CHAPTER 29 Anesthesia for Conjoined Twins
This rare but fascinating congenital problem is of considerable importance to anesthesiologists. In recent medical literature, there are ever-increasing reports of anesthesia for conjoined twins, both general and regional. From the twins’ initial admission, anesthesiologists should be involved in the care and decision-making procedures planned for these infants. At any stage of their time in the hospital, anesthesiologists may be called on to provide resuscitation for the infants or to anesthetize them for investigations or procedures. These may be related to their conjoined state or for some other pathology in one or the other twin, such as pyloric stenosis, or adenoidectomy for upper airway obstruction (Thomas, 2004). Management of conjoined twins is a multidisciplinary exercise involving many specialties, and the anesthesiologist is an integral part of this team. Likewise, it is essential that the anesthesiologists establish a team approach within their own discipline, so that each specialist knows precisely the roles of all individuals at the various stages of the procedures. The separation of a set of conjoined twins is the best example of teamwork that any hospital staff will experience.
Four major centers in the world have described their experiences and outcomes in their management of conjoined twins (Spitz and Kiely, 2003; O’Neill, 1998; Rode et al., 2006; Millar et al., 2009; Rabeeah, 2006) (Table 29-1). Many of the cases and examples used and described in this chapter are from the Red Cross War Memorial Children’s Hospital in Cape Town, South Africa, where over a period of 44 years (1964 to 2009) 49 sets of symmetric and asymmetric conjoined twins have been managed.
Incidence
Because of the significant number of conjoined twins who are aborted or who are stillborn, the exact incidence is not known, but the reported incidence worldwide is estimated at 1:50,000 to 1:100,000 live births, with a higher incidence of 1:14,000 to 1:25,000 experienced in Africa and Asia (Hoyle, 1990). Of those that are born, many may have congenital abnormalities that are incompatible with life. The presence of associated congenital anomalies in live twins is common. Congenital diaphragmatic hernia, pulmonary hypoplasia, congenital heart disease, anomalous hepatic arterial and venous drainage, biliary tree anomalies, bowel atresia, Meckel’s diverticulum, complex urogenital anatomy, and spinal dysraphism have all been reported.
Historical perspectives
Conjoined twins are one of nature’s greatest enigmas. Cave drawings, pottery, figurines, and folklore indicate that conjoined twins have occurred since prehistoric times. Embryonic duplication also occurs in plant and other animal forms of life (Fig. 29-1) (Thomas and Lopez, 2004). The famous and most celebrated pair of conjoined twins, Chang and Eng Bunker, who were joined at their xiphisterna, was born in Siam (now Thailand) in 1811. Because of their fame and the rarity of this pathology, the term Siamese twins has become synonymous with conjoined twins. Over time, the bridge of skin and tissue between them stretched, and they were able to stand side by side. The twins were taken to America, where they were on display by Phineas T. Barnum in his circus. Surgery for separation was deemed too risky unless one of the twins was to die. The Bunker twins lived very interesting lives—they married sisters and fathered 22 children between them. They died within hours of each other at the age of 63 (Spitz, 2003). Interestingly, the twins who have become most famous have all remained conjoined, whereas there is very little literature on the lives of those who have been separated. Votteler and Lipsky (2005) have reported on the long-term results of ten separations, as have Hoyle and Thomas (1989) with their experiences studying ischiopagus twins over 23 years. The first set of omphalopagus twins separated at the Red Cross Children’s Hospital now each has children of their own (Cywes and Louw, 1967; Cywes et al., 1982).
The first successful separation of conjoined twins was performed by Konig in 1689, when he separated omphalopagus twins by slowly tightening an encircling band around the connection until it necrosed the connecting bridge. The first successful separation of thoracopagus twins with a conjoined heart was reported in 1979 when, after the interatrial bridge was interrupted, there was a single survivor (Synhorst et al., 1979).
During the 1960s and 1970s, emphasis in the perioperative management of conjoined twins was on preoperative discussions and planning, dress rehearsals, and on the then-new techniques of invasive intraoperative monitoring for blood loss and hemodynamic status (Diaz and Furman, 1987). From the 1980s onward, most improvements have occurred in obstetric and fetal ultrasound; fetal and neonatal surgery; magnetic resonance imaging (MRI); contrast computed tomography (CT); numerous radiographic technological options to help delineate anatomic variations in different twins; in the anesthetic advances of oximetry, capnography, agent analysis; and in techniques for monitoring brain oxygenation and blood flow.
For the induction of omphalopagus twins, Allen et al. (1959) described the use of cyclopropane and oxygen, supplemented by ether via the open-drop method. He describes the attempts at intubation by holding one infant above the other to facilitate easier intubation as a “near fatal mistake.” In this position, the upper twin became pale and apneic, whereas the condition of the lower infant rapidly deteriorated and it became plethoric. Once they were returned to their normal position, they were successfully intubated. He comments that “there are few vital signs available for monitoring infants of this size. The most important signs are respirations, color, and muscle tone.” Both infants survived the surgical separation.
The anesthesia provided to separate the first set of twins in 1967 in Cape Town, South Africa, was described as “uneventful” (Cywes and Louw, 1967). Preoperative investigations in these omphalopagus twins indicated that there was no cross-circulation between the infants and this was confirmed at the induction of anesthesia, when the pancuronium given to one infant had no effect on the other.
In Furman’s report (Furman et al., 1971), intubation on a set of xiphopagus twins was performed when both infants were awake, and vascular cannulation was done when they were under local anesthetic. Jarem (Jarem et al., 1977), in his classic paper, describes the double breathing circuit fashioned from readily available parts of anesthetic equipment.
Roy (1980) described his experience with craniopagus twins. His group used two anesthetic teams, but only one anesthetic machine with a non–rebreathing Jackson-Rees breathing system from the gas outlet leading to two T pieces. Monitoring included: electrocardiograph (ECG), Doppler arm-cuff pressures, esophageal stethoscopes, and rectal telethermometers. Each infant received preoperative atropine, and they were induced simultaneously with halothane, nitrous oxide, and oxygen. He continued to describe the 15-month preparation time before their separation. This was successfully achieved, but he describes the anesthetic challenges as severe hemorrhage and maintenance of a clear airway with obstruction from secretions, kinking, or accidental extubation. Harrison and his team (1985) commented on the need for modification of the breathing circuit to accommodate the process of ventilating neonatal conjoined twins simultaneously and stated that adequate intravascular fluid replacement was essential to prevent hypotension at separation. They also commented on the intraoperative challenges of these infants.
Diaz and Furman, (1987), in their well-known paper on the perioperative management of conjoined twins, highlighted many factors that are now integral to the care of these patients. Advances in anesthetic equipment, monitoring, and drugs (inhalational or intravenous) give us options for anesthesia that earlier anesthetic specialists did not have. Similarly, outcomes for surgical procedures in these patients have improved. The developments in pediatric intensive care have had a positive impact on both the preoperative and postoperative care and outcomes of these children.
Embryology
Two theories have been proposed as to the etiology of conjoined twins: fusion and fission. Although the differences between these theories have yet to be resolved, fission is the generally accepted and older theory, where the fertilized egg fails to split completely (Kaufman, 2004). The theory of fusion, proposed by Spencer (2000a, 2000b, 2003), suggests that the fertilized egg splits completely, but a secondary union of two separate embryonic discs at the dorsal neural tube or ventral yolk sac takes place at 3 to 4 weeks’ gestation. This theory was also held by Aristotle (Millar et al., 2009).
Conjoined twins are identical, with what has always been thought to be an identical chromosomal pattern. With new molecular genetics, this has been shown to not necessarily be the case, and the twins may neither be completely identical nor have the same chromosome pattern (Hall, 2003). It has been hypothesized that monozygotic twinning occurs at four possible phases: up to 3 days’ postconception, after 4 to 6 days, after 7 to 12 days, and finally after 13 to 17 days. It is during this latter period that conjoined twins, fetus-in-fetu, or a teratoma may arise (Hall and Lopez-Rangel, 1996). Parasitic twins are thought to result from the embryonic death of one twin, with the remaining parts of the body vascularized by the surviving autocyte. Fetus-in-fetu are asymmetric monozygotic diamniotic intraparasitic twins (Hall and Lopez-Rangel, 1996). Conjoined triplets and quadruplets have been documented, although all have been aborted (O’Neill, 1998; Rode et al., 2006). These cases are exceptionally rare, with an even more obscure pathogenesis.
Conjoined twins are always the same gender. There is a 3:1 female-to-male incidence in live births, but more males are stillborn (Hoyle, 1990; Rejjal et al., 1992). They are monozygotic, monoamniotic, and monochorionic. Despite this, the infants usually vary in size, appearance, personality, internal anatomy, and degree of organ-sharing and duplication. Many of the challenges for all disciplines relate to the vast diversity in the anatomic variations that may possibly occur. Organs may be conjoined, duplicated, or absent. Investigations are crucial to clarifying these differences in order to make decisions about surgery, interventions, and survival. Even then, surprises occur at the time of surgery, and innovative techniques may be necessary.
Survival depends on the site and complexity of conjunction, the degree of organ sharing, and the presence of other congenital anomalies, with the extent of cardiac fusion and lung development being major determining factors (Andrews et al., 2006).
Classification, nomenclature, and terminology
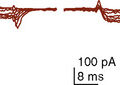
Conjoined twins are typically classified by the anatomic site of conjunction (which may be complex) followed by the suffix -pagus—the Greek word meaning that which is joined (Box 29-1). There is an international collaboration attempting to create a more uniform classification of conjoined twins (Spencer, 1996). This considers a ventral (front, caudal, or lateral) or dorsal (back-to-back, sacrum-to-sacrum, or head-to-head) fusion, with further classification described in the following paragraphs (Fig. 29-2).
| VENTRAL UNION (87%) | DORSAL UNION (13%) |
| Rostral (48%) | Craniopagus (5%) |
| Cephalopagus (11%) | Rachipagus (2%) |
| Thoracopagus (19%) | Pygopagus (6%) |
| Omphalopagus (18%) | |
| Caudal (11%) | |
| Ischiopagus | |
| Lateral (28%) | |
| Parapagus | |
| Dipagus | |
| Dicephalus |
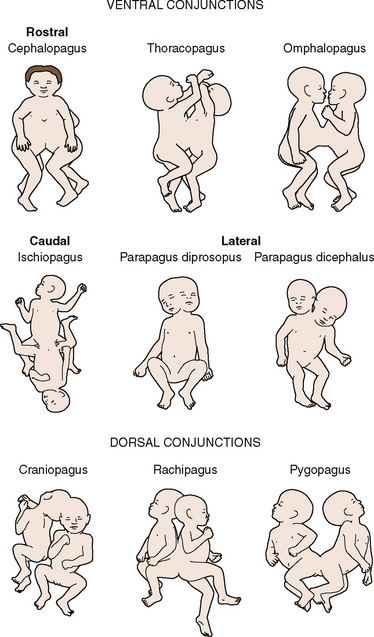
FIGURE 29-2 Illustrations depicting the anatomic relationships of the different types of symmetric conjoined twins.
(Modified from Spencer R: Conjoined twins: developmental malformation and clinical implications, Baltimore, 2003, John Hopkins University Press.)
Twins are described as being symmetric or asymmetric, although these terms are often misleading, because even when the twins are fully formed they are seldom truly symmetric. According to the most prominent site of conjunction, the following sites of conjunction are the most common: the chest (thoracopagus twins), the abdomen (omphalopagus twins), sacrum (pygopagus twins), pelvis (ischiopagus twins), and back (rachipagus twins) (Table 29-2).
| Type | Area(s) Conjoined | Consequences |
| Thoracopagus Thoracoomphalopagus |
Upper to lower chest Heart and pericardium always involved Chest and abdomen |
High mortality Usually face-to-face Complex anatomy of heart, diaphragm, GI tract |
| Thoraco-omphaloischiopagus | Chest, abdomen, and pelvis | Face-to-face Four arms; two, three, or four legs; variable genitalia |
| Xiphopagus | Xiphoid cartilage, upper abdomen | Possible liver fusion Simple conjunction |
| Omphalopagus | Lower chest, upper abdomen | Heart not involved Liver, proximal GI tract, diaphragm, other organs variable |
| Ischiopagus | Fused lower bodies, spinal and urogenital system involvement | Four arms; two, three, or four legs |
| Pygopagus (pyopagus/ileopagus) | Back-to-back at pelvis Sacrum and coccyx fused |
Spine often involved, distal GI tract often fused |
| Parapagus | Fused side-by-side with a shared pelvis Dithoracic: fused abdomen pelvis, not thorax Diprosopic: one trunk, one head, two faces with varying fusion Dicephalic: one trunk, two heads, two, three, or four arms |
Organ sharing variable Limbs variable |
| Craniopagus | Fused skulls, separate bodies Fused back (occipital), front (frontal), side (temporoparietal) or top (parietal) of head, not face or base of skull |
Extent of brain fusion variable Venous connections important |
| Rachipagus | Dorsal fusion, back to back Face away from each other |
Extremely rare Spine involvement variable; fusion terminates above sacrum Occiput possibly involved |
GI, Gastrointestinal.
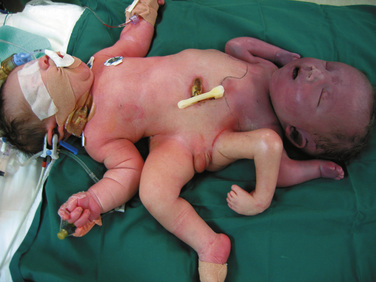
Symmetric twins are two anatomically identifiable infants with a physical appearance suggestive of two possible survivors. With asymmetric, or parasitic, twins, one twin is a potential survivor and usually appears normal, but the other is incomplete and attached as a parasite. This may be externally visible, or it may be internal. Where there is an attachment of an anatomically identifiable part, but not of a total individual, the term heteropagus is used. It is in this group, that regional anesthesia is particularly useful. Within a classified group, especially with complex conjunctions, the external physical appearance and spatial arrangement of the infants may be very different (Figs. 29-3 and 29-4).
FIGURE 29-3 These ischiopagus twin girls were born via vaginal delivery to a very surprised mother and her midwife.

FIGURE 29-4 This set of twin boys has a complex conjunction at the thoracic, abdominal and pelvic levels, and despite also being ischiopagus, show no physical resemblance to the twins in Figure 29-3.
The degree of cardiac fusion, or degree of cardiopagus, can be considered as follows (Andrews et al., 2006):
A: Separate hearts and pericardium
B: Separate hearts and a common/shared pericardium
C: Fused atria and separate ventricles
Outcomes for separation in groups C and D are poor.
Twins Joined at the Head
There are three types of twins joined at the head (Spencer, 2003).
Craniopagus twins are united only at the cranial vault, with two completely separate faces and bodies. O’Connell (1976) described a practical approach to classifying craniopagus twins—partial and total. In the partial form, union is limited and separation is feasible with a good chance of survival; however, in the total form, significant intracranial abnormalities may be present and make attempted separation hazardous. Blood loss may be considerable, making death(s) on the operating table highly likely. Bucholz et al. (1987) reported higher perioperative mortality in the temporoparietal and occipital junctions, with parietal junctions having an intermediate mortality, and frontal craniopagus having the lowest mortality. Winston (1987) described a classification based on the deepest structures shared:
Ethics
In broad terms, ethics are a set of standards of professional conduct (Atkinson, 2004). Whenever planned separation of conjoined twins takes place, and when there is a possibility or probability that one twin may not survive the procedure, moral, ethical, and legal arguments are raised. Many of these issues have been addressed in the literature (Pearn, 2001; Unknown author, 2000; Spitz and Kiely, 2000; Spitz, 2000; Bratton and Chetwynd, 2004). The Hastings report identified three cardinal issues (London and Knowles, 2001):
In his review of this subject, Atkinson (2004) commented that the birth of a handicapped child is an immense burden to any parents. If they are to avoid greater challenges, the clinicians require sensitivity and patience as they steer the family along a pathway to survival. The question most often asked is whether it is justified to sacrifice one life to save another, or should both infants be allowed to die? Answers are seldom simple, and each case should be assessed on its own merits. Decisions depend on the type and complexity of the conjunction, the overall health of both infants, the laws of the country, and the religion and beliefs of the parents. If the sacrifice of one twin is necessary for the other twin to survive, it is important to recognize that both would die without separation. If they were separated, at least one would live (O’Neill et al., 1988).
Ian Aird (1954, 1959) wrote that if there was a possibility of at least one twin surviving, surgery should be performed. The operation does not decide which twin will survive; this is determined by their relative conditions (O’Neill et al., 1988).
Great Ormond Street Ethical Guidelines for Conjoined Twin Separation has been adopted by a number of units. Where separation is feasible with a reasonable chance of success, it should be carried out. When surgery is not possible, custodial care should be offered, and nature should be allowed to take its course. When one twin is dead or has a lethal abnormality and cannot survive independently from its normal twin, and if there is no surgery both twins would die, separation to save the healthy twin should be attempted (Rode et al., 2006; Millar et al., 2009).
Perinatal considerations
Improved techniques of antenatal diagnosis and fetal imaging have allowed the diagnosis of conjoined twins to be made during pregnancy, so that counseling of the parents may allow them the option to have the pregnancy terminated. Conjoined twins can be identified as early as 11 weeks’ gestation, and in this series of cases studied by Sebire et al. (2000), all the parents opted for termination. Complex craniopagus and cardiopagus anatomy in particular may tilt the decision in this direction. Conjoined hearts are easier to study via ultrasound in utero, because the amniotic fluid acts as a buffer, whereas after birth the lungs inflate with air and prevent optimal visibility (Kingston et al., 2001).
Obstructed labor with difficult vaginal delivery may necessitate an emergency cesarean section, but this can be avoided by antenatal diagnosis and elective cesarean section at 36 to 38 weeks’ gestation (Millar et al., 2009). In many developing countries, the birth of conjoined twins may come as a surprise to the mother and the attending midwife or medical practitioner (Thomas, 2004; Thomas and Lopez, 2004).
Careful prenatal investigations may identify cases in which emergency separation at birth is life saving (MacKenzie et al., 2002). Advances in prenatal ultrasound, as well as the use of color-flow Doppler and prenatal MRI, have improved the antenatal diagnosis of conjoined twins. In particular, prenatal and postnatal echocardiography has been shown to accurately delineate the extent of cardiac fusion, the intracardiac anatomy, and the ventricular function (Andrews et al., 2006). Planning of the antenatal course and the perinatal management of twins can be facilitated by identifying those twins at particular risk, such as twins with twin reversed–arterial perfusion sequence. In this situation, vascular communications between the two fetuses allows deoxygenated blood from one fetus (the pump twin) to perfuse the other fetus (the perfused twin), resulting in reversed flow in the umbilical vessels and the development of multiple anomalies, including acardia, in the perfused twin (Norwitz et al., 2000). Antenatal surgical intervention, removing the acardiac twin in utero, may allow for the survival of the remaining (pump) twin. If they are conjoined, however, this is not possible, and immediate surgical intervention at birth is necessary. In order to make rational and sound decisions as to the anesthetic management, anesthesia for fetoscopic fetal surgery requires knowledge of the pathophysiology of the fetus, the fetoplacental unit, and the condition of the mother (Galankin et al., 2000).
Plans may be necessary, in those units providing that option, for an ex utero intrapartum treatment (EXIT) procedure (Bouchard et al., 2002). In this operation, access to the fetus is achieved when the fetus is brought into the surgical field while it is still attached to the mother’s placenta. The planned procedure, whether for access to the fetal airway in the case of an airway tumor, or to allow separation of a conjoined twin from its non–survivable twin, is then carried out. Once it has been completed, the infant is then delivered from the mother and separated from its placenta. This procedure has also been used for airway control of both twins when the antenatal airway and cardiopulmonary status of the twins was not known (Ossowski and Suskind, 2005).
Cardiopulmonary resuscitation (CPR) in conjoined twins has significant limitations. Not only can it be physically challenging, but it may also be unreliable because the anatomy is distorted and access to the heart, especially in thoracopagus twins, is limited. Damage to other organs, particularly the upper gastrointestinal tract and liver, may occur (Millar et al., 2009). The basics of neonatal and pediatric resuscitation should be followed.
Medical and surgical management
Spitz and Kiely (2002) describe the management of conjoined twins in the prenatal and postnatal stages. When complex thoracopagus or craniopagus twins are diagnosed, termination of pregnancy is recommended. The postnatal management involves the following options:
Indications for emergency separation include the following (O’Neill, 1998, Millar et al., 2009; Rode et al., 2006; Cywes et al., 1997):
Elective separation for simple conjunctions can be performed in the neonatal period with minimal problems. No benefit is gained from waiting for the infants to grow or for further investigations to be done. The maternal hormonal influences are still present; thus, the skin is more pliable to cover the wound defect. In general, separation is planned for some time between 4 and 11 months of age when the infants are bigger and their investigations are more meaningful and when they have had adequate tissue expansion to allow closure of the skin defect (O’Neill et al., 1988).
Investigations
Accurate preoperative imaging is essential to surgical planning and prognosis, and the area(s) of fusion determine the imaging modality used. MRI and CT provide very good anatomic and bone detail and show organ positions, shared structures, and limited vascular anatomy (Kingston et al., 2001). Radiography with contrast material provides excellent gastrointestinal and urogenital evaluation. When there is liver conjunction, its anatomy, the vascular supply, and assessment of the biliary tree are required. Angiography helps clarify the vascular supply of organs and determine blood supply between the twins. Careful use of contrast material is important, because there may be overenthusiastic administration of this in an attempt to achieve better views. Evaluation of vascular shunts and cross circulation is vital for anesthesiologists—loss of blood from these during surgery can be catastrophic.
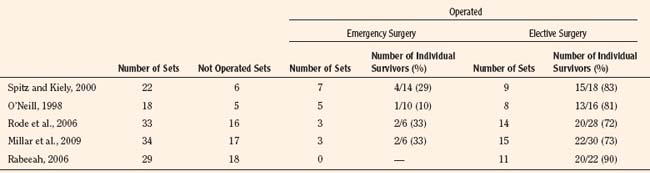
Cardiac catheterization is required to identify intracardiac connections and to clarify cardiac chambers. Echocardiographic technology is constantly improving, so the need for catheterization (and therefore anesthesia) is becoming less common. When cardiac surgery is required, however, this is the preferred investigation (Fig. 29-5).
Results of these tests clarify anatomic variations in each infant. Understanding the complexity of organ conjunctions enables investigators to identify those structures that are present or missing. The results of complex investigations are best evaluated by a multidisciplinary team, because many factors are raised that anesthesiologists do not consider but that are important for both anesthetic management and good overall care of the infants. Each test has its own role for all of these requirements. Box 29-2 details investigations for craniopagus twins. The order in which procedures occur is also determined by the results of the investigations.
Box 29-2 Investigations for Craniopagus Twins
Sedation Techniques for Investigations
Options for simple sedation used include a feeding before CT or MRI, sucrose on a pacifier, swaddling and physical support, chloral hydrate, trimeprazine (with or without droperidol), and midazolam (Thomas, 2004; Thomas and Lopez, 2004). Two pediatric anesthesiologists should be present from the beginning of these investigations, rather than them having to be called in an emergency situation.
Commonly used agents include propofol, ketamine, fentanyl, dexmedetomidine, and inhalational agents. For MRI evaluations, the safest option may be a general anesthetic to ensure airway control for the procedure (Sury et al., 1994). The use of laryngeal mask airways may not always provide the ideal airway management, and rescue maneuvers in this environment are fraught with potential complications (Shank et al., 2005; Szmuk et al., 2005).
Planning before surgery
Preparation of Parents
Congenital abnormalities in singleton children are challenging enough for parents; parenting a set of conjoined twins can be daunting, with the burden of decision making being overwhelming (Atkinson, 2004). This is especially problematic when one infant is not expected to survive or when both may die. Even for infants with a simple conjunction, every effort should be made to make this experience as atraumatic as possible. This may include the involvement of child-life specialists, play therapists, physiotherapists, occupational therapists, and social workers. Where physical challenges and deformities will be present after surgery, involvement of these caregivers before surgery is essential. These principles of care are often overlooked, and it may be necessary during planning meetings to remind the multidisciplinary team of their importance.
Preparation of the Environment and Equipment
When procedures on these twins are to be performed outside the operating rooms, these venues or areas should be visited and scrutinized for suitability for sedation, general anesthesia, and appropriate monitoring. If general anesthesia is planned, the supply of two sources of oxygen, medical air, nitrous oxide, suctioning, and scavenging must be available. It may be necessary to have the maintenance department make up equipment to facilitate this, such as splitters from the wall gas supply. Innovative techniques have been devised to provide ventilation of both twins with one ventilator using a Carlen’s Y-adaptor for ECG-gated MRI angiography (Szmuk et al., 2006). The use of bispectral index (BIS) for detecting cross circulation in thoracopagus twins is also described (Szmuk et al., 2006).
Multidisciplinary Team Preparation
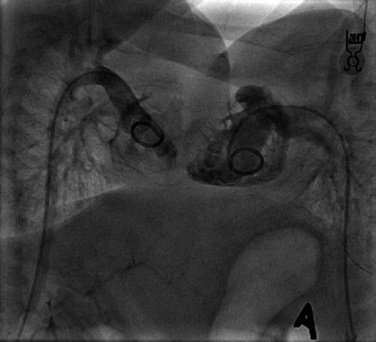
In multidisciplinary meetings, the following should be discussed: the results of all investigations of all disciplines; an assessment of how they overlap in the different specialties; the order in which these surgical specialties will operate; any planned changes in the positions of the infants; locations where intravenous lines may or may not be placed; any anticipated problems or concerns; and whether or not intensive care will be necessary after the procedure. An example to illustrate the importance of such a discussion is a pair of ischiopagus twins who require defunctioning colostomies for intestinal obstruction. The pediatric general surgeon needs to perform the colostomies, the urologist needs to do an examination of the urogenital system under anesthesia, the orthopedic surgeons and neurosurgeons may require input for assessment of the sacrum and pelvis, and finally, the otolaryngologic surgeon has to assess one of the twins for upper airway obstruction and possible adenoidectomy. The use of diagrams of the anatomy, three-dimensional or CT-reconstructed models, and rehearsals in the operating room allow the process to proceed as smoothly as possible (Fig. 29-6).
Anesthetic care of conjoined twins
General Principles
Anesthesia services for conjoined twins are required for various situations (Box 29-3). Anesthetizing conjoined twins is demanding and complex. The anesthesiologists must treat each child as a separate individual. The general principles of pediatric anesthesia, including factors predicting a difficult anesthesia, apply in all of these cases. Any deterioration in the condition of the twins should be anticipated, so intubation and emergency resuscitation measures can be taken in a controlled manner. Two anesthetic teams are always involved in the management of the two patients, with duplication of all equipment necessary for their care.
Anesthetic Challenges in the More Common Types of Conjoined Twins
Thoracopagus Conjunctions
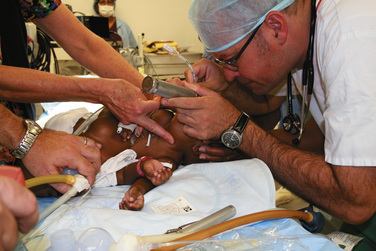
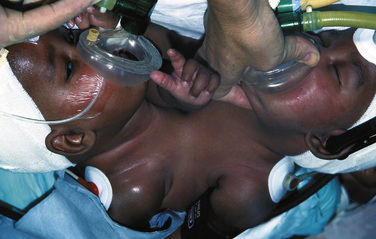
Airway management is difficult because of the extreme lordosis and hyperextension of the infants, with variability of the proximity of their faces (Fig. 29-7). The higher the conjunction, the more difficult airway access becomes. This improves as they grow. Emergency intubation in either or both twins should be avoided. Their deterioration and the need for intervention should be anticipated, and the procedures should be carried out in a quiet and controlled way. Intubation should be done on one infant at a time, taking care not to damage the other twin in the process. It is not advisable to paralyze either infant until there is airway support and control in both infants. Except in moribund patients, awake intubation may be very difficult. It is usually easier to intubate the infants when they are on their sides, turned with the face upward. Positioning one infant above the other causes adverse hemodynamic and respiratory consequences in both infants and is not recommended.
The use of laryngeal mask airways is not as successful in this group as in those twins whose heads face away from each other or in twins whose heads are at opposite ends of the body. Once the infants have been anesthetized, fiberoptic intubation has been used with success, but most intubations can be performed without it. It may be useful to insert a nasopharyngeal tube (a short endotracheal tube [ETT]) into one nostril to provide oxygen and an inhalational agent while attempting to intubate the trachea, either orally or nasally. Direct laryngoscopy and distal airway evaluation may be of value in the work-up before separation, but it is often difficult to insert a rigid bronchoscope without causing damage to the infants (Strocker et al., 2005). A fiberoptic technique is easier. There is little emphasis placed on investigating the respiratory systems of these infants. Lung abnormalities have been identified and include tracheomalacia and the presence of aberrant bronchi (Strocker et al., 2005).
Cardiac complications, especially cardiac failure in the neonatal period, are common. Anatomic possibilities include twins with a single normal heart, twins with two conventional hearts, or twins with compound hearts. The anatomy of major vessels may be extremely variable. With compound hearts, the extent of sharing may vary from minimal to almost complete (Gerlis et al., 1993). Coronary artery sharing usually coexists, even with minor venous sharing, and fusion may occur at the level of the sinus venosus, at the atrial level, or at the atrial and ventricular levels. Cruciate connections from the right atrium of one infant to the right atrium of the other, or from the left ventricle of one to the left ventricle of the other, may be present (Gerlis et al., 1993). Many of the components may only be determined at surgery (or postmortem). For technical reasons, it is often difficult to differentiate whether the ventricles are contiguous or fused.
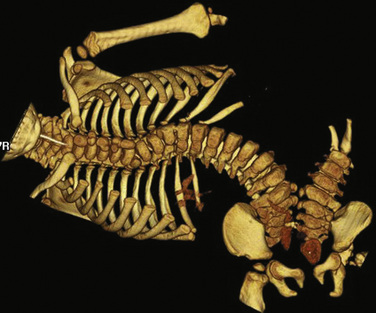
Electrocardiograms (ECGs) in these infants are interesting, but are not conclusive. The presence of two complexes does not rule out significant abnormalities, and a single complex does not necessarily mean significant sharing of heart structures. Reciprocal complexes, or those appearing as a mirror image, usually indicate shared hearts. (Fig. 29-8)
After separation, postoperative ventilation is generally the rule rather than the exception. The repair of deficits in the anterior chest wall needs to be planned, because most require some sort of structural support to facilitate normal ventilatory mechanics and to avoid the development of a flail segment (Hoshina et al., 1987).
Ischiopagus and Pygopagus Conjunctions
Especially in twins where posterior osteotomies are required for pelvic ring closure, blood loss may be extensive. This may also occur suddenly and unexpectedly with dural incision and when vascular structures are unpredictable (Fieggen et al., 2004).
Tethering of the cord, spinal dysraphism, cord fusion, and syringomyelia, in association with anorectal and urogenital abnormalities, are often encountered in ischiopagus, pygopagus, and dipagus twins (Peter et al., 1996). This may preclude the use of spinal or epidural anesthesia in these twins unless ultrasound views clearly identify the anatomy.
Neurosurgeons are experts in the management of craniopagus twins, but their expertise is also required for both ischiopagus and pygopagus conjoined twins who may have a variety of spinal abnormalities (Peter and Fieggen, 2004). These may present challenges both at the time of separation as well as for long-term management. In the presence of hemivertebrae, asymmetric or small chest cavities, or spinal anomalies, progressive scoliosis may develop and will require diligent follow-up care and possible intervention (Fig. 29-9) (Fieggen et al., 2004; Rode et al., 2006).
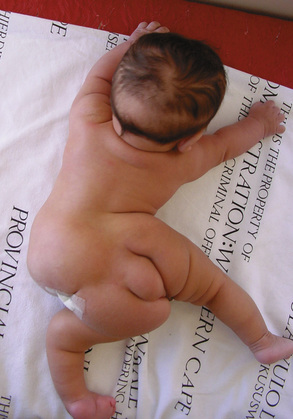
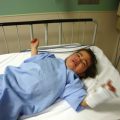
FIGURE 29-9 This is the surviving infant of ischiopagus twins with spinal deformities who requires follow-up surgery. His CT is shown in Figure 29-6.
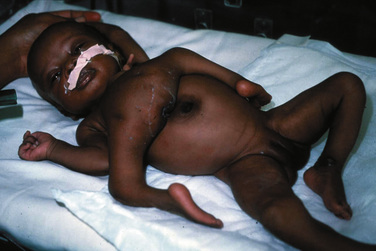
Duplication of an anatomic area (e.g., ischiopagus dipagus, a single trunk but four legs) is amenable to surgical correction with a good outcome (Fig. 29-10). Anesthetic problems in this group are similar to those that would be experienced with tumors of that region, namely vagal stimulation with manipulation of the mass and blood loss.
Craniopagus Conjunctions
Because of the proximity of the infants’ heads and faces, airway management of these twins is often a challenge. Stabilization of the ETT requires particular attention, and fluid or blood from the surgical field can result in dislodgement or displacement during surgery with potentially catastrophic consequences. Especially if significant flexion is anticipated, a reinforced ETT may be the best option. Many of the principles of pediatric neurosurgery apply, including the potential for venous air embolism, and these are affected by the complexity of the conjunction (Girshin et al., 2006). Walker and Browd (2004) provide a comprehensive approach to the embryology, classification, surgical anatomy, and separation of craniopagus twins.
If the venous sinuses of the twins are in communication in the fusion, blood loss may be enormous, and it is often this factor that limits the possibility of surgery for separation. The abnormal development of the arteriovenous system is one of the greatest challenges faced during the separation procedure. To reduce blood loss and allow each child to develop adequate independent vascular drainage and to prevent perioperative cerebral edema, staged separation may be indicated (Walker and Browd, 2004; Girshin et al., 2006). These staged operations have been attempted to encourage the development of a collateral circulation before final separation and to improve outcome.
Central vascular access may be limited to the femoral route.
Preoperative Evaluation
A thorough goal-directed review that includes the history of perinatal events and growth since birth, exposure to infectious diseases (e.g., human immunodeficiency virus), and whether or not and what prophylaxis was taken (mother-to-child transmission medication), as well as previous intubation experience for respiratory or cardiovascular pathology, is required. An understanding of the complexities of each investigation and the implications of these for surgery (and therefore anesthesia) is vital. It is especially important to understand the degree of cross circulation between the twins and at what anatomic level this may be present (e.g., heart, liver, brain, or bowel) (Table 29-3).
TABLE 29-3 Summary of Multidisciplinary Investigations Commonly Required for Conjoined Twins
| System | Investigation |
| Cardiorespiratory Cross circulation |
Radiography: total body X-ray Electrocardiogram Echocardiography/Doppler ultrasound in all conjoined twins MRI/CT with contrast Angiogram Radioisotope scan Tc-99-DMSA, radiolabeled albumin, or red blood cells45 |
| Alimentary tract | Contrast meal and enema Ultrasound Radioisotope scans53 (liver); technetium Tc-99m-(Sn), colloid and excretion Tc-99m mebrofenin Radioisotope scintigraphy |
| Genitourinary | Ultrasound Isotope renography Micturating cystourethrography Genitogram |
| Skeletal system | Radiography MRI (spinal cord) Ultrasound |
| Vascular | Doppler ultrasound Angiography |
Modified from Di Rocco C, Caldarelli M, Tamburrini G, et al: Craniopagus: the Thessaloniki-Rome experience, Childs Nerv Syst 20:576–586, 2004; Mann M, Coutts JP, Kaschula ROC, et al: The use of radionucleotides in the investigation of conjoined twins, J Nucl Med 24:479–484, 1984; Rode H et al: Four decades of conjoined twins at Red Cross Children’s Hospital: lessons learned, S Afr Med J 96(9):931, 2006; Rutka JT, Souweidane M, ter Brugge K, et al: Separation of craniopagus twins in the era of modern neuroimaging, interventional neuroradiology, and frameless stereotaxy, Childs Nerv Syst 20:587–592, 2004.
Physical Examination
Importance must be given to assessing the following:
The Airway
Thoracopagus Twins
Thoracopagus twins have difficult airways until proven otherwise. There is invariably some degree of opisthotonus and hyperextension of the head and neck, making visualization of the vocal cords very difficult. Plans for fiberoptic-assisted intubation may be necessary. In order to facilitate long-term ventilation, tracheostomy has been described in one set of twins, but this should not be regarded as the routine recommendation of airway management (Mair and Mair, 2006). Depending on the relative positions of the heads and faces, craniopagus airway access may also be problematic.
Mechanisms of Ventilation
It is important to ascertain whether or not the diaphragm is involved in the junction, or whether its function will be affected by surgery. In those twins where conjunction involves the chest wall and its contents, serious consideration needs to be given to breathing and ventilation. Lung compliance is affected, areas of atelectasis develop because of the limited space between the two infants and the abnormal anatomy of thoracic structures, the hearts are usually abnormal, and cardiac failure is common in thoracopagus twins. As one twin develops cardiorespiratory compromise with tachycardia, tachypnea, and coughing, the other is also affected. This has significant short- and long-term consequences and should be a major consideration when planning for separation. New synthetic substances that enable reconstruction of an unstable sternum and anterior chest wall are now available. Muscle flaps and skin grafts may be needed (Rabeeah, 2006). In the postoperative period, these grafts avoid the development of a flail chest, which causes significant respiratory compromise and affects long-term survival and quality of life. In cases where one twin has not survived, autologous tissue has been used to cover the deficit caused at separation (Rode et al., 2006; Spitz and Kiely, 2002; Fisherman et al., 2002).
Gastroesophageal Reflux
While they are waiting for separation, good nutrition is crucial to the infants’ growth. The body composition differs between the two twins, as does their resting energy expenditure and caloric intake. This difference stabilizes after separation, with both twins having similar requirements (Powis et al., 1999; Spitz and Kiely, 2002).
Skin Cover: Tissue Expanders
Tissue expanders are inserted to facilitate skin closure when surgery will leave a significant area uncovered. There are numerous reports in the literature of the use of tissue expanders in many sets of all types of conjoined twins. The advantage of their use includes the provision of autologous tissue to cover the defect, thus avoiding the need for the use of foreign material. The disadvantages are the effects of pressure exerted by the expanders in all directions on contiguous tissue where pressure is not desirable. Areas of skin covering the expanders may become necrotic or infected, and the expanders may need to be removed. The inward pressure may affect cardiorespiratory function and result in twins with preexisting lung disease requiring ventilation before separation (Losee et al., 2009). Immediately after insertion, intraabdominal tissue expanders may initially cause an ileus, and they may cause anorexia as the volume in the expanders increase. For craniopagus twins’ skin expansion, especially in younger infants, the pressure effects may impact the growth of underlying bone, resulting in a deformed skull. Depending on the age of the infants, the site chosen for placement of the expanders, the number of expanders inserted, the length of time they will be in place vary, as does the volume of fluid injected (100 to 1,000 mL) to expand the tissue sufficiently. The smaller the infant, the more critical is the volume injected. The shape of the expanders are determined by the age of the infants and the sites chosen for their placement. Depending on the decision of the surgeon, the expanders may remain in situ for anything from 6 weeks to several months.
Wound closure in craniopagus twins is critical, because skin, dura, and bone cover are necessary to prevent perioperative wound breakdown, cerebrospinal leaks, and meningitis. Skull defects may be left to granulate closed, or bone from other sites such as ribs may be used. Fascia lata may be used for dural cover, and prosthetic material (e.g., a titanium plate) may be considered. Malformation in the skull configuration may not allow or accommodate tissue expanders. The risks associated with the prolonged time necessary for tissue expansion may preclude their use (Walker and Browd, 2004).
The size of the deficit can be measured preoperatively by physical assessment of the size of the potential defect (measuring with a tape measure and three-dimensional CT reconstruction), as well as by the use of acrylic models (Campbell, 2004). This information may then be used to facilitate reconstruction.
Anesthesia for non–separation procedures
A variety of preseparation or palliative procedures and investigations can be expected. These may include surgery to remove an inappropriately sited limb, place tissue expanders, perform laparotomy for intestinal obstruction, divert stomas, repair an inguinal hernia in one twin, perform gastroschisis, complete urologic procedures such as vesicostomy, do an adenoidectomy for adenoidal hypertrophy and obstructive sleep apnea in one twin, and to repair necrotizing enterocolitis (Hoyle, 1990; O’Neill, 1998; Jaffray et al., 1999; Seefelder et al., 2003; Lonnee et al., 2007). The possible investigations are listed in Table 29-3. Anesthesia preparation and preoperative evaluation for these procedures should be based on the same principles of management as for separation, but the planning for separation involves a more comprehensive approach to the intraoperative and postoperative care.
The questions that need to be answered preoperatively are listed in Box 29-4. Routine pediatric anesthetic preoperative blood tests are required, and blood should be cross-matched according to the procedure planned.
A dead or dying twin, if not quickly separated from its twin, aggravates the acidosis and causes deterioration and death of both infants (Fig. 29-12). That error was made in one set of twins. By the time they were prepared for surgery nearly 24 hours after birth, one of the twins was regarded as moribund and was not resuscitated or ventilated in the intensive care unit, and this nearly resulted in the death of his brother. As the ill twin deteriorates and dies, there is extensive vasodilation and acidosis, so the intravascular volume of the surviving infant transfuses into the dead twin, causing the healthier twin to succumb. The biochemical challenges are similar to those of a reperfusion syndrome.
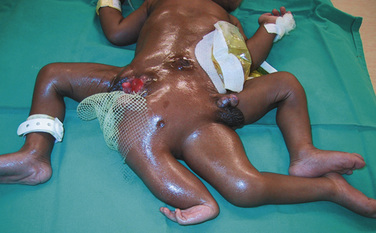
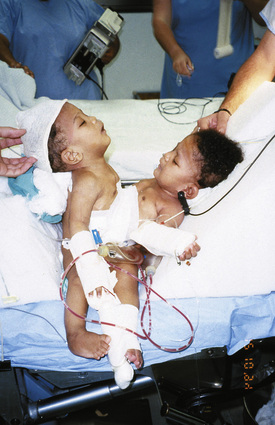
Airway difficulties are more common in those infants where the conjunction is high on the chest, where the heads face each other and are close together, where the head and facial anatomy is abnormal, when hyperextension of the head and neck with exaggerated lordosis is present, and where the parasite of a heteropagus twin is around the head, neck, or upper chest (Fig. 29-13). Vascular access is anticipated to be difficult in this group.
The need for cardiopulmonary bypass brings with it technical difficulties in inserting cannulae, space in the operating room, and all the consequences and complications of open heart surgery (Suan et al., 1998). Preoperative planning is critical for successful outcomes.
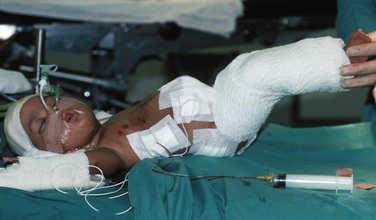
Support materials, such as sponge supports for upright twins or a special chair, may take time to prepare (Fig. 29-14) (Mair and Mair, 2006). When extra limbs or abnormally sited limbs are present, careful protection of these limbs during surgery should be ensured.
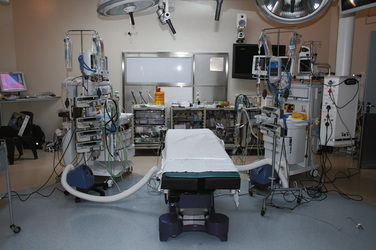
The operating room should be arranged to suit the type of twins; thoracopagus and craniopagus twins tend have the anesthetic machines at the same end of the table, whereas ischiopagus twins’ surgery necessitates that the machines be at opposite ends of the operating room table (Fig. 29-15).
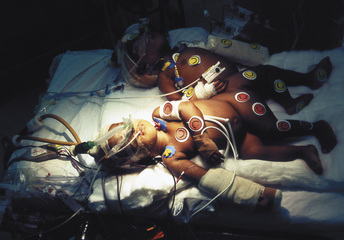
Color coding of each infant’s equipment with all the lines, monitors, equipment, and drugs is very useful (Fig. 29-16). Color coding for each team is also valuable, especially when there are two surviving infants to be operated on in two operating rooms or on two separate operating tables.
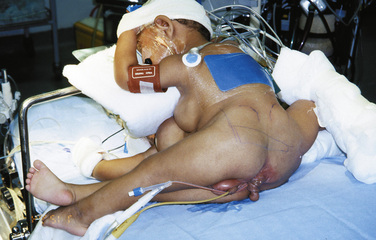
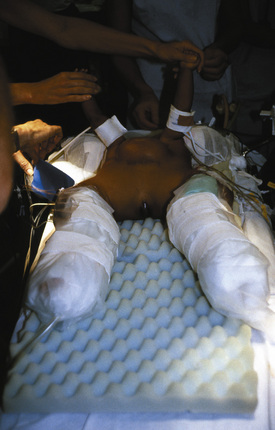
At the separation of ischiopagus or pygopagus twins, posterior osteotomies may be performed to facilitate pelvic ring closure (Verrier et al., 2000). The approach to the iliac spines is from the back of each infant, and appropriate positioning may be a problem. Positions are rehearsed when the infants are awake, and innovative plans may be necessary to achieve the correct positioning of the infants for surgery (Fig. 29-17). Postoperatively, the legs of the infants are anteromedially rotated and strapped in position for 6 weeks; this will impact their diaphragmatic excursions with the potential for atelectasis and pneumonia (Fig. 29-18). Physiotherapy is crucial to their recovery.
Anesthesia for separation
Nonemergency surgical separation is usually planned for between 4 and 11 months, when the infants are larger and the investigations have given information that is clearer and more meaningful. After this age, psychological issues with separation become more problematic (O’Neill et al., 1988). This operation is the culmination of a multidisciplinary preparation that hopefully will end in the successful separation of a pair of conjoined twins.
Premedication
Sedative or anxiolytic premedication is generally not required, but when the infants are older this may be a consideration. Each set of twins should be assessed individually. In older sets of twins, sedation options include midazolam, trimeprazine, chloral hydrate—each of these has been used successfully in some twins over 6 months of age (Thomas and Lopez, 2004). Atropine has been used for neonatal twins, but this is only necessary when vagal stimulation is likely to occur (e.g., with laryngoscopy or bronchoscopy) or when the use of ketamine is planned.
Transport
Transportation of the infants to operating room may well be a simple process, and they may be brought to the operating room door by the parents and accompanied by a nurse. In ill twins who have been in intensive care and who are intubated with all invasive catheter lines in place, transportation to the operating room involves the use of a transport monitor and a ventilator for each twin, and the accompaniment of two anesthesiologists. Maintaining normothermia in transit is essential, and meticulous attention to the airways and intravascular lines reduces the possible risk of disconnection or dislodgement at this time. Each anesthesiologist must clearly understand his or her role during this process. Box 29-5 gives a preparatory checklist for the anesthesiologists before caring for conjoined twins.
Box 29-5 Preoperative Conjoined Twin Checklist
Induction
Techniques for induction of anesthesia are determined by the airway, the availability of intravenous access at induction, the state of health of each infant, the drugs available, and the preferences of the attending anesthesiologists. In those twins with potentially difficult airways, spontaneous respiration with inhalational induction with sevoflurane or the intravenous use of ketamine is helpful (Fig. 29-20) (Thomas, 2004; Thomas and Lopez, 2004; Diaz and Furman, 1987). In this same group, a lidocaine “gargle” before induction to partially anesthetize the airway is a useful technique (2% lidocaine sprayed into the infant’s mouth before starting the anesthetic). In infants with cyanotic congenital heart disease or in those with complex anatomy, intravenous ketamine is a safe option (Thomas and Lopez, 2004; Diaz and Furman, 1987).
Intraoperative Management
The use of local anesthesia should always be considered. Epidural, caudal, and combined epidural-spinal anesthesia have all been used successfully for surgery and postoperative pain relief (Greenberg et al., 2001). There are definite advantages and disadvantages to the use of neuroaxial blocks and regional anesthesia, and each set of twins needs to be assessed individually as to the feasibility of this option. Where regional anesthesia is not possible, the use of infiltration of local anesthetic, together with a vasoconstrictor, should be considered and also helps reduce blood loss.
Circulatory collapse at separation was described in early reports, and adrenal suppression was cited as the cause. This led to the recommendation for use of perioperative steroids (Hoyle and Thomas, 1989; Synhorst et al., 1979; Aird, 1959), but this is no longer relevant, and steroids are only advised for specific surgical specialties such as neurosurgery. Other causes of circulatory collapse at separation are considered to be unappreciated blood loss resulting in an inadequate intravascular volume a vagal response (especially with bony separations of ischiopagus, pygopagus, or dipagus twins) and undiagnosed cardiac abnormalities. Where intravascular blood volume replacement is sufficient and vagal responses is preempted, this has not been a problem.
Postoperative Care
Good pain relief is obligatory and may include the use of intravenous acetaminophen (paracetamol), which can be given orally or rectally, as well as the more traditional options of morphine or clonidine. If chronic pain syndromes are anticipated, the early use of gabapentin should be considered. Further anesthetics may be required for vascular access, secondary wound closure, and wound dehiscence (Rode et al., 2006).
Prognosis
Hidden long-term morbidity and mortality occur with unresolved aspiration after thoracopagus separation; bronchopneumonia, arrhythmias, and embolic cerebrovascular pathology occurred in one survivor when the entire cardiac complex had been assigned to one infant (Rode et al., 2006; Millar et al., 2009).
For many years after their separation, some patients must undergo further surgery to reconstruct shared pelvises and urogenital systems, to improve wound closure with skin grafts, and to revise their anatomic abnormalities in an ongoing effort to improve the quality of their lives. Some survivors will be disabled and require lifelong follow-up care. For many others, life goes on with minimal sequelae (Figs. 29-21 and 29-22).
Conclusion
Many lessons have been learned over generations, and these include the need to consult widely—locally and internationally. The Internet makes this extraordinarily easy. It is important to search using both the American and English spellings of anesthesia and anaesthesia, because different references appear as the result of a search, and there is very little overlap. Descriptions of anesthetic challenges are being reported from all over the world in different socioeconomic and political circumstances, and each article teaches something of value (Bloch and Karis, 1980; Norsidah et al., 1996; Huanc et al., 2004; Leelanukrom et al., 2004). Whether surgery for separation is an option or not, an experienced multidisciplinary team approach to the management of these infants results in the overall improved handling of the patients and their parents. Success requires an experienced team functioning in a tertiary referral center with the full range of medical, surgical, and anesthetic specialties (Spitz and Kiely, 2003).
Aird I. The conjoined twins of kano. BMJ. 1954:831-837.
Aird I. Conjoined twins: further observations. BMJ. 1959:1313-1315.
Allen H.L., Metcalf D.W., Giering C.G. Anesthetic management for separation of conjoined twins. Anesth Analg. 1959;38(2):109-113.
Andrews R.E., McMahon, Yates R.W.M., et al. Echocardiographic assessment of conjoined twins. Heart. 2006;92:382-387.
Atkinson L. Ethics and conjoined twins. Childs Nerv Syst. 2004;20:504-507.
Bloch E.C., Karis J.H. Cardiopagus in neonatal thoracopagus twins: anesthetic management. Anesth Analg. 1980;49:4304-4307.
Bouchard S., Johnson M.P., Flake A.W., Howell, et al. The EXIT procedure: experience and outcome in 31 cases. J Pediatr Surg. 2002;37:418-426.
Bratton M.Q., Chetwynd S.B. One into two will not go: conceptualising conjoined twins. J Med Ethics. 2004;30:279-285.
Bucholz R.D., Yoon K.W., Shively R.E. Temporoparietal craniopagus twins and review of the literature. J Neurosurg. 1987;66:72-79.
Campbell S. Separation of craniopagus twins: the Brisbane experience. Childs Nerv Syst. 2004;20:601-606.
Cywes S., Davies M.R., Rode H. Conjoined twins: the Red Cross War Memorial Children’s Hospital experience. S Afr J Surg. 1982;20:105-118.
Cywes S., Louw J.H. The Siyepu conjoined (Siamese) twins. S Afr Med J. 1967:1050-1057.
Cywes S., Millar A.J.W., Rode H., Brown R.A. Conjoined twins: the Cape Town experience. Pediatr Surg Int. 1997;12:234-248.
Di Rocco C., Caldarelli M., Tamburrini G., et al. Craniopagus: the Thessaloniki-Rome experience. Childs Nerv Syst. 2004;20:576-586.
Diaz J.H., Furman E.B. Perioperative management of conjoined twins. Anesthesiology. 1987;67:965-973.
Fieggen A.G., Dunn R.N., Pitcher R.D., et al. Ischiopagus and pygopagus conjoined twins: neurosurgical considerations. Childs Nerv Syst. 2004;20:640-651.
Fisherman S.J., Puder M., Geva T., et al. Cardiac relocation and chest wall reconstruction after separation of thoracopagus conjoined twins with a single heart. J Pediatr Surg. 2002;37:3515-3517.
Furman E.B., Roman D.G., Hairabet J., et al. Management of anesthesia for surgical separation of newborn conjoined twins. Anesthesiology. 1971;34:95-101.
Galankin J.L., Gaiser R.R., Cohen D.E., et al. Anesthesia for fetoscopic fetal surgery: twin reverse arterial perfusion sequence and twin-twin transfusions syndrome. Anesth Analg. 2000;91:61394-61397.
Gerlis L.M., Seo J.W., Ho S.Y., Chi J.G. Morphology of the cardiovascular system in conjoined twins: spatial and sequential segmental arrangements in 36 cases. Teratology. 1993;47(2):91-108.
Girshin M., Broderick C., Patel D., et al. Anesthetic management of staged separation of craniopagus conjoined twins. Pediatr Anesth. 2006;16:347-351.
Greenberg M., Frankville D.D., Hilfiker M. Separation of omphalopagus twins using combined caudal epidural-general anesthesia. Can J Anaesth. 2001;48:478-482.
Hall J. G: Twinning. Lancet. 2003;362:735-743.
Hall J.G., Lopez-Rangel E.. Twins and twinning. ed 3. Rimoin D.L., Connor J.M., Pyeritz R.E., editors. Emery and Rimoin’s principles and practice of medical genetics. vol 1. New York: Churchill Livingstone; 1996:395-404.
Harrison V.L., Keneally J.P., Gold P.D., et al. Anesthesia for separation of conjoined twins in the neonatal period. Anaesth Intensive Care. 1985;13(1):8-85.
Hoshina H., Tanaka O., Obara H., Iwai S. Thoracopagus conjoined twins: management of anesthetic induction and postoperative chest wall defect. Anesthesiology. 1987;66:424-426.
Hoyle M., Thomas C.G. Twenty-three-year follow-up of separated ischiopagus tetrapus conjoined twins. Ann Surg. 1989;210:5673-5679.
Hoyle R.M. Surgical separation of conjoined twins: collective review. Surg Gynecol Obstet. 1990;170:549-561.
Huanc W.Q., Fang J.Y., Xaio L.C., et al. Anesthetic management for separation of craniopagus twins. Acta Anesthesia Scand. 2004;48:919-921.
Jaffray B., Russell S.A., Bianchi A., Dickson A.P. Necrotising enterocolitis in omphalopagus conjoined twins. J Pediatr Surg. 1999;34(8):130-1306.
Jarem B.J., Flewellen E.H., Tyson K.R.T., et al. Anesthetic management for separation of conjoined twins. Anesthesiol Rev. 1977:17-22.
Kaufman M.H. The embryology of conjoined twins. Childs Nerv Syst. 2004;20:508-525.
Kingston K., McHugh K., Kumaradevan J., Kiely E.M. Imaging in the preoperative assessment of conjoined twins. Radiographics. 2001;21:1187-1208.
Leelanukrom R., Somboonviboom W., Bunburaphong P., Kiatkungwanklai P. Anesthetic experiences in three sets of conjoined twins in King Chulalongkorn Memorial Hospital. Pediatr Anes. 2004;14:176-183.
London A.J., Knowles L.P. The Maltese conjoined twins: two views of their separation. The Hastings Centre Report. 2001;31(Jan-Feb):48-52.
Lonnee H.A., Chetty V., Rawailiu V. Anesthesia for repair of gastroschisis in thoracopagus twins: a case report. Paediatr Anaesth. 2007;17:278-280.
Losee J., Natali M., Grundwaldt L., et al. Induced restrictive lung disease secondary to tissue expansion in ischiopagus conjoined twins. Plast Reconstr Surg. 2009;123(4):1378-1383.
MacKenzie T.C., Crombleholme T.M., Johnson M.P., et al. The natural history of prenatally diagnosed conjoined twins. J Pediatr Surg. 2002;37(3):30-309.
Mair A., Mair E. Two infants, one heart, and no airway. Int J Ped Otorhinolaryngol. 2006;70:1489-1493.
Mann M., Coutts J.P., Kaschula R.O.C., et al. The use of radionucleotides in the investigation of conjoined twins. J Nucl Med. 1984;24:479-484.
Millar A.J.W., Rode H., Thomas J., Hewitson J. Thoracopagus conjoined twins. In: Parikh D.H., Crabbe D.C.G., Auldist A.W., Rothenberg S.S., editors. Pediatric thoracic surgery. London Limited: Springer-Verlag, 2009.
Norsidah A.M., Lim S.K., Ibtisan I., Misiran K. Anesthetic management of conjoined twins: experience with six sets of twins. Med J Malaysia. 1996;51(4):42-425.
Norwitz E.R., Hoyte L.P.J., Jenkins K.J., et al. Separation of conjoined twins with the twin reversed-arterial-perfusion sequence after prenatal planning with three-dimensional modelling. NEJM. 2000;343:6399-6402.
O’Connell J.E.A. Craniopagus twins: surgical anatomy and embryology and their implications. J Neurol Neurosurg Psychiatry. 1976;39:1-22.
O’Neill J.A., Holcomb G.W., Schnaufer L., et al. Experience with thirteen conjoined twins. Ann Surg. 1988;208:299-312.
O’Neill J.A. Conjoined twins. In: O’Neill J.A., Rowe M., Grosfeld J.L., Fonkalsrud E.W., Coran A.G., editors. Pediatric Surgery. ed 5. St Louis: Mosby; 1998:1925-1938.
Ossowski K., Suskind D.L. Airway management in conjoined twins: a rare indication for the EXIT procedure. Arch Otolaryngol Head Neck Surg. 2005;131:58-60.
Paterson N.A. Validation of a theoretically derived model for the management of massive blood loss in pediatric patients: a case report. Paediatr Anaesth. 2009;19:535-540.
Pearn J. Bioethical issues in caring for conjoined twins and their parents. Lancet. 2001;357:1968-1971.
Peter J.C., Fieggen A.G., editors. Conjoined twins and the central nervous system. Childs Nerv Syst. 2004;20:503-651.
Peter J.C., Millar A.J.W., Rode H., et al. Anorectal and urogenital malformations, conjoined twins and myelodysplasia. Childs Nerv Syst. 1996;12:356.
Powis M., Spitz L., Pierro A. Differential energy metabolism in conjoined twins. J Pediatr Surg. 1999;34:71115-71117.
Rabeeah A.A. Conjoined twins: past, present and future. J Pediatr Surg. 2006;41:1000-1004.
Rejjal A-LR, Nazer H.M., Abu-Osaba Y.K., et al. Conjoined twins: medical, surgical and ethical challenges. Aust N Z Surg. 1992;62:287-291.
Rode H., Fieggen A.G., Brown R.A., et al. Four decades of conjoined twins at Red Cross Children’s Hospital: lessons learned. S Afr Med J. 2006;96(9):93-941.
Roy M. Anesthetic management for separation of craniopagus twins. Anesth Analg. 1980;59:883-886.
Rutka J.T., Souweidane M., ter Brugge K., et al. Separation of craniopagus twins in the era of modern neuroimaging, interventional neuroradiology, and frameless stereotaxy. Childs Nerv Syst. 2004;20:587-592.
Sebire N.J., Souka A., Skentou H., et al. First trimester diagnosis of monoamniotic twin pregnancies. Ultrasound Obstet Gynecol. 2000;16:223-225.
Seefelder C., Hill D.R., Shamberger R.C., Holzman R.S. Awake caudal anesthesia for inguinal surgery in one conjoined twin. Anesth Analg. 2003;96:412-413.
Shank E., Manohar N., Schmidt U. Anesthetic management for thoracopagus twins with complex cyanotic heart disease in the magnetic resonance imaging suite. Anesth Analg. 2005;100:361-364.
Spencer R. Anatomic description of conjoined twins: a plea for standardized terminology. Pediatr Surg. 1996;31:941-944.
Spencer R. Conjoined twins: developmental malformation and clinical implications. Baltimore and London: John Hopkins University Press, 2003.
Spencer R. Theoretical and analytical embryology for conjoined twins: Part 1: embryogenesis. Clin Anat. 2000;13:36-53.
Spencer R. Theoretical and analytical embryology for conjoined twins: Part 2: adjustments to union. Clin Anat. 2000;13:97-120.
Spitz L. Success rate for surgery of conjoined twins. Lancet. 2000;356(18):1765.
Spitz L. Hunterian lecture: surgery for conjoined twins. Ann R Coll Surg Engl. 2003;83:230-235.
Spitz L., Kiely E.M. Separation of conjoined twins. Lancet. 2000;356(17):953.
Spitz L., Kiely E.M. Experience in the management of conjoined twins. Br J Surg. 2002;89:1188-1192.
Spitz L., Kiely E.M. Conjoined twins. JAMA. 2003;289:1307-1310.
Strocker A., Ford R., Patel S., et al. Airway evaluation of conjoined twins. Ann Otol Rhinol Laryngol. 2005;114:15-18.
Suan C., Ojeda R., Gacia-Perla J.L., et al. Anesthetic management of the surgical separation of a pair of thoracopagus-cardiopagus twins. Paediatr Anaesth. 1998;8:255-257.
Sury M.R.J., Brown J.L., Aitken K. Anesthesia for conjoined twins during magnetic resonance imaging. Eur J Anesthesiol. 1994;11:139-142.
Synhorst D., Matlak M., Road Y., et al. Separation of conjoined thoracopagus twins joined at the right atrium. Am J Cardiol. 1979;24:662-665.
Szmuk P., Ghelber O., Shank E.S., et al. Letters to the editor. Anesth Analg. 2005;101:1238.
Szmuk P., Rabb M.F., Curry B., et al. Anesthetic management of thoracopagus twins with complex cyanotic heart disease for cardiac assessment: special considerations related to ventilation and cross-circulation. BJA. 2006;96:3341-3345.
Thomas J.M. Anesthesia for conjoined twins. Childs Nerv Syst. 2004;20:538-546.
Thomas J.M., Lopez T.J. Conjoined twins: the anesthetic management of 15 sets from 1991–2002. Pediatr Anesth. 2004;14:117-129.
Tirotta C.F., Lagueruela R., Munro H.M., Zahn E.M., Burke R.P. Anesthetic management of conjoined twins presenting for palliative open-heart surgery. Anesth Analg. 2005;101:44-47.
Unknown author. Editorial. Lancet. 2000;356:953.
Verrier M.D., Hastings C.J., Hoffman E.B. Posterior iliac osteotomy in ischiopagus tetrapus conjoined twins. J Pediatr Orthop. 2000;20:807-811.
Votteler T.P., Lipsky K. Long-term results of 10 conjoined twin separations. J Pediatr Surg. 2005;40:618-629.
Walker M., Browd S.R. Craniopagus twins: embryology, classification, surgical anatomy, and separation. Child Nerv Syst. 2004;20:554-566.
Winston K.R. Cranoipagi: anatomical characteristics and classification. Neurosurgery. 1987;21:769-781.