Chapter 44 Airway Management in Burn Patients
I Introduction
The National Burn Repository reports 181,000 hospital admissions for burn injury for 1998 through 2007 in 73 U.S. burn centers. The overall mortality rate during this period was 4.9%.1 A 2006 report of the National Burn Repository indicated an incidence of inhalational trauma of 5.7% during the previous 10 years.2 Inhalational burn trauma was associated with a 27.3% mortality rate, compared with a rate of 4.5% for burned patients without inhalational injury.2 A review of 850 children admitted with inhalational injury during a 10-year period at the four Shriner’s Pediatric Burn Centers in the United States found a mortality rate of 16.4%.3 Consequently, inhalational burns contribute significantly to mortality among the burn population. Although the outcomes for survival after burns and the quality of life of burn victims have improved, respiratory complications are an ongoing source of burn morbidity and mortality.
II Airway Management in the Acutely Burned Patient
A Evaluation of the Patient after Acute Burn Injury and Indication for Airway Management
1 Assessment at the Scene
First responders have a crucial role in the early management of burn patients. In addition to the usual trauma assessments, emergency medical service (EMS) staff must determine whether the patient’s condition warrants immediate intubation in the field or the patient can be observed. Indications for intubation at the trauma scene include the following4,5:
• Unconsciousness and altered mental status (with incumbent aspiration risk)
• Respiratory distress (e.g., desaturation with supplemental oxygen [O2], tachypnea)
• Hoarseness, stridor, dysphagia, or drooling
• Burn injury to the neck and face after fire or smoke exposure in a closed space or carbonated sputum
• Prolonged transport to the hospital of the patient with possible airway injury
The practice of prehospital intubation of the burned patient has been questioned. Eastman and colleagues6 reviewed the charts of 1272 patients admitted after field intubation over a period of 23 years and found that 69% of them survived. However, 30% of the survivors were extubated on admission or on the second day after the burn. None required reintubation. Klein and associates7 reviewed the charts of patients admitted to the Washington Burn Center after a transport of more than 90 miles for the period of 2000 through 2003. They examined parameters such as duration of transport, error in burn severity estimation, fluid management, appropriateness of intubation, and transport complications. Of 1877 patients, 424 were transported more than 90 miles to the burn center. No patient died during transport, and 111 patients arrived intubated, with only 61% having inhalational burns. More than 50% of patients were extubated within the first 24 hours after admission.
2 Assessment in the Hospital
Roughly 10% of burn patients also present with other traumatic injuries. All burn patients are considered trauma patients and consequently undergo a primary trauma survey (i.e., the ABCDE algorithm: airway, breathing, circulation, disability, and exposure).8 The extent and surface area of the burn are noted, other traumatic injuries identified and treated, and the airway secured if indicated. The incidence of inhalational trauma, length of hospital stay, and mortality rate are increased for patients presenting with burns and other traumatic nonburn injuries.9
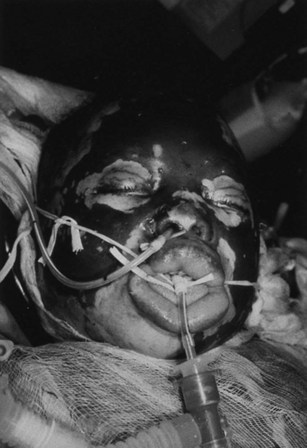
On arrival of the patient at the burn center, the tube position is confirmed with carbon dioxide (CO2) monitoring and auscultation of the lung fields. Uncertainty about endotracheal tube (ETT) placement should be remedied by direct laryngoscopy or fiberoptic bronchoscopy. The mouth, pharynx, and larynx are examined by laryngoscopy to assess edema and identify any burned mucosa and the presence of soot (Fig. 44-1). A radiograph is obtained to verify the ETT position and identify other potential injuries, such as a pneumothorax. Lung parenchymal injuries usually are not immediately detectable by radiography immediately after injury. Patients presenting with a supralaryngeal airway (SLA) on arrival at the hospital require endotracheal intubation or a surgical airway.
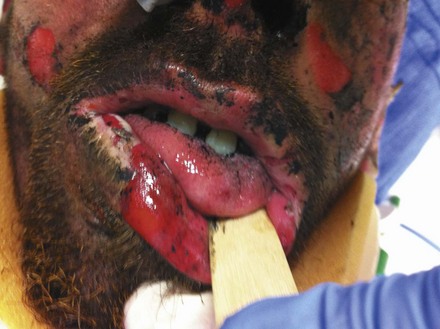
Figure 44-1 Carbonaceous sputum, singed nasal hairs, and facial burns indicate possible upper airway thermal injury.
In the unintubated patient, the presence of soot in the sputum, dyspnea, tachypnea, hoarseness, and stridor are signs of impending airway obstruction. Fiberoptic endoscopy is the gold standard for the diagnosis of inhalational trauma.10–17 In the awake patient, a nasal fiberoptic examination under local anesthesia can be performed to evaluate the larynx and confirm the presence or absence of edema and soot. Patients with altered mental status, dyspnea, hoarseness, or stridor require immediate intubation.
A relevant history for airway management after burn injury includes the following information:
3 Altered Mental Status
An isolated burn injury usually does not produce a mental status change early in the disease course. A disoriented, stuporous, or unconscious burn patient should be closely examined for additional trauma. Burn-related reasons for altered mental status include inhalation-induced hypoxemia, CO poisoning, CN toxicity, and electrical injury. Other sources of altered mental status include head trauma, alcohol or drug ingestion, metabolic disorders, seizures, and psychosis. Patients with altered mental status should be intubated and ventilated with 100% O2 until CO poisoning and CN toxicity can be ruled out. Intubation should be performed using maneuvers to stabilize the cervical spine (C-spine) if neck injury has not been ruled out.10,12,17,20,21
4 Cardiovascular Abnormalities
Cardiovascular instability, dysrhythmia, and cardiac arrest can be consequences of CO intoxication. Cardiac disturbances can also result from innate cardiac disease combined with the response to traumatic injury. Hypotension is often caused by fluid loss associated with burns. Hemorrhagic and neurogenic shock can also lead to hypotension in these patients as a consequence of nonburn injuries. The airway should be secured in hemodynamically unstable patients.10,17,22
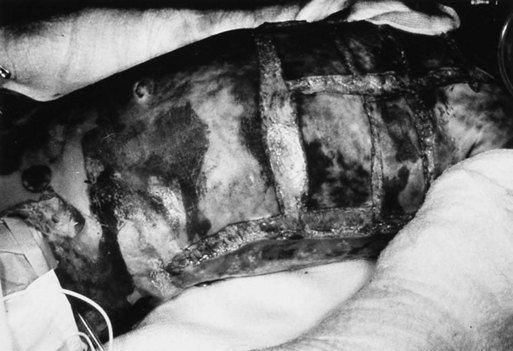
5 Neck and Face Burns
Extensive burns of the face and neck produce facial edema, making direct laryngoscopy difficult or impossible. These patients may have pharyngeal and laryngeal edema, further complicating intubation (Fig. 44-2). Delay in securing the airway can lead to a “cannot intubate, cannot ventilate” situation. Surgical airways must be performed in this instance.10,17,21,23
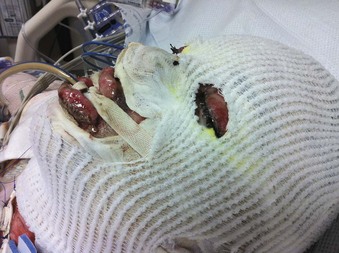
6 Extensive Burns
Patients with large body surface area burns frequently have airway burns as well (Fig. 44-3). Edema after resuscitative efforts can make intubation impossible. After massive burns, patients develop a hypermetabolic state leading to increased CO2 production requiring ventilator support. Some physicians suggest prophylactic intubation for total body surface area burns greater than 30%.10,12,17,22
B Inhalational Injury
Approximately 20% to 30% of patients admitted to regional burn centers have some degree of inhalational injury and are at risk from toxic gases.19,24 Edelman and colleagues25 reviewed 829 patients admitted to a burn center between 2000 and 2004 and found that 28% had an inhalational injury. Although the mortality rate for patients with solely a thermal injury was 3% and was 12% for those with an isolated inhalational injury, patients with combined thermal and inhalational injuries had a mortality rate of 14.6%.25 Box 44-1 summarizes smoke inhalational injuries.
Box 44-1 Smoke Inhalational Injuries
• Inhalation injury occurs in 20% to 30% of burn patients.
• Inhalation injury increases burn mortality.
• The leading cause of immediate death from burns is inhalational injury, not the burn itself.
• Heat injury is confined above the vocal cords.
• Inhalation damage is caused by toxic chemicals and thermal injury.
• Sequelae of inhalational burns include pulmonary edema, 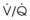 mismatch, atelectasis, airway obstruction, and pneumonia.
mismatch, atelectasis, airway obstruction, and pneumonia.
• Carbon monoxide (CO) and cyanide (CN) systemic toxicities should be suspected with burns in confined areas.
1 Injury to the Respiratory Tract
a Upper and Lower Airway Inhalation Smoke Injury
Thermal injury and inhaled chemical toxins cause burn injuries by different mechanisms. In an enclosed environment, temperatures can exceed 800° C, with an O2 concentration of just 10% and CO concentrations greater than 0.5%.26,27 Injuries are often described by the area of the tracheobronchial tree affected. The upper airway lies above the vocal cords, whereas the lower airway consists of the tracheobronchial tree, including the terminal bronchi and alveoli.
The lower airway includes the tracheobronchial system and the lung parenchyma. Most injuries of the upper and lower airway result from the chemical toxins produced by combustion. Burned rubber and plastics release ammonia, chloride, sulfur, and nitrogen dioxides. Cotton and wool fires produce toxic aldehydes, including formaldehyde. Laminated structures produce cyanide.16,21,22,28–30
b Pathophysiology of Smoke Inhalational Injury
NO formed from arginine by nitrous oxide synthetase (NOS) has an important role in pulmonary changes after smoke injury. Smoke inhalation stimulates vasomotor and sensory nerve endings in the tracheobronchial tract, releasing neuropeptides with bronchoconstricting properties. Neutrophil activation produces reactive oxygen species (ROS), increasing the activity of NOS.31
Increased NO in the lung reduces hypoxic pulmonary vasoconstriction, leading to ventilation-perfusion (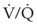 )) mismatch and edema formation. O2 and NO react to form peroxynitrite. Peroxynitrite causes cellular injury and damage to cell membranes that results in increased pulmonary vascular permeability and pulmonary edema. Peroxynitrite, other reactive O2 species, and reactive nitrogen (N2) species damage DNA. In an effort to repair damaged DNA, poly(ADP-ribose) polymerase (PARP) is activated. PARP uses ATP energy reserves to repair damaged DNA. Depletion of ATP by PARP activity results in cell death.32–35
)) mismatch and edema formation. O2 and NO react to form peroxynitrite. Peroxynitrite causes cellular injury and damage to cell membranes that results in increased pulmonary vascular permeability and pulmonary edema. Peroxynitrite, other reactive O2 species, and reactive nitrogen (N2) species damage DNA. In an effort to repair damaged DNA, poly(ADP-ribose) polymerase (PARP) is activated. PARP uses ATP energy reserves to repair damaged DNA. Depletion of ATP by PARP activity results in cell death.32–35
The coagulation cascade is activated, leading to fibrin deposition in the airway. Airway casts are formed, often obstructing the airway and impairing gas exchange in the lungs. Inhibition of surfactant leads to alveolar collapse, atelectasis, and 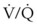 mismatch.11,16,21,28,34,36
mismatch.11,16,21,28,34,36
c Symptoms
Absence of facial burns does not rule out an airway injury. However, carbonated sputum does not necessarily indicate injury below the vocal cords, because it can be drained from the nasal cavity.10,12,13,16,17,21,23
d Diagnosis
The gold standard for the diagnosis of an inhalational injury is direct or fiberoptic inspection of the larynx and tracheobronchial tree. Initial evaluation should document the presence and extent of injury to establish a baseline of the airway injury. Recurrent examinations are needed to evaluate the course of injury and to remove necrotic tissue to prevent airway obstruction and atelectasis.10–17
Flow-volume curves are difficult to obtain and depend on the patient’s effort, making them less useful for discerning airway obstruction and compromise in the acute burn patient.10,16 The arterial blood gas determination may not demonstrate any abnormalities during the early stage of airway injury,10,16 and the radiograph obtained at presentation may not reveal any pathologic changes caused by the burn. Previous changes and nonburn injuries, however, are evident. Radioisotope evaluation of the lungs can demonstrate injury to the lung parenchyma and small airways. After intravenous injection of xenon 133 or inhalation of technetium 99, the healthy lung can rapidly clear the isotopes, but injured parenchyma clears isotopes in a delayed and uneven manner. This type of testing is not practical in the acutely injured patient.16,17,21
2 Systemic Toxicity from Inhalational Injury
a Carbon Monoxide Poisoning
CO is a product of combustion. It is a colorless, odorless, tasteless gas. Burn victims injured in a confined space should always be evaluated for possible CO poisoning. CO has great avidity for the iron of hemoglobin and cytochrome oxidase. CO’s affinity for hemoglobin is 200 times greater than that of O2. Moreover, carboxyhemoglobin shifts the O2 dissociation curve to the left, impeding the delivery of O2 to the tissues. CO binds to cytochrome oxidase in the mitochondria, impairing O2 use on a cellular level and producing tissue hypoxia and acidosis.6,10,13,21,28
CO poisoning is the leading cause of poisoning death in the United States.37 It is often missed because the signs and symptoms are nonspecific, including fatigue, headache, weakness, dizziness, confusion, and loss of consciousness.10,38 A history, examination, and laboratory studies are required to make the diagnosis of CO poisoning and to plan treatment.39–43
Laboratory tests include pulse oximetry and O2 saturation, but O2 partial pressure does not reflect CO poisoning.10,13,17 Carboxyhemoglobin is measured and normally is less than 2% in nonsmokers. The carboxyhemoglobin level is slightly higher in pregnant patients due to fetal CO. The concentration of carboxyhemoglobin is chronically 3% to 8% in smokers and more than 10% to 15% after smoking.
Treatment includes 100% O2 administered to all burn victims. This level of O2 accelerates the dissociation of CO from hemoglobin by 50% every 30 minutes. Patients with CO-hemoglobin levels between 20% and 25% should be intubated. Hyperbaric O2 therapy increases the elimination of CO and can be considered for unconscious patients.11,13,22,28 No data suggest that hyperbaric O2 therapy mitigates any long-term sequelae of CO poisoning. Limited availability of these chambers and difficulties accessing the patient while in the chamber impede the use of hyperbaric therapy for acute care of burned patients.
Sequelae include delayed neurologic symptoms such as ataxia, mental degradation, and incontinence, which are seen in 12.5% of patients with high initial CO-hemoglobin levels. Computed tomography (CT) can demonstrate decreased density of the globus pallidus.10,11,13,16,17,21
b Cyanide Poisoning
CN is the product of burning plastics containing high amounts of nitrogen, such as polyurethane, polyacrylonitrile, and acrocyanate. CN causes asphyxia at the cellular level by inhibiting cytochrome oxidase and preventing mitochondrial respiration. O2 consumption is reduced through interruption of the tricarboxylic acid cycle, and anaerobic metabolism occurs with the development of a lactic acidosis.10,11,13,16,44
A history, examination, and diagnostic studies are required to determine CN poisoning and its treatment. Inhalational injury in a confined space makes CN poisoning more likely, as does an increased CO-hemoglobin concentration. Measurement of blood CN concentration can be made but may not be immediately available. Levels between 0.5 and 1 mg/L are considered toxic, and levels above 1 mg/L are thought to be lethal.13,44,45
Therapy includes amyl nitrite and sodium nitrite to induce methemoglobinemia, which binds with CN. Thiosulfate is a substrate in the metabolism of CN into less toxic thiocyanate by hepatic rhodanese. Hydroxycobalamin also binds with CN, forming nontoxic cyanocobalamin. Hydroxycobalamin has minimal side effects, whereas methemoglobinemia reduces tissue O2 delivery to patients with impaired use of O2 at the cellular level.44,46–51
C Airway Management Approaches
1 Airway Management in the Field
If intubation is difficult in the upper airway patent, an SLA (e.g., laryngeal mask airway [LMA], King LT, Easytube, Combitube) can be employed as needed. Unfortunately, none of these devices protects the airway from aspiration. Moreover, with time, airway and laryngeal edema make ventilation with an SLA progressively difficult, and when necessary, a surgical airway should be employed.30
2 Airway Management in the Hospital
Rapid-sequence intubation techniques should be employed because the patients are unlikely to have been fasted before their burn. Succinylcholine is considered safe to administer in the first 24 hours after a burn. After this 24-hour window, succinylcholine use is contraindicated due to the development of immature nicotinic receptors over the muscle membrane, which can lead to hyperkalemia after succinylcholine administration.52 These receptors occur at the neuromuscular junction and over the entire muscle membrane. Moreover, the prolonged channel opening time of these receptors contributes to the release of potassium from the myocyte. Over time, the number of receptors returns to baseline as the burn heals; however, the patient may be at risk for hyperkalemia for up to 2 years.53,54
In patients presenting with a LMA in place, fiberoptic intubation through the LMA can be performed. The Aintree intubation catheter can be placed with fiberoptic endoscopy into the trachea, the LMA removed, and an ETT passed into the trachea. Chapter 50 further explains this technique.
3 Airway Management in Burned Children
Loss of airway and aspiration is the fourth most common cause of death cited in a study of pediatric burn patients.55,56 Intubation may be indicated when the amount of resuscitative fluid is predicted to be more than 180 mL/kg.57 Intubation with a Miller blade is often successful in children because the high position of the larynx requires lifting of the epiglottis. Cuffed ETTs are preferred because they allow the delivery of higher ventilator pressures and eliminate the need for repeated laryngoscopy and exchanges of the endotracheal tube if the leak is too great.56,58
4 Tracheotomy
Tracheotomy is performed in burn patients when laryngoscopy and intubation attempts fail. Elective tracheotomy for burn patients who need prolonged periods of positive-pressure ventilator support has been associated with airway strictures and stenosis after recovery.10,13,37,59 However, most studies do not identify an increased risk of tracheotomies in burn patients compared with other populations.60–63 In a retrospective study, Namdar and colleagues64 demonstrated that tracheotomy permitted the use of lung-protective ventilation strategies in burn patients. However, Saffle and assocates65 found no benefit for early tracheotomy as far as duration of ventilator support, length of stay, infection complications, or survival. In the pediatric population, tracheotomy caused no increased risk of complications.38,66,67 The potential loss of the airway is less in children with a tracheotomy than those with an ETT in place. Some studies have found that percutaneous tracheotomy has reduced mechanical ventilation, hospital stay, and cost compared with traditional surgical tracheotomy.61,68–71
5 Securing the Artificial Airway
Securing the ETT of the burned patient can be challenging. Ideally, the ETT should be fixed without producing additional facial injuries and adjust to facial swelling. Taping usually is ineffective in securing the ETT. Suturing the tube to the gums, wiring it to a tooth, wiring it to brackets anchored to the enamel of the incisors, and circumferential fixation devices have all been employed.72 Tying the tube with sling ribbon (umbilical tape) or even use of a disposable surgical free mask can secure it to the head. Care must be taken so that the items tied about the head do not lacerate the corners of the mouth or the ears. The tape stops must be checked routinely because facial edema can expand and pull the tube out of the trachea. If a leak develops, fiberoptic laryngoscopy can be used to guide the ETT back into the trachea. The ETT’s position and taping should be checked frequently, especially during changes in patient position and after patient transport.
6 Extubation of the Burn Patient
a Extubation of the Burned Patient after 12 Hours of Intubation
Before extubation, the upper airway should be assessed for patency. Fiberoptic inspection of the larynx and airway is useful to identify potential sources of immediate extubation airway loss. The patient’s other critical illnesses also should be corrected before considering extubation. Any gastric feedings should be discontinued at least 4 hours before extubation, and the patient should be free of ileus and gastrointestinal bleeding. All burn patients should pass a CPAP trial, fulfill routine extubation criteria, and have a leak around the deflated ETT cuff.73
After extubation, speech and swallowing consultants can help to restore the voice and assist in preventing aspiration. Because airway stenosis can occur, prolonged extubation follow-up and fiberoptic evaluation is indicated.14,16,20,28,60,69,74–76 Video stroboscopic vocal cord evaluation can be used as a screening tool to decide whether further airway evaluation by an ear, nose, and throat specialist is required.
Extubation failure occurs with a higher incidence in the burn population than among other critically ill patients (30% versus 23%).77 Usually, the cause of failure is poor pulmonary toilet. Extubation failure is associated with burn size, inhalational trauma, and age of the patient.
b Extubation after Burn Surgery
Patients without airway injuries can be managed according to usual anesthesia practices. If significant edema exists in the face or airway, extubation after surgery is delayed until it subsides. If other airway difficulties occur, extubation in a controlled manner is undertaken as described earlier.13,16,78
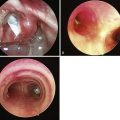
III Airway Management during the Later Stages of Burn Management
Burn patients require multiple procedures after their initial intensive care course. Close airway evaluation is required in this population. SLAs and regional anesthetics can be employed when indicated.79–81 As always, the patient’s airway history is reviewed and an examination completed. Even weeks after extubation following a prolonged intubation, a patient can develop strictures and granulomas, which can complicate airway management.71 Availability of adjuvant airway management devices should be considered when the recovering burn patient is brought to surgery during a long-term convalescence (Fig. 44-4).
V Clinical Pearls
• Additional traumatic injuries should be ruled out in every burn patient.
• Intubation in the acutely burned patient with a traumatic C-spine injury should be performed using C-spine precautions.
• A variety of devices, including video laryngoscopes and fiberoptic endoscopes, should be available for intubation of the acutely burned patient and the previously burned patients; the ASA difficult airway algorithm should be applied in treating a patient with a difficult airway.
• Direct thermal injury to the upper airway can result in a fast-developing massive edema, compromising the airway and impeding intubation.
• Inhalation smoke injury causes direct injury to the upper and lower airways and can cause systemic toxicity through toxic smoke components, such as carbon monoxide and cyanide.
• Fiberoptic endoscopy is the gold standard for the diagnosis of inhalation smoke injury.
• Airway obstruction through edema formation can rapidly develop in children because of their smaller-diameter airways.
• Meticulous endotracheal tube (ETT) fixation and adjustment is crucial in the presence of facial edema formation. Loss of airway at the peak of edema formation can easily result in a “cannot ventilate, cannot intubate” situation.
• Before extubation, pathologic changes such as scars, stenosis, and webs should be excluded in patients after direct thermal or inhalation smoke injury to the upper airway.
• Airway management in the later stages after a burn injury can be complicated by pathologic changes such as scars and webs in the airway and by scars and contractures of the face and the neck.
All references can be found online at expertconsult.com.
3 Palmieri TL, Warner P, Mlcak RP, et al. Inhalation injury in children: A 10 year experience at Shriners Hospital for Children. J Burn Care Res. 2009;30:206–208.
6 Eastman AL, Arnoldo BA, Hunt JL, et al. Pre-burn center management of the burned airway: Do we know enough? J Burn Care Res. 2010;31:701–705.
9 Santaniello JM, Luchette FA, Esposito TJ, et al. The year experience of burn, trauma and combined burn/trauma injuries comparing outcomes. J Trauma. 2004;57:696–701.
32 Maybauer MO, Rehberg S, Traber DL, et al. Pathophysiology of acute lung injury in severe burn and smoke inhalation injury. Anaesthesist. 2009;58:805–812.
50 Barillo DJ. Effects/treatment of toxic gases: diagnosis and treatment of cyanide toxicity. J Burn Care Res. 2009;30:148–152.
51 Borron SW, Baud FJ, Barriot P, et al. Prospective study of hydroxycobalamin for acute cyanide poisoning in smoke inhalation. Ann Emerg Med. 2007;49:794–801.
53 Martyn JA, Fukushima Y, Chon J-Y, et al. Muscle relaxants in burns, trauma, and critical illness. Int Anesthesiol Clin. 2006;44:123–142.
55 Gore DC, Hawkins HK, Chinkes DL, et al. Assessment of adverse events in the demise of pediatric burn patients. J Trauma. 2007;63:814–818.
56 Fidkowski CW, Fuzaylov G, Sherdan RL, Coté CJ. Inhalation burn injury in children. Paediatr Anaesth. 2009;19:147–154.
58 Silver GM, Freiburg C, Halerz M, et al. A survey of airway and ventilator management strategies in North American pediatric burn units. J Burn Care Rehabil. 2004;25:435–440.
1 Miller SF, Bessey PQ, Lentz CW, et al. National Burn Repository 2007 report: A synopsis of the 2007 call for data. J Burn Care Res. 2008;29:862–870.
2 Latenser BA, Miller SF, Bessey PQ, et al. National Burn Repository 2006: A ten-year review. J Burn Care Res. 2007;28:635–658.
3 Palmieri TL, Warner P, Mlcak RP, et al. Inhalation injury in children: A 10 year experience at Shriners Hospital for Children. J Burn Care Res. 2009;30:206–208.
4 Cochran A. Inhalation injury and endotracheal intubation. J Burn Care Res. 2009;30:190–195.
5 Madnani MD, Steele NP, de Vries E. Factors that predict the need for intubation in patients with smoke inhalation injury. Ear Nose Throat. 2006;85:278–280.
6 Eastman AL, Arnoldo BA, Hunt JL, et al. Pre–burn center management of the burned airway: do we know enough? J Burn Care Res. 2010;31:701–705.
7 Klein MB, Nathens AB, Emerson D, et al. An analysis of the long-distance transport of burn patients to a regional burn center. J Burn Care Res. 2007;28:49–55.
8 Grunwald TB, Garner WT. Acute burns. Plast Reconstr Surg. 2008;121:311e–319e.
9 Santaniello JM, Luchette FA, Esposito TJ, et al. The year experience of burn, trauma and combined burn/trauma injuries comparing outcomes. J Trauma. 2004;57:696–701.
10 Blanding R, Stiff J. Perioperative anesthetic management of patients with burns. Anesth Clin North Am. 1999;17:237–249.
11 Cahalane M, Demling RH. Early respiratory abnormalities from smoke inhalation. JAMA. 1984;251:771–773.
12 Herndon DN, Spies M. Modern burn care. Semin Pediatr Surg. 2001;10:28–31.
13 MacLennan N, Heimbach DM, Cullen BF. Anesthesia for major thermal injury. Anesthesiology. 1998;89:749–770.
14 Minamihaba O, Nakamura H, Sata M, et al. Progressive bronchial obstruction associated with toxic epidermal necrolysis. Respirology. 1999;4:93–95.
15 Moylan JA, Chan CK. Inhalation injury—An increasing problem. Ann Surg. 1978;188:34–37.
16 Sheridan RL. Airway management and respiratory care of the burn patient. Int Anesthesiol Clin. 2000;38:129–145.
17 Yowler CJ, Fratienne RB. Current status of burn resuscitation. Clin Plast Surg. 2000;27:1–10.
18 Baud FJ, Barriot P, Toffis V, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med. 1991;325:1761–1766.
19 Brough MD. The king’s Cross fire. Part I: The physical injuries. Burns. 1991;17:6–9.
20 Gaissert HA, Lofgren RH, Grillo HC. Upper airway compromise after inhalation injury. Ann Surg. 1993;218:672–678.
21 Kohn D. Burn trauma. Preclinical and clinical care from an anesthesiologist’s point of view [in German]. Anaesthesist. 2000;49:359–370.
22 Gueugniaud PY, Carsin H, Bertin-Maghit M, et al. Current advances in the initial management of major thermal burns. Intensive Care Med. 2000;26:848–856.
23 Mlcak R, Cortiella J, Desai MH, et al. Emergency management of pediatric burn victims. Pediatr Emerg Care. 1998;14:51–54.
24 Mayes RW. The toxicological examination of the victims of the British Air Tours Boeing 737 accident at Manchester in 1985. J Forensic Sci. 1991;36:179–184.
25 Edelman DA, White MT, Tyburski JG, et al. Factors affecting prognosis of inhalation injury. J Burn Care Res. 2006;27:848–853.
26 Woolley WD, Ames SA, Smith PG. The Manchester Woolworths store fire, May 1979: Burning characteristics of the furniture. Fire Saf J. 1980;3:55–65.
27 Woolley WD, Smith PG, Fardell PJ, et al. The Stardust Disco fire, Dublin 1981. Studies of combustion products during simulated experiments. Fire Saf J. 1984;7:267.
28 Madden MR, Finkelstein JL, Goodwin CW. Respiratory care of the burn patient. Clin Plast Surg. 1986;13:29–38.
29 McCAll JE, Cahill TJI. Respiratory care if the burn patient. J Burn Care Rehabil. 2005;26:200–206.
30 Miller K, Chang A. Acute inhalation injury. Emerg Med Clin North Am. 2003;21:533–557.
31 Rehberg S, Maybauer MO, Enkbaatar P, et al. Pathophysiology, management and treatment of smoke inhalation injury. Expert Rev Respir Med. 2009;3:283–297.
32 Maybauer MO, Rehberg S, Traber DL, et al. Pathophysiology of acute lung injury in severe burn and smoke inhalation injury. Anaesthesist. 2009;58:805–812.
33 Enkhbaatar P, Traber DL. Pathophysiology of acute lung injury in combined burn and smoke inhalation injury. Clin Sci. 2004;107:137–143.
34 Murakami K, Traber DL. Pathophysiological basis of smoke inhalation injury. News Physiol Sci. 2003;18:125–129.
35 Traber DL, Hawkins HK, Enkhbaatar P, et al. The role of the bronchial circulation in the acute lung injury resulting from burn and smoke inhalation. Pulm Pharmacol Ther. 2007;20:163–166.
36 Nguyen TT, Gilpin A, Meyer N, et al. Current treatment of severely burned patients. Ann Surg. 1996;223:14–25.
37 Ames WA. Management of the major burn. Update Anesth. 1999;10:10.
38 Barret JP, Desai MH, Herndon DN. Effects of tracheostomies on infection and airway complications in pediatric burn patient. Burns. 2000;26:190–193.
39 Dolan MC. Carbon monoxide poisoning. CMAJ. 1985;133:392–399.
40 Meredith TJ, Vale JA. Carbon monoxide poisoning. Br Med J. 1988;296:77–79.
41 Olson KR. Carbon monoxide poisoning: Mechanisms, presentation, and controversies in management. J Emerg Med. 1984;1:233–243.
42 Kirkpatrick JN. Acute carbon monoxide poisoning. West J Med. 1987;146:52–56.
43 Ilano AL, Raffin TA. Management of carbon monoxide poisoning. Chest. 1990;97:165–169.
44 Baud FJ. Cyanide: Critical issues in diagnosis and treatment. Hum Exp Toxicol. 2007;26:191–201.
45 Silverman SH, Purdue GF, Hunt JL, et al. Cyanide toxicity in burned patients. J Trauma. 1988;28:171–178.
46 Maybauer DM, Traber DL, Radermacher P, et al. Treatment strategies for acute smoke inhalation injury. Anaesthesist. 2006;55:980–982. 984–988
47 Cancio LC. Airway management and smoke inhalation injury in the burn patient. Clin Plastic Surg. 2009;36:555–567.
48 Rehberg S, Maybauer MO, Enkhbaatar P, et al. Pathophysiology, management and treatment of smoke inhalation injury. Expert Rev Respir Med. 2009;3:283–297.
49 Fortin JL, Giocanti JP, Ruttimann M, et al. Prehospital administration of hydroxycobalamin for smoke inhalation associated cyanide poisoning: Years of experience in the Paris fire brigade. Clin Toxicol. 2006;44(Suppl):37–44.
50 Barillo DJ. Effects/treatment of toxic gases: Diagnosis and treatment of cyanide toxicity. J Burn Care Res. 2009;30:148–152.
51 Borron SW, Baud FJ, Barriot P, et al. Prospective study of hydroxycobalamin for acute cyanide poisoning in smoke inhalation. Ann Emerg Med. 2007;49:794–801.
52 Schneider G. Muscle relaxants in the ICU [in German]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2009;44:358–368.
53 Martyn JA, Richtsfeld M. Succinylcholine induced hyperkalemia in acquired pathological states. Etiologic factors and molecular mechanism. Anesthesiology. 2006;104:158–169.
54 Martyn JA, Fukushima Y, Chon J-Y, et al. Muscle relaxants in burns, trauma, and critical illness. Int Anesthesiol Clin. 2006;44:123–142.
55 Gore DC, Hawkins HK, Chinkes DL, et al. Assessment of adverse events in the demise of pediatric burn patients. J Trauma. 2007;63:814–818.
56 Fidkowski CW, Fuzaylov G, Sherdan RL, et al. Inhalation burn injury in children. Pediatr Anaesth. 2009;19:147–156.
57 Armour AD, Billmire DA. Pediatric thermal injury: Acute care and reconstruction update. Plast Reconstr Surg. 2009;124(Suppl):117e–127e.
58 Silver GM, Freiburg C, Halerz M, et al. A survey of airway and ventilator management strategies in North American pediatric burn units. J Burn Care Rehabil. 2004;25:435–440.
59 Prater ME, Deskin RW. Bronchoscopy and laryngoscopy findings as indications for tracheostomy in the burned child. Arch Otolaryngol Head Neck Surg. 1998;124:1115–1117.
60 Lund T, Goodwin CW, McManus WF, et al. Upper airway sequelae in burn patients requiring endotracheal intubation or tracheostomy. Ann Surg. 1985;201:374–382.
61 Caruso DM, Al-Kasspooles MF, Matthews MR, et al. Rationale for ‘early’ percutaneous dilatational tracheostomy in patients with burn injuries. J Burn Care Rehabil. 1997;18:424–428.
62 Lujan HJ, Dries DJ, Gamelli RL. Comparative analysis of bedside and operating room tracheostomies in critically ill patients with burns. J Burn Care Rehabil. 1995;16:258–261.
63 Aggarwal S, Smailes S, Dziewulski P. Tracheotomy in burned patients revisited. Burns. 2009;35:962–966.
64 Namdar T, Stollwerck PL, Stang FH, et al. Early postoperative alteration of ventilation parameters after tracheostomy in major burn injuries. Ger Med Sci. 2010;8:1–7.
65 Saffle J, Morris SE, Edelman L. Early tracheostomy does not improve outcome in burn patients. J Burn Care Rehabil. 2002;23:431–438.
66 Coln CE, Purdue GF, Hunt JL. Tracheostomy in the young pediatric burn patient. Arch Surg. 1998;133:537–540.
67 Palmieri TL, Jackson WRRT, Greenhalgh DG. Benefits of early tracheostomy in severely burned children. Crit Care Med. 2002;30:922–924.
68 Gravvanis AI, Tsoutsos DA, Iconomou TG, et al. Percutaneous versus conventional tracheostomy in burned patients with inhalational injury. World J Surg. 2005;29:1571–1575.
69 Cobley TDD, Hart WJ, Baldwin DL, et al. Complete fusion of the vocal cords; an unusual case. Burns. 1999;25:361–363.
70 Xiao SC, Zhu SH, Li HY, et al. Treatment of tracheal stenosis with an extended tracheal cannula in a patient with extensive burn. J Burn Care Res. 2010;31:210–213.
71 Schlossmacher P, Martineta O, Testudb R, et al. Emergency percutaneous tracheostomy in a severely burned patient with upper airway obstruction and circulatory arrest. Resuscitation. 2005;68:301–305.
72 Sakata S, Hallett KB, Brandon MS, McNride CA. Easy come, easy go: A simple and effective orthodontic enamel anchor for endotracheal tube stabilization in a child with extensive facial burns. Burns. 2009;35:983–986.
73 Epstein SK. Weaning from ventilatory support. Curr Opin Crit Care. 2010;15:36–43.
74 Timon CI, McShane D, McGovern E, et al. Treatment of combined subglottic and critically low tracheal stenoses secondary to burn inhalation injury. J Laryngol Otol. 1989;103:1083–1086.
75 Valova M, Konigova R, Broz L, et al. Early and late fatal complications of inhalational injury. Acta Chir Plast. 2002;44:51–54.
76 Yang JY, Yang WG, Chang LY, et al. Symptomatic tracheal stenosis in burns. Burns. 1999;25:72–80.
77 Smailes ST, Martin RV, McVicar AJ. The incidence and outcome of extubation failure in burn intensive care patients. J Burn Care Res. 2009;30:386–392.
78 Karam R, Ibrahim G, Tohme H, et al. Severe neck burns and laryngeal mask airway for frequent general anesthetics. Middle East J Anesthesiol. 1996;13:527–535.
79 Hagberg CA, Johnson S, Pillai D. Effective use of the esophageal tracheal Combitube following severe burn injury. J Clin Anesth. 2003;15:436–466.
80 McCall JE, Fischer CG, Schomaker E, et al. Laryngeal mask airway use in children with acute burns: Intraoperative airway management. Paediatr Anaesth. 1999;9:515–520.
81 Cha SI, Kim CH, Lee JH, et al. Isolated smoke inhalation injuries: Acute respiratory dysfunction, clinical outcomes and short-term evolution of pulmonary function with the effects of steroids. Burns. 2007;33:200–208.