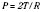CHAPTER 12 Airway Management
Developmental anatomy*
The Upper Airway
Formation of the Cranial Vault and Base
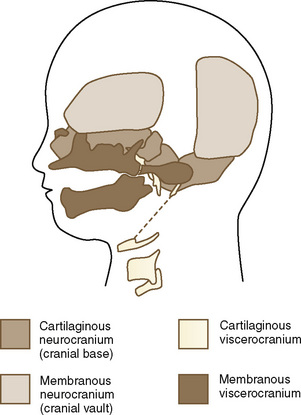
The skull is a critical factor in the development of the face and therefore the upper airway. The skull develops from a membranous and cartilaginous neurocranium (Fig. 12-1). The membranous neurocranium gives rise to the flat bones of the cranial vault, and the cartilaginous neurocranium (chondrocranium) forms the skull base. The flat bones of the neurocranium, which form sutures from edge to edge, also form fontanels where more than two bones meet. The base of the skull is formed from the cartilaginous neurocranium, which then becomes the base of the occipital bone, the sphenoid, the ethmoid and petrous bones, and portions of the temporal bone.
Craniovertebral Development
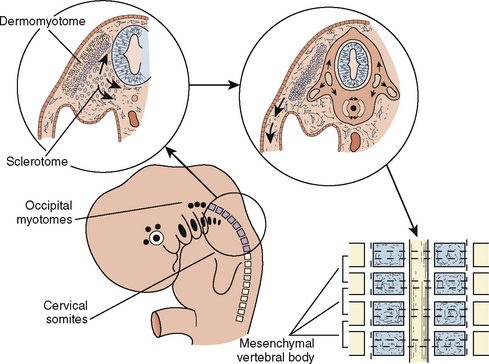
The paraxial mesoderm, a column of tissue on either side of the midline of the embryo, becomes divided into blocks of tissue (somites) at about the fourth week of development. Whereas most of the muscles of the head are derived from mesenchyme of the branchial arches, the cervical somites form the vertebrae of the neck that, under normal circumstances, undergo segmentation (Fig. 12-2). Failure of such segmentation can result in fusion and shortening with severely limited neck movement.
Clinical Correlation
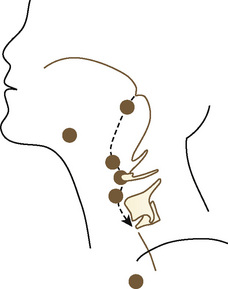
Klippel-Feil syndrome is the result of varying combinations of fusions of the cervical vertebrae, such that the head appears to sit on the shoulders. The normal development of separate cervical vertebrae may be impaired, and fusion of adjacent vertebral bodies may occur. The degree of severity is variable; type I patients have a single-level fusion; type II patients have multiple, noncontiguous fused segments; and type III patients have multiple, contiguous fused segments (Samartzis et al., 2006). Klippel-Feil syndrome can occur with fetal alcohol syndrome, hemifacial microsomia (Goldenhar’s syndrome), and anomalies of the extremities. The neck is short, and the hairline is low. In addition, the neck can be webbed. There may be atlanto-occipital fusion. Laryngoscopy and intubation can be extremely difficult, although laryngeal mask airways (LMAs) have been used successfully (Naguib et al., 1986; Nargozian, 2004).
The Face
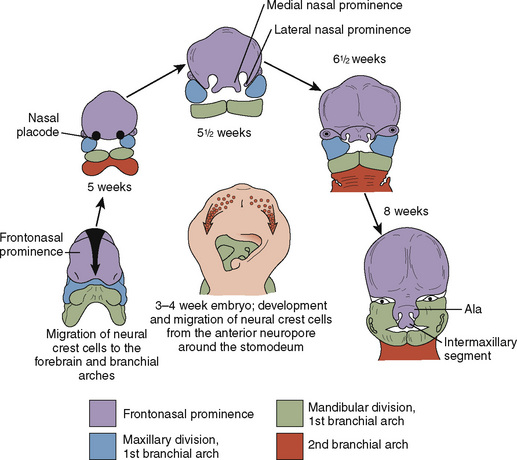
Just as the neurocranium forms the cranial vault and base, the viscerocranium forms the face and is derived mainly from cartilage of the first two branchial arches (Fig. 12-3). Ectodermally-derived neural crest cells of the developing 3- to 4-week-old embryo migrate to branchial arch mesoderm, and the face develops as a result of these massive cell migrations and their interactions. Those cells forming the frontonasal process are derived from the forebrain fold and migrate a relatively short distance as they pass into the nasal region. Those cells that form the mesenchyme of the maxillary and mandibular processes have a considerably longer distance to migrate, because they must move into the branchial arches. At 28 days postconception, the face barely shows its eventual relation to the five primordia from which it is derived: the frontonasal prominence, which is the cranial boundary of the primitive mouth (stomodeum); the paired maxillary prominences (the first branchial arch); and the paired mandibular prominences (also the first branchial arch).
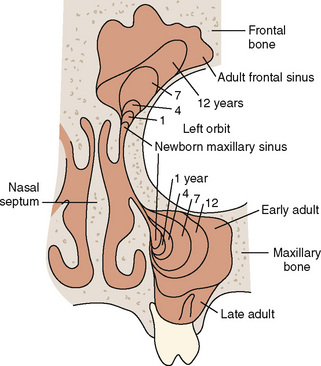
The paranasal sinuses begin developing at approximately 40 weeks of gestational age. The completion of turbinate development signals the beginning of sinus development, which continues until early adult life (Fig. 12-4). Although the exact function of the paranasal sinuses is not well understood, inflammatory, infectious, and neoplastic diseases of the sinuses are of major significance to the anesthesiologist, particularly if there is functional impairment before anesthesia and surgery. Sinus disorders are often comorbidities of asthma, immunoglobulin deficiencies, cystic fibrosis, or Kartagener’s syndrome.
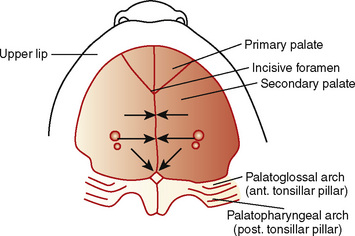
The oral cavity—a structure without structures—where much of the anesthesiologist’s attention and skills are focused, has a complex developmental heritage. The mouth (stomodeum) appears as a slight depression in the surface ectoderm, separated from the oral cavity by the oropharyngeal membrane. This membrane ruptures at about 24 to 26 days’ gestation and the primitive foregut then communicates with the amniotic cavity. The involved germ layers are the endoderm internally and the ectoderm externally. The tongue surface arises primarily from first arch mesenchyme, with significant contributions from the third and fourth arches, hence its complex innervation by the facial nerve in the anterior two thirds and the hypoglossal nerve in the posterior one third (Fig. 12-5). The muscle bulk of the tongue arises primarily from occipital somites, explaining the hypoglossal nerve (XII) innervation and its susceptibility to injury from errant placement of dental rolls and pressure injury from overinflated LMA cuffs. The upper lip is formed by the merging of the maxillary prominences with the medial nasal prominences, with the lateral basal prominences forming the alae. The intermaxillary segment in the central portion of the upper lip area consists of a labial component (forming the philtrum), a maxillary component (associated with the four incisor teeth), and a palatal component (which becomes the primary palate).
The palate divides the nasomaxillary complex from the oral cavity (Fig. 12-6). The palatal processes advance in a medial direction from the maxillary processes of the first branchial arch, fusing in the midline in an anterior-to-posterior sequence and uniting with the premaxilla and the developing nasal septum. The soft palate forms from continued growth of the posterior edges of these palatal processes, ending with the formation and fusion of the two halves of the uvula.
Clinical Correlation
Closure of the cleft palate may result in insufficient tissue for development of normal length or function of the soft palate and require a posterior pharyngeal flap. Velopharyngeal insufficiency is the cause of the hypernasal speech, nasal emission, and nasal turbulence (Sidman and Muntz, 2000).
The Branchial Apparatus
Branchial Arches
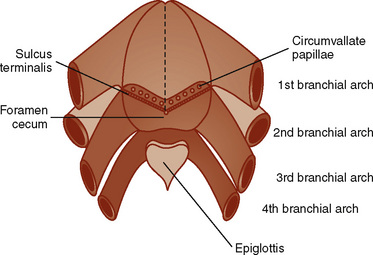
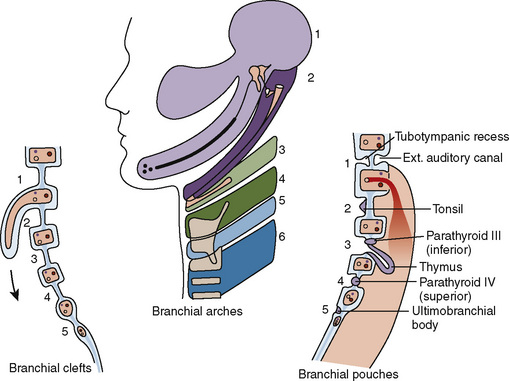
The branchial apparatus consists of four branchial arches visible on the surface of the embryo, as well as fifth and sixth arches that cannot be seen on the surface. Branchial pouches and clefts are likewise numbered craniocaudally (Fig. 12-7). The first branchial arch (Meckel’s) cartilage is the position of the future mandible, as well as the eventual malleus and incus. The second branchial arch cartilage produces the stapes, the styloid process, the stylohyoid ligament, and the superior portion of the body of the hyoid. The other branchial arch cartilages contribute to the inferior portion of the hyoid as well as the thyroid cartilage.
Branchial Pouches
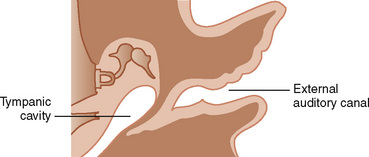
The first branchial pouch develops into the tubotympanic recess, becoming the auditory tube and the middle ear cavity (Fig. 12-8). The cavity of the second branchial pouch is largely obliterated as the palatine tonsil develops, but part of it remains as the tonsillar fossa. The endoderm of the second branchial pouch becomes the surface epithelium of the tonsil and the lining of its crypts, with the mesenchyme around the pouch differentiating into lymphoid tissue. The endoderm of the dorsal part of the third branchial pouches differentiates into the inferior parathyroids, and the ventral parts unite to become the thymus. The paired parathyroid glands develop from separate pouches, with one pair derived from the third and the other from the fourth branchial pouch. At the seventh week of development, the parathyroid glands migrate caudally from their respective branchial pouches, with the third pouch parathyroids moving more caudally than the parathyroids of the fourth pouch. Accessory parathyroid tissue may be left along the line of migration. The endoderm of the fourth branchial pouches differentiates into the superior parathyroid glands, and the ventral parts develop into the ultimobranchial bodies, the calcitonin-secreting portion of the thyroid.
Clinical Correlation
The thyroid begins as a thickening of the endoderm of the floor of the pharynx, in the midline between the first and second pouches, at the foramen cecum. A thin connection, the thyroglossal duct, remains attached to the oral cavity, and its point of attachment marks the origin of the thyroid gland. The thyroid descends along the thyroglossal duct and reaches the level of the first tracheal ring at about the seventh week of gestation (Fig. 12-9). The thyroglossal duct is then normally obliterated. Accessory thyroid tissue may be deposited anywhere along this path; on the other hand, failure of the thyroid to descend may result in a lingual thyroid.
The Larynx
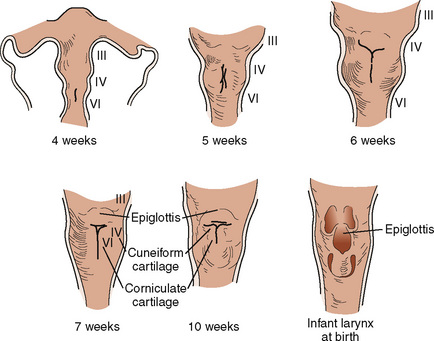
Development of the larynx begins at approximately 3 weeks of gestational age with the formation of the laryngotracheal tube from the ventral wall of the foregut. The laryngotracheal tube then grows caudally into the splanchnic mesoderm on the ventral surface of the foregut, dividing into the right and left lung buds. The epiglottis begins to form from the hypobranchial eminence of the third and fourth arches at approximately 30 to 32 days’ gestation. The aryepiglottic folds develop from the lateral boundaries of the fourth arch along a line from the hypobranchial eminence (epiglottis) to the arytenoid eminence of the sixth arch. Incomplete development at this stage may produce varying degrees of persistent laryngeal cleft (Fig. 12-10). A definite larynx may be seen by 41 days’ gestation. (Fig. 12-11).
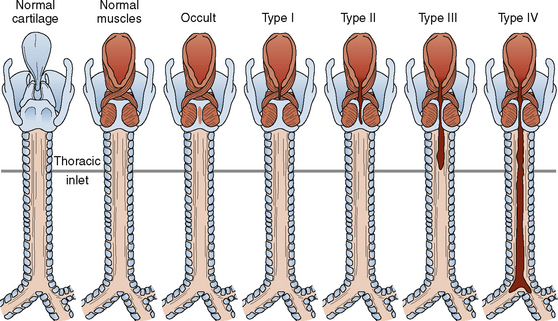
Eckenhoff’s (1951) review of the anatomy of the pediatric larynx has influenced more than a generation of pediatric anesthesiologists with regard to the selection of endotracheal tube size based on the concept of the narrowest (fixed) portion of the upper airway being the level of the cricoid cartilage, or the “funnel-morphing-into-cylinder” concept. Litman et al. (2003) examined laryngeal shape in sedated children undergoing MRI and determined that under sedated conditions with tonic maintenance of laryngeal shape, it is more cylindric than funnel-shaped, as it is in adults, and that the narrowest portion of the airway is at the level of the vocal cords. Furthermore, they concluded that there is no change in this relationship from childhood to adulthood. This finding has been confirmed by video-bronchoscopic imaging in anesthetized and paralyzed children as well and provides an intriguing challenge to traditional teaching (see Chapter 3, Respiratory Physiology) (Dalal et al., 2008, 2009). The adjudication of these disparate concepts, as well as the confirmation in both reports that the cricoid opening is elliptic rather than circular in shape, with the narrowest transverse diameter, help support the transition over the last 10 to 15 years to the common use of cuffed endotracheal tubes with a smaller diameter rather than tightly (or “appropriately”) fitted, uncuffed endotracheal tubes. Moreover, advances in materials and design have allowed the development of thin-walled tubes with shorter, polyurethane cuffs, which provide an equivalent seal at a lower mucosal pressure (Dullenkopf et al., 2005). At this point, the increasingly routine use of cuffed endotracheal tubes, with appropriate care, appears to result in fewer repeated laryngoscopies and reintubations, less postintubation croup and less contamination in the operating room, and lower total fresh-gas flows.
Developmental physiology
The Upper Airway
Isono (2006) has reviewed the physiology of airway maintenance at the pharyngeal level and described the pharynx as a collapsible air-filled tube surrounded by soft tissues enclosed in a rigid box of bony structures, the mandible and vertebrae, with the lumen of the tube determined by the balance of the soft-tissue mass and the size of the surrounding rigid box. This simple approach explains the success of routine clinical airway maneuvers—continuous positive airway pressure (CPAP) serving as a pneumatic stent for the collapsed air-filled tube and surrounding soft tissue, and anterior displacement of the jaw serving to enlarge the rigid box. An oral airway displaces the base of the tongue anteriorly, thereby increasing the transpharyngeal luminal space and moving the base of the tongue away from the prevertebral bodies. Anesthetics, of course, depress the integrity of pharyngeal muscle tone through a variety of mechanisms, including the attenuation of neural input caused by the loss of consciousness and the decrease in tone of the diaphragm and intercostal musculature. The tongue and its muscular attachments such as the genioglossus, geniohyoid, sternohyoid, sternothyroid, and thyrohyoid are affected as well. All of this conspires to narrow the pharyngeal airway, more so in infants and small children than adults, especially during the first year of life (Isono et al., 2000). Adults are more equipped than children to defend against these effects, because they possess a more competent negative pressure reflex serving to augment pharyngeal tone, although the negative pressure reflex has been shown in infants younger than 1 year old as well (Thach et al., 1989; Horner et al., 1991). In addition, the progressive increase in pharyngeal cross-sectional area during the first year of life is positively influenced by the growth of the mandible and maxilla.
Pharyngeal airway obstruction has long been recognized as a significant component of the clinical entity called laryngospasm, and the application of CPAP, whether applied transnasally or transorally, depresses pharyngeal muscle tension and provides a mechanical pneumatic stent for the upper airway (Fink, 1956; Alex et al., 1987). This is a subtle component of the routine technique used by experienced pediatric anesthesiologists in applying gentle amounts of positive pressure during the induction phase, especially with infants and small children.
The fetus is an experienced swallower, well practiced from the age of 10 to 11 weeks’ gestation, with suckling following at 18 to 24 weeks’ gestation. This is fortunate, because coordination of this highly complex task is dependent on practice in utero and learning in pre- and postnatal life. However, breathing, even in utero, is a relatively late event, occurring at about 32 to 37 weeks’ gestational age, and premature infants show their lack of experience with significant discoordination between swallowing and breathing, as do children who are neurologically impaired (Goldson, 1987; Arvedson et al., 1994).
Protective airway reflexes are initiated via adduction of the true and false vocal cords, closing the laryngeal vestibule. The epiglottis deflects posteriorly to cover the laryngeal inlet, diverting food into the pyriform sinuses. Finally, the larynx elevates through the effort of the suprahyoid musculature, contracting prior to the entrance of the food bolus into the hypopharynx, which aids in opening the cricopharyngeal sphincter. Moreover, activation of sensory receptors in the pharynx and anterior tonsillar pillars inhibits respiration during swallowing. Finally, the esophageal phase, from the upper esophageal sphincter to the lower esophageal sphincter (LES), is mediated by contractions that occur throughout the length of the esophagus via a coordinated peristaltic wave. The cricopharyngeal sphincter returns to a tonic state (+5 mm Hg) from its relaxed state (−15 mm Hg), as do the diaphragmatic crura, the angle of the gastroesophageal junction, and the diaphragm, thereby preventing reflux into the hypopharynx. Reflex protection of the airway occurs via two pathways: anterograde protection accomplished during normal swallowing, and retrograde protection accomplished by antireflux mechanisms. Laryngeal and nasopharyngeal closure occur via the influence of the superior laryngeal nerve on laryngeal mucosal receptors. Clinically, the adverse effects of laryngeal secretions manifest themselves as reflex closure of the larynx and sustained apnea (Perkett and Vaughan, 1982; Bartlett, 1985). With maturity, coughing replaces apnea as a protective mechanism (Miller et al., 1952; Leith, 1985). As far as gastrointestinal reflux is concerned, increasing attention has been devoted to its contribution to recurrent pneumonia, reactive airways disease, apnea, and respiratory failure. The upper and lower esophageal sphincters maintain a resting tone of 10 to 30 mm Hg over intragastric pressure, and in combination with crural support and the acute gastroesophageal angle, act to prevent regurgitation of stomach contents. However, premature infants have a lower LES pressure and a less acute gastroesophageal angle; central nervous system (CNS) immaturity is related to abnormalities of the LES relaxation pattern (Hillemeier, 1996).
Developmental airway physics
Because of the variable diameter and length of the trachea, as well as the common (mainly in toddlers) problems of foreign body aspiration and anatomic or functional airway obstruction, understanding the physics of gas flow is even more crucial in pediatric anesthesia than in any other anesthesiology subspecialty. Noisy breathing is often an ominous sign in infants and small children. Stridor is noisy breathing coupled with increased inspiratory efforts, such as nasal and rib-cage flaring and suprasternal and sternal retraction. Severe airway obstruction may result in cyanosis, respiratory distress and fatigue, pneumothorax, pneumomediastinum, and death. Purely inspiratory stridor usually indicates lesions in the upper part of the airway. Lesions distal to the vocal cords usually produce expiratory stridor. Biphasic stridor is most characteristic of obstruction at the level of the subglottic space. Physical laws not only help explain the basis of these signs but provide guidance about effective clinical intervention (see Chapter 3, Respiratory Physiology in Infants and Children).
Laminar and Turbulent Flow
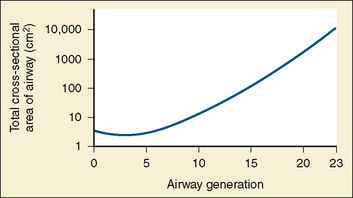
Upper and lower conducting-airway air flow is turbulent, whereas the peripheral airways are characterized by laminar air flow, enhancing alveolar ventilation. The conducting airways bifurcate progressively (but not symmetrically). Cartilaginous support is characteristic of the main, lobar, and segmental bronchi, and more distal conducting airways have bronchial muscle forming a geodesic network. The total cross-sectional area of the respiratory tract is minimal at the third generation (Fig. 12-12). Small bronchi extend through about seven generations, and the total cross-sectional area increases by about sevenfold by generation 11. After the eleventh generation, cartilage disappears from the airway wall. With these noncartilaginous walls embedded within the lung parenchyma, contiguous elastic tissue serves to hold the airways open, and therefore lung volume plays a substantial role in the maintenance of conducting airway patency. In the terminal bronchioles, the total cross-sectional area is about 30 times the area at the level of the large bronchi. Flow resistance in these small airways is about 10% of lower airway resistance (Macklem and Mead, 1967).
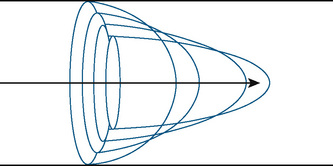
Flow in laminar profiles enhances diffusivity, because flow at the center of the shear increases the rate of spread in that direction (Fig. 12-13). Fluids or gas molecules moving through a tube under constant pressure separate into a gradient of velocity profiles, with the fastest component in the center and the slowest components near the wall. When the cross-sectional area is reduced, the velocity must increase (and the pressure decrease) in order to maintain the same volume flow rate; resulting gas flow is much more turbulent. With abrupt narrowing, complete separation of the stream profiles occurs, the center stream constricts to a minimum value, and laminar flow may not be able to resume because of the turbulence. Pragmatically, when difficulty with positive pressure ventilation occurs, the natural response of the anesthesiologist is to apply more pressure to the rebreathing bag. For the patient whose airway is compromised, this results in more turbulence in the upper airways and less effective air flow downstream. Passive exhalation may also be impaired, and air trapping may result. However, if the pressure gradient (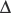 P) across the obstruction is reduced (e.g., changing to spontaneous breathing from controlled ventilation in order to reduce the high pressure proximal to the obstruction), then air flow may be enhanced distally. This applies to airway narrowing, regardless of intrinsic or extrinsic causes.
P) across the obstruction is reduced (e.g., changing to spontaneous breathing from controlled ventilation in order to reduce the high pressure proximal to the obstruction), then air flow may be enhanced distally. This applies to airway narrowing, regardless of intrinsic or extrinsic causes.
Clinical Correlation
Causes of stridor include congenital, inflammatory, traumatic, and foreign bodies; a congenital cause is found in 85% of children younger than 2.5 years of age (Holzman, 2000). Severe airway obstruction may result in cyanosis, respiratory distress and fatigue, pneumothorax, pneumomediastinum, and death.
Bubble Stability and Breathing: Laplace’s Law
where P equals pressure within the sphere, T equals surface tension of the liquid, and R equals the radius of the sphere. In addition to the amazing ability of pulmonary surfactant to dramatically reduce the surface tension at the alveolar surface (gas-liquid interface), the surfactant stabilizes the airspace by its ability to change the surface tension as the surface expands and contracts during the respiratory cycle; it decreases surface tension as the surface expands and increases the surface tension as the surface expands, affecting Laplace’s Law so as to maintain and stabilize air space pressure during the respiratory cycle and prevent alveolar collapse (see Chapter 3, Respiratory Physiology in Infants and Children).
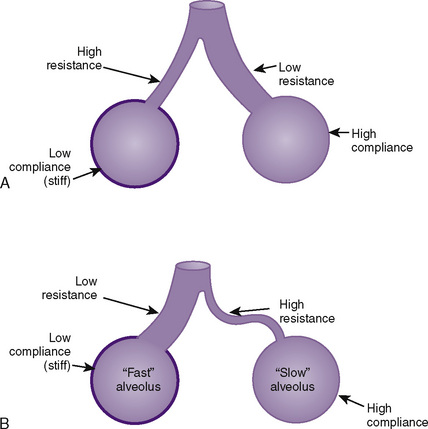
Understanding this relationship also helps explain the improvement in ventilation/perfusion matching with spontaneous breathing when there are two functional lung units of unequal compliance and resistance; an unusually high surface tension, along with interstitial fluid accumulation (edema) and fibrosis, would decrease compliance of the segment. Two functional units (e.g., lungs) of equal compliance and resistance increase their volumes equally when mouth pressure is increased to a constant level; the time course of wash-in is identical for both units. If the compliances of the two units are identical but the resistance of one is twice that of the other, however, the time constant is also twice normal and the unit with the higher resistance fills more slowly, although the volume increase in both units is the same if inflation is prolonged indefinitely. If inspiration ceases after 2 seconds, the pressure is higher in the unit with the lower resistance. Although it is true that paralysis and artificial ventilation do not greatly alter gas exchange ventilation/perfusion (V/Q) matching, dead space to tidal volume ratio (VD/VT), and shunt in young, healthy patients in comparison with spontaneous ventilation. This is not true for patients with inequalities in the distribution of ventilation because of asymmetric resistances between lung units (Fig. 12-14). Slow or sustained inflation permits increased distribution of gas to slow alveoli and so tends to distribute gas in accordance with the compliance of the different functional units. Such a situation is the principle underlying frequency-dependent compliance.
Clinical Correlation
Infantile (or idiopathic) respiratory distress syndrome (IRDS), or hyaline membrane disease, is a major cause of respiratory distress in preterm infants because of surfactant deficiency, as well as ongoing respiratory embarrassment in bronchopulmonary dysplasia. Surfactant is a complex mixture of lipids and proteins secreted in type II respiratory epithelial cells that lowers alveolar surface tension and therefore decreases the pressure needed to keep alveoli open and inflated. Surfactant deficiency and the consequent alteration in the ability of alveoli to remain optimally inflated results in reduced compliance, reduced functional residual capacity, unevenly increased airway resistance, and decreased alveolar ventilation. These anatomic changes therefore result in an increased physiologic dead space, ventilation/perfusion imbalance, hypoxemia, hypercarbia, and mixed respiratory and metabolic acidosis. In addition, for children with peripheral airways obstruction who require assisted ventilation, the work of breathing during spontaneous breaths is decreased by the application of positive end-expiratory pressure (Graham et al., 2007).
Airway equipment
Routine Pediatric Airway Equipment
Face Masks
Because the cushion-seal face masks used for anesthetizing children have dead spaces from 50 to 80 mL, they add substantially to rebreathing. For this reason, Rendell-Baker and Soucek (1962) introduced a molded, low–dead-space face mask specifically for use with spontaneously breathing infants and children at a time when the administration of halothane anesthesia by mask was popular. However, it is easier to seal to the patient’s face with a cushioned face mask than a Rendell-Baker model, and a good mask seal is particularly useful for the application of CPAP during the induction or emergence from general anesthesia.
Airway Adjuncts
Oropharyngeal airway devices are often used as “bite blocks” after a patient’s trachea has been intubated, in order to prevent the clenching of the teeth on the endotracheal tube. This maneuver may, however, be hazardous in children between 5 and 10 years of age with loose deciduous teeth. Oropharyngeal airway devices are responsible for up to 55% of anesthesia-related dental complications (Clokie et al., 1989). Furthermore, when an oropharyngeal airway device is used as a bite block during long cases, it may cause necrosis of the tongue, uvular edema, or lip damage (Moore and Rauscher, 1977; Shulman, 1981). A gauze pad that has been rolled up and placed between the patient’s upper and lower molar teeth is a better method of preventing the teeth from clenching on an endotracheal tube and minimizing dental trauma. Caution must be exercised, however, that the roll not slip and place undue pressure on the lateral aspect of the tongue (paraglossal sulcus), where the hypoglossal nerve runs.
The LMA is used successfully for routine pediatric anesthetics and even for adenotonsillectomies (Webster et al., 1993; Williams and Bailey, 1993). LMAs are currently manufactured in several sizes, for patients ranging from neonates to large adults. With minimal inflation of the mask’s cushion and thorough lubrication of the nonlaryngeal surface, an LMA should be seated at the laryngeal inlet and cause minimal discomfort to the patient postoperatively. Following its blind passage through the oral cavity, the proper seating of an LMA is generally heralded by a slight rise of the device when the mask’s cushion is inflated with air. Care should be taken to use the minimal effective inflation pressure for the cuff, typically up to 60 cm H2O. The routine use of a manometer is advocated. In some patients, the cushion of the LMA overrides the proximal portion of the esophagus, thereby exposing the patient to the risk of the aspiration of gastric contents, with the LMA serving as a conduit to the lungs (Nanji and Maltby, 1992). Nevertheless, although ideal positioning of an LMA appears to be achieved in only 50% of cases, the vast majority of patients fare very well (Rowbottom et al., 1991; Goudsouzian et al., 1992; Mizushima et al., 1992).
The more cephalad and anterior position of the larynx of a child as compared with an adult has prompted the use of an alternate insertion technique in children. In this case, the LMA is inserted with its cushion placed against the hard palate. The device is then rotated through 180 degrees until the cushion is seated at the laryngeal inlet (McNicol, 1991). This method for the insertion of an LMA appears to be especially useful in preschool and young school-age children.
Endotracheal Tubes
The most definitive method for airway management in children remains intubation of the trachea. Polyvinylchloride is still the most popular material employed for the production of endotracheal tubes, although other materials continue to be used and newer technologies are evolving. For example, Weiss and Dullenkof (2007) have the reexamined design requirements of pediatric endotracheal tubes, including cuff placement below the cricoid cartilage, which requires a smaller, more distally placed cuff. Among other features, with polyurethane replacing the polyvinylchloride cuff, the sealing pressures for children are in the range of 6 to 14 cm H2O (Weiss and Dullenkof, 2007). Recommended performance specifications, as well as detailed standards, for endotracheal tubes have been published (Carroll et al., 1973; Shupak and Deas, 1981).
Table 12-1 lists estimated values for the appropriate endotracheal tube sizes and lengths as well as those for pediatric LMAs (Cole, 1957; Penlington, 1974; Morgan and Steward, 1982; Steven and Cohen, 1990). In general, the size of the tube is more related to the age rather than size of the patient. When selecting an endotracheal tube for a child, it is important to remember that the presence of a deflated cuff adds about 0.5 mm to the tube’s external diameter. However, the external diameters of endotracheal tubes differ widely among the manufacturers of the tubes. Cuffs also differ in their shapes and positions along the endotracheal tube. In addition, because nitrous oxide diffuses into the closed airspace of an endotracheal tube’s cuff when the inflation valve is closed, this valve should be rendered incompetent during long procedures. Otherwise, the intracuff pressure should be monitored and maintained at a level below 25 cm H2O (18.4 mm Hg). A variety of endotracheal tubes are available for special needs, including preformed oral or nasotracheal tubes (Ring-Adair-Elwyn [RAE] tubes) for oral or dental surgery and wire-reinforced (anode) endotracheal tubes for head and neck surgery or laryngotracheal reconstruction, when the tube may be inserted through the tracheal stoma and sutured to the anterior chest wall. The special precautions that must be taken with endotracheal tubes during laser surgery of the aerodigestive tract have been described (Sosis 1989, 1992; Sosis and Dillon, 1990, 1991, 1993). The majority of laser airway surgery in pediatrics is for the treatment of juvenile laryngeal papillomatosis or other laryngeal anomalies, and these are cases that lend themselves well to Venturi jet ventilation of the lungs. However, wrapped endotracheal tubes may be required for pediatric laser airway surgery when the laryngeal inlet is too narrow to permit a sufficient entrainment of pharyngeal gas for the Venturi technique (Holzman, 1991, 1992).
Since the seminal report by Koka et al. (1977), an air leak around an endotracheal tube has been strongly advocated for the prevention of postintubation croup in infants and children younger than 10 years of age. Surprisingly, a great deal of variability exists in the ability of a clinician to recognize an air leak around an uncuffed endotracheal tube (Schwartz et al., 1993). However, cuffed endotracheal tubes are advantageous for certain abdominal or thoracic procedures. Also, patients with pulmonary pathologic conditions with poor lung compliance may require high peak inspiratory pressures to assure adequate ventilation of the lungs, and a cuffed endotracheal tube may be necessary for these cases. Because of an increasingly sophisticated understanding of developing laryngeal anatomy and vastly improved materials science in the manufacture of endotracheal tubes, there is a currently a greatly expanded use of cuffed endotracheal tubes in all ages (see The Larynx, p. 350) Accordingly, the use of a cuffed endotracheal tube should be individualized (Table 12-2). Likewise, children who are susceptible to croup during a viral illness of the respiratory tract may benefit from tracheal intubation with a smaller endotracheal tube than is normally used, with or without a cuff, as long as the lungs can be ventilated adequately. A throat pack may help make a seal when an uncuffed endotracheal tube is employed.
TABLE 12-2 Advantages of Cuffed vs. Uncuffed Endotracheal Tubes
| Cuffed | Uncuffed | |
| Advantages | Not important for subglottic stenosis | Larger tube, therefore less resistance for spontaneous breathing and mechanical ventilation |
| Fewer repeat laryngoscopies and reintubations | Lower risk of occlusion | |
| Less contamination | No cuff = no ridges | |
| Lower fresh-gas flow | No concern about tip-to-cuff border distance | |
| Better protection against aspiration | No requirement for pressure monitoring | |
| Indications | High risk of aspiration | Minus the concerns on the left, most likely does not matter much |
| Preexisting or impending impaired pulmonary compliance | ||
| Patient with poor lung compliance undergoing minimally invasive abdominal or chest surgery | ||
| Cardiopulmonary bypass | ||
| Requirement for precisely controlled mechanical ventilation |
Endotracheal tubes are available with or without a special opening known as a Murphy eye in the wall opposite the tube’s distal bevel. Endotracheal tubes fabricated without the Murphy eye are known as Magill tubes, whereas those that have this opening are called Murphy tubes. The Murphy eye was designed to provide an alternate pathway for the flow of ventilatory gases if the distal opening of the endotracheal tube was occluded—a common situation, especially in infants (Murphy, 1941). However, there are potential disadvantages to the presence of a Murphy eye on an endotracheal tube, including a tendency for accumulation of secretions and the possibility that a stylet, catheter, or bronchoscope may get stuck, requiring the removal of the entire assembly. Cuffed endotracheal tubes manufactured without a Murphy eye can have the cuff located closer to the tube’s tip. Nevertheless, pediatric anesthesiologists generally favor the use of an endotracheal tube that is equipped with a Murphy eye because of the obstruction risk. Recently, Weiss and Dullenkof (2007) have reexamined the design requirements of a more anatomically suitable pediatric endotracheal tube, including cuff placement below the cricoid cartilage, thus requiring a smaller, more distally placed cuff and removal of the Murphy eye. Among other features, with a polyurethane as opposed to a polyvinylchloride cuff, the sealing pressures for children are in the range of 6 to 14 cm H2O, well under the 20 to 25 cm H2O for the leak test.
The concerns about anatomic differences between children and adults were typically partisan enough that while an emerging acceptance of endotracheal intubation was obvious beginning in the 1950s and 1960s, it remained a relatively rare technique in pediatric anesthesia. Clinicians were warned about the narrowest part of the pediatric airway located at the cricoid cartilage. Outcome studies began to suggest the safety and efficacy of endotracheal intubation in infants and children, and pediatric anesthesia clinicians were becoming more vocal in defense of this emerging technique. See related video online at www.expertconsult.com
![]() .
.
Equipment for the Management of a Difficult Airway in a Child
Line-of-sight devices have been the mainstay of direct laryngoscopy techniques, yet difficulties with laryngoscopy and intubation are not rare. Although it is typically acknowledged as a rare event, there is emerging literature that difficulty with laryngoscopy may be encountered in 5% to 20% of adult patients; although this may be substantially less of an issue in children and it may be age dependent (0.57% in newborns and toddlers, 0.12% in preschool, and 0.05% in school-age patients) (Rose and Cohen, 1994, 1996; Ezri et al., 2003; Schmidt and Koch, 2008).
Anterior commissure laryngoscopes, because of their tubular construction, are particularly useful with a difficult-to-visualize “anterior” (cephalad) larynx in the patient with a small oral cavity. They require a fiberoptic external light source. In addition, long bronchoscopy forceps must be available. The 15-mm connector is removed from the endotracheal tube, and the laryngoscope is inserted. With the epiglottis lifted directly from the laryngeal surface, the tip of the laryngoscope should be placed at the glottic opening. The endotracheal tube is inserted through the tubular laryngoscope blade. Although direct visualization of the glottis is usually lost at this point, correct positioning and the tubular structure of the blade guide the tube into the trachea. Using the forceps, the tube is held in place while the laryngoscope is removed over the tube and forceps (Fig. 12-15). The endotracheal tube connector is then replaced, and the tube position is verified.
With fiberoptic and video modifications, flexible, malleable, and rigid devices have been developed that have added to the laryngoscopy tool shed in recent years. Optical stylets may be rigid, flexible, or malleable; resemble a conventional laryngoscope; or be more like a flexible fiberoptic bronchoscope (Liem et al., 2003). The basic design consists of a fiberoptic rod with an eyepiece at the user end that allows the anesthesiologist to view the advancement of the endotracheal tube, which is threaded over the device. Examples of these devices include the Bonfils Retromolar Intubation Fiberscope (Karl Storz Endoscopy; Culver City, California), the Shikani Optic Stylet (Clarus Medical LLC; Minneapolis, Minnesota), the StyletScope (Nihon Kohden Corp.; Tokyo, Japan), the Video Optical Intubation Stylet (VOIS, Acutronic Medical Systems, AG; Baar, Switzerland), and the Levitan FPS Scope (Clarus Medical LLC; Minneapolis, Minnesota) (see Chapter 10, Equipment, Fig. 10-24). The Bonfils laryngoscope is a nonmalleable scope, whereas the Shikani laryngoscope has a distally malleable stylet. It can accommodate a size 2.5 endotracheal tube in its pediatric version. All of these stylets can be used alone or in conjunction with rigid direct laryngoscopy. In the absence of direct laryngoscopy, a jaw lift or jaw thrust should be used to increase the anteroposterior distance of the hypopharyngeal space and move the base of the tongue away from the laryngeal inlet.
Rigid fiberoptic laryngoscopes have a fiberoptic bundle encased in a closed structure. They serve the same function as conventional rigid laryngoscopes, in that they are intended for viewing the larynx and confirming the insertion of the endotracheal tube. They cannot view the subglottic space or be inserted by any route other than transorally. Examples include the Bullard laryngoscope, UpsherScope, and WuScope. Only the Bullard laryngoscope is available in pediatric versions (see Chapter 10, Equipment, Fig. 10-30). The Acutronic Video Intubating laryngoscope (Acutronic Medical Systems, AG; Baar, Switzerland) was developed by Weiss and resembles a conventional laryngoscope and blade with a fiberoptic bundle that can be coupled to a video camera.
Rigid video laryngoscopes allow the image to be displayed to an eyepiece or a video screen. The video camera may be located within the handle, mounted on the handle, or displayed remotely. Other rigid video laryngoscopes include the Glidescope (Verathon, Inc.; Bothell, Washington) (see Chapter 10, Equipment, Fig. 10-31; see related video online at www.expertconsult.com)![]() . It is available in pediatric and neonatal versions. Because the larynx is not in the line of sight, the Glidescope should be used with a stylet. Exposure of the vocal cords on the image does not necessarily occur from exposure of the larynx. Antifogging solutions are not required, because a charge-coupled video chip covered by a heated glass window is incorporated into the blade, making it fog resistant.
. It is available in pediatric and neonatal versions. Because the larynx is not in the line of sight, the Glidescope should be used with a stylet. Exposure of the vocal cords on the image does not necessarily occur from exposure of the larynx. Antifogging solutions are not required, because a charge-coupled video chip covered by a heated glass window is incorporated into the blade, making it fog resistant.
The flexible fiberoptic laryngoscopes that are used for children are generally smaller than adult scopes, and not all small fiberscopes are designed with a channel for suctioning or oxygen insufflation. Also, pediatric fiberscopes may be limited in the extent of their tip excursion in comparison with larger fiberoptic laryngoscopes. A pediatric fiberoptic laryngoscope is often used to intubate the trachea of a child who is breathing spontaneously with a volatile anesthetic. In such cases, an airway intubation (Frei) mask or one with a similar design, which incorporates a grommet through which a fiberscope may be passed, is very useful (Frei, 1995) (see related video online at www.expertconsult.com)![]() .
.
It may seem counterintuitive to advocate blind intubation techniques amidst these advances in technology; however, in skilled and thoughtful hands, these methods are worthy of consideration because they have stood the test of time, and simply put, they work! Devices for blind intubation include the gum elastic bougie (e.g., Eschmann Stylet, Portex Limited; Hythe, United Kingdom) and the light wand (e.g., Trachlight, Laerdal Medical; Wappingers Falls, New York). In one study, children whose tracheas were found to be extremely difficult to intubate by conventional means were intubated without difficulty with the use of a light wand (Holzman et al., 1988). Finally, tactile intubation can be accomplished by using the index and middle fingers of the nondominant hand intraorally to palpate the epiglottis while the endotracheal tube or stylet is advanced along the palmar surface of the index finger to the larynx, laryngeal inlet, and intratracheally.
Breathing Systems
The valved-circle anesthesia system or variations of Ayre’s valveless T piece are the most common breathing circuits for pediatric patients (Mapleson, 1954). For years, the T piece was recommended for children weighing less than 10 kg because of the decreased resistance to spontaneous breathing, the better “feel” of the rebreathing bag in the hand of the anesthetist, and the faster anesthesia induction and emergence times using higher nonrebreathing fresh-gas flows. Improvements in machine components have resulted in low-resistance valves, reduced dead space at connections, improved CO2-absorbent canister design, and the availability of capnography, as well as changes in philosophy that favor controlled pulmonary ventilation in small children; such improvements have rendered these arguments less durable. Likewise, modern, less-soluble inhalation agents make the influence of the breathing circuit less of an issue.
Clinical airway care
Expert airway care is the most important skill in pediatric anesthesiology, and skill in understanding and controlling anesthetic depth is arguably the most important aspect of providing safe and expert airway care. It is critical to the success of spontaneous breathing techniques for diagnostic examination of the native airway, dynamic changes such as extrinsic compression or tracheomalacia, and for interventions such as the removal of a foreign body from the airway (see videoclip of infant bronchoscopy online at www.expertconsult.com)![]() . Aside from the assessment of heart rate, blood pressure, and patient movement, depth of anesthesia can also be evaluated by continuous monitoring of the heart sounds, the “classic” eye signs, and repeated examination of muscle tone. The arms and legs should feel completely relaxed, and the abdominal muscles should be soft and easily compressible, like bread dough, distinct from the boardlike rigidity of the excitement phase or the mild to moderate muscle tone of the awake or sedated state. Disconjugate gaze and pupillary dilation shortly after inhalation induction support the assessment of light anesthesia, whereas midsize pupils in midposition, along with other physical findings of acceptable anesthetic depth, add to the clinician’s assessment. All signs together should be used to form a composite, so that ideally the anesthesiologist should be able to ensure lack of patient movement in response to stimulation. Facility in determining anesthetic depth is also important for successful “deep” extubations—that is, the removal of the endotracheal tube while the patient is deeply anesthetized and breathing spontaneously. The presumption is that the patient will have less coughing, bucking, and airway irritability during the emergence process because of the lack of an endotracheal tube. In experienced hands, this technique is at least as safe as the more typical “awake” extubation (Patel et al., 1991; Pounder et al., 1991; Karam et al., 1995).
. Aside from the assessment of heart rate, blood pressure, and patient movement, depth of anesthesia can also be evaluated by continuous monitoring of the heart sounds, the “classic” eye signs, and repeated examination of muscle tone. The arms and legs should feel completely relaxed, and the abdominal muscles should be soft and easily compressible, like bread dough, distinct from the boardlike rigidity of the excitement phase or the mild to moderate muscle tone of the awake or sedated state. Disconjugate gaze and pupillary dilation shortly after inhalation induction support the assessment of light anesthesia, whereas midsize pupils in midposition, along with other physical findings of acceptable anesthetic depth, add to the clinician’s assessment. All signs together should be used to form a composite, so that ideally the anesthesiologist should be able to ensure lack of patient movement in response to stimulation. Facility in determining anesthetic depth is also important for successful “deep” extubations—that is, the removal of the endotracheal tube while the patient is deeply anesthetized and breathing spontaneously. The presumption is that the patient will have less coughing, bucking, and airway irritability during the emergence process because of the lack of an endotracheal tube. In experienced hands, this technique is at least as safe as the more typical “awake” extubation (Patel et al., 1991; Pounder et al., 1991; Karam et al., 1995).
Profound surface analgesia of the oropharynx and laryngeal apparatus can be achieved by a combination of topical anesthesia of the tongue and posterior pharyngeal wall, glossopharyngeal nerve block, and superior laryngeal nerve block, as well as a transtracheal injection of local anesthetic (Cooper and Watson, 1975). Because most children will not tolerate this strategy well when they are awake, it is usually accomplished with sedation.
Care of the child with a difficult airway
General Issues
There is some evidence that the toll of providing care for children, especially resuscitation, may occasionally impose a substantially larger psychological burden on the pediatric specialist. For this reason, a color-coded resuscitation aid such as the Broselow-Luten system may decrease practitioner anxiety and afford more time for critical thinking during crises (Luten and Broselow, 1999, Luten et al., 2002). Although the evaluation of difficult airway anatomy in a child is, in principle, very similar to that of an adult, children often do not cooperate. With regard to the difficult airway, patients with a difficult airway as a “syndrome” are not usually the challenge; they have been previously identified, are often well categorized, and may even have a tracheostomy; the challenge is the patient who may look somewhat challenging to the less experienced evaluator but indeed has a difficult airway.
Specific Issues Unique to Children with Difficult Airways
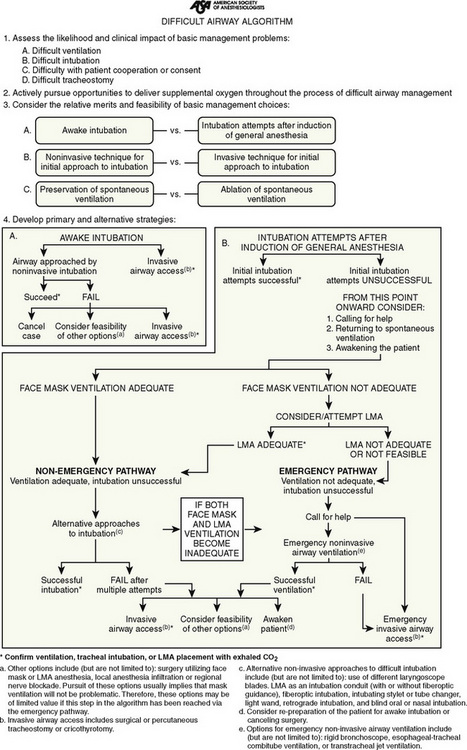
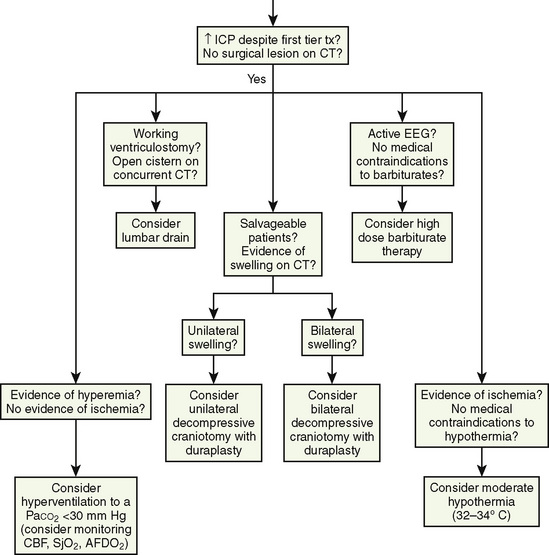
Decision algorithms about the difficult airway are available, and most of the issues that are specific to the anticipated difficult pediatric airway are not that different from those for adults, including a broad range of appropriate equipment, occasional imaging studies, and preparation with sedation and topical local-anesthetic techniques (Fig. 12-19). If a spontaneous-breathing anesthetic technique is chosen, pediatric anesthesiologists are already comfortable with the assessment of anesthetic depth in children. Patient cooperation is a key crucial difference in the recognized difficult airway and in children is influenced by the chronologic, developmental, emotional, and intellectual components of the preoperative assessment. Surgical airway access, whether by needle cricothyroidotomy, open cricothyrotomy, or tracheostomy, is hazardous, especially in inexperienced hands, yet it may be the only option available. If this is a possible part of the airway plan, collaboration with a surgeon is essential.
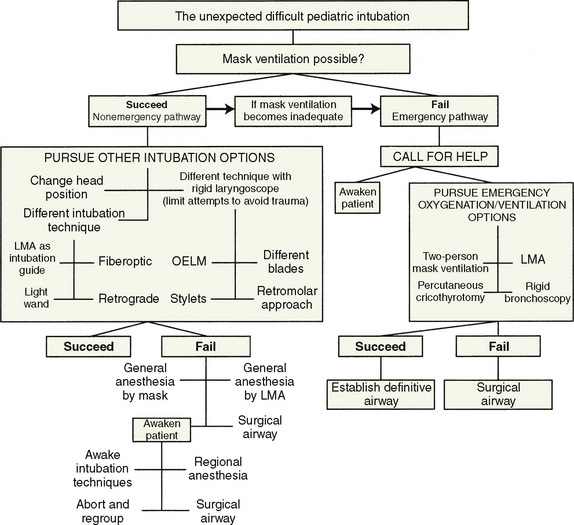
The unexpected difficult pediatric airway patient is a different matter. Wheeler proposed a very reasonable modification to the well-accepted adult difficult airway algorithm (Fig. 12-20) (Wheeler, 1998). The key to this, or any other, algorithm is that it presents a reasoned and organized approach to a high-risk situation.
Care of the Patient with a Surgical or Reconstructed Airway
Pharyngeal Flap Surgery
Following cleft-palate repair, pharyngeal flap and oronasal fistula repair may be needed for velopharyngeal insufficiency. Different types of palatoplasties are performed to alleviate the hypernasality, depending on the cause and dynamics of the insufficiency. The length and width of the flap are tailored to the degree of velopharyngeal incompetence, and it is important for the anesthesiologist to know which kind of repair was done, especially if for subsequent surgery a nasal endotracheal tube or nasogastric tube will be placed, both of which are more problematic if a patient has had a superiorly based flap (Fig. 12-21). Direct vision with a fiberoptic laryngoscope is often the best technique to minimize trauma to the reconstructed area.
External Distraction Devices
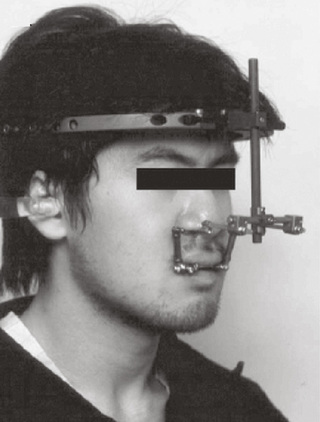
Distraction osteogenesis for unilateral or bilateral advancement of maxillary or mandibular structures has become the major technique in craniofacial surgery. The use of distraction allows the surgeon better control in positioning the bony segments after the osteotomy, enables the segments to be moved a greater distance, and eliminates the need for bone grafts. However, the device may contribute to trismus and also decrease jaw opening. Its presence may interfere with routine airway techniques, and therefore advanced airway-management skills may be needed (Fig. 12-22). Midface advancement using a halo distractor or rigid external distraction (RED) device is favored by some surgeons, whereas others may use an internal midface distractor. Both types have drawbacks. For the anesthesiologist, the initial placement is not as much of a problem as the subsequent procedures to remove them. Limited ability to open the mouth may occur once the distraction is finished. This may be the result of the new position of the maxilla in relation to the mandible, or it may be caused from pain and swelling of the muscles through which the devices pass. The anesthesiologist must therefore be prepared for a more difficult intubation after the initial procedure.
Restoring the Natural Airway
Airway Device Removal
The literature does not provide an extubation strategy for the difficult airway; however, it should probably be closely related to the intubation strategy. Awake extubation vs. extubation before the return of consciousness is a principal consideration, as are general clinical factors that may produce an adverse impact on ventilation after the patient has been extubated. Backup plans for airway management if the patient is not able to maintain adequate ventilation after extubation are a must, as is availability of a device that can serve as a guide for expedited reintubation such as a bougie or exchange catheter (Mort, 2007). Occasionally, a difficult extubation because of patient anxiety is facilitated by a sedation strategy (Arpino et al., 2008).
Summary
For questions and answers on topics in this chapter, go to “Chapter Questions” at www.expertconsult.com.
Alex C., Aronson R., Onal E., et al. Effects of continuous positive airway pressure on upper airway and respiratory muscle activity. J Appl Physiol. 1987;62:2026.
Arpino P., Kalafatas K., Thompson B. Feasibility of dexmedetomidine in facilitating extubation in the intensive care unit. J Clin Pharm Ther. 2008;33:25.
Arvedson J., BR, Buck G., et al. Silent aspiration prominent in children with dysphagia. Int J Pediatr Otorhinolaryngol. 1994;28:173.
Bartlett D. Ventilatory and protective mechanisms of the infant larynx. Am Rev Resp Dis. 1985;131:49. (Suppl)
Carroll R.G., Kamen J.M., Grenvik A., et al. Recommended performance specifications for cuffed endotracheal and tracheostomy tubes: a joint statement of investigators, inventors, and manufacturers. Crit Care Med. 1973;1:155.
Clokie C., Metcalf I., Holland A. Dental trauma in anaesthesia. Can J Anaesth. 1989;36:675.
Cole F. Pediatric formulas for the anesthesiologist. Am J Dis Child. 1957;94:472.
Cooper M., Watson R.L. An improved regional anesthetic technique for peroral endoscopy. Anesthesiology. 1975;43:372.
Dalal P., Murray D., Feng A., et al. Upper airway dimensions in children using rigid video-bronchoscopy and a computer software: description of a measurement technique. Paediatr Anaesth. 2008;18:645.
Dalal P., Murray D., Messner A., et al. Pediatric laryngeal dimensions: an age-based analysis. Anesth Analg. 2009;108:1475.
Dullenkopf A., Gerber A., Weiss M. Fit and seal characteristics of a new paediatric tracheal tube with high volume-low pressure polyurethane cuff. Acta Anaesthiol Scand. 2005;49:232.
Eckenhoff J. Some anatomic considerations of the infant larynx influencing endotracheal anesthesia. Anesthesiology. 1951;12:401.
Ezri T., Weisenberg M., Khazin V., et al. Difficult laryngoscopy: incidence and predictors in patients undergoing coronary artery bypass surgery versus general surgery patients. J Cardiothorac Vasc Anesth. 2003;17:321.
Fink B. The etiology and treatment of laryngeal spasm. Anesthesiology. 1956;17:569.
Goldson E. Suck and swallow in the premature infant. Pediatr. 1987;423:96.
Goudsouzian N.G., Denman W., Cleveland R., et al. Radiologic localization of the laryngeal mask airway in children. Anesthesiology. 1992;77:1085.
Graham A., Chandrashekharaiah G., Citak A., et al. Positive end-expiratory pressure and pressure support in peripheral airways obstruction: work of breathing in intubated children. Intensive Care Med. 2007;33:20.
Hillemeier A. Gastroesophageal reflux: diagnostic and therapeutic approaches. Pediatr Clin North Am. 1996;43:197.
Holzman R.S. Anatomy and embryology of the pediatric airway. In: Riazi J., editor. The difficult pediatric airway. Philadelphia: W.B. Saunders; 1998:707.
Holzman R.S. Anesthesia in the child and adolescent. In: Wetmore R.F., Muntz H.R., McGill T.J., editors. Pediatric otolaryngology: principles and practice pathways. New York, NY: Thieme Medical Publishers; 2000:31.
Holzman R.S. Advances in pediatric anesthesia: implications for otolaryngology. Ear Nose Throat J. 1992;71:99.
Holzman R.S. Anesthesia for pediatric subglottic laser surgery. Surv of Anes. 1991;35:191.
Holzman R.S., Nargozian C.D., Florence F.B. Lightwand intubation in children with abnormal upper airways. Anesthesiology. 1988;69:784.
Horner R., Innes J., Murphy K., et al. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol. 1991;436:15.
Isono S. Developmental changes of pharyngeal airway patency: implications for pediatric anesthesia. Paediatr Anaesth. 2006;16:109.
Isono S., Tanaka A., Ishikawa T., et al. Developmental changes in collapsibility of the passive pharynx during infancy. Am J Resp Crit Care Med. 2000;162:832.
Karam R., Najm J., Kattar M., et al. Respiratory complications in children emerging from halothane anesthesia: awake vs deep extubation. Middle East J Anesthesiol. 1995;13:221.
Koka B.V., Jeon I.S., Andre J.M., et al. Postintubation croup in children. Anesth Analg. 1977;56:501.
Leith D. The development of cough. Am Rev Resp Dis. 1985;131(Suppl):39.
Liem E., Bjoraker D., Gravenstein D. New options for airway management: intubating fibreoptic stylets. Br J Anaesth. 2003;91:408.
Litman R., Weissend E., Shibata D., et al. Developmental changes of laryngeal dimensions in unparalyzed, sedated children. Anesthesiology. 2003;98:41.
Luten R., Broselow J. Rainbow care: the Broselow-Luten system: implications for pediatric patient safety. Ambul Outreach. 1999:14. Fall
Luten R., Wears R., Broselow J. Managing the unique size-related issues of pediatric resuscitation: reducing cognitive load with resuscitation aids. Acad Emerg Med. 2002;9:840.
Macklem P., Mead J. Resistance of central and peripheral airways measured by a retrograde catheter. J Appl Physiol. 1967;22:395.
Mapleson W.W. The elimination of rebreathing in various semiclosed anesthetic systems. Br J Anaesth. 1954;26:323.
McNicol L.R. Insertion of the laryngeal mask airway in children. Anaesthesia. 1991;46:330.
Miller H., Proud G., Berhle F. Variations in the gag, cough and swallow reflexes and tone of the vocal cords as determined by direct laryngoscopy in newborn infants. Yale J Biol Med. 1952;24:284.
Mizushima A., Wardall G.J., Simpson D.L. The laryngeal mask airway in infants. Anaesthesia. 1992;47:849.
Moore M.W., Rauscher L. A complication of oropharyngeal airway placement. Anesthesiology. 1977;47:526.
Morgan G.A., Steward D.J. Linear airway dimensions in children: including those from cleft palate. Can Anaesth Soc J. 1982;29:1.
Mort T. Continuous airway access for the difficult extubation: the efficacy of the airway exchange catheter. Anesth Analg. 2007;105:1357.
Motoyama E.K. The shape of the pediatric larynx: cylindrical or funnel shaped? Anesth Analg. 2009;108:1379-1381.
Murphy F. Two improved intratracheal catheters. Anesth Analg. 1941;20:102.
Naguib M., Farag H., Ibrahim A. Anaesthetic considerations in Klippel-Feil syndrome. Can Anaesth Soc J. 1986;33:66.
Nanji G.M., Maltby J.R. Vomiting and aspiration pneumonitis with the laryngeal mask airway. Can J Anaesth. 1992;39:69.
Nargozian C. The airway in patients with craniofacial abnormalities. Paediatr Anaesth. 2004;14:53.
Patel R., Hannallah R., Norden J., et al. Emergence airway complications in children: a comparison of tracheal extubation in awake and deeply anesthetized patients. Anesth Analg. 1991;73:266.
Penlington G.N. Endotracheal tube sizes for children. Br J Anaesth. 1974;29:494.
Perkett E., Vaughan R. Evidence for laryngeal chemoreflex in some human preterm infants. Acta Paediatr Scand. 1982;71:969.
Pounder D., Blackstock D., Steward D. Tracheal extubation in children: halothane versus isoflurane, anesthetized versus awake. Anesthesiology. 1991;74:653.
Rendell-Baker L., Soucek D.H. New pediatric face masks and anaesthetic equipment. Br Med J. 1962;i:1690.
Rose D., Cohen M. The airway: problems and predictions in 18,500 patients. Can J Anaesth. 1994;41:372.
Rose D., Cohen M. The incidence of airway problems depends on the definition used. Can J Anaesth. 1996;43:30.
Rowbottom S.J., Simpson D.L., Grubb D. The laryngeal mask airway in children: a fibreoptic assessment of positioning. Anaesthesia. 1991;46:489.
Sanders R.D. Two ventilating attachments for bronchoscopes. Del Med J. 1967;39:170.
Samartzis D., Herman J., Lubicky J. Classification of congenitally fused cervical patterns in Klippel-Feil patients: epidemiology and role in the development of cervical spine-related symptoms. Spine. 2006;31:E798.
Schmidt J., Koch T. Incidence of a difficult airway in 19,500 children aged 0–17 years. Anesthesiology. 2008;109:A1244.
Schwartz R.E., Stayer S.A., Pasquariello C.A. Tracheal tube leak test: is there inter-observer agreement? Can J Anaesth. 1993;40:1049.
Shulman M.S. Uvular edema without endotracheal intubation. Anesthesiology. 1981;55:82.
Shupak R.C., Deas T.C.. The ideal tracheal tube. Proceedings of a Workshop on Tracheal Tubes, April 30–May 1, 1981. 1981:103. Valley Forge, PA
Sidman J., Muntz H. Cleft Lip and Palate. In: Wetmore R., Muntz H., McGill T., editors. Pediatric otolaryngology: principles and practice pathways. New York: Thieme; 2000:563.
Sosis M.B., Dillon F. Reflection of CO2 laser radiation from laser-resistant endotracheal tubes. Anesth Analg. 1991;73:338.
Sosis M.B., Dillon F. What is the safest foil tape for endotracheal tube protection during Nd-YAG laser surgery? A comparative study. Anesthesiology. 1990;72:553.
Sosis M.B. What is the safest endotracheal tube for Nd-YAG laser surgery? A comparative study. Anesth Analg. 1989;69:802.
Sosis M.B. Which is the safest endotracheal tube for use with the CO2 laser? A comparative study. J Clin Anesth. 1992;4:217.
Sosis M.B., Dillon F.X. A comparison of CO2 laser ignition of the Xomed, plastic, and rubber endotracheal tubes. Anesth Analg. 1993;76:391.
Steven J., Cohen D. Anesthesia equipment and monitoring. In: Motoyama E.K., Davis P.J., editors. Smith’s anesthesia for infants and children. ed 5,. St. Louis: C. V. Mosby Company; 1990:227.
Thach B., Menon A., Scefft G. Effects of negative upper airwawy pressure on pattern of breathing in sleeping infants. J Appl Physiol. 1989;66:1599.
Webster A.C., Morley-Forster P.K., Dain S., et al. Anaesthesia for adenotonsillectomy: a comparison between tracheal intubation and the armoured laryngeal mask airway. Can J Anaesth. 1993;40:1171.
Weibel E.R. Morphometry of the human lung. New York: Academic Press, 1963.
Weiss M., Dullenkof A. Cuffed tracheal tubes in children: past, present and future. Expert Rev Med Devices. 2007;4:73.
Wheeler M. Management strategies for the difficult pediatric airway. In: Riazi J., editor. The difficult pediatric airway. Philadelphia: W.B. Saunders; 1998:743.
Williams P.J., Bailey P.M. Comparison of the reinforced laryngeal mask airway and tracheal intubation for adenotonsillectomy. Br J Anaesth. 1993;70:30.

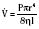
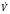 equals the flow of the gas, P equals the total pressure drop, r equals the radius of the tube, η equals viscosity, and l equals length.
equals the flow of the gas, P equals the total pressure drop, r equals the radius of the tube, η equals viscosity, and l equals length.
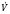 equals the flow of the gas, P equals the total pressure drop, r equals the radius of the tube, μ equals density, l equals length, and f equals Fanning’s friction factor.
equals the flow of the gas, P equals the total pressure drop, r equals the radius of the tube, μ equals density, l equals length, and f equals Fanning’s friction factor.