Chapter 95 Adult Pseudotumor Cerebri Syndrome
Pseudotumor cerebri syndrome (PTCS) is characterized by (1) intracranial pressure (ICP) elevated to at least 250 mm H2O, (2) normal or small-sized ventricles, (3) no evidence of an intracranial mass, and (4) normal cerebrospinal fluid (CSF) composition. Most patients also have papilledema on funduscopic examination.1–3 About 10% of patients with PTCS have an identifiable cause for the condition; however, 90% of patients are young, obese women in whom no definite explanation can be found. Such individuals are said to have idiopathic intracranial hypertension (IIH). As implied by the term “pseudotumor cerebri,” intracranial masses may present in a similar fashion. Patients with PTCS (including those with IIH) commonly present with headaches, tinnitus, horizontal diplopia, and progressive visual field defects. Headache, which is the most common symptom, is present in 94% patients and is dull in character and usually not localized.4 Diurnal variations in headaches with early morning aggravation are common. Straining and maneuvers like Valsalva that elevate ICP also exacerbate headaches. Lhermitte sign (electric shock-like neck pain precipitated by neck flexion) has also been reported.4,5 Pulsatile tinnitus is reported by up to 58% of patients.4 Visual deterioration in patients with IIH occurs almost exclusively in the setting of severe papilledema, beginning with enlargement of the physiologic blind spot and then progressing to nasal constriction, followed by temporal constriction, and, eventually, loss of central vision and color vision. As this occurs, the papilledema resolves, leaving optic atrophy and blindness. Transient visual obscurations occur in about 68% of patients and can be monocular or binocular. They usually occur at the peak of headache and are often precipitated upon bending or stooping.4 Metamorphopsia caused by distortion of the macula due to subretinal collections of fluid from a swollen optic disc usually occurs with very severe and chronic papilledema but may occur earlier in the course of IIH.6
Etiology and Causative Agents
Elevated dural venous pressures leading to disturbances in CSF absorption at the level of the arachnoid granulations is a widely proposed pathophysiology for IIH.7 Secondary intracranial hypertension as a cause of PTCS has been reported to occur following cerebral venous thrombosis in thrombophilic states, such as systemic lupus erythematosus (SLE),8 protein C and protein S deficiencies,9,10 malignancies, oral contraceptive use,11 pregnancy, polycystic ovary disease,9 and from infections including meningitis and mastoiditis. Jugular valve insufficiency has also been found in association with PTCS/IIH.12 Thrombophlebitis due to an indwelling central venous catheter has also been reported to cause PTCS.13 Other associations with PTCS include metabolic and endocrine disturbances/conditions such as Addison disease, hyperthyroidism, hypothyroidism, menarche, and pregnancy. A variety of drugs have been implicated in the development of PTCS, most commonly tetracycline, vitamin A, and lithium carbonate (Table 95-1).
| Metabolic Disorders and Endocrinopathies |
| Pseudohypoparathyroidism |
| Hypophosphatasia |
| Prolonged corticosteroid therapy or too rapid corticosteroid withdrawal |
| Addison disease |
| Galactosemia |
| Pregnancy |
| Oral Contraceptives |
| Anabolic Steroids |
| Norplant System (Levonorgestrel Implants) |
| Drug Toxicities |
| Nalidixic acid |
| Doxycycline |
| Minocycline |
| Hypervitaminosis A |
| Lithium |
| Clomiphene |
| Venous Sinus Thrombosis and Elevated Central Venous Pressures |
| Guillain-Barré syndrome |
| Chronic otitis media and mastoiditis |
| Sinusitis |
| Jugular venous thrombosis |
| Thrombophilic States (Including Hematologic Disorders and Hyperestrogenic States) |
| Systemic lupus erythematosus (SLE) |
| Chronic obstructive pulmonary disease (COPD) |
| Anemia |
| Iron deficiency anemia |
| Acquired aplastic anemia |
| Paroxysmal nocturnal hemoglobinuria |
| Megaloblastic anemia |
| Sickle cell disease |
| Wiskott-Aldrich syndrome |
| Other |
| Head injury |
| Sleep apnea |
Diagnosis
Patients who present with headaches with features suggestive of elevated ICP should undergo a funduscopic examination to assess the optic discs for papilledema. Magnetic resonance imaging (MRI) or computed tomographic (CT) scanning should be performed in all patients with papilledema to evaluate for intracranial masses and to assess ventricular size. Patients with increased ICP may not only have normal or small-sized ventricles,14 but those with long-standing increased ICP may have an empty appearance of the sella turcica (26.7% sensitive, 94.6% specific), and patients with severe papilledema may show flattening of the posterior aspect of the eye (43.3% sensitive, 100% specific) (Fig. 95-1).15 The diagnosis of IIH thus should be suspected when no intracranial lesion is found and the ventricles are not dilated. In addition, MR or CT venography should be performed to determine if venous sinus stenosis or thrombosis is present, even if the patient appears to have a physical appearance typical of IIH. Neurologic examination in patients with PTCS/IIH usually reveals no focal deficits except for occasional unilateral or bilateral sixth nerve pareses.
A lumbar puncture to measure opening pressure and to assess CSF content is required to establish the diagnosis of PTCS/IIH. It is important however, that no patient undergoes lumbar puncture until an MRI or CT scan of the brain is performed to exclude an intracranial mass. CSF opening pressures greater than 250 mm H2O and normal CSF composition and cytology are required for diagnosis. Lumbar opening pressures should be always be measured with patients lying in the lateral decubitus position with legs extended and relaxed. Patients lying prone during the insertion of the LP needle should be repositioned to the lateral decubitus position before opening pressures are measured to prevent falsely high measurements that result from an increase in abdominal pressure when patients lie prone.1,3 This precaution should be observed especially when a lumbar drain is placed under fluoroscopic guidance during which patients are typically positioned prone. Similarly, patients whose lumbar puncture is performed with the patient sitting upright should be repositioned to the lateral decubitus position, as measurements of ICP in the sitting position are different from those in the lateral decubitus position, because of the relative positions of the heart and head.
Frequently, a single measurement of opening pressure and the presence of other clinical features fulfilling the criteria described above are sufficient to diagnose a patient with PTCS or IIH; however, patients with atypical presentations, such as chronic persistent headaches without papilledema and normal opening pressures, but in whom there is a high suspicion of IIH (obese patients or those with other conditions associated with IIH), require a 24- to 48-hour continuous recording of ICP, as intermittent elevations of ICP, mostly during sleep, can occur.16,17
Management
The treatment approach to patients of IIH is determined based on assessment of visual function. Weight loss, salt restriction, and analgesics for headaches should be recommended to patients with mild-to-moderate papilledema, but without any decrease in visual acuity or impairment of visual fields other than enlargement of the blind spot. Although weight loss is often difficult to achieve, it is clearly beneficial in attaining control of headaches and visual symptoms. Kupersmith et al. reported improvement in headaches and papilledema in 58 obese females with IIH who lost 7% to 10% of their weight over a 3-month period, and others have reported similar results with surgically produced weight loss.18,19 Acetazolamide should be initiated at doses of 250 mg QID or 500 mg (sequels) BID to lower ICP and decrease papilledema. Paresthesias, dizziness, dysgusia, and so on are common side effects of acetazolamide and may limit compliance or any increment in dose, particularly if patients are not warned about these effects before starting the drug. Visual function and fundus appearance should be monitored at regular intervals in all patients on medical treatment, so that any evidence of onset of progression of papilledema or development or progression of optic nerve dysfunction can be detected and treated with ONSF or shunt surgery. Patients with more severe papilledema or rapid decline in visual function should be monitored more frequently (e.g., every 1 to 2 weeks) than other patients. In such patients, the dose of acetazolamide may be increased up to 4 g/day. Some physicians are reluctant to use acetazolamide in patients with a sulfa allergy; however, this drug is not the same as typical sulfa drugs, and there is no evidence that patients allergic to sulfa drugs will have a similar allergic response to acetazolamide. For patients who cannot be treated with, cannot tolerate, or do not respond to acetazolamide, furosemide, triamterene or spironolactone may be used or added to the regimen. Topiramate is an antiepileptic agent that is being increasingly used in patients with IIH for its ability to lower ICP, reduce weight, and control headache, but its efficacy is not yet proven. A single open-label study reported equivalent visual field outcomes for topiramate and acetazolamide.32 Corticosteroids may be added to regimens of patients with autoimmune conditions such as sarcoidosis or SLE or in patients with recent venous sinus thrombosis; however, they have no place in the treatment of typical IIH and may, in fact, aggravate the condition by causing weight gain. In addition, withdrawal of steroids prescribed for other reasons has been associated with the development of PTCS.
In patients with moderate or severe papilledema who experience deterioration of visual acuity or worsening of their visual fields (other than enlargement of the blind spot) despite medical therapy or who cannot tolerate medical therapy, consideration should be given to more aggressive therapy. Surgical approaches to treatment of IIH include CSF diversion to lower ICP, ONSF, stenting of venous sinuses, and bariatric surgery for obese patients. Surgical treatment also should be considered in patients who are noncompliant with medications and in those with uncertain follow-up.
Shunt Surgery
Shunt surgery allows egress of CSF, thereby normalizing ICP, improving visual symptoms, and resolving papilledema. Reversible causes of PTCS should be investigated and treated before shunt surgery is considered. Shunt placement, in addition to preserving or improving vision, also benefits other symptoms of PTCS, particularly headache. This is not the case with ONSF, which, although it may result in resolution of papilledema, may not improve the headaches associated with PTCS. Lumboperitoneal (LP) shunts are preferred by some neurosurgeons, as it is relatively easier to access the lumbar subarachnoid space than to place a shunt in small or normal-sized ventricles as are present in patients with PTCS; however, several authors have reported a high incidence of tonsillar herniation following LP shunts, higher failure rates than occurring with other types of shunts,20,21 and complication of the proximal end of the shunt, such as radiculopathy. LP shunts are also associated with other complications commonly associated with other types of shunts, such as infection and malposition.
Tulipan et al. reported that ventricular shunts can be accurately placed under stereotactic guidance in patients of IIH.22 In their series, ventriculoperitoneal (VP) shunts were successfully placed in seven patients who had failed medical therapy for IIH without any complications. Papilledema resolved in five patients, and two patients had mild residual papilledema at last follow-up. Headaches resolved in all but one patient whose headache persisted despite a patent shunt. Bynke et al. reported successful insertion of a VP shunt in 17 patients without any stereotactic guidance.23 Papilledema resolved within 0.6 to 7 months (mean, 3.1 months) in all patients, and visual field defects disappeared in 11 eyes, were better than prior to shunt placement in 11 eyes, and stabilized in 12 eyes. Over the follow-up period, seven patients required nine revisions, one because of peritoneal malposition, two due to suspected shunt infection, and six for shunt obstruction.
In our experience of 115 shunts placed in 42 patients between 1973 and 2003, 36 ventricular shunts were successfully placed under stereotactic guidance, with no procedure having to be aborted. Ventricular and lumbar shunts were equally efficacious in resolving headache and visual symptoms, with 95% of patients responding in 1 month. Headaches and visual symptoms responded to shunting best in patients with shorter duration of symptoms. Revision rates were higher for lumbar shunts (86%) than for ventricular shunts (44%) over a mean follow-up of 49 months. The risk of LP shunt revision due to obstruction was three times that for ventricular shunts, even though both shunt types were similar as regards other causes of malfunction. The overall incidence of shunt overdrainage was 14%, distal catheter migration was 5%, shunt infection was 3.5% and CSF leakage was 3%. Severe complications such as back pain (possibly due to radiculopathy) and tonsillar herniation were observed in 4% and 5% of patients, respectively.24 We therefore prefer ventricular shunts over lumbar shunts as they are less likely to become obstructed and are free from severe complications such as tonsillar herniation. Ventricular shunts can be placed in ventriculoatrial (VA) or ventriculoperitoneal configurations. VP shunts are preferred over VA shunts by some surgeons as the latter may cause complications such as arrhythmias, thromboembolism, pulmonary hypertension, and perforation. Furthermore, migration of the distal catheter or malposition of the catheter into the superior vena cava or innominate artery may also occur with VA shunts. VA shunt placement is therefore recommended primarily in patients in whom poor peritoneal absorption is suspected, such as patients with a history of peritonitis, abdominal surgery, or abdominal or pelvic masses. In our experience, however, VA shunts are safe and have acceptable rates of complications, including obstruction (22%), infection (5.5%), malposition/migration (5.5%), and CSF leakage (0.9%). VP shunts are associated with similar risks of obstruction and malposition,23 as well as some uncommon complications such as abdominal pain due to peritoneal irritation and, rarely, perforation. Rare instances of delayed intra-cerebral hemorrhage following insertion of ventricular shunts have been reported from 5th to 18th postoperative day.25–27 The site of hemorrhage is often in the parenchyma surrounding the catheter but may occur at any site. The hemorrhage can be caused by diverse etiologies such as bleeding disorders, anticoagulation, shunt-induced disseminated intravascular coagulopathy (DIC) related to the release of plasminogen activators from the choroid plexus in response to shunting, erosion of the parenchymal vessels by the catheter due to transmitted pulsations from the CSF, and arterial vasculature, bleeding from an occult vascular aneurysm, and so on.25,28
Despite the specific features of most low-pressure headaches, it often is difficult to distinguish low-pressure conditions from shunt obstruction using clinical assessment and results of imaging. Under such circumstances, it is important to perform a diagnostic procedure such as a radionuclide shunt patency study (described later) or ICP monitoring. Radionuclide studies can evaluate accurately the patency of the distal catheter; however, they cannot diagnose overdrainage, which often requires ICP monitoring. ICP recordings within the normal range suggest a normal-functioning shunt, in which case, other causes of headaches should be considered and evaluated. Alternatively, in an emergency setting where suspicion of shunt obstruction and visual deterioration is feared, a lumbar tap will not only aid diagnosis by measuring lumbar CSF opening pressure but also provide relief from symptoms until shunt revision is performed.
Optic Nerve Sheath Fenestration
Optic nerve sheath fenestration decompresses the optic nerve by making one or more openings (or a window) in the dura and arachnoid of the optic nerve, and usually results in resolution of papilledema.29–32 Banta et al. described the outcomes of 158 ONSFs performed in 86 patients with IIH.29 Improvement or stabilization of visual acuity and visual fields was observed in 94% and 88% patients, respectively. Complications were often self-limiting, the most common being diplopia (34.86%) and anisocoria (7.5%). One patient had total loss of vision and ophthalmoplegia following surgery, which resolved after a month with corticosteroid treatment, whereas another patient had worse vision postoperatively due to intraoperative damage to the optic nerve. Sergott et al.32 reported the use of a modified ONSF wherein multiple longitudinal fenestrations were created and subarachnoid adhesions lysed. These authors noted improvement in 21 of 23 patients (91.3%) with IIH who underwent the procedure for progressive decline of vision in one or both eyes. Two patients who did not respond to initial surgery improved following the modified procedure. Interestingly, 12 of the patients had improvement in both eyes following unilateral ONSF,32 presumably because the procedure resulted not only in decompression of the operated optic nerve but also reduction in ICP. It is therefore recommended that most patients with bilateral papilledema undergo unilateral surgery first, with the contralateral eye being operated on only after observing the response of both eyes to the first surgery.
Despite the observations described previously and although ONSF is usually an efficacious treatment for visual symptoms in patients with IIH, it does not always reduce ICP or relieve headaches. Thus, concurrent medical treatment with acetazolamide and analgesics or even shunt surgery may be required in some cases. As both ONSF and shunt surgery can prevent loss of vision, either of the two can be offered as treatment; however, ONSF is preferred in patients with predominantly visual manifestations and mild-to-moderate headaches that do not require treatment or that can be managed medically. Early failure of ONSF characterized by progressive loss of vision may occur immediately or within a month after surgery due to intraoperative bleeding into the sheath and formation of a fibrous plug that occludes the surgically made openings.33 These can sometimes be treated with corticosteroids; however, in other cases, shunt placement is required. Mauriello et al. described five patients who experienced loss of vision shortly after ONSF.33 One patient who presented with loss of vision on the sixth postoperative day had restoration of normal vision after treatment with high-dose corticosteroids followed by LP shunt placement. A second patient presented with visual loss and conjunctival injection on the third postoperative day and recovered after treatment for presumed infectious optic neuropathy. The vision stabilized after insertion of an LP shunt in three patients who had gradual loss of vision. Progressive visual loss despite a functioning shunt also can be successfully treated with ONSF.34 The complications of ONSF include blindness from damage to the optic nerve, diplopia, bleeding, and infection, among others. Feldon et al. performed a meta-analysis of the visual outcome of 423 eyes in 252 patients who underwent ONSF for IIH.35 Visual improvement occurred in 80% of patients over a mean follow-up of 21.1 months. Specifically, visual acuity improved in 113 eyes (50%), and visual field deficits improved in 226 (72%) eyes. Worsening of vision was observed in 42 (10%) eyes. Further surgery was required in 50 (12%) eyes, including repeat ONSF in 26 (6%) cases, shunt surgery in 19 (4%) cases, and both ONSF and shunt surgery in four (1%) cases.
Long-term failure of ONSF is common due to occlusion of the openings by scarring and formation of subarachnoid adhesions. Spoor and McHenry reported a series of 75 eyes in 54 patients.31 In their series, 24 (32%) eyes in 20 patients failed during 6 to 60 months of follow-up. The authors calculated a 35% failure rate within 5 years of surgery. All patients were treated with repeat procedures, resulting in visual improvement in 5 (21%) eyes, stabilization in 13 (54%) eyes, and deterioration in 6 (25%) eyes. Spoor and McHenry emphasized that such repeat procedures are difficult to perform with less favorable visual outcomes than occur with primary ONSF.31 In addition, they pointed out that in rare cases, particularly those in which severe visual loss has been present for some time, visual function may not improve despite CSF diversion after a failed ONSF.31
Venous Sinus Stenting
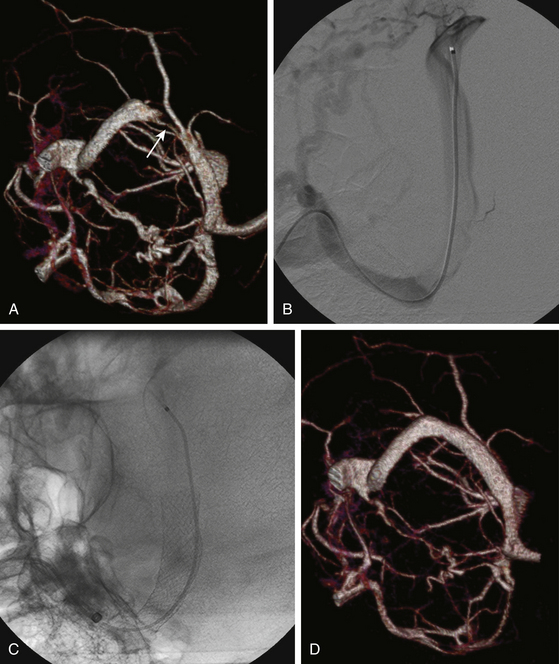
Elevation of intracranial venous pressure is known to raise ICP by impeding CSF absorption. Stenosis of transverse venous sinuses and, to a lesser extent, the superior sagittal sinus, increasingly is being diagnosed in patients with otherwise typical IIH, and it is believed by many that stenting of such sinuses may directly address the primary pathology of IIH. Bussiere et al. reported transverse sinus stenosis in 13 patients with IIH, 10 of whom underwent stenting of the affected sinus.36 Nine patients had successful stent placement via femoral access. One patient required a temporo-occipital craniectomy to place the stent after an unsuccessful attempt to stent via femoral route due to undue tortuosity of sigmoid-jugular junction. Immediate improvement in symptoms was observed in four patients whereas others improved gradually over subsequent 2 to 6 weeks. Almost complete resolution of papilledema was seen in eight patients and significantly improved in two others. Significant improvement of visual acuity occurred in three patients who had pretreatment decline in vision. One patient did not improve significantly due to pre-existing optic nerve atrophy.
Higgins et al. diagnosed venous sinus stenosis associated with an elevated, trans-stenosis pressure gradient in 12 patients with refractory IIH using MRV.37 All 12 patients underwent dilation and stenting of the stenosed segment of the sinus. Complete resolution of clinical symptoms occurred in 5 patients, 2 patients improved, and 5 remained unchanged over the follow-up period. Furthermore, of 8 patients with papilledema at baseline, 4 (50%) had resolution of disc swelling and 2 had a decrease in swelling. No clinical response was noted in 4 patients whose papilledema failed to resolve after stenting even though measurements of sinus venous pressure before and after stenting showed resolution of venous hypertension in all 8 patients.
Similar to these findings, Owler et al. reported the presence of venous sinus stenosis in five of nine patients with presumed IIH who underwent direct retrograde cerebral venography.38 Stenting of the stenosed segment was performed in four patients, all of whom experienced improvement in vision and other symptoms related to increased ICP. These and other series underscore the high prevalence of venous sinus stenosis and the potential utility of stenting these patients.
Agid R., Farb R., Willinsky R., et al. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology. 2006;48:521-527.
Banta J., Farris B. Pseudotumor cerebri and optic nerve sheath decompression. Ophthalmology. 2000;107:1907-1912.
Bynke G., Zemack G., Bynke H., Romner B. Ventriculoperitoneal shunting for idiopathic intracranial hypertension. Neurology. 2004;63:1314-1316.
Chumas P., et al. Tonsillar herniation: the rule rather than the exception after lumboperitoneal shunting in the pediatric population. J Neurosurg. 1993;78:568-573.
Corbett J., Mehta M. Cerebrospinal fluid pressure in normal obese subjects and patients with pseudotumor cerebri. Neurology. 1983;33:1386-1388.
Feldon S. Visual outcomes comparing surgical techniques for management of severe idiopathic intracranial hypertension. Neurosurg Focus. 2007;23:E6.
Friedman D., Jacobson D. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492-1495.
Giuseffi V., Wall M., Siegel P., Rojas P. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): a case-control study. Neurology. 1991;41:239-244.
Higgins J., Cousins C., Owler B., et al. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry. 2003;74:1662-1666.
Karabatsou K., Quigley G., Buxton N., et al. Lumboperitoneal shunts: are the complications acceptable? Acta Neurochir (Wien). 2004;146:1193-1197.
Karahalios D., Rekate H., Khayata M., Apostolides P. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology. 1996;46:198-202.
Kelman S., et al. Modified optic nerve decompression in patients with functioning lumboperitoneal shunts and progressive visual loss. Ophthalmology. 1991;98:1449-1453.
Kelman S., Heaps R., Wolf A., Elman M. Optic nerve decompression surgery improves visual function in patients with pseudotumor cerebri. Neurosurgery. 1992;30:391-395.
Kupersmith M., et al. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50:1094-1098.
Lim M., Pushparajah K., Jan W., et al. Magnetic resonance imaging changes in idiopathic intracranial hypertension in children. J Child Neurol. 2010;25(3):294-299.
Mascalchi M. Delayed intracerebral hemorrhage after CSF shunt for communicating “normal-pressure” hydrocephalus. Case report. Ital J Neurol Sci. 1991;12:109-112.
Mauriello J.J., Shaderowfsky P., Gizzi M., Frohman L. Management of visual loss after optic nerve sheath decompression in patients with pseudotumor cerebri. Ophthalmology. 1995;102:441-445.
McGirt M., et al. Cerebrospinal fluid shunt placement for pseudotumor cerebri-associated intractable headache: predictors of treatment response and an analysis of long-term outcomes. J Neurosurg. 2004;101:627-632.
Owler B., et al. Pseudotumor cerebri syndrome: venous sinus obstruction and its treatment with stent placement. J Neurosurg. 2003;98:1045-1055.
Sergott R., Savino P., Bosley T. Modified optic nerve sheath decompression provides long-term visual improvement for pseudotumor cerebri. Arch Ophthalmol. 1988;106:1384-1390.
Spence J., Amacher A., Willis N. Benign intracranial hypertension without papilledema: role of 24-hour cerebrospinal fluid pressure monitoring in diagnosis and management. Neurosurgery. 1980;7:326-336.
Spoor T., McHenry J. Long-term effectiveness of optic nerve sheath decompression for pseudotumor cerebri. Arch Ophthalmol. 1993;111:632-635.
Sugerman H., Felton W.R., Salvant J.J., et al. Effects of surgically induced weight loss on idiopathic intracranial hypertension in morbid obesity. Neurology. 1995;45:1655-1659.
Torbey M., Geocadin R., Razumovsky A., et al. Utility of CSF pressure monitoring to identify idiopathic intracranial hypertension without papilledema in patients with chronic daily headache. Cephalalgia. 2004;24:495-502.
Whiteley W., Al-Shahi R., Warlow C., et al. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67:1690-1691.
1. Whiteley W., Al-Shahi R., Warlow C., et al. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67:1690-1691.
2. Corbett J., Mehta M. Cerebrospinal fluid pressure in normal obese subjects and patients with pseudotumor cerebri. Neurology. 1983;33:1386-1388.
3. Friedman D., Jacobson D. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492-1495.
4. Giuseffi V., Wall M., Siegel P., Rojas P. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): a case-control study. Neurology. 1991;41:239-244.
5. Comabella M., Montalbán J., Lozano M., Codina A. Lhermitte’s sign in pseudotumour cerebri. J Neurol. 1995;242:610-611.
6. Warner J., Katz B. Metamorphopsia as an initial complaint of idiopathic intracranial hypertension. Arch Ophthalmol. 2005;123:1003-1006.
7. Karahalios D., Rekate H., Khayata M., Apostolides P. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology. 1996;46:198-202.
8. Dave S., Longmuir R., Shah V., et al. Intracranial hypertension in systemic lupus erythematosus. Semin Ophthalmol. 2008;23:127-133.
9. Glueck C., Iyengar S., Goldenberg N., et al. Idiopathic intracranial hypertension: associations with coagulation disorders and polycystic-ovary syndrome. J Lab Clin Med. 2003;142:35-45.
10. Confavreux C., et al. Congenital protein C deficiency and superior sagittal sinus thrombosis causing isolated intracranial hypertension. J Neurol Neurosurg Psychiatry. 1994;57:655-657.
11. Walsh F., Clark D., Thompson R., Nicholson D. Oral contraceptives and neuro-ophthalmologic interest. Arch Ophthalmol. 1965;74:628-640.
12. Nedelmann M., Kaps M., Mueller-Forell W. Venous obstruction and jugular valve insufficiency in idiopathic intracranial hypertension. J Neurol. 2009;256:964-969.
13. Birdwell B., Yeager R., Whitsett T. Pseudotumor cerebri. A complication of catheter-induced subclavian vein thrombosis. Arch Intern Med. 1994;154:808-811.
14. Lim M., Pushparajah K., Jan W., et al. Magnetic resonance imaging changes in idiopathic intracranial hypertension in children. J Child Neurol. 2010;25:294-299.
15. Agid R., Farb R., Willinsky R., et al. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology. 2006;48:521-527.
16. Torbey M., Geocadin R., Razumovsky A., et al. Utility of CSF pressure monitoring to identify idiopathic intracranial hypertension without papilledema in patients with chronic daily headache. Cephalalgia. 2004;24:495-502.
17. Spence J., Amacher A., Willis N. Benign intracranial hypertension without papilledema: role of 24-hour cerebrospinal fluid pressure monitoring in diagnosis and management. Neurosurgery. 1980;7:326-336.
18. Sugerman H., Felton W.r., Salvant J.J., et al. Effects of surgically induced weight loss on idiopathic intracranial hypertension in morbid obesity. Neurology. 1995;45:1655-1659.
19. Kupersmith M., et al. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50:1094-1098.
20. Chumas P., et al. Tonsillar herniation: the rule rather than the exception after lumboperitoneal shunting in the pediatric population. J Neurosurg. 1993;78:568-573.
21. Karabatsou K., Quigley G., Buxton N., et al. Lumboperitoneal shunts: are the complications acceptable? Acta Neurochir (Wien). 2004;146:1193-1197.
22. Tulipan N., Lavin P., Copeland M. Stereotactic ventriculoperitoneal shunt for idiopathic intracranial hypertension: technical note. Neurosurgery. 1998;43:175-176. discussion 176-177
23. Bynke G., Zemack G., Bynke H., Romner B. Ventriculoperitoneal shunting for idiopathic intracranial hypertension. Neurology. 2004;63:1314-1316.
24. McGirt M., et al. Cerebrospinal fluid shunt placement for pseudotumor cerebri-associated intractable headache: predictors of treatment response and an analysis of long-term outcomes. J Neurosurg. 2004;101:627-632.
25. Alcázar L., et al. Delayed intracerebral hemorrhage after ventriculoperitoneal shunt insertion. Case report and literature review. Neurocirugia (Astur). 2007;18:128-133.
26. Mascalchi M. Delayed intracerebral hemorrhage after CSF shunt for communicating “normal-pressure” hydrocephalus. Case report. Ital J Neurol Sci. 1991;12:109-112.
27. Peker S., Özduman K., Özgen S., Pamir M.N. Delayed intracerebral hemorrhage after ventriculoperitoneal shunting. Marmara Med J. 2004;17:28-31.
28. Snow R., Zimmerman R., Devinsky O. Delayed intracerebral hemorrhage after ventriculoperitoneal shunting. Neurosurgery. 1986;19:305-307.
29. Banta J., Farris B. Pseudotumor cerebri and optic nerve sheath decompression. Ophthalmology. 2000;107:1907-1912.
30. Kelman S., Heaps R., Wolf A., Elman M. Optic nerve decompression surgery improves visual function in patients with pseudotumor cerebri. Neurosurgery. 1992;30:391-395.
31. Spoor T., McHenry J. Long-term effectiveness of optic nerve sheath decompression for pseudotumor cerebri. Arch Ophthalmol. 1993;111:632-635.
32. Sergott R., Savino P., Bosley T. Modified optic nerve sheath decompression provides long-term visual improvement for pseudotumor cerebri. Arch Ophthalmol. 1988;106:1384-1390.
33. Mauriello J.J., Shaderowfsky P., Gizzi M., Frohman L. Management of visual loss after optic nerve sheath decompression in patients with pseudotumor cerebri. Ophthalmology. 1995;102:441-445.
34. Kelman S., et al. Modified optic nerve decompression in patients with functioning lumboperitoneal shunts and progressive visual loss. Ophthalmology. 1991;98:1449-1453.
35. Feldon S. Visual outcomes comparing surgical techniques for management of severe idiopathic intracranial hypertension. Neurosurg Focus. 2007;23:E6.
36. Bussière M., et al. Unilateral transverse sinus stenting of patients with idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2010;31:645-650.
37. Higgins J., Cousins C., Owler B., et al. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry. 2003;74:1662-1666.
38. Owler B., et al. Pseudotumor cerebri syndrome: venous sinus obstruction and its treatment with stent placement. J Neurosurg. 2003;98:1045-1055.