Chapter 279 American Trypanosomiasis (Chagas Disease; Trypanosoma cruzi)
Etiology
American trypanosomiasis is a caused by Trypanosoma cruzi, a parasitic, flagellated kinetoplastid protozoan (Fig. 279-1). The main vectors for T. cruzi are insects of the order Triatominae, which includes Triatoma infestans, Rhodnius prolixus, and Panstrongylus megistus.
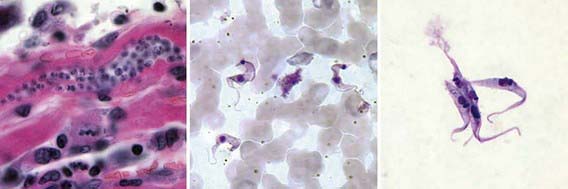
Figure 279-1 Stages of Trypanosoma cruzi. From left to right: amastigote, trypomastigote, and epimastigote.
(From the Centers for Disease Control and Prevention: Laboratory identification of parasites of public health concern. Trypanosomiasis, American [website]. www.dpd.cdc.gov/dpdx/HTML/ImageLibrary/TrypanosomiasisAmerican_il.htm. Accessed August 30, 2010.)
Life Cycle
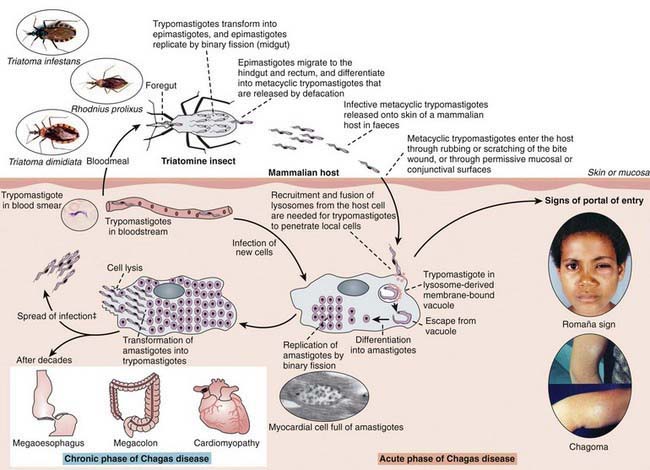
T. cruzi has 3 recognizable morphogenetic phases: amastigotes, trypomastigotes, and epimastigotes (Figures 279-1 and 279-2). Amastigotes are intracellular forms found in mammalian tissues that are spherical and have a short flagellum but form clusters of oval shapes (pseudocysts) within infected tissues. Trypomastigotes are spindle-shaped, extracellular, nondividing forms that are found in blood and are responsible for both transmission of infection to the insect vector and cell-to-cell spread of infection. Epimastigotes are found in the midgut of the vector insect and multiply in the midgut and rectum of arthropods, differentiating into metacyclic trypomastigotes. Metacyclic trypomastigotes are the infectious form for humans and are released onto the skin of a human when the insect defecates close to the site of a bite, entering via the damaged skin or mucous membranes. Once in the host, these multiply intracellularly as amastigotes and are released into the circulation when the cell dies. Blood-borne trypomastigotes circulate until they enter another host cell or are taken up by the bite of another insect, completing the life cycle.
Epidemiology
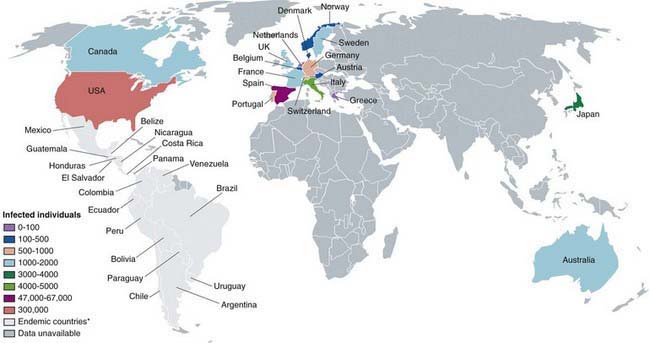
Chagas disease is found only in the Western hemisphere, specifically the Americas, particularly in Southern Patagonia (Fig. 279-3). Natural transmission only occurs within this region, but the disease may arise elsewhere due to migration and transmission through contaminated blood. Multilateral efforts coordinated by the World Health Organization (WHO) in large-scale vector control, blood donor screening to prevent transmission through transfusion, and case-finding and treatment of chronically infected mothers and newborn infants have effectively halted transmission in a number of areas of South America. The number of cases has dropped from a peak of 24 million in 1984 to a current estimate of 8-10 million. The lack of international consensus guidelines on case definition, mass screening, and treatment recommendations for the different stages of the disease severely hamper the prospects for elimination of this disease. Moreover, the lack of clear therapeutic endpoints, logistic difficulties in drug procurement, and substantial drug toxicity presents substantial barriers to the formulation and adoption of universal treatment guidelines.
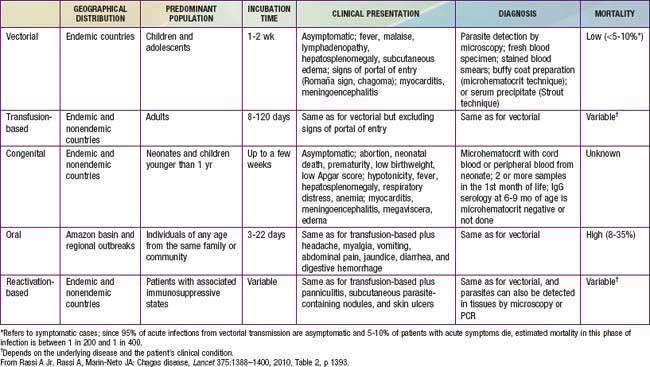
Infection is divided into 3 main phases: acute (Table 279-1), indeterminate, and chronic. Acute infection is the most amenable to treatment. Indeterminate infection is asymptomatic but associated with a positive antibody titer. Up to 30% of infected persons proceed to chronic T. cruzi infection and develop symptoms. While it was initially believed that chronic infection without treatment does not clear, at least 3 well-documented cases of spontaneous resolution without treatment have been reported. It is still unclear how this parasitic protozoan escapes the immune system because, unlike African trypanosomiasis (Chapter 278), antigenic variation is not observed.
Humans can be infected transplacentally, occurring in 10.5% of infected mothers and causing congenital Chagas disease. Transplacental infection is associated with premature birth, fetal wastage, and placentitis. Previously, up to 1,000 neonates infected with T. cruzi were born every year in Argentina; this number has substantially decreased since widespread control programs were initiated. Disease transmission can occur through blood transfusions in endemic areas from asymptomatic blood donors. Seropositivity rates in endemic areas are as high as 20%. The risk for transmission through a single blood transfusion from a chagasic donor is 13-23%. A blood screening test for T. cruzi was approved by the U.S. Food and Drug Administration in December of 2006, and the American Red Cross began routinely screening donated blood in January 2007. Since then, nearly 800 cases of Chagas disease have been detected and confirmed in the USA blood supply (www.aabb.org/Content/Programs_and_Services/Data_Center/Chagas/). Percutaneous injection as a result of laboratory accidents is also a documented mode of transmission. Oral transmission through contaminated food has been reported. Although breast-feeding is a very uncommon mode of transmission, women with acute infections should not nurse until they have been treated.
Pathogenesis
Acute Disease
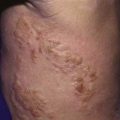
At the site of entry or puncture site, neutrophils, lymphocytes, macrophages, and monocytes infiltrate. T. cruzi organisms are engulfed by macrophages and are sequestered in membrane-bound vacuoles. Trypanosomes lyse the phagosomal membrane, escape into the cytoplasm, and replicate. A local tissue reaction, the chagoma, develops, and the process extends to a local lymph node (see Fig. 279-2). Blood forms appear, and the process disseminates. Immune recognition of parasites is incompletely understood but probably involves toll-like receptor (TLR)-independent mechanisms.
Chronic Disease
T. cruzi strains demonstrate selective parasitism for certain tissues. Most strains are myotropic and invade smooth, skeletal, and heart muscle cells. Attachment is mediated by specific receptors on the trypomastigotes that attach to complementary glycoconjugates on the host cell surface. Attachment to cardiac muscle results in inflammation of the endocardium and myocardium, edema, focal necrosis in the contractile and conducting systems, periganglionitis, and lymphocytic inflammation. The heart becomes enlarged, and endocardial thrombosis or aneurysm may result. Right bundle branch block is also common. Trypanosome parasites also attach to neural cells and reticuloendothelial cells. In patients with gastrointestinal tract involvement, myenteric plexus destruction leads to pathologic organ dilatation. Immunologic mechanisms for control of parasitism and resistance are not fully understood. Despite strong acquired immunity, parasitologic cure in chronic infection is exceedingly rare. Antigenic variation that is typical of African trypanosomiasis (Chapter 278) is not seen with American trypanosomiasis. Antibodies involved with resistance to T. cruzi are related to the phase of infection. Immunoglobulin G antibodies, probably to several major surface antigens, mediate immunophagocytosis of T. cruzi by macrophages. Conditions that depress cell-mediated immunity increase the severity of T. cruzi infection. Macrophages likely play a major role in protection against T. cruzi infection, especially in the acute phase. Interferon-γ stimulates macrophage killing of amastigotes through oxidative mechanisms.
Clinical Manifestations
Acute Chagas disease in children is usually asymptomatic or is associated with a mild febrile illness characterized by malaise, facial edema, and lymphadenopathy (Table 279-1). Infants often demonstrate local signs of inflammation at the site of parasite entry, which is then referred to as a chagoma. Approximately 50% of children come to medical attention with the Romaña sign (unilateral, painless eye swelling), conjunctivitis, and preauricular lymphadenitis. Patients complain of fatigue and headache. Fever can persist from 4-5 weeks. More severe systemic presentations can occur in children younger than 2 yr old and may include lymphadenopathy, hepatosplenomegaly, and meningoencephalitis. A cutaneous morbilliform eruption can accompany the acute syndrome. Anemia, lymphocytosis, hepatitis, and thrombocytopenia have also been described.
Diagnosis
A careful history with attention to geographic origin and travel is important. A peripheral blood smear or a Giemsa-stained smear during the acute phase of illness may show motile trypanosomes, which is diagnostic for Chagas disease (see Fig. 279-1). These are only seen in the 1st 6-12 wk of illness. Buffy coat smears may improve yield.
Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis. 2009;49:e52-e54.
Bern C, Montgomery SP, Herwaldt BL, et al. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA. 2007;298:2171-2181.
Chessler AD, Ferreira LR, Chang TH, et al. A novel IFN regulatory factor 3-dependent pathway activated by trypanosomes triggers IFN-beta in macrophages and fibroblasts. J Immunol. 2008;181:7917-7924.
Dias JC, Dias E, Filho OM, et al. Further evidence of spontaneous cure in human Chagas disease. Rev Soc Bras Med Trop. 2008;41:505-506.
Jannin J, Villa L. An overview of Chagas disease treatment. Mem Inst Oswaldo Cruz. 2007;102:S95-S97.
Médecins Sans Frontières International meeting. new diagnostic tests are urgently needed to treat patients with Chagas disease. Campaign for Access to Essential Medicines. Rev Soc Bras Med Trop. 2008;41:315-319.
Medei EH, Nascimento JH, Pedrosa RC, et al. Role of autoantibodies in the physiopathology of Chagas’ disease. Arq Bras Cardiol. 2008;91:257-262. 281–286
Milei J, Guerri-Guttenberg RA, Grana DR, et al. Prognostic impact of Chagas disease in the United States. Am Heart J. 2009;157:22-29.
Rassi AJr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388-1400.
Salazar-Schettino PM, Perera R, Ruiz-Hernandez AL, et al. Chagas disease as a cause of symptomatic chronic myocardiopathy in Mexican children. Pediatr Infect Dis J. 2009;28:1011-1012.
Villar JC, Marin-Neto JA, Ebrahim S, et al: Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst Rev 1:CD003463, 2002.
Yadon ZE, Schmunis GA. Congenital Chagas disease: estimating the potential risk in the United States. Am J Trop Med Hyg. 2009;81:927-933.