Chapter 25 Airway management and acute upper-airway obstruction
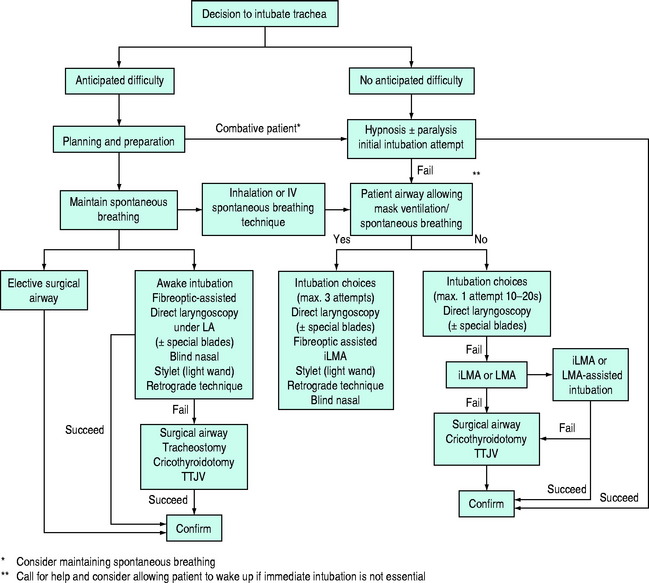
The primary objective of airway management is to secure unobstructed gas exchange and protect the lungs from soiling. Because of the critical importance of maintaining gas exchange, upper-airway obstruction is a life-threatening emergency. Upper-airway obstruction results from a wide range of pathophysiological processes, and therefore rapid assessment and establishment of a patent airway must take priority, even in the absence of a specific diagnosis. As no single airway management modality is universally applicable, the intensive care unit (ICU) physician must be capable of performing a variety of airway management techniques and instituting them in a logical and systematic way (Figure 25.1).
AIRWAY MANAGEMENT TECHNIQUES
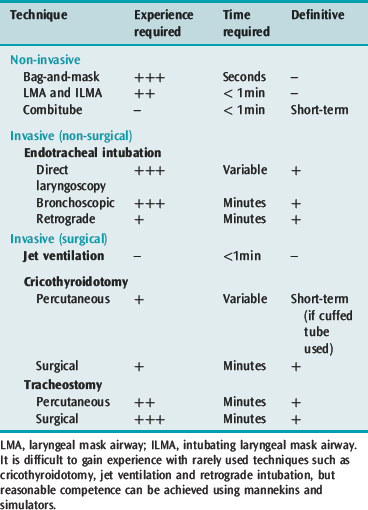
Airway management techniques are generally classified as non-invasive or invasive, depending on whether instrumentation occurs above or below the glottis, surgical or non-surgical, and definitive (Table 25.1). Definitive techniques secure the trachea and provide some protection from macroscopic aspiration and soiling. While bag-and-mask ventilation and direct laryngoscopic tracheal intubation remain the routine methods of airway management in ICU, the use of fibreoptic bronchoscopy is increasingly common, especially in special circumstances. Management of failed intubation and ventilation by various alternative techniques, particularly the use of the intubating laryngeal mask airway (iLMA) and cricothyroidotomy, is well described.1,2
The technique of choice will depend on each individual situation and is determined by the interaction of patient and clinical factors, which partially determine the appropriate technique (Table 25.2), and the clinician’s experience in applying the chosen technique. Other factors include availability of help, levels of training and supervision and accessibility of equipment. A portable storage unit with a wide choice of equipment appropriate for difficult airway management should be available in every ICU (Table 25.3).
Table 25.2 Commonly recommended applications of described airway management techniques. Examples of common alternatives are given in approximate order of choice
| Difficult direct laryngoscopic intubation | With difficult spontaneous/mask ventilation | |
|---|---|---|
| Awake | Fibreoptic bronchoscopic intubation Direct laryngoscopic intubation* Blind nasal intubation Retrograde intubation | Percutaneous cricothyroidotomy* Surgical tracheostomy* |
| Anaesthetised or comatose (empty stomach) | Bag-and-mask ventilation Direct laryngoscopic intubation Different blade Fibreoptic bronchoscopic intubation Intubating LMA/LMA Lighted stylet Blind nasal intubation | Laryngeal mask airway (LMA) Transtracheal jet ventilation Rigid ventilating bronchoscope Percutaneous cricothyroidotomy Surgical tracheostomy |
| (full stomach) | Maintain cricoid pressure with all techniques Intubating LMA/Proseal LMA Combitube | Percutaneous cricothyroidotomy Surgical tracheostomy Combitube |
The technique(s) chosen should also depend on the clinician’s knowledge and ability. Cricoid pressure should be applied with a force of approximately 30 N, but applied force can be temporarily reduced to assist airway manoeuvres.
Table 25.3 Suggested contents of a portable storage facility for difficult airway management
| Masks |
| Face and nasal masks of differing make and size variety |
| Airways |
| Oropharyngeal airways |
| Nasopharyngeal airways |
| Airway intubator guide for oral endoscopic intubation |
| Laryngeal mask airway (LMA) and intubating LMA with appropriate endotracheal tubes |
| Rigid laryngoscope with a variety of designs and sizes |
| Short handle or variable angle (Patil-Syracuse) laryngoscope |
| Curved blades: Macintosh, Bizarri-Guiffrida |
| Straight blades: Miller |
| Bent blade: Belscope |
| Articulating-tip blade: McCoy |
| Fibreoptic stylet laryngoscope or Bullard laryngoscope |
| Endotracheal tubes of assorted size |
| Murphy tubes |
| Microlaryngoscopy tubes |
| Endotracheal tube stylets |
| Gum elastic bougie (Eschmann stylet) |
| Malleable stylet |
| Tube changer, hollow tube changer (jet stylet) |
| Lighted stylet (light wand) |
| Fibreoptic intubation equipment |
| Patil endoscopic mask, oral airways or blocks to facilitate oral endoscopic intubation |
| Fibreoptic endoscopes with light source, adult and paediatric-sized |
| Combitube |
| Emergency surgical airway access |
| Percutaneous cricothyroidotomy set |
| Transtracheal jet ventilation – cannula and high-pressure O2 source connectors |
| Regulated central wall O2 pressure (Sanders-type injector) |
| Unregulated central wall O2 pressure device |
| Exhaled carbon dioxide monitor |
| Capnometer/capnograph |
| Chemical indicators |
NON-INVASIVE TECHNIQUES
BAG-MASK VENTILATION
Some considerations when performing mask ventilation include the following:
ORO- AND NASOPHARYNGEAL AIRWAYS
A nasopharyngeal airway is a soft rubber or plastic tube inserted into the nostril and advanced along the floor of the nose (in the direction of the occiput). Correctly positioned, the tube traverses the posterior pharynx. It is better tolerated by semiconscious patients than the oropharyngeal airway. Complications include epistaxis, aspiration and, rarely, laryngospasm or oesophageal placement.
LARYNGEAL MASK AIRWAY (LMA) AND INTUBATING LMA
The LMA is a reusable device that consists of a silicone rubber tube connected to a distal elliptical spoon-shaped mask with an inflatable rim, which is positioned blindly into the pharynx to form a low-pressure seal against the laryngeal inlet.3 LMAs are useful to achieve non-definitive airway patency in many emergency situations (see Figure 25.1), and can be used to provide positive-pressure ventilation.2 Once positioned the LMA has been used to guide the passage of stylets, bougies, the bronchoscope and an endotracheal tube into the trachea, but with difficulty.2,4 The iLMA or FasTrach is a modification of the LMA with several features to facilitate intubation once the iLMA is placed.5 There is a guiding ramp and epiglottic elevating bar at the aperture to direct the endotracheal tube to the glottis. It also has an anatomically curved, rigid shaft and handle to allow easy and firm manipulation during placement and when the endotracheal tube is passed.6 The iLMA is the laryngeal mask of choice if intubation is required, as is frequently the case in ICU patients.
Although preparation and patient positioning techniques for placement of a LMA and iLMA are similar, the insertion technique is quite different. The mask airway is prepared for insertion by deflating and smoothing out the cuffed rim to be wrinkle-free, and the posterior surface and patient hard palate are lubricated with water soluble jelly. The patient is positioned as for endotracheal intubation, with slight flexion of the neck and extension of the atlanto-occipital joint (sniffing-the-morning-air position). The LMA is inserted with the tip of the cuff continuously applied to the hard palate, and with the right index finger guiding the tube to the back of the tongue until a firm resistance is encountered. The cuff is then inflated with 20–40 ml of air (adult sizes) before attachment of the breathing circuit.
COMBITUBE (OESOPHAGEAL–TRACHEAL DOUBLE-LUMEN AIRWAY)
The oesophageal–tracheal Combitube is a double-lumen tube that is blindly inserted into the oropharynx up to the indicated markings.7 The oesophageal lumen is blocked at the distal end and has side perforations at the pharyngeal level whereas the tracheal lumen has a hole at the distal end. It has two balloon cuffs, a distal one and a proximal pharyngeal balloon. The patient is ventilated through the oesophageal lumen initially as the Combitube usually enters the oesophagus,7 with the distal cuff sealing the oesophagus and the proximal balloon sealing the proximal pharynx. Gas exits the perforations and enters the pharynx and larynx. In the event of failure of ventilation, tracheal intubation may have occurred and then the tracheal lumen is ventilated while the distal cuff seals the trachea. Although demonstrated to be a useful airway management adjunct, its role in resuscitation and management of the difficult airway in the ICU environment is yet to be established. Barotrauma, especially oesophageal rupture, has been reported.
INVASIVE TECHNIQUES
ENDOTRACHEAL INTUBATION
Endotracheal intubation remains the ‘gold standard’ of definitive airway management, allowing for spontaneous and positive-pressure ventilation, with good macroscopic protection from aspiration. Indications include acute airway obstruction, facilitation of tracheal suctioning, protection of the airway in those without protective reflexes, and respiratory failure requiring ventilatory support with high inspired concentrations of oxygen and PEEP.
Preparation
Prior to proceeding with any attempts at intubation, regardless of the technique chosen, preparation and checking of all relevant equipment are essential. Tracheal intubation should be preceded by adequate preoxygenation, particularly in ICU patients who frequently have pulmonary or cardiac pathology. Difficult airway management equipment (see Table 25.3) should also be accessible within a few minutes. Food, vomitus, blood or sputum may obstruct the airway and therefore suction, able to generate at least 300 mmHg (40 kPa) and a flow rate of 30 l/min, should always be available. Excessively vigorous suctioning should be avoided as it can cause laryngospasm, vagal stimulation, mucosal injury and bleeding.
Direct laryngoscopy
Although an essential skill for all intensivists, direct laryngoscopy and intubation is difficult to master.8 It can be learned by simulation, exposure to patients in a controlled environment such as the operating room and subsequently practised under supervision in the ICU setting. A detailed description of the technique is beyond the scope of this chapter; however certain problems and complications commonly encountered in ICU patients should be anticipated and prevented.
The route of intubation may be orotracheal or nasotracheal. The orotracheal route is preferred because it has fewer complications. Nasotracheal intubation is contraindicated in the presence of fracture of the base of skull. Other complications include epistaxis, turbinate cartilage and nasal septal damage, and in the long-term an increased risk of nosocomial pneumonia and sinusitis.9
Rigid indirect fibreoptic technique
The Bullard laryngoscope is a rigid, indirect fibreoptic instrument that is shaped to follow the hard palate. The learning curve appears reasonable and it has been shown to be a viable alternative for intubation in the difficult airway and reduces cervical spine movement during laryngoscopy compared with the Macintosh or Miller laryngoscope.10
Fibreoptic bronchoscopic technique
This technique offers advantages of direct visualisation, immediate diagnosis of upper-airway lesions and immobility of the neck during the procedure.11 It also allows reasonably comfortable intubation of a cooperative, awake patient under local anaesthesia, and use of the sitting position. Experience and skill are necessary, especially for dealing with emergent situations, but success rates > 96% are expected.12 Fibreoptic oral intubation may also be performed in anaesthetised patients, using a modified face mask with diaphragm. Nasal intubation is usually performed through an endotracheal tube placed in the nasopharynx, with the tip just above the glottis. The fibreoptic bronchoscope tip is guided into the trachea and the tube is advanced over the bronchoscope. Correct placement is visually checked before the scope is removed. In ICU the fibreoptic bronchoscope can be used to improve the safety of airway procedures such as endotracheal tube changes and percutaneous tracheostomy.13,14 A number of specially designed oral airways are available to assist oral fibreoptic intubation. The most common cause of failure is obstructed vision from blood or secretions.
Less commonly used techniques of endotracheal intubation
The retrograde intubation technique consists of the percutaneous introduction of a J-tip guidewire through the cricothyroid membrane, which is then advanced into the retropharynx. The tip is retrieved from the oral cavity, and the wire is used to guide an oral endotracheal tube past the obstruction and into the trachea.15 The procedure is a relatively simple and safe alternative if other techniques fail or are not possible. Commercial kits are available.
Confirmation of tracheal tube placement
Confirming correct intratracheal tube placement is essential. Direct visualisation and measurement of expired CO2 by capnography are the most reliable methods.16 Capnography may produce false-positive results with the first few breaths after oesophageal intubation (i.e. detectable end-tidal PCO2), if gastric insufflation from mask ventilation has occurred. A false-negative (decreased PCO2, despite correct position) may occur with cardiac arrest and low-cardiac-output states. Position can also be reliably confirmed by fibreoptic confirmation and the use of oesophageal detectors or self-inflating bulbs.1 Other clinical signs, such as auscultation of breath sounds over both sides of the chest and epigastrium, visualisation of condensed water vapour in the tube and chest wall movement, are less reliable.
TRANSTRACHEAL JET VENTILATION (TTJV)
Percutaneous TTJV, using a large-bore intravenous (IV) catheter inserted through the cricothyroid membrane, can be used to provide temporary ventilation when other techniques have failed.17 Ventilation through the cannula with a standard manual resuscitator bag is inadequate, and a jet ventilation system is necessary. A high-pressure (up to 50 psi or 344 kPa) oxygen source is required for adequate ventilation through a 14 FG IV cannula with a manually regulated jet injector. Expiratory gases must be able to escape via the glottis. Appropriate chest movements during expiration must be noted. The consequence of expiratory obstruction is severe and potentially fatal barotrauma.
CRICOTHYROIDOTOMY
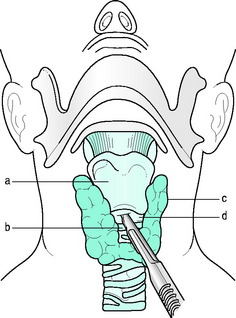
Cricothyroidotomy, by surgery or percutaneously, is a reliable, relatively safe and easy way of providing an emergency airway.18 It is the method of choice if severe or complete upper-airway obstruction exists. The simplest, fastest and most proven method uses a horizontal incision through the cricothyroid membrane with the space held wide open by the scalpel handle or forceps. This is followed by insertion of a small tracheostomy or endotracheal tube (Figure 25.2). If available, a small surgical hook is useful to hold down the inferior margin of the incision to facilitate cannulation. Commercial cricothyroidotomy sets, using the Seldinger technique, are available. A tube with internal diameter of 3.0 mm will allow adequate gas flow for self-inflating bag ventilation provided supplemental oxygen is used. Since the diameter of the cricothyroid space is 9 × 30 mm, tubes of 8.5 mm outer diameter or less should avoid laryngeal and vocal cord damage. Commercially available percutaneous tracheostomy sets that meet the above requirements are available. Complications such as subglottic stenosis (1.6%), thyroid fracture, haemorrhage and pneumothorax are acceptably low. Cricothyroidotomy is generally contraindicated in complete laryngotracheal disruption and age < 12 years.
TRACHEOSTOMY
There is little agreement on the indications, best technique or optimal timing of tracheostomy in ICU patients. Suggested indications for tracheostomy include bypass of glottic and supraglottic obstruction, access for tracheal toilet, provision of a more comfortable airway for prolonged ventilatory support and protection of the airways from aspiration.19 In uncomplicated patients, percutaneous tracheostomy performed by an intensivist at the bedside is at least as safe as surgical tracheostomy performed in the operating room, and is probably associated with a lower incidence of infectious complications.20,21 The added convenience and cost savings have made percutaneous tracheostomy the procedure of choice in many institutions. However, the percutaneous technique is best avoided in the presence of coagulapathy – an international normalised ratio (INR) > 2 or platelet count < 40 × 109/l; significant anatomical abnormality in the anterior neck in the region of the trachea, vessels or thyroid gland; previous tracheostomy scar; or a cervical spinal injury that is considered unstable.
Ciaglia’s percutaneous technique was described in 1985.22 After making an adequate skin incision and using blunt dissection with forceps the trachea is gently exposed. The endotracheal tube is then withdrawn so that its cuff lies just above the vocal cords. The operator confirms tube position to be above the stoma site by palpation of the trachea. A J-wire is placed in the trachea through a needle inserted through the membrane above or below the second tracheal ring. A series of curved dilators is used to enlarge the stoma progressively. A tracheostomy tube is then inserted into the trachea and the endotracheal tube removed. Ciaglia later introduced a modified tapered dilator to avoid the use of multiple dilators. Although quicker, the single dilator may cause more tracheal wall injuries and ring fractures.22 The Griggs technique utilises a Kelly forceps, modified to allow it to be guided by the J-wire, to dilate the tract before insertion of tracheostomy tube.23 Although the Griggs technique is marginally faster (by 2–3 minutes) than the Ciaglia technique, most, but not all, prospective studies have suggested that the Griggs technique may cause marginally more bleeding and functional complications, and cannula insertion may be somewhat more difficult.24,25 Fibreoptic bronchoscopy during percutaneous tracheostomy may help to prevent incorrect guidewire placement and tracheal ring rupture or herniation, but definitive evidence supporting its routine use is lacking. Definitive identification of the best technique still requires further investigation and long-term follow-up.
| Immediate |
| Procedural complications |
| Haemorrhage |
| Surgical emphysema, pneumothorax, air embolism |
| Cricoid cartilage damage |
| Misplacement in pretracheal tissues or right main bronchus |
| Compression of tube lumen by cuff herniation |
| Occlusion of the tip against the carina or tracheal wall |
| Delayed |
| Blockage with secretions |
| Infection of the tracheostomy site, tracheobronchial tree and larynx |
| Pressure on tracheal wall from the tracheostomy tube or cuff |
| Mucosal ulceration and perforation |
| Deep erosion into the innominate artery |
| Tracheo-oesophageal fistula |
| Late |
| Granulomata of the trachea |
| Tracheal and laryngeal stenosis |
| Persistent sinus at tracheostomy site |
| Tracheomalacia and tracheal dilatation |
LOCAL ANAESTHESIA
Instrumentation of the upper airway in awake patients requires good local anaesthesia to increase comfort, improve cooperation, attenuate cardiovascular responses and reduce the risk of laryngospasm. Rapid transcricoid injection and either sprayed or nebulised lidocaine to the nares, posterior pharynx and tongue are effective (Table 25.5).26 Nerve block techniques may improve analgesia but are not essential. Cocaine has been a popular choice for its vasoconstrictor properties, but it is toxic and its supply is regulated. Systemic absorption of topically applied lidocaine (maximum dose 4 mg/kg) is variable, and the clinician should be alert for signs and symptoms of toxicity.
| Technique | Drug dosage |
|---|---|
| Nerve block | |
| Internal branch of superior laryngeal nerve | Lidocaine 1–2% (2 ml/side) |
| Glossopharyngeal nerve | Lidocaine 1–2% (3 ml/side) |
| Topical anaesthesia of the tongue and oropharynx | |
| Gargle | Lidocaine viscous 4% (5 ml) |
| Spray | Lidocaine 10% (5–10 sprays = 50–100 mg) |
| Nebulised | Lidocaine 4% |
| Topical anaesthesia of the nasal mucosa | |
| Cocaine spray or paste | Cocaine 4–5% (0.5–2 ml) |
| Gel | Lidocaine 2% gel (5 ml) |
| Lidocaine spray | Lidocaine 10% (10 sprays = 100 mg) |
| Lidocaine + phenylephrine spray | Lidocaine 3% + phenylephrine 0.25% (0.5 ml) |
| Topical anaesthesia of glottis and trachea | |
| Spray-as-you-go through bronchoscope | Lidocaine 1–4% (3 mg/kg) |
| Cricothyroid membrane puncture | Lidocaine 2% (5 ml) |
| Nebulised | Lidocaine 4% (4 ml) ± phenylephrine 1% (1 ml) |
THE DIFFICULT AIRWAY
The difficult airway has been described as one in which a conventionally trained anaesthesiologist experiences difficulty with mask ventilation, tracheal intubation or both. Difficult intubations may be expected in 1–3% of patients presenting for general anaesthesia, and the incidence is likely to be considerably higher in ICU patients.
ASSISTANCE AND ENVIRONMENT
Because the patient’s condition may rapidly deteriorate as a consequence of a poorly managed airway emergency, the most senior help available should be immediately summoned. If the situation allows, the patient should be moved to the best location for emergency airway interventions, usually the operating theatre or ICU, and difficult airway equipment requested (see Table 25.3). A senior assistant can help in gaining IV access, administering drugs, setting up equipment and managing the airway. A skilled intensivist or ear, nose and throat surgeon (gowned and standing by) can help to provide a surgical airway or perform rigid bronchoscopy to remove foreign bodies.
ANTICIPATING AND GRADING A DIFFICULT AIRWAY
FAILED INTUBATION AND VENTILATION ALGORITHMS
A pre-prepared plan in the event of failed intubation and/or ventilation is essential and a number have been described in the form of single or multiple algorithms.1,2 The algorithm shown here is an example of a single algorithm applicable to some anticipated airway difficulty scenarios in ICU patients (see Figure 25.1). Although not comprehensive, the algorithm is complex and only one or two pathways are applicable to an individual clinical scenario. The relevant pathway should be identified as early as possible in each individual case and then applied. Ultimately effective implementation depends on the skill of the operator and the appropriate use of the airway techniques described above.
If the initial attempt at intubation fails, call for help immediately. Repeated attempts at direct laryngoscopy should be avoided unless a more experienced operator intervenes, or a potentially helpful manoeuvre has been carried out (e.g. significant repositioning, externally applied laryngeal pressure or change of laryngoscope blade). Hypoxia will quickly result if the patient is inade-quately ventilated between attempts (see above). In addition, oedema and bleeding caused by repeated laryngoscopy attempts may impair both mask ventilation and the use of alternative techniques such as fibreoptic intubation. When indicated to prevent hypoxia a surgical airway should not be delayed (see Figures 25.1 and 25.2).
UPPER-AIRWAY OBSTRUCTION
AETIOLOGY
Acute upper-airway obstruction may result from functional or mechanical causes (Table 25.6). Functional causes include central nervous system and neuromuscular dysfunction. Mechanical causes may occur within the lumen, in the wall or extrinsic to the airway.
Table 25.6 Clinical conditions associated with acute upper-airway obstruction
| Functional causes |
| Central nervous system depression |
| Head injury, cerebrovascular accident, cardiorespiratory arrest, shock, hypoxia, drug overdose, metabolic encephalopathies |
| Peripheral nervous system and neuromuscular abnormalities |
| Recurrent laryngeal nerve palsy (postoperative, inflammatory or tumour infiltration), obstructive sleep apnoea, laryngospasm, myasthenia gravis, Guillain–Barré polyneuritis, hypocalcaemic vocal cord spasm |
| Mechanical causes |
| Foreign-body aspiration |
| Infections |
| Epiglottitis, retropharyngeal cellulitis or abscess, Ludwig’s angina, diphtheria and tetanus, bacterial tracheitis, laryngotracheobronchitis |
| Laryngeal oedema |
| Allergic laryngeal oedema, angiotensin-converting enzyme inhibitor-associated, hereditary angioedema, acquired C1 esterase deficiency |
| Haemorrhage and haematoma |
| Postoperative, anticoagulation therapy, inherited or acquired coagulation factor deficiency |
| Trauma |
| Burns |
| Inhalational thermal injury, ingestion of toxic chemical and caustic agents |
| Neoplasm |
| Pharyngeal, laryngeal and tracheobronchial carcinoma, vocal cord polyposis |
| Congenital |
| Vascular rings, laryngeal webs, laryngocele |
| Miscellaneous |
| Cricoarytenoid arthritis, achalasia of the oesophagus, hysterical stridor, myxoedema |
CLINICAL PRESENTATION
Signs of sudden complete upper-airway obstruction are characteristic and progress rapidly. The victim cannot breathe, speak or cough, and may hold the throat between the thumb and index finger – the universal choking sign.29 Agitation, panic and vigorous breathing efforts are rapidly followed by cyanosis. Respiratory efforts diminish as consciousness is lost, and death results within 2–5 min if obstruction is not relieved.
SPECIAL EVALUATION OR INVESTIGATIONS
LARYNGOSCOPY AND BRONCHOSCOPY
Indirect laryngoscopy in a stable, cooperative patient is useful to diagnose foreign bodies, retropharyngeal or laryngeal masses and other glottic pathology.30
RADIOGRAPHIC IMAGING
Patients with potentially unstable airways should not be transported from a ‘safe’ environment like the emergency room, operating theatre or ICU for radiological investigation until the airway is secure. Anteroposterior and lateral plain neck X-rays are useful to detect radiopaque foreign bodies. The lateral view in the upright position in inspiration with the neck fully extended may reveal swelling of the epiglottis and supraglottic tissues. Ballooning of the hypopharynx is a classical signs of epiglottitis, but is not always present. Soft tissues and the extent of space-occupying lesions are best visualised by computed tomography (CT) scan, which can also assess the thyroid, cricoid and arytenoid cartilages and the airway lumen in stable patients, or in those in whom the airway has been secured.31 Although magnetic resonance imaging (MRI) has been used to image the upper airway, its use in acute airway obstruction is unproven.
MANAGEMENT
PREPARATION
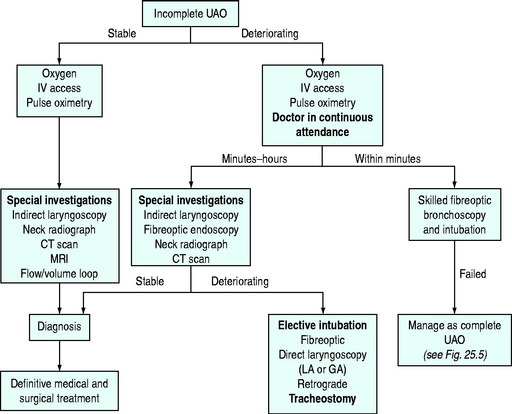
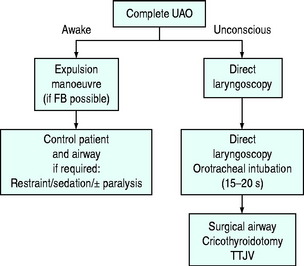
Simplified algorithms to assist the management of partial and complete upper-airway obstruction are shown in Figures 25.4 and 25.5 and an appropriate management path should be chosen. Improvisation may be required for certain difficult problems. Special techniques in patients with suspected cervical spine instability are discussed elsewhere in this volume. Initial management follows:
AIRWAY MANAGEMENT TECHNIQUES IN AIRWAY OBSTRUCTION
THE UNCONSCIOUS PATIENT
If the upper airway is obstructed by the tongue and retropharyngeal tissues in an unconscious patient, airway patency is initially achieved by using standard airway manoeuvres33 and oropharyngeal and nasopharyngeal airways. Definitive airway control should follow if consciousness does not immediately return.
ENDOTRACHEAL INTUBATION
COMMON CLINICAL CONDITIONS AND THEIR MANAGEMENT
FOREIGN-BODY OBSTRUCTION
Foreign-body obstruction is the most common cause of acute airway obstruction. The elderly, especially those in institutions, are at risk. The use of dentures, alcohol and depressant drugs increases risk. Fatal food asphyxiation or ‘café coronary’ should be considered in any acute respiratory arrest where the victim cannot be ventilated.35
Patients who are still able to cough or speak clearly should be given the opportunity to expel the foreign body spontaneously. If not, expulsion of the foreign body can be attempted with one or a combination of chest thrusts, abdominal thrusts (Heimlich manoeuvre29) or back blows applied in rapid sequence in the most convenient order.33 The abdominal thrust is performed from behind: the rescuer encircles his or her arms around the victim, placing the thumb of one fist between the umbilicus and xiphisternum. The fist is gripped by the other hand, and an inward, upward thrust is applied. Chest thrusts are similar (rescuer’s arms should encircle the chest, and a fist placed over midsternum) and are convenient for use in pregnancy and obesity. Unwanted effects, such as vomiting, aspiration, fractured ribs, barotrauma and ruptured organs, have been reported. Extract visible solid material with a finger sweep only in unconscious patients.33 If these manoeuvres fail, management immediately proceeds as shown in Figures 25.4 and 25.5. If cardiac arrest occurs, proceed with cardiopulmonary resuscitation.
EXTRINSIC AIRWAY COMPRESSION
Partial airway obstruction caused by retropharyngeal abscess is best managed by drainage under local anaesthesia. Gentle fibreoptic examination and intubation or direct laryngoscopy and intubation in the lateral, head-down position are favored by some.30 Risks are related to inadvertent rupture of the abscess, with subsequent flooding of the airway.
Ludwig’s angina is a mixed infection of the floor of the mouth resulting in an inflammatory mass in the space between the tongue and the muscles and anterior neck fascia. The supraglottic airway is compressed and becomes narrowed.36 Direct laryngoscopy is difficult, as the tongue cannot be anteriorly displaced. Awake fibreoptic-guided intubation or a surgical airway, along with antibiotic therapy, is required.
INTRINSIC AIRWAY COMPRESSION
Burn inhalation and ingestion injury
Patients with large burn area (more than 40%), or with severe facial burns or inhalation injury (soot in the nostrils, burns of the tongue and pharynx, stridor or hoarseness) are at risk pf developing progressive supraglottic oedema, usually within 24–48 hours. Such patients usually require early prophylactic tracheal intubation. If not prophylactically intubated, subsequent need for tracheal intubation can best be decided by frequent (2–4-hourly), repeated awake fibreoptic laryngoscopy as well as regular charting of known signs and symptoms of obstruction.37 Careful inspection by direct laryngoscopy is an alternative to fibreoptic laryngoscopy. Ingestion of hot fluids or corrosive agents can also cause delayed oedema and airway swelling and should be managed similarly.38
Adult epiglottitis
Epiglottitis is an uncommon but increasingly recognised infectious disease in adults.39 It involves the epiglottis and supraglottic larynx, causing swelling with consequent airway obstruction. Haemophilus influenzae, H. parainfluenzae, Streptococcus pneumoniae, haemolytic streptococci and Staphylococcus aureus are common causative organisms. Reported mortality varies in adults (0%–7%), due to difficult diagnosis and non-standardised treatment.40 Clinical features are sudden onset of sore throat (pain often greater than suggested by clinical findings), muffled voice, dysphagia, stridor, dyspnoea and respiratory distress. Systemic toxaemia is common. Gentle indirect laryngoscopy, fibreoptic laryngoscopy or lateral neck X-ray confirms the diagnosis.
Airway management is controversial.39–41 Some experts recommend securing a definitive airway on presentation while others suggest close observation in ICU. There are, however, reports of sudden obstruction and death with the latter approach.40 Onset of dyspnoea is an important sign predicting the need for intubation. Both tracheal intubation and tracheostomy are acceptable, but tracheal intubation may result in better long-term outcome. Prior to securing the airway, patient positioning is important, and changing from a sitting to supine position may induce complete obstruction. In more stable patients, awake fibreoptic intubation is preferable if a skilled operator is available. Endotracheal intubation following gaseous induction is recommended by some authorities, but complete obstruction can occur, even when this procedure is undertaken by a skilled anaesthetist.41 A skilled assistant, scrubbed and ready to secure a surgical airway, may prevent disaster. Rapid-sequence induction using muscle relaxants is dangerous and should be avoided. Tracheostomy under local anaesthesia is a safe alternative.
Angioedema
Allergic responses involving the upper airway may be localised or part of a systemic anaphylactic reaction. Angioedema is characterised by subepithelial swelling. Angioedema of the lips, supraglottis, glottis and infraglottis may result in airway obstruction. The systemic reaction consists of variable combinations of urticaria (79%), bronchospasm (70%), shock, cardiovascular collapse and abdominal pain.42 Common causative agents are Hymenoptera stings, shellfish ingestion and drugs. Treatment consists of immediately ensuring an adequate airway (see Figures 25.4 and 25.5), and administration of oxygen, adrenaline (epinephrine) and steroids. As it is likely to recur, the patient should be kept under close supervision and fully investigated.
Hereditary angioedema is a rare, inherited disorder of the complement system, caused by functionless or low levels of C1 esterase inhibitor.43 Non-pruritic, non-painful angioedema involving skin and subcutaneous tissue occurs in various locations, including the upper airway.44 Precipitating causes include stress, physical exertion and localised trauma (including dental or maxillofacial surgery and laryngoscopy). Acute attacks do not respond to adrenaline, antihistamines or corticosteroids. Management consists of establishing a secure airway and infusion of C1 esterase inhibitor concentrate (25 U/kg) which has an onset of action of 30–120 minutes.44,45 If not available, fresh frozen plasma (2–4 units) may be considered. Stanozolol 1–4 mg daily or danazol 50–600 mg/day has been shown to be effective in decreasing frequency and severity of attacks.46 Antifibrinolytic agents (e.g. tranexamic acid) are less effective. Danazol, C1 esterase inhibitor and fresh frozen plasma (2–4 units) can be used as preoperative prophylaxis.45
Angiotensin-converting enzyme inhibitor-related angioedema is increasingly seen and is possibly the result of reduced bradykinin metabolism.47 Treatment focuses on airway support.
Postextubation laryngeal oedema
Laryngeal oedema following extubation occurs in about 20% of adults, but is not usually severe enough to precipitate reintubation.48 Risk of severe oedema is increased after excessive airway manipulation, traumatic or prolonged tracheal intubation and if high cuff pressures are used. Treatment in adults is conservative, with close observation and humidified oxygen therapy. Nebulised plain epinephrine (1–2 ml 1:1000 solution diluted with 2 ml saline or undiluted 1:1000 solution 4–5 ml) or racemic epinephrine (0.25–0.5 ml 2.25% solution in 2–4 ml saline) has been used. Nebulisation may need to be repeated every 30–60 minutes. The prophylactic use of steroids to reduce post extubation laryngeal oedema remains controversial. Optimal dose, duration and target groups for prophylaxis have not yet been clearly defined. A recent study demonstrated that methylprednisolone 20 mg IV four-hourly started 12 hours prior to extubation (80 mg total dose) reduced the incidence of early re-intubation in patients intubated for more than 36 hours.48
POSTOBSTRUCTION PULMONARY OEDEMA
Postobstruction pulmonary oedema may occur in up to 11% of cases of airway obstruction.49 Oedema appears to be caused by the markedly decreased intrathoracic and interstitial tissue pressure resulting from forced inspiration against a closed upper airway. The resulting increased hydrostatic gradient causes transudation of fluid from pulmonary capillaries to the interstitium. In addition, increased venous return may increase pulmonary blood flow and pressure, further worsening oedema. Hypoxia and the hyperadrenergic stress state may also affect capillary hydrostatic pressure, although pulmonary capillary occlusion pressure is often normal. The oedema usually occurs within minutes after the relief of the obstruction, but may be delayed up to 2.5 hours.50 Management includes maintenance of airway patency, oxygen therapy, diuretics, morphine and fluid restriction. Application of continuous positive airways pressure or ventilation with PEEP may be necessary in severe cases. Pulmonary artery catheterisation need only be used for complicated cases.
1 Practice Guidelines for Management of the Difficult Airway. An updated report by the American Society of Anesthesiologists Task Force on management of the difficult airway. Anesthesiology. 2003;98:1269-1277.
2 Henderson JJ, Popat MT, Latto IP, et al. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia. 2004;59:675-694.
3 Brain AIJ. The laryngeal mask: a new concept in airway management. Br J Anaesth. 1983;55:801-805.
4 McNamee CJ, Meyns B, Pagliero KM. Flexible bronchoscopy via the laryngeal mask: a new technique. Thorax. 1991;46:141-142.
5 Brain AIJ, Verghese C. The Intubating Laryngeal Mask (FasTrach) Instruction Manual. San Deigo, CA: LMA North America, 1998.
6 Ferson DZ, Rosenblatt WH, Johansen MJ, et al. Use of the intubating LMA-Fastrach in 254 patients with difficult-to-manage airways. Anesthesiology. 2001;95:1175-1181.
7 Frass M, Frenzer R, Rauscha F, et al. Evaluation of esophageal tracheal combitube in cardiopulmonary resuscitation. Crit Care Med. 1986;15:609-611.
8 Konrad C, Schupfer G, Witlisbach M, et al. Learning manual skills in anesthesiology: is there a recommended number of cases for anesthetic procedures? Anesth Analg. 1998;86:635-639.
9 Holzapfel L, Chastang C, Demingeon G, et al. A randomized study assessing the systematic search for maxillary sinusitis in nasotracheally mechanically ventilated patients. Influence of nosocomial maxillary sinusitis on the occurrence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1999;159:695-701.
10 Hastings R, Vigil CA, Hanna R, et al. Cervical spine movement during laryngoscopy with the Bullard, Macintosh, and Miller laryngoscopes. Anesthesiology. 1995;82:859-869.
11 Giudice JC, Komansky H, Gordon R, et al. Acute upper airway obstruction – fibreoptic bronchoscopy in diagnosis and therapy. Crit Care Med. 1981;9:878-879.
12 Ovassapian A. Fibreoptic assisted airway management. Acta Anaesthesiol Scand. 1997;110(Suppl):46-47.
13 Bapat P. Use of a fibreoptic bronchoscope to change endotracheal tubes. Anesthesiology. 1997;86:509.
14 Reilly PM, Schapiro MB, Malcynski JT. Percutaneous dilation tracheostomy under the microscope: justification for intra-procedural bronchoscopy? Intens Care Med. 1999;25:3-4.
15 McNamara RM. Retrograde intubation of the trachea. Ann Emerg Med. 1987;16:680-682.
16 Tinker JH, Dull DL, Caplan RA. Role of monitoring devices in prevention of anesthetic mishaps: a closed claim analysis. Anesthesiology. 1989;71:541.
17 Benumof JL, Scheller MS. The importance of transtracheal jet ventilation in the management of the difficult airway. Anesthesiology. 1989;71:769-778.
18 Kress TD, Balasubramaniam S. Cricothyroidotomy. Ann Emerg Med. 1982;11:197-201.
19 Pryor JP, Reilly PM, Schapiro MB. Surgical airway management in the intensive care unit. Crit Care Clin. 2000;16:473-488.
20 Delaney A, Bagshaw SM, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta-analysis. Crit Care. 2006;10:R55.
21 Silvester W, Goldsmith D, Uchino S, et al. Percutaneous versus surgical tracheostomy: a randomized controlled study with long-term follow-up. Crit Care Med. 2006;34:2145-2152.
22 Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy. Chest. 1985;87:715-719.
23 Griggs WM, Worthley LIG, Gilligan JE, et al. A simple percutaneous tracheostomy technique. Surg Gynecol Obstet. 1990;170:543-545.
24 Anon JM, Escuela MP, Gomez V, et al. Use of percutaneous tracheostomy in intensive care units in Spain. Results of a national survey. Intens Care Med. 2004;30:1212-1215.
25 Nates JL, Cooper DJ, Myles PS, et al. Percutaneous tracheostomy in critically ill patients: a prospective, randomized comparison of two techniques. Crit Care Med. 2000;28:3734-3739.
26 Gross JB, Hartigan M, Schaffer DW. A suitable substitute for 4% cocaine before blind nasotracheal intubation: 3% lidocaine–0.25% phenylephrine nasal spray. Anesth Analg. 1984;63:915-918.
27 Mallampatti SR, Gugino LD, Desai SP, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can J Anaesth. 1985;32:429-434.
28 Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105-1111.
29 Heimlich HJ. A life saving maneuver to prevent food-choking. JAMA. 1975;234:398-401.
30 Bogdonoff DL, Stone DJ. Emergency management of the airway outside the emergency room. Can J Anaesth. 1992;39:1069-1089.
31 Angood PB, Attia EL, Brown RA, et al. Extrinsic civilian trauma to the larynx and cervical trachea – important predictors of long term morbidity. J Trauma. 1986;26:869-873.
32 Miller RD, Hyatt RE. Evaluation of obstructing lesions of the trachea and larynx by flow volume loops. Am Rev Respir Dis. 1973;108:475-481.
33 ECC Committee, Subcommittees and Task Forces of the American Heart Association. American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112(Suppl 1):IV1-IV203.
34 Afilalo M, Guttman A, Stern E, et al. Fibreoptic intubation in the emergency department: a case series. J Emerg Med. 1993;11:387-391.
35 Mittleman RE, Wetli CV. The fatal café coronary: foreign body airway obstruction. JAMA. 1982;247:1285-1288.
36 Barakate MS, Jensen MJ, Hemli JM, et al. Ludwig’s angina: report of a case and review of management issues. Ann Otol Rhinol Laryngol. 2001;110:453-456.
37 Muehlberger T, Kunar D, Munster A, et al. Efficacy of fibreoptic laryngoscopy in the diagnosis of inhalation injuries. Arch Otolaryngol Head Neck Surg. 1998;124:1003-1007.
38 Joynt GM, Ho KM, Gomersall CD. Delayed upper airway obstruction. A life-threatening complication of Dettol poisoning. Anaesthesia. 1997;52:261-263.
39 Park KW, Darvish A, Lowenstein E. Airway management for adult patients with acute epiglottitis: a 12-year experience at an academic medical center (1984–1995). Anesthesiology. 1998;88:254-261.
40 Mayo-Smith M. Fatal respiratory arrest in adult epiglottitis in the intensive care unit. Chest. 1993;104:964-965.
41 Ames WA, Ward VM, Tranter RM, et al. Adult epiglottitis: an under-recognized, life-threatening condition. Br J Anaesth. 2000;85:795-797.
42 Corren J, Schocket AL. Anaphylaxis: a preventable emergency. Postgrad Med. 1990;87:167-178.
43 Donaldson VH, Evans RR. A biochemical abnormality in hereditary angioneurotic edema: absence of serum inhibitor of C’1-esterase. Am J Med. 1963;35:37-44.
44 Joynt GM, Abdullah V, Wormald PJ. Hereditary angioedema: report of a case. Ear Nose Throat J. 2001;80:321-324.
45 Bork K, Barnstedt SE. Treatment of 193 episodes of laryngeal edema with C1 inhibitor concentrate in patients with hereditary angioedema. Arch Intern Med. 2001;161:714-718.
46 Niels JF, Weiler JM. C1 esterase inhibitor deficiency, airway compromise, and anesthesia. Anesth Analg. 1998;87:480-488.
47 Agostoni A, Cicardi M, Cugno M, et al. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology. 1999;44:21-25.
48 François B, Bellissant E, Gissot V, et al. 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomised double-blind trial. Lancet. 2007;369:1083-1089.
49 Tami TA, Chu F, Wildes TO, et al. Pulmonary edema and acute upper airway obstruction. Laryngoscope. 1986;96:506-509.
50 Willms D, Shure D. Pulmonary edema due to upper airway obstruction in adults. Chest. 1988;94:1090-1092.