Chapter 180 Anesthesia
Preoperative Assessment
Laboratory Studies
Routine laboratory screening tests, including coagulation studies, rarely reveal abnormalities that were not apparent from the history and physical examination. It is reasonable to obtain a preoperative hemoglobin and hematocrit in patients with coexisting disease or a history of anemia and serum electrolytes in patients being treated with diuretics. Chronic hypokalemia, to a serum potassium of 3 mmol/L, is not a contraindication to elective surgery in the absence of cardiac comorbidity, symptomatic arrhythmias, or digitalis therapy.1 Symptomatic chronic hypokalemia in the perioperative period requires oral replacement on an outpatient basis because rapid IV supplementation may increase morbidity and mortality.2 Preoperative electrocardiograms and chest radiographs should be limited to elderly patients or to those with known or suspected cardiopulmonary disease.
Considerations in Patients with Spinal Cord Injury
Preoperative considerations in spinal cord injury (SCI) patients vary with the timing of surgery in relation to the time of and the anatomic level of injury. Patients who show symptoms of neurologic deficits secondary to acute SCI are the most challenging. SCI patients must have sufficient respiratory muscle strength to oxygenate and ventilate effectively. They may have impaired coughing ability and significant ventilation-perfusion mismatch.3 Pneumonias are common in patients with acute or chronic SCIs due to the high incidence of aspiration and pulmonary dysfunction with lesions above T7.4 The potential for associated injuries related to trauma, including rib fractures, pneumothoraces, closed-head injuries, and pelvic fractures, must be considered. Most of these patients show symptoms of varying degrees of hypotension as well as impaired myocardial contractility resulting from acute sympathetic denervation. They require judicious volume loading and, often, vasopressors and/or inotropes to maintain adequate organ perfusion pressure. Depolarizing muscle relaxants may result in fatal hyperkalemia.
Considerations in Patients with Scoliosis
It is important to evaluate the degree of preoperative pulmonary compromise in the patient with scoliosis. Pulmonary function tests are quite useful in this patient population. The forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) are the best indicators of the extent of restrictive lung disease caused by the thoracic deformity. Baseline arterial blood gas measurement or preoperative oximetry may also be helpful in guiding postoperative care.
Considerations in Patients with Rheumatoid Arthritis
Patients with rheumatoid arthritis must be carefully evaluated for the extent of their systemic disease so that the risks of surgery and anesthesia may be minimized. Deformities produced by articular involvement may make intravascular catheter placement difficult and increase the risk of positioning-related injury. Cervical spine films should be obtained because up to 30% of these patients may have asymptomatic cervical instability.5 Cervical spine instability or significant temporomandibular joint disease may require awake fiberoptic airway management and strict attention to positioning. The electrocardiogram should be examined for the presence of conduction abnormalities, and an echocardiogram should be obtained if there are any history or physical examination findings compatible with valvular dysfunction. The serum blood urea nitrogen (BUN) and creatinine should be checked to assess renal function in patients taking high doses of nonsteroidal anti-inflammatory drugs. Liver function tests are useful in patients taking cytotoxic drugs. Finally, stress-dose steroids should be ordered for all patients with a recent history of steroid use.
Pharmacology
Preoperative medications serve a variety of functions, including sedation, amnesia, anxiolysis, and aspiration prophylaxis. The goal of premedication in the neurosurgical patient is to provide anxiolysis with minimal sedation at the termination of surgery. Benzodiazepines have largely supplanted barbiturates and anticholinergics for this purpose. The reliability of midazolam in the immediate preoperative period has greatly reduced the need for longer-acting premedicants. H2 blockers raise the pH of gastric fluid6 and usually decrease gastric volume in patients at risk for aspiration. However, the routine use of histamine blockers for aspiration prophylaxis in patients not at risk is difficult to justify, given their cost.
Induction
Agents used to induce anesthesia include barbiturates, narcotics, benzodiazepines, and a variety of other unclassified drugs. Since the early 1940s, the barbiturates have been used for this purpose and reliably decrease cerebral blood flow, cerebral metabolic rate of oxygen (CMRO2), and intracranial pressure (ICP). Treatment with barbiturates after a global ischemic event does not appear to provide neuronal protection. Barbiturate administration after focal or partial ischemic events, however, seems to provide some protection from neurologic injury.7 These agents have limited use in maintaining anesthesia secondary to their prolonged effects. Most of them depress cardiac output and systemic vascular resistance, so care must be taken when they are given to a hypovolemic or traumatized patient.
Propofol
Propofol, a sedative-hypnotic agent, possesses all the benefits of the barbiturates with regard to reduction of cerebral blood flow and CMRO28 Propofol is cleared rapidly and produces prompt awakening in patients shortly after an infusion is discontinued. The autoregulatory capacity of the cerebral circulation remains intact during propofol anesthesia.9 To date, there is little experimental evidence indicating that propofol provides a significant degree of neurologic protection in temporary focal ischemia models. The only animal study suggesting a protective benefit of propofol in burst-suppressive doses failed to measure or control cerebral perfusion pressure.10
Ketamine
Ketamine, a phencyclidine derivative, differs from most induction drugs in that it raises CMRO2, blood flow, and ICP.11 It is thus less ideal for neuroanesthesia, but these are desirable properties for use in the hypovolemic patient. Ketamine preserves central circulating volume and afterload in patients with traumatic spinal cord lesions secondary to the release of endogenous catecholamines. However, in severely hypovolemic patients who have exhausted their sympathetic reserve, the bolus administration of ketamine may result in hemodynamic collapse because of its unopposed direct myocardial depressant effects.
Inhalation Agents
Inhalation anesthetics are the agents used most commonly for the maintenance of general anesthesia. Their mode of delivery and pharmacokinetics allow for controlled, predictable action and easy reversal. They are typically mixed with inspired gases via vaporizers, which are devices that make adjustments for temperature, flow rate, and anesthetic vapor pressure so that a known quantity can be delivered over a wide range of conditions. The inhalation agents act on the brain via an unknown mechanism. Hypothesized mechanisms include membrane protein inhibition and membrane depolarization through membrane swelling or carrier protein inhibition.12 Anesthetic potency parallels the lipid solubility of the agent. A standard known as the minimum alveolar concentration (MAC) is used as a guide to compare anesthetics of different potency. One MAC of any anesthetic is the end-tidal concentration that will render 50% of patients immobile to the surgical incision. The MAC for different anesthetic agents is additive; 0.5 MAC of nitrous oxide mixed with 1 MAC of isoflurane yields 1.5 MAC of anesthetic.
A number of factors determine the rate of increase of the partial pressure of an anesthetic in the brain, and hence its speed of onset. These factors include the concentration of the anesthetic delivered, solubility of the anesthetic in both the blood and the brain, alveolar ventilation, cardiac output, and presence of intrapulmonary or intracardiac shunts.13 For example, nitrous oxide is a poorly soluble gas with a MAC of 105% that is routinely delivered in high concentrations (50–70%) and has the most rapid onset of action. Isoflurane has intermediate solubility, a MAC of 1.2%, and a slower onset.
The inhalation anesthetics currently in common use include isoflurane, desflurane, and sevoflurane. They all possess cerebral vasodilator properties and decrease blood pressure by reducing either cardiac output or systemic vascular resistance. The increased cerebral blood flow seen with isoflurane can be attenuated by hyperventilation and a reduction in the partial pressure of carbon dioxide (Pco2).14 Desflurane and sevoflurane are both less soluble in blood than isoflurane and possess the theoretic advantage of more rapid emergence. Their effects on the cerebral vasculature parallel those of isoflurane,15 although sevoflurane appears to preserve the autoregulatory ability of the cerebral vasculature at higher MAC levels than either isoflurane or desflurane. Nitrous oxide is the least potent and most used inhalation agent and exhibits a favorable safety profile in spine surgery. It causes a mild rise in blood pressure and ICP when used alone. It is not clear if any inhalation agent confers specific advantages in spinal cord surgery, and agent choice should be dictated by the overall anesthetic plan.
Narcotics
Remifentanil, one of our newest narcotic agents, is unique in that it does not demonstrate any significant accumulation over prolonged periods of infusion. Recovery from remifentanil is essentially dose-independent because of its rapid esterase metabolism.16 Remifentanil is particularly useful in cases involving somatosensory-evoked potential (SSEP) or motor-evoked potential (MEP) monitoring as well as any case requiring a total intravenous anesthetic (TIVA). Because of the rapid offset of remifentanil, which is faster than the onset of most other analgesics, care must be taken to provide supplemental analgesics prior to stopping remifentanil in cases where substantial postoperative pain is anticipated.17
Muscle Relaxants
The use of muscle relaxants in spine surgery optimizes the conditions for intubation, provides an immobile surgical field, and reduces the risk of patient coughing and straining. Muscle relaxants may be broadly classified into two groups: depolarizing and nondepolarizing. Succinylcholine, the only depolarizing agent approved for use in the United States, has a rapid onset and short duration, qualities that make it useful when rapid intubation conditions are desired. This agent actively depolarizes the muscle at the myoneural junction until it becomes refractory to further stimulation. Typically, the administration of succinylcholine produces a 0.5-mEq/L rise in serum potassium.18 Succinylcholine also depolarizes extrajunctional acetylcholine receptors in patients with burns or denervation injuries. These receptors are more numerous and have a greater ionic permeability, leading to acute, profound hyperkalemia when stimulated.19 The risk of hyperkalemia is greatest after 3 to 7 days after injury and may persist for several years.20 Life-threatening succinylcholine-induced hyperkalemia has been hypothesized but not reported after immobilization or disuse atrophy in the absence of other causal factors.21 Succinylcholine is also a triggering agent for malignant hyperthermia and is contraindicated in any patient with a family history of malignant hyperthermia or a history of degenerative muscular disease. The routine use of succinylcholine is also contraindicated in children based on several reports of postadministration hyperkalemic cardiac arrest presumed secondary to unrecognized or undiagnosed muscular dystrophy.
The nondepolarizing muscle relaxants, including pancuronium, vecuronium, rocuronium, and cisatracurium, differ from one another primarily in onset and duration of action. These agents all bind to the myoneural junction and competitively inhibit the binding of acetylcholine. The extent of neuromuscular blockade is monitored intraoperatively in a number of ways. The most reliable method is with the use of a train-of-four (TOF) monitor. This device allows for subjective or objective comparison of the ratio of the first and fourth muscle stimuli, which correlates well with the density of receptor occupation. A ratio less than 0.25 correlates with dense paralysis, and a ratio greater than 0.75 correlates well with the patient’s ability to maintain protective airway reflexes after extubation.22
Muscle relaxation is reversed by the administration of anticholinesterase agents. These agents reliably reverse a blockade when the effects of the nondepolarizing muscle relaxant have begun to fade. Because these compounds increase acetylcholine levels at all cholinergic receptors, they are usually given in conjunction with a muscarinic anticholinergic drug (e.g., atropine or glycopyrrolate) to prevent unwanted bradycardia, salivation, and bronchial secretions.
The most important factors that affect the ability to reverse muscle relaxation are the depth of block at the time of reversal, choice and method of administration of relaxant, and dose of reversal agent. Other factors that may antagonize the ability to reverse a nondepolarizing blockade include hypothermia, metabolic acidosis, respiratory alkalosis, and the administration of certain antibiotics.23 As previously mentioned, reversal is followed with the TOF monitor. The best clinical assessment of adequate reversal is the ability of the patient to sustain an unassisted head lift for at least 5 seconds. The assessment of less cooperative patients can be carried out by observing the negative inspiratory force generated during spontaneous ventilation. A negative inspiratory force of at least –25 cm H2O correlates well with adequate reversal but not airway protection.24
Monitoring
General Monitoring
Patients with both acute and chronic cervical spine injuries may show symptoms of a variety of specific electrocardiographic abnormalities. These abnormalities have been attributed to the autonomic imbalance created by disruption of sympathetic pathways located in the cervical cord. Severe acute cervical spine injury is frequently associated with marked sinus bradycardia. It also carries an increased incidence of ventricular and supraventricular arrhythmias, as well as cardiac arrest, when compared with injury of the thoracolumbar spine.25 Multilead ST-segment elevation has been noted in a significant percentage of patients with chronic, complete SCI. These alterations in ventricular repolarization are hypothesized to be manifestations of central sympathetic dysfunction and, indeed, resolve with low-dose isoproterenol infusion.26
Central monitoring of venous or pulmonary artery pressure may be indicated in patients with a history of ischemic heart disease or left ventricular dysfunction, particularly in the setting of anticipated large blood loss or fluid shifts. In patients with normal cardiac function, central venous pressures provide an adequate estimate of left ventricular end-diastolic volume. A pulmonary artery catheter, however, may more accurately assess left ventricular volume in patients with ventricular dysfunction. Acute cervical spine injury with spinal shock is associated with substantial hemodynamic lability and a high incidence of left ventricular dysfunction.27 Spinal shock patients are less tolerant of aggressive fluid replacement and more prone to develop pulmonary edema. The acutely quadriplegic patient qualified for surgery should be monitored with both an arterial line and either a central venous or pulmonary artery catheter.
Neurophysiologic Monitoring
Awake Patient
The awake patient is the ultimate spinal cord monitor. Several case reports describe the use of local anesthesia for spine surgery in the awake patient, although it is not a common means of neurologic monitoring. Chang28 and Drummond et al.29 both describe the use of anesthesia by local infiltration for dorsal cervical osteotomy. From these descriptions it appears that at least a short period of unconsciousness may be required because of significant discomfort associated with the fracturing of the anterior longitudinal ligament. Zigler et al.30 presented a series of 34 consecutive cases of dorsal cervical stabilization and fusion in patients with unstable cervical spines and variable degrees of neurologic injury using local anesthesia in conjunction with light sedation. They encountered no untoward complications and found that the technique was well tolerated by patients, although occasionally bone graft harvesting under local anesthesia was uncomfortable.
Wake-Up Test
In 1973, Vauzelle et al.31 described their use of an intraoperative “wake-up” with observation of limb movement for the assessment of spinal cord function. This simple test is an excellent monitor of gross motor function and is used most commonly during surgical procedures involving spinal column instrumentation and distraction. Its use is based on clinical evidence that neural impairment resulting from distraction is reversible when the distracting forces are modified during its early phase.29,32 Currently, an awake patient is the only available monitoring modality to provide unequivocal intraoperative documentation of intact motor function.
An advantage of the wake-up test over more highly technical forms of neurophysiologic monitoring is that specialized equipment or ancillary monitoring personnel are unnecessary. Two limitations are (1) that the patient can only be awakened intermittently and, therefore, the anesthesiologist and surgeon are restricted to a few spot checks of the integrity of motor pathways; and (2) it is possible that neurologic impairment may occur despite a successful wake-up test. Diaz and Lockhart33 reported one case of unresolved paraplegia after a normal wake-up test. This test may be difficult or impossible to perform in young children, patients with cognitive difficulties, and those with significant hearing impairment. A number of complications of this technique have been described, including dislodgement of spinal hardware, displacement of IV lines and monitors, accidental extubation, air embolism, and the possibility of intraoperative recall. These complications appear to be uncommon in the clinical setting, although they are always a reason for concern.32,34
Somatosensory Spinal-Evoked Potentials
Interpretation of SSEP data is based on changes in response amplitude and latency. Amplitude is defined as the vertical dimension of the waveform and can be measured as the difference between two peaks of opposite polarity or between a specific peak and a reference point of zero potential. Latency is defined as the elapsed time between the stimulus and the response. Apel et al.35 considered a clinically significant change in SSEPs to have occurred if latency was increased by 10% or amplitude was reduced by at least 50%. Owen,36 however, believes that an interpretation criterion of 50% for amplitude reduction may be too sensitive and may result in unnecessary false-positive findings. He recommends that the surgeon be informed of degraded data if latency increases more than 10% or amplitude decreases more than 60% relative to baseline.
The clinical utility of SSEPs lies in their ability to demonstrate the functional integrity of neural pathways in an anesthetized and presumably unresponsive patient. Grundy et al.37,38 have shown that intraoperative SSEPs are very sensitive indicators of hypoxia and ischemia associated with spine manipulation. Numerous cases have been reported in which early recognition of SSEP changes appeared to have prevented permanent neurologic damage by alerting the surgeon to the need for appropriate corrective action. There are also several case reports of false-negative SSEPs in which postoperative neurologic deficits occurred with preserved intraoperative waveforms.39,40
In the operating room, baseline SSEPs should be recorded after the induction of anesthesia and skin incision. Serial intraoperative recordings are obtained and should always be interpreted relative to these baseline measurements. If significant changes are noted in the SSEPs, a review of the monitoring equipment and all temporally related surgical and anesthetic events should ensue. Physiologic alterations that may affect SSEPs include hypotension, hypothermia, anemia, hypoxemia, and changes in arterial Pco2. Changes in the depth of anesthesia may have profound effects on evoked potential waveforms. Every effort should be made to avoid alterations in the inhaled gas concentration and/or bolus injection of hypnotic agents during periods of risk. If the SSEP changes persist without adequate explanation, the possibility of injury to neural tissues exists and a wake-up test should be performed.
The preferred anesthetic for SSEP monitoring is one that allows for the recording of adequate baseline waveforms and avoids rapid alterations in anesthetic depth during the course of the surgical procedure. Many techniques satisfy these requirements but a commonly used anesthetic is a balanced O2/N2O/narcotic technique with low-dose volatile anesthetic supplementation (≤1%). Although this regimen decreases the amplitude of SSEPs, most believe that it is compatible with effective monitoring. The use of a continuous narcotic infusion will have the least effect on evoked potential monitoring and provides additional hemodynamic stability to any anesthetic regimen. As noted in Table 180-1, IV agents generally have only modest effects on SSEPs, whereas volatile agents, such as isoflurane, attenuate the SSEP waveforms in a dose-dependent fashion. Care must be taken to maintain isoflurane concentrations at the lowest practical level.
TABLE 180-1 Effects of Anesthetic Agents on Somatosensory Spinal-Evoked Potentials
| Agent | Amplitude | Latency |
|---|---|---|
| Isoflurane | Decrease | Increase |
| Nitrous oxide | Decrease | Minimal change |
| Propofol | Minimal change | Increase |
| Pentothal | Mild decrease | Increase |
| Ketamine | Increase | Increase |
| Etomidate | Marked increase | Increase |
| Fentanyl | Minimal increase | Mild increase |
| Midazolam | Decrease | Minimal increase |
SSEP monitoring has been associated with several unique complications. Legatt and Frost41 reported electrocardiogram artifact produced by the triggering of the pacer enhancement circuitry in the electrocardiogram monitor by the somatosensory stimuli. Merritt et al.42 described a case of pacemaker-mediated tachycardia induced by intraoperative SSEP stimuli. The tachycardia and associated hypotension were a result of mistaken interpretation by the programmable pacemaker of the SSEP stimulus as its own intrinsic atrial event.
Motor-Evoked Potentials
MEPs may be categorized as either transcranial or spinal, depending on the location of the stimulating electrode. The stimulus may be either electrical or magnetic, with electrical stimulation being the most commonly used. The same interpretation criteria used for SSEPs of 60% loss of amplitude and 10% prolongation of latency may also be used for MEPs.43
MEPs are extraordinarily sensitive to anesthetic agents, with transcranial MEPs being more sensitive than spinal cord MEPs. The anesthetic techniques normally used during the monitoring of SSEPs are not compatible with MEP monitoring. Volatile anesthetics, nitrous oxide, and sodium pentothal all cause significant depression of MEPs.44–47 Ketamine, etomidate, propofol, and narcotic agents appear to produce less significant changes.48 The bolus injection of any of these agents during MEP monitoring may cause a transient decrease in amplitude, suggesting that continuous infusion techniques are preferable to repeated boluses.49 The most common anesthetic currently used at our institution to provide optimal monitoring conditions for MEPs is a total IV anesthetic technique using propofol and remifentanil infusions.
A controlled level of neuromuscular blockade (90% reduction in twitch), as provided by an infusion of a neuromuscular blocking agent, permits recording of compound muscle action potentials while eliminating motor activity that could interfere with surgery.50 However, our neuromonitoring team feels that the complete avoidance of neuromuscular blockade provides the best conditions for monitoring MEPs.
Anesthetic Techniques
Regional Anesthesia
Regional analgesia is a useful adjunct for the management of postoperative pain after thoracic or lumbar spine surgery. Epidural or intrathecal morphine provides long-lasting analgesia and improved pulmonary mechanics with fewer systemic side effects than if given intravenously. Patients receiving neuraxial opioids require some sort of monitoring for respiratory depression, a rare but potentially fatal side effect.
Airway Management
The inability to manage a patient’s airway successfully has resulted in as many as 30% of intraoperative deaths attributable to anesthesia.51 Effective airway management in the patient undergoing spine surgery must account for any abnormalities in airway anatomy as well as any potential or known instability in the cervical spine.
Preoperative Evaluation
Preoperative evaluation of the airway can be accomplished using three relatively simple tests that predict the difficulty of orotracheal intubation. How well the examiner can visualize the dorsal pharynx when the patient is facing the examiner in a neutral position with tongue protruded predicts the difficulty of visualization of the airway during laryngoscopy.52,53 In addition, if the distance between the thyroid cartilage and the end of the mandible is greater than 7 cm, adequate space is usually available for ventral displacement of the tongue during visualization.54 The third parameter is an assessment of atlanto-occipital mobility. Adequate neck extension ensures that proper alignment of the oral, pharyngeal, and laryngeal axes can be obtained.
Difficult Intubation
The American Society of Anesthesiologists has developed an algorithm that is designed to facilitate appropriate airway management during rapidly evolving clinical situations55(Fig. 180-1). Patients who are known to be difficult to intubate are much less likely to suffer morbidity due to airway management if proper preparations can be made before the induction of anesthesia. Awake fiberoptic intubation under light sedation and administration of topical local anesthetic to the airway allows the anesthesiologist to definitively secure the airway while the patient is spontaneously ventilating. In addition, retrograde intubation via a guidewire fed through the cricothyroid membrane and threaded through the mouth or naris may be used as a guide for intubation during spontaneous ventilation. The retrograde technique is most useful in patients with significant maxillofacial trauma in whom visualization of the airway with a fiberoptic scope may be quite difficult because of the presence of blood and secretions.
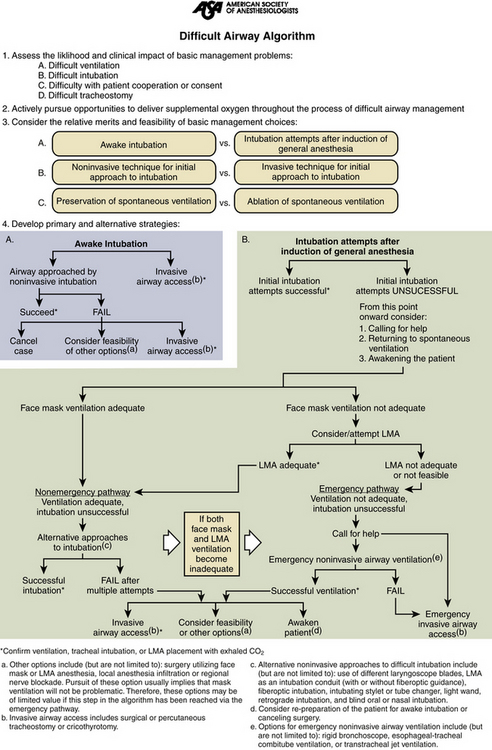
FIGURE 180-1 American Society of Anesthesiologists’ difficult airway algorithm. LMA, laryngeal mask airway.
One potential disadvantage of the LMA in patients qualified for spine surgery is the need for cervical spine extension during placement, although in experienced hands the success rate of placement of the device with the head in a neutral position has been reported to be as high as 95%.56 The LMA does not provide protection against the aspiration of stomach contents and is, therefore, contraindicated in elective airway management for the patient at risk for aspiration.
Patient with Cervical Spine Injury
In patients with potential or known cervical spine injury, airway management must be dictated by the acuity of the situation. Patients who require immediate intervention secondary to hemodynamic instability, acute respiratory failure, inability to protect their airway, or elevated intracranial pressure probably are best treated with bag-mask assisted ventilation followed by tracheal intubation with direct laryngoscopy. Usually, this can be safely accomplished with in-line stabilization of the neck in a neutral position. The risk of neurologic complications from direct laryngoscopy in patients with unstable cervical spines has not been quantified, but is probably quite low. Atlantoaxial extension with minimal movement of the lower cervical spine has been demonstrated during laryngoscopy in anesthetized patients.57 Movement of the cervical spine is also significantly reduced when in-line stabilization is performed during laryngoscopy.58 Several studies in the literature have shown no neurologic deterioration in patients with known cervical spine injuries after direct laryngoscopy with stabilization of the cervical spine,59–62 and Holly and Jordon63 reported similar safety with nasotracheal intubation. Although the risk of neurologic injury secondary to direct laryngoscopy is low, it is not zero. Recent studies of different intubation strategies on cervical spine motion suggest potential advantages with the use of fiberoptic bronchoscopy as well as the Bullard laryngoscope.64–66
Patient with Rheumatoid Arthritis
Airway abnormalities in patients with rheumatoid arthritis deserve special mention. In addition to having atlanto-occipital instability, these patients may exhibit scoliotic deformities of the larynx and trachea, making intubation difficult even with a fiberoptic bronchoscope.67 Arthritic changes of the cricoarytenoid joint associated with this disease have been reported as a source of upper airway obstruction after extubation.68
Extubation
Extubation of the patient with a difficult airway should proceed with caution. Extra care must be taken to ensure that the effects of all respiratory depressant anesthetics are eliminated and that the patient has fully recovered from any neuromuscular blockade. This can be assessed most effectively by examining the patient’s ability to maintain a voluntary head lift for greater than 5 seconds. This maneuver correlates well with the ability to maintain protective airway reflexes after extubation and to cough effectively.24 When in doubt about the integrity of the airway resulting from surgical trauma, altered anatomy, hematoma formation, facial edema, or neurologic injury, prudence would dictate a period of postoperative mechanical ventilation with the patient in a head-elevated position until recovery is complete. Then it is possible to anesthetize the trachea with the topical administration of local anesthetic and extubate the patient under direct vision using the fiberoptic bronchoscope to assess airway integrity. A guidewire or intubating stylet may be left in place in the patient’s airway after extubation to facilitate reintubation, if needed.55
There are several case reports of upper airway obstruction after multilevel cervical corpectomies.69 The cause of the airway compromise is unclear but may relate to severe hypopharyngeal and supraglottic swelling secondary to either disruption of lymphatic drainage during the operative dissection, inflammation, and/or venous obstruction from small blood clots. Some centers have developed guidelines for minimizing postoperative airway complications in these patients. This includes 48 hours of postoperative intubation if a multilevel corpectomy is performed, or if operative time exceeds 5 hours.70 Intermittent retractor release throughout the surgical procedure may help prevent this complication.
Positioning
Cranial Nerve Injury
Recurrent laryngeal nerve injury remains an important cause of postoperative morbidity following anterior cervical spine procedures and is generally felt to be related to retractor-mediated injury. The reported incidence of paresis and paralysis is 15% to 20%, whereas the incidence of paralysis alone is 3%.71 Failure to perform direct laryngoscopy on asymptomatic patients in some studies may contribute to underestimation in cases of mild paresis. A predilection for injury during right-sided procedures has been noted and may be related to the anatomic pathway of the right recurrent laryngeal nerve lying outside the tracheoesophageal groove. A commonly used maneuver to help mitigate injury to the nerve (i.e., deflation and reinflation of the endotracheal tube cuff following retractor placement) does not appear to significantly affect the frequency of recurrent laryngeal nerve injury.71
Postoperative Visual Loss
Postoperative visual loss (POVL) after spine surgery is a relatively rare event that has been reported with increasing frequency over the past decade.72–74 Concern regarding the apparent increased reporting frequency of this event led the ASA Committee on Professional Liability to establish the ASA Postoperative Visual Loss Registry in July 1999. The registry collects detailed information on cases of POVL in an effort to better define intraoperative risk factors and patient characteristics that may predispose to this perioperative complication.72 Anonymous reporting is encouraged, and the standardized, anonymous case report form can be accessed via the Internet at www.asaclosedclaims.org under the POVL Registry subheading.72
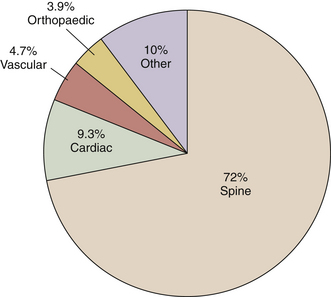
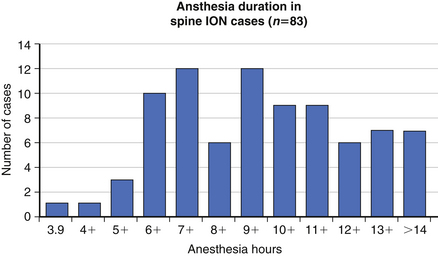
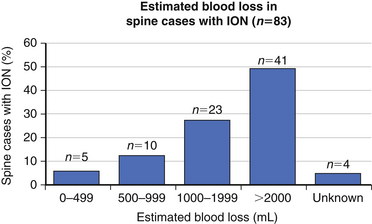
The precise cause of POVL is not completely defined but is most likely a multifactorial phenomenon. Suggested contributing factors include hypotension, large blood loss, high-volume fluid resuscitation, anemia, direct ocular pressure, head-dependent position, long operative time, and the presence of vascular disease. Review of the POVL Registry data as of Winter 200172 shows that the majority of cases reported involved patients in the prone position for spine procedures (Fig. 180-2). The most common type of ophthalmic lesion associated with POVL is ischemic optic neuropathy (89%), with only 6% of cases being associated with central retinal artery occlusion. Based on registry data, the incidence of ischemic optic neuropathy appears to increase when time spent in the prone position exceeds 6 hours in duration (Fig. 180-3). Blood loss of 1 L or more was associated with ischemic optic neuropathy in 86% of spine cases in the registry (Fig. 180-4). Ischemic optic neuropathy occurs across all age ranges, and younger age does not appear to guarantee protection against it. It should be noted that the registry does not contain denominator data of all cases of prone spine surgery; therefore, definitive conclusions regarding risk cannot be made.72
Sitting Position
Venous air embolism is one of the most feared complications of the sitting position. The incidence of air embolism during spine surgery in the sitting position appears to be substantially less than that during intracranial procedures in the sitting position (7% vs. 43%).75 The most sensitive monitors for venous air embolism include end-tidal partial pressure of carbon dioxide (ETco2), end-tidal nitrogen concentration (ETn2), the precordial Doppler sonogram, and, most sensitive of all, the transesophageal echocardiogram. A long-arm central venous line is routinely placed for aspiration of air should an embolus occur. It may be accurately positioned just below the junction of the superior vena cava and the right atrium under electrocardiographic guidance within a matter of minutes.76 Treatment of an air embolus involves the identification of the source, flooding the field to prevent further influx of air, aspiration of air through the central venous line, discontinuation of nitrous oxide to avoid expansion of the air bubbles, and supportive therapy with fluids and pressors.
Fluid and Blood Therapy
Goals of fluid management in anesthesia for spine surgery include the maintenance of adequate plasma volume to preserve spinal cord perfusion while avoiding decreases in plasma oncotic pressure and interstitial fluid accumulation. Fluid replacement can be accomplished with isotonic crystalloid solutions, colloid preparations, or blood products to satisfy these goals. Glucose-containing solutions should be avoided in the absence of documented hypoglycemia because of their demonstrated ability to worsen neurologic outcome following temporary focal ischemia.77 Hypertonic saline has been used with a safety record similar to isotonic fluids and provides more rapid restoration of intravascular volume after trauma.78
Blood Loss and Replacement
When blood losses become greater than one blood volume, many patients develop a progressive increase in bleeding. Presumably, this occurs due to dilution of circulating procoagulants and platelets. At a 1984 National Institutes of Health (NIH) consensus conference, the committee concluded that during massive transfusion, the component required most frequently when increased bleeding occurred was not fresh-frozen plasma but platelets.79 Thrombocytopenia, or a qualitative platelet dysfunction, is probably the most likely cause of bleeding even in the presence of elevated prothrombin and partial thromboplastin times.80 With the exception of surgical hemostasis, disseminated intravascular coagulation is the most common unanticipated cause for major bleeding in patients without an underlying coagulopathy.
There is debate over the optimal hematocrit for surgical procedures,81 and this mystical value has drifted downward over time.82 Acute normovolemic hemodilution remains a safe and effective means of intraoperative blood conservation in those patients expected to have blood losses of 1 to 2 L. Reduction in hematocrit reduces the oxygen-carrying capacity of blood, thus decreasing the “margin of safety” of effective oxygen transport. However, this reduction in hematocrit also causes a reduction in viscosity and an increase in cardiac output. A normovolemic decrease in hematocrit from 40% to 20% induces a 50% drop in oxygen-carrying capacity, but only a 10% drop in oxygen delivery secondary to increased cardiac output. This change parallels the decrease in oxygen consumption seen with the institution of general anesthesia or mild hypothermia. This form of therapy should be reserved for moderately healthy patients without preexisting cardiac, cerebrovascular, or pulmonary disease. There is no evidence that hemodilution has adverse effects on wound healing, infection rates, or immunologic status. Conversely, hemoconcentration may have detrimental effects based on animal studies of focal ischemia.83
Replacement of blood losses during surgery with the patient’s own shed blood is also an attractive conservation option. Most cell-saving devices currently in use mix salvaged blood with an anticoagulant, then wash and concentrate it to a hematocrit of about 50%. The effluent, which contains plasma fractions, platelets, leukocytes, free hemoglobin, anticoagulant, saline, and other debris, is discarded. Induction of disseminated intravascular coagulation does not appear to be a problem with these devices.84 Their use is contraindicated in the presence of tumor cells or bacterial contamination. Residual anticoagulation is not a problem with sufficient washing.
Induced Hypotension
Induced hypotension may be useful in helping to minimize blood loss and transfusion therapy during spine surgery, although its efficacy has not been consistently demonstrated.85 With the use of high concentrations of volatile anesthetics or direct-acting vasodilators such as nitroprusside or nicardipine, the mean arterial pressure may be dropped to the lower limits of autoregulatory control of blood flow, thereby reducing capillary venous pressure and blood loss. Obviously, this technique should be reserved for the otherwise healthy patient.
Pharmacologic Aids
Agents that attempt to reduce bleeding pharmacologically have also been used. Desmopressin (DDAVP), an analogue of the natural hormone vasopressin, induces endothelial cells to release von Willebrand factor, tissue-type plasminogen activator, and certain prostaglandins.86 With these effects in mind, DDAVP has been used in two human trials during scoliosis surgery with variable results.87,88 Aprotinin is a serine protease inhibitor that preserves platelet integrity and adhesiveness. It has shown promise as an agent for the prevention of blood loss during cardiac surgery in multiple studies and appears to reduce blood loss and transfusion requirements in spine fusion procedures as well.89 However, recent studies have suggested an increased risk of long-term mortality following cardiac surgery in patients treated with aprotinin.90
A meta-analysis was conducted in 2008 to determine if antifibrinolytic agents reduced blood transfusions in patients undergoing spine surgery and to compare their effects.91 Aprotinin, tranexamic acid, and ε-aminocaproic acid were all found to be effective in reducing blood loss and the need for transfusions. With the exception of aprotinin, side effects of these agents have not been shown to cause substantial morbidity or to increase the rate of thromboembolic events. Their use should be considered for patients undergoing spinal procedures in which significant blood loss is anticipated. Note, however, that this is not an FDA-approved indication for these agents.91
Audu P., Artz G., Scheid S., et al. Recurrent laryngeal nerve palsy after anterior cervical spine surgery. Anesthesiology. 2008;105:898-901.
Benumof J.L. Practice guidelines for management of the difficult airway. Anesthesiology. 2003;98:1269-1277.
Caldwell M.W., O’Hara R.C., Bloom M.J., et al. Postoperative airway complications in patients undergoing anterior cervical corpectomy with graft placement: a review of the Pittsburgh experience. J Neurosurg Anesthesiol. 1994;6:311.
Gill J.B., Chin Y., Levin A., et al. The use of antifibrinlytic agents in spine surgery. J Bone Joint Surg [Am]. 2008;90:2399-2407.
Guay J., Reinberg C., Poitras B., et al. A trial of desmopressin to reduce blood loss in patients undergoing spinal fusion for idiopathic scoliosis. Anesth Analg. 1992;75:405-410.
Keenan M.A., Stiles C.M., Kaufman R.L. Acquired laryngeal deviation associated with cervical spine disease in erosive polyarticular arthritis. Anesthesiology. 1983;58:441-449.
Lee L.A., Roth S., Posner K.L., et al. The American Society of Anesthesiologists postoperative visual loss registry—analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2008;105:652-659.
1. Wong K.C., Schafer P.G., Schultz J.R. Hypokalemia and anesthetic implications. Anesth Analg. 1993;77:1238-1260.
2. Lawson D.H. Adverse reactions to potassium chloride. Q J Med. 1974;43:433-440.
3. McMichan J.C., Michel L., Westbrook P.R. Pulmonary dysfunction following traumatic quadriplegia. Recognition, prevention, and treatment. JAMA. 1980;243:528-531.
4. Giffen J.P. Anesthesia for patients with spinal cord injury: acute and chronic. ASA Refresher Course Lectures. 1991;22:152.
5. Skues M.A., Welchew E.A. Anaesthesia and rheumatoid arthritis. Anaesthesia. 1993;48:989-997.
6. Coombs D.W., Hooper D., Colton T. Acid aspiration prophylaxis by use of preoperative oral administration of cimetidine. Anesthesiology. 1979;51:352-356.
7. Hayek D.A., Veremakis C. Cerebral resuscitation. In: Pinsky M.R., Dhainault J.F., editors. Pathophysiologic foundations of critical care. Baltimore: Williams & Wilkins; 1993:753-777.
8. Ravussin P., Guinard J.P., Ralley F., et al. Effect of propofol on cerebrospinal fluid pressure and cerebral fluid pressure and cerebral perfusion pressure in patients undergoing craniotomy. Anaesthesia. 1988;43(Suppl):37-41.
9. Craen R.A., Gelb A.W., Murkin J.M., Chong K.Y. Human cerebral autoregulation is maintained during propofol air/O2 anesthesia. Anesthesiology. 1992;77:A220.
10. Young Y., Menon D.K., Tisavipat N., et al. Propofol neuroprotection in a rat model of ischaemic reperfusion injury. Eur J Anaesthesiol. 1997;14:320-326.
11. Shapiro H.M., Wyte S.R., Harris A.B., et al. Acute intraoperative intracranial hypertension in neurosurgical patients: mechanical and pharmacologic factors. Anesthesiology. 1972;37:399-405.
12. Kublin D.D. Mechanisms of action. In: Miller R.D., editor. Anesthesia. New York: Churchill–Livingstone; 1993:51-83.
13. Pharmacokinetics and pharmacodynamics of inhaled and injected drugs. In: Stoelting, editor. Pharmacology and physiology of anesthetic practice. Philadelphia: Lippincott-Raven; 1991:1-32.
14. Entrei C., Lesczniewski W., Carlsson C. Local application of 133 Xenon for measurement of cerebral blood flow during halothane, enflurane and isoflurane anesthesia in humans. Anesthesiology. 1985;63:391-399.
15. Hanson T.D., Warner D.S., Todd M.M., Vust L.J. The role of cerebral metabolism in determining the local cerebral blood flow effects of volatile anesthetics: evidence for persistent flow-metabolism coupling. J Cereb Blood Flow Metab. 1989;9:323-328.
16. Dershwitz M., Randel G.I., Rosow C.E., et al. Initial clinical experience with remifentanil. Anesth Analg. 1995;81:619-623.
17. Albrecht S., Schuttler J., Yarmush J. Postoperative pain management after intraoperative remifentanil. Anesth Analg. 1998;89:S40-S45.
18. Fung D.L., White D.A., Jones B.J., Gronert G.A. The onset of disuse–related potassium efflux to succinylcholine. Anesthesiology. 1991;55:547-549.
19. Fambrough D.M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979;59:165-227.
20. Martyn J.J., White D.A., Gronert G.A., et al. Up and down regulation of skeletal muscle acetylcholine receptors. Anesthesiology. 1992;76:822-843.
21. Azar I. The response of patients with neuromuscular disorders to muscle relaxants: a review. Anesthesiology. 1984;61:173-187.
22. Ali H.H., Utting J.E., Kitz R.J. Evaluation of recovery from nondepolarizing neuromuscular block using a digital neuromuscular transmission analyzer. Anesth Analg. 1973;52:740-745.
23. Miller R.D., Roderick L.L. Acid base balance and neostigmine antagonism of pancuronium neuromuscular blockade. Br J Anaesth. 1978;50:317-324.
24. Pavlin K.G., Holle R.H., Schoene R.B. Recovery of airway protection compared with ventilation in humans after paralysis with curare. Anesthesiology. 1989;70:381-385.
25. Lehmann K.G., Lane J.G., Piepmeier J.M., Batsford W.P. Cardiovascular abnormalities accompanying acute spinal cord injury in humans: incidence, time course and severity. JACC. 1987;10:46-52.
26. Lehmann K.G., Shandling A.H., Yusi A.U., Froelicher V.F. Altered ventricular repolarization in central sympathetic dysfunction associated with spinal cord injury. Am J Cardiol. 1989;63:1498-1504.
27. Mackenzie C.F., Shin B., Krishnaprasad D., et al. Assessment of cardiac function during surgery on patients with acute quadriplegia. J Neurosurg. 1985;62:843-849.
28. Chang J. Anaesthesia for cervical osteotomy. Can Anaesth Soc J. 1974;21:83-91.
29. Drummond J.C., Abitbol J.J., Sanford T.J., Garfin S.R. Transient paraplegia during posterior cervical osteotomy. Anesthesiology. 1991;74:628-630.
30. Zigler J., Rockowitz N., Capen D., et al. Posterior cervical fusion with local anesthesia: the awake patient as the ultimate spinal cord monitor. Spine (Phila Pa 1976). 1987;12:206-208.
31. Vauzelle C., Stagnara P., Jouvinoux P. Functional monitoring of the spinal cord during surgery. Clin Orthop Relat Res. 1973;93:173-178.
32. Hall J.E., Levine C.R., Sudhir K.G. Intraoperative awakening to monitor spinal cord function during Harrington instrumentation and spine fusion. J Bone Joint Surg [Am]. 1978;60:533-536.
33. Diaz J.H., Lockhart C.H. Postoperative quadriplegia after spinal fusion for scoliosis with intraoperative awakening. Anesth Analg. 1987;66:1039-1042.
34. Sudhir K.G., Smith R.M., Hall J.E., Hansen D.D. Intraoperative awakening for early recognition of possible sequelae during Harrington–rod spinal fusion. Anesth Analg. 1976;55:526-528.
35. Apel D.M., Marrero G., King J., et al. Avoiding paraplegia during anterior spinal surgery: the role of SSEP monitoring with temporary occlusion of segmental spinal arteries. Spine (Phila Pa 1976). 1991;16:S365-S370.
36. Owen J.H. Update on evoked potentials during spinal surgery. Curr Opin Orthop. 1993;4:12-20.
37. Grundy B.L., Heros R.C., Tung A.S. Intraoperative hypoxia detected by evoked potential monitoring. Anesth Analg. 1981;60:437-439.
38. Grundy B.L., Nash C.L., Brown R.H. Deliberate hypotension for spinal fusion: a prospective randomized study with evoked potential monitoring. Can Anaesth Soc J. 1982;29:452-461.
39. Ben-David B., Haller G., Taylor P. Anterior spinal fusion complicated by paraplegia. Spine (Phila Pa 1976). 1987;12:536-539.
40. Lesser R.P., Raudzens P., Luders H., et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1986;19:22-25.
41. Legatt A.D., Frost E.A.M. EKG artifacts during intraoperative evoked potential monitoring. Anesthesiology. 1989;70:559-560.
42. Merritt W.T., Brinker J.A., Beattie C. Pacemaker–mediated tachycardia induced by intraoperative somatosensory evoked potential stimuli. Anesthesiology. 1988;69:766-768.
43. Owen J.H., Bridwell K.H., Grubb R., et al. The clinical application of neurogenic motor evoked potentials to monitor spinal cord function during surgery. Spine (Phila Pa 1976). 1991;16:S385-S390.
44. Ghaly R., Stone J., Kartha R., et al. Effect of neuroleptanalgesia on motor potentials evoked by transcranial magnetic stimulation in primates. Anesthesiology. 1991;75:A595.
45. Kalkman C.J., Drummond J.C., Ribberink A.A. Low concentrations of isoflurane abolish motor evoked responses to transcranial electrical stimulation during N2O/opioid anesthesia in humans. Anesth Analg. 1991;73:410-415.
46. Kalkman C.J., Drummond J.C., Ribberink A.A. Effects of propofol, etomidate, midazolam and fentanyl on motor evoked responses to transcranial electrical or magnetic stimulation in humans. Anesthesiology. 1992;76:502-509.
47. Losasso T.J., Boudreaux J.K., Muzzi D.A., et al. The effect of anesthetic agents on transcranial magnetic motor evoked potentials in neurosurgical patients. Anesthesiology. 1991;75:A1032.
48. Lee V.C. Spinal and cortical evoked potential studies in the ketamine-anesthetized rabbit: fentanyl exerts component-specific, naloxone-reversible changes dependent on stimulus intensity. Anesth Analg. 1994;78:280-286.
49. Ubags L.H., Kalkman C.J., Been H.D., et al. The use of ketamine or etomidate to supplement sufentanil/N2O anesthesia does not disrupt monitoring of myogenic transcranial motor-evoked responses. J Neurosurg Anesthesiol. 1997;9:228-233.
50. Adams D.C., Emerson R.G., Heyer E.J., et al. Monitoring of intraoperative motor-evoked potentials under conditions of controlled neuromuscular blockade. Anesth Analg. 1993;77:913-918.
51. Caplan R.A., Posner K.L., Ward R.J., Cheney F.W. Adverse respiratory events in anesthesia: a closed claims analysis. Anesthesiology. 1990;72:828-833.
52. Cormack B.S., Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105-1111.
53. Mallampati S.R., Gatt S.P., Gugino L.D., et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can J Anaesth. 1985;32:429-434.
54. Frerk C.M. Predicting difficult intubation. Anaesthesia. 1991;46:1005-1008.
55. Benumof J.L. Practice guidelines for management of the difficult airway. Anesthesiology. 2003;98:1269-1277.
56. Brimacombe J., Berry A. Laryngeal mask airway insertion: a comparison of the standard versus neutral position in normal patients with a view to its use in cervical spine instability. Anaesthesia. 1993;48:670-671.
57. Majernick T., Bieniek R., Houston J., Hughes H. Cervical spine movement during orotracheal intubation. Ann Emerg Med. 1986;15:417-420.
58. Hastings R.H., Wood P.R. Head extension and laryngeal view during laryngoscopy with cervical spine stabilization maneuvers. Anesthesiology. 1994;80:825-831.
59. Chekan R., Weber S. Intubation with or without neuromuscular blockade in trauma patients with cervical spine injury. Anesth Analg. 1990;70:S54.
60. Rhee K.J., Green W., Holcroft J.W., Mangilli J.A. Oral intubation in the multiply injured patient: the risk of exacerbating spinal cord injury. Ann Emerg Med. 1990;19:511-514.
61. Stellin G.P., Barker S., Saboe L., Miller J. Etiology and clinical course of missed spine fractures. J Trauma. 1986;27:980-986.
62. Talcucci R.C., Shaikh K.A., Schwab C.W. Rapid sequence induction with oral endotracheal intubation in the multiply injured patient. Am Surg. 1988;54:185-187.
63. Holly J., Jordon R. Airway management in patients with unstable cervical spine fractures. Ann Emerg Med. 1989;18:1237-1239.
64. Cohn A.I., Zornow M.H. Awake endotracheal intubation in patients with cervical spine disease: a comparison of the Bullard laryngoscope and the fiberoptic bronchoscope. Anesth Analg. 1995;81:1283-1286.
65. Hastings R.H., Vigil A.C., Hana R., et al. Cervical spine movement during laryngoscopy with the Bullard, Macintosh, and Miller laryngoscopes. Anesthesiology. 1995;82:859-869.
66. Watts A.D., Gleb A.W., Bach D.B., Pelz D.M. Comparison of the Bullard and Macintosh laryngoscopes for endotracheal intubation of patients with a potential cervical spine injury. Anesthesiology. 1997;87:1335-1342.
67. Keenan M.A., Stiles C.M., Kaufman R.L. Acquired laryngeal deviation associated with cervical spine disease in erosive polyarticular arthritis. Anesthesiology. 1983;58:441-449.
68. Phelps J.A. Laryngeal obstruction due to cricoarytenoid arthritis. Anesthesiology. 1966;27:518-519.
69. Emery S.E., Smith M.D., Bohlman H.H. Upper-airway obstruction after multilevel cervical corpectomy for myelopathy. J Bone Joint Surg [Am]. 1991;73:544-551.
70. Caldwell M.W., O’Hara R.C., Bloom M.J., et al. Postoperative airway complications in patients undergoing anterior cervical corpectomy with graft placement: a review of the Pittsburgh experience. J Neurosurg Anesthesiol. 1994;6:311.
71. Audu P., Artz G., Scheid S., et al. Recurrent laryngeal nerve palsy after anterior cervical spine surgery. Anesthesiology. 2008;105:898-901.
72. Lee L: ASA postoperative visual loss (POVL) registry. Anesthesia Patient Safety Foundation Newsletter 16(4):56–57, 2001–2002.
73. Stevens W.R., Glazer P.A., Kelley S.D., et al. Ophthalmic complications after spine surgery. Spine (Phila Pa 1976). 1997;22:1319-1324.
74. Warner M.E., Warner M.A., Garrity J.A., et al. The frequency of perioperative vision loss. Anesth Analg. 2000;93:1417-1421.
75. Losasso T.J., Muzzi D.A., Dietz N.M., Cucchiara R.F. Fifty percent nitrous oxide does not increase the risk of venous air embolism in neurosurgical patients operated upon in the sitting position. Anesthesiology. 1992;77:21-39.
76. Cucchiara R.F., Messick J.M., Gronert G.G., Michenfelder J.D. Time required and success rate of percutaneous right atrial catheterization: description of a technique. Can Anaesth Soc J. 1980;27:572-573.
77. Drummond J.C., Moore S.S. The influence of dextrose administration on neurologic outcome after temporary spinal cord ischemia in the rabbit. Anesthesiology. 1989;70:64-70.
78. Vassar M.J., Perry C.A., Gannaway W.L., Holcroft J.W. 7.5% sodium chloride/dextran for resuscitation of trauma patients undergoing helicopter transport. Arch Surg. 1991;126:1065-1072.
79. NIH Consensus Conference. Fresh frozen plasma indications and risks. JAMA. 1984;253:551-553.
80. Murray D.J., Olson J., Strauss R., Tinkder J.H. Coagulation changes during packed red cell replacement of major blood loss. Anesthesiology. 1988;69:839-845.
81. Robertie P.G., Gravlee G.P. Safe limits of isovolemic hemodilution and recommendations for erythrocyte transfusion. Int Anesthesiol Clin. 1992;28:197-204.
82. Woerkens E.C.S.M., Trouwborst A., Lanschot J.J.B. Profound hemodilution: what is the critical level of hemodilution at which oxygen delivery-dependent oxygen consumption starts in an anesthetized human? Anesth Analg. 1992;75:818-821.
83. Kiyohara Y., Fujishima M., Ishitsuka T., et al. Effects of hematocrit on brain metabolism in experimentally induced cerebral ischemia in spontaneously hypertensive rats. Stroke. 1985;16:835.
84. Thurer R.L., Lytle B.W., Cosgrove D.M. Autotransfusion following cardiac operations: a randomized, prospective study. Ann Thorac Surg. 1979;27:500-507.
85. Nuttall G.A., Horlocker T.T., Santrach P.J., et al. Predictors of blood transfusions in spinal instrumentation and fusion surgery. Spine (Phila Pa 1976). 2000;25(5):596-601.
86. Richardson D.W., Robinson A.G. Desmopressin. Ann Intern Med. 1980;103:228-239.
87. Guay J., Reinberg C., Poitras B., et al. A trial of desmopressin to reduce blood loss in patients undergoing spinal fusion for idiopathic scoliosis. Anesth Analg. 1992;75:405-410.
88. Kobinsky N.L., Letts R.M., Patel L.R., et al. 1-Desamino-8-D-arginine vasopressin (desmopressin) decreases operative blood loss in patients having Harrington rod spinal fusion surgery. Ann Intern Med. 1987;107:446-450.
89. Lentschener C., Cottin P., Bouaziz H., et al. Reduction of blood loss and transfusion requirements by aprotinin in posterior lumbar spine fusion. Anesth Analg. 1999;89:590-597.
90. Shaw A.D., Stafford-Smith M., White W.D., et al. The effects of aprotinin on outcome after coronary-artery bypass grafting. N Engl J Med. 2008;358:784-793.
91. Gill J.B., Chin Y., Levin A., Feng D. The use of antifibrinlytic agents in spine surgery. J Bone Joint Surg [Am]. 2008;90:2399-2407.