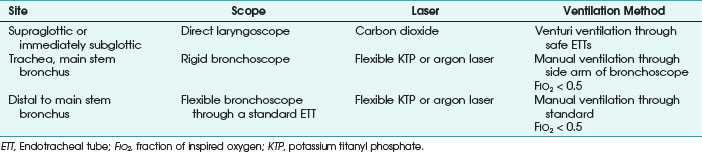
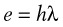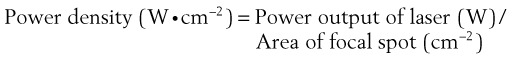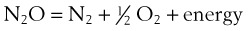Chapter 40 Anesthesia for Laser Airway Surgery
II. Principles of Laser Technology
VI. Anesthetic Techniques for Laser Airway Surgery
VII. Anesthesia Management for Laryngeal Laser Surgery
VIII. Anesthetic Management for Tracheobronchial Tree Laser Surgery
I Introduction
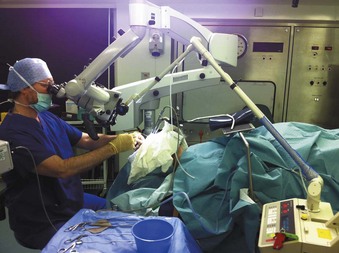
Airway surgery is unique in that it involves the anesthesiologist and surgeon working in the same anatomic field. Even without the use of a laser in a shared airway, procedures are challenging when the airway is compromised. The addition of a laser beam (i.e., ignition source) into a shared airway can cause a catastrophic fire, and to avoid these disasters, all operating room staff must recognize that a team approach is essential (Fig. 40-1).1–39 If a high-risk situation exists, all team members must proactively agree on how a fire will be prevented and managed.1 The importance of a team approach cannot be overstated.
II Principles of Laser Technology
A History
The history and development of lasers parallels our understanding of electromagnetic radiation, ions, molecules, and atoms. In 1900, Max Planck revised his earlier work on radiation and the absorption of light and heat by a black body (i.e., a perfect absorber).40 He proposed that electromagnetic energy could be emitted only in quantized form. In this process, called the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from short-wavelength electromagnetic radiation, such as visible or ultraviolet light. In 1905, Albert Einstein described how the photoelectric effect was caused by absorption of quanta of light (i.e., photons) and explained that the energy of these quanta was directly related to the frequency of the radiation absorbed. From these observations, Einstein developed his ideas on quantum mechanics, and in 1917, he developed the quantum theory of radiation,41,42 in which he discussed the interactions of atoms, ions, molecules, photons, and electromagnetic radiation. According to the quantum theory of radiation, electrons, atoms, molecules, and photons interact with electromagnetic radiation of quantum units by absorption, spontaneous emission, and stimulated emission.43
In 1954, Charles Townes described the first amplification and generation of electromagnetic waves by stimulated emission by using microwaves, which he called microwave amplification by the stimulated emission of radiation (MASER). In 1960, Theodore Maiman produced light amplification by the stimulated emission of radiation (LASER) using a ruby crystal and red light. Throughout the 1960s and 1970s, many substrates and ions were tried as the laser medium. Some, such as the carbon dioxide (CO2) laser, were successfully developed, found to be useful in medical practice, and are still used.44
B Physics
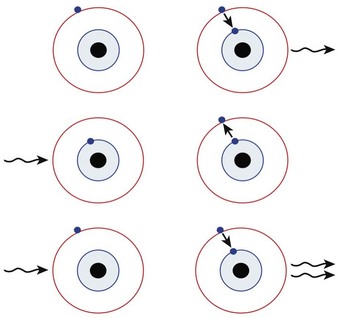
In 1917, Einstein postulated that if a photon released from an excited atom collided with another atom already in the excited state, the second atom would release two photons with the same wavelength, phase, and direction (i.e., identical photons). If these two photons stimulated more excited atoms, more identical photons would be released. Einstein called this process stimulated emission of radiation, and it is the basic principle of laser physics (Fig. 40-2).45
All lasers consist of a laser medium (e.g., CO2, argon) contained in an optical cavity between two mirrors, one totally reflecting and one partially reflecting mirror. An external energy source continuously excites or pumps the laser medium, causing many of the atoms to reach a higher energy level (Fig. 40-3). When more than one half of the atoms are in an excited state, a population inversion has taken place. In this state, spontaneous emission occurs in every direction, but photons emitted in the direction of the optical cavity strike the mirror and are reflected back, striking excited atoms, and resulting in stimulated emission. This process repeats itself at an increasing rate with each passage of the photons reflected off the mirrors through the laser medium, generating a huge number of identical photons that all have the same wavelength, phase, and direction. The partially reflecting mirror lets a small amount of this energy escape through the aperture, creating the laser beam.
C Laser Parameters
2 Spot Size
After the laser light has emerged from the optical cavity of a laser system, it may pass through a lens that focuses the beam to a very small diameter, or spot size, ranging from 0.1 to 2.0 mm. The optical properties of each focusing lens determine the focal length, or distance from the lens to the intended target tissue.46
3 Power
When using lasers in clinical practice, power often is measured in watts (W), although a more useful term is the power density. For a given power, the effects vary according to the spot size and the time of exposure. The relationship between power and depth of tissue injury becomes logarithmic when the power and exposure time are kept constant and the spot size is varied.47 Power density describes the amount of power (W) arriving at a surface area (W/cm2).
D Laser-Tissue Interaction
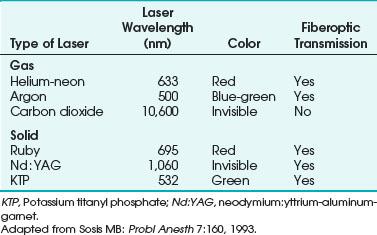
When a laser interacts with tissues, the energy can be reflected, absorbed, conducted as heat, or scattered. The extent to which these processes occur depends on the type of laser used and the tissue with which it interacts. Laser wavelengths depend on the medium, and the degree of absorption depends on the target tissue components. The CO2 laser (10,600-nm wavelength) is particularly well absorbed by water, whereas the neodymium : yttrium-aluminum-garnet (Nd : YAG) laser (1064-nm wavelength) is absorbed less by water and creates more energy scatter. The argon laser (488- and 514-nm wavelengths) and potassium titanyl phosphate (KTP) laser (532-nm wavelength) are in the visible spectrum, and their energies are absorbed well by pigments such as melanin and hemoglobin. For the most commonly used lasers in medicine, absorption of laser energy excites the atoms within tissues, which produces heat (Fig. 40-4).
The CO2 laser heats the water within cells, and as the temperature rises to about 65° C, proteins denature and thermal necrosis occurs. As the heating continues and the temperature reaches 100° C, the water within the cell boils and produces water vapor; this leads to an explosive vaporization, and the cell explodes. The tissue surface in contact with the CO2 laser disintegrates and undergoes charring, with carbon debris deposited at about 350° C. There is little damage to the underlying structures. Surrounding the charred area is a zone of thermal necrosis, and around this is an area of thermal conductivity and repair.48
E Laser Classification
Class 1. These lasers are considered safe, with no risk when viewed by the naked eye. Class 1M denotes that the beam is potentially hazardous if it is viewed with an optical instrument, such as a microscope.
Class 2. These lasers emit low-power visible radiation in the range of 400 to 700 nm. An aversion reaction or blink reflex limits exposure to less than 0.25 seconds and provides protection from equipment such as a laser pointer. Class 2M devices are the same as class 2, but they may be hazardous if viewed with an optical instrument.
Class 3. A class 3R laser is considered safe with restricted beam viewing, and if the maximum permissible exposure is exceeded, the risk of injury is low. Class 3B lasers are hazardous if the eye is exposed directly, and protective eyewear is required if direct viewing of a laser beam may occur. A key switch and safety interlock must be present on all class 3B lasers.
Class 4. Most medical lasers belong in this class and pose dangers to the eye, skin, and combustible material. They are a fire risk and produce air contaminants and plumes. All class 3B and class 4 lasers must be operated by trained, authorized personnel and be equipped with a key switch and a safety interlock.
F Types of Laser
Lasers are named according to their medium, which can be a gas, solid, liquid, or semiconductor. Many types of lasers exist, and their clinical application depends on their wavelength, tissue absorptive characteristics, cost, ease of use, and safety. Lasers have differences in their emitted wavelength, output power, mode of operation (i.e., pulsed or continuous mode), and application (i.e., contact or noncontact). For laser airway surgery, the CO2, argon, Nd : YAG, KTP, and diode lasers are the most commonly used (Table 40-1).
1 Carbon Dioxide Laser
The CO2 laser has been used for many otolaryngology and head and neck surgical procedures and in gynecologic surgery for the treatment of intraepithelial neoplasms, condylomata acuminata, and other lesions. It is the most commonly used laser for airway surgery and is particularly suited to laryngeal and head and neck surgical procedures (Fig. 40-5).
The CO2 laser can be used in a continuous, pulsed, or superpulsed mode. The superpulsed mode reduces the exposure time to a few nanoseconds while delivering high energies of 400 to 500 W with each peak (Fig. 40-6). The rest time between each peak allows the tissues to cool and reduces thermal injury to adjacent tissues.
The CO2 laser has advantages that make it an effective surgical tool. Its use with an operating microscope allows the surgeon to precisely destroy targets approximately 2 mm in diameter under binocular vision. This degree of precision may be impossible to achieve with conventional cautery. During CO2 laser surgery, the surgical field is usually bloodless because of the laser’s considerable hemostatic action. Vessels up to 0.5 mm in diameter usually can be sectioned without bleeding.49 The CO2 laser has been used successfully to excise vascular lesions, even in patients with a bleeding diathesis.50 The use of more traditional surgical tools, such as cautery, usually results in considerable postoperative edema. Edema does not usually occur after using the CO2 laser because of the sharp line of tissue destruction, with virtually no injury to surrounding tissue. Ninety percent of the laser’s energy is absorbed within 0.03 mm.51 There is no manipulation of tissues because laser treatment is usually a noncontact technique. Microscopic examination of tissue after CO2 laser surgery reveals a discrete line of destruction, with preservation of capillaries and normal features of adjacent tissues. The preservation of adjacent tissues is thought to account for the rapid healing, minimal scarring, and reduced pain often observed after CO2 laser surgery.49
The CO2 laser is a safe and extremely useful surgical modality in the airways and the digestive tract.52 It is best suited for widening benign tracheal stenoses and for removal of granulation tissues.53
2 Flexible-Fiber Carbon Dioxide Laser
A drawback of CO2 lasers is that they are fixed to a line-of-site delivery mechanism and cannot be transmitted through ordinary fiberoptic bundles, which limits their use to areas accessible by a straight approach. Flexible-fiber CO2 lasers (Omniguide, Cambridge, MA; Fiberlase CO2 Fiber, Lumenis, CA) have been developed for use in airway procedures, including base of tongue tumors, laryngeal tumors, tracheal tumors, laryngeal papillomas, tracheal stenosis, and laryngeal lesions.54–59
3 Potassium Titanyl Phosphate Laser
The KTP laser is strongly absorbed by hemoglobin and melanin, and it is used for otolaryngologic lesions, vascular diseases, and hemorrhages.60 The KTP laser has been used to treat several skin conditions, including port wine stains, hemangioma, telangiectasia, spider nevi, and red scars of the skin (Fig. 40-7).
The KTP laser is generated by passing the light of a rapidly pulsed Nd : YAG laser through a potassium titanyl phosphate crystal, which doubles the frequency and halves the wavelength to 532 nm, producing a beam in the bright green visible spectrum that has a tissue penetration of 0.5 to 2 mm.60 The radiation is able to pass through a flexible fiberoptic bundle and is used in a pulsed mode. It is used in vascular lesions within the airway, and because it can be transmitted through clear substances and can pass through a flexible fiberoptic fiber, it can be used in areas where a direct line-of-site laser cannot. The KTP laser has been used for laryngotracheal stenosis, laryngeal paralysis, and choanal atresia. The convenience of fiber delivery, concomitant telescopic control, and low-grade edematous reaction were the main advantages over the CO2 laser. Healing time was longer for the KTP laser than for the CO2 laser.
4 Argon Laser
The argon laser contains argon gas and produces a visible blue-green beam with wavelengths of 488 nm and 514 nm, which are absorbed selectively by hemoglobin, melanin, and other pigments that lie under the retina. The beam is readily transmitted down a fiberoptic bundle, allowing endobronchial surgery (Fig. 40-8).
Applications of the argon laser include ophthalmologic surgery, especially for retinal and anterior chamber procedures. Because the laser passes readily through fluid inside the eye without damage, it is used in the treatment of retinal vascular lesions, including diabetic retinopathy, retinal detachment, glaucoma, and macular degeneration. Its applications in dermatologic and plastic surgery include the removal of port wine stains, hemangiomas, and tattoos because of the laser’s absorption by hemoglobin and other pigments. Because the tissue penetration is 0.5 to 2 mm, it is useful for superficial coagulation of capillary vessels. Port wine stains are lightened without scarring after treatment with the argon laser.50 To vaporize tissues, the power density is increased to produce a small focal spot; this allows argon laser stapedotomy. The argon laser has been used in infants to remove obstructive endobronchial lesions due to traumatic suction catheter injuries by passing argon laser fibers through the suction port of the fiberoptic bronchoscope (FOB) and targeting the obstructive endobronchial lesion with the argon laser beam.
5 Neodymium : Yttrium-Aluminum-Garnet Laser
The Nd : YAG laser has been used for photocoagulation and deep thermal necrosis in the treatment of gastrointestinal bleeding and obstructing bronchial lesions. The Nd : YAG laser uses a clear, solid, crystalline medium that emits radiation in the infrared region of the electromagnetic spectrum; the radiation has a wavelength of 1060 nm and is invisible (see Table 40-1). Conventional fiberoptic bundles readily transmit Nd : YAG laser radiation at high power, which is applied in a continuous or pulsed mode. The radiation is more readily absorbed by dark tissue. Blue or black pigmentation enhances Nd : YAG absorption, whereas pale colors enhance its penetration.61,62 Nd : YAG radiation penetrates tissues to a depth of 2 to 6 mm and provides good homeostasis for blood vessels up to 0.5 cm in diameter. However, the depth of penetration is less predictable than that of the CO2 laser. The power density below the tissue surface depends on the color of the surface, which makes laser penetration more difficult with the Nd : YAG laser than with the CO2 laser.
The Nd : YAG laser is recommended for lesions distal to the larynx and for bulky, vascular endobronchial neoplasms. The advantage of the Nd : YAG laser is that the radiation can be transmitted through fiberoptic bundles. Excision of lesions that are located distal to the larynx is complicated because it is difficult to reach the tumor with the laser beam. In these situations, the Nd : YAG laser is preferred because it can be used with a rigid bronchoscope or an FOB.62 Nd : YAG lasers are less precise in cutting, penetrate the tissue deeper, and have improved photocoagulation and superior hemostasis compared with other lasers.
Dumon and coworkers,63 reporting a large series of cases, recommended the use of rigid rather than flexible bronchoscopy for treating obstructive pulmonary lesions by means of endoscopic Nd : YAG laser surgery. Brutinel and associates reported difficulty ventilating and oxygenating patients’ lungs through an endotracheal tube (ETT) during Nd : YAG surgery with an indwelling FOB in place.64 Casey and colleagues reported combustion of the FOB and ETT in a patient undergoing Nd : YAG laser airway surgery.65 The use of a rigid bronchoscope for the treatment of obstructing pulmonary lesions facilitates the removal of tissue and the treatment of complications.66 Power levels less than 50 W given in short pulsations decrease the chances of impingement on vital underlying structures.66 McDougall and Cortese reported two patients who died during Nd : YAG laser endoscopic treatment of airway obstructions using very high power.67 At high power, the penetration of tissues by the Nd : YAG laser cannot be readily controlled, and perforation of a large blood vessel is possible.
6 Ruby Laser
The ruby laser uses a solid medium of a crystal aluminum oxide (i.e., sapphire) containing chromium ions. It emits visible red radiation at a wavelength of 695 nm (see Table 40-1). The ruby laser is used only in the pulse mode. The radiation is not readily absorbed by water but is significantly absorbed by pigments such as melanin and hemoglobin. The ruby laser can easily penetrate the anterior structures of the eye. It is used to photocoagulate vascular and pigmented retinal lesions. Use of this laser has decreased with the availability of newer types, and the ruby laser is not commonly used for laser airway surgery.
7 Diode Laser
The diode laser is another addition to a growing array of tools for laser surgery. This laser is effective for treatment of hyperplastic inferior nasal turbinates and provides good hemostasis and a sufficient reduction of tumors in otolaryngology practice.60 The continuous-wave, semiconductor diode laser emits radiation at wavelengths of 810 and 940 nm in the near-infrared spectrum. The radiation is delivered by a flexible fiber coupled with an aiming beam. The diode laser penetrates tissue to a depth of 1 to 3 mm, and it is suitable for vascular lesions. It is used to remove and debulk airway pathology, including tumors throughout the airway.
III Laser Hazards
A General Laser Hazards
1 Eye Damage
Lasers can easily damage the eyes by direct or indirect exposure, causing serious corneal and retinal injuries that may be irreversible. Eye protection is essential for anyone within the operating room, including the patient, auxiliary staff, nursing staff, surgeon, and anesthesiologist (Fig. 40-9). General precautions for the patient include taping the eyes closed and avoiding the use of petroleum-based eye lubricants. Further protection includes saline-soaked eye pads, protective eye glasses, or metal eye goggles, depending on the wavelength and type of laser used.
All laser glasses should be labeled clearly with the optical density value, the wavelengths against which protection is afforded, and maximum radiant exposure, or irradiance, to which the eyewear can be exposed (Figs. 40-10 and 40-11).68 The correct protective eye glasses must be used for the type of laser used for the procedure; failure to do so provides no protection for the eyes. In hospitals where only one type of laser is in use, this is less of a problem, but care must be taken in hospitals with many types of lasers and protective eye goggles.
For CO2 lasers, the damage to the eye is limited to the cornea because its radiation is largely absorbed by water, and the cornea is more than 75% water.61,69 There is no risk to the retina. For airway use, the patient’s eyes should be taped, protective saline-soaked eye pads placed, and surgical drapes applied and covered with another layer of saline-soaked swabs placed over the drapes. For the operating room staff, CO2 laser–protective eyeglasses should be worn. Contact lenses protect only the area covered and provide inadequate protection. Regular conventional eyeglasses can protect against the CO2 laser beam if the beam has been directed or reflected straight onto the eyeglass; however, if the beam is reflected accidentally into the side of the eyeglasses, damage to the eye can occur. When working with CO2, lasers, all operating room staff should use eyeglasses that wrap around the eye, protecting from CO2 laser entry from the side. The surgeon does not need to use CO2 laser protective eyeglasses when working with the operating microscope because the optics of the microscope provide protection70; however, if the surgeon uses the CO2 laser with a handpiece, the surgeon must also wear protective eyeglasses.
For Nd : YAG lasers, unprotected eyes may absorb and focus laser light at the ocular fundus, resulting in irreparable damage. Radiation from the KTP and other lasers such as the Nd : YAG can penetrate the cornea and lens of the eye, resulting in severe retinal damage. The endoscopist is at greatest risk because of the Nd : YAG laser’s potential for backscatter.62 The patient and all operating room staff should wear protective eyeglasses. Filters that absorb the Nd : YAG wavelength of 1.06 µm can be placed into rigid and flexible bronchoscopes. If the fibers break during laser surgery, the beam may be deflected to anywhere in the operating room.
For KTP and argon lasers, all operating room personnel require protective amber-colored eyeglasses, and for laser procedures on the face, metal goggles are used (Fig. 40-12). For rigid and flexible endoscopes and operating microscopes, filters that absorb the KTP wavelength can be introduced.
2 Skin and Drape Damage
Laser burns to the skin can occur, and the face or exposed areas should be protected with wet towels and drapes. For laser airway procedures involving a suspension laryngoscope, all of the area around the surgical laryngoscope and face should be completely covered with wet towels, which must be kept wet throughout the procedure. Care should be taken, particularly around the draping of the proximal portion of the surgical laryngoscope, to ensure that the lips and nose are fully protected because this region is more likely to be struck by a reflected laser beam from the proximal rim of the surgical laryngoscope (Fig. 40-13).
Preparation solution should not contain alcohol. Oxygen saturation monitors are a standard of care and should be used because detection of cyanosis is difficult with the patient’s face covered. Disposable surgical drapes are a potential fire hazard. They are treated with flame-retardant chemicals and are water resistant, but all types of surgical drapes are potentially flammable. Many cases of drapes catching fire have been reported. When the drapes catch fire, it is difficult to extinguish because the drapes are water resistant, and the water rolls off them. A CO2 fire extinguisher should be available. Drapes, once ignited, go up in flames immediately, causing the operating room to be inundated with smoke and making it difficult for everyone to see and breathe.71–73 It is important to keep the towels moist, or they can become flammable.
3 Laser Plume
The plume of smoke produced by vaporization of tissues in electrocautery or laser surgery may be hazardous. The smoke contains fine particles (mean size, 0.31 µm; range, 0.1 to 0.8 µm) that can be efficiently transported and deposited in the alveoli.74 In rat lungs, the deposition of laser plume particles could produce interstitial pneumonia, bronchiolitis, a reduction in mucociliary clearance, inflammation, and emphysema.75,76 The laser’s smoke plume acting as a vector for viruses is a controversial idea. Viral DNA has been detected in plumes from condylomas and skin warts but not from laryngeal papillomas.77–80 CO2 lasers seem to produce the most smoke from vaporization of tissue. Operating room personnel can be protected by using an efficient smoke evacuator at the surgical site (Fig. 40-14).81,82 Ordinary operating room masks can filter particles no smaller than 3.0 µm. Laser masks that are more efficient should be used to protect the operating room personnel from plume particles.
4 Gas Embolism
Gas embolism has been reported with Nd : YAG laser resection of tracheal and bronchial tumors, and it may be a risk factor for flexible-fiber CO2 lasers introduced into the bronchial tree.83,84 Gas embolism has been reported in laparoscopic surgery that used Nd : YAG laser probes that required a gas cooling system.85,86
5 Misdirected Laser and Laser Protocol
Lasers should always be set to the standby mode, except when they are ready to fire, to prevent inadvertent actuation. They should be used in the pulsed (shuttered) mode rather than the continuous mode whenever possible to limit the energy delivered by the laser and to allow the area being lasered to cool between firings. The laser radiation should never be allowed to strike highly polished or mirror-like surfaces. Most instrumentation for use with lasers has a dull or matte finish, and blackened instruments are widely used. This is important because reflection of the coherent laser beam may not disperse it, and injury to the patient or operating room personnel is possible, even from a reflected laser beam. Any instruments that become hot as a result of laser radiation may cause burns.68,87 Tracheal laceration, tooth damage, injury to soft tissue, and cutaneous burns to operating room personnel have been described during laser surgery.88
The Nd : YAG and argon lasers can penetrate glass, and any windows in the operating room should have an opaque covering to prevent penetration by laser radiation. A warning sign should be placed on the operating room door so that anyone entering is informed that the laser is in use (Figs. 40-15 and 40-16). To prevent personnel inadvertently entering the operating room during laser surgery, the doors may be automatically locked when the operating room is in laser mode (Fig. 40-17). Extra goggles should be available for personnel entering the operating room.
B Airway Laser Hazards
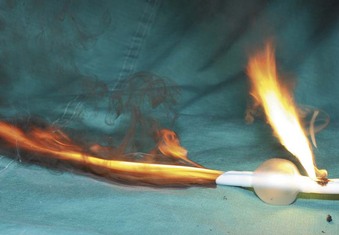
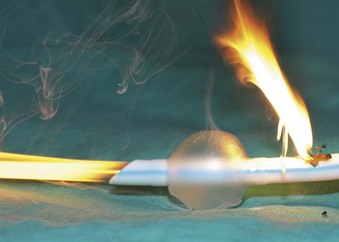
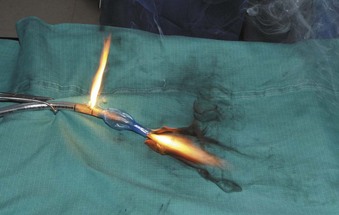
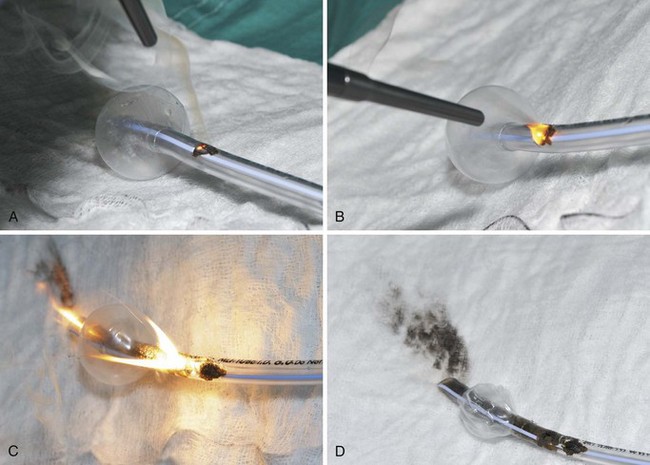
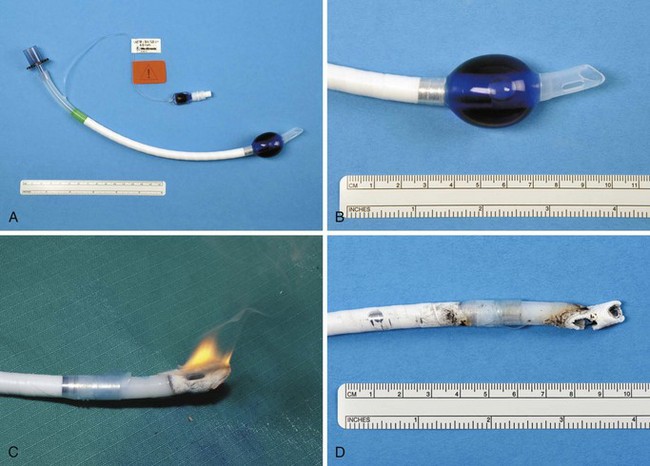
The high energy of the laser and its potential for combustion can cause an airway fire when the surgical field is near to the airway (Fig. 40-18). When a laser strikes the unprotected external surface of a tracheal tube during laser airway surgery, the surface starts to disintegrate and can catch fire. If the fire is not recognized and the laser continues to be applied, it can produce a hole in the tracheal tube and expose the burning surface to the oxidant-rich gas within the anesthesia system. At this stage, an explosive blowtorch-like fire may occur and rapidly spread in a distal and proximal manner. Any airway fire is a life-threatening complication, but the blowtorch fire is especially feared (Fig. 40-19).
If the cuff of an ETT is punctured, the oxidant-rich gas within the circuit becomes exposed to the external surface of the ETT, and the risk of an airway fire, including a blowtorch fire, is increased significantly. Examination of an ETT after a blowtorch laser fire reveals total or near-total destruction of the ETT, with molten material, smoke, and other particulate material spreading out from the distal end of the tube (Figs. 40-20 and 40-21).
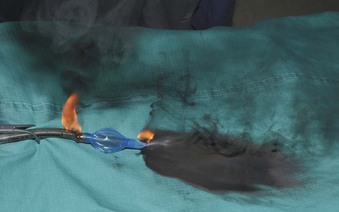
Figure 40-20 Smoke, molten material, and other particulate material spread out after an airway fire.
It is thought that operating room fires are under-reported and that there are probably 100 to 200 operating room fires in the United States per year. Of the reported fires, 20% result in serious injury to the patient. One or two deaths per year are caused by airway fires.89
Many options are available for anesthesia management during airway laser surgery. Selection of the ventilation method and type of laser depends on the nature and location of the lesion, the condition of the patient, and the availability of equipment and expertise. Different anesthesia management techniques have been described in the treatment of recurrent respiratory papillomatosis using the CO2 laser.90 In a 1995 survey, 92% of otolaryngologists preferred using a CO2 laser for removal of recurrent respiratory papillomas. However, there is no consensus with respect to anesthesia management. About 46% preferred using a laser-safe ETT, 26% favored using jet ventilation, 16% preferred an apneic technique, and 12% preferred spontaneous ventilation.91 Other options include awake with topical anesthesia, general anesthesia through an ETT, rigid bronchoscopy with general anesthesia, and general anesthesia by a laryngeal mask airway (LMA). The possibility of complete airway collapse or an inability to ventilate must be taken into consideration when deciding on spontaneous or positive-pressure ventilation.
If ventilation through an ETT is proposed, prevention of an ETT fire or explosion requires the use of special techniques and appropriate ETTs. Surveys of otolaryngologists active in this type of surgery concerning the complications of CO2 laser laryngeal surgery have found ETT fires or explosions to be the most common major complication.52,92 Historically, the estimated incidence of airway fires was between 0.4% and 0.57% of the patients undergoing laser airway surgery.39 The one or two deaths per year are caused by airway fires. As reported by Cozine and colleagues, patients have died because of combustion of an ETT during CO2 laser surgery.93
IV Preventing Airway Fires
A Fuel Source Considerations
1 Use of Metallic Foil Tapes to Protect Endotracheal Tubes
Metal foil tape wrapped around a standard ETT that is used during laser surgery in adults is of historical interest, but it is not commonly used for laser airway surgery. Foil tapes were first suggested as a simple, inexpensive means of protecting the shafts of combustible ETTs from laser beams by Strong and Jako in 1972.49 Their use during laser surgery was described during the 1970s and 1980s,94–97 when specially manufactured laser-resistant ETTs were not available or performed poorly.
Metallic foil tapes provide protection only from the direct impact of the laser beam. Indirect combustion caused by sparks or heat from gaseous or tube combustion is still possible because the ETTs used typically were combustible and usually had an enriched concentration of oxygen flowing through them. Problems with the use of metallic foil tape have included obstruction of the airway when the tape came loose from a wrapped ETT98,99 and aluminum foil tape becoming trapped in the trachea after laser airway supraglottoplasty.100
No metallic foil tapes are manufactured for medical applications, and the U.S. Food and Drug Administration has not sanctioned their use. The physician who wraps an ETT with metal foil tape incurs some product liability risk as a noncertified “manufacturer” if injury occurs.101 One manufacturer, when questioned, cautioned against the use of its aluminum tape for medical purposes.102
Wrapping the metal foil tape in a spiral, overlapping manner should cover the shaft from the cuff to the most proximal region possible and should include the pilot tube. Poor wrapping may cause some areas of the shaft to be unprotected, and bending of the tube may expose unprotected areas. Foil-wrapped tubes may loose flexibility, and their foil edge surface may traumatize. The type of metallic tape used to protect combustible ETTs is important.102
The possibility of changes in the composition of any metallic foil tape requires every batch of tape to be evaluated for its incendiary characteristics before use.95 Metal foil tape wrapped around a standard ETT is not recommended for laser airway surgery.
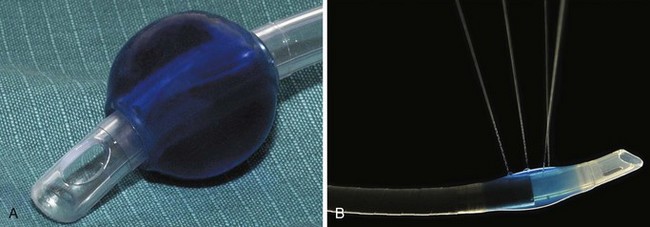
3 Prevention of Endotracheal Tube Cuff Fires with Saline
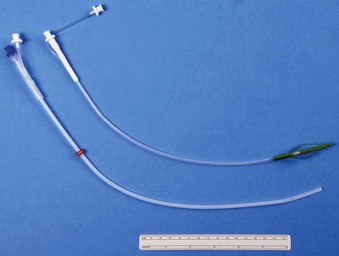
During the 1970s and 1980s, attempts were made to improve the laser-resistant properties of the shaft of combustible ETTs. In 1982, LeJeune and colleagues suggested that filling ETT cuffs with saline could protect them from the CO2 laser, because a laser strike on the cuff results in a jet of water that acts as a “built-in fire extinguisher” (Fig. 40-22).103 The saline also can act as a heat sink. Sosis and Dillon compared air-filled and saline-filled cuffs, and they found the saline-filled cuffs,104 although perforated by the laser beam as rapidly as air-filled cuffs, were significantly slower to deflate, allowing more time before reaching the point at which airway pressure could no longer be maintained. Saline-filled cuffs prevented ETT ignition by the CO2 laser set to 40 W (Table 40-2) in a statistically significant number of cases compared with the control group of air-filled cuffs, and saline filling of ETT cuffs was recommended for laser airway surgery. A small amount of dye, such as methylene blue, should be added to the saline so that laser-induced ETT cuff perforation becomes obvious to the surgeon, who can immediately terminate operation of the laser. Further protection of the ETT cuffs can be obtained by placing moistened pledgets above them and keeping the pledgets moist throughout the procedure.105
TABLE 40-2 Incidence of Endotracheal Tube Combustion with Air- and Saline-Filled Cuffs with the Carbon Dioxide Laser Set to 40 W
| Cuff Type | Number of Cases | Combustion (%) |
|---|---|---|
| Air | 5 | 100* |
| Saline | 5 | 20* |
* P < 0.05, Mann-Whitney U test.
From Sosis MB, Dillon FX: Saline-filled cuffs help prevent laser-induced polyvinylchloride endotracheal tube fires. Anesth Analg 72:187, 1991.
4 Special Endotracheal Tubes for Laser Airway Surgery
Laser proof implies that irrespective of the oxidant environment and the power of the laser, the ETT cannot catch fire. With a laser-proof ETT, a continuous laser strike with extremely high power in a 100% oxygen environment does not produce a fire of the tube. Only one laser-proof tracheal tube (Norton) has been designed, and it is discussed later.106
All other manufactured ETTs for laser airway surgery are only laser resistant. Laser-resistant tubes provide some degree of protection against a laser strike, but these tubes vary in their materials, protective coating, relative size, and number of cuffs used. Any laser-resistant tube can result in an airway fire or blowtorch fire (Fig. 40-23) if it is used outside of its limits, such as a laser strike on an unprotected area between the tube shaft distal and the cuff, at the unprotected proximal part of the shaft, or at the cuff itself. The anesthesiologist should appreciate the maximum power settings for which a laser-resistant tube has been tested, the range within which ignition does not occur, and the effect the oxidant environment has on the laser resistance characteristics. All laser-resistant tubes are not laser proof, and they must not be used outside of their limits.
a Norton Laser Endotracheal Tube
The Norton ETT (V. Mueller, Baxter Healthcare Corp., Niles, IL), first described in 1978 by Norton and De Vos, was the only laser-proof tracheal tube produced (Fig. 40-24).106 It was constructed from interlocking, spiral-wound, stainless steel parts. It is no longer manufactured. Because it was made of steel, it was extremely resistant to multiple uses and autoclaving, and it may still exist in some hospitals.
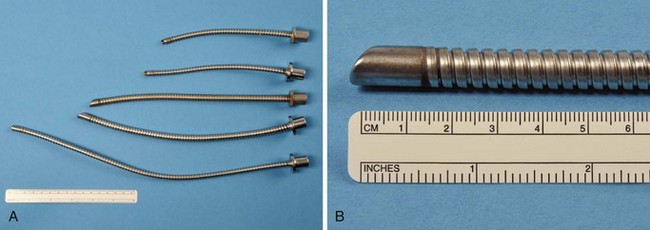
Figure 40-24 A and B, In the selection of Norton laser tubes, notice the absence of a cuff and ribbed exterior.
The Norton ETT had a matte or sand-blasted finish, rather thick walls, and a ribbed exterior. It came in three sizes: 4.0-, 4.8-, and 6.4-mm internal diameter (ID) (Table 40-3).107 The matte finish diffused reflected laser beams.106 A 4.8-mm-ID Norton ETT has a wall thickness of 1.4 mm. The thick wall of this ETT is considered a disadvantage because the tube can obscure the surgeon’s view more than an ETT with the same ID but with thinner walls—an important consideration during laser airway surgery. This ETT’s large size and stiffness may make surgical exposure and laryngoscope positioning difficult, and it can make passage through the glottis traumatic. It is usually necessary to use a stylet to introduce the Norton ETT.
TABLE 40-3 Internal Diameter, External Diameter, and Wall Thickness for the Norton Laser Endotracheal Tube
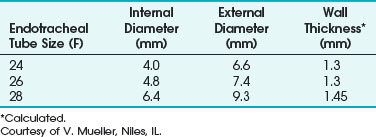
The relatively small diameter and ridged internal surface produce turbulent airflow and an increased resistance to airflow. Woo and Strong suggested Venturi ventilation through the Norton tube in an attempt to overcome the ventilation issues of increased resistance,108 airflow, and air leaks.
b Xomed Laser-Shield I Endotracheal Tube
The Xomed Laser-Shield I ETT (Xomed-Treace, Jacksonville, FL) is no longer manufactured and is of historical interest only. The silicon rubber tube was coated with a silicon elastomer to which metallic particles had been added, and it was designed to be used only with a CO2 laser. Several airway fires were reported with its use.109–112 It is a useful reminder of the limitations of a laser-resistant ETT and the dangers of using a device outside of its intended range.
c Bivona Laser Endotracheal Tube
The Bivona (Gary, IN) laser ETT is no longer available in many parts of the world and is mainly of historical interest. It was designed to limit the effect of cuff perforation by a laser strike, because the polyurethane foam cuff with its silicone envelope maintained the cuff seal even when penetrated. The Bivona laser ETT was designed to be used only with a CO2 laser. Blowtorch airway fires were reported with this tube.112
d Xomed Laser-Shield II Endotracheal Tube
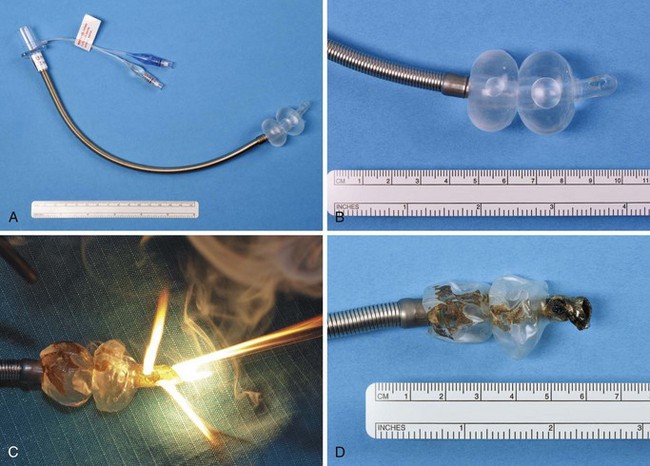
The Xomed Laser-Shield II ETT (Xomed Surgical Products, Jacksonville, FL) has a laser-resistant overwrap of aluminum foil tape and a Teflon cover. The proximal and distal ends of the silicon elastomer shaft and cuff are not protected and therefore are not laser resistant (Fig. 40-25). The metallic tape provides protection against laser impact. The Teflon tape gives the tube a smooth surface to minimize mucosal injury from tube manipulation. No adhesives are used because adhesives with metallic tape increase the risk of an ETT fire.98 Dry methylene blue dye has been placed in the cuff inflation valve to enable detection of a cuff rupture. The Xomed Laser-Shield II comes packaged with neurosurgical cottonoid, which is used wet to protect the distal shaft and cuff for an added margin of safety.
Dillon and associates evaluated the combustibility of the Xomed Laser-Shield II ETT and compared it with 3M’s no. 425 aluminum foil–wrapped, combustible, polyvinyl chloride (PVC) ETTs used with a CO2 laser and an Nd : YAG laser.113 Exposure of the bare silicon rubber shaft of the Laser Shield-II ETT to CO2 laser radiation resulted in combustion in 2.1 ± 0.7 seconds; Nd : YAG laser radiation–induced combustion occurred at 3.3 ± 4.5 seconds (P = 0.05). The silicon rubber burned with a bright flame and disintegrated. It was difficult to extinguish. Dillon and colleagues concluded that the foil-wrapped shaft of the Laser Shield-II ETT provided adequate protection against high-power, continuous-mode Nd : YAG and CO2 laser radiation.113
Ossoff and colleagues also studied the Laser-Shield II for combustibility with CO2 and KTP lasers. They evaluated the Laser-Shield II dry, with blood, with a blood/K-Y jelly mixture, and with K-Y jelly alone on the ETT. Each tube was clamped, and 100% oxygen was delivered at 3 L/min through it. All trials were performed with 3 minutes of continuous output. The maximum power output was 40 W for the CO2 laser and 15 W for the KTP laser. These settings far exceed the clinical settings used. With the KTP laser (13.5 to 15 W), no fires were observed, regardless of surface penetration. For the CO2 laser, no fires were observed at 40 W for 3 minutes on continuous mode with the dry ETT. Blood or KY-jelly, or both, decreased the resistance of the ETT to CO2 laser radiation. For the tube with blood alone, 25 W was the highest power that the tubes withstood without ignition. For the K-Y jelly/blood mixture, the maximum power was reduced to 19 W. With K-Y jelly alone, no fire occurred at the maximum power setting of 40 W.114
Medtronic Xomed recommends only the Xomed Laser-Shield II for all surgical procedures involving the use of CO2 or KTP lasers in a normal-pulsed or continuous mode of noncontact delivery. It is contraindicated for use with any Nd : YAG laser, argon laser, or any laser other than the CO2 or KTP. When the laser beam hits the Laser-Shield II Teflon, the reflective aluminum wrapping may be exposed, and it is possible for the beam to be reflected into the patient’s tissue. The sizes of Xomed Laser-Shield II ETTs are given in Table 40-4.
TABLE 40-4 Internal Diameter, External Diameter, and Wall Thickness for the Xomed Laser-Shield II Endotracheal Tube
| Internal Diameter (mm) | External Diameter (mm) | Wall Thickness (mm)* |
|---|---|---|
| 4.0 | 6.6 | 1.3 |
| 4.5 | 7.3 | 1.4 |
| 5.0 | 8.0 | 1.5 |
| 5.5 | 8.6 | 1.55 |
| 6.0 | 9.0 | 1.5 |
| 6.5 | 10.0 | 1.75 |
| 7.0 | 10.5 | 1.75 |
| 7.5 | 11.0 | 1.75 |
| 8.0 | 11.5 | 1.75 |
Courtesy of Xomed-Surgical Products, Jacksonville, FL.
e Mallinckrodt Laser-Flex Endotracheal Tube
Mallinckrodt (Glens Falls, NY) Laser-Flex ETTs have corrugated stainless steel shafts. They are designed as a single-use item, and the manufacturer states that this type of ETT should be used only with the CO2 and KTP lasers. The adult version of this ETT incorporates two PVC cuffs. The manufacturer suggests that the distal cuff can be used if the laser damages the proximal one. The adult tube’s distal end, including its Murphy eye and the proximal 15-mm connector, is constructed from combustible PVC (Fig. 40-26). The Laser-Flex cuffs are inflated by means of two 1-mm-diameter PVC pilot tubes that are located on the inside of the ETT. An Nd : YAG or other laser fiber should never be inserted through this tube. Heyman and colleagues found that prolonged laser impingement on the shaft of a Mallinckrodt Laser-Flex ETT could prevent cuff deflation, and they stressed the importance of aspirating the saline from the cuff in a slow, gentle manner.115
The adult sizes of Mallinckrodt Laser-Flex ETTs available with the double-cuff system are 4.5-, 5.0-, 5.5-, and 6.0-mm ID. Three pediatric sizes (3.0-, 3.5-, and 4.0-mm ID) of uncuffed Mallinckrodt Laser-Flex ETTs are also available. They are all stainless steel, except for a PVC 15-mm adapter. They are not equipped with Murphy eyes (Table 40-5).
TABLE 40-5 Internal Diameter, External Diameter, and Wall Thickness for the Mallinckrodt Laser-Flex Endotracheal Tube
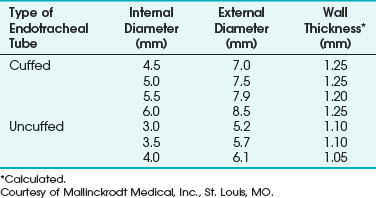
Unlike the all-stainless-steel Norton ETT, the Mallinckrodt Laser-Flex ETT has an airtight shaft. However, the walls of both types of tubes are somewhat rough. In their product information for the uncuffed pediatric tubes, Mallinckrodt states, “Due to the spiral design of the tube, the airflow resistance for a given size will be approximately equal to a PVC tube, which is 0.5 mm smaller.” They add, “Due to the bore size of the tube, patients should be monitored closely to guard against overinflation of the respiratory system and a build-up of expiratory gases.”116
Sosis and coworkers studied the resistance to CO2 laser radiation of size 4.5-mm-ID Mallinckrodt Laser-Flex ETTs.117 The tubes were positioned horizontally on a stainless steel tabletop covered with a wet towel. An oxygen flow of 5 L/min passed through the tubes as a Sharplan (Tel Aviv, Israel) model 734 CO2 laser was aimed at an ETT’s shaft. A laser power setting of 35 W was used with a beam diameter of 0.6 mm. This resulted in a power density of 13,400 W/cm2. The laser, set to the continuous mode of operation, was activated for 90 seconds or until combustion occurred. Blowtorch combustion occurred in one of five Laser-Flex ETTs studied. However, when human blood was applied to the shafts of four 4.5-mm-ID Mallinckrodt Laser-Flex ETTs, blowtorch combustion occurred in all cases.
In another study, Sosis and Dillon noted that there is less danger of a reflected laser beam causing damage when the Mallinckrodt Laser-Flex ETT is used compared with foil-wrapped ETTs.118 In an evaluation of the Laser-Flex ETT with the Nd : YAG laser, Sosis found that the shaft of the Laser-Flex ETT could be ignited in all cases by the Nd : YAG laser when operated at high power.97 Clinical Laser Monthly reported the occurrence of an airway fire during a case in which a Mallinckrodt Laser-Flex ETT was used during a laser excision of vocal cord polyps.119 They stated that the patient had minor burns. They reported that, at the time of the fire, the cuffs of the ETT were not inflated with saline as recommended by the manufacturer.
f Sheridan Laser-Trach Endotracheal Tube
Sosis and associates compared 6.0-mm-ID Sheridan Laser-Trach ETTs with plain (bare) Rüsch red rubber ETTs of the same internal diameter.120 Five liters per minute of oxygen flowed through the tubes being studied. The tubes were subjected to continuous radiation at 40 W from a Sharplan CO2 laser or 40 W of continuous output from a Laserphotonics (Orlando, FL) Nd : YAG laser. The Nd : YAG laser radiation was propagated by a 600-µm fiber bundle. Each type of laser was directed perpendicular to the ETT being studied. The laser’s output was continued until a blowtorch fire occurred or 50 seconds elapsed. No ignition occurred after 60 seconds of CO2 laser fire to the shafts of eight Sheridan Laser-Trach ETTs tested. However, blowtorch ignition of all eight bare rubber ETTs tested occurred after 0.87 ± 21 (mean ± SD) seconds of CO2 laser fire. Nd : YAG laser contact with the Sheridan copper and fabric-covered rubber ETTs resulted in perforation and blowtorch ignition in all tubes tested after 18.79 ± 7.83 seconds. This was significantly (P < 0.05) longer than the 5.45 ± 4.75 seconds required for blowtorch ignition of all eight plain red rubber ETTs tested with the Nd : YAG laser.
It was concluded that under the conditions of the study, the Sheridan Laser-Trach ETT was resistant to CO2 laser radiation. It is not recommended for use with the Nd : YAG laser. Table 40-6 lists the sizes of the Sheridan Laser-Trach ETTs available.
TABLE 40-6 Internal Diameter, External Diameter, and Wall Thickness for the Sheridan Laser-Trach Endotracheal Tube
| Internal Diameter (mm) | External Diameter (mm) | Wall Thickness* |
|---|---|---|
| 4.0 | 8.2 | 2.10 |
| 5.0 | 9.5 | 2.25 |
| 6.0 | 10.6 | 2.30 |
Courtesy of Sheridan Catheters, Argyle, NY.
g Lasertubus Endotracheal Tubes
Lasertubus (Rüsch, Duluth, GA) is a laser-resistant ETT with a shaft made of soft white rubber and a laser guard approximately 17 cm long, consisting of a Merocel sponge and silver foil with a double cuff. A cuff within a cuff arrangement exists and the sponge surface should be soaked to reduce the risk of an airway fire in a manner similar to that for the Sheridan Laser-Trach. According to the manufacturer, the Lasertubus offers resistance to all types of medical lasers, such as argon, Nd : YAG, and CO2, with wavelengths ranging from 488 to 1060 nm. Sosis and colleagues evaluated the Rüsch Lasertubus in vitro.121
Jacobs and colleagues reported crimping of the Lasertubus, resulting in hypoxemia in a patient.122 After placement of the Lasertubus, the surgeon extended the patient’s head and neck and within 30 seconds, peak inspiratory pressures increased, oxygen saturation decreased, and no end-tidal CO2 was evident. Immediate direct laryngoscopy confirmed that the ETT was correctly placed. On inspection, no kinks or obvious obstruction was seen. Ultimately, the laser tube was removed and the patient reintubated with a PVC ETT. The patient’s oxygen saturations returned to 100%. On inspection of the Lasertubus, the tube had crimped under the tape. Although this was a small defect not obvious to cursory inspection, it resulted in complete obstruction to airflow. The investigators then experimented by bending unused laser tubes and found that a weakness within the wall of the tube remained, predisposing to crimping with minimal force and producing complete obstruction to airflow.122
B Oxidant Source Considerations
1 Effect of Anesthetic Gases on Endotracheal Tube Flammability
In an experiment analogous to one with oxygen, a glowing match thrust into a vessel containing nitrous oxide bursts into flames. Nitrous oxide supports combustion to approximately the same extent as oxygen, and the addition of nitrous oxide in an attempt to dilute oxygen makes no difference to the flammability.123
Chilcoat and coworkers stressed that the reduction in oxygen concentration on dilution with nitrous oxide did not provide any additional safety when performing laryngeal laser surgery.124 They recommended the use of either nitrogen, air, or helium when it was desired to reduce the oxygen concentration in anesthetic gas mixtures to levels of 30% or less for laser surgery.124 Wolf and Simpson showed that nitrous oxide and oxygen are linearly additive in their ability to sustain combustion.125 Nitrous oxide should not be considered safe, and careful consideration is required before using it with laser surgery.
Helium and nitrogen are inert gases, and when added to oxygen, they can delay ETT flammability (Table 40-7). Pashayan and Gravenstein and Osoff found that helium was more protective than nitrogen in retarding laser-ignited ETT fires.110,126 The protective effect of helium is probably due to its high thermal diffusivity (i.e., quantity of heat passing through 1 cm2 of cross-sectional area per unit of time) or thermal conductivity (i.e., time rate of transfer of heat by conduction). Presumably, helium diffusivity prevents a rise in temperature around the site of laser exposure, preventing the laser-irradiated ETT from reaching its temperature of spontaneous ignition.
TABLE 40-7 Physical Properties of Helium and Nitrogen at 26° C
| Physical Properties | Helium | Nitrogen |
|---|---|---|
| Thermal conductivity (cal [sec·cm2 °C·cm−1·10−6]−1) |
360.36 | 62.40 |
| Thermal capacity (cal·g−1·K−1) |
1.24 | 0.249 |
| Density (g·L−1) |
0.179 | 1.25 |
| Thermal diffusivity (cm2·sec−1) |
1.621 | 0.199 |
From Pashayan AG, Gravenstein JS: Helium retards endotracheal tube fires from carbon dioxide lasers. Anesthesiology 62:274, 1985.
Pashayan and Gravenstein studied the CO2 laser–induced combustibility of 2-cm segments of PVC ETTs in various oxygen, helium, and nitrogen environments.126 At oxygen concentrations of 30% and 40%, the mean time to ignition with nitrogen was significantly shorter than the time for the same concentration of oxygen in helium. Pashayan and Gravenstein concluded that helium in concentrations of 60% or greater delayed CO2 laser–induced combustion of PVC ETTs if the laser power output was 10 W or less.126 They also concluded that the radiopaque barium stripe on the PVC ETT was more combustible than the clear portions of the ETT and that they should be positioned away from the laser. Reflected beams may reach a stripe that appears to be out of the direct line of sight, and using an ETT without such stripes is preferred.
Ossoff compared the incendiary characteristics of three ETTs in various oxygen concentrations diluted with either helium or nitrogen using a CO2 laser. They found that helium was better than nitrogen as an inert mixture with use of the CO2 laser to decrease flammability. They also showed that the safest anesthetic gas mixture was 30% oxygen in helium.110
Al Haddad and Brenner studied the combustion of 1- to 2-inch PVC ETT segments with the KTP laser and CO2 laser in helium-oxygen and nitrogen-oxygen atmospheres.127 They compared these two lasers because of their different wavelengths and penetration characteristics that are commonly used in airway laser surgery. The CO2 laser mean ignition time in helium was prolonged compared with nitrogen (P < 0.001), whereas increasing the oxygen concentration reduced the ignition time for both nitrogen and helium. With KTP, there was no significant difference for ignition among the five groups of oxygen concentrations or between nitrogen and helium. Al Haddad and Brenner reconfirmed that helium, as part of the mixture with 30% oxygen, is safest for the CO2 laser but concluded that the use of helium instead of nitrogen conferred no added safety during KTP laser surgery.
Simpson and colleagues determined the flammability of four types of ETTs in mixtures of helium and oxygen or nitrous oxide compared with mixtures of nitrogen and oxygen or nitrous oxide.128 The ETTs were initially ignited in oxygen and nitrogen, and the nitrogen was quickly discontinued and replaced with helium. The helium concentration was increased until the candle-like flame on the ETT was extinguished. The oxygen concentrations just before the flame’s extinction with helium were defined as the oxygen/helium index of flammability by the researchers. A similar experimental design was used with mixtures of nitrogen and oxygen until the propane torch–induced combustion of the four types of ETTs was extinguished. This was defined as the oxygen/nitrogen index of flammability. Next, the oxygen was replaced by nitrous oxide, and the procedure was repeated to determine the nitrous oxide/helium and nitrous oxide/nitrogen indices of flammability. The indices of flammability of each type of ETT studied were averaged, and the results were compared using Bonferroni corrected t-tests. These results show that the PVC ETT is flammable in 27.4% oxygen with the remainder consisting of helium and in 25.4% oxygen with the remainder nitrogen. In all cases, the oxidant oxygen/helium values were statistically significantly higher than the oxygen/nitrogen values for the same type of ETT (P < 0.05). The oxidant nitrous oxide/helium indices were statistically higher than the nitrous oxide/nitrogen indices for all except the Xomed ETT.
2 Flammability Limits of Potent Inhaled Anesthetics
In 1850 in Boston, Massachusetts, the first recorded fire occurring in the operating theater was reported during facial surgery. With the use of ether, acetylene, ethylene, and cyclopropane, many more reports followed.129 The range of flammability of potent inhaled anesthetics used in modern practice is well above the alveolar concentrations that would be applied to patients in clinical practice.
Leonard,130 in investigating the lower limits of flammability of halothane, enflurane, and isoflurane, observed that halogenation rendered these compounds less flammable but might not prevent their combustion under all circumstances. He found that it was possible to ignite a mixture of 4.75% halothane in 30% oxygen with the remainder composed of nitrous oxide (Table 40-8). In 20% oxygen with the remainder nitrous oxide, halothane concentrations greater than 3.25% were combustible. In oxygen–nitrous oxide mixtures of 20% and 30% oxygen, enflurane could be ignited at concentrations greater than 4.25% and 5.75%, whereas isoflurane required 5.25% and 7.0%, respectively. These values were obtained under laboratory conditions designed to encourage flammability. A closed combustion vessel was used that contained no water vapor, CO2, or nitrogen. Ignition was initiated with a 15-kV transformer that delivered a 60-mA current across a 0.25-inch gap. However, because the spark duration was not specified, the total energy delivered could not be calculated. The ignition power used (900 W) was, however, higher than the maximum power output delivered by most electrosurgical equipment. Leonard observed that the energy used in this experiment was much greater than that of a static discharge in the operating room. He concluded that even if the fraction of nitrous oxide administered to the patient exceeds 70%, the lowest flammable concentration of each of the three volatile halogenated anesthetic agents is above that which would be used clinically, except perhaps at the beginning of an inhalation induction.
TABLE 40-8 Minimum Flammable Concentrations of Halothane, Enflurane, and Isoflurane
| Anesthetic Agent | MFC of Agent in 20% O2/Remainder N2O (%)* | MFC of Agent in 30% O2/Remainder N2O (%)* |
|---|---|---|
| Halothane | 3.25 | 4.75 |
| Enflurane | 4.25 | 5.75 |
| Isoflurane | 5.75 | 7.0 |
* The minimum flammable concentration (MFC) was determined by igniting each anesthetic in a mixture of 20% or 30% oxygen (O2,), with the remainder composed of nitrous oxide (N2O).
Adapted from Leonard PF: The lower limits of flammability of halothane, enflurane, and isoflurane. Anesth Analg 54:238, 1975.
A study by Pashayan and Gravenstein showed that the addition of 2% halothane to a mixture of 40% oxygen and 60% helium significantly decreased the mean time to combustion of PVC ETT segments that were subjected to CO2 laser radiation.126 Ossoff and colleagues found that the addition of 2% halothane significantly retarded the ignition of Rüsch red rubber ETTs in atmospheres of 30%, 40%, and 50% oxygen–balance helium at power settings of 10, 15, and 20 W.95
The ratio (3.3) of the percentage of desflurane at the lower limits of flammability in 70% nitrous oxide to the minimum alveolar concentration (6%) is not markedly different from what Leonard reported with enflurane (3.4), halothane (6.3), or isoflurane (6.1).96,110 Sevoflurane is considered to be nonflammable over the entire anesthetizing concentration range in the presence of air, oxygen, and nitrous oxide.96,131
C American Society of Anesthesiologists Practice Advisory for the Prevention and Management of Operating Room Fires
In 2008, a report by the American Society of Anesthesiologists (ASA) Task Force on operating room fires published a practice advisory for the prevention and management of operating room fires.1 Practice advisories are systematically developed reports that are intended to assist decision making in areas of patient care.1 They are based on scientific literature, expert opinion, clinical feasibility data, open forum commentary, and consensus surveys. They are not intended as standards, guidelines, or absolute requirements and can be adopted, modified, or rejected according to clinical needs and constraints.1 This practice advisory looked at operating room fires, surgical fires, airway fires, and high-risk procedures.
The practice advisory defined an operating room fire as a fire that occurred on or near patients who are under anesthesia care and included surgical fires, airway fires, and fires within the airway circuit. A surgical fire was defined as a fire that occurred on or in a patient. An airway fire was a special type of surgical fire that occurred in a patient’s airway and that may or may not include fire in the attached breathing circuit.1 A procedure was designated as high risk when an ignition source came close to an oxidizer-enriched atmosphere (e.g., tracheostomy, laryngeal surgery).
The ASA Task Force’s primary findings commented on education, operating room fire drills, preparation for every case, and prevention and management of operating room fires. The anesthesiologist should collaborate with all members of the procedure team throughout the procedure to minimize the presence of an oxidizer-enriched atmosphere in proximity to an ignition source.1
For all procedures, surgical drapes should be configured to minimize the accumulation of oxidizers (i.e., oxygen and nitrous oxide) under the drapes and to keep them from flowing into the surgical site. Flammable skin prepping solutions should be dry before draping. Gauze and sponges should be moistened before use near an ignition source.1
For high-risk procedures, the anesthesiologist should notify the surgeon whenever there is a potential for an ignition source to be in proximity to an oxidizer-enriched atmosphere or when there is an increase in oxidizer concentration at the surgical site. Any reduction in supplied oxygen to the patient should be assessed by monitoring pulse oximetry and, if feasible, inspired, exhaled, and delivered oxygen concentrations.1
For laser procedures, a laser-resistant ETT should be used. The laser-resistant ETT used should be chosen to be resistant to the laser used for the procedure (e.g., CO2, Nd : YAG, argon, erbium : YAG, KTP). The ETT cuff of the laser tube should be filled with saline and colored with an indicator dye such as methylene blue.1
Before activating a laser, the surgeon should give the anesthesiologist adequate notice that the laser is about to be activated.1 The anesthesiologist should reduce the delivered oxygen concentration to the minimum required to avoid hypoxia, stop the use of nitrous oxide, and wait a few minutes after reducing the oxidizer-enriched atmosphere before approving activation of the laser.1
For cases involving an ignition source and surgery inside the airway, cuffed tracheal tubes should be used when clinically appropriate.1 The anesthesiologist should advise the surgeon against entering the trachea with an ignition source (e.g., electrosurgery unit).
Before activating an ignition source inside the airway, the surgeon should give the anesthesiologist adequate notice that the ignition source is about to be activated. The anesthesiologist should reduce the delivered oxygen concentration to the minimum required to avoid hypoxia, stop the use of nitrous oxide, and wait a few minutes after reducing the oxidizer-enriched atmosphere before approving the activation of the ignition source.1 In some cases (e.g., surgery in the oropharynx), scavenging with suction may be used to reduce oxidizer enrichment in the operative field.1
For a fire in the airway or breathing circuit, remove the ETT as fast as possible, stop the flow of all airway gases, remove all flammable and burning materials from the airway, and pour saline or water into the patient’s airway.1 If the airway or breathing circuit fire is extinguished, reestablish ventilation by mask, avoiding supplemental oxygen and nitrous oxide, if possible. Extinguish and examine the ETT to assess whether fragments were left in the airway. Consider bronchoscopy (preferably rigid) to look for ETT fragments, assess the injury, and remove residual debris. Assess the patient’s status and devise a plan for ongoing care.1
D Management of an Airway Fire
Schramm and colleagues discussed immediate management of an airway fire.132 The management protocol calls for immediate removal of the ETT.132 Sosis wrote a laser airway fire protocol in which certain steps should be taken simultaneously by the anesthesiologist and surgeon (Box 40-1).133 In the event of an airway fire, the protocol calls for immediate cessation of ventilation and turning off of all anesthetic gases, especially oxygen, by disconnecting the hose from the common gas outlet, detaching the ETT from the anesthetic circuit, or clamping the ETT or turning off the flow meters. At the same time, the flames should be extinguished with a sterile saline or water solution. Presuming an easy airway, the protocol calls for the ETT to be removed immediately because it no longer provides an airway and may be on fire. After the ETT has been removed and the fire completely extinguished, the patient’s lungs should be ventilated with 100% oxygen by bag and mask.
Box 40-1
Laser Airway Fire Protocol
• Cease ventilation and turn off all anesthetic gases, including oxygen.*
• Extinguish flames with a saline solution.*
• Remove endotracheal tube (ETT) after deflating cuff,* and ensure the whole ETT has been removed.
• Ventilate the patient’s lungs by mask after all burning material has been removed and extinguished.
• Examine the airway for burns and foreign bodies such as fragments of the ETT or packing materials.
Adapted from Sosis MB: Probl Anesth 7:160, 1993.
Chee and Benumof described “a patient scheduled for an elective tracheostomy whose airway evaluation revealed an in situ 8-mm-ID PVC ETT, swollen lips, an edematous tongue protruding out of the mouth and an oropharynx filled with secretions.”134 General anesthesia was induced with 300 mg of intravenous propofol and maintained with 0.4% inspired isoflurane (Forane) and a 35% oxygen-air mixture. The vital signs remained stable. Electrocautery was used for coagulation by the surgeons. The patient was administered 100% oxygen immediately before insertion of the tracheostomy tube. Suddenly, the surgeons reported a blue flame shooting up vertically from the patient’s neck. The breathing circuit was disconnected immediately from the ETT, and 20 mL of 0.9% saline was flushed in the ETT. The fire was extinguished promptly. The ETT was not removed because the ability to reintubate was uncertain. Despite a leak around the perforated cuff, the seal was sufficient to generate a peak inspiratory pressure of 20 cm H2O. Meanwhile, the surgeons were able to insert a tracheostomy tube into the trachea. Fiberoptic bronchoscopy was performed in the operating room and postoperatively revealed generalized upper airway edema consistent with prolonged intubation, no distal airway burn injury, and minimal burn injury to the proximal aspect of the tracheostomy site. The patient experienced no sequelae from the airway fire.
Each patient before laser surgery must be evaluated for risk of possible difficult intubation. If the patient is difficult to intubate or potentially a high risk for difficult intubation and an airway fire occurs, the patient will still be difficult to intubate. In these circumstances, the risk of removing the ETT and not being able to reestablish an airway outweighs the risk of leaving the tube in after the airway fire is extinguished. Van Der Spek and coworkers suggested that if the patient is not easy to intubate,135 a long stylet can be passed through the existing tube before removing it to facilitate the passage of a new one. Chee and Benomuf also suggested that the ETT can serve as a conduit for a tube exchanger.134 For reintubation, an airway exchange catheter is placed through the ETT with the assistance of a laryngoscope, if possible, to facilitate the passage of a new ETT over the airway exchange catheter. To minimize the risk of failing to pass an ETT over the airway exchange catheter, the operator can use a relatively small ETT over a relatively large airway exchange catheter. The airway exchange catheter allows ventilation, which allows time for an alternative reintubation strategy, such as a surgical airway or FOB.136
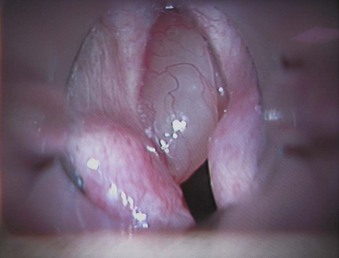
V Airway Pathology
1. Nodules are benign lesions of the vocal fold (Fig. 40-27). They can be bilateral and are usually caused by vocal abuse. They can be resected by fine surgical instruments augmented by a laser.
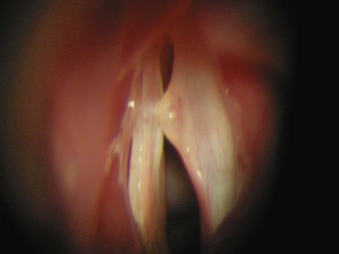
2. Polyps are benign lesions of the vocal fold (Fig. 40-28). They are common in adults, are usually unilateral, and can be resected surgically augmented by a laser.
3. Cysts are benign lesions of the vocal folds (Fig. 40-29). They are commonly seen in professional voice users. They are caused by obstruction of a glandular duct, resulting in a mucous retention cyst. Surgery involves careful resection and may involve a laser.
4. Granulomas are benign lesions induced by healing granulomatous tissue that develops after microtrauma. They are usually found on the posterior third of the vocal process after trauma during intubation or extubation. Removal involves surgical instruments augmented by a laser.
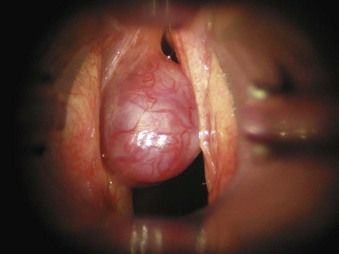
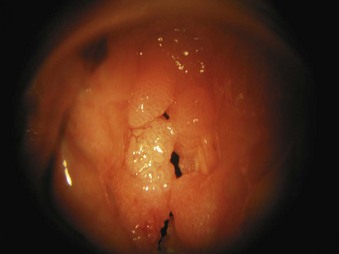
5. Papillomas of the vocal folds are caused by human papillomavirus infection (Fig. 40-30). Most are benign lesions, but they rarely can undergo malignant transformation. In adults, hoarseness is usually the main symptom, although in severe cases, significant airway compromise with stridor can occur. In children, any airway obstruction has a significant effect on airflow, and near-total airway obstruction can occur. Laser resection usually involves a CO2 laser, and after surgery, the airway obstruction and airflow are improved. Papillomas can recur, and some patients require repeated laser surgery at 1- to 12-month intervals.
6. Malignant tumors of the vocal cord are usually unilateral and occur mainly in the middle third of the vocal fold. Eighty percent are found in men 45 to 65 years old. More than 90% of patients are smokers with a high alcohol intake. Treatment depends on tumor staging and involves local resection, radiotherapy, transoral laser resection, hemilaryngectomy, or laryngectomy.
7. Other lesions on the vocal cord are caused by hemangiomas, submucous hemorrhage, Reinke’s edema, chronic laryngitis, amyloidosis, sarcoidosis, tuberculosis, and rheumatoid arthritis. These lesions can be resected by a laser and any subsequent bleeding controlled with a laser.
VI Anesthetic Techniques for Laser Airway Surgery
A Laryngeal Mask Airway
The LMA was first described in 1983.137 It is used in routine anesthetic practice and for management of the difficult airway.119 The LMA and reinforced flexible laryngeal mask airway (fLMA) have been used for awake and anesthetized patients undergoing fiberoptic bronchoscopy for diagnostic and airway evaluation.138,139 It has also been used for laser pharyngoplasty.140 Carinal obstruction was managed using a combination of Nd : YAG laser therapy through a FOB inserted through an LMA and subsequent Nd : YAG through an FOB with a rigid bronchoscope.141 Other reports described the use of a LMA and high-frequency jet ventilation for resection in tracheal surgery.142
The LMA and fLMA are constructed from silicon rubber that contains silica filler, which provides high-temperature resistance and some flame-retardation ability. The same material has been used to manufacture laser-resistant ETTs.137 Pennant and colleagues did a limited study of the incendiary characteristics of the LMA and fLMA, suggesting that they are resistant to CO2 lasers at power densities below 1.25 to 2.35 × 103 W/cm2, but 1.2 × 104 W/cm2 produced immediate ignition.143 Brimacombe looked at the incendiary characteristics of the LMA and fLMA with the CO2 laser and compared them with two standard PVC ETTs: Mallinckrodt reinforced armored tube and Mallinckrodt RAE tube. Continuous firing led to light smoke production and penetration of the tube in 20 to 30 seconds. Both PVC tubes were penetrated in 0.5 to 2 seconds, and ignition occurred at 2 to 6 seconds with 100% O2 and 2 to 8 seconds with an O2-N2O mixture.144
Pandit and colleagues showed that at laser outputs and distances of the KTP fiber tip from the fLMA normally encountered in clinical practice, the fLMA was not penetrated or ignited.145 Keller and associates looked at the standard LMA (silicon based) and fLMA (silicon based with metal wire) and at a disposable LMA (PVC based) and intubating LMA (silicon and steel based) and PVC-based ETTs using KTP and Nd : YAG lasers at two power densities used commonly in airway surgery (570 and 1140 W/cm2).146 Each airway device was fixed to a table in room air and attached to a closed-circuit breathing system with 30% oxygen in air delivered at 3 L/min. The laser was fired at the same distance of 3 mm. They also evaluated the marked and unmarked parts of the airway device and the unmarked device after application of 0.1 mL of unclotted fresh human blood. They evaluated the cuff with air or undiluted methylene blue dye.
There was no ignition of any airway device. The impact sites of the silicon-based LMA for both lasers revealed a layer of silica ash just like that found by Pandit and colleagues. The silicon-based tubes were less easily penetrated than the PVC-based tubes, except for the intubating LMA, which flared sooner than with the KTP laser at both densities. The disposable LMA cuff was more resistant to penetration than the silicon LMA cuff or PVC ETT. The cuffs filled with air were penetrated more rapidly than those with methylene blue.99
Pandit and Keller and their colleagues showed that the cuff was more vulnerable to a laser strike with the CO2 and KTP lasers, especially when filled with air. Pandit filled the cuff with saline, and Keller filled the cuff with nondilute methylene blue, and both found increased resistance to the laser strike. The disadvantage of using saline in the cuff is that the manufacturers recommend filling the cuff only with air. It has been reported that if not all the saline is withdrawn from the cuff after use in the standard reusable LMA, the cuff may rupture during autoclaving.147,148 Coorey and colleagues reported a technique in which they were able to empty the saline-inflated LMA reliably by a syringe without the plunger with the cuff held above the syringe to facilitate gravitational drainage. The cuff was then manually squeezed. The LMA with the syringe barrel attached was placed in a warming cupboard at 60° C for 12 hours. They found that filling the LMA cuff with saline was a viable option for laser airway surgery.149
The other option is the disposable LMA, for which filling the cuff with saline is not a concern because it is used only one time. The only concern is that the disposable LMA shaft is more easily penetrated than the silicon-based LMA, but the disposable LMA cuff was more resistant to penetration than the silicon-based LMA or PVC ETT cuffs. When filled with methylene blue dye and with the Nd : YAG laser power density at 570 W/cm2, none of the cuffs was affected after 30 seconds.146
B Management of Anesthesia
During laser airway surgery, Rontal and colleagues used a video monitor during all laser endoscopic procedures so that the entire operating room team could observe the procedure.150 The use of a video monitor to observe the impact of the laser beam on tissues in shared airway procedures is used by many surgical units and allows the operating room nurses to anticipate the surgeons’ needs and the anesthesiologist to communicate better, adjust the anesthesia accordingly, and act quickly if there is an airway fire (Fig. 40-31). Without a video monitor, only the surgeon can see the surgical site and the impact of the laser beam; vital seconds may be lost before the team recognizes an airway fire is happening, but it would have been obvious on a monitor screen. The presence of a video monitor has no disadvantage, but its absence may be detrimental, and some units regard it as a standard requirement for shared laser airway surgery.
1 Preoperative Evaluation
At the end of the preoperative assessment, the anesthesiologist should have some idea of the size, mobility, and location of the lesion. Standard airway assessments to predict the ease of ventilation, visualization of the laryngeal inlet, and tracheal intubation should be performed. Airway pathology and its impact on airway management should be assessed. The severity and size of lesions at the glottic level are assessed by direct or indirect laryngoscopy, undertaken by surgeons in an outpatient setting, and a photograph or image of the findings is often added to the record. Information about subglottic and tracheal lesions is provided by chest radiography, computed tomography (CT), and magnetic resonance imaging (MRI). The size of the lesion gives an indication of potential airflow obstruction. Stridor indicates a significantly narrowed airway. In the adult, stridor implies an airway diameter of less than 4 to 5 mm, but the absence of stridor does not exclude a significantly narrowed airway (Box 40-2).
Box 40-2 Preoperative Evaluation
1. History of endoscopic procedures, previous difficulties, medical record, and chosen technique
2. Hoarse voice: occurs with minor vocal fold lesions and significant pathology
3. Voice changes: nonspecific symptom
5. Altered breathing position: significant
6. Inability to lie flat: significant
7. Difficulty breathing during sleep: significant
9. Stridor on exertion: suggests obstruction becoming critical
10. Stridor at rest: indicates critical airway obstruction
11. Inspiratory stridor: suggests extrathoracic airway obstruction
12. Expiratory stridor: suggests intrathoracic airway obstruction
1. Airway or head and neck problems: The anesthesiologist should know the underlying pathology of the lesion, anatomic characteristics, and severity of obstruction. An extensive evaluation of the degree of airway obstruction should be undertaken. A history should be obtained of signs of airway obstruction possibly related to edema or tumor, such as obstructive sleep apnea, difficulty breathing, difficulty swallowing, shortness of breath, snoring, stridor, wheezing, or difficulty clearing secretions. The airway should be examined for the Mallampati classification, mouth opening, prognathia, size of the tongue, neck range of motion, prominent teeth, and tracheal deviation from external compression. The precise location and extent of the tumor should be defined by chest radiography, CT, and MRI.151 However, the patient’s general condition may preclude obtaining imaging. The test results should be reviewed for the luminal size of the trachea and tracheobronchial obstruction. These studies should include chest radiography (i.e., anteroposterior and lateral for possible pulmonary collapse and consolidation, presence of air-filled lung bullae, or pneumothorax), CT or MRI, flow-volume loops, and barium swallow.
2. Respiratory problems: The concerns are upper airway obstruction and coexisting pulmonary problems, such as a history of chronic obstructive pulmonary disease, emphysema, or newly diagnosed asthma; wheezing; and decreased exercise tolerance. Whether the patient requires home oxygen should be determined. Physical examination includes auscultation of the lungs for wheezing or decreased breath sounds and looking for clubbing or cyanosis. The examiner should review the chest radiograph, percent of oxygen saturation, baseline arterial blood gas determination, flow-volume loop, and pulmonary function tests to determine the amount of pulmonary reserve and involvement of the extrabronchial structures.
3. Cardiovascular problems: The concerns are related to potential cardioarterial disease. History of chest pain, shortness of breath with exertion, decreased exercise tolerance, and previous myocardial infarction or congestive heart failure are of interest. Examination includes auscultation of the heart for rate, rhythm, and heart murmurs and assessment of the lungs for rales. The examiner should look for jugular venous distention, for congestive heart failure, and for possible subclavian venous occlusion due to the tumor. Tests that may be needed are a 12-lead electrocardiogram, cardiac echocardiogram, dipyridamole (Persantine) thallium, and exercise stress test.
4. Other system problems: The gastrointestinal system should be assessed for aspiration potentials, such as hiatal hernia, acid reflux, nighttime cough, and obesity. Routine blood work should be done unless otherwise indicated.
2 Premedication
Patients should be monitored with a three- or five-lead electrocardiogram, depending on the cardiac history, pulse oximetry, capnography, oxygen analyzer, precordial stethoscope, peripheral nerve stimulator, and noninvasive blood pressure cuff. An invasive arterial blood pressure line should be considered for patients with cardiovascular instability to monitor closely hemodynamic changes and arterial blood gases intraoperatively.152
3 Induction and Maintenance of Anesthesia
a General Anesthesia
When airway compromise is an issue, traditional teaching suggests any technique that uses inhalational induction and maintains spontaneous ventilation is safest in the presence of airway obstruction.111,114 Spontaneous breathing has traditionally been thought of as critical with intrathoracic obstructive lesions of the large airways. During spontaneous breathing, large airways are stented open during inspiration by the negative intrathoracic pressure that is generated. This permits some inspired tidal volume to get past the obstruction. When the chest wall expands during inspiration, the negative pressure generated is beneficial in preventing collapse of the tumor mass onto the large airways and vessels. These two safeguards are removed when spontaneous ventilation ceases.
Traditional teaching for inhalational induction and maintenance of spontaneous ventilation in obstructed airways has been questioned. Inhalational induction for advanced laryngeal tumors with airway obstruction is difficult and challenging. Physiologic problems include a reduction in airflow with spontaneous ventilation, an increased collapsibility of the airway, increased work of breathing, critical instability at points of narrowing leading to further airway collapse, and a reduction in functional residual capacity.113 Induction is slow with apneic periods, and episodes of obstruction are common. Controversy exists about the suitability of “taking over” the patient’s own spontaneous ventilation with bag-mask ventilation. The advantage of retaining spontaneous ventilation throughout is the theoretical inherent advantage of this technique. However, taking over ventilation allows a suitable depth of anesthesia for laryngoscopy to be achieved quicker. This avoids the long periods of spontaenous ventilation while waiting for an adequate depth of anesthesia, during which the patient may become unstable.
The administration of a muscle relaxant provides optimal ventilation and intubating conditions. Calder and Yentis commented on the perceived safe practice of not giving muscle relaxants until face mask ventilation has been documented and argued the theoretical advantage is not fulfilled in practice.153 They considered two studies and looked at the incidence and prediction of difficult and impossible mask ventilation in which no patient was awakened and suggested that when a patient has an obstructed airway,154,155 it was not feasible to await awakening and something had to be done to ventilate the lungs before catastrophic desaturation occurred.153
General anesthesia is used in pediatric patients. In small children with airway obstruction, attempting bronchoscopic procedures with sedation alone is not recommended. General anesthesia using inhalation agents allows better airway control and permits the operator to concentrate on the procedure.156 In a retrospective review of pediatric patients undergoing diagnostic bronchoscopic procedures in the intensive care unit with sedation, ketamine was associated with a 20% rate of adverse reactions. Complications were mostly in children younger than 3 years with severe underlying disease.157
A tubeless spontaneous respiration technique has been used for pediatric microlaryngeal surgery. It allows an unrestricted view and surgical access to the larynx. This can be accomplished by delivering inhalation agents proximal to the larynx or by using propofol. Halothane (0% to 5% at 4 to 10 L/min) is delivered by the suction channel of the microlaryngoscope. Propofol (2 mg/kg) as a bolus is followed by an infusion of 100 µg/kg/min. In pediatric microsurgery, satisfactory results were obtained in two groups of children using halothane or propofol.158
General anesthesia is preferred in most adults to the same extent. Airway control that is possible with general anesthesia is fundamental in situations of airway obstruction and severe respiratory distress. There is better control of the airway and easier clearance of blood and mucus with use of a rigid bronchoscope.159 For the insertion of a rigid bronchoscope, general anesthesia is necessary.
Rigid bronchoscopy can be performed in a spontaneously breathing patient using an inhalation technique. This technique requires experience, particularly in the presence of significant airway obstruction. A deep plane of anesthesia is needed to prevent coughing. Laryngospasm or bronchospasm can complicate induction if the instrumentation is attempted too hastily. This can disastrously worsen the situation, causing total airway obstruction resulting in hypoxemia, hypotension, and trauma to the trachea. Because the uptake of any inhalation agent is delayed by airway obstruction, induction times are always longer than usual. Sufficient time must be spent making sure that the patient is “deep enough” for intubation. Judging the appropriate depth of anesthesia is often difficult. Excess anesthesia may result in hypoventilation in a spontaneously breathing patient.160 Caution should be exercised in monitoring the patient when using inhalation agents at such critical periods. The concentration required for an adequate depth of anesthesia to permit instrumentation of the trachea may not be tolerated by the cardiovascular system, causing hypotension and hemodynamic instability. Consequently, the margin of safety for inhalation agents may be very narrow. This is especially true in infants and in elderly patients. In children, sevoflurane seems to cause fewer complications, such as dysrhythmias and laryngospasm, than halothane.
Patients with significant tracheal obstruction benefit from sitting up and receiving supplementary humidified oxygen. A coughing spell may critically exacerbate the obstruction and should be avoided. In the operating room glycopyrrolate (0.2 to 0.3 mg) may be helpful in controlling secretions, and midazolam (2 to 4 mg intravenously) may be used for sedation. If an inhalation induction is chosen, the patient is preoxygenated with 100% oxygen, gradually induced with sevoflurane, and allowed to breathe spontaneously. For total intravenous anesthesia, the patient is induced with a sleep bolus of 2 mg/kg of propofol followed by a propofol infusion of 100 to 150 µg/kg/min. Before administering a muscle relaxant, it has traditionally been thought prudent to establish the ability to ventilate the patient by positive pressure through a face mask, although this approach has been questioned.153 Positive-pressure ventilation may become impossible in the presence of certain large lesions in the airway. Urgent measures are necessary when the situation deteriorates to acute central airway obstruction. An ETT may be inserted to bypass the obstruction. Positive-pressure ventilation and suctioning may not be enough in certain situations. Rigid bronchoscopy may be required urgently to force the tube and establish an airway under direct vision.161
b Sedation and Topical Anesthesia
Selected adult populations undergoing flexible fiberoptic laser excision can be managed by experienced individuals with sedation and topical anesthesia. Topical anesthesia with sedation is suitable for adults who are cooperative and for a short endoscopy period with a flexible bronchoscope. This is a favored method for lesions in the middle or lower third of the trachea. Complications are rare. Bleeding seems the most life-threatening complication.162
Sedative drugs can decrease ventilation and should be used judiciously in small children and elderly patients. Two distinct mechanisms are accountable for this reduction: decreased respiratory drive and upper airway obstruction. Sedatives alter normal respiratory responses to hypercarbia and hypoxia, thereby decreasing the respiratory drive. Brief hypercarbia and acidosis that may develop under these circumstances are ordinarily inconsequential. However, airway obstruction is a real and more dangerous outcome of sedation because it can quickly lead to oxygen desaturation. Hypoxemia from airway obstruction can have devastating consequences.163 The exact mechanism of upper airway obstruction during sedation or anesthesia is not clear. It may be caused by decreased muscular tone and increased collapsibility of the upper airway. Midazolam induces upper airway obstruction during deep sedation. Flumazenil relieves this obstruction in addition to reversing the sedative effects of midazolam.71
Introduction of additional obstruction to a patient with an airway that is already narrowed by a fixed lesion is fraught with danger. A perilous scenario can ensue, with progressively increasing obstruction resulting in total loss of the airway. Prompt measures must be undertaken to relieve the obstruction. They include asking the patient to take deep breaths, jaw thrust, chin lift, and insertion of an oral or a nasal airway. Chin lift or jaw thrust may not always relieve the obstruction. However, when these two maneuvers are combined with positive airway pressure, the pharyngeal airway is widened, counteracting airway narrowing.164 If a procedure being performed under sedation becomes prolonged, additional, unplanned drugs have to be administered, increasing the risk of excessive intraoperative sedation and of postoperative somnolence.
VII Anesthesia Management for Laryngeal Laser Surgery
B Spontaneous Ventilation and Insufflation Techniques
The limitations of spontaneous ventilation or insufflation techniques include the lack of control over ventilation and the potential for airway soiling. Operating room pollution due to insufflation of volatile agents is also an issue and requires high-intensity suction catheters near the mouth (see Fig. 40-14). Insufflation techniques may not be suitable for large, soft, floppy lesions, particularly in the supraglottis or glottis, which may obstruct the airway after the onset of general anesthesia with spontaneous ventilation.
Rita and associates used the CO2 laser successfully to excise subglottic stenosis in infants,165 avoiding a tracheostomy, and reported that ETTs of any size in this group make surgery in infants difficult or impossible because the operative field is obstructed. They reported that in some infants with subglottic stenosis, even a 2.5-mm-ID ETT, was difficult to pass through the stenotic area. Rita and associates concluded that although the insufflation technique is potentially hazardous,165 if it is done correctly, it provides an appropriate anesthetic technique for surgery on the larynx of infants.166 They reported good results with this technique over the course of many years.
C Intermittent Apneic Technique
Weisberger and Miner described the use of an intermittent apneic technique for laser laryngeal surgery in the resection of juvenile papillomatosis of the larynx without the use of an ETT.167 The investigators stated that a clear, unobstructed view of the airway and complete immobility of the surgical field were essential for the surgeon during these cases (see Fig. 40-30). During a 2-year period, 51 procedures were performed by the investigators on nine patients who had juvenile laryngeal papillomatosis. Their average age was 10.5 years (range, 3.5 to 37 years). After induction of general anesthesia, the patients were paralyzed with atracurium or vecuronium and anesthesia maintained with halothane or enflurane in 100% oxygen. The patients’ tracheas were intubated with small-caliber ETTs wrapped with foil tape. In cases of extensive papillomatosis, resection was started with the ETT in situ while the patients’ lungs were ventilated with 40% oxygen. Otherwise, the ETTs were removed and the surgery started. The surgery was interrupted when the oxygen saturation decreased. The patients’ tracheas were then reintubated, and they were hyperventilated with 100% oxygen. This resulted in a rapid rise in the oxygen saturation so that repeated extubations and surgery could continue. The median number of apneic episodes required for each procedure was two, with a range of one to five. The duration of apneic episodes was 2.6 minutes, with a range of 1 to 4.5 minutes. A suspension laryngoscope and microscope, which provided excellent visualization of the larynx, were used in these cases so that endotracheal intubation could be readily accomplished without moving the laryngoscope. The apneic technique removes all the flammable material from the larynx during laser actuation and is thought to decrease greatly the possibility of an airway fire. Weisberger and Miner stated that the apneic period should be shortened for small children because of their decreased functional residual capacity.167 The technique is contraindicated in patients in whom visualization of the larynx is difficult.
D Jet Ventilation Techniques
Jet ventilation techniques are suitable for ventilation during laser surgery of most adult vocal cord lesions. The main limitation for their use is the experience of the anesthesiologist and the absence of suitable equipment. Jet ventilation techniques involve the intermittent administration of high-pressure jets of air, oxygen, or oxygen-air mixtures that entrain room air and lower the delivered pressures. The risk of life-threatening barotrauma is the most serious complication, and it occurs when entrainment of room air is absent. This results in the delivery of very high driving pressures, leading to pneumomediastinum, pneumothorax, and surgical emphysema.168–170
In 1967, Sanders first described a jet ventilation technique using a 16-gauge, high-pressure source (jet) placed down the side arm of a rigid open bronchoscope.171 Sanders used intermittent manual ventilation with a rate of 8 L/min and a driving pressure of 700 torr to entrain room air and showed the technique could maintain supranormal oxygen pressure and prevent a rise in the CO2 partial pressure. Since 1967, modifications to Sanders’ original jet ventilation technique have been made for endoscopic airway surgery. These modifications include frequency of ventilation (i.e., low-frequency, high-frequency, or superimposed frequency jet ventilation) and the site at which the jet emerges in the airway (i.e., supraglottic, subglottic, or transtracheal).
There is no clear consensus about the ideal ventilation modes or the laser techniques. Selection must be done on the basis of a thorough understanding of the pathophysiology of airway obstruction and individual experience with use of these devices. Hunsaker introduced a laser-safe subglottic monojet ventilation tube.109,172
1 Site of Jet Delivery
b Subglottic Jet Ventilation
Subglottic jet ventilation involves placing a small (2 to 3 mm external diameter) catheter or specifically designed tubes (e.g., Benjet, Hunsaker, Acutronic, Carl Reiner) through the glottis and into the trachea, which allows delivery of a jet of gas directly into the trachea (Fig. 40-32). This means a fuel source is present within the airway, and care must be taken during laser airway surgery. Many of the subglottic catheters that are commercially available have some laser-resistant properties, but if their tolerance is exceeded, the catheters can degrade and fracture. If after a fracture of the catheter and after a laser strike, jet ventilation is resumed, the distal fragment may be forced into the distal airways. Some catheters (e.g., Hunsaker) have metal wires within the catheter to reduce the chances of this distal fragmentation.
Subglottic jet ventilation is more efficient than supraglottic jet ventilation and results in reduced driving pressures, minimal vocal cord movements, a good surgical field, and no time constraints for the surgeon in the placement of the rigid laryngoscope. The main disadvantage is the potential for a laser-induced airway fire due to the presence of a potential fuel source within the airway and a greater risk of barotrauma than in supraglottic jet techniques (Fig. 40-33).
c Transtracheal Jet Ventilation
Elective transtracheal catheter placement under local anesthesia in individuals with significant airway pathology or under general anesthesia for elective laryngeal surgery has been described.173 Transtracheal jet techniques carry the greatest risks of barotrauma. Other potential problems include blockage, kinking, infection, bleeding, and failure to site the catheter. None of the commercially available transtracheal catheters is specifically designed for laser use, and transtracheal jet ventilation techniques for endoscopic laser surgery of the larynx require a careful evaluation of the potential risks and benefits.
2 Jet Ventilation Frequency
a Low-Frequency Jet Ventilation
During supraglottic jet ventilation, there is considerable movement of the laryngeal structures, and the timing of each breath can be coordinated with the surgeon operating the laser. Even small movements are magnified under the operating microscope. This movement is much less of an issue with subglottic jet ventilation. Intravenous agents, such as propofol infusions, are used to maintain anesthesia. Short-acting muscle relaxants are used to prevent movements of the patient that can be dangerous during a laser operation. Gastric distention with possible regurgitation may occur with a misaligned cannula,174 and a nasogastric tube may be required. Adequate fluids are given intraoperatively, and gases may be humidified in the postoperative period to prevent mucosal drying.
Jet ventilation is helpful in many situations in which a laser is not used. Intermittent jet ventilation has been applied through the instrument channel of the flexible FOB to expand atelectatic lungs and through the Hunsaker jet ventilation tube for one-lung ventilation for lobectomy.175 However, extreme care should be taken to avoid pulmonary barotrauma.
b High-Frequency Jet Ventilation
During high-frequency jet ventilation (HFJV), small tidal volumes are delivered at a rate of 60 to 150 cycles per minute from a high-pressure source (5 to 50 psi), with inspiration taking 20% to 40% of each cycle. This is an effective ventilation method that provides adequate gas exchange. This method has been used in rigid bronchoscopy. Hautmann and colleagues described a technique of delivering HFJV through a noncompliant insufflation catheter.176 The catheter can be threaded through the sidearm of the bronchoscope and positioned in the trachea or in the main stem bronchus. Medici and coworkers used an insufflation catheter with a side hole in the contralateral main stem to deliver high-frequency positive-pressure ventilation during endobronchial surgery using an Nd : YAG laser.177 HFJV catheters can be placed transtracheally; however, in a prospective multicenter study, the rate of subcutaneous emphysema was 8.4%.178
Although the reported incidence of pulmonary barotrauma in HFJV is low, it can lead to serious complications. Pulmonary barotrauma can result from high driving gas pressures and air trapping. Ventilators should initially be set at the lowest possible driving pressures and increased incrementally while carefully observing chest movements. There is a potential risk of barotrauma if the air pressures are not monitored. In addition to an injector catheter, Unzueta and associates used a second identical catheter placed 7 cm distal to the injector site to measure airway pressure continually.146 Continuous monitoring of end-expired CO2 and O2 by conventional methods is difficult to interpret because of fast respiratory rates and high gas flow rates. However, there is a well-defined relationship between arterial CO2 concentrations and end-tidal CO2 concentration when expiratory gas is obtained by sidestream sampling during jet ventilation.179 Observation of chest wall movements and blood gas analysis are reliable methods for assessing the adequacy of ventilation. Klein and coworkers suggested the use of a double-lumen jet catheter for respiratory monitoring.168
Air trapping and stacking of breaths are inherent problems in high-frequency ventilation because of short expiratory times.180 In airways distal to the obstruction, injurious excess pressure can easily build up. Obstruction to gas outflow can easily occur because of the tumor and because of the presence of surgical instruments, bleeding, and mucosal edema. Light anesthesia or inadequate muscle relaxation can cause laryngospasm, closing the glottis and further obstructing the airflow. To prevent barotrauma, it is essential to ensure that there is adequate outflow.
c Combined Frequency Jet Ventilation
Oxygenation and CO2 elimination may be improved by using low-frequency and high-frequency ventilation in combination (Fig. 40-34). In this method using combined (superimposed) frequencies of ventilation, the location of the gas inflow in the airway seems to influence the efficiency of jet ventilation. Supraglottic, combined-frequency ventilation seem to be superior to subglottic, monofrequent jet ventilation in providing an unobstructed view and access to glottis.181 With intratracheal jet ventilation, aspiration and air entrapment are virtually absent. There is no clear advantage of one ventilation technique over the other.182 In a study of 37 adult patients, supraglottic, combined-frequency ventilation was more efficient than subglottic, low-frequency or subglottic, combined-frequency ventilation.181 However, in a bench model of laryngotracheal stenosis, supraglottic HFJV was associated with significantly larger end-expiratory pressures and peak inspiratory pressures.183 Two risks are built in because of the location of the jet cannula: aspiration at the supraglottic location and barotrauma at the subglottic location.
High-frequency ventilation should be reserved for patients with severe airway obstruction or severe pulmonary dysfunction. Most cases do well with a variety of simpler techniques that are well established. They include ventilation with a laser-resistant tube or microlaryngoscopy tube, supraglottic jet ventilation with a jet needle, and subglottic jet ventilation with a catheter.184
There is no perfect mode of ventilation that suits all clinical conditions. Complications have been associated with various ventilation techniques in laser resections (Table 40-9). Table 40-10 suggests a general approach that is used in the pediatric population.
TABLE 40-9 Complications Associated with Jet Ventilation of the Lungs
| Ventilation Related (n)* |
Ventilation Unrelated (n)* |
|---|---|
| Major | |
| Pneumothorax (9) | None |
| Hypoxemia during anesthesia (6) | |
| Mediastinal air (2) | |
| Tension pneumothorax (1) | |
| Minor | |
| Carbon dioxide retention during anesthesia (3) | Prolonged mask support (10) |
| Subcutaneous emphysema (2) | Prolonged endotracheal intubation (5) |
| Gastric dilation (2) | Laryngospasm (5) |
| Airway bleeding (2) | Prolonged sleep or paralysis (2) |
| Recall of procedure (1) |
* Data from 15,701 patients were analyzed.
Adapted from Cozine K, Stone JG, Shulman S, Flaster ER: Ventilatory complications of carbon dioxide laser laryngeal surgery. J Clin Anesth 3:20, 1991.
VIII Anesthetic Management for Tracheobronchial Tree Laser Surgery
Laser resection is the preferred method for removal of obstructive lesions from the trachea and bronchus. It is a safe and effective means of relieving central airway obstruction. With more than 4000 published interventions, the safety and efficacy of laser bronchoscopy has been well established.142 Providing safe anesthesia for laser excision of lesions that are in the tracheobronchial tree can be challenging. Maintaining unhampered views of the operative field, gaining free and safe access to the laser, and supplying adequate ventilation are essential in choosing a method of anesthetic management, and these are not easy measures to provide for patients with an obstructed airway. The challenge is to preserve adequate gas exchange while maintaining good visualization and surgical access to the lesion.
The primary advantage of using a laser for recurrent benign tumors is that multiple resections can be safely performed in outpatients. This approach avoids the increased morbidity of open thoracotomy procedures. In cancer patients, endoscopic palliation helps in evaluation and staging of malignant tumors. These treatments reduce morbidity during chemotherapy without increasing surgical complications.185 In cases of severe obstruction by unresectable tumors, a patent airway can be cored with a laser.186,187 The combination of laser and rigid bronchoscopy is ideal for treating central airway obstruction.188 Relief of acute ventilatory distress is a major benefit of bronchoscopic laser resections.189 Therapeutic bronchoscopy should be considered even in individuals with cancer requiring intubation and mechanical ventilation. Often, mechanical ventilation is successfully discontinued and death from suffocation postponed.190
When anesthesiologists and surgeons simultaneously handle the airway, they must have a predetermined plan for intraoperative and postoperative management. Good communication and cooperation are important, and development of a team approach is necessary for successful results.191 Devising such a plan is made easier if the current problems are conceptualized in two broad categories: mechanical and patient-related issues. Mechanical issues deal with attaining good surgical access while simultaneously providing adequate gas exchange and anesthesia. Distal lesions pose a greater challenge because they can cause complete airway obstruction and are difficult to reach with laser treatment. An ideal treatment modality for endoscopic ablation provides secure airway protection, excellent visualization of the lesion, and delivery of safe and effective method of treatment. Use of a contact Nd : YAG fiberoptic laser system through a rigid bronchoscope has performed well in meeting these criteria.192 Patient-related topics such as age, size, pulmonary function, and general health status should be the basis for all final decisions. It is important first to decide on the type of laser therapy, type of scope, and technique of ventilation. An anesthesia management plan can then be formulated on the basis of patient-related factors, available equipment, and expertise.
A Rigid Ventilating Bronchoscopy
Although the rigid bronchoscope is an old instrument, it continues to play a major role in newer therapies for access to the airway.193 Rigid bronchoscopy is the standard technique for anesthesia management during laser resection of severely obstructing tracheal masses.194 The main advantage of the rigid bronchoscope is complete control of the airway. The bronchoscope ultimately functions as a rigid ETT. The area and clarity of the view of the surgical field with rigid scopes are superior to those with flexible scopes. However, the view is limited to the trachea, carina, and the main bronchi. Subglottic lesions can be visualized through a rigid bronchoscope. Compared with flexible bronchoscopes, rigid bronchoscopes have large-diameter instrument side channels. A variety of baskets, forceps, and grabbers that are used for a wide range of therapeutic procedures can pass easily through these wide channels. In the event of significant bleeding or tenacious secretions, the telescope can be withdrawn and suction catheters passed directly down the bronchoscope while maintaining oxygenation and control of the airway.
Rigid bronchoscopy can be invaluable and life-saving in cases of severe obstruction because the bronchoscope can be pushed past the obstruction.141,195 It is unsurpassed in evaluation, control, and therapeutic manipulation of the tracheobronchial tree.196 A retrospective review of 300 patients over a 12-year period concluded that the use of rigid bronchoscopy offers certain advantages in ventilation during general anesthesia. Rigid therapeutic bronchoscopic intervention is increasingly accepted to treat patients with central airway obstruction. Laser resection is applicable when the obstruction is caused by malignant exophytic tracheobronchial lesions, benign granulation tissue, or tracheobronchial stenosis with scar tissue formation.190 In one study, rigid bronchoscopy was preferred for proximal lesions and FOB used for distal lesions. Often, the rigid and flexible bronchoscopes were used together to maximum advantage.197
Drawbacks of rigid bronchoscopy include increased secretions and finite access to the distal airways and upper lobes. Conditions such as ankylosis of the jaw or rigid cervical spine, which make direct visualization of the larynx difficult, may preclude the use of a rigid bronchoscope. General anesthesia is often necessary for rigid bronchoscopy, whereas flexible bronchoscopy can be performed with sedation and topical anesthesia. Patients do not tolerate pressure on the soft tissues, and significant neck extensions are painful.198
B Flexible Bronchoscopy
Flexible bronchoscopy is used to reach lesions in distal airways. Flexible fiberoptic scopes are more suited for procedures under sedation and local anesthesia.161 Flexible bronchoscopes are built with bundles of optical fibers or a camera at the distal end. The fibers deliver light to the tip and transmit pixels. An image is constituted when all the pixels are reassembled. However, the image formed is not as good as the view from direct laryngoscopy. It is possible to visualize the entire airway because the tip of the bronchoscope can be flexed through an arc of 220 degrees. There is usually a working channel, which can be used for suction and for passing instruments. In adults, 5.5- to 6.0-mm flexible bronchoscopes are used, and in children, 3.6- and 2.2-mm scopes are popular. The disadvantage of the smaller scopes is that there is no instrument channel or, if present, it is too narrow. As technologic improvements occur in the design of smaller instruments and improved forceps, the therapeutic and diagnostic role of flexible bronchoscopes will become more prevalent in clinical practice.199
X Clinical Pearls
• Good communication, cooperation, and the development of a team approach are essential requirements for successful laser airway surgery. The surgeon and anesthesiologist should be familiar with the principles of a laser, the types of laser available, hazards of laser use, the management of an airway fire, and the advantages and disadvantages of the various anesthetic techniques available.
• For an airway fire, three components of the fire triad are needed: fuel source, oxidant sources, and ignition source. Removal of one of these components prevents or reduces the risk of an airway fire, which in practice means removing or protecting the fuel sources, minimizing the oxidant sources, and not using an ignition source.
• Oxygen and nitrous oxide support combustion very well, and dilution of oxygen by nitrous oxide makes no difference to the flammability risk. In clinical practice, air and oxygen are most frequently used during laser airway surgery, with the oxygen concentration kept as low as clinically feasible while the laser is near the tube.
• All manufactured laser tubes have laser-resistant properties, providing some degree of protection against a laser strike, and these limits are provided by the manufacturer in the product literature, which describes the types of laser the tube has protection from and the limits of power settings for different lasers. Laser tubes vary in the material used, protective coating, relative size, and the number of cuffs. All laser tubes can result in an airway fire or blowtorch fire if used outside of their limits.
• The preoperative evaluation attempts to identify factors that may cause difficulty during the perioperative course. It involves a thorough medical evaluation of all patients before surgery. At the end of the preoperative assessment, the anesthesiologist should have some idea of the size, mobility, and location of the lesion. Recognition of a compromised or anatomically distorted upper airway is paramount in the preoperative assessment of these patients.
• Premedication to reduce secretions and anxiety may be useful in patients with minor airway lesions, but it should be avoided in patients with severe airway compromise. Thick secretions may block narrowed airways further, may be difficult to cough out by the patient, and may in extreme circumstances totally obstruct critically narrowed airways.
• Jet ventilation techniques are suitable for ventilation during laser surgery of most adult vocal cord lesions. The main limitation for their use is the experience of the anesthesiologist and the absence of suitable equipment. Life-threatening barotrauma is the most serious complication, and it occurs when room air is not entrained. This results in the delivery of very high driving pressures, leading to pneumomediastinum, pneumothorax, and surgical emphysema.
All references can be found online at expertconsult.com.
1 Caplan RA, Barker SJ, Connis RT, et al. Practice advisory for the prevention and management of operating room fires. A report by the American Society of Anesthesiologists task force on operating room fires. Anesthesiology. 2008;108:786.
43 Puttick N. Anesthesia for laser airway surgery. In: Oswal V, Remacle M. Principles and practice of lasers in otolaryngology and head and neck surgery. The Hague: Krugrer; 2002:63–76.
101 Rampil IJ. Anesthesia for laser surgery. In: Miller RD, ed. Miller’s anesthesia. ed 7. Philadelphia: Churchill Livingstone; 2010:2405–2418.
109 Hunsaker DH. Anesthesia for microlaryngeal surgery: The case for subglottic jet ventilation. Laryngoscope. 1994;104(Suppl 65):1.
110 Ossoff RH. Laser safety in otolaryngology–head and neck surgery: Anesthetic and educational considerations for laryngeal surgery. Laryngoscope. 1989;99(Suppl 48):1.
134 Chee WK, Benumof JL. Airway fire during tracheostomy: Extubation may be contraindicated. Anesthesiology. 1998;89:1576.
135 Van Der Spek AF, Spargo PM, Norton ML. The physics of lasers and implications for their use during airway surgery. Br J Anaesth. 1988;60:709.
140 Sher M, Brimacombe J, Laing D. Anaesthesia for laser pharyngoplasty—A comparison of the tracheal tube with the reinforced laryngeal mask airway. Anaesth Intensive Care. 1995;23:149.
153 Calder I, Yentis SM. Could “safe practice” be compromising safe practice? Should anaesthetists have to demonstrate that face mask ventilation is possible before giving a neuromuscular blocker? Anaesthesia. 2008;63:113.
161 Conacher ID. Anaesthesia and tracheobronchial stenting for central airway obstruction in adults. Br J Anaesth. 2003;90:367.
1 Caplan RA, Barker SJ, Connis RT, et al. Practice advisory for the prevention and management of operating room fires. A report by the American Society of Anesthesiologists task force on operating room fires. Anesthesiology. 2008;108:786.
2 Pennsylvania Patient Safety Authority. Airway fires during surgery. PA PSRS Patient Saf Advis. 2007;4:1. Available at http://patientsafetyauthority.org/ADVISORIES/AdvisoryLibrary/2007/mar4(1)/Pages/01b.aspx (accessed May 2012)
3 Prasad R, Quezado Z, St. Andre A: Fires in the operating room and intensive care unit: Awareness is the key to prevention. Anesth Analg. 2006;102:172.
4 Singla AK, Campagna JA, Wright CD, et al. Surgical field fire during a repair of bronchoesophageal fistula. Anesth Analg. 2005;100:1062.
5 Paugh DH, White KW. Fire in the operating room during tracheotomy: A case report. AANA J. 2005;73:97.
6 Katz JA, Campbell L. Fire during thoracotomy: A need to control the inspired oxygen concentration. Anesth Analg. 2005;101:612.
7 Varcoe RL, MacGowan KM, Cass AJ. Airway fire during tracheostomy. ANZ J Surg. 2004;74:507.
8 Ng JM, Hartigan PM. Airway fire during tracheostomy: Should we extubate? Anesthesiology. 2003;98:1303.
9 Awan MS, Ahmed I. Endotracheal tube fire during tracheostomy: A case report. Ear Nose Throat J. 2002;81:90.
10 Rogers ML, Nickalls RW, Brackenbury ET, et al. Airway fire during tracheostomy: Prevention strategies for surgeons and anaesthetists. Ann R Coll Surg Engl. 2001;83:376.
11 Lai A, Ng KP. Fire during thoracic surgery. Anaesth Intensive Care. 2001;29:301.
12 Barker SJ, Polson JS. Fire in the operating room: A case report and laboratory study. Anesth Analg. 2001;93:960.
13 Handa KK, Bhalla AP, Arora A. Fire during the use of Nd-Yag laser. Int J Pediatr Otorhinolaryngol. 2001;60:239.
14 Santos P, Ayuso A, Luis M, et al. Airway ignition during CO2 laser laryngeal surgery and high frequency jet ventilation. Eur J Anaesth. 2000;17:204.
15 Baur DA, Butler RC. Electrocautery-ignited endotracheal tube fire: Case report. Br J Oral Maxillofac Surg. 1999;37:142.
16 Martin L, Dolman P. Fire!. Can J Anaesth. 1999;46:909.
17 Ortega RA. A rare cause of fire in the operating room. Anesthesiology. 1998;89:1608.
18 Galapo S, Wolf GL, Sidebotham GW, et al. Laser ignition of surgical drapes in an oxygen enriched atmosphere. Anesthesiology. 1998;89:A580.
19 Chee WK, Benumof JL. Airway fire during tracheostomy: Extubation may be contraindicated. Anesthesiology. 1998;89:1576.
20 Thompson JW, Colin W, Snowden T, et al. Fire in the operating room during tracheostomy. South Med J. 1998;91:243.
21 Waldorf HA, Kauvar NB, Geronemus RG, et al. Remote fire with the pulsed dye laser: Risk and prevention. J Am Acad Dermatol. 1996;34:503.
22 Macdonald MR, Wong A, Walker P, et al. Electrocautery-induced ignition of tonsillar packing. J Otolaryngol. 1994;23:426.
23 Bennett JA, Agree M. Fire in the chest. Anesth Analg. 1994;78:406.
24 Michels AM, Stott S. Explosion of tracheal tube during tracheostomy. Anaesthesia. 1994;49:1104.
25 Marsh B, Riley RH. Double-lumen tube fire during tracheostomy. Anesthesiology. 1992;76:480.
26 Keller C, Elliott W, Hubbell RN. Endotracheal tube safety during electrodissection tonsillectomy. Arch Otolaryngol Head Neck Surg. 1992;118:643.
27 Wegrzynowicz ES, Jensen NF, Pearson KS, et al. Airway fire during jet ventilation for laser excision of vocal cord papillomata. Anesthesiology. 1992;76:468.
28 Aly A, McIlwain M, Duncavage JA. Electrosurgery-induced endotracheal tube ignition during tracheotomy. Ann Otol Rhinol Laryngol. 1991;100:131.
29 Lew EO, Mittleman RE, Murray D. Endotracheal tube ignition by electrocautery during tracheostomy: Case report with autopsy findings. J Forensic Sci. 1991;36:1586.
30 Bailey MK, Bromley HR, Allison JG, et al. Electrocautery-induced airway fire during tracheostomy. Anesth Analg. 1990;71:702.
31 Mandych A, Mickelson S, Amis R. Operating room fire. Arch Otolaryngol Head Neck Surg. 1990;116:1452.
32 Le Clair J, Gartner S, Halma G. Endotracheal tube cuff ignited by electrocautery during tracheostomy. AANA J. 1990;58:259.
33 Pashayan AG, Gravenstein JS. Airway fires during surgery with the carbon dioxide laser. Anesthesiology. 1989;71:478.
34 Simpson JI, Wolf GL. Endotracheal tube fire ignited by pharyngeal electrocautery. Anesthesiology. 1986;65:76.
35 Casey KR, Fairfax WR, Smith SJ, et al. Intratracheal fire ignited by the Nd-YAG laser during treatment of tracheal stenosis. Chest. 1983;84:295.
36 Meyers A. Complications of CO2 laser surgery of the larynx. Ann Otol Rhinol Laryngol. 1981;90:132.
37 Cozine K, Rosenbaum LM, Askanazi J, et al. Laser-induced endotracheal tube fire. Anesthesiology. 1981;55:583.
38 Hirshman CA, Smith J. Indirect ignition of the endotracheal tube during carbon dioxide laser surgery. Arch Otolaryngol. 1980;106:639.
39 Burgess GE, III., LeJeune FE, Jr. Endotracheal tube ignition during laser surgery of the larynx. Arch Otolaryngol. 1979;105:561–562.
40 Planck M. On an improvement of Wien’s equation for the spectrum. Verh Dtsch Phys Ges. 1900;2:202.
41 Einstein A. Zur Electrodynamik bewegter. Korper. Ann Phys. 1905;17:132.
42 Einstein A. A Zar Quanten theorie der. Strahlung. Physiology. 1917;18:121.
43 Puttick N. Anesthesia for laser airway surgery. In: Oswal V, Remacle M. Principles and practice of lasers in otolaryngology and head and neck surgery. The Hague: Krugrer; 2002:63–76.
44 Patel CKN. Continuous-wave laser action on vibrational-rotational transitions of CO2. Phys Rev. 1964;136:A1187.
45 Fuller TA. The physics of surgical lasers. Lasers Surg Med. 1980;1:5.
46 Garrett CG, Ossof RH, Reinisch L. Laser surgery: basic principles and safety considerations. Flint PW, Haughey BH, Lund VJ, et al. Cummings otolaryngology–head and neck surgery, ed 5, Philadelphia: Elsevier, 2010.
47 Karlan MS, Ossoff RH. A new laser-laryngoscope coupler. Otolaryngol Head Neck Surg. 1984;92:717.
48 Mihasi S, Jako GJ, Incze J, et al. Laser surgery in otolaryngology: interaction of CO, laser and soft tissue. Ann N Y Acad Sci. 1976;267:263.
49 Strong MS, Jako GJ. Laser surgery in the larynx. Early clinical experience with continuous CO2 laser. Ann Otol Rhinol Laryngol. 1972;81:791.
50 Council on Scientific Affairs. Lasers in medicine and surgery. JAMA. 1986;256:900.
51 Alberti PW. The complications of CO2 laser surgery in otolaryngology. Acta Otolaryngol. 1981;91:375.
52 Healy GB, Strong MS, Shapshay S, et al. Complications of CO2 laser surgery of the aerodigestive tract: Experience of 4416 cases. Otolaryngol Head Neck Surg. 1984;92:13.
53 Beamis JF, Jr., Vergos K, Rebeiz EE, et al. Endoscopic laser therapy for obstructing tracheobronchial lesions. Ann Otol Rhinol Laryngol. 1991;100:413.
54 Solares CA, Strome M. Transoral robot-assisted CO2 laser supraglottic laryngectomy: Experimental and clinical data. Laryngoscope. 2007;117:817.
55 Holsinger FC, Weber RS. Swing of the surgical pendulum: A return to surgery for treatment of head and neck cancer in the 21st century? Int J Radiat Oncol Biol Phys. 2007;69:129–131.
56 Devaiah AK, Shapshay SM, Desai U, et al. Surgical utility of a new carbon dioxide laser fiber: Functional and histological study. Laryngoscope. 2005;115:1463.
57 Zeitels SM, Kobler JB, Heaton JT, et al. Carbon dioxide laser fiber for laryngeal cancer surgery. Ann Otol Rhinol Laryngol. 2006;115:535.
58 Shires CB, Shete MM, Thompson JW. Management of suprastomal tracheal fibroma: introduction of a new technique and comparison with other techniques. Int J Pediatr Otorhinolaryngol. 2008;73:67.
59 Jacobson AS, Woo P, Shapshay SM. Emerging technology: Flexible CO2 laser WaveGuide. Otolaryngol Head Neck Surg. 2006;135:469.
60 Janda P, Sroka R, Baumgartner R, et al. Laser treatment of hyperplastic inferior nasal turbinates: A review. Lasers Surg Med. 2001;28:404.
61 Leibowitz PG. Corneal injury produced by carbon dioxide laser radiation. Arch Ophthalmol. 1969;81:713.
62 Shapshay SM, Beamis JF, Jr. Safety precautions for bronchoscopic Nd-YAG laser surgery. Otolaryngol Head Neck Surg. 1986;94:175.
63 Dumon JF, Shapshay S, Bourcereau J, et al. Principles for safety in application of neodymium-YAG laser in bronchology. Chest. 1984;86:163.
64 Brutinel WM, Cortese DA, McDougall JC. Bronchoscopic phototherapy with the neodymium-YAG laser. Chest. 1984;86:158.
65 Casey KR, Fairfax WR, Smith SJ, et al. Intratracheal fire ignited by the Nd-YAG laser during treatment of tracheal stenosis. Chest. 1983;84:295.
66 Brutinel WM, McDougall JC, Cortese DA. Bronchoscopic therapy with neodymium-yttrium-aluminum-garnet laser during intravenous anesthesia: Effect on arterial blood gas levels, pH, hemoglobin saturation, and production of abnormal hemoglobin. Chest. 1983;84:518.
67 McDougall JC, Cortese DA. Neodymium-YAG laser therapy of malignant airway obstruction. A preliminary report. Mayo Clin Proc. 1983;58:35.
68 Carruth JA, McKenzie AL, Wainwright AC. The carbon dioxide laser: Safety aspects. J Laryngol Otol. 1980;94:411.
69 Rampil I. Anesthetic considerations for laser surgery. Anesth Analg. 1992;74:424.
70 Ossoff RH. Safety protocol. Laser Med Surg News Adv. 1983;1:6.
71 Litman RS, Hayes JL, Basco MG, et al. Use of dynamic negative airway pressure (DNAP) to assess sedative-induced upper airway obstruction. Anesthesiology. 2002;96:342.
72 Milliken RA, Bizzarri DV. Flammable surgical drapes—A patient and personnel hazard. Anesth Analg. 1985;64:54.
73 Ott AE. Disposable surgical drapes—A potential fire hazard. Obstet Gynecol. 1983;61:667.
74 Nezhat C, Winer WK, Nezhat F, et al. Smoke from laser surgery: Is there a health hazard? Lasers Surg Med. 1987;7:376.
75 Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260.
76 Freitag L, Eugene J. Chemical composition of laser tissue interaction smoke plume. J Laser Appl. 20, 1989.
77 Adelsmayr E, Keller C, Erd G, et al. The laryngeal mask and high-frequency jet ventilation for resection of high tracheal stenosis. Anesth Analg. 1998;86:907.
78 Ferenczy A, Bergeron C, Richart RM. Carbon dioxide laser energy disperses human papillomavirus deoxyribonucleic acid onto treatment fields. Am J Obstet Gynecol. 1990;163:1271.
79 Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser–generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114.
80 Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: Detection and protection. J Am Acad Dermatol. 1989;21:41.
81 Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276.
82 Smith JP, Topmiller JL, Shulman S. Factors affecting emission collection by surgical smoke evacuators. Lasers Surg Med. 1990;10:224.
83 Golish JA, Pena CM, Mehta AC. Massive air embolism complicating Nd-YAG laser endobronchial photoresection. Lasers Surg Med. 1992;12:338.
84 Peachey T, Easton J, Moxham J, et al. Systematic air embolism during laser bronchoscopy. Anaesthesia. 1988;43:872.
85 Baggish MS, Daniel JF. Catastrophic injury secondary to the use of coaxial gas-cooled fibres and artificial sapphire tips for intrauterine surgery: A report of five cases. Lasers Surg Med. 1989;9:581.
86 Challener RC, Kaufman B. Fatal venous air embolism following sequential unsheathed (bare) and sheathed quartz fiber Nd-YAG laser endometrial ablation. Anesthesiology. 1990;73:548.
87 Levine DL. More laser accidents: One state health department reacts. J Clin Laser Med Surg. 1990;8:8.
88 Ossoff RH, Hotaling AJ, Karlan MS, et al. CO2 laser in otolaryngology–head and neck surgery: A retrospective analysis of complications. Laryngoscope. 1983;93:1287.
89 Wolf LG. Danger from OR fires still a serious problem; APSF Newsletter. Journal of Clinical Monitoring and Computing. 2000;16:237–238.
90 Shykhon M, Kuo M, Pearman K. Recurrent respiratory papillomatosis. Clin Otolaryngol Allied Sci. 2002;27:237.
91 Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg. 1995;121:1386.
92 Fried MP. A survey of the complications of laser laryngoscopy. Arch Otolaryngol. 1984;110:31.
93 Cozine K, Stone JG, Shulman S, Flaster ER. Ventilatory complications of carbon dioxide laser laryngeal surgery. J Clin Anesth. 1991;3:20.
94 Andrews AH, Jr., Goldenberg RA, Moss HW, et al. Carbon dioxide laser for laryngeal surgery. Surg Ann. 1974;6:459.
95 Cork RC. Anesthesia for otolaryngologic surgery involving use of a laser. In: Brown BR, Jr. Anesthesia and ENT surgery. Philadelphia: FA Davis, 1987.
96 Edelist G. Anaesthesia for endoscopy and laser surgery. Can Anaesth Soc J. 1984;31:1.
97 Sosis MB. What is the safest endotracheal tube for Nd-YAG laser surgery? A comparative study. Anesth Analg. 1989;69:802.
98 Hirshman CA, Smith J. Indirect ignition of the endotracheal tube during carbon dioxide laser surgery. Arch Otolaryngol. 1980;106:639.
99 Kaeder CS, Hirshman CA. Acute airway obstruction: A complication of aluminum tape wrapping of tracheal tubes in laser surgery. Can Anaesth Soc J. 1979;26:138.
100 Soong WJ, Lee YS, Soong YH, et al. Tracheal foreign body after laser supraglottoplasty: A hidden but risky complication of an aluminium foil tape-wrapped endotracheal tube. Int J Pediatr Otorhinolaryngol. 2010;74:1432.
101 Rampil IJ. Anesthesia for laser surgery. In: Miller RD, ed. Miller’s anesthesia. ed 7. Philadelphia: Churchill Livingstone; 2010:2405–2418.
102 Sosis MB. Evaluation of five metallic tapes for protection of endotracheal tubes during CO2 laser surgery. Anesth Analg. 1989;68:392.
103 LeJeune FE, Jr., Guice C, LeTard F, et al. Heat sink protection against lasering endotracheal cuffs. Ann Otol Rhinol Laryngol. 1982;91:606.
104 Sosis MB, Dillon FX. Saline-filled cuffs help prevent laser-induced polyvinylchloride endotracheal tube fires. Anesth Analg. 1991;72:187.
105 Sosis MB. Saline soaked pledgets prevent carbon dioxide laser–induced endotracheal tube cuff ignition. J Clin Anesth. 1995;7:395.
106 Norton ML, de Vos P. New endotracheal tube for laser surgery of the larynx. Ann Otol Rhinol Laryngol. 1978;87:554.
107 Skaredoff MN, Poppers PJ. Beware of sharp edges in metal endotracheal tubes. Anesthesiology. 1983;58:595.
108 Woo P, Strong MS. Venturi ventilation through the metal endotracheal tube: A nonflammable system. Ann Otol Laryngol. 1983;92:405.
109 Hunsaker DH. Anesthesia for microlaryngeal surgery: The case for subglottic jet ventilation. Laryngoscope. 1994;104(Suppl 65):1.
110 Ossoff RH. Laser safety in otolaryngology–head and neck surgery: Anesthetic and educational considerations for laryngeal surgery. Laryngoscope. 1989;99(Suppl 48):1.
111 Sosis M. Airway fire during carbon dioxide laser surgery using a Xomed laser endotracheal tube. Anesthesiology. 1990;72:747.
112 Sosis MB. Which is the safest endotracheal tube for use with the CO2 laser? A comparative study. J Clin Anesth. 1992;4:217.
113 Dillon F, Sosis M, Heller S. Evaluation of a new foil wrapped endotracheal tube for laser airway surgery. Anesthesiology. 1991;75:A392.
114 Ossoff RH, Aly A, Gonzales D, et al. A new endotracheal tube for carbon dioxide and KTP laser surgery of the aerodigestive tract. Otolaryngol Head Neck Surg. 1993;108:96.
115 Heyman DM, Greenfeld AL, Rogers JS, et al. Inability to deflate the distal cuff of the laser-flex tracheal tube preventing extubation after laser surgery of the larynx. Anesthesiology. 1994;80:236.
116 Mallinckrodt anesthesiology products. St Louis: Mallinckrodt, 1990.
117 Sosis MB, Pritikin JB, Caldarelli DD. The effect of blood on laser-resistant endotracheal tube combustion. Laryngoscope. 1994;104:829.
118 Sosis M, Dillon F. Reflection of CO2 laser radiation from laser-resistant endotracheal tubes. Anesth Analg. 1991;73:338.
119 Safety design can’t eliminate endotracheal tube fires. Clin Laser Mon. 1990;8:148.
120 Sosis M, Braverman B, Ivanovich AD. Evaluation of a new laser-resistant fabric and copper foil wrapped endotracheal tube. Anesthesiology. 1993;79:A536.
121 Sosis M, Kelanic S, Caldarelli DD. An in vitro evaluation of a new laser resistant endotracheal tube: The Rusch Lasertubus. Anesthesiology. 1997;87:A483.
122 Jacobs JS, Lewis MC, DeSouza GJ, et al. Crimping of a laser tube resulting in hypoxemia. Anesthesiology. 1999;91:898.
123 Macintosh R, Mushin WW, Epstein HG. Physics for the anaesthetist. Oxford: Blackwell; 1963.
124 Chilcoat RT, Byles PH, Kellman RM. The hazard of nitrous oxide during laser endoscopic surgery. Anesthesiology. 1983;59:258.
125 Wolf GL, Simpson JI. Flammability of endotracheal tubes in oxygen and nitrous oxide enriched atmosphere. Anesthesiology. 1987;67:236.
126 Pashayan AG, Gravenstein JS. Helium retards endotracheal tube fires from carbon dioxide lasers. Anesthesiology. 1985;62:274.
127 Al Haddad S, Brenner J. The effect of helium on endotracheal tube flammability during KTP/532 laser use. Anesthesiology. 1990;73:A491.
128 Simpson JI, Schiff GA, Wolf GL. The effect of helium on endotracheal tube flammability. Anesthesiology. 1990;73:538.
129 MacDonald AG. A short history of fires and explosions caused by anaesthetic agents. Br J Anaesth. 1994;72:710.
130 Leonard PF. The lower limits of flammability of halothane, enflurane, and isoflurane. Anesth Analg. 1975;54:238.
131 Wallin RF, Regan BM, Napoli MD, et al. Sevoflurane: A new inhalational anesthetic agent. Anesth Analg. 1975;54:758.
132 Schramm VL, Jr., Mattox DE, Stool SE. Acute management of laser-ignited intratracheal explosion. Laryngoscope. 1981;91:1417.
133 From Sosis MB. Probl Anesth. 1993;7:160.
134 Chee WK, Benumof JL. Airway fire during tracheostomy: Extubation may be contraindicated. Anesthesiology. 1998;89:1576.
135 Van Der Spek AF, Spargo PM, Norton ML. The physics of lasers and implications for their use during airway surgery. Br J Anaesth. 1988;60:709.
136 Benumof JL. Airway exchange catheters for safe extubation: The clinical and scientific details that make the concept work. Chest. 1997;111:1483.
137 Brain AI. The laryngeal mask—A new concept in airway management. Br J Anaesth. 1983;55:801.
138 Brimacombe J, Tucker P, Simons S. The laryngeal mask airway for awake diagnostic bronchoscopy: A retrospective study of 200 consecutive patients. Eur J Anaesthesiol. 1995;12:357.
139 Ferson DZ, Nesbitt JC, Nesbitt K, et al. The laryngeal mask airway: A new standard for airway evaluation in thoracic surgery. Ann Thorac Surg. 1997;63:768.
140 Sher M, Brimacombe J, Laing D. Anaesthesia for laser pharyngoplasty—A comparison of the tracheal tube with the reinforced laryngeal mask airway. Anaesth Intensive Care. 1995;23:149.
141 Slinger P, Robinson R, Shennib H, et al. Case 6–1992. Alternative technique for laser resection of a carinal obstruction. J Cardiothorac Vasc Anesth. 1992;6:749.
142 Duhamel DR, Harrell JH, 2nd. Laser bronchoscopy. Chest Surg Clin North Am. 2001;11:769.
143 Pennant JH, Gajraj NM, Miller JF. Resistance of the laryngeal mask airway to CO2 laser. Anesthesiology. 1993;79:A1055.
144 Brimacombe J. The incendiary characteristics of the laryngeal and reinforced laryngeal mask airway to CO2 laser strike—A comparison with two polyvinyl chloride tracheal tubes. Anaesth Intensive Care. 1994;22:694.
145 Pandit JJ, Chambers P, O’Malley S. KTP laser-resistant properties of the reinforced laryngeal mask airway. Br J Anaesth. 1997;78:594.
146 Keller C, Brimacombe J, Coorey A, et al. Liability of laryngeal mask airway devices to thermal damage from KTP and Nd:YAG lasers. Br J Anaesth. 1999;82:291.
147 Asai T, Koga K, Morris S. Damage to the laryngeal mask by residual fluid in the cuff. Anaesthesia. 1997;52:977.
148 Biro P. Damage to laryngeal masks during sterilization. Anesth Analg. 1993;77:1079.
149 Coorey A, Brimacombe J, Keller C. Saline as an alternative to air for filling the laryngeal mask airway cuff. Br J Anaesth. 1998;81:398.
150 Rontal M, Rontal E, Wenokur ME, et al. Anesthetic management for tracheobronchial laser surgery. Ann Otol Rhinol Laryngol. 1986;95:556.
151 Parsons RB, Milestone BN, Adler LP. Radiographic assessment of airway tumors. Chest Surg Clin North Am. 2003;13:63.
152 Pinsonneault C, Fortier J, Donati F. Tracheal resection and reconstruction. Can J Anaesth. 1999;46:439.
153 Calder I, Yentis SM. Could ‘safe practice’ be compromising safe practice? Should anaesthetists have to demonstrate that face mask ventilation is possible before giving a neuromuscular blocker? Anaesthesia. 2008;63:113.
154 Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology. 2006;105:885.
155 Langeron O, Massa E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1217.
156 Stacey S, Hurley E, Bush A. Sedation for pediatric bronchoscopy. Chest. 2001;119:316.
157 Slonim AD, Ognibene FP. Amnestic agents in pediatric bronchoscopy. Chest. 1999;116:1802.
158 Quintal MC, Cunningham MJ, Ferrari LR. Tubeless spontaneous respiration technique for pediatric microlaryngeal surgery. Arch Otolaryngol Head Neck Surg. 1997;123:209.
159 George PJ, Garrett CP, Nixon C, et al. Laser treatment for tracheobronchial tumours: Local or general anaesthesia? Thorax. 1987;42:656.
160 Meretoja OA, Taivainen T, Raiha L, et al. Sevoflurane–nitrous oxide or halothane–nitrous oxide for paediatric bronchoscopy and gastroscopy. Br J Anaesth. 1996;76:767.
161 Conacher ID. Anaesthesia and tracheobronchial stenting for central airway obstruction in adults. Br J Anaesth. 2003;90:367.
162 Vilaseca-González I, Bernal-Sprekelsen M, Blanch-Alejandro JL, et al. Complications in transoral CO2 laser surgery for carcinoma of the larynx and hypopharynx. Head Neck. 2003;25:382.
163 Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med. 2001;344:740.
164 Meier S, Geiduschek J, Paganoni R, et al. The effect of chin lift, jaw thrust, and continuous positive airway pressure on the size of the glottic opening and on stridor score in anesthetized, spontaneously breathing children. Anesth Analg. 2002;94:494.
165 Rita L, Seleny F, Holinger LD. Anesthetic management and gas scavenging for laser surgery of infant subglottic stenosis. Anesthesiology. 1983;58:191.
166 Eger E, II., Weiskopf RB, Eisendraft JB. The pharmacology of inhaled anesthetics. Round Lake, IL: Baxter Healthcare; 2002.
167 Weisberger EC, Miner JD. Apneic anesthesia for improved endoscopic removal of laryngeal papillomata. Laryngoscope. 1988;98:693.
168 Klein U, Karzai W, Gottschall R, et al. Respiratory gas monitoring during high-frequency jet ventilation for tracheal resection using a double-lumen jet catheter. Anesth Analg. 1999;88:224.
169 Oliverio R, Jr., Ruder CB, Fermon C, et al. Pneumothorax secondary to ball-valve obstruction during jet ventilation. Anesthesiology. 1979;51:255.
170 Shikowitz MJ, Abramson AL, Liberatore L. Endolaryngeal jet ventilation: A 10-year review. Laryngoscope. 1991;101:455.
171 Sanders RD. Two ventilating attachments for bronchoscopes. Del Med J. 1967;39:170.
172 Orloff LA, Parhizkar N, Ortiz E. The Hunsaker Mon-Jet ventilation tube for microlaryngeal surgery: Optimal laryngeal exposure. Ear Nose Throat J. 2002;81:390.
173 Ross-Anderson DJ, Ferguson C, Patel A. Transtracheal jet ventilation in 50 patients with severe airway compromise and stridor. Br J Anaesth. 2011;106:140.
174 Chang JL, Meeuwis H, Bleyaert A, et al. Severe abdominal distention following jet ventilation during general anesthesia. Anesthesiology. 1978;49:216.
175 Robinson RJ. One-lung ventilation for thoracotomy using a Hunsaker jet ventilation tube. Anesthesiology. 1997;87:1572.
176 Hautmann H, Gamarra F, Henke M, et al. High frequency jet ventilation in interventional fiberoptic bronchoscopy. Anesth Analg. 2000;90:1436.
177 Medici G, Mallios C, Custers WT, et al. Anesthesia for endobronchial laser surgery: A modified technique. Anesth Analg. 1999;88:298.
178 Bourgain JL, Desruennes E, Fischler M, et al. Transtracheal high frequency jet ventilation for endoscopic airway surgery: A multicentre study. Br J Anaesth. 2001;87:870.
179 Gottschalk A, Mirza N, Weinstein GS, et al. Capnography during jet ventilation for laryngoscopy. Anesth Analg. 1997;85:155.
180 Spackman DR, Kellow N, White SA, et al. High frequency jet ventilation and gas trapping. Br J Anaesth. 1999;83:708.
181 Bacher A, Pichler K, Aloy A. Supraglottic combined frequency jet ventilation versus subglottic monofrequent jet ventilation in patients undergoing microlaryngeal surgery. Anesth Analg. 2000;90:460.
182 Baer GA. No need for claims: Facts rule performance of jet ventilation. Anesth Analg. 2000;91:1040.
183 Ng A, Russell WC, Harvey N, et al. Comparing methods of administering high-frequency jet ventilation in a model of laryngotracheal stenosis. Anesth Analg. 2002;95:764.
184 Patel A, Randhawa N, Semenov RA. Transtracheal high frequency jet ventilation and iatrogenic injury. Br J Anaesth. 2002;89:184.
185 Venuta F, Rendina EA, De Giacomo T, et al. Nd:YAG laser resection of lung cancer invading the airway as a bridge to surgery and palliative treatment. Ann Thorac Surg. 2002;74:995.
186 Morris CD, Budde JM, Godette KD, et al. Palliative management of malignant airway obstruction. Ann Thorac Surg. 2002;74:1928.
187 Wood DE. Management of malignant tracheobronchial obstruction. Surg Clin North Am. 2002;82:621.
188 Stanopoulos IT, Beamis JF, Jr., Martinez FJ, et al. Laser bronchoscopy in respiratory failure from malignant airway obstruction. Crit Care Med. 1993;21:386.
189 Prakash UB. Advances in bronchoscopic procedures. Chest. 1999;116:1403.
190 Colt HG, Harrell JH. Therapeutic rigid bronchoscopy allows level of care changes in patients with acute respiratory failure from central airways obstruction. Chest. 1997;112:202.
191 Rimell FL. Pediatric laser bronchoscopy. Int Anesthesiol Clin. 1997;35:107.
192 McQueen CT, Cullen RD. Endoscopic ablation of distal tracheal lesions using Nd:YAG contact laser. Int J Pediatr Otorhinolaryngol. 2003;67:181.
193 Ayers ML, Beamis JF, Jr. Rigid bronchoscopy in the twenty-first century. Clin Chest Med. 2001;22:355.
194 Brownlee KG, Crabbe DC. Paediatric bronchoscopy. Arch Dis Child. 1997;77:272.
195 Benumof J. Anesthesia for thoracic surgery. Philadelphia: WB Saunders; 1987.
196 Wain JC. Rigid bronchoscopy. The value of a venerable procedure. Chest Surg Clin North Am. 2001;11:691.
197 Chan AL, Tharratt RS, Siefkin AD, et al. Nd:YAG laser bronchoscopy. Rigid or fiberoptic mode? Chest. 1990;98:271.
198 Plummer S, Hartley M, Vaughan RS. Anaesthesia for telescopic procedures in the thorax. Br J Anaesth. 1998;80:223.
199 Cohen S, Pine H, Drake A. Use of rigid and flexible bronchoscopy among pediatric otolaryngologists. Arch Otolaryngol Head Neck Surg. 2001;127:505.