Alternative Methods of Drug Administration
The rapid administration of lifesaving, pain-relieving, and sedative medications lies at the core of the practice of emergency medicine. The intravenous (IV) route is usually the delivery method of choice. However, there are circumstances in which vascular access is either not available or contraindicated. Thus, emergency providers need to have a working knowledge of alternative routes of drug administration. This chapter describes the endotracheal (ET), intranasal, and rectal routes. Intraosseous (IO) access is covered in Chapter 25.
ET Administration of Medication
Historical Perspective
ET drug administration dates back to 1857, when Bernard1 demonstrated that the lung could rapidly absorb a solution of curare. In this historical experiment he instilled a fatal solution into the upper respiratory tract of dogs by way of a tracheostomy. Over the following decades, other investigators expanded this work and demonstrated that solutions containing salicylates, atropine, potassium iodide, strychnine, and chloral hydrate were also rapidly absorbed from the lung and excreted in urine after injecting aqueous solutions into the tracheas of experimental animals.2 The use of intrapulmonary medication for the treatment of lung disease gained further acceptance when studies demonstrated that inhaling epinephrine mist dramatically relieved the symptoms of asthma.3
In the late 1930s and 1940s, several important observations were made concerning ET drug therapy: (1) penicillin delivered by the ET route demonstrated a depot effect, which resulted in therapeutic blood levels that lasted twice as long as those with intramuscular injection4; (2) various diluents mixed with penicillin affected both the rate and the degree of absorption from the lungs5; and (3) higher serum drug levels were attained with direct ET drug administration than with aerosolized administration.5 In the 1950s it was noted that drugs delivered endotracheally were absorbed much more rapidly than those applied to the posterior part of the pharynx. Drugs applied locally to the larynx and trachea were absorbed rapidly and even resulted in blood levels significant enough to cause adverse anesthetic reactions.6
In 1967, Redding and coworkers7 studied the use of ET administration as a route of drug delivery in a canine model of cardiopulmonary arrest. They administered epinephrine by the IV, intracardiac, and intratracheal routes to resuscitate dogs that had undergone both respiratory and circulatory arrest secondary to hypoxia. They then evaluated the effectiveness of the epinephrine after administration of the drug by all three of these routes. Their study revealed that all three routes of drug administration were equally effective in restoring the circulation of dogs in hypoxia-induced cardiac arrest, again demonstrating that the ET route of drug delivery provides effective access to the systemic circulation.
In the late 1970s, Roberts, Greenberg, and colleagues8–11 studied ET drug delivery in a series of laboratory experiments and clinical applications of ET epinephrine. Since that time, a number of important animal and human studies, as well as case reports, have been published in which the various aspects of ET drug administration were investigated. These studies have addressed (1) the appropriate dose of drug to administer; (2) the effect of the drug solution’s volume; (3) the effect of different diluent solutions; (4) the role of different ET drug delivery techniques; and (5) the effects of hypoxia, hypotension, shock, and cardiopulmonary arrest on the absorption, distribution, and efficacy of endotracheally administered drugs.
Recommendations For ET Drug Delivery
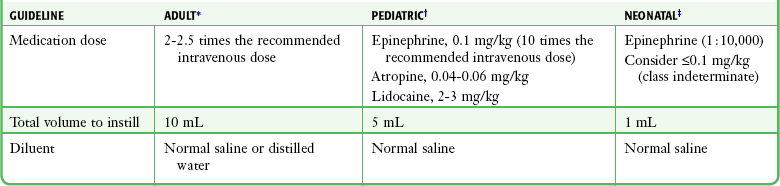
It is imperative to remember that ET drug delivery is not the delivery method of choice. The American Heart Association (AHA) recommends that if IV access is not available, IO access should be obtained.12 Although the AHA makes specific recommendations regarding the use of ET drug delivery for cardiac resuscitation (Table 26-1),12–14 much of the existing literature is controversial and contradictory at times. It is therefore possible that some of these issues will continue to be the subject of future investigations.
TABLE 26-1
American Heart Association Guidelines for Endotracheal Drug Administration
*Adult data from American Heart Association. Guidelines 2010 for cardiopulmonary resuscitation and emergency cardiovascular care, part 8: advanced cardiac life support. Circulation. 2010;122:S729.
†Pediatric data from American Heart Association. Guidelines 2010 for cardiopulmonary resuscitation and emergency cardiovascular care, part 14: Pediatric advanced life support. Circulation. 2010;122:S876.
‡Neonatal data from American Heart Association. 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care: neonatal resuscitation. Circulation. 2010;122:S909.
Appropriate Dose
All investigators agree that the ET dose of a medication should be at least equal to the IV dose of the same drug when given for the same indication, but most studies indicate that higher doses are needed when administering drugs endotracheally. For advanced cardiac life support (ACLS) medications in adults, the AHA recommends a dose that is 2.0 to 2.5 times the usual IV dose when administered endotracheally.12 This would be 2.0 to 2.5 mg (twice the standard IV dose of 1.0 mg). This recommendation is supported by the results of a study of epinephrine administered immediately after intubation in the out-of-hospital setting.15 Other studies of endotracheally administered lidocaine indicate that a 3-mg/kg dose (twice the standard IV dose of 1.5 mg/kg of lidocaine) was needed to obtain therapeutic serum levels.16,17
Some animal studies18 and case reports10 have reported conflicting results, with positive effects or recovery from cardiovascular collapse when epinephrine was used in doses equal to the recommended IV doses. In other animal19 and human20 studies, however, epinephrine administered endotracheally at doses of approximately 0.01 and 0.02 mg/kg, respectively, were shown to be unreliable in producing a physiologic response. In addition, studies using both normotensive and cardiac arrest canine models have shown that epinephrine doses of 0.01 mg/kg administered endotracheally produce serum levels approximately a 10th of that produced when the same dose is given intravenously.9,21,22 These studies recommend increasing the ET epinephrine dose to 0.1 mg/kg and are the basis for the 2010 AHA recommendation to use a 10-fold increased dose when administering ET epinephrine to pediatric patients.13 Some studies have shown that ET epinephrine at all doses causes a significant decrease in diastolic blood pressure immediately after instillation and that a dose of 0.3 mg/kg increases diastolic blood pressure after 1 minute. This may be due to β-adrenergic blockade at lower doses.23,24
ET drug delivery is associated with a depot effect, with ET drugs being “stored” and released slowly over time, similar to a continuous IV drip. This presumably occurs as a result of local vasoconstriction and lymphatic storage of the drug8 or pooling in lung tissue because of poor lung perfusion.25 With epinephrine use, the depot effect can produce postresuscitative dysrhythmias, hypertension, and tachycardia together with resultant increased myocardial oxygen demand. Given these conflicting data, it seems reasonable in adults to start with a dose 2.0 to 2.5 times the usual IV dose. If this is ineffective, higher doses may be used subsequently.
Volume for a Single Dose
For ET drugs, the AHA recommends a total volume of 10 mL in adults,12 5 mL in pediatric patients,13 and 1 mL in neonates.14 In studies involving dogs, Mace26 compared undiluted lidocaine with diluted lidocaine (volume ≈6.5 mL) and found significantly higher plasma lidocaine levels in the animals receiving diluted lidocaine. There were no changes in arterial blood gas values before or after ET drug administration. In another animal study, lidocaine diluted with normal saline to volumes of up to 25 mL produced no changes in arterial blood gases or the clinical condition and no change in the gross anatomy or histology of the lung.27 In contrast, a study comparing normal saline with distilled water revealed decreased arterial oxygen partial pressure (Pao2) with both solutions (water producing the greatest effect), but this study used large volumes (2 mL/kg) of solution.28
Studies of endotracheally administered lidocaine in human subjects reveal that dilution with distilled water to a total volume of 10 mL results in higher plasma lidocaine levels but also produces a decrease in Pao2 of approximately 40 mm Hg that persists for longer than 1 hour.29 A total volume of 5 mL yields lower plasma levels and a shorter period of hypoxemia. Data and volume recommendations in the setting of multiple doses of the drug are lacking.
Appropriate Diluent
Both normal saline and distilled water may be used as diluents for ET drug administration, but it remains unclear which is preferred. In one study, intubated dogs were administered epinephrine via ET tube, and peak serum epinephrine levels were 13 times higher when distilled water was used as a diluent instead of normal saline.30 In addition, mean arterial blood pressure increased significantly only in dogs that were administered epinephrine diluted with distilled water. Greenberg and coworkers,28 however, reported that normal saline administered via the ET route produced fewer detrimental effects on arterial blood gases than distilled water did. Another study found no changes in pulmonary status (arterial blood gas, oxygen saturation, gross anatomy, or histology) in dogs given lidocaine diluted with normal saline in total volumes ranging between 6 and 25 mL.27 Still another study found no difference in arterial blood gases after the delivery of either diluent solution.31 Hence, the optimal diluent is controversial; saline may produce less pulmonary dysfunction, but distilled water appears to deliver a greater amount of drug.
Technique for ET Drug Delivery
Techniques for ET drug administration include direct instillation into the proximal end of the ET tube, administration via a catheter that extends just beyond the distal tip of the ET tube, deep endobronchial administration using a longer catheter, administration via ET tube monitoring ports, administration with equipment developed specifically for ET atomized drugs, and injection through the side of the ET tube with a needle. Several studies have indicated that the use of a catheter or feeding tube may not be needed to enhance the drug’s effectiveness. Greenberg and Spivey32 instilled radiopaque contrast material directly into the proximal end of the ET tube and compared its distribution with that of contrast material instilled via a catheter extending out the distal end of the tube. Both techniques were equally effective in distributing the contrast agent to the peripheral lung fields as long as five rapid manual ventilations followed the instillation. In addition, using a porcine cardiopulmonary arrest model, Jasani and colleagues33 showed no difference in resuscitation rates or physiologic responses between epinephrine administered by direct injection into the ET tube, via a catheter extending out the distal end of the ET tube, or via a monitoring lumen built into the side wall of the ET tube. Rehan and associates34 demonstrated that there was no difference in the amount of drug delivered via catheter versus direct instillation in neonates. In studies of patients with normal perfusion, some support “deep bronchial” ET drug administration, whereas others do not.35–37
Some studies have suggested that drug absorption with direct instillation into the ET tube is inconsistent during cardiopulmonary arrest.17,20 To address one specific question, one study found no difference in plasma epinephrine levels when epinephrine was instilled during apnea versus instillation during the ventilator inspiratory cycle.38
Effects of Hypoxia, Hypotension, and Cardiopulmonary Arrest
Despite concerns that medications might not be absorbed in states of hypoxia or low blood flow, the data available reveal the opposite to be true. In a hemorrhagic shock model, Mace39 demonstrated that higher plasma lidocaine levels were obtained via the ET route during shock than during nonshock states. In a lamb model, when epinephrine was administered endotracheally, higher plasma epinephrine levels were achieved during hypoxia-induced low pulmonary blood flow than during baseline, normal pulmonary blood flow.40 Finally, plasma lidocaine levels rose earlier when lidocaine was administered endotracheally to dogs that were hypoxemic than to dogs that were not.41
Significant questions remain regarding the efficacy of endotracheally administered medications.17,19,20,42–44 The 2010 guidelines for neonatal resuscitation14 discourage the routine use of ET epinephrine. More importantly, these studies serve to emphasize that ET drug administration should not be used in lieu of attempts to obtain definitive access to the systemic circulation. ET drug administration should not be performed when more direct means of accessing the central circulation are available.
Indications
ET drug therapy is indicated when emergency pharmacologic intervention is needed and other access, either IV or IO, is not available. This most frequently occurs during cardiovascular collapse. Though intuitively and experimentally attractive, randomized, controlled trials of the efficacy of ET drug therapy are lacking.45 Niemann and colleagues, in a retrospective cohort study, examined the outcomes of 596 cardiac arrest patients who received IV or ET medications. There were no survivors to hospital discharge in the 101 patients who received ET medications versus a 5% survival rate in patients who received IV medications. However, more patients in the ET group were asystolic, a sign that these patients were already in much worse condition.45
Specific indications for the delivery of a drug endotracheally are the same as those for IV and IO administration. However, only a limited number of emergency drugs can be given safely by the ET route (Boxes 26-1 and 26-2). Medications that are appropriate for ET administration based on animal and human studies include epinephrine,8,10,47 atropine,48–50 lidocaine,36,39,41,51 and naloxone.52,53 ET naloxone is not currently recommended in neonates.14
Diazepam has also been shown to be effective.54,55 However, in one animal model, diazepam produced pneumonitis when 0.5 mg/kg was administered via the ET route.46 Because diazepam is sparingly soluble in water, it is available only in a solution of propylene glycol, ethanol, and benzyl alcohol. It is unknown whether the reported pneumonitis was due to the direct effect of diazepam or the diluent. The AHA has removed diazepam from its list of medications that can be given safely via the ET route; other routes may be more appropriate for this drug.13,56,57
Experimental studies of vasopressin,58,59 midazolam,60 flumazenil,61 propranolol,48 and metaraminol62 in animal models suggest that these medications may also be effective when administered endotracheally, but no clinical studies in humans have been conducted to verify these findings. Efrati and coworkers’ study59 demonstrated that ET vasopressin had greater effects on diastolic blood pressure than did ET epinephrine with little effect on the heart rate, but no clinical trials have evaluated its efficacy. Based on a study by Wenzel and colleagues,58 the 2010 ACLS guidelines added vasopressin to the list of cardiac resuscitation drugs that can be administered via the ET route (in addition to lidocaine, epinephrine, atropine).12 It is interesting to note that in the study of midazolam, no pathologic changes were seen in lung sections after the administration of midazolam.60 In addition, midazolam is available commercially in aqueous solution and could therefore be diluted with normal saline or distilled water for ET administration. However, given that midazolam is approved for intramuscular use, it seems unlikely that ET administration would ever be necessary. Palmer and associates61 demonstrated that therapeutic blood levels of flumazenil were obtained within a minute after ET delivery of 1 mg of the drug diluted in 10 mL of saline. This is 10 times the recommended IV dose of 0.1- to 0.2-mg aliquots. The role of flumazenil by ET administration remains to be determined.
Contraindications
At present, the only true contraindication to the ET delivery of an appropriate drug is the presence of another form of access to the systemic circulation through which the needed drug can be delivered rapidly and effectively. A complete list of drugs that are contraindicated for ET delivery is not available, but specific medications that have been shown to be ineffective or unsafe when given via the ET route include sodium bicarbonate, amiodarone, isoproterenol, and bretylium. In dogs, sodium bicarbonate was shown to inactivate lung surfactant.63 Isoproterenol, even when given in doses 10 times the IV dose, failed to produce significant changes in arterial blood pressure or heart rate.49 Studies of bretylium also indicate low serum levels after ET administration, even when administered at doses of 20 mg/kg.64 Amiodarone induces pneumonitis and pulmonary fibrosis in animals and is therefore not recommended for ET administration.65
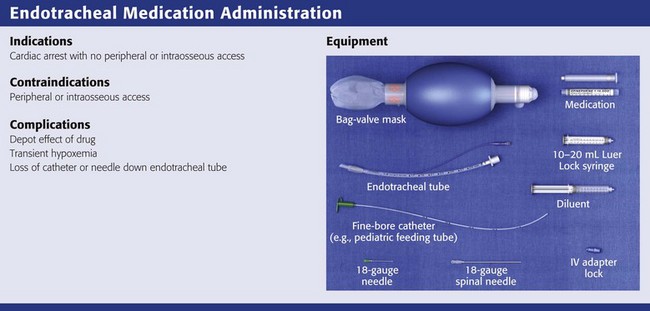
Equipment
The patient must first be intubated endotracheally. It should be noted that in studies in which the recommended ET tube doses of medications were administered by Combitube (Kendall-Sheridan, Argyle, NY) or laryngeal mask airway (LMA North America, San Diego, CA), absorption of drugs was found to be subtherapeutic.66–68 A Combitube, when placed in the esophagus (requiring medications to travel out the side holes to reach the trachea), needs 10 times more epinephrine than that used with an ET tube to obtain the same serum concentration and hemodynamic effects.66 Presumably, a Combitube that enters the trachea directly would function equivalently to an ET tube, but no studies have been done to support this assumption.
1. Manual bag ventilation device capable of delivering a fraction of inspiratory oxygen (Fio2) of at least 50%. When ET drug delivery is indicated, the patient’s condition almost always warrants supplemental oxygen. Although the technique may not result in any significant deterioration in respiratory function, it is still advisable to administer additional oxygen after drug delivery. Use the bag ventilation device to also deliver several rapid insufflations immediately after drug delivery to assist in delivery of the drug distally, where it may be absorbed more rapidly and effectively.32 It should be noted, however, that the priorities of drug administration via the ET route must be balanced against the potential deleterious effects that such rapid insufflation might have on hemodynamics and cerebral perfusion. Excessive hyperventilation of victims of out-of-hospital cardiac arrest is common and associated with poor outcomes.69
2. A fine-bore catheter or special ET tube designed to deliver the drug at or beyond the distal end of the ET tube. For adults, select a catheter that is at least 8 Fr in size and 35 cm (14 inches) in length. It should be long enough to protrude past the distal end of the ET tube. The diameter of the catheter should be large enough to allow rapid delivery of 10 mL of solution. Several different types of tubes and catheters commonly available in the emergency department (ED) can be used for this purpose:
a. A 16-gauge central venous pressure or cutdown catheter. Because most are only 30 cm in length, the proximal end of the ET tube should be shortened so that the catheter can protrude past the end.
b. An 8- or 10-Fr polyethylene pediatric feeding tube (e.g., Argyle, St. Louis). These tubes are much longer than needed, so cut them to reduce dead space. Luer-Lok ends fit onto the proximal end of the tube. For neonates, use a 5-Fr feeding tube with a syringe and an IV adapter.
c. An 8-Fr (or larger) pediatric pulmonary suction catheter without the control port. Because this catheter is designed to extend past the tip of the ET tube, it is an ideal length. However, with some brands it is difficult to attach a syringe or IV adapter lock after the suction control port is removed.
3. An IV adapter lock. This can be placed as needed onto the proximal end of the irrigation lumen of the Hi-Lo Jet Tracheal Tube or on the catheters described previously to convert them for use with prefilled syringes. This adapter is generally unnecessary if a standard syringe is used.
4. A 10- to 20-mL syringe, preferably a Luer-Lok type, large enough to deliver the desired volume of drug solution plus an additional 5 mL of air. Most of the medications now prescribed for emergency situations come in prefilled syringes. This type of apparatus does not usually allow one to draw up diluent or an additional volume of air to empty the syringe of solution. In addition, depending on the manufacturer and model, some prefilled syringes have either needles or a needleless system that may require an IV adapter lock to use them for ET injection.
5. Diluent solution. Keep an adequate volume of diluent available, such as normal saline or distilled water.
6. Medications to be instilled (see Box 26-1).
7. An 18- or 19-gauge needle to draw up the medication and inject it. Use an 18-gauge, 8.9-cm (3.5-inch) spinal needle for direct instillation of medications into the proximal end of the ET tube.
8. Alcohol wipes to clean the vials and injection ports.
9. Gloves, mask, and eye protection. After instillation, the solution often refluxes out of the ET tube, which makes blood and body fluid precautions critical.
Procedure
Direct Instillation into the ET Tube
While the patient is being ventilated, draw up the desired drug into a syringe (or use a prefilled syringe) (Fig. 26-1, step 1). Dilute the drug to a final volume of 10 mL (adults), 5 mL (children), or 1 mL (neonates) with normal saline or distilled water. Attach an 18- or 19-gauge needle. Some authors recommend using an 8.9-cm (3.5-inch) spinal needle. If using a prefilled syringe, draw up an appropriate volume of diluent in a second syringe so that the total instillation volume (drug plus diluent) equals 10 mL (adults), 5 mL (children), or 1 mL (neonates). Attach an 18- or 19-gauge needle, which will be used to flush the ET tube after instillation of the drug.
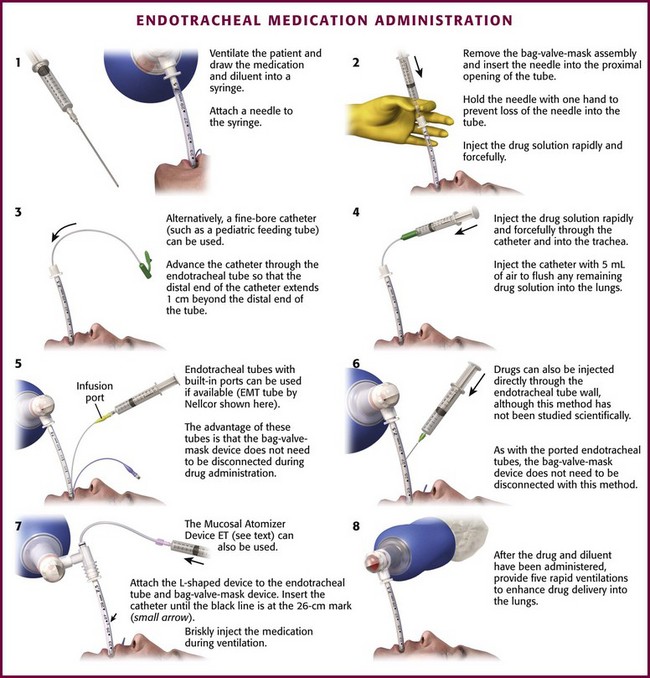
Figure 26-1 Endotracheal administration of medication.
Interrupt the connection between the proximal end of the ET tube and the bag ventilation device. Insert the needle of the syringe into the proximal opening of the ET tube (see Fig. 26-1, step 2). Hold the proximal end of the needle with one hand to prevent loss of the needle into the tube. Inject the drug solution rapidly and forcefully. If using a prefilled syringe, flush the tube immediately with the diluent in a second syringe. If the patient makes an effort to cough, place a thumb over the opening of the ET tube to prevent expulsion of the solution. Reattach the bag ventilation device and deliver five rapid insufflations.
Use of a Catheter
Draw the plunger back to add 5 mL of air to the liquid in the syringe. If the drug to be delivered is in a prefilled syringe, place an IV adapter lock on the catheter if necessary to accommodate the syringe needle or needleless tip. Attach the syringe to the catheter at this time or once the catheter has been placed within the ET tube. In addition, draw up the appropriate volume of diluent (normal saline or distilled water) plus 5 mL air into a second syringe to flush the catheter after instillation of the drug from the prefilled syringe. The air flush presumably forces out any medication adhering to the walls of the catheter’s lumen. Rehan and colleagues34 determined that when using a catheter in a neonatal model, more medication was delivered with an additional air flush than without an air flush.
Disconnect the proximal end of the ET tube from the bag ventilation device. Place the catheter into the lumen of the ET tube in such a manner that the distal end of the catheter extends approximately 1 cm beyond the distal end of the ET tube (see Fig. 26-1, step 3). For the catheter to reach deep enough, the proximal end of the ET tube may need to be cut to a shorter length. Hold the proximal ends of the catheter and ET tube at all times during the procedure. If it has not already been done, attach the syringe to the catheter. Inject the drug solution rapidly and forcefully through the catheter into the trachea followed by the 5 mL of air needed to flush the catheter of any remaining drug solution (see Fig. 26-1, step 4). If using a prefilled syringe, use the second syringe to promptly flush with the diluent and air. Immediately remove the syringe and catheter from the ET tube. Reconnect the bag ventilation device with supplemental oxygen to the ET tube and deliver five rapid ventilations.
Use of ET Tubes with Irrigation and Drug Delivery Lumens
The following tubes have built-in ports:
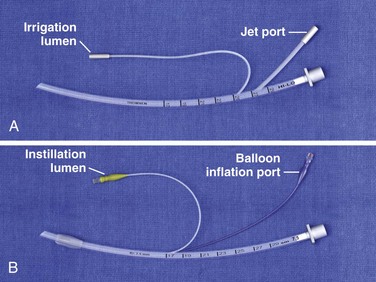
1. ET tubes designed for bronchoscopy (e.g., Hi-Lo Jet Tracheal Tube; Nellcor, Pleasanton, CA) (Fig. 26-2A). These tubes have two additional ports, one for monitoring or irrigation (opaque lumen) and one for jet ventilation (transparent lumen). They are available in only uncuffed sizes. In a porcine cardiopulmonary arrest model, successful resuscitation with this tube was comparable to resuscitation with other forms of ET drug administration.33 The major disadvantage of this ET tube is the need to be familiar with the specific ports before use. If one has never seen the tube previously, determining which port is used for irrigation could prove to be time-consuming. In addition, the port requires placement of an IV adapter lock or Luer-Lok to use a prefilled syringe.
2. ET tube with a side port (ETSP; e.g., EMT Emergency Medicine Tube, Nellcor, Pleasanton, CA) (see Figs. 26-1, step 5, and 26-2B). This tube is designed specifically for ET drug administration but is available in cuffed sizes only. The instillation lumen opens into the tube at Murphy’s eye, approximately 1 cm from the end of the tube. The injection port has an IV adapter lock, which makes it amenable to use with prefilled syringes. The ETSP is not available in pediatric sizes. In addition, in one study comparing the administration of lidocaine via the ETSP with administration through the proximal end of the standard ET tube, serum lidocaine measurements never reached therapeutic levels in the ETSP group, in contrast to the ET group and IV control group.70
3. Uncuffed tracheal tube with a monitoring lumen (Nellcor, Pleasanton, CA). This tube contains a separate monitoring lumen in the wall of the tube that opens inside the distal tip. A three-way stopcock with a Luer-Lok adapter provides access to the monitoring lumen. The major disadvantage of this ET tube is the need to be familiar with the additional port.
The advantage of these specialized ET tubes is that they eliminate the need to disconnect the bag ventilation device and the ET tube.
Injection through the Wall of the ET Tube
This method of drug delivery has not yet been evaluated scientifically but has been used clinically.71,72 As with ET tubes with drug delivery lumens, this technique requires no interruption of the connection between the bag ventilation device and the ET tube (see Fig. 26-1, step 6). In addition, placing an IV adapter lock on the needle allows it to be left inserted in the ET tube for use with additional medications.72
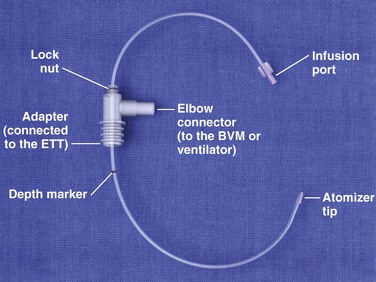
Use of the ET Atomizer
The Mucosal Atomizer Device-Endotracheal Tube (MADett, LMA North America, San Diego, CA) is an L-shaped port that attaches to both the ventilator bag and the ET tube (Fig. 26-3; also see Fig. 26-1, step 7). A catheter is inserted into the adapter and a mark is aligned at the 26-cm line of the ET tube. The catheter is then locked into place in the adapter. The L shape allows ventilation of the patient to be uninterrupted while medication is administered via the catheter and atomized into the patient’s lung mucosa at the distal tip that protrudes from the end of the ET tube. This device can be used only with ET tubes 7.0 or larger and longer than 28 cm.
Complications
After ET drug administration, the well-described systemic effects of drugs administered in emergency situations may produce adverse effects. Administration of epinephrine during cardiopulmonary resuscitation has been noted in case reports to produce prolonged hypertension, tachycardia, and arrhythmias after the return of a perfusing rhythm.10,21 It appears that these side effects are related to the depot effect, in which larger doses of drugs administered endotracheally are released slowly over time. In addition to epinephrine, atropine and lidocaine also exhibit a depot effect when administered endotracheally.63 No serious long-term sequelae, however, have been reported to result from this effect.
Intranasal Administration of Medication
Anatomy and Physiology
The human nasal cavity is a convenient and readily available site to deliver medications. It has been used for centuries and has recently been introduced into the ED. It has a volume of 15 to 20 mL and a total surface area of approximately 150 cm2.2,73 The nasal cavity is divided into two mostly symmetric halves by the nasal septum. Each half consists of four anatomically and histologically distinct regions: the vestibule, atrium, and respiratory and olfactory regions (Fig. 26-4). The respiratory and olfactory regions are areas of high vascularity and good permeability. The respiratory region has the largest surface area at approximately 130 cm2.2,74 Blood flow in the nasal mucosa is higher, per cubic centimeter, than in muscle, brain, or liver tissue.75
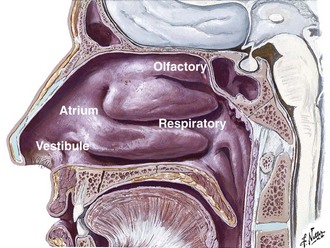
Figure 26-4 Regions of the nasal cavity. (Netter illustration from www.netterimages.com. © Elsevier Inc. All rights reserved.)
The olfactory mucosa has been theorized to have a direct connection to the brain and bypasses the first-pass metabolism of orally administered medications.76–78 Drugs that can be delivered via the nasal mucosa are generally small in molecular weight, stabile, and dissolvable in the watery mucus of the nasal passages.79
Indications and Contraindications
The intranasal route may be very useful in circumstances where placing an IV line is not possible or practical. This is especially true in patients at the extremes of age. Thus, knowledge of this painless, needleless route of drug administration is important for practicing emergency physicians. Intranasal administration of medication has been shown for more than 30 years to be effective.80 Many medications are routinely administered nasally in the outpatient setting for both local and systemic delivery,79 but the majority of these medications have little clinical application in the ED. Drugs used routinely in the ED that have been studied intranasally include the opioid antagonist naloxone, the benzodiazepine midazolam, the sedative-hypnotic ketamine, and the opioids fentanyl and sufentanil. Most of these studies have focused on the pediatric patient population as an alternative to IV medication.
Narcotic Overdose
Intranasal naloxone at a dose of 2 mg was shown to be as effective as IV naloxone in patients with known or suspected opioid overdose.81 Patients receiving intranasal naloxone had an increase in the respiratory rate that was statistically equivalent to that with IV naloxone. Intranasal naloxone was shown to reduce the need to initiate an IV line in narcotic overdose patients.82 This could help reduce the risk for needlestick injuries in the prehospital setting in this high-risk patient population. Similarly, nebulized naloxone has also been used to treat opioid intoxication in the non-apneic patient.
Seizures
Bhattacharyya and colleagues studied intranasal midazolam versus rectal diazepam in pediatric seizure patients. Midazolam, 0.2 mg/kg delivered intranasally, was found to have a significantly more rapid onset of seizure cessation with minimal effect on respiration and oxygen saturation.83 The authors suggested that this route could be used in both the prehospital setting and the ED to deliver antiepileptic medication until an IV line can be initiated.
Sedation
Klein and coworkers studied intranasal, buccal, and oral administration of midazolam for pediatric procedural sedation.84 They demonstrated that 0.3 mg/kg of midazolam administered intranasally exhibited a faster onset of sedation, achieved adequate sedation in a greater proportion of patients, and resulted in more parents of pediatric patients stating that they would chose the same regimen again. However, more patients complained of local irritation of the nasal mucosa. This is probably due to the acidity of midazolam. Local irritation has been noted especially when drops are used to instill the midazolam.85 Premedication with intranasal lidocaine may decrease the local irritation associated with midazolam.86
Ketamine has been studied in the pediatric population both as a stand-alone sedative and in combination with midazolam.87,88 Ketamine, 3 mg/kg, was found to be very safe and effective when administered intranasally and had good sedation scores and no significant adverse events. Ketamine, 5 mg/kg, in combination with midazolam, 0.3 mg/kg, was administered intranasally as an anesthesia preinduction agent and had rapid onset of sedation. However, this study used a higher dose of ketamine, which could result in prolonged sedation or sedation deeper than desired.
Pain Management
Fentanyl is widely used for the management of acute pain. Intranasal fentanyl has been shown to have desirable pharmacokinetics and high bioavailability in the treatment of acute pain.89,90 Foster, Upton, and colleagues demonstrated that intranasal fentanyl (75 to 200 µg) had only a slight, nonstatistically significant lag in the onset of analgesia but equivalent pain reduction.89
Intranasal fentanyl at 1.7 µg/kg concentrated to 150 µg/mL was compared with IV morphine in pediatric patients with long-bone fractures.91 Intranasal fentanyl was shown to be as effective as IV morphine and had a similar side effect profile. Intranasal fentanyl can be used to commence pain control in these patients before initiating an IV line or as a noninvasive alternative to IV or intramuscular injection.
Procedure
Two methods to deliver drugs to the nasal mucosa can be used: drops or aerosol. Nasal drops require a cooperative patient and correct positioning to enhance drug delivery.92,93 Some authors have noted that much of the drug is lost to the environment by dripping out the nose or into the throat and then swallowed.92,93 This can result in metabolism of the drug in the liver through first-pass metabolism. Nasal atomization has been found to be the optimal method of enhancing mucosal coverage and increasing plasma concentrations of intranasal medications.92–94 Regardless of the method, the relatively small volume of the nasal cavity limits the volume of medication to approximately 1 mL per naris. If the volume is greater than 1 mL, split the dose and instill half into each naris. Use concentrated forms of medications to decrease the overall volume.79
Nasal Drops
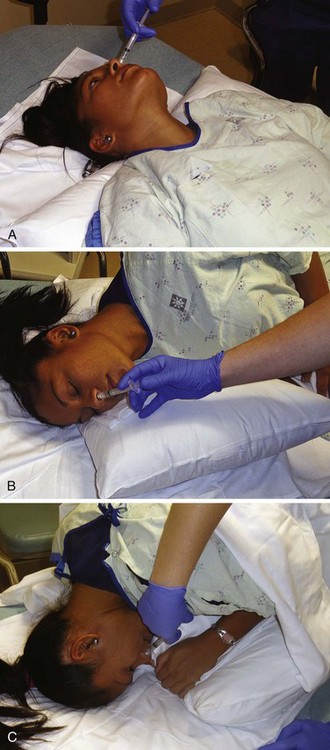
One position is to place the patient on the back with the head down and nose pointing up. Slowly instill the drops into each naris along the nasal septum and allow the medication to flow into the turbinates92,93 (Fig. 26-5A).
A second position is the lateral decubitus position with the head angled downward92,93 (see Fig. 26-5B). Use pillows or towels to elevate the body at the shoulders if necessary. Instill the drops into the naris that is “up” so that the medication runs along the nasal septum and turbinates.
An alternative, though more uncomfortable position is to place the patient on the knees with the head down and the vertex parallel to the bed, essentially in a position similar to starting a forward roll92,93 (see Fig. 26-5C). Instill the medication against the septum and let it flow to the turbinates.
Nasal Atomization
Many commercial devices are available for home and outpatient use to deliver medications intranasally. The Mucosal Atomization Device (LMA North America, San Diego, CA) is small, easy to use, and attaches to nearly any syringe (Fig. 26-6). Atomization of midazolam was found to achieve higher plasma concentrations than nebulized midazolam.95 Atomization results in very fine particles that distribute over the surface and can be absorbed more readily.
Draw up an appropriate volume of medication into a syringe with an additional amount to accommodate for the dead space of the device (0.1 mL). Insert the device approximately 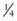 to
to 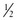 inch into the vestibule. Rapidly depress the plunger. If the total volume is greater than 1 mL, repeat this in the other naris (Fig. 26-7).
inch into the vestibule. Rapidly depress the plunger. If the total volume is greater than 1 mL, repeat this in the other naris (Fig. 26-7).
Complications
Aside from the side effects of the medications (allergy, nausea, apnea, etc.), there are few to no complications when using intranasal medications. As mentioned previously, midazolam has been noted to cause short-term local irritation. A single case of anosmia following long-term use of intranasal ketamine has been reported.96
Nebulized Naloxone
Naloxone has been administered via nebulization, as an alternative to more traditional routes, to reverse opioid intoxication.97 The proposed benefit of nebulized naloxone is that intravenous access is not required, the reversal effect may be prolonged or continued as long as the nebulizer is used, and there may be a more gradual, but often not complete, reversal of opioid intoxication, reversing opioid effects without producing acute withdrawal. Most reports are anecdotal experience or case reports, and there are little data on the specific use. The procedure may be applicable to nonapneic patients and can be used by both prehospital and ED clinicians. Rescue intramuscular or IV naloxone should be available for those not responding appropriately. Empirical dosing is 2 to 4 mg of naloxone in 3 mL of saline, delivered by an oxygen driven nebulizer, such as those used to deliver aerosolized beta-agonists to asthmatics.
Weber et al98 reported success with nebulized naloxone in approximately 80% of spontaneously breathing patients with suspected opioid intoxication when the medication was administered by paramedics. Nebulized naloxone is a reasonable option for nonapneic patients with suspected opioid-induced depressed mental status or respiratory depression; however, intravenous or intramuscular naloxone must be used if the desired reversal is not accomplished.
Rectal Administration of Medication
Drug absorption from the rectum is a simple diffusion process across the lipid membrane. In general, the rate of absorption rises with increasing lipid solubility of the drug and, when applicable, with increased rate of drug release from its carrier (e.g., time to liquefaction of suppository preparations). Other factors affecting transmucosal rectal absorption include the volume of liquid, concentration of the drug, length of the rectal catheter (i.e., site of rectal drug delivery), presence of stool in the rectal vault, pH of the rectal contents, rectal retention of the drug or drugs administered, and differences in venous drainage within the rectosigmoid region.99
Anatomy and Physiology
The rectum is the terminal portion of the large intestine; it begins at the confluence of the three taeniae coli of the sigmoid colon and ends at the anal canal. In adults, the anal canal is about 5 cm in length and the rectum is approximately 10 to 15 cm in length. In children, the size of the rectum varies with age (see Table 26-2).
TABLE 26-2
Age-Related Changes in Rectal Dimensions
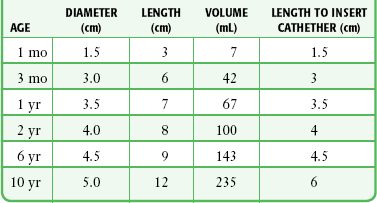
From Smith S, Sharkey I, Campbell D. Guidelines for rectal administration of anticonvulsant medication in children. Pediatr Perinatal Drug Ther. 2001;4:140–147.
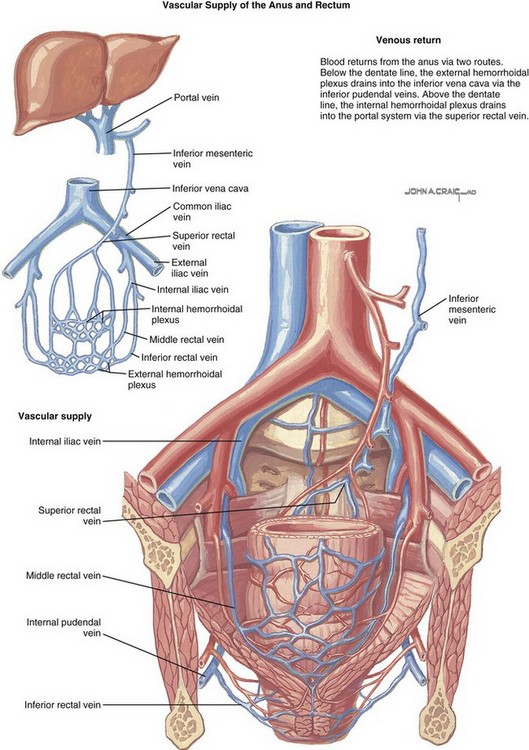
The rectum has two main routes of venous drainage (Fig. 26-8). The superior rectal vein drains into the portal circulation (by way of the inferior mesenteric vein), whereas the middle and inferior rectal veins drain into the caval system (by way of the inferior iliac vein). This pattern of venous drainage has a significant impact on the peak serum concentrations achieved by rectally administered drugs. Drugs administered high in the rectum (the area drained by the superior rectal vein) are carried directly to the liver via the portal vein and are subject to first-pass metabolism. In contrast, drugs administered low in the rectum are delivered systemically into the inferior vena cava, thereby avoiding first-pass elimination in the liver.
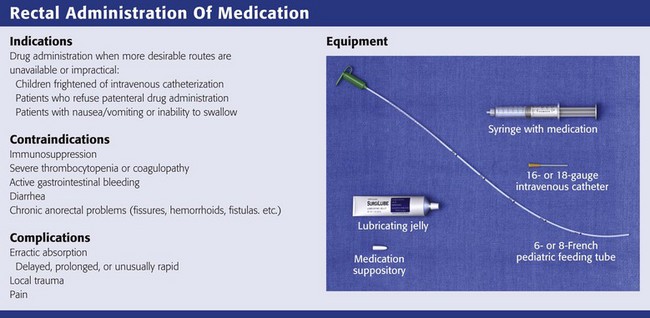
Indications and Contraindications
There are few, if any absolute contraindications to rectal drug administration. Rectal administration should be avoided in immunosuppressed patients, in whom even minimal trauma could lead to abscess formation, and in patients with severe thrombocytopenia or coagulopathy to avoid difficult-to-control bleeding.99,100 Patients with active lower gastrointestinal bleeding and those with severe diarrhea are not generally good candidates for rectal drug administration. Finally, patients with a variety of acute or chronic anorectal problems such as fissures, hemorrhoids, or perianal abscesses or fistulas may not tolerate rectal drug administration.
Procedure
Place adults and large children in a lateral recumbent position with the upper part of the leg flexed at the knee and hip and the lower part of the leg extended. Infants, because of their small size, can be placed in almost any position. Place the lubricated suppository at the rectal opening and gently push it into the rectum toward the umbilicus until the gloved index finger has been inserted approximately 7.5 cm in adults, 3.5 cm in children, or 1.5 cm in infants (see Table 26-2). To help prevent expulsion of the suppository, do not allow the patient to get up for approximately 10 to 15 minutes after insertion.
Most suppositories have an apex at one end (pointed end) and taper to a blunt base at the other end. For ease of insertion, manufacturers recommend inserting the tapered end first. However, in 1991, Abd-El-Maeboud and colleagues found that inserting suppositories blunt end first resulted in greater retention within the rectum and a lower expulsion rate.101 Nonetheless, these findings have not been corroborated and have been challenged by nursing educators as insufficient evidence on which to base clinical practice.102
Liquids and Gels
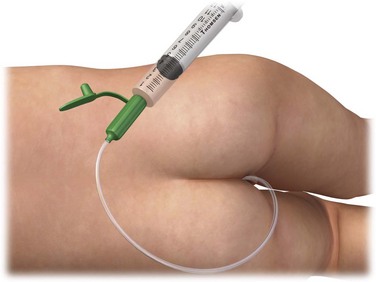
Position the patient as described for insertion of a suppository. Draw up the desired dose of medication into an appropriately sized syringe attached to an 6- or 8-Fr rubber feeding tube (adults and large children) or an 18- or 16-guage IV catheter (infants and small children) with the needle removed (Fig. 26-9). The goal is to deposit the drug in the low to midportion of the rectum to avoid first-pass elimination by the liver. For adults, insert the rubber feeding tube approximately 7.5 to 10.0 cm and slowly inject the drug. In children, catheter depth varies with age (see Table 26-2). When administering rectal medication to infants and young children, be sure to squeeze the buttock cheeks closed after withdrawing the catheter to prevent expulsion of the medication. A 3-inch piece of tape placed across the buttocks also works well and frees the clinician to perform other duties.
Medications
Analgesics and Antipyretics
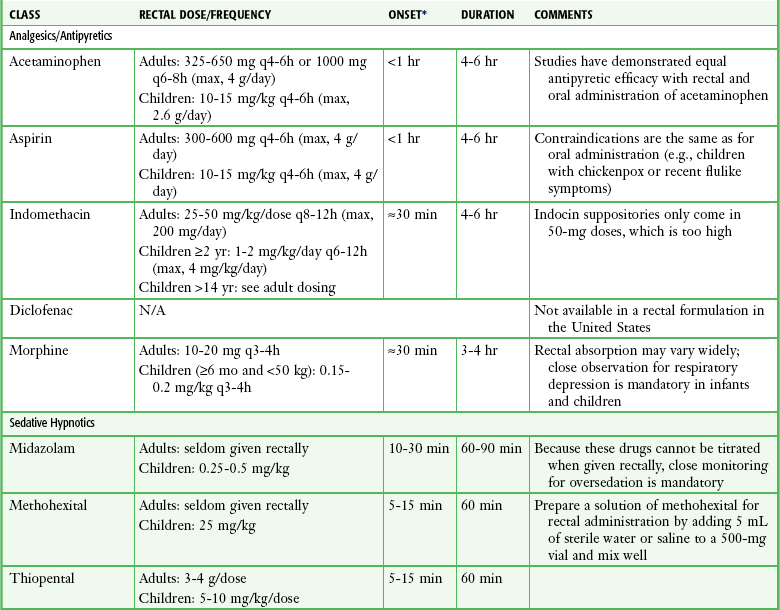
Acetaminophen is frequently administered rectally in children for both fever and pain. Common reasons for rectal administration include refusal to take the medication orally, vomiting, and altered mental status. Acetaminophen is commercially available in suppository form and is easy to obtain and administer. Studies comparing oral and rectal administration of acetaminophen have demonstrated equal antipyretic effectiveness.103,104 Rectal dosing of acetaminophen is the same as oral dosing (Table 26-3).
TABLE 26-3
Drugs Commonly Administered Rectally in the ED
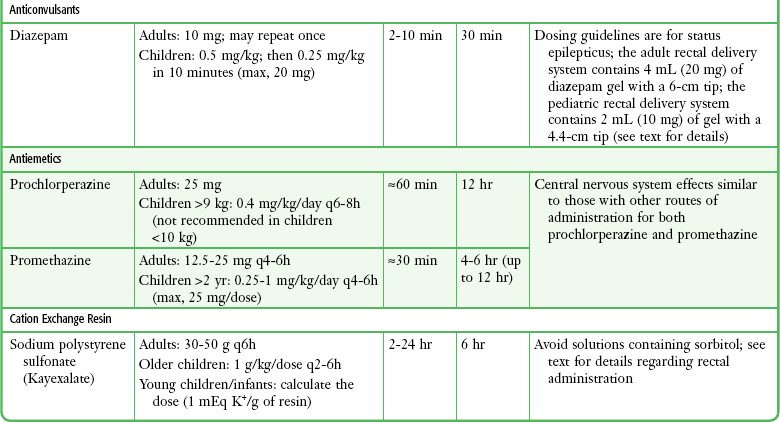
*The onset of action of rectally administered medications may vary widely because of erratic absorption and first-pass elimination in the liver.
Though not commonly administered rectally, aspirin, nonsteroidal antiinflammatory drugs (NSAIDs), including indomethacin and diclofenac (diclofenac suppositories are not available in the United States), and morphine also come in rectal formulations and can be very useful in a variety of ED encounters. For example, aspirin is commonly administered rectally to adults with symptoms of a transient ischemic attack, an acute stroke, or an acute coronary syndrome who may have an impaired swallowing mechanism or are too unstable to take medication orally. Like acetaminophen, the oral and rectal doses of aspirin are similar (see Table 26-3). Rectal NSAIDs and morphine may be an alternative for patients discharged from the ED who require ongoing analgesia but cannot tolerate oral medications.105–109 Rectal doses of indomethacin and morphine are provided in Table 26-3.
Sedative-Hypnotic Agents
Sedative-hypnotic agents, including midazolam, methohexital, and thiopental, may be administered rectally in children requiring sedation in the ED.110–113 This occurs most often in pediatric patients in whom IV access may be problematic or for procedures that may require only minimal sedation in conjunction with the use of local anesthetics (see Chapter 33 for a detailed discussion of sedation and analgesia in children). Rectal administration of methohexital and thiopental is particularly useful for sedating children before advanced imaging studies.111,113 The major drawback of rectal administration is an inability to titrate these medications to the desired level of sedation.
Midazolam, methohexital, and thiopental are administered rectally using the IV formulations of each agent as described previously. The rectal doses of midazolam, methohexital, and thiopental in children are 0.25 to 0.5 mg/kg, 25 mg/kg, and 5 to 10 mg/kg, respectively (see Table 26-3). To prepare a solution of methohexital for rectal administration, add 5 mL of sterile water or saline to a 500-mg vial of methohexital and mix well; this provides a methohexital solution of 100 mg/mL.
Anticonvulsants
Anticonvulsants are generally administered orally or intravenously in the ED. However, there may be situations, such as in actively seizing patients, in which oral or IV administration is impossible or unlikely to be accomplished in a reasonable period. In these cases, rectal administration may be an effective alternative.114 Studies have demonstrated that diazepam, because of its high lipid solubility, is rapidly absorbed from the rectum and can quickly halt seizures.114,115 Lorazepam has much lower lipid solubility and is not recommended for rectal use.
Diazepam is commercially available in a gel formulation that is preloaded in a rectal delivery system (Diastat AcuDial). However, the undiluted parenteral formulation can also be used. The preloaded rectal delivery system is available for both pediatric and adult use. The adult device contains 4 mL (20 mg) of diazepam gel and has a 6-cm tip for rectal administration. This device is designed to deliver set doses of 10, 12.5, 15, 17.5, and 20 mg of diazepam. Two pediatric devices are available: one contains 0.5 mL of a 5-mg/mL gel, and the other contains 2 mL of a 5-mg/mL gel. The latter is designed to deliver set doses of 5, 7.5, and 10 mg of diazepam. Both pediatric devices have a 4.4-cm tip for rectal administration. The recommended dose of diazepam rectal gel for treating actively seizing children and those in status epilepticus is 0.5 mg/kg, followed by 0.25 mg/kg in 10 minutes if needed (maximum dose, 20 mg); the adult dose is 10 mg, which may be repeated once (see Table 26-3).
Antiemetics
The two most common antiemetics administered rectally in the ED are prochlorperazine and promethazine. Both come in suppository formulations, which makes rectal administration easy. Prochlorperazine requires a higher dose when given rectally, whereas promethazine dosing is the same regardless of the route of administration (see Table 26-3).
Cation Exchange Resin
Sodium polystyrene sulfonate may be given orally or rectally as a retention enema. Oral dosing is more effective if intestinal motility is not impaired. Common reasons for rectal administration include an inability or refusal to swallow (the oral solution is not very palatable), vomiting, and altered mental status. The resin comes in two forms: a powder that must be reconstituted and a premixed suspension containing sorbitol. The latter should be avoided whenever possible because it has been linked to the development of intestinal necrosis.116,117 Nevertheless, sodium polystyrene sulfonate in sorbitol remains the most frequently used preparation in the ED.118
Before drug administration, perform a cleansing enema with warm tap water. Prepare a sodium polystyrene sulfonate enema by dissolving 50 g of the resin in 100 to 150 mL of tap water warmed to body temperature. In adults, administer the resin emulsion via a 6- or 8-Fr rubber feeding tube placed about 20 cm from the rectum with the tip well into the sigmoid colon. Retain the enema in the colon for at least 30 to 60 minutes and for several hours if possible. Once retention is complete, irrigate the colon with 50 to 100 mL of a non-sodium-containing fluid. The recommended dose of sodium polystyrene sulfonate for rectal administration in adults is 30 to 50 g every 6 hours. For older children, the dose is 1 g/kg per dose every 2 to 6 hours. Use lower doses in small children and infants. To calculate this dose, use the exchange ratio of 1 mEq K+/g of resin (see Table 26-3).
Complications
Absorption of a rectally administered drug may be delayed or prolonged, or uptake may be almost as rapid as though the agent were administered intravenously.99 Unusually rapid absorption has resulted in the death of a  -month-old child receiving rectal morphine for postoperative pain.119 In addition to erratic absorption, peak serum concentrations may vary considerably depending on how much of the drug undergoes first-pass elimination in the liver. These factors make rectal drug administration less desirable in most cases when parenteral administration is possible.
-month-old child receiving rectal morphine for postoperative pain.119 In addition to erratic absorption, peak serum concentrations may vary considerably depending on how much of the drug undergoes first-pass elimination in the liver. These factors make rectal drug administration less desirable in most cases when parenteral administration is possible.
Insertion of a suppository or catheter into the rectum may cause mild pain and mucosal irritation, which is usually well tolerated by most patients. Similarly, bleeding from local trauma is usually of no clinical consequence, except in patients with a clinically significant coagulopathy or severe thrombocytopenia.100 Development of a perianal or perirectal abscess is a theoretical concern in neutropenic patients, and thus rectal drug administration is best avoided in such patients.
References
1. Bernard, C. Leçons sur les Effets des Substances Toxiques et Medicamenteuses. Paris: JB Bailliere; 1857. [286].
2. Mutch, N. Inhalation of chemotherapeutic substances. Lancet. 1944;2:775.
3. Graeser, JB, Rowe, AH. Inhalation of epinephrine for the relief of asthmatic symptoms. J Allergy. 1935;6:415.
4. Gaensler, EA, Beakey, JF, Segal, MS. Pharmacodynamics of pulmonary absorption in man: I. Aerosol and intratracheal penicillin. Ann Intern Med. 1949;31:582.
5. Beakey, JF, Gaensler, EA, Segal, MS. Pharmacodynamics of pulmonary absorption in man: II. The influence of various diluents on aerosol and intratracheal penicillin. Ann Intern Med. 1949;31:805.
6. Campbell, D, Adriani, J. Absorption of local anesthetics. JAMA. 1958;168:873.
7. Redding, JS, Asuncion, JS, Pearson, JW. Effective routes of drug administration during cardiac arrest. Anesth Analg. 1967;46:253.
8. Roberts, JR, Greenberg, MI, Knaub, M, et al. Comparison of the pharmacological effects of epinephrine administered by the intravenous and endotracheal routes. JACEP. 1978;7:260.
9. Roberts, JR, Greenberg, MI, Knaub, MA, et al. Blood levels following intravenous and endotracheal epinephrine administration. JACEP. 1979;8:53.
10. Roberts, JR, Greenberg, MI, Baskin, SI. Endotracheal epinephrine in cardiorespiratory collapse. JACEP. 1979;8:515.
11. Greenberg, MI, Roberts, JR, Krusz, JC, et al. Endotracheal epinephrine in a canine anaphylactic shock model. JACEP. 1979;8:500.
12. Newmar, RW, Otto, CW, Link, MS, et al. Part 8: adult advanced cardiovascular life support: American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S729–S767.
13. Kleinman, ME, Chameides, L, Schexnayder, SM, et al. Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S876–S906.
14. Kattwinkel, J, Periman, JM, Aziz, K, et al. Part 15: neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S909–S919.
15. Schüttler, J, Bartsch, A, Ebling, BJ, et al. Endobronchial administration of adrenaline in preclinical cardiopulmonary resuscitation. Anesth Intensivther Notfallmed. 1987;22:63.
16. Chu, SS, Rah, KH, Brannan, MD, et al. Plasma concentration of lidocaine after endotracheal spray. Anesth Analg. 1975;54:438.
17. McDonald, JL. Serum lidocaine levels during cardiopulmonary resuscitation after intravenous and endotracheal administration. Crit Care Med. 1985;13:914.
18. Chernow, B, Holbrook, P, D’Angona, DS, et al. Epinephrine absorption after intratracheal administration. Anesth Analg. 1984;63:829.
19. Orlowski, JP, Gallagher, JM, Porembka, DT. Endotracheal epinephrine is unreliable. Resuscitation. 1990;19:103.
20. Quinton, DN, O’Byrne, G, Aitkenhead, AR. Comparison of endotracheal and peripheral intravenous adrenaline in cardiac arrest: is the endotracheal route reliable? Lancet. 1987;1:828.
21. Ralston, SH, Tacker, WA, Showen, L, et al. Endotracheal versus intravenous epinephrine during electromechanical dissociation with CPR in dogs. Ann Emerg Med. 1985;14:1044.
22. Crespo, SG, Schoffstall, JM, Fuhs, LR, et al. Comparison of two doses of endotracheal epinephrine in a cardiac arrest model. Ann Emerg Med. 1991;20:230.
23. Manisterski, Y, Vaknin, Z, Ben-Abraham, R, et al. Endotracheal epinephrine: a call for larger doses. Anesth Analg. 2002;95:1037.
24. Vaknin, Z, Manisterski, Y, Ben-Abraham, R, et al. Is endotracheal adrenaline deleterious due to the beta-adrenergic effect? Anesth Analg. 2001;92:1408.
25. Wenzel, V, Prengel, AW, Lindner, KH. A strategy to improve endobronchial drug administration [editorial]. Anesth Analg. 2000;91:255.
26. Mace, SE. The effect of dilution on plasma lidocaine levels with endotracheal administration. Ann Emerg Med. 1987;16:522.
27. Mace, SE. Differences in plasma lidocaine levels with endotracheal drug therapy secondary to total volume of diluent administered. Resuscitation. 1990;20:185.
28. Greenberg, MI, Baskin, SI, Kaplan, AM, et al. Effects of endotracheally administered distilled water and normal saline on the arterial blood gases of dogs. Ann Emerg Med. 1982;11:600.
29. Hahnel, JH, Lindner, KH, Schurmann, C, et al. What is the optimal volume of administration for endobronchial drugs? Am J Emerg Med. 1990;8:504.
30. Naganobu, K, Hasebe, Y, Uchiyama, Y, et al. A comparison of distilled water and normal saline as diluents for endobronchial administration of epinephrine in the dog. Anesth Analg. 2000;91:317.
31. Yang, LY, He, CQ, Zhang, ZG. Endotracheal administration of epinephrine during cardiopulmonary resuscitation. Chin Med J. 1991;104:986.
32. Greenberg, MI, Spivey, W. Comparison of deep vs shallow endotracheal medication administration in dogs. Ann Emerg Med. 1985;14:209.
33. Jasani, MS, Nadkarni, VM, Finkelstein, MS, et al. Effects of different techniques of endotracheal epinephrine administration in pediatric porcine hypoxic-hypercarbic cardiopulmonary arrest. Crit Care Med. 1994;22:1174.
34. Rehan, V, Garcia, M, Kao, J, et al. Epinephrine delivery during neonatal resuscitation: comparison of direct endotracheal tube vs catheter inserted into endotracheal tube administration. J Perinatol. 2004;24:686.
35. Prengel, AW, Lindner, KH, Hahnel, J, et al. Endotracheal and endobronchial lidocaine administration: effects on plasma lidocaine concentration and blood gases. Crit Care Med. 1991;19:911.
36. Prengel, AW, Lindner, KH, Hahnel, JH, et al. Pharmacokinetics and technique of endotracheal and deep endobronchial lidocaine administration. Anesth Analg. 1993;77:985.
37. Mazkereth, R, Paret, G, Ezra, D, et al. Epinephrine blood concentrations after peripheral bronchial versus endotracheal administration of epinephrine in dogs. Crit Care Med. 1992;20:1582.
38. Jasani, MS, Nadkarni, VM, Finkelstein, MS, et al. Inspiratory-cycle instillation of endotracheal epinephrine in porcine arrest. Acad Emerg Med. 1994;1:340.
39. Mace, SE. Plasma lidocaine levels occurring with endotracheal administration during hemorrhagic shock. Resuscitation. 1990;19:291.
40. Lucas, VW, Preziosi, MP, Burchfield, DJ. Epinephrine absorption following endotracheal administration: effects of hypoxia-induced low pulmonary blood flow. Resuscitation. 1994;27:31.
41. Mace, SE. Effect of hypoxemia on pharmacokinetics of endotracheal lidocaine in dogs. Resuscitation. 1990;20:41.
42. Schneider, SM, Yealy, DM, Michaelson, EA, et al. Endotracheal versus intravenous epinephrine in the prehospital treatment of cardiac arrest. Prehosp Disaster Med. 1990;5:341.
43. Niemann, JT, Statton, SJ. Endotracheal versus intravenous epinephrine and atropine in out-of-hospital “primary” and postcountershock asystole. Crit Care Med. 2000;28:1815.
44. Kleinman, ME, Oh, W, Stonestreet, BS. Comparison of intravenous and endotracheal epinephrine during cardiopulmonary resuscitation in newborn piglets. Crit Care Med. 1999;27:2748.
45. Niemann, J, Stratton, S, Cruz, B, et al. Endotracheal drug administration during out-of-hospital resuscitation: where are the survivors? Resuscitation. 2002;53:153.
46. Rusli, M, Spivey, WH, Bonner, H, et al. Endotracheal diazepam: absorption and pulmonary pathologic effects. Ann Emerg Med. 1987;16:314.
47. Lindemann, R. Resuscitation of the newborn: endotracheal administration of epinephrine. Acta Paediatr Scand. 1984;73:210.
48. Bray, BM, Jones, HM, Grundy, EM. Tracheal versus intravenous atropine. Anaesthesia. 1987;42:1188.
49. Scott, B, Martin, FG, Matchett, J, et al. Canine cardiovascular responses to endotracheally and intravenously administered atropine, isoproterenol, and propranolol. Ann Emerg Med. 1987;16:1.
50. Howard, RF, Bingham, RM. Endotracheal compared with intravenous administration of atropine. Arch Dis Child. 1990;65:449.
51. Boster, SR, Danzl, DF, Madden, RJ, et al. Translaryngeal absorption of lidocaine. Ann Emerg Med. 1982;11:461.
52. Greenberg, MI, Roberts, JR, Baskin, SI. Endotracheal naloxone reversal of morphine-induced respiratory depression in rabbits. Ann Emerg Med. 1980;9:289.
53. Tandberg, D, Abercrombie, D. Treatment of heroin overdose with endotracheal naloxone. Ann Emerg Med. 1982;11:443.
54. Barsan, WG, Ward, JT, Otten, EJ. Blood levels of diazepam after endotracheal administration in dogs. Ann Emerg Med. 1982;11:242.
55. Pasternak, SJ, Heller, MB. Endotracheal diazepam in status epilepticus [letter]. Ann Emerg Med. 1985;14:485.
56. Johnston, C. Endotracheal drug delivery. Pediatr Emerg Care. 1992;8:94.
57. Zaritsky, A. Pediatric resuscitation pharmacology. Ann Emerg Med. 1993;22:445.
58. Wenzel, V, Lindner, KH, Prengel, AW, et al. Endobronchial vasopressin improves survival during cardiopulmonary resuscitation in pigs. Anesthesiology. 1997;86:1375.
59. Efrati, O, Barak, A, Ben-Abraham, R, et al. Should vasopressin replace adrenaline for endotracheal drug administration? Crit Care Med. 2003;31:572.
60. Jaimovich, DG, Osborne, JS, Shabino, CL. Comparison of intravenous and endotracheal administration of midazolam and the effect on pulmonary function and histology in the lamb model. Ann Emerg Med. 1992;21:480.
61. Palmer, RB, Mautz, DS, Cox, K, et al. Endotracheal flumazenil: a new route of administration for benzodiazepine antagonism. Am J Emerg Med. 1998;16:170.
62. Grube, JA, Administration of Metaraminol by Intravenous and Intrapulmonary Routes: A Comparative Study. [thesis], Madison, University of Wisconsin, 1981. [1].
63. Elam, JO. The intrapulmonary route for CPR drugs. In: Safar P, ed. Advances in Cardiopulmonary Resuscitation. New York: Springer-Verlag; 1977:132.
64. Murphy, KM, Caplen, SM, Nowak, RM, et al. Endotracheal bretylium tosylate in a canine model. Ann Emerg Med. 1984;13:87.
65. Daniels, JM, Brien, JF, Massey, TE. Pulmonary fibrosis induced in the hamster by amiodarone and desethylamiodarone. Toxicol Appl Pharmacol. 1989;100:350.
66. Kofler, J, Sterz, F, Hofbauer, R, et al. Epinephrine application via an endotracheal airway and via the Combitube in esophageal position. Crit Care Med. 2000;28:1445.
67. Palmer, RB, Mautz, DS, Cox, K, et al. Endotracheal lidocaine administration via an esophageal Combitube. J Emerg Med. 2000;18:153.
68. Prengel, AW, Rembecki, M, Wenzel, V, et al. A comparison of the endotracheal tube and the laryngeal mask airway as a route for endobronchial lidocaine administration. Anesth Analg. 2001;92:1505.
69. Bobrow, BJ, Ewy, GA. Ventilation during resuscitation efforts for out-of-hospital primary cardiac arrest. Curr Opin Crit Care. 2009;15:228–233.
70. Storrow, AB, Walthall, JW, Magoon, MR, et al. The impact of an endotracheal side port on the absorption of lidocaine. Acad Emerg Med. 1997;4:793.
71. Feferman, I, Leblanc, L. A simple method for administering endotracheal medication [letter]. Ann Emerg Med. 1983;12:196.
72. Stewart, RD, Lacovey, DC. Administering endotracheal medication [letter]. Ann Emerg Med. 1985;14:711.
73. Nygind, N, Dahl, R. Anatomy, physiology and function of the nasal cavities in health and disease. Adv Drug Deliv Rev. 1998;29:3.
74. Illum, L. Nasal drug delivery: possibilities, problems and solutions. J Control Release. 2003;87:187.
75. Shelly, K, Peach, MJ. The clinical applications of intranasal opioids. Curr Drug Deliv. 2008;5:55.
76. Vyas, TK, Shahiwala, A, Marathe, S, et al. Intranasal drug delivery for brain targeting. Curr Drug Deliv. 2005;2:165.
77. Talegaonkar, S, Mishra, PR. Intranasal delivery: an approach to bypass the blood brain barrier. Indian J Pharmacol. 2004;36:140.
78. Westin, UE, Bostrom, E, Gasjo, J. Direct nose-to-brain transfer of morphine after nasal administration in rats. Pharm Res. 2006;23:565.
79. Pires, A, Fortuna, A, Gilberto, A, et al. Intranasal drug delivery: how, why and what for? J Pharm Pharmaceut Sci. 2009;12:288.
80. Freestone, DS, Weinber, AL. The administration of drugs and vaccines by the intranasal route. Br J Clin Pharmacol. 1976;3:827.
81. Merlin, M, Saybolt, M, Kapitanyan, R, et al. Intranasal naloxone delivery is an alternative to intravenous naloxone for opioid overdose. Am J Emerg Med. 2010;28:296–303.
82. Barton, E, Colwell, C, Wolfe, T, et al. Efficacy of intranasal naloxone as a needleless alternative for treatment of opioid overdose in the pre-hospital setting. J Emerg Med. 2005;29:265.
83. Bhattacharyya, M, Kalra, V, Gulatie, S. Intranasal midazolam vs rectal diazepam in acute childhood seizures. Pediatr Neurol. 2006;34:355.
84. Klein, E, Brown, J, Kobayashi, A, et al. A randomized clinical trial comparing oral, aerosolized intranasal, and aerosolized buccal midazolam. Ann Emerg Med. 2011;58:323.
85. al-Rakaf, H, Bello, LL, Turkustani, A, et al. Intranasal midazolam in conscious sedation of young paediatric dental patients. Int J Pediatr Dent. 2001;11:33.
86. Chiaretti, A, Barone, G, Rigante, D, et al. Intranasal lidocaine and midazolam for procedural sedation in children. Arch Dis Child. 2011;96:160.
87. Abrams, R, Morrison, J, Villasenor, A, et al. Safety and effectiveness of intranasal administration of sedative medications (ketamine, midazolam, or sufentanil) for urgent brief pediatric dental procedures. Anesth Prog. 1993;40:63.
88. Roelofse, JA, Shipton, EA, de la Harpe, CJ, et al. Intranasal sufentanil/midazolam versus ketamine/midazolam for analgesia/sedation in the pediatric population prior to undergoing multiple dental extractions under general anesthesia: a prospective, double-blind, randomized comparison. Anesth Prog. 2004;51:114.
89. Foster, D, Upton, R, Christrup, L, et al. Pharmacokinetics and pharmacodynamics of intranasal versus intravenous fentanyl in patients with pain after oral surgery. Ann Pharmacother. 2008;42:1380.
90. Prommer, E, Thompson, L. Intranasal fentanyl for pain control: current status with a focus on patient considerations. Patient Prefer Adherence. 2011;5:157–164.
91. Borland, M, Jacobs, I, King, B, et al. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Ann Emerg Med. 2007;49:335.
92. Merkus, P, Ebbens, FA, Muller, B, et al. Influence of anatomy and head position on intranasal drug deposition. Eur Arch Otorhinolaryngol. 2006;263:827.
93. Merkus, P, Ebbens, FA, Muller, B, et al. The ‘best method’ of topical nasal drug delivery: comparison of seven techniques. Rhinology. 2006;44:102.
94. Nygind, N, Vesterharge, S. Aerosol distribution in the nose. Rhinology. 1978;16:79.
95. McCormick, AS, Thomas, VL, Berry, D, et al. Plasma concentrations and sedation scores after nebulized and intranasal midazolam in healthy volunteers. Br J Anaesth. 2008;100:631–666.
96. Mayell, A, Natusch, D. Anosmia—a potential complication of intranasal ketamine. Anesthesia. 2009;64:457.
97. Mycyk, MB, Szyszko, AL, Aks, SE. Nebulized naloxone gently and effectively reverses methadone intoxication. J Emerg Med. 2003;24(2):185.
98. Weber, JM, Tataris, KL, Hoffman, JD, et al. Can nebulized naloxone be used safely and effectively by emergency medical services for suspected opioid overdose? Prehosp Emerg Care. 2011. [Dec 22; [e-pub ahead of print]].
99. American Academy of Pediatrics. Alternative routes of drug administration—advantages and disadvantages (subject review). Pediatrics. 1997;100:143–152.
100. Pasero, CP. Perioperative rectal administration of non-opioid analgesics. J Perianesth Nurs. 2010;25:5–6.
101. Abd-el-Maeboud, KH, el-Naggar, T, el-Hawi, EM, et al. Rectal suppository: common sense and motor insertion. Lancet. 1991;338:798–800.
102. Bradshaw, A. Rectal suppository insertion: the reliability of the evidence as a basis for nursing practice. J Clin Nurs. 2007;16:98–103.
103. Goldstein, LH, Berlin, M, Berkovitch, M, et al. Effectiveness of oral versus rectal acetaminophen: a meta-analysis. Arch Pediatr Adolesc Med. 2008;162:1042–1046.
104. Karbasi, SA, Modares-Mosadegh, M, Golestan, M. Comparison of antipyretic effectiveness of equal doses of rectal and oral acetaminophen in children. Pediatrics (Rio J). 2010;86:228–232.
105. Achariyapota, V, Titapant, V. Relieving perineal pain after perineorrhaphy by diclofenac rectal suppositories: a randomized double-blinded placebo controlled trial. J Med Assoc Thai. 2008;91:799–804.
106. Dhawan, N, Das, S, Kiran, U, et al. Effect of rectal diclofenac in reducing postoperative pain and rescue analgesia requirement after cardiac surgery. Pain Pract. 2009;9:385–393.
107. Rhendra Hardy, MZ, Zayuah, MS, Baharudin, A, et al. The effects of topical viscous lignocaine 2% versus per-rectal diclofenac in early post-tonsillectomy pain in children. Int J Pediatr Otorhinolaryngol. 2010;4:374–377.
108. Bahar, NM, Jangjoo, A, Soltani, E, et al. Effect of preoperative rectal indomethacin on postoperative pain reduction after open cholecystectomy. J Perianesth Nurs. 2010;25:7–10.
109. Radbruch, L, Trottenberg, P, Elsner, F, et al. Systemic review of the role of alternative application routes for opioid treatment for moderate to severe cancer pain: an EPCRC opioid guidelines project. Palliat Med. 2011;25:578–596.
110. Shane, SA, Fuchs, SM, Khine, H. Efficacy of rectal midazolam for the sedation of preschool children undergoing laceration repair. Ann Emerg Med. 1994;24:1065.
111. Pomeranz, ES, Chudnofsky, CR, Lozon, MM, et al. Rectal methohexital sedation for CT imaging of pediatric patients in the ED. Pediatrics. 2000;105:110.
112. Okutan, V, Lenk, MK, Sarici, SU, et al. Efficacy and safety of rectal thiopental sedation in outpatient echocardiographic examination of children. Acta Paediatr. 2000;89:1340.
113. Akhlaghpoor, S, Shabestari, AA, Moghdam, MS. Low dose of rectal thiopental sodium for pediatric sedation in spiral computed tomography study. Pediatr Int. 2007;49:387–391.
114. Cereghino, JJ, Cloyd, JC, Kuzniecky, RI. Rectal diazepam gel for treatment of acute repetitive seizures in adults. Arch Neurol. 2002;59:1915–1920.
115. Fitzgerald, BJ. Treatment of out-of-hospital status epilepticus with diazepam rectal gel. Seizure. 2003;12:52–55.
116. Sterns, RH, Rojas, M, Bernstein, P, et al. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733–735.
117. McGowan, CE, Saha, S, Chu, G, et al. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J. 2009;102:493–497.
118. Joshi, P, Beaulieu, J, Shemin, D. The effects of a single dose of polystyrene sulfonate (SPS) in hyperkalemic patients with kidney disease [abstract]. J Am Soc Nephrol. 2008;19:335A.
119. Gourlay, GK, Boas, RA. Fatal outcome with use of rectal morphine for postoperative pain control in an infant. Br Med J. 1992;304:766–767.