The annual incidence of out-of-hospital sudden cardiac arrest in the United States is estimated to be 350,000 to 400,000, with approximately 200,000 individuals being treated for in-hospital cardiac arrest. The incidence of ventricular fibrillation (VF) as initial rhythm has declined to less than 30% of the sudden cardiac arrests, both in and out of the hospital, possibly because, as the care of cardiac disease has improved, people who arrest have more severe disease.
Survival to hospital discharge varies greatly among communities in the United States: from 1% to 2% to greater than 20% for patients who receive cardiopulmonary resuscitation (CPR) for any abnormal rhythm out of hospital, and 24% for in-hospital adult cardiac arrests from Get With the Guidelines-Resuscitation 2012 data. The key to successful resuscitation is a well-organized community program, consisting of early recognition and activation, early institution of effective CPR, early defibrillation, and postresuscitation care. Survival has been discouragingly unchanged over decades despite factors such as the number of education programs, the increased availability of defibrillators in public places, and the number of first responders. However, over the last several years, there have been reports of improvements due to changes in CPR, most of which have been compiled in the American Heart Association’s 2010 CPR guidelines, which focus on the most important components of resuscitation—the delivery and effectiveness of those components—and postresuscitation care.
The 2010 guidelines place more emphasis on chest compressions of sufficient depth and rate with minimal interruptions because studies have demonstrated that, in the past, approximately one third of compressions were performed at less than the recommended depth, and pauses in compressions frequently accounted for half of the CPR time. Inadequate compression depth and compression pauses decrease the chance of successful defibrillation occurring, whereas periods of continuous chest compressions before shocks are delivered have been reported to increase the defibrillation success rate for ambulance response times of more than 4 to 5 min. Compressions should be at least 2 inches deep at a rate of 100/min, allowing full chest recoil after each compression.
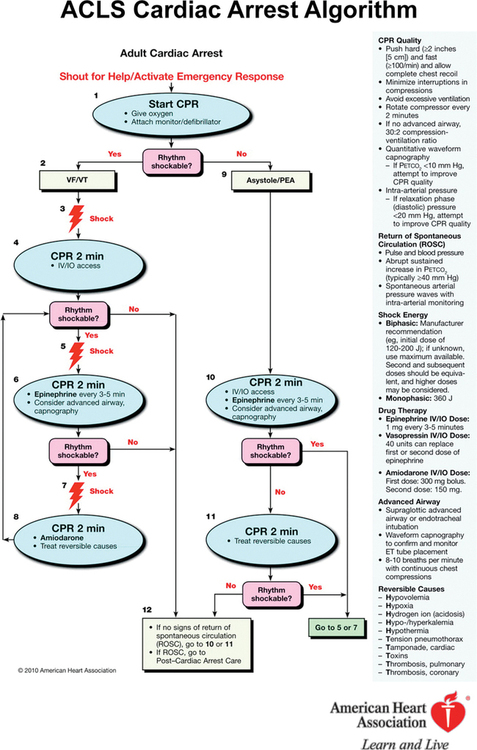
Other changes during the past decade have been in the type of defibrillator used and in the recommendations for defibrillation. Termination of VF is higher with biphasic defibrillators. Delivering only one shock is recommended, not stacks of three, and the shock should be immediately followed by 2 min of CPR before rhythm or pulse checks are performed ( Figure 222-1 ). In patients with continuous electrocardiographic and hemodynamic monitoring (as might occur in the hospital setting), performance of defibrillation may be modified by the physician in charge, especially for arrests of very short duration, such as in the operating room or intensive care unit. If the patient does not have a secured airway, chest compressions and ventilations are given in a 30:2 ratio, but the importance of ventilation is being questioned, particularly for patients with cardiac arrest of cardiac origin. If the patient has an advanced airway device in place (supraglottic airways are increasingly being recommended, particularly for paramedics and emergency medical technicians), compressions are given without pausing for ventilations, which can be interposed at a rate of 8 to 10/min while 100% O2 is delivered. No consensus has been reached on whether the use of mechanical chest compressions or other adjuncts increase survival, as compared with performance of standard manual CPR. To avoid deterioration in the quality of CPR when performing manual CPR, the person performing compressions should change every 2 min (see Figure 222-1 ).
Figure 222-1 Advanced Cardiac Life Support (ACLS) cardiac arrest algorithm. CPR, Cardiopulmonary resuscitation; ET, endotracheal; IO, intraosseous; IV, intravenous; PEA, pulseless electrical activity; VF, ventricular fibrillation; VT, ventricular tachycardia. (Reprinted with permission from 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care, Part 8: Adult Advanced Cardiovascular Life Support. Circulation. 2010;122[Suppl 3]:S729-S767, © 2010 American Heart Association, Inc.)
Both amiodarone, 300 mg, given in shock-refractory VF and epinephrine, 1 mg, given every 3 to 5 min, increase the rate of return of spontaneous circulation (ROSC), but no drug (including these, other vasopressors, fibrinolytic agents, or buffers) administered during CPR has been shown to improve final outcome.
The high rate of noncompliance with CPR guidelines has triggered a search for technologies that provide real-time feedback on CPR effectiveness. During CPR, end-tidal CO2 (ETCO 2 ) correlates more with chest compressions than with adequacy of effective ventilation and is therefore an indicator of cardiac output. In addition, return to normal levels of ETCO 2 during CPR is a good indicator of ROSC. As an indicator of correct tracheal tube placement, ETCO 2 is not a good measure of CO2 returned to the lungs during CPR. For example, ventilation through a tracheal tube placed in the esophagus can result in relatively high ETCO 2 with the initial breaths because it is actually measuring exhaled breath forced into the stomach during mouth-to-mouth resuscitation before the tracheal tube was placed, providing an erroneous measurement, which may be interpreted as correct tube placement. Carotid or femoral pulses cannot be used to indicate adequate coronary perfusion pressure (aortic–right atrial decompression or “diastolic” pressure). With invasive monitoring in place, coronary perfusion pressure can guide therapy and should be kept at a minimum of 15 mm Hg, which has been shown to increase ROSC rate. Most modern defibrillators used for CPR collect data on chest compressions, ventilations, and changes in VF frequency spectra, which are very useful for evaluating CPR quality in debriefing sessions. Some defibrillators use the same data to provide automated real-time auditory and visual feedback during the actual CPR event. Both uses have been shown to improve quality of CPR.
The importance of the postresuscitation phase is increasingly recognized. Therapeutic hypothermia improved neurologic outcome in randomized studies of patients with initial VF who remained unconscious after ROSC. The optimal target temperature or duration is not known, but the present recommendation is to cool to 32º C to 34º C for 12 to 24 h. However, the recommendation may change when new guidelines are developed that include the results of two recent studies that call into question the benefit of hypothermia following cardiac arrest. Few well-controlled studies have evaluated other factors after ROSC, but some hospital systems report doubling of survival to hospital discharge with good neurologic outcome after introduction of well-defined standardized post-ROSC protocols. Such protocols usually recommend (1) using therapeutic hypothermia in all unconscious patients regardless of initial pulseless rhythm if active treatment is required; (2) undertaking coronary angiography with coronary revascularization, as indicated, as soon as possible after ROSC; (3) avoiding high levels of glucose (>180-200 mg/dL); (4) treating seizures recognized clinically or on electroencephalography; and (5) optimizing vital organ perfusion. Reversible postresuscitation myocardial dysfunction (myocardial stunning) is normally present in the early phase after ROSC, requiring several efforts to optimize organ perfusion, such as a positive fluid balance and the use of vasopressor agents, inotropes, an intraaortic balloon pump, or a combination thereof. Echocardiography should initially be undertaken daily. Unless the patient is conscious after ROSC, ventilation should be controlled, but hyperventilation (PaCO 2 < 35 mm Hg) might worsen cerebral perfusion, and, thus, neurologic outcome, and should be avoided. The optimal level of oxygenation has not been established, but with recognized increased production of reactive O2 species in the reperfusion phase, it is reasonable to monitor arterial O2 saturation and avoid hyperoxygenation.
Because unconscious patients after ROSC require high levels of care, prognostication with high predictive accuracy is desirable as early as possible so that futile efforts can be discontinued. Before the introduction of therapeutic hypothermia, absence of corneal or pupillary light reflexes, motor response to pain no better than extension, bilateral absence of somatosensory evoked potentials, or high levels of neuron-specific enolase and protein S-100B 48 to 72 h after cardiac arrest have been used as prognosticators for poor outcome (severe brain damage, permanent coma, or death) with 100% specificity and tight confidence intervals. Studies from the hypothermia era are sparse, but data so far indicate that futility decisions are more difficult and should probably not be undertaken until 72 h after rewarming in still-comatose patients. Self-fulfilling prophecies might lead to early treatment withdrawal in potentially successful survivors.