CHAPTER 16 Amenorrhoea, oligomenorrhoea and hypothalamic–pituitary dysfunction
Introduction
Amenorrhoea and oligomenorrhoea are symptoms of ovarian and reproductive dysfunction. Patients thus commonly present to the gynaecologist with complaints of problematic menstruation or fertility delay. This chapter provides an overview of the current understanding and general management of the associated disorders of the hypothalamic–pituitary–ovarian (HPO) axis that result in amenorrhoea and oligomenorrhoea. These symptoms are also features of the polycystic ovary syndrome (PCOS), and a detailed description of the symptoms, diagnosis and management of PCOS is provided in Chapter 18.
Definitions
Normal menstruation
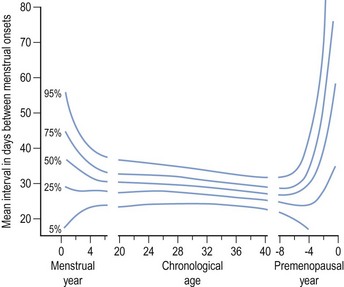
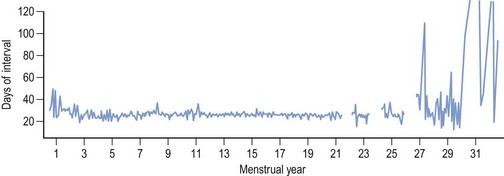
Regular monthly menstruation is a phenomenon of modern society. Furthermore, the availability of effective contraception has enabled couples to choose both the size and timing of their desired family. The classic data (derived from 22,754 calendar years of experience) from the studies of Treloar et al (1967) demonstrated that each woman has her own central trend and variation in menstrual cycle length, both of which change with age (Figure 16.1). The first (and last) few years of menstrual life are marked by a variable pattern of mixed short and long intervals, with a characteristic transition into and out of the more regular pattern of middle life (Figure 16.2). The length, regularity and frequency of normal menstrual cycles have been described in both population and observational studies (Harlow and Ephross 1995, Fraser and Inceboz 2000). Mean menstrual cycle length between the ages of 20 and 34 years varies between 28 and 30.7 days (range 19.7–43.5 between the 5th and 95th centiles). Physiologically regular menses indicate cyclical ovarian activity, in turn dependent upon an intact HPO axis. Aberrations in menstrual pattern are thus an indication of disorder of ovarian function. The average age of the menarche is 12.8 years, but this has been gradually decreasing. Factors such as ethnic origin, socio-economic status and nutrition can affect age of menarche. With obesity presently such a prominent problem, it is interesting to note that there is a relationship between age of menarche and body mass index (BMI), with early menarche associated with raised BMI (Golden and Carlson 2008).
Causes of Amenorrhoea
Causes of amenorrhoea are classified according to a systematic endocrine approach and are listed in Box 16.1 (Baird 1997). Disorders in other endocrine systems, such as thyroid disease and adrenal disease, may result in amenorrhoea.
Box 16.1
Classification of causes of amenorrhoea
Adapted from Baird, Amenorrhoea. Lancet 350:275–279. © The Lancet Ltd 1997.
Physiological amenorrhoea
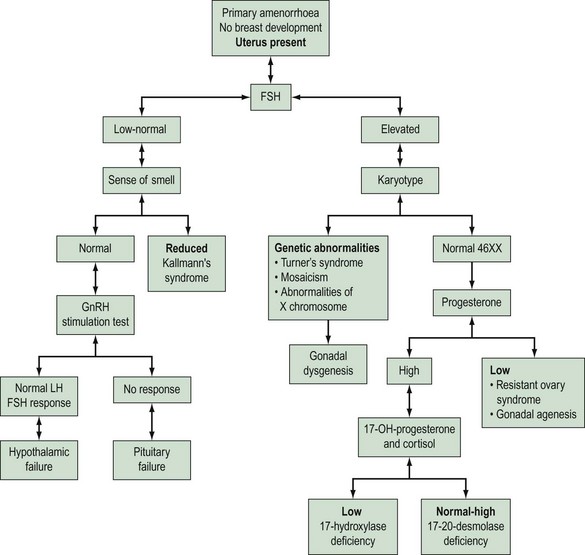
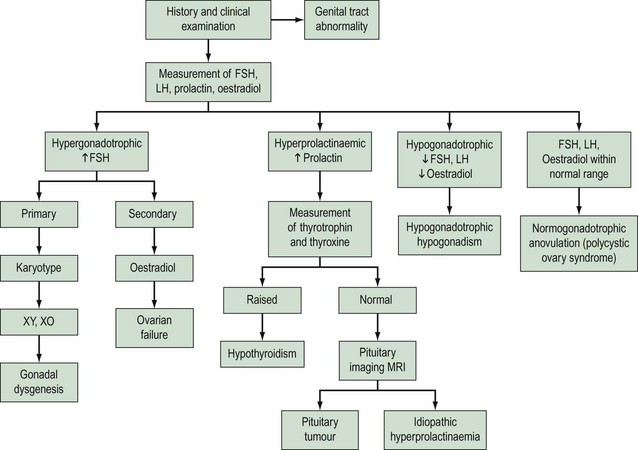
Amenorrhoea is physiological at certain critical times in a woman’s life, these being prepuberty, during pregnancy and lactation, and in the postmenopausal period. Amenorrhoea, if not physiological, has an estimated prevalence in the female population of reproductive age of 1.8–3% (Pettersson et al 1973). Amenorrhoea may be either primary or secondary. The prevalence of secondary amenorrhoea is in the order of 2–5% (Singh 1981). The aetiology of primary and secondary amenorrhoea may be similar. Causes of primary amenorrhoea are listed in Box 16.2. In practice, investigation of primary amenorrhoea is usually initiated by the age of 14 years if there is evidence of delayed puberty (absent secondary sexual characteristics and absent menses), or no menstruation within 4 years of the onset of adrenarche and thelarche (Zreik and Olive 1998). The diagnosis of cause of primary amenorrhoea may be categorized depending on whether or not the uterus is present, and whether or not there is breast development. Zreik and Olive (1998) described a very useful scheme to aid diagnosis of primary amenorrhoea (Figure 16.3).
Anatomical causes
Anatomical abnormalities of the genital tract account for approximately 1% of cases of amenorrhoea. The anatomical causes of amenorrhoea are summarized in Box 16.3. In girls with breast development but evidence of an absent uterus, two disorders need to be considered. First, congenital absence of the uterus (Müllerian agenesis, Mayer–Rokitansky–Kuster–Hauser syndrome) is due to an early development failure of the Müllerian system. Affected girls have a normal XX karyotype, normal ovaries and secondary sexual characteristics. The vagina is absent or hypoplastic. Magnetic resonance imaging is a useful adjunct diagnostic test for establishment of the diagnosis, thereby avoiding laparoscopy. Since anomalies of the Wolffian duct system may be present in these patients, an intravenous urogram is an important investigation for this condition. The second disorder to consider is testicular feminization or androgen insensitivity, an X-linked inherited disorder. Patients have a 46XY karyotype, a female phenotype and undescended testes. The uterus is absent and there is a short, blind vaginal pouch. The X-linked androgen receptor is essential for androgen action, leading to normal primary male sexual development prior to birth (masculinization). Thus, androgen receptor dysfunction in XY individuals results in androgen insensitivity syndrome. Diagnosis of this syndrome is established on clinical findings, endocrine investigations and, if possible, family history. There are three phenotypes: complete androgen insensitivity syndrome (CAIS), partial androgen insensitivity syndrome (PAIS) and mixed androgen insensitivity syndrome (MAIS). CAIS is most often diagnosed on clinical findings and laboratory investigations, and PAIS and MAIS usually require a family history consistent with X-linked inheritance (Gottlieb et al 1999). Androgen insensitivity may be caused by several mutations of the androgen receptor (Gottlieb et al 1999), resulting in a lack of androgenization during sexual differentiation (Imperato-McGinley 1995). Development of the uterus and upper vagina is inhibited, as the secretion of anti-Müllerian hormone (AMH) by the testes is normal in these patients. Recently, due to nationwide cooperation between paediatric endocrinologists in the UK, an extensive database (278 cases) of phenotypic features, androgen receptor binding, and mutational analysis of cases of intersex and ambiguous genitalia has been established (Ahmed et al 2000). All cases of PAIS presented within the first month of life. The median age for presentation of individuals with CAIS was 1 year. The gonads were removed before puberty in 66% of cases with CAIS, and after puberty in 29% of cases. The indication for gonadal removal is the high incidence of neoplasia (5%).
Box 16.3 Anatomical causes of amenorrhoea
A further anatomical cause of primary amenorrhoea is the presence of an imperforate hymen that obstructs the outflow of menses. Girls commonly present with cyclical pelvic pain and a delayed menarche. Secondary amenorrhoea can result from anatomical abnormalities; the endometrium may be destroyed as a result of infection (e.g. pelvic tuberculosis) or iatrogenically, as a result of an endometrial ablation procedure. Complications of uterine curettage can also result in amenorrhoea. An example of this is Asherman’s syndrome (Asherman 1948), which is defined by the presence of intrauterine permanent adhesions, obliterating the uterine cavity either partially or completely. The main cause of this syndrome is the procedure of endometrial curettage required to treat secondary postpartum haemorrhage due to retained placental products. It has also been suggested that missed miscarriages are an important risk factor for the development of adhesions.
Hypothalamic–pituitary dysfunction (endocrine causes)
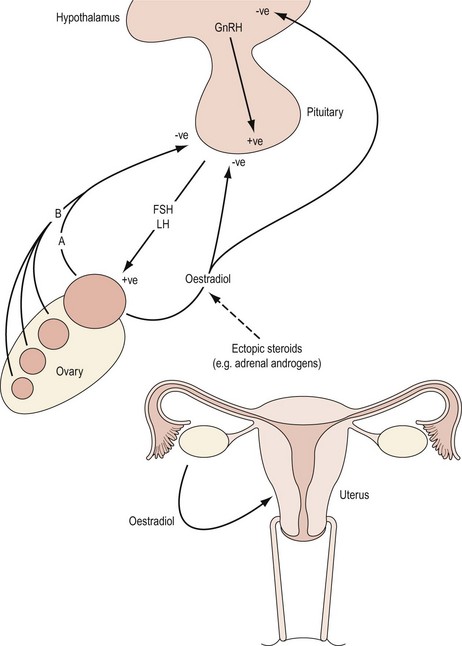
The hypothalamic–pituitary axis is regulated at two levels. At a higher level, gonadotrophin-releasing hormone (GnRH) neurones within the hypothalamus are stimulated by afferent inputs from the central nervous system. Furthermore, there is endocrine control of GnRH synthesis and secretion by means of gonadal feedback mechanisms and GnRH itself. At a lower level, the output of FSH and luteinizing hormone (LH) by the gonadotropes in the anterior pituitary reflects GnRH activity. Gonadotrophin synthesis and secretion are, in turn, influenced by endocrine feedback from the ovaries as well as paracrine mechanisms and other extrinsic factors. Endocrine causes of oligomenorrhoea and amenorrhoea include disorders of the HPO axis that are summarized in Box 16.4. Disturbances in menstrual pattern will be the consequence of either a structural or functional defect in the tightly controlled feedback system involving the hypothalamus, anterior pituitary and ovary (Baird 1997; Figure 16.4). If the uterus is present and there is no breast development, absence of menstruation may be due to failure of secretion of GnRH or to failure of pituitary or gonadal function (see Figures 16.3 and 16.4). Hypothalamic amenorrhoea suggests an intact HPO axis.
Kallman’s syndrome
Kallman’s syndrome, occurring in 1 : 50,000 girls (Kallman et al 1944), has the associated symptom of anosmia and is an inherited autosomal-dominant or X-linked autosomal-recessive anomaly. The impairment of olfactory sensation is often subtle. Women with this condition typically present with primary amenorrhoea and poor development of secondary sexual characteristics. If untreated, infertility results. The gonadotrophin deficiency is due to an inability to activate pulsatile GnRH secretion (Leiblich et al 1982). Five Kallman’s syndrome genes have been identified to date. The syndrome appears to result from insufficient cell signalling through fibroblast growth factor receptor 1 (FGFR1), in which integrins play a role in signalling, and the prokineticin receptor 2, a G-protein coupled receptor essential for normal development of the olfactory bulbs and sexual maturation (Abreu et al 2008). Some cases are due to a mutation in the KAL gene situated on the p22.3 region of the X chromosome (tip of the short arm) that codes for an adhesion-molecule-like X chromosome (Legouis et al 1991). This protein has homology with fibronectin and plays a role in the migration of GnRH-like neurones from the nasal pit to the hypothalamus (Rugarli and Ballabio 1993). KAL1 gene mutations account for 33–70% of cases of the X-linked form of Kallman’s syndrome. Ovulation induction can achieve pregnancy for these women, but requires both exogenous FSH and LH to successfully stimulate follicular maturation and ovarian steroidogenesis.
Hypogonadotrophic hypogonadism
Female hypogonadism refers to deficient or abnormal function of the HPO axis that clinically presents with menstrual cycle disturbances. Female hypogonadism can be due to a congenital or acquired cause, and the defect can be at the level of the hypothalamus, pituitary or ovary. It is characterized by reduced secretion of FSH (although this can be normal) and LH. There is a consequent failure of follicular development and oestradiol production by the ovaries. A hypo-oestrogenic state thus prevails. If the situation is present before puberty, the girl will present with primary amenorrhoea and a lack of secondary sexual features. Usually, no organic lesion is identified in the hypothalamus or anterior pituitary, and the situation is considered to be idiopathic. Mutations in three genes account for 15–20% of all cases of idiopathic hypogonadotrophic hypogonadism: KAL1, GNRHR and FGFR1. Nearly all mutations are point mutations (Pederson-White et al 2008). Severe weight loss, psychological stress and chronic debilitating disease are all associated with a cessation of hypothalamic function, and are conditions that will be addressed later.
Hypothalamic and pituitary lesions
The most likely hypothalamic lesion to present to a gynaecologist is a craniopharyngioma. Compression of the hypothalamus will suppress GnRH secretion and interrupt portal flow of GnRH in the pituitary stalk. The peripubertal period is the most common age for presentation. The lesions are cystic and often calcified, and are readily recognizable on a lateral skull radiograph or with imaging techniques, such as magnetic resonance imaging. Other tumours which may affect hypothalamic–pituitary function are gliomas (which may arise from the optic tract), meningiomas, endodermal sinus tumour (yolk sac carcinoma), which secretes α-fetoprotein, and congenital hamartomas composed of GnRH neurosecretory cells which can lead to precocious puberty (Yen 1999). Congenital absence of the anterior pituitary gland along with other midline structural defects is extremely rare. Primary deficiency of pituitary hormone secretion is also very uncommon. Growth hormone deficiency may occur in isolation or with panhypopituitary dwarfism. Pituitary failure may be secondary to other organic disease, such as pituitary adenoma, mumps, encephalitis, infarction (Sheehan’s syndrome, secondary to major obstetric haemorrhage) and irradiation. Pituitary cells are relatively resistant to irradiation compared with other brain tissues that are more radiosensitive. Disturbance of pituitary function may therefore be indirectly due to hypothalamic damage. Subjects with gonadal failure are oestrogen deficient and have elevated gonadotrophins (hypergonadotrophic hypogonadism). Causes of gonadal failure are listed in Box 16.5 and will be addressed in detail later. One of the most common chromosomal causes of primary amenorrhoea is Turner’s syndrome. Enzyme-deficiency states associated with primary amenorrhoea include galactosaemia and 17-hydroxylase deficiency.
Central nervous system–hypothalamic disturbance
The modulating influence of extrahypothalamic brain centres on the pulsatile nature of hypothalamic GnRH secretion is addressed in Chapter 15. Psychological disorders account for approximately one-third of cases of amenorrhoea due to central nervous system–hypothalamic disturbance. Functional disorders of the hypothalamic–pituitary axis that cause amenorrhoea result from weight loss, extreme exercise and psychological stress. In each of these situations, there is a decrease in GnRH neuronal activity in the hypothalamus with a subsequent decrease in gonadotrophin secretion (FSH and LH). Ovarian follicular development and ovulation fail to occur if LH pulsatility is less frequent than every 2 h (Baird 1997).
Weight-related amenorrhoea
Marked weight loss, such as that occurring with anorexia nervosa, may result in amenorrhoea. However, menstrual irregularity is an associated feature of all eating disorders rather than being restricted to anorexia nervosa alone. The amount of weight loss that may result in cessation of menstruation varies from a few kilograms in an adolescent who is dieting to a loss of up to 50% of body weight in women with anorexia nervosa (Warren 1995). Regular menses are unlikely to occur in subjects with BMI <19 kg/m2 (Balen et al 1995). It has been reported that nearly one-fifth of the body mass should be adipose tissue for ovulatory cycles to be sustained (Frisch 1984). The rate of loss of weight seems to be important, and rapid loss is frequently associated with psychological disturbance. The chronic hypo-oestrogenic state that becomes established in long-standing amenorrhoea carries significant risk of premature osteoporosis and cardiovascular disease. Irregular cycles and anovulation are also common in PCOS and may be due to the commonly associated raised BMI. The insulin resistance with PCOS which is exacerbated by an increase in BMI affects the intraovarian response to gonadotrophins (Greer et al 1980).
Exercise-related amenorrhoea
Irregularities of menstrual pattern are reported in association with many competitive sporting activities. There is typically a progressive failure of regular menses with anovulatory cycles and amenorrhoea and, in prepubertal girls, a delayed menarche. Usually, the degree of menstrual aberration reflects the intensity and length of sporting activity (Yen 1999). Secondary amenorrhoea and oligomenorrhoea are also common in professional dancers (Warren et al 1986). Women with hypothalamic amenorrhoea associated with an excessive exercise habit, weight loss and stress have hypercortisolism, on account of raised corticotrophin-releasing hormone (CRH) and adrenocorticotrophic hormone. Consequently, there is likely to be disruption of reproductive function as CRH directly inhibits GnRH secretion, possibly via increased endogenous opioid secretion (Speroff et al 1999a). A detailed study was reported by Laughlin and Yen (1996) which addressed the interactions between energy balance and regulators of metabolic fuel and the association with reduced LH pulsatility in women with exercise-induced menstrual dysfunction. Notable differences were observed in nutritional intake, insulin/glucose dynamics, the somatotrophic axis and LH pulsatility with both degree of exercise and menstrual pattern. Those athletes who were amenorrhoeic demonstrated an increase in insulin sensitivity and a reduced hypoglycaemic effect of insulin-like growth factor 1 (IGF-1), along with raised growth hormone and cortisol concentrations. The authors suggest that there is a cascade of glucoregulatory adaptations designed to redistribute metabolic fuel and thereby conserve protein. Furthermore, the reduced GnRH/LH pulse generator activity is in response to both a reduced stimulatory effect of IGF-1 (due to increased IGF-binding protein 1) and central negative effects of corticotrophin-releasing factor. The outlook for women with stress-/exercise-related amenorrhoea is very good if recognized early. Most women see a return of ovulatory cycles with weight gain or reduction in levels of stress or exercise habit (Speroff et al 1999a). Hormone replacement may be indicated for women with long-standing hypothalamic amenorrhoea as there will be a risk of bone loss and cardiovascular changes.
Metabolic hormones regulating reproductive function
Kisspeptins, and their cognate receptor gpr-54, were first found to regulate the hypothalamic–pituitary–gonadal axis in 2003, when two groups demonstrated that mutations in gpr-54 cause idiopathic hypogonadotropic hypogonadism characterized by delayed or absent puberty (Roseweir and Millar 2009). KiSS1 neurones have recently been suggested to mediate leptin’s effect on the reproductive system by encoding hypothalamic neuropeptides. Leptin is the primary product of the ob gene and is a 167 amino acid peptide made exclusively in adipose tissue; it acts on the hypothalamus. It is likely that leptin plays a central role in energy production, reproduction and weight, and it has been proposed as a mediator between adipose tissue and the gonads (Matkovic et al 1997). In both eating disorders and excessive exercise, amenorrhoea results from an adaptive response to an energy deficit, partially mediated by leptin. A critical blood leptin level has been reported as necessary to trigger reproductive function in women, suggesting a threshold effect. In this context, severe weight loss is known to result in subnormal gonadotrophin concentrations. Leptin receptors have been identified in the hypothalamus, and leptin inhibits neuropeptide Y (NPY) synthesis and release. A link between leptin and GnRH neurones is, in part, mediated by NPY. Secondary leptin deficiency can present as weight-related amenorrhoea in women (Conway and Jacobs 1997). Speroff et al (1999a) noted that CRH is elevated in stress- and, particularly, weight-loss-related amenorrhoea. It has been proposed that the decrease in leptin and increase in NPY described in stress-related weight loss are inadequate to suppress the stress-induced increase in CRH. Moreover, the increase in CRH and resulting hypercortisolism exacerbate the increase in metabolism and weight loss. Research leading to a better understanding of the metabolic regulation of reproductive function has implications for the prevention and management of reproductive dysfunction and its associated comorbidities.
Neurological and psychiatric disorders
Epilepsy, bipolar disorder and migraines are common disorders which can be associated with disturbances in menstrual function in adolescent girls. With epilepsy, it is thought that both the disease itself and the treatment medications contribute to the aetiology of the menstrual abnormality by disturbing the HPO axis. Many antipsychotic drugs are also dopamine antagonists, and can cause prolactin levels to increase up to 10-fold, again resulting in inhibition of the HPO axis (see Box 16.6).
Idiopathic delayed puberty
The limiting factor for increasing amounts of gonadotrophin secretion as normal puberty approaches is hypothalamic GnRH release (Yen 1999). Delayed puberty may be recognized by the age of 14 years if breast development is still absent. Most cases are due to idiopathic hypothalamic failure and usually resolve spontaneously. There may often be a family history of late puberty. Enquiry should seek any history of impaired olfaction, marked weight loss, extremes of exercise and ill health. Other causes of amenorrhoea with oestrogen deficiency should be excluded with a lateral skull radiograph/hypothalamic–pituitary imaging (non-endocrine tumours, e.g. craniopharyngioma), and serum prolactin (prolactinoma) and FSH measurements (ovarian dysgenesis). If the diagnosis is idiopathic delayed puberty, it remains difficult to distinguish whether the failure is purely delayed or permanent, and thus treatment should commence without delay. Once the patient’s secondary sexual characteristics are mature, treatment may be periodically interrupted to establish whether there is spontaneous HPO activity.
Hyperprolactinaemia
In girls with primary amenorrhoea with normal breast development and a uterus, hyperprolactinaemia must be considered. Approximately 15–20% of women with secondary amenorrhoea have elevated serum prolactin concentrations (Jacobs et al 1976). Unlike the other trophic hormones secreted by the anterior pituitary, prolactin secretion is regulated primarily by inhibition from the hypothalamus by dopamine. Prolactin secretion is not subject directly or indirectly to negative feedback by peripheral hormones. Regulation of secretion is via a short-loop feedback on hypothalamic dopamine by means of a countercurrent flow in the hypophyseal portal system (as well as inhibition of GnRH pulsatile secretion). Prolactin secretion is stimulated by peripheral oestrogen, and this is derived from the placenta in pregnancy.
Circulating concentrations of prolactin rise during pregnancy; at term, the levels are four to 20 times higher than those in non-pregnant women. After delivery, prolactin levels decline in non-lactating women over a 15–20-day period. In lactating women, circulating levels of prolactin are maintained by suckling, and hyperprolactinaemia may last for up to 2 years. During breastfeeding, there is an increase in the sensitivity of the HPO axis to the negative feedback effect of oestradiol (Illingworth et al 1995). The duration and causes of this period of hypersensitivity to oestrogen are unknown. Data are available to support the concept that the suckling-induced suppression of the GnRH system during lactation is associated with enhancement of the negative effects of oestradiol on the hypothalamic GnRH system (Perheentupa et al 2000). Pathological hyperprolactinaemia is associated with amenorrhoea. There is no ovarian function, reduced or absent pulsatility of LH secretion, and an absence of positive feedback response to oestrogen. Treatment of raised prolactin concentrations restores ovulation in 80–90% of women (Randeva et al 2000), and normal cyclical activity returns once prolactin levels are reduced. Pathological hyperprolactinaemia may be induced by drugs that inhibit dopamine action or production. A list of potential compounds is given in Box 16.6. Pharmacological agents account for 1–2% of cases (see Box 16.6). The most common causes of hyperprolactinaemia are pituitary prolactinomas (40–50% of cases) and idiopathic hypersecretion. Primary hypothyroidism will be the coincident diagnosis in 3–5% of cases. Rare causes are ectopic production from a distant extrapituitary tumour, and chronic renal failure may be associated with hyperprolactinaemia, due to both decreased excretion of the hormone and central mechanisms affecting dopamine secretion. Only about half of patients who present with hyperprolactinaemia describe galactorrhoea. Moreover, only about half of the women who report galactorrhoea have raised prolactin levels (Baird 1997). Serum prolactin measurements are essential. Usually, a second sample is required if the first is raised. A common problem is the misinterpretation of the upper normal limit of a geometric (skewed) distribution of normal values. The upper limit is taken at approximately 800 mU/l. The functional relevance of borderline hyperprolactinaemia is unclear. It is most important to consider the prolactin concentration in the context of the associated ovulatory disorder. Significant hyperprolactinaemia is usually associated with oligomenorrhoea or amenorrhoea. Mild hyperprolactinaemia is identified with some cases of PCOS. The phenomenon is likely to be the consequence of excess oestrogenic stimulation. Conversely, prolactin levels are low in hypothalamic amenorrhoea and are likely to be due to lack of oestrogenic stimulation. Thyroid-releasing hormone stimulates prolactin. This can be raised in hypothyroidism, so thyroid function tests should be checked in hyperprolactinaemia. In addition, serotonin can increase prolactin with resulting possible effects from the use of drugs such as selective serotonin reuptake inhibitors.
The mechanism by which hyperprolactinaemia interferes with ovarian function is not clearly defined. It has been suggested that in hyperprolactinaemia, endogenous opioids may have an inhibitory effect on hypothalamic GnRH-secreting neurones (Grossman et al 1982). However, this is not a consistent observation, and prolactin may, in fact, have a direct effect on hypothalamic activity. Although serum levels of prolactin in women with idiopathic hyperprolactinaemia or with pituitary tumours may normalize after treatment with a dopamine agonist (or after pituitary surgery), there are many issues about treatment and long-term follow-up that remain unresolved. Recently published guidelines have advised that the minimal length of dopamine agonist therapy should be 1 year (Casanueva et al 2006). A recent report indicates that dopamine agonists can be safely withdrawn in patients with long-term normalization of prolactin levels and no evidence of tumour on magnetic resonance imaging (Colao et al 2003). The natural history of hyperprolactinaemia is not known. Interestingly, as many as 10% of patients may have a spontaneous remission (Glasier et al 1987). In a longitudinal prospective 3–7 year study of 30 women with untreated hyperprolactinaemia, with annual clinical, radiographic and hormonal evaluation, progression of the disease was observed to be unlikely; in fact, some women showed clinical and radiographic improvement (Schlechte et al 1989).
Ovarian failure
It is well established that the number of follicles in the human ovary declines steadily from midlife onwards. The onset of menopause is prompted by the number of ovarian follicles falling below a critical number as the consequence of the programmed disappearance of a limited store of follicles. The process is irreversible as oogonal stem cells disappear after birth. The loss of follicles is age dependent, and the rate of disappearance increases with age. There is a reported acceleration of loss from the age of 37.5 years (Faddy et al 1992). In this context, Richardson et al (1987) observed that entry into the perimenopausal phase of life was associated with a marked decline in follicle reserve, and that the reserve of follicles was nearly exhausted as menopause approached.
Premature ovarian failure (POF), or more appropriately primary ovarian insufficiency, is defined as the triad of amenorrhoea, oestrogen deficiency and elevated concentrations of FSH and LH in women less than 40 years old. It also includes women with primary amenorrhoea; that is, those who have no known prior ovarian function. Secondary ovarian failure occurs in women who have previously menstruated. The causes of both are similar. In patients diagnosed with POF, particularly with a recent diagnosis or a fluctuating FSH, there has been a reported pregnancy rate of 5–10% (van Kasteran and Schoemaker 1999). For those less fortunate, oocyte donation is a possible alternative.
The age that best separates ‘premature’ from ‘normal’ menopause is arbitrary. The definition of POF assumes that those patients with the disorder constitute a group with specific characteristics that distinguish them from patients with normal menopause (Alper et al 1986). Forty years is the preferred age for definition for practical purposes. Many women with POF at 40 years or younger are concerned with fertility. If one were to define abnormality as those values greater than, or less than, two standard deviations (SD) from the mean (where 95% of the observations of a normally distributed variable are found), then 40 years is also an appropriate age. If the age of menopause is assumed to be a normally distributed variable, Walsh (1978) reported the mean age of menopause as 50.4 years (SD 3.72 years), and 2.5% of women reach menopause at 43 years of age or younger. There are no unique features that unequivocally establish the diagnosis of POF. Indeed, the diagnosis of POF is often delayed, even with the classic symptoms of menopause. At least 4 months with missing or irregular periods may indicate carrying out a serum FSH which, if elevated in the menopausal range on at least two occasions a few weeks apart, is a useful diagnostic test; ultrasound and ovarian biopsy have no role. A delay in diagnosis may result in preventable bone loss (Neale 2009). Ovarian failure itself is an irreversible pathological process with major implications for the patient (in particular, reduced bone mineral density, increased cardiovascular risk and infertility), and thus its diagnosis is an important responsibility for the gynaecologist.
The incidence of POF is not precisely known. Calculations based on the incidence of permanent secondary amenorrhoea suggest 2–3% (Bachmann and Kemmann 1982). Coulam et al (1986) reported POF in 1–3% of the general population. These latter authors conducted a detailed assessment of age-specific incidence rates of natural menopause for a cohort of 1858 women during a 4-year period, and identified a 1% risk at 40 years of age. The incidence at 30 years of age is 0.1% and decreases as age decreases (Anasti 1998). As such, the condition is not rare.
Advances in modern molecular biology have enabled major contributions to be made to understanding the aetiology of POF; however, despite this, only one-third of women have an identifiable pathology (Conway 1997). POF indicates absent ovarian function; it is not a disease but a clinical state and is often a fluctuating condition. Ovarian failure may be primary or secondary, and the chromosomes of the patient may be normal or abnormal. Common causes of POF are summarized in Box 16.5. Genetic disorders are the most common identifiable causes. Chromosomal anomalies are found in women with primary amenorrhoea and ovarian dysgenesis. In Turner’s syndrome, there is an X chromosome deletion. Typically, these patients have streak ovaries, are of short stature and have a characteristic phenotype, including one or some of the following features: neck webbing, widely spaced nipples, increased carrying angle and coarctation of the aorta. Approximately two-thirds of Turner’s patients have the total loss of one X chromosome, and the rest either exhibit a structural abnormality in one X chromosome or display mosaicism with an abnormal X chromosome (Speroff et al 1999b). Menstrual function and pregnancy are possible among patients with XO mosaicism. In Turner’s girls, 10–20% experience spontaneous puberty and 2–5% experience spontaneous menstruation (Pasquino et al 1997). Girls with ovarian dysgenesis may be grouped according to their karyotype (Speroff et al 1999c). Fifty percent of girls will have Turner’s syndrome and 25% will be mosaics (45X/46XX). Patients may have gonadal dysgenesis and a normal XX chromosome complement (25%). This is pure gonadal dysgenesis and the girls invariably have streak ovaries (Sohval 1965), diagnosis of the latter only being made after direct visualization, usually at laparoscopy. In XY gonadal dysgenesis, the testis fails to develop due to loss or mutation of the sex-determining region of the Y chromosome (Tdy or SRY). The testis-determining gene, Tdy, is situated on the short arm of the Y chromosome, and the gene responsible for the H-Y antigen is on the long arm (Goodfellow and Lovell-Badge 1993). If an XY karyotype is detected, the gonadal streaks should be removed since there is a risk of neoplastic transformation. Recent attention has focused on other genetic determinants of POF. Gonadotrophin resistance as a consequence of an FSH receptor mutation has been described (Aittomaki et al 1995). It has been known for some time that fragile X mental retardation 1 gene (FMR1) is characterized by a dynamic CGG repeat expansion in the 5’ untranslated region. Female carriers of the FMR1 premutation allele are at risk of passing the FMR full mutation (>200 repeats) to their offspring, resulting in mental retardation known as fragile X (De Caro et al 2008). This is the most common inherited form of mental retardation. Women who carry one X chromosome with a fragile X premutation display an increased prevalence of POF (Conway et al 1995). This is estimated to be approximately 15–24% compared with the incidence of 1% in the general population. The US National Society of Genetic Counselors does not currently recommend routine testing of minors for the FMR1 premutation allele. There is ongoing discussion regarding the most appropriate terminology to describe the reproductive abnormalities associated with the FMR1 mutation, sometimes known as fragile X-associated primary ovarian insufficiency.
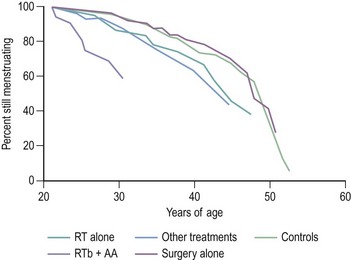
It is felt that the term ‘primary ovarian insufficiency’ may be preferable to ‘POF’ as it better describes what is, in fact, a spectrum of ovarian changes rather than what is sometimes not complete dysfunction of the ovary. Family history remains a predictor of early menopause. In a case–control study of 344 cases of early menopause, a family history of earlier menopause (before 46 years) was associated with an increased risk of early menopause, with the link being strongest in women with a history of menopause before 40 years of age (Cramer et al 1995). Within the last few years, a mutation in the FOXL2 gene, located on chromosome 3 and essential for proper reproductive function in females, has been implicated in POF (Crisponi et al 2001). The authors indicate that this is the first human gene to be identified that may play a role in the maintenance of ovarian follicles. Those carrying mutations in FOXL2 display blepharophimosis/ptosis/epicanthus inversus syndrome, an autosomal-dominant disease associated with eyelid defects and POF. It is quite remarkable that FOXL2 encodes a protein important to both eye and ovarian development. FOXL2 mutations and other gene mutations will only account for a small fraction of cases of POF, but the identification of genes regulated by FOXL2 will contribute to understanding of the biochemical pathways that may be aberrant in POF, and hence potentially offer novel treatments for female infertility in the future. Another gene thought to be related to POF is BMP15. Recent studies have demonstrated heterozygous BMP15 missense mutations in women with POF. Patients with galactosaemia treated in early life with a galactose-free diet exhibit a high frequency of ovarian failure. Affected individuals have mutations of the galactose 1-phosphate uridyltransferase gene (Kaufman et al 1981). The associated feature of ovarian failure is considered to be a consequence of accumulated galactose disturbing the migration of the germ cells from the urogenital ridge to the gonad in fetal life. There is an association between autoimmune disease and ovarian failure, and between 10% and 20% of women with POF have intercurrent autoimmune conditions (Conway et al 1996). These include Addison’s disease, hypothyroidism, pernicious anaemia, Hashimoto’s disease, idiopathic thrombocytopenia, rheumatoid arthritis with vitiligo, alopecia areata, Cushing’s disease, autoimmune haemolytic anaemia and myaesthenia gravis. Among patients in whom the aetiology of POF is obscure, there are other lines of evidence to suggest autoimmune mechanisms. Circulating antibodies to ovarian tissue have been demonstrated in the sera of women with POF. Organ-specific autoimmunity may be directed against the intracellular enzymes involved in hormone production. 3-β-hydroxysteroid dehydrogenase (3-β-HSD) has been identified as an autoantigen in one-fifth of women with POF. This observation now offers a potential marker for autoimmune ovarian damage, although the presence of anti-3-β-HSD antibodies may be the consequence of ovarian inflammation rather than the causal antigen (Arif et al 1996). Infections associated with loss of ovarian function are mumps and pelvic tuberculosis. Environmental factors contributing to an early menopause include cigarette smoking. As a consequence of the improved survival for patients with certain neoplastic conditions treated with radiotherapy and chemotherapy, an increasing number of survivors are facing the absence of ovarian function. Irradiation is known to induce POF and was once a method of castration. The reproductive system is one of the major sites of secondary effects of anticancer treatment (Ogilvy-Stuart and Shalet 1993). Different therapeutic insults will be associated with different risks for early menopause. It is important to have accurate information on the gonadotoxicity of different cancer treatment regimens to improve patient information and optimize approaches for fertility preservation. For survivors of treatment for cancer, treatment with radiotherapy below the diaphragm and alkylating agent chemotherapy, the average age at menopause was approximately 31 years. Other modes of treatment conferred an excess risk of ovarian failure, although not as great (Byrne 1999; Figure 16.5).
Anderson and Cameron (2007) detected changes in the ultrasound markers of the ovarian reserve (OR), with decreases in both ovarian volume and antral follicle count during chemotherapy. Anderson and Cameron (2007) assessed the OR markers in premenopausal women to investigate and compare the effects of chemotherapy and long-term gonadotrophin withdrawal on ovarian function, and detected changes in ultrasound markers of the OR, with decreases in both ovarian volume and antral follicle count during chemotherapy. These data confirm the value of AMH concentration as an early indicator of ovarian ageing, including assessment of chemotherapy-induced ovarian follicle loss. FSH and AMH concentration measurements may be useful for the comparison of ovarian toxicity of different chemotherapy regimens. Adverse effects on female reproductive function may be mediated through effects at one or more levels of the HPO axis (Wallace et al 1989) or at the uterus (Critchley et al 1992). Commonly, anticancer treatment (chemotherapy, particularly alkylating agents, and scatter radiation to the ovary) affects the ovary as a consequence of depletion of the stock of primordial follicles, thereby advancing or inducing menopause (Critchley and Wallace 2005). Effects on the hypothalamus and pituitary may be subtle. High-dose cranial irradiation is known to have direct damaging effects, but the effects of the lower doses used in the management of childhood leukaemia are, as yet, uncertain. Data concerning ovarian function after treatment of standard risk leukaemia have been reassuring (Wallace et al 1993). A recent study by Bath et al (2001) examined HPO function in 12 women in first remission from childhood acute lymphoblastic leukaemia. This study revealed a high prevalence of short luteal phases (<11 days) among these women compared with a normal control group. A reduced LH excretion (probably secondary to cranial irradiation) and ovarian oestrogen production (probably reflecting a reduction in gonadotrophic stimulus) were observed in the women with acute lymphoblastic leukaemia. Such disturbances in LH secrection may have an effect on reproductive potential (Box 16.7).
Ovarian reserve
The OR, a term that has evolved in the era of assisted reproductive technology, refers to the residual oocyte-granulosa cell repertoire. An improved ascertainment of OR status may help to optimize the planned therapeutic intervention, and thus minimize the emotional and financial strain placed upon couples seeking fertility treatment. A spectrum of markers prognostic of the OR are validated to varying degrees in the infertile population. These include biochemical markers (FSH, prostaglandin E2, inhibin B, AMH and FSH:LH ratio) and ovarian morphometeric markers (ovarian volume, antral follicle count and mean ovarian diameter) assessed in the early follicular phase (basal) of the menstrual cycle (Bowen et al 2007).
Raised follicle-stimulating hormone (FSH) is important as it is the first measurable parameter that changes in reproductive ageing. Pituitary FSH production is co-regulated by inhibin and oestradiol, acting via negative endocrine feedback (Muttukrishna et al 2000). Anti-Mullerian hormone (AMH) has recently been suggested as a more accurate indicator of the presence of ovarian follicles (Durlinger et al 2002). Since there is no change in AMH levels in response to gonadotrophins, AMH can be measured throughout the cycle in contrast to the other parameters, which can only be determined during the early follicular phase: an advantage for both patients and clinicians. It reflects earlier stages of follicle development than inhibin B and oestradiol, and thus is believed to more closely approximate the number of primordial follicles in the ovary. Inhibin B is a product of the granulosa cells. It has recently been shown that the value of inhibin B as a measure of the OR is greatly increased following administration of a single dose of FSH to stimulate granulosa cell function in small healthy follicles.
A nationwide prospective cohort study in the Netherlands concluded that, compared with inhibin B and antral follicle count, AMH was more consistently correlated with the clinical degree of follicle pool depletion in young women presenting with elevated FSH levels (Knauff et al 2009). AMH may provide a more accurate assessment of the follicle pool in young hypergonadotropic patients, especially in the clinically challenging subgroups of patients with elevated FSH and regular menses (i.e. incipient ovarian failure), and in hypergonadotropic women with cycle disturbances not fulfilling the diagnostic criteria for POF (i.e. transitional ovarian failure). Ultrasound measurement of both ovarian volume and antral follicle count (the number of small follicles present) is also of value.
Whilst all individual ovarian parameters (width, length or an average of the two) reliably reflect the OR in premenopausal infertile women, the mean ovarian width (as determined by ultrasound) exhibits a more robust relationship with OR status compared with ovarian length or the average of the two dimensions (Fratarelli et al 2002).
Endocrine disorders arising outside the HPO axis leading to chronic anovulation
Other endocrine disorders may interfere with the normal feedback loops of the HPO axis, thereby causing a disturbance in cyclical ovarian activity and manifesting as amenorrhoea or oligomenorrhoea (Baird 1997). Thyroid disease may cause amenorrhoea and oligomenorrhoea. There is an increase in circulating concentrations of oestrogen and testosterone in hyperthyroidism as levels of sex-hormone-binding globulin increase. In hypothyroidism, there may be associated raised levels of prolactin due to stimulation with thyrotrophic-releasing hormone. In adrenal disease, the excessive secretion of sex steroids leads to amenorrhoea, as gonadotrophin secretion will be suppressed.
Diagnosis and Management of Oligomenorrhoea and Amenorrhoea
The successful management of oligomenorrhoea and amenorrhoea is dependent upon the correct diagnosis and an assessment of the requirements of the patient. Patients will articulate different needs which may include advice about future fertility prospects, fertility control, symptoms of hirsutism, delayed secondary sexual development, protection from osteoporosis and endometrial protection from unopposed oestrogen action. A thorough history and clinical examination (as appropriate) are of paramount importance in establishing the diagnosis of an endocrine disorder, complemented with an appropriate examination and conduct of straightforward endocrine investigations. Since the withdrawal of sex steroids results in endometrial bleeding, a detailed menstrual history will be extremely valuable in the determination of endogenous ovarian activity. Provided that the patient has not been administered exogenous hormone preparations, a report of only light menstrual bleeding will indicate sufficient ovarian production of oestrogen to produce endometrial proliferation. Hence if a history is given of oligomenorrhoea, there must be some capacity for ovarian activity. It is essential to exclude an anatomical cause of amenorrhoea. The bimanual assessment of a young woman who has never been sexually active is inappropriate. The examination of secondary sexual characteristics (breast development) and the external genitalia of an adolescent should be performed in the presence of the patient’s parent. Indeed, a deferred examination separate from the initial consultation may be most appropriate, thereby providing an opportunity for confidence to be established in the subsequent doctor–patient relationship. Modern imaging techniques, particularly transabdominal ultrasound, are valuable non-invasive modes of establishing information about reproductive anatomy. In the context of a history of amenorrhoea, an enquiry about experience of hot flushes may elicit a diagnosis of ovarian failure. A detailed drug history (see Box 16.6) and information about diet, exercise habit and weight change are essential. Furthermore, enquiry about life events producing psychological stress should be made. Signs of hyperandrogenism may be evident, such as hirsutism, acne and balding. Enquiry about the presence of galactorrhoea should be made. A distinction is required between hyperandrogenism and virilization, and acanthosis nigricans is a feature of severe insulin resistance. An assessment of the development of secondary sexual characteristics and BMI is important. Baseline endocrine investigations should include the exclusion of pregnancy (most common cause of secondary amenorrhoea), measurement of serum gonadotrophin (FSH and LH), oestradiol and prolactin concentrations, and markers of thyroid function (thyroxine and/or thyroid-stimulating hormone) if there are clinical signs of thyroid disease or any signs of hyperprolactinaemia. It is also possible to assess endogenous ovarian activity over the cycle with once- or twice-weekly serial measurements of oestradiol and progesterone concentrations in serum (or plasma) or their metabolites in urine. Serum androgens (testosterone) should be measured in subjects with hirsutism or androgenization. The possible use of transabdominal (and, where appropriate, transvaginal) ultrasound as mentioned above may be a useful adjunct to clinical examination. These baseline endocrine investigations will permit the categorization of patients into essentially one of four diagnostic groups: hypergonadotrophic hypogonadism, hyperprolactinaemia, hypogonadotrophic hypogonadism and normogonadotrophic anovulation (Baird 1997; Figure 16.6).
Hyperprolactinaemia
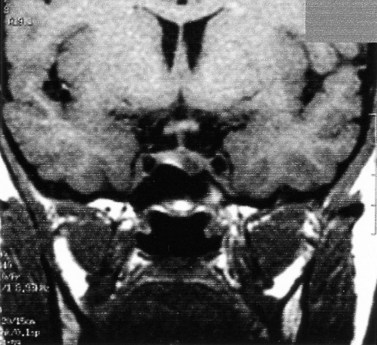
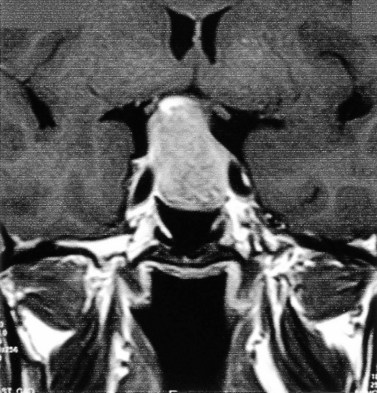
Although the true prevalence of hyperprolactinemia is difficult to establish, it is estimated that among women presenting with reproductive disorders, approximately 15% with anovulation and 43% with anovulation and galactorrhea have hyperprolactinemia. Hyperprolactinaemia (raised serum prolactin) is diagnosed when concentrations of prolactin are outwith the normal range (up to 500 mU/l). It should be noted that serum prolactin levels may be transiently and moderately raised (>700 mU/l) at times of stress. A persistent, albeit moderate, elevation of prolactin will be recorded in the presence of hypothyroidism, and is a common feature among women with PCOS. In the latter group, levels as high as 2500 mU/l have been reported (Baird 1997, Balen 2000). Thyroid disease may be excluded with estimation of serum thyrotrophin concentrations. Patients with PCOS-related hyperprolactinaemia may be distinguished from women with unrelated hyperprolactinaemia and polycystic ovaries on ultrasound by means of a progestogen challenge test (Lunenfeld and Insler 1974), which will induce a withdrawal bleed from an oestrogen-primed endometrium. Serum prolactin measurements >1500 mU/l will require more detailed investigations. Imaging of the pituitary fossa is best undertaken with either magnetic resonance imaging or computed tomography. Serum prolactin concentrations >5000 mU/l are associated with macroprolactinomas (>1 cm diameter). Diagnostic imaging will detect the presence of a hypothalamic tumour or a non-functioning pituitary tumour causing compression of the hypothalamus or a pituitary microadenoma (Figures 16.7 and 16.8).
Hypogonadotrophic hypogonadism
Hypogonadotrophic hypogonadism is characterized by reduced FSH and LH secretion, and consequent absence of follicular development and oestradiol production. Before puberty, the situation will manifest as primary amenorrhoea and lack of development of secondary sexual characteristics. Usually, however, no organic disease is identified in the hypothalamus or anterior pituitary gland. On rare occasions, it may be necessary to distinguish hypothalamic causes from pituitary causes with a GnRH stimulation test (100 µg s.c.) following 1 week of ethinyl oestradiol orally at a dose of 5–10 µg/day. The administration of this supraphysiological dose of GnRH assesses the responsiveness and capacity of the pituitary to secrete gonadotrophins (Yen et al 1973). A better index of ‘pituitary reserve’ is provided by assessment of the response to repeated physiological doses of GnRH (5–10 µg) at 2–3-h intervals (rather than a single supraphysiological injection). This assessment of pituitary secretory capacity correlates better with the degree of spontaneous secretion, as indicated by the amplitude and frequency of LH pulses. Raised FSH and LH concentrations in response to GnRH stimulation may indicate hypothalamic failure. Such patients require imaging to exclude a craniopharyngioma and consideration of Kallman’s syndrome.
Therapeutic Issues
Oestrogen deficiency
In addition to a thorough explanation to patients about the diagnosis and its consequences, women with ovarian failure require sex steroid replacement therapy with cyclic oestrogen and progestogen. Lack of oestrogen after the normal age of menopause has an adverse effect on bone and blood vessel health. Premenopausal levels of oestradiol in women with normal ovarian function protect the female skeleton from demineralization (Howell and Shalet 1999). The cardiovascular protective effects of oestrogen are well documented in older women who have had endogenous protection for many years before the menopause (Mendelsohn and Karas 1999). In Turner’s syndrome, there is evidence to suggest that women do not achieve peak bone mass and have a higher rate of fractures (Davies et al 1995). In girls with Turner’s syndrome, the rate of bone mineral acquisition on the oral contraceptive pill is less than is seen in normal adolescents, with a 25% reduction in bone mineral content from that predicted for age, height, weight and bone size. It is possible that this may be a reflection of suboptimal oestrogen replacement. Research to date has delivered well-evaluated hormone replacement regimens for older women designed to mitigate the adverse effects of lack of oestrogen and taken for somewhere in the order of 10 years. Some women seek alternatives to conventional hormone replacement therapy (HRT) which can include antidepressants (e.g. serotonin) and herbal preparations. A recent systematic review looking at black cohosh as a treatment for menopausal symptoms concluded that the efficacy was uncertain and that further trials are warranted (Borrelli and Ernst 2008). Currently, younger women with a premature menopause are offered combined sex steroid replacement in the convenient form of the combined oral contraceptive pill or HRT, which should be continued at least until the average age of natural menopause (52 years in the UK). This view is endorsed by regulatory bodies such as the Committee on Safety of Medicines (now the Commission on Human Medicines) in the UK (Pitkin et al 2007). These preparations are not designed to achieve physiological replacement of oestrogen or progesterone, either in dosage or biochemical structure. The optimal mode and formulation of sex steroid replacement have not yet been established for young women with ovarian failure. The well-documented risks with HRT relate to the postmenopausal age category and not those with premature menopause. Indeed, recent guidelines from the British Menopause Society (2008) confirm that there is no increase in the risk of breast cancer or cardiovascular disease with the use of HRT for patients with premature menopause. There may also be a need for topical oestrogen in the form of vaginal cream or pessary. In addition, testosterone replacement may need to be considered where there are marked symptoms of reduced libido; this is delivered in a patch. Patients should be warned about possible side-effects of excess hair growth and acne. A recent randomized, double-blind, placebo-controlled trial showed transdermal testosterone therapy to be very effective (Shifren et al 2006). Early menopause is a risk factor for osteoporosis, so women with an early menopause should have bone density testing performed within 10 years of menopause so that osteopenia or osteoporosis will be diagnosed early and appropriate antiresorptive therapy initiated.
Management of hyperprolactinaemia
The management of women with hyperprolactinaemia has to take into account the individual’s desire or otherwise for pregnancy, her oestrogen status, and the presence/absence and size of a pituitary tumour. Women who describe regular menses and who are found to have raised prolactin concentrations do not require treatment unless their menstrual cycles are anovulatory and they wish for pregnancy (Soule and Jacobs 1995). Dopaminergic agents are recommended as the primary therapy for prolactin-secreting adenomas and idiopathic hyperprolactinaemia (Webster et al 1994). The effects induced by dopamine agonists are suppressive but not tumoricidal. Thus, the therapeutic effect is only maintained for as long as the drug is administered. Consequently, in most cases, treatment has to be continued life-long with a few exceptions, in whom normoprolactinemia persists even after discontinuation of dopamine agonists. Surgery (trans-sphenoidal adenectomy) is usually reserved for dopamine-resistant conditions, intolerable side-effects of therapy and where there is failure to adequately shrink a macroadenoma (Balen 2000). Dopamine agonists include bromocriptine and cabergoline, the latter being better tolerated than the former, which may be explained in part by the longer half-life which results in fewer changes in drug concentration in the blood. There have been recent guidelines from the European Medicines Agency (2008) regarding the use of dopamine agonists and the risk of cardiac fibrosis. Prescribing recommendations include avoiding using such drugs in any patient with a history of cardiac fibrosis, monitoring for signs of fibrosis throughout treatment by blood tests or chest X-rays, and ensuring adherence to a daily maximum dose of 30 mg bromocriptine. The fall in circulating prolactin concentrations with therapy is accompanied by an increase in frequency of LH pulses. The return of menstruation is usually associated with anovulatory cycles. Since normal ovarian cycles have usually returned by 6 months, it may be necessary to provide contraception unless immediate fertility is desired. A tumour >1 cm in diameter should be surgically removed or reduced in size by dopaminergic therapy prior to pregnancy, since there is a small risk of tumour enlargement during pregnancy. Once pregnancy is diagnosed, dopaminergic therapy should be discontinued. There is no evidence to indicate that bromocriptine is teratogenic. Bromocriptine therapy should be recommenced if there was evidence of tumour enlargement during the pregnancy. Should hyperprolactinaemia be a consequence of drug administration itself (see Box 16.6), cessation of the offending preparation should be recommended after due consultation with other health professionals involved in the patient’s care. If continuation of a psychotrophic preparation is essential for the patient’s good health (e.g. in the case of schizophrenia), administration of a low-dose oral contraceptive preparation, in addition to continuation of the required dopaminergic therapy, will provide protection against coincident symptoms of oestrogen deficiency. Serum concentrations of prolactin should continue to be monitored. Surgical intervention is necessary for non-functioning tumours. These tumours are detected by a combination of imaging and a serum prolactin concentration <3000 milliunits/l (Balen 2000). A suprasellar extension of the tumour will also be an indication for surgery when dopaminergic therapy has failed to produce regression, or there is a wish for pregnancy. Surgery may carry a risk of hypopituitarism and, if present, usually manifests promptly following surgery. Pituitary irradiation (although not common) has a risk of incipient hypopituitarism and therefore necessitates long-term surveillance (Soule and Jacobs 1995).
Fertility Issues
There may be reasonable optimism about future fertility prospects for women with amenorrhoea, provided that they do not have POF. Women with oligomenorrhoea, however, have a slightly reduced chance overall (Hull et al 1982). In women with normal gonadotrophin concentrations, it is relatively easy to restore fertility with induction of ovulation with an antioestrogen preparation such as clomiphene citrate. Some women will also require administration of human chorionic gonadotrophin to induce ovulation, despite a response to clomiphene in terms of follicular development. Patients with low gonadotrophin concentrations will require ovulation induction with gonadotrophins or pulsatile GnRH therapy. Detail about ovulation induction regimes is addressed in Chapter 17. The assessment of oestrogen status is a useful index of likely responsiveness to ovulation induction agents, such as clomiphene citrate. Furthermore, it identifies the requirement for long-term hormone replacement, where there is a risk of osteoporosis due to oestrogen deficiency, or for endometrial protection with a progestogen, where there is continuous unopposed oestrogen stimulation. The progestogen challenge test utilizes the endometrial response to administration and withdrawal of progestogen. The oestrogen-primed endometrium will exhibit a withdrawal bleed some 24–48 h following cessation of progestogen administration. An alternative mode of assessment of oestrogen status is the measurement of endometrial thickness with ultrasound. The restoration of fertility may be possible in young women with ovarian failure with HRT and assisted reproduction which includes the use of donor oocytes and embryo transfer. More controversial is the use of assisted reproductive technology and surrogacy for the fulfilment of family desires among women who have congenital absence of the uterus or who have undergone hysterectomy for cancer at a young age (Brinsden et al 2000).
Contraceptive Advice
KEY POINTS
Abreu AP, Trarbach EB, de Castro M, et al. Loss-of-function mutations in the genes encoding prokineticin-2 or prokineticin receptor-2 cause autosomal recessive Kallmann syndrome. Journal of Clinical Endocrinology and Metabolism. 2008;93:4113-4118.
Ahmed SF, Cheng A, Dovey L, et al. Phenotypic features, androgen receptor binding, and mutational analysis in 278 clinical cases reported as androgen insensitivity syndrome. Journal of Clinical Endocrinology and Metabolism. 2000;85:658-665.
Aittomaki K, Lucena JL, Pakarinen P, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotrophic ovarian failure. Cell. 1995;82:959-968.
Alper MM, Garner PR, Seibel MM. Premature ovarian failure: current concepts. Journal of Reproductive Medicine. 1986;31:699-708.
Anasti JN. Premature ovarian failure: an update. Fertility and Sterility. 1998;70:1-15.
Anderson RA, Cameron DA. Assessment of the effect of chemotherapy on ovarian function in women with breast cancer. Journal of Clinical Oncology. 2007;25:1630-1631.
Arif S, Vallian S, Farazneh F, et al. Identification of 3 beta-hydroxysteroid dehydrogenase as novel target of steroid cell autoantibodies: association of autoantibodies with endocrine autoimmune disease. Journal of Clinical Endocrinology and Metabolism. 1996;81:4439-4445.
Asherman JG. Amenorrhoea traumatica (atretica). Journal of Obstetrics and Gynaecology of the British Empire. 1948;55:23-30.
Bachmann GA, Kemmann E. Prevalence of oligomenorrhoea and amenorrhoea in a college population. American Journal of Obstetrics and Gynecology. 1982;144:98-102.
Baird DT. Amenorrhoea. The Lancet. 1997;350:275-279.
Balen AH. Amenorrhoea, oligomenorrhoea and polycystic ovarian syndrome. In: O’Brien S, Cameron I, Maclean A, editors. Disorders of the Menstrual Cycle. London: RCOG Press, 2000.
Balen AH, Conway GS, Kaltsas G, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Human Reproduction. 1995;10:2017-2111.
Bath LE, Anderson RA, Critchley HO, Kelnar CJ, Wallace WH. Hypothalamic–pituitary–ovarian dysfunction after pre-pubertal chemotherapy and cranial irradiation for acute leukaemia. Human Reproduction. 2001;16:1838-1844.
Borrelli F, Ernst E. Black cohosh (Cimicifuga racemosa) for menopausal symptoms: a systematic review of its efficacy. Pharmacological Research. 2008;58:8-14.
Bowen S, Norian J, Santoro N, Pal L. Simple tools for assessment of ovarian reserve (OR): individual ovarian dimensions are reliable predictors of OR. Fertility and Sterility. 2007;88:390-395.
Brinsden PR, Appleton TC, Murray E, Hussein M, Akagbosu F, Marcus SF. Treatment by in vitro fertilisation with surrogacy: experience of one British centre. BMJ (Clinical Research Ed.). 2000;320:924-928.
British Menopause Society. Premature Menopause. Available at http://www.thebms.org.uk/factdetail.php?id=1, 2008.
Byrne J. Infertility and premature menopause in childhood cancer survivors. Medical and Pediatric Oncology. 1999;33:24-28.
Byrne J, Fears TR, Gail MH, et al. Early menopause in long-term survivors of cancer during adolescence. American Journal of Obstetrics and Gynecology. 1992;166:788-793.
Casanueva F, Molitch M, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clinical Endocrinology. 2006;65:265-273.
Colao A, Di Sarno A, Cappabianca P, Di Somma C, Pivonello R, Lombardi G. Withdrawal of long-term cabergoline therapy for tumoural and nontumoural hyperprolactinaemia. New England Journal of Medicine. 2003;349:2023-2033.
Conway GS. Premature ovarian failure. Current Opinion in Obstetrics and Gynecology. 1997;9:202-206.
Conway GS, Hettiarachchi S, Murray A, Jacobs PA. Fragile X permutations in familial premature ovarian failure. The Lancet. 1995;346:309-310.
Conway GS, Kaltsas G, Patel A, Davies MC, Jacobs HS. Characteristics of idiopathic premature ovarian failure. Fertility and Sterility. 1996;65:337-341.
Conway GS, Jacobs HS. Leptin: a hormone of reproduction. Human Reproduction. 1997;12:633-635.
Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstetrics and Gynecology. 1986;67:604-606.
Cramer DW, Xu H, Harlow BL. Family history as predictor of early menopause. Fertility and Sterility. 1995;64:740-745.
Crisponi L, Deiana M, Loi A, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nature Genetics. 2001;27:159-166.
Critchley HO, Wallace WH, Shalet SM, Mamtora H, Higginson J, Anderson DC. Abdominal irradiation in childhood: the potential for pregnancy. BJOG: an International Journal of Obstetrics and Gynaecology. 1992;99:392-394.
Critchley HO, Wallace WH. Impact of cancer treatment on uterine function. Journal of the National Cancer Institute Monographs. 2005;34:64-68.
Davies MC, Gulekli B, Jacobs HS. Osteoporosis in Turner’s syndrome and other forms of primary amenorrhoea. Clinical Endocrinology (Oxford). 1995;43:741-746.
De Caro JJ, Dominguez C, Sherman SL. Reproductive health of adolescent girls who carry the FMR1 premutation: expected phenotype based on current knowledge of fragile x-associated primary ovarian insufficiency. Annals of the New York Academy of Sciences. 2008;1135:99-111.
Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction. 2002;124:601-609.
European Medicines Agency. Questions and Answers on the Review of Ergot-derived Dopamine Agonists. Available at http://www.emea.europa.eu/pdfs/general/direct/pr/31905408en.pdf, 2008.
Faddy MJ, Gosden RG, Gougeon A, Richarson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Human Reproduction. 1992;7:1342-1346.
Fraser IS, Inceboz US. Defining disturbances of the menstrual cycle. In: O’Brien S, Cameron I, Maclean A, editors. Disorders of the Menstrual Cycle. London: RCOG Press, 2000.
Frattarelli JL, Levi AJ, Miller BT. A prospective novel method of determining ovarian size during in vitro fertilization cycles. Journal of Assisted Reproduction and Genetics. 2002;19:39-41.
Frisch RE. Body fat, puberty and fertility. Biological Reviews of the Cambridge Philosophical Society. 1984;59:161-188.
Glasier AF, Hendry RA, Seth J, Baird DT. Does treatment with bromocriptine influence the course of hyperprolactinaemia? Clinical Reproduction and Fertility. 1987;5:359-366.
Golden NH, Carlson JL. The pathophysiology of amenorrhoea in the adolescent. Annals of the New York Academy of Sciences. 2008;1135:163-178.
Goodfellow PN, Lovell-Badge R. SRY and sex determination in mammals. Annual Review of Genetics. 1993;27:71-92.
Gottlieb B, Pinsky L, Beitel LK, Trifiro M. Androgen insensitivity. American Journal of Medical Genetics. 1999;89:210-217.
Greer ME, Moraczewski T, Rakoff JS. Prevalence of hyperprolactinemia in anovulatory women. Obstetrics and Gynaecology. 1980;56:65-69.
Grossman A, Moult PJ, McIntyre H, et al. Opiate mediation of amenorrhoea in hyperprolactinaemia and in weight loss related amenorrhoea. Clinical Endocrinology (Oxford). 1982;17:379-388.
Harlow SD, Ephross SA. Epidemiology of menstruation and its relevance to women’s health. Epidemiologic Reviews. 1995;17:265-286.
Howell SJ, Shalet SM. Aetiology-specific effect of premature ovarian failure on bone mass — is residual ovarian function important? Clinical Endocrinology (Oxford). 1999;51:531-534.
Hull MG, Savage PE, Bromham DR. Anovulatory and ovulatory infertility: results with simplied management. BMJ (Clinical Research Ed.). 1982;284:1681-1685.
Illingworth PJ, Seaton JEV, McKinlay C, Reid-Thomas V, McNeilly AS. Low dose transdermal oestradiol suppresses gonadotrophin secretion in breast-feeding women. Human Reproduction. 1995;10:1671-1677.
Imperato-McGinley J. Male pseudohermaphroditism. In: Adashi EY, Rock JA, Rosenwalks Z, editors. Reproductive Endocrinology, Surgery and Technology, vol. 1. Philadelphia: Oxford University Press; 1995.
Jacobs HS, Franks S, Murray MA, Hull MG, Steele SJ, Nabarro JDN. Clinical and endocrine features of hyperprolactinemic amenorrhoea. Clinical Endocrinology (Oxford). 1976;5:439-454.
Kallman F, Schonfield WA, Barrera SE. The genetic aspects of primary eunuchoidism. American Journal of Mental Deficiency. 1944;48:203-236.
Kaufman FR, Kogut MD, Donnell GN, Goebelsmann U, March C, Koch R. Hypergonadotropic hypogonadism in female patients with galactosemia. New England Journal of Medicine. 1981;304:994-998.
Knauff EA, Eijkemans MJ, Lambalk CB, et al. Anti-Müllerian hormone, inhibin B, and antral follicle count in young women with ovarian failure. Journal of Clinical Endocrinology and Metabolism. 2009;94:786-792.
Laughlin GA, Yen SS. Nutritional and endocrine–metabolic aberrations in amenorrheic athletes. Journal of Clinical Endocrinology and Metabolism. 1996;81:4301-4309.
Legouis R, Hardelin JP, Levilliers J, et al. The candidate gene for the X-linked Kallman syndrome encodes a protein related to adhesion molecules. Cell. 1991;67:423-435.
Leiblich JM, Rogol AD, White BJ, Rosen SW. Syndrome of anosmia with hypogonadotropic hypogonadism (Kallman’s syndrome): clinical and laboratory studies in 23 cases. American Journal of Medicine. 1982;73:506-519.
Lunenfeld B, Insler V. Classification of amenorrhoea states and their treatment by ovulation induction. Clinical Endocrinology (Oxford). 1974;3:223-237.
Matkovic V, Ilich JZ, Skugor M, et al. Leptin is inversely related to age at menarche in human females. Journal of Clinical Endocrinology and Metabolism. 1997;82:3239-3245.
Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. New England Journal of Medicine. 1999;340:1801-1811.
Muttukrishna S, Child T, Lockwood GM, Groome NP, Barlow DH, Ledger WL. Serum concentrations of dimeric inhibins, activin A, gonadotrophins and ovarian steroids during the menstrual cycle in older women. Human Reproduction. 2000;15:549-556.
Neale T. Missed menstrual cycles in young women may signal ovarian insufficiency, 2009.
Ogilvy-Stuart AL, Shalet SM. Effect of radiation on the human reproductive system. Environmental Health Perspectives. 1993;101(Suppl 2):109-116.
Pasquino AM, Passeri F, Pucarelli I, Segni M, Municchi G. Spontaneous pubertal development in Turner’s syndrome. Italian Study Group for Turner’s Syndrome. Journal of Clinical Endocrinology and Metabolism. 1997;82:1810-1813.
Pederson-White JR, Chorich LP, Bick DP, Sherins RJ, Layman LC. The prevalence of intragenic deletions in patients with idiopathic hypogonadotrophic hypogonadsim and Kallmann syndrome. Molecular Human Reproduction. 2008;14:367-370.
Perheentupa A, Critchley HO, Illingworth PJ, McNeilly AS. Enhanced sensitivity to steroid-negative feedback during breast-feeding: low dose estradiol (transdermal estradiol supplementation) suppresses gonadotropins and ovarian activity assessed by inhibin B. Journal of Clinical Endocrinology and Metabolism. 2000;85:4280-4286.
Pettersson F, Fries H, Nillius SJ. Epidemiology of secondary amenorrhoea. I. Incidence and prevalence rates. American Journal of Obstetrics and Gynecology. 1973;117:80-86.
Pitkin J, Rees MC, Gray S, et al. Management of premature menopause. Menopause International. 2007;13:44-45.
Randeva HS, Davis M, Prelevic GM. Prolactinoma and pregnancy. BJOG: an International Journal of Obstetrics and Gynaecology. 2000;107:1064-1068.
Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. Journal of Clinical Endocrinology and Metabolism. 1987;65:1231-1237.
Roseweir AK, Millar RP. The role of kisspeptin in the role of gonadotrophin secretion. Human Reproduction Update. 2009;15:203-212.
Rugarli EI, Ballabio A. Kallman syndrome from genetics to neurobiology. JAMA: the Journal of the American Medical Association. 1993;270:2713-2716.
Schlechte J, Dolan K, Sherman B, Chapler F, Luciano A. The natural history of untreated hyperprolactinemia: a prospective analysis. Journal of Clinical Endocrinology and Metabolism. 1989;68:412-418.
Shifren JL, Davis SR, Moreau M, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 Study. Menopause. 2006;13:770-779.
Singh KB. Menstrual disorders in college students. American Journal of Obstetrics and Gynecology. 1981;140:299-302.
Sohval AR. The syndrome of pure gonadal dysgenesis. American Journal of Medicine. 1965;38:615-625.
Soule SG, Jacobs HS. Prolactinomas: present day management. BJOG: an International Journal of Obstetrics and Gynaecology. 1995;102:178-181.
Speroff L, Glass RH, Kase NG. Amenorrhoea. In Speroff L, Glass RH, Kase NG, editors: Clinical Gynecologic Endocrinology and Infertility, 6th edn, Baltimore: Lippincott, Williams and Wilkins, 1999.
Speroff L, Glass RH, Kase NG. Amenorrhoea. In Speroff L, Glass RH, Kase NG, editors: Clinical Gynecologic Endocrinology and Infertility, 6th edn, Baltimore: Lippincott, Williams and Wilkins, 1999.
Speroff L, Glass RH, Kase NG. Normal and abnormal sexual development. In Speroff L, Glass RH, Kase NG, editors: Clinical Gynecologic Endocrinology and Infertility, 6th edn, Baltimore: Lippincott, Williams and Wilkins, 1999.
Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. International Journal of Fertility. 1967;12:77-126.
van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Human Reproduction Update. 1999;5:483-492.
Wallace WH, Shalet SM, Crowne EC, Morris-Jones PH, Gattameneni HR. Ovarian failure following abdominal irradiation in childhood: natural history and prognosis. Clinical Oncology (Royal College of Radiologists). 1989;1:75-79.
Wallace WH, Shalet SM, Tetlow LJ, Morris-Jones PH. Ovarian function following the treatment of childhood acute lymphoblastic leukaemia. Medical and Pediatric Oncology. 1993;21:333-339.
Walsh RJ. The age of the menopause of Australian women. Medical Journal of Australia. 1978;2:181-182. 215
Warren MP. Anorexia nervosa. In de Groot LJ, editor: Endocrinology, 3rd edn, Philadelphia: WB Saunders, 1995.
Warren MP, Brooks-Gunn J, Hamilton LH, Warren LF, Hamilton WG. Scoliosis and fractures in young ballet dancers: relation to delayed menarche and secondary amenorrhoea. New England Journal of Medicine. 1986;314:1348-1353.
Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinaemic amenorrhoea. New England Journal of Medicine. 1994;331:904-909.
Yen SS, Rebar R, Vandenberg G, Ehara Y, Siler T. Pituitary gonadotrophin responsiveness to synthetic LRF in subjects with normal and abnormal hypothalamic–pituitary–gonadal axis. Journal of Reproduction and Fertility. 1973;20(Suppl):137-161.
Yen SSC. Chronic anovulation due to CNS–hypothalamic–pituitary dysfunction. In Yen SSC, Jaffe RB, Barbieri RL, editors: Reproductive Endocrinology, Physiology, Pathophysiology, and Clinical Management, 4th edn, Philadelphia: WB Saunders, 1999.
Zreik TG, Olive D. Amenorrhoea. In: O’Brien S, Cameron I, Maclean A, editors. Disorders of the Menstrual Cycle. London: RCOG Press, 1998.