Chapter 46 Airway Management in Intensive Care Medicine
II. Noninvasive Airway Management
III. Endotracheal Intubation in Intensive Care
IV. Difficult Airway Management in Intensive Care
V. Complications of Translaryngeal Intubation
VI. Airway Management for Prolonged Mechanical Ventilation (Tracheostomy)
VII. Routine Airway Care in Mechanically Ventilated Patients
I Introduction
This chapter briefly describes noninvasive and “invasive” airway management with special respect on the recommendations of the American Society of Anesthesiologists Task Force on Management of the Difficult Airway (ASA algorithm) first presented in 1991.1 The basic principles of the ASA algorithm are meticulous patient evaluation before sedation or anesthesia induction, awake intubation if problems are suspected, and preparation of an alternative approach in case of failure.
II Noninvasive Airway Management
Invasive ventilation using an endotracheal tube (ETT) was once the gold standard for ventilatory support of critically ill patients. However, longer duration of invasive ventilation increases the risk of complications such as ventilator-associated pneumonia (VAP).2 Noninvasive ventilation (NIV) preserves the patient’s ability to speak and cough and has been shown to reduce complications related to intubation, especially VAP.3–5 For this reason, use of NIV has become widely accepted, especially in patients with chronic obstructive pulmonary disease (COPD) and patients with cardiac failure, since weaning from respirator and ETT may become extremely difficult. In selected patients with respiratory failure, noninvasive positive pressure ventilation helps to reduce an increased respiratory rate, augments tidal volumes and reduces work of breathing.6,7 Therefore, NIV is a promising approach to ventilatory support of ICU patients and might become even more important as a “weaning as well as a therapeutic procedure” in the near future.
A Indications and Contraindications of Noninvasive Ventilation
1 Acute Respiratory Failure
Over the past decade, noninvasive positive-pressure ventilation (NPPV) has increased in popularity in the patient with acute exacerbations of COPD. In these patients, based on evidence derived from multiple randomized trials, NIV should be considered as first-line therapy.8–12
In a systematic review, Keenan and colleagues13 assessed the effect of NPPV on rate of endotracheal intubation, length of hospital stay, and in-hospital mortality in patients with an acute exacerbation of COPD. The addition of NPPV to standard care in patients with COPD exacerbation decreased the rate of endotracheal intubation (risk reduction [RR], 28%; 95% confidence interval [CI], 15%-40%), length of hospital stay (absolute reduction, 4.57 days; CI, 2.30-6.83 days), and in-hospital mortality rate (RR, 10%; CI, 5%-15%). However, subgroup analysis showed that these beneficial effects occurred only in patients with severe exacerbations, not in those with milder exacerbations of COPD.
A similar approach was used by the Cochrane Database group, presenting a systematic review on the effectiveness of NPPV in management of acute COPD exacerbations.14 Only randomized controlled trials (RCTs) were selected by two independent reviewers. NPPV not only decreased mortality (RR 0.41; 95% CI 0.26, 0.64), but also decreased the need for intubation (RR 0.42; CI 0.31, 0.59) and treatment failures (RR 0.51; CI 0.39, 0.67). In addition, complications associated with treatment (RR 0.32; CI 0.18, 0.56) and length of hospital stay (−3.24 days; CI −4.42, −2.06) were also reduced in the NPPV group. The reviewers concluded that NPPV should be used as the first-line intervention in all patients with respiratory failure secondary to an acute exacerbation of COPD. NPPV should be considered early in the course of respiratory failure, to avoid endotracheal intubation, reduce mortality, and avoid treatment failure.
Carlucci and associates15 evaluated the changes in the practice of NIV for the treatment of COPD patients between 1992 and 1999. In this special patients’ collective, the failure rate of NPPV was constant over the years (17%). Although the severity of acute respiratory failure (ARF) episodes increased (defined by pH and APACHE II at admission), the risk of failure for a patient with pH less than 7.25 was threefold lower in the period 1997-99 compared with 1992-96. Furthermore, a significantly higher percentage of patients (pH >7.28) were treated at the normal ward and not in the ICU, which allowed a significant cost reduction.
Based on strong evidence (level 1), recent clinical studies support the use of NIV to treat ARF related to COPD exacerbations, treat cardiogenic pulmonary edema, and facilitate weaning and extubation in patients with COPD and immunosuppressed patients.8,16 The future will show whether this trend away from “invasive” toward noninvasive ventilatory support in routine patient care will be sustained.
2 Weaning Strategy
Weaning from the ventilator is difficult in up to 20% of all patients. Already the consequent use of standardized weaning strategies (regardless of their content) has increased the success rates after extubation. In this context, Udwadia and coauthors17 described the use of NIV as a weaning strategy first in 1992. In 2003, Burns and coworkers18 performed the first meta-analysis of NIV as a weaning strategy, including five clinical trials and 171 patients. NIV showed significant benefit for duration of in-hospital-stay, total duration of ventilation, reduced mortality, and lower VAP rate compared with conventional weaning using endotracheal intubation. In a 2010 systematic review that included 12 randomized and quasi-randomized studies, Burns and others19 compared NIV and invasive weaning strategy in 530 COPD patients.19 This meta-analysis showed that NIV weaning in COPD patients is associated with decreased mortality, decreased incidence of VAP, reduced length of stay in ICU and hospital, and decreased total duration of ventilation and duration of endotracheal intubation.
Current studies on NIV weaning, predominantly focused on COPD patients, propose a potential reduction in mortality and VAP.20 In fact, further clinical research is necessary to clarify potential benefits of NIV as weaning strategy versus conventional weaning via ETTs or cannulas.
3 Difficult Airway Management
Critically ill patients are characterized by limited physiologic reserves, possibly resulting in catastrophic complications during airway management, including cardiac arrest. After a systematic review of airway management in critically ill patients in 2007, Walz and colleagues21 proposed adaptations to the ASA Difficult Airway Algorithm. In this algorithm, NIV serves as a valuable adjunct to conventional invasive airway management.
B Different Types of Noninvasive Devices
Various types of face masks and helmets are widely used for administering NIV (Fig. 46-1).
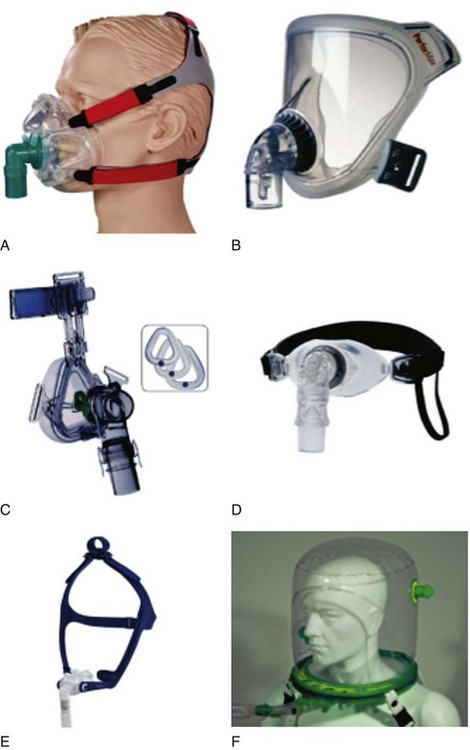
Figure 46-1 A, Full face mask; B, total face mask; C, nasal mask; D, mouthpiece; E, nasal pillows; F, helmet.
(From Nava S, Hill N: Noninvasive ventilation in acute respiratory failure. Lancet 374:250–259, 2009.)
1 Noninvasive Positive-Pressure Ventilation via Face Mask
Noninvasive PPV can be administered by face masks covering the mouth and nose (and eyes) or by nasal masks covering only the nose. Either technique includes a mask that is pressed against the patient’s face using an elastic band (e.g., Classen band). The patient may now be supported by either continuous positive airway pressure (CPAP), pressure support ventilation (PSV), or volume-cycled or pressure-cycled systems, such as bi-level positive airway pressure (BiPAP). However, dyspneic ARF patients tend to breathe through their mouth, causing air leaking and reducing the efficacy of nasal NPPV. Face masks are preferable in these patients, and application seems to be easy. However, the pressure of the face mask against the face, especially the root of the nose, causes significant discomfort and skin lesions, and several patients refused prolonged application.22,23 Further disadvantages are potential gastric inflation followed by vomiting (risk of aspiration), claustrophobia, and difficult speaking.16
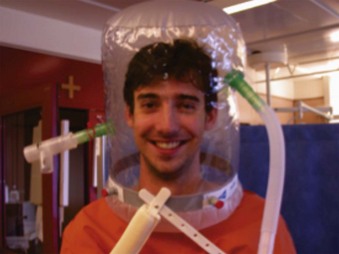
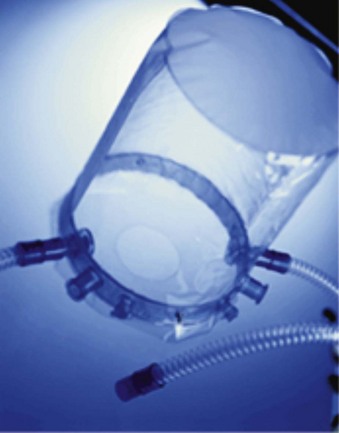
2 Noninvasive Positive-Pressure Ventilation via Helmet
The helmet consists of a cylindrical transparent part that is drawn over the patient’s head (e.g., CaStar, Starmed, Italy). While the helmet is closed, the lower part contains an elastic ring to ensure a tight seal at the patient’s neck (Fig. 46-2). Two openings with sideways fittings to ventilatory tubing allow supportive ventilation such as continuous flow or pressure support. The helmet is a noninvasive means of ventilation; the patient has a free view through the transparent helmet and can even wear glasses, with no fogging from the circulating air. A new model of the Castar helmet includes an additional round opening with a diameter of about 10 cm for nursing the patient’s face. This helmet was used as first-line intervention to treat patients with hypoxemic ARF, compared with NPPV via standard face mask.24 Thirty-three consecutive patients without COPD and with hypoxemic ARF (defined as severe dyspnea at rest, respiratory rate >30 breaths/min, PaO2/FIO2 <200, and active contraction of the accessory muscles of respiration) were enrolled. The 33 patients and the 66 controls had similar characteristics at baseline. Eight patients (24%) in the helmet group and 21 patients (32%) in the facial mask group (P = .3) failed NPPV and were intubated. No patients failed NPPV because of intolerance of the technique in the helmet group, versus eight patients (38%) in the mask group (P = .047). Complications related to the technique (skin necrosis, gastric distention, eye irritation) were fewer in the helmet group compared with the mask group (no patients vs. 14 patients (21%), P = .002). The helmet allowed continuous application of NPPV for a longer period (P = .05). NPPV by helmet successfully treated hypoxemic ARF, with better tolerance and fewer complications than face mask NPPV.
In another prospective clinical investigation in a general ICU, the feasibility and safety of fiberoptic bronchoscopy (FOB) with bronchoalveolar lavage (BAL) was tested during NPPV delivered by helmet in patients with ARF and suspected pneumonia.25 Four adult patients with ARF underwent NPPV via the helmet and required fiberoptic BAL for suspected pneumonia. NPPV was delivered through the helmet in the pressure support ventilation mode. The specific seal connector placed in the plastic ring of the helmet allowed the passage of the bronchoscope, maintaining assisted ventilation. Arterial blood gas levels, pH, oxygen saturation, respiratory rate, heart rate, and mean arterial blood pressure (BP) were monitored during the study. Helmet NPPV avoided gas exchange deterioration during FOB and BAL, with good tolerance. During the procedure, heart rate increased by 5% and mean arterial BP by 7% over baseline; these levels returned to prebronchoscopy values immediately after the withdrawal of the bronchoscope. Endotracheal intubation was never required during the 24 hours after the procedure. BAL yielded diagnostic information in three of four patients. NPPV through the helmet allows a safe diagnostic FOB with BAL in patients with hypoxemic ARF, avoiding gas exchange deterioration, and endotracheal intubation.
Several special aspects of the helmet, such as the effects of direct exposure of external and middle ear to positive airway pressure, remain to be determined. Cavaliere and others26 recommended earplugs in select NPPV patients treated with the helmet, especially during long-term support at high airway pressures. NPPV has become an integral part of daily ICU care for many patients with acute and chronic respiratory failure. However, the potential effectiveness of NPPV varies among different patient populations. The greatest benefit is seen in patients with pure hypercapnic respiratory failure, as seen in COPD patients. The more severe hypoxemia becomes, the less beneficial the effects observed. Clearly, further studies are necessary to identify patients with hypoxemic ARF who might benefit from NPPV.27
The Rüsch 4Vent (Kernen, Germany) allows unrestricted field of view and minimum level of noise. Tubing and accessories are connected to the rigid ring of the 4Vent. Diffusers prevent the stream of gas from blowing directly into the patient’s face. Filters reduce the noise inside the helmet. Similar to the Castar helmet, the patient is able to speak and take liquid food and drinks. The supple material allows the helmet also to be worn lying down (Fig. 46-3).
Advantages of NPPV include few air leaks, minimal cooperation required, and absence of nasal or facial skin damage. Disadvantages include rebreathing, gastric inflation followed by vomiting, dry eyes, noise, and asynchrony with pressure support ventilation, as well as shoulder discomfort induced by the straps.16
III Endotracheal Intubation in Intensive Care
A Prelaryngoscopy Airway Assessment
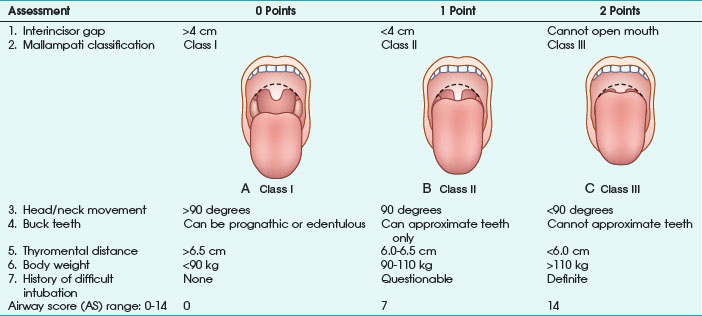
Any factor in the patient’s history suggesting a difficult airway must be known before anesthesia induction, especially all diseases resulting in pharyngeal or laryngeal deformities (congenital abnormalities, surgical or traumatic deformities, laryngeal trauma, edema or tumors, goiter). As a first and rapid orientation, the simple “rule of threes” can be applied: if three fingers can be placed between the teeth, between the mandible and the hyoid bone, and between the thyroid cartilage and the sternal notch in neutral position, direct laryngoscopy should be straightforward.28 Furthermore, more or less complex tests and scoring systems have been suggested to predict a difficult airway before anesthesia induction. The most frequently used tests are the Mallampati classification, thyromental distance, mobility of the cervical spine, and combined scoring systems (e.g., Wilson test) or multivariate risk indices29–31 (Table 46-1).
Mallampati classification is the most common diagnostic test and is based on the evaluation of the relationship of tongue size relative to pharynx size, as follows31:
Class I: uvula, faucial pillars, and soft palate visible
The operator should be aware that the risk of difficult airway is increased in class II and especially class III patients. In the original publication, Mallampati and colleagues31 reported excellent statistical results on the performance of the classification system. However, later investigations could not replicate these findings and report a high incidence of false-positive results.32,33
Thyromental distance (according to Patil) is defined as distance between thyroid cartilage (Adam’s apple) and the mandible with the patient’s head and neck maximally extended. The authors measured thyromental distance before anesthesia induction in 75 patients and reported a minimum distance of 6.5 cm being necessary for a successful laryngoscopic approach. At a thyromental distance less than 6.0 cm, direct laryngoscopy is impossible; between 6.0 and 6.5 cm, the operator should be prepared for a difficult airway. Sensitivity of this test varies according to definition of the “danger zone” (i.e., thyromental distance <7.0 or <6.5 cm) and is reported in the range of 30% to 90%. In most studies, specificity ranges between 80% and 97%.32,34 Thyromental distance is a useful diagnostic test with a relatively low intra- and inter-observer variation. However, the lacking stratification for patient height and weight makes data interpretation difficult.
Direct laryngoscopy without extension of the atlanto-occipital joint is impossible. Decreased mobility of the upper cervical spine (e.g., in patients with rheumatoid arthritis) is a major factor responsible for failed attempts of endotracheal intubation.35 Nichol and Zuck30 clearly demonstrated that a reduced atlanto-occipital distance is a major factor for a limited extension of head and neck. Assessment of cervical spine mobility is performed with the patient looking straight ahead and maximum mouth opening. The patient is now urged to flex and extend the head and neck. The angle between the extreme positions should be at least 90 degrees; otherwise, a difficult laryngoscopy must be assumed, with high specificity in the 90% range.
The range and freedom of mandibular movement and the architecture of the teeth have pivotal roles in facilitating laryngoscopic intubation, so Khan and colleagues36 proposed the upper lip bite test. Patients were grouped according to the following findings: lower incisors can bite the upper lip above the vermilion line (class I); lower incisors can bite the upper lip below the vermilion line (class II); and lower incisors cannot bite the upper lip (class III). In 300 elective patients, the authors evaluated the new test with the Mallampati classification. Negative predictive value for both test groups was similar (98%), but the positive predictive value of the lip bite test was better (29% vs. 13%). To improve positive and negative predictive values of those screening tests, several authors proposed the combination of various risk scores. In 1988, Wilson and associates29 published a screening test system, including patient weight, mobility of the cervical spine, and anatomic-physiologic parameters of the stomatognathic system (Table 46-2). According to the authors, difficult airways must be suspected with 2 or more cumulative points. This scoring system requires more extensive evaluation but results in improved statistical performance. In patients with a cumulative result of 2 points, sensitivity of 75% was observed.29 Oates and coauthors32 reported specificity of 92%.
| Risk Factor | Criterion | Points |
|---|---|---|
| Body weight | <90 kg | 0 |
| 90-110 kg | 1 | |
| >110 kg | 2 | |
| Head and neck movement | >90 degrees 90 degrees (±10) <90 degrees |
0 1 2 |
| Jaw movement | MMO >5 cm or LJ in front of UJ | 0 |
| MMO <5 cm and LJ = UJ | 1 | |
| MMO <5 cm and LJ behind UJ | 2 | |
| Receding mandible | Normal Moderate Severe |
01 2 |
| Buck teeth | Normal | 0 |
| Moderate | 1 | |
| Severe | 2 |
MMO, Maximum mouth opening; LJ, lower jaw; UJ, upper jaw.
From Wilson ME et al: Predicting difficult intubation. Br J Anaesth 61:211–216, 1988.
El-Ganzouri and associates37 proposed a multivariate risk index, similar to the Wilson test. The authors recommended the following approach:
• Airway score (AS) of 0: proceed with routine management, no difficulties are expected.
• AS of 1 to 2 points: proceed with routine management, check fiberoptic availability, and prepare a special plan B.
• AS of 3 to 4 points: have the fiberoptic scope on standby at bedside, and call for help; prepare for asleep or awake fiberoptic intubation.
• AS greater than 5 points: perform awake intubation (most practitioners prefer awake fiberoptic intubation).
Statistical results demonstrate a relatively weak performance in clinical routine. Even combinations of tests or scoring systems combining several risk factors are prone to misjudgment for the individual patient.34 The upper airway is a complex structure and consists of many anatomically relevant variables. Also, statistical performance of these tests is still unsatisfying; the operator might be warned of trouble. Closely evaluating the patient before anesthesia induction might prevent catastrophic “cannot intubate, cannot ventilate” situations.
B Oral vs. Nasal Endotracheal Intubation
Oral intubation and especially nasal intubation are characterized by special advantages and complications, with orotracheal intubation being currently favored by most anesthesiologists. Box 46-1 lists the contraindications for nasal intubation in critically ill patients.
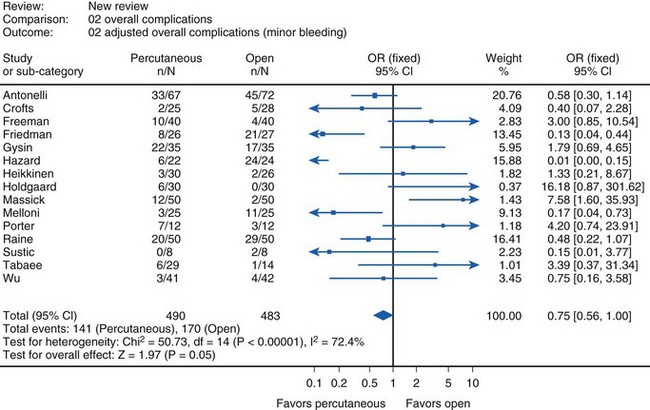
The most common complication of nasotracheal intubation is nasal bleeding, occurring in about 45% of patients.38 The incidence of bleeding can be reduced with adequate preparation (vasoconstrictors). In most cases, bleeding is self-limited, and no further intervention is needed. Other complications are necrosis of the tip or wing of the nose (up to 4% of patients) and complications induced by the impaired drainage of paranasal sinus secretions.39 Nasotracheal intubation impairs the drainage of the maxillary sinus, resulting in congestion of secretions, followed within a few days by bacterial overgrowth and sinusitis. This typical complication of intensive care occurs within 8 days in 25% to 100% of transnasally intubated patients.40,41 The incidence is closely related to the duration of endotracheal intubation, from 37% after 3 days to 100% after 1 week, with the majority resolving within 1 week after extubation.42 Therefore, in all ICU patients presenting with fever of unknown origin, a high index of suspicion for sinusitis must be maintained. Radiologic or ultrasound studies, including computed tomography (CT) scans, may be necessary to demonstrate the presence of fluid or inflammation. Bacterial sinus infection may ultimately lead to bacteremia and systemic inflammatory response. These infections tend to be polymicrobial, but often display a predominance of gram-negative bacilli (particularly Pseudomonas aeruginosa), Staphylococcus aureus, or fungi. Treatment includes removal of all nasal tubes and institution of appropriate antibiotic therapy, along with decongestant therapy. In some cases, surgical drainage may be necessary. Serious, but rare complications after nasotracheal intubation are accidental turbinectomy, intracranial placement, and retropharyngeal dissection.43–45
Oral intubation, on the other hand, interferes greatly with oral and pharyngeal hygiene, and even small lesions of oral soft tissues may result in extensive bacterial or viral soft tissue infections (e.g., herpetic lesions).46 Most authors agree that all complications typically seen with nasal intubation may also occur during orotracheal intubation, although at a significantly lower incidence. Therefore, oral endotracheal intubation is the preferred route of tube passage, and nasal intubation is reserved for special indications, mainly short-term ventilatory support in oral surgery. For patients requiring intubation for more than 7 days, the nasotracheal route should always be avoided.47
C Choosing the Correct ETT Size
According to literature, there is a clear association between endotracheal tube diameter and the incidence of laryngeal complications. Injury is located mainly in the posterior part of the glottis, where pressures up to 200 or 400 mm Hg are exerted by poorly deformable ETTs.48 This pressure is reduced by the use of softer ETTs with a smaller diameter. In routine anesthesia patients, use of a smaller ETT reduces the incidence of postoperative sore throat, presumably because of the decreased pressure at the ETT-mucosal interface. Stout and associates49 studied 101 patients randomized to larger (9 mm for male, 8.5 for female) or smaller (7 mm for male, 6.5 mm for female) endotracheal tubes, and the incidence of postoperative sore throat was 48% (large ETT) versus 22% (small ETT). No ventilatory difficulties were observed in either group.
The limiting factor for minimizing ETT diameter is the increased flow resistance and work of breathing. The pressure gradient required to generate gas flow can be calculated according to the Hagen-Poiseuille relationship, in which the rapidity of gas flow is directly proportional to the square of the ETT diameter (D) and the pressure (P) and indirectly to the ETT length and gas viscosity (valid only in laminar flow conditions). In other words, the pressure gradient through the airways proportionally rises with flow, viscosity, and ETT length but increases exponentially when ETT radius decreases. Viscosity of the gas administered has clinical consequences; with lower viscosity, a lower pressure gradient necessarily results in a lower airflow resistance. For example, gas mixtures with a high percentage of helium are often used to overcome upper airway obstruction (e.g., from tracheal compression or stenosis) and to treat the most severe status asthmaticus.50,51
In all patients, endotracheal intubation artificially increases airway resistance, because ETT inner diameter (ID) is smaller than tracheal diameter. Usually, ETT length exceeds length of the natural airways. Resistance is further increased by the curved design of the tubes necessary to resemble the patient’s airway. Resistance measured using curved tubes is about 3% higher compared with straight tubes.52 Therefore, the pressure gradient to generate gas flow is minimized by using an ETT with a larger diameter, short length, and straight design. Flow resistance can be translated in work of breathing, which is inversely proportional to ETT diameter (Fig. 46-4). The non-elastic work of breathing is increased twofold by using 7.0 mm–ID ETT and onefold by using an 8.0 mm–ID ETT versus a 9.0 mm–ID ETT.53 With respect to gas flow, it is warranted to use as large an ETT as is practical for patients presenting with respiratory dysfunction; a short tracheal cannula with ID of 9.0 to 10 mm is best.
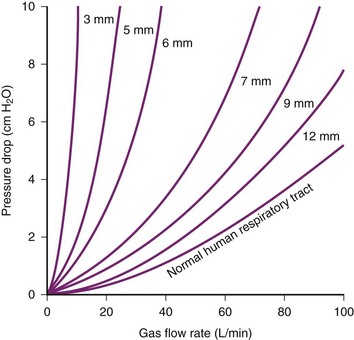
Figure 46-4 Driving pressure required to produce a flow rate of air versus internal diameter (ID) of endotracheal tubes.
(From Nunn JF: Applied respiratory physiology, ed 3, Boston, 1987, Butterworths.)
Clinical Implications
An interesting aspect of ETT selection is airway management for professional singers. Powner54 sent a written survey to all physician members of the Voice Foundation on airway management in professional singers. A strong consensus (76%) favored a smaller ETT for singers (6- to 7-mm ID for males and 6.0-mm ID for females) via the oral (46%) versus nasal (36%) route. Intubation/extubation by the most expert/experienced personnel was emphasized to minimize laryngeal trauma. Preferences for an early tracheostomy (6 days) versus their usual time (10 days) were approximately equal (44% vs. 50%, respectively).
The tracheal cuff has also been implicated as a cause of tracheal damage following long-term ventilatory support. Red-rubber ETTs equipped with low–residual volume, high-pressure cuffs exert high pressures to the tracheal mucosa and are thought to be damaging.55 Using an experimental study design in rabbits, Nordin and coauthors56 investigated tracheal mucosal perfusion and cuff-to-tracheal wall pressures exerted by low–residual volume, high-pressure cuffs and compared the results to those obtained with high-volume, low-pressure cuffs. Blood flow to the tracheal mucosa adjacent to the high-pressure cuff ceased beyond 30 mm Hg. Using high-volume cuffs, mucosal blood flow did not cease up to intracuff pressures of 80 to 120 mm Hg. However, cautious recommendations were made, and cuff pressure should be maintained below 30 cm H2O.55
Prolonged airway support using ETT or tracheal cannula might result in severe damage to the tracheal mucosa and increased incidence of late complications. Tracheal stenosis might be induced by direct trauma or by the pressure exerted by the ETT cuff. The presence of an ETT sutured to canine tracheal mucosa induced erythematous laryngeal mucosa in less than 1 day, proceeding to mucosal ulceration and loss of airway architecture in less than 1 week.57 Damage was generally severe after 1 week, but no further tendency to aggravation after 1 week was observed.
The tracheal vascular supply to the tracheal submucosa is oriented in a circumferential direction anteriorly and longitudinally in the posterior part of the trachea. Seegobin and van Hessalt58 investigated tracheal mucosal blood flow in 40 routine surgery patients using an endoscopic photographic technique while varying cuff inflation pressure. They suggested that tracheal blood flow is almost normal at 25 cm H2O. Increasing cuff pressure results in a pale appearance of the tracheal mucosa at 40, blanched at 50, and flow is completely absent at 60 cm H2O. Thus, it was recommended that cuff inflation pressure should not exceed 30 cm H2O (22 mm Hg).
Joh and colleagues59 observed similar results, measuring tracheal mucosal blood flow in an experimental model using the hydrogen clearance method. No significant depression in mucosal blood flow was observed with the cuff inflated to a maximum of 20 mm Hg. Increasing cuff pressures to 30 or even 45 mm Hg resulted in significant reduction in mucosal blood flow, and the authors concluded that cuff pressures should be kept at or below 20 mm Hg. Incorrect cuff pressure settings may result in tracheomalacia and subglottic stenosis (too high pressures) or late-onset nosocomial pneumonia (insufficient pressures). Several authors suggested that microaspirations might thereby be facilitated and recommended the use of automatic cuff-pressure regulators.60 In a randomized study in 130 patients, Pothmann and associates61 reported a significant reduction in late-onset pneumonias by a microprocessor-controlled automatic cuff-pressure regulator. However, the few studies covering small numbers of patients means that the devices described cannot currently be recommended for routine patient care.60,62
D Drugs Used for Sedation and Analgesia
The presumption of a “full stomach” in emergency intubations dictates the use of a rapid-sequence induction (RSI) technique. Cricoid pressure and laryngoscopy at the earliest possible moment place increased demands on the physician preparing to intubate the patient.63 The ICU patient presents with even more risk factors than the routine OR patient. With facial distortion, swelling, secretions, and mandibular or cervical spine injury, ICU patients present the most challenging airway management problems.
Stimulation of the airway with a laryngoscope and ETT presents an extremely noxious stimulus.64 This stimulation causes “pressor response” and results in hypertension and tachycardia. In critically ill patients with limited reserves for adequate tissue oxygenation, this pressor response may induce myocardial and cerebrovascular injury.65 This physiologic stress after airway management may unmask relative hypovolemia or vasodilatation and may result in post-intubation hypotension.66 Endotracheal intubation also can provoke bronchospasm and coughing, possibly aggravating underlying conditions such as asthma, intraocular hypertension, and intracranial hypertension. Patients at risk for adverse events from airway manipulation benefit from pre-induction drugs.67
We prefer the combination of low-dose midazolam (0.1 µg/kg) and an opioid such as fentanyl (2 µg/kg). Fentanyl is the most frequently used opioid because of its rapid onset and short duration of action. Fentanyl and its derivatives, sufentanil and alfentanil, can cause rigidity of chest wall, but this rigidity seems to be associated with higher doses and rapid opioid injection. Fentanyl can be antagonized rapidly using naloxone,68 whereas midazolam can be antagonized using flumazenil.
Remifentanil is a novel µ-receptor agonist that has been used in surgical and obstetric analgesia for more than a decade. Its pharmacokinetics suggests that remifentanil may be an ideal opioid. Notably, its unique features include rapid onset of action (~1 minute), rapid degradation by tissue and plasma esterases, and half-life of approximately 3 minutes.69,70 Infusion of 0.05 to 0.1 µg/kg/min produces blood concentrations of 1 to 3 ng/mL. Rapid onset and elimination of remifentanil facilitate its effective and safe use during airway management in the ICU. Several studies focused on remifentanil as a sole sedative and analgesic agent in the ICU setting. Puchner and coworkers71 prospectively studied remifentanil (0.1-0.5 µg/kg/min) alone versus fentanyl plus midazolam for fiberoptic intubation. Fiberoptic nasal intubation was possible in all patients, with a better-suppressed hemodynamic response and tolerance of ETT advance in the remifentanil group. Recall was significantly more common in the remifentanil group. A combination of remifentanil and midazolam or propofol is a valuable approach.72
Lallo and coworkers73 recently studied 60 patients requiring fiberoptic nasotracheal intubation, randomized to target-controlled infusions of propofol or remifentanil. Both agents can be rapidly titrated to achieve good intubating conditions and patient comfort. In addition, remifentanil preserves patient cooperation, making it safer when spontaneous ventilation is paramount. Yeganeh and associates74 enrolled 22 patients who had cervical trauma and semi-elective maxillofacial surgery and administered remifentanil with target-controlled or manually controlled infusion techniques. They recommended remifentanil infusion to provide good conscious sedation, but target-controlled remifentanil infusion seems to provide better conditions compared with manually controlled remifentanil infusion and is easier to use.
Alternative drugs suitable for ICU airway management and even awake fiberoptic intubation are (S+)-ketamine and propofol.75 Propofol is a rapid-acting, lipid-soluble induction drug that induces hypnosis in a single arm-brain circulation time. In patients with cardiac comorbidities and limited physiologic reserve, the use of propofol can be associated with significant hypotension. Causes of hypotension are reduced systemic vascular resistance and possibly depressed inotropy.76 In an analysis of 4096 patients undergoing general anesthesia, Reich and associates77 reported that the use of propofol was a statistically significant predictor of hypotension. Furthermore, in 2406 patients with retrievable outcome data, prolonged in-hospital stay and death were more common in those who experienced hypotension.77 Therefore, use of propofol may have special risks in high-risk patients with known cardiac dysfunction.78
Ketamine is a rapid-acting, dissociative anesthetic agent (similar to phencyclidine) with potent amnestic, analgesic, and sympathomimetic effects.67 Ketamine can cause realistic hallucinations and extreme emotional distress. This may be prevented with the obligatory combination of a benzodiazepine. The sympathomimetic effects of ketamine may produce cardiac ischemia by increasing cardiac output. Consequently, use of ketamine should be avoided in patients with cardiac dysfunction. Leykin and others79 found that the administration of ketamine resulted in excellent intubation conditions in patients undergoing elective surgery compared with sodium pentothal. In a prospective, randomized clinical trial, Ledowski and Wulf80 reported that the combination of ketamine with rocuronium and etomidate resulted in superior intubation conditions. This combination may be useful in treating hemodynamically unstable patients in the ICU setting, when succinylcholine is contraindicated.21 The use of ketamine in patients with increased intracranial pressure is only recommended in mechanically ventilated patients.81 Ketamine is associated with realistic and truthful nightmares. In this context, the combination with midazolam seems to be an obligatory recommendation. Currently, an S(+) enantiomer of ketamine is available. This enantiomer seems to be free of these clinically relevant side effects although use of these S(+) enantiomers is still recommended in combination with midazolam.
Etomidate provides good intubation conditions with only moderate hemodynamic depression in patients undergoing RSI.82 Etomidate inhibits adrenosteroid production through the inhibition of mitochondrial hydroxylase, after continuous or even single administration.83–85 Cuthbergson and colleagues86 recruited 500 patients to a multicenter, randomized, double-blind, placebo-controlled trial. The use of bolus etomidate was associated with an increased incidence of inadequate response to corticotrophin and is likely associated with an increase in mortality. Therefore, the authors recommend extreme caution in the use of etomidate in critically ill patients with septic shock.86 Roberts and Redman87 and Bloomfield and Noble88,89 also questioned any use of etomidate in critically ill patients.
Dexmedetomidine is increasingly used as a sedative for monitored anesthesia. Advantages include its analgesic properties, “cooperative sedation,” and lack of respiratory depression. Dexmedetomidine is also increasingly used in RSI and awake intubation, with promising studies showing a beneficial effect.90,91 However, long-term observational studies are still lacking, and the current scientific level is too weak to recommend routine use of dexmedetomidine.
Succinylcholine is a very-rapid-acting, polarizing muscle relaxant enabling excellent intubation conditions in less than 1 minute.92 Unfortunately, it has serious side effects, including the possibility of triggering malignant hyperthermia. The use of succinylcholine is contraindicated in patients with acute major burns, upper or lower neuronal lesions, prolonged immobility, massive crush injuries, and various myopathies.21 Benumof and associates93 clearly demonstrated that even the short duration of action of succinylcholine is too long, and critical hemoglobin desaturation will occur before return to an unparalyzed state, even after a 1-mg/kg dose.93
Rocuronium is the most widely used non-depolarizing neuromuscular blocking agent. The recommended dosage of “normal” intubation is 0.6 mg/kg. For RSI, higher dosages up to 1.2 mg/kg are necessary and provide excellent intubation conditions within 1 minute.94 A meta-analysis by the Cochrane Collaborative Group concluded that succinylcholine created superior intubation conditions compared with rocuronium.95 The only absolute contraindication against the use of rocuronium is allergy to aminosteroid neuromuscular drugs. A major advantage of rocuronium is the possibility of rapid and complete reversal of even deep neuromuscular block by the administration of sugammadex.96 Based on a systemic review by Chambers and others,97 the use of sugammadex in combination with high-dose rocuronium is efficacious.
E Awake Endotracheal Intubation
Once the patient is properly prepared, any of the intubation techniques listed in Box 46-2 can be chosen according to the operator’s preference.98–102
Box 46-2 Nonsurgical Techniques for Awake Intubation
A valuable alternative approach is the insertion of the intubating laryngeal mask airway (ILMA) under local anesthesia and light sedation. Intubation is then performed blindly or using a fiberscope inserted through the LMA. Langeron and coworkers103 prospectively compared ILMA intubation with fiberoptic intubation in patients with difficult airway and reported similar success rates (>90%) and durations for both techniques. Another interesting approach is the combination of Fastrach and lighted stylet. Dimitriou and associates104 studied 44 of 11,621 patients in whom three attempts of direct laryngoscopy failed. In all these patients, an ILMA was inserted and sufficient ventilation was possible in all patients after a maximum of two attempts. Thereafter, a well-lubricated silicon ETT loaded with a flexible lightwand was introduced. Lightwand intubation was successful at the first attempt in 38 of 44 patients, at the second attempt in three patients, and at the third to fifth attempt in two. Intubation failed in only one patient. Finally, if all those alternatives fail, the establishment of a surgical airway should be considered.
F Assessment of Correct ETT Placement
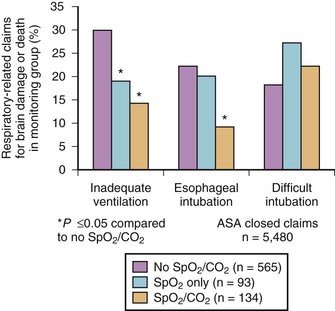
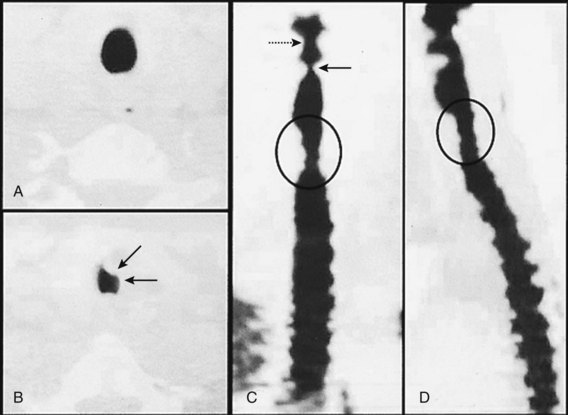
Esophageal intubation is a major complication of airway management and can result in severe brain damage or even death. Evaluating the U.S./American closed-claims database (5480 claims), Cheney and colleagues105 reported the changing trends in anesthesia-related death and permanent brain damage. In the 1980s, 11% of claims associated with death or brain damage involved esophageal intubation, fortunately decreasing to 3% in the 1990s. This reduction was attributed to the introduction of end-tidal carbon dioxide partial pressure (PETCO2) monitoring in the 1990s (Fig. 46-5).
Esophageal intubation is an issue even in sufficiently equipped ICUs. Schwarz and colleagues106 studied almost 300 emergency intubations in an ICU setting and reported esophageal intubation in 8% of cases (ETT misplacement was corrected in all patients before the onset of hypoxemia).
Techniques for clinical evaluation of ETT position include the following:
• Direct visualization of the ETT between the vocal cords107,108
• Ventilation-bag compliance109
• Auscultation of breath sounds over lungs and epigastrium110
• Observation of symmetrical chest excursions
• Lighted stylet (lightwand)112
• Esophageal detector device and self-inflating bulb113–115
• Acoustic reflectometry116,117
• Colorimetric end-tidal CO2 detector and PETCO2 measurement115,118
Radiographic investigation is of limited value because of the delay in diagnosis. Fiberoptic evaluation needs special equipment and preparation and is time-consuming. Acoustic reflectometry is a promising new approach with excellent results, at least in experienced hands. Rafael and coworkers117 studied acoustic reflectometry in 200 adult intubated patients and reported a 99% correct tracheal and a 100% correct esophageal identification rate. Currently, CO2 monitoring comes closest to the ideal monitor of correct ETT positioning. CO2 monitoring can be performed either colorimetrically by relatively inexpensive single-use CO2 detectors (e.g., Easy Cap, Tyco Healthcare; or Colibri, ICOR AB, Bromma, Sweden) or by capnography using infrared absorption.118,119 Ventilation is assessed as PETCO2 approximates arterial CO2 partial pressure (PaCO2) within 2 to 6 mm Hg in normal lungs. Unfortunately, in many disease states, this discrepancy increases significantly. The concentration of CO2 expired is determined by CO2 production (e.g., body temperature, muscle tone), CO2 transport (e.g., circulation, pulmonary perfusion), and CO2 elimination (pulmonary and airway integrity). Therefore, absence of PETCO2 indicates esophageal intubation, circuit disconnection, cardiac arrest, or airway obstruction. A false-positive result may be obtained after gastric inflation with CO2 containing gas or digestion of carbohydrate-enriched beverages. ETT-misplacement can be excluded by observing a normal PETCO2 waveform for three to six consecutive breaths.
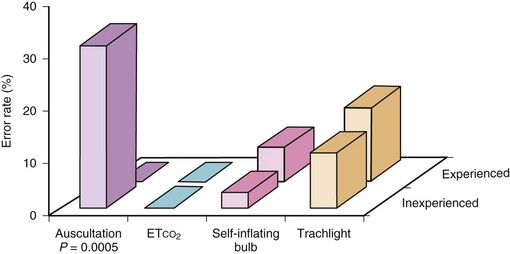
Knapp and associates120 investigated four different methods—auscultation, capnographic PETCO2 determination, self-inflating bulb, and transillumination using Trachlight—for the verification of correct ETT positioning in 152 examinations in an ICU setting. Only PETCO2 monitoring was reliable for the verification of tracheal ETT placement (Fig 46-6). A poorer performance was only reported for prehospital and in-hospital cardiac arrest patients. However, end-tidal CO2 concentrations are closely correlated with resuscitation outcome in this special setting.121
IV Difficult Airway Management in Intensive Care
Critically ill patients with preexisting hypoxia and poor cardiopulmonary reserve have a higher risk of adverse events during airway management.122 Consequently, emergency endotracheal intubation in critically ill patients is associated with a significant incidence of major complications. Airway management in intensive care patients differs significantly from endotracheal intubation done for routine surgical procedures in the OR. Airway management in the ICU is often carried out in deteriorating patients in respiratory failure, shock, or cardiopulmonary arrest, and little time may be available for patient evaluation, examination, and preparation. Therefore, the ICU physician should approach the patient with the following four questions in mind123:
• Will mask ventilation likely to be successful, when general anesthesia is induced before securing the airway?
• Will endotracheal intubation likely to be successful, when general anesthesia is induced before securing the airway?
• Are there any obstacles making awake fiberoptic intubation difficult?
In a prospective investigation of 297 endotracheal intubations in an emergency ICU setting, Schwartz and coauthors106 reported major complications in a significant number of patients. Among the problems encountered were difficult intubations (8%), esophageal intubations (8%), pulmonary aspiration (4%), and an associated mortality of 3%. The authors also reported that 3% of hospitalized critically ill patients die within 30 minutes of emergency intubation.
In another observational multicenter study performed in French ICUs, at least one severe complication occurred in 28%: severe hypoxemia (26%), hemodynamic collapse (25%), and cardiac arrest (2%).124 The other complications were difficult intubation (12%), cardiac dysrhythmia (10%), esophageal intubation (5%), and aspiration (2%).
Difficult Mask Ventilation
Difficult ventilation has been defined as the inability of a trained anesthetist to maintain the oxygen saturation greater than 90% using a face mask for ventilation and 100% inspired oxygen, provided that the preventilation oxygen saturation level was within the normal range.125 Langeron and coauthors126 prospectively studied 1502 patients and observed difficult mask ventilation (DMV) in 75 patients (5%). DMV was anticipated by the anesthesiologist in only 13 patients (17% of DMV cases). Using a multivariate analysis, five criteria were recognized as independent factors for a DMV (age >55, BMI >26 kg/m2, beard, lack of teeth, history of snoring), with the presence of two indicating high likelihood of DMV (sensitivity, 0.72; specificity, 0.73). The implication for clinical practice is to avoid the administration of intravenous induction agents in patients with a high risk of DMV and secure the airway awake (Table 46-3). The risk factors for DMV include age over 55 years, body mass index (BMI) greater than 26 kg/m2, lack of teeth, male gender, Mallampati class IV airway, presence of beard, and a history of snoring.21,126,127
TABLE 46-3 Anatomic Factors Associated with Difficult Ventilation
| Site | Airway Issue | Intervention |
|---|---|---|
| Face | Facial wasting, beard, edentulous, snoring history | Patient positioning: sniffing position, and/or jaw thrust; ensure proper fit of mask to face; variety of different mask sizes; oropharyngeal and nasopharyngeal airways; team ventilation, with one person “bagging” while other ensures proper seal; leave in dentures while ventilating patient. |
| Upper airway | Abscess, hematoma, neoplasm, epiglottitis | Assist ventilation and avoid neuromuscular paralysis; awake intubation, possible fiberoptic with preparation for emergency cricothyrotomy; call for help if upper airway obstruction is suspected. |
| Lower airway | Reactive airways | Preinduction administration of bronchodilators, nitrates, and diuretics |
| Airspace disease: pneumonia, ARDS, pulmonary edema, hemo/pneumothorax | PEEP valve for oxygenation in pulmonary edema, ARDS, and pneumonia; decompress pneumothorax if you are going to apply positive-pressure ventilation. | |
| Thorax-abdomen | Ascites, obesity, hemoperitoneum, abdominal compartment syndrome | Use of bag-valve-mask with PEEP valve may help oxygenation and ventilation. |
ARDS, Adult respiratory distress syndrome; PEEP, positive end-expiratory pressure.
From Reynolds SF, Heffner J: Airway management of the critically ill patient: rapid-sequence intubation. Chest 127:1397–1412, 2005.
Difficult or Impossible Intubation
Difficult intubation (DI) has been defined by the need for more than three intubation attempts or attempts at intubation that last more than 10 minutes. Difficult laryngoscopy and intubation are common phenomena even during routine patient care in the OR. Several investigations clearly demonstrated that minor problems necessitating a second intubation attempt are encountered in up to 8% of patients.128–130 Grade 3 laryngoscopy, requiring multiple attempts at intubation, occurs in 1% to 4% among all patients.131–133 Inability to intubate due to grade 3 or 4 laryngoscopic views is present in 0.05% to 0.35% of routine anesthesia cases.131,134,135 Fortunately, the “cannot ventilate, cannot intubate” situation is rare in the OR and occurs in approximately 2 in 10,000 cases.125,136,137 Christie and others138 recapitulated several clinical indicators (Table 46-4).
| Airway Exam Component | Non-Reassuring Findings |
|---|---|
| Length of upper incisors | Relatively long |
| Relation of maxillary and mandibular incisors during normal jaw closure | Prominent “overbite” (maxillary incisors anterior to mandibular incisors) |
| Relation of maxillary and mandibular incisors during voluntary protrusion | Patient cannot bring mandibular incisors anterior to (in front of) maxillary incisors |
| Interincisor distance | <3 cm |
| Visibility of uvula | Not visible when tongue is protruded with patient in sitting position (e.g., Mallampati class >II) |
| Shape of palate | Highly arched or very narrow |
| Compliance of mandibular space | Stiff, indurated, occupied by mass, or nonresilient |
| Thyromental distance | Less than three ordinary finger breadths |
| Length of neck | Short |
| Thickness of neck | Thick |
| Range of motion of head and neck | Patient cannot touch tip of chin to chest or cannot extend neck. |
From Christie JM et al: Unplanned endotracheal extubation in the intensive care unit. J Clin Anesth 8:289–293, 1996.
In addition, Reed and colleagues139 demonstrated that patients with large incisors, a reduced mouth opening and a reduced thyroid-to-floor-of-mouth distance are more likely to have a poor glottic view during laryngoscopy.
Management of the difficult airway in the emergency department (ED) and in the ICU has not been studied as well as in the OR. Sakles and associates140 performed a 1-year study on 610 patients requiring airway control in the ED. Rapid-sequence induction was used in 84%, and a total of 98.9% were successfully intubated. In 33 patients (5.4%), inadvertent esophageal intubation occurred. Seven (1.2%) patients could not be intubated and underwent cricothyrotomy. Three patients experienced sustained cardiac arrest after intubation. In a prospective observational study on failed intubation attempts in the ED, Bair and colleagues102 identified 7712 patients undergoing emergency intubation. In seven patients, a definitive airway could never be established. A total of 207 patients (2.7%) with failed endotracheal intubation were then included in the study. The majority of failed intubations occurred when RSI was not used as first choice (i.e., oral intubation under sedation, oral intubation without sedation, or blind nasotracheal intubation). The most common rescue technique was RSI (49%), followed by a surgical airway (21%). The authors concluded that invasive airway techniques are still important, and rescue airway techniques should be emphasized in ongoing medical education.
Schwartz and others106 investigated the complications of 297 consecutive endotracheal intubations in 238 adult critically ill patients in the ICU, studied prospectively over 10 months; 89% of intubations were accomplished at the first attempt, and 8% of patients met criteria for DI. Four percent of intubations were only possible after four or more attempts. Esophageal intubation was observed in 8% of intubations but all were recognized before any adverse sequelae resulted. New and/or unexplained pulmonary infiltrates were regarded as indicative for pulmonary aspiration of gastric contents and occurred in 4% of patients. Seven patients (3%) died during or within 30 minutes of the procedure.
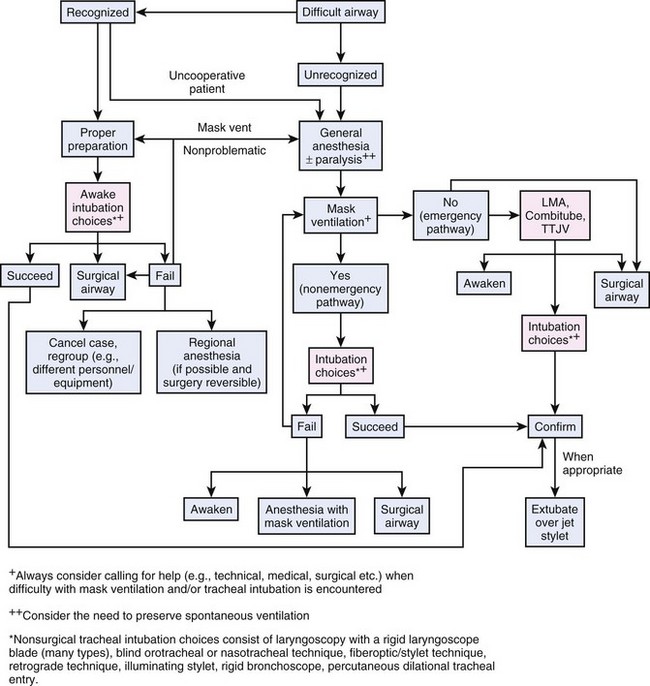
The ASA Difficult Airway Algorithm helps reduce the incidence of severe or catastrophic events during airway management, especially for anesthesia patients.1 The basic principles of this approach are meticulous patient evaluation before anesthesia induction, awake intubation if problems are suspected, and preparation of an alternative approach in case of failure (Fig. 46-7). Those recommendations are also valuable for the management of critically ill patients. As mentioned, the ASA algorithm has characteristics that prevent direct application to the practice of ICU airway management. Airway management in the ICU is usually urgent or emergent, often depriving the physician of the time necessary to evaluate the patient and plan the life-saving intervention. The patient is presumed to have a full stomach and therefore must undergo awake airway management or RSI. The option of reemergence from anesthesia to resume spontaneous ventilation if difficulty is encountered is often impossible. Therefore, modified algorithms with more specific considerations of the special circumstances encountered in ICU and emergency patients have been suggested (“crash airway” or “failed airway” algorithm).141 Although lacking prospective evaluation, these guidelines might represent a more appropriate application of principles and constraints to airway management in the ED setting.63
The best means for coping with the problem of failed airway is to prevent its occurrence. This requires the optimization of the initial attempt of laryngoscopy, to prevent multiple attempts causing bleeding and swelling. The net result might be a vicious circle, with each attempt leading to greater likelihood of failed intubation and ventilation, with potentially disastrous consequences. Optimizing laryngoscopy requires appropriate positioning of the patient, use of a laryngoscope blade best fitting the situation, optimal external laryngeal pressure (BURP maneuver: backward, upward, rightward pressure on cricoid cartilage), and eventually effective muscle relaxation.63 When all these factors are taken into account, the first attempt of laryngoscopy is likely to be the best attempt.
According to the ASA Difficult Airway Algorithm and most other recommendations, there are only four appropriate options for the management of “cannot mask-ventilate, cannot intubate” situations142 (Fig. 46-7): immediate insertion of laryngeal mask airway or esophageal-tracheal Combitube, manual transtracheal jet ventilation, or surgical access to the patient’s airway (tracheostomy or cricothyrotomy).1 A rational and well-considered approach in difficult airway management is elementary. Strict adherence to airway management guidelines (e.g., ASA algorithm) is essential and reduces failed airway attempts.
A Esophageal-Tracheal Combitube
The esophageal-tracheal Combitube (ETC) is a double-lumen airway (“pharyngeal” and “distal” lumen) invented by Frass and coworkers,143 equipped with a pharyngeal balloon and a distal cuff (Covidien, Mansfield, Mass). The ETC is designed for blind insertion into the patient’s esophagus or trachea. In more than 95% of all blind insertions, the ETC enters the esophagus. After insertion, the pharyngeal balloon is inflated (maximum, 85/100 mL air) and seals against the oral cavity, while the distal cuff (5-12/15 mL air) prevents gastric inflation. Test ventilation is started via the pharyngeal lumen (i.e., supraglottic ventilation). Absence of breath sounds and gastric inflation indicate the rare case where the ETC has blindly entered the trachea. Ventilation is attempted via the distal lumen, the distal cuff blocked with the minimum amount of air providing adequate seal, and the ETC used as a conventional ETT. Two different ETC sizes are available: a small-adult model (37 French, Combitube SA) for women and men ranging in height from 120 to 200 cm and a 41-F model for taller patients.144,145
The ETC has been used extensively in emergency situations, as well as during routine surgery.144,146,147 The authors recommend sufficient training during elective surgery before using the airway in emergency situations.145,148,149 In routine cases, the ETC might be inserted using a standard laryngoscope to reduce the risk of tissue injury.150,151 Contraindications for the use of the ETC are esophageal pathologies, ingestion of caustic substances, and central airway pathologies. Disadvantages are the lack of access to the patient’s airways (suctioning of tracheal secretions impossible), lack of a pediatric ETC, and risk of venous stasis and soft tissue (tongue) swelling after prolonged use. The former limitations will be eliminated by the introduction of a redesigned ETC enabling fiberoptic access to the patient’s airways and a pediatric version of the ETC.152,153
Advantages of the Combitube include protection against aspiration of gastric contents and the applicability of high airway pressures.154 The ETC might stay in place for up to 8 hours, providing time for further decision making.155 Changing the ETC for a definitive airway (regular ETT or tracheal cannula) might be done by an attempt of direct laryngoscopy, fiberoptically, or surgically (elective tracheostomy or cricothyrotomy).152,156
Recently, the EasyTube has been released (Teleflexmedical Ruesch). It is similar to the Combitube but offers certain advantages: The “pharyngeal” lumen of the EasyTube ends just below the oropharyngeal balloon. Therefore, the “tracheo-esophageal” lumen is thinner than that of the Combitube, which carries the two lumens down to the end. Since the distal single lumen of the EasyTube is significantly thinner, the potential danger of mucosal damage is minimized. Furthermore, the oropharyngeal balloon is latex free. The EasyTube comes in two sizes. The 28-F EasyTube (“pediatric size”) is available for patients ranging in height from 90 to 130 cm; the 41-F EasyTube is designed for patients taller than 130 cm. A fiberscope can be passed through the so-called pharyngeal lumen because it is open at the distal end. This feature allows inspection of the trachea and possible replacement of the EasyTube using a guidewire, in addition to a larger suction catheter via both lumens (14-F suction catheter in 41-F EasyTube). The EasyTube has been used in a few cases of difficult intubation at the ICU (see Chapter 27).
B Laryngeal Mask Airway
The laryngeal mask airway (LMA North America, San Diego) was presented in 1983, gained widespread popularity, and is currently used extensively during general anesthesia.142,157,158 The LMA may be placed for three different reasons: as a routine airway and ventilatory device, as an emergency airway in cannot-intubate, cannot-ventilate situations or as a conduit for endotracheal intubation. Advantages of LMA use as a routine airway have been demonstrated clearly over conventional face mask ventilation. Tidal volumes administered were higher and problems associated with airway management (difficulties in maintaining airway or SpO2 ≥95%) less frequently encountered during LMA use compared with regular face mask.159 LMA works well under these circumstances, because exact positioning is not crucial for a clinically acceptable patent airway.
Furthermore, the potential use of this recent airway in respiratory emergency situations has been recognized early.160 Leach and associates161 described use of the LMA as the immediate airway in cardiopulmonary resuscitation, and their findings were confirmed by a larger investigation.162 A multicenter study was undertaken to assess the potential value of the LMA when inserted by ward nurses during resuscitation as a method of airway management, prior to the arrival of the advanced life support team with endotracheal intubation capability; 130 nurses were trained and 164 cases of cardiac arrest studied. The LMA was inserted at the first attempt in 71% and at the second attempt in 26% of patients. Satisfactory chest expansion occurred in 86%. The mean interval between cardiac arrest and LMA insertion was 2.4 minutes. Regurgitation of gastric contents occurred before airway insertion in 20 patients (12%) and during insertion in three (2%).162
Therefore, the LMA has been incorporated into the ASA algorithm and might be inserted as a conduit for fiberoptic endotracheal intubation in the awake or anesthetized patient who cannot be conventionally intubated (mask ventilation may or may not be possible) and as a non-emergency or emergency airway in the anesthetized patient142 (Fig. 46-8).
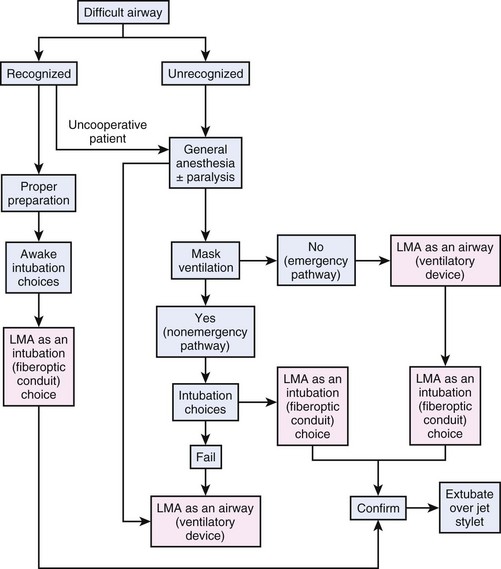
Figure 46-8 Laryngeal mask airway (LMA) and ASA DA algorithm.
(From Benumof JL: Laryngeal mask airway and ASA Difficult Airway Algorithm. Anesthesiology 84:686–699, 1996.)
In the meantime, several modifications of the LMA have been proposed and have been or will be introduced into clinical routine. The ILMA differs from conventional LMAs by having a wider, shorter stainless steel tube; a handle to steady the device; and an epiglottic elevating bar (movable flap fixed to upper rim of mask). The ILMA is accepting cuffed ETTs up to ID of 8.5 mm, enabling blind or fiberoptic intubation using the correctly placed ILMA as a conduit. Success rates for blind ILMA intubation in the 75% to 90% or greater range have been reported.103,163,164
The ILMA has therefore been recommended as a rescue device for emergency airway management. Ferson and colleagues163 used the ILMA in 254 patients with different types of difficult airway. Insertion of the ILMA was accomplished in three attempts or fewer in all patients. The overall success rates for blind and fiberoptically guided intubations through the ILMA were 96.5% and 100.0%, respectively. Repeated “blind” intubation attempts with the ILMA resulted in esophageal perforation in one elderly patient.165 Therefore, for blind ILMA intubation, the specially designed silicon tube with rounded bevel should be preferred, because success rates exceed those encountered with standard reinforced tubes.166 Muscle relaxation is not needed for blind ILMA intubation but increases success rates.166
Laryngeal Mask Airway vs. Combitube
Sealing capacities and protection against regurgitation of gastric contents of the intubating LMA seem to be inferior to those of the Combitube (ETC), with average leak fractions of the LMA of 20% to 25% during positive-pressure ventilation and airway pressures of 20 to 30 cm H2O.167 Use of the ETC offers higher peak airway pressures and permits positive end-expiratory pressure (PEEP) ventilation, thereby enabling higher tidal ventilation and maintenance of adequate gas exchange, even in those patients with severe underlying pulmonary pathology (e.g., aspiration of gastric contents).168 In contrast to the ETC, blind or fiberoptic intubation is possible through the LMA lumen (using conventional ETTs up to ID of 6.5 mm) and especially through the ILMA (up to 8.5 mm). With the currently available ETC model, no blind or fiberoptic access to the patient’s airway is necessary. Krafft and others152 modified the standard ETC model and created a larger ventilation hole, which can be used for fiberoptic intubation or tracheal cleansing. However, the redesigned model is currently not available, and it is uncertain whether it will be produced in the near future.
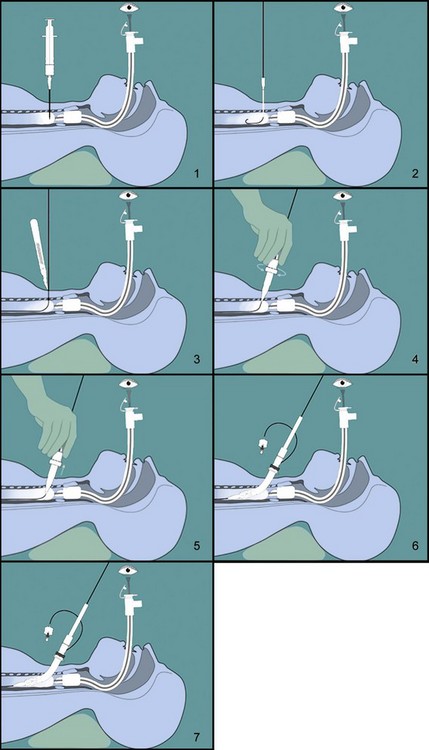
C Transtracheal Jet Ventilation
In brief, transtracheal jet ventilation (TTJV) is performed using a 16- or 14-gauge catheter, and a 1.5- to 3.5-bar oxygen source (equipped with pressure regulator) in combination with noncompliant tubing. The TTJV catheter is inserted through a cricothyroid puncture, and correct tracheal placement is verified by the aspiration of 20 mL of free air. Noncompliant tubing is then connected and manual oxygen inflation started at an inspiratory rate less than 1 second (~0.5 second) and an inspiratory/expiratory time ratio of 1 : 3 (to ensure enough time for passive exhalation). Alveolar ventilation is achieved by bulk flow of oxygen through the cannula, as well as translaryngeal entrainment of room air (Venturi effect). For example, use of a 16-gauge cannula and a driving pressure of 3.5 bar results in a gas flow of approximately 500 mL/sec.169 Therefore, facilitation of passive exhalation by maintaining patency of natural airways is of major importance to avoid overinflation and pulmonary barotrauma. Therefore, nasopharyngeal and oropharyngeal airways are inserted, maximum jaw thrust is well maintained throughout the entire procedure, and expiratory chest movements are observed continuously.
Performed in elective as well as emergency situations, the major indications for TTJV are lack of equipment for conventional airway management, the “cannot ventilate, cannot intubate” situation, ventilatory support during upper airway surgery, or support during endotracheal intubation by other techniques (e.g., ventilation during prolonged fiberoptic intubation). Complications of TTJV during elective application are rare but occur frequently during emergency TTJV (mainly limited to tissue emphysema). Monnier and colleagues170 used high-frequency TTJV for ventilatory support during elective laser surgery for laryngeal and subglottic lesions and observed only one complication in 65 patients (mediastinal emphysema). Smith and coworkers,171 however, reported a 29% complication rate in 28 emergency TTJV patients (exhalation difficulty in 14%, subcutaneous emphysema in 7%, mediastinal emphysema in 4%, arterial perforation in 4%). Other complications of TTJV (e.g., esophageal puncture, bleeding, hematoma formation) have been reported but rarely occur.
D Emergency Surgical Airway
Rapid-sequence induction is the standard for emergency airway management in ICUs and EDs. Reported success rates of RSI are 97% to 99%, and an emergency surgical airway is only necessary in 0.5% to 2% of patients.140,172 Sakles and associates140 evaluated 610 patients requiring emergency airway management in the ED during a 1-year period; RSI was used in 515 (84%). A total of 603 patients (98.9%) were successfully intubated, and only seven patients could not be intubated and underwent cricothyrotomy. Therefore, anesthesiologists and intensive care physicians have only limited experience with surgical airway management. The ASA algorithm has proved to be an invaluable tool, but only briefly addresses the circumstances when a patient must be intubated immediately regardless of the difficulties presented. Since performance of a surgical airway is the final pathway of all airway algorithms, cricothyrotomy must be mastered by all clinicians involved in emergency airway management. Surgical airway management comprises percutaneous dilatational cricothyrotomy or tracheostomy using the Seldinger technique, surgical cricothyrotomy, or surgical tracheostomy. In emergency situations and in cases of unexpected intubation failure in the ICU, surgical or percutaneous cricothyrotomy using the Seldinger technique is preferred over tracheostomy and enables rapid and easy airway access and restoration of adequate gas exchange and ventilation.
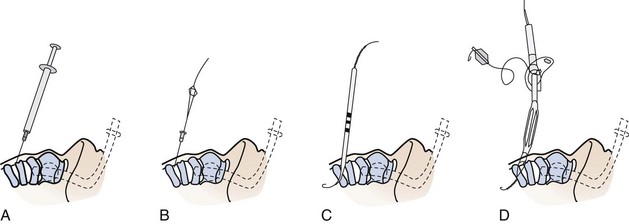
1 Percutaneous Cricothyrotomy
Several commercial kits are available for emergency cricothyrotomy, implying easy insertion and high success rates (e.g., Melker or Arndt set, Cook Critical Care, Bloomington, Ind). Seldinger technique cricothyrotomy is achieved by performing cricothyroid puncture with a medium-sized needle, passing a wire through the needle, and then passing a dilator and airway over the wire (analogous to insertion of central venous line). Advertisement and promotion claim that percutaneous cricothyrotomy is a quick and easy procedure; in fact, after identification of the cricothyroid membrane, the Seldinger approach is no faster than open cricothyrotomy.173 Furthermore, none of the percutaneous techniques provides a cuffed tube within the trachea.
Eisenburger and colleagues174 compared Seldinger technique emergency cricothyrotomy with conventional surgical approach in human cadavers. Tracheal placement of the tube was achieved in 60% (n = 12) in the Seldinger group versus 70% (n = 14) in the open group (P = ns). Furthermore, five attempts in the Seldinger group had to be aborted because of kinking of the guidewire. No differences in the time necessary to perform the procedures were registered. Chan and coworkers173 performed a similar study in cadavers but observed higher success rates. Airway placement was accurate in 13 of 15 cases for the standard technique (87%) and in 14 of 15 cases for the wire-guided technique (93%). Comparing wire-guided versus standard techniques, no differences were seen in complication rates or performance times. However, one must remember that all these results are obtained in an unstressed elective situation in the morgue; one assumes that results in true emergency situations might be even poorer.
A newer cricothyrotomy device, the Portex Cricothyrotomy Kit (PCK), is based on the concept of mechanical detection of the posterior wall of the larynx (see Chapter 30, Fig. 30-15). In a comparison of two emergency cricothyrotomy kits in 40 human cadavers, the use of PCK was responsible for more lesions and more failures than the standard set, in which cricothyrotomy was based on the Seldinger technique.175
2 Surgical Cricothyrotomy
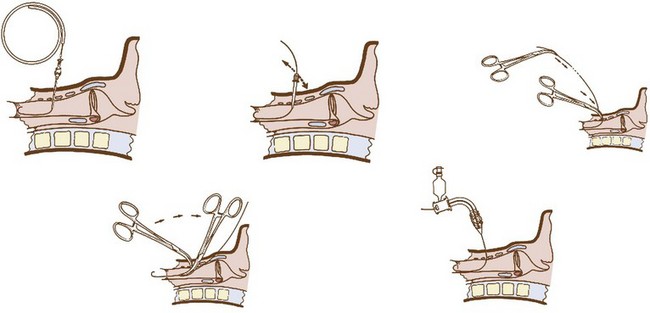
The vasculature is rich overlying the cervical trachea but is strikingly absent over the cricothyroid membrane (CTM). Surgical cricothyrotomy can be performed only after correct identification of the CTM between thyroid and cricoid cartilage; the vertical length of the membrane is about 0.7 to 1.0 mm. The set of instruments used should be simple: scalpel with no. 11 blade, tracheal hook, Trousseau dilator, and cuffed tracheostomy tube. The most common technique is the “no drop” technique, where the larynx and trachea are immobilized by the operator’s hand throughout the procedure. Other techniques have been proposed (e.g., four-step technique), but the no-drop technique has proved its value with the following six steps (Fig. 46-9)176:
Step 1: Identify the CTM. Key landmark is the laryngeal prominence (Adam’s apple), which is easier to palpate in men than in women. It can be identified in the midline at the junction of upper and middle third of the anterior neck. Thumb and long finger stabilize the larynx, while the index finger is run down the anterior surface of the thyroid cartilage until the concavity of the CTM is reached.
Step 2: Immobilize the larynx by holding the superior horns of the thyroid between thumb and long finger, and incise the skin vertically (vertical incision 2-3 cm in length provides maximum flexibility and minimum trauma). Vertical skin incision should be made through skin and subcutaneous tissues and not into the airway. Thereafter, the CTM is again identified using the index finger. Several authors prefer to leave the index finger in the wound even during the dissection of the membrane (enables the membrane incision directly caudal to the index finger).
Step 3: Incise the CTM transversely in its distal third whenever possible (avoidance of superior thyroid artery). The incision should be wide enough to permit easy insertion of the cannula (1.5-2 cm).
Step 4: Insert the tracheal hook transversely into the incised membrane, then rotate it 90 degrees to the midline (some authors waive use of tracheal hook in open cricothyrotomy). The hook strictly immobilizes the larynx and brings the opening within the membrane closer into the field of view.
Step 5: Insert the Trousseau dilator into the airway for several millimeters, then open it to dilate the airway. Some prefer opening the branches in a rostrocaudal direction because most resistance to cannulation is encountered between thyroid and cricoid. However, transverse dilation is also possible.
Step 6: Insert the respective tracheal cannula with the right hand between the prongs opened with the operator’s left hand. Slight twisting of the cannula, with a 90-degree rotation of the Trousseau dilator, may be necessary to advance the tracheal cannula safely.
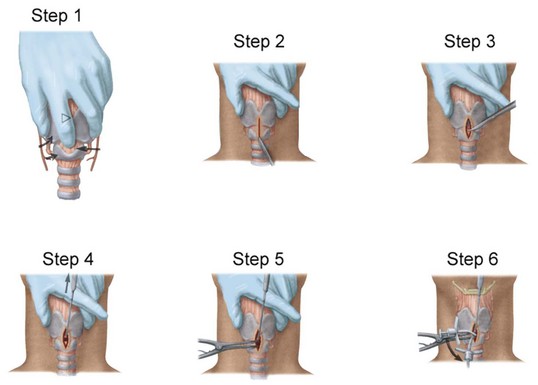
Figure 46-9 Six steps in performing surgical cricothyrotomy.
(From Walls RM, Gibbs MA: Surgical airway. Semin Anesth Periop Med Pain 20:183–192, 2001.)
In conclusion, the ASA Difficult Airway Algorithm was approved by the ASA House of Delegates in October 1992 and became effective July 1993.1 Every patient needs to be screened for difficult airways before anesthesia induction, and when difficulties are expected, airways need to be secured with the patient still awake (using fiberoptic scope in most cases). If difficulties are encountered with the patient already anesthetized, refrain from repeated and forceful attempts at direct laryngoscopy, and instead consider alternative methods. If a “cannot ventilate, cannot intubate” situation is encountered, either LMA or Combitube must be inserted immediately. Further alternatives are institution of TTJV or immediate surgical access to the patient’s airway. The most important point is to be alert and have a plan B and C prepared in case difficulties arise.
V Complications of Translaryngeal Intubation
In a recent report on 3423 emergent non-OR endotracheal intubations, Martin and associates177 found that the total incidence of difficult intubations was 10.3%. Complications occurred in 4.2%, including aspiration (2.8%), esophageal intubation (1.3%), dental injury (0.2%), and pneumothorax (0.1%). This report confirms that airway management in the hospital environment is also associated with increased risk of airway complications.
Hoarseness is a common complication after translaryngeal intubation and is reported to occur in 20% to 42% of patients.55,178 Jones and colleagues179 prospectively studied the incidence of hoarseness in 167 patients undergoing anesthesia, endotracheal intubation, and surgery. Fifty-four patients (32%) complained about postoperative hoarseness, with all except five returning to normal within 7 days. Vocal cord granuloma was observed in two patients. The site of granuloma is typically at the tip of the vocal processes of the arytenoid cartilages because of their incessant movement.180
In a prospective study of 226 endotracheal intubations in 143 adult patients, Stauffer and associates46 reported that 62% of all patients developed at least one complication of long-term translaryngeal intubation. The main complications, in anatomic order, are nasal and paranasal as well as laryngeal and tracheal injuries. Laryngeal injury is a common complication even after short-term translaryngeal intubation. Kambic and Radsel181 examined 1000 patients at the end of anesthesia. Severe intubation lesions were registered in 6.2% of patients, with most injuries resolving within a few days. However, 1% of patients sustained vocal cord dysfunction even after short-term translaryngeal intubation (Table 46-5).
In about 4% to 10% of patients, severe damage to the vocal apparatus is encountered (e.g., vocal cord paralysis, granuloma, subluxation of arytenoid cartilage).182 Most severe complications are observed within the trachea itself (e.g., ulcerations, hematomas up to necrosis, tracheomalacia).39,46 These complications occur infrequently but may require extensive surgical interventions (i.e., resection of trachea)183 (Figs. 46-10 and 46-11).
Laryngeal or tracheal trauma is even more common during acute airway management in emergency situations. Maxeiner184 performed postmortem examinations involving 294 cases of emergency intubation. Acute macroscopic sequelae (mucosal hemorrhage or injury, deeper tissue hemorrhage in false vocal cords) were observed in 18% of those resuscitated outside the hospital and in 16.9% of in-hospital patients. Soft tissue hematomas were observed at the laryngeal opening (31%), vocal cords (37%), laryngeal opening and vocal cords (17%), in the subglottic region (17%), and in the hypopharynx (29%). Lesions at the laryngeal aperture were mainly located at the right part of the larynx.
Other serious complications of translaryngeal intubation have also been reported. Lim and coworkers185 reported three cases of recurrent laryngeal nerve palsy in three patients after short-term intubation for surgery unrelated to the neck. Pressure neuropraxia resulted from an overinflated ETT cuff compressing the peripheral anterior branches of the right recurrent laryngeal nerve. Frink and Pattison186 and Castella and coworkers187 reported posterior arytenoid dislocation after uneventful endotracheal intubation and anesthesia. Furthermore, both pharyngeal and esophageal perforation have been reported after repeated attempts at intubation, especially using a rigid stylet.188 The tip of the rigid stylet therefore should never protrude from the distal tip of the ETT.
In addition, endotracheal intubation may result in reduced clearance and retention of mucous secretions, bacterial colonization, and ventilator-associated pneumonia.189 In a survey of 9080 ventilated patients, VAP developed in 842 patients (9.3%).190 The mean interval between ICU admission and diagnosis of VAP was 4.5 ±7.5 days, and independent risk factors for the development of VAP were male gender and trauma admission. However, VAP seems to be a complication more of endotracheal intubation and not of ventilatory support, because recent studies show a reduced incidence in patients undergoing noninvasive mechanical ventilation.191 A prospective, randomized study evaluated whether a closed suctioning (CS) system (TrachCare) influences crossover contamination between bronchial system and gastric juices, as well as frequency of VAP, compared with an open suctioning (OS) system. Five cross-contaminations were observed in the OS group on day 3 versus day 1; the 5 strains shared common genotypes as determined by random amplification of polymorphic DNA. No cross-contaminations were seen in the CS group (P = 0.037). VAP occurred in five patients of the OS group but in none of the CS group patients (P = 0.037). Arterial oxygen saturation (SpaO2) decreased significantly in the OS group compared with presuctioning values, the opposite of the CS group. Whereas presuctioning values were comparable between groups, postsuctioning SpaO2 was significantly higher in the CS group. CS significantly reduced cross-contamination between bronchial system and gastric juices and reduced the incidence of VAP when compared with OS. Hypoxic phases can be reduced by the help of CS.192
VI Airway Management for Prolonged Mechanical Ventilation (Tracheostomy)
Tracheostomy is one of the oldest surgical procedures performed in history. Transcutaneous insertion of a cannula into the patient’s trachea was first described in Egyptian and Hinduistic literature between 2000 and 1000 BC.193 The first description of “modern” tracheostomy was by the Italian surgeon Fabricius of Aquapendente in the 17th century using a tracheal cannula.194 Development of tracheostomy continued, with many case reports on alternative techniques reported in the next three centuries.195,196
The currently used technique for “conventional” surgical tracheostomy was first described by Jackson197 in 1909. Until several years ago, “open” surgical tracheostomy was the one and only method and therefore the gold standard for the long-term airway management in intensive care medicine. However, the conventional approach has several disadvantages, such as need to transfer the patient to the OR and early and late complications (e.g., bleeding, stoma infection). Therefore, Shelden and colleagues198 reported a percutaneous access to the trachea. However, this procedure was relatively complicated and was followed by deadly complications.199 Not until 1985 did Ciaglia and associates200 propose a percutaneous approach using the Seldinger technique and increasingly larger-sized dilators for progressive tracheal dilation, as supposed by Sanctorius201 in the 17th century.
Note the following definitions:
Tracheostomy: Surgical incision of the upper third of the anterior tracheal wall to insert a cannula.
Tracheostoma: Artificial opening into the trachea through the neck to the outside created by tracheostomy or tracheotomy.
Tracheotomy: Surgical technique to create a permanent epithelialized tracheostoma.
Cricothyrotomy: Surgical incision of the cricothyroid membrane for the insertion of a cannula.
Coniotomy: Percutaneous emergency cricothyrotomy using special single-use sets.
A Reasons for Performing Tracheostomy
Tracheostomy (or tracheotomy) is one of the most frequently performed elective surgical procedures in ICU patients, done in almost 10% of intubated patients. Tracheostomy is generally done for the following four reasons202:
• To relieve upper-airway obstruction caused by tumor, surgery, trauma, foreign body, or infection
• To prevent laryngeal and upper airway damage caused by prolonged translaryngeal intubation
• To allow easy or frequent access to the lower airway for suctioning and secretion removal
• To provide a stable airway in a patient who requires prolonged mechanical ventilation or oxygenation support203 (Table 46-6)
TABLE 46-6 Major Complications of Prolonged Translaryngeal Intubation
| Complication | Rate (%) | Reference |
|---|---|---|
| Supraglottic Laryngeal Injury (Ulceration, Scarring, Stenosis) | ||
| Laryngitis | 3 | 323 |
| Mucosal ulceration/edema of epiglottis | 7-12 | 46 |
| Mucosal ulceration/edema of larynx | 29-51 | 46 |
| Submucosal hemorrhage of epiglottis/larynx | 5-12 | 46 |
| Supraglottic laryngeal stenosis | 12* | 260 |
| Glottic Injury | ||
| Glottic ulceration | 51 | 46 |
| Glottic scarring and stenosis | 12-18* | 260 |
| Bilateral vocal cord paralysis (rare) | Few reported cases | 323 |
| Posterior commissure syndrome | 6 | 260 |
| Subglottic Injury | ||
| Subglottic stenosis/scarring | 12* | 260 |
| Tracheal injury | ||
| Tracheal stenosis (<50% stenosis) | 19 | 46 |
| Tracheal dilation/tracheomalacia | N/A | N/A |
| Tracheoesophageal fistula† | 0.5-5* | 324 |
NA, Not available.
* After 10 days of endotracheal intubation.
† From 0.5% to 5% of all tracheoesophageal fistulas are caused by endotracheal intubation.
From Shirawi N, Arabi Y: Bench-to-bedside review: early tracheostomy in critically ill trauma patients. Crit Care 10:201, 2006.
The issue of long-term translaryngeal intubation versus tracheostomy now favors secondary tracheostomy, although the adequate time point is still under discussion. In 2010, Durbin202 listed benefits of changing from a translaryngeal ETT to a tracheostomy tube in patients who require prolonged intubation. However, the evidence level for most topics discussed is “poor” and comes mainly from uncontrolled trials (Table 46-7).
TABLE 46-7 Benefits of Changing from Translaryngeal Endotracheal Tube to Tracheostomy Tube in Patient Requiring Prolonged Intubation
| Benefit | Type/Quality of Evidence Showing Benefit |
|---|---|
| Improved patient comfort | Uncontrolled reports, clinical opinion |
| Less need for sedation | Several RCTs |
| Lower work of breathing | Theoretical analysis, one small study |
| Improved patient safety | Clinical belief but minimal data, some contradictory |
| Improved oral hygiene | Clinical observation |
| Oral intake more likely | Opinion only |
| Earlier ability to speak | Uncontrolled reports |
| Better long-term laryngeal function | Large uncontrolled reports |
| Faster weaning from mechanical ventilation | One RCT |
| Lower risk of ventilator-associated pneumonia | Controversial, data support for both sides |
| Lower mortality | One RCT supports, many do not, but large RCT supports no increased mortality with tracheostomy |
| Shorter intensive care unit and hospital stay | Several meta-analyses |
RCT, Randomized controlled trial.
From Durbin CG Jr: Tracheostomy: why, when, and how? Respir Care 55:1056–1068, 2010.
B Timing of Tracheostomy
In 1989, at the Consensus Conference on Artificial Airways in patients receiving mechanical ventilation, the indications for nasal or oral ETTs and tracheostomy tubes were forged; “if the need for an artificial airway is anticipated to be greater than 21 days, a tracheostomy should be preferred.”204 With the introduction of percutaneous methods, the issue of translaryngeal intubation versus tracheostomy shifted to the best time point to perform tracheostomy.
In a prospective multicenter randomized trial, Rumbak and coworkers205 assigned 120 patients to early (within 48 hours after arrival at ICU) and delayed (14-16 days after arrival) percutaneous dilatational tracheostomy. Early tracheostomy was associated with significantly less mortality, nosocomial pneumonia, unplanned extubation, oral and laryngeal trauma, and a shorter duration of mechanical ventilation and ICU admission (Tables 46-8 and 46-9). Prospective studies by Arabi,206 Bouderka,207 and Rodriguez208 and their associates approved the shorter duration of mechanical ventilation in patients with earlier tracheostomy. In a systemic review and meta-analysis of five randomized trials, Griffith and colleagues209 found that early tracheostomy is associated with 8.5 fewer days of mechanical ventilation and 15 fewer days in the ICU. However, there were no significant differences in mortality or nosocomial pneumonia rates.
TABLE 46-8 Early vs. Delayed Percutaneous Dilatational Tracheostomy
| Outcome Measurement | Early Tracheotomy (n = 60) | Prolonged Translaryngeal Intubation (n = 60) |
|---|---|---|
| Died (%) | 19 (31.7) | 37 (61.7)* |
| Pneumonia (5) | 3 (5) | 15 (25)* |
| Days in intensive care unit (±SD) | 4.8 (±1.4) | 16.2 (±3.8)† |
| Days mechanically ventilated (±SD) | 7.6 (±4.0) | 17.4 (±5.3)† |
| Days sedated (±SD) | 3.2 (±0.4) | 14.1 (±2.9)† |
| Days on high-dose pressors | 3.5 (±4) | 3.0 (±4.5) |
| Organism(s) causing pneumonia: Methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa mixture | 1 | 5 |
There was a significant difference between the early tracheotomy groups and the prolonged translaryngeal intubation group in outcome measures. Some patients were sent to a step-down while still on mechanical ventilation.
From Rumbak MJ et al: A prospective, randomized study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med 32:1689-1694, 2004.
TABLE 46-9 Cause of Death in Critically Ill Medical Patients Receiving Early Tracheotomy or Delayed Translaryngeal Intubation*
| Cause of Death | Early Tracheotomy (n = 19) | Delayed Translaryngeal Intubation (n = 37) |
|---|---|---|
| Ventilator-associated pneumonia | 2 | 9 |
| Gastrointestinal bleed | 1 | 3 |
| Acute myocardial infarction | 2 | 4 |
| Pulmonary embolus | 1 | 1 |
| Intractable septic shock | 4 | 8 |
| Withdrawal of life support | 2 | 1 |
| Respiratory failure | 7 | 11 |
* More patients died of ventilator-associated pneumonia in the prolonged translaryngeal group than the early tracheotomy group.
From Rumbak MJ et al: A prospective, randomized study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med 32:1689–1694, 2004.
In contrast, most studies could not demonstrate definitive advantages of early (days 3-5) versus later (days 10-14) tracheostomy.210,211 In a systematic review, Maziak and colleagues212 identified 48 possibly relevant articles of 8153 citations; five of the 48 articles met the inclusion criteria. After data review, the authors found no significant advantages of early (days 2-7) versus late (>days 4-14) tracheostomy, concluding insufficient evidence exists to support that the timing of tracheostomy alters the duration of mechanical ventilation or extent of airway injury. In a 2006 meta-analysis comparing early and late tracheostomy in ICU trauma patients, Dunham and Ransom213 found no differences in mortality, nosocomial pneumonia, duration of mechanical ventilation, laryngotracheal pathology rates, or length of stay in hospital.
In a questionnaire answered by 48 Swiss ICUs (1995-1996; 90,412 patients and 243,921 ventilator-days), 10% of all patients who were ventilated longer than 24 hours underwent tracheostomy.214 The majority of tracheotomies (35%) were performed in the second week of critical illness. However, 68% of all interviewed physicians would also perform an early (<day 7) tracheostomy if indicated (expected longer ICU course). The authors concluded that tracheostomy in Swiss ICUs is far from being standardized with regard to indication, timing, and choice of technique.
A randomized prospective study is required to examine the need for and the timing of tracheostomy in patients requiring prolonged mechanical ventilation. The findings of such a study would have significant impact on the clinical practice and hospital costs.212 Until then, the following recommendations of the Consensus Conference of 1989 are still valid204:
• Patients with an estimated duration of intubation longer than 7 to 10 days are managed by translaryngeal intubation.
• Reevaluation of the patient at day 7: extubation possible within the next 7 days? If yes, continue translaryngeal intubation (cumulative duration of translaryngeal intubation <14 days).
• All patients with slow recovery or deterioration with an estimated duration of intubation longer than 14 days should undergo tracheostomy.
• Early elective tracheostomy (days 3-5) in patients with an estimated duration of intubation longer than 21 days
• Patients in whom estimation of ventilator days is difficult should undergo daily evaluation of the pros and cons of tracheostomy and continued translaryngeal intubation.215
C Tracheostomy Techniques
1 Conventional Surgical Tracheostomy
Tracheal dissection to provide relief from airway obstruction resurfaced in the diphtheria epidemic in the 19th century. The current technique for “conventional” ST was first described by Jackson197 in 1909. Many variations exist in technique, but ST is preferably performed in the OR.216 In critically ill patients for whom transport seems too risky, tracheostomy can also be performed in the ICU bed, although mainly under inferior surgical conditions. Positioning the patient with head and neck extended and a small pillow under the shoulders is a major part of successful technique. Overextension of the head should be avoided; the airway might be narrowed further, and the tracheostomy site might be erroneously too low (near innominate artery). The operating field should be prepared according to aseptic conditions. Patients who do not tolerate this position may assume a sitting or semi-recumbent position.
A permanent tracheostoma is necessary for patients who indefinitely require secure transluminal access (e.g., head injury). For the creation of a permanent stoma, a small portion of the anterior tracheal wall is resected, and skin flaps are sutured to the rectangular tracheal opening (Fig. 46-12).
2 Percutaneous Dilatational Tracheostomy Techniques
During the 1990s, a dramatic change in airway management for long-term ventilatory support occurred with the introduction of percutaneous approaches to the trachea. Percutaneous tracheostomy was first described by Shelden and colleagues198 in 1955, but the approach never became popular because of severe complications.199 With the introduction of the Seldinger technique by Ciaglia and others,200 a triumphant advance of percutaneous tracheostomy began because of presumed advantages (Box 46-3).
Simpson and associates217 showed that the number of critically ill patients at their facility undergoing tracheostomy had increased from 8.5% to 16.8% of all critically ill patients since the introduction of PDT. This represents a doubling of the proportion of ICU patients undergoing tracheostomy. Tracheostomy was also performed significantly earlier, at a median of 4 days versus 8 days of mechanical ventilation. Of approximately 31,000 tracheostomies performed in Germany (~80 million population), more than 50% were PDTs.
a Percutaneous dilatational tracheostomy according to Ciaglia
Pasquale Ciaglia introduced PDT in 1985 as an alternative approach to conventional ST.200 Using the original Ciaglia technique, the correct puncture site in the midline between first or third tracheal ring is identified, the trachea punctured (free aspiration of 20 mL air possible), and a guidewire inserted. Over this guidewire, special dilators are introduced, and the anterior wall of the trachea is dilated until an opening with a diameter of approximately 36 French is achieved (Fig. 46-13). Advantages of this method are short duration of the procedure and bedside performance. However, contraindications must be considered (Box 46-4).
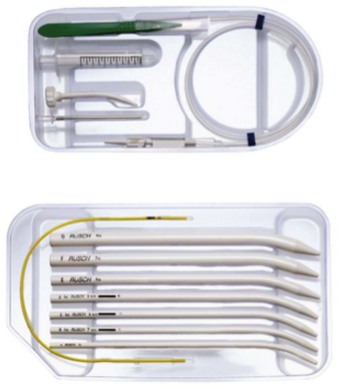
Figure 46-13 PercuQuick set for percutaneous tracheostomy.
(Courtesy of Rüsch, Essilengen, Germany.)
To enhance patient safety, Marelli and colleagues218 proposed that the entire procedure be monitored fiberoptically. The use of a FOB prevents endotracheal insertion out of the anterior midline or injury to the posterior tracheal wall, particularly during dilatation. A drawback of FOB control is that its insertion into the airway results in a partial obstruction of the ventilatory path thereby compromising ventilation.219
For performing PDT, the anesthetized patient is positioned with the head and neck extended. The anterior part of the neck is cleaned and draped in accordance with thyroid surgery guidelines. The FOB is then introduced through the tracheal tube in place, and the correct puncture site is identified. For this purpose, the ETT must be drawn back toward the glottic opening, and the FOB must be inside the ETT to prevent erroneous puncture of the scope (after the procedure, the scope must undergo a complete evaluation to prevent water intrusion).220
After puncturing the trachea in the midline between the first and third tracheal ring, a guidewire is inserted under fiberoptic vision (Fig. 46-14A). Puncturing must be done in the midline, in the middle between the tracheal cartilages. Puncture near a tracheal ring must be prevented because cartilage damage or fractures are associated with late complications such as tracheal stenosis (Fig. 46-14B). Thereafter, a guidance cannula is slid over the guidewire and a 1.5-cm skin incision made. The puncture site is then dilated with seven (hydrophilic) plastic dilators up to a diameter of 36 F (12 mm) (Fig. 46-14C). Lastly, the tracheal cannula (maximum ID of 9 mm) is inserted as guided by a medium-sized dilator (Fig. 46-14D). A slight resistance is often encountered during cannula insertion, which is overcome by using sufficient lubricating fluid and rotating movements during insertion. After cannula insertion, the FOB is removed from the ETT and inserted into the tracheal cannula, to verify correct placement, optimize cannula insertion depth, and remove bloody secretions from trachea and bronchi.
b Percutaneous dilatational tracheostomy according to Griggs
In 1989, Schachner and associates221 presented a single-dilatation tracheostomy forceps over a guidewire (Rapitrach; Fresenius, Runcom, Cheshire, UK), which carried a relatively high incidence of injury of the posterior tracheal wall. In 1990, Griggs and coauthors222 developed another guidewire dilating forceps (Portex, Kent, England) with a smooth rounded tip (modified Howard-Kelly forceps with blunt edge). The procedure is characterized by a similar approach as the Ciaglia technique with transillumination (diaphanoscopy), puncturing of the trachea (18-gauge needle), guidewire insertion, and skin incision (Fig. 46-15). All neck structures are slightly dilated by one small dilator. Next, the specially designed forceps is inserted through the guidewire until the trachea is reached, and the skin and pretracheal tissues are dilated by opening the blades. The forceps is carefully advanced into the trachea under fiberoptic control, with the tip of the forceps pointing toward the carina. By pulling both branches of the forceps apart, the trachea is also dilated. According to the Ciaglia technique, the tracheal cannula chosen is then inserted by means of an adequate dilator over the guidewire.
c Translaryngeal Tracheostomy According to Fantoni
The translaryngeal approach for tracheostomy (TLT) was introduced in 1996 by Fantoni and coworkers.223,224 In contrast to all other percutaneous techniques, the initial puncture of the trachea is directed cranially (Fig. 46-16). After tracheal puncture, a guidewire is passed through the needle and passed toward the oropharynx alongside or inside the tracheal tube. The patient is then reintubated with the kit’s 5-mm-ID tube, and the wire is connected with the tracheal cannula. The cannula is then advanced through the pharynx into the trachea by constantly pulling on the guidewire. The pointed head is then pulled out the anterior tracheal wall and soft tissues of the neck. Next, the pointed head is cut off, and the cannula is to be rotated 180 degrees downward using a plastic obturator. Thereafter, the fiberoptic scope is inserted through the cannula to check correct positioning in the trachea.
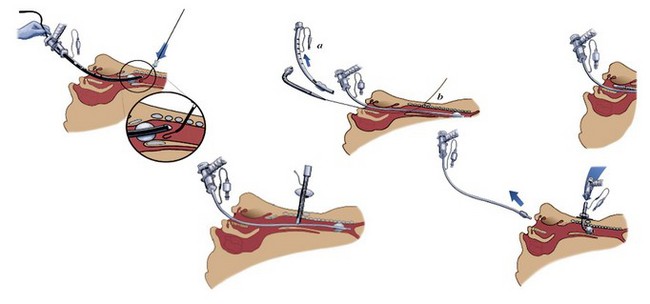
Figure 46-16 Percutaneous tracheostomy according to Fantoni.
(Courtesy of Rüsch, Vienna.) (Modified from Fantoni A, Ripamonti D: A non-derivative, non-surgical tracheostomy: the translaryngeal method. Intensive Care Med 23:386–392, 1997.)
Byhahn and coworkers225 investigated practicability and early complications of Fantoni’s TLT technique in 47 ICU patients. No severe complications (aspiration or bleeding) were observed; only oxygenation deteriorated in about one fourth of the patients. The authors concluded that TLT is a valuable alternative technique to Ciaglia’s approach, but should be performed only in patients requiring a fraction of inspired oxygen concentration (FIO2) of less than 0.8. Westphal and associates226 compared TLT with the Ciaglia approach in a prospective study of 90 patients. Although success and complication rates were similar between the groups, problems during the procedure were far more common with the TLT technique, especially in positioning the guidewire correctly. Cantais and others227 prospectively compared forceps-dilational and translaryngeal technique in 100 adult critically ill patients. Fantoni’s translaryngeal technique took nearly twice as long as forceps-dilation (13 vs. 7 minutes, respectively). Although minor bleeding episodes were less in the Fantoni group, major complications were more common, and unsolvable technical problems were encountered in 23% of patients. Nevertheless, the authors concluded that both techniques are safe, and physicians should be able to choose their preferred technique.
d Rotating dilatational tracheostomy (PercuTwist)
The PercuTwist technique (Teleflexmedical Ruesch, Kernen, Germany) offers an alternative real single-step percutaneous tracheostomy approach (Figs. 46-17 and 46-18). Preparation, puncturing of the trachea, and guidewire insertion are performed according to the Ciaglia and Griggs techniques (Fig. 46-17, 1 and 2). After needle removal, an 8-mm to 10-mm skin incision is made (Fig. 46-17, 3), and a special hydrophilic dilation screw (suited for either 8 or 9 mm ID tracheal cannulas) is slid over the guidewire until the skin incision is reached (Fig. 46-17, 4). Using slight pressure, the dilator is turned clockwise until the first threads are advanced into the pretracheal soft tissues (Fig. 46-17, 5). The PercuTwist is then carefully advanced without pushing until the tip of the screw is fiberoptically visualized inside the trachea (Fig. 46-19). The screw is then advanced into the trachea twist by twist under gentle elevation of the anterior tracheal wall. The dilator is removed by counterclockwise rotation, and the tracheal cannula is inserted using an insertion dilator guided by the in situ guidewire (Fig. 46-17, 6 and 7).
Figure 46-19 Fiberoptic control of the PercuTwist technique.
(Courtesy of Teleflexmedical Ruesch, Vienna.)
Frova and Quintel228 reported the use of the PercuTwist system in 50 consecutive ICU patients and found a 100% success rate without serious complications. Tracheal ring fractures were observed in 4 of 50 patients (8%), but no posterior tracheal wall injury. Westphal and coworkers229 studied 10 consecutive patients and reported no early complications besides minor bleeding (<20 mL) in two patients. In a prospective randomized study in 70 consecutive patients, Byhahn and associates230 compared safety and efficacy of PercuTwist and Blue Rhino tracheostomy. In the PercuTwist group, complications occurred in 12 patients, including posterior wall tears, tracheoesophageal fistula, false cannula passage, and four failures to insert the cannula, compared with seven complications in the Blue Rhino group. However, no statistically significant differences in minor and overall complications were observed between the two techniques. This major complication rate of 33% in the PercuTwist group was in vast contrast with the results of Sengupta and coauthors,231 who observed about 90 patients over 20 months of routine use of PercuTwist. All procedures performed were graded as having absent or minimal bleeding, although six patients had abnormal clotting profiles. Grundling and colleagues232 also reported no severe complications in 54 patients of a prospective observational clinical study. A rare complication of tracheostomy is tracheal ring fracture, which was also described with the PercuTwist technique.233
Yurtseven and others234 reported on 130 patients undergoing tracheostomy using three different techniques. The main finding was that PercuTwist (n = 45) was associated with minimal complications (two patients with longitudinal tracheal abrasion) and appears to be an easy to perform and practical alternative to standard PDT (n = 44, four longitudinal abrasions, one posterior tracheal wall injury, and one tracheal ring rupture) and the guidewire dilating forceps (GWDF) group (n = 41; two cases of longitudinal tracheal abrasions, one posterior tracheal wall injury). Furthermore, operating times using PercuTwist were significantly shorter than in the other groups (9.9 ±1.1, 6.2 ±1.4, and 5.4 ±1.2 minutes in PDT, GWDF, and PercuTwist groups, respectively).
Imperiale and associates235 reported on intracranial pressure changes during tracheostomy using PercuTwist. Use of PercuTwist did not cause secondary intracranial hypertension insult and therefore could also be considered safe in a select population of brain-injured patients.
e Ciaglia Blue Rhino
The technique of PDT has constantly been evolving. In 1998 a modification was introduced called Ciaglia Blue Rhino (Percutaneous Tracheostomy Introducer Kit, Cook Critical Care). The standard multiple dilators were replaced by a single, sharply tapered dilator shaped similar to the horn of a rhino (Fig. 46-20). The dilation process can be performed in a single step, without the need to change dilators (Fig. 46-21). Therefore, the risk of injury to the posterior tracheal wall, bleeding episodes, and impaired oxygenation by repeated airway obstruction during the dilation process may be reduced.236
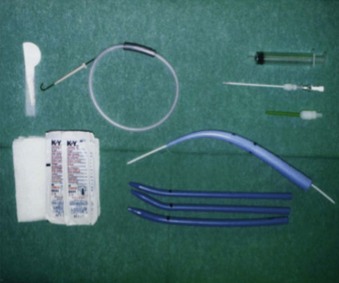
Figure 46-20 Ciaglia Blue Rhino dilator set for percutaneous tracheostomy.
(Courtesy of Rüsch, Vienna.)
The Percutaneous Tracheostomy Introducer Set consists of a puncture needle, guidewire, small dilator and special Blue Rhino dilator, and three curved stylets for placement of the tracheostomy tube. Instructions are as follows237:
1. Withdraw the ETT under fiberoptic control to a level above the assumed puncture site.
2. Make a horizontal skin incision and puncture the trachea in the midline between the second and third tracheal cartilage rings.
3. Introduce the guidewire using the Seldinger technique.
4. Withdraw the needle, and introduce the guiding catheter.
5. Predilate the puncture canal with the small dilator.
6. To increase the dilator’s external smoothness, moisten the Blue Rhino dilator with a few milliliters of saline solution or distilled water.
7. Advance the wet dilator over the guidewire and guiding catheter through the soft tissues and into the trachea up to its marking of 38-F external diameter. The dilation should require a minimum of force because of the dilator’s smoothness (Fig. 46-21A and B).
8. Ensure the Blue Rhino PDT set contains three hard-rubber stylets of different sizes with cone-shaped tips. The tracheostomy tube should be armed with its corresponding stylet.
9. Advance this perfectly fitting unit into the trachea (Fig. 46-21C).
10. Afterward, withdraw the stylet and confirm the correct position of the tracheostomy tube using fiberoptic bronchoscopy.
11. Connect the tube with the respirator, and remove the ETT.
In a small, prospective, randomized study in 2000, Byhahn and others237 compared the classic PDT with the Blue Rhino in 50 critically ill patients and reported a relatively high incidence of cartilage damage (9 of 25 cases), but no life-threatening complications with either method. In 2002, Fikkers and associates238 assessed perioperative, early, and late complications in 100 consecutive Blue Rhino tracheostomies. Success rate was 98%, with minor and major complications observed in 30% and 12%, respectively. Tracheal stenosis was observed in one patient (over 6-12 months). In 2003, Dongelmans and others239 showed that the use of Blue Rhino in an ICU environment is associated with a very low rate of major and minor complications.240 In a retrospective study of 318 tracheostomies, Bhatti and associates240 reported a low complication rate similar to open surgical tracheostomy, concluding that the complication rate of PDT can be reduced further with experience and use of strict protocols, making it even safer than an open ST.
In 2005, Kost and colleagues241 evaluated 500 adults; 309 underwent PDT using Ciaglia Blue Rhino (single dilator), and 191 used the Ciaglia Percutaneous Tracheostomy Introducer Kit (multiple dilator). Total complication rate was 9.2% (6.5% in Blue Rhino and 13.6% in multidilator group). The most frequent intraoperative complications were brief oxygen desaturation (14 patients) and bleeding (12 patients). This study also identified ASA class IV patients as having a significantly increased risk of complications. Johnson242 and Ambesh243 and coworkers showed that the single-dilator technique decreased operative time compared with the serial-dilator technique. In both studies, complication rates were not increased by single-step dilation. In 2007, De Leyn and colleagues244 performed a systematic review and recommended the modified Blue Rhino as the technique of choice, based on its simplicity and short procedure time (level 2c). In 2010, Dempsey and colleagues245 reported a prospective evaluation of the single-tapered-dilator technique, with tracheostomies performed in 589 patients (2003-2009). PDT was attempted in 576 patients and successfully completed in 572. Four attempts were stopped due to bleeding. In 149 patients (26%) intraoperative technical difficulties were encountered. Early complications occurred in 16 patients (3%). Significant late complications occurred in four patients; in two patients, a trachea-innominate fistula was found and led to the death of both patients. The mortality directly attributable to PDT was 0.35%.
Based on clinical studies and personal experience, the single-step PDT currently is the most widely used technique.245–248
f Ciaglia Blue Dolphin
In 2009, Gromann and associates249,250 studied a modified PDT called Blue Dolphin, a balloon dilation technique that exerts mainly radial force to widen the tracheostoma. This force may reduce complications such as fracture of tracheal cartilage rings or injuries to the posterior tracheal wall (Fig. 46-22).
Surgical Procedure
1. Ensure optimal placement of patient with full extension of head and neck.
2. Perform surgical disinfection and sterile covering of the operative area.
3. Manually examine the cricoid cartilage.
4. Make a 1-cm to 2-cm, horizontal surgical incision.
5. Puncture the trachea at the level between the second and third tracheal cartilages.
6. Ensure puncture of the trachea using bronchoscopy.
7. Place the guidewire using the Seldinger technique.
8. Enlarge the puncture canal with the blue, short introducing dilators.
9. Attach the prepared inflation device to the balloon port on the balloon-catheter assembly.
10. Remove the introducing dilators; the guidewire remains in situ.
In the primary study by Gromann,250 20 patients underwent this procedure at the ICU bedside, and surgery averaged 3.3 ±1.9 minutes. There were no injuries of the posterior tracheal wall, no bleeding requiring treatment, or wound infections. One patient had a fracture of a tracheal ring, and another patient had subcutaneous emphysema.
In 2010, Cianchi and others251 compared this new Blue Dolphin system with the Ciaglia Blue Rhino system in 70 ICU patients. Surgical procedure time in the Blue Rhino group was significantly shorter than in the Blue Dolphin group (1.5 vs. 4 minutes). Limited intratracheal bleeding requiring no intervention was more frequent in the Dolphin group. Although Blue Rhino was associated with fewer tracheal injuries and time for procedure was shorter, the authors concluded that PDT using the Ciaglia Blue Dolphin technique is a feasible and viable option in ICU patients.
D Airway Management During Tracheostomy
During puncture of the trachea, the endotracheal tube must be withdrawn just below the vocal cords to protect the bronchoscope and tube cuff. Therefore, alternative approaches such as ventilatory support using a laryngeal mask airway or a Fastrach have been suggested.252–254 In an RCT, Dosemeci and colleagues255 studied LMA as an alternative airway for management during PDT in 60 patients, concluding that LMA is an effective and successful ventilatory device. It improves visualization of the trachea and larynx during fiberoptic-assisted PDT and prevents the difficulties associated with use of the ETT, such as cuff puncture, tube transection by the needle, and accidental extubation. However, Ambesh and associates256 clearly demonstrated that changing ETT to LMA might result in potentially life-threatening complications, including loss of the airway during PDT. In a prospective randomized study, they compared the safety and efficacy of ETT versus LMA ventilation during PDT in 60 critically ill patients. Although the complication rate was low in the ETT group (7% ETT impalement, 7% cuff puncture, and 3% accidental extubation), a high rate of complications was observed in the LMA group (33% incidence of airway loss, inadequate ventilation, and gastric distention). A change of the ETT for an LMA during the performance of tracheostomy therefore cannot be recommended and may only be an alternative method for patients not intubated before the procedure.
E Perioperative and Early Complications of Tracheostomy
A number of potential complications may occur during or immediately after PDT (Box 46-5). Bleeding is the most common perioperative complication, with incidence up to 4%.257 After controversial statements, routine chest radiography for identifying complications at an early stage is not longer recommended after tracheostomy placement, unless there are signs of unexpected compromise of air exchange.258,259 Ambesh and associates243 compared two percutaneous single-dilator techniques (Blue Rhino, Griggs dilating forceps) in 60 consecutive patients. Percutaneous access to the trachea was possible in all patients, with significantly more difficult cannulations in the Griggs group (30% vs. 7%). Overdilatation of the stoma occurred more frequently in the Griggs group (23% vs. 0%), whereas Blue Rhino was often associated with tracheal ring fractures (30% vs. 0%). Some variation in complications is seen with different techniques for PDT. The single-dilator technique (Blue Rhino) appears to be fast and incurs no more risk than the multiple-dilator technique of Ciaglia.238,242
F Late Complications of Tracheostomy
Many mechanisms may cause late complications after tracheostomy.46 The most important factors may be placement of the tube, especially prolonged intubations; leaving the tube in place for prolonged periods, possibly related to inflated cuff; and abnormal healing at the site of injured tracheal mucosa. Clinically relevant late complications after tracheostomy occur in up to 65% of patients.260–263 The most common and important complication is development of granulation tissue. Granulation tissue occurs subclinically or presents as failure to wean patients from ventilator, failure to decannulate, or as upper airway obstruction with respiratory failure after decannulation.264 Further late complications include tracheal stenosis, aspiration pneumonia, tracheomalacia, and tracheoesophageal fistula265 (Table 46-10).
TABLE 46-10 Long-Term Complications of Surgical Tracheostomy (ST) vs. Percutaneous Dilatational Tracheostomy (PDT) in Critically Ill Patients
After its introduction by Ciaglia in 1985,200 many investigators have reported their experience with the technique and have concluded that PDT is safe and cost-effective. Vigliaroli and others266 reported their clinical experience with Ciaglia’s dilational tracheostomy over 6 years in 304 patients, 41 of whom were evaluated for late complications. No perioperative death occurred, and early complication rate was 5%. No late complications (e.g., tracheal stenosis) were observed in any of the fiberoptically investigated patients during follow-up to 180 days.
Escarment and associates267 evaluated the safety and complication rates of the Griggs technique in a consecutive series of 162 ICU patients. Early intraoperative complications occurred in 17%, being minor technical difficulties without morbidity (e.g., inadvertent extubation, difficult cannulation, hemorrhage). Average cannulation duration was 25 days, and stoma closure was effective in all patients after about 3 to 4 days. Follow-up was available in 81 patients, and endoscopic evaluation of the airways 3 months after decannulation was performed in 73 patients; 62 (85%) were normal, seven (10%) had granulation tissue at the stoma site, and four (5%) had tracheal stenosis, with dyspnea in two patients (3%).
Norwood and colleagues268 determined the incidence of tracheal stenosis and other late complications after PDT in 422 patients undergoing tracheostomy between 1992 and 1999 (Ciaglia approach). Of the 340 survivors, 100 were interviewed and further evaluated by fiberoptic laryngotracheoscopy and tracheal computed tomography. CT scans identified mild stenosis (11%-25%) in 21% of patients (all asymptomatic), moderate stenosis (26%-50%) in 8%, and severe stenosis (>50%) in 2% of patients (Fig. 46-23; see also Fig. 46-11A).
Hotchkiss and coworkers269 performed a cadaver study to evaluate the stoma and surrounding insertion site for laryngotracheal injury in six fixed cadaver specimens. Puncture and dilation were performed using the Blue Rhino technique, and no fiberoptic control of the procedure was used. Puncture level was accurately predicted in only 50% of cadavers. Anterior tracheal internal wall mucosal injury was observed in all specimens. Cartilaginous injury was severe in five of six specimens that sustained multiple comminuted injuries to two or more adjacent tracheal rings. The authors concluded that the injuries observed may significantly contribute to the development of tracheal stenosis. However, no data are presented on the potential impact of bronchoscopic guidance on the incidence of these complications.
Leonard and others270 assessed the late outcome following forceps tracheostomy in a prospective observational study of 49 patients. Tracheal stenosis was observed in one patient (2%), whereas other, minor complications (e.g., change in voice, 49%) were observed frequently. However, whether this was caused by the preceding translaryngeal intubation or the technique of tracheostomy could not be determined.
The main problem of PDT leading to tracheal stenosis is that tracheal rings are forced into a position that creates the opening for the cannula. This maneuver is capable of deforming the tracheal cartilage so that it protrudes into the airway (Fig. 46-24).
Figure 46-24 Suprastomal stenosis. Anterior tracheal wall is invaginated into the lumen.
(From Koitschev A, et al: Tracheal stenosis and obliteration above the tracheostoma after percutaneous dilational tracheostomy. Crit Care Med 31:1574–1576, 2003.)
Koitschev and associates271 reported on three patients with severe tracheal stenosis/obliteration after PDT (Figs. 46-25 and 46-26). In all patients, tracheal narrowing occurred above the level of the stoma. The authors speculated the cause was a procedure-related mechanism (tracheal ring invagination and consecutive development of granulation tissue) rather than a mechanism based on the duration of cannulation (normally producing stenosis below the stoma).
Figure 46-25 Endotracheal view revealing invagination of the cartilage rings.
(From Koitschev A, et al: Tracheal stenosis and obliteration above the tracheostoma after percutaneous dilational tracheostomy. Crit Care Med 31:1574–1576, 2003.)
Figure 46-26 Anatomic specimen of resected trachea: front (A) and back (B) views. Arrows indicate scar tissue above the stoma.
(From Koitschev A, et al: Tracheal stenosis and obliteration above the tracheostoma after percutaneous dilational tracheostomy. Crit Care Med 31:1574–1576, 2003.)
In conclusion, PDT seems to be a safe, relatively simple, and quick procedure that can performed at bedside. Early complications and infection rate (~2%) might be even lower and cosmetic results better than with surgical tracheostomy. However, PDT must be performed by an experienced operator at the correct puncture site, straight in the midline between two tracheal rings, and may never be performed without fiberoptic guidance.272 All special techniques proposed are characterized by special drawbacks or complications. The Blue Rhino technique is associated with a relatively high incidence of tracheal ring fractures (~30%), whereas the PercuTwist approach has a higher risk of posterior tracheal wall injury. It is therefore difficult to compare “percutaneous dilatational tracheostomy” to the conventional surgical approach, since these are completely different techniques with different success and complication rates. Much larger studies over a longer time studying clearly defined patients and techniques are necessary to clarify the incidence of late complications after PDT. Currently, it is too early to draw definitive conclusions on PDT versus surgical tracheostomy.
G Conventional (Surgical) vs. Percutaneous Tracheostomy
Comparing PDT with historical data of complications of ST is erroneous and may give a biased picture. Moreover, varying definitions and different techniques used by the authors make interpretation of data difficult. Complication rates for ST vary widely, between 6% and 66%, with mortality of 2%.273 To reduce this incidence of complications and to perform a bedside procedure, the different percutaneous techniques have been proposed.
Special reference to early complications shows that serious bleeding can occur with either ST or PDT, but appears to be less frequent with PDT.274 Considerable danger arises in the significant rate of posterior tracheal wall perforation with development of tension pneumothoraces when using PDT.275 To avoid posterior tracheal wall injury, guidewire and guiding catheter should be firmly stabilized during PDT, and fiberoptic visualization of the entire process is obligatory. In common, pneumothorax is infrequent when using PDT but occurs in 1% to 3% when using ST.276
Numerous studies suggest minimal difference in acute serious complications between ST and PDT,277 while others propose a learning curve and a significantly lower complications rate thereafter. Petros257 clearly showed a learning curve in performing tracheostomy after prospectively evaluating 234 PDTs (Fig. 46-27).
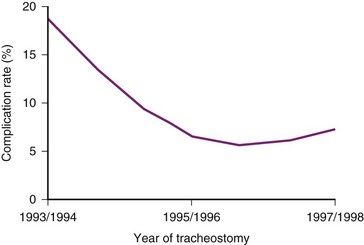
Figure 46-27 Learning curve for perioperative complications during percutaneous dilatational tracheostomy.
(From Petros S: Percutaneous tracheostomy. Crit Care 3:R5–R10, 1999.)
Massick and colleagues278 performed a prospective analysis of complication incidence for the first 100 PDTs (Ciaglia) performed in a local community hospital. The authors clearly demonstrated a learning curve, with the first 20 patients exhibiting a significantly higher incidence of perioperative as well as late complications. Furthermore, patients with suboptimal anatomy were found to have significantly increased complication rates independent of operator and institutional experience. PDT has a steep learning curve, with most perioperative, postoperative, and late complications occurring in the first 20 patients.
Beiderlinden and others279 assessed the incidence of early complications of fiberoptically guided PDT beyond the learning curve (all investigators performed >100 PDTs). With 133 consecutive patients undergoing PDT (114 conventional Ciaglia technique and 22 Blue Rhino), tubes were fixed at the skin, and no routine tube exchanges were performed. Insertion of the tracheal tube was easy or modestly difficult in 87%, and no procedure-related deaths occurred. The incidence of tube-related complications (e.g., tracheal wall lesion, bleeding, cannula misplacement) was low, 0.7%. Fracture of tracheal rings was observed in 24%. Despite this finding, the authors concluded that with experience in performing PDT, fixation of the tracheal cannula, and omission of routine tube changes, the complication rate is low.
Several studies compared ST and PDT technique, in addition to multiple reviews and four meta-analyses.265,280–282 At present, no consensus exists as the optimal approach in terms of minimizing complications.
Dulguerov and associates280 performed a meta-analysis comparing surgical and percutaneous access to the trachea. Study patients were grouped as having surgical tracheostomy from 1960 to 1984 (17 investigations in 4185 patients), surgical tracheostomy from 1985 to 1996 (21 studies in 3512 patients), or PDT (27 studies in 1817 patients). The highest perioperative and postoperative complication rates were observed in the “older” surgical studies (9% and 33%, respectively). Although perioperative complication rates were higher in the percutaneous studies (10% vs. 3%), postoperative complications were lower compared with the surgical approach (7% vs. 10%). However, this meta-analysis was criticized because studies on percutaneous tracheostomy without fiberoptic control were also included. Furthermore, it is difficult to perform a meta-analysis on this topic because not only percutaneous versus surgical approaches were compared. The percutaneous group is a mix of several different procedures and techniques used (i.e., with or without fiberoptic control, Ciaglia or Griggs technique).
Subsequently, Delaney and coauthors265 performed a systematic review and meta-analysis, including 17 RCTs involving 1212 patients. Pooled analysis showed no statistical difference in mortality or major complications. The rate of wound and stoma infections was low, 6.6%, but infections were significantly less common in the PDT group. Unfortunately, studies rarely include long-term follow-up, and significant late complications may be missed.
In a meta-analysis, Freeman and associates281 examined five studies (236 patients) and found that PDT was associated with a lower overall postoperative complication rate, less perioperative bleeding, and lower incidence of stomal bleeding and stomal infection. In addition, PDT was quicker than ST by 9.84 minutes. The meta-analysis of Higgins and Punthakee282 included 973 patients from 15 RCTs (490 PDTs and 483 STs). The author found significantly fewer complications in PDT with respect to wound infection and unfavorable scarring. Consequently, overall complications trended toward favoring the percutaneous technique. PDT appeared to be more cost-effective by freeing up OR resources, including time and personnel; providing greater feasibility in terms of bedside capability; and allowing nonsurgeons to perform the procedure safely282,283 (Tables 46-11 and 46-12). Most authors reported lower complication rates with the individual percutaneous technique used272,284 (Table 46-11). Tracheostomy is increasingly performed outside the OR and by PDT rather than ST technique.285
TABLE 46-11 Comparison of Overall Complications for Open vs. Percutaneous Tracheostomy, Including Adjusted Values for Minor Hemorrhage
From Higgins KM, Punthakee X: Meta-analysis comparison of open versus percutaneous tracheostomy. Laryngoscope 117:447–454, 2007.
OR, Odds ratio; CI, confidence interval.
TABLE 46-12 Mortality and Complication Rates for Surgical Tracheostomy (Open) and Percutaneous Dilatational Tracheostomy (PDT)
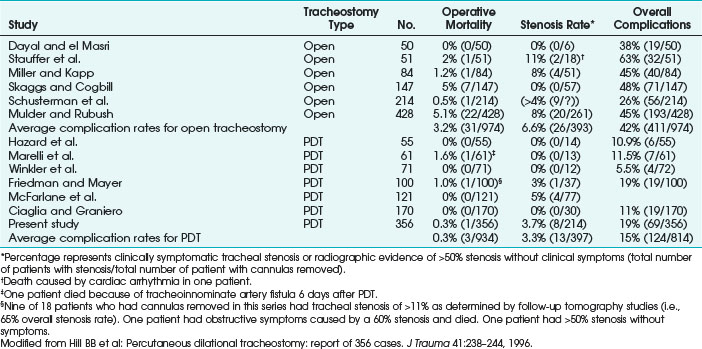
Bowen and colleagues286 performed a retrospective medical chart review. Over 23 months, 213 patients received ST (n = 74) or PDT (n = 139). Perioperative complications occurred in five of 74 patients (6.76%) during PDT (whereas three patients, 4.1%, required emergent operative exploration of the neck) versus three of 139 patients (2.2%) during ST. Surgical time for PDT in the ICU was significantly shorter and cost less than ST performed in the OR.
In conclusion, all studies clearly show that adequate training and teaching is the major factor reducing complications with PDT. Until those issues are cleared, PDT can be recommended with some exceptions. Especially in patients presenting with a short thick neck, goiter, or difficult airway, PDT should be performed only by an experienced physician, with a physician prepared for ST present.287 The most important part is fiberoptic control of the entire procedure, since erroneous punctures or direct trauma of the esophagus or tracheal wall are prevented or recognized early.288