Alcaligenes, Bordetella (Non-pertussis), Comamonas, and Similar Organisms
1. Describe the normal habitat of the organisms discussed in this chapter and the means of transmission for human infection.
2. List the general characteristics of the bacteria discussed in this chapter.
3. Identify unusual biochemical reactions and incubation conditions required of organisms discussed in this chapter.
4. Outline the major tests used to identify the organisms in these groups.
5. Compare the appearance of the different genera in Gram stain preparations.
6. Describe the colonial appearance of the clinically significant species.
Epidemiology
The habitats of the species listed in Table 25-1 vary from soil and water environments to the upper respiratory tract of various mammals. Certain species have been exclusively found in humans, whereas the natural habitat for other organisms remains unknown.
TABLE 25-1
| Species | Habitat (Reservoir) | Mode of Transmission |
| Achromobacter xylosoxidans | Environment, including moist areas of hospital. Transient colonizer of human gastrointestinal or respiratory tract in patients with cystic fibrosis | Not often known. Usually involves exposure to contaminated fluids (e.g., intravenous fluids, hemodialysis fluids, irrigation fluids), soaps, and disinfectants |
| Achromobacter piechaudii | Environment | Unknown. Rarely found in humans |
| Alcaligenes faecalis | Environment; soil and water, including moist hospital environments. May transiently colonize the skin | Exposure to contaminated medical devices and solutions |
| Bordetella bronchiseptica | Normal respiratory flora of several mammals, including dogs, cats, and rabbits. Not part of human flora | Probably by exposure to contaminated respiratory droplets during close contact with animals |
| Comamonas spp. | Environment, soil and water; can be found in hospital environment. Not part of human flora | Nosocomial opportunistic pathogens because of their ability to survive in aqueous environments |
| CDC group IVc-2 | Uncertain. Probably water sources, including those in the hospital setting. Not part of human flora | Usually involves contaminated dialysis systems or exposure of wounds to contaminated water |
| Delftia acidovorans | Environment, soil and water; can be found in hospital environment. Not part of human flora | Uncertain. Rarely found in humans. Probably involves exposure to contaminated solutions or devices |
| Ignatzchineria spp. | Unknown. Probably environmental. Not part of human flora | Unknown. Rarely found in humans |
| Oligella urethralis Oligella ureolytica |
Unknown. May colonize distal urethra | Manipulation (e.g., catheterization) of urinary tract |
| Psychrobacter spp. | Unknown | Unknown |
| Roseomonas spp. | Unknown | Unknown. Rarely found in humans |
Pathogenesis and Spectrum of Disease
Identifiable virulence factors are not known for most of the organisms listed in Table 25-2. However, because infections usually involve exposure of compromised patients to contaminated materials, most of these species are probably of low virulence. Among the environmental organisms listed, Achromobacter spp. are most frequently associated with various infections, including bacteremia, meningitis, pneumonia, and peritonitis. They also have been implicated in outbreaks of nosocomial infections. Achromobacter piechaudii has been isolated from pharyngeal swabs, wounds, blood, and ear discharge. Achromobacter xylosoxidans increasingly has been recovered from patients with cystic fibrosis. However, it is unclear whether the organism is implicated in causing clinical disease in patients with cystic fibrosis or whether it simply colonizes the respiratory tract. A. denitrificans has been recovered from urine, prostrate secretions, the buccal cavity, pleural fluid, and eye secretions. A. faecalis has been isolated from a wide range of clinical specimens and has been identified in bacteremia, ocular infections, pancreatic abscesses, bone infections, urine, and ear discharge. Comamonas spp. have been identified in cases of endocarditis, meningitis, and catheter-associated bacteremia. They have also been recovered from sputum in patients with cystic fibrosis. Other organisms, such as O. urethralis and O. ureolytica have been isolated predominantly from the human urinary tract. Pseudomonas alcaligenes and Pseudomonas pseudoalcaligenes rarely have been identified in clinical samples.
TABLE 25-2
Pathogenesis and Spectrum of Disease
| Species | Virulence Factors | Spectrum of Disease and Infections |
| Achromobacter dentrificans | Unknown. Survival in hospital the result of inherent resistance to disinfectants and antimicrobial agents | Infections usually involve compromised patients and include bacteremia, urinary tract infections, meningitis, wound infections, pneumonia, and peritonitis; occur in various body sites; can be involved in nosocomial outbreaks. |
| Achromobacter xylosoxidans | Unknown. Survival in hospital the result of inherent resistance to disinfectants and antimicrobial agents | Infections usually involve compromised patients and include meningitis, pneumonia, otitis media, urinary tract infections, surgical wound infections, and bacteremia. |
| Alcaligenes faecalis | Unknown | Infections usually involve compromised patients. Often a contaminant; clinical significance of isolates should be interpreted with caution. Has been isolated from blood, respiratory specimens, and urine. |
| Alcaligenes piechaudii | Unknown | Rare cause of human infection. |
| Bordetella bronchiseptica | Unknown for humans. Has several factors similar to B. parapertussis | Opportunistic infection in compromised patients with history of close animal contact. Infections are uncommon and include pneumonia, bacteremia, urinary tract infections, meningitis, and endocarditis. |
| CDC group IVc-2 | Unknown | Rare cause of human infection. Infections in compromised patients include bacteremia and peritonitis. |
| Comamonas testosteroni Comamonas spp. |
Unknown | Isolated from respiratory tract, eye, and blood but rarely implicated as being clinically significant. |
| Cupriavidus spp. | Unknown | Recovered from cystic fibrosis patients. Additional infections include bacteremia, peritonitis and tenosynovitis. |
| Delftia acidovorans | Unknown | Isolated from respiratory tract, eye, and blood but rarely implicated as being clinically significant. |
| Ignatzchineria spp. | Unknown | Clinical significance uncertain, has been isolated from wounds, urine, and blood. |
| Oligella urethralis | Unknown | Urinary tract infections, particularly in females. |
| Oligella ureolytica | Unknown | Also isolated from kidney, joint, and peritoneal fluid. |
| P. alcaligenes P. pseudoalcaligenes |
Unknown; low virulence associated with administration of contaminated solutions and medicines | Recovered from the respiratory secretions of patients with cystic fibrosis. |
| Psychrobacter spp. | Unknown | Rare cause of human infection |
| Roseomonas spp. | Unknown; uncommon isolates from humans | Clinical significance uncertain. Typically opportunistic infections. Most isolated from blood, wounds, exudates, abscesses, or genitourinary tract of immunocompromised or debilitated patients. |
Laboratory Diagnosis
Specimen Collection and Transport
No special considerations are required for collection and transport of the organisms discussed in this chapter. Refer to Table 5-1 for general information on specimen collection and transport.
Cultivation
Media of Choice
Colonial Appearance
Table 25-3 describes the colonial appearance and other distinguishing characteristics (e.g., pigment and odor) of each genus on 5% sheep blood and MacConkey agars.
TABLE 25-3
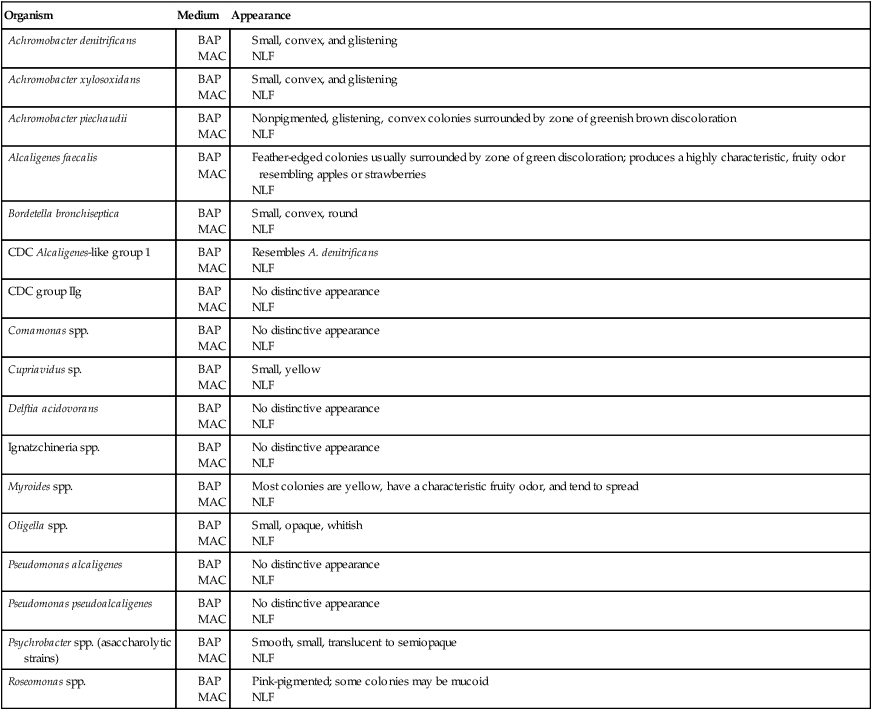
BA, 5% sheep blood agar; Mac, MacConkey agar; NLF, non–lactose fermenter.
Approach to Identification
Many microbiologists groan at the mere mention of having to perform a flagella stain, but the method described in Procedure 13-16 is a wet mount that is easy to perform. At the very least, a simple wet mount to observe cells for motility helps distinguish between the motile and nonmotile genera. The pseudomonads and Brevundimonas, Burkholderia, and Ralstonia species described in Chapter 22 are motile by means of single or multiple polar flagella; the motile organisms described in this chapter have peritrichous flagella (e.g., B. bronchiseptica, Alcaligenes spp., and Achromobacter spp.), or polar flagella (e.g., Delftia, Comamonas spp.).
Organisms are first categorized on the basis of Gram stain morphology (i.e., coccoid [Table 25-4] or rod shaped [Tables 25-5 through 25-7]). They are then further characterized based on whether the organisms are nonmotile (see Table 25-5), peritrichously flagellated (see Table 25-6), or flagellated by polar tufts (see Table 25-7).
TABLE 25-4
Key Biochemical and Physiologic Characteristics for Coccoid Species
| Organisms | Motility | Urea Hydrolysis | Nitrate Reduction | Nitrite Reduction |
| Oligella ureolytica | + or (+)* | + | + | + |
| Oligella urethralis | nm | – | – | + |
| Psychrobacter phenylpyruvicus† | nm | + | v | – |
| Psychrobacter immobilis‡ (asaccharolytic strains) | nm | v | v | ND |

ND, No data available; nm, nonmotile; v, variable; +, >90% of strains are positive; –, >90% of strains are negative; (+), positive delayed.
*Petrichous flagella but motility may be delayed or difficult to demonstrate,
Data compiled from Holt JG, Krieg NR, Sneath PH et al, editors: Bergey’s manual of determinatative bacteriology, ed 9, Baltimore, 1994, Williams & Wilkins; and Versalovic J: Manual of clinical microbiology, ed 10, Washington, DC, 2011, ASM Press.
TABLE 25-5
Key Biochemical and Physiologic Characteristics for Rod-Shaped Nonmotile Species
| Organisms | Insoluble Pigment | Indole | Urea Hydrolysis | Nitrite Reduction |
| CDC group IIg | v, tan or salmon | + | – | + |
| Myroides spp. | v, yellow | – | + | v |

v, Variable; +, >90% of strains are positive; –, >90% of strains are negative
Data compiled from Holt JG, Krieg NR, Sneath PH et al, editors: Bergey’s manual of determinatative bacteriology, ed 9, Baltimore, 1994, Williams & Wilkins; and Versalovic J: Manual of clinical microbiology, ed 10, Washington, DC, 2011, ASM Press.
TABLE 25-6
Key Biochemical and Physiologic Characteristics for Rod-Shaped Motile Species with Polar Flagella
| Organism | Number of Flagella | Oxidizes Mannitol | Insoluble Pigment | Growth at 42°C | Nitrate Reduction |
| Delftia acidovorans | >2 | + | – | v | + |
| Comamonas spp. | >2 | – | – | v | + |
| Pseudomonas alcaligenes | 1-2 | – | va | v | v |
| Pseudomonas pseudoalcaligenesb | 1-2 | – | – | + | + |
| Roseomonas spp.c | 1-2d | v | pink | v | v |
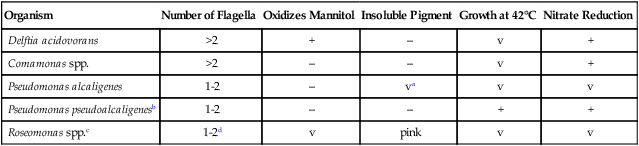
v, Variable; +, >90% of strains are positive; –, >90% of strains are negative.
aSome strains have a yellow-orange insoluble pigment.
cRepresents composite of several species and genomospecies.
dGenomospecies 5 is nonmotile.
Data compiled from Holt JG, Krieg NR, Sneath PH, et al, editors: Bergey’s manual of determinatative bacteriology, ed 9, Baltimore, 1994, Williams & Wilkins; and Versalovic J: Manual of clinical microbiology, ed 10, 2011, Washington, DC, ASM Press.
TABLE 25-7
| Organism | Urea Hydrolysis | Nitrate Reduction | Gas from Nitrate | Growth on Cetrimide | Jordan’s Tartrate* |
| Achromobacter denitrificans | – | + | + | v | + |
| Achromobacter xylosoxidans | + | + | + | ||
| Achromobacter piechaudii |
– | + | – | + | + |
| Alcaligenes faecalis† | – | – | – | v | – |
| CDC Alcaligenes-like group 1 | v | + | + | – | – |
| Bordetella bronchiseptica | + | + | – | – | – |
| Cupriavidus pauculus | + | v | – | – | + |
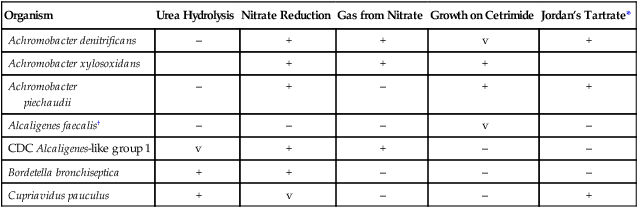
v, Variable; +, >90% of strains are positive; –, >90% of strains are negative.
*Jordan’s tartrate agar deeps is a medium used to differentiate gram-negative enteric microorganisms based on the utilization of tartrate.
Data compiled from Holt JG, Krieg NR, Sneath PH et al, editors: Bergey’s manual of determinatative bacteriology, ed 9, Baltimore, 1994, Williams & Wilkins; and Versalovic J: Manual of clinical microbiology, ed 10, 2011, Washington, DC, ASM Press.
Antimicrobial Susceptibility Testingand Therapy
Validated susceptibility testing methods do not exist for these organisms. Although they will grow on the media and under the conditions recommended for testing the more commonly encountered bacteria (see Chapter 12 for more information regarding validated testing methods), this does not necessarily mean that interpretable and reliable results will be produced. Chapter 12 should be reviewed for preferable strategies that can be used to provide susceptibility information when validated testing methods do not exist for a clinically important bacterial isolate.
The lack of validated in vitro susceptibility testing methods does not allow definitive treatment and testing guidelines to be given for most organisms listed in Table 25-8. If antimicrobial sensitivity testing is required for Achromobacter and Alcaligenes spp., methods include broth macrodilution and microdilution, agar dilution, breakpoint methods, and Etest. Bordetella parapertussis is an exception; significant clinical experience indicates that erythromycin is the antimicrobial agent of choice for whooping cough caused by this organism (see Chapter 37 for more information on therapy for Bordetella pertussis and B. parapertussis infections). Standardized testing methods do not exist for this species, but the recent recognition of erythromycin resistance in B. pertussis indicates that development of such testing may be warranted for the causative agents of whooping cough.
TABLE 25-8
Antimicrobial Therapy and Susceptibility Testing
| Species | Therapeutic Options | Potential Resistance to Therapeutic Options | Validated Testing Methods* | Comments |
| Achromobacter denitrificans |
Oligella ureolytica
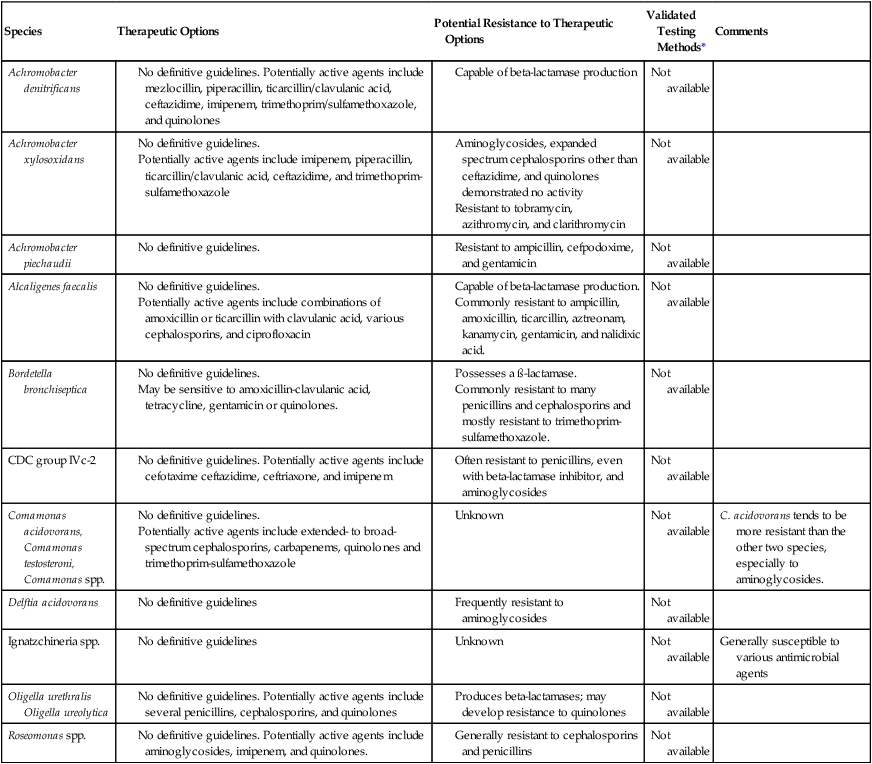
*Validated testing methods include standard methods recommended by the Clinical and Laboratory Standards Institute (CLSI) and commercial methods approved by the U.S. Food and Drug Administration (FDA).
Even though standardized methods have not been established for the other species discussed in this chapter, in vitro susceptibility studies have been published, and antimicrobial agents that have potential activity are noted, where appropriate, in Table 25-8. A. xylosoxidans demonstrates variable susceptibility to β-lactams, ureidopenicillins, and carbapenems; the organism is resistant to narrow-spectrum penicillins and cephalosporins, including cefotaxime.







