Anatomic and Physiologic Aspects of Airways
Structure
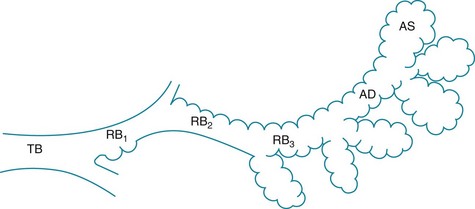
The trachea, bronchi, and bronchioles down to the level of the terminal bronchioles constitute the conducting airways. Their functions are to transport gas and protect the distal lung from inhaled contaminants. Beyond the terminal bronchioles are the respiratory bronchioles. They mark the beginning of the respiratory zone of the lung, where gas exchange takes place. Respiratory bronchioles are considered part of the gas-exchanging region of lung because alveoli are present along their walls. With successive generations of respiratory bronchioles, more alveoli appear along the walls up to the site of the alveolar ducts, which are entirely “alveolarized” (Fig. 4-1). The discussion in this chapter is limited to the conducting airways and those aspects of the more distal airways that affect air movement but not gas exchange. Alveolar structure is discussed further in Chapter 8.
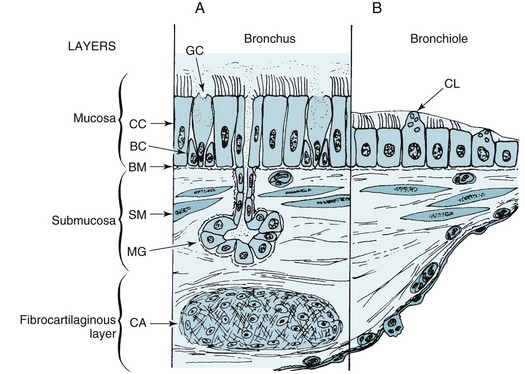
The airways are composed of several layers of tissue (Fig. 4-2). Adjacent to the airway lumen is the mucosa, beneath which is a basement membrane separating the epithelial cells of the mucosa from the submucosa. Within the submucosa are mucous glands (the contents of which are extruded through the mucosa), smooth muscle, and loose connective tissue with some nerves and lymphatic vessels. Surrounding the submucosa is a fibrocartilaginous layer that contains the cartilage rings that support several generations of airways. Finally, a layer of peribronchial tissue with fat, lymphatics, vessels, and nerves encircles the rest of the airway wall. Each of these layers is considered here, with a description of the component cells and the way the structure changes in the distal progression through the tracheobronchial tree.
The surface layer (mucosa) consists predominantly of pseudostratified columnar epithelial cells. The mucosa appears to be several cells thick in the trachea and large bronchi, owing to the columnar shape and variable positions of the nuclei; however, each cell is resting on the basement membrane (see Fig. 4-2, A). The cilia that line the airway lumen are responsible for protecting the deeper airways by propelling tracheobronchial secretions (and inhaled particles) toward the pharynx. The cilia of airway epithelial cells have the characteristic ultrastructure seen in other ciliated cells: a central pair of microtubules and an outer ring of nine double microtubules (see Fig. 22-1). Small side arms called dynein arms, which contain the adenosine triphosphatase (ATPase) dynein, are found on the outer double microtubules. Proper configuration and function of dynein arms are necessary for normal ciliary functioning, and patients with cilia lacking the dynein side arms have impaired ciliary action and recurrent bronchopulmonary infections.
The surface epithelium appears to have other important functions that may be altered in certain clinical conditions. By virtue of tight junctions between epithelial cells at the luminal surface, the epithelium prevents access of inhaled foreign material to deeper levels of the airway wall. Whether a disturbance in this barrier function is important in asthma—perhaps by allowing inhaled foreign material to penetrate the epithelial surface—is not known with certainty. Another important function involves active transport of ions, particularly chloride, to maintain a favorable ionic environment in the mucous layer lining the airway wall. In cystic fibrosis, an abnormality in chloride transport by surface epithelial cells plays a crucial role in the pathogenesis of the disease (see Chapter 7).
The submucosal layer has two major components: bronchial mucous glands and bronchial smooth muscle. Mucus is a gel-like substance composed mostly of water (97%) and mucins. Other proteins including immunomodulators are also present, as well as electrolytes, lipids, and cellular debris. The mucous glands are located between bands of smooth muscle. The base of the glands is lined by mucous cells and serous cells and is connected to the airways by ducts lined by ciliated cells. The duct transports the secretions through the mucosa and discharges them into the airway lumen. As noted earlier, the primary mucin produced by mucous glands is MUC5B. In disease states such as chronic obstructive pulmonary disease and cystic fibrosis, where there is mucous gland hyperplasia, MUC5B becomes more prominent than MUC5AC in bronchial secretions. However, whether the functions of MUC5AC and MUC5B are different is not yet understood. Serous cells also line the mucous gland; these cells secrete proteoglycans and numerous antimicrobial substances involved in innate immunity (see Chapter 22). Airway smooth muscle is present from the trachea down to the level of the bronchioles and even the alveolar ducts. Disturbances in the quantity and function of the smooth muscle are important in disease, particularly in the case of bronchial asthma.
We have thus far described the general structure of the airways, but structure varies considerably at different levels. Some of these differences are illustrated in Figure 4-2. In the progression distally through the tracheobronchial tree, the following changes are normally seen:
1. The epithelial layer of cells becomes progressively thinner until there is a single layer of cuboidal cells at the level of the terminal bronchioles.
2. Goblet cells decrease in number until they disappear about at the level of the terminal bronchiole. Domed-shaped Clara cells appear in the smaller airways, where they contribute to mucus production and other functions.
3. Mucous glands, which are present in the trachea and large bronchi, are most numerous in the medium-sized bronchi. They become progressively fewer in number more distally and are absent from the bronchioles.
4. Smooth muscle changes in configuration at different levels of the tracheobronchial tree. In the trachea and large bronchi, smooth muscle is found either as bands or a spiral network, whereas in the smaller bronchi and bronchioles, a continuous layer of smooth muscle encircles the airway. As airway size decreases distally in the tracheobronchial tree, smooth muscle generally occupies a larger portion of the total thickness of the airway wall. The proportion of smooth muscle to airway wall thickness becomes maximal at the level of the terminal bronchiole.
5. Cartilage also changes in configuration. In the trachea, the cartilaginous rings are horseshoe shaped, with the posterior aspect of the trachea being free of cartilage. In the bronchi, plates of cartilage become smaller and less numerous distally until cartilage is absent in the bronchioles.
The preceding discussion describes many of the structural features of normal airways. However, a variety of changes occur with chronic exposure to an irritant like cigarette smoke. Some of these changes, particularly in the epithelial cells, are important because of the potential for eventual malignancy (see Chapter 20). Other changes are apparent in the mucus-secreting structures (bronchial mucous glands and goblet cells) and are important features of chronic bronchitis. With chronic irritation, the mucous glands hypertrophy, and goblet cells become more numerous and are found more distally than usual, even in the terminal bronchioles. The implications of these changes in disease states are discussed in Chapter 6.
Neural Control of Airways
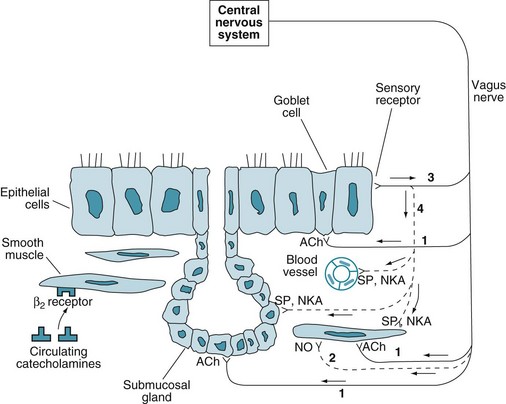
Innervation (neural control) of airways is an important aspect of airway structure, with particular clinical relevance in asthma (see Chapter 5). Neural control of airways affects not only contraction and relaxation of bronchial smooth muscle but also the activity of bronchial mucous glands. An understanding of the innervation, receptors, and mediators involved in neural control of airway function is important both because of the potential role of neural control in the pathogenesis of asthma and because of the well-established role of pharmacotherapy in stimulating or blocking airway receptors. The following discussion focuses on three components of the neural control of airways: the parasympathetic (cholinergic) system, the sympathetic (adrenergic) system, and the nonadrenergic, noncholinergic inhibitory system (Fig. 4-3).
The parasympathetic nervous system provides the primary bronchoconstrictor tone to the airways. This innervation comes from branches of the vagus nerve; stimulation of these branches causes contraction of smooth muscle in the airway wall. In addition, vagal fibers innervate bronchial mucous glands and goblet cells, resulting in increased secretions from both components of the mucus-secreting apparatus. The receptors on smooth muscle and the mucus-secreting apparatus are muscarinic cholinergic receptors; the neurotransmitter is acetylcholine. These cholinergic receptors are more dense in central than in peripheral airways. Identification of multiple muscarinic receptors, elucidation of a variety of effects on both airway smooth muscle and on nerves supplying smooth muscle, and evidence of “cross-talk” among muscarinic and adrenergic receptors have demonstrated that muscarinic receptor signaling actually is much more complicated, but the simplified schema just described provides a practical framework for later discussions about pathophysiology and treatment of airway diseases. The inhaled anticholinergic medications ipratropium and tiotropium block the muscarinic cholinergic receptors, resulting in bronchodilation and decreased mucus production (see Chapter 6.)
The role of the sympathetic (adrenergic) nervous system in controlling airway tone is much less clear because there is sparse if any adrenergic innervation of human airways. Despite the paucity of innervation by sympathetic nerves, there are adrenergic, primarily β2, receptors on bronchial smooth muscle. These receptors are stimulated by circulating catecholamines. When stimulated, β2 receptors activate adenylate cyclase, increasing the intracellular concentration of cyclic adenosine monophosphate (cAMP) and causing relaxation of bronchial smooth muscle. In contrast, stimulation of the less important α-adrenergic receptors results in bronchoconstriction. Receptor density of β2-adrenergic receptors is opposite that of cholinergic receptors; β2-adrenergic receptors are more dense in peripheral than in central airways. Inhaled β-adrenergic agonists cause bronchodilation and are a critical part of asthma treatment (see Chapter 6).
Thus far, only the neural output to (i.e., efferent control of) the airways has been discussed. In addition, there are airway receptors with sensory (afferent) nerve innervation. These receptors, which are located in the airway epithelial layer and responsive to various chemical and mechanical stimuli, include myelinated cough (“irritant”) receptors and unmyelinated C fibers. Neural traffic is carried from these sensory endings in afferent fibers of the vagus nerve. This sensory information not only is communicated to the central nervous system via the afferent vagal fibers but is also responsible for activation of local reflexes causing release of mediators called tachykinins from nerve endings in the airway wall. The tachykinins, which include substance P and neurokinin A, can cause bronchoconstriction, increased submucosal gland secretion, and increased vascular permeability (see Fig. 4-3). However, the magnitude of their importance in disease states (e.g., asthma) is not known with certainty.
Function
Airway Resistance
Table 4-1 lists the total cross-sectional area of the airways at different levels of the tracheobronchial tree. The major site of resistance (the smallest total cross-sectional area) is at the level of medium-sized bronchi. The small or peripheral airways, generally defined as airways less than 2 mm in diameter, contribute only approximately 10% to 20% of the total resistance. Hence, these airways are frequently called the “silent zone” because disease in them can affect their size without significantly altering the total airway resistance. Unfortunately, despite a great deal of work by physiologists to develop methods capable of detecting increased resistance in small airways, the usefulness of such tests has not met original expectations. The correlation between these functional studies and histopathologic confirmation of disease in small airways has been inconsistent; consequently, these tests are used infrequently.
Table 4-1
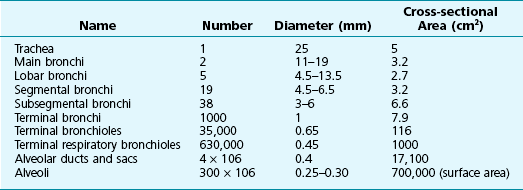
Adapted from Thurlbeck WM: Chronic obstructive lung disease. In Sommers SC, editor: Pathology annual, vol 3, New York, 1968, Appleton-Century-Crofts.
Maximal Expiratory Effort
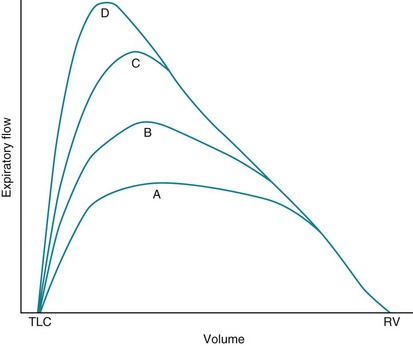
The next important aspect of the physiology of airflow is the distinction between normal breathing and forced or maximal respiratory efforts. A great deal of information can be obtained by looking at flow during a forced expiration (i.e., breathing out from total lung capacity down to residual volume as hard and as fast as possible). In a discussion of this concept, it is useful to consider the flow-volume curve mentioned in Chapter 3 and shown again in Figure 4-4. In this figure, a series of expiratory curves shows the kind of flow rates generated by progressively greater expiratory efforts. Curve A shows expiratory flow with a relatively low effort, whereas curve D shows flow with a maximal expiratory effort.
Two unanswered questions about maximal expiratory flow remain. First, why does critical narrowing of the airways occur such that increasing effort proves fruitless in augmenting flow? Second, at what level in the airways does this critical narrowing occur? Answers to these questions, which have been of great interest to pulmonary physiologists, must be distilled from a large amount of theory and research.*
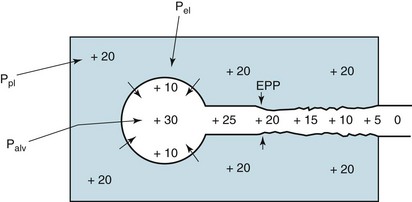
During a forced expiration, there are several determinants of airway diameter. First and most obvious is the inherent size of the airway, which depends on its level in the tracheobronchial tree and the tone of the airway smooth muscle. In disease, smooth muscle tone may be increased (as in asthma), or secretions in the airway may narrow the lumen (as in asthma or chronic bronchitis). Second is the amount of radial traction exerted by surrounding lung tissue on the airway walls. Airways are not isolated structures but are surrounded by a supporting framework of alveolar walls that are constantly “pulling” or “tethering” the airways open. When lung parenchyma is destroyed, as in emphysema, the airways lose some of their normal support and are more likely to collapse (see Chapter 6). Third, and perhaps most difficult to understand, is the combination of pressures acting on the airway from without and within. This balance of pressures is crucial in determining whether a particular airway remains open or closed during a forced expiration.
The external pressure acting on an airway is determined to a large extent by pleural pressure (Fig. 4-5). When pleural pressure is strongly positive, as with a forced expiration, the airway becomes compressed. It is only because of a counteracting pressure within the airways that they are able to remain open in the face of a strongly positive external pressure. Two factors contribute to the internal airway pressure: (1) the elastic recoil of the lungs and (2) pleural pressure transmitted to the alveoli and airways. Figure 4-5 shows that the alveolar wall is like a stretched balloon trying to expel its air. In the same way a balloon, in trying to collapse, exerts pressure on the air inside, the alveolar wall has its elastic recoil that exerts pressure on the gas within. This pressure results in flow from the alveoli through the airways. However, remember that flow through an airway is accompanied by a pressure drop along the airway. At a certain point along the airway, the pressure falls enough so that pressure within the airway becomes equal to the pressure outside the airway (i.e., pleural pressure). This point where the pressure inside the airway is the same as the pressure outside the airway is called the equal pressure point. Increased effort causes increased pleural pressure, which is exerted both internally on the alveolus and externally on the airway wall. The increased pressure on the alveoli (which would increase flow) is therefore matched by the increased external pressure on the airway. Thus, the driving pressure (i.e., the difference between alveolar pressure and the pressure at the equal pressure point) is determined only by the elastic recoil pressure of the lung. With additional effort the increased alveolar driving pressure is exactly balanced by the increased external pressure on the airway, which promotes airway collapse (see Fig. 4-5). As a net result, the elastic recoil pressure, not the pleural pressure produced by a maximal expiratory effort, is the important determinant of maximal expiratory flow, at least in the effort-independent or latter part of a forced expiration. Subsequent chapters show that in diseases with altered elastic recoil, maximal expiratory flow rates are affected by this change in the effective driving pressure for airflow.
Albertine, KH. Anatomy of the lungs. In: Mason RJ, Broaddus VC, Martin TR, et al, eds. Textbook of respiratory medicine. ed 5. Philadelphia: WB Saunders; 2010:3–25.
Barnes, PJ. Neurogenic inflammation in the airways. Respir Physiol. 2001;125:145–154.
Barnes, PJ. Pharmacologic principles. In: Mason RJ, Broaddus VC, Martin TR, et al, eds. Textbook of respiratory medicine. ed 5. Philadelphia: WB Saunders; 2010:159–197.
Canning, BJ, Fischer, A. Neural regulation of airway smooth muscle tone. Respir Physiol. 2001;125:113–127.
Fahy, JV, Dickey, BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247.
Hall, IP. Second messengers, ion channels and pharmacology of airway smooth muscle. Eur Respir J. 2000;15:1120–1127.
Kajstura, J, Rota, M, Hall, SR, et al. Evidence for human lung stem cells. N Engl J Med. 2011;364:1795–1806.
Leslie, KO, Wick, MR. Lung anatomy. In: Leslie KO, Wick MR, eds. Practical pulmonary pathology. A diagnostic approach. Philadelphia: Churchill Livingstone; 2005:1–17.
Parker, D, Prince, A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201.
Proskocil, BJ, Fryer, AD. β2-Agonist and anticholinergic drugs in the treatment of lung disease. Proc Am Thorac Soc. 2005;2:305–310.
Reynolds, SD, Malkinson, AM. Clara cell: progenitor for the bronchiolar epithelium. Int J Biochem Cell Biol. 2010;42:1–4.
Schwartzstein, RM, Parker, MJ. Respiratory physiology: a clinical approach. Philadelphia: Lippincott Williams & Wilkins; 2006.
Tam, A, Wadsworth, S, Dorscheid, D, et al. The airway epithelium: more than just a structural barrier. Ther Adv Respir Dis. 2011;5:255–273.
West, JB. Respiratory physiology—the essentials, ed 9. Philadelphia: Lippincott Williams & Wilkins; 2011.