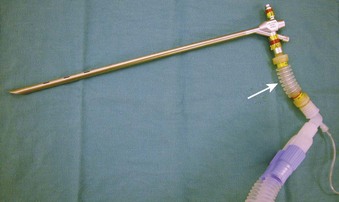
Supraglottic JV (i.e., jet nozzle above the glottic opening) for microlaryngeal surgery can be performed through the side port of a suspension operating laryngoscope, with the jet cannula attached to the lumen of the laryngoscope (Fig. 38-15; see Fig. 38-4)1,24,59,112 or through a specialized jet laryngoscope.113,114
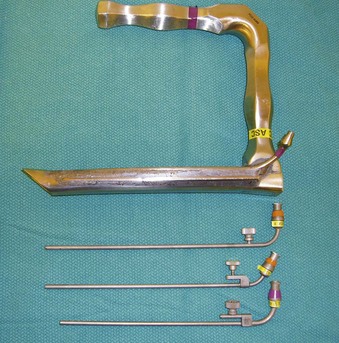
Figure 38-15 A Dedo operating laryngoscope with the integrated side port (top) can be used for supraglottic jet ventilation, and different jetting metal cannulas (bottom) can be inserted through the side ports of the operating laryngoscopes or directly through their lumen (see Fig. 38-4).
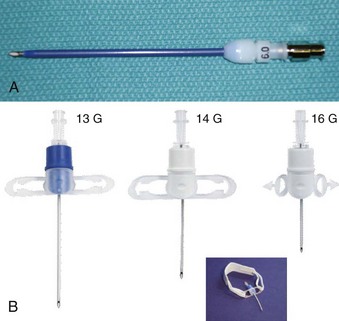
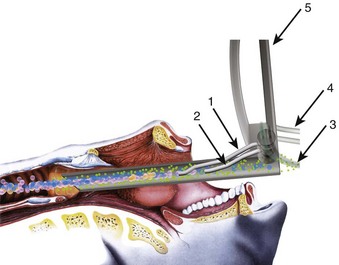
Subglottic JV (i.e., jet nozzle below the glottic opening) is established by bypassing the larynx from above (i.e., translaryngeal or transglottal approach) or below (i.e., percutaneous approach) through the CTM or the upper TTJV rings.74,86,87,111 Transglottal JV typically employs specialized, laser-safe, small-diameter, orally placed, double-lumen catheters (Fig. 38-16),24,81,115,116 in which the large port is used for jetting and the smaller lumen for monitoring the distal airway pressure and respiratory gases. Long, single-lumen catheters (typically 1.5- to 3-mm ID), some of which are laser resistant, may be used and can be placed through the oral or nasal route24,34,87,102,117–119; however, they lack concurrent monitoring capability. Alternatively, a small-diameter, movable, metal jet cannula can be passed through the glottis by the surgeon after the suspension laryngoscope is in position (Fig. 38-17).87,120 For transglottal JV, midtracheal placement of the catheter or cannula is usually preferred. TTJV is typically administered through a long catheter or Ravussin-type cannula (Fig. 38-18).74,86,121 For TTJV catheter or cannula placement, the use of an FFB or a rigid bronchoscope may be advocated to monitor the procedure87,111,122 and to minimize the risk of unnoticed posterior tracheal wall laceration, which may lead to submucosal gas injection and barotrauma.87,111 Use of a rigid bronchoscope with the bevel turned posteriorly may be especially efficacious, because the posterior tracheal wall is protected by the bronchoscope from the needle entry.87 For transglottal JV and TTJV, endoscopic control also allows adjustment of the position of the distal end of the catheter or cannula to optimize HFJV.24,86,87,122
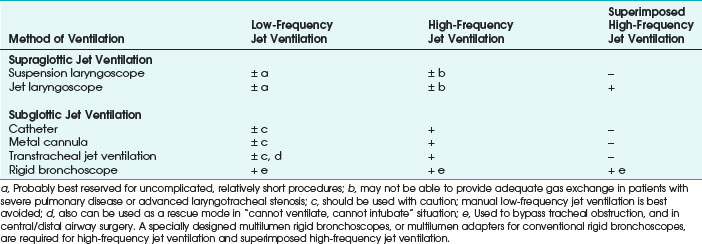
Compared with endotracheal intubation, supraglottic and subglottic JV techniques have distinct advantages of providing the surgeon with an enlarged, clear or minimally impeded, and undistorted view of the endolarynx, facilitating surgical access and eliminating flammable material (i.e., ETT) from the patient’s airway during laser surgery.24,115 Although supraglottic and subglottic ventilation techniques can use low-frequency jet ventilation (LFJV), HFJV, or superimposed high-frequency jet ventilation (SHFJV) modes,34,86,111,123–125 the use of these modes in clinical practice is usually more restrictive (Table 38-1).
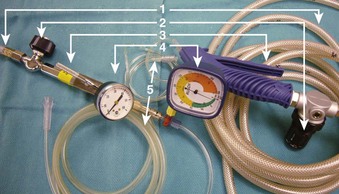
The use of manual supraglottic LFJV (i.e., Venturi jet ventilation) at a rate of less than 60 breaths/min continues to predominate in clinical practice,111 probably because of the low cost and easy accessibility of manual JV devices (Fig. 38-19) (a manual mode also can be preset on commercially available jet ventilators, where available).59,111,126,127 Although an overall incidence of complications with manual supraglottic LFJV may be low (0.42%),120 a survey of 229 U.K. centers revealed that it was responsible for most major complications (e.g., significant hypoxemia, barotrauma, unplanned admission to the intensive care unit) and for all deaths, especially when applied subglottically.111 This suggests that LFJV should be reserved for uncomplicated, elective procedures of short duration and that it may not be regarded as a standard of practice for microlaryngeal surgery.24 For increased safety, LFJV should be started with a low driving pressure (≤10 psi), which is gradually increased until visible chest excursions are observed, and adequate oxygen saturation is maintained.24,59,111
The subglottic HFJV mode (respiratory rate of 100 to 300 breaths/min; tidal volumes [VT] of 1 to 3 mL/kg), delivered through specialized automated jet ventilators, is typically used.24,34 Compared with supraglottic LFJV, in which intermittent apnea is frequently required due to significant vocal cord movement, subglottic HFJV significantly reduces laryngeal motion and affords a quiet surgical field without the need for interrupting ventilation.125 If vocal cord movement becomes a problem, HFJV driving pressure can be decreased, and the respiratory frequency can be increased to provide a smoother gas flow, or the ventilator can be turned off during particularly delicate parts of the procedure.24,125
Despite very small VT values, CO2 elimination during subglottic HFJV is facilitated by the upstream turbulent convective flow of CO2 along the decreasing gradient from the alveoli to the conducting airways.34 The alveolar-arterial CO2 gradient in patients with normal lung function is largely maintained within normal range.34,128 Monitoring EtCO2 during HFJV can be accomplished by briefly switching to LFJV mode on the ventilator to get a reliable signal or by using transcutaneous Pco2 monitoring.24 Administration of subglottic HFJV also results in a higher inspired O2 concentration, because entrainment of air is reduced deep inside the airway.24,86 Improved oxygenation is further enhanced by generation of continuous positive end-expiratory pressure in small airways (auto-PEEP), leading to alveolar recruitment and increased functional residual capacity (FRC).24,34,119 Nevertheless, patients with severely restricted lung compliance, 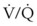 mismatch or shunting, and reduced FRC (e.g., morbid obesity) remain at increased risk for hypoxemia.86
mismatch or shunting, and reduced FRC (e.g., morbid obesity) remain at increased risk for hypoxemia.86
In contrast to supraglottic LFJV, with which contamination of the lower airway due to air entrainment is possible,34,81 a continuous, upward-directed flow of gas during subglottic HFJV creates a positive-pressure build-up, preventing blood and surgical debris from being directed down an unprotected airway.111,119,129 However, increased airway pressure creates concern about air trapping and barotrauma, mandating maintenance of adequate gas outflow at all times.130
Before the suspension laryngoscope is secured, with the patient anesthetized and a subglottic JV catheter or cannula in place, strategies for minimizing the risk of barotrauma on initiation of subglottic HFJV may include starting ventilation with low driving pressures (<15 to 30 psi), allowing sufficient exhalation time by avoiding high-frequency ventilation (i.e., starting with LFJV first), and maintaining the patient’s airway patency with the assistance of an oral airway or providing a jaw lift, if needed.74,86,87,125,131 Alternatively, initiation of the subglottic HFJV can be held off until the suspension laryngoscope is deployed, and ventilation is supported conventionally through a face mask or the LMA. It may be prudent to confirm absence of the subglottic catheter or cannula obstruction by the EtCO2 return and to check the catheter or cannula position endoscopically before subglottic HFJV commences.86,125
On emergence from anesthesia, small VT values and low peak and mean airway pressures associated with subglottic HFJV enable the patient to breathe spontaneously, facilitating a transition to adequate spontaneous ventilation.24,34,86,132,133 This transition can be further assisted at the end of surgery by increasing the frequency of ventilation to 300 breaths/min, increasing FIO2 to 1.0, and setting a ventilator driving pressure at about 10 psi (0.8 bar), which enables almost continuous flow of O2 and apneic oxygenation, as well as a rise in the carbon dioxide (CO2) level.24 If the conversion to spontaneous ventilation through a small subglottic catheter proves difficult, the patient’s airway can be supported through a face mask, LMA, or ETT, as required; these conventional bridge airway strategies equally apply to transitioning from supraglottic JV. If obstructive airway lesions exist, subglottic HFJV must be used with extreme caution. If upper airway obstruction is greater than 50%, the position of the jet nozzle should be proximal to the site of the obstruction to prevent barotrauma, or the obstruction must be bypassed by a rigid bronchoscope first.24,79
Total outflow obstruction with resultant barotrauma during subglottic HFJV can be quickly precipitated by surgical instrumentation, glottic edema, laryngospasm, or closure of the vocal cords due to inadequate depth of anesthesia or inadequate muscle relaxation.81,86,130 Modern automated jet ventilators (e.g., Monsoon III Universal Jet Ventilator [Acutronic Medical Systems AG, Hirzel, Switzerland], Twin Stream [Carl Rainer GmbH, Vienna, Austria]) incorporate multiple safety features, including automatic ventilator shutdown, if the user-preset pressure limits are exceeded.24,74 This design has enabled some experienced providers to successfully use high-frequency TTJV in patients with massive supraglottic lesions and severe airway compromise,74 for which the use of supraglottic or subglottic JV was not possible or surgically feasible. The presence of a second anesthesiologist to facilitate monitoring and maintenance of an upper airway was required and deemed an important safety factor in preventing intraoperative pressure-related complications in all cases.74 Although no major complications were observed in this series of 50 patients,74 the incidence of minor complications reached 20%, a more than threefold increase compared with the instances when high-frequency TTJV has been used in patients with less severe airway compromise.87 Study results hold some promise that a simple,134,135 portable expiratory ventilation assistance (EVA) device may be able to facilitate egress of gas through the jet catheter during TTJV, thereby increasing the safety of this technique; however EVA suitability for microlaryngeal surgery remains to be established.
Compared with the transglottal approach, high-frequency TTJV is associated with a significantly higher combined major and minor (e.g., transient hypoxemia) complication rate (see “Intraoperative Complications”),86,87 and it represents an independent risk factor for complications during JV for microlaryngeal surgery.87 Modern automated JV may not be able to remediate all possible causes of barotrauma associated with high-frequency TTJV; complications may be related to the TTJV catheter insertion problems, laryngospasm, and high-pressure episodes (e.g., coughing, active expiration) during the recovery period.80,86 Notwithstanding the attractive features of high-frequency TTJV, such as a motionless surgical field and a particularly easy transition to spontaneous respiration,86 it may be advisable to reserve the elective use of this technique (especially in cases of severe supraglottic airway obstruction) for the most complicated patients74 and to designate operators with significant clinical experience and expertise.74,80,86,111
SHFJV, which combines high-frequency and low-frequency ventilation modes, has been used effectively in surgical treatment of high-grade laryngeal and tracheal stenosis, even with a remaining glottic opening as small as 2 to 3 mm.136 SHFJV is delivered supraglottically through a specialized jet laryngoscope, which incorporates welded low-frequency and high-frequency jet nozzles (Fig. 38-20).113,136,137 As the streams (LFJV of 12 to 20 breaths/min; HFJV of 100 to 900 breaths/min) get simultaneously directed from the ventilator toward the center of the distal end of the jet laryngoscope, LFJV entrains air (Fig. 38-21) and produces cyclic changes in VT (similar to supraglottic LFJV), facilitating maintenance of PaCO2 at near-normal limits and allowing HFJV to be adjusted as needed.136,137 HFJV builds up a continuous PEEP and promotes alveolar recruitment, maintaining PaCO2 even in the presence of the low FIO2 required for laser surgery.136–139 Safety of SHFJV is enhanced by an integrated port for continuous pressure (PIP and PEEP) and gas (FIO2 and EtCO2) monitoring at the end of the jet laryngoscope (see Fig. 38-21)137 and of an automatic pressure-triggered ventilator shutdown feature, similar to an isolated HFJV mode.
To achieve adequate SHFJV, it appears to be sufficient to generate a PIP of 15 to 30 cm H2O, as measured at the end of the jet laryngoscope, which closely correlates with the PIP at the glottic and tracheal levels (i.e., no further increase in pressure occurs in the distal airway).136,138 The PEEP values may not exceed 2.5 to 5 cm H2O.137,138 As a result, no adverse hemodynamic effects and barotrauma were observed in more than 1500 adult and pediatric patients who had undergone supraglottic SHFJV for laryngotracheal surgery, and endotracheal intubation was required in only 3 patients (0.2%), with concomitant significant restrictive or obstructive pulmonary disease.136 Due to the HFJV component, vocal cord movement is greatly attenuated during SHFJV; if a perfectly still surgical field is requested by the surgeon, HFJV can be further increased, LFJV decreased or stopped, or a short period of full apnea instituted.24,136,137
SHFJV is a completely tubeless, laser-safe, open breathing system that allows a fully unobstructed surgical field (Fig. 38-22). It enables an easy switch between different JV modes and parameters, and it offers greater versatility and ventilation capabilities over the single-frequency JV techniques, especially in patients with preexisting compromised gas exchange. However, its effective use requires optimal laryngoscope alignment and adjustability in relation to the glottic opening.
Despite the increased safety profile of SHFJV, clinical monitoring of the patient to prevent barotrauma should remain the standard of care for all JV techniques (Fig. 38-23).86,87 Close cooperation between the surgeon and the anesthesiologist is essential; if the operating laryngoscope moves or is removed and obstructs the airway without a warning to the anesthesia team, major barotrauma may result.16 Ensuring an adequate level of anesthesia, analgesia, and muscle relaxation; painstaking attention to maintaining unobstructed exhalation; and close monitoring of vital signs and chest excursions are essential for the patient’s safety.59,86,87,138 The main advantages and disadvantages of the ventilation techniques used for microlaryngeal surgery are compared in Table 38-2.
TABLE 38-2 Advantages and Disadvantages of Ventilation Techniques Used for Microlaryngeal Surgery
| Technique | Advantages | Disadvantages |
|---|---|---|
| Awake airway surgery |
Success depends on adequate topical and regional anesthesia of the airway
Local anesthetic–induced side effects
Lack of precision afforded by a still surgical field
May require conscious sedation
Loss of patient cooperation may result in injury
Limited ability to handle major intraoperative complications, such as bleeding and edema
Limited amount of tissue biopsied or excised
May not be suitable for patients with significant PD or CVD or for young pediatric patients
Precision surgery difficult or impossible in the moving surgical field
Contamination of operating room environment with anesthetic gases, if insufflation technique is used
Difficulty controlling adequate depth of anesthesia and absence of patient movement
Inability to continuously and reliably monitor FIO2, EtCO2, and anesthetic gases
May not be suitable for patients with significant PD or CVD or for very young pediatric patients
Possible airway trauma and disruption of the surgical field due to repeated passage of the endotracheal tube
Possible propensity for intraoperative laryngospasm
Inability to continuously and reliably monitor FIO2, EtCO2, and anesthetic gases
May not be suitable for patients with significant PD or CVD or for very young pediatric patients
Sole dependence on total intravenous anesthesia
Association with most major (e.g., barotrauma) and minor intraoperative anesthesia-related complications
Dependence on sophisticated automated jet ventilators for safe use
Limitations of manual JV and transtracheal JV
Significant experience and presence of two operators often required
CVD, Cardiovascular disease; EtCO2, end-tidal carbon dioxide; FIO2, fraction of inspired oxygen; JV, jet ventilation; NMB, neuromuscular blockade; PD, pulmonary disease; PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure.
C General Anesthesia Management for Panendoscopy and Microlaryngeal Surgery
1 Premedication and Monitoring
The main anesthesia objectives and special considerations for microlaryngeal surgery are discussed in “Special Considerations and Anesthesia Objectives.” Premedication can frequently be omitted. Administration of an antisialogogue (e.g., 0.2 mg of glycopyrrolate given intravenously) may be desired by the surgeon to facilitate the surgical field, especially in patients with copious secretions,3,10 but it does not constitute the standard of care.43 Mucosal intake of drying agents may result in increased vocal cord viscosity and impaired vibration, markedly changing the patient’s voice postoperatively and contributing to postoperative dysphonia.140 Steroids (e.g., 8 to 12 mg of dexamethasone given intravenously) are frequently used to prevent or minimize excessive postoperative swelling,16,34 although controlled studies documenting this beneficial effect appear to be lacking.34
Standard OR monitors are used. Invasive arterial blood pressure monitoring is rarely indicated, and its use should be dictated by the patient’s medical history, physical examination results, and special considerations such as prolonged JV or intraoperative problems (see “Jet Ventilation Problems”), for which intermittent sampling of arterial blood gases may become necessary. Monitoring the degree of neuromuscular blockade (NMB) with a conventional nerve stimulator should constitute the standard of care during panendoscopy and microlaryngeal surgery (see “Neuromuscular Blockade”).
2 Anesthesia Induction and Maintenance
Conventional and advanced airway management is discussed in “Conventional and Advanced Airway Management.” In most patients, standard intravenous induction can be safely performed. Difficulty in maintaining the airway during inhalational induction in patients with large, pedunculated tumors, granulomas and cysts should be anticipated, even if preoperative symptoms of airway obstruction are mild,38 and early application of continuous positive airway pressure (CPAP) can help to stent the airway open. Another useful strategy involves preparing the patient’s nares with a mixture of a vasoconstrictor and a topical anesthetic before induction, which will allow early passage of a nasal airway if the airway obstructs.17,33
Sevoflurane is most commonly used for inhalational induction in adult and pediatric patients.34,141 The minimum alveolar concentration (MAC) of sevoflurane required to provide adequate endotracheal intubating (EI) conditions in 50% of unpremedicated adult patients (MACEI50) is 4.5% (95% confidence interval [CI], 3.9% to 5.2%), and the 95% effective dose (ED95) for endotracheal intubation is 8%.142 Although the ED95 time for achieving this target concentration in patients with normal airway is approximately 7 minutes,143 up to 20 minutes may be required in patients with partial airway obstruction due to preexisting increase in minute ventilation and 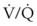 mismatch.17,34 This time can be shortened with the addition of small intravenous doses of midazolam or fentanyl,141,144 although at the expense of an increased risk of apnea and loss of the airway.
mismatch.17,34 This time can be shortened with the addition of small intravenous doses of midazolam or fentanyl,141,144 although at the expense of an increased risk of apnea and loss of the airway.
If the patient’s airway is reassuring, TIVA with propofol and an opioid is most commonly used during induction and maintenance of anesthesia for panendoscopy and microlaryngeal surgery.34,74,86,87,111 TIVA offers many practical advantages, such as delivering a stable, consistent level of anesthesia in cases of JV and other settings in which the delivery of inhalational anesthetics is compromised. TIVA facilitates maintenance of induced hypotension, resulting in improved surgical visibility; ensures rapid return of protective airway reflexes; promotes rapid awakening and early postanesthesia recovery; and decreases the incidence of postoperative nausea and vomiting.70,145–150 A propofol-based anesthetic results in profound depression of pharyngeal and laryngeal musculature and reflexes, and it effectively blocks the catecholamine release and hyperdynamic cardiovascular responses.145,151–153 Full synergistic effect of propofol with rapidly acting opioids (e.g., remifentanil, alfentanil) allows rapid titration of anesthetic to the desired clinical effect.154–156
Remifentanil and alfentanil are widely used because of their favorable pharmacokinetic profile. Remifentanil is superior to fentanyl and alfentanil in promoting intraoperative hemodynamic stability, improving respiratory and general recovery, and facilitating patients’ discharge after outpatient surgery.157–162 The recommended induction doses are an intravenous bolus of 0.5 to 2 µg/kg of remifentanil and intravenous bolus dose of 1 to 2 mg/kg of propofol, followed by continuous maintenance infusions of 0.1 to 0.5 µg/kg/min of remifentanil and 80 to 180 µg/kg/min of propofol.48,156–158,160,163 For alfentanil, an intravenous bolus of 20 to 30 µg/kg is commonly used for induction, followed by a continuous maintenance infusion of 0.25 to 1 µg/kg/min.159,160,164,165
Compared with conventional weight-based manual infusions, target-controlled infusions allow easier and more rapid titration of analgesia to the individual patient’s responses, avoiding overshoot, improving the time course of the drug effect, and facilitating perioperative hemodynamic control.166–168 Targeting propofol concentrations of 3 to 3.5 µg/mL and remifentanil concentrations of 2 to 5 ng/mL should be sufficient for most otherwise young (18 to 65 years old) and healthy (ASA physical class I or II) patients during the maintenance stage of anesthesia if adequate NMB is maintained.166,169–171
Severe bradycardic response to remifentanil should be treated promptly: activation of afferent parasympathetic fibers during direct and suspension laryngoscopy and endotracheal intubation or instrumentation may result in severe cardiac arrhythmias and asystole.172,173 Pediatric patients and adults with high vagal tone may be at highest risk for these grave complications,172 and pretreatment with anticholinergic agents may prevent or attenuate vagus-mediated cardiac arrhythmias. Treatment of reflex bradycardia and asystole during direct or suspension laryngoscopy must include immediate cessation of the offending stimulus, prompt administration of anticholinergics (e.g., atropine), and cardiopulmonary resuscitation, if necessary; infusion of isoproterenol or cardiac pacing may be required.172
Sevoflurane can be effectively combined with remifentanil and alfentanil for microlaryngeal surgery, producing good operating conditions, cardiovascular stability, and rapid emergence from anesthesia.162 Compared with other inhalational anesthetics, sevoflurane may be preferred for outpatient microlaryngeal surgery. It improves the quality of postoperative patient recovery and shortens the discharge time, is associated with a reduced incidence of coughing compared with desflurane,174 and produces less somnolence and postoperative nausea and vomiting compared with isoflurane.175 Compared with TIVA, sevoflurane decreases salivary gland excretion, potentially promoting better visibility for the surgeon,176 although clinical significance of this effect is unknown.
3 Neuromuscular Blockade
Maintenance of adequate NMB for microlaryngeal surgery is recommended,1,34,38,86,111 but this advice is not universally followed.111,177 Lack of appreciation for the benefits of adequate muscle relaxation constitutes a significant area for improvement in anesthesia care in general177 and microlaryngeal surgery in particular.
Full muscle relaxation facilitates smooth endotracheal intubation, which should be accomplished in patients presenting for microlaryngeal surgery without the use of an ETT stylet when possible.1 Maintenance of adequate NMB can prevent disastrous consequences of sudden patient movement, such as bucking or coughing while in suspension or during bronchoscopy or esophagoscopy,43 and it can facilitate respiratory compliance during JV.34 For some short surgical interventions, for which adequate muscle relaxation is only transiently required, combined administration of 2 µg/kg of remifentanil, 2 mg/kg of propofol, 1.5 mg/kg of lidocaine, and only one half of an intubating dose of rocuronium (0.3 mg/kg) can be administered, producing intubating conditions similar to intravenous administration of 1.5 mg/kg of succinylcholine.178 For very short diagnostic procedures, for which muscle relaxants can be completely avoided, a bolus dose of 3 to 4 µg/kg of remifentanil (or intravenous bolus of 40 to 60 µg/kg of alfentanil), administered over 90 seconds and followed by an intravenous bolus of 2 to 2.5 mg/kg of propofol should provide excellent conditions for endotracheal intubation179–181 or a quick “surgical look.”
For most adult microlaryngeal surgical and panendoscopy procedures, maintenance of a high degree of NMB is strongly preferred and is most commonly achieved by administration of intermittent intravenous bolus doses of intermediate-acting, non-depolarizing neuromuscular blockers or succinylcholine infusion.86 In the absence of contraindications to succinylcholine, the choice may be largely influenced by the duration of the procedure, the anesthesiologist’s preference, and special surgical requirements. If rapid or intermittent return of spontaneous ventilation is required intraoperatively, an intravenous bolus of succinylcholine followed by an infusion may be preferred.34 A succinylcholine infusion at a rate of 0.1 mg/kg/min (95% CI, 0.06 to 0.14 mg/kg/min) provides 100% twitch depression to the train-of-four (TOF) stimulation of the ulnar nerve (adductor pollicis muscle) and can be easily titrated to effect due to its linear kinetics.182 The infusion should be started after the intubating dose of succinylcholine has dissipated and a twitch response has reappeared.182,183 For practical purposes, it should be titrated to a barely visible single twitch during TOF stimulation of the ulnar nerve, which corresponds to 95% to 98% of twitch depression.182 The observed increased succinylcholine requirements (i.e., tachyphylaxis) should alert the anesthesiologist to a rapidly developing transition from phase 1 to phase 2 NMB.183,184 Similar phenomena are observed in children.185
In the absence of a phase 2 block, succinylcholine infusions may avoid postoperative residual curarization (PORC). The incidence of PORC with the use of the intermediate-acting, non-depolarizing muscle relaxants reaches 20% to 30% in the general surgical population.186,187 PORC is associated with delayed discharge from the recovery room, even in the absence of respiratory compromise.186 The use of mivacurium, despite its short duration of action, is associated with an almost 10% incidence of residual block, unless its action is pharmacologically reversed.188 This highlights the need for vigilant intraoperative monitoring of NMB and routine reversal of NMB after microlaryngeal surgery to ensure adequate TOF recovery to a level of more than 0.9.189–192 Even small degrees of residual paralysis (TOF of 0.7 to 0.9) are associated with impaired pharyngeal function and increased risk of aspiration, significant attenuation of the hypoxic ventilatory response, unpleasant symptoms of muscle weakness, and weakness of the laryngeal and upper airway muscles, which may contribute to the possibility of acute upper airway obstruction postoperatively (see “Postoperative Complications”).192
During microlaryngeal surgery, access to the patient’s arms is frequently difficult or impossible, and the anesthesiologist is faced with an alternative choice of monitoring NMB at the temporal branch of the facial nerve (i.e., orbicularis oculi muscle) or posterior tibial nerve (i.e., flexor hallucis brevis muscle). Monitoring TOF at the orbicularis oculi correlates best with NMB at the laryngeal adductor muscles, which are responsible for vocal cord movement and glottic closure193 and may constitute the surgeon’s preference.43 For adequate orbicularis oculi monitoring, the following guidelines should be followed194,195:
1. The electrodes should be positioned just lateral to the eye or with one electrode lateral to the eye and one in front of the ear.
2. Small currents (20 to 30 mA) and small electrodes should be used to avoid stimulation of other facial muscles.
3. The responses to stimulation should be observed in the middle of the eyebrow.
The onset of and the recovery from non-depolarizing NMB at the larynx and orbicularis oculi are similar and faster than at the ulnar nerve.191,193–196 Significantly quicker recovery of NMB is observed in adults at the flexor hallucis brevis muscle (i.e., great toe) compared with the hand muscles.191 Assessment of the residual block at the end of the surgery must still be guided by a twitch recovery at the ulnar nerve, because the adductor pollicis muscle recovers last.191,194,196
The anesthesiologist must maintain constant communication with the surgeon to match the maintenance of adequate NMB with the duration of surgery. If additional muscle relaxation is requested at the end of the procedure to abolish vocal cord movement, administration of non-depolarizing muscle relaxants should be avoided, and deepening the level of anesthesia and/or administration of additional analgesia (e.g., intravenous bolus of remifentanil) should be performed instead.43 Pharmacologic reversal of non-depolarizing NMB should be routinely done at the end of microlaryngeal surgery.
Sugammadex, a selective relaxant binding drug, can antagonize any level of NMB, including the profound blockade induced by rocuronium, adding flexibility to the use of non-depolarizing relaxants.197 The recommended dose is 2 to 16 mg/kg, depending on the level of the block.188,197 Profound NMB induced by rocuronium can be reversed in less than 3 minutes.198 Sugammadex, however, has no affinity for atracurium, cisatracurium, or succinylcholine; is not widely available; and is very expensive.197
4 Conventional and Operative Direct Laryngoscopy
The intense sympathoadrenal response observed during direct laryngoscopy and endotracheal intubation (see “Special Considerations and Anesthesia Objectives”) may be further accentuated by continuous deployment of the suspension laryngoscope and intralaryngeal or intratracheal surgical manipulations, resulting in a 10% to 17% incidence of transient intraoperative myocardial ischemia.199 The administration of 1.5 to 2 mg/kg of esmolol by intravenous bolus, followed by continuous intravenous infusion at a rate of 100 to 300 µg/kg/min, may be particularly effective in promoting hemodynamic stability and preventing intraoperative myocardial ischemia.200–202 Potentiation of the action of opioids and anesthetic agents by esmolol further results in decreased postoperative opioid analgesic requirements,203–206 facilitates emergence from anesthesia, and shortens a discharge time after outpatient surgical procedures.207–210
5 Jet Ventilation Problems24,34
b Insufficient Carbon Dioxide Elimination
Intraoperative hypercapnia can be observed in patients with severe COPD and reduced lung compliance. Slightly increasing the driving pressure is recommended until the problem is corrected. If EtCO2 or PaCO2 levels continue to rise despite maximal driving pressure, permissive hypercapnia can be allowed in selected patients. Studies indicate that PaCO2 values as high as 100 mm Hg may be well tolerated intraoperatively.211 A switch to conventional endotracheal intubation and intermittent positive-pressure ventilation always remains an option.
The higher ASA physical status correlates with the higher incidence of complications with transglottal and TTJV techniques,87 and these patients should be given special attention if JV is planned. Patients with previous neck radiation therapy are at higher risk for multiple attempts at TTJV catheter placement and subsequent risk of developing intraoperative barotrauma.86
6 Bronchoscopy and Esophagoscopy
If rigid bronchoscopy is planned first, general anesthesia is induced in a standard manner, and the patient’s lungs are hyperventilated through the face mask with an FIO2 of 1.0. With the onset of complete muscle relaxation, the patient is immediately turned over to the surgeon without securing an airway.43 Quick and gentle direct laryngoscopy for the purpose of applying topical laryngotracheal anesthesia (3 to 4 mL of 4% lidocaine) before rigid bronchoscopy can be tried to help blunt hemodynamic responses to subsequent surgical manipulation; however, the risk of residual laryngeal anesthesia should be kept in mind if the procedure is short.212
After the rigid bronchoscope is introduced into the patient’s trachea, JV can be instituted, or ventilation can commence manually through the OR anesthesia circuit by using the Racine universal adapter connected to the side arm of the bronchoscope (see Fig. 38-10). A rigid telescope and many accessory instruments such as forceps, suction catheters, laser fibers, or silicone stent delivery systems can be placed by the surgeon through the central lumen of the bronchoscope.213 Although balanced inhalational anesthesia has been used successfully in this setting,34 TIVA can provide a more stable plane of anesthesia. With manual ventilation, high gas flows are usually required because of the variable leak around the end of the bronchoscope. Close communication with the surgeon is essential for decreasing the manual inflating pressures when the bronchoscope is introduced into a main stem bronchus and to ensure complete exhalation.43
After rigid bronchoscopy is completed, if endotracheal intubation is planned, a resumption of adequate mask or LMA ventilation with an FIO2 of 1.0 is advisable before direct laryngoscopy. If necessary, the airway can be exchanged with the ETT through the in situ rigid bronchoscope (see Fig. 38-11).72,73
If endotracheal intubation is chosen, a small-diameter (e.g., 6.0 mm ID), wire-reinforced ETT may be preferred for rigid esophagoscopy to avoid possible compression of the ETT lumen; however, if suspension laryngoscopy with endotracheal intubation is planned next, a 5.0 mm ID MLT tube should be used instead. The ETT should be moved over to the left side of the patient’s mouth to facilitate introduction of the surgical instruments and securely taped to the lower jaw, facilitating full opening of the patient’s mouth by the surgeon.43
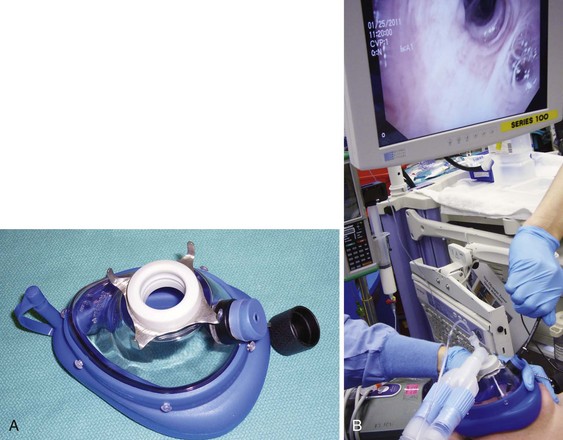
Intraoperative flexible fiberoptic bronchoscopy can be performed by the surgeon through an appropriately sized ETT, alongside the small ETT with its cuff deflated; through the LMA; or through Patil-Syracuse endoscopy mask (Ambu Inc., Glen Burnie, MD) (Fig. 38-24). If flexible fiberoptic bronchoscopy is performed through the ETT (7.5 to 8.0 mm ID) using a swivel bronchoscopy adapter, ventilation is better controlled manually because of increased resistance to the gas flow and high PIP. Gentle, manual ventilation with small tidal volumes and an FIO2 of 1.0 is usually well tolerated by the patient over the short course of the procedure. The efficacy of ventilation through the Patil-Syracuse mask during flexible fiberoptic bronchoscopy is greater than through the Classic LMA or iLMA,214 and the Patil-Syracuse mask can be used to facilitate flexible esophagoscopy.
7 Emergence from General Anesthesia
Although highly stimulating intraoperatively, panendoscopy and microlaryngoscopic surgical procedures are typically characterized by low postoperative pain scores, even when the operation is prolonged.43 Use of remifentanil infusions frequently allows reduction of the total dose of supplemental intravenous fentanyl to 1 to 2 µg/kg or avoidance of intraoperative use of fentanyl completely. In the recovery room, intermittent intravenous bolus doses of fentanyl in combination with oral analgesics are usually sufficient for pain control.43 Remifentanil-induced hyperalgesia has not been an issue in our experience, possibly because of the short duration of most cases and the relatively low infusion rates that are typically required intraoperatively (0.1 to 0.3 µg/kg/min). If increased pain occurs postoperatively, it should raise an alert about possible surgical complications, such as esophageal perforation (see “Intraoperative and Immediately Postoperative Complications”).
Antiemetic prophylaxis should be routine,43 and it is most commonly achieved by intravenous administration of a serotonin 5HT3 receptor antagonist (e.g., ondansetron). Multimodal antiemetic prophylaxis should be employed for patients at high risk for postoperative nausea and vomiting.
If endotracheal intubation was performed, smooth, nonstimulating emergence from anesthesia constitutes one of the most challenging tasks.1,43 Patient’s straining, bucking, or coughing with the ETT in situ results in an attempted forceful glottic closure, which may provoke additional trauma to and ulceration of the mucosal surface of the vocal cords, leading to wound formation.140,215,216 Emergence phenomena such as a patient’s agitation and uncontrolled head movements and post-extubation laryngospasm may exacerbate the surgically compromised vocal cords further.140 Subsequent vocal cord wound healing leads to remodeling of the superficial layer of the vocal cord lamina propria and the epithelium, which may result in formation of vocal cord nodules, polyps, and cysts.215
Three strategies can facilitate smooth tracheal extubation. First, the patient’s trachea can be extubated at a deep plane of anesthesia, and the airway supported by a mask until the patient resumes spontaneous ventilation and emerges from anesthesia. Although this may be a viable approach, it is time and labor consuming, carries an increased risk of post-extubation laryngospasm, and leaves the patient’s airway unprotected. It should be undertaken only if airway management on induction was uncomplicated. The second approach (i.e., Bailey maneuver), with the patient still anesthetized, involves insertion of a supralaryngeal airway (usually an LMA) behind the existing ETT, removal of the ETT, and administration of the supraglottic ventilatory support until the patient resumes spontaneous ventilation and awakens from anesthesia.217 With the third, pharmacologic approach, the anesthesiologist relies on a low-dose remifentanil infusion to blunt the tracheal responses and to promote smooth extubation and awakening at the end of surgery.
Remifentanil is ideally suited for the control of tracheal extubation, because the return of consciousness and the cough reflex occur almost simultaneously.17 Although the optimal dose of remifentanil required to blunt tracheal responses to extubation remains to be determined, current data indicate that a remifentanil infusion of 0.05 to 0.06 µg/kg/min (target concentration of 1.5 ng/mL) during emergence is likely sufficient; it reliably and effectively suppresses the cough reflex in awake intubated patients while promoting hemodynamic stability.218,219
IV Intraoperative and Immediately Postoperative Complications
Most common, clinically relevant major and minor complications of panendoscopy and microlaryngeal surgery are summarized in Table 38-3. The incidence of complications is small and is largely related to the experience of the anesthesiologist and the surgeon, as well as their cohesive team work, the characteristics of the patient population treated, and the status of the treating institution (e.g., academic, tertiary care, private practice).2,40,220–222
TABLE 38-3 Complications of Panendoscopy and Microlaryngeal Surgery
| Major Complications | Minor Complications |
|---|---|
| Intraoperative | Intraoperative |
Data from references 1, 2, 13, 33, 38, 40, 43, 213, 220–222.
A Intraoperative Complications
Panendoscopy and microlaryngeal surgery remain very safe procedures. The mortality rate is exceedingly low (0.02% to 0.6%).221,223 In a large, single-institution, retrospective review of 1093 endoscopic laryngeal surgery cases, Jaquet and colleagues87 reported no intraoperative deaths, an incidence of major complications of 0.37% (all related to barotrauma during subglottic JV), and no major complications for 281 pediatric patients between the ages of less than 1 year and 16 years.
Loss of the airway on induction, requiring emergent cricothyrotomy or tracheostomy, can be sudden, especially in patients with critical airway obstruction. Proper preparation of the OR team is the key to promptly and efficiently dealing with intraoperative airway emergencies.38 An overall incidence of major barotrauma complications (e.g., cervicomediastinal emphysema, pneumothorax, tension pneumothorax) is small (0.2% to 0.5%),87,111,120 and these complications are most frequently observed during TTJV (1.1%).87 Intraoperative bronchial or transbronchial biopsy represents an independent risk factor for intraoperative pneumothorax (about 10%).221 These patients also carry a higher risk of developing pulmonary edema after transbronchial biopsy.221 Although the overall incidence of cardiovascular compromise may reach 20% to 50%,2,83,142 major cardiac and cerebrovascular complications are rarely observed (0% to 2.2%).86,87,111,221 Massive bleeding is rare and may be encountered in patients while coring out friable vascular tumors or during inadvertent perforation of the tracheal or bronchial wall with the laser or rigid bronchoscope.71,213 Ensuing respiratory failure with an inability to wean the patient from the ventilator has been described.71 The rigid bronchoscope should be used by experienced operators, because improper technique frequently results in dental or oropharyngeal trauma.213 Particular care must be exercised when introducing a rigid bronchoscope into the airway of patients considered at high risk for cervical spine (C-spine) dislocation during neck extension (e.g., elderly, patients with rheumatoid arthritis, those with congenital C-spine abnormalities).71 Prospective trials identify the incidence of dental trauma after suspension laryngoscopy at 0% to 6.5%,40,224 depending on the operator’s experience, methodology of the study, dental injury criteria, preexisting dentition status of the patient, and suspension technique used.40
Esophageal perforation is a rare event if flexible esophagoscopy is used.2 A significantly higher complication rate (2.6%) was reported for rigid esophagoscopy, in which case patients with a history of head and neck cancer present a particular risk.220 Pulmonary aspiration remains a particular concern when the patient’s airway is left unprotected (e.g., JV), especially in patients at increased risk for aspiration of gastric contents. Intraoperative airway soiling may occur from aspiration of blood, secretions, surgical debris, or tumor cell contamination of the lower airway.38
Minor intraoperative anesthesia-related complications happen infrequently (2.6%).87 The incidence of minor intraoperative laryngospasm during microlaryngeal surgery has been reported by Jaquet and colleagues as 3.1%87; it was exclusively related to the use of AIV and was associated with a light plane of anesthesia. In contrast, prospectively recorded, surgery-related minor complications are common (37.5% to 73%).40,222 Minor surgical complications, such as sore throat, mucosal injury (e.g., cuts, edema, hematoma), and cranial nerve dysfunction (e.g., lingual, glossopharyngeal, hypoglossal), are most commonly observed.1,40,221,222 The latter are likely caused by direct pressure or stretch injury associated with laryngoscopy or suspension of the laryngoscope,40 and they are related to the size of the operating laryngoscope used and the duration of suspension.1,40 Presenting symptoms in the recovery room may include dysesthesia and taste alteration, swallowing problems, and deviation of the tip of the tongue.40,222
B Postoperative Complications
The risk of postoperative airway compromise is significantly greater among the patients who underwent diagnostic laryngoscopy and panendoscopy than those in the general surgical population.225 The residual effects of anesthetics, analgesics, and inadequately reversed NMB may further contribute to the development of hypoventilation, atelectases, and poor mobilization of secretions in the early postoperative period.34 Airway surgery invariably produces a certain degree of traumatic edema, which may precipitate acute airway obstruction postoperatively in an already compromised airway.13 Development of airway obstruction should be suspected if the patient has symptoms such as dyspnea, respiratory distress, and particularly inspiratory stridor.34 Aggressive early treatment with humidified oxygen and nebulized racemic epinephrine constitutes the first reasonable therapeutic intervention.13,34 Postoperative laryngospasm may precede or accompany airway obstruction226,227 and quickly lead to development of acute negative-pressure pulmonary edema.34,226 Although the incidence of these complications in the general surgical population is small (0.3% and 0.09%, respectively),226,228 negative-pressure pulmonary edema is preceded by laryngospasm in more than 50% of cases.227 Treatment is supportive and consists of reestablishment of airway patency, O2 supplementation, ventilatory support (i.e., CPAP or endotracheal intubation with positive-pressure ventilation and PEEP), management of fluid shifts, and maintenance of normal intravascular volume.34,226–228 Postoperative hemoptysis is usually associated with interventional bronchoscopy, for which the incidence may reach as high as 41%.221 It is common for the suspension laryngoscopy to aggravate preexisting temporomandibular joint disease, and these patients should be advised accordingly before surgery.1
V Anesthesia for Pediatric upper Airway Endoscopy and Microlaryngeal Surgery
A General Considerations
The general principles of adult airway endoscopy can be applied to infants and children, although important anatomic, physiologic, and pathologic differences in the airway exist (see Chapter 36). Some of the most common indications for flexible and rigid airway endoscopy in pediatric patients and neonates are listed in Table 38-4. Usually, examination of the entire airway, including the nasopharynx, larynx, the subglottic region, and the remaining tracheobronchial tree is indicated, because abnormalities at more than one site are found in 10% to 20% of patients.229–231
TABLE 38-4 Indications for Flexible and Rigid Airway Endoscopy in Pediatric Patients
| Flexible Endoscopy | Rigid Endoscopy |
|---|---|
B Patient Preoperative Evaluation and Preparation
Preoperative assessment of the pediatric patient should consider the likely diagnosis,232,233 the extent of airway compromise, and the degree of urgency of the procedure. A complete history, including a history of prematurity, any concurrent diseases, and an overview of the child’s progress since birth, should be obtained.
Preterm infants, infants whose gestational age at birth is less than 37 weeks, are at higher risk for postoperative respiratory depression and apnea when exposed to anesthesia.234–238 The postconceptual age (PCA; i.e., gestational age plus postnatal age) is inversely related to the risk of postoperative apnea,234,235,237 with infants younger than 44 weeks’ PCA at the greatest risk.238,239 The former preterm infant with a PCA of more than 60 weeks can be sent home safely using standard discharge criteria.238,239 Infants with a PCA of less than 60 weeks should be admitted for monitoring at least 12 hours postoperatively or overnight.239 Comorbidities to consider when assessing increased risk of postoperative apnea include apnea at baseline, anemia, neurologic disease, and chronic lung disease.235,237,238,240,241 Elective procedures in preterm infants should be postponed until more than 60 weeks’ PCA.238,239 Apnea after anesthesia has also been reported in term infants,242–244 and elective procedures should be postponed until the infant is more than 44 weeks’ PCA.239
Routine laboratory studies are not indicated for most children. Preoperative hemoglobin levels should be considered for procedures with the potential for blood loss for children with hemoglobinopathies, former preterm infants, and those younger than 6 months old.239 Preoperative fasting guidelines should be followed unless the procedure is deemed emergent.
C Operative Pediatric Airway Endoscopy and Microlaryngeal Surgery
1 Special Considerations and Anesthesia Objectives
Some of the most commonly used general purpose (diagnostic or intubating) operating laryngoscopes are the Storz and Parsons laryngoscopes (Storz, Tuttlingen, Germany), which are available in different sizes.245 The Parsons laryngoscope (Fig. 38-25) can also be used for bronchoscopy, and after suspension, for some types of microlaryngeal surgery.245 The Benjamin-Lindholm laryngoscope (Storz) provides a more panoramic view of the larynx and facilitates access for microlaryngeal surgery.246 The Parsons and Benjamin-Lindholm laryngoscopes allow continuous insufflation of O2 or anesthetic gases through a channel on the left side of the device (see Fig. 38-13). The pediatric rigid bronchoscopes manufactured by Storz are used almost universally.229 The OD of the bronchoscope typically is larger than an ETT with the same ID, and careful attention to choosing a bronchoscope with the appropriate OD is important to avoid damage to laryngeal structures.247
The anesthesia objectives in pediatric patients are similar to those in adults (see “Special Considerations and Anesthesia Objectives”). Because of the shared airway, communication between the surgeon and the anesthesiologist before and during the procedure is critical, and it is probably the most important factor in avoiding complications.248
2 Intraoperative Ventilation Techniques and Strategies
Pediatric patients undergoing upper airway endoscopy and microlaryngeal surgery frequently present with difficult airway problems. Alternative techniques for pediatric difficult airway management are addressed in Chapter 36. The approach to ventilation of the pediatric patient usually follows the one used for adults (see “Intraoperative Ventilation Techniques and Strategies for Microlaryngeal Surgery”).
a Awake Approach
In pediatric patients, the awake approach is typically limited to a diagnostic evaluation of the upper airway and its dynamic function with a nasopharyngolaryngoscope or a FFB.249 It can be safely performed in an office setting under topical anesthesia without sedation in children of all ages, even neonates,249–251 but it may also be performed in the OR as part of a more complete examination. The limitation of performing this procedure in an office setting without sedation is the inability to pass the scope below the vocal cords.249,251
b Spontaneous Ventilation
Under general anesthesia, spontaneous ventilation is generally preferred over controlled ventilation for endoscopy of the pediatric airway.229,247,252 However, the multiple techniques in use indicate that no single method is universally accepted.229,247
Spontaneous breathing techniques in neonates and infants should be limited to shorter examinations. Under anesthesia, a combination of reduced FRC, high compliance of the pediatric airways and chest wall, increased O2 consumption, and further airflow limitations imposed by the surgical instrumentation inside and around the airway can lead to a rapid hypoxemia if spontaneous ventilation is maintained.229,253–255 Nevertheless, successful intraoperative spontaneous ventilation has been reported in neonates as young as 2 weeks,256–258 and it is more easily maintained during the entire procedure in older infants and children. If spontaneous ventilation fails due to apnea or hypoventilation, institution of alternative ventilation techniques must be promptly implemented.
d Laser-Safe Endotracheal Tube
Small, laser-safe ETTs are not commercially available for infants and small children.255 Regular pediatric ETTs can be wrapped with a fire-resistant foil for laser surgery.254 Preparation of these wrapped ETTs is becoming less common because it is time consuming and cumbersome. Potentially dangerous gaps in the wrapping may allow ignition of the ETT, and fragments of wrapping material can serve as foreign bodies.
e Jet Ventilation
In children, supraglottic JV is probably most commonly used.255,259,260 Subglottic JV is used less frequently,87 because the smaller, compliant airways of infants and small children may not allow adequate exhalation, potentially leading to a higher incidence of barotrauma.254 In infants, even a small cannula can obstruct the surgical field. The use of TTJV in infants and small children is uncommon.87
3 General Anesthesia Management for Pediatric Airway Endoscopy and Microlaryngeal Surgery
a Premedication and Monitoring
Routine, noninvasive monitoring usually is sufficient. If quantitative assessment of CO2 is not available due to the method of airway management, the anesthesiologist should visually monitor chest excursions and auscultate breath sounds using a precordial stethoscope.261
The goals of premedication in children include alleviation of anxiety, a smooth separation from parents, and facilitation of the induction of anesthesia. If the child is symptomatic and there is a possibility of airway obstruction or respiratory compromise, premedication using a smaller amount than customary should be considered. Premedication can decrease the likelihood of crying and struggling on induction and thereby prevent worsening of the airway symptoms. Infants 9 to 10 months old typically benefit from premedication.262 Recommended dosages for midazolam are 0.25 to 0.5 mg/kg taken orally or 0.05 to 0.1 mg/kg administered intravenously.263
If intravenous access has already been established, an antisialagogue can be given to decrease secretions. Glycopyrrolate is preferred in children because it has no central effects, and it causes less tachycardia than atropine.264,265 Intramuscular injections of any medication are painful and should be avoided if possible.266 Administration of 0.5 mg/kg of dexamethasone, with a maximum dose of 10 to 20 mg, is recommended to minimize postoperative airway edema.247
b Anesthesia Induction and Maintenance
For children without intravenous access, gentle inhalational induction with sevoflurane is recommended, and the intravenous access is secured after sufficient depth of anesthesia is reached. Preserving spontaneous respiration initially is critical to allow dynamic assessment of the vocal cords, larynx, and tracheobronchial tree,229,261,267 and it is strongly preferred when a high-grade obstruction of the upper airway is suspected or a difficult airway is anticipated.261,268 If only a short endoscopic examination is planned, the nasopharyngolaryngoscope or FFB is passed through the nose into the pharynx by way of a bronchoscopic swivel adapter attached to a standard anesthesia mask.269 Alternatively, the anesthesia mask can be removed to facilitate the examination. The FFB can also be passed through a laryngeal mask or an ETT if visualization of the upper airway is not required.269
When the depth of anesthesia is sufficient to tolerate laryngoscopy, the surgeon performs direct laryngoscopy and topicalizes the airway with lidocaine, which typically is applied to the vocal cords by atomizer or sprayed with a 3-mL Luer-Lok syringe fitted with a plastic or metal cannula.247 The usual concentration of lidocaine is 2% for children (1% for small infants), and the maximum dose is 3 to 5 mg/kg229,270; toxicity is possible with excessive doses, and seizures have been reported.271 When appropriately applied, the lidocaine provides surface analgesia to the glottis, subglottis, and proximal trachea, decreasing the chance of laryngospasm or coughing when the bronchoscope is inserted and reducing the general anesthetic requirements. After the airway has been successfully topicalized, the surgeon performs a second laryngoscopy to evaluate the larynx and the vocal cords more completely.
Depending on the pathology, the surgeon may choose to pass the telescope for a rapid assessment. This has the advantage of producing less airway trauma than by the larger bronchoscope. If there is significant narrowing, the telescope may pass beyond the narrowing more easily. The surgeon may choose to place the child in suspension at this point. A rigid bronchoscope is then passed through the vocal cords. A thinner telescope with an optical eyepiece is subsequently placed through the bronchoscope, allowing the subglottis and trachea to be examined. After the bronchoscope is introduced past the vocal cords, the anesthesia circuit is connected to the side arm of the bronchoscope, allowing gas exchange and continued ventilation, similar to the technique used in adults (see Fig. 38-10). With the optical telescope in place, gas exchange occurs in the space between the telescope and the bronchoscope sheath. Partial occlusion of the bronchoscope lumen by the optical telescope increases resistance to ventilation, especially when the 2.5-, 3.0-, and 3.5-mm ID bronchoscopes are used.247 The smallest optical telescope should be used to prevent increased airflow resistance.259
After an appropriate diagnosis has been established, the surgeon may choose to immediately initiate treatment of the pathology. Endoscopic surgery can include the use of microdebriders, dilating balloons, stents, or lasers.272,273 Many pediatric airway centers are using laser less frequently, because alternatives such as microdebriders and balloon dilation become preferred.272,273
TIVA with propofol and remifentanil is used most frequently for maintenance of anesthesia, even during spontaneous ventilation. Infusions of 150 to 500 µg/kg/min of propofol and 0.05 to 0.2 µg/kg/min of remifentanil have been reported.258,267 A bolus dose of propofol (1 to 5 mg/kg) may be administered to achieve steady-state conditions rapidly.258,274 Remifentanil infusion may require a more dilute solution in neonates and infants to provide sufficient delivery volume.275 Infusions must be carefully titrated to effect to avoid apnea, and they should be delivered as close to the intravenous catheter as possible to minimize delays in response to changes in infusion rates.275
Dexmedetomidine may be a particularly useful adjunct to TIVA when the spontaneous ventilation technique is used, because its administration is characterized by lack of respiratory depression and stable hemodynamic profile, even at high doses.276–278 MRI of the upper airway in spontaneously breathing children with a high-dose intravenous infusion of dexmedetomidine (3 µg/kg/hr) showed only small reductions in upper airway diameter and no clinical evidence of upper airway obstruction.279 For the preservation of spontaneous ventilation, a combination of an intravenous bolus (0.25 to 1 µg/kg) and intravenous infusion (2 to 2.5 µg/kg/hr) of dexmedetomidine with an intravenous infusion (200 to 300 µg/kg/min) of propofol appears to be well tolerated.277,278
Several manual infusion schemes exist for TIVA in children. Devices that allow target-controlled intravenous infusions of anesthetics in children are not widely available,274,280 and the target concentrations for neonates and infants or for critically ill children have not been fully investigated.281 Significantly higher propofol doses per unit of body weight are necessary in healthy children for induction and maintenance of anesthesia.274 Compared with adults, to achieve blood concentration of propofol of 3 µg/mL, the bolus dose should be increased by more than 50%, and the infusion rate should be almost doubled (19 mg/kg/hr for 10 minutes, followed by 15 mg/kg/hr for 10 minutes and 12 mg/kg/hr thereafter).274 Similarly, the reported target concentration of remifentanil necessary to block the response to a skin incision under target-controlled propofol anesthetic in pediatric patients between the ages of 3 and 11 years is almost twice that needed in adults (0.15 µg/kg/min, 95% CI, 0.13 to 0.17 versus 0.08 µg/kg/min, 95% CI, 0.06 to 0.12).282
c Emergence from Anesthesia in the Pediatric Patient
After airway endoscopy is completed, an ETT can be placed in the trachea to control the airway, or more commonly, the child can be allowed to emerge breathing 100% O2 by mask.247,267 If the child is intubated, the usual criteria for extubation should be applied. In the recovery room, these patients should be carefully monitored for signs of airway edema or respiratory distress.
4 Complications of Pediatric Airway Endoscopy
Major and minor complications of pediatric airway endoscopy largely replicate those observed in adult patients (see “Intraoperative and Immediately Postoperative Complications”).249,267,283 No major complications were recorded with different intraoperative ventilatory techniques in more than 280 pediatric patients.87 Intraoperatively, adverse airway events are most likely to occur with an inadequate depth of anesthesia.267 Interventions that seem to decrease complications are careful and effective topicalization of the airway, close observation of the child for any movement or response to initial stimulation, and immediate withdrawal of the laryngoscope or bronchoscope with patient response until an adequate depth of anesthesia is reestablished.267 In two large series of pediatric patients undergoing rigid bronchoscopy, the complication rate was 2% to 3%.232,284 Stridor due to airway edema is the most commonly seen complication in the immediate postoperative period, and it should be treated with nebulized racemic epinephrine.
5 Recurrent Respiratory Papillomatosis
Recurrent respiratory papillomatosis (RRP) (Fig. 38-26) is the most common benign neoplasm of the respiratory tract in children, and it is characterized by the proliferation of exophytic squamous papillomas within the respiratory tract.285–287 Because of the recurrent nature of the disease, most children return frequently for treatment. The average enrollee in the National Registry for Juvenile-Onset Recurrent Respiratory Papillomatosis underwent a mean of 5.1 operations annually.288 Repeated surgical treatment may lead to laryngeal web formation and irreversible damage to the vocal cords.287,288
Figure 38-26 Recurrent respiratory papillomatosis.
(Courtesy of Alan Cheng, MD, Stanford University Medical Center, Stanford, CA.)
The most common symptoms of RRP are related to laryngeal obstruction and include hoarseness, abnormal cry or voice change, and stridor.187,289 The larynx and glottis are affected in almost all cases, followed by supraglottic involvement and subglottic involvement. Distal spread to the trachea, bronchi, and lungs has been reported.286,287 Pulmonary lesions can cause bronchiectasis, pneumonia, and declining pulmonary status.286
Anesthesia and airway management for patients with RRP can employ tubeless spontaneous ventilation,290 AIV, and JV247,267,291; the advantages and disadvantages of each technique have been discussed (see “Intraoperative Ventilation Techniques and Strategies for Microlaryngeal Surgery”). A 2002 survey of members of the American Society of Pediatric Otolaryngology showed a preference for spontaneous ventilation or AIV (63.5%), followed by JV (24.3%) and laser-safe ETTs (9.6%),285 although a theoretical risk of carrying the human papillomavirus (HPV) particles into the distal airways with AIV or JV exists.267,291 A higher incidence of apnea and laryngospasm may be observed during spontaneous ventilation in RRP patients under TIVA compared with positive-pressure ventilation through an ETT.292
Current surgical preference for debulking RRP lesions appears to favor the use of the microdebrider over the CO2 laser,285,286 because it shortens the operating time, eliminates the risk of a laser fire, and is associated with decreased postoperative pain.293 With microdebrider surgery, the ETT can be used for the entire procedure, although it limits the surgeon’s access to the posterior glottis.
D Foreign Bodies in the Airway and Esophagus
1 Foreign Body Aspiration
Foreign body aspiration in the airway is most common in children 1 to 3 years old289,294 and is a leading cause of accidental death in children younger than 5 years.295 Food products (especially nuts and seeds) are the most commonly aspirated airway foreign bodies (77% to 86%).296 Nuts with oils on the surface can be particularly problematic because they may stimulate a significant inflammatory response.297
Foreign body aspiration is suspected when a child has an acute choking event or severe coughing with respiratory distress, but in many instances, the diagnosis is delayed.297–299 Foreign bodies in children with a delayed diagnosis may be more difficult to extract,300 and these children are also at higher risk for complications.300,301 Symptoms and signs associated with bronchial aspiration include coughing, wheezing, dyspnea, and decreased breath sounds on the affected side, whereas coughing, stridor, dyspnea, and cyanosis are more common with laryngeal or tracheal foreign bodies.302
Most airway foreign bodies lodge in the bronchial tree (85% to 91%), preferentially on the right side, with the remainder catching in the larynx or trachea233,296 (Fig. 38-27). A foreign body in the bronchus can cause a ball-valve obstruction with hyperinflation of the distal lung, and if the obstruction is complete, the distal lung collapses.229
The treatment of choice for airway foreign bodies is prompt endoscopic removal under conditions of maximal safety and minimal trauma.297,301 Following preoperative fasting guidelines is recommended if the procedure is not an emergency.299 An intravenous catheter should be inserted in most cases. In an emergency situation or with a distressed infant, establishing intravenous access immediately after inhalation induction is acceptable.299 As with most pediatric endoscopy cases, glycopyrrolate should be administered to decrease secretions, and intravenous dexamethasone is recommended to minimize postoperative airway edema.247
Before induction of anesthesia, the possibility of complete airway obstruction should be anticipated. Distal airway foreign bodies are more difficult to remove, whereas proximal foreign bodies are more likely to obstruct the airway.299 A backup plan should include emergency rigid bronchoscopy, deliberate advancement of the foreign body into a main stem bronchus, tracheotomy, and thoracotomy in selected cases.
Induction and maintenance of anesthesia are similar to anesthesia described earlier for direct laryngoscopy and rigid bronchoscopy. The choice between maintaining spontaneous respiration and controlling ventilation remains controversial, with both techniques producing equally good results.289,299,303 For airway foreign bodies, pediatric anesthesiologists seem to prefer maintenance of spontaneous ventilation,304 because positive pressure can force the foreign body deeper into the smaller airways.299,303 For the esophageal foreign bodies, controlled ventilation with an ETT is the preferred technique.304
When the airway foreign body is too large to withdraw through the lumen of the bronchoscope, the bronchoscope, forceps, and foreign body are removed as a single unit.289,294 If the foreign body is dropped in the proximal airway and cannot be immediately retrieved, it should be pushed distally into the bronchus. If the foreign body is pushed into the contralateral bronchus, there is a potential for complete obstruction from edema in the original bronchus.305 The FFB can be preferentially used when the foreign body is lodged far into the periphery of the lung.297
Esophageal foreign bodies (usually coins) are considered less serious than airway foreign bodies, and most pass through the gastrointestinal tract harmlessly.306–308 For asymptomatic esophageal coins, an observation period of 8 to 16 hours is considered appropriate309; batteries and magnets are exceptions and should be removed expeditiously.310,311 Esophageal foreign bodies most commonly lodge at the level of the cricopharyngeal muscle,294 and foreign body impaction is most frequently associated with vomiting, gagging, odynophagia, dysphagia, poor feeding, and excessive salivation.308 A large esophageal foreign body may cause symptoms of airway obstruction by compression or irritation of the upper airway, necessitating emergent intervention.301
2 Complications of Endoscopy for Foreign Body Removal
a Intraoperative Complications
Intraoperative complications during foreign body removal are usually minor, and include laryngospasm, bronchospasm, and O2 desaturations. In a large review of almost 13,000 tracheobronchial foreign bodies in children, major complications, such as tracheal or bronchial laceration requiring repair, pneumothorax or pneumomediastinum, failed bronchoscopy requiring tracheostomy or thoracotomy, cardiac arrest, and hypoxic brain damage, occurred in about 1% of cases.296 Fatal complications have been reported, but they are rare (0.4%).297,298
b Postoperative Complications
The most common immediate postoperative complications include laryngeal edema and traumatic laryngitis.297 Long-term complications, such as granulation tissue and stricture formation, can occur at the site of the foreign body.301 Persistent pneumonia and atelectasis may be seen after removal of long-standing foreign bodies.294,297 After esophagoscopy, the most common complications are vomiting, aspiration, fever, and a second, missed foreign body.301
VI Conclusions
The field of laryngeal surgery is complex and continues to expand rapidly. It is likely that the mounting complexity of microlaryngoscopic procedures will benefit from perioperative involvement of a dedicated anesthesia team. Lack of collaboration and planning between the surgeon and the anesthesiologist can turn even a simple microlaryngoscopic case into a chaotic, life-threatening airway crisis.1 As anesthetic challenges continue to grow, so will the demand for a wide range of unique skills and expertise from the anesthesiologist to ensure patient safety and favorable surgical outcomes.
VII Clinical Pearls
• Close communication between the anesthesiologist and the surgeon throughout the perioperative period is critical for the success of the operation and for patient safety.
• A structured and stepwise approach to a tenuous and partially obstructed airway is necessary for successful management.
• Core surgical and anesthesia objectives include an anesthetic plan suitable for variable or unpredictable duration of surgery, an immobile and clear surgical field, absence of the patient’s movement, maintenance of a stable plane of anesthesia, competent execution of different intraoperative ventilation techniques and strategies, full return of the patient’s protective airway reflexes, smooth and rapid emergence from anesthesia, and fast-tracking patients for discharge.
• The principles and practice of jet ventilation must be well understood and adhered to by the anesthesiologist and the surgeon.
• Total intravenous anesthesia (TIVA) is preferred, especially when delivery of inhalational anesthetics is compromised.
• Neuromuscular blockade (NMB) should be adequately maintained.
• In pediatric patients, spontaneous ventilation technique typically is preferred over controlled ventilation.
• The possibility of post-extubation airway edema, laryngospasm, and postoperative airway obstruction should be anticipated and treated appropriately.
All references can be found online at expertconsult.com.
1 Rosen CA, Simpson CB. Operative techniques in laryngology. Berlin: Springer-Verlag; 2008.
18 Xiao P, Zhang XS. Adult laryngotracheal surgery. Anesthesiol Clin. 2010;28:529–540.
24 Biro P. Jet ventilation for surgical interventions in the upper airway. Anesthesiol Clin. 2010;28:397–409.
33 Mason RA, Fielder CP. The obstructed airway in head and neck surgery. Anaesthesia. 1999;54:625–628.
86 Bourgain JL, Desruennes E, Fischler M, et al. Transtracheal high frequency jet ventilation for endoscopic airway surgery: A multicentre study. Br J Anaesth. 2001;87:870–875.
87 Jaquet Y, Monnier P, Van Melle G, et al. Complications of different ventilation strategies in endoscopic laryngeal surgery: A 10-year review. Anesthesiology. 2006;104:52–59.
111 Cook TM, Alexander R. Major complications during anaesthesia for elective laryngeal surgery in the UK: A national survey of the use of high-pressure source ventilation. Br J Anaesth. 2008;101:266–272.
229 Benjamin B. Anesthesia for pediatric airway endoscopy. Otolaryngol Clin North Am. 2000;33:29–47.
232 Hoeve LJ, Rombout J. Pediatric laryngobronchoscopy: 1332 procedures stored in a data base. Int J Pediatr Otorhinolaryngol. 1992;24:73–82.
267 Collins CE. Anesthesia for pediatric airway surgery: Recommendations and review from a pediatric referral center. Anesthesiol Clin. 2010;28:505–517.
296 Fidkowski CW, Zheng H, Firth PG. The anesthetic considerations of tracheobronchial foreign bodies in children: A literature review of 12,979 cases. Anesth Analg. 2010;111:1016–1025.
1 Rosen CA, Simpson CB. Operative techniques in laryngology. Berlin: Springer-Verlag; 2008.
2 Rosen CA, Amin MR, Sulica L, et al. Advances in office-based diagnosis and treatment in laryngology. Laryngoscope. 2009;119(Suppl 2):S185–S212.
3 Atkins JH, Mirza N. Anesthetic considerations and surgical caveats for awake airway surgery. Anesthesiol Clin. 2010;28:555–575.
4 Sataloff RT. Laryngology: State of the art. Laryngoscope. 2003;113:1477–1478.
5 Koufman JA. Introduction to office-based surgery in laryngology. Curr Opin Otolaryngol Head Neck Surg. 2007;15:383–386.
6 Hillel AT, Kapoor A, Simaan N, et al. Applications of robotics for laryngeal surgery. Otolaryngol Clin North Am. 2008;41:781–791.
7 Chi JJ, Mandel JE, Weinstein GS, O’Malley BW, Jr. Anesthetic considerations for transoral robotic surgery. Anesthesiol Clin. 2010;28:411–422.
8 Webster AC. Anesthesia for operations on the upper airway. Int Anesthesiol Clin. 1972;10:61–122.
9 Agosti L. Anaesthetic technique for microsurgery of the larynx. Anaesthesia. 1977;32:362–365.
10 Welty P. Anesthetic concerns and complications during suspension microlaryngoscopy procedures. CRNA. 1992;3:113–118.
11 Kim MK, Deschler DG, Hayden RE. Flexible esophagoscopy as part of routine panendoscopy in ENT resident and fellowship training. Ear Nose Throat J. 2001;80:49–50.
12 Dhooge IJ, De Vos M, Albers FW, Van Cauwenberge PB. Panendoscopy as a screening procedure for simultaneous primary tumors in head and neck cancer. Eur Arch Otorhinolaryngol. 1996;253:319–324.
13 Lyon ST, Holinger LD. Endoscopic evaluation of the patient with head and neck cancer. Clin Plast Surg. 1985;12:331–341.
14 Falcone MT, Garrett CG, Slaughter JC, Vaezi M. Transnasal esophagoscopy findings: Interspecialty comparison. Otolaryngol Head Neck Surg. 2009;140:812–815.
15 Rubin AD, Sataloff RT. Vocal fold paresis and paralysis. Otolaryngol Clin North Am. 2007;40:1109–1131.
16 Sataloff RT, Hawkshaw MJ, Divi V, Heman-Ackah YD. Voice surgery. Otolaryngol Clin North Am. 2007;40:1151–1183.
17 Rees L, Mason RA. Advanced upper airway obstruction in ENT surgery. Br J Anaesth CEPD Rev. 2002;2:134–138.
18 Xiao P, Zhang XS. Adult laryngotracheal surgery. Anesthesiol Clin. 2010;28:529–540.
19 Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997;257:59–84.
20 McKillop IH, Schrum LW. Alcohol and liver cancer. Alcohol. 2005;35:195–203.
21 Sweeney BP, Bromilow J. Liver enzyme induction and inhibition: Implications for anaesthesia. Anaesthesia. 2006;61:159–177.
22 Sweeney BP, Grayling M. Smoking and anaesthesia: The pharmacological implications. Anaesthesia. 2009;64:179–186.
23 Ziser A, Plevak DJ, Wiesner RH, et al. Morbidity and mortality in cirrhotic patients undergoing anesthesia and surgery. Anesthesiology. 1999;90:42–53.
24 Biro P. Jet ventilation for surgical interventions in the upper airway. Anesthesiol Clin. 2010;28:397–409.
25 Arne J, Descoins P, Fusciardi J, et al. Preoperative assessment for difficult intubation in general and ENT surgery: Predictive value of a clinical multivariate risk index. Br J Anaesth. 1998;80:140–146.
26 Burkle CD, Walsh MT, Harrison BA, et al. Airway management after failure to intubate by direct laryngoscopy: Outcomes in a large teaching hospital. Can J Anaesth. 2005;52:634–640.
27 Rose DK, Cohen MM. The airway: Problems and predictions in 18,500 patients. Can J Anaesth. 1994;41:372–383.
28 Connelly NR, Ghandour K, Robbins L, et al. Management of unexpected difficult airway at a teaching institution over a 7-year period. J Clin Anesth. 2006;18:198–204.
29 Crosby ET, Cooper RM, Douglas MJ, et al. The unanticipated difficult airway with recommendations for management. Can J Anaesth. 1998;45:757–776.
30 Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology. 2006;105:885–891.
31 Kheterpal S, Martin L, Shanks AM, Tremper KK. Prediction and outcomes of impossible mask ventilation: A review of 50,000 anesthetics. Anesthesiology. 2009;110:891–897.
32 Bainton CR. Difficult intubation–what’s the best test? Can J Anaesth. 1996;43:541–543.
33 Mason RA, Fielder CP. The obstructed airway in head and neck surgery. Anaesthesia. 1999;54:625–628.
34 McRae K. Anesthesia for airway surgery. Anesthesiol Clin North Am. 2001;19:497–541.
35 Williams A, Patel A, Ferguson C. High frequency jet ventilation through the laryngeal mask airway in a critically obstructed airway. Anaesthesia. 2008;63:1369–1371.
36 Liess BD, Scheidt TD, Templer JW. The difficult airway. Otolaryngol Clin North Am. 2008;41:567–580.
37 Fitzwilliams B, Volikas I. An unexpectedly difficult intubation following repeated endoscopy. Anaesth Intensive Care. 2004;32:265–267.
38 Benjamin B, Lindholm CE. Systematic direct laryngoscopy: The Lindholm laryngoscopes. Ann Otol Rhinol Laryngol. 2003;112(Pt 1):787–797.
39 Benjamin B. Anesthesia for laryngoscopy. Ann Otol Rhinol Laryngol. 1984;93(Pt 1):338–342.
40 Rosen CA, Andrade Filho PA, Scheffel L, Buckmire R. Oropharyngeal complications of suspension laryngoscopy: A prospective study. Laryngoscope. 2005;115:1681–1684.
41 Pollard BJ. Anaesthesia for laryngeal microsurgery. Anaesthesia. 1968;23:534–542.
42 Glass PS, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg. 1999;89:S7–S14.
43 Nekhendzy V, Guta C, Champeau M. Otolaryngology–head and neck surgery. In: Jaffe RA, Samuels SI. Anesthesiologist’s manual of surgical procedures. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2009:174–258.
44 Shribman AJ, Smith G, Achola KJ. Cardiovascular and catecholamine responses to laryngoscopy with and without tracheal intubation. Br J Anaesth. 1987;59:295–299.
45 Hassan HG, el-Sharkawy TY, Renck H, et al. Hemodynamic and catecholamine responses to laryngoscopy with vs. without endotracheal intubation. Acta Anaesthesiol Scand. 1991;35:442–447.
46 Kaplan JD, Schuster DP. Physiologic consequences of tracheal intubation. Clin Chest Med. 1991;12:425–432.
47 Siddiqui N, Katznelson R, Friedman Z. Heart rate/blood pressure response and airway morbidity following tracheal intubation with direct laryngoscopy, GlideScope and Trachlight: A randomized control trial. Eur J Anaesthesiol. 2009;26:740–745.
48 Thompson JP, Hall AP, Russell J, et al. Effect of remifentanil on the haemodynamic response to orotracheal intubation. Br J Anaesth. 1998;80:467–469.
49 Kahl M, Eberhart LH, Behnke H, et al. Stress response to tracheal intubation in patients undergoing coronary artery surgery: Direct laryngoscopy versus an intubating laryngeal mask airway. J Cardiothorac Vasc Anesth. 2004;18:275–280.
50 Agro F, Barzoi G, Montecchia F. Tracheal intubation using a Macintosh laryngoscope or a GlideScope in 15 patients with cervical spine immobilization. Br J Anaesth. 2003;90:705–706.
51 Kaplan MB, Ward DS, Berci G. A new video laryngoscope—An aid to intubation and teaching. J Clin Anesth. 2002;14:620–626.
52 Kaplan MB, Hagberg CA, Ward DS, et al. Comparison of direct and video assisted views of the larynx during routine intubation. Clin Anesth. 2006;18:357–362.
53 Sun DA, Warriner CB, Parsons DG, et al. The GlideScope Video Laryngoscope: Randomized clinical trial in 200 patients. Br J Anaesth. 2005;94:381–384.
54 Cooper RM, Pacey JA, Bishop MJ, et al. Early clinical experience with a new videolaryngoscope (GlideScope) in 728 patients. Can J Anaesth. 2005;52:191–198.
55 Takenaka I, Aoyama K, Nakamura M, et al. Oral styletted intubation under video control in a patient with a large mobile glottic tumour and a difficult airway. Can J Anaesth. 2002;49:203–206.
56 Lange M, Frommer M, Redel A, et al. Comparison of the Glidescope and Airtraq optical laryngoscopes in patients undergoing direct microlaryngoscopy. Anaesthesia. 2009;64:323–328.
57 Aziz MF, Healy D, Kheterpal S, et al. Routine clinical practice effectiveness of the Glidescope in difficult airway management: An analysis of 2,004 Glidescope intubations, complications, and failures from two institutions. Anesthesiology. 2011;114:34–41.
58 Zeitels SM, Burns JA, Dailey SH. Suspension laryngoscopy revisited. Ann Otol Rhinol Laryngol. 2004;113:16–22.
59 Crockett DM, Scamman FL, McCabe BF, et al. Venturi jet ventilation for microlaryngoscopy: Technique, complications, pitfalls. Laryngoscope. 1987;97:1326–1330.
60 Kanagalingam J, Hurley R, Grant HR, Patel A. A new technique for the management of inaccessible anterior glottic lesions. J Laryngol Otol. 2003;117:302–306.
61 Chang CH, Bai SJ, Kim MK, Nam SB. The usefulness of the laryngeal mask airway Fastrach for laryngeal surgery. Eur J Anaesthesiol. 2010;27:20–23.
62 Mandel JE. Laryngeal mask airways in ear, nose, and throat procedures. Anesthesiol Clin. 2010;28:469–483.
63 Ferson DZ, Rosenblatt WH, Johansen MJ, et al. Use of the intubating LMA-Fastrach in 254 patients with difficult-to-manage airways. Anesthesiology. 2001;95:1175–1181.
64 Kihara S, Watanabe S, Taguchi N, et al. A comparison of blind and lightwandguided tracheal intubation through the intubating laryngeal mask airway. Anaesthesia. 2000;55:427–431.
65 Joo HS, Kapoor S, Rose FK, et al. The intubating laryngeal mask airway after induction of general anesthesia versus awake fiberoptic intubation in patients with difficult airways. Anesth Analg. 2001;92:1342–1346.
66 Dimitriou V, Voyagis GS, Brimacombe JR. Flexible lightwand-guided tracheal intubation with the intubating laryngeal mask Fastrach in adults after unpredicted failed laryngoscope-guided tracheal intubation. Anesthesiology. 2002;96:296–299.
67 Van Zundert AA, Hermans B, Kuczkowski KM. Successful use of a videolaryngoscope in a patient with carcinoma of the oropharynx and obstructed airway. Minerva Anestesiol. 2009;75:475–476.
68 American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277.
69 Sofferman RA, Johnson DL, Spencer RF. Lost airway during anesthesia induction: Alternatives for management. Laryngoscope. 1997;107:1476–1482.
70 Abernathy JH, 3rd., Reeves ST. Airway catastrophes. Curr Opin Anaesthesiol. 2010;23:41–46.
71 Theodore PR. Emergent management of malignancy-related acute airway obstruction. Emerg Med Clin North Am. 2009;27:231–241.
72 Nekhendzy V, Simmonds PK. Rigid bronchoscope-assisted endotracheal intubation: Yet another use of the gum elastic bougie. Anesth Analg. 2004;98:545–547.
73 Nekhendzy V, Simmons P. The Eschmann tracheal tube introducer is not an airway exchange device. Anesth Analg. 2004;99:1269–1270. author reply 1270
74 Ross-Anderson DJ, Ferguson C, Patel A. Transtracheal jet ventilation in 50 patients with severe airway compromise and stridor. Br J Anaesth. 2011;106:140–144.
75 Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229–1236.
76 Moorthy SS, Gupta S, Laurent B, Weisberger EC. Management of airway in patients with laryngeal tumors. J Clin Anesth. 2005;17:604–609.
77 Shaw IC, Welchew EA, Harrison BJ, Michael S. Complete airway obstruction during awake fibreoptic intubation. Anaesthesia. 1997;52:582–585.
78 Ho AM, Chung DC, To EW, Karmakar MK. Total airway obstruction during local anesthesia in a non-sedated patient with a compromised airway. Can J Anaesth. 2004;51:838–841.
79 Chao YK, Liu YH, Hsieh MJ, et al. Controlling difficult airway by rigid bronchoscope—An old but effective method. Interact Cardiovasc Thorac Surg. 2005;4:175–179.
80 Gerig HJ, Schnider T, Heidegger T. Prophylactic percutaneous transtracheal catheterisation in the management of patients with anticipated difficult airways: A case series. Anaesthesia. 2005;60:801–805.
81 Brooker CR, Hunsaker DH, Zimmerman AA. A new anesthetic system for microlaryngeal surgery. Otolaryngol Head Neck Surg. 1998;118:55–60.
82 Ku PK, Tong MC, Kwan A, van Hasselt CA. Modified tubeless anesthesia during endoscopy for assessment of head and neck cancers. Ear Nose Throat J. 2003;82:121–125.
83 Yung KC, Courey MS. The effect of office-based flexible endoscopic surgery on hemodynamic stability. Laryngoscope. 2010;120:2231–2236.
84 Drummond GB. Comparison of sedation with midazolam and ketamine: Effects on airway muscle activity. Br J Anaesth. 1996;76:663–667.
85 Hillman DR, Platt PR, Eastwood PR. Anesthesia, sleep, and upper airway collapsibility. Anesthesiol Clin. 2010;28:443–455.
86 Bourgain JL, Desruennes E, Fischler M, et al. Transtracheal high frequency jet ventilation for endoscopic airway surgery: A multicentre study. Br J Anaesth. 2001;87:870–875.
87 Jaquet Y, Monnier P, Van Melle G, et al. Complications of different ventilation strategies in endoscopic laryngeal surgery: A 10-year review. Anesthesiology. 2006;104:52–59.
88 Cinar SO, Coskun BU, Cinar U, et al. Blood gas changes in patients undergoing laryngeal microsurgery. Auris Nasus Larynx. 2006;33:299–302.
89 Nicelli E, Gemma M, De Vitis A, et al. Feasibility of standard mechanical ventilation with low FIO2 and small endotracheal tubes during laser microlaryngeal surgery. Head Neck. 2010;32:204–209.
90 Kawaida M, Fukuda H, Kohno N. Microlaryngosurgery for benign posterior glottal lesions using a posterior glottis direct laryngoscope. ORL J Otorhinolaryngol Relat Spec. 2001;63:127–130.
91 Strong MS. Microscopic laryngoscopy. A review and appraisal. Laryngoscope. 1970;80:1540–1552.
92 Ossoff RH, Duncavage JA. Adult subglottiscope for laser surgery. Ann Otol Rhinol Laryngol. 1988;97(Pt 1):552–553.
93 Talmage EA. Safe combined general and topical anesthesia for laryngoscopy and bronchoscopy. South Med J. 1973;66:455–459.
94 Talmage EA. Endotracheal tube is not necessary for laryngeal microsurgery [letter]. Anesthesiology. 1981;55:332.
95 Judelman H. Anaesthesia for laryngoscopy and microsurgery of the larynx. S Afr Med J. 1979;55:698.
96 Williams SR, van Hasselt CA, Aun CS, et al. Tubeless anesthetic technique for optimal carbon dioxide laser surgery of the larynx. Am J Otolaryngol. 1993;14:271–274.
97 Aun CS, Houghton IT, So HY, et al. Tubeless anaesthesia for microlaryngeal surgery. Anaesth Intensive Care. 1990;18:497–503.
98 Beattie C. The modified nasal trumpet maneuver. Anesth Analg. 2002;94:467–469.
99 Metz S. Perioperative use of the modified nasal trumpet in 346 patients. Br J Anaesth. 2004;92:694–696.
100 Mahajan R. Simple tracheal tube—An alternative to the modified nasal trumpet [letter]. Br J Anaesth. 2003;91:759. author reply 759–760
101 Bund M, Walz R, Lobbes W, et al. A tube in the pharynx for emergency ventilation. Acta Anaesthesiol Scand. 1997;41:529–530.
102 Rontal M, Rontal E, Wenokur M. Jet insufflation anesthesia for endolaryngeal surgery. Laryngoscope. 1980;90:1162–1168.
103 Hadaway EG, Page J, Shortridge RT. Anaesthesia for microsurgery of the larynx. Ann R Coll Surg Engl. 1982;64:279–280.
104 Sun KO. Doxapram in tubeless anaesthesia for microlaryngeal surgery. Anaesth Intensive Care. 1993;21:250–251.
105 MacGillivray RG, Fernandes CM. Microlaryngeal surgery without a tracheal tube. Anaesthesia. 1995;50:832–833.
106 Weigand H. Circulatory response to endolaryngeal microsurgery under light general anesthesia and the influence of surface anesthesia. Anesth Analg. 1969;48:953–957.
107 Weisberger EC, Emhardt JD. Apneic anesthesia with intermittent ventilation for microsurgery of the upper airway. Laryngoscope. 1996;106:1099–1102.
108 Nelson RA, Miller T. Apneic anesthesia for microlaryngeal surgery. Laryngoscope. 1973;83:1228–1233.
109 Barr AM, Wong RM. Awareness during general anaesthesia for bronchoscopy and laryngoscopy using the apnoeic oxygenation technique. Br J Anaesth. 1973;45:894–900.
110 Sebel PS, Bowdle TA, Ghoneim MM, et al. The incidence of awareness during anesthesia: A multicenter United States study. Anesth Analg. 2004;99:833–839.
111 Cook TM, Alexander R. Major complications during anaesthesia for elective laryngeal surgery in the UK: A national survey of the use of high-pressure source ventilation. Br J Anaesth. 2008;101:266–272.
112 Albert SN, Shibuya J, Albert CA. Ventilation with an oxygen injector for suspension laryngoscopy under general anesthesia. Anesth Analg. 1972;51:866–870.
113 Aloy A, Schachner M, Cancura W. Tubeless translaryngeal superimposed jet ventilation. Eur Arch Otorhinolaryngol. 1991;248:475–478.
114 Grasl MC, Donner A, Schragl E, Aloy A. Tubeless laryngotracheal surgery in infants and children via jet ventilation laryngoscope. Laryngoscope. 1997;107:277–281.
115 Orloff LA, Parhizkar N, Ortiz E. The Hunsaker Mon-Jet ventilation tube for microlaryngeal surgery: Optimal laryngeal exposure. Ear Nose Throat J. 2002;81:390–394.
116 Barakate M, Maver E, Wotherspoon G, et al. Anaesthesia for microlaryngeal and laser laryngeal surgery: Impact of subglottic jet ventilation. J Laryngol Otol. 2010;124:641–645.
117 Hautmann H, Gamarra F, Henke M, et al. High frequency jet ventilation in interventional fiberoptic bronchoscopy. Anesth Analg. 2000;90:1436–1440.
118 Young SS, Wang SJ, Lin SY, et al. An urgent technique of applying high frequency jet ventilation in patients with extreme periglottic stenosis. Acta Anaesthesiol Sin. 1995;33:63–68.
119 Borg U, Eriksson I, Sjöstrand U. High-frequency positive pressure ventilation (HFPPV): A review based upon its use during bronchoscopy and for laryngoscopy and microlaryngeal surgery under general anesthesia. Anesth Analg. 1980;59:594–603.
120 Shikowitz MJ, Abramson AL, Liberatore L. Endolaryngeal jet ventilation: A 10-year review. Laryngoscope. 1991;101:455–461.
121 Ravussin P, Freeman J. A new transtracheal catheter for ventilation and resuscitation. Can Anaesth Soc J. 1985;32:60–64.
122 Gerig HJ, Heidegger T, Ulrich B, et al. Fiberoptically guided insertion of transtracheal catheters. Anesth Analg. 2001;93:663–666.
123 Vourch G, Fischler M, Michon F, et al. High frequency jet ventilation v. manual jet ventilation during bronchoscopy in patients with tracheo-bronchial stenosis. Br J Anaesth. 1983;55:969–972.
124 Bacher A, Lang T, Weber J, et al. Respiratory efficacy of subglottic low-frequency, subglottic combined-frequency, and supraglottic combined-frequency jet ventilation during microlaryngeal surgery. Anesth Analg. 2000;91:1506–1512.
125 Patel A, Rubin JS. The difficult airway: The use of subglottic jet ventilation for laryngeal surgery. Logoped Phoniatr Vocol. 2008;33:22–24.
126 Sanders RD. Two ventilating attachments for bronchoscopes. Del Med J. 1967;192:170–175.
127 Gaitini LA, Fradis M, Vaida SJ, et al. Pneumomediastinum due to Venturi jet ventilation used during microlaryngeal surgery in a previously neck-irradiated patient. Ann Otol Rhinol Laryngol. 2000;109:519–521.
128 Bourgain JL, McGee K, Cosset MF, et al. Carbon dioxide monitoring during high frequency jet ventilation for direct laryngoscopy. Br J Anaesth. 1990;64:327–330.
129 Jawan B, Lee JH. Aspiration in transtracheal jet ventilation. Acta Anaesthesiol Scand. 1996;40:684–686.
130 Ihra G, Aloy A. On the use of Venturi’s principle to describe entrainment during jet ventilation. J Clin Anesth. 2000;12:417–419.
131 Davies JM, Hillel AD, Maronian NC, et al. The Hunsaker Mon-Jet tube with jet ventilation is effective for microlaryngeal surgery. Can J Anaesth. 2009;56:284–290.
132 Young JD, Sykes MK. Assisted ventilation. 1. Artificial ventilation: history, equipment and techniques. Thorax. 1990;45:753–758.
133 Ng A, Russell WC, Harvey N, et al. Comparing methods of administering high-frequency jet ventilation in a model of laryngotracheal stenosis. Anesth Analg. 2002;95:764–769.
134 Hamaekers AE, Götz T, Borg PA, et al. Achieving an adequate minute volume through a 2 mm transtracheal catheter in simulated upper airway obstruction using a modified industrial ejector. Br J Anaesth. 2010;104:382–386.
135 Hamaekers AE, Borg PA, Götz T, et al. Ventilation through a small-bore catheter: Optimizing expiratory ventilation assistance. Br J Anaesth. 2011;106:403–409.
136 Rezaie-Majd A, Bigenzahn W, Denk DM, et al. Superimposed high-frequency jet ventilation (SHFJV) for endoscopic laryngotracheal surgery in more than 1500 patients. Br J Anaesth. 2006;96:650–659.
137 Lanzenberger-Schragl E, Donner A, Grasl MC, et al. Superimposed high-frequency jet ventilation for laryngeal and tracheal surgery. Arch Otolaryngol Head Neck Surg. 2000;126:40–44.
138 Ihra G, Hieber C, Adel S, et al. Tubeless combined high-frequency jet ventilation for laryngotracheal laser surgery in paediatric anaesthesia. Acta Anaesthesiol Scand. 2000;44:475–479.
139 Kraincuk P, Körmöczi G, Prokop M, et al. Alveolar recruitment of atelectasis under combined high-frequency jet ventilation: A computed tomography study. Intensive Care Med. 2003;29:1265–1272.
140 Hamdan AL, Sibai A, Rameh C, et al. Short-term effects of endotracheal intubation on voice. J Voice. 2007;21:762–768.
141 Woods AW, Allam S. Tracheal intubation without the use of neuromuscular blocking agents. Br J Anaesth. 2005;94:150–158.
142 Kimura T, Watanabe S, Asakura N, et al. Determination of end-tidal sevoflurane concentration for tracheal intubation and minimum alveolar anesthetic concentration in adults. Anesth Analg. 1994;79:378–381.
143 Muzi M, Robinson BJ, Ebert TJ, et al. Induction of anesthesia and tracheal intubation with sevoflurane in adults. Anesthesiology. 1996;8:536–543.
144 Muzi M, Colinco MD, Robinson BJ, et al. The effects of premedication on inhaled induction of anesthesia with sevoflurane. Anesth Analg. 1997;85:1143–1148.
145 Sonne NM, Clausen TG, Valentin N, et al. Total intravenous anaesthesia for direct laryngoscopy: Propofol infusion compared to thiopentone combined with midazolam and methohexitone infusion. Acta Anaesthesiol Scand. 1992;36:250–254.
146 Atkins JH, Mandel JE. Current topics in anesthesia for head and neck surgery. Anesthesiol Clin. 2010;28:xv–xvi.
147 Gupta A, Stierer T, Zuckerman R, et al. Comparison of recovery profile after ambulatory anesthesia with propofol, isoflurane, sevoflurane and desflurane: A systematic review. Anesth Analg. 2004;98:632–641.
148 Montes FR, Trillos JE, Rincón IE, et al. Comparison of total intravenous anesthesia and sevoflurane-fentanyl anesthesia for outpatient otorhinolaryngeal surgery. J Clin Anesth. 2002;14:324–328.
149 Larsen B, Seitz A, Larsen R. Recovery of cognitive function after remifentanil-propofol anesthesia: A comparison with desflurane and sevoflurane anesthesia. Anesth Analg. 2000;90:168–174.
150 Visser K, Hassink EA, Bonsel GJ, et al. Randomized controlled trial of total intravenous anesthesia with propofol versus inhalation anesthesia with isoflurane-nitrous oxide: Postoperative nausea with vomiting and economic analysis. Anesthesiology. 2001;95:616–626.
151 McKeating K, Bali IM, Dundee JW. The effects of thiopentone and propofol on upper airway integrity. Anaesthesia. 1988;43:638–640.
152 Mustola ST, Baer GA, Metsä-Ketelä T, et al. Haemodynamic and plasma catecholamine responses during total intravenous anaesthesia for laryngomicroscopy. Thiopentone compared with propofol. Anaesthesia. 1995;50:108–113.
153 Ewalenko P, Deloof T, Gerin M, et al. Propofol infusion with or without fentanyl supplementation for microlaryngoscopy. Acta Anaesthesiol Belg. 1990;41:297–306.
154 Mustola ST, Baer GA, Neuvonen PJ, et al. Requirements of propofol at different end-points without adjuvant and during two different steady infusions of remifentanil. Acta Anaesthesiol Scand. 2005;49:215–221.
155 Pavlin JD, Colley PS, Weymuller EA, Jr., et al. Propofol versus isoflurane for endoscopic sinus surgery. Am J Otolaryngol. 1999;20:96–101.
156 Vuyk J. Clinical interpretation of pharmacokinetic and pharmacodynamic propofol-opioid interactions. Acta Anaesth Belg. 2001;52:445–451.
157 Twersky RS, Jamerson B, Warner DS, et al. Hemodynamics and emergence profile of remifentanil versus fentanyl prospectively compared in a large population of surgical patients. J Clin Anesth. 2001;13:407–416.
158 Wuesten R, Van Aken H, Glass PS, et al. Assessment of depth of anesthesia and postoperative respiratory recovery after remifentanil- versus alfentanil-based total intravenous anesthesia in patients undergoing ear-nose-throat surgery. Anesthesiology. 2001;94:211–217.
159 Wiel E, Davette M, Carpentier L, et al. Comparison of remifentanil and alfentanil during anaesthesia for patients undergoing direct laryngoscopy without intubation. Br J Anaesth. 2003;91:421–423.
160 Ozkose Z, Yalcin Cok O, Tuncer B, et al. Comparison of hemodynamics, recovery profile, and early postoperative pain control and costs of remifentanil versus alfentanil-based total intravenous anesthesia (TIVA). J Clin Anesth. 2002;14:161–168.
161 Alper I, Erhan E, Ugur G, Ozyar B. Remifentanil versus alfentanil in total intravenous anaesthesia for day case surgery. Eur J Anaesthesiol. 2003;20:61–64.
162 Pandazi AK, Louizos AA, Davilis DJ, et al. Inhalational anesthetic technique in microlaryngeal surgery: A comparison between sevoflurane-remifentanil and sevoflurane-alfentanil anesthesia. Ann Otol Rhinol Laryngol. 2003;112:373–378.
163 McAtamney D, O’Hare R, Hughes D, et al. Evaluation of remifentanil for control of haemodynamic response to tracheal intubation. Anaesthesia. 1998;53:1223–1227.
164 Miller DR, Martineau RJ, O’Brien H, et al. Effects of alfentanil on the hemodynamic and catecholamine response to tracheal intubation. Anesth Analg. 1993;76:1040–1046.
165 Stanski DR, Shafer SL. Quantifying anesthetic drug interaction. Implications for drug dosing. Anesthesiology. 1995;83:1–5.
166 Albertin A, Casati A, Federica L, et al. The effect-site concentration of remifentanil blunting cardiovascular responses to tracheal intubation and skin incision during bispectral index-guided propofol anesthesia. Anesth Analg. 2005;101:125–130.
167 Björkman S, Wada DR, Stanski DR. Application of physiologic models to predict the influence of changes in body composition and blood flows on the pharmacokinetics of fentanyl and alfentanil in patients. Anesthesiology. 1998;88:657–667.
168 Passot S, Servin F, Allary R, et al. Target-controlled versus manually-controlled infusion of propofol for direct laryngoscopy and bronchoscopy. Anesth Analg. 2002;94:1212–1216.
169 Stanski DR, Shafer SL. Quantifying anesthetic drug interaction. Implications for drug dosing. Anesthesiology. 1995;83:1–5.
170 Leone M, Rousseau S, Avidan M, et al. Target concentrations of remifentanil with propofol to blunt coughing during intubation, cuff inflation, and tracheal suctioning. Br J Anaesth. 2004;93:660–663.
171 Troy AM, Huthinson RC, Easy WR, et al. Tracheal intubating conditions using propofol and remifentanil target-controlled infusions. Anaesthesia. 2002;57:1204–1207.
172 Sutera PT, Smith CE. Asystole during direct laryngoscopy and tracheal intubation. J Cardiothorac Vasc Anesth. 1994;8:79–80.
173 Ko HB, Lee DY, Lee YC. Severe bradycardia during suspension laryngoscopy performed after tracheal intubation using a direct laryngoscope with a curved blade. A case report. Korean J Anesthesiol. 2010;59:116–118.
174 White PF, Tang J, Wender RH, et al. Desflurane versus sevoflurane for maintenance of outpatient anesthesia: The effect on early versus late recovery and perioperative coughing. Anesth Analg. 2009;109:387–393.
175 Philip BK, Kallar SK, Bogetz MS, et al. A multicenter comparison of maintenance and recovery with sevoflurane or isoflurane for adult ambulatory anesthesia. The Sevoflurane Multicenter Ambulatory Group. Anesth Analg. 1996;83:314–319.
176 Kang JG, Kim JK, Jeong HS, et al. A prospective, randomized comparison of the effects of inhaled sevoflurane anesthesia and propofol/remifentanil intravenous anesthesia on salivary excretion during laryngeal microsurgery. Anesth Analg. 2008;106:1723–1727.
177 Naguib M, Kopman AF, Lien CA, et al. A survey of current management of neuromuscular block in the United States and Europe. Anesth Analg. 2010;111:110–119.
178 Siddik-Sayyid SM, Taha SK, Kanazi GE, et al. Excellent intubating conditions with remifentanil-propofol and either low-dose rocuronium or succinylcholine. Can J Anaesth. 2009;56:483–488.
179 Scheller MS, Zornow MH, Saidman LJ. Tracheal intubation without the use of muscle relaxants: A technique using propofol and varying doses of alfentanil. Anesth Analg. 1992;75:788–793.
180 Erhan E, Ugur G, Alper I, et al. Tracheal intubation without muscle relaxants: Remifentanil or alfentanil in combination with propofol. Eur J Anaesthesiol. 2003;20:37–43.
181 Erhan E, Ugur G, Gunusen I, et al. Propofol—not thiopental or etomidate—with remifentanil provides adequate intubating conditions in the absence of neuromuscular blockade. Can J Anaesth. 2003;50:108–115.
182 Carnie J. Continuous suxamethonium infusion for microlaryngeal surgery. Br J Anaesth. 1982;54:11–14.
183 Ramsey FM, Lebowitz PW, Savarese JJ, et al. Clinical characteristics of long-term succinylcholine neuromuscular blockade during balanced anesthesia. Anesth Analg. 1980;59:110–116.
184 Lee C. Dose relationships of phase II, tachyphylaxis and train-of-four fade in suxamethonium-induced dual neuromuscular block in man. Br J Anaesth. 1975;47:841–845.
185 DeCook TH, Goudsouzian NG. Tachyphylaxis and phase II block development during infusion of succinylcholine in children. Anesth Analg. 1980;59:639–643.
186 Butterly A, Bittner EA, George E, et al. Postoperative residual curarization from intermediate-acting neuromuscular blocking agents delays recovery room discharge. Br J Anaesth. 2010;105:304–309.
187 Futter M, Gin T. Neuromuscular block: Views from the Western pacific. Anesth Analg. 2010;111:11–12.
188 Caldwell JE, Miller RD. Clinical implications of sugammadex. Anaesthesia. 2009;64(Suppl 1):66–72.
189 Eriksson LI, Sundman E, Olsson R, et al. Functional assessment of the pharynx at rest and during swallowing in partially paralyzed humans: Simultaneous videomanometry and mechanomyography of awake human volunteers. Anesthesiology. 1997;87:1035–1043.
190 Miller RD, Ward TA. Monitoring and pharmacologic reversal of a nondepolarizing neuromuscular blockade should be routine. Anesth Analg. 2010;111:3–5.
191 Hemmerling TM, Le N. Brief review: Neuromuscular monitoring: An update for the clinician. Can J Anaesth. 2007;54:58–72.
192 Murphy GS, Brull SJ. Residual neuromuscular block: Lessons unlearned. Part I: Definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg. 2010;111:120–128.
193 Ungureanu D, Meistelman C, Frossard J, et al. The orbicularis oculi and the adductor pollicis muscles as monitors of atracurium block of laryngeal muscles. Anesth Analg. 1993;77:775–779.
194 Hemmerling TM, Donati F. Neuromuscular blockade at the larynx, the diaphragm and the corrugator supercilii muscle: A review. Can J Anaesth. 2003;50:779–794.
195 Gätke MR, Larsen PB, Engbaek J, et al. Acceleromyography of the orbicularis oculi muscle. I: Significance of the electrode position. Acta Anaesthesiol Scand. 2002;46:1124–1230.
196 Larsen PB, Gätke MR, Fredensborg BB, et al. Acceleromyography of the orbicularis oculi muscle II: Comparing the orbicularis oculi and adductor pollicis muscles. Acta Anaesthesiol Scand. 2002;46:1131–1136.
197 Brull SJ, Murphy GS. Residual neuromuscular block: Lessons unlearned. Part II: Methods to reduce the risk of residual weakness. Anesth Analg. 2010;111:129–140.
198 Chambers D, Paulden M, Paton F, et al. Sugammadex for the reversal of muscle relaxation in general anaesthesia: A systematic review and economic assessment. Health Technol Assess. 2010;14:1–211.
199 Matot I, Sichel JY, Yofe V, et al. The effect of clonidine premedication on hemodynamic responses to microlaryngoscopy and rigid bronchoscopy. Anesth Analg. 2000;91:828–833.
200 Ayuso A, Luis M, Sala X, et al. Effects of anesthetic technique on the hemodynamic response to microlaryngeal surgery. Ann Otol Rhinol Laryngol. 1997;106(Pt1):863–868.
201 Figueredo E, Garcia-Fuentes EM. Assessment of the efficacy of esmolol on the haemodynamic changes induced by laryngoscopy and tracheal intubation: A meta-analysis. Acta Anaesthesiol Scand. 2001;45:1011–1022.
202 Yu SK, Tait G, Karkouti K, et al. The safety of perioperative esmolol: A systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2011;112:267–281.
203 Miller DR, Martineau RJ, Wynands JE, et al. Bolus administration of esmolol for controlling the haemodynamic response to tracheal intubation: The Canadian Multicentre Trial. Can J Anaesth. 1991;38:849–858.
204 Menigaux C, Guignard B, Adam F, et al. Esmolol prevents movement and attenuates the BIS response to orotracheal intubation. Br J Anaesth. 2002;89:857–862.
205 Oda Y, Nishikawa K, Hase I, et al. The short-acting beta1-adrenoceptor antagonists esmolol and landiolol suppress the bispectral index response to tracheal intubation during sevoflurane anesthesia. Anesth Analg. 2005;100:733–737.
206 Korpinen R, Saarnivaara L, Siren K, et al. Modification of the haemodynamic responses to induction of anaesthesia and tracheal intubation with alfentanil, esmolol and their combination. Can J Anaesth. 1995;42:298–304.
207 Louizos AA, Hadzilia SJ, Davilis DI, et al. Administration of esmolol in microlaryngeal surgery for blunting the hemodynamic response during laryngoscopy and tracheal intubation in cigarette smokers. Ann Otol Rhinol Laryngol. 2007;116:107–111.
208 White PF, Wang B, Tang J, et al. The effect of intraoperative use of esmolol and nicardipine on recovery after ambulatory surgery. Anesth Analg. 2003;97:1633–1638.
209 Coloma M, Chiu JW, White PF, et al. The use of esmolol as an alternative to remifentanil during desflurane anesthesia for fast-track outpatient gynecologic laparoscopic surgery. Anesth Analg. 2001;92:352–357.
210 Smith I, Van Hemelrijck J, White PF. Efficacy of esmolol versus alfentanil as a supplement to propofol-nitrous oxide anesthesia. Anesth Analg. 1991;73:540–546.
211 Morisaki H, Serita R, Innami Y, Kotake Y, et al. Permissive hypercapnia during thoracic anaesthesia. Acta Anaesthesiol Scand. 1999;43:845–849.
212 Gaumann DM, Tassonyi E, Fathi F, et al. Effects of topical laryngeal lidocaine on the sympathetic response to rigid panendoscopy under general anesthesia. J Otorhinolaryngol Relat Spec. 1992;54:49–53.
213 Ayers ML, Beamis JF, Jr. Rigid bronchoscopy in the twenty-first century. Clin Chest Med. 2001;22:355–364.
214 Aoyama K, Yasunaga E, Takenaka I, et al. Positive pressure ventilation during fibreoptic intubation: Comparison of the laryngeal mask airway, intubating laryngeal mask and endoscopy mask techniques. Br J Anaesth. 2002;88:246–254.
215 Johns MM. Update on the etiology, diagnosis, and treatment of vocal fold nodules, polyps, and cysts. Curr Opin Otolaryngol Head Neck Surg. 2003;11:456–461.
216 Altman KW. Vocal fold masses. Otolaryngol Clin North Am. 2007;40:1091–1108.
217 Verghese C. Laryngeal mask airway devices: Three maneuvers for any clinical situation. Available at http://www.anesthesiologynews.com/download/3Maneuvers_ANGAM10_WM.pdf (accessed February 2012)
218 Machata AM, Illievich UM, Gustorff B, et al. Remifentanil for tracheal tube tolerance: A case control study. Anaesthesia. 2007;62:796–801.
219 Nho JS, Lee SY, Kang JM, et al. Effects of maintaining a remifentanil infusion on the recovery profiles during emergence from anaesthesia and tracheal extubation. Br J Anaesth. 2009;103:817–821.
220 Tsao GJ, Damrose EJ. Complications of esophagoscopy in an academic training program. Otolaryngol Head Neck Surg. 2010;142:500–504.
221 Facciolongo N, Patelli M, Gasparini S, et al. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis. 2009;71:8–14.
222 Corvo MA, Inacio A, Mello MB, et al. Extra-laryngeal complications of suspension laryngoscopy. Braz J Otorhinolaryngol. 2007;73:727–732.
223 Robinson PM. Complications of microlaryngeal surgery. Clin Otolaryngol Allied Sci. 1989;14:545–549.
224 Klussmann JP, Knoedgen R, Wittekindt C, et al. Complications of suspension laryngoscopy. Ann Otol Rhinol Laryngol. 2002;111:972–976.
225 Hill RS, Koltai PJ, Parnes SM. Airway complications from laryngoscopy and panendoscopy. Ann Otol Rhinol Laryngol. 1987;96:691–694.
226 Westreich R, Sampson I, Shaari CM, et al. Negative-pressure pulmonary edema after routine septorhinoplasty: Discussion of pathophysiology, treatment, and prevention. Arch Facial Plast Surg. 2006;8:8–15.
227 Chuang YC, Wang CH, Lin YS. Negative pressure pulmonary edema: Report of three cases and review of the literature. Eur Arch Otorhinolaryngol. 2007;264:1113–1116.
228 Mamiya H, Ichinohe T, Kaneko Y. Negative pressure pulmonary edema after oral and maxillofacial surgery. Anesth Prog. 2009;56:49–52.
229 Benjamin B. Anesthesia for pediatric airway endoscopy. Otolaryngol Clin North Am. 2000;33:29–47.
230 Friedman EM, Williams M, Healy GB, et al. Pediatric endoscopy: A review of 616 cases. Ann Otol Rhinol Laryngol. 1984;93:517–519.
231 Wood RE. Evaluation of the upper airway in children. Curr Opin Pediatr. 2008;20:266–271.
232 Hoeve LJ, Rombout J. Pediatric laryngobronchoscopy: 1332 procedures stored in a data base. Int J Pediatr Otorhinolaryngol. 1992;24:73–82.
233 Cohen S, Pine H, Drake A. Use of rigid and flexible bronchoscopy among pediatric otolaryngologists. Arch Otolaryngol Head Neck Surg. 2001;127:505–509.
234 Kurth CD, LeBard SE. Association of postoperative apnea, airway obstruction, and hypoxemia in former premature infants. Anesthesiology. 1991;75:22–26.
235 Malviya S, Swartz J, Lerman J. Are all preterm infants younger than 60 weeks postconceptual age at risk for postanesthetic apnea? Anesthesiology. 1993;78:1076–1081.
236 Welborn LG, Greenspun JC. Anesthesia and apnea. Perioperative considerations in the former preterm infant. Pediatr Clin North Am. 1994;41:181–198.
237 Coté CJ, Zaslavsky A, Downes JJ, et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy. A combined analysis. Anesthesiology. 1995;82:809–822.
238 Walther-Larsen S, Rasmussen LS. The former preterm infant and risk of post-operative apnoea: Recommendations for management. Acta Anaesthesiol Scand. 2006;50:888–893.
239 Ghazal EA, Mason LJ, Coté CJ. Preoperative evaluation, premedication, and induction of anesthesia. In: Coté CJ, Lerman J, Todres ID. A practice of anesthesia for infants and children. ed 4. Philadelphia: Saunders Elsevier; 2009:37–69.
240 Welborn LG, Hannallah RS, Luban NL, et al. Anemia and postoperative apnea in former preterm infants. Anesthesiology. 1991;74:1003–1006.
241 Gollin G, Bell C, Dubose R, et al. Predictors of postoperative respiratory complications in premature infants after inguinal herniorrhaphy. J Pediatr Surg. 1993;28:244–247.
242 Tetzlaff JE, Annand DW, Pudimat MA, et al. Postoperative apnea in a full-term infant. Anesthesiology. 1988;69:426–428.
243 Coté CJ, Kelly DH. Postoperative apnea in a full-term infant with a demonstrable respiratory pattern abnormality. Anesthesiology. 1990;72:559–561.
244 Noseworthy J, Duran C, Khine HH. Postoperative apnea in a full-term infant. Anesthesiology. 1989;70:879–880.
245 Benjamin B. Direct laryngoscopy. In: Endolaryngeal surgery. London: Martin Dunitz; 1998:69–81.
246 Benjamin B, Lindholm CE. Systematic direct laryngoscopy: The Lindholm laryngoscopes. Ann Otol Rhinol Laryngol. 2003;112:787–797.
247 Hannallah RS, Brown KA, Verghese ST. Otorhinolaryngologic procedures. In: Coté CJ, Lerman J, Todres ID. A practice of anesthesia for infants and children. ed 4. Philadelphia: Saunders Elsevier; 2009:657–683.
248 Yellon RF. Prevention and management of complications of airway surgery in children. Paediatr Anaesth. 2004;14:107–111.
249 Casselbrant ML, Alper CM. Methods of examination. In: Bluestone CD, Stool SE, Alper CM, et al. Pediatric otolaryngology. ed 4. Philadelphia: Saunders; 2003:1379–1394.
250 O’Sullivan BP, Finger L, Zwerdling RG. Use of nasopharyngoscopy in the evaluation of children with noisy breathing. Chest. 2004;125:1265–1269.
251 Botma M, Kishore A, Kubba H, et al. The role of fibreoptic laryngoscopy in infants with stridor. Int J Pediatr Otorhinolaryngol. 2000;55:17–20.
252 Ayari-Khalfallah S, Froehlich P. Airway endoscopy and assessment in children. In: Graham JM, Scadding GK, Bull PD. Pediatric ENT. New York: Springer; 2008:177–182.
253 Motoyama EK. Anesthesia and the upper airway in infants and children. Int Anesthesiol Clin. 1992;30:17–19.
254 Werkhaven JA. Microlaryngoscopy-airway management with anaesthetic techniques for CO(2) laser. Paediatr Anaesth. 2004;14:90–94.
255 Mausser G, Friedrich G, Schwarz G. Airway management and anesthesia in neonates, infants and children during endolaryngotracheal surgery. Paediatr Anaesth. 2007;17:942–947.
256 Shukry M, Kennedy K. Dexmedetomidine as a total intravenous anesthetic in infants. Pediatr Anesth. 2007;17:581–583.
257 Quintal MC, Cunningham MJ, Ferrari LR. Tubeless spontaneous respiration technique for pediatric microlaryngeal surgery. Arch Otolaryngol Head Neck Surg. 1997;123:209–214.
258 Malherbe S, Whyte S, Singh P, et al. Total intravenous anesthesia and spontaneous respiration for airway endoscopy in children—A prospective evaluation. Paediatr Anaesth. 2010;20:434–438.
259 Landsman IS, Werkhaven JA, Motoyama EK. Anesthesia for pediatric otorhinolaryngologic surgery. In: Motoyama EK, Davis PJ. Smith’s anesthesia for infants and children. ed 7. Philadelphia: Mosby Elsevier; 2006:789–822.
260 Dilos BM. Anesthesia for pediatric airway endoscopy and upper gastrointestinal endoscopy. Int Anesthesiol Clin. 2009;47:55–62.
261 Swanson VC, Gries H, Koh J. Anesthesia in pediatric otolaryngology. Flint PW, Haughey BH, Lund VJ, et al. Cummings otolaryngology: Head and neck surgery, ed 5, St Louis: Mosby Elsevier, 2010.
262 Litman RS. Preanesthetic preparation of the pediatric patient. In: Litman RS, ed. Pediatric anesthesia: The requisites in anesthesiology. Philadelphia: Elsevier Mosby; 2004:87–98.
263 Cladis F. Appendix A: Pediatric drug dosages. In: Motoyama EKDP, ed. Smith’s anesthesia for infants and children. ed 7. Philadelphia: Mosby Elsevier; 2006:1199–1202.
264 McCubbin TD, Brown JH, Dewar KM, et al. Glycopyrrolate as a premedicant: Comparison with atropine. Br J Anaesth. 1979;51:885–889.
265 Krane EJ, Davis PJ. Preoperative preparation for infants and children. In: Motoyama EK, Davis PJ. Smith’s anesthesia for infants and children. ed 7. Philadelphia: Mosby Elsevier; 2006:255–271.
266 Steward DJ. Preoperative evaluation and preparation for surgery. In: Gregory GA, ed. Pediatric anesthesia. ed 4. Philadelphia: Churchill Livingstone; 2004:175–190.
267 Collins CE. Anesthesia for pediatric airway surgery: Recommendations and review from a pediatric referral center. Anesthesiol Clin. 2010;28:505–517.
268 Brooks P, Ree R, Rosen D, et al. Canadian pediatric anesthesiologists prefer inhalational anesthesia to manage difficult airways. Can J Anaesth. 2005;52:285–290.
269 Midulla F, de Blic J, Barbato A, et al. Flexible endoscopy of paediatric airways. Eur Respir J. 2003;22:698–708.
270 Brett CM, Zwass MS. Eyes, ears, nose, throat,and dental surgery. In: Gregory GA, ed. Pediatric anesthesia. ed 4. Philadelphia: Churchill Livingstone; 2004:663–705.
271 Wu FL, Razzaghi A, Souney PF. Seizure after lidocaine for bronchoscopy: Case report and review of the use of lidocaine in airway anesthesia. Pharmacotherapy. 1993;13:72–78.
272 Kirse DJ. Use of the microdebrider in pediatric endoscopic airway surgery. Curr Opin Otolaryngol Head Neck Surg. 2009;17:477–482.
273 Rutter MJ, Cohen AP, de Alarcon A. Endoscopic airway management in children. Curr Opin Otolaryngol Head Neck Surg. 2008;16:525–529.
274 Mani V, Morton NS. Overview of total intravenous anesthesia in children. Paediatr Anaesth. 2010;20:211–222.
275 Ma H, Lovich MA, Peterfreund RA. Quantitative analysis of continuous intravenous infusions in pediatric anesthesia: Safety implications of dead volume, flow rates, and fluid delivery. Paediatr Anaesth. 2011;21:78–86.
276 Yuen VM. Dexmedetomidine: Perioperative applications in children. Paediatr Anaesth. 2010;20:256–264.
277 Quezado ZM, Groblewski JC, Gelfand HJ, et al. Dexmedetomidine and proprofol in complex microlaryngeal surgery in infants. Int J Pediatr Otorhinolaryngol. 2009;73:1311–1312.
278 Seybold JL, Ramamurthi RJ, Hammer GB. The use of dexmedetomidine during laryngoscopy, bronchoscopy, and tracheal extubation following tracheal reconstruction. Paediatr Anaesth. 2007;17:1212–1214.
279 Mahmoud M, Radhakrishman R, Gunter J, et al. Effect of increasing depth of dexmedetomidine anesthesia on upper airway morphology in children. Paediatr Anaesth. 2010;20:506–515.
280 Lerman J, Jöhr M. Inhalational anesthesia vs total intravenous anesthesia (TIVA) for pediatric anesthesia. Paediatr Anaesth. 2009;19:521–534.
281 Morton N. Total intravenous anesthesia and target-controlled infusion. In: Coté CJ, Lerman J, Todres ID. A practice of anesthesia for infants and children. ed 4. Philadelphia: Saunders Elsevier; 2009:147–157.
282 Munoz HR, Cortinez LI, Ibacache ME, et al. Remifentanil requirements during propofol administration to block the somatic responses to skin incision in children and adults. Anesth Analg. 2007;104:77–80.
283 Wu KH, Man TT, Wong KL, et al. Bronchoscopy and anesthesia for preschool-aged patients: A review of 228 cases. Int Surg. 2002;87:252–255.
284 Wiseman NE, Sanchez I, Powell RE. Rigid bronchoscopy in the pediatric age group: Diagnostic effectiveness. J Pediatr Surg. 1992;27:1294–1297.
285 Schraff S, Derkay CS, Burke B, et al. American Society of Pediatric Otolaryngology members’ experience with recurrent respiratory papillomatosis and the use of adjuvant therapy. Arch Otolaryngol Head Neck Surg. 2004;130:1039–1042.
286 Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: A review. Laryngoscope. 2008;118:1236–1247.
287 Pransky SM, Kang DR. Tumors of the larynx, trachea, and bronchi. In: Bluestone CD, Stool SE, Alper CM, et al. Pediatric otolaryngology. ed 4. Philadelphia: Saunders; 2003:1558–1572.
288 Reeves WC, Ruparelia SS, Swanson KI, et al. National registry for juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2003;129:976–982.
289 Verghese ST, Hannallah RS. Pediatric otolaryngologic emergencies. Anesthesiol Clin North Am. 2001;19:237–256.
290 Stern Y, McCall JE, Willging JP, et al. Spontaneous respiration anesthesia for respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2000;109:72–76.
291 Derkay CS, Faust RA. Recurrent respiratory papillomatosis. Flint PW, Haughey BH, Lund VJ, et al. Cummings otolaryngology: Head and neck surgery, ed 5, St Louis: Mosby Elsevier, 2010.
292 Lei W, Wen W, Su Z, et al. Comparison of intravenous general anaesthesia vs endotracheal intubation in the surgical management of juvenile onset recurrent respiratory papillomatosis. Acta Otolaryngol. 2010;130:281–285.
293 Pasquale K, Wiatrak B, Woolley A, et al. Microdebrider versus CO2 laser removal of recurrent respiratory papillomas: A prospective analysis. Laryngoscope. 2003;113:139–143.
294 Friedman EM. Tracheobronchial foreign bodies. Otolaryngol Clin North Am. 2000;33:179–185.
295 Doody D. Foreign body aspiration. In: Grillo H, ed. Surgery of the trachea and bronchi. Hamilton, Ontario: BC Decker; 2004:707–718.
296 Fidkowski CW, Zheng H, Firth PG. The anesthetic considerations of tracheobronchial foreign bodies in children: A literature review of 12,979 cases. Anesth Analg. 2010;111:1016–1025.
297 Darrow DH, Holinger LD. Foreign bodies of the larynx, trachea, and bronchi. In: Bluestone CD, Stool SE, Alper CM, et al. Pediatric otolaryngology. Philadelphia: Saunders; 2003:1543–1557.
298 Metrangelo S, Monetti C, Meneghini L, et al. Eight years’ experience with foreign-body aspiration in children: What is really important for a timely diagnosis? J Pediatr Surg. 1999;34:1229–1231.
299 Farrell PT. Rigid bronchoscopy for foreign body removal: Anaesthesia and ventilation. Paediatr Anaesth. 2004;14:84–89.
300 Tomaske M, Gerber AC, Weiss M. Anesthesia and periinterventional morbidity of rigid bronchoscopy for tracheobronchial foreign body diagnosis and removal. Paediatr Anaesth. 2006;16:123–129.
301 Holinger LD, Poznanovic SA. Foreign bodies of the airway and esophagus. Flint PW, Haughey BH, Lund VJ, et al. Cummings otolaryngology: Head and neck surgery, ed 5, St Louis: Mosby Elsevier, 2010.
302 Blazer S, Naveh Y, Friedman A. Foreign body in the airway. A review of 200 cases. Am J Dis Child. 1980;134:68–71.
303 Litman RS, Ponnuri J, Trogan I. Anesthesia for tracheal or bronchial foreign body removal in children: An analysis of ninety-four cases. Anesth Analg. 2000;91:1389–1391.
304 Kain ZN, O’Connor TZ, Berde CB. Management of tracheobronchial and esophageal foreign bodies in children: A survey study. J Clin Anesth. 1994;6:28–32.
305 Pawar DK. Dislodgement of bronchial foreign body during retrieval in children. Paediatr Anaesth. 2000;10:333–335.
306 Singh B, Kantu M, Har-El G, et al. Complications associated with 327 foreign bodies of the pharynx, larynx, and esophagus. Ann Otol Rhinol Laryngol. 1997;106:301–304.
307 Soprano JV, Fleisher GR, Mandl KD. The spontaneous passage of esophageal coins in children. Arch Pediatr Adolesc Med. 1999;153:1073–1076.
308 Athanassiadi K, Gerazounis M, Metaxas E, et al. Management of esophageal foreign bodies: A retrospective review of 400 cases. Eur J Cardiothorac Surg. 2002;21:653–656.
309 Waltzman ML. Management of esophageal coins. Curr Opin Pediatr. 2006;18:571–574.
310 Kay M, Wyllie R. Pediatric foreign bodies and their management. Curr Gastroenterol Rep. 2005;7:212–218.
311 Litovitz T, Whitaker N, Clark L, et al. Emerging battery-ingestion hazard: Clinical implications. Pediatrics. 2010;125:1168–1677.