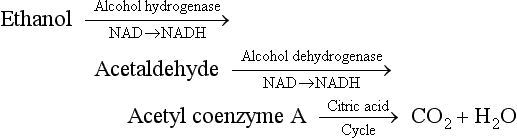Alcohol-Related Disease
Perspective
Epidemiology
The disastrous effects and widespread incidence of alcoholism are well known to the emergency physician. Almost all societies that consume alcohol show related health and social problems.1 Motor vehicle collisions, drowning, suicides, homicides, divorce, violent crime, child abuse, unemployment, and disruption of the family are often either directly or indirectly associated with excessive alcohol consumption. The tragic effects of alcohol not only affect the individual drinker but also have far-reaching implications for the family, community, and workplace. In the United States, an estimated 7.6 million visits to the emergency department (ED) a year are related to alcohol, accounting for 7.9% of all ED visits.2 Table 185-1 lists the causes of death related to alcohol abuse.
Table 185-1
Causes of Death Related to Alcohol Abuse
| 1 | Mouth and oropharynx cancer |
| 2 | Alcohol use disorders |
| 3 | Ischemic heart disease |
| 4 | Liver cirrhosis |
| 5 | Road traffic accidents |
| 6 | Poisonings |
| 7 | Falls |
| 8 | Intentional injuries |
Modified from Miranda-Mendez A, Lugo-Baruqui A, Armendariz-Borunda J: Molecular basis and current treatment for alcoholic liver disease. Int J Environ Res Public Health 7:1872-1888, 2010.
Alcohol is the most common recreational drug taken by Americans, and per capita consumption is increasing. An estimated 18 million alcoholics live in the United States; with more than 85,000 alcohol-related deaths each year, resulting in 2.3 million years of potential life lost,3 alcohol is the third leading cause of preventable death in the United States.4 Alcoholism permeates all levels of society and is a preventable cause of morbidity and mortality, with a cost to the nation estimated to be greater than $185 billion annually.5
Diagnosis
Alcohol dependence is associated with major physiologic consequences and life impairment. Dependence can be identified as repetitive problems, affecting three or more areas of life, and about 80% of people who are diagnosed with dependence at any point still have alcohol-related problems when they are assessed a year or more later.6 Dependence criteria are reliable across different ages, sexes, and most cultural groups. Alcohol abuse is defined by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), as one or more problems with functioning in a 12-month period in a person without dependence: failure in obligations; alcohol use in hazardous situations; recurrent legal problems; or continued use despite social or interpersonal problems.7
Alcohol Screening Questionnaires
The AUDIT-C Screening Questionnaire:
1. How often do you have a drink containing alcohol?
Never (0 points), monthly or less (1 point), two to four times a month (2 points), two or three times a week (3 points), four or more times a week (4 points)
2. How many drinks containing alcohol do you have on a typical day when you are drinking?
3. How often do you have six or more drinks on one occasion?
Never (0 points), less than monthly (1 point), monthly (2 points), weekly (3 points), daily or almost daily (4 points)
Scoring: The sum of scores for the three questions results in an AUDIT-C score of 0 to 12 points.
Blood tests can be useful if the history is in doubt and can also help patients recognize that alcohol has adversely affected their health. One such marker is γ-glutamyltransferase, an enzyme important in amino acid transport. Results of at least 35 units/L indicate the probability of heavy drinking. A second test is for carbohydrate-deficient transferrin, which measures a change in the structure of a proportion of transferrin that is likely to occur with heavy drinking during a long period; a result of 20 units/L or more indicates heavy drinking. Tests of liver function that measure aspartate transaminase (AST) and alanine transaminase (ALT) can identify heavy drinking and alcohol-use disorders with sensitivities of 25 to 45% and specificities as high as 90%. A ratio of AST to ALT higher than 2, especially if concentrations of these enzymes do not exceed 400 units/L, suggests alcoholic hepatitis. Although many newer biomarkers are not yet available, these newer markers (acetaldehyde adducts) rely on protein modifications by acetaldehyde and play an important role in the pathogenesis of tissue damage in alcoholics (Tables 185-2 and 185-3).
Table 185-2
Current Biomarkers in Alcoholism
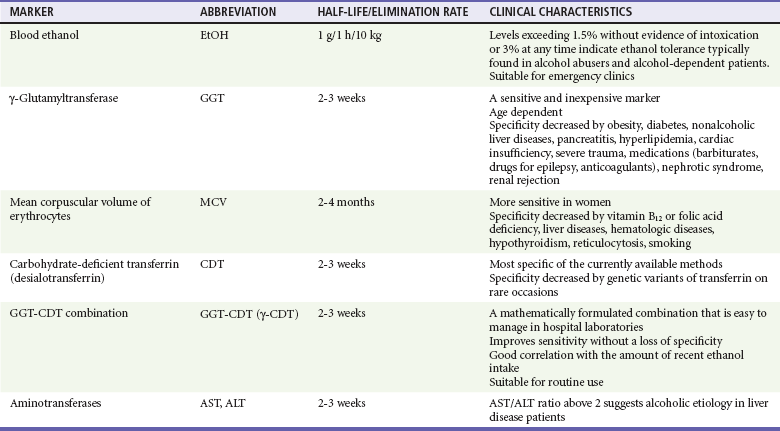
From Niemelä O: Biomarkers in alcoholism. Clin Chim Acta 377:39-49, 2007.
Table 185-3
Emerging Biomarkers in Alcoholism
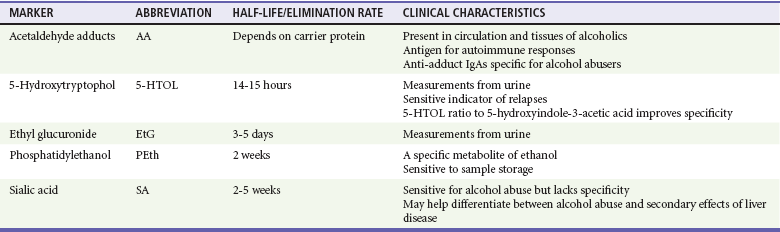
From Niemelä O: Biomarkers in alcoholism. Clin Chim Acta 377:39-49, 2007.
Definition and Natural History
A precise definition of alcoholism is difficult. A proposed definition encompassing the features of alcoholism is “a primary chronic disease with genetic, psychosocial, and environmental factors influencing its development and manifestations.” The disease is often progressive and fatal. It is characterized by impaired control over drinking, preoccupation with and use of alcohol despite adverse consequences, and distortions in thinking, most notably denial. Each of these symptoms may be periodic or continuous.8 Alcoholism is present when drinking adversely affects an individual’s physical health, ability to function in society, or interpersonal relationships.
Men: more than 14 drinks per week or more than 4 drinks per occasion
Women: more than 7 drinks per week or more than 3 drinks per occasion
Age older than 65 years: more than 7 drinks per week or more than 1 drink per occasion
Harmful drinkers present with negative consequences related to alcohol.
The natural history of alcoholism is variable, and it may appear in any patient despite age or social status. The age at onset of alcoholism continues to decrease. Up to 6% of high school seniors drink daily, and it is not unusual to see children younger than 16 years who have already graduated from an alcohol detoxification program.9 Many individuals also begin drinking heavily after the age of 60 years.
The DSM-IV has two categories for substance disorders that include alcohol abuse: (1) substance abuse and (2) substance dependence.7 The chronic substance abuse of alcohol eventually leads to acquired tolerance, a condition in which larger and larger doses of alcohol are required for the same effect.
Principles of Disease: Metabolism of Alcohol
The absorption and elimination rates of alcohol vary by individual and depend on many factors: diet, gender, body weight and habitus, speed of consumption, gastric motility, presence of food in the stomach, smoking history, age, whether the person is a chronic alcohol consumer with enzyme induction and high-activity MEOS, advanced cirrhosis, presence of ascites, and state of nourishment.10 There is enormous variation among patients in the rate of disappearance of ethanol from the blood, ranging from 9 to 36 mg/dL per hour in published data. Although the clearance rate may be as high as 36 mg/dL per hour in some chronic drinkers, 20 mg/dL per hour is a reasonable rate to assume in a typical intoxicated ED patient. This holds true for adults, adolescents, and children.11
Physiologic effects vary directly with the blood alcohol level (Table 185-4). Diminished fine motor control and impaired judgment appear with alcohol concentrations as low as 20 mg/dL (0.02 mg%), but wide individual variability exists. Chronic alcoholics can exhibit impressive tolerance. The blood alcohol concentration of a person cannot be accurately determined without quantitative testing. More than 50% of the adult population is obviously intoxicated with a level of 150 mg/dL (0.15 mg%). As the ethanol level rises, the patient’s level of consciousness declines, eventually ending in coma. Death is caused by aspiration or respiratory depression.
Table 185-4
Physiologic Effects and Blood Alcohol Levels
| BLOOD ALCOHOL CONCENTRATION (mg/dL) | EFFECTS* |
| 20-50 | Diminished fine motor control |
| 50-100 | Impaired judgment; impaired coordination |
| 100-150 | Difficulty with gait and balance |
| 150-250 | Lethargy; difficulty sitting upright without assistance |
| 300 | Coma in the novice drinker |
| 400 | Respiratory depression |
*These effects are for the occasional drinker. Chronic drinkers can function at much higher alcohol concentrations because of tolerance. On the other hand, patients may become comatose with low levels of alcohol in mixed alcohol-drug overdose.
Alcohol through passive diffusion will be present anywhere there is water in the body. Hence, expired breath alcohol or saliva can be used to obtain a reliable approximation of blood alcohol concentration in a cooperative patient. This value can be used as a rapid screen for alcohol intoxication.12,13
Management
Glucose (dextrose, 25 g IV) produces a dramatic response in alcohol-induced hypoglycemic patients. Unlike hypoglycemia of other causes, alcohol-induced hypoglycemia may be unresponsive to glucagon because of depleted liver glycogen stores. Although Wernicke’s encephalopathy is a medical emergency, alcohol-induced hypoglycemia is a much more common condition with serious and permanent morbidity if it is left untreated. Therefore, thiamine can be given in a timely fashion, but glucose therapy should not be delayed.14
Alcohol Withdrawal Syndrome
The neurophysiology of alcohol withdrawal is complex and not fully understood. Chronic alcohol consumption has a depressant effect on the central nervous system (CNS). The hallmark of alcohol withdrawal is CNS excitation with increased cerebrospinal fluid, plasma, and urinary catecholamine levels. Chronic alcohol consumption affects central adrenergic alpha receptors, glutamate, central adrenergic beta receptors, the inhibitory neurotransmitter γ-aminobutyric acid (GABA), and dopamine turnover.15
Clinical Features
Isbell’s classic study in 1955 confirmed the relationship between alcohol and the withdrawal syndrome.16 He documented that the severity of signs and symptoms depends on both the dose and the duration of ethanol consumption. The withdrawal syndrome may occur any time after the blood alcohol level starts to fall. Therefore, only a reduction, not the abrupt cessation, of ethanol intake may result in withdrawal.
Minor alcohol withdrawal occurs as early as 6 hours and usually peaks at 24 to 36 hours after cessation of or significant decrease in alcohol intake. It is characterized by mild autonomic hyperactivity: nausea, anorexia, coarse tremor, tachycardia, hypertension, hyper-reflexia, sleep disturbances (e.g., insomnia, vivid dreams), and anxiety.17
Major alcohol withdrawal occurs after more than 24 hours and usually peaks at 50 hours but occasionally takes up to 5 days to be manifested after the decline or termination of drinking. The syndrome is characterized by pronounced anxiety, insomnia, irritability, tremor, anorexia, tachycardia, hyper-reflexia, hypertension, fever, decreased seizure threshold, auditory and more commonly visual hallucinations, and finally delirium.18
Management
Benzodiazepines.: The benzodiazepines have superior anticonvulsant activity, have the least respiratory and cardiac depressive effect of all the CNS depressants, and can be given parenterally in the uncooperative patient. By interacting with receptors linked to the GABA-associated chloride ion channel, benzodiazepines substitute for the withdrawal of the GABA-potentiating effect of alcohol and abate withdrawal signs and symptoms.19 Numerous benzodiazepines have been studied, but there is no evidence of clear superiority of any one benzodiazepine.
As one dosing regimen, diazepam, 5 mg IV every 5 to 10 minutes (2.5 mg/min), can be given in major withdrawal until the patient is calm. The dose can be repeated in 5 to 10 minutes. If the second dose of 5 mg is not working, consider 10 mg for the third and fourth doses every 5 to 10 minutes. If this is not effective, consider 20 mg for the fifth and subsequent dose until adequate sedation is obtained.20,21
Butyrophenones.: Haloperidol, a dopamine antagonist, can be considered in patients with major alcohol withdrawal or delirium tremens not responding to intravenous benzodiazepines. Haloperidol has little effect on myocardial function or respiratory drive, and its safety and efficacy by the intravenous, intramuscular, or oral route in the ED have been established. Haloperidol has no anticonvulsant properties; however, extrapyramidal effects may be seen. Caution should be used in patients who may be susceptible to a prolonged QTc. Droperidol has effects similar to those of haloperidol. Despite the 2001 Food and Drug Administration black box warning for QTc prolongation and torsades de pointes after droperidol use, droperidol remains a safe and effective treatment for agitated patients.22
Alcohol-Related Seizures
Among the many medical problems related to alcohol abuse, the differential diagnosis and management of seizures remain among the most challenging and controversial (Box 185-1). Patients presenting to the ED with seizures should be questioned about alcohol intake. Of seizure patients presenting to an ED, 20 to 40% will have their seizures related to alcohol use or abuse.23 Alcohol is a causative factor in 11 to 24% of patients with status epilepticus.24,25 In states where alcohol sales are restricted on Sundays, EDs see a spike in alcohol-related seizures on Mondays.26
The primary consideration in the initial care of seizure patients who use alcohol is the recognition of treatable, life-threatening causes. These causes include but are not limited to CNS infection, metabolic disorders, and intracranial hemorrhage. Alcohol may act in one of several ways to produce seizures in patients with or without underlying foci: by its partial or absolute withdrawal after a period of chronic intake, by an acute alcohol-related metabolic disorder (e.g., hypoglycemia, hyponatremia), by creation of a situation leading to cerebral trauma, by precipitation of seizures in patients with idiopathic or post-traumatic epilepsy, or by lowering of the seizure threshold in patients with prior existing intracerebral disease states. Moreover, alcoholics are more susceptible to other disorders associated with seizures, including neurosyphilis, acquired immunodeficiency syndrome (AIDS), brain abscess, and meningitis.27–29
Alcohol Withdrawal Seizures
Descriptions of alcohol withdrawal seizures are based on data collected by Victor and Brausch.30 Seizures occurred 6 to 48 hours after the cessation of drinking. Ninety percent had one to six generalized tonic-clonic seizures. Sixty percent experienced multiple seizures within a 6-hour period. However, seizure recurrence can be reduced to 3% with lorazepam administration after the initial seizure.31 The incidence of partial seizures, common with post-traumatic epilepsy, is increased in alcohol withdrawal. The term alcohol withdrawal seizure is reserved for seizures with the characteristics described by Victor and Brausch.30 The term alcohol-related seizure is used to refer to all seizures in the aggregate associated with alcohol use, including this subset of alcohol withdrawal seizure.
Patients Presenting with Normal Findings on Neurologic Examination
New-Onset Alcohol-Related Seizures
Patients with new-onset alcohol-related seizure should be thoroughly evaluated. This includes alcoholics who claim to have had seizures but for whom no documentation or an appropriate work-up is available. Metabolic disorders, toxic ingestion, infection, and structural abnormalities should be considered. Laboratory and radiographic testing to include electrolyte values and blood urea nitrogen, creatinine, glucose, and anticonvulsant levels and brain computed tomography (CT) scan may be necessary. Of 259 patients presenting with their first alcohol-related seizure, clinical management was changed in 3.9% on the basis of head CT results.32
Seizures in the Alert Patient with a History of Seizures during Prior Withdrawal
The risk of seizure increases significantly in alcoholic patients with manifestations of alcohol withdrawal who relate a history of alcohol withdrawal seizure.33,34 Detoxification with benzodiazepines reduces alcohol withdrawal seizure and should be initiated early because most seizures occur within the first 24 hours after alcohol withdrawal. An initial dose of 2 mg of lorazepam or 5 mg of diazepam can be given intravenously. These doses frequently need to be repeated.35 The patient should be observed for 4 to 6 hours before discharge is considered. The prescription of benzodiazepines or antiepileptic drugs on discharge carries its own hazards. Benzodiazepines (other than a short 3- to 6-day tapering dose for withdrawal) may increase the potential risk of addiction. In a noncompliant patient, antiepileptic drugs, such as phenytoin, may paradoxically increase the number of seizures. The poorly compliant alcoholic patient may do better without outpatient anticonvulsants for a concurrent seizure disorder.33 The ideal disposition is referral to a detoxification or rehabilitation unit.
Patients with an Abnormal Neurologic Presentation
Partial seizures account for 24 to 51% of alcohol-related seizures.36 Conversely, studies have shown that 17 to 21% of patients with partial alcohol-related seizure have structural lesions: hematomas, tumors, vascular abnormalities, or stroke.37 These primary causes of partial alcohol-related seizure, such as prior head trauma, may be easily missed in the history taking. As a result, an emergent CT scan is indicated to evaluate new-onset partial seizures. The patient with a history of a focal alcohol-related seizure who has been previously evaluated does not require an emergency CT scan, provided a return to baseline occurs promptly. A patient presenting with a focal alcohol-related seizure with subsequent normal neuroimaging findings can be managed with supportive care, observation for 4 to 6 hours, and a benzodiazepine for withdrawal signs or symptoms. Appropriate follow-up should be arranged.
Patients Taking Phenytoin-Anticonvulsant
Phenytoin has no significant benefit over placebo in prevention of recurrence of uncomplicated (e.g., no old subdural) alcohol withdrawal seizure. Considering the risks of phenytoin and no demonstrated benefit in the setting of alcohol withdrawal seizure, it is not indicated for the treatment of alcohol withdrawal seizure. The sudden withdrawal of phenytoin may potentiate the convulsive effects of alcohol withdrawal.23,38
A patient currently taking antiepileptic drugs for an antecedent seizure disorder who presents with a seizure while intoxicated falls into a different category. Such an episode could be an isolated event in a usually compliant patient without a history of chronic alcohol abuse. In this patient, a seizure in the setting of a subtherapeutic antiepileptic drug level may represent the consequences of noncompliance with antiepileptic medication or sleep deprivation versus alcohol withdrawal seizure.33
Other Clinical Features and Management
Acute and chronic ethanol consumption can affect the mechanical function of the heart, produce dysrhythmias, and exacerbate coronary artery disease (CAD). It may alter myocardial function by direct toxic effects, by associated hypertension, or indirectly by altering specific electrolytes. Acute intoxication can decrease cardiac output in both alcoholic and nonalcoholic patients with preexisting cardiac disease.38
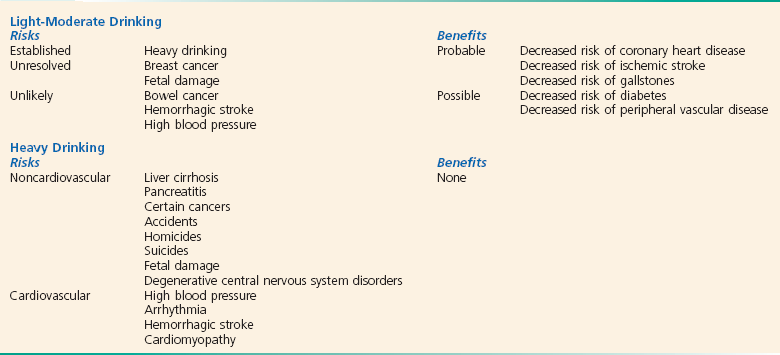
Studies have linked moderate alcohol consumption (two to four drinks per day in men and one or two in women) to a protective effect from CAD. Low to moderate alcohol consumption decreases platelet aggregation, raises plasma levels of endogenous tissue plasminogen activator,39 and lowers insulin resistance. Experimental data suggest that alcohol may have antioxidant properties, produce effects on smooth muscles through interactions with nitric oxide, and alter plasma total homocysteine levels.40,41 The red versus white wine hypothesis rests on human studies showing short-term cardiovascular benefits achieved by de-alcoholized red but not white wine and many studies on isolated tissues or organs assessing the effects of polyphenols in red wine, especially resveratrol. Therefore, red wine potentially has beneficial effects beyond alcohol content.42
Studies suggest that moderate alcohol consumption, through a reduced risk of CAD, may also protect individuals from congestive heart failure (Box 185-2).43 All of these beneficial effects are lost in heavy drinkers, in whom chronic alcoholism is associated with hypertension and congestive cardiomyopathy.
Up to one third of chronic alcoholic patients have left ventricular dysfunction demonstrated by radionuclide ventriculography, usually coexisting with skeletal muscle disease. Those who stop using alcohol may have an improved ejection fraction during the course of 3 years.44 Although the primary functional abnormality of alcoholic cardiomyopathy was thought to be a depression in systolic function, it is now appreciated that an impairment in diastolic function is present in one third of alcoholics who have a normal systolic function; in many, systolic dysfunction and diastolic dysfunction coexist. Excess alcohol consumption affects not only the cardiomyocytes but striated skeletal muscle as well. Women appear to be more sensitive than men to the toxic effects of alcohol on striated muscle and are at greater risk for cardiomyopathy and myopathy as well as for skeletal myopathy for any given lifetime amount of alcohol.44
Heavy alcohol consumption has a detrimental effect on those with preexisting CAD. It can reduce exercise tolerance, induce coronary vasoconstriction, and raise heart rate and blood pressure.45 Additive cardiovascular effects of ethanol and nicotine contribute to dysrhythmias and sudden death in patients with CAD. In one study, nearly half the patients with alcohol withdrawal had prolongation of the QT interval. Prolonged QT can precipitate a dysrhythmia, resulting in sudden death.46 There is an increased incidence of sudden death among heavy drinkers regardless of concomitant CAD or smoking.
Alcohol also affects cardiac function indirectly by lowering potassium and magnesium levels. Data from the Framingham Heart Study indicate that patients with lower levels of potassium and magnesium have higher rates of dysrhythmias.47
Pulmonary Effects
Alcohol reduces the mobilization of alveolar macrophages and their bactericidal capacity. Their impairment is greatest in alcoholics with hepatic cirrhosis. In alcoholic patients, the lungs are more vulnerable to oxidative stress and injury. There is evidence that chronic alcohol consumption decreases the level of glutathione, promoting inflammation and remodeling of the lung tissue.48 These effects, along with aspiration, decreased airway sensitivity, concomitant smoking, and malnutrition, probably account for the increased incidence of pneumonia, particularly lobar pneumonia, among alcoholic patients.49 Alcohol abuse is also associated with an increased likelihood of intensive care unit admission and a longer hospital length of stay than for non-alcoholic patients with community-acquired pneumonia.50
At least 80% of alcoholics are smokers, making it difficult to distinguish between alcohol-induced and tobacco-induced injury to the lungs. The high prevalence of respiratory disease in alcoholics is largely caused by smoking. Patients with sepsis and chronic alcohol abuse are at least twice as likely to require mechanical ventilation and have a twofold to fourfold risk for development of the acute respiratory distress syndrome.50
Alcohol induces bronchospasm in some asthmatics and increases ventricular ectopy and sleep apnea in patients with chronic obstructive pulmonary disease. Alcoholic patients with hepatic cirrhosis can have hypoxemia as a result of precapillary shunting in their lungs. Hyperventilation and respiratory alkalosis are also seen with hepatic cirrhosis. One or two drinks per day has been found to decrease the risk of pulmonary embolus and deep venous thrombosis in elderly patients.51
Gastrointestinal and Hepatic Effects
Alcoholic patients have a higher incidence of esophagitis, gastric cancer, and esophageal carcinoma than that in the general population. Acute alcohol ingestion also decreases lower esophageal sphincter pressure, delays gastric emptying, and disrupts the normal gastric mucosal barrier. Alcohol consumption, because of its inherent toxicity, has been shown to eliminate infection of the gastric mucosa by Helicobacter pylori.52 Vomiting is common among drinkers. Forceful or persistent emesis can lead to a Mallory-Weiss tear or Boerhaave’s syndrome.
Gastrointestinal Bleeding
Alcohol is closely associated with gastrointestinal bleeding. Causes and contributing factors include Mallory-Weiss tears, esophagitis, esophageal varices, acute and chronic gastritis, thrombocytopenia, portal hypertensive gastropathy, qualitative and quantitative platelet disorders, and prolonged clotting times. Alcohol may exacerbate gastric mucosal damage when it is combined with nonsteroidal anti-inflammatory drugs (NSAIDs), but ethanol itself is not a risk factor for peptic ulcer disease. An inverse relationship exists between consumption of alcohol, particularly wine, and active H. pylori infection. Peptic ulcer disease is the most common cause of bleeding in alcoholic patients with upper gastrointestinal hemorrhage as well as in those who do not drink.53
Liver
Hepatic damage has been recognized for centuries as the hallmark of chronic alcohol abuse. Obesity potentiates the severity of alcohol-induced liver damage.54 The activation of the immune system with the production of cytokines such as tumor necrosis factor alpha is one of the earliest events in many types of liver injury.55 This cascade stimulates Kupffer cells and the production of other cytokines that together enlist inflammatory cells, kill hepatocytes, and initiate healing through fibrogenesis. There is no single test that can be used to diagnose alcoholic liver disease reliably. However, the ratio of AST to ALT higher than 2 suggests that alcohol is the cause of liver injury.56 Alcoholic liver disease is the most common liver disorder in the Western world and along with hepatitis C is a leading cause of liver transplantation.57
The earliest, mildest, and most common liver change in alcoholism is the accumulation of macrovesicular fat in the hepatocytes, predominantly involving triglycerides. Alcoholic fatty liver is usually asymptomatic, associated with mild elevations of AST and ALT. It is detected by the finding of hepatomegaly on physical examination or abnormalities on ultrasonography or CT but is confirmed by liver biopsy. Fatty liver is a reversible disorder if the patient can refrain from drinking.58
Alcoholic Hepatitis
Alcoholic hepatitis is more serious than fatty infiltration and develops in up to 35% of heavy drinkers.59 These individuals usually have right upper quadrant pain, tender enlarged liver, fever, jaundice, leukocytosis, and altered liver function test results. AST levels are usually less than 400 IU/L, and ALT levels are typically less than half the AST level. Alcoholic hepatitis has a range of clinical manifestations, from mildly symptomatic hepatomegaly to fulminant hepatic failure. The severity of the disease can be estimated in the ED by a prolonged prothrombin time/international normalized ratio (INR)60 or with the use of discriminant factor.61 The ABIC (age, bilirubin, INR, creatinine) score and the Model for End-stage Liver Disease (MELD) are also helpful in predicting mortality in these patients.59,62
Evaluation of symptomatic patients includes complete blood count, electrolyte values, blood urea nitrogen and creatinine concentrations, glucose concentration, prothrombin time/INR, liver function tests, and urinalysis. If the patient has an abnormal prothrombin time/INR and is actively bleeding, fresh frozen plasma should be started in the ED. Steroids are indicated in severe cases (encephalopathy, coagulopathy).63 Steroids are relatively contraindicated in patients with gastrointestinal bleeding or concurrent infection. In those for whom steroid treatment is contraindicated, pentoxifylline has been shown to be beneficial.64 Up to 80% of patients with alcoholic hepatitis who continue to drink eventually have cirrhosis.
Alcoholic Cirrhosis
Hepatitis C antibodies are found in one third to one half of alcoholics with alcoholic liver disease, presumably from similar risk factors. Patients with alcoholic liver disease and hepatitis C have histologically more severe disease, shorter survival, and tenfold increased rates of cirrhosis and liver cancer.65
No specific medical therapy exists for alcoholic liver disease other than abstinence, proper diet, and management of the subsequent hepatic decompensation (i.e., ascites and encephalopathy). A decrease in the amount of alcohol consumed during 1 year is associated with a 60% decrease in mortality.66
Pancreatitis and Malabsorption
The diagnosis of alcoholic pancreatitis can be difficult because asymptomatic alcoholics may have an elevated amylase level. Conversely, up to 30% of patients with acute alcoholic pancreatitis have an amylase value within normal limits. Serum lipase rises after amylase, remains elevated longer, and is a more reliable indicator of alcoholic pancreatitis, especially when it is more than three times normal.67 Alcohol is the leading cause of chronic pancreatitis.
Diarrhea and impaired intestinal absorption are common problems of the chronic alcoholic. Alcohol increases small intestine transit time and decreases brush border enzyme activity. Thiamine, vitamin B12, amino acids, folic acid, and glucose have impaired absorption in alcoholics. Dietary deficiencies in folic acid and protein, pancreatic insufficiency, abnormal biliary secretion, and direct toxic effects of ethanol on the gastrointestinal tract contribute to malabsorption. Abstinence and adequate nutrition reverse the diarrhea and much of the malabsorption.58
Neurologic Effects
Wernicke-Korsakoff Syndrome
Although they are similar pathologically and caused by thiamine deficiency, Wernicke and Korsakoff syndromes are clinically distinct. Wernicke’s encephalopathy, a medical emergency with a mortality rate of 10 to 20%, remains a clinical diagnosis and is often unrecognized. Contemporary criteria require two of these signs: dietary deficiencies, oculomotor abnormalities (nystagmus is most common), cerebellar dysfunction, and either an altered mental state or mild memory impairment.68 Mental abnormalities include lethargy, inattentiveness, abulia, and impaired memory, progressing without treatment to coma.
Genetic and environmental factors may play a part in the pathogenesis of this disorder. A thiamine-dependent enzyme, transketolase, is deficient or less active in some patients who have Wernicke-Korsakoff syndrome. This may explain why the disorder develops in only a few alcoholics. Persons with transketolase deficiency are asymptomatic until they are stressed by thiamine deficiency. Protracted vomiting, inadequate diet, and malabsorption contribute to thiamine deficiency in the alcoholic.69
Korsakoff’s psychosis or amnesic state, also called alcohol-induced persisting amnestic disorder, is a disorder with recent memory impairment, inability to learn new information or to recall previously learned information, apathy, and confabulation. Although it is common, confabulation is not essential for the diagnosis. Whereas 80% of patients with acute Wernicke’s encephalopathy have Korsakoff’s syndrome, age older than 40 years and many years of heavy alcohol use are additional risk factors.70
Movement Disorders
Alcohol withdrawal is associated with tremor, ataxia, and myoclonus. Acute alcohol consumption ameliorates essential tremor and myoclonus. Persistent tremor is occasionally seen in chronic alcoholism. This alcoholic tremor may persist up to 1 year after abstinence. Although the pathophysiologic mechanism is poorly understood, studies have confirmed that essential tremor and alcoholic tremor are distinct entities.71
Alcoholic Cerebellar Degeneration
Characterized by ataxia of the extremities, cerebellar ataxia of alcoholism results in a wide-based stance and uncoordinated gait. Lower extremity involvement predominates, although the arms may rarely be involved. Pathologic changes consist of degeneration of elements in the cerebellum, especially the Purkinje cells. The diagnosis is based on history, physical examination, and magnetic resonance imaging or CT (which shows severe cerebellar atrophy). Treatment consists of abstinence, adequate nutrition, and thiamine.72
Dementia
Some studies suggest that ethanol may offer direct neuroprotection to the brain, bearing on the apparent risk reduction of dementia in selected drinkers.73 Once again, a J-shaped curve emerges, with moderate intake reducing the risk of cognitive impairment and heavy drinking increasing it.74 Whereas small amounts of ethanol probably protect against dementia and Alzheimer’s disease but not against vascular dementia or cognitive decline, these conclusions are controversial.75
Infections
Experimental and clinical data support the conclusion that alcohol is a potent immunomodulatory agent. Chronic alcohol abuse (≥8 drinks/day) leads to immunosuppression.76 Animal and human studies have implicated acute and chronic ethanol ingestion in causing decreased serum bactericidal activity, impaired mononuclear phagocyte function, diminished cell-mediated immune functions, reduced delayed hypersensitivity reaction, and defective polymorphonuclear neutrophils. Neutropenia may be found in up to 8% of hospitalized alcoholics.77
The most common infection in alcoholism is pneumonia. Associated risk factors for pneumonia in alcoholics include smoking, decreased ciliary function, decreased surfactant production, depressed cough reflex, malnutrition, and poor oral hygiene. Although alcoholic patients may contract a variety of bacterial pneumonias, Streptococcus pneumoniae is still the most common organism. Periods of alcoholic stupor with incomplete glottic closure and subsequent aspiration can lead to aspiration pneumonia or lung abscess. Klebsiella pneumoniae, classically associated with alcoholism, is currently more common in patients with cytotoxic chemotherapy, hematologic malignant disease, and transplantation than in the chronic alcoholic. In addition, these infections now tend to be nosocomial rather than community acquired.77
Chronic alcoholism is also associated with a threefold increase of tuberculosis.78 Alcoholism itself does not seem to influence the long-term relapse rates in tuberculous patients if they have closely supervised therapy of adequate duration. Homeless alcoholic patients are an important reservoir of tuberculosis in the United States.
Hepatitis C appears to be related to concomitant injection drug use rather than the direct effect of alcohol abuse. Alcoholism is associated with a high prevalence of unsafe sexual behavior and human immunodeficiency virus (HIV) seropositivity,79 with greater immunologic changes in HIV-1–positive patients who also consume alcohol.80
Endocrine Effects
Alcohol dependence adversely affects many endocrine systems. Both peripheral thyroid hormone dysfunction and central hypothalamic-pituitary-thyroid axis deregulation are seen.81 Male hypogonadism and feminism are seen in chronic male alcoholics. Alcohol’s effects on both the testes and the hypothalamus decrease testosterone production in men.82 Alcohol may cause impotence by CNS sedation, secondary depression, or decreased testosterone production. Decreased testosterone, increased estrogen (in patients with liver disease), and increased prolactin can lead to decreased libido, feminization, and gynecomastia in male alcoholics and to abnormalities in lactation and menstruation in women. In female alcoholics, increased levels of testosterone and estrogen are found. Estrogen replacement therapy may increase hormonal levels threefold and thus increase the risk of cholelithiasis and breast cancer.83
Metabolic Effects
Alcohol-induced hypoglycemia occurs in 1 to 4% of intoxicated ED patients. It is more frequently seen in chronic alcoholics.84 Coma, seizures, hemiparesis, and a variety of other neurologic signs have been described in patients presenting with alcohol-induced hypoglycemia. Starvation, depletion of liver glycogen stores, decreased plasma cortisol levels, impaired release of growth hormone, and inhibition of gluconeogenesis contribute to this phenomenon.
Hyperglycemia and diabetes may be found in chronic alcoholism. Alcohol abuse can lead to chronic pancreatitis, resulting in underproduction of insulin by the damaged pancreatic cells. Alcohol also impairs peripheral glucose utilization, causing a relative insulin resistance (similar to type 2 diabetes). In diabetic patients, alcohol can induce hypoglycemia and also mask the signs of hypoglycemia. This is more prominent in the fasting state.85
Lipids
A reversible hypertriglyceridemia occurs in many chronic alcoholics. Ethanol increases hepatic synthesis of triglycerides. Abstention is necessary to reduce elevated triglyceride levels. Except for its relationship to fatty infiltration of the liver, the clinical significance of this hyperlipidemia is unknown.86
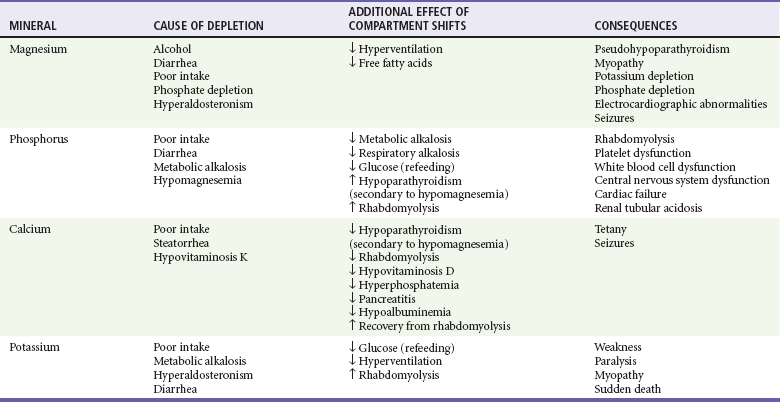
Electrolytes
Ethanol has numerous effects on electrolytes and mineral metabolism as summarized in Table 185-5. Hyponatremia and hypokalemia are common in active drinkers. Vomiting, diarrhea, magnesium depletion, malnutrition, and metabolic alkalosis contribute to these abnormalities.
Table 185-5
Effect of Ethanol on Mineral Metabolism
↓ out of plasma; ↑ into plasma.
From Kaysen G, Noth R: The effects of alcohol on blood pressure and electrolytes. Med Clin North Am 1984; 68:239.
Alcoholism is the most common cause of severe magnesium deficiency in adult outpatients. Thirty percent of alcoholics are magnesium deficient as a result of malabsorption, malnutrition, diarrhea, vomiting, and increased urinary losses. Oral magnesium supplementation in chronic alcoholics improves liver function test findings, electrolyte balance, and muscle strength.87 Multivitamin preparations may be considered for chronic malnutrition. Although their clinical benefit is not proved, they carry no significant risk or cost.
Hypocalcemia is common in alcoholic patients with magnesium depletion. The mechanism is related to diminished parathyroid hormone secretion, decreased tissue responsiveness to parathyroid hormone, decreased vitamin D metabolism, and decreased calcium release from bone independent of parathyroid hormone. Correction of magnesium depletion is necessary to restore calcium to normal levels. Hypoalbuminemia, pancreatitis, or vitamin D deficiency also contributes to low serum calcium or low total body stores of calcium in alcoholic patients.88
Hypophosphatemia is found in 30 to 50% of hospitalized patients with alcoholism. Phosphorus depletion results from malnutrition, vomiting, respiratory alkalosis, diarrhea, enhanced release of calcitonin, phosphate-binding antacids, and urinary loss (related to vitamin D deficiency and secondary hyperparathyroidism). Hypophosphatemic patients often have low magnesium levels.89 Rehydration, carbohydrate repletion, and parenteral alimentation further exacerbate phosphorus depletion. Glucose bolus and infusion have been shown to produce a significant fall in serum inorganic phosphate levels. Severe hypophosphatemia (<1 mg/dL) has been associated with acute respiratory failure; myocardial depression; dysfunction of erythrocytes, leukocytes, and platelets; CNS irritability; and rhabdomyolysis.
Alcoholic Ketoacidosis
Alcoholic ketoacidosis most frequently occurs in severe chronic alcoholics who have had a recent binge followed 1 to 3 days later by protracted vomiting, decreased food intake, dehydration, and abstinence. Nausea, vomiting, and abdominal pain are common presenting complaints.90 These patients have tachypnea, dehydration, ketonuria, and little or no glucosuria. Serum glucose levels are usually less than 200 mg/dL. Normal blood pH may be found despite ketonemia because of coexisting respiratory alkalosis and metabolic alkalosis.
The alcoholic patient with metabolic acidosis presents an interesting dilemma because most of these patients have an increased anion gap acidosis. Glucosuria may suggest diabetes; crystalluria can be seen in ethylene glycol poisoning; low specific gravity, proteinuria, and casts can be seen in renal failure; leukocytes and bacteria are present with urosepsis; and significant ketones in an otherwise normal urine may indicate starvation or alcoholic ketosis. Elevated levels or a very high osmolal gap (>25 mOsm/kg) is specific (88%) for methanol or ethylene glycol ingestion.91
Hematologic Effects
The alcoholic presents with myriad hematologic abnormalities. The direct toxic effect of ethanol and its metabolites, secondary nutritional deficiency, and hepatic disease, individually or in combination, affect red blood cells, white blood cells, platelets, hemostasis, and the immune system.92 Macrocytosis is the most common hematologic manifestation of the chronic alcoholic. It may be caused by folate deficiency, reticulocytosis (the younger reticulocytes are larger), liver disease (producing an abnormal lipid coating of the red blood cell membrane), or vitamin B12 deficiency. The most common condition is idiopathic macrocytosis of alcoholism.
Platelet Disorders
Thrombocytopenia can occur with folate deficiency, sepsis, disseminated intravascular coagulation, or splenic sequestration. The direct toxic effects of alcohol decrease measured survival time and impair production of platelets in the bone marrow, but marrow toxicity will rarely reduce the platelet count below 30,000. Qualitative platelet function is also impaired. Binge drinking is associated with a reactive thrombocytosis potentially responsible for acute stroke and sudden death.93
Oncologic Effects
Worldwide, 389,000 annual cases of cancer representing 3.6% of all cancers are alcohol related.94,95 Although alcohol itself is not carcinogenic, its metabolite, acetaldehyde, is emerging as an important contributor, being able to form stable DNA adducts, to trigger mutations in tumor suppressors and oncogenes, and to interfere with DNA repair. Smoking certainly has an additional role as a cause of neoplasia and is difficult to isolate in these studies.
Chronic alcohol use is associated with an increased incidence of upper alimentary and respiratory tract cancers with a clear dose-response relationship. Specifically, alcohol increases the risk of cancer of the mouth, pharynx, larynx, lung, esophagus, liver, and pancreas.96 Chronic hepatitis B infection may sensitize the liver to alcohol, producing hepatocellular carcinoma. Women who drink two to five drinks per day have a relative risk of 1.41 for invasive breast cancer compared with nondrinkers.97 There is also a significant increase in endometrial cancer risk among postmenopausal women who consume more than two alcoholic drinks/day.98 Moderate alcohol consumption leads to an increased risk of colorectal and prostate cancer.99
Hypothermia
Acute alcohol ingestion is one of the most common precipitating factors for accidental hypothermia and occurs in 33 to 73% of patients presenting with a core temperature below 35° C.100 Alcohol exacerbates hypothermia of other causes with depressed hypothalamic thermoregulation, peripheral vasodilation producing heat loss, CNS depression, sepsis, inability to shiver, hypoglycemia, and increased risk of environmental exposure. Hypothermia may be the presentation of Wernicke’s syndrome, possibly caused by lesions of the posterior hypothalamus, hypoglycemia, or sepsis. Intoxicated patients may have slower rewarming rates.
Psychiatric Effects
Depression and antisocial personality are the two most common psychiatric disorders that correlate with alcoholism, with a prevalence of 30 to 60% in most studies. Chronic alcohol use can produce an imbalance in the serotoninergic system. This imbalance may lead to increased anxiety, aggression, and depression. Interestingly, aggressive behavior is more strongly linked to depression than to alcohol dependence.101 Secondary depression may be caused by alcoholism, or the primary affective disorder may be present with secondary alcoholism. Mild depressive symptoms are also common in alcohol withdrawal. Antisocial individuals are at high risk for alcoholism and drug dependence, although an unstable, unhappy childhood environment appears to be more important than alcohol to the development of sociopathy. Alcohol increases the lifetime risk of suicide, with 17% of all alcoholics eventually dying by suicide.102,103 Alcoholism, major depression, and antisocial personality all predispose to suicide, and interaction among the three is particularly dangerous, but the acute risk on any day is difficult to assess.
An alcoholic patient who presents with an apparent psychiatric disorder poses a diagnostic dilemma. Does the patient have a primary affective disorder with secondary alcoholism, or is the patient manifesting anxiety or depressive behavior because of alcoholism? In general, alcoholic patients with psychiatric disorders are best treated with medications that are specific for their psychiatric condition.104 Many alcoholics with mild depression have spontaneous resolution of symptoms with abstinence. Nevertheless, depression meeting DSM-IV criteria is best treated with antidepressants.105,106 If abstinence can be achieved, underlying psychiatric disorders are more easily diagnosed and treated.
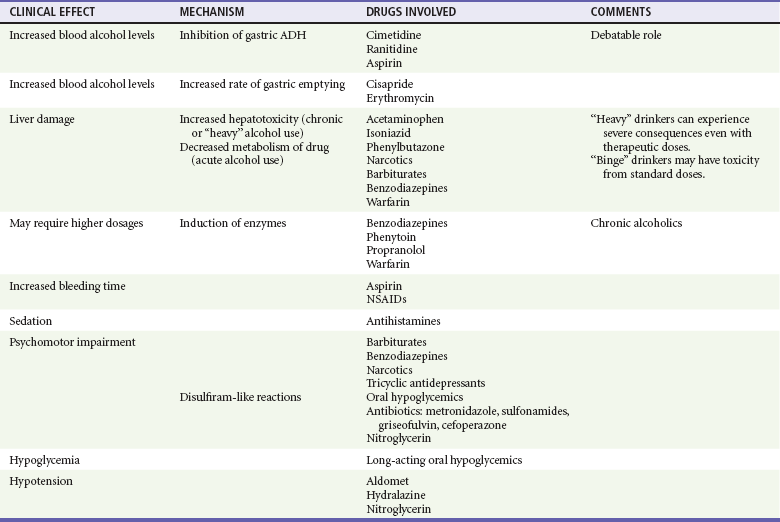
Alcohol-Drug Interactions
Alcohol is associated with a vast number of drug interactions (Table 185-6). These may occur through several mechanisms: altered absorption; enhanced metabolism and activated toxic metabolites through the hepatic CYP2E1 pathway; additive or synergistic effects; disulfiram-ethanol–like reactions; and congeners, compounds found in alcoholic beverages. In general, chronic alcoholism is associated with an increased rate of drug clearance as a result of enhanced metabolism and enzyme induction (cytochrome P450 system). Conversely, acute alcohol intoxication reduces clearance for other drugs, increasing their serum concentration because of competition for a shared detoxification pathway.107
Table 185-6
Effects and Mechanisms of Alcohol-Drug Interactions
ADH, alcohol dehydrogenase; NSAIDs, nonsteroidal anti-inflammatory drugs.
Data from Fraser AG: Pharmacokinetic interactions between alcohol and other drugs. Clin Pharmacokinet 1997; 33:79; and Adams WL: Interactions between alcohol and other drugs. Int J Addict 30:1903, 1995.
Enhanced Metabolism and Toxic Metabolites
Most alcohol is metabolized by ADH. A small percentage of alcohol is metabolized by the MEOS–cytochrome P450 system, which is also responsible for the metabolism of many drugs. The MEOS metabolic pathway is enhanced in chronic alcoholics, which is associated with the acceleration of the degradation of these other drugs. For example, the half-lives of warfarin, phenytoin, and isoniazid are 50% shorter in abstaining alcoholics than in nondrinkers. Barbiturates, diazepam, propranolol, and rifampin may have increased rates of clearance and shorter half-lives when they are taken by chronic alcoholics. This effect can persist for days to weeks after the cessation of drinking.58,108
Acetaminophen is the most widely used analgesic in the United States and is often recommended to alcoholic patients instead of NSAIDs to prevent gastritis. Chronic alcohol ingestion may enhance acetaminophen hepatotoxicity by accelerating the biotransformation to a toxic metabolite on the basis of a few case reports of severe or lethal acetaminophen toxicity in alcoholics taking therapeutic doses. Increased vulnerability seems to occur immediately after cessation of drinking. A synergistic effect apparently occurs with alcohol, fasting, and acetaminophen use in combination with depleted glutathione stores. A prospective observational study suggested that acute ethanol intake may be associated with a lower risk of hepatotoxicity after acetaminophen overdose.109 Greatly elevated AST levels (3000-48,000 in one series), increased ALT levels, and greatly elevated prothrombin/INR times help distinguish acetaminophen hepatotoxicity from alcoholic hepatitis.107
Additive or Synergistic Effects
When cocaine and ethanol are taken concomitantly, the unique metabolite, cocaethylene, is a neurologically active compound that is significantly more toxic than cocaine to the heart, liver, and brain and more addicting and more lethal than cocaine alone. Cocaethylene produces a higher incidence of confusion, lower mean Glasgow Coma Scale (GCS) scores, and a higher incidence of violent trauma and more often requires endotracheal intubation.110 Hemodynamically, these patients demonstrate an elevated heart rate (1.5-5 times normal) and blood pressure higher than with either drug alone. Sudden death is increased 18- to 25-fold above that associated with use of cocaine alone. Plasma levels of cocaine in this combined group were higher than in those who used cocaine alone.111
Ethanol increases aspirin-induced prolongation of bleeding time and reduces the metabolism of warfarin, leading to increased anticoagulant effects. There is an increased risk of upper gastrointestinal bleeding when alcohol is combined with NSAIDs. This may be the most dangerous additive or synergistic effect of alcohol.112
Disulfiram and Similar Reactions
A similar but milder disulfiram-ethanol–like reaction has been described when alcohol combines with several different drugs. The reaction may occur days to weeks after the last dose of medication. Four cephalosporins (cefamandole, cefoperazone, cefotetan, moxalactam), metronidazole, chloramphenicol, griseofulvin, nitrofurantoin, sulfonamides, all sulfonylureas, and chloral hydrate have produced this reaction in combination with alcohol. Life-threatening toxic reactions between griseofulvin and small amounts of alcohol have been reported.113 Treatment for disulfiram reaction is usually just observation, an antiemetic for symptoms and intravenous fluids, and rarely dopamine for severe hypotension.
Oral Hypoglycemics
Profound hypoglycemia can occur when alcohol and oral hypoglycemic agents are combined. Patients taking metformin may have an increased risk for development of lactic acidosis when it is combined with heavy drinking. A disulfiram-ethanol–like reaction has been described with many hypoglycemic agents.108
Adolescent Patient
Although a significant percentage of children drink alcohol, there are as yet few established screening instruments for this population. The usual age at first drinking, independently of the family, is about 15 years (although this varies across cultural groups) and has not changed much in decades.6 The period of heaviest drinking is usually between 18 and 22 years of age and also does not differ between those with future alcohol-use disorders and the general population. More than 60% of teenagers, even those without alcohol-use disorders, have experienced drunkenness by the age of 18 years, and about 30% have given up events such as school or work to drink or have driven while intoxicated.
Alcohol is often associated with the three leading causes of death among youth: unintentional injury, homicide, and suicide.114 Adolescent drinking is associated with many negative consequences including suicide, carrying weapons, driving under the influence and resultant fatalities, unsafe or increased sexual activity, sexual assault, and date rape. Seventy-eight percent of high school students have tried alcohol, and 30% admit to binge drinking at least once a month.115 Binge drinking, typically defined as drinking more than five drinks on an occasion, accounts for 90% of the alcohol consumed by 12- to 17-year-old youths.114
The age at drinking onset may be an indicator for increased risk of alcohol-related injury. Adolescents who began drinking regularly before the age of 14 years are at least three times more likely to be diagnosed with alcohol dependence than are those who began drinking at the age of 21 years. In addition, adolescents who began drinking before the age of 21 years were significantly more likely, during their lives, to be injured while under the influence of alcohol.116
Elderly Patient
Unhealthy drinking is found in up to 15% of elderly (older than 65 years) ED patients.117 Fifty percent of older people drink alcohol, and 2 to 4% meet criteria for alcohol abuse or dependence. Common screening tests (e.g., CAGE) tend to be less sensitive in this age group. Alcohol may exacerbate underlying disease by masking anginal chest pain, worsening hypertension, and inducing dysrhythmias.118 However, elderly persons consuming low to moderate levels of alcohol may have a decreased risk for development of dementia and heart failure.119 More than 90% of people aged 65 years or older use more than one prescribed medication.120 Aging alters gastrointestinal absorption, lowers volume of distribution, diminishes homeostatic responses, and reduces renal and hepatic function.108 Elderly persons also demonstrate increased end-organ sensitivity, particularly involving the CNS, with concomitant drug use increasing their risk for alcohol and drug interactions.
A protective effect on cognitive functioning has been shown with moderate alcohol consumption, particularly in women.121 Moderate consumption is associated with a 38% reduced risk of dementia.122 Still, elderly patients are more likely to have neuropsychiatric complications of alcoholism: sleep problems, anxiety, depression, and dementia. Alcohol is involved in one third of suicides in elderly persons. Older subjects also perform less well than younger subjects on tests of perception and attention at all blood alcohol levels. This may result in an increased risk of fractures from falling and osteoporosis. However, recent evidence suggests that compared with abstinence, consumption of up to one drink per day is associated with a decreased risk of osteoporotic hip fracture and a beneficial effect of moderate alcohol consumption on bone density.123
Pregnancy
Many scientific reports confirm alcohol’s teratogenic effects. According to the National Institute on Drug Abuse, almost 19% of all children born in the United States have been exposed to alcohol during gestation.124 Pregnant women who report use of any alcohol, binge drinking, or frequent drinking are more likely to be older than 30 years, employed, and unmarried.125
Fetal alcohol syndrome is characterized by a triad of CNS defects, including mild to moderate mental retardation; dysmorphology, involving mostly facial structures; and growth deficiencies, usually consisting of short stature and microcephaly.126 Fetal alcohol syndrome is now considered the most common identifiable source of mental retardation. Children exposed to prenatal alcohol exhibit increased activity levels, cognitive and attention deficits, perseverative behavior, and language and motor problems, which persist into adulthood.127
Whereas there is no known safe amount of consumption during pregnancy, in a cohort study of more than 5000 patients observed for 14 years, an average of less than one drink per day in early or late pregnancy showed no measurable impact on a child’s learning or cognitive functioning. Adverse outcomes in this study were associated with an average of more than one drink per day, binge drinking, and consumption of alcohol later in pregnancy.128 The American Academy of Pediatrics recommends abstinence from alcohol for women who are pregnant or who are planning a pregnancy.129
Trauma
The single greatest contributor to alcohol-related mortality in the United States is unintentional injury, accounting for approximately 26,000 deaths per year.130 The importance of alcohol misuse as a precursor to serious injury is widely accepted enough that since 2006 the American College of Surgeons Committee on Trauma requires screening for problem drinking for designation as a level I or level II trauma center. In addition, level I trauma centers must provide an intervention for problem drinkers identified. Alcohol and trauma are inextricably linked. Independently, the tragic effects of each are numerous; in combination, they are staggering. Injury is the leading cause of death between the ages of 1 and 44 years, accounting for more than 50 million injuries per year. In the United States, alcohol is the major risk factor for virtually all categories of intentional and unintentional injury. Besides increasing the frequency and severity of injury, alcohol significantly complicates the management of the trauma victim.131 Alcohol intoxication often complicates the initial assessment of injury severity, resulting in an increased need for invasive diagnostic and therapeutic procedures (e.g., intubation, CT scan, intracranial pressure monitoring).132
Alcohol may diminish the patient’s capacity to respond to hemorrhagic shock by altering hemodynamic effects and acid-base balance. Volume depletion as a result of the diuretic effect of alcohol or vomiting can impair the reserve of the intoxicated trauma patient. Peripheral vasodilation caused by alcohol may contribute to hypotension and hypothermia. Although these effects may be minimal, they underscore the need for early and adequate fluid resuscitation in these patients. Intoxicated patients with severe non-neurologic trauma may have lower blood pressures and carbon dioxide levels (indicative of a compensatory hyperventilation) on hospital arrival compared with sober patients. More important, a poorly understood cardiac depressant effect also increases the depth of shock and volume requirements for resuscitation. Alcohol-induced skin vasodilation may be accompanied by an increase in skeletal muscle, mesenteric, and renal bed constriction and left ventricular stroke work. Thus the overall effect on systemic vascular resistance and blood pressure may be balanced.133
Alcohol intoxication predisposes to abdominal wall laxity and thus less protection from blunt trauma.133 These patients are also likely to have full stomachs, increasing the risk of gastric injury after trauma and predisposing to vomiting and aspiration, especially during airway management. The fatty liver changes of alcoholism can result in hepatomegaly. Portal hypertension in alcoholics may produce splenomegaly. These organs can become more vulnerable to the effects of trauma because of their enlarged size, protrusion beneath the protection of the ribs, and increased intracapsular pressure.
Alcohol intoxication contributes to CNS injury in many ways. It is associated with aggressive behavior, impaired reflexes and coordination, and inappropriate avoidance responses. A higher degree of trauma to the spinal cord and much worse neurologic and functional recovery occur in patients who are intoxicated during trauma compared with sober patients.134 Experimental evidence suggests that alcohol acts synergistically with mechanical injury of the spinal cord to amplify the trauma response by increasing edema formation within the contused tissue.135
No consensus exists on the indications for an emergency CT scan in patients with “minor head injury” (loss of consciousness, post-traumatic amnesia, GCS score of 14 to 15, normal findings on neurologic examination). One disturbing prospective study found that the GCS score and 1 hour of observation were unable to predict abnormal head CT scans in intoxicated patients with minor head trauma. Patients with signs of head trauma and focal or generalized seizures need an urgent CT scan. CT scans of the head should be performed for any patient with deteriorating mental status, focal neurologic findings, new-onset seizures even without obvious signs of history of trauma, failure to improve over time, or mental status changes out of proportion to the degree of intoxication.136
The good news is that during the past 20 years, there has been a decline in the number of alcohol-related fatalities. Five states (Hawaii, Illinois, Indiana, Pennsylvania, and Utah) have enacted mandatory hospital and provider reporting laws.137
Admission Guidelines and Disposition
Psychiatric and Social Problems
Patients who are no longer able to care for themselves may also require admission. Although these patients’ ultimate destination is a rehabilitation center or a board-and-care program, hospitalization may be necessary to rule out medical or psychiatric illness and to treat impending withdrawal symptoms. Patients who wish to stop drinking are usually admitted to a detoxification unit for treatment of impending withdrawal. Data and interest are increasing for outpatient drug therapy in alcohol dependence. The U.S. Food and Drug Administration has approved disulfiram, naltrexone, acamprosate, and topiramate for treatment of alcohol dependence.104 There is growing evidence that patients with alcohol dependence who carry a particular variant of an opioid receptor gene are more likely to respond to naltrexone, raising the possibility that genetic tests may one day guide medication selection.138 Naltrexone, ondansetron, acamprosate, and acamprosate plus naltrexone have had mixed results facilitating abstinence.139,140 The role of medications in combination with behavioral therapy is actively investigated.141,142
Several other medications are under active study and are sometimes prescribed for alcoholism treatment on an unapproved or off-label basis. Baclofen, because of its anticraving action and safety, could have an important role for treatment of alcohol-dependent patients with advanced liver disease.143 Gabapentin is used as monotherapy or as an add-on pharmacotherapy in outpatient settings in the control of alcohol consumption and craving and in helping patients achieve abstinence.144 Ondansetron may show benefit in early-onset but not in late-onset alcoholics.145
Brief intervention has reduced alcohol consumption in some well-designed ED studies but not in others.146,147 Alcohol dependence and a positive screen for alcohol-related injuries are decreased after screening and brief intervention.148 Internet-based interventions show promise for reducing alcohol consumption, especially among those meeting criteria for hazardous or harmful drinking.149 Telephone contact after the ED visit may be another effective tool to screen injured patients for hazardous drinking and to offer brief intervention while avoiding interruptions to patient flow.150 Referral and brief intervention are warranted.151 Most communities have either an Alcoholics Anonymous (AA) chapter or a treatment center for anyone who desires help with alcohol. In smaller communities, clergy or social workers can usually arrange rehabilitation.
Alcohol kills—it kills the alcoholic and it kills unintended victims by the acts of inebriated persons. Whereas medical, psychological, or social problems bring the alcoholic to the ED, the underlying problem is alcoholism and the ultimate goal is abstinence. This disease surely progresses if alcoholism is not first recognized and the patient is never given the opportunity to participate in a rehabilitation program.152 The emergency physician can intervene on behalf of the patient and the public.
References
1. Rehm, J, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233.
2. Miner, JR, Gaetz, A, Biros, MH. The association of a decreased level of awareness and blood alcohol concentration with both agitation and sedation in intoxicated patients in the ED. Am J Emerg Med. 2007;25:743–748.
3. Bernstein, SL. The clinical impact of health behaviors on emergency department visits. Acad Emerg Med. 2009;16:1054–1059.
4. Seventh Special Report to the U.S. Congress on Alcohol and Health from the Secretary of Health and Human Services. Rockville, Md: National Institute on Alcohol Abuse and Alcoholism, Department of Health and Human Services, 1990.
5. Pitzele, HZ, Tolia, VM. Twenty per hour: Altered mental state due to ethanol abuse and withdrawal. Emerg Med Clin North Am. 2010;28:683–705.
6. Schuckit, MA. Alcohol-use disorders. Lancet. 2009;373:492–501.
7. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC:American Psychiatric Association; 1994.
8. Morse, RM, Flavin, DK. The definition of alcoholism. The Joint Committee of the National Council on Alcoholism and Drug Dependence and the American Society of Addiction Medicine to Study the Definition and Criteria for the Diagnosis of Alcoholism. JAMA. 1992;268:1012–1014.
9. Werner, MJ, Adger, HJr. Early identification, screening, and brief intervention for adolescent alcohol use. Arch Pediatr Adolesc Med. 1995;149:1241–1248.
10. Jones, AW. Disappearance rate of ethanol from the blood of human subjects: Implications in forensic toxicology. J Forensic Sci. 1993;38:104–118.
11. Brennan, DF, Betzelos, S, Reed, R, Falk, JL. Ethanol elimination rates in an ED population. Am J Emerg Med. 1995;13:276–280.
12. Christopher, TA, Zeccardi, JA. Evaluation of the Q.E.D. Saliva Alcohol Test: A new, rapid, accurate device for measuring ethanol in saliva. Ann Emerg Med. 1992;21:1135–1137.
13. Degutis, LC, Rabinovici, R, Sabbaj, A, Mascia, R, D’Onofrio, G. The saliva strip test is an accurate method to determine blood alcohol concentration in trauma patients. Acad Emerg Med. 2004;11:885–887.
14. Krishel, S, SaFranek, D, Clark, RF. Intravenous vitamins for alcoholics in the emergency department: A review. J Emerg Med. 1998;16:419–424.
15. McKeon, A, Frye, MA, Delanty, N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry. 2008;79:854–862.
16. Isbell, H, Fraser, HF, Wikler, A, Belleville, RE, Eisenman, AJ. An experimental study of the etiology of rum fits and delirium tremens. Q J Stud Alcohol. 1955;16:1–33.
17. Turner, RC, Lichstein, PR, Peden, JG, Jr., Busher, JT, Waivers, LE. Alcohol withdrawal syndromes: A review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4:432–444.
18. Berk, W, Todd, K. Relationship of abstinence to the presentation and peak intensity of signs and alcohol withdrawal. Ann Emerg Med. 1993;22:339.
19. Mayo-Smith, MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278:144–151.
20. Daeppen, JB, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: A randomized treatment trial. Arch Intern Med. 2002;162:1117–1121.
21. Mayo-Smith, MF, et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med. 2004;164:1405–1412.
22. Shale, JH, Shale, CM, Mastin, WD. A review of the safety and efficacy of droperidol for the rapid sedation of severely agitated and violent patients. J Clin Psychiatry. 2003;64:500–505.
23. Rathlev, NK, et al. The lack of efficacy of phenytoin in the prevention of recurrent alcohol-related seizures. Ann Emerg Med. 1994;23:513–518.
24. Alldredge, BK, Lowenstein, DH. Status epilepticus related to alcohol abuse. Epilepsia. 1993;34:1033–1037.
25. Lowenstein, DH, Alldredge, BK. Status epilepticus at an urban public hospital in the 1980s. Neurology. 1993;43(Pt 1):483–488.
26. Rathlev, NK, et al. Etiology and weekly occurrence of alcohol-related seizures. Acad Emerg Med. 2002;9:824–828.
27. Brust, JC. Acute neurologic complications of drug and alcohol abuse. Neurol Clin. 1998;16:503–519.
28. Lacy, JR, Filley, CM, Earnest, MP, Graff-Radford, NR. Brain infarction and hemorrhage in young and middle-aged adults. West J Med. 1984;141:329–334.
29. Ng, SK, Hauser, WA, Brust, JC, Susser, M. Alcohol consumption and withdrawal in new-onset seizures. N Engl J Med. 1988;319:666–673.
30. Victor, M, Brausch, C. The role of abstinence in the genesis of alcoholic epilepsy. Epilepsia. 1967;8:1–20.
31. D’Onofrio, G, Rathlev, NK, Ulrich, AS, Fish, SS, Freedland, ES. Lorazepam for the prevention of recurrent seizures related to alcohol. N Engl J Med. 1999;340:915–919.
32. Smith, BJ. Treatment of status epilepticus. Neurol Clin. 2001;19:347–369.
33. Hillbom, ME, Hjelm-Jager, M. Should alcohol withdrawal seizures be treated with anti-epileptic drugs? Acta Neurol Scand. 1984;69:39–42.
34. Marx, J, Berner, J, Bar-Or, D, Gorayeb, M. Prophylaxis of alcohol withdrawal seizures: A prospective study [abstract]. Ann Emerg Med. 1986;15:637.
35. Kahan, M, Borgundvaag, B, Borsoi, D, Edwards, C, Ladhani, N. Treatment variability and outcome differences in the emergency department management of alcohol withdrawal. CJEM. 2005;7:87–92.
36. Brathen, G, Brodtkorb, E, Helde, G, Sand, T, Bovim, G. The diversity of seizures related to alcohol use. A study of consecutive patients. Eur J Neurol. 1999;6:697–703.
37. Earnest, MP, et al. Intracranial lesions shown by CT scans in 259 cases of first alcohol-related seizures. Neurology. 1988;38:1561–1565.
38. Segel, L. Alcohol and the heart. Med Clin North Am. 1984;68:147.
39. Ridker, PM, Vaughan, DE, Stampfer, MJ, Glynn, RJ, Hennekens, CH. Association of moderate alcohol consumption and plasma concentration of endogenous tissue-type plasminogen activator. JAMA. 1994;272:929–933.
40. Agarwal, DP. Cardioprotective effects of light-moderate consumption of alcohol: A review of putative mechanisms. Alcohol Alcohol. 2002;37:409–415.
41. Fagrell, B, et al. The effects of light to moderate drinking on cardiovascular diseases. J Intern Med. 1999;246:331–340.
42. Opie, LH, Lecour, S. The red wine hypothesis: From concepts to protective signalling molecules. Eur Heart J. 2007;28:1683–1693.
43. Walsh, CR, et al. Alcohol consumption and risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2002;136:181–191.
44. Kloner, RA, Rezkalla, SH. To drink or not to drink? That is the question. Circulation. 2007;116:1306–1317.
45. Mukamal, KJ, Maclure, M, Muller, JE, Sherwood, JB, Mittleman, MA. Prior alcohol consumption and mortality following acute myocardial infarction. JAMA. 2001;285:1965–1970.
46. Otero-Anton, E, et al. Prolongation of the QTc interval during alcohol withdrawal syndrome. Acta Cardiol. 1997;52:285–294.
47. Tsuji, H, Venditti, FJ, Jr., Evans, JC, Larson, MG, Levy, D. The associations of levels of serum potassium and magnesium with ventricular premature complexes (the Framingham Heart Study). Am J Cardiol. 1994;74:232–235.
48. Thakur, L, et al. Alcohol consumption and development of acute respiratory distress syndrome: A population-based study. Int J Environ Res Public Health. 2009;6:2426–2435.
49. Thomsen, JL. Diseases of the airways and lungs in forensic autopsy material of alcoholics. Med Sci Law. 1997;37:23–26.
50. de Wit, M, Best, AM, Gennings, C, Burnham, EL, Moss, M. Alcohol use disorders increase the risk for mechanical ventilation in medical patients. Alcohol Clin Exp Res. 2007;31:1224–1230.
51. Pahor, M, et al. Alcohol consumption and risk of deep venous thrombosis and pulmonary embolism in older persons. J Am Geriatr Soc. 1996;44:1030–1037.
52. Gao, L, Weck, MN, Stegmaier, C, Rothenbacher, D, Brenner, H. Alcohol consumption, serum gamma-glutamyltransferase, and Helicobacter pylori infection in a population-based study among 9733 older adults. Ann Epidemiol. 2010;20:122–128.
53. Brenner, H, Rothenbacher, D, Bode, G, Adler, G. Inverse graded relation between alcohol consumption and active infection with Helicobacter pylori. Am J Epidemiol. 1999;149:571–576.
54. Alatalo, PI, et al. Effect of moderate alcohol consumption on liver enzymes increases with increasing body mass index. Am J Clin Nutr. 2008;88:1097–1103.
55. Nguyen-Khac, E, Houchi, H, Daoust, M, Dupas, JL, Naassila, M. The −308 TNFα gene polymorphism in severe acute alcoholic hepatitis: Identification of a new susceptibility marker. Alcohol Clin Exp Res. 2008;32:822–828.
56. Diehl, AM. Liver disease in alcohol abusers: Clinical perspective. Alcohol. 2002;27:7–11.
57. Breitkopf, K, et al. Current experimental perspectives on the clinical progression of alcoholic liver disease. Alcohol Clin Exp Res. 2009;33:1647–1655.
58. Lieber, CS. Hepatic and other medical disorders of alcoholism: From pathogenesis to treatment. J Stud Alcohol. 1998;59:9–25.
59. Dominguez, M, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–2756.
60. Alcoholic liver disease. The ACG’s recommendations. J Crit Illn. 14(264), 1999.
61. Maddrey, WC, et al. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199.
62. Dunn, W, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–358.
63. Mathurin, P, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): Individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:480–487.
64. Akriviadis, E, et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: A double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648.
65. Khan, KN, Yatsuhashi, H. Effect of alcohol consumption on the progression of hepatitis C virus infection and risk of hepatocellular carcinoma in Japanese patients. Alcohol Alcohol. 2000;35:286–295.
66. Sigal, SH, Lieber, CS. Treating the most severe manifestations of alcoholic liver disease. J Crit Illn. 11(155), 1996.
67. Gumaste, V, Dave, P, Sereny, G. Serum lipase: A better test to diagnose acute alcoholic pancreatitis. Am J Med. 1992;92:239–242.
68. Harper, C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998;57:101–110.
69. Reuler, JB, Girard, DE, Cooney, TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312:1035–1039.
70. Donnino, MW, Vega, J, Miller, J, Walsh, M. Myths and misconceptions of Wernicke’s encephalopathy: What every emergency physician should know. Ann Emerg Med. 2007;50:715–721.
71. Cardoso, F, Jankovic, J. Movement disorders. Neurol Clin. 1993;11:625–638.
72. Charness, ME, Simon, RP, Greenberg, DA. Ethanol and the nervous system. N Engl J Med. 1989;321:442–454.
73. Brust, JC. Ethanol and cognition: Indirect effects, neurotoxicity and neuroprotection: A review. Int J Environ Res Public Health. 2010;7:1540–1557.
74. Peters, R, Peters, J, Warner, J, Beckett, N, Bulpitt, C. Alcohol, dementia and cognitive decline in the elderly: A systematic review. Age Ageing. 2008;37:505–512.
75. Lobo, E, et al. Is there an association between low-to-moderate alcohol consumption and risk of cognitive decline? Am J Epidemiol. 2010;172:708–716.
76. Goral, J, Karavitis, J, Kovacs, EJ. Exposure-dependent effects of ethanol on the innate immune system. Alcohol. 2008;42:237–247.
77. Cook, RT. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res. 1998;22:1927–1942.
78. Lonnroth, K, Williams, BG, Stadlin, S, Jaramillo, E, Dye, C. Alcohol use as a risk factor for tuberculosis—a systematic review. BMC Public Health. 2008;8:289.
79. Avins, AL, et al. HIV infection and risk behaviors among heterosexuals in alcohol treatment programs. JAMA. 1994;271:515–518.
80. Szabo, G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34:830–841.
81. Ozsoy, S, Esel, E, Izgi, HB, Sofuoglu, S. Thyroid function in early and late alcohol withdrawal: Relationship with aggression, family history, and onset age of alcoholism. Alcohol Alcohol. 2006;41:515–521.
82. Noth, RH, Walter, RMJr. The effects of alcohol on the endocrine system. Med Clin North Am. 1984;68:133–146.
83. Ginsburg, ES, et al. Effects of alcohol ingestion on estrogens in postmenopausal women. JAMA. 1996;276:1747–1751.
84. Sucov, A, Woolard, RH. Ethanol-associated hypoglycemia is uncommon. Acad Emerg Med. 1995;2:185–189.
85. Bell, DS. Alcohol and the NIDDM patient. Diabetes Care. 1996;19:509–513.
86. Baraona, E, Lieber, CS. Alcohol and lipids. Recent Dev Alcohol. 1998;14:97–134.
87. Gullestad, L, et al. Oral magnesium supplementation improves metabolic variables and muscle strength in alcoholics. Alcohol Clin Exp Res. 1992;16:986–990.
88. Vamvakas, S, Teschner, M, Bahner, U, Heidland, A. Alcohol abuse: Potential role in electrolyte disturbances and kidney diseases. Clin Nephrol. 1998;49:205–213.
89. Elisaf, MS, Siamopoulos, KC. Mechanisms of hypophosphataemia in alcoholic patients. Int J Clin Pract. 1997;51:501–503.
90. Fulop, M. Alcoholic ketoacidosis. Endocrinol Metab Clin North Am. 1993;22:209–219.
91. Almaghamsi, AM, Yeung, CK. Osmolal gap in alcoholic ketoacidosis. Clin Nephrol. 1997;48:52–53.
92. Lindenbaum, J. Hematologic complications of alcohol abuse. Semin Liver Dis. 1987;7:169–181.
93. Renaud, SC, Ruf, JC. Effects of alcohol on platelet functions. Clin Chim Acta. 1996;246:77–89.
94. Toriola, AT, Kurl, S, Dyba, T, Laukkanen, JA, Kauhanen, J. The impact of alcohol consumption on the risk of cancer among men: A 20-year follow-up study from Finland. Eur J Cancer. 2010;46:1488–1492.
95. Watters, JL, Park, Y, Hollenbeck, A, Schatzkin, A, Albanes, D. Alcoholic beverages and prostate cancer in a prospective US cohort study. Am J Epidemiol. 2010;172:773–780.
96. Thygesen, LC, et al. Cancer incidence among patients with alcohol use disorders—long-term follow-up. Alcohol Alcohol. 2009;44:387–391.
97. Dumitrescu, RG, Shields, PG. The etiology of alcohol-induced breast cancer. Alcohol. 2005;35:213–225.
98. Setiawan, VW, et al. Alcohol consumption and endometrial cancer risk: The multiethnic cohort. Int J Cancer. 2008;122:634–638.
99. Sesso, HD, Paffenbarger, RS, Jr., Lee, IM. Alcohol consumption and risk of prostate cancer: The Harvard Alumni Health Study. Int J Epidemiol. 2001;30:749–755.
100. Kortelainen, ML. Drugs and alcohol in hypothermia and hyperthermia related deaths: A retrospective study. J Forensic Sci. 1987;32:1704–1712.
101. Bacaner, N, Kinney, TA, Biros, M, Bochert, S, Casuto, N. The relationship among depressive and alcoholic symptoms and aggressive behavior in adult male emergency department patients. Acad Emerg Med. 2002;9:120–129.
102. Bourgeois, JA, Nelson, JL, Slack, MB, Ingram, M. Comorbid affective disorders and personality traits in alcohol abuse inpatients at an Air Force Medical Center. Mil Med. 1999;164:103–106.
103. O’Connor, PG, Schottenfeld, RS. Patients with alcohol problems. N Engl J Med. 1998;338:592–602.
104. Swift, RM. Drug therapy for alcohol dependence. N Engl J Med. 1999;340:1482–1490.
105. Mason, BJ, Kocsis, JH, Ritvo, EC, Cutler, RB. A double-blind, placebo-controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. JAMA. 1996;275:761–767.
106. Nunes, EV, Levin, FR. Treatment of depression in patients with alcohol or other drug dependence: A meta-analysis. JAMA. 2004;291:1887–1896.
107. Wootton, FT, Lee, WM. Acetaminophen hepatotoxicity in the alcoholic. South Med J. 1990;83:1047–1049.
108. Fraser, AG. Pharmacokinetic interactions between alcohol and other drugs. Clin Pharmacokinet. 1997;33:79–90.
109. Waring, WS, Stephen, AF, Malkowska, AM, Robinson, OD. Acute ethanol coingestion confers a lower risk of hepatotoxicity after deliberate acetaminophen overdose. Acad Emerg Med. 2008;15:54–58.
110. Andrews, P. Cocaethylene toxicity. J Addict Dis. 1997;16:75–84.
111. Vanek, VW, et al. Concurrent use of cocaine and alcohol by patients treated in the emergency department. Ann Emerg Med. 1996;28:508–514.
112. Henry, D, Dobson, A, Turner, C. Variability in the risk of major gastrointestinal complications from nonaspirin nonsteroidal anti-inflammatory drugs. Gastroenterology. 1993;105:1078–1088.
113. Fett, DL, Vukov, LF. An unusual case of severe griseofulvin-alcohol interaction. Ann Emerg Med. 1994;24:95–97.
114. Miller, JW, Naimi, TS, Brewer, RD, Jones, SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85.
115. Foster, SE, Vaughan, RD, Foster, WH, Califano, JAJr. Alcohol consumption and expenditures for underage drinking and adult excessive drinking. JAMA. 2003;289:989–995.
116. Hingson, RW, Heeren, T, Jamanka, A, Howland, J. Age of drinking onset and unintentional injury involvement after drinking. JAMA. 2000;284:1527–1533.
117. Merrick, EL, et al. Unhealthy drinking patterns in older adults: Prevalence and associated characteristics. J Am Geriatr Soc. 2008;56:214–223.
118. Adams, WL. Potential for adverse drug-alcohol interactions among retirement community residents. J Am Geriatr Soc. 1995;43:1021–1025.
119. Ruitenberg, A, et al. Alcohol consumption and risk of dementia: The Rotterdam Study. Lancet. 2002;359:281–286.
120. Moore, AA, Whiteman, EJ, Ward, KT. Risks of combined alcohol/medication use in older adults. Am J Geriatr Pharmacother. 2007;5:64–74.
121. McGuire, LC, Ajani, UA, Ford, ES. Cognitive functioning in late life: The impact of moderate alcohol consumption. Ann Epidemiol. 2007;17:93–99.
122. Peters, R, Peters, J, Warner, J, Beckett, N, Bulpitt, C. Alcohol, dementia and cognitive decline in the elderly: A systematic review. Age Ageing. 2008;37:505–512.
123. Berg, KM, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121:406–418.
124. Young, NK. Effects of alcohol and other drugs on children. J Psychoactive Drugs. 1997;29:23–42.
125. Centers for Disease Control and Prevention. Alcohol use among women of childbearing age—United States, 1991-1999. JAMA. 2002;287:2069–2071.
126. Gottlieb, LD, Horwitz, RI, Kraus, ML, Segal, SR, Viscoli, CM. Randomized controlled trial in alcohol relapse prevention: Role of atenolol, alcohol craving, and treatment adherence. J Subst Abuse Trea. 1994;11:253–258.
127. O’Connor, MJ, et al. Psychiatric illness in a clinical sample of children with prenatal alcohol exposure. Am J Drug Alcohol Abuse. 2002;28:743–754.
128. O’Callaghan, FV, O’Callaghan, M, Najman, JM, Williams, GM, Bor, W. Prenatal alcohol exposure and attention, learning and intellectual ability at 14 years: A prospective longitudinal study. Early Hum Dev. 2007;83:115–123.
129. American Academy of Pediatrics Committee on Substance Abuse and Committee on Children with Disabilities. Fetal alcohol syndrome and fetal alcohol effects. Pediatrics. 1993;91:1004–1006.
130. Shults, RA, Elder, RW, Hungerford, DW, Strife, BJ, Ryan, GW. Emergency department visits for alcohol-related unintentional traumatic injuries, United States, 2001. J Safety Res. 2009;40:329–331.
131. Kitchens, JM. Does this patient have an alcohol problem? JAMA. 1994;272:1782–1787.
132. Jurkovich, GJ, et al. Effects of alcohol intoxication on the initial assessment of trauma patients. Ann Emerg Med. 1992;21:704–708.
133. Marx, J. Alcohol and trauma. Emerg Med Clin North Am. 1990;8:929–938.
134. Kiwerski, J, Krasuski, M, Wozniak, E. Sequelae of injuries of thoracic vertebrae with spinal cord trauma in persons under the influence of alcohol [in Polish]. Pol Tyg Lek. 1989;44:910–912.
135. Brodner, RA, Van Gilder, JC, Collins, WFJr. Experimental spinal cord trauma: Potentiation by alcohol. J Trauma. 1981;21:124–129.
136. Stein, SC, O’Malley, KF, Ross, SE. Is routine computed tomography scanning too expensive for mild head injury? Ann Emerg Med. 1991;20:1286–1289.
137. McManus, J, Magaret, ND, Hedges, JR, Rayner, NB, Rice, M. A survey of Oregon emergency physicians to assess mandatory reporting knowledge and reporting patterns regarding intoxicated drivers in the state of Oregon. Acad Emerg Med. 2005;12:896–899.
138. Anton, RF, et al. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence. Arch Gen Psychiatry. 2008;65:135–144.
139. Kenna, GA, McGeary, JE, Swift, RM. Pharmacotherapy, pharmacogenomics, and the future of alcohol dependence treatment, part 2. Am J Health Syst Pharm. 2004;61:2380–2388.
140. Krystal, JH, Cramer, JA, Krol, WF, Kirk, GF, Rosenheck, RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–1739.
141. Anton, RF, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. JAMA. 2006;295:2003–2017.
142. Bazargan-Hejazi, S, et al. Evaluation of a brief intervention in an inner-city emergency department. Ann Emerg Med. 2005;46:67–76.
143. Addolorato, G, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: Randomised, double-blind controlled study. Lancet. 2007;370:1915–1922.
144. Furieri, FA, Nakamura-Palacios, EA. Gabapentin reduces alcohol consumption and craving: A randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691–1700.
145. Myrick, H, et al. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475.
146. Ahmed, M. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. Is emergency department based brief intervention worthwhile in adults presenting with alcohol related events? Emerg Med J. 2007;24:785–788.
147. Daeppen, JB, et al. Brief alcohol intervention and alcohol assessment do not influence alcohol use in injured patients treated in the emergency department: A randomized controlled clinical trial. Addiction. 2007;102:1224–1233.
148. Field, CA, Caetano, R. The effectiveness of brief intervention among injured patients with alcohol dependence: Who benefits from brief interventions? Drug Alcohol Depend. 2010;111:13–20.
149. Cunningham, JA, Wild, TC, Cordingley, J, Van Mierlo, T, Humphreys, K. Twelve-month follow-up results from a randomized controlled trial of a brief personalized feedback intervention for problem drinkers. Alcohol Alcohol. 2010;45:258–262.
150. Diguiseppi, C, Goss, C, Xu, S, Magid, D, Graham, A. Telephone screening for hazardous drinking among injured patients seen in acute care clinics: Feasibility study. Alcohol Alcohol. 2006;41:438–445.
151. Crawford, MJ, et al. Screening and referral for brief intervention of alcohol-misusing patients in an emergency department: A pragmatic randomised controlled trial. Lancet. 2004;364:1334–1339.
152. Davidson, P, Koziol-McLain, J, Harrison, L, Timken, D, Lowenstein, SR. Intoxicated ED patients: A 5-year follow-up of morbidity and mortality. Ann Emerg Med. 1997;30:593–597.