CHAPTER 41 Advanced Techniques
PART A Arthroscopic Management of Lateral Epicondylitis
INTRODUCTION
Lateral epicondylitis, or tennis elbow, is the most common affliction of the elbow.
The origin of the extensor carpi radialis brevis (ECRB) has been implicated as the source of pathology in this condition.2,5,7–10,13–16,18 Reported histopathologic findings in the affected tendon origin include vascular proliferation and hyaline degeneration, which are consistent with a chronic, degenerative process.10,14,18,20 Most commonly, surgical treatment is directed at excision of this pathologic tissue through an open approach or more recently arthroscopic methods.1,3,5,6,11,15,17,19,21,22
This chapter covers the anatomy of the extensor tendon origins at the humeral epicondyle based on anatomic dissections.4 The location of the ECRB tendon origin is defined relative to intra-articular landmarks. Using these data, a technique for arthroscopic lateral epicondylitis surgery is presented with early clinical results.
ANATOMY
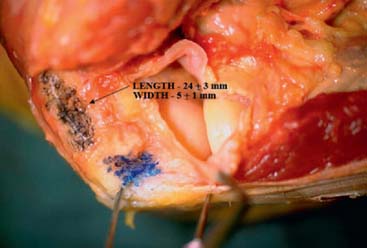
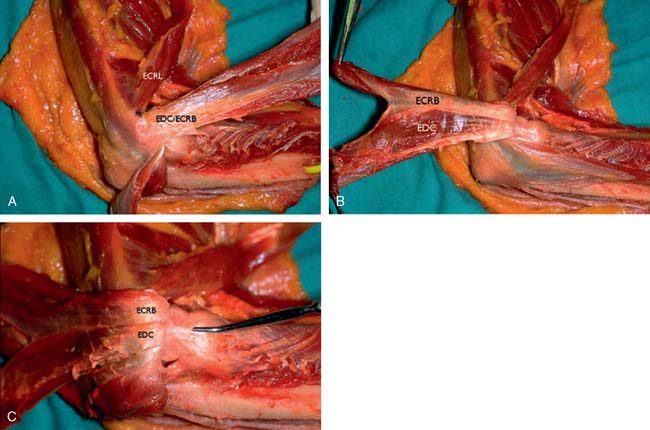
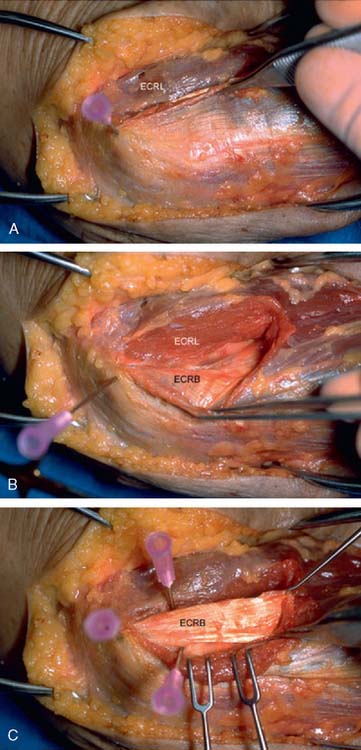
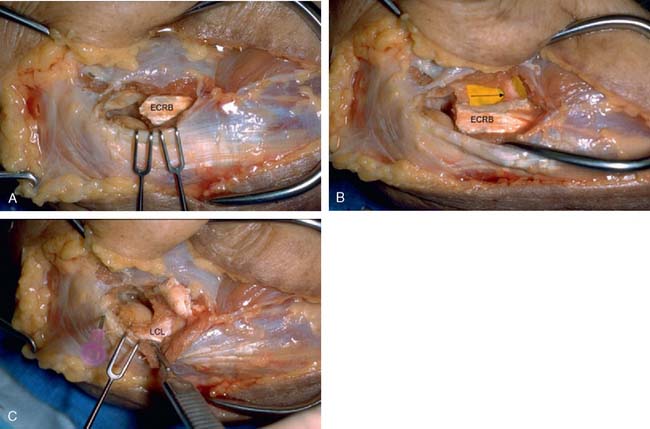
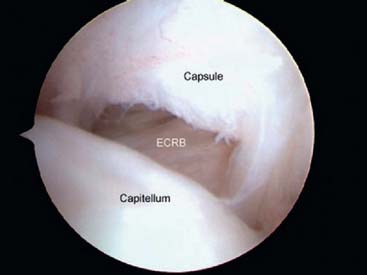
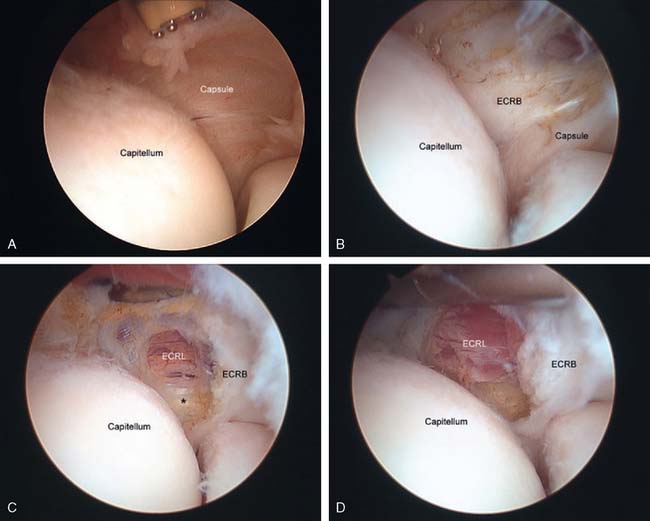
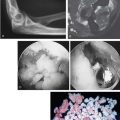
The origin of the ECRL is entirely muscular along the lateral supracondylar ridge of the humerus (Fig. 41-1). The muscle origin has a triangular configuration, with the apex pointing proximally. In contrast, the origin of the ECRB is entirely tendinous. Although it blends with the origin of the extensor digitorum communis (EDC), when dissected from a distal to proximal direction and using the tendon undersurface, it can be separated from the EDC back to the humerus (Fig. 41-2). The anatomic origin of the ECRB is located just beneath the distal most tip of the lateral supracondylar ridge (Fig. 41-3). The footprint is diamond shaped, measuring approximately 13 by 7 mm (Fig. 41-4). At the level of the radiocapitellar joint, the ECRB is intimate with the underlying anterior capsule of the elbow joint, but it is easily separable at this level.4
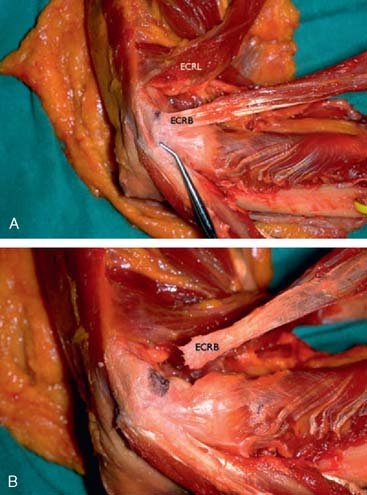
In an effort to develop a strategy for arthroscopic identification and release of the tendon origins, we performed a cadaveric study. The ECRB was identified at the level of the radiocapitellar joint by first separating the ECRL anteriorly from the extensor aponeurosis posteriorly (Fig. 41-5). The EDC origin was then dissected from anterior to posterior to expose the underlying ECRB tendon. The anterior and posterior borders of the ECRB were marked with intra-articular 18-gauge needles, and the ECRL/EDC interval and the skin were repaired (Fig. 41-5).
Dissection of the specimens revealed an intact extensor aponeurosis and a complete release of the underlying ECRB in all cases (Fig. 41-6). The capsule was identified as a separate layer beneath the ECRB release. The collateral ligament origin was not violated, and the PIN was well distal to the release and not compromised in any case (see Fig. 41-6). Using these data, an arthroscopic technique was designed for lateral epicondylar release.4
TECHNIQUE
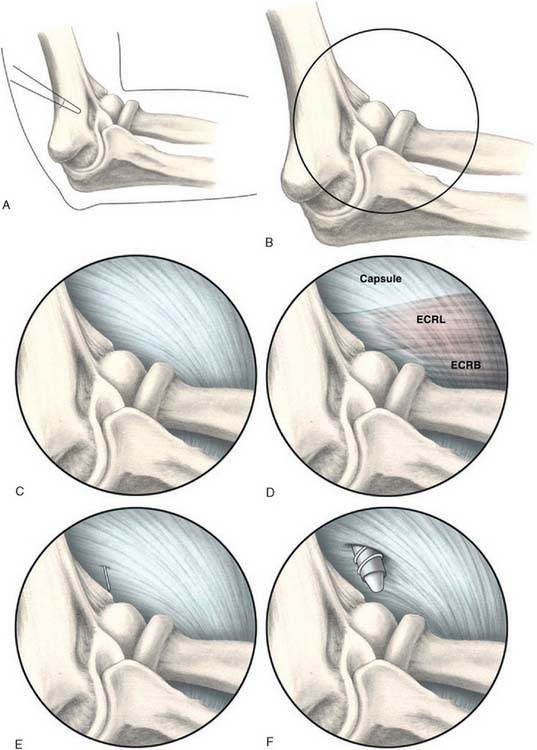
Next, a standard anteromedial portal is established (Fig. 41-7). This is started several centimeters proximal and anterior to the medial epicondyle and well anterior to the palpable intermuscular septum. Care is taken to slide along the anterior humerus, and the joint is entered with a blunt introducer or a switching stick. This medial portal allows one to view the lateral joint including the radial head, capitellum, and the lateral capsule. It is often helpful at this point to open the inflow to allow distension of the capsule. If visualization is a problem, a retractor can be introduced through a proximal anterolateral portal 2 to 3 cm proximal and just anterior to the lateral supracondylar ridge. A simple freer elevator is useful for this purpose. By tensioning the capsule anteriorly, improved visualization of the lateral capsule and soft tissues can be achieved.
A modified anterolateral portal is established using an inside-out technique. This is started 2 to 3 cm above and anterior to the lateral epicondyle (see Fig. 41-7). The portal is slightly more proximal than a standard anterolateral portal. This allows instrumentation down to the tendon origin rather than entering the joint through the ECRB tendon itself. If lateral synovitis is present, this can be débrided with a resector.
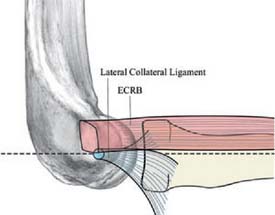
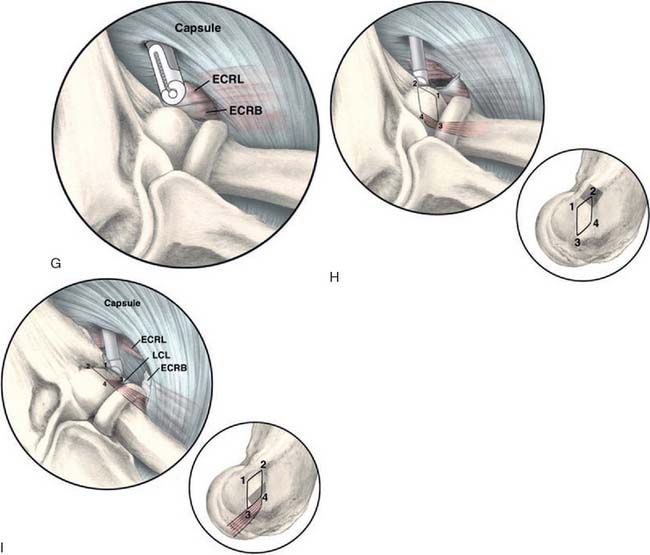
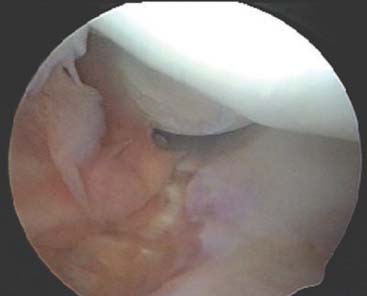
The capsule is next released. Occasionally in epicondylitis, one can find a disruption of the underlying capsule from the humerus (Fig. 41-8). Most commonly, the capsule is intact although small linear tears can be present (Fig. 41-9). We have found it easier to release the lateral soft tissues in layers using a monopolar thermal device. In this way, the capsule is first incised or released from the humerus. When it retracts distally, one can appreciate the ECRB tendon posteriorly and the ECRL, which is principally muscular, more anterior. As noted earlier, the ECRB tendon spans from the top of the capitellum to the midline of the radiocapitellar joint.
Once the capsule is adequately resected, the ECRB origin is released from the epicondyle (see Figs. 41-7 and 41-9). This is started at the top of the capitellum and carried posteriorly. The lateral collateral ligament is not at risk if the release is kept anterior to the midline of the radiocapitellar joint.19 On average, adequate resection of the ECRB must include approximately 13 mm of tendon origin from anterior to posterior.4 Care is taken to drive the scope in adequately to view the release down to the midline of the radiocapitellar joint. Typically, the entire ECRB retracts distally away from the humerus.
Care is taken not to release the extensor aponeurosis, which lies behind the ECRB tendon. This can be visualized as a stripped background of transversely oriented tendon and muscular fibers much less distinct than the ECRB (see Fig. 41-9). It is located posterior to the ECRL, which again is principally muscular in origin. If the aponeurosis is violated, one will débride into the subcutaneous tissue about the lateral elbow.
Discussion
In recent years, there has been an interest in arthroscopic treatment of lateral epicondylitis.1,3,6,11,17,19,21,22 A cadaveric study demonstrated that arthroscopic release of the ECRB was a safe, reliable, and reproducible procedure for refractory lateral epicondylitis.11 However, the results of arthroscopic treatment of this condition have been variable. Tseng reported satisfactory results in 9 of 11 patients.22 However, he also had a 33% complication rate. Stapleton and Baker compared five patients treated arthroscopically with 10 patients treated by open débridement.21 They reported similar results and complication rates between the two groups. Later, Baker et al.1 reported on 39 elbows treated arthroscopically, with 37 reporting being “better” or “much better” at follow-up. Peart et al17 reported on 33 arthroscopic procedures for lateral epicondylitis, with 28% of patients failing to achieve good or excellent outcomes.
The variable results reported using various arthroscopic techniques may be related to increased difficulty in identifying the ECRB origin through the arthroscope.6 The tendon is extra-articular, and capsular release is required to visualize its origin. The tendon footprint is diamond shaped and located between midline of the radiocapitellar joint and the top of the humeral capitellum averaging 13 by 7 mm (see Fig. 41-4). The posterior interosseous nerve should be well medial and distal to the area of dissection. The lateral collateral ligament is not compromised, as long as the release is kept anterior to the midline of the radiocapitellar joint. Care is taken not to release the extensor aponeurosis, which lies superficial to the ECRB tendon.
We reviewed a consecutive series of 36 patients with recalcitrant lateral epicondylitis treated with arthroscopic release using the aforementioned technique.12 There were 24 men and 12 women, with an average age of 42 years at the time of surgery. The cohort had symptoms for an average of 19 months before surgical intervention. Intraoperative findings revealed significant lateral intra-articular synovitis in approximately 30% of patients. Approximately 75% of cases had an intact elbow capsule or a minor linear capsular tear, whereas 25% had a significant proximal capsular disruption. All patients were evaluated by independent examiners for the purposes of this study at a minimum 2-year follow-up. On average, patients required 4 weeks to return to regular activities and 7 weeks to return to full work duties. No major complications were reported. One patient had a neurapraxia of the superficial radial nerve that resolved by 2 weeks postoperatively. The average functional component of the Mayo Elbow Performance Score at follow-up averaged 11.1 out of 12 (range, 5-12). Grip strength averaged 91% of the opposite, uninvolved side. Subjective pain ratings as measured on a visual analog scale improved from 8.5 + 2.4 to 1.9 + 1.3 (P < 0.01). However, 10 patients reported continued pain with strenuous activities and repetitive use of the affected arm. Two patients continued to have significant pain and were considered failures.12
1 Baker C.L., Murphy K.P., Gottlob C.A., Curd D.T. Arthroscopic classification and treatment of lateral epicondylitis: two-year clinical results. J. Shoulder Elbow Surg. 2000;9:475.
2 Bunata R.E., Brown D.S., Capelo R. Anatomic factors related to the cause of tennis elbow. J. Bone Joint Surg. 2007;89A:1955.
3 Cohen M.S., Romeo A.A. Lateral epicondylitis: open and arthroscopic treatment. J. Am. Soc. Surg. Hand. 2001;3:172.
4 Cohen, M. S., Romeo, A. A., Hennigan, S. P., and Gordon, M.: Lateral epicondylitis: anatomic relationships of the extensor tendon origins and implications for arthroscopic treatment. J. Shoulder Elbow Surg. (in press).
5 Coonrad R.W., Hooper W.R. Tennis elbow: its course, natural history, conservative and surgical management. J. Bone Joint Surg. 1973;55A:1177.
6 Cummins C.A. Lateral epicondylitis: in vivo assessment of arthroscopic debridment and correlation with patient outcomes. Am. J. Sports Med. 2006;34:1486.
7 Cyriax J.H. The pathology and treatment of tennis elbow. J. Bone Joint Surg. 1936;18:921.
8 Garden R.S. Tennis elbow. J. Bone Joint Surg. 1961;43-B:100.
9 Gardner R.C. Tennis elbow: diagnosis, pathology, and treatment. Clin. Orthop. Relat. Res. 1970;72:248.
10 Kraushaar B.S., Nirschl R.P. Tendinosis of the elbow (tennis elbow. Clinical features and findings of histological, immunohistochemical, and electron microscopy studies). J. Bone Joint Surg. 1999;81A:259.
11 Kuklo T.R., Taylor K.F., Murphy K.P., Islinger R.B., Heekin R.D., Baker C.L.Jr. Arthroscopic release for lateral epicondylitis: a cadaveric model. Arthroscopy. 1999;15:259.
12 Lattermann, C., Anbari, A., McCarty, L. P., Cole, B. J., Romeo, A. A., and Cohen, M. S.: Three-year follow-up of arthroscopic treatment for recalcitrant lateral epicondylitis, Arthroscopy (under review).
13 Morrey B.F. Reoperation for failed surgical treatment of refractory lateral epicondylitis. J. Shoulder Elbow Surg. 1992;1:47.
14 Nirschl R.P. Elbow tendinosis/tennis elbow. Clin. Sports Med. 1992;11:851.
15 Nirschl R.P., Pettrone F.A. Tennis elbow. The surgical treatment of lateral epicondylitis. J. Bone Joint Surg. 1979;61A:832.
16 Organ S.W., Nirschl R.P., Kraushaar B.S., Guidi E.J. Salvage surgery for lateral tennis elbow. Am. J. Sports Med. 1997;25:746.
17 Peart R.E., Strickler S.S., Schweitzer K.M.Jr. Lateral epicondylitis: a comparative study of open and arthroscopic lateral release. Am. J. Orthop. 2004;33:565.
18 Regan W., Wold L.E., Coonrad R., Morrey B.F. Microscopic histopathology of chronic refractory lateral epicondylitis. Am. J. Sports Med. 1992;20:746.
19 Smith A.M., Castle J.A., Ruch D.S. Arthroscopic resection of the common extensor origin: anatomic considerations. J. Shoulder Elbow Surg. 2003;12:375.
20 Spencer G.E., Herndon C.H. Surgical treatment of epicondylitis. J. Bone Joint Surg. 1953;35-A:421.
21 Stapleton T.R., Baker C.L. Arthroscopic treatment of lateral epicondylitis. Arthroscopy. 1996;10:335.
22 Tseng V. Arthroscopic lateral release for treatment of tennis elbow. Arthroscopy. 1994;10:335.
PART B Arthroscopic Radial Ulnohumeral Ligament Reconstruction for Posterolateral Rotatory Instability of the Elbow
INTRODUCTION
Since the original description by O’Driscoll and Morrey in 1991, there has been increasing attention and research directed toward the diagnosis and management of posterolateral rotatory instability (PLRI) of the elbow. PLRI has been described as an instability pattern of the elbow that results from an incompetent radial ulnohumeral ligament (RUHL).6 Recent anatomic studies have attempted to further define the involved tissue. Dunning et al. state that both the RUHL and the radial collateral ligament must be sectioned to achieve PLRI. They also state that they could not differentiate visually the two ligaments at their humeral origin. They could only differentiate the RUHL from the radial collateral ligament (RCL) by identifying the distal extent of the RUHL at the supinator crest.2 Seki et al10 were able to show that sectioning just the anterior band of the lateral collateral ligament was insufficient to induce instability. These and other more recent studies demonstrate that the entity of PLRI is, in fact, a spectrum of injury.3–5,8,9,12 Although originally described as either the result of an elbow dislocation or due to extensive tennis elbow surgery, these anatomic studies help support the concept that there is a continuum of injury between mild, grade 1 PLRI and frank elbow dislocation.6,11
EXAMINATION
The history is usually one of a minor trauma. The symptoms are vague pain and feelings of minor and inconsistent shifting with certain activities. PLRI is best demonstrated clinically with the pivot shift test of the elbow. This test as first described by O’Driscoll and Morrey may elicit gross instability or simply pain and apprehension.6 Two other very useful clinical tests described by Regan8 include pain when pushing up from an arm chair with the palms facing inward. A second test involves having the patient push up from a prone position first with the forearms maximally pronated and with the thumbs pointing toward each other. Repeating the test with the thumbs pointed outward and the forearms maximally supinated causes symptoms that were not present with the forearms pronated. This can alternately be performed standing while performing a wall pushup.12
Imaging studies for PLRI can be helpful. Radiographs may reveal an avulsion fragment from the humeral lateral epicondyle. However, radiographs often are normal. A stress radiograph or fluoroscopy performed while conducting the pivot shift test will show the radial head and proximal ulnar moving together in a subluxed and posterolaterally rotated position. Examination under anesthesia will show similar findings. Contrast-enhanced magnetic resonance imaging of the elbow has been described to identify a lesion in the RUHL.7 It is our experience that the best interpretation occurs when the radiologist and orthopedist interpret the films together.
Although much has been written about the pathoanatomy and biomechanics of the lesion, little has been reported on the arthroscopic treatment of these patients. Chapter 40 reports on the management by a conventional open procedure, whereas this chapter section reviews the technique and results of the authors’ arthroscopic technique previously described by Smith et al.11
SURGICAL TECHNIQUE
The development of an arthroscopic technique for the treatment of PLRI was described by Smith et al11 in 2001. The instability can be readily seen during arthroscopic evaluation. While viewing from the proximal anteromedial portal, the ulna and radial head can be seen to subluxate posterolaterally during the performance of a pivot shift test. Additionally, an arthroscope in the posterolateral portal placed down the posterolateral gutter can be driven into and across the lateral aspect of the ulnohumeral joint, the arthroscopic “drive through” sign of the elbow, which we believe is analogous to the “drive-through” sign of multidirectional laxity in shoulder instability (Fig. 41-10). This ability to translate the arthroscope through the ulnohumeral joint from lateral to medial is not possible in stable elbows, nor after the instability is corrected in patients with PLRI.
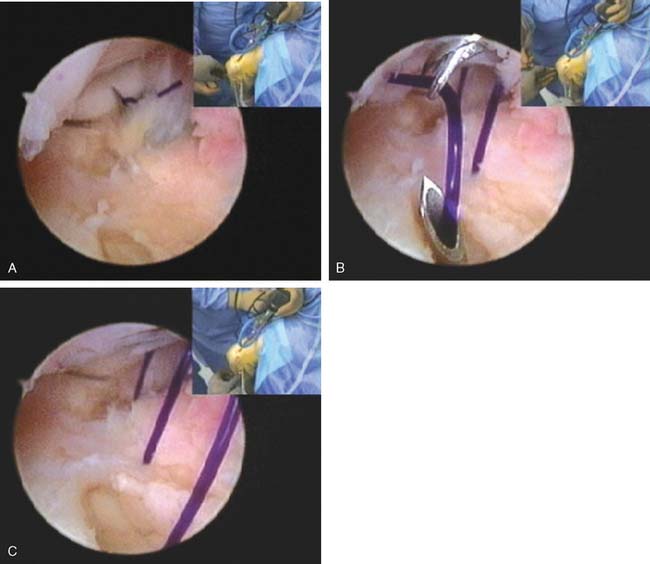
The instability from an injured lateral collateral ligament complex can often be effectively treated with an arthroscopic plication. This is performed by placing six to seven absorbable sutures obliquely from the radial border of the ulna to the radial border of the humerus. The sutures are introduced through a spinal needle. The first two sutures are delivered into the joint through the annular ligament (Fig. 41-11A) and retrieved through the humeral attachment of the capsule. Subsequent sutures are brought into the joint in a progressively more proximal position. Each suture is immediately retrieved with a suture retriever that passes into the joint adjacent to the posterior aspect of the lateral epicondyle (Fig. 41-11B). These sutures are then retrieved subcutaneously through the soft spot skin portal and pulled to tension. The arthroscope is driven out of the lateral gutter as the sutures are tensioned. The sutures are then tied individually from distal to proximal either under or over the anconeus muscle.
If there is a humeral avulsion, a suture anchor is placed under direct arthroscopic visualization into the normal site of origin of the RUHL from the posterior epicondyle. The suture from the anchor is retrieved under all the transfixing sutures before they are tied and are pulled out of the skin distal to the most distal suture. The sutures are then tied and the anchor suture retrieved subcutaneously over the sutures and then tied, pulling the entire reconstructed ligament back to the normal origin (see Fig. 41-11C).
RESULTS
The average follow-up was 41 months (range 12-103 months). Overall, Andrews-Carson scores for all repairs improved from 145 to 180 P < 0.0001.1 Subjective scores from 57 to 85 P < 0.0001 and objective scores improved from 88 to 95 P = 0.008. This study, along with that of Smith et al.11 reveals that arthroscopic plication of the RUHL can be as successful as open repair. Arthroscopic suture plication has become a useful technique in shoulder instability, and this report shows that the same ideas can translate to the elbow. The technique is technically demanding and requires a thorough understanding of elbow anatomy.
1 Andrews J.R., Carson W.G. Arthroscopy of the elbow. Arthroscopy. 1985;1:97.
2 Dunning C.E., Zarzour Z.D., Patterson S.D., Johnson J.A., King G.J. Ligamentous stabilizers against posterolateral rotator instability of the elbow. J. Bone Joint Surg. Am. 2001;83-A:1823.
3 Kalainov D.M., Cohen M.S. Posterolateral rotatory instability of the elbow in association with lateral epicondylitis. A report of three cases. J. Bone Joint Surg. 2005;87A:1120.
4 King G.J., Dunning C.E., Zarzour Z.D., Patterson S.D., Johnson J.A. Single-strand reconstruction of the lateral ulnar collateral ligament restores varus and posterolateral rotatory stability of the elbow J. Shoulder Elbow Surg. 2002;11:60.
5 Mehta J.A., Bain G.I. Posterolateral instability of the elbow. J. Am. Acad. Orthop. Surg. 2004;12:405.
6 O’Driscoll S.W., Bell D.F., Morrey B.F. Posterolateral rotatory instability of the elbow. J. Bone Joint Surg. Am. 1991;73:440.
7 Potter H.G., Weiland A.J., Schatz J.A., Paletta G.A., Hotchkiss R.N. Posterolateral rotator instability of the elbow: usefulness of MR imaging in diagnosis. Radiology. 1997;204:185.
8 Regan W., Lapner P.C. Prospective evaluation of two diagnostic apprehension signs for posterolateral instability of the elbow. J. Shoulder Elbow Surg. 2006;15:344.
9 Sanchez-Sotelo J., Morrey B.F., O’Driscoll S.W. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J. Bone Joint Surg. 2005;87B:54.
10 Seki A., Olsen B.S., Jensen S.L., Eygendaal D., Sojbjerg J.O. Functional anatomy of the lateral collateral ligament complex of the elbow: configuration of Y and its role. J. Shoulder Elbow Surg. 2002;11:53.
11 Smith J.P., Savoie F.H., Field L.D. Posterolateral rotatory instability of the elbow. Clin. Sports Med. 2001;20:47.
12 Yadao M.A., Savoie F.H.3rd, Field L.D. Posterolateral rotatory instability of the elbow. Inst. Course Lect. 2004;53:607.